Antioxidantes para la subfertilidad masculina
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised crossover trial. Single centre | |
| Participants | Country: Japan Male infertility Mean age: 36 years (treatment group age range 24 to 49, control 30 to 37 years) N = 10 recruited Inclusion criteria: male infertility (ROS > 5 x 10,000 counts/10,000,000 viable spermatozoa) Exclusion criteria: azoospermia, pyospermia Duration of study: 8 months | |
| Interventions | Ethylcysteine 600 mg/day for 3 months (n = 5) versus Vitamin E 600 mg/day (n = 5) With a one month wash out, then crossover for another 3 months. Only data from the first phase were used in data analysis | |
| Outcomes | Semen parameters | |
| Notes | In Japanese. Data extraction translated by Ichiro, a colleague of Samantha Roberts, 29 January 2009 Author contacted 'no further information is available' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were divided randomly" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Sperm parameters reported |
| Methods | Open label randomised controlled trial | |
| Participants | Country: Egypt Isolated idiopathic athenozospermia Prior to intrauterine insemination (IUI) Mean age: unknown, "both treatment groups were homogenous at the time of randomization regarding the type and duration of infertility" N = 60 Inclusion criteria: only couples with idiopathic athenozospermia (progressive motility < 32%) with normal other seminal criteria and normal infertility workup for female partner Trial duration: unknown | |
| Interventions | N‐acetylcysteine (NAC) 600 mg (n = 30) versus No treatment (n = 30) Duration of treatment: 12 weeks prior to IUI | |
| Outcomes | Mean sperm concentration Percentage of progressive sperm motility Clinical pregnancy rate | |
| Notes | Conference abstract Couples were randomised ‐ attempted to contact authors 4 February 2014, unable to find e‐mail address. Letter posted 12 February 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Couples were randomised" ‐ does not describe methods |
| Allocation concealment (selection bias) | Unclear risk | No description of methods |
| Blinding (performance bias and detection bias) | High risk | "Open‐labelled" |
| Incomplete outcome data (attrition bias) | Unclear risk | No description of dropouts |
| Selective reporting (reporting bias) | Unclear risk | Unknown ‐ conference abstract |
| Methods | Randomised study, double blind placebo‐controlled ‐ 4‐arm trial | |
| Participants | Country: Iran Infertile subjects (N = 160) with varicocelectomy only 112 completed the study Mean age: age ranges and duration of infertility of male partners were from 20 to 43 years (mean ± SD: 29.07 ± 6.8) and 1 to 10 years (mean ± SD: 3.32 ± 2.4) respectively Inclusion criteria: the presence of a grade III varicocele, on I to III scale, was the criteria to enter the study. It was assessed by clinical parameters and was confirmed by Doppler ultrasound scanning Exclusion criteria: patients with the evidence of leukocytospermia, low testicular volume < 15 mL, congenital urogenital abnormalities and urogenital infections were excluded from the study Duration of study: May 2008 to November 2010, 2.5 years Duration of treatment: 6 months | |
| Interventions | Zinc (n = 32) versus Folic acid (n = 26) versus Zinc and folic acid (n = 29) versus Placebo (n = 25) Patients in each group took one capsule orally per day after dinner following varicocelectomy for 6 months. The dosage of the zinc sulfate (Alhavi pharmaceutical Co, Tehran, Iran) and folic acid (Iran Daru, Tehran, Iran) was 66 mg and 5 mg per capsule, respectively. Patients in placebo group received the same capsules without the effective drug | |
| Outcomes | Sperm parameters; number, morphology, halo formation rate, motility, forward progressive motility | |
| Notes | IRCT registration no: IRCT138802261910N1 Contact details: SN Nematollahi‐mahani email: [email protected] or [email protected] E‐mailed the author 3 March 2014. Author replied 6 March 2014 with information included in the ROB table. Author e‐mailed again to ask about pregnancy data and dropouts from which group. The author informed us that Azizollahi 2011 was part of this trial and gave pregnancy and dropout data (there were originally 40 in each group). "At that time we observed 2 pregnancies in zinc/folic acid group, 1 pregnancy in zinc group, and no pregnancy in placebo and folic acid group. These data were just 6 months after the start of the trial." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | For randomisation we used a table with 200 numbers (1 to 200). Before the trial we gave each group a number between 1 and 4 and allocated each group into the table. By this method the first, fifth, ninth, 13th and ... patients were allocated into the group 1 and the same manner was applied to the other groups |
| Allocation concealment (selection bias) | Low risk | "We used sealed containers with the randomization number on them. Drugs or placebo were in opaque capsules" |
| Blinding (performance bias and detection bias) | Low risk | "Our study was double blind. Neither the urologist nor the patient or examiner in the lab were aware of the arrangement of the study" |
| Incomplete outcome data (attrition bias) | Low risk | Information gained from communication with the author explained the dropouts numbers |
| Selective reporting (reporting bias) | Low risk | Clinical pregnancy rate data gained from email correspondence with the author |
| Methods | Randomised trial, double blind. No details of randomisation or concealment | |
| Participants | Country: Italy Infertile men recruited from an andrology clinic Mean age 30 (range 24 to 38 years) N = 60 recruited Inclusion criteria: primary infertility > 2 years after regular intercourse with a fertile woman; 20 to 40 years of age; normal rheologic characteristics; sperm count > 20 x 106 /mL; sperm motility < 50%; normal sperm morphological features > 30%; seminal WBC < 1 x 106 /mL; negative sperm culture and chlamydia and mycoplasma urealyticum; normal serum gonadotropins; T, E2 and PRL; absence of infectious or genital disease; no anatomic abnormalities of the genital tract; absence of systemic diseases or treatment with other drugs within the 3 months before enrolment in the study. Absence of smoking, alcohol or recreational drug use or of occupational chemical exposure Total duration of study 9 months | |
| Interventions | L carnitine 3 g/day orally (n = 15) versus L acetyl carnitine 3 g/day orally (n = 15) versus L carnitine 2 g/day + L acetyl carnitine 1 g/day (n = 15) versus placebo (n = 15) Duration of treatment: 6 months | |
| Outcomes | Semen parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Placebo controlled double blind randomised trial". No methods of randomisation mentioned |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Low risk | "double blind" study. Patients blinded but no details as to who else |
| Incomplete outcome data (attrition bias) | Low risk | 1 withdrawal from the L carnitine 2g/day + L acetyl carnitine 1 g/day group |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Double blind, placebo‐controlled randomised trial | |
| Participants | Country: Italy (University of Marche, Ancona) Infertile men recruited from andrology clinic Mean age: 32 years (range 27 to 32) N = 60 men recruited Inclusion criteria: age 20 to 40 years, infertility > 2 years, regular sexual intercourse with a potentially fertile female; normal rheologic characteristics (appearance, consistency and liquefaction) of semen and volume and pH in normal range, sperm count > 20 x 106 /mL, sperm motility < 50% (WHO 1999), normal morphology > 30%, seminal white blood cells < 1 x 106 /mL and a negative sperm culture and chlamydia and M.urealyticum detection. Normal levels of gonadotropins absence of genital disease and anatomical abnormalities of the genital tract including variocoele and antibodies. Absence of systemic disease or treatment with other drugs within 3 months of being enrolled in the study. Absence of smoking, alcohol and drug addiction and exposure to occupational chemicals Exclusion criteria: transient decrease in semen quality during run in and those who had sudden improvement in semen parameters during run in | |
| Interventions | Coenzyme Q10 100 mg 2 times /day (n = 30) versus Placebo (n = 30) Duration of study: 9 months | |
| Outcomes | Primary: semen parameters Secondary: pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" ‐ no details. At end of trial the paper mentions ‐ "after opening randomisation list" page 1789 |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Low risk | "Double blind" ‐ placebo used therefore low risk for performance bias |
| Incomplete outcome data (attrition bias) | Low risk | "5 patients dropped out of the study", 2 from the treatment group and 3 from the placebo group; this was discovered after opening the randomisation list at the end of the study. Intention to treat was carried out |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised study ‐ conference proceeding | |
| Participants | Country: Italy, Andrology clinic in Bologna Population: severe idiopathic oligoasthenospermia (sperm concentration < 5000 /μl) Mean age: group a and b 35 (range 30 to 40 years), Group c 31 (range 24 to 34) N= 42 Inclusion criteria: severe idiopathic oligoasthenospermia (sperm concentration < 5000 /μl) Exclusion criteria: Genomic, hormonal or inflammatory diseases Duration of study: ? | |
| Interventions | a. Acetyl‐carnitine 1 g/day + L‐carnitine 2 g/day + cinnoxicam (n = 14) versus b. ALC + LC (n = 14) versus c. No therapy (n = 14) Duration of treatment: ? | |
| Outcomes | Semen parameters | |
| Notes | Conference abstract ‐ no data given. Contacted authors but no reply re questions as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomised (1patient = 1 block) analysis of variance" Was this at the time of sequence generation or at data analysis? |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | High risk | Not mentioned. Control is no treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear conference abstract |
| Methods | Randomised controlled trial. Allocation concealment: anonymous colour coded boxes | |
| Participants | Country: Italy Population: idiopathic plus variocoele associated oligo‐asthenospermia (OAT) Mean age: 34 years (range 27 to 40) N = 325 Inclusion criteria: OAT men with deficiencies in all sperm patterns whose chief complaint was primary couple infertility > 12 months with regular intercourse. Normal sperm appearance, consistency, liquefaction, volume, pH. Female partner without fertility problems. Varicoceles. Exclusion criteria: azoospermia, seminal white blood cell concentration more than 1000,000/mL, positive urethral chlamydia swab test, oligospermia < 5,000,000 /ml, hormonal alterations, age > 40 yrs, presence of anti‐sperm antibodies, drug, tobacco or alcohol abuse, ongoing medical treatments, presence of hydrocoele, diabetes,hypertension, x‐ray exposure in previous 8 months, peptic ulcer, unexplained gastric pain, previous hypersensitivity to NSAIDS or carnitines, carnitine metabolism deficiency, bilateral variocoele, prostate abnormalities, previous or current testicular pathology, testicle echographic abnormalities Duration of study: 9 months | |
| Interventions | Group 1: placebo, starch tablets 2 times /day + glycerine suppository (1 every 4 days) (n = 47) Group 2: L‐carnitine 1 x 2 g/day plus acetyl‐L‐carnitine 500 x 2 mg/day plus glycerine suppository (n = 39) Group 3: L‐carnitine 1x 2 g/day plus acetyl‐L‐carnitine 500 x 2 mg/day plus glycerine suppository plus cinnoxicam suppository 1 x 30 mg (every 4 days) (n =44) Cinnoxicam is a non‐steroidal ant‐inflammatory therefore this arm (Group 3) was not included in meta‐analysis as per protocol Duration of treatment: 6 months | |
| Outcomes | Primary: sperm parameters Secondary: pregnancy, side effects | |
| Notes | Continuous data taken from Cavallini 2004a 'excluded conference abstract' no data for placebo group Unit of analysis variocoele therefore cannot extract data that were presented as median (interquartile range) Author contacted regarding uneven numbers and missing placebo and continuous data Author replied that raw data were not available due to computer crash | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "casual random tables" |
| Allocation concealment (selection bias) | Low risk | "drug placebos identical in appearance", "anonymized carnitine and cinnoxicam and glycerine suppository containers; and filled and sealed anonymous color coded boxes", "the color code was disclosed to physicians by pharmacists and by IRB at the end of the research" |
| Blinding (performance bias and detection bias) | Low risk | "All study personnel and participants were blinded to treatment assignment for the duration of the study" |
| Incomplete outcome data (attrition bias) | High risk | 325 randomised but only 185 accounted for; 55 dropouts from 185 (42%), 53 reasons given for the dropouts |
| Selective reporting (reporting bias) | Low risk | Sperm parameters were primary outcome. Intention to collect biochemical pregnancy data as secondary outcome recorded in the methods |
| Methods | Randomised controlled trial Allocation concealment: sealed envelopes | |
| Participants | Country: Turkey Population: men attending fertility clinic with idiopathic infertility. Normal sperm parameters Mean age: treatment group 33.1 ± 4.5, control 32.8 ± 3.7 years N = 120 recruited Inclusion criteria: men attending fertility clinic with idiopathic infertility. Normal sperm parameters Exclusion criteria: cryptorchidism, vasectomy, abnormal liver functioning, smoking, alcohol consumption Duration of study: 3 months | |
| Interventions | N‐acetylcysteine 600 mg/day (n = 60) versus placebo (n = 60) Duration of treatment: 3 months | |
| Outcomes | Primary outcomes: Total antioxidant capacity, peroxide levels, oxidative stress Secondary outcomes: Other semen parameters | |
| Notes | Attempt made to contact author regarding data reported in SDs or SEs, e‐mail sent 24 September 2010 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly allocated to the study group (60 men) or control group (60 men)" No mention of how the randomisation was carried out |
| Allocation concealment (selection bias) | Low risk | "Using sealed envelopes, these patients were randomly allocated..." |
| Blinding (performance bias and detection bias) | Low risk | "The patients in the study and control groups were unaware of whether they were receiving the drug or placebo." ? researchers blinded |
| Incomplete outcome data (attrition bias) | Low risk | All men recruited were analysed. No withdrawals |
| Selective reporting (reporting bias) | Low risk | Report includes all expected outcomes Baseline data were similar for both groups |
| Methods | Randomised placebo controlled trial, 3 arms | |
| Participants | Country: Canada Population: healthy astheno‐zoospermic individuals who were patients of an infertility clinic Mean age: placebo = 35.2, DHA 400 mg = 38.3, DHA 800 mg = 34.4 N = 28 Inclusion criteria: Astheno‐zoospermic, sperm motility < 50% of total sperm Exclusion criteria: Not stated Duration of study: Not stated | |
| Interventions | Fatty acid omega 3 Docosahexaenoic acid (DHA) 400 mg/day (n = 9) versus DHA 800 mg/day (n = 10) versus placebo (n = 9) Duration of treatment: 3 months | |
| Outcomes | Sperm parameters | |
| Notes | Data with SEs converted to SDs Placebo arms split | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 28 subjects were randomly assigned to ..." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Placebo capsules made with "corn oil/soy oil" however blinding not stated |
| Incomplete outcome data (attrition bias) | Low risk | All men randomised were in the analysis, no dropouts. All specified outcomes were reported |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised controlled trial | |
| Participants | Country: USA Population: males with sperm agglutination Mean age: age range 25 to 45 years N = 30 Inclusion criteria: sperm agglutination over 25%, negative sperm antibodies, physically normal, no inflammatory disease Exclusion criteria: unclear Duration of study: 4 weeks | |
| Interventions | Ascorbic acid (AA) 1000 mg (n = 10) versus AA 200 mg (n = 10) versus placebo (n = 10) Duration of treatment: 3 weeks | |
| Outcomes | Seminal parameters | |
| Notes | Placebo numbers split by 2 Data were given in SE converted to SD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "By random selection, three groups of 10 subjects each.." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | "Each subject was told he was receiving AA and expected improvement in sperm quality" |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All specified outcomes were reported |
| Methods | Randomised controlled trial | |
| Participants | Country: Japan Population: infertile men with oligoasthenospermia Mean age: unclear N = 96 Inclusion criteria: unclear Exclusion criteria: unclear Duration of study: 12 weeks | |
| Interventions | L‐carnitine 1000 mg/day (n = 26) versus No treatment (n = 22) Other groups randomised but not appropriate for this review are: vardenafil (n = 23) and sildenafil (n = 25) | |
| Outcomes | Seminal parameters | |
| Notes | Tried multiple times to contact authors for randomisation details and methods. No response. Last contacted in Feburary 2014. E‐mail addresses tried: saitomo@kochi‐u.ac.jp, [email protected] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not given in paper and no response from authors |
| Allocation concealment (selection bias) | Unclear risk | Details not given in paper and no response from authors |
| Blinding (performance bias and detection bias) | High risk | Control no treatment. Details not given in paper and no response from authors |
| Incomplete outcome data (attrition bias) | Low risk | All data points accounted for |
| Selective reporting (reporting bias) | Low risk | All data points accounted for |
| Methods | Randomised controlled trial ‐ triple blind | |
| Participants | Country: Iran Population: astheno‐zoospermic infertile men Mean age: unclear N = 50 Inclusion criteria: patients interest in contribution aged 20‐45 who have passed at least one year from the date they have decided to have a baby, not to using pregnancy protection methods, affected by idiopathic asthenozoospermia based on WHO criteria, normal serum gonadotropin, testosterone and prolactin values Exclusion criteria: affected by genital system infection or taking drug for the infection during past three months, affected by anatomical anomalies in genital system such as varicocoele, surgical history on testicles and vasdeferane Duration of study: 12 weeks | |
| Interventions | 465 mg of DHA plus 600 IU of vitamin E (n = 25) versus Placebo (n = 25) | |
| Outcomes | Seminal parameters ‐ serum fatty acid concentration and sperm membrane fatty acid concentration | |
| Notes | In Arabic ‐ translated. Tried multiple times to contact authors for further study details with no response. Last tried to contact Feburary 2014: [email protected] | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Cans containing capsules marked as A1, A2, B1, B2 and patients, researchers and physician were unaware of the types of drugs |
| Blinding (performance bias and detection bias) | Low risk | Cans containing capsules marked as A1,A2,B1, B2 and patients, researchers and physician were unaware of the types of drugs |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals and exclusions: Intervention group (3 withdrawals): one man could not refer to the clinic in sixth week, the wife of the other one got pregnant, and another one was excluded because he have not taken more than 10% of the capsules Control group (6 withdrawals): two men could not refer to the clinic in sixth week, one man could not refer to the clinic in 12th week. One man used complementary Coenzyme Q10, and another one was excluded because he have not taken more than 10% of the capsules |
| Selective reporting (reporting bias) | Low risk | Sperm parameters reported |
| Methods | Randomised controlled, intention to treat, single centre study. Central allocation ‐ pharmacy, blinded Power calculation performed | |
| Participants | Country: Italy Population: men with persistent oligospermia (5 to 20 m/ml) Mean age: treatment group 32 years (27.5 to 35.5), control 33 (23 to 36) N = 42 Inclusion criteria: having performed a retrograde embolization with concomitant oligospermia, persistent oligospermia and infertility > 12 months Exclusion criteria: smoking, alcohol consumption, taking any fertility drugs within 3 months prior to the study, serious medical or psychiatric condition, abnormal hormonal profile, sperm infection | |
| Interventions | N‐acetylcysteine (NAC) 600 mg and vitamins‐minerals (vitamin C, vitamin E, vitamin A, thiamine, riboflavin, piridoxin, nicotinamide, pantothenate, biotin, cyanocobalamin, ergocalciferol, calcium, magnesium, phosphate, iron, manganese, copper, zinc (n = 20) versus no treatment (n = 22) Duration: 12 months after end of study which ran for 90 days | |
| Outcomes | Primary: seminal parameters Secondary: pregnancy (undefined) and adverse effects | |
| Notes | Attempted to contact author regarding median data. No response as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to either antioxidant therapy or no medical therapy. Randomisation number was assigned by random allocation software using a block randomisation design" |
| Allocation concealment (selection bias) | Low risk | 'All steps of randomisation process were performed blindly in the pharmacy of our hospital.' |
| Blinding (performance bias and detection bias) | High risk | Control is no treatment |
| Incomplete outcome data (attrition bias) | Low risk | "intention to treat" |
| Selective reporting (reporting bias) | Low risk | Does not appear to be any selective reporting |
| Methods | Double blind randomised controlled trial. Trialists and patients blinded. Methods were unclear | |
| Participants | Country: France Population: infertile males Mean age: ? N = 64 Inclusion criteria: TUNEL assay showed a presence of fragmented DNA ≥ 15% of ejaculated spermatozoa Exclusion criteria: variocele, genitourinary inflammation, infection, smoking Duration of study: ? | |
| Interventions | Vitamin C 500 mg 2 x /day + vitamin E 500 mg 2 x /day (n = 32) versus placebo (n = 32) Duration of treatment: 2 months | |
| Outcomes | Sperm parameters | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study participants were randomised into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | Low risk | "The study was double‐blinded with both the authors and the patients unaware of which of the patients was in the treatment or control arm of the study" |
| Incomplete outcome data (attrition bias) | Low risk | All specified outcomes are assessed. No dropouts |
| Selective reporting (reporting bias) | Low risk | Does not appear to be any selective reporting |
| Methods | Randomised controlled trial ‐ open label | |
| Participants | Country: Tunisia Population: infertile men Mean age: 35.5 ± 6.8 (SD) Recruited: N = 78 Randomised: N = 54 Inclusion criteria: infertile men who had been married one year Exclusion criteria: ? Duration of study: 10 months | |
| Interventions | Vitamin E (400 mg/day) + selenium (225 mg/day) for 3 months (n = 12) versus vitamin B (4.5 g/day) (n = 8) Duration of treatment: 3 months | |
| Outcomes | Semen parameters | |
| Notes | Attempted to contact authors regarding high attrition rate > 50% ?78 men randomised or 78 recruited then only 54 (28 in intervention and 26 in control). Then only 20 analysed due to non‐compliance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed with random numbers" and the numeric code was withheld from researchers and patients" |
| Allocation concealment (selection bias) | Low risk | "the numeric code was withheld from researchers and patients" |
| Blinding (performance bias and detection bias) | High risk | "The trial was randomised and open" |
| Incomplete outcome data (attrition bias) | Low risk | High attrition rate was due to non‐compliance, no intention to treat |
| Selective reporting (reporting bias) | Low risk | Sperm parameters reported |
| Methods | Double blinded randomised placebo crossover trial Power calculation performed | |
| Participants | Country: UK, Sheffield Population: men with high levels of reactive oxygen species (ROS) attending Obstetrics and Gynecology department for infertility Couples were undergoing IVF Mean age: ? Median age: 32 years Recruited: N = 30 Inclusion criteria: attending fertility clinic, high levels of ROS in semen. Female partner has patent tubes and is ovulating Exclusion criteria: men with antisperm antibodies, > 20% spermatozoa with I g (immunoglobulin A) or IgG antibodies and sperm concentration < 5 x 06 mL Duration of study: 2 years | |
| Interventions | Vitamin E 300 mg 2 x /day (n = 15) versus identical placebo (n = 15) Duration of treatment: 3 months | |
| Outcomes | Primary outcomes: semen parameters Secondary outcomes: adverse effects. Live birth | |
| Notes | Attempted to contact author regarding median data, no response as yet Only first phase data used in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study was a randomised double blind placebo controlled trial". "The randomisation was performed by the manufacturer" |
| Allocation concealment (selection bias) | Unclear risk | "The randomisation was performed by the manufacturer" |
| Blinding (performance bias and detection bias) | Low risk | "the code was blind for the researcher and patients. The code was broken at the end of the trial" |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes are reported |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as stated in the methods section |
| Methods | Double‐blind randomised parallel trial | |
| Participants | Country: Japan, 25 centres Population: male patients with abnormal sperm count or motility Mean age: average 32.8 (SD 4.8) Recruited: 375 Inclusion criteria: 1. Average sperm count ≤ 40 × 106 /mL measured on ≥ 2 occasions OR 2. Average sperm count ≥ 40 count ≤ 40 × 106 /mL measured on ≥ 2 occasions AND sperm motility < 50% Exclusion criteria: 1. Sperm count only measured at 1 occasion 2. Average sperm count ≤ 2 × 106/mL 3. Sperm motility = 0% 4. Testicular size < 8 mL using orchidometer bilaterally 5. Use of hormone or anti‐hormone drug within preceding 3 months before the study period 6. WBC > 5/HPF in the semen or the presence of possible genito‐urinary infection 7. Presence of hypoganadism or endocrine disease Presence of undescended testes, genito‐uninary tract obstruction, varicocele or any other serious associated condition also included concomitant use of anti‐hormonal and hormonal treatment and the 2 patients with polypharmacy were excluded from the data analysis Duraton of trial: January 1985 to June 1986 Duration of treatment: 12 weeks | |
| Interventions | 1. Mecobalamin group 6,000 micrograms/day (n = 125) (vitamin B12) versus 2. Mecobalamin group 1,500 micrograms/day (n = 124) versus 3. Placebo (n = 126) | |
| Outcomes | Sperm concentration Sperm motility | |
| Notes | Paper translated by Dr Tomoko Kumaga and Tan Wantao. No contact details available for authors. No useable data available. Data in spreadsheet to be added to other tables No ITT completed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 396 patients were divided into 3 groups (6000ug/day, 1500ug/day, placebo) by randomization. The implementation of randomization and allocation concealment was carried out by two people (Doctor Yamamoto, Doctor Shimizu) |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) | Low risk | Double blind ‐ placebo used |
| Incomplete outcome data (attrition bias) | High risk | No ITT |
| Selective reporting (reporting bias) | High risk | Subgroup analysis performed as an addition post‐treatment |
| Methods | Randomised placebo‐controlled, double blind crossover trial Power calculation performed | |
| Participants | Country: Italy Population: male factor infertility ‐ oligoasthenoteratozoospermia (OAT) Mean age: ? Range: 20 to 40 years N = 100 Inclusion criteria: men are aged between 20 to 40 years with infertility lasting longer than 2 years. Regular sexual intercourse with a gynaecologically normal female partner with no female infertility. Absence of endocrine disease, genital infections, obstructive cryptorchism, antisperm antibodies, normal sperm parameters with no significant differences after 3 tests. Mild oligospermia. Sperm concentration 10 to 20 x 106/mL and motility 10% to 30% Exclusion criteria: not mentioned Duration of study: 10 months | |
| Interventions | L‐carnitine 2 g/day (n = 43) versus placebo (n = 43) Duration of treatment: 6 months | |
| Outcomes | Semen parameters and pregnancy rate | |
| Notes | First phase data only used in analysis Attempted to contact author regarding standard deviations, how many were in each group for the first phase and how many of the 4 who went to assisted reproduction did so in the first phase and what do they mean by 172 cycles. No response yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "we report on a randomised placebo controlled cross over trial". No mention of method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No mention |
| Blinding (performance bias and detection bias) | Low risk | Double blinded, "seemingly identical placebo" |
| Incomplete outcome data (attrition bias) | Low risk | 14 withdrew ‐ 4 went onto assisted reproduction, 6 did not return for second period and 4 due to pregnancy in first phase. Therefore should only be ?4 at the most lost from first phase. No intention to treat All withdrawals accounted for for whole trial however how many were lost in the first phase in first phase |
| Selective reporting (reporting bias) | Low risk | All outcomes are reported |
| Methods | Placebo‐controlled, double blind randomised trial. No mention of method of randomisation or allocation concealment "When codes were broken at the end of the study" Power calculation performed | |
| Participants | Country: Italy Population: infertile males with oligoasthenoteratozoospermia Mean age: not stated. Age range 20 to 40 years N = 60 Duration of study: 8 months Inclusion criteria: oligoasthenoteratospermia, age between 20 to 40 years, infertility > 2 years with regular intercourse. No endocrine disease, cryptorchidism, genital infections or obstructions, variocoele or testicular hypertrophy, antisperm antibodies Exclusion criteria: none Duration of study: 8 months | |
| Interventions | L‐carnitine 2 g/day + L‐acetyl‐carnitine 500 mg 2 x /day (n = 30) versus placebo (n = 26) Duration of treatment: 6 months | |
| Outcomes | Semen parameters and pregnancy rate | |
| Notes | Attempted to contact author regarding 8 month follow up data. No reply as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "placebo controlled double blind randomised trial" |
| Allocation concealment (selection bias) | Unclear risk | Mentions coding ‐ "When codes were broken at the end of the study" |
| Blinding (performance bias and detection bias) | Low risk | "Double blind" ‐ placebo used therefore low risk for performance bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 4 men withdrew from the placebo group. 60 randomised 56 analysed. No intention to treat |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Double blinded randomised parallel trial | |
| Participants | Country: Eastern China Population: infertile men with oligoasthenospermia Mean Age: Treatment 30 ± 5.5 (23 to 45 years), Control 32 ± 3.5 (24 to 46 years) N = 150 Inclusion criteria: no smoking or alcohol. Any fertility medication needed to be stopped 2 weeks before Exclusion criteria: nil Duration: 3 months | |
| Interventions | L‐carnitine 2 g/day + acetyl‐L‐carnitine 1 g/day (n = 85) (90 with intention to treat) versus vitamin E (100 mg tid) + vitamin C (100 mg tid) (n = 53) (60 with intention to treat) Duration of treatment: 3 months | |
| Outcomes | Seminal parameters and pregnancy rate per couple | |
| Notes | Withdrawal: 5 from treatment group and 7 from control Contact author re methods of randomisation, concealment and whether SD or SEs used and query that this is the same trial as Li 2005a Translated by Shaofu Li 10 November 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial. Methods not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) | Unclear risk | Double blind but unclear who is blinded as the control is another antioxidant i.e. not placebo |
| Incomplete outcome data (attrition bias) | Low risk | Attrition explained |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data given in standard deviations or standard errors. Assumed to be SDs |
| Methods | Randomised trial. No information on concealment | |
| Participants | Country: Eastern China Population: infertile men with oligoasthenospermia Mean age: 29 ± 3.5 (23 to 40 years) N = 80 Inclusion criteria: no smoking or alcohol. Any fertility medication needed to be stopped 2 weeks before Exclusion criteria: nil Duration: not stated | |
| Interventions | L‐carnitine 2g/day (n = 40) versus vitamin E 100 mg + vitamin C 100 mg tid (n = 40) Duration of treatment: 3 months | |
| Outcomes | Seminal parameters and pregnancy | |
| Notes | Attempted to contact author re methods of randomisation, concealment and whether SD or SEs used and whether this is the same trial as Li 2005. Also asked whether there were any data on pregnancy rate. Translator replied 22.09.09 no pregnancy data were available in the text of the trial Translated by Shaofu Li 10/11/08 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawal: 8 from treatment (n = 32) and 9 from control (n = 31). 21% loss to follow up. No intention to treat |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether data given in SDs or SEs |
| Methods | Randomised controlled crossover trial | |
| Participants | Country: Italy Population: male infertility, oligoasthenospermia Mean age: not stated N = 100 Inclusion criteria: age 20 to 40 years. Infertility > 2 years. 3 baseline semen analysis demonstrating concentration 10 to 20 106/mL, 10% to 30% total motility, forward progression < 15%, abnormal morphological forms < 70%, curvilinear velocity 10 to 30 /second + linearity < 4 Exclusion criteria: not mentioned Duration: 10 months | |
| Interventions | L‐carnitine 2 g/day (n=?) versus placebo (n =?) Duration of treatment: 2 months | |
| Outcomes | Semen parameters | |
| Notes | Abstract only Attempted to contact author re first phase data, outcomes, randomisation, concealment and whether there was a full publication of the trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | randomised ‐ "all patients had an initial 2 months run in period and then randomised to 2 months of carnitine or placebo." |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | Low risk | "double blind crossover trial" ‐ placebo used therefore low risk for performance bias |
| Incomplete outcome data (attrition bias) | Unclear risk | 86 patients completed the trial out of 100. Need to see full trial for the reasons for withdrawals and intention to treat |
| Selective reporting (reporting bias) | Unclear risk | Unclear (conference abstract) |
| Methods | Randomised double‐blind study controlled trial | |
| Participants | Country: Spain Population: infertile men Mean age: DHA group 35.23, placebo 36.10 – overall average age 35 N = 42 – abstract, n = 64 from author Inclusion criteria: men suffering from male factor infertility, according to the World Health Organization guidelines (WHO 1999), and who were undergoing infertility evaluation during the period 2009 to 2011 Exclusion criteria: oncological patients, those suffering from metabolic disease, chromosomal or genetic alterations, and patients on anticoagulant treatment Duration: 10 weeks | |
| Interventions | Brudy Plus ‐ enzymatic nutraceutical triglyceride oil – 1500 mg/day of DHA‐enriched oil (the patients ingested 1000 mg of DHA and 135 mg of eicosapentaenoic acid (EPA) per day) (n = 35) versus placebo (n = 29) Duration of treatment: 10 weeks | |
| Outcomes | Sperm DNA fragmentation, seminal parameters, lipid composition, antioxidant capacity | |
| Notes | Pharmaceutical funding: Intervention Brudy plus and trial was supported by Brudy technology S.L Abstract from conference Contacted author multiple times via e‐mail, [email protected], for further study details. Clarified that the abstract details were different from that in the final study, a copy of the unofficial manuscript was submitted to the review authors. Last contact was on 26 February 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list with a computer program |
| Allocation concealment (selection bias) | Low risk | Closed and numerated envelopes with allocation group |
| Blinding (performance bias and detection bias) | Low risk | Participants knew that they was included in group A or B but only Brudy technology knew the assignation to the control group or experimental group |
| Incomplete outcome data (attrition bias) | Low risk | Unable to be ascertained from study |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised controlled trial | |
| Participants | Country: Mexico Population: male idiopathic asthenozoospermia attending a fertility clinic Mean age: 30.8 ± 6 (20 to 40 years) N = 47 Inclusion criteria: free of urogenital symptoms, idiopathic asthenozoospermia, not received drugs in prior 6 months and healthy Exclusion criteria: variocoele, inflammatory diseases, endocrine disorders Duration of study: 6 months | |
| Interventions | Pentoxifylline 1200 mg to 400 mg /3 x day (n = 25) versus placebo (n = 22) Duration of intervention: 6 months | |
| Outcomes | Seminal parameters and hormonal assays | |
| Notes | Attempted to contact author to ask if had data in means + SD and not medians + range as published. Letter sent 15 September 2009. Letter returned to sender | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The 47 men were divided at random" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No mention of blinding however the trial is placebo controlled |
| Incomplete outcome data (attrition bias) | Unclear risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | Randomised controlled trial Methods not stated. No mention on methods of concealment | |
| Participants | Country: Belgrade, Hungary Population: idiopathic oligoasthenospermia Mean age: 29 ± 2 in treatment group, 26 ± 3 in control group N = 90 Inclusion criteria: idiopathic oligoasthenospermia and no pregnancy for 2 years Exclusion criteria: variocoele, inflammatory diseases, endocrine disorders Duration of study: 3 months | |
| Interventions | Pentoxifylline 1200 mg/day (n = 51) versus no treatment (n = 39) Duration of treatment: 3 months | |
| Outcomes | Seminal parameters | |
| Notes | Attempted to contact author regarding extractable data for pregnancy rate plus methods of of randomisation and concealment also don't know if adverse events are single or multiple events. No reply as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomised group of 90 men" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | No mention of blinding. The control is no treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Don't know if adverse events are single or multiple. Pregnancy data not extractable |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Methods | Randomised controlled trial | |
| Participants | Country: Italy Population: Infertile men with with asthenospermia Mean age: Between 25 and 49 N = 180 Inclusion criteria: man age between 28 and 45, sperm concentration < 20 x 106 spermatozoa /mL, sperm progressive motility < 30%, normal morphology < 30%, leucocyte < 1 x 106 /mL, no infections Exclusion criteria: men younger than 28 and over 45, sperm concentration > 20 x 106 spermatozoa /mL, sperm progressive motility > 30%, normal morphology > 30%, leucocyte > 1 x 106 /mL, current infections, history of testicular pathology: cryptorchidism, varicocele, surgical operations, radiotherapy or chemotherapy, use of anabolic steroids, deficiency of hypothalamic‐pituitary‐gonadal axis, genital tract infections Duration: 3 months | |
| Interventions | L‐arginine 1660 mg, carnitine 150 mg, acetyl‐carnitine 50 mg and ginseng 200 mg in one vial (n = 90) versus no treatment (n = 90) Duration of treatment: 3 months | |
| Outcomes | Semen parameters Study also measured sexual satisfaction | |
| Notes | Contacted author via email, [email protected], to clarify study details, recruitment, randomisation, blinding, ethics approval, study population, withdrawals and to clarify progressive mortality. Last response was on 12.03.14 'Total motility and progressive motility are similar terms for the same definition: all the spermatozoa that have progressive or not linear motility.' Motility has been excluded from this analysis Initially translated from Italian by Roberto D'Amico | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised' unable to ascertain risk |
| Allocation concealment (selection bias) | Unclear risk | Unable to ascertain risk |
| Blinding (performance bias and detection bias) | High risk | Control is no treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Unable to ascertain risk |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised controlled trial Power calculation performed | |
| Participants | Country: Iran Population: infertile men with oligoasthenoteratozoospermia who have been trying for pregnancy for > 1 yr unprotected intercourse Mean age: 34 N = 60 Inclusion criteria: seminal white blood cells < 1,000,000 /mL, absence of anatomical abnormalities of the genital tract, absence of infectious genital diseases or systemic diseases, absence of treatment with other drugs and dietary supplement during the 3 months before enrolling in the study, at last absence of smoking, drug, and alcohol use or occupational chemical exposure Exclusion criteria: seminal white blood cells > 1,000,000 /mL, presence of anatomical abnormalities of the genital tract, presence of infectious genital diseases or systemic diseases, presence of treatment with other drugs and dietary supplement during the 3 months before enrolling in the study, currently smoking, using drug, or alcohol use or occupational chemical exposure Duration: 3 months | |
| Interventions | CoQ10 ‐ 200 mg/day orally (n = 23) versus placebo (n = 24) Duration of treatment: 3 months | |
| Outcomes | Sperm motility and concentration Also measure progression, total antioxidant capacity (TAC) | |
| Notes | Contacted regarding methods, randomisation, allocation concealment, recruitment, blinding and dropouts. Response from Azadeh Nadjarzadeh, [email protected], October 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using block randomization. It was done by Dr Motevallian who is epidemiologist and it has done before study |
| Allocation concealment (selection bias) | Low risk | Before the trial a colleague, that had not role in the study, coded the bottles of Coenzyme Q10 and placebo (that were similar) in A and B and give them to one of the staff of Avicenna Research centre. Only that person has a list of randomisation and give A or B bottles to the participants according to their code |
| Blinding (performance bias and detection bias) | Low risk | Both participants and investigators blinded – The appearance and the bottles of capsules were similar and none of outcome assessors knew group, because everyone had a code after being allocated group A and B |
| Incomplete outcome data (attrition bias) | Low risk | 13 dropped out (22%) ‐ 7 from treatment group and 6 from the control group |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised comparative study No mention of allocation concealment Abstract only | |
| Participants | Country: Tunisia Population: Infertile males with oligoasthenoteratozoospermia Mean age: not stated N=? Inclusion criteria: males with oligoasthenoteratozoospermia. Exclusion criteria: none mentioned Duration of study: not stated | |
| Interventions | Vitamin E (400 mg) + selenium (200 μg) /day (n = 12) versus vitamin B (B2, B6 and B12) (n = 8) Duration of treatment: 3 months | |
| Outcomes | Seminal parameters | |
| Notes | Attempted to contact authors regarding methods of randomisation and data ‐ no extractable data from the abstract. No reply as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In a prospective randomised comparative study" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | No mention of blinding. Control is no treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised controlled trial Open trial ‐ control is no treatment | |
| Participants | Country: Kuwait Population: men with asthenozoospermia attending infertility and andrology clinic Mean age: 37.8 ± 7.9 in treatment group, 38.1 ± 8.2 in control N = 100 Inclusion criteria: men with asthenozoospermia. Spermatozoal motility impaired with >4 0% non‐motile sperm. Have been trying to conceive for at least one year. Plus no obvious female factor Exclusion criteria: none mentioned Duration of study: 12 months | |
| Interventions | Zinc 250 mg 2 x day (n = 49) versus no treatment (n = 48) Duration of treatment: 3 months | |
| Outcomes | Seminal parameters | |
| Notes | Attempted to contact authors regarding methods randomisation and concealment questioned. No reply as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised. "100 men with Asthenozoospermia were randomised into two groups" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | Control is no treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | 100 men randomised, 97 analysed, dropouts are accounted for |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised controlled 4‐armed trial, open as one arm of the trial is no therapy | |
| Participants | Country: Kuwait Population: men with asthenozoospermia attending infertility clinic in Kuwait Mean age: 35 ± 1 N = 45 Inclusion criteria: asthenozoospermia with normal sperm concentration (20 to 250 million/mL) but with 40% or more immotile sperm Exclusion criteria: asthenozoospermia but sperm concentration of < 20 million/mL Duration of study: No stated | |
| Interventions | Zinc 200 mg 2 x /day (n = 11) versus zinc 200 mg + vitamin E 10 mg 2 x /day (n = 12) versus zinc 200 mg + vitamin E 10 mg + vitamin C 5 mg 2 x /day (n = 14) versus no therapy (n = 8) Duration of intervention: 3 months | |
| Outcomes | Seminal parameters | |
| Notes | Attempted to contact author re methods of randomisation ‐ states that "8 men served as non‐ therapy control". No reply as yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the 45 asthenozoospermic men were randomised into four groups" |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | No mention of blinding. Control is another antioxidant or no treatment |
| Incomplete outcome data (attrition bias) | Low risk | All outcomes are reported. No dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Double blind randomised crossover trial | |
| Participants | Country: Iran Population: infertile men with at least two abnormal spermiograms N = 30 Exclusion criteria: variocoele, testicular atrophy, ejaculatory disorders, use of medications, azoospermia, endocrinological disorders, ICSI candidacy or other causes of infertility Duration of first phase: 8 weeks | |
| Interventions | L‐carnitine (2 g/day) (n = 15) versus placebo (n = 15) | |
| Outcomes | Sperm parameters | |
| Notes | Abstract in English, full text in Arabic. Contacted the author and he is filling out the data extraction sheets. Author responded but data queries remain contacted again re SDs and pregnancies in first phase of crossover. Author responded saying that the data was given in SDs and there were 3 pregnancies in the first phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "patients were randomly allocated to two groups of A and B" |
| Allocation concealment (selection bias) | Low risk | "sealed opaque envelopes" |
| Blinding (performance bias and detection bias) | Low risk | "Double blind" "outcome assessor was blinded". Placebo controlled |
| Incomplete outcome data (attrition bias) | Unclear risk | 'loss to follow up was not accounted for" |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | A placebo‐controlled double blind randomised trial | |
| Participants | Country: Panama Infertile healthy men (N = 60) "60 patients completed the study ‐?how may were randomised. "There was no statistical differences in age, body mass index or initial sperm parameters between groups" Inclusion criteria: infertile healthy men without previous treatments, non smokers, no alcoholics or drug users Exclusion criteria: varicocele and leukocyte‐spermia were excluded Duration of study: January 2012 to March 2013 Duration of treatment: 13 weeks No power calculation described | |
| Interventions | Group I: L‐carnitine (Cardispan, Gossman de C.V) 1 g pill each 12 hours (n = ?) Group II: Spermotrend (Catalysis) 1 pill each 8 hours (n = ?) Group III: Maca extract (NatureWay Products, Inc) 1 g pill each 12 hours (n = ?) Group IV: placebo 1 pill each 12 hours (n = ?) | |
| Outcomes | Sperm motility Sperm concentration Normal sperm (morphology) | |
| Notes | The study was approved by the National Bioethical Committee. Funded by a grant given by the Ministry of Economy and Finances. Panama, Republic of Panama Unknown withdrawal numbers, age Conference abstract. Letter written and posted regarding methods and data 12 February 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unknown methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unknown methods of allocation concealment |
| Blinding (performance bias and detection bias) | Low risk | Double blind and placebo‐controlled |
| Incomplete outcome data (attrition bias) | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Double blind randomised crossover trial | |
| Participants | Country: UK (two centres) Population: men with severe oligozoospermia Mean age: ? Randomised: N = 64 Inclusion criteria: 1 sperm count of less than 10 million per ejaculate on each of 2 occasions immediately preceding the trial 2. No uncorrected varicoceles or testicular maldescent 3. Testicular biopsy already performed (Johnsen 1970) 4. No drugs taken in past 3 months which were known to affect spermatogenesis 5. No history of biliary disease owing to a suggestion that arginine might interfere with the metabolism of bile salts The wives of all these men had been fully investigated with regard to fertility Duration of study: unknown Duration of treatment: 12 weeks for 1st phase."Each treatment period lasted 12 weeks with no intervening wash‐out period" Exclusion criteria: men with varicocoele | |
| Interventions | Arginine 4 g/day (n = 35) versus placebo (n = 29) | |
| Outcomes | Total sperm motility Hormone levels | |
| Notes | No mention of funding sources. E Merck Ltd supplied the capsules of arginine and placebo. Unclear if consent sought. Dropouts explained but unclear from which group No data available for sperm parameters Pregnancy not stated in the methods section as an outcome of interest but reported in the results 10 withdrew reasons were given but unsure from which group, the paper stated that they used ITT but data not presented The study didn't report the outcomes for the different phases of the trial (i.e. not separated into phase 1 phase 2). Pregnancy data is separated into phase one data but probably biochemical and will be used in biochemical pregnancy table. Unable to contact author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of randomisation not explained |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Double blind ‐ placebo controlled |
| Incomplete outcome data (attrition bias) | Unclear risk | 10 withdrew but unsure from which group |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised placebo‐controlled double blind study No mention of allocation concealment Power calculation performed | |
| Participants | Country: Munster Germany Population: men with infertility for over one year. Mean age: treatment 36.1 ± 5.0, placebo 35.2 ± 4.8 N = 33 Inclusion criteria: asthenozoospermia (< 50% motile) diagnosed after 2 examinations, normal or reduced sperm concentration (> 20 x 106 per ejaculate) and without infection of access glands Exclusion criteria: none mentioned Duration of study: 8 weeks | |
| Interventions | Vitamin C 1000 mg + vitamin E 800 mg/day (n = 15) versus placebo (n = 16) Duration of treatment: 8 weeks | |
| Outcomes | Primary: semen parameters Secondary: pregnancy rate and adverse effects | |
| Notes | Contacted author about the allocation concealment and pregnancy and adverse effects were outcomes in their protocol. Rolf replied saying that pregnancy and adverse effects were stated in the protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed with random numbers without further stratification by the pharmacist and the code was withheld from researchers and patients" |
| Allocation concealment (selection bias) | Unclear risk | Pharmacist performing randomisation and code withheld from patients and researchers. However no mention of type of containers or envelopes |
| Blinding (performance bias and detection bias) | Low risk | Double ‐ patients and researchers |
| Incomplete outcome data (attrition bias) | Low risk | All data reported, 2 patients withdrew from the trial ‐ "results from two patients were rejected from analysis." 1 from the treatment group due to poor compliance and 1 from the placebo group due to genital tract infection. Intention to treat |
| Selective reporting (reporting bias) | Low risk | All semen outcomes reported and author states (e‐mail 22.09.09) that pregnancy and adverse effects were set a priori in the protocol |
| Methods | Double blind placebo‐controlled randomised study. Randomised using permuted blocks Allocation concealment: sealed envelopes Blinding: double, identical coating of tablets Power calculation performed | |
| Participants | Country: Iran Population: idiopathic oligoasthenoteratospermia, asthenospermia or teratospermia of 2 years duration Mean age: 31 years (25 to 48 years) Recruited: N = 548 Randomised: N = 468 Inclusion criteria: sperm count > 5 x 106 /ml, over 2 years of failed conception, no female fertility problems, no history of possible cause for male infertility Exclusion criteria: abnormal testes, history of cancer or chemotherapy, testosterone or antiandrogen use, use of selenium or N‐acetyl‐cystine supplements, abnormal hormone levels, genital disease, genital inflammation or variocoele, history of genital surgery, major surgery, central nervous system injury, a known sperm defect or retrograde ejaculation. Y chromosome abnormalities, sexually transmitted disease, genitourinary infection, leukocytospermia, smoking, any environmental exposures to reproductive toxins. Medical, neurological or psychological problems. A history of drug or alcohol abuse, hepatobiliary disease or significant renal insufficiency. Any endocrine abnormality, a body mass index (BMI) of 30 kg/m2 or over, participation in another investigational study and a likelihood of being unavailable for follow up Duration of study: 56 weeks | |
| Interventions | Selenium 200 µg/day (n = 116) versus N‐acetylcysteine 600 mg/day (n = 118) versus selenium 200 µg/day + N‐acetylcysteine 600 mg/day (n = 116) versus placebo (n = 118) Duration of treatment: 26 weeks or 6.5 weeks Analysed: n = 105 in selenium group (loss 11), n = 106 in placebo group (loss 12), n = 105 in N‐acetylcysteine group (loss 13) and n = 104 in selenium + N‐acetylcysteine group (loss 12) | |
| Outcomes | Primary outcome: semen parameters Secondary outcomes: adverse events | |
| Notes | Attempted to contact authors regarding side effect data that had not yet been added to the review due to the query of multiple comparisons. Also to ask whether data is in SD (as reported in the text) or SE, as requested by statistician 24.09.10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation table generated by the method of random permuted blocks. Patient randomisation numbers were allocated to each site in ascending sequence in blocks." |
| Allocation concealment (selection bias) | Low risk | "Assignment to treatment groups was performed using a sealed envelope technique." |
| Blinding (performance bias and detection bias) | Low risk | "Eligible patients were randomly assigned to double blind.." "Placebo pills were coated with titanium oxide to ensure an identical appearance and smell." |
| Incomplete outcome data (attrition bias) | Low risk | All withdrawals were accounted for in each treatment group. Withdrawal was mainly due to withdrawal of consent followed by lost to follow up and lastly for reasons of missing data. No intention to treat |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes |
| Methods | Randomised controlled trial, double blind Allocation concealment: Not mentioned Power calculation performed | |
| Participants | Country: Tehran, Iran Population: infertile males between 21 and 42 years with idiopathic oligoasthenoteratospermia Mean age: treatment group 28 ± 9, placebo 28 ± 10 (range 21 to 42 years) Recruited: N = 268 Randomised: N = 212 Inclusion criteria: minimum 2 years unprotected intercourse with 2 years unwilling childlessness. male infertility diagnosed if 1 or more standard semen parameters were below cutoff levels accepted by World Health Organisation (WHO). A fertile female partner. No known medical condition that could account for infertility, testicular volume 12 ml or greater. No medical therapy for at least 12 weeks before the study begins. Only patients seeking medical attention for infertility were included Exclusion criteria: azoospermia or severe oligospermia (sperm count less than 5 million/ml. An history of epypidymo‐orchitis, prostatitis, genital trauma, testicular torsion, inguinal or genital surgery. Any genital or central nervous system disease, endocrinopathy, cytotoxic drugs, immunosuppressants, anticonvulsives, androgens, antiandrogens, a recent history of Sexually transmitted disease. Psychological or physiological abnormalities that would impair sexual functioning or ability to produce sperm samples. Drug, alcohol or substance abuse. Liver disease, renal insufficiency or chromosome abnormalities. occupational and environmental exposures to reproductive toxins. A body mass index (BMI) of 30 kg/m2 or over, participation in another investigational study and a likelihood of being unavailable for follow up Duration of study: 20 months, from February 2005 until October 2006 | |
| Interventions | Coenzyme Q10 (CoQ10) 300 mg/day (n = 106) versus placebo (n = 106) Duration of treatment: 26 weeks or 6.5 months Analysed n = 98, 8 withdrawals in treatment group, analysed n = 96, 10 withdrawals in the placebo group | |
| Outcomes | Primary outcomes: semen parameters and testicular volume Secondary outcomes: adverse effects and hormone levels | |
| Notes | Attempted to contact authors to ask whether data is in SD (as reported in the text) or SE, as requested by statistician 24.09.10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each eligible patient received a randomisation number, which was determined by a computer generated schedule. Therafter a randomisation table was generated by the method of random permuted blocks. Individuals who were geographically and operationally independent of the study investigator performed the study randomisation" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment: not mentioned |
| Blinding (performance bias and detection bias) | Low risk | "The clinician prescriber and the patients were blinded to the treatment condition. To maintain and guarantee blinding CoQ10 and placebo were identical in appearance. Participant data collected during this trial were kept confidential and locked in a secure office area. Randomisation codes were opened only after all patients had completed the whole study protocol." |
| Incomplete outcome data (attrition bias) | Low risk | All patients who dropped out of the trial were accounted for ‐ 8 from treatment group and 10 from placebo group for reasons such as withdrawal of consent, missing data and loss to follow up |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised controlled trial Power calculation performed | |
| Participants | Setting: Iran ‐ fertility clinic Recruitment October 2006 to December 2008 N = 254 infertile men attending a fertility work‐up (referred or self referred) having been infertile for at least 2 years Mean age PTX 32.1 ± 4.3 years, placebo 32.8 ± 4.6 years. Mean duration of infertility in PTX 5.1 ± 2.8 years, placebo 5.2 ± 2.6 years Inclusion criteria: ≤ 45 years old. unable to conceive after 2 years. Normal basal serum of testosterone, luteinising hormone (LH), follicle‐stimulating hormone (FSH), thyroid‐stimulating hormone (TSH), prolactin (PRL), and inhibin B (> 150 pg/ml); no known medical condition that could account for OAT, total testicular volume ≥ 12 mL, and a normal fertile female partner Exclusion criteria: history of cancer chemotherapy, androgens and antiandrogens, or history of the following: cryptorchidism, orchitis, testicular torsion or trauma, variocoele, relevant genitourinary infection, sexually transmitted disease, reproductive hormone levels outside of normal limits, hyperprolactinemia, alcohol or substance abuse, hepatobiliary disease, significant renal insufficiency, presence of antisperm antibodies, Y chromosome microdeletions, and karyotypic abnormalities. Men with severe oligozoospermia (< 5 million/mL), leukocytospermia (more than 106 white blood cells /mL), tobacco use, and occupational and environmental exposures to potential reproductive toxins were also excluded. Any drugs that might be affecting the spermatogenesis courses should be discontinued 6 months before enrolment | |
| Interventions | Pentoxyfiline (n = 127) 400mg PTX (Apo‐Pentoxifylline, Apotex Inc. Toronto, Canada), twice daily, orally for 24 weeks versus placebo (n = 127) components not stated, twice daily, orally for 24 weeks | |
| Outcomes | Semen volume, sperm motility, sperm count, sperm morphology, sperm density, adverse events, seminal plasma antioxidant status SOD and CAT, % acrosome reaction reproductive hormones and genetic analyses | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via a central computerised voice response system |
| Allocation concealment (selection bias) | Low risk | Central computerised voice response system |
| Blinding (performance bias and detection bias) | Low risk | 'Study medication was over‐encapsulated so that they appeared the same. Patients appear to be blinded' and 'Posttreatment semen analyses were carried out in a blinded fashion with regard to treatment.' |
| Incomplete outcome data (attrition bias) | Low risk | 254 randomised, 127 to each group PTX 11 excluded during treatment protocol (protocol violation 4, withdrawn consent 2, lost to follow up 4, adverse events 1) after 12 week follow up 4 more excluded due to loss to follow up Placebo 10 excluded during treatment protocol (protocol violation 4, withdrawn consent 2, lost to follow up 4) after 12 week follow up 4 more excluded due to loss to follow up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported appear to have been analysed |
| Methods | Randomised controlled trial Power calculation performed | |
| Participants | Setting: single urology centre/private clinic in Iran Duration of trial from June 2010 to January 2011 N = 228 infertile men; mean age ubiquinol 31 years and placebo 32 years. Primary infertility for at least 2 years Inclusion criteria: history of primary infertility of more than 2 years, Abnormal sperm count and motility according to WHO criteria, Wife age between 20 and 40 years, Documentation of fertile female partner, No known medical or surgical condition which can result in infertility Exclusion criteria: history of cancer chemotherapy or radiotherapy; History of genital disease such as cryptorchidism and varicocoele; History of genital surgery; Body mass index 30 kg/m2 or greater; Any endocrinopathy; Y chromosome microdeletion or karyotype abnormalities; Leukocytospermia (more than 106 white blood cells per mL); Drug, alcohol or substance abuse; tobacco use; use of anticonvulsants, androgens or antiandrogens; Significant liver (serum bilirubin greater than 2.0 mg/dl) or renal function (serum creatinine greater than 2.0 mg/dl) impairment; Occupational and environmental exposure to reproductive toxins Severe oligozoospermia (less than 5 x 106 /mL), azoospermia and testicular volume less than 12 mL | |
| Interventions | Coenzyme Q10 (Ubiquinol) (n = 114) 200 mg ubiquinol (New Life CoEnz QH, Istanbu, Turkey), orally, once daily after a meal for 26 weeks versus placebo (n = 114) content not specified orally once daily after a meal for 26 weeks | |
| Outcomes | Semen volume, sperm density, sperm motility, sperm morphology, seminal plasma antioxidant status | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number table |
| Allocation concealment (selection bias) | Low risk | The randomisation codes were centrally assigned by the co‐ordination centre after checking the main eligibility criteria |
| Blinding (performance bias and detection bias) | Low risk | All investigators and study staff were blinded to treatment allocation during the whole study period, All of the participants were naive for treatment |
| Incomplete outcome data (attrition bias) | Low risk | 228 were randomised of 264 eligible Ubiquinol group – 13 excluded at end of treatment (3 protocol violations, 4 withdrawal of consent and 6 lost to follow up). At 12 weeks follow up a further 5 were lost to follow up Placebo group – 12 excluded at end of treatment (4 protocol violations, 4 withdrawal of consent, 6 lost to follow up. At 12 weeks follow up a further 7 were lost to follow up |
| Selective reporting (reporting bias) | High risk | The authors do not pre‐specify which outcome measures will be reported. The primary outcome is a % change from baseline at the end of the treatment period |
| Methods | Double blind randomised trial Allocation concealment: no mention | |
| Participants | Country: Glasgow, UK Population: men attending subfertility clinic with low sperm motility Mean age: 33.3 years ± 0.64 Recruited: N = 69 Analysed: N = 64 Inclusion criteria: low sperm motility Exclusion criteria: not mentioned Duration of study: 3 months and two weeks | |
| Interventions | Selenium 100 µg/day (n = 16) versus selenium 100 µg + vitamin A 1 mg + vitamin C 10 mg + vitamin E 15 mg/day (n = 30) versus placebo (n = 18) Duration of treatment: 3 months Analysed = 64, 5 withdrew 1 from selenium group, 4 from selenium + vitamin A, C and E group. All withdrawals were due to non‐compliance | |
| Outcomes | Primary outcome: semen parameters Secondary outcome: pregnancy rates | |
| Notes | Uneven numbers, multivitamin numbers are double the other groups Need to ask author if they have separate numbers for pregnancy data. Currently have 5 pregnancies in the 2 treatment groups and none in placebo Who was blinded, was the placebo identical when group 2 contained so many different vitamins Was there any allocation concealment? Author has retired and is not able to be contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "As the patients entered the trial they were randomly allocated to one of three treatments, which had in turn been randomised within each block of four numbers and 'blinded' using a numeric code." Unclear as to why the uneven nature of the numbers in the groups i.e. 30 in multivitamin group and 16 in selenium, 18 in placebo |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | Double blind, placebo controlled |
| Incomplete outcome data (attrition bias) | Low risk | Numbers of withdrawals and reasons (non compliance) were reported |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Randomised double blind trial Allocation concealment: adequate | |
| Participants | Country: Minneapolis, USA Population: males aged 18 to 65 years IVF Mean age: 36.2 ± 5.8 (SD), 35.3 ± 7.5 (SD) Recruited: N = 26 Analysed: N = 21, 5 withdrawals Inclusion criteria: males 18 to 65 years with infertility of at least six months duration, sperm concentration of at least 5 million sperm/mL, motility of 10% to 50%, absent pyospermia and normal FSH and testosterone levels Exclusion criteria: history of post‐pubertal mumps, cryptorchism, vasal or epididymal surgery, history of medication or chemotherapy. recent alcohol, chronic marijuana. Use of testosterone or steroids. Exposure to environmental toxins. Recent history of fever or diabetes, liver failure, renal failure, endocrine disorder, untreated variocoele, urogenital infection, or prior vasectomy reversal Duration of study: 24 weeks | |
| Interventions | Carnitine (L‐carnitine 2000 mg +L‐acetylcarnitine 1000 mg/day) (n = 12) versus placebo (n = 9) | |
| Outcomes | Primary outcome: semen parameters Secondary outcomes: pregnancy ‐ 2 pregnancies, 1 in the treatment arm after IVF and one in placebo through intercourse | |
| Notes | Author replied 21.09.09 saying: 'The published 2006 trial is the published version of the 2003 abstract (Pryor 2003)' and giving details of randomisation and concealment. Author says he will try and find out about the 5 patients that dropped out. Why did ‐ "5 additional patients entered the study but dropped out before completion" ‐ when did these patients enter and were they randomised? "One of these 5 dropped out because of pregnancy three months after starting carnitine" Pryor paper excluded as it is the same study as Sigman, author also gave details of randomisation and allocation concealment, author will try to find info on 5 patients who dropped out. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised to receive carnitine or placebo" "The randomisation was done by a third party a company that oversaw the trial. We sent the patient number of new recruited patients in to them, they assigned them a study number that was associated with a collection of medication/placebo." The author replied to randomisation query 23.09.09 saying that the protocol stated that ‐ "treatments will be assigned randomly to a subject number. The numbers will range from 1‐84 for study centre 1 and 85‐168 for study centre 2. Randomisation of treatments for each centre will be done independently. One half of subject numbers will be placebo, the other half, active ingredient." |
| Allocation concealment (selection bias) | Low risk | "The investigators and study sites had the study medication/placebo packets identified by number only. They were blinded to what was in the medication/placebo packets. We were sent the code at the conclusion of the trial." The author replied to a query on allocation concealment on 23.09.09 saying that the protocol stated that ‐ " Integrated Data Solutions, Inc. will keep the randomisation code in a separate sealed envelope for each site until the end of the study. The randomisation lists will be provided to the packaging company for packaging of the packets into patient medication boxes.” |
| Blinding (performance bias and detection bias) | Low risk | "Both the investigators and the patient were blinded to the treatment arm assignment." |
| Incomplete outcome data (attrition bias) | Low risk | "5 additional patients entered the study but dropped out before completion. One of these dropped out because of pregnancy three months after starting carnitine." Author replied to query re drop outs ‐ "I have data on one drop out at my site ‐ the drop out occurred after randomisation to carnitine. The drop out occurred before the first follow‐up study visit. The other four drop outs were from the other study site ‐ I am trying to get that data for you" (23.09.09) |
| Selective reporting (reporting bias) | Low risk | All outcomes of interest were reported |
| Methods | Randomised controlled study ‐ open label | |
| Participants | Population: men with chronic prostatitis and abnormal fertility, for more than 6 months Age: 18 to 40 years Randomised: N = 30 Duration of treatment: 3 months | |
| Interventions | Selznic (selenium + zinc and vitamins) (n = 15) versus placebo (n = 15) | |
| Outcomes | Sperm motility Sperm concentration | |
| Notes | No standard deviations available. Need to contact authors regarding methods, standard deviations, type of control and any pregnancy data. Paper translated from Russian by Vasya Vlassov. Vasya 17.02.14 saying that the control was placebo and SD's not given. Emailed the institution 18.02.14 regarding methods and data, no reply as of 7.03.13. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not explained |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | 'Open labelled' however placebo used maybe a translation problem |
| Incomplete outcome data (attrition bias) | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Double blind randomised controlled trial No mention of allocation concealment | |
| Participants | Country: Saudi Arabia Population: men attending a fertility centre Mean age: 34.8 (27 to 52 years), placebo 33.2 (22 to 45 years) N = 110 Inclusion criteria: asthenospermic (≥ 20 x 106 /mL). sperm motility ≤ 40%, normal sperm count, leucocyte concentration < 5%, normal fructose concentration Normal female Exclusion criteria: none mentioned Duration of study: 6 months | |
| Interventions | Vitamin E 100 mg 3 x /day (n = 52) versus placebo 3 x /day (n = 35) Duration of treatment: 6 months | |
| Outcomes | Primary outcome: motility and MDA concentration Secondary outcome: live birth, pregnancy, miscarriage | |
| Notes | Method of randomisation not stated. Large imbalance between the treatment (n = 52) and placebo group (n = 35) at analysis. Dropouts were accounted for in text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | " Either 100mg vitamin E or a placebo was prescribed in a random double blind fashion". Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | "Double blinded". Does not report on who was blinded, however placebo‐controlled |
| Incomplete outcome data (attrition bias) | High risk | The exact dropout figures for each group is unclear ‐ "A total of 110 patients were enrolled in the study, but some of the patients dropped out and some left the region and failed to continue. When the experiment was terminated, 52 patients were found to have taken vitamin E and 35 patients to have taken the placebo." Assuming the groups were equal initially then the placebo group lost 20 men and the intervention lost 3 |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods were reported in results |
| Methods | Randomised double blind controlled trial. randomisation by computer generated blocks. Using a 2:1 ratio (treatment 2: placebo 1) Allocation concealment: numbered bottles delivered to the site with all members of the trial blinded to sequence, identical placebo Power calculation performed | |
| Participants | Country: Australia Population:male factor infertility undergoing IVF Mean age: treatment group ‐ 37.1 ± 5.1, placebo group ‐ 35.5 ± 4.3 Recruited: N = 82 Randomised: N = 60 Inclusion criteria: men with sperm samples showing oxidative stress and a significant level of DNA fragmentation (> 25% TUNEL positive) Exclusion criteria: female partner with diminished ovarian reserve or if the female partner is aged over 39 years Duration of study: 1.5 years | |
| Interventions | Menevit (folate 0.5 mg, garlic 1000 mg, lycopene 6 mg, vitamin E 400 IU, vitamin C 100 mg, zinc 25 mg, selenium 26 μg, palm oil). One capsule per day (n = 40) versus placebo (identical in appearance and taste ‐ containing palm oil) (n = 20) Duraton of treatment: 3 months prior to IVF cycle | |
| Outcomes | Primary outcome: embryo quality Secondary outcomes: pregnancy, multiple pregnancy, fertilisation rate, side effects | |
| Notes | Associate Professor Tremellen provided live birth data in December 2014 "Only one pregnancy failed in the Menevit arm after 13 weeks (late miscarriage 19 weeks of male infant). All other pregnancies, including the twin pregnancies went on to live birth and all babies appear to be doing well from the records". There were three sets of twins in the combined antioxidants group and nil in the placebo group. Each twin pregnancy and live birth was counted as one event in the data analyses due to the protocol specifications of the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation schedule was computer generated in blocks of six by Bayer Consumer Care Australia". Using a 2:1 ratio "There were no significant differences between the active and the placebo group in terms of important baseline prognostic characteristics..." |
| Allocation concealment (selection bias) | Low risk | "the appropriately numbered bottles of capsules delivered to the clinical site without any participant knowing the treatment sequence. Patients were allocated the next numerical treatment package (one to sixty as they became eligible for enrolment" |
| Blinding (performance bias and detection bias) | Low risk | "double blind placebo controlled trial" |
| Incomplete outcome data (attrition bias) | Low risk | All withdrawals were accounted for, 2 from the intervention group, 4 from placebo all due to the couples not going through to embryo transfer |
| Selective reporting (reporting bias) | Low risk | All specified outcomes are reported |
| Methods | Randomised placebo controlled trial | |
| Participants | Country: Hong Kong Population: new patients attending infertility clinic Mean age: 33.7 ± 4.4 years (28 to 45 years) N = 46 Inclusion criteria: men with idiopathic oligospermia attending fertility clinic with normal hormones. They had not received treatment with pharmacologic agents for 1 year prior to entry into the trial. None of the female partners had irreversible causes of female infertility Exclusion criteria: not mentioned Duration of study: 6 to 9 months | |
| Interventions | Pentoxifylline 400 mg 3 x /day (n = 11) versus placebo (n = 7) Duration of treatment: 6 months Other interventions not included in meta analysis were clomiphene citrate, mesterolone and testosterone enanthate | |
| Outcomes | Primary outcome: semen parameters and pregnancy | |
| Notes | Pentoxifylline supplied by Hoechst Aktiengesellshaft Attempted to contact the author re motility data as it was stated in the paper that this was an outcome. Also asked about control data for post‐treatment period and methods of randomisation + allocation concealment. Author replied saying she no longer has the data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the subjects were randomly assigned to one of the therapy groups.." No mention of methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | No blinding ‐ one of the interventions was an intra muscular injection while the others were oral |
| Incomplete outcome data (attrition bias) | High risk | Sperm motility data not given when motility was discussed as an outcome of interest |
| Selective reporting (reporting bias) | High risk | No post‐treatment control data given however there is post‐treatment given for treatment group No intention to treat reported Side effects reported in the results but this was not stated as an initial outcome |
| Methods | Random controlled trial | |
| Participants | Country: China Population: male infertility ‐ asthenozoospermia Age: 23 to 26 years old. "Balanced in age, course of disease, and Semen parameters" Recruited: all male asthenozoospermia outpatients in the Second Hospital of HeBei Medical University from August 2007 to August 2009 Randomised: N = 135 Inclusion criteria: male asthenozoospermia patients, aged 23 to 26 years old, with a history of infertility for about 1 to 10 years, and with no contraception measures after marriage at least 12 months, has normal sex life, the wife’s fertility is normal Semen analysis for at least twice based on WHO criteria: Forward mobile sperm (a + b level) < 50%, and fast forward movement sperm (a level) < 25% Sperm density > 20 x 106 /mL Tests for peripheral blood chromosome and reproductive hormones (FSH, LH, PRL, T) were normal The tests for semen ureaplasma mycoplasma and chlamydia trachomatis were negative Semen WBC < 1 x 106 /mL Exclusion criteria: cryptorchidism, testicular dysplasia, varicoceles, reproductive tract infection Duration of trial: August 2007 to August 2009 Duration of treatment: 3 months | |
| Interventions | L‐carnitine 2g/day plus vitamin E (n = 68) versus vitamin E (n = 67) | |
| Outcomes | Pregnancy rates Adverse effects % forward motile sperm Sperm density % sperm normal morphology | |
| Notes | Funding source: population and family planning commission of HeBei Province, China. Paper translated by Liu Qin 22 patients lost during the study ‐ no reasons given for dropouts, 7 from the intervention and 15 from the control, the trial did not use ITT. E‐mailed Qin (translator) regarding pregnancy and adverse event data, then may need to write to the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A total of 135 patients with asthenozoospermia were randomly divided into Groups". Methods are unknown |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) | High risk | 22 dropouts. Numbers from each group are given but no reasons are provided for the withdrawals. Intention to treat not used in the trial analysis |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | Double blind randomised placebo‐controlled interventional study. Computer generated randomisation Allocation concealment: central pharmacy coding | |
| Participants | Country: Netherlands Population: Fertile and subfertile men Mean age: 34.3 ± 3.9 years Recruited: N = 258 subfertile Randomised: N = 103 Inclusion criteria for subfertile group: failure to conceive after 1 year regular unprotected intercourse and sperm concentration of 5 to 20 million/mL Exclusion criteria for subfertile group: chromosomal disorders, cryptorchidism, vasectomy, use of folic acid or zinc supplements in the previous 3 months, vitamin B deficiency Duration of study: 1 year | |
| Interventions | Folic acid 5 mg/day (n = 22) versus zinc sulphate 66 mg/day (n = 23) versus zinc sulphate 66 mg/day + folic acid 5 mg/day (n = 24) versus placebo (n = 25) Duration of treatment: 26 weeks | |
| Outcomes | Semen parameters | |
| Notes | Data in median and range. Attempted to contact authors regarding means and standard deviations. Letter returned to sender | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "eligible fertile and subfertile men were randomly assigned according to a simple computer‐generated randomisation schedule in four blocks to receive folic acid and placebo, zinc sulphate and placebo, zinc sulphate and folic acid, or placebo and placebo, which resulted in eight subgroups." "At the end of the trial, the research fellow received the randomisation list that matched the codes from the hospital pharmacy." |
| Allocation concealment (selection bias) | Low risk | "capsules were coded by the hospital pharmacy according to the randomisation list." |
| Blinding (performance bias and detection bias) | Low risk | Double blind placebo‐controlled "Neither the research fellow and the participants knew whether the participants received folic acid, zinc sulphate or placebo capsules" "Folic acid and placebo capsules were yellow and identical in appearance. Zinc sulphate and placebo capsules were white and identical in appearance" |
| Incomplete outcome data (attrition bias) | Unclear risk | 9 men withdrew from the subfertile arm of the trial, 1 due to side effects (gastrointestinal) and 8 due to lack of motivation. It is unclear which treatment groups these men were randomised to |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Methods | A randomised pilot study Allocation concealment not mentioned | |
| Participants | Country: Belgium Population: men attending andrology clinic Mean age: not given N = 22 Inclusion criteria: none given Exclusion criteria: none given Duration of study: ?4 to 6 months | |
| Interventions | Acetylcysteine 600 mg/day (n = 5) versus EFA (DHA 1 g, y‐linolenic acid, arachidonic acid 100 mg) 100 mg/day + antioxidant oil mixture and tocopherol + B‐carotene (n = 12) versus acetylcysteine + EFA + antioxidants (n = 5) Duration of treatment: 4 to 6 months | |
| Outcomes | Sperm parameters | |
| Notes | Abstract only. No extractable data. Attempted to contact authors re availability of data as means, if published?, methods of randomisation and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A prospective randomised pilot study" No details of randomisation given |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | No details |
| Selective reporting (reporting bias) | Unclear risk | Unclear, abstract only |
| Methods | Randomised placebo controlled clinical study Allocation concealment not mentioned | |
| Participants | Country: Hungary Population: subfertile men attending andrology Department of Obstetrics and Gynaecology, University of Szeged Mean age: treatment group 29.6, placebo group 28.3 years Recruited: N = 26 Randomised: N = 20 Inclusion criteria: unsuccessful attempt at pregnancy for over one year. A healthy female partner examined by a gynaecologist. Sperm volume < 2 mL and/or sperm concentration < 20 million/mL and/or morphology ratio < 30% and/or motility < 50%. No genital tract infection, no bacteria or fungi in urine or semen. Hormones are within physiological range. Intact renal function. No excessive magnesium intake Exclusion criteria: none mentioned Duration of study: 3 months | |
| Interventions | Magnesium 3000 mg/day (n = 10) versus placebo (n = 10) Number analysed: N = 14 Duration of treatment: 90 days | |
| Outcomes | Primary: semen parameters Secondary: pregnancy and side effects | |
| Notes | Attempted to contact authors regarding methods of randomisation and allocation concealment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised and divided into Magnesium or Placebo groups" Methods of randomisation are not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | "The members of Group P received the same number of placebo tablets which closely resembled the Magnerot tablets." No mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 20 were randomised and 14 were analysed. "To date 26 patients have participated in the study and 20 men (10 in both groups) have completed the program of treatment. Six patients (2 in group M and 4 in group P were excluded from the program, including five cases for poor compliance, since they did not attend the control meeting at the end of treatment. One patient from Group M experienced severe diarrhoea and so his treatment was halted." |
| Selective reporting (reporting bias) | Low risk | All sperm data for outcomes in the trial were given, however clinical pregnancy only reported in the results section and not mentioned in methods |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study population normozoospermic men and donors | |
| Study population normo‐zoospermic men and donors | |
| Study population not subfertile men | |
| Protocol exclusion criteria regarding exclusion of interventions this includes fertility drugs like tamoxifen. Group a tamoxifen + vitamin E, Group b tamoxifen | |
| Used non‐randomised controls recruited from another unrelated trial | |
| Inappropriate population, polymorphisms | |
| Antioxidant given to the women and not to the men | |
| Intervention clomiphene + vitamin E versus placebo. Protocol exclusion criteria ‐ “trials that included men taking other fertility enhancing drugs” | |
| Excluded due to incorrect intervention ‐ plant extracts, herbal formulation | |
| Not randomised "men were classified into groups". Numbers of men in the groups were uneven | |
| Female participants not men | |
| Used a herbo‐mineral supplement | |
| Route of supplementation was intramuscular not oral | |
| Population was women, not men | |
| Not randomised, 105 men in the treatment group and 35 in control. Abstract only | |
| A commentary on Ghanem 2010 | |
| Trial is not randomised, allocation method is by alternation. Translated from Bulgarian by Ivan Sola. "50 of them were randomly invited to participate depending on their order of attendance to the clinic" | |
| Not a randomised controlled trial | |
| The population is women not men | |
| Incorrect intervention ‐ saffron, herbal not a supplement | |
| Population is not subfertile men | |
| Trial design is not random. The trial uses alternate allocation, odd and even numbers. Appears to be a report of the trial Nikolova 2007 | |
| Protocol exclusion criteria (tamoxifen + Q10 versus tsamoxifen) “trials that included men taking other fertility enhancing drugs” | |
| Spirulina platensis (4 g) and Resveratrol (500 mg) are plant extracts not antioxidant supplements | |
| Inappropriate control (anti‐inflammatory). Treatment is not compared to placebo or another antioxidant | |
| Inappropriate comparison. The same antioxidant is compared at different times ‐ L‐carnitine + acetyl‐carnitine versus L‐carnitine + acetyl‐carnitine | |
| Inappropriate control (anti‐inflammatory). Treatment is not compared to placebo or another antioxidant | |
| Protocol exclusion criteria regarding exclusion of interventions this includes fertility drugs like tamoxifen. Group a L carnitine and tamoxifen, Group b L carnitine, Group c tamoxifen | |
| Probably not randomised, no mention of randomisation in the abstract and uneven numbers between the groups, attempted to contact authors with no reply |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised |
| Participants | Male infertility |
| Interventions | DHA |
| Outcomes | Sperm parameters |
| Notes | Definitely same trial as excluded Anarte 2013 (checked 04.09.14) |
| Methods | Randomised |
| Participants | Idiopathic oligoasthenozoospermia |
| Interventions | Combination antioxidants |
| Outcomes | Sperm parameters and pregnancy |
| Notes |
| Methods | Randomised |
| Participants | male idiopathic infertility |
| Interventions | Combination therapy of antiestrogen and a natural composite containing tribulus terrestris, alga ecklonia bicyclis, biovis and myo‐inositol |
| Outcomes | Sperm parameters and pregnancy |
| Notes | Probably exclude due to incorrect intervention |
| Methods | Randomised |
| Participants | Male subfertility |
| Interventions | Coenzyme Q10 |
| Outcomes | Sperm parameters |
| Notes |
| Methods | Unknown |
| Participants | Pathospermia |
| Interventions | Spematon (L‐carnitine, zinc and vitamin E) |
| Outcomes | Acrosome reaction |
| Notes | Maybe a combination antioxidant. Needs translation (Russian) |
| Methods | Randomised |
| Participants | Varicocelectomy |
| Interventions | Folic acid and zinc |
| Outcomes | Sperm parameters |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Effect of Treatment With Myo‐inositol on Human Semen Parameters in Patients Undergoing In Vitro Fertilization Cycles |
| Methods | Interventional, Phase 4 |
| Participants | 18 years to 40 years male |
| Interventions | Dietary Supplement: myo‐inositol + folic acid |
| Outcomes | Primary outcome measures: Semen volume |
| Starting date | March 2012 |
| Contact information | Italy, University of Catania ‐ Department of Surgery ‐ Section of Obstetrics and Gynecology ‐Center of Physiopathology of Reproduction, AGUNCO Obstetrics and Gynecology Centre |
| Notes | ClinicalTrials.gov identifier: NCT01560065 |
| Trial name or title | Assessment of the efficacy of dietary supplement Spermotrend in the treatment of male infertility |
| Methods | Randomised, double blind (subject, caregiver, investigator), placebo control, parallel assignment, efficacy study |
| Participants | Subfertile men |
| Interventions | Spermotrend (vitamins plus other antioxidants) versus placebo |
| Outcomes | Parameters of seminal analysis at weeks 24 Fertilisation achievement Presence of mild or severe adverse effects |
| Starting date | September 2009 |
| Contact information | Rogelio Gonzalez Sanchez, MD 53 7 838 2626 ext 277 Gynecologic and Obstetric Hospital Havana, Cuba, 10400 |
| Notes | Havana, Cuba, 10400 NCT00975117 |
| Trial name or title | Vitamin D Supplementation and Male Infertility: The CBG‐study a Randomized Clinical Trial |
| Methods | Double blinded randomised clinical trial |
| Participants | Male 18 years and over low sperm concentration and motility |
| Interventions | Drug: Cholecalciferol and calcium |
| Outcomes |
|
| Starting date | 2011 recruiting 2014 |
| Contact information | Further study details as provided by Rigshospitalet, Denmark |
| Notes | NCT01304927 |
| Trial name or title | To compare the effectiveness of antioxidants versus no treatment for male partner for improving pregnancy rates in couples undergoing In vitro fertilization (IVF) for abnormal semen analysis |
| Methods | Randomised: Permuted block randomisation, variable method of allocation concealment: sequentially numbered, sealed, opaque envelopes. Blinding and masking: Open label |
| Participants | Inclusion criteria: Couples undergoing ART due to male factor infertility with the following parameters Exclusion criteria: Severe oligozoospermia < 1 million/mL |
| Interventions | Intervention1: Tablet Vitamin C 500 mg, Capsule Vitamin E 400 mg and Tablet Zinc 140 mg OD for 3 months versus no treatment |
| Outcomes | Clinical pregnancy rate. Timepoint: February 2015 Ongoing pregnancy rate |
| Starting date | 01.02.13 |
| Contact information | Mohan S Kamath Address: Dr Mohan S Kamath, MS,DNB, Fellow ( Reproductive Medicine) Associate Professor, Reproductive Medicine Unit, Christian Medical College and Hospital, Vellore �?? 632004 632004 Vellore, TAMIL NADU India Telephone: 04162283301 Email: [email protected] Affiliation: Christian Medical College and Hospital |
| Notes | CTRI/2013/02/003431 Email sent 26.03.14. Dr Kamath replied 3.04.14 saying that they were still in the recruitment phase and were hoping to finish the trial in 2015. |
| Trial name or title | Effect of Treatment With Myo‐inositol on Human Semen Parameters in Patients Undergoing IVF Cycles |
| Methods | Allocation: Randomized |
| Participants | Asthenozoospermia Male 25 years to 65 years |
| Interventions | Dietary Supplement: Myo‐inositol OAT |
| Outcomes | Samples of seminal fluid were obtained from two groups of patients undergoing to an IVF cycle: healthy normospermic subjects and subjects with oligoasthenoteratospermia (OAT, < 15 mil/ml). Semen volume, spermatozoa number and motility were evaluated during the initial semen analysis and after density gradient separation method. These parameters were evaluated before and after the administration of 4000mg/die of myo‐inositol associated to 400 µg of folic acid (Inofolic lolipharma Rome) for three months. A third group of healthy normospermic subject were traded with 400 µg of folic acid for three months and was consider a control group. |
| Starting date | March 2013 |
| Contact information | Sponsor: AGUNCO Obstetrics and Gynecology Centre Information provided by (Responsible Party):AGUNCO Obstetrics and Gynecology Centre. Italy |
| Notes | Marco Palumbo, M.D.University of Catania ‐ Department of Surgery ‐ Section of Obstetrics and Gynecology ‐ Centre of Physiopathology of Reproduction Gianfranco Carlomagno, A.G.Un.Co. Obstetrics and Gynaecology Center NCT01828710 |
| Trial name or title | Vitamin D supplementation and male infertility: a randomized double blinded clinical trial |
| Methods | Interventional clinical trial of medicinal product Study design: Controlled: yes |
| Participants | semen concentration < 20 mio./ml or < 40 progressive motility, or < 7% morphologically normal sperm. Exclusion criteria: |
| Interventions | Cholecalciferol |
| Outcomes | Main objective: To investigate whether vitamin D supplementation to vitamin D deficient or insufficient men improves semen quality and male fertility Primary end point(s): change in semen quality and fertility potential Secondary objective: Reproductive or sex hormones hormones |
| Starting date | 11/02/2011 |
| Contact information | Not Known |
| Notes | Denmark EUCTR2010‐024588‐42‐DK |
| Trial name or title | Sadeghi M. Effects of coenzymeQ10 (CoQ10) supplementation on semen quality and seminal oxidative stress of idiopathic oligoasthenoteratozoospermic (iOAT) infertile men. World Health Organization International Clinical Trials Registry Platform Search Portal 2008. [ISRCTN: ISRCTN29954277] |
| Methods | Randomised double blind placebo controlled trial |
| Participants | Men with idiopathic oligoasthenoteratozoospermia with at least one year infertility |
| Interventions | Coenzyme10 (ubiquinone) 200mg 2x/day for 3 months |
| Outcomes | Sperm morphology, motility and sperm DNA fragmentation |
| Starting date | 1.10.08 |
| Contact information | |
| Notes | letter sent to author regarding data 17.09.09. Email sent 26.03.14 [ISRCTN: ISRCTN29954277] |
| Trial name or title | Effect of Ubiquinone Supplementation on Semen Quality, Antioxidant Enzyme, Oxidative Stress and DNA Fragmentation in infertile men |
| Methods | Randomisation: randomised. Blinding: Double blind. Placebo: used. Assignment: Parallel. Purpose: Treatment. |
| Participants | Inclusion criteria: Inclusion : idiopathic oligoasthenoteratospermia, normal profile for hormones, normal genital anatomy Exclusion criteria: Age minimum: 20 |
| Interventions | Co Q10 supplement: 2 (100 mg) per day (200 mg), 3 month. Intervention 2: Placebo (lactose): 2 capsule per day, 3 month . |
| Outcomes | activity of seminal plasma catalase. Timepoint: 45 days , 90 days. Method of measurement: spectrophotometry activity of seminal plasma superoxide dysmutase. Time point: 45 days , 90days. Method of measurement: spectrophotometry DNA fregmentation . Timepoint: 45 days , 90 days. Method of measurement: Flow cytometry semen analysis. Timepoint: 45 days , 90 days. Method of measurement: HPLC, flourometry , spectrophotometry, ELIZA seminal plasma ubiquinone concentration. Timepoint: 45 days, 90 days. Method of measurement: HPLC sperm Isoprostan concentration. Timepoint: 45 days, 90 days. Method of measurement: ELIZA |
| Starting date | 09.08.2011 |
| Contact information | Mohammad Reza Sadeghi Address: Avicenna Research Institute, Shahid Beheshti University , Evin 1983969411 Tehran Iran, Islamic Republic Of Telephone: 00982122432024 Email: [email protected] Affiliation: Avicenna Research Institute |
| Notes | Is this the same trial as Sadeghi 2008? NB the trials have different IRCT numbers [ISRCTN: IRCT138706031079N1] Email sent 26.03.14 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Live birth; type of antioxidant Show forest plot | 4 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.21 [2.08, 8.51] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 1 Live birth; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 Vitamin E versus placebo | 2 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.44 [1.72, 24.04] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 Zinc versus no treatment | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.74 [1.02, 13.74] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 Combined antioxidants versus placebo | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.42 [1.15, 10.13] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Live birth; IVF/ICSI Show forest plot | 2 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.61 [1.27, 10.29] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 2 Live birth; IVF/ICSI. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Clinical pregnancy; type of antioxidant Show forest plot | 7 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.43 [1.92, 6.11] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 3 Clinical pregnancy; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 Combined antioxidants versus placebo | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [0.84, 7.13] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 Magnesium versus placebo | 1 | 26 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.73 [0.17, 445.08] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 Vitamin E versus placebo | 2 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.71 [1.98, 22.69] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3.4 Zinc versus placebo or no treatment | 2 | 153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.43 [1.39, 14.14] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3.5 N‐acetylcysteine versus no treatment | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.42, 6.16] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3.6 Zinc plus folic acid versus placebo | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.86 [0.15, 99.84] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3.7 Folic acid versus placebo | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Clinical pregnancy; IVF/ICSI Show forest plot | 2 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.64 [0.94, 7.41] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 4 Clinical pregnancy; IVF/ICSI. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Adverse events Show forest plot | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 5 Adverse events. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 Miscarriage | 3 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.40, 7.60] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 5.2 Gastrointestinal | 6 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.47, 5.50] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 5.3 Euphoria | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.16, 9.01] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 5.4 Ectopic pregnancy | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.48 [0.07, 286.49] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 6 Sperm DNA fragmentation; type of antioxidant Show forest plot | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐13.85 [‐17.28, ‐10.41] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.6  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 6 Sperm DNA fragmentation; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6.1 Vitamin C + vitamin E versus placebo at 2 months | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐13.80 [‐17.50, ‐10.10] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 6.2 Docosahexaenoic acid (DHA) 1000mg/day versus placebo at 10 weeks | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐14.12 [‐23.23, ‐5.01] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7 Total sperm motility at 3 months or less; type of antioxidant Show forest plot | 16 | 1039 | Mean Difference (IV, Random, 95% CI) | 10.02 [3.79, 16.25] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.7  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 7 Total sperm motility at 3 months or less; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7.1 Docosahexaenoic acid (DHA) 400mg/day versus placebo | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐7.80 [‐27.79, 12.19] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.2 Docosahexaenoic acid (DHA) 800mg/day vs placebo | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐15.20 [‐30.92, 0.52] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.3 Docosahexaenoic acid (DHA) 1000mg/day versus placebo at 10 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐17.64, 4.74] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.4 Vitamin C acid 200mg/day versus placebo | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐18.82, 22.82] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.5 Vitamin C 1000mg/day versus placebo | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 45.0 [19.72, 70.28] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.6 Vitamin C + Vitamin E versus placebo at 2 months | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 1.46 [‐5.82, 8.74] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.7 Carnitines versus placebo or no treatment at 3 months | 3 | 99 | Mean Difference (IV, Random, 95% CI) | 15.32 [‐3.70, 34.35] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.8 Selenium versus placebo at 3 months | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 14.9 [1.14, 28.66] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.9 Combined antioxidants versus placebo or no treatment at 3 months | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 15.13 [13.56, 16.69] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.10 N‐acetylcysteine versus placebo/no treatment at 3 months | 2 | 180 | Mean Difference (IV, Random, 95% CI) | 7.65 [0.68, 14.62] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.11 Magnesium versus placebo at 90 days | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 14.5 [‐6.01, 35.01] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.12 Zinc versus no treatment or placebo at 3 months | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 14.66 [‐5.91, 35.24] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.13 Zinc + Vitamin E versus no treatment at 3 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 26.0 [12.85, 39.15] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.14 Zinc + Vitamin E + Vitamin C versus no treatment at 3 months | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 26.0 [12.62, 39.38] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.15 Coenzyme Q10 versus placebo | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 3.58 [‐6.16, 13.32] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.16 Zinc plus folic acid versus placebo | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐2.84, 16.44] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 7.17 Folic acid versus placebo | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 8.40 [‐0.99, 17.79] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 8 Total sperm motility at 3 months or less (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.8
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 8 Total sperm motility at 3 months or less (data not suitable for meta analysis). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.1 L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.2 Combined antioxidants versus no treatment | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.3 Vitamin E versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.4 L‐carnitine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 8.5 Selenium + Zinc versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 Total sperm motility at 6 months; type of antioxidant Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.9  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 9 Total sperm motility at 6 months; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9.1 Carnitines versus placebo at 6 months | 3 | 107 | Mean Difference (IV, Random, 95% CI) | 7.28 [‐9.47, 24.02] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.2 Selenium versus placebo at 26 weeks (6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 3.20 [2.28, 4.12] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.3 N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.98, 2.82] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.4 Selenium plus N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 139 | Mean Difference (IV, Random, 95% CI) | 6.30 [5.38, 7.22] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.5 Coenzyme Q10 versus placebo at 6 months | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 6.58 [1.80, 11.37] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.6 Vitamin E versus placebo at 6 months | 1 | 87 | Mean Difference (IV, Random, 95% CI) | 13.0 [7.02, 18.98] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.7 Zinc versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐10.19, 10.19] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.8 Zinc plus folic acid versus placebo | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐8.82, 14.02] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 9.9 Folic acid versus placebo | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐8.49, 11.89] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 10 Total sperm motility at 6 months(data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.10
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 10 Total sperm motility at 6 months(data not suitable for meta analysis). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.1 L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.2 Folic acid versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.3 Zinc versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 10.4 Zinc + folic acid versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 Total sperm motility at 9 months or more; type of antioxidant Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.11  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 11 Total sperm motility at 9 months or more; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11.1 L‐carnitine versus placebo at 9 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 11.54 [1.66, 21.42] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 11.2 L‐acetyl carnitine versus placebo at 9 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 7.84 [‐1.41, 17.09] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 11.3 L‐carnitine + L‐acetyl carnitine versus placebo at 9 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 6.27 [‐3.36, 15.90] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 11.4 Coenzyme Q10 versus placebo at 9 months | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐1.58, 5.34] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 12 Total sperm motility over time Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.12  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 12 Total sperm motility over time. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 12.1 Total sperm motility at 3 months or less | 16 | 832 | Mean Difference (IV, Random, 95% CI) | 9.55 [2.12, 16.97] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 12.2 Total sperm motility at 6 months | 9 | 964 | Mean Difference (IV, Random, 95% CI) | 6.86 [3.78, 9.94] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 12.3 Total sperm motility at 9 months or more | 4 | 509 | Mean Difference (IV, Random, 95% CI) | 3.17 [‐0.10, 6.45] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13 Sperm concentration at 3 months or less; type of antioxidant Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.13  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 13 Sperm concentration at 3 months or less; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 13.1 Docosahexaenoic acid (DHA) 400g/day versus placebo | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐41.09, 30.49] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.2 Docosahexaenoic acid (DHA) 800g/day versus placebo | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐35.23, 38.23] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.3 Docosahexaenoic acid (DHA) 1000mg/day versus placebo at 10 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐18.78, 16.02] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.4 Magnesium versus placebo at 90 days | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 5.20 [‐2.61, 13.01] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.5 Vitamin C + Vitamin E versus placebo at 2 months | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐10.01, 12.72] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.6 N‐acetylcysteine versus placebo at 3 months | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐6.70, 5.76] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.7 Carnitines versus placebo | 2 | 78 | Mean Difference (IV, Random, 95% CI) | 14.29 [‐15.50, 44.08] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.8 Coenzyme Q10 versus placebo | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐12.39, 12.15] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.9 N‐acetylcysteine versus no treatment | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.72 [‐0.31, 9.75] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.10 Combined antioxidants versus placebo or no treatment | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.84, 0.06] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.11 Zinc plus folic acid versus placebo | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 18.0 [1.13, 34.87] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.12 Folic acid versus placebo | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 22.20 [3.79, 40.61] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.13 Zinc versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 16.9 [0.53, 33.27] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 13.14 Selenium versus placebo | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 21.20 [‐11.45, 53.85] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 14 Sperm concentration at 3 months or less (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.14
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 14 Sperm concentration at 3 months or less (data not suitable for meta analysis). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.1 L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.2 Vitamin E versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 14.3 L‐carnitine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 Sperm concentration at 6 months; type of antioxidant Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.15  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 15 Sperm concentration at 6 months; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15.1 Carnitines versus placebo at 6 months | 2 | 116 | Mean Difference (IV, Random, 95% CI) | 2.59 [‐3.11, 8.30] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 15.2 Selenium versus placebo at 26 weeks (6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 4.10 [1.82, 6.38] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 15.3 N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 3.30 [1.13, 5.47] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 15.4 Selenium plus N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 139 | Mean Difference (IV, Random, 95% CI) | 8.60 [6.28, 10.92] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 15.5 Coenzyme Q10 versus placebo at 6 months | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 6.88 [1.20, 12.56] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 15.6 Zinc plus folic acid versus placebo | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 17.70 [‐1.88, 37.28] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 15.7 Folic acid versus placebo | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 19.20 [4.74, 33.66] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 15.8 Zinc versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.70 [‐7.01, 26.41] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 16 Sperm concentration at 6 months(data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.16
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 16 Sperm concentration at 6 months(data not suitable for meta analysis). | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16.1 L‐carnitine + acetyl‐carnitine versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 16.2 Folic acid versus Placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 16.3 Zinc versus Placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 16.4 Zinc + folic acid versus placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 Sperm concentration at 9 months; type of antioxidant Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.17  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 17 Sperm concentration at 9 months; type of antioxidant. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17.1 Carnitines versus placebo at 9 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.12 [‐1.74, 9.99] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 17.2 Coenzyme Q10 versus placebo at 9 months or more | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 2.74 [‐1.56, 7.05] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 18 Sperm concentration over time Show forest plot | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.18  Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 18 Sperm concentration over time. | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18.1 Sperm concentration at 3 months or less | 13 | 746 | Mean Difference (IV, Random, 95% CI) | 5.32 [‐0.62, 11.26] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 18.2 Sperm concentration 6 months | 8 | 851 | Mean Difference (IV, Random, 95% CI) | 5.46 [1.81, 9.11] | ||||||||||||||||||||||||||||||||||||||||||||||||
| 18.3 Sperm concentration at 9 months or more | 4 | 509 | Mean Difference (IV, Random, 95% CI) | 3.66 [‐0.31, 7.64] | ||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||
| 1 Total sperm motility at 3 months or less; type of antioxidant Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 Head to head antioxidant(s), Outcome 1 Total sperm motility at 3 months or less; type of antioxidant. | ||||||||||||||||||||||||||||||||||||
| 1.1 Ethylcysteine 600mg/day vs Vitamin E | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐41.97, 38.17] | ||||||||||||||||||||||||||||||||
| 1.2 Docosahexaenoic acid (DHA) 400g/day vs Docosahexaenoic acid 800mg/day | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 7.40 [‐11.35, 26.15] | ||||||||||||||||||||||||||||||||
| 1.3 Vitamin C 200mg/day versus vitamin C 1000mg/day | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐43.0 [‐67.10, ‐18.90] | ||||||||||||||||||||||||||||||||
| 1.4 Vitamin E + Selenium versus Vitamin B at 3 months | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐10.71, 10.71] | ||||||||||||||||||||||||||||||||
| 1.5 Zinc versus Zinc + Vitamin E at 3 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐13.00, 13.00] | ||||||||||||||||||||||||||||||||
| 1.6 Zinc versus Zinc + Vitamin E + Vitamin C at 3 months | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐19.66, 17.66] | ||||||||||||||||||||||||||||||||
| 1.7 Zinc + Vitamin E versus Zinc + Vitamin E + Vitamin C at 3 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐18.97, 18.97] | ||||||||||||||||||||||||||||||||
| 1.8 Selenium versus combined antioxidants | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐10.13, 16.53] | ||||||||||||||||||||||||||||||||
| 1.9 L acetyl carnitine + L carnitine versus Vitamin E + Vitamin C | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | 23.05 [20.09, 26.01] | ||||||||||||||||||||||||||||||||
| 1.10 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐7.74, 6.54] | ||||||||||||||||||||||||||||||||
| 1.11 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐12.91, 7.31] | ||||||||||||||||||||||||||||||||
| 1.12 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐14.21, 5.41] | ||||||||||||||||||||||||||||||||
| 2 Total sperm motility at 6 months; type of antioxidant Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||
| Analysis 2.2  Comparison 2 Head to head antioxidant(s), Outcome 2 Total sperm motility at 6 months; type of antioxidant. | ||||||||||||||||||||||||||||||||||||
| 2.1 L‐acetyl carnitine + L‐carnitine versus L‐carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.46 [‐9.72, 2.80] | ||||||||||||||||||||||||||||||||
| 2.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐6.37, 7.65] | ||||||||||||||||||||||||||||||||
| 2.3 Selenium versus N‐acetyl‐cysteine | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.56, 2.04] | ||||||||||||||||||||||||||||||||
| 2.4 Selenium versus selenium plus N‐acetyl‐cysteine | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐3.85, ‐2.35] | ||||||||||||||||||||||||||||||||
| 2.5 N‐acetyl‐cysteine vs selenium plus N‐acetyl‐cysteine | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐5.14, ‐3.66] | ||||||||||||||||||||||||||||||||
| 2.6 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐5.45, 7.25] | ||||||||||||||||||||||||||||||||
| 2.7 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐9.13, 3.93] | ||||||||||||||||||||||||||||||||
| 2.8 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.42, 3.02] | ||||||||||||||||||||||||||||||||
| 3 Total sperm motility at 6 months Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| Analysis 2.3
Comparison 2 Head to head antioxidant(s), Outcome 3 Total sperm motility at 6 months. | ||||||||||||||||||||||||||||||||||||
| 3.1 Zinc versus Folic acid | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| 3.2 Zinc versus Zinc + folic acid | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| 3.3 Folic acid versus Zinc + folic acid | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| 4 Total sperm motility at 9 months or more; type of antioxidant Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||
| Analysis 2.4  Comparison 2 Head to head antioxidant(s), Outcome 4 Total sperm motility at 9 months or more; type of antioxidant. | ||||||||||||||||||||||||||||||||||||
| 4.1 L‐aceytl carnitine + L‐carnitine versus L‐carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐11.28, 0.74] | ||||||||||||||||||||||||||||||||
| 4.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐6.46, 3.32] | ||||||||||||||||||||||||||||||||
| 5 Sperm concentration at 3 months or less; type of antioxidant Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||
| Analysis 2.5  Comparison 2 Head to head antioxidant(s), Outcome 5 Sperm concentration at 3 months or less; type of antioxidant. | ||||||||||||||||||||||||||||||||||||
| 5.1 Ethylcysteine 600mg/day vs Vitamin E | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐16.65, 21.05] | ||||||||||||||||||||||||||||||||
| 5.2 Docosahexaenoic acid (DHA) 400g/day versus Docosahexaenoic acid (DHA) 800g/day | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐41.87, 28.27] | ||||||||||||||||||||||||||||||||
| 5.3 L‐carnitine versus Vitamin E + Vitamin C | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 15.5 [12.49, 18.51] | ||||||||||||||||||||||||||||||||
| 5.4 L‐carnitine plus vitamin E versus vitamin E | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐10.52, 14.32] | ||||||||||||||||||||||||||||||||
| 5.5 Selenium versus combined antioxidants | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 14.70 [‐6.51, 35.91] | ||||||||||||||||||||||||||||||||
| 5.6 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐22.21, 13.81] | ||||||||||||||||||||||||||||||||
| 5.7 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐18.63, 16.43] | ||||||||||||||||||||||||||||||||
| 5.8 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐23.38, 12.78] | ||||||||||||||||||||||||||||||||
| 6 Sperm concentration at 6 months; type of antioxidant Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||
| Analysis 2.6  Comparison 2 Head to head antioxidant(s), Outcome 6 Sperm concentration at 6 months; type of antioxidant. | ||||||||||||||||||||||||||||||||||||
| 6.1 L‐aceytl carnitine +L‐carnitine versus L‐carnitine at 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐8.13 [‐21.79, 5.53] | ||||||||||||||||||||||||||||||||
| 6.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine at 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐15.26, 10.92] | ||||||||||||||||||||||||||||||||
| 6.3 Selenium versus N‐acetyl‐cysteine at 26 weeks (6 months) | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.71, 2.31] | ||||||||||||||||||||||||||||||||
| 6.4 Selenium versus selenium plus N‐acetyl‐cysteine at 26 weeks (6 months) | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐6.20, ‐2.80] | ||||||||||||||||||||||||||||||||
| 6.5 N‐acetyl‐cysteine vs selenium plus N‐acetyl‐cysteine at 26 weeks | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐6.86, ‐3.74] | ||||||||||||||||||||||||||||||||
| 6.6 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐15.06, 12.06] | ||||||||||||||||||||||||||||||||
| 6.7 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐23.69, 7.69] | ||||||||||||||||||||||||||||||||
| 6.8 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐20.31, 1.31] | ||||||||||||||||||||||||||||||||
| 7 Sperm concentration at 6 months Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| Analysis 2.7
Comparison 2 Head to head antioxidant(s), Outcome 7 Sperm concentration at 6 months. | ||||||||||||||||||||||||||||||||||||
| 7.1 Zinc versus Folic acid | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| 7.2 Zinc versus Zinc + Folic acid | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| 7.3 Folic acid versus Zinc + folic acid | Other data | No numeric data | ||||||||||||||||||||||||||||||||||
| 8 Sperm concentration at 9 months or more; type of antioxidant Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||
| Analysis 2.8  Comparison 2 Head to head antioxidant(s), Outcome 8 Sperm concentration at 9 months or more; type of antioxidant. | ||||||||||||||||||||||||||||||||||||
| 8.1 L‐acetyl carnitine + L‐carnitine versus L‐carnitine at 9 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐6.13 [‐15.99, 3.73] | ||||||||||||||||||||||||||||||||
| 8.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine at 9 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.06 [‐6.09, 10.21] | ||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||
| 1 Total sperm motility at 3 months or less; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 12.77 [9.23, 16.31] | ||||||||||||||||
| Analysis 3.1  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 1 Total sperm motility at 3 months or less; pentoxifylline versus placebo or no treatment. | ||||||||||||||||||||
| 2 Total sperm motility at 3 months or less (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||||||||||||||||||
| Analysis 3.2
Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 2 Total sperm motility at 3 months or less (data not suitable for meta analysis). | ||||||||||||||||||||
| 2.1 Pentoxifylline versus placebo | Other data | No numeric data | ||||||||||||||||||
| 3 Total sperm motility at 6 months; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 229 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [9.09, 11.11] | ||||||||||||||||
| Analysis 3.3  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 3 Total sperm motility at 6 months; pentoxifylline versus placebo or no treatment. | ||||||||||||||||||||
| 4 Total sperm motility at 6 months (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||||||||||||||||||
| Analysis 3.4
Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 4 Total sperm motility at 6 months (data not suitable for meta analysis). | ||||||||||||||||||||
| 4.1 Pentoxifylline versus placebo | Other data | No numeric data | ||||||||||||||||||
| 5 Total sperm motility at 9 months; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 221 | Mean Difference (IV, Random, 95% CI) | 3.10 [1.93, 4.27] | ||||||||||||||||
| Analysis 3.5  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 5 Total sperm motility at 9 months; pentoxifylline versus placebo or no treatment. | ||||||||||||||||||||
| 6 Total sperm motility over time Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||
| Analysis 3.6  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 6 Total sperm motility over time. | ||||||||||||||||||||
| 6.1 Total sperm motility at 3 months or less | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 12.77 [9.23, 16.31] | ||||||||||||||||
| 6.2 Total sperm motility at 6 months | 1 | 229 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [9.09, 11.11] | ||||||||||||||||
| 6.3 Total sperm motility at 9 months or more | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [1.93, 4.27] | ||||||||||||||||
| 7 Sperm concentration at 3 months or less; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.30 [‐0.69, 9.29] | ||||||||||||||||
| Analysis 3.7  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 7 Sperm concentration at 3 months or less; pentoxifylline versus placebo or no treatment. | ||||||||||||||||||||
| 8 Sperm concentration at 6 months; pentoxifylline versus placebo Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||
| Analysis 3.8  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 8 Sperm concentration at 6 months; pentoxifylline versus placebo. | ||||||||||||||||||||
| 9 Sperm concentration at 9 months; pentoxifylline versus placebo Show forest plot | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.62, 2.78] | ||||||||||||||||
| Analysis 3.9  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 9 Sperm concentration at 9 months; pentoxifylline versus placebo. | ||||||||||||||||||||
| 10 Sperm concentration over time Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |||||||||||||||||
| Analysis 3.10  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 10 Sperm concentration over time. | ||||||||||||||||||||
| 10.1 Sperm concentration at 3 months or less | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 4.30 [‐0.69, 9.29] | ||||||||||||||||
| 10.2 Sperm concentration at 6 months | 2 | 247 | Mean Difference (IV, Random, 95% CI) | 6.90 [‐0.09, 13.89] | ||||||||||||||||
| 10.3 Sperm concentration at 9 months | 1 | 221 | Mean Difference (IV, Random, 95% CI) | 1.70 [0.62, 2.78] | ||||||||||||||||
| 11 Adverse events Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |||||||||||||||||
| Analysis 3.11  Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 11 Adverse events. | ||||||||||||||||||||
| 11.1 Vomiting | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.98 [1.32, 18.81] | ||||||||||||||||
| 11.2 Dyspepsia | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.68 [1.15, 19.07] | ||||||||||||||||
| 11.3 Headache | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.41 [0.54, 10.78] | ||||||||||||||||
| 11.4 Diarrhoea | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.63 [1.30, 44.67] | ||||||||||||||||
| 11.5 Tremor | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.45 [0.46, 119.73] | ||||||||||||||||
| 11.6 Dizziness | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.45 [0.46, 119.73] | ||||||||||||||||
| 11.7 Vertigo | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.20, 18.99] | ||||||||||||||||
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
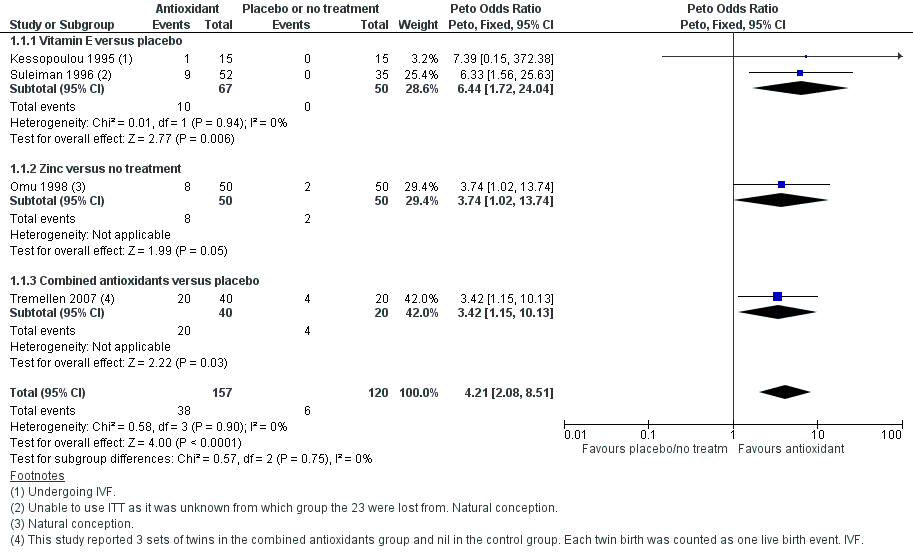
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.1 Live birth; type of antioxidant.
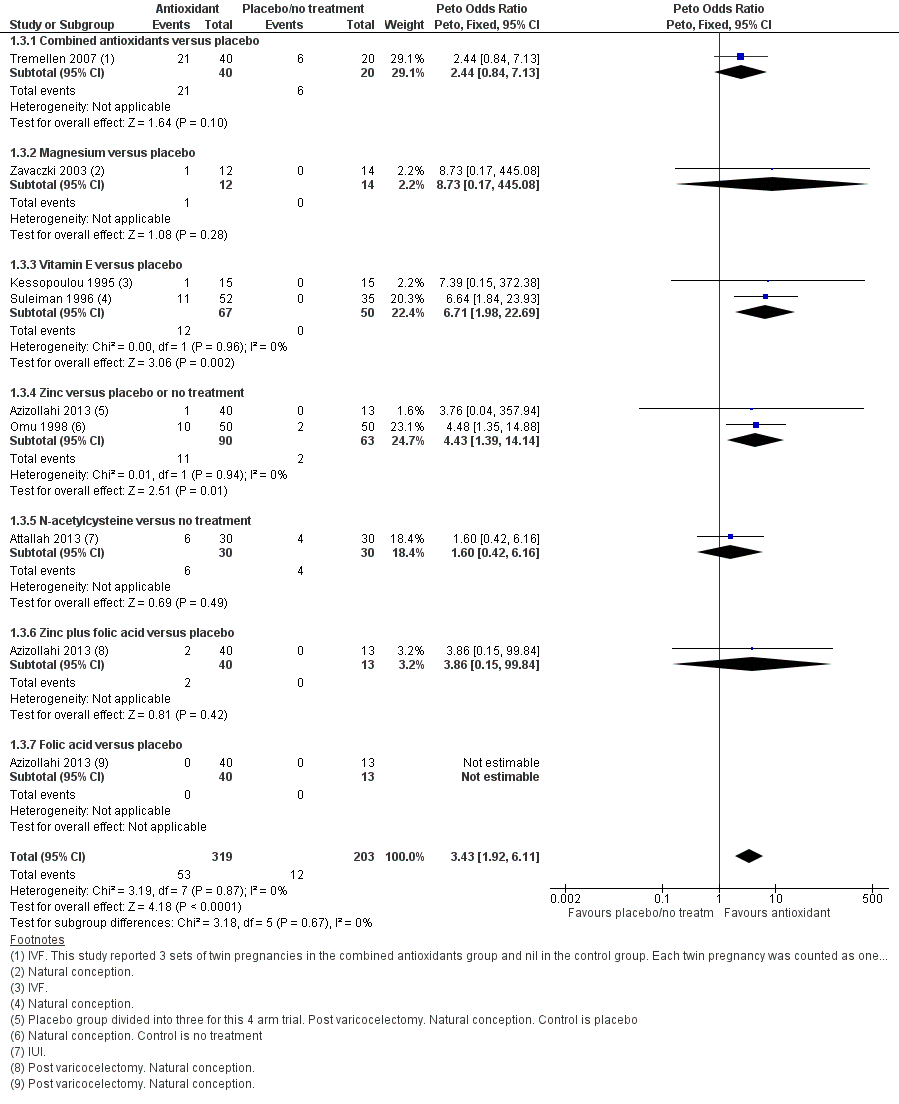
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.3 Clinical pregnancy; type of antioxidant.
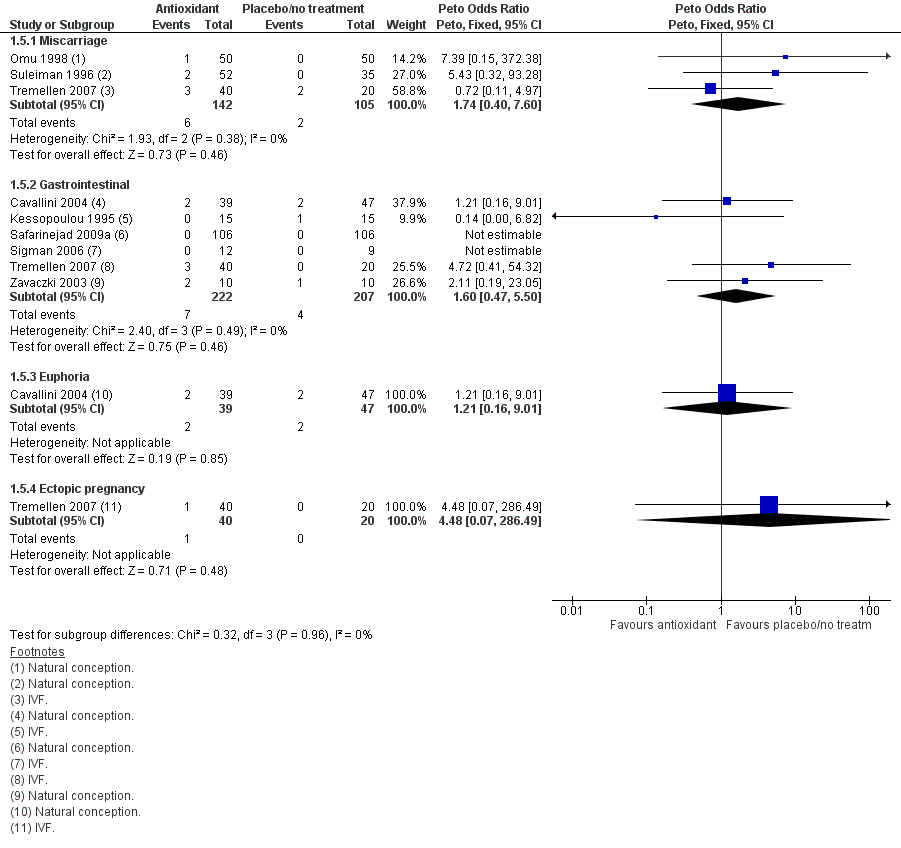
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.5 Adverse events.
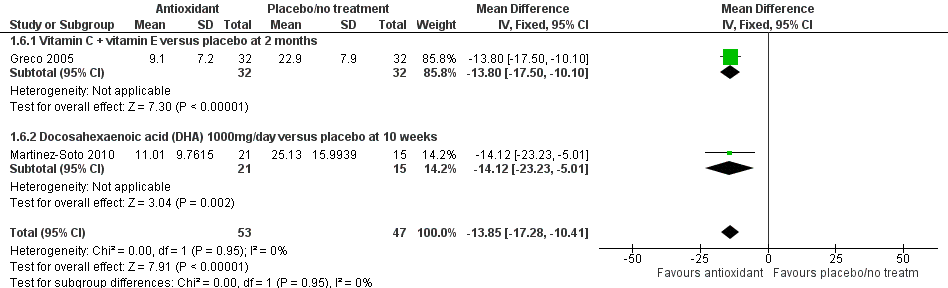
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.6 Sperm DNA fragmentation; type of antioxidant.
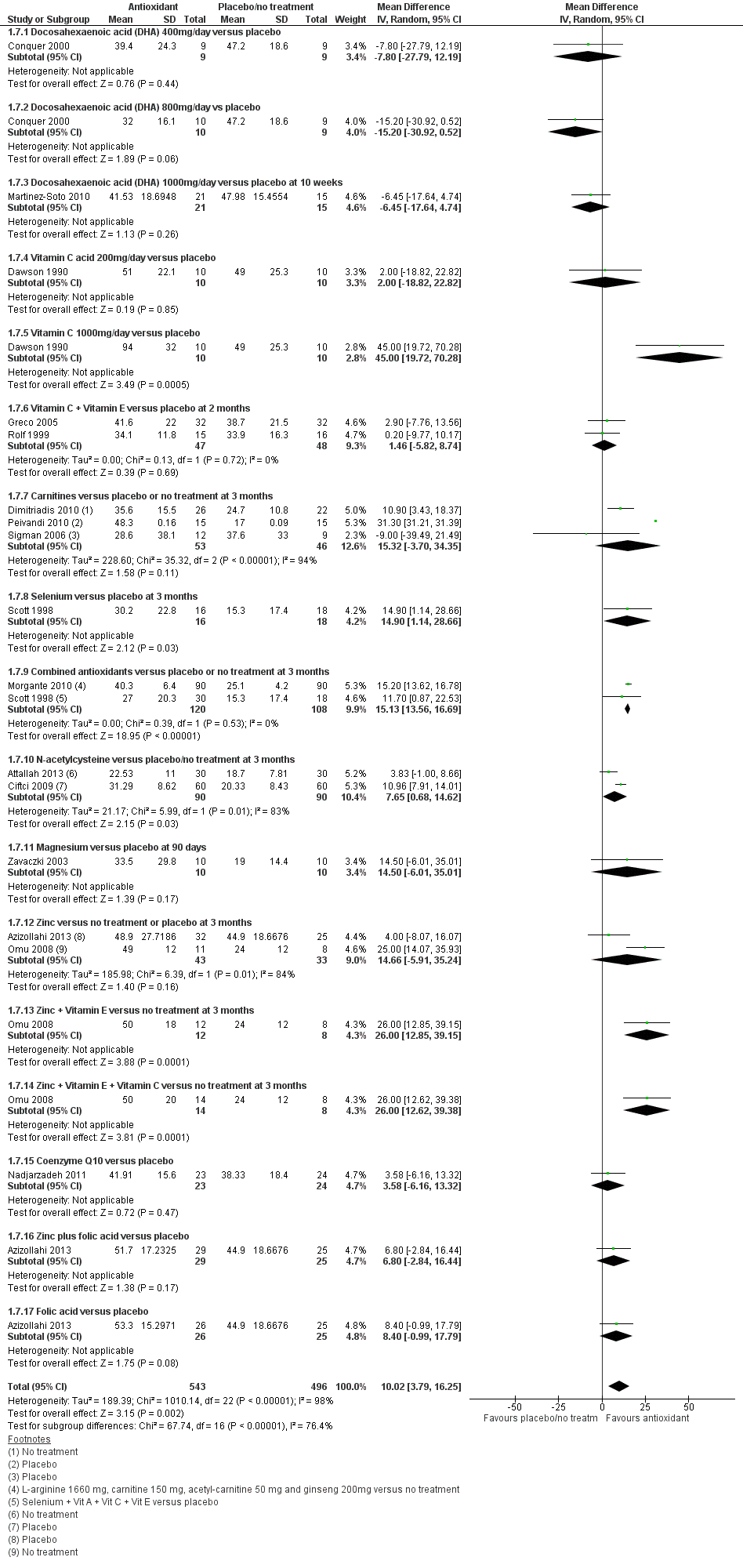
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.7 Total sperm motility at 3 months or less; type of antioxidant.

Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.12 Total sperm motility over time.
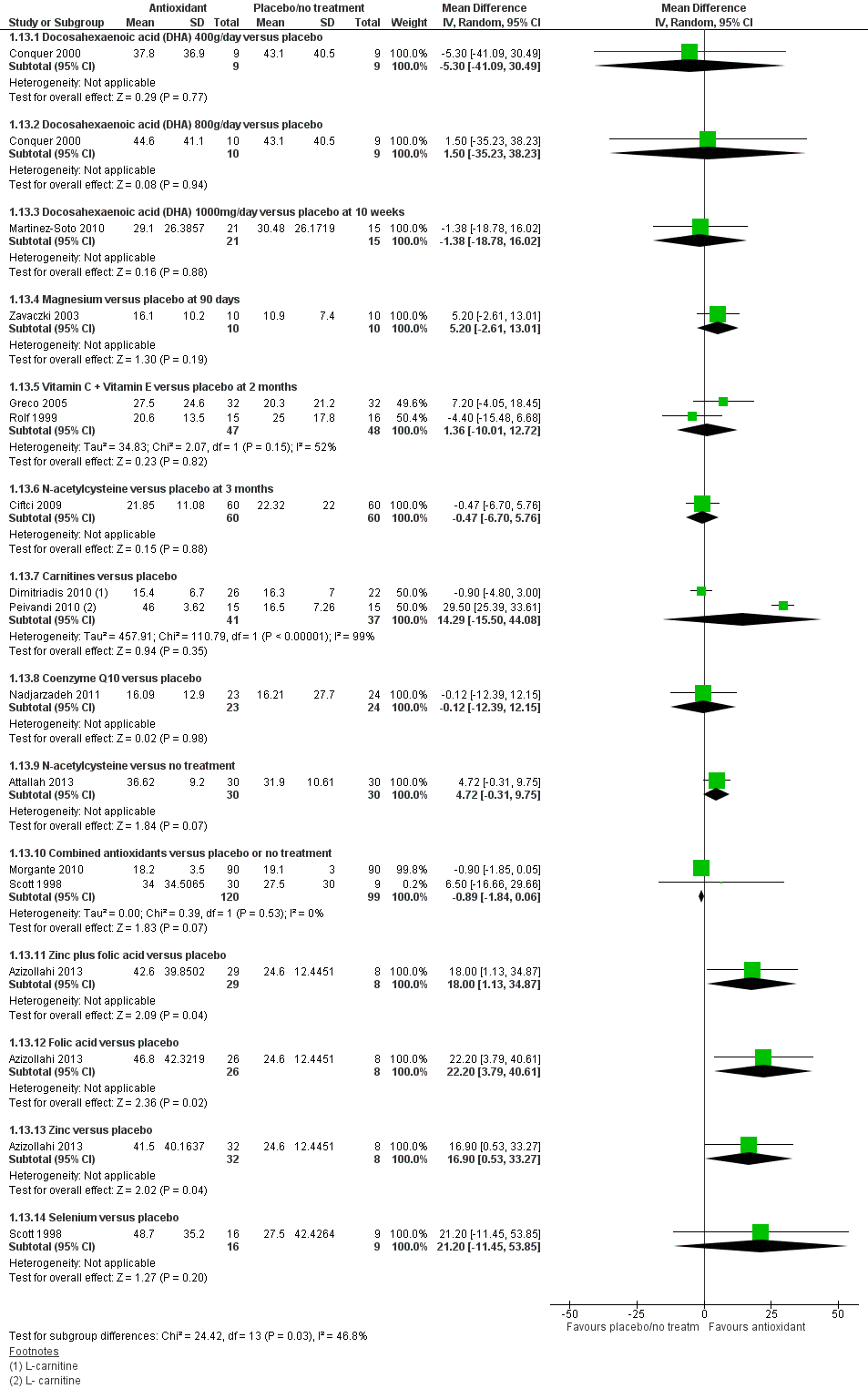
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.13 Sperm concentration at 3 months or less; type of antioxidant.

Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment, outcome: 1.18 Sperm concentration over time.
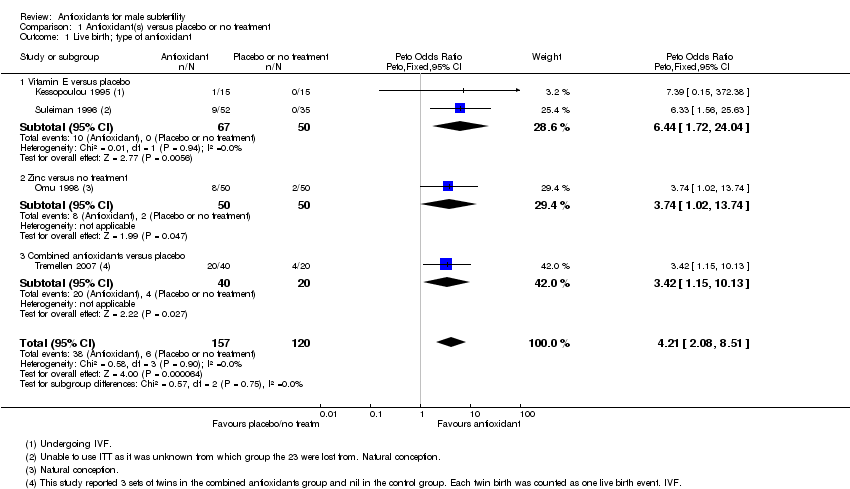
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 1 Live birth; type of antioxidant.
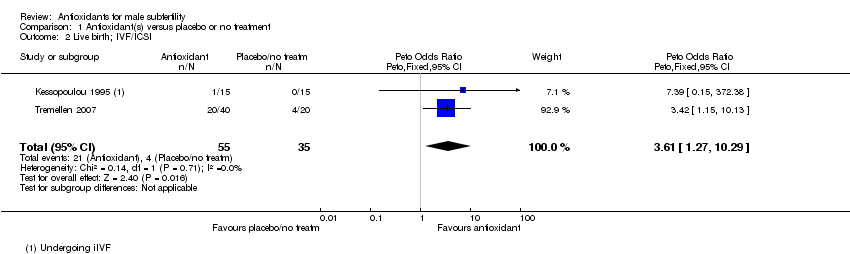
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 2 Live birth; IVF/ICSI.
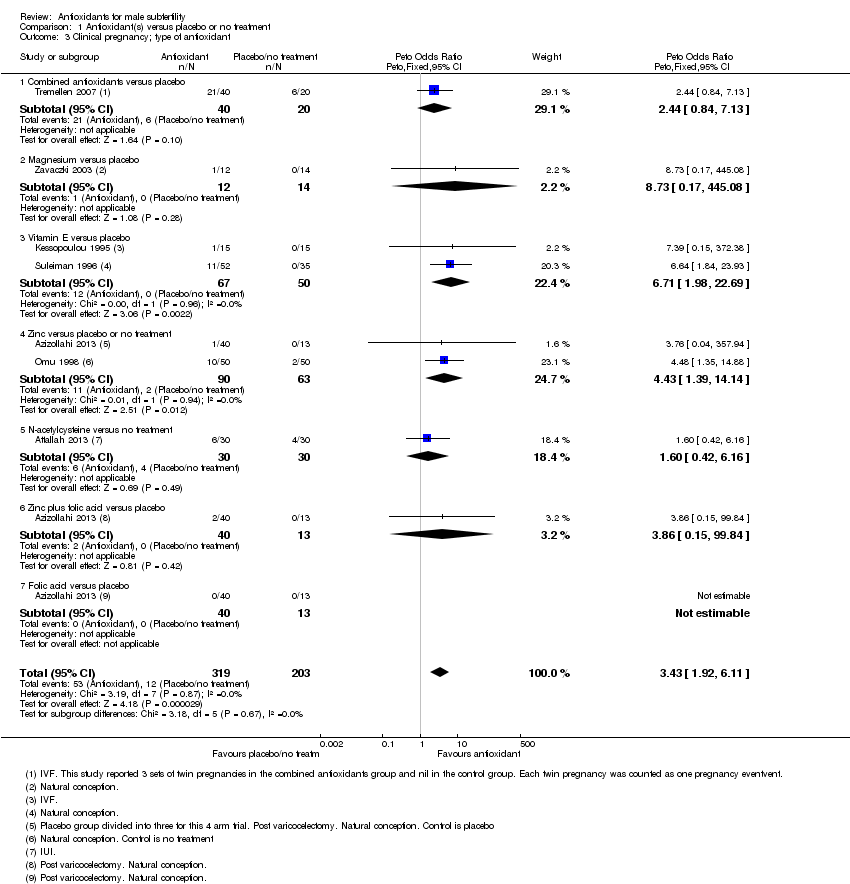
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 3 Clinical pregnancy; type of antioxidant.
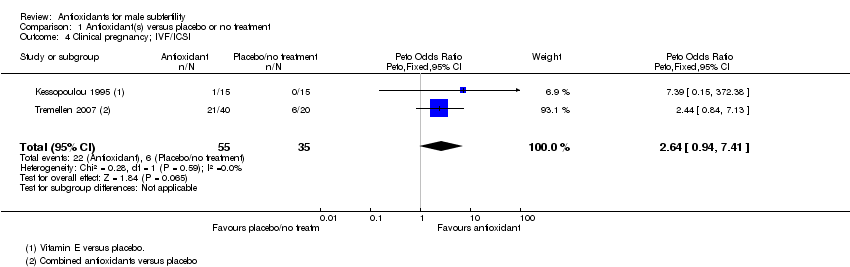
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 4 Clinical pregnancy; IVF/ICSI.
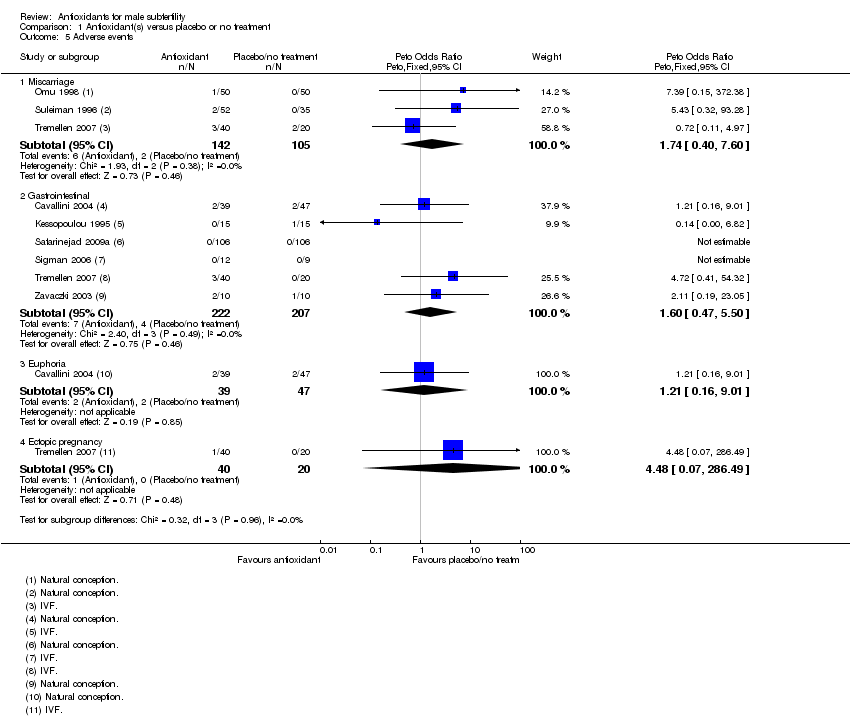
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 5 Adverse events.
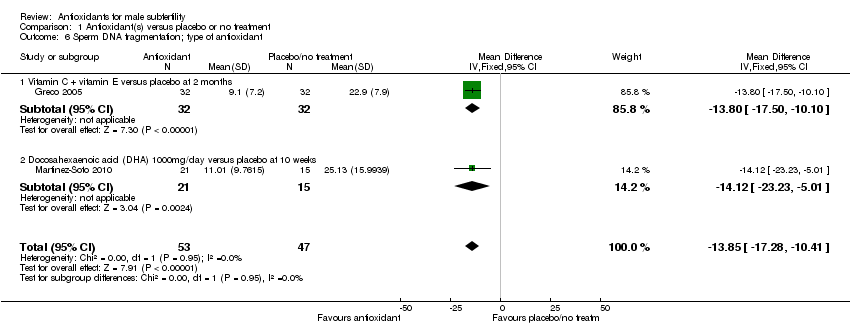
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 6 Sperm DNA fragmentation; type of antioxidant.

Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 7 Total sperm motility at 3 months or less; type of antioxidant.
| Study | Intervention | Control | P value |
| L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | |||
| Cavallini 2004 | L‐carnitine + Acetyl‐carnitine Median=22.3 (n=39) Interquartile range =28.4‐15.2 | Placebo Median= 14.0 (n=47) Interquartile range=17.4‐5.1 | Not provided |
| Combined antioxidants versus no treatment | |||
| Galatioto 2008 | Combined antioxidants % of motile sperm =58% (n=20) | No treatment % of motile sperm =51% (n=22) | P=0.847 |
| Vitamin E versus placebo | |||
| Kessopoulou 1995 | Vitamin E Median=7 (n=15) min/max= ‐27‐34 | Placebo Median=7 (n=15) min/max= ‐33‐36 | Not provided |
| L‐carnitine versus placebo | |||
| Lenzi 2003 | L‐carnitine Mean=11(n=43) no sd given | Placebo Mean=8.8 (n=43) no sd given | P=.04 |
| Selenium + Zinc versus placebo | |||
| Sivkov 2011 | Selenium + Zinc Mean =38.3 (n=15) no sd given | Placebo Mean =38 (n=15) no sd given | Not provided |
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 8 Total sperm motility at 3 months or less (data not suitable for meta analysis).
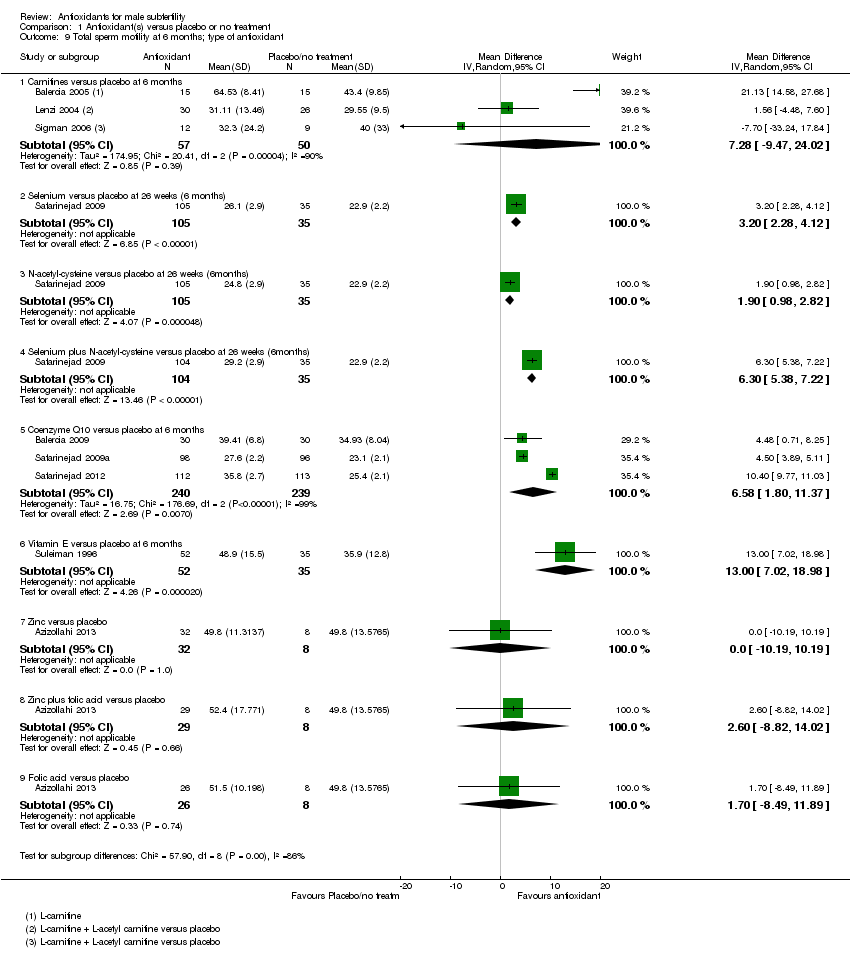
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 9 Total sperm motility at 6 months; type of antioxidant.
| Study | Intervention | Control | P value |
| L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | |||
| Cavallini 2004 | L‐carnitine + acetyl‐carnitine Median=23.6 (n=39) Interquartile range=28.9‐16.0 | Placebo Median=13.2 (n=47) Interquartile range=18.6‐9.0 | Not provided |
| Folic acid versus placebo | |||
| Wong 2002 | Folic acid Median=35 (n=22) Range=5‐65 | Placebo Median=30 (n=25) Range=5‐80 | |
| Zinc versus placebo | |||
| Wong 2002 | Zinc Median=35 (n=23) Range=10‐65 | Placebo Median=30 (n=25) Range=5‐80 | |
| Zinc + folic acid versus placebo | |||
| Wong 2002 | Zinc + folic acid Median=35 (n=24) Range 5‐70 | Placebo Median=30 (n=25) Range=5‐80 | |
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 10 Total sperm motility at 6 months(data not suitable for meta analysis).
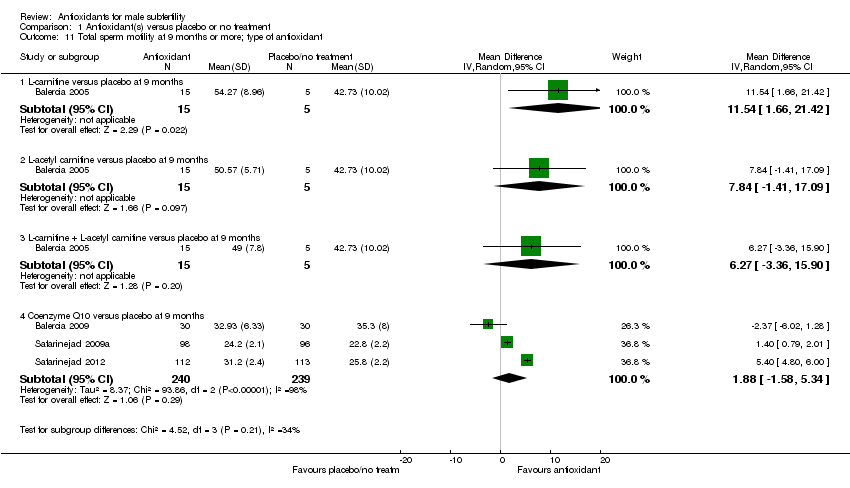
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 11 Total sperm motility at 9 months or more; type of antioxidant.
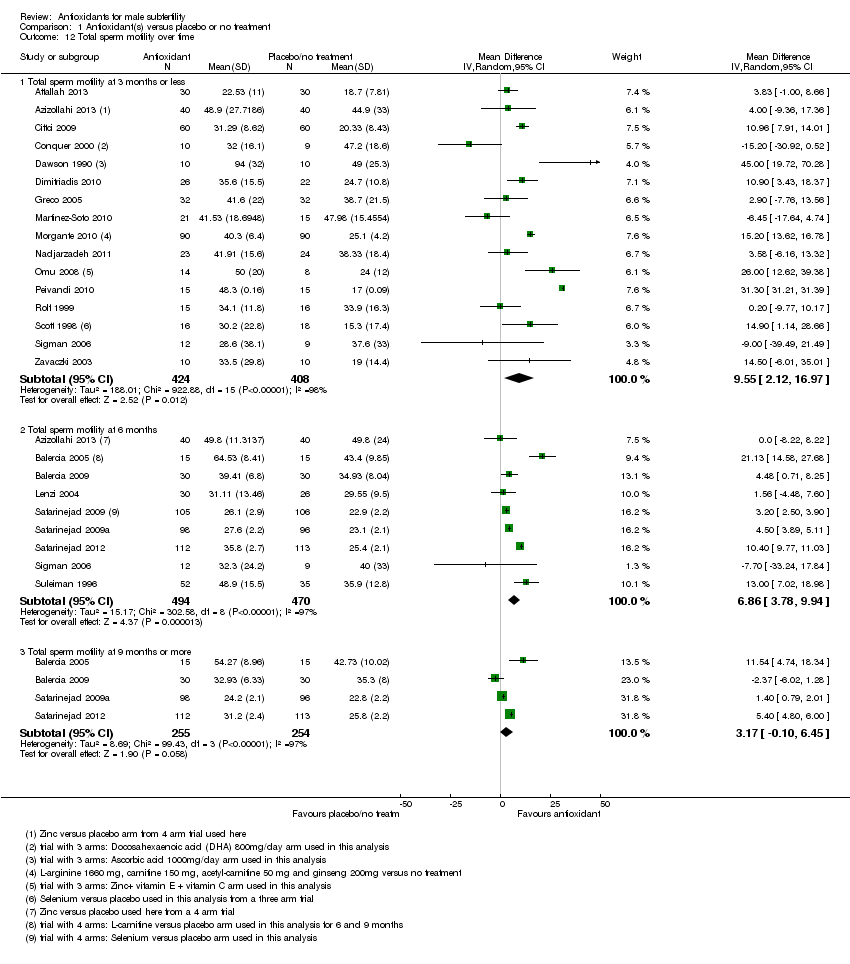
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 12 Total sperm motility over time.

Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 13 Sperm concentration at 3 months or less; type of antioxidant.
| Study | Intervention | Control | P value |
| L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | |||
| Cavallini 2004 | L‐carnitine + acetyl‐carnitine Median=20.9 (n=39) Interquartile range=25.6‐14.8 | Placebo Median=12.3 (n=47) Interquartile range=16.0‐9.1 | Not provided |
| Vitamin E versus placebo | |||
| Kessopoulou 1995 | Vitamin E Median= ‐15 (n=15) min/max= ‐58‐59 | Placebo Median=0 (n=15) min/max= ‐37‐160 | Not provided |
| L‐carnitine versus placebo | |||
| Lenzi 2003 | L‐carnitine Mean= 9 (1st phase data) (n=43) no sd given | Placebo Mean=5.3 (n=43) no sd given | P=0.03 |
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 14 Sperm concentration at 3 months or less (data not suitable for meta analysis).

Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 15 Sperm concentration at 6 months; type of antioxidant.
| Study | Intervention | Control | P value |
| L‐carnitine + acetyl‐carnitine versus placebo | |||
| Cavallini 2004 | L‐carnitine + acetyl‐carniitne Median=20.6 (n=39) Interquartile range=24.9‐15.1 | Placebo Median=10.9 (n=47) Interquartile range=15.1‐9.0 | Not provided |
| Folic acid versus Placebo | |||
| Wong 2002 | Folic acid Median=14 (n=22) Range=0.9‐130 | Placebo Median=9 (n=25) Range=0.8‐80 | Not provided |
| Zinc versus Placebo | |||
| Wong 2002 | Zinc Median=16 (n= 23) Range=0.6‐80 | Placebo Median=9 (n= 25) Range=0.8‐80 | Not provided |
| Zinc + folic acid versus placebo | |||
| Wong 2002 | Zinc + folic acid Median= 12 (n=24) Range= 0.5‐180 | Placebo Median=9 (n= 25) Range=0.8‐80 | Not provided |
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 16 Sperm concentration at 6 months(data not suitable for meta analysis).
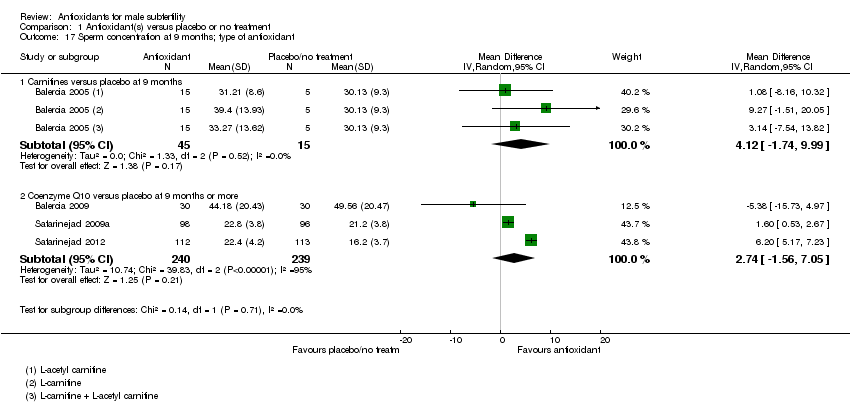
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 17 Sperm concentration at 9 months; type of antioxidant.
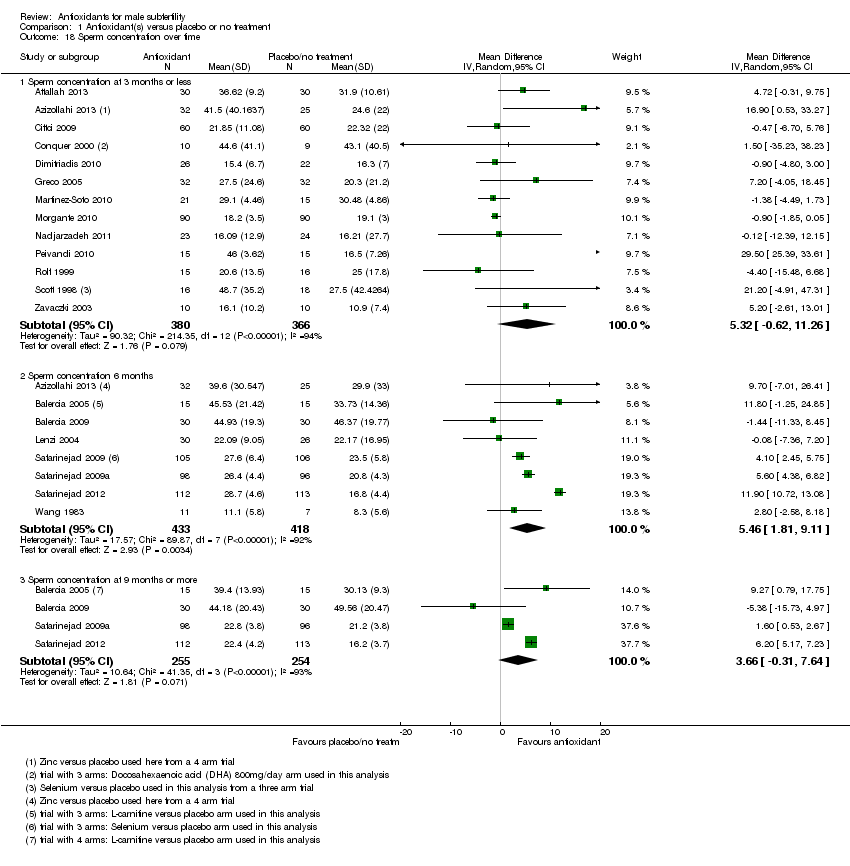
Comparison 1 Antioxidant(s) versus placebo or no treatment, Outcome 18 Sperm concentration over time.
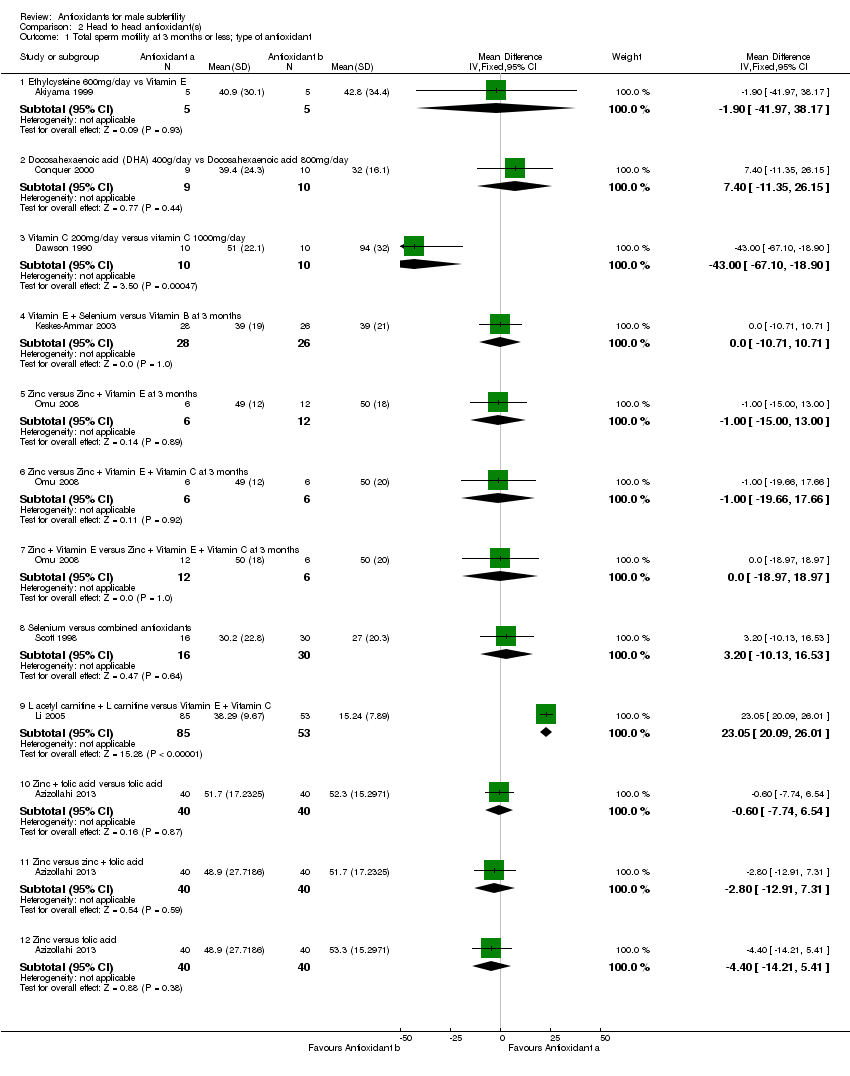
Comparison 2 Head to head antioxidant(s), Outcome 1 Total sperm motility at 3 months or less; type of antioxidant.
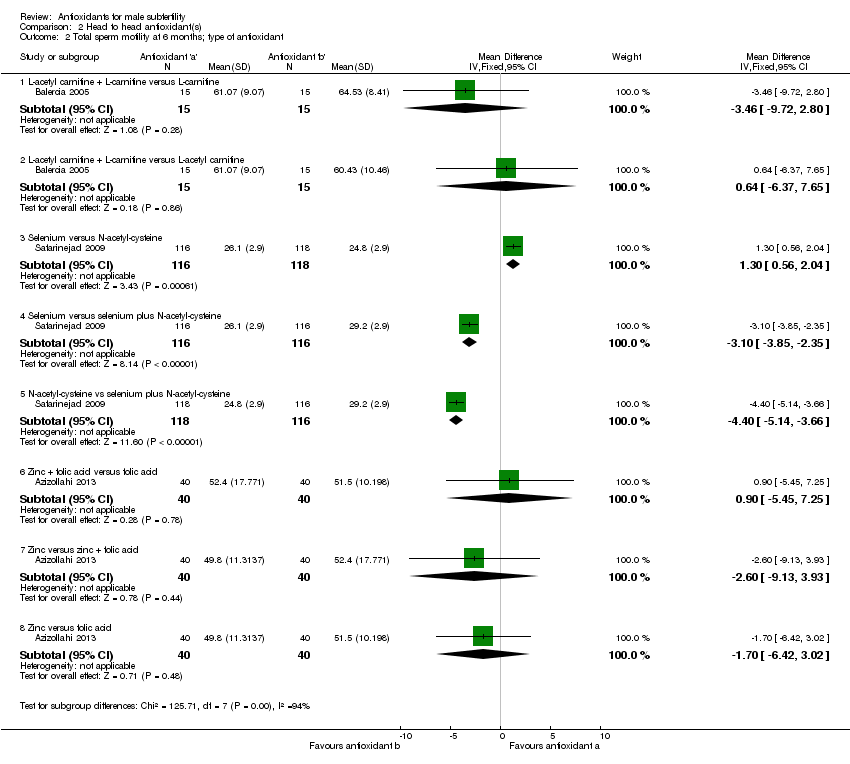
Comparison 2 Head to head antioxidant(s), Outcome 2 Total sperm motility at 6 months; type of antioxidant.
| Study | Antioxidant a | Antioxidant b | P value |
| Zinc versus Folic acid | |||
| Wong 2002 | Zinc Median=35 Range=10‐65 | Folic acid Median=35 Range=5‐65 | Not provided |
| Zinc versus Zinc + folic acid | |||
| Wong 2002 | Zinc Median=35 Range=10‐65 | Zinc + folic acid Median=35 Range=5‐70 | Not provided |
| Folic acid versus Zinc + folic acid | |||
| Wong 2002 | Folic acid Median=35 Range=5‐65 | Zinc + folic acid Median=35 Range=5‐70 | Not provided |
Comparison 2 Head to head antioxidant(s), Outcome 3 Total sperm motility at 6 months.

Comparison 2 Head to head antioxidant(s), Outcome 4 Total sperm motility at 9 months or more; type of antioxidant.
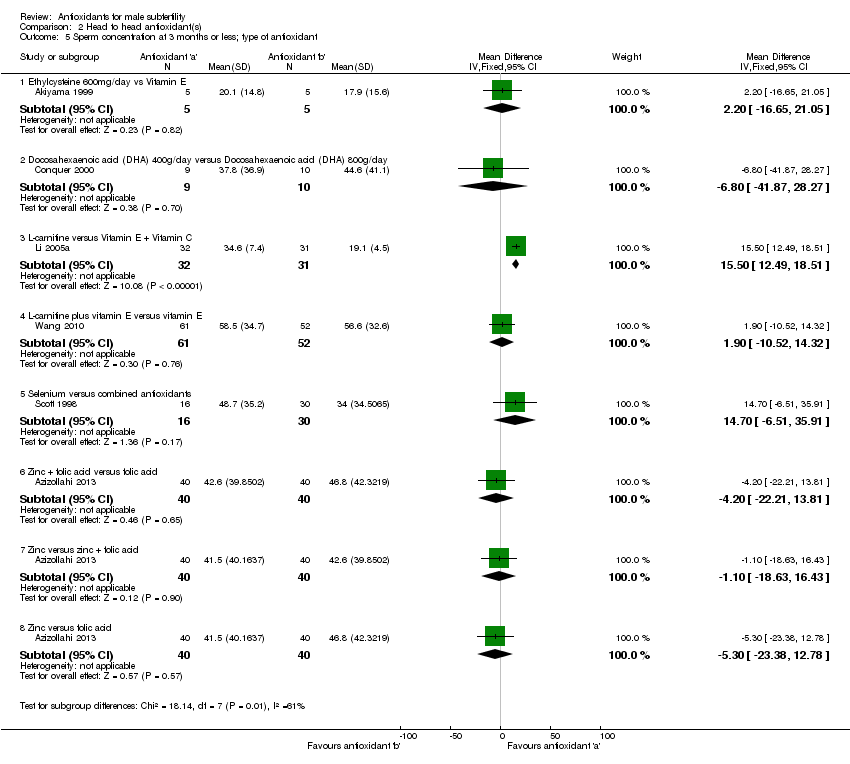
Comparison 2 Head to head antioxidant(s), Outcome 5 Sperm concentration at 3 months or less; type of antioxidant.

Comparison 2 Head to head antioxidant(s), Outcome 6 Sperm concentration at 6 months; type of antioxidant.
| Study | Antioxidant a | Antioxidant b | P value |
| Zinc versus Folic acid | |||
| Wong 2002 | Zinc Median=16 Range=0.6‐80 | Folic acid Median=14 Range=0.9‐130 | Not provided |
| Zinc versus Zinc + Folic acid | |||
| Wong 2002 | Zinc Median= 16 Range=0.6‐80 | Zinc + Folic acid Median=12 Range=0.5‐180 | Not provided |
| Folic acid versus Zinc + folic acid | |||
| Wong 2002 | Folic acid Folic acid Median=14 Range=0.9‐130 | Zinc + Folic acid Median=12 Range=0.5‐180 | Not provided |
Comparison 2 Head to head antioxidant(s), Outcome 7 Sperm concentration at 6 months.

Comparison 2 Head to head antioxidant(s), Outcome 8 Sperm concentration at 9 months or more; type of antioxidant.

Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 1 Total sperm motility at 3 months or less; pentoxifylline versus placebo or no treatment.
| Study | Antioxidant | Placebo | P value |
| Pentoxifylline versus placebo | |||
| Merino 1997 | Pentoxifylline Median=35.5 (n=25) Range=31.5‐39.5 | Placebo Median=33.5 (n=22) Range=28.5‐37.5 | P<0.01 |
Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 2 Total sperm motility at 3 months or less (data not suitable for meta analysis).
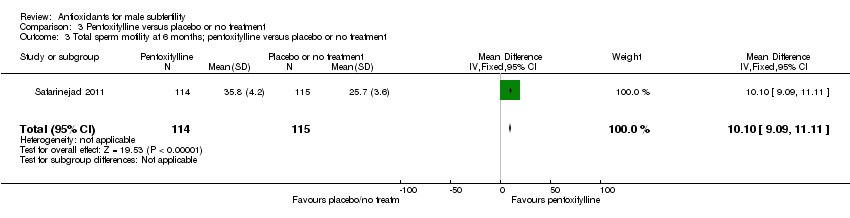
Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 3 Total sperm motility at 6 months; pentoxifylline versus placebo or no treatment.
| Study | Antioxidant | Control | P value |
| Pentoxifylline versus placebo | |||
| Merino 1997 | Pentoxifylline Median=42 (n=25) Range=38‐46 | Placebo Median=31.5 (22) Range=28‐35 | P<0.00001 |
Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 4 Total sperm motility at 6 months (data not suitable for meta analysis).

Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 5 Total sperm motility at 9 months; pentoxifylline versus placebo or no treatment.

Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 6 Total sperm motility over time.

Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 7 Sperm concentration at 3 months or less; pentoxifylline versus placebo or no treatment.

Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 8 Sperm concentration at 6 months; pentoxifylline versus placebo.
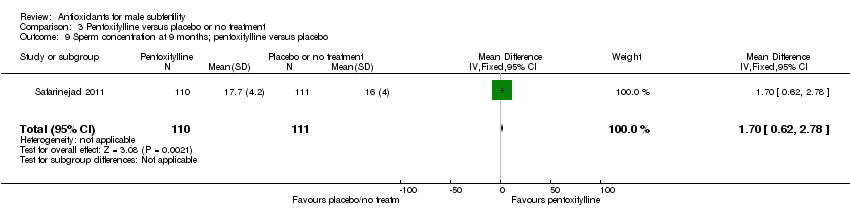
Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 9 Sperm concentration at 9 months; pentoxifylline versus placebo.
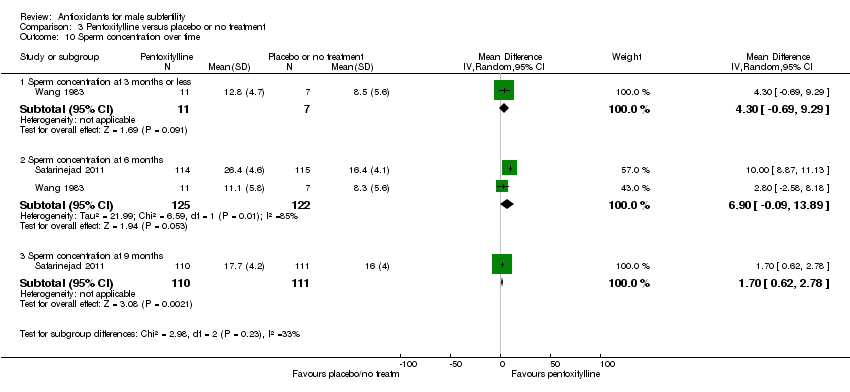
Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 10 Sperm concentration over time.

Comparison 3 Pentoxifylline versus placebo or no treatment, Outcome 11 Adverse events.
| Antioxidants versus placebo or no treatment for male subfertility | ||||||
| Patient or population: patients with male subfertility | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antioxidants versus placebo or no treatment | |||||
| Live Birth per couple randomised | 50 per 1000 | 181 per 1000 | OR 4.21 | 277 | ⊕⊕⊝⊝ | |
| Clinical Pregnancy rate per couple randomised | 59 per 1000 | 177 per 1000 | OR 3.43 | 522 | ⊕⊕⊝⊝ | |
| Adverse event: Miscarriage rate per couple randomised | 19 per 1000 | 33 per 1000 | OR 1.74 | 247 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Inadequate explanations of methodology and large unexplained dropouts in one study | ||||||
| Undefined or biochemical pregnancy | Events Intervention | Total 1 | Events Control | Total 2 | Effect Estimate | CI | |
| Antioxidant(s) versus placebo or no treatment | |||||||
| Combined antioxidants | |||||||
| Galatioto 2008 | 1 | 20 | 0 | 22 | |||
| Arginine versus placebo | |||||||
| Pryor 1978 | 2 | 35 | 2 | 29 | 0.82 | 0.82 [0.11, 6.16] | |
| Carnitines versus placebo or no treatment | 25 | 154 | 3 | 145 | 5.33 | ||
| Sigman 2006 | 1 | 12 | 1 | 9 | 0.74 | 0.74 [0.04, 13.02] | |
| Peivandi 2010 | 3 | 15 | 0 | 15 | 8.57 | 8.57 [0.82, 89.45] | |
| Lenzi 2004 | 4 | 30 | 0 | 26 | 7.20 | 7.20 [0.95, 54.34] | |
| Lenzi 2003 | 6 | 43 | 0 | 43 | 8.37 | 8.37 [1.61, 43.58] | |
| Cavallini 2004 | 9 | 39 | 1 | 47 | 7.50 | 7.50 [2.01, 27.98] | |
| Balercia 2005 | 2 | 15 | 1 | 5 | 0.61 | 0.61 [0.04, 9.64] | |
| Coenzyme Q10 versus placebo | 6 | 136 | 3 | 136 | 2.16 | ||
| Safarinejad 2009a | 0 | 106 | 0 | 106 | 0 | Not estimable | |
| Balercia 2009 | 6 | 30 | 3 | 30 | 2.16 | 2.16 [0.53, 8.82] | |
| Pentoxifylline versus placebo | |||||||
| Wang 1983 | 0 | 11 | 0 | 7 | 0 | Not estimable | |
| Vitamin C plus vitamin E versus placebo | |||||||
| Rolf 1999 | 0 | 15 | 0 | 16 | 0 | Not estimable | |
| Head to head antioxidant(s) | CI Start | CI End | |||||
| L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine | |||||||
| Balercia 2005 | 2 | 7 | 2 | 15 | 2.66 | 0.27 | 25.80 |
| L‐acetyl carnitine + L‐carnitine versus L‐carnitine | |||||||
| Balercia 2005 | 3 | 8 | 2 | 15 | 3.89 | 0.51 | 29.76 |
| L‐acetyl carnitine + L‐carnitine versus vitamin E + vitamin C | |||||||
| Li 2005 | 10 | 85 | 2 | 53 | 2.72 | 0.81 | 9.14 |
| L‐carnitine plus vitamin E versus vitamin E | |||||||
| Wang 2010 | 21 | 68 | 3 | 67 | 6.01 | 2.49 | 14.47 |
| Study ID | Population, design | Outcomes described in methods section | Outcomes reported on in results | In meta‐analysis Y or N | Results | Conclusions + = positive effect ‐ = negative or no effect |
| Infertile men ‐ high ROS levels N = 10 Crossover Head to head Japanese | Sperm parameters | Sperm parameters | Y | Sperm density and motility did not improve but "sperm function" increased and ROS levels decreased | + Ethylcysteine shown to be effective when compared to vitamin E for ROS associated infertility | |
| Idiopathic athenozospermia IUI N = 30 parallel, no treatment conference abstract | Chemical and clinical pregnancy sperm parameters | Clinical pregnancy Sperm parameters | Y | NAC increased sperm concentration and motility Clinical pregnancy was not significantly different between the groups | + NAC improves semen quality and improves pregnancy rates prior to IUI | |
| Men post‐varicocelectomy N = 160 4‐armed trial | Sperm parameters | Sperm parameters | Y sperm parameters reported but clinical pregnancy from correspondence with author ‐ men were asked at their last semen assessment session about pregnancy if yes ultrasound was used to confirm | Mild improvement in sperm parameters with the use of antioxidants | + Co‐administration of zinc and folic acid improved sperm parameters and increased varicocelectomy outcomes | |
| Infertile men or unexplained infertility N = 60 Placebo and head to head | Sperm parameters | Sperm parameters Spontaneous pregnancies 5 LC + LAC 2 LC 2 LAC 3 placebo | Y ‐ sperm N ‐ pregnancy | Improvement in motility in LAC group. 12 spontaneous pregnancies (unknown if biochemical or clinical) | + Long term carnitine is effective in increasing sperm motility | |
| Infertile and unexplained N = 60 | Sperm parameters | Sperm parameters Spontaneous pregnancies 6 Q10 3 placebo | Y ‐ sperm N ‐ pregnancy | Co enzyme Q10 increased sperm motility. 9 spontaneous pregnancies (unknown if biochemical or clinical) | + Q10 effective in improving sperm kinetic features in asthenospermia | |
| Severe idiopathic oligoasthenospermia conference abstract N = 42 | Sperm parameters | Sperm | N ‐ no data available | A significant improvement in morphology concentration, motility in the carnitine group No side effects | + Quality of semen is positively associated with fertilisation and implantation rates in assisted reproduction | |
| Idiopathic and varicocoele associated infertility N = 325 | Sperm parameters pregnancies side effects | Sperm parameters pregnancies at 6 months post‐treatment and assumed to be clinical | N Medians only given for sperm parameters in full paper Analysis 1.8 , .Analysis 1.10; Analysis 1.13; Analysis 1.14; Analysis 1.16. Means in conference abstract but no data given for placebo group and data for group 3 (carnitine + cinoxacin) versus group 2 (carnitines) unable to be used as 3 includes cinoxacin an antiinflammatory drug Y clinical pregnancy | Significant increase in sperm parameters for carnitines when compared to placebo. Carnitine groups had a significantly higher pregnancy rate than placebo group | + The antioxidant plus antiinflammatory group was more effective in improving sperm parameters and pregnancy than those of carnitines alone or placebo however carnitines alone were more effective than placebo | |
| Idiopathic infertility N = 120 | Sperm parameters | Sperm parameters | Y sperm parameters | NAC showed significant improvement in sperm parameters when compared with placebo | + Sperm parameters improved after the use of NAC | |
| Asthenozoospermic men N = 28 | Sperm parameters | Sperm parameters | Y sperm parameters (SEs converted to SDs) | DHA showed no effect on sperm motility or concentration | ± DHA supplementation increased DHA levels in the sperm but not motility or concentration | |
| Agglutination associated infertility N = 30 | Sperm parameters | Sperm parameters | Y sperm parameters (SEs converted to SDs) | The group receiving 1000 mg of AA showed more improvement in parameters than the 200mg group and the placebo | + | |
| Oligoasthenospermia N = 75 4 arm trial only 2 arms able to be used | Sperm parameters | Sperm parameters | Y sperm parameters | An improvement in sperm concentration with carnitine versus no treatment | + Enhancement of Leydig cell secretory function may increase sperm concentration and motility | |
| Asthenoszoospermic men N = 50 | Sperm parameters | sperm parameters ‐ sperm membrane and serum fatty acids | N outcomes not included in this review | Sperm parameters improved with DHA + vitamin E supplementation | + | |
| Oligospermia post‐embolisation of varicocoele N = 42 | Sperm parameters Pregnancy Adverse events | Sperm parameters Pregnancy Adverse events | N medians only given for sperm parameters Analysis 1.8 Y pregnancy at 12 months post‐treatment assumed to be clinical Adverse events | Significant difference in sperm count in combined antioxidant group but not in motility. One pregnancy in the NAC group No significant adverse effects | ± | |
| Male infertility ‐ high DNA fragmentation N = 64 | Sperm parameters | Sperm parameters | Y sperm parameters | No significant difference in concentration or motility however DNA fragmentation was significantly reduced in the vitamin C + E when compared to placebo | + A short oral treatment of VitC + E can reduce DNA fragmentation | |
| Men with high levels of ROS in semen N = 78 Head to head | Sperm parameters | Sperm parameters | Y sperm parameters | Treatment with Vit E and selenium increased sperm motility when compared to vitamin B | + | |
| Male infertility Crossover N = 30 | Sperm parameters | Sperm parameters Live birth Clinical pregnancy | N medians only given for sperm parameters Analysis 1.8; Analysis 1.14 Y Pregnancy | No differences in sperm outcomes were seen between the groups. 1 pregnancy in the vitamin E group and nil in the placebo (first phase data) | ‐ No difference in semen parameters | |
| Male patients with abnormal sperm count and motility 3‐armed trial N = 396 | Sperm parameters | Sperm parameters | N scales given | No statistical difference in sperm outcomes in vitamin B 12 groups or placebo | ‐ | |
| Male factor infertility N = 100 Crossover | Sperm parameters Pregnancy rates | Sperm parameters Pregnancy rates | N no SDs given for sperm parameters Analysis 1.8; Analysis 1.14 N no definition of pregnancy given see Table 1 | The patient groups showed no differences in sperm outcomes between therapy (carnitine) and placebo groups. Six pregnancies in the carnitine group and nil in the placebo (first phase) | + The pregnancies obtained during the carnitine therapy period could suggest that carnitines may also lead to improvement in sperm function and fertilisation | |
| Infertile males ‐ oligoasthenoteratozoospermia N = 60 | Sperm parameters Adverse events | Sperm parameters Pregnancy rates | Y sperm parameters N no definition of pregnancy given see analysis for biochemical pregnancy Table 1 N adverse events | Four participants taking carnitine induced a pregnancy in their partner and nil in the placebo | + | |
| Infertile males ‐ oligoasthenoteratozoospermia (150) Head to head | Sperm parameters Pregnancy rates Adverse events | Sperm parameters Pregnancy rates | Y sperm parameters N no definition of pregnancy given see analysis for biochemical pregnancy Table 1 | Y 10 pregnancies in the carnitine group and 2 in the vitamin E + C group | + Lcarnitine and acetyl carnitine more effective than vitamin E + vitamin C for pregnancy, sperm parameters and no evidence of adverse events | |
| Infertile males ‐ oligoasthenoteratozoospermia (80) Head to head | Sperm parameters | Sperm parameters | Y | + Staistical significance for carnitines over vitamin E + C | ||
| Infertile males Conference abstract Crossover (N = 100) | Sperm parameters | Sperm parameters | N no data | + Sperm parameters (concentration, motility) carnitines versus placebo | ||
| Infertile males (N = 50) Conference abstract + communication with author | Sperm parameters | Sperm parameters | Y | + DNA fragmentation | ||
| Idiopathic asthenospermia (N = 47) | Sperm parameters | N medians only given for sperm parameters Analysis 3.2; Analysis 3.4 | + | |||
| Idiopathic asthenospermia (N = 90) | Sperm parameters | Y | + Significant improvement in sperm motility in pentoxifylline versus no treatment | |||
| Idiopathic asthenospermia (N = 180) | Sperm parameters | Y | + Sexual satisfaction Significant improvement in sperm motility | |||
| Idiopathic oligoasthenospermia (N = 60) | Sperm parameters | Y | ‐ | |||
| Oligoasthenospermia head to head (N = 20) | Sperm parameters | N no data available | + Vitamin E + selenium associated with improved sperm motility when compared with vitamin B | |||
| Asthenospermia (N = 100) | Sperm parameters | Sperm parameters pregnancy and live birth | Y pregnancy and live birth only N sperm parameters not appropriate for review | + Pregnancy or live birth and sperm parameters | ||
| Asthenospermia (N = 100) | Sperm parameters | Y | + | |||
| Infertile men (N = 30) (crossover) | Sperm parameters | Y Y biochemical pregnancies Table 1 | + Sperm outcomes + biochemical pregnancies | |||
| Infertile men (N = 60) conference abstract | Sperm parameters | N | + Sperm concentration and motility with L‐carnitine and spermotrend | |||
| Oligozoospermia (N = 64) crossover | Sperm parameters Pregnancy rates | N bar graph of % patients showing an increase in motility and density Y pregnancy data included in biochemical analysis Table 1 | ‐ Arginine was no more effective than placebo for sperm parameters and biochemical pregnancy rates | |||
| Asthenospermia (N = 33) | Sperm parameters Pregnancy rates | Y | No adverse events or pregnancies in either group | ‐ No difference vitamin E + C versus placebo | ||
| Idiopathic oligozoospermia (N = 468) | Sperm parameters | Y | + N acetylcysteine, selenium | |||
| Idiopathic oligozoospermia (N = 212) | Sperm parameters | Y | + Coenzyme Q10 | |||
| Idiopathic infertility (N = 254) | Sperm parameters | Y | Adverse events, sperm concentration and motility | + Pentoxifylline | ||
| Idiopathic infertility (N=228) | Sperm parameters | Y | + Coenzyme Q10 | |||
| Reduced sperm motility (N = 69) | Sperm parameters Pregnancy | Y N due to pregnancy data pooled in the two intervention groups | + Sperm motility and pregnancy, combined antioxidants and selenium | |||
| Low sperm motility (N = 26) | Sperm parameters Pregnancy | Y | _ Carnitine | |||
| Subnormal spermatogenesis ‐ prostatitis (N = 30) Russian | Sperm parameters | N no sd given see Analysis 1.8 | + Selenium + zinc | |||
| Asthenospermia (N = 110) | Sperm parameters | Sperm parameters Pregnancy Live birth Miscarriage | Y Y Y Y | + Vit E | ||
| Male factor infertility (N = 60) | Pregnancy Side effects | Pregnancy Side effects | Y | + Menevit | ||
| Idiopathic oligozoospermia (N = 46) | Sperm parameters Pregnancy | Y no data on motility available | ‐ Pentoxifylline | |||
| Asthenospermia (N = 135) Chinese | Sperm parameters Pregnancy | Sperm parameters pregnancy | Y | + Sperm motility, pregnancy ‐ Sperm density and normal morphology | ||
| Subfertile males (N = 103) | Sperm parameters | N Medians only see Analysis 1.10;and Analysis 1.16 | + Folic acid + zinc | |||
| Men attending andrology clinic (N = 22) conference abstract | Sperm parameters including DNA fragmentation | N only before and after median data given | + DNA fragmentation but ‐ Other sperm parameters Combined antioxidants and fatty acids (DHA) | |||
| Idiopathic infertility (N = 20) | Sperm parameters Clinical pregnancy | Y | ‐ Magnesium |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth; type of antioxidant Show forest plot | 4 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.21 [2.08, 8.51] |
| 1.1 Vitamin E versus placebo | 2 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.44 [1.72, 24.04] |
| 1.2 Zinc versus no treatment | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.74 [1.02, 13.74] |
| 1.3 Combined antioxidants versus placebo | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.42 [1.15, 10.13] |
| 2 Live birth; IVF/ICSI Show forest plot | 2 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.61 [1.27, 10.29] |
| 3 Clinical pregnancy; type of antioxidant Show forest plot | 7 | 522 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.43 [1.92, 6.11] |
| 3.1 Combined antioxidants versus placebo | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [0.84, 7.13] |
| 3.2 Magnesium versus placebo | 1 | 26 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.73 [0.17, 445.08] |
| 3.3 Vitamin E versus placebo | 2 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.71 [1.98, 22.69] |
| 3.4 Zinc versus placebo or no treatment | 2 | 153 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.43 [1.39, 14.14] |
| 3.5 N‐acetylcysteine versus no treatment | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.42, 6.16] |
| 3.6 Zinc plus folic acid versus placebo | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.86 [0.15, 99.84] |
| 3.7 Folic acid versus placebo | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Clinical pregnancy; IVF/ICSI Show forest plot | 2 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.64 [0.94, 7.41] |
| 5 Adverse events Show forest plot | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Miscarriage | 3 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.40, 7.60] |
| 5.2 Gastrointestinal | 6 | 429 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.47, 5.50] |
| 5.3 Euphoria | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.16, 9.01] |
| 5.4 Ectopic pregnancy | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.48 [0.07, 286.49] |
| 6 Sperm DNA fragmentation; type of antioxidant Show forest plot | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐13.85 [‐17.28, ‐10.41] |
| 6.1 Vitamin C + vitamin E versus placebo at 2 months | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐13.80 [‐17.50, ‐10.10] |
| 6.2 Docosahexaenoic acid (DHA) 1000mg/day versus placebo at 10 weeks | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐14.12 [‐23.23, ‐5.01] |
| 7 Total sperm motility at 3 months or less; type of antioxidant Show forest plot | 16 | 1039 | Mean Difference (IV, Random, 95% CI) | 10.02 [3.79, 16.25] |
| 7.1 Docosahexaenoic acid (DHA) 400mg/day versus placebo | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐7.80 [‐27.79, 12.19] |
| 7.2 Docosahexaenoic acid (DHA) 800mg/day vs placebo | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐15.20 [‐30.92, 0.52] |
| 7.3 Docosahexaenoic acid (DHA) 1000mg/day versus placebo at 10 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.45 [‐17.64, 4.74] |
| 7.4 Vitamin C acid 200mg/day versus placebo | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐18.82, 22.82] |
| 7.5 Vitamin C 1000mg/day versus placebo | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 45.0 [19.72, 70.28] |
| 7.6 Vitamin C + Vitamin E versus placebo at 2 months | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 1.46 [‐5.82, 8.74] |
| 7.7 Carnitines versus placebo or no treatment at 3 months | 3 | 99 | Mean Difference (IV, Random, 95% CI) | 15.32 [‐3.70, 34.35] |
| 7.8 Selenium versus placebo at 3 months | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 14.9 [1.14, 28.66] |
| 7.9 Combined antioxidants versus placebo or no treatment at 3 months | 2 | 228 | Mean Difference (IV, Random, 95% CI) | 15.13 [13.56, 16.69] |
| 7.10 N‐acetylcysteine versus placebo/no treatment at 3 months | 2 | 180 | Mean Difference (IV, Random, 95% CI) | 7.65 [0.68, 14.62] |
| 7.11 Magnesium versus placebo at 90 days | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 14.5 [‐6.01, 35.01] |
| 7.12 Zinc versus no treatment or placebo at 3 months | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 14.66 [‐5.91, 35.24] |
| 7.13 Zinc + Vitamin E versus no treatment at 3 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 26.0 [12.85, 39.15] |
| 7.14 Zinc + Vitamin E + Vitamin C versus no treatment at 3 months | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 26.0 [12.62, 39.38] |
| 7.15 Coenzyme Q10 versus placebo | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 3.58 [‐6.16, 13.32] |
| 7.16 Zinc plus folic acid versus placebo | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 6.80 [‐2.84, 16.44] |
| 7.17 Folic acid versus placebo | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 8.40 [‐0.99, 17.79] |
| 8 Total sperm motility at 3 months or less (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||
| 8.1 L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | Other data | No numeric data | ||
| 8.2 Combined antioxidants versus no treatment | Other data | No numeric data | ||
| 8.3 Vitamin E versus placebo | Other data | No numeric data | ||
| 8.4 L‐carnitine versus placebo | Other data | No numeric data | ||
| 8.5 Selenium + Zinc versus placebo | Other data | No numeric data | ||
| 9 Total sperm motility at 6 months; type of antioxidant Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Carnitines versus placebo at 6 months | 3 | 107 | Mean Difference (IV, Random, 95% CI) | 7.28 [‐9.47, 24.02] |
| 9.2 Selenium versus placebo at 26 weeks (6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 3.20 [2.28, 4.12] |
| 9.3 N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.98, 2.82] |
| 9.4 Selenium plus N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 139 | Mean Difference (IV, Random, 95% CI) | 6.30 [5.38, 7.22] |
| 9.5 Coenzyme Q10 versus placebo at 6 months | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 6.58 [1.80, 11.37] |
| 9.6 Vitamin E versus placebo at 6 months | 1 | 87 | Mean Difference (IV, Random, 95% CI) | 13.0 [7.02, 18.98] |
| 9.7 Zinc versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐10.19, 10.19] |
| 9.8 Zinc plus folic acid versus placebo | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐8.82, 14.02] |
| 9.9 Folic acid versus placebo | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐8.49, 11.89] |
| 10 Total sperm motility at 6 months(data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||
| 10.1 L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | Other data | No numeric data | ||
| 10.2 Folic acid versus placebo | Other data | No numeric data | ||
| 10.3 Zinc versus placebo | Other data | No numeric data | ||
| 10.4 Zinc + folic acid versus placebo | Other data | No numeric data | ||
| 11 Total sperm motility at 9 months or more; type of antioxidant Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 L‐carnitine versus placebo at 9 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 11.54 [1.66, 21.42] |
| 11.2 L‐acetyl carnitine versus placebo at 9 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 7.84 [‐1.41, 17.09] |
| 11.3 L‐carnitine + L‐acetyl carnitine versus placebo at 9 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 6.27 [‐3.36, 15.90] |
| 11.4 Coenzyme Q10 versus placebo at 9 months | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 1.88 [‐1.58, 5.34] |
| 12 Total sperm motility over time Show forest plot | 23 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Total sperm motility at 3 months or less | 16 | 832 | Mean Difference (IV, Random, 95% CI) | 9.55 [2.12, 16.97] |
| 12.2 Total sperm motility at 6 months | 9 | 964 | Mean Difference (IV, Random, 95% CI) | 6.86 [3.78, 9.94] |
| 12.3 Total sperm motility at 9 months or more | 4 | 509 | Mean Difference (IV, Random, 95% CI) | 3.17 [‐0.10, 6.45] |
| 13 Sperm concentration at 3 months or less; type of antioxidant Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Docosahexaenoic acid (DHA) 400g/day versus placebo | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐5.30 [‐41.09, 30.49] |
| 13.2 Docosahexaenoic acid (DHA) 800g/day versus placebo | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 1.50 [‐35.23, 38.23] |
| 13.3 Docosahexaenoic acid (DHA) 1000mg/day versus placebo at 10 weeks | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐18.78, 16.02] |
| 13.4 Magnesium versus placebo at 90 days | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 5.20 [‐2.61, 13.01] |
| 13.5 Vitamin C + Vitamin E versus placebo at 2 months | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐10.01, 12.72] |
| 13.6 N‐acetylcysteine versus placebo at 3 months | 1 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐6.70, 5.76] |
| 13.7 Carnitines versus placebo | 2 | 78 | Mean Difference (IV, Random, 95% CI) | 14.29 [‐15.50, 44.08] |
| 13.8 Coenzyme Q10 versus placebo | 1 | 47 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐12.39, 12.15] |
| 13.9 N‐acetylcysteine versus no treatment | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.72 [‐0.31, 9.75] |
| 13.10 Combined antioxidants versus placebo or no treatment | 2 | 219 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.84, 0.06] |
| 13.11 Zinc plus folic acid versus placebo | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 18.0 [1.13, 34.87] |
| 13.12 Folic acid versus placebo | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 22.20 [3.79, 40.61] |
| 13.13 Zinc versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 16.9 [0.53, 33.27] |
| 13.14 Selenium versus placebo | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 21.20 [‐11.45, 53.85] |
| 14 Sperm concentration at 3 months or less (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||
| 14.1 L‐carnitine + Acetyl‐carnitine versus placebo (median and interquartile range) | Other data | No numeric data | ||
| 14.2 Vitamin E versus placebo | Other data | No numeric data | ||
| 14.3 L‐carnitine versus placebo | Other data | No numeric data | ||
| 15 Sperm concentration at 6 months; type of antioxidant Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Carnitines versus placebo at 6 months | 2 | 116 | Mean Difference (IV, Random, 95% CI) | 2.59 [‐3.11, 8.30] |
| 15.2 Selenium versus placebo at 26 weeks (6 months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 4.10 [1.82, 6.38] |
| 15.3 N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 140 | Mean Difference (IV, Random, 95% CI) | 3.30 [1.13, 5.47] |
| 15.4 Selenium plus N‐acetyl‐cysteine versus placebo at 26 weeks (6months) | 1 | 139 | Mean Difference (IV, Random, 95% CI) | 8.60 [6.28, 10.92] |
| 15.5 Coenzyme Q10 versus placebo at 6 months | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 6.88 [1.20, 12.56] |
| 15.6 Zinc plus folic acid versus placebo | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 17.70 [‐1.88, 37.28] |
| 15.7 Folic acid versus placebo | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 19.20 [4.74, 33.66] |
| 15.8 Zinc versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.70 [‐7.01, 26.41] |
| 16 Sperm concentration at 6 months(data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||
| 16.1 L‐carnitine + acetyl‐carnitine versus placebo | Other data | No numeric data | ||
| 16.2 Folic acid versus Placebo | Other data | No numeric data | ||
| 16.3 Zinc versus Placebo | Other data | No numeric data | ||
| 16.4 Zinc + folic acid versus placebo | Other data | No numeric data | ||
| 17 Sperm concentration at 9 months; type of antioxidant Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Carnitines versus placebo at 9 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 4.12 [‐1.74, 9.99] |
| 17.2 Coenzyme Q10 versus placebo at 9 months or more | 3 | 479 | Mean Difference (IV, Random, 95% CI) | 2.74 [‐1.56, 7.05] |
| 18 Sperm concentration over time Show forest plot | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Sperm concentration at 3 months or less | 13 | 746 | Mean Difference (IV, Random, 95% CI) | 5.32 [‐0.62, 11.26] |
| 18.2 Sperm concentration 6 months | 8 | 851 | Mean Difference (IV, Random, 95% CI) | 5.46 [1.81, 9.11] |
| 18.3 Sperm concentration at 9 months or more | 4 | 509 | Mean Difference (IV, Random, 95% CI) | 3.66 [‐0.31, 7.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total sperm motility at 3 months or less; type of antioxidant Show forest plot | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Ethylcysteine 600mg/day vs Vitamin E | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐41.97, 38.17] |
| 1.2 Docosahexaenoic acid (DHA) 400g/day vs Docosahexaenoic acid 800mg/day | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | 7.40 [‐11.35, 26.15] |
| 1.3 Vitamin C 200mg/day versus vitamin C 1000mg/day | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐43.0 [‐67.10, ‐18.90] |
| 1.4 Vitamin E + Selenium versus Vitamin B at 3 months | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐10.71, 10.71] |
| 1.5 Zinc versus Zinc + Vitamin E at 3 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐13.00, 13.00] |
| 1.6 Zinc versus Zinc + Vitamin E + Vitamin C at 3 months | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐19.66, 17.66] |
| 1.7 Zinc + Vitamin E versus Zinc + Vitamin E + Vitamin C at 3 months | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐18.97, 18.97] |
| 1.8 Selenium versus combined antioxidants | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐10.13, 16.53] |
| 1.9 L acetyl carnitine + L carnitine versus Vitamin E + Vitamin C | 1 | 138 | Mean Difference (IV, Fixed, 95% CI) | 23.05 [20.09, 26.01] |
| 1.10 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐7.74, 6.54] |
| 1.11 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐12.91, 7.31] |
| 1.12 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐14.21, 5.41] |
| 2 Total sperm motility at 6 months; type of antioxidant Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 L‐acetyl carnitine + L‐carnitine versus L‐carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐3.46 [‐9.72, 2.80] |
| 2.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐6.37, 7.65] |
| 2.3 Selenium versus N‐acetyl‐cysteine | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.56, 2.04] |
| 2.4 Selenium versus selenium plus N‐acetyl‐cysteine | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐3.85, ‐2.35] |
| 2.5 N‐acetyl‐cysteine vs selenium plus N‐acetyl‐cysteine | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐5.14, ‐3.66] |
| 2.6 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐5.45, 7.25] |
| 2.7 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐9.13, 3.93] |
| 2.8 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.42, 3.02] |
| 3 Total sperm motility at 6 months Show forest plot | Other data | No numeric data | ||
| 3.1 Zinc versus Folic acid | Other data | No numeric data | ||
| 3.2 Zinc versus Zinc + folic acid | Other data | No numeric data | ||
| 3.3 Folic acid versus Zinc + folic acid | Other data | No numeric data | ||
| 4 Total sperm motility at 9 months or more; type of antioxidant Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 L‐aceytl carnitine + L‐carnitine versus L‐carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐5.27 [‐11.28, 0.74] |
| 4.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐6.46, 3.32] |
| 5 Sperm concentration at 3 months or less; type of antioxidant Show forest plot | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Ethylcysteine 600mg/day vs Vitamin E | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [‐16.65, 21.05] |
| 5.2 Docosahexaenoic acid (DHA) 400g/day versus Docosahexaenoic acid (DHA) 800g/day | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐41.87, 28.27] |
| 5.3 L‐carnitine versus Vitamin E + Vitamin C | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 15.5 [12.49, 18.51] |
| 5.4 L‐carnitine plus vitamin E versus vitamin E | 1 | 113 | Mean Difference (IV, Fixed, 95% CI) | 1.90 [‐10.52, 14.32] |
| 5.5 Selenium versus combined antioxidants | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 14.70 [‐6.51, 35.91] |
| 5.6 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐4.20 [‐22.21, 13.81] |
| 5.7 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐18.63, 16.43] |
| 5.8 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐23.38, 12.78] |
| 6 Sperm concentration at 6 months; type of antioxidant Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 L‐aceytl carnitine +L‐carnitine versus L‐carnitine at 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐8.13 [‐21.79, 5.53] |
| 6.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine at 6 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.17 [‐15.26, 10.92] |
| 6.3 Selenium versus N‐acetyl‐cysteine at 26 weeks (6 months) | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.71, 2.31] |
| 6.4 Selenium versus selenium plus N‐acetyl‐cysteine at 26 weeks (6 months) | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐6.20, ‐2.80] |
| 6.5 N‐acetyl‐cysteine vs selenium plus N‐acetyl‐cysteine at 26 weeks | 1 | 234 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐6.86, ‐3.74] |
| 6.6 Zinc + folic acid versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐15.06, 12.06] |
| 6.7 Zinc versus zinc + folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐23.69, 7.69] |
| 6.8 Zinc versus folic acid | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐9.5 [‐20.31, 1.31] |
| 7 Sperm concentration at 6 months Show forest plot | Other data | No numeric data | ||
| 7.1 Zinc versus Folic acid | Other data | No numeric data | ||
| 7.2 Zinc versus Zinc + Folic acid | Other data | No numeric data | ||
| 7.3 Folic acid versus Zinc + folic acid | Other data | No numeric data | ||
| 8 Sperm concentration at 9 months or more; type of antioxidant Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 L‐acetyl carnitine + L‐carnitine versus L‐carnitine at 9 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐6.13 [‐15.99, 3.73] |
| 8.2 L‐acetyl carnitine + L‐carnitine versus L‐acetyl carnitine at 9 months | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.06 [‐6.09, 10.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total sperm motility at 3 months or less; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 12.77 [9.23, 16.31] |
| 2 Total sperm motility at 3 months or less (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||
| 2.1 Pentoxifylline versus placebo | Other data | No numeric data | ||
| 3 Total sperm motility at 6 months; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 229 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [9.09, 11.11] |
| 4 Total sperm motility at 6 months (data not suitable for meta analysis) Show forest plot | Other data | No numeric data | ||
| 4.1 Pentoxifylline versus placebo | Other data | No numeric data | ||
| 5 Total sperm motility at 9 months; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 221 | Mean Difference (IV, Random, 95% CI) | 3.10 [1.93, 4.27] |
| 6 Total sperm motility over time Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Total sperm motility at 3 months or less | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 12.77 [9.23, 16.31] |
| 6.2 Total sperm motility at 6 months | 1 | 229 | Mean Difference (IV, Fixed, 95% CI) | 10.10 [9.09, 11.11] |
| 6.3 Total sperm motility at 9 months or more | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [1.93, 4.27] |
| 7 Sperm concentration at 3 months or less; pentoxifylline versus placebo or no treatment Show forest plot | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 4.30 [‐0.69, 9.29] |
| 8 Sperm concentration at 6 months; pentoxifylline versus placebo Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9 Sperm concentration at 9 months; pentoxifylline versus placebo Show forest plot | 1 | 221 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.62, 2.78] |
| 10 Sperm concentration over time Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Sperm concentration at 3 months or less | 1 | 18 | Mean Difference (IV, Random, 95% CI) | 4.30 [‐0.69, 9.29] |
| 10.2 Sperm concentration at 6 months | 2 | 247 | Mean Difference (IV, Random, 95% CI) | 6.90 [‐0.09, 13.89] |
| 10.3 Sperm concentration at 9 months | 1 | 221 | Mean Difference (IV, Random, 95% CI) | 1.70 [0.62, 2.78] |
| 11 Adverse events Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 Vomiting | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.98 [1.32, 18.81] |
| 11.2 Dyspepsia | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.68 [1.15, 19.07] |
| 11.3 Headache | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.41 [0.54, 10.78] |
| 11.4 Diarrhoea | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.63 [1.30, 44.67] |
| 11.5 Tremor | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.45 [0.46, 119.73] |
| 11.6 Dizziness | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.45 [0.46, 119.73] |
| 11.7 Vertigo | 1 | 254 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.20, 18.99] |

