| Study | Interventions | Left study because of adverse events (n, %) | Hospitalised (n, %) | Symptoms reported (n, %) | Notes |
| Amsterdam 2009 | I1: black cohosh | I1: 1 (7%) | I1: NR | I1: light headedness (2, 15%), difficulty falling asleep (2, 15%), dry mouth (1, 8%), diaphoresis (1, 8%), pain (1, 8%), oedema, GI bloating (1, 8%), diarrhoea (1, 8%), abdominal cramping (1, 8%), vaginal bleeding (1, 8%), mid‐night wakening (1, 8%), anxiety (1, 8%). | |
| C1: placebo | C1: 0 (0%) | C1: NR | C1: menstrual flow (2, 15.4%), irritability (1, 8%), listlessness (1, 8%), flu symptoms (1, 8%), breast tenderness (1, 8%), constipation (1, 8%), vaginal spotting (1, 8%). | |
| | Total: 1 (4%) | Total: NR | | |
| Bai 2007 | I1: black cohosh | I1: 5 (6%) | I1: NR | I1: breast pain/enlargement (32, 21%), abdominal pain (12, 10%), vaginal bleeding (6, 5%), vaginal spotting (11, 7%), oedema (7, 5%), leucorrhoea (7, 6%) | |
| C1: tibolone | C1: 9 (7%) | C1: NR | C1: breast pain/enlargement (48, 35%), vaginal bleeding (40, 23%), abdominal pain (30, 24%), leucorrhoea (27, 18%), vaginal spotting (21, 13%), oedema (17, 12%) | |
| | Total: 14 (6%) | Total: NR | | |
| Bebenek 2010 | I1: exercise + black cohosh | I1: NR | I1: NR | I1: NR | |
| I2: exercise only | I2: NR | I2: NR | I2: NR | |
| C1: wellness control | C1: NR | C1: NR | C1: NR | |
| | Total: NR | Total: NR | | |
| Carlisle 2008 | I1: black cohosh + calcium and vitamin D supplement | I1: NR | I1: NR | I1: NR | |
| C1: placebo + calcium and vitamin D supplement | C1: NR | C1: NR | C1: NR | |
| Frei‐Kleiner 2005 | I1: black cohosh | I1: NR | I1: NR | I1: NR | |
| C1: placebo | C1: NR | C1: NR | C1: NR | |
| | Total: NR | Total: NR | | |
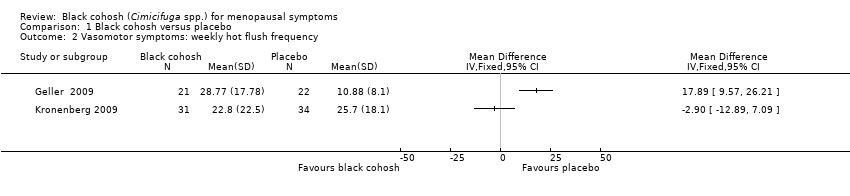
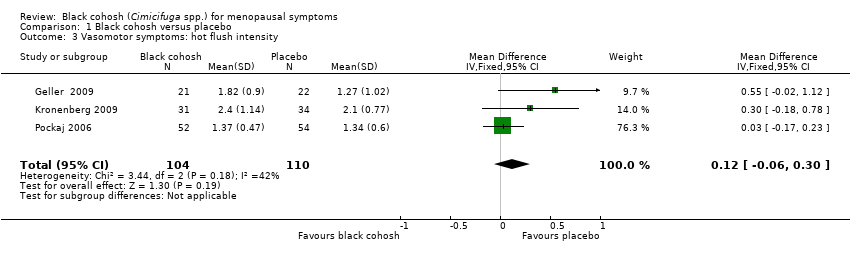
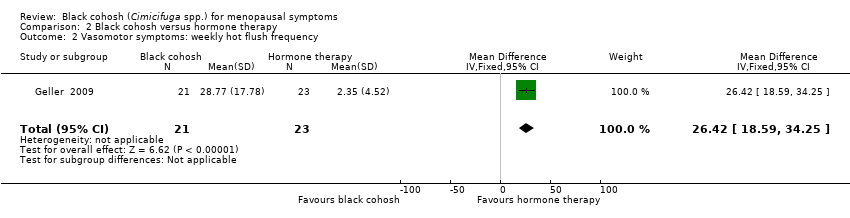
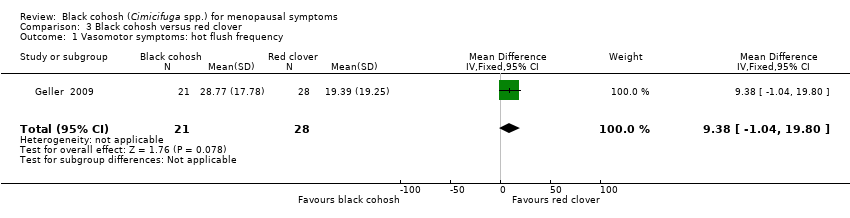
| Geller 2009 | I1: black cohosh | I1: 0 (0%) | I1: NR | I1: NR | |
| I2: red clover | I2: 0 (0%) | I2: NR | I2: NR | |
| I3: conjugated oestrogen + MDP | I3: 1 (4%) | I3: NR | I3: NR | |
| C1: placebo | C1: 0 (0%) | C1: NR | C1: NR | |
| | Total: | Total: NR | | |
| Jacobson 2001 | I1: black cohosh | I1: 3 (7%) | I1: NR | I1: hysterectomy (1, 2%), breast cancer recurrence (1, 2%), constipation (1, 2%), arrhythmia (1, 2%), weight gain (1, 2%), endometrial hyperplasia (1, 2%), dilatation and curettage (1, 2%), cramping (1, 2%), indigestion (1, 2%), vaginal bleeding (1, 2%) | The majority of participants were also taking tamoxifen |
| C1: placebo | C1: 1 (2%) | C1: NR | C1: appendectomy (1, 2%), swollen finger (1, 2%), abdominal rash (1, 2%) | |
| | Total: 4 (5%) | Total: NR | | |
| Kronenberg 2009 | I1: black cohosh | I1: NR | I1: NR | I1: upper respiratory infection (5, 8%), skin complaints (4, 7%), vaginal bleeding (4, 7%), vaginitis (1, 2%), abnormal ECG (2, 3%), increased endometrial thickness (3, 5%) | |
| C1: placebo | C1: NR | C1: NR | C1: upper respiratory infection (12, 18%), skin complaints (11, 16%), vaginitis (4, 6%), abnormal ECG (3, 4%), elevated liver enzymes (2, 3%), vaginal bleeding (1, 2%), increased endometrial thickness (1, 2%) | |
| | Total: NR | Total: NR | | |
| Lehmann‐Willenbrock 1988 | I1: black cohosh | | I1: NR | I1: NR | |
| C1: oestriol | C1: NR | C1: NR | C1: NR | |
| C2: conjugated oestrogen | C2: NR | C2: NR | C2: NR | |
| C3: oestradiol/ norethisterone acetate | C3: NR | C3: NR | C3: NR | |
| | Total: NR | Total: NR | | |
| Nappi 2005 | I1: black cohosh | I1: NR | I1: NR | I1: NA | |
| C1: oestradiol + dihydrogesterone | C1: NR | C1: NR | C1: vaginal spotting (2, 6%) | |
| | Total: NR | Total: NR | | |
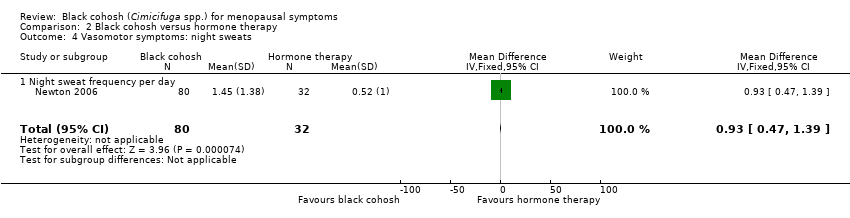
| Newton 2006 / Reed 2008 | I1: black cohosh | I1: NR | I1: NR | I1: menstrual disorders (10, NR), GI upset (12, NR), headache (12, NR), fatigue (12, NR), myalgia / arthralgia (11, NR) | |
| I2: multi‐botanical | I2: NR | I2: NR | I2: menstrual disorders (8, NR), breast discomfort (1, NR), GI upset (11, NR), headache (8, NR), fatigue (7, NR), myalgia / arthralgia (9, NR) | |
| I3: mult‐ibotanical + dietary soy | I3: NR | I3: NR | I3: menstrual disorders (14, NR), breast discomfort (2, NR), GI upset (8, NR), headache (12, NRI4: menstrual disorders (19, NR), breast discomfort (5, NR), GI upset (4, NR), headache (6, NR), fatigue (6, NR), myalgia / arthralgia (1, NR)), fatigue (12, NR), myalgia / arthralgia (9, NR) | |
| I4: conjugated oestrogen + MDP | I4: NR | I4: NR | I4: menstrual disorders (19, NR), breast discomfort (5, NR), GI upset (4, NR), headache (6, NR), fatigue (6, NR), myalgia / arthralgia (1, NR) | |
| C1: placebo | C1: NR | C1: NR | C1: menstrual disorders (17, NR), headache (16, NR), GI upset (13, NR), myalgia / arthralgia (10, NR), fatigue (8, NR), breast discomfort (3, NR) | |
| | Total: NR | Total: NR | | |
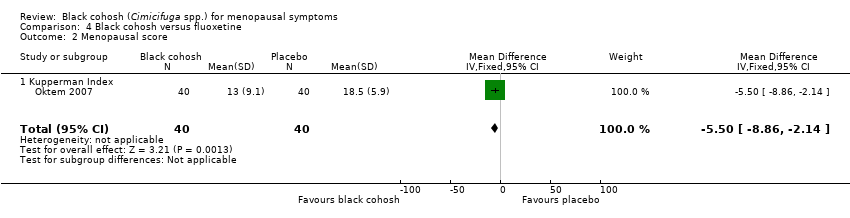
| Oktem 2007 | I1: black cohosh | I1: NR | I1: NR | I1: dyspepsia (2, 5%), constipation (2, 5%), tiredness (1, 3%), skin allergy (1, 3%), irritability (1, 3%) | |
| C1: fluoxetine | C1: NR | C1: NR | C1: dyspepsia (1, 3%), constipation (1, 3%), sleep disturbance (3, 8%), dry mouth (2, 5%), tiredness (2, 5%), skin allergy (2, 5%), irritability (1, 3%), headache (1, 3%) | |
| | Total: NR | Total: NR | | |
| Osmers 2005 | I1: black cohosh | I1: 7 (5%) | I1: NR | I1: musculoskeletal disorder (15, 10%) infection (13, 9%), GI disorder (8, 5%), nervous system disorder (4, 3%), reproductive / breast disorder (4, 3%), skin disorder (3, 2%), psychiatric disorder (2, 1%), tachycardia (2, 1%), metabolic / nutrition disorder (2, 1%), blood disorder (1, 1%), renal/urinary disorder (1, 1%), vascular disorder (1, 1%) | |
| C1: placebo | C1: 5 (3%) | C1: NR | C1: infection (19, 13%), musculoskeletal disorder (10, 7%) GI disorder (7, 5%), nervous system disorder (5, 3%), psychiatric disorder (5, 3%), reproductive / breast disorder (4, 3%), skin disorder (3, 2%), blood disorder (1, 1%), ear/labyrinth disorder (1, 1%), vascular disorder (1, 1%), respiratory disorder (1, 1%) | |
| | Total: 12 (4%) | Total: NR | | |
| Pockaj 2006 | I1: black cohosh | I1: NR | I1: NR | I1: NR | |
| C1: placebo | C1: NR | C1: NR | C1: NR | |
| | Total: NR | Total: NR | | |
| Stoll 1987 | I1: black cohosh | I1: 1 (3%) | I1: NR | I1: NR | |
| C1: oestrogen | C1: 2 (7%) | C1: NR | C1: NR | |
| C2: placebo | C2: 2 (10%) | C2: NR | C2: NR | |
| | Total: 5 (6%) | Total: NR | | |
| Wuttke 2003/2006a/2006b | I1: black cohosh | I1: 0 (0%) | I1: NR | I1: vaginal spotting (3, 15%), vertigo (1, 5%), hypertension (1, 5%), headache (1, 5%), bronchitis (1, 5%), rhinitis (1, 5%), viral infection (1, 5%) | |
| C1: conjugated oestrogens | C1: 0 (0%) | C1: NR | C1: bronchitis (2, 9%), toothache (2, 9%), vaginal spotting (1, 5%), diarrhoea (1, 5%), dermatitis (1, 5%), viral infection (1, 5%), elevated ALT (1, 5%) | |
| C2: placebo | C2: 0 (0%) | C2: NR | C2: vaginal spotting (2, 10%), hyperglycaemia (1, 5%), arthritis (1, 5%), local skin reaction (1, 5%), rhinitis (1, 5%), back pain (1, 5%), breast pain (1, 5%) | |
| | Total: 0 (0%) | Total: NR | | |