Anestesia raquídea‐epidural combinada versus anestesia raquídea para la cesárea
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐blind, prospective, randomised controlled trial carried out at a single site in Spain The trial has 4 arms | |
| Participants | 224 women for elective CS. Exclusions were contra‐indications to neuraxial block, and pre‐existing neurological disease. Average age of the women was 32, with a BMI of 28.4 | |
| Interventions | Women were randomised to
All blocks were done sitting at the level of the intercristal line. The doses of drugs used were not stated. | |
| Outcomes | The stated objective was to determine the incidence of paraesthesia with different spinal puncture techniques using a 27 gauge Whitacre needle. Outcomes were: rate of paraesthesia the dermatomes affected number of attempts at insertion. Number of women with PDPH also reported. | |
| Notes | University teaching hospital based in La Paz, Madrid, Spain Trial ran from January 2005 ‐ November 2006 Source of funding not stated No author declaration of interest stated Study had consent from the Ethics Committee of the hospital, and informed consent of the women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that a randomisation sequence was used |
| Allocation concealment (selection bias) | High risk | An anaesthetist who was not involved with the procedures communicated the allocation |
| Blinding of participants and personnel (performance bias) | High risk | The proceduralists were aware of the technique |
| Blinding of outcome assessment (detection bias) | High risk | The assessor was aware of the group allocation |
| Incomplete outcome data (attrition bias) | Low risk | All women were included |
| Selective reporting (reporting bias) | Low risk | All the proposed outcomes were reported |
| Other bias | High risk | This is a technical study assessing paraesthesia on needle insertion with subtle differences in needle placement. Further information requested from authors about drugs administered and any other secondary outcomes, including long‐term follow‐up if done, but the authors could not be contacted |
| Methods | Prospective randomised controlled trial was conducted at a single site in Italy The trial has 2 arms | |
| Participants | 100 women for elective CS, full‐term, healthy; no specific exclusions stated. No further information on the women was stated | |
| Interventions | Women were randomised to
The spinal component was with 5 mg 0.5% levobupivacaine plus sufentanil 5 mcg, followed by epidural of 0.25% levobupivacaine, either 10 or 12 mL depending on height greater or less than 162 cm, given through the needle; a 20 G epidural catheter was then placed for postoperative pain management. All blocks were at L1‐2. All women were in the sitting position and then placed supine with wedge under right hip immediately after the block. All women were pre‐hydrated with 500 mL of plasma expander | |
| Outcomes | There were no statements on pre‐study outcomes or power analysis. Reported outcomes were: number of women with hypotension defined as fall in BP of ≥ 20% of baseline or systolic BP < 100 mmHg; number with intra‐operative vomiting; number with bradycardia of < 60 bpm; number with intra‐operative discomfort; number with motor block, defined as inability to straight leg raise 50 minutes after the block | |
| Notes | National hospital based in Bari, Italy No information about the dates the trial Source of funding not stated No author declaration of interest stated Technique of L1‐2 spinal injection in both groups No information stated about ethics or informed consent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | All women were included |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised and double‐blinded controlled study conducted at single site in Seoul, Korea. There were 2 arms to the trial | |
| Participants | 100 women for elective CS, ≥ 36 weeks' gestation; exclusions were contra‐indications to neuraxial block, multiple pregnancy, placenta praevia and cardiac disease Participant characteristics and demographics were provided, with an average age of 30 years old, weight of 68.8 kg and height of 160.2 cm | |
| Interventions | Women were randomised to
The spinal component was with 6 mg 0.5% hyperbaric bupivacaine plus fentanyl 20 mcg, followed by epidural 10 mL of 0.25% bupivacaine 5 minutes after the intrathecal injection. All blocks were done in the right lateral position and all women were pre‐hydrated with 20 mL/kg of Ringer's lactate immediately before the block | |
| Outcomes | The pre‐study power analysis was based on the incidence of hypotension. Outcomes reported were: number of women with hypotension, defined as fall in BP of greater than or equal to 20% of baseline or systolic BP < 95 mmHg; amount of ephedrine used to treat hypotension; time to effective anaesthesia from intrathecal injection; number with intra‐operative nausea or vomiting; number with dizziness; time for sensory block regression to T10; time to onset of postoperative pain | |
| Notes | University hospital based in Seoul, Korea Institutional review board approval and written informed consent was obtained No information about the dates of the trial Source of funding not stated No author declaration of interest stated The pre‐study null hypothesis and outcomes were stated in the form of comparing CSE with SSS "in terms of feasibility and incidences of side effects such as hypotension and nausea" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated to have used computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Only statement is of "patients were allocated" |
| Blinding of participants and personnel (performance bias) | Low risk | The operator would have been aware of the allocation but the investigator was not; it is reasonable to conclude that the recording and management of their primary outcomes would not have been significantly influenced |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessor came into the room after the procedure; the protocol included a "sham" epidural catheter taped to the woman's back |
| Incomplete outcome data (attrition bias) | Low risk | 2 CSE women were excluded because of failure to complete the spinal component; although there were 2 failures of the CSE technique, they were recorded and can be identified against the outcome of complications of the interventions |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised and single‐blinded controlled study conducted at single site in Zagrab, Croatia There were 2 arms to the trial | |
| Participants | 77 ASA I/II women undergoing elective CS under regional anaesthesia with a singleton uncomplicated pregnancy > 37 weeks' gestation Participant characteristics and demographics were provided, with an average age of 31 years old, weight of 80 kg, height of 166 cm, gestation of 38.9 weeks | |
| Interventions | Women were randomised into 2 groups
The blocks in both groups were performed in the sitting position. The CSE group were placed in the lateral tilt supine position after the epidural catheter was secured. The SSS group were kept sitting for 3 minutes after spinal block before being positioned in the lateral tilt supine position. All women received fentanyl in their spinal dose: 15 mcg if height < 165 cm and 25 mcg if 165 ‐ 175 cm tall. All women received 500 mL of Ringers solution and were premedicated with metoclopramide 1 mg iv and ranitidine 75 mg iv. Intra‐operative pain was treated with 100 mg of sodium thiopentone iv | |
| Outcomes | There were no statements about pre‐study power analysis. The primary outcomes were stated as "haemodynamic changes during intraoperative time". These changes included: hypotension, defined as systolic BP < 90 mmHg or > 20% below baseline and bradycardia (HR < 50 bpm). Mean and diastolic BPs were also recorded. Total ephedrine use was also recorded (administered when hypotension as defined previously was observed). The secondary outcome was "the sensory block height, 10 minutes after spinal injection". Other reported outcomes commented on included lower limb motor block, intra‐operative pain ("failure of block"), nausea and vomiting, ephedrine consumption, total administered infusions, neonatal outcome (umbilical pH and Apgar scores at 1 and 10 minutes) Time to recovery of motor function and active mobilisation; pruritus and first request for analgesia was also reported. PDPH rates were also reported | |
| Notes | The Ethical Committee of General Hospital Pula approved the study protocol and written consent was obtained for the study which was conducted at the University hospital based in Zagreb, Croatia No information about the dates of the trial Source of funding not stated No author declaration of interest stated While pruritis was reported, no intervention was required in either group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "prospective, randomized study...". No comments relating to randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No details stated |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were blinded to group allocation. Proceduralist was not blinded as this is not feasible |
| Blinding of outcome assessment (detection bias) | High risk | Primary outcome assessment of haemodynamic effects and ephedrine use was not blinded. Assessment of the following outcomes were blinded: time for motor recovery and first analgesic request |
| Incomplete outcome data (attrition bias) | High risk | 1 woman was excluded after she had a recognised unintentional dural puncture during CSE placement Intention‐to‐treat analysis not used |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised and double‐blinded controlled study conducted at single site in Stanford, California, USA There were 2 arms to the trial | |
| Participants | 30 ASA I/II women, 18 ‐ 45 years of age, for elective CS under regional. Specific exclusions were BMI > 40 kg/m2, height < 150 cm, contraindications to regional block, spinal deformity or surgery, multiple gestations, labour, pre‐eclampsia, diabetes and cardiac conditions. Participant characteristics and demographics were provided, with an average age of 34 years, weight of 78 kg, height of 161 cm, gestation of 39 weeks | |
| Interventions | Women were randomised to
The spinal component was with the same solution as the SSS group, followed by immediate removal of the epidural and spinal needles without any medication epidurally nor placement of an epidural catheter. All blocks were done in the right lateral position and all women were pre‐hydrated with 1000 mL of Ringer's lactate plus 500 mL of Hetastarch immediately before the block | |
| Outcomes | The pre‐study power analysis was based on the difference in block height. Reported outcomes were: peak sensory level to pinprick, cold and touch CSF pressure immediately before intrathecal injection number requiring supplementation during the surgery amount of phenylephrine used to maintain BP within 10% of baseline | |
| Notes | Institutional review board approval and written informed consent were obtained University hospital based in Stanford, California, USA Women were enrolled over a 2‐month period from August ‐ October 2006 Quote: "This study was funded internally by the Department of Anesthesia, Stanford University School of Medicine, Stanford, California. The authors involved in this study received no external financial support. The authors share no relationship with any company or organisation with a vested interest in the outcome of this study." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated to have used computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Stated to have used sealed opaque envelopes opened on arrival in operating suite |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and primary anaesthetist were not blinded but the primary outcome of block characteristics is unlikely to be influenced by this |
| Blinding of outcome assessment (detection bias) | Low risk | Sensory level assessment was blinded; use of phenylephrine for BP control was not; need for supplementation not blinded |
| Incomplete outcome data (attrition bias) | High risk | 2 women were excluded from the SSS group due to inability to perform spinal in lateral position. 1 woman in the SSS group did not have CSF pressure results |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised double‐blinded controlled trial in Singapore There were 2 arms to the trial | |
| Participants | 30 ASA I women for elective CS under regional. Specific exclusions were BMI < 20 or > 35 kg/m2, height < 145 or greater than 180 cm, contra‐indications to regional block, allergy to study drugs, multiple gestations, labour, pre‐eclampsia, and placenta praevia Participant characteristics and demographics were provided, with an average age of 32 years old, weight of 71 kg, height of 154 cm, and a BMI of 30 | |
| Interventions | Women were randomised to
The CSE spinal component was with the same solution as the SSS group followed by immediate removal of the epidural and spinal needles without any medication epidurally nor placement of an epidural catheter. All blocks were done in the right lateral position and all women were pre‐hydrated with 500 mL of Ringer's lactate immediately before the block | |
| Outcomes | The pre‐study power analysis was based on the difference in sensory block height Reported outcomes were: peak sensory level to cold; time taken to achieve the maximal block height after intrathecal injection; number of women achieving a sensory level of T4 or higher; maximal motor block from Bromage score; number of women with hypotension defined as fall in systolic BP of > 20% from baseline; amount of ephedrine used to treat hypotension; maximum decrease in systolic pressure; maximum decrease in heart rate; number with intra‐operative nausea or vomiting; number with shivering; time for sensory block regression to T10; number requiring supplementation during the surgery | |
| Notes | Hospital research ethics committee and informed written consent were obtained National hospital based in Singapore No information about the dates of the trial Source of funding not stated No author declaration of interest stated There are a large number of secondary outcomes reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized into one of the two groups using sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and primary anaesthetist were not blinded but the primary outcomes of block characteristics are unlikely to be influenced by this |
| Blinding of outcome assessment (detection bias) | Low risk | The primary outcome of sensory level assessment was blinded. The use of phenylephrine for BP control and the need for supplementation were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Sensory levels were reported for all the enrolled women |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised controlled trial at a single study site conducted in Korea There were 2 arms to the trial | |
| Participants | 30 healthy women with an mean gestational date of 38 weeks, 15 in each group Participant characteristics and demographics were provided, with an average age of 30 years old, weight of 67 kg, height of 157 cm, gestational age 38.8 weeks and a birthweight of 3.3 kg | |
| Interventions |
If the block in the sequential CSE group did not reach the T(4) level in 15 minutes, it was extended by fractionated doses of 0.5% bupivacaine administered through the epidural catheter | |
| Outcomes | The time intervals from induction of block to start of surgery and delivery time, total duration of the surgery and the interval between first analgesia and first request of postoperative pain relief were measured The level of analgesia measured by pinprick at 15 minutes as well as post‐operatively Degree of muscle relaxation, as assessed by the surgeon and women's assessment of surgical anaesthesia Apgar at 1 min and 5 min post‐delivery Complications reported were hypotension, PDPH | |
| Notes | No information stated about ethics approval or informed written consent University hospital based in Seoul, Korea No information about the dates of the trial Source of funding not stated No author declaration of interest stated All women in sequential CSE group needed epidural bupivacaine, 51.3 ± 3.5 mg (mean ± SEM) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thirty healthy parturients were randomly divided." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | All women were included in this study. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised controlled trial at a single study site conducted in Korea There were 2 arms to the trial | |
| Participants | 50 healthy women with a mean gestational date of 39 weeks Participant characteristics and demographics were provided, with an average age of 30 years old, weight of 66 kg, height of 159 cm, gestational age 39 weeks | |
| Interventions |
| |
| Outcomes | Maternal hypotension time to T4 sensory block and peak sensory block mean duration of block time of the end of the injection to delivery and time of surgery changes in sensory block over time women's assessment of analgesia complications which included nausea, vomiting, pruritus and headaches | |
| Notes | No information stated regarding ethics approval or informed written consent University hospital based in Seoul, Korea No information about the dates of the trial Source of funding not stated No author declaration of interest stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Fifty parturients were randomly divided." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Quote: "four people from CSE were excluded as analgesia was not bearable." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised controlled trial conducted in Korea There were 2 arms to the trial | |
| Participants | 46 women having a caesarean birth | |
| Interventions |
| |
| Outcomes | Upper block height assessed with alcohol swab, sharp pinprick and pinprick touch at 5, 10, 15, 20, 30, 60, 90 and 120 minutes after intrathecal injection. There were no outcomes of relevance to this review. | |
| Notes | No information stated regarding ethics approval or informed written consent University hospital based in Seoul, Korea No information about the dates of the trial Source of funding not stated No author declaration of interest stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "parturients were randomly given (spinal anaesthesia)" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | All women were included |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Free from other source of bias |
| Methods | Prospective randomised double‐blinded controlled trial in Singapore There were 2 arms to the trial | |
| Participants | 62 ASA I/II women for elective CS, 50 ‐ 95 kg, height 150 ‐ 170 cm, singleton; exclusions were emergency CS, contra‐indications to neuraxial block, hypertensive disorders and peripartum haemorrhagic conditions Participant characteristics and demographics were provided, with an average age of 33 years old, weight of 69 kg, height of 157 cm | |
| Interventions | Women were randomised to
All blocks were done in the right lateral position and all women were pre‐hydrated with 500 mL of Ringer's lactate immediately before the block. Any subsequent breakthrough pain defined as VAS > 30 (on a 101‐point scale) was treated in the SSS group with intravenous fentanyl or ketorolac and in the CSE group with epidural 3 mL of 1.5% lignocaine | |
| Outcomes | The pre‐study power analysis was based on the incidence of hypotension and motor block duration based on a pilot study. Outcomes reported were: number of women with hypotension defined as fall in MAP of ≥ 20% of baseline or systolic BP < 100 mmHg amount of ephedrine used to treat hypotension lowest systolic BP maximal sensory block height maximal modified Bromage score and time for regression to a score of 0 time for sensory block regression to T10 Also reported were: the need for intervention for intra‐operative pain including conversion to general anaesthesia, satisfaction on a 4‐point scale, number with PDPH, number requiring intervention for high block or respiratory depression. Also commented upon but not directly reported were: maternal side effects of nausea and vomiting, pruritus, shivering and neonatal Apgar scores and cord pH | |
| Notes | The hospital research ethics committee and informed written consent were obtained for a trial to be conducted at a National hospital based in Singapore No information about the dates of the trial Source of funding not stated No author declaration of interest stated Outcomes of maternal side effects of nausea and vomiting, pruritus, shivering and neonatal Apgars and cord pH have been requested from author but the data could not be found | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated to have used computer‐generated random number tables |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and primary anaesthetist were not blinded but the primary outcomes of hypotension and block characteristics are unlikely to be influenced by this |
| Blinding of outcome assessment (detection bias) | Low risk | The outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | Low risk | All women were included |
| Selective reporting (reporting bias) | Low risk | All the primary outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised double‐blinded controlled trial in Singapore. There were 2 arms to the trial | |
| Participants | 40 ASA I women in established labour for emergency CS under regional anaesthesia. Specific exclusions were contra‐indications to regional block and allergy to study drugs Participant characteristics and demographics were provided, with an average age of 32 years old, weight of 70 kg, height of 157 cm, BMI of 28.2 | |
| Interventions | Women were randomised to
The CSE spinal component was with the same solution as the SSS group, followed by immediate removal of the epidural and spinal needles without any medication epidurally nor placement of an epidural catheter. All blocks were done in the right lateral position | |
| Outcomes | The pre‐study power analysis was based on the difference in sensory block height. Reported outcomes were: peak sensory level and maximal motor block from Bromage score; time taken to achieve the maximal sensory block height and motor block after intrathecal injection time for sensory block regression to T10 and for the motor block to recede to Bromage score of 2 number of women with hypotension amount of phenylephrine used to treat hypotension maximum decrease in systolic pressure maximum decrease in heart rate number with intra‐operative nausea or vomiting number with shivering number requiring supplementation during the surgery | |
| Notes | The hospital research ethics committee and informed written consent were obtained for a trial to be conducted at a National hospital based in Singapore No information about the dates of the trial Source of funding not stated No author declaration of interest stated Subject to sensitivity analysis, as participants were in labour. Analyses for which data were available were 1.1, 1.2, 1.3 and 1.6. and as there were zero events for the first 3 of these, sensitivity was only undertaken for outcome 1.6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized using sealed opaque envelopes". |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes." |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and primary anaesthetist were not blinded but the primary outcomes of block characteristics are unlikely to be influenced by this |
| Blinding of outcome assessment (detection bias) | Low risk | The investigator for block characteristics and haemodynamic change was blinded |
| Incomplete outcome data (attrition bias) | Low risk | All women were accounted for |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported |
| Other bias | High risk | Hypotension is not defined, nor protocol for phenylephrine, nor any fluid loading |
| Methods | Prospective randomised single‐blinded controlled trial in Glasgow, Scotland There were 2 arms to the trial | |
| Participants | 70 ASA I/II women for elective CS with singleton pregnancy. Specific exclusions were < 36 weeks' gestation, contra‐indications to regional block, fetal compromise, maternal cardiac, renal or respiratory disease, pregnancy‐induced hypertension or previous abdominal surgery Participant characteristics and demographics were provided, with an average age of 32 years old, weight of 79 kg, height of 162 cm and a gestational age of 39 weeks | |
| Interventions | Women were randomised to
The CSE spinal component was with the same solution as the SSS group, followed by immediate removal of the epidural and spinal needles without any medication epidurally nor placement of an epidural catheter. All blocks were done in the sitting position and all women were pre‐hydrated with 500 mL of Hartmann's solution immediately before the block | |
| Outcomes | The pre‐study power analysis was based on the amount of ephedrine used to manage hypotension. The reported outcomes were: peak sensory level and maximal motor block from Bromage score time taken to achieve the maximal sensory block height and motor block after intrathecal injection time for sensory block regression to T10 and for the motor block to recede to Bromage score of 2 number of women with hypotension amount of ephedrine used to treat hypotension maximum decrease in systolic pressure maximum decrease in heart rate number with intra‐operative nausea or vomiting number with shivering number requiring supplementation during the surgery | |
| Notes | Hospital research ethics committee and informed written consent was obtained National hospital based in Glasgow, Scotland No information about the dates of the trial Source of funding not stated No author declaration of interest stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised into 2 groups "using a computer generated randomization list". |
| Allocation concealment (selection bias) | Low risk | Stated to have used "sealed envelopes". |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and primary anaesthetist were not blinded but the primary outcomes of hypotension are unlikely to be influenced by this. The management of the hypotension was by a blinded anaesthetist |
| Blinding of outcome assessment (detection bias) | Low risk | The BP measurements and hypotension management were performed by a blinded observer |
| Incomplete outcome data (attrition bias) | Low risk | All the women were accounted for |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised single‐blinded controlled trial in Brisbane, Australia There were 2 arms to the trial | |
| Participants | 89 women for elective CS. Specific exclusions were failure to obtain consent, emergency procedure and contraindication to neuraxial block. Participant characteristics and demographics were provided, with an average age of 32 years old, weight of 79 kg, height of 162 cm and a gestational age of 39 weeks | |
| Interventions | Women were randomised to
The same brand and needle designs and sizes were used on all women. All blocks were performed in the midline but there was a mixture of sitting and lateral positioning and of loss of resistance techniques at anaesthetist's preference. The 3rd or 4th lumbar interspace was used for the blocks. The SSS was performed using a 26 G pencil‐point needle through an introducer while the CSE was performed as a needle‐through‐needle technique with a 16 G Tuohy needle and 26 G spinal needle. An undisclosed dose of 0.5% hyperbaric bupivicaine plus opioid was used in the spinal component of each block | |
| Outcomes | The primary outcome was incidence of paraesthesia during spinal needle insertion Also reported the number requiring further interventions including general anaesthesia | |
| Notes | Hospital research ethics committee and informed consent were obtained National hospital based in Brisbane, Australia Women were enrolled from July 2001 ‐ March 2002 Source of funding not stated No author declaration of interest stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated number sequence." |
| Allocation concealment (selection bias) | High risk | Allocation was "via sealed envelopes" immediately prior to neuraxial block insertion. The allocation of operator was not randomised or concealed and there were 3 grades of operator experience involved; it is possible that some selection bias may have been generated at this point |
| Blinding of participants and personnel (performance bias) | Low risk | None of participants, proceduralists or observers were blinded. It is unlikely that this lack of blinding significantly influenced the conduct of the procedure |
| Blinding of outcome assessment (detection bias) | Unclear risk | The use of a blinded observer was attempted but this proved unworkable. The endpoint of paraesthesia as defined is unlikely to be significantly influenced by this lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | All the data were reported |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported |
| Other bias | High risk | There was a mixture of sitting and lateral positioning and of loss of resistance techniques. There was a higher proportion of high‐BMI women in the SSS group |
| Methods | Prospective randomised and single‐blinded controlled study conducted at single site in North Carolina, USA There were 2 arms to the trial | |
| Participants | 44 parturients weighing > 100 kg presenting for elective CS. Specific exclusions were any contra‐indication to neuroaxial anaesthesia and associated spinal drugs. 3 participants were eventually excluded due to protocol violation Participant characteristics and demographics were provided, with an average age of 30 years old, weight of 136 kg, height of 166 cm, BMI of 49.3 and a gestational age of 38 weeks | |
| Interventions | Women were randomised to
All blocks were performed in the sitting position. Upon confirming free aspiration of cerebrospinal fluid, both groups were administered a subarachnoid dose of 12 mg of hyperbaric bupivicaine with 20 mcg of fentanyl and 200 mcg of preservative free morphine All SSS blocks were done with Whitacre needles, or similar non‐cutting needles if longer needles were required. CSE was performed using loss of resistance (not stated to air or saline) after which a range of different non‐cutting needles to the SSS group were used to perform the block. In the CSE group, after administering the spinal dose, the epidural catheter was inserted and secured after the spinal needle was removed. Testing or dosing of the epidural catheter was withheld unless supplement surgical anaesthesia was needed. Both groups were positioned supine with left lateral tilt once the neuroaxial block was performed | |
| Outcomes | A pre‐study power analysis was performed. The primary outcome was time taken from insertion of introducer (SSS) or epidural needle (CSE) to successful intrathecal injection Secondary outcomes included proportion of first operators to complete the procedure in under 10 minutes total number of attempts time from intrathecal injection to T4 block women's verbal numerical pain score of procedure number of unintentional dural punctures with Tuohy needle number of PDPHs Apgar scores at 1 and 5 minutes requirement for epidural supplementation in CSE group excessively high blocks/vasopressor use between groups | |
| Notes | University hospital based in Winston‐Salem, North Carolina, USA Institutional review board approval and written informed consent were obtained Women were enrolled from September 2004 ‐ August 2007 No additional funding other than department funds The authors declare no conflicts of interest High block and vasopressor use were reported as not different between groups but no data were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization sequence and allocation (generated with Microsoft Office Exels's random number function [RAND] and roup sorting function) were created for the 2 groups". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "listed sequentially on the pages inside a binder securely stored in the research file cabinet". Comment: Not stated if pages were sealed, opaque or interchangeable |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and interventionalists not feasible and deemed low risk of introducing bias by review authors |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "Research personnel, not involved with the medical care of the subject, recorded...". Comment: Not stated if the research personnel were blinded to the technique used |
| Incomplete outcome data (attrition bias) | High risk | Missing outcome data (1 participant in SSS group and 2 participants in CSE group) similar across intervention groups, with same reasons for missing data in both groups |
| Selective reporting (reporting bias) | High risk | A number of secondary outcomes were first mentioned in the Results or Discussion section |
| Other bias | Low risk | No other sources of bias |
| Methods | Prospective randomised non‐blinded controlled trial in Antalya, Turkey There were 3 arms to the trial | |
| Participants | ASA I/II women aged 18 ‐ 40 years who were full‐term gestation (37 ‐ 42 weeks) scheduled for CS. Specific exclusions were allergy to local anaesthetic, diabetes mellitis, weight > 100 kg, height < 155 cm, pre‐eclampsia, placenta praevia, fetal abnormalities, fetal bradycardia, or maternal neurological or psychiatric disorders Participant characteristics and demographics were provided, with an average age of 30 years old, weight of 77 kg, height of 163 cm, BMI of 28.9 and a gestational age of 38 weeks | |
| Interventions | Women were randomised to 3 groups
Spinal drugs and dosing regimens were identical amongst all 3 groups, using 0.5% levobupivicaine and 20 mcg of fentanyl. Spinal dose was determined according to woman's height with ≤ 160 cm receiving 10 mg, 161 ‐ 164 cm receiving 12 mg, 165 ‐ 169 receiving 14 mg and ≥ 170 receiving 15 mg. Blocks were performed in the right lateral recumbent position. All women received 1000 mL of Ringers lactate prior and all women received a "prophylactic" dose of ephedrine intravenously immediately following block completion. The principal author performed all blocks. Spinals were performed with 27 G Quincke needles. CSEs performed with 18 G Tuohy needles and 27 G spinal needles (needle‐through‐needle technique, type of spinal needle not specified). Women were placed in supine position with left lateral tilt immediately after completion of block | |
| Outcomes | The pre‐study power analysis was based on the time to reach maximum sensory block. Reported outcomes included time to onset of sensory block time for sensory block to reach T10 the level of maximum sensory block 2 segment‐regression time of sensory block regression of sensory block to T10 time to onset of motor block time to reach maximum motor block time to recover from motor block quality of intra‐operative anaesthesia including failed block or inadequate block requiring GA or need for adjuvant anaesthesia Heart rate and total ephedrine use for intra‐operative hypotension (< 20% of baseline) were recorded, as were Apgar scores at 1 and 5 minutes | |
| Notes | University research ethics committee and informed written consent were obtained University hospital based in Antalya, Turkey No information about the dates of the trial This study was supported by a Grant from the Akdeniz University School of Medicine’s Research and Application Centre The authors declare no conflicts of interest Time to effective anaesthesia not reported as an outcome. Further details on the Apgars and hypotension data were requested from the authors but we could not reach them | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Care providers in the labour room generated the random sequence generation". |
| Allocation concealment (selection bias) | Low risk | Quote: "The patients were randomly assigned to one of the three groups using sealed opaque envelopes...". |
| Blinding of participants and personnel (performance bias) | High risk | The participants and proceduralists were unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "anesthesiologist who was unaware of the technique and drug used received by each patient recorded hemodynamic status and block profile". Comment: No comment on blinding for neonatal outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All women were accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | High risk | Spinal doses varied according to height so "high dose vs low dose" categorisation was problematic. Most women were > 160 cm so received high‐dose spinal; we therefore ranked this study as a 'high‐dose' spinal study. The 2 CSE arms of the trial were compared independently to the spinal arm as either low‐dose sequential or EVE |
| Methods | Prospective randomised controlled study conducted at single site in Orebro, Sweden There were 2 arms to the trial | |
| Participants | 42 ASA I women, at term, singleton pregnancies without complications for elective CS under regional Participant characteristics and demographics were provided, with an average age of 30 years old, weight of 75 kg, height of 165 cm, gestational age of 38 weeks and gestational weight of 3602 g | |
| Interventions | Women were randomised to
The spinal component was with 7.5 mg of 0.5% hyperbaric bupivacaine followed by immediate removal of the epidural and spinal needles and insertion of an epidural catheter; if the block had not achieved a sensory level of T4 after 15 minutes, a bolus of 10 mg of bupivacaine per unblocked segment was administered epidurally. All blocks were done in the sitting position and all women were pre‐hydrated with 1500 to 2000 mL of Ringer's lactate immediately before the block All the spinals were performed with Quincke needles. The prehydration varied | |
| Outcomes | Level of sensory loss of pinprick 15 minutes after intrathecal injection number of women achieving a sensory level of T4 or higher at 15 minutes number of women with hypotension defined as fall in systolic BP of greater than 20% from baseline or systolic pressure less than 100 mmHg women's assessment of analgesia before and after delivery | |
| Notes | Institutional review board approval and written informed consent were obtained National hospital based in Orebro, Sweden No information about the dates of the trial Source of funding not stated No author declaration of interest stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomized into two groups". Comment: Insufficient information on randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No statement about this |
| Blinding of participants and personnel (performance bias) | High risk | The participants, proceduralists and observers were all unblinded |
| Blinding of outcome assessment (detection bias) | High risk | All the observations except the neonatal assessments were unblinded |
| Incomplete outcome data (attrition bias) | Low risk | All the women were included |
| Selective reporting (reporting bias) | Low risk | All the outcomes were reported |
| Other bias | Low risk | No other sources of bias |
| Methods | Prospective randomised controlled study conducted at single site in Delhi, India There were 3 arms to the trial | |
| Participants | 60 ASA I/II women of ≥ 37 weeks' gestation for elective CS under regional. Specific exclusions were BMI < 20 or > 35 kg/m2, height < 145 or > 180 cm, contra‐indications to regional block, multiple gestations, labour and placenta praevia Participant characteristics and demographics were provided, with an average age of 25 years old, weight of 57 kg, height of 152 cm | |
| Interventions | Women were randomised to
All blocks were done in the sitting position and all women were pre‐hydrated with 10 mL/kg of Ringer's lactate immediately before the block | |
| Outcomes | The pre‐study power analysis was based on the difference in sensory block height Reported outcomes were: peak sensory level to pinprick time taken from intrathecal injection to the following levels ‐ maximal block height, onset to T10, onset to T6, 2‐segment regression from maximum, regression to T10 number of women achieving a sensory level of T6 or higher maximal motor block by Bromage score time to maximum motor block time to complete motor block regression number of women with hypotension defined as fall in systolic BP of greater than 20% from baseline amount of ephedrine used to treat hypotension in 6 mg boluses number with intra‐operative nausea or vomiting number with shivering number with pruritus number requiring supplementation during the surgery 1 and 5 minute Apgar scores | |
| Notes | Institutional review board approval and written informed consent were obtained University hospital based in Delhi, India No information about the dates of the trial Source of funding not stated No author declaration of interest stated There are a large number of secondary outcomes reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Women were randomised" Comment: There is no randomisation method stated. |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) | Low risk | The participants and proceduralists were not blinded, but this probably did not influence the primary outcomes pertaining to block characteristics |
| Blinding of outcome assessment (detection bias) | Low risk | The block assessments were done by a blinded anaesthetist and the epidural catheter was concealed |
| Incomplete outcome data (attrition bias) | Low risk | All women included |
| Selective reporting (reporting bias) | Low risk | All the primary outcomes have data reported |
| Other bias | Low risk | Free from other sources of bias |
| Methods | Prospective randomised controlled study conducted at single site in Izmir, Turkey There were 2 arms to the trial | |
| Participants | 40 ASA I/II women for elective CS, healthy, full‐term, singleton; exclusions were contra‐indications to neuraxial block, known allergies to medications used, fetal pathology Participant characteristics and demographics were provided, with an average age of 29 years old, weight of 76 kg, height of 163 cm and a gestational age of 39 weeks | |
| Interventions | Women were randomised to
The spinal component was with 7.5 mg 0.5% hyperbaric bupivacaine, followed 10 minutes after the spinal with 2 mL of 0.5% bupivacaine per segment of block height below T4 level via the epidural catheter. All blocks were done in the sitting position at lumbar spine level L2‐3 or L3‐4. All women were pre‐hydrated with 15 mL/kg of balanced electrolyte solution. Any subsequent breakthrough pain defined as VAS ≥ 3 (on a scale of 0 ‐ 10) was treated in the SSS group with intravenous fentanyl and in the CSE group with epidural 2 mL of 0.5% bupivacaine | |
| Outcomes | There were no statements regarding pre‐study outcomes and power analysis Reported outcomes were: number of hypotensive episodes, defined as fall in BP of ≥ 20% of baseline or systolic BP < 90 mmHg amount of ephedrine used to treat hypotension in 5 ‐ 10 mg boluses maximal sensory height of block to pinprick time taken from intrathecal injection to achieve T4 sensory level and subsequent time to regression to T10 maximal degree of motor block and time of motor block regression from modified Bromage score 3 to 1 Also reported were: number with intra‐operative pain; nausea or vomiting; 1‐ and 5‐minute Apgar scores; umbilical vein and artery pH and base deficit NACS. Women were also followed up for PDPH | |
| Notes | Institutional ethics committee approval and informed consent were obtained University hospital based in Izmir, Turkey No information about the dates of the trial Source of funding not stated No author declaration of interest stated All the spinals were performed with Quincke needles. There were no opioids used intrathecally. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated except for neonatal which were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | A number of outcomes have percentage but no absolute numbers reported |
| Selective reporting (reporting bias) | Low risk | All outcomes have been reported |
| Other bias | Low risk | No other sources of bias |
ASA: American Society of Anesthesiologists; BP: blood pressure; CS: caesarean section; CSE: combined spinal‐epidural; iv: intravenous; PDPH: post‐dural puncture headache; SSS: single‐shot spinal; VAS: visual analogue score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This is a study involving SSS and CSE for caesarean section which evaluated post‐operative pain relief only, which is not relevant for our review | |
| This is a study involving PCEA versus epidural or intrathecal opioid bolus administration for caesarean section which evaluated post‐operative pain relief only, which is not relevant for our review | |
| This is a study involving SSS and CSE for caesarean section which evaluated post‐operative pain relief only, which is not relevant for our review |
CSE: combined spinal‐epidural
SSS: single‐shot spinal
PCEA: patient‐controlled epidural anaesthesia
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Prospective randomised study The aim of the study was to evaluate the influence of epidural restriction (injection of saline) on the distribution of anaesthesia as well as the incidence of hypotension during the spinal anaesthesia |
| Participants | 60 full‐term parturient women (ASA I or II) with uncomplicated pregnancies |
| Interventions | Single‐shot spinal anaesthesia and CSE‐EVR blocks were performed at the L2/3 or L3/4 level in a sitting position. In the CSE‐EVR group using the needle‐through‐needle technique |
| Outcomes | Height of the block was assessed by the pinprick method and the motor block by the Bromage scale, 10 minutes after spinal injection, during the operation time and at the end of surgery. Haemodynamic monitoring (NIBP, HR) was assessed every 2 minutes until the childbirth, then every 5 minutes during operative time. Anaesthetic efficacy was evaluated for breakthrough pain by visual analogue pain score, Apgar score at birth, umbilical artery pH, and epinephrine consumption |
| Notes | Further clarity from authors required on this study compared to Fabris 2013 Consideration for inclusion in the next update |
| Methods | Prospective randomised study with 2 arms, based in Tunisia |
| Participants | 24 pre‐eclamptic women undergoing non‐emergency caesarean sections |
| Interventions | Spinal group (n = 11) received intrathecal injection of 10 mg bupivacaine with fentanyl and morphine. The CSE group (n = 13) received 5 mg bupivacaine with the same doses of fentanyl and morphine, with further supplementation with bupivacaine administered by the epidural catheter |
| Outcomes | Incidence and severity of hypotension; nausea and vomiting; women's satisfaction |
| Notes | Abstract only; awaiting more detail |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade Show forest plot | 7 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.05, 1.97] |
| Analysis 1.1  Comparison 1 CSE versus high‐dose spinal, Outcome 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade. | ||||
| 1.1 No ED use CSE versus high dose spinal | 5 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.05, 1.97] |
| 1.2 Sequential CSE versus high dose spinal | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion Show forest plot | 7 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.19, 8.43] |
| Analysis 1.2  Comparison 1 CSE versus high‐dose spinal, Outcome 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion. | ||||
| 2.1 No ED use CSE versus high dose spinal | 4 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Sequential CSE versus high dose spinal | 3 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.18, 21.49] |
| 2.3 EVE CSE versus high dose spinal | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.28] |
| 3 Number of women requiring intra‐operative conversion to general anaesthesia Show forest plot | 7 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| Analysis 1.3  Comparison 1 CSE versus high‐dose spinal, Outcome 3 Number of women requiring intra‐operative conversion to general anaesthesia. | ||||
| 3.1 No ED use CSE versus high dose spinal | 4 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Sequential CSE versus high dose spinal | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 3.3 EVE CSE versus high dose spinal | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of women satisfied with anaesthesia Show forest plot | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| Analysis 1.4  Comparison 1 CSE versus high‐dose spinal, Outcome 4 Number of women satisfied with anaesthesia. | ||||
| 4.1 Sequential CSE versus high dose spinal | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 5 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 CSE versus high‐dose spinal, Outcome 5 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery. | ||||
| 6 Number of women with hypotension Show forest plot | 4 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.33] |
| Analysis 1.6  Comparison 1 CSE versus high‐dose spinal, Outcome 6 Number of women with hypotension. | ||||
| 7 Number of women with nausea or vomiting or both Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.02, 4.61] |
| Analysis 1.7  Comparison 1 CSE versus high‐dose spinal, Outcome 7 Number of women with nausea or vomiting or both. | ||||
| 8 Number of women with a post‐dural puncture headache Show forest plot | 3 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.32, 2.15] |
| Analysis 1.8  Comparison 1 CSE versus high‐dose spinal, Outcome 8 Number of women with a post‐dural puncture headache. | ||||
| 9 Mean umbilical artery pH at delivery Show forest plot | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| Analysis 1.9  Comparison 1 CSE versus high‐dose spinal, Outcome 9 Mean umbilical artery pH at delivery. | ||||
| 10 Number of neonates with Apgar less than 7 at 5 minutes Show forest plot | 4 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.10  Comparison 1 CSE versus high‐dose spinal, Outcome 10 Number of neonates with Apgar less than 7 at 5 minutes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade Show forest plot | 3 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [0.24, 97.80] |
| Analysis 2.1  Comparison 2 CSE versus low‐dose spinal, Outcome 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade. | ||||
| 1.1 No ED use CSE versus low dose spinal | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Sequential CSE versus low dose spinal | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [0.24, 97.80] |
| 1.3 EVE CSE versus low dose spinal | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion Show forest plot | 4 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.78, 3.92] |
| Analysis 2.2  Comparison 2 CSE versus low‐dose spinal, Outcome 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion. | ||||
| 2.1 No ED use CSE versus low dose spinal | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Sequential CSE versus low dose spinal | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 EVE CSE versus low dose spinal | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.78, 3.92] |
| 3 Number of women requiring intra‐operative conversion to general anaesthesia Show forest plot | 3 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.3  Comparison 2 CSE versus low‐dose spinal, Outcome 3 Number of women requiring intra‐operative conversion to general anaesthesia. | ||||
| 3.1 No ED use CSE versus low dose spinal | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Sequential CSE versus low dose spinal | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 EVE CSE versus low dose spinal | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery Show forest plot | 2 | 160 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.52, 1.18] |
| Analysis 2.4  Comparison 2 CSE versus low‐dose spinal, Outcome 4 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery. | ||||
| 5 Number of women with hypotension Show forest plot | 4 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.93] |
| Analysis 2.5  Comparison 2 CSE versus low‐dose spinal, Outcome 5 Number of women with hypotension. | ||||
| 6 Number of women with nausea or vomiting or both Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.89] |
| Analysis 2.6  Comparison 2 CSE versus low‐dose spinal, Outcome 6 Number of women with nausea or vomiting or both. | ||||
| 7 Number of women with a post‐dural puncture headache Show forest plot | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.7  Comparison 2 CSE versus low‐dose spinal, Outcome 7 Number of women with a post‐dural puncture headache. | ||||
| 8 Mean umbilical artery pH at delivery Show forest plot | 1 | 76 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.68, 1.66] |
| Analysis 2.8  Comparison 2 CSE versus low‐dose spinal, Outcome 8 Mean umbilical artery pH at delivery. | ||||
| 9 Number of neonates with Apgar less than 7 at 5 minutes Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.9  Comparison 2 CSE versus low‐dose spinal, Outcome 9 Number of neonates with Apgar less than 7 at 5 minutes. | ||||
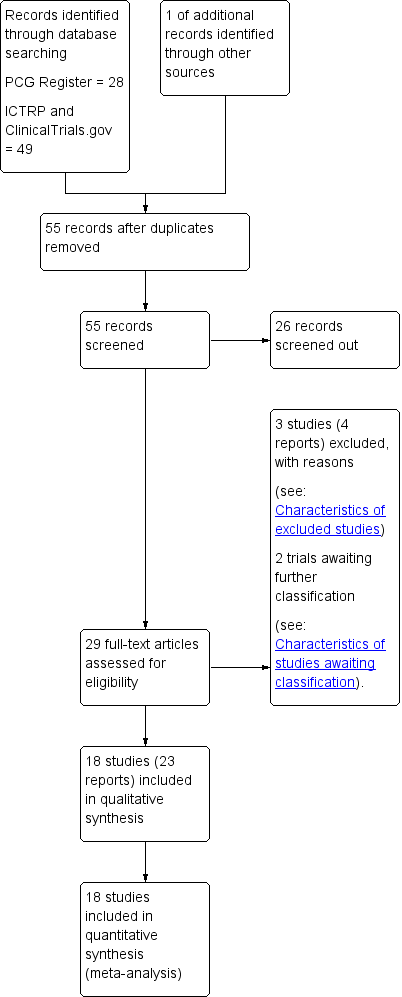
Study flow diagram.
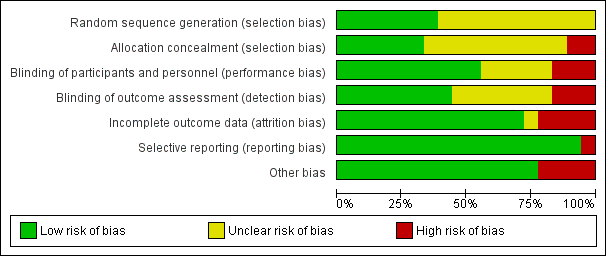
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 CSE versus high‐dose spinal, Outcome 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade.

Comparison 1 CSE versus high‐dose spinal, Outcome 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion.
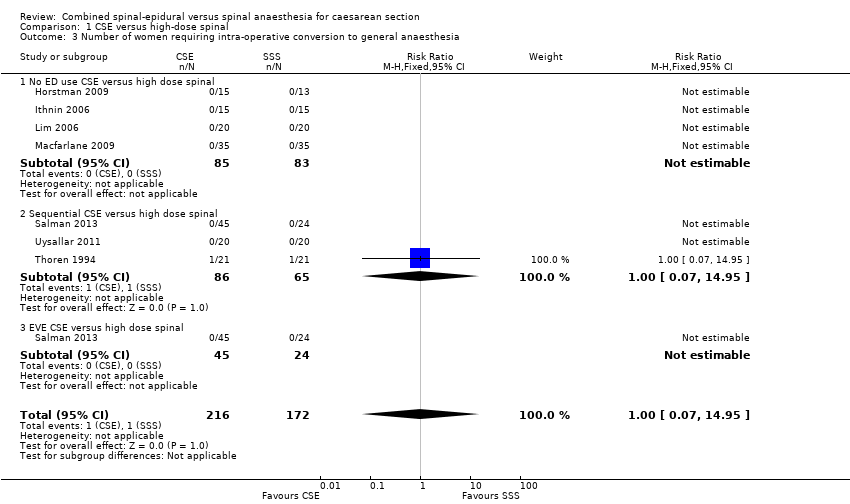
Comparison 1 CSE versus high‐dose spinal, Outcome 3 Number of women requiring intra‐operative conversion to general anaesthesia.
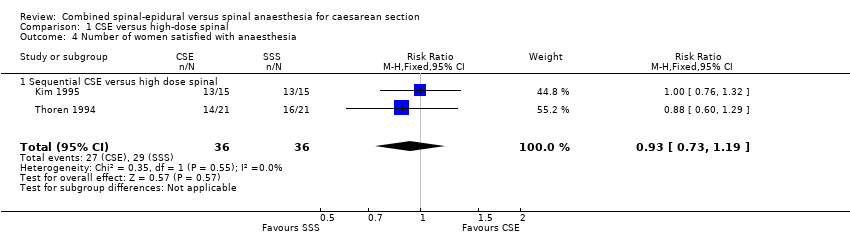
Comparison 1 CSE versus high‐dose spinal, Outcome 4 Number of women satisfied with anaesthesia.
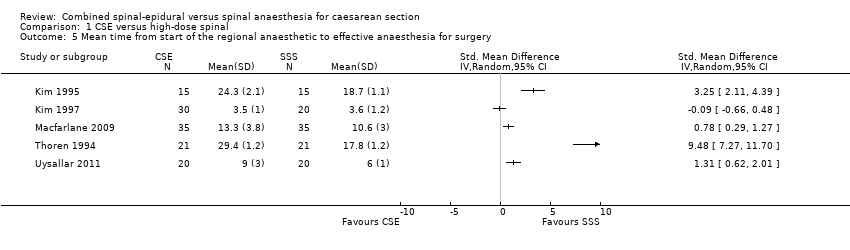
Comparison 1 CSE versus high‐dose spinal, Outcome 5 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery.

Comparison 1 CSE versus high‐dose spinal, Outcome 6 Number of women with hypotension.
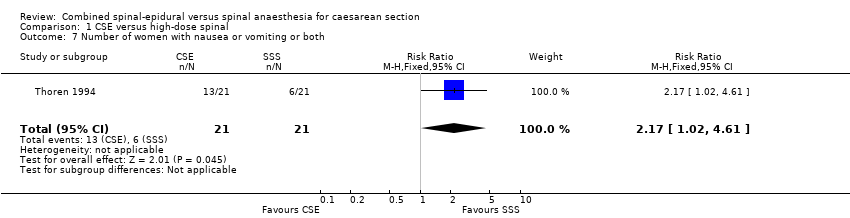
Comparison 1 CSE versus high‐dose spinal, Outcome 7 Number of women with nausea or vomiting or both.
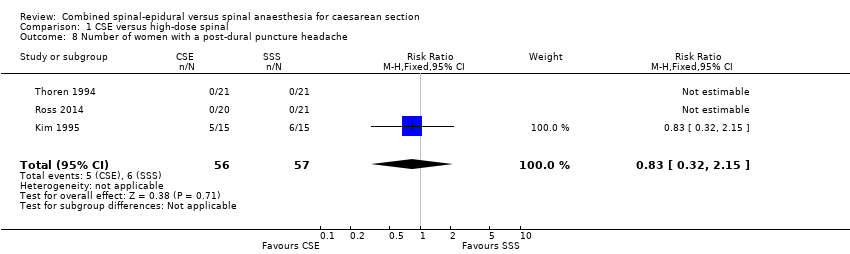
Comparison 1 CSE versus high‐dose spinal, Outcome 8 Number of women with a post‐dural puncture headache.
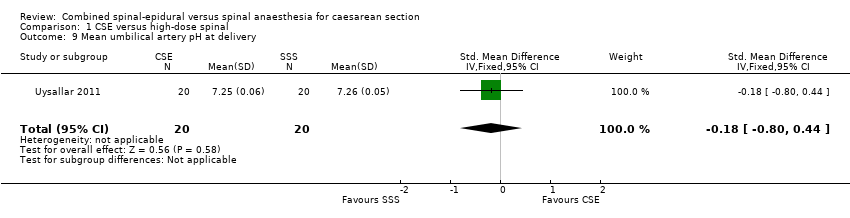
Comparison 1 CSE versus high‐dose spinal, Outcome 9 Mean umbilical artery pH at delivery.
Comparison 1 CSE versus high‐dose spinal, Outcome 10 Number of neonates with Apgar less than 7 at 5 minutes.

Comparison 2 CSE versus low‐dose spinal, Outcome 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade.
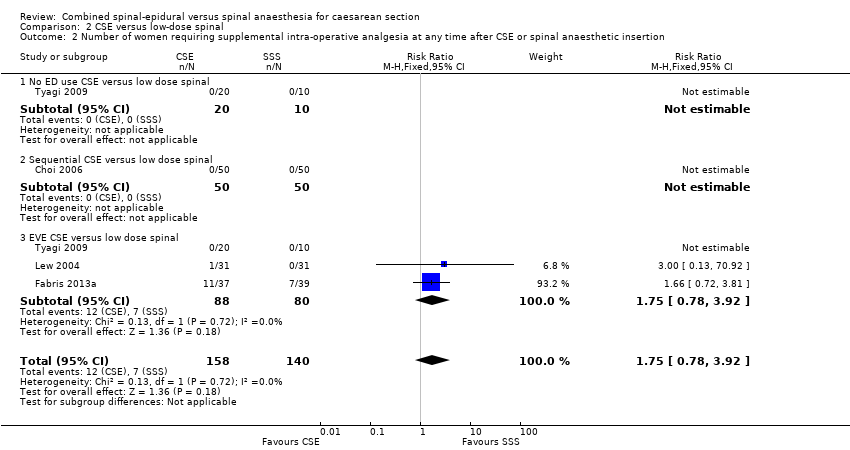
Comparison 2 CSE versus low‐dose spinal, Outcome 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion.

Comparison 2 CSE versus low‐dose spinal, Outcome 3 Number of women requiring intra‐operative conversion to general anaesthesia.
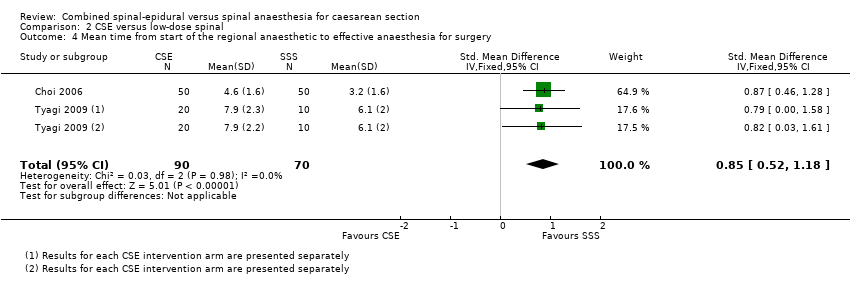
Comparison 2 CSE versus low‐dose spinal, Outcome 4 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery.

Comparison 2 CSE versus low‐dose spinal, Outcome 5 Number of women with hypotension.

Comparison 2 CSE versus low‐dose spinal, Outcome 6 Number of women with nausea or vomiting or both.

Comparison 2 CSE versus low‐dose spinal, Outcome 7 Number of women with a post‐dural puncture headache.
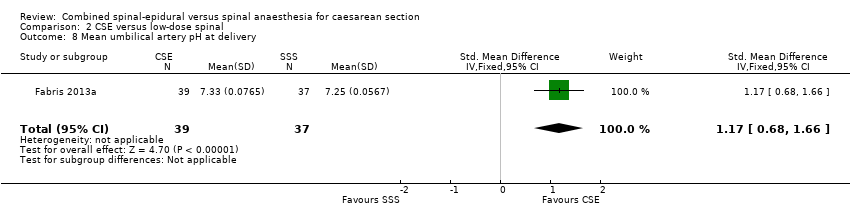
Comparison 2 CSE versus low‐dose spinal, Outcome 8 Mean umbilical artery pH at delivery.

Comparison 2 CSE versus low‐dose spinal, Outcome 9 Number of neonates with Apgar less than 7 at 5 minutes.
| CSE compared to high‐dose spinal for caesarean section | ||||||
| Patient or population: women having a caesarean section Setting: university or national hospitals | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with high‐dose spinal | Risk with CSE | |||||
| Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade | Study population | RR 0.32 | 341 | ⊕⊕⊝⊝ | ‐ | |
| 69 per 1000 | 22 per 1000 | |||||
| Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion | Study population | Average RR 1.25 | 390 | ⊕⊝⊝⊝ | ‐ | |
| 124 per 1000 | 154 per 1000 | |||||
| Number of women requiring intra‐operative conversion to general anaesthesia | Study population | RR 1.00 | 388 | ⊕⊝⊝⊝ | ‐ | |
| 48 per 1000 | 48 per 1000 | |||||
| Number of women satisfied with anaesthesia | Study population | RR 0.93 (0.73 to 1.19) | 72 | ⊕⊝⊝⊝ | ‐ | |
| 806 per 1000 | 749 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSmall sample size and wide confidence interval crossing the line of no effect (imprecision −1). | ||||||
| CSE compared to low‐dose spinal for caesarean section | ||||||
| Patient or population: women having a caesarean section Setting: university or national hospitals | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with low‐dose spinal | Risk with CSE | |||||
| Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade | Study population | RR 4.81 | 224 | ⊕⊕⊝⊝ | ‐ | |
| 0 per 1000 | 0 per 1000 | |||||
| Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion | Study population | RR 1.75 | 298 | ⊕⊕⊕⊝ | ‐ | |
| 100 per 1000 | 175 per 1000 | |||||
| Number of women requiring intra‐operative conversion to general anaesthesia | Study population | ‐ | 222 | ⊕⊕⊝⊝ | Effect is uncertain. No women in these 3 RCTs required a conversion to general anaesthetic | |
| see comment | see comment | |||||
| Number of women satisfied with anaesthesia | Study population | ‐ | (0 studies) | ‐ | No data available in the included studies | |
| see comment | see comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aSmall sample size and very wide confidence interval crossing the line of no effect (imprecision −2). | ||||||
|
| CSE: Needle‐through‐needle | CSE: Two injection | ||||
| SPINAL | No ED use | Sequential | EVE | No ED use | Sequential | EVE |
| High‐dose | ‐ | ‐ | ‐ | |||
| Low‐dose | ‐ | ‐ | ||||
| Abizanda 2007 is not included in this table as the doses of drugs used and the mode of administration were not stated | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade Show forest plot | 7 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.05, 1.97] |
| 1.1 No ED use CSE versus high dose spinal | 5 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.05, 1.97] |
| 1.2 Sequential CSE versus high dose spinal | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion Show forest plot | 7 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.19, 8.43] |
| 2.1 No ED use CSE versus high dose spinal | 4 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Sequential CSE versus high dose spinal | 3 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.18, 21.49] |
| 2.3 EVE CSE versus high dose spinal | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.28] |
| 3 Number of women requiring intra‐operative conversion to general anaesthesia Show forest plot | 7 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 3.1 No ED use CSE versus high dose spinal | 4 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Sequential CSE versus high dose spinal | 3 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.95] |
| 3.3 EVE CSE versus high dose spinal | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Number of women satisfied with anaesthesia Show forest plot | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 4.1 Sequential CSE versus high dose spinal | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.73, 1.19] |
| 5 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery Show forest plot | 5 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Number of women with hypotension Show forest plot | 4 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.33] |
| 7 Number of women with nausea or vomiting or both Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.02, 4.61] |
| 8 Number of women with a post‐dural puncture headache Show forest plot | 3 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.32, 2.15] |
| 9 Mean umbilical artery pH at delivery Show forest plot | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.80, 0.44] |
| 10 Number of neonates with Apgar less than 7 at 5 minutes Show forest plot | 4 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of women requiring a repeat regional block or a general anaesthetic as a result of failure to establish adequate initial blockade Show forest plot | 3 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [0.24, 97.80] |
| 1.1 No ED use CSE versus low dose spinal | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Sequential CSE versus low dose spinal | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.81 [0.24, 97.80] |
| 1.3 EVE CSE versus low dose spinal | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Number of women requiring supplemental intra‐operative analgesia at any time after CSE or spinal anaesthetic insertion Show forest plot | 4 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.78, 3.92] |
| 2.1 No ED use CSE versus low dose spinal | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Sequential CSE versus low dose spinal | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 EVE CSE versus low dose spinal | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.78, 3.92] |
| 3 Number of women requiring intra‐operative conversion to general anaesthesia Show forest plot | 3 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 No ED use CSE versus low dose spinal | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Sequential CSE versus low dose spinal | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 EVE CSE versus low dose spinal | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Mean time from start of the regional anaesthetic to effective anaesthesia for surgery Show forest plot | 2 | 160 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.85 [0.52, 1.18] |
| 5 Number of women with hypotension Show forest plot | 4 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.38, 0.93] |
| 6 Number of women with nausea or vomiting or both Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.89] |
| 7 Number of women with a post‐dural puncture headache Show forest plot | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Mean umbilical artery pH at delivery Show forest plot | 1 | 76 | Std. Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.68, 1.66] |
| 9 Number of neonates with Apgar less than 7 at 5 minutes Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

