Tratamiento adyuvante con lamotrigina para las crisis convulsivas tonicoclónicas generalizadas refractarias
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, randomised, double‐blind, cross‐over study. Two treatment arms: 1 placebo, 1 lamotrigine. Randomization concealment methods not stated. Baseline = 8 weeks. Treatment I and II = 8 weeks each. Washout = 4 weeks including 1 week taper. | |
| Participants | Multicentre Australian study with 26 participants with refractory generalized epilepsy. There were 11 males and 15 females. The mean age was 29 years (range 15 to 50). Up to 4 other AEDs were permitted. | |
| Interventions | Add‐on lamotrigine or placebo. Daily lamotrigine regimes were 150 mg or 75 mg, designed to achieve optimal plasma concentrations. Participants taking an enzyme‐inhibiting AED received the higher dosing regime. | |
| Outcomes | 1. 50% responder rate. 2. Withdrawal from treatment for any reason. 3. Adverse effects. | |
| Notes | Four participants were excluded from the efficacy analysis, but included in the safety analysis. Four participants withdrew from the study; 1 withdrew after the first treatment phase and 2 during the treatment phase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
| Methods | Randomized, double‐blind, parallel study. Two treatment arms: 1 lamotrigine, 1 placebo. Randomization concealment methods not stated. Pre‐randomization baseline = 8 weeks. Treatment = 7 weeks escalation phase for adolescent and adult participants (> 12 years), 12 weeks escalation for paediatric participants (< 13 years). Followed by 12 week maintenance phase. No washout period. Ongoing continuation phase = 1 year. | |
| Participants | Single‐centre study from the US. 121 randomized participants with primary generalized tonic‐clonic seizures, mean ages 24.9 (placebo) and 26.9 years (lamotrigine), age range from 2‐55 years. 55 female and 62 male participants. 4 did not receive medication. 58 were allocated to lamotrigine and 59 to placebo in the treatment phase. Maximum number of other AEDs = 2. | |
| Interventions | Add‐on lamotrigine or placebo. Lamotrigine was introduced and titrated on schedule based on age and concurrent AED regimen. Participants taking concurrent valproate had to achieve a target of 200 mg/day; those taking enzyme inducing AEDs to achieve targets of 400 mg/day; and those taking an AED other than valproate or an enzyme inducing AED for 300 mg/day. | |
| Outcomes | 1. % change in PGTC seizure frequency monthly. 2. % change in other generalized seizure types monthly. 3. Median seizure counts monthly. 4. Proportion of people greater than 25% reduction in frequencies of PGTC seizures and all generalized seizures. 5. Withdrawal from study for any reason. 6. Adverse effects. | |
| Notes | No participants were excluded from analysis. 4 did not receive the study drug. 34 withdrew from the study; 16 receiving lamotrigine, 14 receiving placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Unclear |
AED = Antiepileptic drugs
PGTC = Primary generalized tonic‐clonic
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Study included participants with Lennox‐Gastaut syndrome. | |
| Included Lennox‐Gastaut syndrome patients. | |
| Majority of participants included in study had partial and secondarily generalized seizures. | |
| An abstract study which sub‐analysed data from an earlier RCT (Biton 2005). It included 38 subjects with a history of absence seizures receiving lamotrigine. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Greater than 50% reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Lamotrigine versus placebo, Outcome 1 Greater than 50% reduction in seizure frequency. | ||||
| 2 Total cessation of seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Lamotrigine versus placebo, Outcome 2 Total cessation of seizure frequency. | ||||
| 3 Proportion of individuals who withdrew from treatment Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Lamotrigine versus placebo, Outcome 3 Proportion of individuals who withdrew from treatment. | ||||
| 4 Proportion of individuals experience any side effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Lamotrigine versus placebo, Outcome 4 Proportion of individuals experience any side effects. | ||||
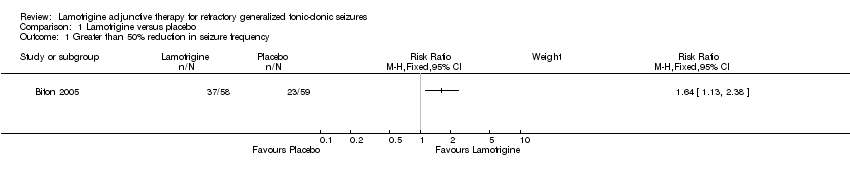
Comparison 1 Lamotrigine versus placebo, Outcome 1 Greater than 50% reduction in seizure frequency.
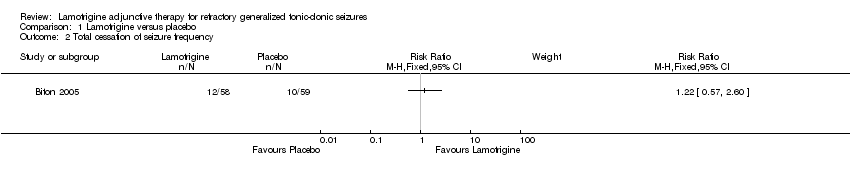
Comparison 1 Lamotrigine versus placebo, Outcome 2 Total cessation of seizure frequency.

Comparison 1 Lamotrigine versus placebo, Outcome 3 Proportion of individuals who withdrew from treatment.
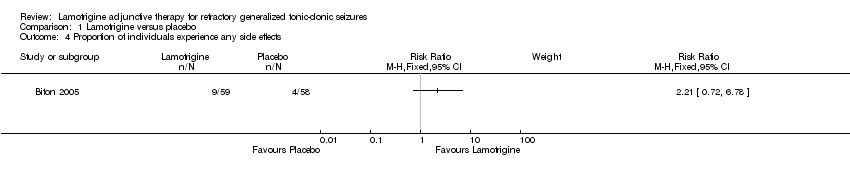
Comparison 1 Lamotrigine versus placebo, Outcome 4 Proportion of individuals experience any side effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Greater than 50% reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2 Total cessation of seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Proportion of individuals who withdrew from treatment Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Proportion of individuals experience any side effects Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

