Oxigenoterapia hiperbárica para el tratamiento de las heridas traumáticas y quirúrgicas agudas
Información
- DOI:
- https://doi.org/10.1002/14651858.CD008059.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 16 diciembre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Heridas
- Copyright:
-
- Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Anne Eskes: conceived the review question and developed the protocol; completed the first draft and edited the protocol; made an intellectual contribution to the protocol and approved the final version prior to submission; updated the original review.
Dirk T Ubbink: conceived the review question, secured funding and co‐ordinated the protocol development; edited and advised on the protocol; made an intellectual contribution and approved the final version prior to submission; updated the original review; and is guarantor of the work.
Cees Lucas: completed the first draft of the revision and advised on part of the protocol; made an intellectual contribution to the protocol, the original review and updated version, approved the final version prior to submission.
Hester Vermeulen: conceived the review question, secured funding and co‐ordinated the protocol development; edited and advised on the protocol; made an intellectual contribution and approved the final version prior to submission; updated the original review; and is guarantor of the work.
Contributions of editorial base
Nicky Cullum: edited the protocol and review; advised on methodology, interpretation and content; and approved the final review prior to submission.
Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content; edited the protocol, review and review update.
Ruth Foxlee: designed the search strategy, edited the search methods section and ran the searches.
Rachel Richardson: edited and checked the review update.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
Infrastructure supplied by the hospital, Academic Medical Centre at the University of Amsterdam, Netherlands.
-
NIHR/Department of Health (England), (Cochrane Wounds Group), UK.
Declarations of interest
None of the authors have any conflict of interest.
Acknowledgements
We thank all the peer reviewers for reviewing the protocol and review for relevance, rigour and readability (Mike Bennett, Andrew Jull, Caroline Main, Frank Peinemann, Joan Webster, Durhane Wong‐Rieger and Gill Worthy). We thank Faridi van Etten for help with developing our search strategy and Jenny Bellorini who copy edited the text of our review. We also thank Maarten Lubbers for his contribution to the original review.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 16 | Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds | Review | Anne Eskes, Hester Vermeulen, Cees Lucas, Dirk T Ubbink | |
| 2010 Oct 06 | Hyperbaric oxygen therapy for treating acute surgical and traumatic wounds | Review | Anne Eskes, Dirk T Ubbink, Maarten Lubbers, Cees Lucas, Hester Vermeulen | |
| 2009 Oct 07 | Hyperbaric oxygen therapy for acute surgical and traumatic wounds | Protocol | Anne Eskes, Dirk T Ubbink, Maarten Lubbers, Cees Lucas, Hester Vermeulen | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICO

Study flow diagram.
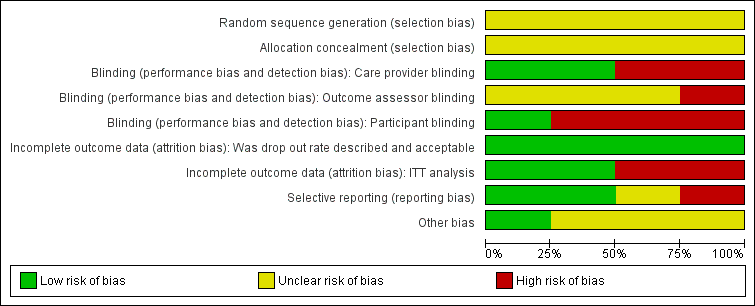
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 HBOT compared with usual care, Outcome 1 Complete survival (defined as at least 95% take) at day 7.

Comparison 2 HBOT compared with sham HBOT, Outcome 1 Complete healing.

Comparison 2 HBOT compared with sham HBOT, Outcome 2 Time to healing (days).
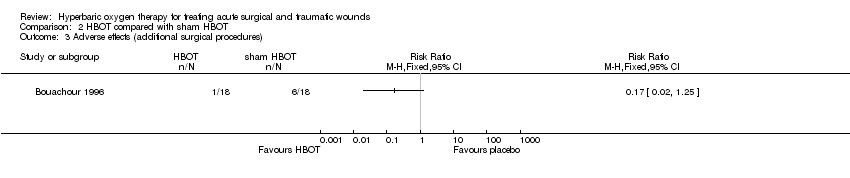
Comparison 2 HBOT compared with sham HBOT, Outcome 3 Adverse effects (additional surgical procedures).

Comparison 2 HBOT compared with sham HBOT, Outcome 4 Adverse effects (tissue necrosis).
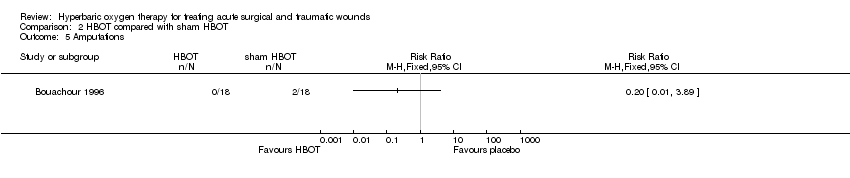
Comparison 2 HBOT compared with sham HBOT, Outcome 5 Amputations.
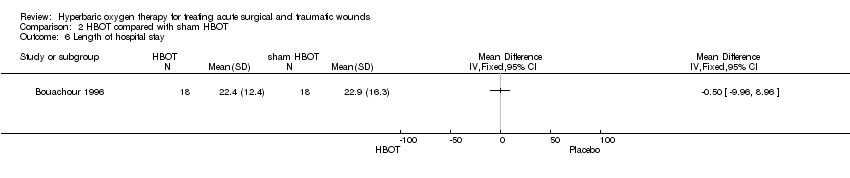
Comparison 2 HBOT compared with sham HBOT, Outcome 6 Length of hospital stay.

Comparison 3 HBOT compared with dexamethasone or heparin, Outcome 1 Complete healing HBOT vs dexamethasone.
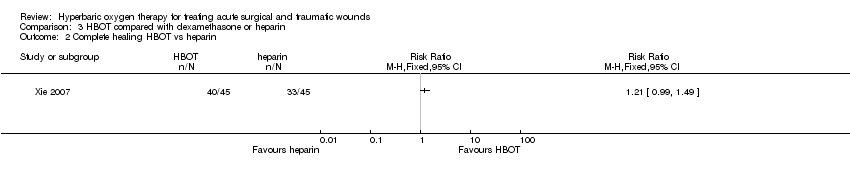
Comparison 3 HBOT compared with dexamethasone or heparin, Outcome 2 Complete healing HBOT vs heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete survival (defined as at least 95% take) at day 7 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete healing Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Time to healing (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects (additional surgical procedures) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse effects (tissue necrosis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Amputations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete healing HBOT vs dexamethasone Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Complete healing HBOT vs heparin Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

