Entrenamiento de las aptitudes sociales para el Trastorno de Hiperactividad y Déficit de Atención (THDA) en niños de cinco a 18 años de edad
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised Clinical Trial | |
| Participants | 103 children( age 7.0 ‐ 9.9 years) participated in the study. Sex: 93% boys, 7% girls Ethnicity: 84% white, 13% African American, 2% Hispanic, and 1% other. Sample size calculation is not reported. Comorbidity: 53.4% had oppositional defiant disorder, 30% had conduct disorder, 16.5% anxiety disorder. All the participants in the study received psychostimulant medication. 84 of the children (81.2%) lived with both parents, 13 (12.6%) with one parent and 6 (5.8%) with their mother and stepfather. Setting: Outpatient clinic in two large medicals Centre in New York and Montreal. Baseline between group differences: No differences except on socioeconomic status, where there were differences between the M alone and M + ACT. Medications for comorbid disorders: no information Inclusion criteria: 1) A diagnosis of ADHD based on the DISC‐P2 conducted by a clinical psychologist. The diagnosis had to be confirmed by a child psychiatrist based on a comprehensive clinical interview with the child, and parent and teacher reports. The children had to, on two different occasions, receive a mean teacher rating of at least 1.5 on the hyperactivity factor or the hyperactivity index of the Conners Teachers Rating Scale. 2) Children had to be medication free for at last 2 weeks before evaluation. 3) Normal IQ (i.e.,WISC‐R ≥ 85). 4) Living with at least one parent, and have telephone access. 5) Positive response to methylphenidate. Exclusion criteria: 1) Children with diagnosable neurological disorders. 2) Psychosis. 3) Significant medical illness. 4) Current physical or sexual abuse. 5) Chronic tic disorder or Tourette´s disorder. 6) DSM‐III‐R based developmental reading or arithmetic disorder, defined as a standard score in reading or mathematics on the Kaufmann Test of Educational Achievement of 85 or less. 7) Children with a diagnosis of conduct disorder. | |
| Interventions | Number of participants allocated per group: 34 children were randomised to methylphenidate (M), 34 to M+ MultiModal Psychosocial Treatment (MPT), and 35 to M+ Attention Control Treatment (ACT). Number of patients lost to follow up per group: 22 children failed to complete the study, 10 from the M alone group, 6 from the combined M+MPT group, and 6 from the M+ ACT group. Format and duration of the intervention: Duration of the trial: 2 years. M+MPT= Methylphenidate + Parent training/family therapy, academic organizational skills training, individualized academic assistance, academic remediation (when necessary), social skills training, and individual psychotherapy. All the treatment modules were fully manual‐based and the manual was developed before the start of the study. Each component was delivered once a week in the first year and once monthly during the second year. M+MPT= Methylphenidate + attention control program (delivered once a week in the first year and once monthly during the second year.) A 75% of attendance was required. Content of the intervention: The Multimodal Psychosocial Treatment (MPT) was manual‐based and integrated several treatment components. Children received individualized academic assistance, organizational skills training, individual psychotherapy, social skills training and, when necessary, reading remediation. Parents received parent management training and counselling. Daily report cards were completed by teachers and formed the basis for a home‐based reinforcement program for targeted school behaviour and academic performance. The Attention Control Psychosocial Treatment consisted of components parallel to those in the MPT but excluded the therapeutic content. Medication: The medical treatment consisted of a medical manual and efforts was made to give each child a maximal dose of methylphenidate. There was a five week open methylphenidate titration trial before randomisation. No between groups difference were found in the methylphenidate medication. | |
| Outcomes | Parent rated: Hyperkinesis Index from Conners Parent Rating Scale. Home situations questionnaire (parents). SSRS ‐ Social Skills Rating Scale(parents). Teacher rated: Hyperactivity and Conduct Problems Factors from the Conners Teacher Rating Scale. School Situations Questionnaire (teachers). Children rated: SSRS ‐ Social Skills Rating Scale (children). Clinician rated: Child Psychiatrists completed a DSM‐III R checklist for ADHD, ODD, conduct disorder symptoms, and a C‐GAS. Observations: School Observations: The Classroom Observation Code. CTRS Hyperkinesis Index(rated by observers). IOWA CTRS(rated by observers). Children were evaluated at baseline, 6, 12, 18, and 24 months after baseline. School observation: Social Interaction Observation Code. | |
| Notes | Authors conclusions: There is no support for adding ambitious long‐term psychosocial interventions to methylphendidate to improve ADHD and ODD symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clarification has been requested from the one of the trial investigators and Hovard Abikoff informed us in an email on 28 January 2011 that they had used a block randomisation scheme with blocks of 4 children. The groups were balanced for age, sex, ODD and ethnicity. |
| Allocation concealment (selection bias) | Low risk | Clarification has been requested from the one of the trial investigators and Howard Abikoff informed in an email that they had used sealed envelops. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | There were blinding on at least one of this reviews primary outcomes, in the rest of the outcomes there were no blinding. |
| Incomplete outcome data (attrition bias) | High risk | 22 out of 103 children failed to complete the study. |
| Selective reporting (reporting bias) | Unclear risk | No prior statement of assessment tools. Design article published at the same time as trial article. |
| Vested interest bias | High risk | The trial was based in two large medical centres and the centres have extensive previous experience with research focused on ADHD and behavioural treatment. |
| Other sources of bias? | Unclear risk | Dr. Klein is a member of a pharmaceutical board. |
| Methods | Randomised Clinical Trial | |
| Participants | 120 children from 8‐12 years with ADHD, Inattentive type (n = 59) or ADHD, Combined type (n = 61). Sex: 90 boys, 30 girls. Ethnicity: 112 children were Caucasian, 6 African American, 2 Asian American. Participants were recruited from newspaper advertisement and from consecutive referrals to a university based behavioural paediatric clinic specialized in ADHD and related disorders. Comorbidity: 53 children had comorbid ODD, 29 had mood disorders, 11 had anxiety disorders, and 5 tic disorders. Al 120 participants were taking stimulant medication (n = 110) or selective Serotonin reuptake inhibitor medications. Sample size calculation not reported. Pre randomisation: 142 Post randomisation:120 No statistical significant between‐groups differences in age, sex, or classroom placement, duration and severity of ADHD symptoms, or comorbid conditions. Setting:Out patient clinic, Kentucky, USA. Co‐medications for comorbid‐disorders: SSRI balanced between groups. Inclusion criteria: 1) A diagnosis ADHD based on DSM‐IV (DICA‐R‐P). Only the children which scored >1.0 SD above the mean on the CBCL Attention subscale were included. Exclusion criteria: 1) Not having an ADHD diagnosis. 2) Ages 8‐12, children with significant cognitive delays (IQ < 70) 3) Children with English as a second language. (Information received in an email from Kevin Antshel, 16 December 2010). | |
| Interventions | Format and duration of the intervention: The treatment groups consisted of 8 weeks treatment and there were 90 minutes group sessions for the children during consecutive weeks. The parents met in 3 parent sessions. Content of the interventions: All sessions were conducted by the same two therapists, a male doctoral student i psychology and a female master´s student in social work. The treatment were videotaped to ensure treatment consistency. The therapist followed a treatment manual. The child groups consisted of different methods to promote generalization of social skills.There were 6 themes which consisted of: Cooperation with peers, learning how to take others perspective, problem solving, recognizing and controlling anger, assertiveness, conversations (giving and receiving complements). The parent sessions consisted of information about the themes and content in the Childrens group and discussion of how to assess and monitor homework completion. Mean attendance at the 8 treatment session was 94% for the diagnostically homogeneous and 92% for the diagnostically heterogenous treatment groups. The control group was a wait list group. Medication: No statistically significant between groups differences on medication type and dosage. | |
| Outcomes | Parent rated: SSRS ‐ Social Skills Rating Scale. (38 items) Higher scores indicate more social skills competences. The scale has indexes which are used as outcomes in this study; cooperation, assertion, self‐control, empathy (child version only) and responsibility (parents version only). Both the child and parent version of the SSRS, social skill domain scores range from 0 (less skilled) to 20 (high level of skills). Child rated: The SSRS ‐ Social Skills Rating Scale (34 items). The outcome assessment were 8 weeks after the pretest and follow up were 3 months after the posttest. There were 100% completion rate at all tree assessment intervals( pre‐, post‐treatment and follow‐up). | |
| Notes | Authors conclusion: The results of this trial do not support the efficacy of social skills training for children with ADHD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They used a computer generated randomisation process. Information received from Kevin Antshel in an email 13 July 2011. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed. Information received from Kevin Antshel in an email 13 July 2011. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinded outcome assessors. High risk of bias. |
| Incomplete outcome data (attrition bias) | Low risk | There is stated that there was 100% completion rate. |
| Selective reporting (reporting bias) | Low risk | All of interest reported. |
| Vested interest bias | Unclear risk | No funding source reported. |
| Other sources of bias? | Unclear risk | Referal of patients, selection of ADHD‐I effort due to low prevalence can skew the data. |
| Methods | Randomised Clinical Trial. | |
| Participants | A multistage identification process based on cut of scores in the CBCL: Child Behaviour Checklist teacher/parent resulted in a group of 64 children who were assessed by the Diagnostic Interview for Children and Adolescents‐ DICA‐R. Finally 52 children with ADHD were randomised to either the multi component CBT condition, a teacher intervention, and a wait list control group. Sex: 36 boys and 16 girls in the age 8‐9 years. Ethnicity: 95% was Caucasian students. Comorbidity: 18 (35%) had also ODD. Sample size calculation not reported. The groups were highly comparable on the descriptive and subjective identification measures; age, IQ, Academic achievement, hyperactivity and self‐control behaviour, externalising, internalising behaviour on baseline. Setting: Three suburban elementary schools in the same school district. Co‐medications for comorbid disorders: no information Inclusion criteria: 1) T≥60 on the CBCL‐Teacher. 2) Signed consent form. 3) TT≥60 on the CBCL‐Parent. 4) An ADHD diagnosis on the basis of DIACA‐R. Exclusion criteria: 1) Mental retardation. 2) Epilepsy. 3) Severe emotional disorder. 4) Pervasive development disorder. | |
| Interventions | Format and duration of the intervention: The intervention consisted of Multicomponent Cognitive‐Behavioral Therapy Intervention(MLB): The intervention included coordinated child, parent, and teacher training components. The child component consisted of two one hour group sessions each week over a 10 week period (20 sessions). The teacher component consisted of one 2 hour in service and six 45‐60 minutes consultation over a 10 week‐ period. The parent intervention component consisted of seven 90 minutes group sessions. Teacher‐only Intervention: This component consisted as the same teacher component as above but without the child only and the parent component. Waiting‐list control: No intervention. Content of the intervention: The intervention based on Braswell and Bloomquist(1991) and Bloomquist and Braswell´s cognitive‐behavioural therapy program for ADHD children. A variety of cognitive‐ behavioural techniques were utilized in the child component such as: didactic instructions, modelling, role‐play exercises and so on.The teacher intervention was focused on, for example, problem solving in the classroom and on reinforcing appropriate behaviour and consequating disruptive behaviour. The parent intervention targeted to teach the parents about ADHD, to establish a positive trusting atmosphere among the parents, and to teach them cognitive/behavioural principles identical to those adressed in the teacher training component. The child group was led by school psychologist, the parents' groups by therapist and the teacher intervention by a consultant. The child and teacher interventions had almost 100% attendance. | |
| Outcomes | Observations: Structured behavioural observations(blinded to treatment assignment) Children rated: Self‐Control Rating Scale (SCRS) (33 item questionnaire, 7 point scale. The higher the score, the more the child lacked self‐control). Teacher rated: Conners Teacher Rating Scale (CTRS) (39 items questionnaire, 4 point Likert scale: from not at all (0) to very much (3)) Teacher Report‐Walker‐McConnell Scale of Social Competance and School Adjustment (43 items) There was a comprehensive treatment manual for the MLB and for the teacher only intervention. | |
| Notes | Key conclusions of the study authors: No difference between groups. Authors refer to another paper by Bloomquist 1991. We cannot find this paper. We do not know if it was ever published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of the randomisation method used. |
| Allocation concealment (selection bias) | Unclear risk | No description of the allocation method used. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | The observers were blinded to treatment assignment but the teachers were not. No blinding on primary outcomes. |
| Incomplete outcome data (attrition bias) | High risk | 16 excluded data sets with much likelihood to bias results. |
| Selective reporting (reporting bias) | Low risk | All of interest reported |
| Vested interest bias | Unclear risk | No funding stated. |
| Other sources of bias? | Unclear risk | No test for compliance of the intervention groups. |
| Methods | Randomised Clinical Trial | |
| Participants | 24 children were randomised to four different groups. Six received cognitive behaviour modification, eight received methylphenidate only, six received both treatments, and four were untreated. Sex: 21 boys and three girls. Age: 5‐6 years. Comorbidity: No information. Co‐medications for comorbid‐disorders: no information Inclusion criteria: 1)Scores ≥1,5 on Conners abbreviated Teacher Rating Scale(CTRS). 2) IQ≥80 on WPPSI. 3)No neurological damage or psychosis. Ethnicity: Canadian. No sample size calculation. | |
| Interventions | Format and duration of the intervention: Cognitive Behaviour Treatment: Individual training 1 hour twice weekly sessions for a total of 20 sessions (10 weeks). Content of the intervention: The aim of the treatment was to teach the children to slow down, developing better problem solving ability, and to evaluate his/hers own performance. Medication: Drug dosage was individually titrated. Dosages ranged from 10 to 30 mg methylphenidate pr. day. No information about medication balance between groups. | |
| Outcomes | Teacher rated: Conners Behaviour Rating Scale(teacher version) PMFFT/MFFT(measuring cognitive impulsivity) Parents rated: Conners Behaviour Rating Scale. Observation: Observations in classrooms. Motor impulsivity. What Happens Next and Preschool Interpersonal Problem Solving Test measured social problem‐solving skills. Nowick‐ Strickland Scale(Locus of control). Richman‐Graham(emotional and social adjustment). | |
| Notes | Key conclusion of the study authors: No difference in treatment effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | High risk | No description of the allocation concealment, but four patients were moved between groups. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | There were blinding on at least one of this reviews primary outcomes, in the rest of the outcomes there were no blinding. |
| Incomplete outcome data (attrition bias) | High risk | Many tables different from text. No explanation. Lack of teacher responses. |
| Selective reporting (reporting bias) | Low risk | All apparent assessments are made. |
| Vested interest bias | Low risk | Funding ok, no previous research on the topic. |
| Other sources of bias? | High risk | The selection procedure of patients not stated in article. |
| Methods | Randomised Clinical Trial | |
| Participants | 576 children with ADHD (DSM‐IV) aged to 9.9 years were randomised to either 14 month of medical treatment, intensive behavioural treatment, the two combined and standard community care. Setting: Six multi site outpatient clinics in USA. Sex: 465 boys and 111 girls. Ethnicity: 61% white, 20% African American, and 8% Hispanic. Comorbidity: 33.5% anxiety disorder, 14.3% conduct disorder, 39.9% oppositional‐defiant disorder, 3.8 % affective disorder, 10.9% tic disorder, 2.2%, other (bulimia, enuresis). Co‐medications for comorbid disorders: balanced between groups. Inclusion criteria: 1) Boys and girls, aged 7.0 ‐ 9.9 years (1 st‐4th grades), residing with primary caretakers for at least 6 months, who meet dimensional criteria for hyperactivity on the basis of parent end teacher rating scales and full diagnostic criteria for ADHD, Combined type. Exclusion criteria: 1) Currently in hospital (inability to obtain school assessments). 2) Currently in another treatment study (confounding of assessments and treatments). 3) Below 80 on WISC‐III Verbal IQ, Performance IQ, or Full Scale IQ scores and on Scales of Independent Behavior (insufficient ability to participate in psychosocial interventions). 4) Bipolar disorder, psychosis, pervasive developmental disorder, severe obsessive‐compulsive disorder (treatment may be incompatible with MTA treatments). 5) Chronic, serious tics or Tourette´s Disorder (possible contraindication for stimulant treatment). 6) Neuroleptic treatment in previous 6 months (may need resumption, which is incompatible with MTA treatments). 7) Major neurological or medical illness that would interfere with study participation or require medications incompatible with MTA medications (inability to participate in MTA treatment). 8) History of intolerance to MTA medications (dangerous if participants assigned to arm involving medications). 9) Suicidal of homicidal (needs more intensive treatment than MTA provides). 10) Ongoing or previously undisclosed child abuse (risk of removal from home precludes parent intervention and consistent parent data). 11) Missed more than 25% of school days in previous 2 months (interference with teacher assessments and school intervention). 12) Another child in household already participating in MTA (cross‐arm contamination if two children in same household randomised to different arms). 13)Same classroom as child already participating in MTA (cross‐arm contamination if two pupils in same classroom are randomised to different arms). 14) Parental stimulant/cocaine abuse in past 2 years (possible co‐opting of child´s medications). 15) Inability of parent to speak English (inability to participate in parent training). 16) No telephone (inability to participate in telephone calls with therapists). | |
| Interventions | There were four treatment conditions; Medication Treatment Group, Psychosocial Treatment Group, Combined Treatment (M+PS), and Community Care Group. Format and duration of the treatment: Medication Treatment Group: 1 month of blind titration. Monthly visits after the titration period, doses adjusted as indicated by monthly monitors. Behavioural Treatment Group: Intense, Multi‐Component, including 27 group & 8 individual sessions of parent training, 16‐20 sessions teacher consultations, 8 week full time Summer Treatment Program, and 12 week of half‐time classroom behavioural specialist. No medication. Combined Treatment Group: Integration of all treatment components in Medication Treatment Group and Behavioral Treatment Group. Community Care Group: Treatment of own choosing in the community. No treatment provided by MTA. Content of the Treatment: Medical Treatment Group: 1 month of blind titration with methylphenidate for best dose, if unsatisfactory, then open titration with d‐amphetamine, pemoline, imipramine, others. Supplementary general advice and selected readings without systematic behavioural intervention. Behavioural Treatment Group: Consisted of three major components: parent training, a two part school intervention component, and a child treatment component anchored in an intensive summer treatment program. | |
| Outcomes | Parent rated: Homework Problems Checklist Social Skills Rating System DISC 3.0 Conners Rating Scale Child Behavior Checklist SNAP‐IV DSM‐IV Conduct Disorder Checklist Consumer Satisfaction Teacher rated: Social Skills Rating System Conners Rating Scale Child Behavior Checklist SNAP‐IV Child rated: WIAT Social Skills Rating System DISC 3.0 Self Report of Antisocial Behaviour Observator rated: Classroom Observations | |
| Notes | The authors states that medication and combined treatment do not differ on teacher and parent rated social skills. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate method used. |
| Allocation concealment (selection bias) | Low risk | Adequate method used. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Blinded and unblinded raters. |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation method? |
| Selective reporting (reporting bias) | Unclear risk | Where is the consumer satisfaction and the CBCL data reported? Clarification has been requested from one of the trial investigators, but we had received no answer when this review was finished. |
| Vested interest bias | Low risk | Funding okay. |
| Other sources of bias? | Low risk | Low risk. |
| Methods | Design: Randomised Clinical Trial. | |
| Participants | Participants were recruited from newspaper advertisement and from consecutive referrals to a university‐based behavioural paediatric clinic specialized in ADHD and related disorders. Children 8‐10 years with an ADHD diagnosis made on the basis of DSM‐III‐R criteria. Sex: 19 boys, 8 girls. Ethnicity: Patients were Caucasian except from 1 boy, who was African American. Socioeconomic status were from middle to upper middle class. Two children were from single‐parent families. Comorbidity: 25 children met criteria for ADHD and 2 met criteria for UADD. 19 children met criteria for comorbid oppositional defiant disorder, 3 for conduct disorder, 4 for separation anxiety disorder, 5 for overanxious disorder, and 2 for dysthymic disorder. Only 12 of the children (44%) were receiving stimulant medication. Sample size calculation not reported. Pre randomisation: 27. Post randomisation: 18. Setting: University based paediatric clinic, USA. Co‐medications for comorbid disorders: no information Inclusion criteria: 1) Diagnosis of ADHD (DSM‐III‐R), a mean score at or above 1,5 on at least one of the parent‐completed sub scales assessing ADHD. behaviour from the CLAM rating scale or the SNAP‐R, a T score of at least 60 on the Attention Problem subscale of the CBCL. Exclusion criteria: Not reported. We have attempted to get information about this from the study investigators but have not succeeded in this attempt. | |
| Interventions | Three conditions: Social skills training for the children (SST), Social skills training for the children with parent mediated generalisation (SST‐PG) and a wait list control group. Format and duration of the intervention: The two treatment groups attended 8 group sessions. There were assessment at pre and post treatment and follow‐up 3‐4 months post treatment. Children in the treatment groups received 90 min group sessions during consecutive weeks. Content of the intervention: The same two therapists were teaching in all the children groups. Six themes/modules were covered during the 8 weeks. 1) good sportsmanship, 2) accepting consequences, 3) assertiveness, 4) ignoring provocations, 5) problem solving, 6) recognising and dealing with feelings. Children were assigned homework to practice at home. The children received points for following the rules of the groups, participate and attend the sessions.The points could be exchanged for child‐selected games and activities during the last 10 minute for each group. In the SST‐PG parent were used as a primary vehicle to program generalisation of the social skills learned in the SST groups to home and school settings. The parents group were led by a licensed psychologist. The parents went through the same group themes or agendas as the children did. The parents met with their childrens teacher and gave the teacher a template for the scorecard, also called the daily report card.The teacher scored the child on a 4 point scale and parents rewarded the child when the child scored high on the scale. There were a protocol for both the SST and the SST‐PG intervention. The intervention groups were led by psychologist. | |
| Outcomes | Parent rated: Social Skils Rating System (SSRS) The Social Skills scale consists of 30 items on a Likert scale (0‐3). Social Skills Scale (UCI) 10 items (5 point scale: 1‐5) (parent). Consumer Satisfaction Questionnaire (12 items, 7 point scale (scores above 4 reflecting satisfaction and scores below 4 reflecting dissatisfaction). Teacher rated: Social Skils Rating System (SSRS). The Social Skills scale consists of 30 items on a Likert scale (0‐3). Interviewers: Test of social skill knowledge (six questions each scored from 1‐15)(scored by blinded raters). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given in the article. Clarification requested from the trial investigators and they reported in an email 26 May 2011 that it is not possible to find this data now. |
| Allocation concealment (selection bias) | Unclear risk | No information given in the article. Clarification requested from the trial investigators and they reported in an email 26 May 2011 that it was not possible to find this data now. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | There was blinding on at least one of this study's primary outcomes; in the rest of the outcomes there was no blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Imputation method used. F.u. scores for 3 participants replaced by m. |
| Selective reporting (reporting bias) | High risk | The author informed in an email that the CLAM and SNAP were used post treatment, but not reported in the article. It was not possible to get the data because they had been lost over time. |
| Vested interest bias | Low risk | Funding okay. No previous publication on the topic. |
| Other sources of bias? | Unclear risk | $12 (f.u.) paid to families. $10, $25 for teachers. 44% of the participant were medicated with stimulant medication, but the number of medicated children in the different groups is not stated. |
| Methods | Design: Randomised Clinical Trial. | |
| Participants | There were 69 children in the age 7‐ 11 years randomised to Child Life and Attention Skills Program (CLAS) or a control group who did not receive the intervention. Setting: Outpatient clinic. Sex: 46 boys, 23 girls. Ethnicity: White 51%, Asian 16%, Hispanic 10%, Afro American 6%, and mixed 17%. There were not any significant difference between group in the form of child age, sex, race, symptoms of hyperactivity/impulsivity, comorbid oppositional defiant disorder, anxiety or depression, IQ or academic achievement. Comorbidity: ODD (23%), depressive (1%), anxiety (12%) Co‐medications for comorbid disorders: no information Inclusion criteria: 1) DSM‐IV diagnosis of ADHD‐I. 2) IQ > 80 (based on Wechsler Abbreviated Scale of Intelligence). 3) Living with at least one parent for the past year. 4) Attending school full time. 5) The school consenting to participate in school‐based treatment. Exclusion criteria: 1) Families expecting to change medication status for their child during the study. 2) Children with visual or hearing impairment. 3) Severe language delay. 4) Major neurological illness. 5) Psychosis, or pervasive development disorder. 6) A child being in the same classroom as another participant or having a sibling who was already enrolled. In the intervention group 7 children were lost to follow‐up and in the control group 8 children were lost to follow‐up. | |
| Interventions | The treatment included three components administrated concurrently over 12 weeks: teacher consultation, parent training, and child skills training. Format and duration of the treatment: Child skills training: 8 (cohort:1‐4) and 10 (cohort 5) 1 ½ hours a week groups with child skills training in the 12 week period. Parent Training: 8 (cohort:1‐4) or 10 (cohort 5) 1½ hour group sessions and 4 to 5 family sessions (cohort 2‐5). Teacher Consultations: 1/2 hour overview of behavioural interventions and classroom‐based accommodations for ADHD followed by 4‐5 1/2 hour meetings of teacher, child, and therapist over the 12 week period. Content of the intervention: Child Skills Training: The training were divided into modules focused on skills for independence and skills for social competance.There were both behavioural interventions (for example, a reward based contingency management program) and cognitive‐behavioural interventions (for example, problem‐solving, the use of cues/verbal mediation strategies to stay on task and focused). Parent Training:The modules in the child group were reviewed each week and the parents were taught methods to promote end reinforce the childs use of skills at home. The parents were also taught methods to managing ADHD. Teacher consultations: A school‐home daily report card was designed and used (Classroom Challenge‐CC). Also a special notebook was created for each child containing copies of CC. All the interventions were manual‐based. There were made some changes to the manuals to refine the interventions based on feedback from clinicians, participants, teachers, and parents. Attendance: Parents in all cohorts participated in more than 95% of the group meetings. | |
| Outcomes | Parent rated: Child Symptom Inventory (parents and teachers) corresponds to DSM‐IV inattention symptoms and are rated on a 4 point scale (0 = never to 3 = very often). The SCT scale (parents and teachers) consists of 15 SCT items rated on a 4‐point scale (0 = never to 3 = very often). SSRS ‐ Social Skills Rating Scale. 30 items rated on a 3 point scale (never, sometimes, very often). Organisational Skills (parents: 58 items, 4 point scale, 1 = never to 4 = just about all time). Clinical Global Impression ‐ Improvement. Teacher rated: Child Symptom Inventory (parents and teachers) corresponds to DSM‐IV inattention symptoms and are rated on a 4 point scale (0 = never to 3 = very often). The SCT scale (parents and teachers) consists of 15 SCT items rated on a 4‐point scale (0 = never to 3 = very often). SSRS ‐ Social Skills Rating Scale. 30 items rated on a 3 point scale (never, sometimes, very often). Organisational Skills (teacher: 38 items, 4 point scale, 1 = never to 4 = just about all time). Clinical Global Impression‐Improvement. Children rated: Test of Life Skill Knowledge Consumer satisfaction: Parents: 100% very satisfied 35% improved or much improved 90% useful or very useful Teachers: 32 of 36 rated the programme as appropriate 73% rated improved attentional difficulties Children: 32 of 36 liked the group. 83% found the programme helped at home. 78% found the programme helped in school. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random table. Information received from Pfiffner in an email 25 May 2011. |
| Allocation concealment (selection bias) | Low risk | Sealed. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Imputation method used. |
| Selective reporting (reporting bias) | Low risk | All apparent assessment are made. |
| Vested interest bias | High risk | Has done previous trials |
| Other sources of bias? | High risk | Changes in treatment protocol. Timing for follow up differ; same school year vs next school year (both approximately 3 months after treatment but summer break between). Families and teachers were paid for each of the post treatment and follow‐up assessment. |
| Methods | Design: Randomised Clinical Trial. | |
| Participants | Children 5‐12 with an ADHD diagnosis made on the basis of DSM‐IV criteria. Sex: 75 boys and 25 girls Ethnicity: White (49% intervention group, 38% control group), Afro‐American (4% IG, 2% CG), Asian (5% IG, 1% CG), Hispanic 1% IG, 0% CG). Sample size calculation not reported. Setting:Outpatient clinic, Washington, USA Pre randomisation: 100 Post randomisation: Blinded follow‐up measures were completed by 97% and 98% of parent or guardian participants at 3 and 6 months after enrolment, respectively. Follow‐up completion rates for teacher participants yielded 92% and 75% for 3 and 6 months after enrolment, respectively. Participants with missing data did not differ from participants with complete data sets across time or any clinical, functional, and demographic variables according to the authors of the study article. For the ADHD Rating Scale outcome 2 children where lost to follow up. For the Child Attention Profile outcome totally 24 children where lost to follow‐up (16 in the IG and 8 in the CG). Co‐medications for comorbid disorders: it was allowed but not stated if it were balanced between groups. Inclusion criteria: Diagnosis of ADHD (DSM‐IV) Exclusion criteria: 1) Conduct disorder 2) Oppositional defiant disorder 3) Tourette syndrome 4) Affective disorder 5) Active alcohol or other substance abuse during previously 90 days 6) Chronic mental ilness 7) Patients enrolled in BSS class at GHC in the past. Baseline characteristics: Mean baseline parented attention‐deficit hyperactivity disorder symptom scores were more symptomatic for the IG than for the CG, as well as the use of parent discipline practice. These between groups differences were adjusted before follow‐up analysis. | |
| Interventions | Two conditions: Behavioural and social skills class versus control group (waiting list). Both groups received psychostimulant treatment. Format and duration of the intervention: The BSS intervention consisted of 8 once a week, 50 minute group sessions. The children were divided into one of three child groups according to age; 5‐7, 8‐10 and 11‐12 years. There was a parent only group at the same time as the child only group. Content of the intervention: The BSS intervention was based on an existing ADHD program previously developed by the CADD clinical team. The intervention are designed to enhance the children's overall understanding of ADHD and how to cope and manage with many of the physical and psychosocial problems connected to this condition. Each BSS session was based on a structured session by session agenda. The intervention was delivered by master levels therapist with at least two years experience. The child and parents were divided into a child only and a parent only group. There was no significant between‐groups difference in psychostimulant use found at 3 (49.01% vs 53,6%; X2= 0.196, P = 0.658) months and 6 months (52,94% vs 39.02% x2 = 1.768, P = 0.184) for both IG and CG participants. Co‐medication: allowed, but not stated if equal in groups. | |
| Outcomes | Outcome assessment were conducted at 3 and 6 months. Clinican rated: The ADHD Rating Scale (18 items, Likert scale) assessed by a blinded research assistant at baseline, 3 months and 6 months. Assessment by telephone interviews of parents. The Child Attention Profile (12 items. Likert scale) assessed by a blinded research assistant at baseline, 3 months and 6 months. Assessment by telephone interviews of teachers. | |
| Notes | There was a third outcome used in this study, but it is not relevant for this review, because it measured the parents' discipline practice. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study used coin toss method performed by the research assistant and this is an adequate method according to the Cochrane Handbook. |
| Allocation concealment (selection bias) | Unclear risk | Information on this is not reported. Clarification about method of allocation concealment has been requested form the trial investigators, but no information on this topic was available at the time the review was prepared. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessment by telephone interviews of parents and teacher performed by a blinded research assistant. The parents not blinded, and therefore not an adequate method. |
| Incomplete outcome data (attrition bias) | Low risk | There were used an ITT method. |
| Selective reporting (reporting bias) | Low risk | All of interest reported. |
| Vested interest bias | Low risk | Funding okay, and no previous trials on the topic. |
| Other sources of bias? | Unclear risk | The co‐medication not specified. |
| Methods | Design: Randomised Clinical Trial. | |
| Participants | Children aged 8‐12 years with ADHD diagnosed according to DSM‐IV. Ethnicity: 40 children(89%) children from Caucasian parents, 1 child(2%) was from Caribbean parents, and 4(9%) from mixed origin. Comorbidity: ODD/CD 61,9% in one group and 41,7% in the other. Sample size calculation not reported. Setting: Five different outpatient clinics in Netherland. Co‐medications for comorbid disorders: no information Inclusion criteria: 1) ADHD diagnosis based on DSM‐IV established with the parent version of DISC‐IV. 2) Total IQ of 75 or above based on the short version of WISC‐R. 3) Parents must give informed consent for their child to participate in the trial. Baseline characteristic: One‐way ANOVAs and Chi2 analyses showed not significant differences between the two conditions in terms of baseline demographic characteristics. Furthermore one‐way ANOVAs showed no significant group differences. Exclusion criteria: 1) Inadequate mastering of the Dutch language by the child or both parents. 2) A history of methylphenidate use. Of the 50 randomised children one declined the methylphenidate only group and two of the children in the methylphenidate plus BT discontinued the intervention. Furthermore, one child was lost to posttest and follow up in the methylphenidate only intervention, one was omitted from analysis in the combined intervention group. Medication:There were used a four‐week, pseudo‐randomised, multiple‐blind placebo‐controlled, crossover medication design, as described in the MTA study. The treatment groups did not differ on dose of methylphenidate either at baseline or posttreatment. | |
| Interventions | Format and duration of the intervention. 50 children were randomised to either methylphenidate (n = 23) or methylphenidate plus multimodal behaviour therapy (n = 27). The multimodal treatment consisted of child cognitive‐behaviour therapy, parent behaviour therapy and teacher behavioural training. The child cognitive‐behaviour therapy consisted of 10 weekly, 75 minutes group sessions, provided by two therapists. The parent behaviour therapy onsite of 10 weekly sessions of 90 minutes group therapy, provided by two therapists. Content of the intervention. There were used a treatment program and there were manuals in all the groups. In the child group there were used cognitive‐ behaviour techniques and the program for this group was adapted from Kendall and Braswell. It consisted of problem solving techniques, relaxation techniques, contingency management techniques, role playing, and guided practice. The parent group was based on Barkley´s training's manual "Defiant children: A clinicians manual for parent training." Components included, for example, psychoeducation on ADHD, structuring the environments, practicing positive attending skills and contingency management skills. The teacher training was based on the teacher training manual by Pelham and consisted of a two hour workshop, which consisted of, for example, psycho‐education on ADHD, structuring the classroom environment, and a daily report card. Mean treatment attendance in the combined condition was 88.6%. To ensure treatment compliance all therapist completed a treatment integrity checklist. | |
| Outcomes | Parent rated: DBDRS consist of 42 items and contains four sub scales: Inattention (9 items), Hyperactivity/Impulsivity (9 items), ODD (8 items), and CD (16 items). Has a 4‐point Likert scale (0‐3). The Inattention and the Hyperactivity/Impulsitivity sub scale were combined into one ADHD score. Higher scores indicate more increased symptoms. SSRS ‐ Social Skills Rating Scale. (The parent version consist of 38 items. Has a 3‐point Likert scale rating from 0‐2.) Parenting Stress Index(PSI) Teacher rated: DBDRS consist of 42 items and contains four sub scales: Inattention(9 items), Hyperactivity/Impulsivity (9 items), ODD (8 items), and CD (16 items).Has a 4‐point Likert scale (0‐3). The Inattention and the Hyperactivity/Impulsitivity sub scale were combined into one ADHD score. Higher scores indicate more increased symptoms. SSRS ‐ Social Skills Rating Scale. The teacher version consist of 30 items. Has a 3‐point Likert scale rating from 0‐2. Child rated: Self‐Perception Profile for Children (SPPC). State Trait Anxiety Inventory for Children (STAIC). | |
| Notes | Authors conclusion is that there are no additive effect of multi modal treatment compared to medical treatment alone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information on this is not reported. Clarification about method of allocation concealment has been requested form the trial investigators, but no information on this topic was available at the time the review was prepared. |
| Allocation concealment (selection bias) | Unclear risk | Information on this is not reported. Clarification about method of allocation concealment has been requested form the trial investigators, but no information on this topic was available at the time the review was prepared. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | All the participants lost to follow up were stated and lost to follow up not believed to influence results. |
| Selective reporting (reporting bias) | Low risk | No. |
| Vested interest bias | Unclear risk | Funding not stated. |
| Other sources of bias? | Unclear risk | Co‐medication not specified. |
| Methods | Design: Randomised Clinical Trial. | |
| Participants | Children aged 6‐12 years with ADHD diagnosed according to DSM‐IV‐TR Ethnicity: 80,4% white, 10.7% African American, and 8,9% mixed. 7 children (12.5%) discontinued the study, 5 in the IG and 2 in the CG. Sample size calculation not reported. Of the 56 children 48 were diagnosed with ADHD‐combined type, 7 were diagnosed with ADHD‐inattentive type, and 1 was diagnosed with ADHD‐hyperactive/impulsive type. 22 children met criteria for comorbid conduct disorder and 24 for oppositional defiant disorder, leaving only 10 children with non comorbid ADHD. Setting: Outpatient (attending school), Buffalo, USA Co‐medications for comorbid disorders: no information Inclusion criteria: ADHD based on DSM‐IV Exclusion criteria: 1) Current or past history of seizures (not including benign febrile seizures). 2) Other physical conditions that precluded administration of atomoxetine (for example, marked cardiac conduction delay). 3) Documented failed trial of atomoxetine, defined as 3 weeks or more on treatment with at least 0.8 mg/kg/d, or a documented inability to tolerate this dose. 4) Serious forms of psychopathology other than ADHD, such as autism, bi‐polar disorder, schizophrenia, or any other psychopathology requiring urgent treatment with psychotropic medication. 5) Any history of major depression requiring treatment, or any past history of self‐harm or serious suicidal ideation. 6) An intelligence quotient of less than 75 (based on Wechsler Intelligence Scale for Children, 3rd edition). 7) No evidence of ADHD‐related impairment at school. Baseline characteristics: 56 children randomised. 45 boys and 11 girls. Medication: All patients received psychostimulant medications. No significant between group differences in mean doses of atomoxetine. | |
| Interventions | 8 week intervention + Medication versus Medication alone. Number of participants allocated per group: 27 (Med); 29 (Med + BT) Number of patents lost to follow up per group: 2 (Med); 5 (BT + Med) Format and duration of the intervention: The 8 week intervention consisted of: Parent group: 8 week group, 2 hours session;1 session/week. Child group ‐ SST: 8 week. 2 hours session;1 session/week. Teacher: Daily report card. Content of the intervention: Parent group: Based on the COPE program and consisted of social learning's principals targeting at the Childrens behaviour and lack of impulse control. Group leaders were advanced graduate students or doctoral level clinicians. Child group: Social skills training program. Group leaders were graduate students in clinical psychology. Treatment compliance: The parent intervention was based on a manual (COPE). It is unclear whether the child group intervention also was based on a manual. 62% of the parents attended 8 sessions, 62% attended 6 or more sessions. The children's attendance in the SST group is not reported. | |
| Outcomes | Parent rated: DBD (45 items, Likert scale 0 (not very much) to 3 (very much)). SSRS (Social Skills Rating Scale) (55 items on the parent version, rated from 0 (not at all) to 2 (very often)). Treatment satisfaction (Likert scale from 1 (strongly disagree) to 7 (strongly agree)). Teacher rated: DBD (45 items, Likert scale 0 (not very much) to 3 (very much)). SSRS (Social Skills Rating Scale) (57 items on the teacher version, rated from 0 (not at all) to 2 (very often) and from 0(lowest 10%) to 5 (highest 10%). APRS (Academic Performance Rating Scale) (19 items scale, 1‐5 Likert scale). DRC (Daily Report Card). Observations: Observations of violence to classroom rules using Student Behaviour Teacher Response Observation Code. Clinician ratings: CGI(Clinical Global Impressions scale). Classroom behaviour. ADHD symptoms and functioning at home and at school. | |
| Notes | Key conclusion of the author: Behavioural therapy improved ADHD symptoms at the home but not at school. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clarification requested from the one of the trial investigators and Dan Wascbusch informed in an email 22 June 2011 that they had used a computer generated randomisation process |
| Allocation concealment (selection bias) | Low risk | Clarification requested from the one of the trial investigators and Dan Wascbusch informed in an email 22 June 2011 that the clinicians did not know the treatment assignment before it was assigned. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | High risk | Clarification requested from the one of the trial investigators and Dan Wascbusch informed in an email that subjects were dropped if there was not sufficient information. Scores in indexes were computed if there were at least 50% of the items in the index answered and counted them missing if they were not. Dan Wascbusch also informed that they had essentially complete data pre‐treatment and nearly complete at post‐treatment. For teachers they had a lower response. They included whatever they had in the analyses and dropped subjects when there was not sufficient information, repeating this for each analysis. |
| Selective reporting (reporting bias) | Unclear risk | Protocol published in Clinicaltrials.gov after trial conduct. Publication and report in Clinicaltrials gov. is not consistent. |
| Vested interest bias | High risk | Fundings from and collaboration with Eli‐Lilly Company. |
| Other sources of bias? | Unclear risk | Co‐medication not specified. |
| Methods | Design: Randomised Clinical Trial | |
| Participants | 90 children with ADHD were randomised to psychosocial treatment plus methylphenidate versus methylphenidate treatment alone. Children aged 7 ‐ 9.9 years. Ethnicity: Chinese children. Sample size calculation was made. Sex: 77 boys, 9 girls. Comorbidity: Anxiety 29%, Depression 6%, ODD 50%, Conduct Disorder 6%. Setting: Community mental health center: Out patient clinic in Hong Kong Socio demographic: No significant differences between the two treatment groups in demographic and social economic status, comorbid conditions, and additional intervention received in the first six months of the treatment. Medication: All participants received methylphenidate treatment. No information about between group differences in the medical treatment. Co‐medications for comorbid disorders: no information Inclusion criteria: 1) ADHD‐Combined Type based on DSM‐IV criteria 2) Children in the age 7‐9.9 years. 3) Studying first to fourth grade. 4) Living with a parent, who is the major caretaker. 5) IQ>80. 6) No significant physical disability. 7) No stimulant medication (methylphenidate) use for more than 2 weeks previously. 8) Their parents willingness to accept stimulant medication and psychosocial intervention of this study. 9) The parents willingness to accept random allocation. 10) No parent suffering from intellectual impairment or current psychosis. | |
| Interventions | There were three components in the psychosocial treatment; child training, cognitive‐ behavioural parent training, and school consultations. Pre randomisation: 146 Post randomisation: 90 Form and duration of the treatment: Child training: 24 weekly sessions. Each group session lasted for 1 hour and 30 minutes to 2 hours. Cognitive‐Behavioral Parent Training: 18 weekly sessions in total. Each session lasted for 1 hour and 30 minutes to 2 hours. School Consultations: There were two telephone consultations, Content of the intervention: The child training:The training provided a rich direct contingency management environment, in which the training of problem solving's skills and anger control management was provided. All sessions were videotaped to check treatment integrity. Themes were, for example, feelings, games, problem solving, stop & think, role play school and home. Parent training: The child training and the parent training were developed to implement concommitantly.Themes in the group were for instance: know yourself, attention rules, stress management, child mood management, homework coaching. School consultations: The therapist in the child groups talked to the teachers about implementations of classroom management strategies and review of the child's progress in school. No protocol violations to both child and parent training treatment program were detected. | |
| Outcomes | Parent rated: SWAN rating scale, (30 items, 7 point scale, 1(slightly below average) to ‐3 (far above average). Teacher rated: SWAN rating scale.(30 items, 7 point scale, 1(slightly below average) to ‐3 (far above average). Clinician rated: MFFT(computer programme to measure impulsivity. Scores time taken to make the response, and total numbers of errors.) Consumer satisfaction: At post treatment: 40% very useful, 60% useful. | |
| Notes | Combined Medication + PST yielded benefits on primary ADHD symptoms and on conduct problems. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers, with block size of two. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding on this reviews primary outcome. |
| Incomplete outcome data (attrition bias) | High risk | Type of imputation method used is unclear. |
| Selective reporting (reporting bias) | Low risk | Low risk. |
| Vested interest bias | Unclear risk | Yuk‐Chi So has done previous research on the topic. |
| Other sources of bias? | High risk | Have done previous research and no statement of funding. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Only parent training programme. | |
| Intervention not eligible. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not children with ADHD. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| Article ‐ not a trial. | |
| Control group without ADHD. | |
| Not social skills training. | |
| Not a randomised clinical trial. | |
| Not a suitable outcome. | |
| Not a control as specified in the review protocol. | |
| A review. | |
| Not adding relevant data to the review. | |
| Not social skills training. | |
| Not all of the children was diagnosed with ADHD. | |
| Not social skills training. | |
| Not social skills training. | |
| Not a suitable outcome for this review. | |
| Participants under five years of age. | |
| Intervention for the teachers, not a clinical ADHD diagnosis. | |
| Not a randomised clinical trial. | |
| Review. | |
| The control group is not suitable. | |
| Not adding necessary data to the review. | |
| Not a randomised clinical trial. | |
| Not a randomised clinical trial. | |
| No relevant data for the review. | |
| Not a randomised clinical trial. | |
| Not social skills training. | |
| Not a randomised clinical trial. | |
| Participants under five years of age. | |
| Participants under five years of age. | |
| Not a randomised clinical trial. | |
| Not social skills training. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Compensatory executive functioning skills training for adolescents with ADHD. |
| Methods | Randomised efficacy study, single blind crossover assignment. |
| Participants | Adolescents from 14 to 18 years diagnosed with ADHD. Both genders. |
| Interventions | Twelve weekly treatment sessions either immediately upon enrolling in the study or after a four month‐waiting period. Cognitive behavioural therapy versus wait list control. This study, adapted from a similar research study for adults with ADHD, will examine whether cognitive behavioural therapy (CBT) plus medication is more effective at treating ADHD than medication therapy alone in adolescents with ADHD. |
| Outcomes | Primary outcome measures: Changes in ADHD symptoms, measured before randomisation, and at 4 months and 8 months follow up. Secondary outcomes measures: Changes in secondary symptoms af ADHD (for example, mood). |
| Starting date | October 2009. Estimated study completion date: May 2012. |
| Contact information | Principal Investigators: Steven A. Safren, Ph.D., Massachusetts General Hospital. Susan E. Sprich, Ph.D., Massachusetts General Hospital. |
| Notes | Clincaltrials.gov Identifier: NCT0109252 |
| Trial name or title | Randomised social‐skills training and parental training plus standard treatment versus standard treatment of children with attention deficit hyperactivity disorder ‐ The SOSTRA trial. |
| Methods | The design is randomised two‐armed, parallel group, assessor‐blinded trial. |
| Participants | Children aged 8‐12 years with a diagnosis of ADHD are randomised to social‐skills training and parental training plus standard treatment versus standard treatment alone. A sample size calculation estimated that at least 52 children must be included to show a 4‐point difference in the primary outcome on the Conners 3rd Edition subscale for 'hyperactivity‐impulsivity' between the intervention group and the control group. |
| Interventions | Social skills training will consist of 8 weeks of group treatment with weekly sessions of one and a half hours and includes role play, exercises and games as well as home work which will include the parents. At the same time the parents are participating in parental training groups, that will focus on supporting the children's social training. Both the children and the parental groups are lead by two group therapists. The intervention will be additional to the received standard treatment. The standard treatment consists of medical treatment, briefing, consulting and supporting conversations with a focus on securing compliance to the treatments and on aiding children and their families with the difficulties arising with the children's illness. Furthermore the parents participate in parental groups three times during the 8 weeks in which the experiment takes place. This group lasts 2 hours and is managed by two nurses who are attached to the ADHD‐ treatment group. |
| Outcomes | The outcomes will be assessed 3 and 6 months after randomisation. The primary outcome measure is ADHD symptoms. The secondary outcome is social skills. Tertiary outcomes include the relationship between social skills and symptoms of ADHD, the ability to form attachment, and parents' ADHD symptoms |
| Starting date | August 2009. Finished August 2011. |
| Contact information | Contact: Ole J. Storebø, psychol., MS [email protected] Contact: Jesper Pedersen, MD, Phd. [email protected] |
| Notes | Clincal Trials.gov Identifier:NCT00937469 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary analysis: Social skills competences Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Social skills (SSRS), Outcome 1 Primary analysis: Social skills competences. | ||||
| 1.1 Primary analysis: Teacher‐rated social skills competences at end of treatment ‐ all eligible trials | 5 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
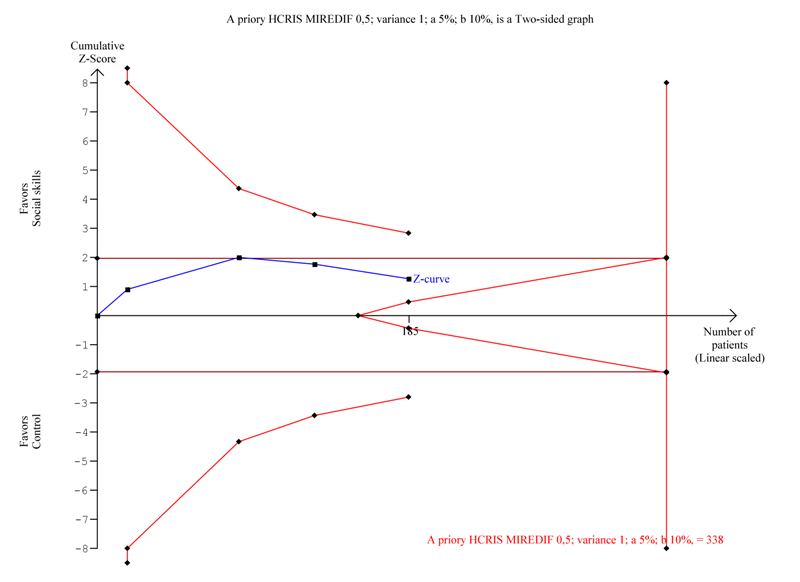
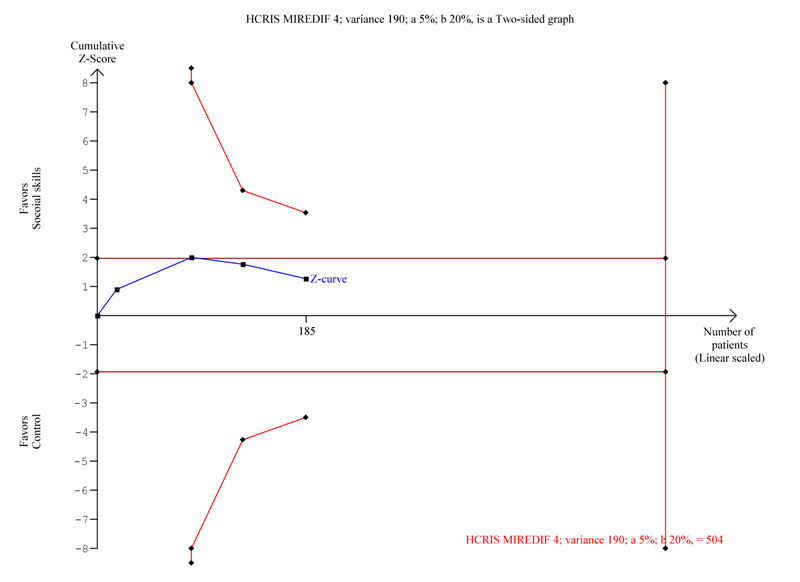
| 1.2 Sensitivity analysis:Teacher‐rated social skills competences ‐ excluding the trial with longest intervention duration (also largest trial) | 4 | 185 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.10, 0.48] |
| 1.3 Teacher‐rated social skills competences ‐ longest follow‐up | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.86, 0.98] |
| 1.4 Secondary analysis: Parent‐rated social skills competences at end of treatment ‐ all eligible trials | 7 | 628 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.04, 0.40] |
| 1.5 Secondary analysis: Participant‐rated social skills competences at end of treatment ‐ all eligible trials | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.09, 0.51] |
| 2 Teacher‐rated social skills (Mean Difference) Show forest plot | 4 | 185 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐1.02, 4.64] |
| Analysis 1.2  Comparison 1 Social skills (SSRS), Outcome 2 Teacher‐rated social skills (Mean Difference). | ||||
| 3 Parent‐rated social skills competences (Mean Difference) Show forest plot | 5 | 313 | Mean Difference (IV, Random, 95% CI) | 2.82 [‐0.92, 6.56] |
| Analysis 1.3  Comparison 1 Social skills (SSRS), Outcome 3 Parent‐rated social skills competences (Mean Difference). | ||||
| 4 Walker‐McConnel Social skills Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐0.47, 2.59] |
| Analysis 1.4  Comparison 1 Social skills (SSRS), Outcome 4 Walker‐McConnel Social skills. | ||||
| 5 Social skills scale (UCI) parent‐rated Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 9.70 [6.07, 13.33] |
| Analysis 1.5  Comparison 1 Social skills (SSRS), Outcome 5 Social skills scale (UCI) parent‐rated. | ||||
| 6 Child social skills knowledge scale Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [1.99, 6.41] |
| Analysis 1.6  Comparison 1 Social skills (SSRS), Outcome 6 Child social skills knowledge scale. | ||||
| 7 Social interaction observation ‐ negative behaviour Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.11, 0.51] |
| Analysis 1.7  Comparison 1 Social skills (SSRS), Outcome 7 Social interaction observation ‐ negative behaviour. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary analysis: Teacher‐rated general behaviour Show forest plot | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 General behaviour, Outcome 1 Primary analysis: Teacher‐rated general behaviour. | ||||
| 1.1 Primary analysis: Teacher‐rated general behaviour at end of treatment ‐ all eligible trials | 3 | 358 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.21, 0.21] |
| 1.2 Sensitivity analysis: Teacher‐rated general behaviour excluding the trial with longest intervention duration | 2 | 290 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.27, 0.19] |
| 1.3 Sensitivity analysis: Teacher‐rated general behaviour excluding the largest trial | 2 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.24, 0.53] |
| 1.4 Teacher‐rated general behaviour ‐ longest follow‐up | 2 | 256 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.48, 0.01] |
| 1.5 Secondary analysis: Parent‐rated general behaviour at end of treatment | 1 | 254 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.51, ‐0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Teacher‐rated ADHD symptoms Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 ADHD symptoms, Outcome 1 Teacher‐rated ADHD symptoms. | ||||
| 1.1 Primary analysis: Teacher‐rated ADHD symptoms at end of treatment ‐all eligible trials | 6 | 515 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.16] |
| 1.2 Sensitivity analysis:Teacher‐rated ADHD symptoms excluding the trial with the longest treatment intervention | 5 | 447 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.25, 0.12] |
| 1.3 Sensitivity analysis: Teacher‐rated ADHD symptoms at end of treatment excluding the largest trial | 5 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.31] |
| 1.4 Teacher‐rated ADHD symptoms ‐ longest follow‐up | 2 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.90, 0.41] |
| 1.5 Teacher‐rated ADHD symptoms ‐ MTA inattention outcome | 1 | 254 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.23, 0.26] |
| 1.6 Teacher‐rated Sluggish cognitive tempo end of treatment | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.78, 0.20] |
| 1.7 Secondary analysis: Parent‐rated ADHD symptoms at end of treatment ‐ all eligible trials | 7 | 654 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.79, ‐0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Teacher‐rated Show forest plot | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 7.42 [‐1.42, 16.25] |
| Analysis 4.1  Comparison 4 Subgroup analysis 1: trials with social skills training without parental training compared to social skills training combined with parental training, Outcome 1 Teacher‐rated. | ||||
| 1.1 Teacher‐rated social skills training with parent training | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐6.78, 17.98] |
| 1.2 Teacher‐rated social skills training without parent training | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 9.30 [‐3.31, 21.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Parent‐rated ADHD symptoms at end of treatment Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Subgroup analysis 2: trials with ADHD including comorbidity compared to trials with ADHD and no comorbidity, Outcome 1 Parent‐rated ADHD symptoms at end of treatment. | ||||
| 1.1 Parent‐rated ADHD symptoms at end of treatment without comorbidity | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.19, ‐0.36] |
| 1.2 Parent‐rated ADHD symptoms at end of treatment ‐ with comorbidity | 6 | 557 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.76, ‐0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Social skills training, parent training, teacher consultations with and without medication Show forest plot | 5 | 331 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.04, 0.56] |
| Analysis 6.1  Comparison 6 Subgroup analysis 3: trials with social skills training, parental training and medication compared to trials with social skills training and parental training without medication, Outcome 1 Social skills training, parent training, teacher consultations with and without medication. | ||||
| 1.1 Social skills training and parent training with medication | 3 | 244 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.10, 0.48] |
| 1.2 Social skills training, parent training without medication | 2 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.48, 1.76] |

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Comparison 1 Social skills (SSRS), Outcome 1 Primary analysis: Social skills competences.
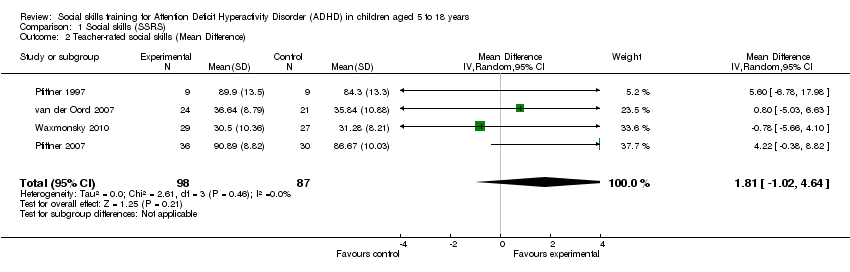
Comparison 1 Social skills (SSRS), Outcome 2 Teacher‐rated social skills (Mean Difference).
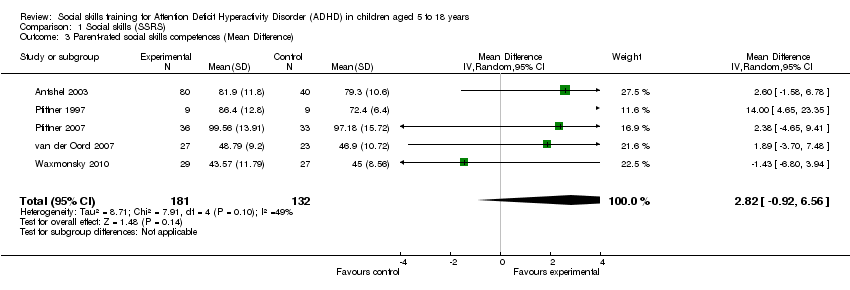
Comparison 1 Social skills (SSRS), Outcome 3 Parent‐rated social skills competences (Mean Difference).

Comparison 1 Social skills (SSRS), Outcome 4 Walker‐McConnel Social skills.
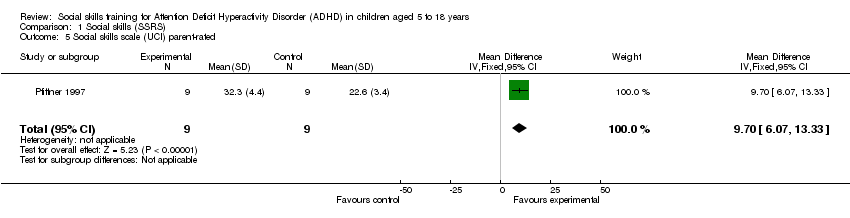
Comparison 1 Social skills (SSRS), Outcome 5 Social skills scale (UCI) parent‐rated.
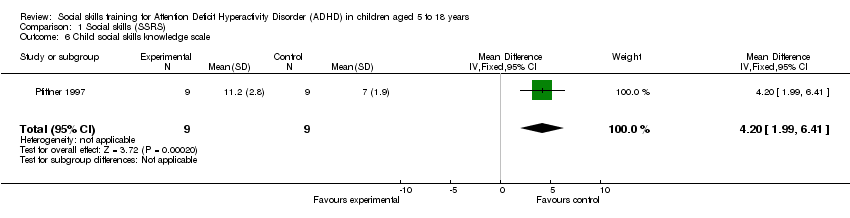
Comparison 1 Social skills (SSRS), Outcome 6 Child social skills knowledge scale.

Comparison 1 Social skills (SSRS), Outcome 7 Social interaction observation ‐ negative behaviour.

Comparison 2 General behaviour, Outcome 1 Primary analysis: Teacher‐rated general behaviour.
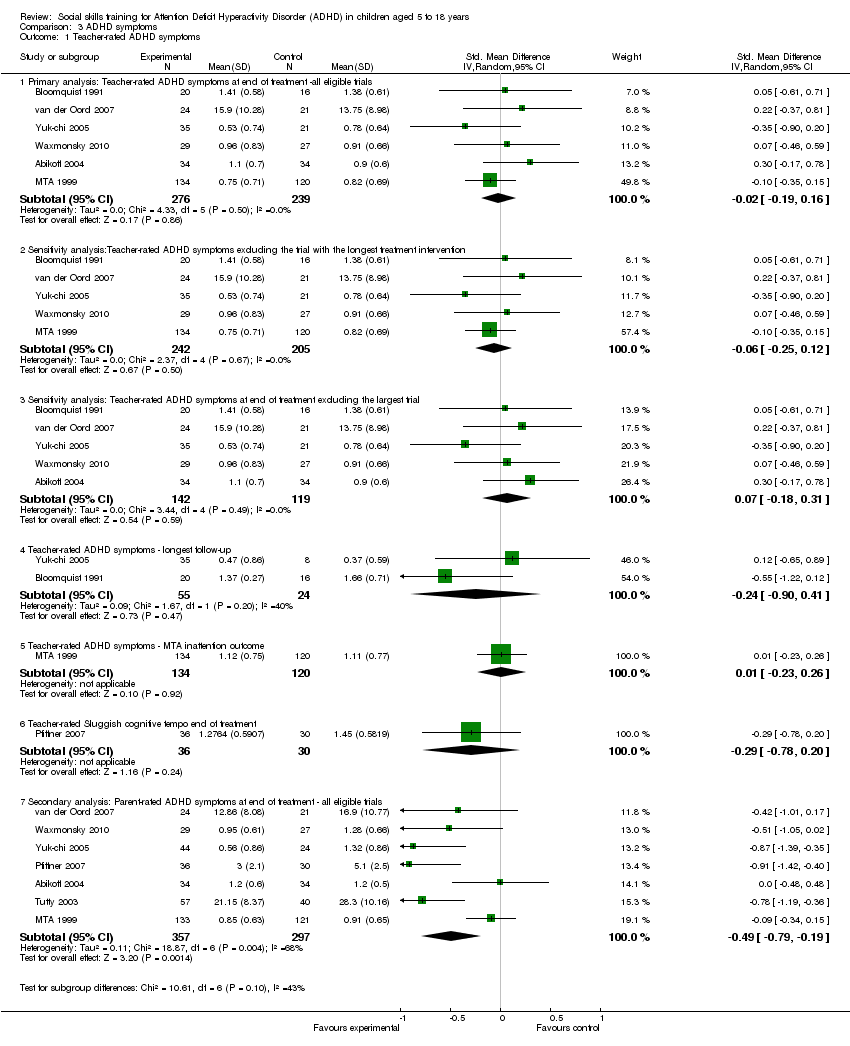
Comparison 3 ADHD symptoms, Outcome 1 Teacher‐rated ADHD symptoms.
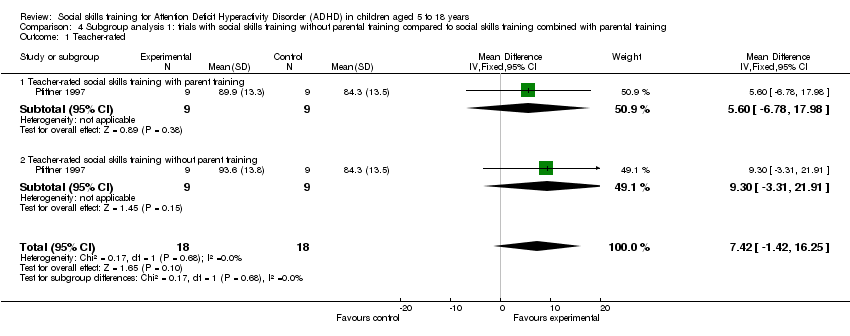
Comparison 4 Subgroup analysis 1: trials with social skills training without parental training compared to social skills training combined with parental training, Outcome 1 Teacher‐rated.
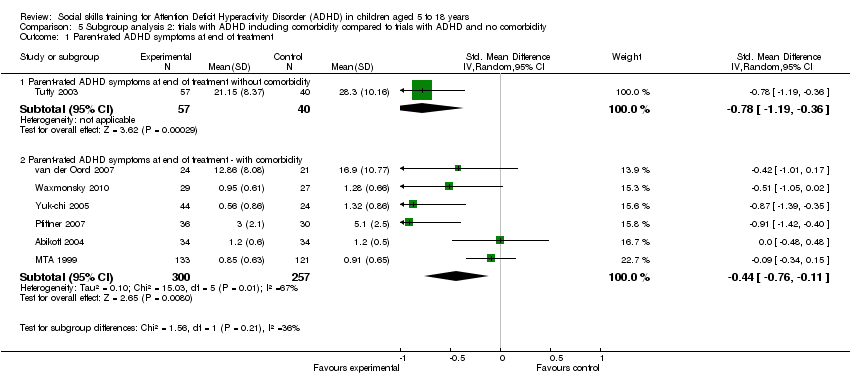
Comparison 5 Subgroup analysis 2: trials with ADHD including comorbidity compared to trials with ADHD and no comorbidity, Outcome 1 Parent‐rated ADHD symptoms at end of treatment.

Comparison 6 Subgroup analysis 3: trials with social skills training, parental training and medication compared to trials with social skills training and parental training without medication, Outcome 1 Social skills training, parent training, teacher consultations with and without medication.
| Social skills training compared to no intervention | ||||||
| Patient or population: Children aged 5 to 18 years with ADHD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Social skills training | ||||||
| Teacher‐rated social skills competences at end of treatment ‐ all eligible trials | The mean score for teacher‐rated social skills competences at end of treatment in the intervention groups was | 392 | ⊕⊕⊝⊝ | |||
| Teacher‐rated general behaviour at end of treatment ‐ all eligible trials | The difference in mean scores for teacher‐rated general behaviour at end of treatment between the intervention and control groups was | 358 | ⊕⊕⊝⊝ | |||
| Teacher‐rated ADHD symptoms ‐ all eligible trials | The mean score for teacher‐rated ADHD symptoms at end of treatment in the intervention groups was 0.02 standard deviations lower | 515 | ⊕⊕⊝⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% confidence interval). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Protocol | Review |
| Outcomes: In the protocol we stated that we would measure the two primary and the first two of secondary outcomes at short term (up to six months), medium term (six to 12 months), and long term (more than 12 months). In the protocol we did not prespecify the most important comparisons for the 'Summary of findings' table. | In the review this has been changed to at the end of treatment and at the longest follow‐up. We have added definition of adverse outcomes according to the International Committee of Harmonization guidelines (ICH 1996). In the review we used our two primary outcomes (teacher‐rated social‐skills and general behaviour) and the first secondary outcome (ADHD symptoms) in the 'Summary of findings' table. |
| Subgroup analysis: In the protocol we planned to perform subgroup analysis according to the following categories: | In the review it was not possible to perform subgroup analysis 1, 2, 5, and 6 due to lack of sufficient data in the included trials. |
| Risk of bias: In the protocol we had not planned to evaluate blinding of participants and personnel. In the protocol we stated that we would only use trials with low risk (or lower risk) of bias in the meta‐analysis. | In the review we also assessed the blinding of participants and personnel. In the review we changed this decision to restrict meta‐analysis to trials with comparable risk of bias (for example, all low risk, all unclear, or all the trials are at high risk of bias), and perform sensitivity analyses accordingly. |
| Sensitivity analysis: In the protocol we stated that we would repeat the analysis taking different methods used to handle the missing data into consideration. | In the review we did not perform this due to lack of necessary data. We have analysed the data 'as reported'. |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary analysis: Social skills competences Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Primary analysis: Teacher‐rated social skills competences at end of treatment ‐ all eligible trials | 5 | 392 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.04, 0.36] |
| 1.2 Sensitivity analysis:Teacher‐rated social skills competences ‐ excluding the trial with longest intervention duration (also largest trial) | 4 | 185 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.10, 0.48] |
| 1.3 Teacher‐rated social skills competences ‐ longest follow‐up | 1 | 18 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.86, 0.98] |
| 1.4 Secondary analysis: Parent‐rated social skills competences at end of treatment ‐ all eligible trials | 7 | 628 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.04, 0.40] |
| 1.5 Secondary analysis: Participant‐rated social skills competences at end of treatment ‐ all eligible trials | 2 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.09, 0.51] |
| 2 Teacher‐rated social skills (Mean Difference) Show forest plot | 4 | 185 | Mean Difference (IV, Random, 95% CI) | 1.81 [‐1.02, 4.64] |
| 3 Parent‐rated social skills competences (Mean Difference) Show forest plot | 5 | 313 | Mean Difference (IV, Random, 95% CI) | 2.82 [‐0.92, 6.56] |
| 4 Walker‐McConnel Social skills Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [‐0.47, 2.59] |
| 5 Social skills scale (UCI) parent‐rated Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 9.70 [6.07, 13.33] |
| 6 Child social skills knowledge scale Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [1.99, 6.41] |
| 7 Social interaction observation ‐ negative behaviour Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.11, 0.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary analysis: Teacher‐rated general behaviour Show forest plot | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Primary analysis: Teacher‐rated general behaviour at end of treatment ‐ all eligible trials | 3 | 358 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.21, 0.21] |
| 1.2 Sensitivity analysis: Teacher‐rated general behaviour excluding the trial with longest intervention duration | 2 | 290 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.27, 0.19] |
| 1.3 Sensitivity analysis: Teacher‐rated general behaviour excluding the largest trial | 2 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.24, 0.53] |
| 1.4 Teacher‐rated general behaviour ‐ longest follow‐up | 2 | 256 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.48, 0.01] |
| 1.5 Secondary analysis: Parent‐rated general behaviour at end of treatment | 1 | 254 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.51, ‐0.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Teacher‐rated ADHD symptoms Show forest plot | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Primary analysis: Teacher‐rated ADHD symptoms at end of treatment ‐all eligible trials | 6 | 515 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.19, 0.16] |
| 1.2 Sensitivity analysis:Teacher‐rated ADHD symptoms excluding the trial with the longest treatment intervention | 5 | 447 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.25, 0.12] |
| 1.3 Sensitivity analysis: Teacher‐rated ADHD symptoms at end of treatment excluding the largest trial | 5 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.18, 0.31] |
| 1.4 Teacher‐rated ADHD symptoms ‐ longest follow‐up | 2 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.90, 0.41] |
| 1.5 Teacher‐rated ADHD symptoms ‐ MTA inattention outcome | 1 | 254 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.23, 0.26] |
| 1.6 Teacher‐rated Sluggish cognitive tempo end of treatment | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.78, 0.20] |
| 1.7 Secondary analysis: Parent‐rated ADHD symptoms at end of treatment ‐ all eligible trials | 7 | 654 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.79, ‐0.19] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Teacher‐rated Show forest plot | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 7.42 [‐1.42, 16.25] |
| 1.1 Teacher‐rated social skills training with parent training | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐6.78, 17.98] |
| 1.2 Teacher‐rated social skills training without parent training | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 9.30 [‐3.31, 21.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Parent‐rated ADHD symptoms at end of treatment Show forest plot | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Parent‐rated ADHD symptoms at end of treatment without comorbidity | 1 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.19, ‐0.36] |
| 1.2 Parent‐rated ADHD symptoms at end of treatment ‐ with comorbidity | 6 | 557 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.76, ‐0.11] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Social skills training, parent training, teacher consultations with and without medication Show forest plot | 5 | 331 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.04, 0.56] |
| 1.1 Social skills training and parent training with medication | 3 | 244 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.10, 0.48] |
| 1.2 Social skills training, parent training without medication | 2 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.64 [‐0.48, 1.76] |