Oxigenoterapia hiperbárica para el tratamiento de las heridas traumáticas y quirúrgicas agudas
Appendices
Appendix 1. Search methods section from the original review
For this original review we searched the following databases for reports of eligible trials over all years:
-
Cochrane Wounds Group Specialised Register (searched 25 August 2010)
-
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2010 Issue 3
-
Ovid MEDLINE ‐ 1950 to August Week 2 2010
-
Ovid MEDLINE ‐ In‐Process & Other Non‐Indexed Citations August 24, 2010
-
Ovid EMBASE ‐ 1980 to 2010 Week 33
-
EBSCO CINAHL ‐ 1982 to 20 August 2010
The following search strategy was used in the Cochrane Central Register of Controlled Trials (CENTRAL):
#1 MeSH descriptor Acute Disease explode all trees
#2 MeSH descriptor Wounds and Injuries explode all trees
#3 (#1 AND #2)
#4 MeSH descriptor Surgical Wound Infection explode all trees
#5 MeSH descriptor Surgical Wound Dehiscence explode all trees
#6 MeSH descriptor Wounds, Penetrating explode all trees
#7 MeSH descriptor Lacerations explode all trees
#8 MeSH descriptor Burns explode all trees
#9 MeSH descriptor Bites and Stings explode all trees
#10 MeSH descriptor Skin Transplantation explode all trees
#11 MeSH descriptor Fractures, Open explode all trees
#12 MeSH descriptor Gas Gangrene explode all trees
#13 ((surgical NEXT wound*) or (incised NEXT wound*)):ti,ab,kw
#14 (laceration* or gunshot or (gun NEXT shot) or stab or stabbing or
stabbed or bite* or bitten):ti,ab,kw
#15 ((traumatic NEXT wound*) or (acute NEXT wound*)):ti,ab,kw
#16 ((mechanical NEXT trauma) or polytrauma):ti,ab,kw
#17 ((thermal or blast or crush or avulsion) NEXT injur*):ti,ab,kw
#18 ("burn" or "burns" or burned or scald*):ti,ab,kw
#19 acute NEXT wound*:ti,ab,kw
#20 acute NEXT ulcer*:ti,ab,kw
#21 ((donor NEXT site*) or (skin NEXT graft*)):ti,ab,kw
#22 ((open NEXT fracture*) or (compound NEXT fracture*)):ti,ab,kw
#23 "gas gangrene":ti,ab,kw
#24 experimental NEXT wound*:ti,ab,kw
#25 "skin infarction":ti,ab,kw
#26 skin NEXT flap*:ti,ab,kw
#27 (#3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26)
#28 MeSH descriptor Hyperbaric Oxygenation explode all trees
#29 (hyperbaric* NEXT oxygen*):ti,ab,kw
#30 (HBO or HBOT):ti,ab,kw
#31 (high NEXT pressure NEXT oxygen*):ti,ab,kw
#32 (#28 OR #29 OR #30 OR #31)
#33 (#27 AND #32)
This strategy was adapted where necessary to search Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL. These search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL are shown in Appendix 2; Appendix 3; Appendix 4 respectively. The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network. There were no restrictions on the basis of date or language of publication. We contacted the Trials Search Co‐ordinator of the Cochrane Wounds Group to assist with the development of the various search strategies.
Appendix 2. Ovid MEDLINE search strategy
1 exp Acute Disease/
2 exp "Wounds and Injuries"/
3 1 and 2
4 exp Surgical Wound Infection/
5 exp Surgical Wound Dehiscence/
6 exp Wounds, Penetrating/
7 exp Lacerations/
8 exp Burns/
9 exp "Bites and Stings"/
10 exp Skin Transplantation/
11 exp Fractures, Open/
12 exp Gas Gangrene/
13 (surgical wound$ or incised wound$).ti,ab.
14 (laceration$ or gunshot or gun shot or stab or stabbing or stabbed or bite$1 or bitten).ti,ab.
15 (traumatic wound$ or acute wound$).ti,ab.
16 (mechanical trauma or polytrauma).ti,ab.
17 ((thermal or blast or crush or avulsion) adj injur$).ti,ab.
18 (burn or burns or burned or scald$).ti,ab.
19 acute wound$.ti,ab.
20 acute ulcer$.ti,ab.
21 (donor site$ or skin graft$).ti,ab.
22 (open fracture$ or compound fracture$).ti,ab.
23 gas gangrene.ti,ab.
24 experimental wound$.ti,ab.
25 skin infarction.ti,ab.
26 skin flap$.ti,ab.
27 or/3‐26
28 exp Hyperbaric Oxygenation/
29 (hyperbaric$ adj oxygen$).ti,ab.
30 (HBO or HBOT).ti,ab.
31 high pressure oxygen$.ti,ab.
32 or/28‐31
33 27 and 32
Appendix 3. Ovid EMBASE search strategy
1 exp Acute Disease/
2 exp Wound/
3 1 and 2
4 exp Surgical Wound/
5 exp Wound Dehiscence/
6 exp Penetrating Trauma/
7 exp Laceration/
8 exp Burn/
9 exp Bite/
10 exp Bite Wound/
11 exp Dog Bite/
12 exp Skin Transplantation/
13 exp Open Fracture/
14 exp Gas Gangrene/
15 (surgical wound$ or incised wound$).ti,ab.
16 (laceration$ or gunshot or gun shot or stab or stabbing or stabbed or bite$1 or bitten).ti,ab.
17 (traumatic wound$ or acute wound$).ti,ab.
18 (mechanical trauma or polytrauma).ti,ab.
19 ((thermal or blast or crush or avulsion) adj injur$).ti,ab.
20 (burn or burns or burned or scald$).ti,ab.
21 acute wound$.ti,ab.
22 acute ulcer$.ti,ab.
23 (donor site$ or skin graft$).ti,ab.
24 (open fracture$ or compound fracture$).ti,ab.
25 gas gangrene.ti,ab.
26 experimental wound$.ti,ab.
27 skin infarction.ti,ab.
28 skin flap$.ti,ab.
29 or/3‐28
30 exp Hyperbaric Oxygen/
31 (hyperbaric$ adj oxygen$).ti,ab.
32 (HBO or HBOT).ti,ab.
33 high pressure oxygen$.ti,ab.
34 or/30‐33
Appendix 4. EBSCO CINAHL search strategy
S32 S26 and S31
S31 S27 or S28 or S29 or S30
S30 TI high pressure oxygen* or AB high pressure oxygen*
S29 TI ( HBO or HBOT ) or AB ( HBO or HBOT )
S28 TI hyperbaric oxygen* or AB hyperbaric oxygen*
S27 (MH "Hyperbaric Oxygenation")
S26 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25
S25 TI skin flap* or AB skin flap*
S24 TI skin infarction or AB skin infarction
S23 TI experimental wound* or AB experimental wound*
S22 TI gas gangrene or AB gas gangrene
S21 TI ( open fracture* or compound fracture* ) or AB ( open fracture* or compound fracture* )
S20 TI ( donor site* or skin graft* ) or AB ( donor site* or skin graft* )
S19 TI ( acute wound* or acute ulcer* ) or AB ( acute wound* or acute ulcer* )
S18 TI ( burn or burns or burned or scald* ) or AB ( burn or burns or burned or scald* )
S17 TI ( thermal injur* or blast injur* or crush injur* or avulsion ) or AB ( thermal injur* or blast injur* or crush injur* or avulsion )
S16 TI ( mechanical trauma or polytrauma ) or AB ( mechanical trauma or polytrauma )
S15 TI ( traumatic wound* or acute wound* ) or AB ( traumatic wound* or acute wound* )
S14 TI ( laceration* or gunshot or gun shot or stab or stabbing or stabbed or bite* or bitten ) or AB ( laceration* or gunshot or gun shot or stab or stabbing or stabbed or bite* or bitten )
S13 TI ( surgical wound* or incised wound* ) or AB ( surgical wound* or incised wound* )
S12 (MH "Gas Gangrene")
S11 (MH "Fractures, Open")
S10 (MH "Skin Transplantation")
S9 (MH "Burns+")
S8 (MH "Bites and Stings+")
S7 (MH "Tears and Lacerations")
S6 (MH "Wounds, Penetrating+")
S5 (MH "Surgical Wound Dehiscence")
S4 (MH "Surgical Wound Infection")
S3 S1 and S2
S2 (MH "Wounds and Injuries+")
S1 MH "Acute Disease")
Appendix 5. Risk of bias definitions
Criteria for judgements about the sources of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process, such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of ‘Yes’ or ‘No’.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of ‘Yes’ or ‘No’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
-
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
-
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
-
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
-
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
-
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following:
-
Insufficient information to permit judgement of ‘Yes’ or ‘No’.
-
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
-
No missing outcome data.
-
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
-
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
-
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following:
-
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
-
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
-
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
-
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation;
-
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following:
-
Insufficient reporting of attrition/exclusions to permit judgement of ‘Yes’ or ‘No’ (e.g. number randomised not stated, no reasons for missing data provided).
-
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following:
-
The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
-
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
-
Not all of the study’s pre‐specified primary outcomes have been reported.
-
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified.
-
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
-
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered into a meta‐analysis.
-
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of ‘Yes’ or ‘No’. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias, for example the study:
-
had a potential source of bias related to the specific study design used; or
-
stopped early due to some data‐dependent process (including a formal‐stopping rule); or
-
had extreme baseline imbalance; or
-
had some other problem.
Unclear
There may be a risk of bias, but there is either:
-
insufficient information to assess whether an important risk of bias exists; or
-
insufficient rationale or evidence that an identified problem will introduce bias

Study flow diagram.
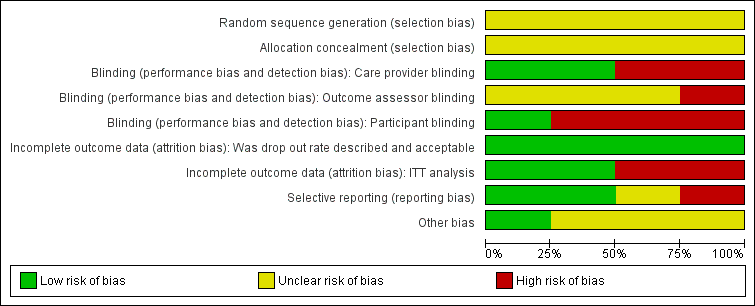
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 HBOT compared with usual care, Outcome 1 Complete survival (defined as at least 95% take) at day 7.

Comparison 2 HBOT compared with sham HBOT, Outcome 1 Complete healing.

Comparison 2 HBOT compared with sham HBOT, Outcome 2 Time to healing (days).
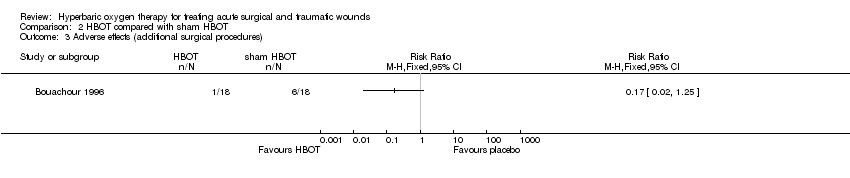
Comparison 2 HBOT compared with sham HBOT, Outcome 3 Adverse effects (additional surgical procedures).

Comparison 2 HBOT compared with sham HBOT, Outcome 4 Adverse effects (tissue necrosis).
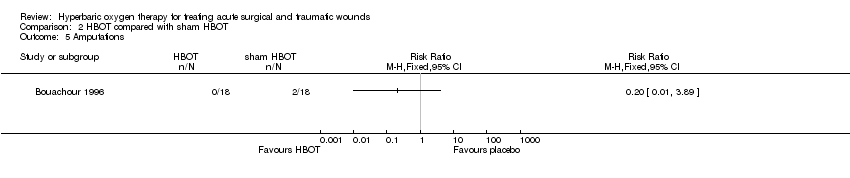
Comparison 2 HBOT compared with sham HBOT, Outcome 5 Amputations.
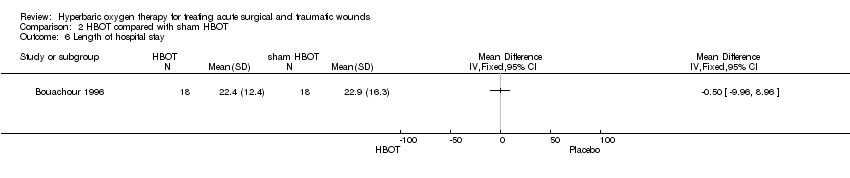
Comparison 2 HBOT compared with sham HBOT, Outcome 6 Length of hospital stay.

Comparison 3 HBOT compared with dexamethasone or heparin, Outcome 1 Complete healing HBOT vs dexamethasone.
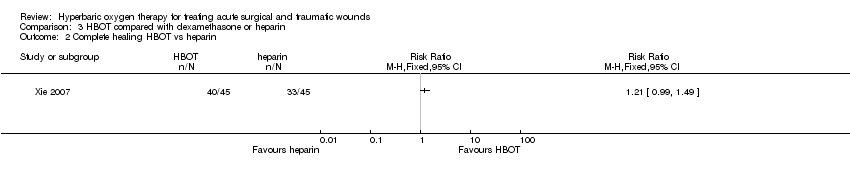
Comparison 3 HBOT compared with dexamethasone or heparin, Outcome 2 Complete healing HBOT vs heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete survival (defined as at least 95% take) at day 7 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete healing Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Time to healing (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects (additional surgical procedures) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse effects (tissue necrosis) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Amputations Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Length of hospital stay Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Complete healing HBOT vs dexamethasone Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Complete healing HBOT vs heparin Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

