Terapia hormonal a largo plazo para pacientes perimenopáusicas y posmenopáusicas
Resumen
Antecedentes
La terapia hormonal (TH) se utiliza ampliamente para controlar los síntomas menopáusicos y también se ha utilizado para el tratamiento y la prevención de las enfermedades cardiovasculares, la osteoporosis y la demencia en mujeres mayores. Ésta es una actualización de una revisión Cochrane publicada por primera vez en 2005.
Objetivos
Evaluar los efectos de la TH a largo plazo (duración de al menos un año) sobre la mortalidad, los resultados cardiovasculares, el cáncer, la colecistopatía, las fracturas y la cognición en pacientes perimenopáusicas y posmenopáusicas durante y después de la interrupción del tratamiento.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos hasta septiembre de 2016: registro de ensayos del Grupo Cochrane de Ginecología y Fertilidad (Cochrane Gynaecology and Fertility Group Trials Register), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, Embase y PsycINFO. Se realizaron búsquedas en los registros de ensayos en curso y listas de referencias proporcionadas en estudios previos y revisiones sistemáticas.
Criterios de selección
Se incluyeron los estudios aleatorios doble ciego de TH versus placebo, con una duración de al menos un año, en pacientes perimenopáusicas o posmenopáusicas. La TH incluyó estrógenos, con o sin progestágenos, por vía oral, transdérmica, subcutánea o intranasal.
Obtención y análisis de los datos
Dos autores de la revisión, de forma independiente, seleccionaron los estudios evaluaron el riesgo de sesgo y extrajeron los datos. Se calcularon los cocientes de riesgos (CR) de los datos dicotómicos y las diferencias de medias (DM) de los datos continuos, con intervalos de confianza (IC) del 95%. Se evaluó la calidad de las pruebas mediante los métodos GRADE.
Resultados principales
Se incluyeron 22 estudios con 43 637 mujeres. Alrededor del 70% de los datos se obtuvieron de dos estudios bien realizados (HERS 1998; WHI 1998). La mayoría de las participantes eran ciudadanas estadounidenses posmenopáusicas con al menos algún grado de comorbilidad, y la edad media de las participantes en la mayoría de los estudios era de más de 60 años. Ninguno de los estudios se centró en mujeres perimenopáusicas.
En las mujeres posmenopáusicas relativamente sanas (generalmente con buen estado físico y sin enfermedad evidente), la TH combinada continua aumentó el riesgo de un evento coronario (después de un año de tratamiento: de 2 por 1000 a entre 3 y 7 por 1000), tromboembolia venosa (después de un año de tratamiento: de 2 por 1000 a entre 4 y 11 por 1000), accidente cerebrovascular (después de tres años de tratamiento: de 6 por 1000 a entre 6 y 12 por 1000), cáncer de mama (después de 5,6 años de tratamiento: de 19 por 1000 a entre 20 y 30 por 1000), colecistopatía (después de 5,6 años de tratamiento: de 27 por 1000 a entre 38 y 60 por 1000) y muerte por cáncer de pulmón (después de 5,6 años de tratamiento más 2,4 años adicionales de seguimiento: de 5 por 1000 a entre 6 y 13 por 1000).
La TH con estrógeno solo aumentó el riesgo de tromboembolia venosa (después de 1 a 2 años de tratamiento: de 2 por 1000 a 2 a 10 por 1000; después de siete años de tratamiento: de 16 por 1000 a 16 a 28 por 1000), accidente cerebrovascular (después de 7 años de tratamiento: de 24 por 1000 a entre 25 y 40 por 1000) y colecistopatía (después de 7 años de tratamiento: de 27 por 1000 a entre 38 y 60 por 1000) pero redujo el riesgo de cáncer de mama (después de 7 años de tratamiento: de 25 por 1000 a entre 15 y 25 por 1000) y fractura clínica (después de 7 años de tratamiento: de 141 por 1000 a entre 92 y 113 por 1000) y no aumentó el riesgo de eventos coronarios en ningún momento del seguimiento.
Las mujeres mayores de 65 años de edad que eran relativamente sanas y recibían la TH combinada continua presentaron un aumento de la incidencia de demencia (después de 4 años de tratamiento: de 9 por 1000 a 11 a 30 por 1000). En las pacientes con enfermedades cardiovasculares, la administración de TH combinada continua aumentó significativamente el riesgo de tromboembolia venosa (después de 1 año de tratamiento: de 3 por 1000 a entre 3 y 29 por 1000). Las pacientes que recibieron TH tuvieron una incidencia significativamente menor de fractura con el tratamiento a largo plazo.
El riesgo de fractura fue el único resultado para el que pruebas sólidas mostraron un beneficio clínico derivado de la TH (después de 5,6 años de TH combinada: de 111 por 1000 a entre 79 y 96 por 1000; después de 7,1 años de TH con estrógeno solo: de 141 por 1000 a entre 92 y 113 por 1000). No hubo pruebas sólidas de que la TH tuviera alguna repercusión clínicamente significativa en la incidencia de cáncer colorrectal.
Un ensayo analizó subgrupos de 2839 mujeres relativamente sanas, de entre 50 y 59 de años de edad, tratadas con TH combinada continua y 1637 con TH con estrógeno solo, versus grupos placebo de tamaños similares. El único aumento significativo del riesgo informado fue el de tromboembolia venosa en las mujeres con TH combinada continua: el riesgo absoluto permaneció bajo, por debajo de 1/500. Sin embargo, no se pueden descartar otras diferencias en el riesgo ya que este estudio no se diseñó con un poder estadístico capaz de detectar diferencias entre los grupos de mujeres diez años después de la menopausia.
En la mayoría de los estudios, el riesgo de sesgo fue bajo en la mayoría de los dominios. La calidad general de las pruebas para las comparaciones principales fue moderada. La limitación principal en la calidad de las pruebas fue que sólo cerca del 30% de las pacientes tenían de 50 a 59 años de edad al inicio del tratamiento, que es la edad a la cual las mujeres tienen grandes probabilidades de considerar la TH para los síntomas vasomotores.
Conclusiones de los autores
Es probable que las pacientes con síntomas menopáusicos intolerables deseen sopesar los beneficios del alivio de los síntomas con respecto al riesgo absoluto menor de efectos perjudiciales debido a la administración a corto plazo de la TH a dosis baja, siempre que no haya contraindicaciones específicas. La TH puede no ser apropiada en algunas mujeres, incluidas las que presentan un mayor riesgo de enfermedad cardiovascular y de enfermedad tromboembólica (como las pacientes con obesidad o antecedentes de trombosis venosa) o un mayor riesgo de algunos tipos de cáncer (como el cáncer de mama, en mujeres con útero). Está bien documentado el riesgo de cáncer endometrial en mujeres con útero que reciben TH con estrógeno solo.
La TH no está indicada para la prevención primaria ni secundaria de las enfermedades cardiovasculares o la demencia, ni para prevenir el deterioro de la función cognitiva en mujeres posmenopáusicas. Si bien la TH se considera efectiva para la prevención de la osteoporosis posmenopáusica, se suele recomendar como una opción sólo en las pacientes con un riesgo significativo, en quienes los tratamientos sin estrógenos no son apropiados. No hay suficientes datos para evaluar el riesgo de la TH a largo plazo en mujeres perimenopáusicas y posmenopáusicas menores de 50 años de edad.
PICO
Resumen en términos sencillos
Terapia hormonal a largo plazo para pacientes perimenopáusicas y posmenopáusicas
Pregunta de la revisión
¿Cuáles son los efectos clínicos de utilizar la terapia hormonal (TH) por un año o más en las mujeres perimenopáusicas y posmenopáusicas?
Antecedentes
La TH se administra para el control de los síntomas menopáusicos. También se ha utilizado para el tratamiento y la prevención de enfermedades crónicas como las enfermedades cardiovasculares, la osteoporosis y la demencia.
Características de los estudios
Esta revisión incluye 22 ensayos controlados aleatorios (ECA) doble ciego (43 637 mujeres). Las pruebas están actualizadas hasta septiembre 2016.
Resultados clave
En las mujeres posmenopáusicas relativamente sanas, la administración de TH combinada continua por un año aumentó el riesgo de un ataque cardíaco de cerca de 2 por 1000 a entre 3 y 7 por 1000, y aumentó el riesgo de trombosis venosa (coágulo sanguíneo) de cerca de 2 por 1000 a entre 4 y 11 por 1000. Con la administración por más tiempo, la TH también aumentó el riesgo de accidente cerebrovascular, cáncer de mama, colecistopatía y muerte por cáncer de pulmón.
La TH con estrógeno solo aumentó el riesgo de tromboembolia venosa (después de 1 a 2 años de tratamiento: de 2 por 1000 a 2 a 10 por 1000. Con la administración por más tiempo también aumentó el riesgo de accidente cerebrovascular y colecistopatía, pero se redujo el riesgo de cáncer de mama (después de siete años de tratamiento) de 25 por 1000 a entre 15 y 25 por 1000.
Entre las mujeres con más de 65 años de edad que recibieron TH combinada continua aumentó la incidencia de demencia.
El riesgo de fracturas fue el único resultado para el cual hubo pruebas sólidas de un beneficio clínico con la TH (ambos tipos).
Es probable que las pacientes con síntomas menopáusicos intolerables deseen sopesar los beneficios del alivio de los síntomas con respecto al riesgo absoluto menor de efectos perjudiciales debido a la administración a corto plazo de la TH a dosis baja, siempre que no haya contraindicaciones específicas. La TH puede no ser apropiada en algunas mujeres, incluidas las que presentan un mayor riesgo de enfermedad cardiovascular y de enfermedad tromboembólica (como las pacientes con obesidad o antecedentes de trombosis venosa) o un mayor riesgo de algunos tipos de cáncer (como el cáncer de mama, en mujeres con útero). Está bien documentado el riesgo de cáncer endometrial en mujeres con útero que reciben TH con estrógeno solo.
La TH no se indica para la prevención primaria ni secundaria de las enfermedades cardiovasculares ni la demencia, ni para prevenir el deterioro de la función cognitiva en mujeres posmenopáusicas. Si bien la TH se considera efectiva para la prevención de la osteoporosis posmenopáusica, se suele recomendar como una opción sólo en las pacientes con un riesgo significativo, en quienes los tratamientos sin estrógenos no son apropiados. No hay suficientes datos para evaluar el riesgo de la TH a largo plazo en mujeres perimenopáusicas o posmenopáusicas menores de 50 años de edad.
Calidad de la evidencia
En la mayoría de los estudios el riesgo de sesgo fue bajo en la mayoría de los dominios y la calidad general de las pruebas fue moderada. La limitación principal fue que sólo cerca del 30% de las mujeres tuvieron de 50 a 59 años de edad al inicio del tratamiento, la edad a la que las mujeres tienen la probabilidad de considerar la TH para los síntomas vasomotores.
Conclusiones de los autores
Summary of findings
| Combined continuous hormone therapy (HT) compared with placebo for perimenopausal and postmenopausal women | ||||||
| Population: relatively healthy postmenopausal women Setting: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk* | Corresponding risk | |||||
| Placebo | Combined continuous hormone therapy (HT) | |||||
| Coronary events (MI or cardiac death) Follow‐up: mean/median 1 year | 2 per 1000 | 4 per 1000 | RR 1.89 | 20,993 | ⊕⊕⊕⊝ | |
| Stroke | 6 per 1000 | 8 per 1000 | RR 146 | 17,585 | ⊕⊕⊕⊝ | |
| Venous thromboembolism (DVT or PE) Follow‐up: mean/median 1 year | 2 per 1000 | 7 per 1000 | RR 4.28 | 20,993 | ⊕⊕⊕⊝ | |
| Breast cancer | 19 per 1000 | 24 per 1000 | RR 1.27 (1.03 to 1.56) | 16,608 | ⊕⊕⊕⊝ | |
| Death from lung cancer Follow‐up: median 8 yearsb | 5 per 1,000 | 9 per 1000 (6 to 13) | RR 1.74 (1.18 to 2.55) | 16,608 | ⊕⊕⊕⊝ | |
| Gallbladder disease Follow‐up: mean 5.6 years | 16 per 1000 | 27 per 1000 (21 to 34) | RR 1.64 (1.30 to 2.06) | 14,203 (1 study) | ⊕⊕⊕⊝ | |
| All clinical fractures | 111 per 1000 | 87 per 1000 | RR 0.78 | 16,608 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level for questionable applicability: Only about 33% of the study sample was 50‐59 years of age at baseline (i.e. the age women are most likely to consider HT for vasomotor symptoms); mean participant age was 63 years. b5.6 years' intervention plus postintervention follow‐up: post hoc analysis. | ||||||
| Oestrogen‐only hormone therapy (HT) compared with placebo for perimenopausal and postmenopausal women | ||||||
| Population: relatively healthy postmenopausal women Setting: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oestrogen‐only hormone therapy (HT) | |||||
| Coronary events (MI or cardiac death) | 41 per 1000 | 38 per 1000 | RR 0.94 | 10,739 | ⊕⊕⊕⊝ | |
| Stroke | 24 per 1000 | 32 per 1000 | RR 1.33 | 10,739 | ⊕⊕⊕⊝ | |
| Venous thromboembolism (DVT or PE) Follow up 1‐2 years | 2 per 1000 | 5 per 1000 (2 to 10) | RR 2.22 (1.12 to 4.39) | 10,739 | ⊕⊕⊕⊝ | |
| Venous thromboembolism (DVT or PE): CEE 0.625 mg (moderate dose) | 16 per 1000 | 21 per 1000 | RR 1.32 | 10,739 | ⊕⊕⊕⊝ | |
| Breast cancer | 25 per 1000 | 20 per 1000 | RR 0.79 | 10,739 | ⊕⊕⊕⊝ | |
| Gallbladder disease Follow‐up: mean 7.1 yearsa | 27 per 1000 | 47 per 1000 (38 to 60) | RR 1.78 (1.42 to 2.24) | 8376 | ⊕⊕⊕⊝ | |
| All clinical fractures | 141 per 1000 | 103 per 1000 | RR 0.73 | 10,739 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aMedian use of CEE 5.9 years (LaCroix 2011). | ||||||
Antecedentes
Descripción de la afección
La mediana de edad de la aparición de la menopausia varía entre las regiones geográficas. En Europa, varía de cerca de 50 a 53 años, en Norteamérica de 50 a 51 años, en América Latina de 44 a 53 años y en Asia de 42 a 49 años (Palacios 2010). La mayoría de las mujeres presenta la menopausia (última menstruación) después de una fase de cambios en la función ovárica (perimenopausia) que puede durar varios años y que se caracteriza por ciclos menstruales irregulares (Greendale 1999). Se considera que una mujer es posmenopáusica cuando transcurren 12 meses desde la última menstruación. Muchas mujeres perimenopáusicas y posmenopáusicas (aunque no todas) informan diversos síntomas que incluyen sofocos y resequedad vaginal, los que probablemente se relacionan con la disminución natural en los niveles de estrógeno. Los síntomas tienden a fluctuar y su gravedad varía enormemente de una persona a otra, algunas experimentan un malestar intenso y una reducción apreciable de la calidad de vida. La mayoría de las investigaciones se han centrado en mujeres de raza blanca, pero la presentación de la menopausia varía según la raza y el grupo étnico, y también según el estadio menopáusico (Avis 2001; Palacios 2010). La duración de los sofocos regulares es muy variable. La mayoría de las pacientes informan que los sofocos persisten de seis meses a dos años (Kronenberg 1994), pero estudios de investigación longitudinales indican que el tiempo desde la aparición hasta la resolución de los síntomas a menudo es considerablemente más prolongado (Guthrie 2005).
Descripción de la intervención
La terapia hormonal (TH) consiste en estrógeno solo (TH con estrógeno solo) o estrógeno combinado con un progestágeno (TH combinada). Se utiliza en varias formulaciones y dosis que pueden administrarse por vía oral, vaginal, transnasal o en forma de implante, parche cutáneo, crema o gel. Los efectos clínicos varían según el tipo de TH y el tiempo de administración.
El agregado de un progestágeno reduce el riesgo de hiperplasia de endometrio asociada con la administración de estrógeno solo en las mujeres con útero (Furness 2012), pero el tema es problemático porque los progestágenos tienen efectos adversos sobre los lípidos sanguíneos y es posible que puedan causar síntomas como cefalea, timpanismo abdominal y sensibilidad mamaria (McKinney 1998). Los progestágenos usados en la TH incluyen los derivados sintéticos de la progesterona, los derivados sintéticos de la testosterona y las progesteronas naturales derivadas de plantas. Éstos difieren en cuanto a la acción metabólica y a la posibilidad de efectos adversos; además, todavía no está claro qué tipo de progestágeno posee el mejor perfil riesgo‐beneficio para su empleo en la TH. En la TH combinada, el progestágeno se puede tomar de forma continua (todos los días), secuencial (en una parte de cada mes) o con una frecuencia menor.
La terapia hormonal (TH) se ha utilizado por más de 50 años para el tratamiento de las pacientes con sofocos y otros síntomas menopáusicos y su eficacia está bien establecida, como se demostró en una revisión sistemática Cochrane de 24 estudios controlados aleatorios de TH para los sofocos que se publicó entre 1971 y 2000 (MacLennan 2004).
Durante los últimos 25 años, la TH también se ha utilizado para el tratamiento o la prevención de las enfermedades crónicas. Los estrógenos y los progestágenos afectan a la mayoría de los sistemas corporales y se han propuesto como agentes potencialmente causales o preventivos de un grupo amplio de enfermedades. Las recomendaciones sobre su uso han variado con el tiempo, pero en la década de 1990 la opinión de los especialistas dominante era que la mayoría de las mujeres posmenopáusicas podrían beneficiarse con la TH (Hemminki 2000a). Este criterio se basó en pruebas observacionales sólidas y consistentes acerca de que la TH reducía el riesgo de cardiopatía coronaria en al menos el 30%. Un metanálisis de 25 estudios de cohortes, de casos y controles y angiográficos publicados hasta 1997, informó una cociente de riesgos de cardiopatía coronaria de 0,70 (intervalo de confianza [IC] del 95%: 0,65 a 0,75) en las usuarias de estrógenos en comparación con las que nunca los usaron.
Otros efectos beneficiosos informados en los estudios observacionales de TH fueron las pruebas sólidas de reducción de fracturas osteoporóticas, un posible efecto preventivo o retardador del deterioro cognitivo o de la demencia, y una reducción de la mortalidad global de las usuarias (Barrett‐Connor 1998).
De qué manera podría funcionar la intervención
El estrógeno tiene un efecto favorable sobre algunos marcadores biológicos, incluidos los indicadores de enfermedades cardiovasculares y trastornos del metabolismo óseo. Han mostrado mejorar la función vasodilatadora endotelial, estimular la angiogénesis y modular la función autonómica. Por lo tanto los efectos beneficiosos cardioprotectores del estrógeno tienen cierta plausibilidad biológica ( Miller 2008). Sin embargo, los marcadores biológicos interactúan mediante vías complejas múltiples y el efecto general del estrógeno sobre los resultados clínicos no se puede predecir con certeza. Por lo tanto, se necesitan ensayos con variables principales de evaluación clínica como el infarto de miocardio (IM) (Banks 2009a).
Por qué es importante realizar esta revisión
Los estudios observacionales también indicaron una serie de efectos adversos de la TH, que incluían una duplicación o triplicación del riesgo de eventos tromboembólicos, un aumento significativo del riesgo de cáncer endometrial en las pacientes que tomaban estrógeno sin progestágeno, una mayor incidencia de colecistopatía y una posible conexión entre la TH y el cáncer de mama. La sugerencia de que la TH podría aumentar el riesgo de cáncer de mama fue apoyada por pruebas de un aumento de la densidad mamaria en una elevada proporción de pacientes que recibían estrógenos, pero los hallazgos fueron inconsistentes y polémicos (Barrett‐Connor 1998). Los resultados de un estudio observacional muy grande realizado en el Reino Unido (Beral 2003) suscitaron inquietudes acerca de que las usuarias actuales de TH combinada y con estrógeno solo tuvieron un mayor riesgo de incidencia de cáncer de mama y cáncer de mama mortal después de períodos relativamente cortos de administración. El aumento del riesgo fue mayor para las usuarias de TH combinada, sin grandes variaciones informadas entre los efectos de los estrógenos específicos y los progestágenos específicos. Los riesgos fueron mayores si la administración de TH comenzó alrededor del momento de la menopausia con respecto a si comenzó después. Las tasas de cáncer de mama fueron más altas entre las usuarias actuales de TH combinada que comenzaron su uso en el transcurso de cinco años de la menopausia (Beral 2011).
La cardiopatía coronaria es la causa más frecuente de mortalidad y de morbilidad en las mujeres mayores y se ha señalado que la reducción significativa del riesgo de cardiopatía coronaria asociada a la TH superaría los posibles efectos adversos. Sin embargo, estos estudios no controlados mostraron una fuerte posibilidad de sesgo de selección o de cumplimiento, o ambos, ya que es más probable que las mujeres que reciben estrógenos sean mujeres sanas, cultas y cumplan con el tratamiento, con un riesgo inicial inferior de enfermedades cardiovasculares. Se ha reconocido la necesidad de realizar ensayos controlados aleatorios (Barrett‐Connor 2001; Hemminki 2000a). Se ha indicado que la amplia prescripción de la TH en la década de 1990, a pesar de la falta de pruebas de estudios aleatorios sobre su eficacia y su seguridad, quizás refleje el conflicto entre los grupos de interés comerciales y profesionales y una adecuada política pública (Hemminki 2000). Los ensayos controlados aleatorios (ECA) no lograron demostrar los notables beneficios de la TH en la cardiopatía coronaria advertidos en los estudios observacionales, y plantearon dudas acerca del perfil riesgo/beneficio global.
Otras revisiones Cochrane encontraron pruebas sólidas acerca de la efectividad de la TH para el tratamiento de las mujeres con síntomas menopáusicos. Una revisión informó una reducción del 75% en la frecuencia de sofocos en mujeres perimenopáusicas y posmenopáusicas que recibieron TH en comparación con placebo, y también encontró una reducción estadísticamente significativa en la gravedad de los síntomas en el grupo de TH (odds ratio [OR] 0,13; IC del 95%: 0,07 a 0,23) (MacLennan 2004). Otra revisión encontró que los estrógenos locales eran más efectivos para el alivio de los síntomas de la atrofia vaginal en las mujeres posmenopáusicas que el placebo o el gel no hormonal (Suckling 2006). Sin embargo, las mujeres que consideran el uso de la TH para los síntomas menopáusicos deben estar al tanto de los hallazgos negativos en otras áreas, que se mencionan a continuación.
Revisiones sistemáticas Cochrane previas y venideras de TH en mujeres perimenopáusicas y posmenopáusicas explorarán los siguientes temas.
-
Enfermedades cardiovasculares (Boardman 2015).
-
Demencia y función cognitiva (Hogervorst 2009; Lethaby 2008).
-
Hiperplasia endometrial (Furness 2012).
-
Sofocos (MacLennan 2004).
-
Prolapso de órganos pélvicos (Ismail 2010).
-
Función sexual (Nastri 2012).
-
Incontinencia urinaria (Cody 2009).
-
Atrofia vaginal (Suckling 2006).
-
Peso y distribución de la grasa corporal (Kongnyuy 1999).
Debido al gran número de revisiones sobre aspectos individuales de la TH, los autores de la revisión reconocieron la necesidad de una revisión sistemática que proporcionara un resumen de todos los resultados clínicos relevantes a largo plazo, para proporcionar ayuda a las mujeres y a los médicos que deben emitir valoraciones informadas acerca de la administración de la TH. Se tomó la decisión a priori de excluir los ensayos de una duración inferior a un año y de no incluir como resultados el control de los síntomas menopáusicos, los efectos secundarios de aparición precoz de la TH y las medidas substitutas como la hiperplasia endometrial y la densidad mineral ósea. Esta revisión no pretende reemplazar otras revisiones Cochrane sobre la TH, como las mencionadas anteriormente. Éstas son aún una fuente importante de pruebas sobre los aspectos individuales de la TH y seguirán actualizándose de forma regular.
Ésta es una versión actualizada de la revisión Cochrane original publicada en 2005.
Objetivos
Evaluar los efectos de la TH a largo plazo (duración de al menos un año) sobre la mortalidad, los resultados cardiovasculares, el cáncer, la colecistopatía, las fracturas y la cognición en pacientes perimenopáusicas y posmenopáusicas durante y después de la interrupción del tratamiento.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Se incluyeron los estudios aleatorios, doble ciego, que proporcionaron un cegamiento a las participantes y a todos los investigadores y los evaluadores de resultado.
Para los ensayos cruzados, se procuró utilizar solamente los resultados del final de la primera fase (antes del tratamiento de cruzamiento) debido al posible efecto residual de la primera fase de la TH. Sin embargo, no se identificaron estudios cruzados para inclusión.
Tipos de participantes
Las participantes elegibles fueron mujeres perimenopáusicas o posmenopáusicas reclutadas de cualquier ámbito de asistencia sanitaria o bien una muestra poblacional.
Las mujeres perimenopáusicas se definieron como las que todavía no habían tenido su período menstrual final pero estaban en el período de transición entre ciclos más o menos regulares de ovulación y menstruación y el cese completo de estos ciclos.
Las mujeres posmenopáusicas se definieron como mujeres con menopausia quirúrgica (extirpación de ambos ovarios) y mujeres con menopausia espontánea y amenorrea de más de 12 meses.
Los estudios incluyeron a mujeres con y sin antecedentes previos de enfermedad (p.ej. enfermedades cardiovasculares, fractura, osteoporosis).
Tipos de intervenciones
Todos los estrógenos, con y sin progestágenos, administrados por vía oral, transdérmica, subcutánea o intranasal, como tratamiento perimenopáusico o posmenopáusico por cualquier motivo durante 12 o más meses, en comparación con placebo.
Criterios de exclusión
Se excluyeron los estudios con cointervenciones que podrían afectar potencialmente los resultados medidos y los estudios de cremas vaginales tópicas de TH, comprimidos tópicos y anillos. Estas intervenciones son tratadas en otra revisión Cochrane (Suckling 2006).
La justificación para la exclusión de los ensayos con una duración menor de un año es que se consideró que era poco probable que dichos ensayos fueran suficientemente prolongados para que los investigadores informaran eventos clínicos relacionados con la intervención.
Tipos de medida de resultado
Sólo se consideraron los estudios que informaron al menos uno de los siguientes resultados para su inclusión en esta revisión.
-
Muerte por cualquier causa (mortalidad total).
-
Mortalidad por causas específicas.
-
Eventos coronarios (infarto de miocardio o muerte coronaria).
-
Accidente cerebrovascular (isquémico o hemorrágico) o ataque isquémico transitorio (AIT).
-
Tromboembolia venosa (embolia pulmonar o trombosis venosa profunda).
-
Cáncer de mama.
-
Cáncer colorrectal.
-
Cáncer de pulmón.
-
Cáncer endometrial.
-
Cáncer de ovario.
-
Colecistopatía.
-
Fracturas (fractura de cadera, fractura vertebral diagnosticada clínicamente, fractura total diagnosticada clínicamente).
-
Función cognitiva (con el uso de medidas globales) o demencia (incluida enfermedad de Alzheimer) como se midió en los estudios incluidos.
Se planificó restringir el foco a los resultados clínicos a largo plazo y no incluir el control de los síntomas menopáusicos ni los efectos secundarios de aparición temprana de la TH como resultados. La TH para el control de los sofocos es el tema de otra revisión sistemática (MacLennan 2004).
La inclusión se limitó a los estudios que informaron uno de los resultados de interés porque la TH se puede estudiar en la misma población para diferentes propósitos y se deseaba asegurar que se incluyeran solamente los estudios relevantes.
Results
Description of studies
Results of the search
Results of search to 2012
We retrieved 57 studies through searches conducted up to 2012 and considered them for inclusion. We included 23 studies and excluded 34 studies.
Search update 2017
We screened 3046 records, discarded 3041 as clearly irrelevant and retained 45 articles, which we checked in full text. From these 44 articles, we identified two new studies: KEEPS 2012 (15 articles) and ELITE 2014 (5 articles). We also identified 20 articles that were additional publications related to studies already included (19 articles for WHI 1998 and one article for EPHT 2006) and five studies that we excluded (AHT 2015; Paoletti 2015; Rasgon 2014; Schierbeck 2012; SMART 2016).
For this update of the review, we also excluded three studies that were included in previous versions of the review but that no longer meet our eligibility criteria because we have decided to report fewer outcomes (Haines 2003; Nielsen 2006; Pefanco 2007). See Differences between protocol and review.
Thus we have included 22 studies and have excluded 42 studies from this review (see Figure 2 for study flow).

Study flow diagram.
Included studies
The 22 eligible studies are based on one very large study (WHI 1998). WHI 1998 incorporated randomised comparisons of two different HT regimens versus placebo and published these results separately. One study (WHI 2002) compared combined oestrogen and progesterone versus placebo and is referred to in this review as WHI 1998 (combined HT arm); the other compared oestrogen‐only HT versus placebo and is referred to in this review as WHI 1998 (oestrogen‐only HT arm). WHI 1998 also included a subgroup study known as the Women's Health Initiative Memory Study (WHIMS), which measured cognitive outcomes in older women (aged 65 to 79 years at study entry) from both arms of WHI 1998 and is referred to in this review as WHI 1998 (WHIMS) (Shumaker 1998). An additional ancillary study ‐ WHI 1998 (WHISCA) ‐ enrolled women from WHI 1998 (WHIMS) who were free of dementia to investigate the effects of HT on domain‐specific cognitive function in older women (Resnick 2004).
The 22 identified studies included 43,637 randomised women: 22,693 randomised to some form of HT and 20,928 to placebo (treatment allocation was unclear for 16 women in one study (Ferenczy 2002)). WISDOM 2007 included 1307 additional women who were randomised to a comparison of two active hormone therapies but are not included in this review. Investigators analysed results for more than 99% of these women by intention to treat. Although some studies used biological measures as their primary outcome (e.g. lumen of carotid artery), we included them because they also reported clinical endpoints relevant to this review as prespecified secondary outcomes.
The studies varied dramatically in size. The largest was WHI 1998, which randomised 27,347 participants, and the other studies varied in sample size from 40 (Tierney 2009) to 5692 (WISDOM 2007) participants. Investigators included 8000 women in each group in WHI 1998 (combined HT arm) and more than 5000 in each group in WHI 1998 (oestrogen‐only HT arm), along with more than 1400 in each group on the oestrogen‐only HT arm of WHI 1998 (WHIMS) and more than 2200 in each group on the combined arm of WHI 1998 (WHIMS). HERS 1998 included about 1380 women in each comparison group, ESPRIT 2002 included more than 500 in each group, EPHT 2006 included around 400 women in each group and KEEPS 2012 included 220 to 275 per group. Otherwise, none of the studies included more than 210 women in each group. Five of the smaller studies were single‐centred (ELITE 2014; EPAT 2001; Nachtigall 1979; Obel 1993; Tierney 2009), and it is unclear whether one study (EVTET 2000) enlisted more than one trial centre. The other 10 studies involved between 7 and 40 trial centres.
Fourteen studies were conducted in the USA, and one in each of the following countries: UK, Estonia, Norway, Canada and Denmark; three studies were international in scope (one in the USA and Canada, one in Canada and the Netherlands and one in the UK, Australia and New Zealand). Two studies (EPHT 2006; WISDOM 2007) were originally planned as part of a larger international project, but planning was beset with delays, and in the meantime, WHI 1998 began in the USA when other countries were no longer prepared to commit funds to a second study with similar objectives. Both of these studies were prematurely closed as a result of publication of early WHI 1998 findings.
We attempted to contact investigators for the following studies to request more information about their methods or outcomes: Barakat 2006; ELITE 2014; EPAT 2001; EVTET 2000; Ferenczy 2002; HERS 1998; KEEPS 2012, Mulnard 2000; Notelovitz 2002; Obel 1993; PEPI 1995; WAVE 2002; WEST 2001; WHI 1998, WISDOM 2007. Investigators from the following studies kindly supplied clarification or additional unpublished data, or both: Barakat 2006; ELITE 2014; ERA 2000; EPHT 2006; HERS 1998; Obel 1993; PEPI 1995; WAVE 2002; WISDOM 2007.
Participants
The women included in these studies were predominantly postmenopausal, spontaneously or surgically. The age of participants ranged from 26 to 91 years, with mean or median age of each study ranging from 48 to 76 years (no age was stated in Obel 1993). In more than half of the studies, mean participant age was over 60 years. Inclusion criteria varied according to the primary objectives of individual studies. Some were designed to investigate the use of HT for treatment of women with menopausal symptoms or for disease prevention and thus enrolled women in reasonably good health. Others were designed to assess whether HT was beneficial for women with a history of cancer or established disease, including heart disease, thromboembolic disease, stroke, Alzheimer's disease or long‐term medical conditions requiring hospitalisation; these studies restricted entry to women who had received a diagnosis of the condition of interest.
Studies of women without established medical conditions
Thirteen studies enrolled relatively healthy women (ELITE 2014; EPAT 2001; EPHT 2006; Ferenczy 2002; Greenspan 2005; KEEPS 2012, Notelovitz 2002; Obel 1993; PEPI 1995; Tierney 2009; WHI 1998; WISDOM 2007; Yaffe 2006). Women in some of these studies had risk factors (such as raised cholesterol), and a small minority within individual studies had a history of cardiovascular disease, but most participants were fit women without overt disease. Most of these studies were interested in the use of HT for disease prevention.
Three studies were large and investigated the use of HT to prevent cardiovascular disease while also reporting a wide range of other endpoints; researchers provided highly detailed lists of inclusion and exclusion criteria (PEPI 1995; WHI 1998, WISDOM 2007). In WHI 1998, enrolment was targeted to establish set fractions for baseline age categories and to achieve representation of racial and ethnic groups in the proportions recorded by the US census for individuals 50 to 79 years of age.
The WHI 1998 (combined HT arm) investigators noted that prevalence of prior cardiovascular disease in participants was low: 4.4% had a history of myocardial infarction, coronary revascularisation, stroke or transient ischaemic attack. They also commented that levels of cardiovascular risk factors were consistent with a generally healthy population of postmenopausal women: 2.9% reported a history of angina, 36% were hypertensive (or were being treated for hypertension), 13% were being treated for high cholesterol, 4.4% were being treated for diabetes and 10.5% were current smokers (Manson 2003). Similarly, in WHI 1998 (oestrogen‐only HT arm), participants in general were considered healthy, although 4.1% had a history of myocardial infarction or coronary revascularisation, 5.8% had a history of angina, 1.4% had a history of stroke,1.6% had a history of venous thrombosis, 48% were hypertensive (or were being treated for hypertension), 15% were receiving treatment for high cholesterol, 7.7% were being treated for diabetes and 10.5% were current smokers (Stefanick 2003).
PEPI 1995 compared the characteristics of their cohort with values returned in large US surveys and concluded that although the PEPI 1995 cohort was generally in better health than the wider US population, these individuals were not so markedly different as to limit the generalisability of study results. Both KEEPS 2012 and ELITE 2014 were designed to test whether menopausal HT initiated soon after menopause could delay progression of atherosclerosis. Two other 'prevention' studies aimed to test the possible beneficial effects of HT on arterial wall density (EPAT 2001) and bone density (Notelovitz 2002). Four much smaller studies also enrolled women without stated health problems who were in early menopause (Obel 1993) or were postmenopausal and aimed to assess the effects of HT on endometrial safety (Ferenczy 2002; Obel 1993) and other clinical outcomes (Greenspan 2005; Tierney 2009).
WISDOM 2007 recruited women with no known major health problems from general practice registers in countries with free or low fee healthcare systems. Investigators designed recruitment to target older women first; as a result, median participant age was 63 years and few women in the younger age group were included when the study closed prematurely.
Studies of women with established medical conditions or a history of cancer
Six studies included women with established cardiovascular disease (ERA 2000; ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002; WEST 2001). ERA 2000 and WAVE 2002 included women who had coronary artery stenosis evident on angiogram. HERS 1998 and ESPRIT 2002 randomised women who had had a myocardial infarction or (in the case of HERS 1998) coronary artery surgery. EVTET 2000 and WEST 2001 included women who had had a thromboembolic (pulmonary embolism (PE) or deep vein thrombosis (DVT)) or cerebrovascular event (stroke or TIA). The largest of these six studies (HERS 1998) compared its cohort of women with a similar group of women presumed to have coronary heart disease, who were participants in a survey designed to produce nationally representative data: The HERS 1998 cohort included significantly fewer smokers, women with hypertension and women with diabetes than the comparison group, but individuals were comparable with respect to blood pressure, body mass index, physical activity and cholesterol levels.
One study (Mulnard 2000) included women with Alzheimer's disease, and an older study (Nachtigall 1979) included women with a range of medical conditions such as diabetes, need for custodial care, arteriosclerosis and chronic neurological disorders: All participants in this study were hospitalised for the duration of the 10‐year study.
One study enrolled women after surgery (including bilateral salpingo‐oophorectomy) for early‐stage endometrial cancer (Barakat 2006).
Interventions
The included studies used a wide variety of oestrogen‐alone or oestrogen and progestogen combinations as interventions; some included more than one intervention arm, each with a different dose, formulation or route of HT. Most comparisons used a moderate dose of oestrogen (e.g. oestradiol 1 mg, conjugated equine oestrogen (CEE) 0.625 mg daily, transdermal oestradiol 0.05 mg twice weekly). Nachtigall 1979 used a much higher dose than the other included studies, reflecting the fact that it was conducted many years earlier than the others.
The range of interventions used follows here.
Oestrogen‐only HTs
These included the following.
-
Oestradiol (17‐B oestradiol), an oestrogen derived from Mexican wild yam, 1 mg orally (ELITE 2014; EPAT 2001; WEST 2001).
-
Oestradiol valerate, which is a pro‐drug for oestradiol (meaning that it is converted in the body into the active form); the dose used was 2 mg (ESPRIT 2002).
-
Transdermal oestradiol skin patches; doses used were 0.014 mg (Yaffe 2006) and 0.025 mg, 0.05 mg or 0.075 mg daily (Notelovitz 2002).
-
Intranasal 17‐B oestradiol, delivered by a puff via each nostril once a day, at a dose of 0.15 mg or 0.3 mg daily (Nielsen 2006).
-
Conjugated equine oestrogen (CEE), a blend of equine oestrogens; 0.625 mg (Barakat 2006; ERA 2000; Greenspan 2005; Mulnard 2000; PEPI 1995; WAVE 2002; WHI 1998 (oestrogen‐only HT arm)) and 1.25 mg daily (Mulnard 2000). One study (Barakat 2006) allowed doubling of the dose for women who were symptomatic. WISDOM 2007 also included an oestrogen‐only arm, but the comparison group was taking combined therapy, and this comparison is not relevant to this review.
Most studies using oestrogen‐only HT did not randomise women to this comparison unless they had had a hysterectomy (Greenspan 2005; Mulnard 2000; Nachtigall 1979; Notelovitz 2002; WAVE 2002; WEST 2001; WHI 1998 (oestrogen‐only HT arm)).
Combined HT regimens
Combined regimens included one of the above types of oestrogen in combination with one of the following progestogens.
-
Medroxyprogesterone acetate (MPA), a synthetic progestogen structurally related to progesterone.
-
Dydrogesterone, a synthetic progestogen structurally related to progesterone.
-
Norethisterone (norethindrone), a synthetic progestogen structurally related to testosterone.
-
Micronised progesterone, a natural progestogen synthesised from plant sources and finely ground to improve its absorption.
-
Drosperinone, a synthetic progestogen structurally related to spironolactone.
Continuous combined regimens
These included the following.
-
CEE 0.625 mg with MPA 2.5 mg daily (EPHT 2006; ERA 2000; Greenspan 2005; HERS 1998; PEPI 1995; WAVE 2002; WHI 1998 (combined arm); WISDOM 2007).
-
CEE 2.5 mg with MPA 10 mg daily (Nachtigall 1979).
-
Oestradiol 2 mg with 1 mg norethisterone daily (EVTET 2000).
Combined sequential regimens
These included the following.
-
Oestradiol 1 mg daily with MPA 5 mg for 12 days once a year (WEST 2001).
-
Oestradiol 1 mg daily for 4 days, oestradiol 1 mg plus 0.35 mg norethindrone daily for 3 days each week (Tierney 2009).
-
Oestradiol 2 mg days 1 to 22, 1 mg days 22 to 28, with norethisterone 1 mg days 13 to 22 (Obel 1993).
-
Oestradiol 1 mg daily with dydrogesterone 5 mg or 10 mg days 14 to 28 (Ferenczy 2002).
-
Oestradiol 2 mg daily with 10 to 20 mg dydrogesterone days 14 to 28 (Ferenczy 2002).
-
Oestradiol 0.05 mg patch with cyclic micronised progesterone 200 mg daily for 12 days a month (KEEPS 2012).
-
CEE 0.425 mg daily with cyclic micronised progesterone 200 mg daily for 12 days a month (KEEPS 2012).
-
CEE 0.625 with MPA 10 mg days 1 to 12 (PEPI 1995).
-
CEE 0.625 mg with micronised progesterone 200 mg days 1 to 12 (PEPI 1995).
-
Oral oestradiol 1 mg daily, plus 40 mg cyclic micronised progesterone as 4% vaginal gel for 10 days per 30‐day cycle for women with an intact uterus only (ELITE 2014).
The control arm of each study received placebo tablets, patches or nasal spray, as appropriate.
The duration of HT use varied, with the longest study lasting 10 years (Nachtigall 1979). Three studies reported outcomes after HT use for around 1 year (EVTET 2000; Mulnard 2000; WISDOM 2007); seven measured outcomes after 2 years (EPAT 2001; ESPRIT 2002; Ferenczy 2002; Notelovitz 2002; Obel 1993; Tierney 2009; Yaffe 2006), eight at around 3 years (Barakat 2006; EPHT 2006; ERA 2000; Greenspan 2005; PEPI 1995; WAVE 2002; WEST 2001) and 1 at 4 years (KEEPS 2012). HERS 1998 measured outcomes after 4.1 years and continued the study unblinded for 2.7 additional years. ELITE 2014 measured outcomes after 2.5 years and subsequently at 5 years of HT use.
Investigators planned that interventions in the WHI study would continue for 8.5 years, but both arms of the study were terminated early. WHI 1998 (combined HT arm) was stopped early owing to net harm. Researchers reported outcomes at 5.6 years and over 4 subsequent months of follow‐up for primary and selected outcomes, incorporating events up to the date that participants were instructed to stop their study pills. WHI 1998 (oestrogen‐only HT arm) was also stopped early when it was decided that the prospect of obtaining more precise evidence about effects of the intervention was unlikely to outweigh potential harms, although no predefined safety boundaries had been crossed. Investigators reported results in the oestrogen‐only arm for a mean follow‐up of 7.1 years for primary outcomes: Median time receiving treatment was 5.9 years in the intervention group and 5.8 years in the placebo group. Additional poststudy follow‐up occurred in WHI 1998, as noted below.
Two other studies also closed prematurely in response to WHI 1998 findings (EPHT 2006; WISDOM 2007).
Outcomes
The outcomes measured by individual studies varied according to study objectives. Major clinical events were not primary outcomes for several of these studies but were measured as adverse effects, for example, cardiovascular events or the incidence of cancer and fracture in the study population, or both. Eight studies used biological measures as their primary outcome (ELITE 2014; EPAT 2001; ERA 2000; KEEPS 2012; Notelovitz 2002; PEPI 1995; WAVE 2002; Yaffe 2006).
The largest study in the review (WHI 1998) was concerned mainly with the cardioprotective role of HT in relatively healthy women, and study authors reported cardiovascular clinical endpoints as the primary outcome. They designated invasive breast cancer as a primary adverse outcome and included the incidence of other cancers, fractures, gallbladder disease and death as secondary outcomes. Two other studies (EPHT 2006; WISDOM 2007) measured similar outcomes.
WHI 1998 also conducted a number of analyses not specified in the study protocol. Lung cancer was not a prespecified outcome but was investigated in both arms of the study in post hoc analyses, which included additional follow‐up periods after the planned completion date of the study.
After the intervention phase of WHI 1998 had been completed, investigators followed up major clinical outcomes in surviving participants (i.e. those who consented), comprising 78% of participants in the oestrogen‐only arm and 83% in the combined HT arm. Median cumulative follow‐up (intervention phase plus extended follow‐up) was 13.2 years in the oestrogen‐only arm (including median postintervention follow‐up of 6.6 years) and 13 years in the combined HT group (including median postintervention follow‐up of 6.6 years) (Manson 2013).
WHI 1998 (WHIMS) comprised a large subset of older women from WHI 1998 who were evaluated for probable dementia (the planned primary outcome) and for mild cognitive impairment (as a planned secondary outcome). Researchers also reported global cognitive function, although this was not a formally preplanned endpoint. WHI 1998 (WHIMS) reported separate results for the two study arms and also pooled study results, but we did not include the pooled results in this review (see Methods).
Two smaller studies reported endometrial cancer as a primary outcome (Barakat 2006; Ferenczy 2002), and two (Obel 1993; Tierney 2009) reported as primary outcomes clinical events that were not of interest for this review, but researchers measured outcomes of interest as adverse events.
Five other studies were concerned with the effect of HT on established clinical disease. Four reported cardiovascular outcomes: Primary outcomes were myocardial infarction or death (ESPRIT 2002; HERS 1998), thromboembolism (EVTET 2000) and stroke (WEST 2001). The larger studies also measured a range of other major clinical events such as the incidence of cancer, fracture and gallbladder disease (ESPRIT 2002; HERS 1998). One study reported the effect of HT on global cognitive function (Greenspan 2005) and one on progression of symptoms in women with Alzheimer's disease (Mulnard 2000); another study measured a wide range of clinical outcomes over a treatment period of 10 years with HT in women who were receiving long‐term hospital care for a range of medical conditions (Nachtigall 1979).
Excluded studies
We excluded 42 studies from this review for the following reasons.
-
29 reported no outcomes of interest for this review.
-
5 were not double‐blinded.
-
4 used an intervention of less than 1 year's duration or reported only short‐term (3‐month) outcomes.
-
3 did not include a placebo group.
-
1 used a co‐intervention in the HT group.
See Excluded studies.
Risk of bias in included studies

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Seventeen of the 22 studies described a satisfactory method of randomisation, which in all cases was computer generated. Sixteen described a satisfactory method of allocation concealment: In these studies, researchers entered information about an eligible participant, or they accomplished this via remote contact between the recruiting centre and the study coordinating centre or pharmacy. One of these studies (EPHT 2006) randomised women who expressed an interest in participating but did not open the randomisation envelope until their eligibility had been checked and they had consented. Two studies described using computer‐generated randomisation but did not provide details of the procedure for allocation to treatment (EVTET 2000; Mulnard 2000). Three studies supplied no detailed information about randomisation nor allocation concealment (Ferenczy 2002; Nachtigall 1979; Notelovitz 2002).
We rated 17 studies as having low risk of bias related to sequence generation and 16 as having low risk of bias related to allocation concealment. We rated remaining studies as having unclear risk of bias in these domains.
Blinding
All studies described themselves as (at least) double‐blinded. Eighteen studies explicitly stated that all participants, clinical staff and outcome assessors or research staff were blinded to treatment allocation, or they reported 'hard' outcomes unlikely to be influenced by blinding. In the WHI study, 331 women randomised to receive active treatment were unblinded and changed arms from WHI 1998 (oestrogen‐only HT arm) to WHI 1998 (combined HT arm) according to a change in protocol. Three studies apparently blinded participants and clinical staff but did not explicitly state whether outcomes assessors were also blinded (Mulnard 2000; Obel 1993; Tierney 2009)
The larger studies described an unblinded mechanism to be used when required for management of adverse effects. PEPI 1995 unblinded 39 women (4%) during the course of the study, 32 of whom were taking oestrogen‐only HT. WHI 1998 (combined HT arm) reported that during 5.6 years of follow‐up, 3444 women in the combined HT group (40%) and 548 women in the placebo group (6%) were unblinded; whereas in WHI 1998 (oestrogen‐only HT arm), only 100 women in the active group (< 2%) and 83 in the placebo group (< 2%) were unblinded. Nachtigall 1979 reported that 13 women in the HT group and 17 in the control group were unblinded. Two women were unblinded in WISDOM 2007. The other studies did not report such information.
One randomised blinded study (HERS 1998) completed 4.1 years of follow‐up and was then extended for a further duration 2.7 years unblinded.
We rated all studies as having low risk of performance bias and 19 as having low risk of detection bias. We rated three studies as having unclear risk of detection bias.
Incomplete outcome data
For the purposes of this review, we defined losses to follow‐up as participants for whom outcomes of interest were unknown (and who may or may not have had outcomes imputed in statistical analysis). We defined drop‐outs as participants who stopped their allocated treatment (and in some cases changed to a different off‐trial treatment) but had known clinical outcomes and were included in the analysis. Adherence to treatment refers to the number of tablets actually taken, which we often assessed by pill counts (Table 1). We defined intention to treat as analysis of all randomised participants in the groups to which they were randomised.
| Study | How defined | Assessment | HT group | Placebo group | Note |
| Discontinuation of therapy for longer than a month (or use of HT in placebo group) | Not stated | 41.1% compliant for whole follow‐up period (median 3 years) | 50.1% compliant for whole follow‐up period (median 3 years) | ||
| > 80% of prescribed treatment taken | Pill counts | Median > 98% over median of 5 years | Median > 98% over median of 5 years | ||
| Percentage of study medication consumed | Pill counts | Level of adherence 95% in the 87% of participants evaluated | Level of adherence 92% in the 92% of participants evaluated | ||
| > 80% of prescribed treatment taken | Number of collected and returned drugs and clinic reports | < 40% compliant at 3 years (estimated from graph) | < 30% compliant at 3 years (estimated from graph) | ||
| Percentage of study medication taken | Pill counts | Level of adherence at 3.2 years: Women on combined HRT, measured in 82% of participants only: 84% | Level of adherence at 3.2 years: | ||
| "Regular tablet use" | Self‐report to family doctor. Self‐report to study nurse at 6 weeks and whenever in contact with trial staff | Number non‐adherent: | Number non‐adherent: | Triallists attribute higher non‐compliance in HRT group to prevalence of vaginal bleeding (reported by 56% in HRT group, 7% in controls) | |
| Adherence not described | |||||
| Adherence not described | |||||
| "Taking at least 80% of medication for at least 80% of entire study period" | Pill counts 6‐monthly | 90% adherent at 3 years | 94% adherent at 3 years | ||
| Taking at least 80% of study medication | Pill counts | 79% adherent at 1 year | 91% adherent at 1 year | Proportion of women who reported taking study medication at 1 year: | |
| Pill or patch counts, percentage used | Pill counts or weights | 94%‐95% in all groups, among women who completed trial at 4 years | |||
| Taking at least 80% of study medication | Plasma oestradiol level evaluation at each visit | No information given in publication | |||
| Adherence not described | |||||
| Adherence not described | |||||
| Adherence not described | |||||
| Taking at least 80% of study medication | Study diary reviewed at clinic visits | Number adherent at 36 months: Women with uterus: | Number adherent at 36 months: Women with uterus: 76% | ||
| Taking at least 80% of study medication | Pill counts weekly | No information given in publication | |||
| Percentage of study medication taken | Pill counts | At 2.8 years: | At 2.8 years: | ||
| Percentage of study medication taken | Self‐report to study nurse 3‐monthly | At 2.8 years: Mean adherence excluding dropouts: 90% | At 2.8 years: Mean adherence excluding dropouts: 90% 24% discontinued medication | ||
| WHI 1998 (unopposed oestrogen arm) | Taking at least 80% of study medication. Temporary discontinuation (e.g. during surgery) permitted | Weighing of returned medication bottles | At 6.8 years, about 53.8% of women were non‐adherent | At 6.8 years, about 53.8% of women were non‐adherent | |
| WHI 1998 (combined arm) | Taking at least 80% of study medication. Temporary discontinuation (e.g. during surgery) permitted | Weighing of returned medication bottles | 42% non‐adherent by 5.2 years | 10.7% crossed to active treatment by 5.2 years | Analyses censoring events 6 months after non‐adherence increased effect sizes |
| Supply of study medication | Time at risk minus temporary interruptions and time after withdrawal from treatment | 73% of time | 86% of time | Women had a 3 month run‐in period on placebo. Only women who took 80% of tablets were randomised | |
| Supply of study medication | Patch counts: 75% use over 2 years counted as compliance | 84% | 84% of time | Women had a 1 week run‐in period. Only compliant women were randomised. |
Drop‐out rates were generally high, particularly in the active treatment groups, and they increased over time. In WHI 1998 (combined HT arm), 42% of the active treatment group and 38% of the placebo group were no longer taking their allocated treatment at 5 years, and a further 10.7% of the placebo group had crossed to active therapy. In WHI 1998 (oestrogen‐only HT arm), 53% of participants overall were no longer taking their allocated treatment at 6.8 years, and a further 5.7% had initiated hormone use outside the study. See the Characteristics of included studies table and Table 1 for details on drop‐outs and non‐adherence in other studies.
Losses to follow‐up were low in most studies, with no women lost to follow‐up in seven studies (EPAT 2001; ERA 2000; ESPRIT 2002; EVTET 2000; Mulnard 2000; Nachtigall 1979; WEST 2001), and 1% to 5.2% lost in five other studies, all of which were large and of long duration (3 to 6.8 years) (Greenspan 2005; HERS 1998; PEPI 1995; WAVE 2002; WHI 1998). Only five women (0.01%) were lost to follow‐up in WISDOM 2007. The Estonian study monitored outcomes by means of linkages to a national health insurance database and national cancer registry, and study authors stated that the probability of missing data in these databases was small (EPHT 2006). However, different publications for this study (EPHT 2006) reported slightly different numbers of randomised participants. In six smaller studies of 1 to 5 years' duration, a higher proportion of women (8.5% to 21%) were lost to follow‐up (ELITE 2014; KEEPS 2012; Notelovitz 2002; Obel 1993; Tierney 2009; Yaffe 2006), and in Ferenczy 2002, results were unavailable for 34% of participants for the outcome of interest for this review. It was unclear whether any women were lost to follow‐up in Barakat 2006 (see Description of studies).
Fourteen of the included studies supplied sufficient data to permit an intention‐to‐treat (ITT) analysis, at least for all reported outcomes of interest for this review (EPAT 2001; ERA 2000; ESPRIT 2002; EVTET 2000; Greenspan 2005; HERS 1998; KEEPS 2012; Mulnard 2000; Nachtigall 1979; Notelovitz 2002; WEST 2001; WHI 1998; WISDOM 2007; Yaffe 2006), or such data were extractable, and a further two studies analysed more than 97% of participants by intention to treat (PEPI 1995; WAVE 2002). Five studies did not include all participants in an ITT analysis for outcomes of interest (ELITE 2014; EVTET 2000; Ferenczy 2002; Obel 1993; Tierney 2009). It was unclear whether one study used ITT analysis because investigators provided no description of participants other than those that were "eligible and assessable" (Barakat 2006), and one study had slightly differing participation rates across trial publications (EPHT 2006).
WHI 1998 (combined HT arm) and WHI 1998 (WHISCA) continued follow‐up beyond the planned study completion date (March 2005) for women who consented to continue follow‐up. All women had already been instructed to stop taking their assigned study medication in July 2002. Seventeen per cent of surviving women in WHI 1998 (combined HT arm) declined to provide re‐consent, and their data were censored for the additional follow‐up period. Baseline characteristics were similar in the two groups, and imputation analyses suggested that this loss to follow‐up did not significantly influence study findings. Fifteen per cent of women in WHI 1998 (WHISCA) declined to continue follow‐up. The study extension phase ran from April 2005 to September 2010. WHI 1998 (oestrogen‐only HT arm) also conducted extended follow‐up (in 78% of surviving participants) from April 2005 to September 2010; among women who provided additional consent, baseline characteristics were similar to those of the original randomised group.
We rated 16 studies as having low risk of attrition bias, four as having unclear risk and three as having high risk.
Selective reporting
All studies reported all expected outcomes, and we rated them as having low risk of selective reporting.
Other potential sources of bias
Eleven of the included studies had other potential sources of bias (ELITE 2014; EPHT 2006; ERA 2000; Greenspan 2005; Mulnard 2000; Nachtigall 1979; Obel 1993; PEPI 1995; Tierney 2009; WAVE 2002; WHI 1998); we rated them as havng unclear risk of this bias. In most cases, potential bias was related to baseline imbalance between participants in individual prognostic characteristics and did not appear likely to have a marked effect on outcomes. We rated the other studies as having low risk of bias in this domain.
Effects of interventions
See: Summary of findings for the main comparison Combined continuous hormone therapy (HT) compared with placebo for postmenopausal women; Summary of findings 2 Oestrogen‐only hormone therapy (HT) compared with placebo for postmenopausal women
We present the results below. In most cases, details of effect measures are reported in the text only when results were statistically significant. For full results of all comparisons, see Data and analyses. See also summary of findings Table for the main comparison and summary of findings Table 2.
We grouped results as follows.
-
By outcome.
-
We grouped outcomes such as death, cardiovascular events, cognitive measures and quality of life according to the clinical status of participant groups, in the following order: relatively healthy women, women with a history of cardiovascular disease, women hospitalised with chronic illness and women with dementia.
-
For outcomes such as cancer, fracture and gallbladder disease, we grouped all participants together as 'all women'.
-
-
By intervention.
-
Oestrogen‐only HT.
-
Combined continuous HT regimens.
-
Combined sequential regimens.
-
Within these categories, we have grouped interventions according to the oestrogen dose used, with equivalence between doses based on the Australian Menopause Society guide to equivalent HT doses (AMS 2016), which classifies HT as low dose (e.g. oral oestradiol 1 mg), medium dose (e.g. oral oestradiol 2 mg, transdermal oestradiol 50 µg, conjugated equine oestrogen 0.065 mg) or higher dose (e.g. transdermal oestradiol 75 µg).
Meta‐analysis
Although comparisons with similar oestrogen doses are grouped together, we pooled comparisons (meta‐analysed) only if they used the same combination of oestrogen and progestogen for the same (or a similar) length of time. WHI 1998 and PEPI 1995 used the same HT regimen and reported several of the same clinical outcomes at 3 years, but in most cases, PEPI 1995 reported no events in either arm. We combined three studies (ERA 2000; HERS 1998; WAVE 2002) for some 3‐year (2.8 to 3.2) outcomes, but otherwise meta‐analysis was inappropriate for most outcomes because the studies used different types or doses of oestrogen or progestogen, or both, and these do not necessarily have the same metabolic effects; or they used different durations of HT, which might have led to different effects as the result of trends over time.
Very few results were suitable for pooling; therefore, statistical heterogeneity was not a major issue in this review. One meta‐analysis displayed statistically significant heterogeneity (I2 = 66.2%), but it involved only two small studies with few events, and we attributed the heterogeneity to chance (Analysis 2.21).
Time points for reporting results
In some cases, we rounded up or down time points for reporting results, as follows.
-
WHI 1998 (oestrogen‐only HT arm) reported results after a mean follow‐up of 7.1 or 7.9 years. Among women who consented (78% of those surviving), follow‐up was extended (for a median of 6.6 years) after the predefined study termination date to achieve a cumulative median follow‐up of 13.2 years. The median duration of active treatment in this arm of the study was 5.8 to 5.9 years (LaCroix 2011). We have reported results at mean or median follow‐up points as reported by the study publications.
-
WHI 1998 (combined HT arm) reported results after a mean of 5.6 years of active treatment (intervention phase) or at a mean of 7.9 years. The 7.9‐year follow‐up included 2.4 years of postintervention follow‐up and continued up to the predefined study termination date (31 March 2005). Among women who consented (83% of those surviving), follow‐up was extended after the predefined study termination date for a median of 6.6 years to achieve median cumulative follow‐up of 13 years. This arm of the study also reported selected clinical outcomes for each year of follow‐up: All women had been enrolled for at least 3.5 years at the time of the study publication, so we used these data to calculate outcomes on an ITT basis after 1, 2 and 3 years of use of HT, with all randomised participants inserted as the denominator (Chlebowski 2009). We have reported results at mean or median follow‐up points as reported by the study publications.
-
EPHT 2006 reported results for most outcomes at a mean follow‐up of 3.43 years, with a range of 2 to 5 years. Results for quality of life were reported at a mean of 3.6 years. We have reported results in our tables as if all women underwent 3 years of follow‐up.
-
WISDOM 2007 reported results after a median follow‐up of 11.9 months (range 7.1 to 19.6). We have reported results in our tables as if all women had undergone 1 year's follow‐up.
-
Barakat 2006 reported results after a median follow‐up of 35.7 months. We have reported results in our tables as if all women had undergone 3 years of follow‐up.
-
HERS 1998 reported results from the blinded portion of the study after a mean follow‐up of 4.1 years, which we mentioned above (see Methods). These results were presented as dichotomous data, and investigators reported selected clinical outcomes for each year of follow‐up. All women had been enrolled for at least 3 years at the time of the report, so for this review, we have used these data to calculate outcomes on an ITT basis after 1, 2 and 3 years of HT use, with all randomised participants inserted as the denominator.
Results for outcomes of interest
We derived all of the statistically significant findings of this review from the two biggest studies ‐ HERS 1998 and WHI 1998 ‐ both of which reported adequate methods of allocation concealment, analysed all participants by intention to treat and reported small losses to follow‐up (1% to 5.2%).
1. Death from any cause (total mortality)
Relevant comparisons
Seven studies (ELITE 2014; EPHT 2006; EPAT 2001; KEEPS 2012; PEPI 1995; WHI 1998; WISDOM 2007) with a total of eight different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome in healthy women.
Five studies of women with cardiovascular disease (ERA 2000; ESPRIT 2002; HERS 1998; WAVE 2002; WEST 2001) with a total of four different interventions, comprising comparisons of oestrogen‐only HT and combined continuous HT versus placebo for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured death from any cause.
Two other studies measured this outcome: one comparing oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer (Barakat 2006), and one (Nachtigall 1979) comparing combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
Results of analysis show no statistically significant difference between HT and placebo for this outcome in any population group (Analysis 1.1; Analysis 1.2; Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 4.1; Analysis 5.1).
2. Cause‐specific mortality
2.1 Death from coronary heart disease
Relevant comparisons
Four studies (EPAT 2001; Tierney 2009; WHI 1998; WISDOM 2007) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome in relatively healthy women.
Five studies of women with cardiovascular disease (ERA 2000; ESPRIT 2002; HERS 1998; WAVE 2002; WEST 2001) with a total of four different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured death from coronary heart disease.
In addition, the study comparing oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer measured this outcome (Barakat 2006).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome in any population group (Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 4.3).
2.2 Death from stroke
Relevant comparisons
Four comparisons of relatively healthy women taking combined continuous HT for 1 year (WISDOM 2007) and for 5.6 years (WHI 1998 (combined HT arm)), or taking oestrogen‐only HT for 7.1 years (WHI 1998 (oestrogen‐only HT arm)) or taking combined sequential HT for 2 years (Tierney 2009), reported this outcome. One study of women with a history of stroke who were taking oestrogen‐only HT (with annual progesterone for women who had a uterus) for 2.8 years (WEST 2001) also reported this outcome.
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 1.7; Analysis 1.9; Analysis 1.8; Analysis 2.8).
2.3 Death from breast cancer
Relevant comparisons
One study of comparatively healthy women taking oestrogen‐only HT for a median of 7.2 years (WHI 1998 (oestrogen‐only HT arm)) with postintervention follow‐up for a median of 4.7 years reported this outcome, as did two studies of relatively healthy women taking combined continuous HT for 1 year (WISDOM 2007) and for 5.6 years (WHI 1998). Follow‐up for breast cancer outcomes was continued for a mean total of 11 years among women in WHI 1998 (combined HT arm) who agreed to continue follow‐up after the planned study completion date (Chlebowski 2010).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome at 1 or 5.6 years.
Among women taking oestrogen‐only HT, after a median of 11.8 years (7.2 years' intervention plus postintervention follow‐up), the death rate from breast cancer was lower in the HT arm (risk ratio (RR) 0.38, 95% confidence interval (CI) 0.15 to 0.98) (WHI 1998 (oestrogen‐only HT; Analysis 1.12).
At 11 years' follow‐up, WHI 1998 (combined HT arm) reported more deaths from breast cancer in the HT group than in the placebo group; this finding was of borderline statistical significance (RR 1.98, 95% CI 1.00 to 3.95; Analysis 1.11). Absolute risk of breast cancer increased from 1 per 1000 in the control group to 3 per 1000 (95% CI 1 to 6) in the HT group.
At 11 years' follow‐up, researchers also found that significantly more deaths resulted from all causes after a breast cancer diagnosis in the combined HT group than in the placebo group (published hazard ratio (HR) 1.57, 95% CI 1.01 to 2.48; P = 0.045) (Chlebowski 2010).
2.4 Death from colorectal cancer
Relevant comparisons
Investigators reported this outcome in relatively healthy women in the oestrogen‐alone group of WHI 1998 after mean follow‐up of 7.1 years, as well as in the WHI 1998 (combined HT arm) at mean follow‐up of 5.6 and 7.1 years. Researchers also reported on this after 11.6 years' follow‐up, including a mean of 5.6 years' intervention plus postintervention follow‐up after the study ended, in 83% of participants (Simon 2012).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 1.10; Analysis 1.13).
2.5 Death from endometrial cancer
Relevant comparisons
The study comparing oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer reported this outcome (Barakat 2006).
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 4.2).
2.6 Death from lung cancer
Relevant comparisons
WHI 1998 reported this outcome in relatively healthy women in the oestrogen‐only HT group in a post hoc analysis after mean follow‐up of 7.9 years (including 8 months' follow‐up post intervention) (Chlebowski 2010b), and in the combined HT arm of WHI 1998 in a post hoc analysis after mean follow‐up of 8 years (including 2.4 years' follow‐up post intervention) (Chlebowski 2009). Study authors reported lung cancer overall, non‐small cell lung cancer and small cell lung cancer separately. One much smaller study (Tierney 2009) reported this outcome in women taking combined sequential HT or placebo.
Results
Results of analysis show no statistically significant differences between HT and placebo for any of these outcomes among women in the oestrogen‐only HT arm of WHI 1998 (Analysis 1.14). However, in the combined HT arm of WHI 1998, women in the intervention group were significantly more likely to die of lung cancer overall (RR 1.74, 95% CI 1.18 to 2.55), or of non‐small cell lung cancer (RR 1.91, 95% CI 1.24 to 2.93), than women in the placebo arm (Analysis 1.15). Absolute risk of lung cancer increased from 5 per 1000 in the control group to 9 per 1000 (95% CI 6 to 13) in the HT group. This finding was independent of smoking status. The mortality rate for small cell lung cancer did not differ significantly between groups. Review authors noted no statistically significant findings in the combined sequential HT study (Analysis 1.16).
2.7 Death from any cancer
Relevant comparisons
Two studies of relatively healthy women taking continuous HT for 1 year (WISDOM 2007) and for 5.6 years (WHI 1998 (combined HT arm)) and one study of women with cardiovascular disease taking combined continuous HT for 4.1 years, with unblinded follow‐up to 6.8 years (HERS 1998) reported this outcome.
Results
Results of analysis showed no statistically significant differences between HT and placebo for this outcome (Analysis 1.17; Analysis 2.9).
3. Coronary events (myocardial infarction or cardiac death)
Relevant comparisons
Eight studies (ELITE 2014; EPAT 2001; EPHT 2006; KEEPS 2012; PEPI 1995; Tierney 2009; WHI 1998, WISDOM 2007) with a total of nine different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for varying durations from 1 year to over 7 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm) (LaCroix 2011) and to 13.2 years in the combined HT arm (Manson 2013), reported this outcome in relatively healthy women.
Six studies (ERA 2000; ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002; WEST 2001) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured coronary events as an outcome in women with cardiovascular disease.
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
WHI 1998 (oestrogen‐only HT arm) reported no statistically significant difference between the two groups for this outcome (Analysis 1.18). However, WHI 1998 (combined HT arm) reported that relatively healthy women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) were at significantly higher risk of a coronary event after taking HT for 1, 2 and 3 years (at 1 year: RR 1.74 (95% CI 1.05 to 2.89); at 2 years: RR 1.49 (95% CI 1.05 to 2.12); at 3 years: RR 1.43 (95% CI 1.05 to 1.95)). At mean follow‐up of 5.6 years, researchers noted no statistically significant differences between groups (RR 1.17, 95% CI 0.95 to 1.44), and they observed no differences between groups after extended follow‐up to 13.2 years. WISDOM 2007 and EPHT 2006 reported data for this outcome at 1 year and at 3 years, respectively. Pooling these data with data from WHI 1998 (combined HT arm) resulted in a risk ratio at 1 year of 1.89 (95% CI 1.15 to 3.10) and at 3 years of 1.45 (95% CI 1.07 to 1.98; Analysis 1.19). Absolute risk of a coronary event increased after 1 year from 2 per 1000 in the control group to 4 per 1000 (95% CI 3 to 7) in the HT group; after 2 years from 6 per 1000 in the control group to 9 per 1000 (95% CI 7 to 13) in the HT group; and after 4 years from 8 per 1000 in the control group to 11 per 1000 (95% CI 8 to 13) in the HT group.
No other studies found statistically significant differences between HT and placebo for this outcome (Analysis 1.20; Analysis 2.10; Analysis 2.7; Analysis 2.11; Analysis 2.12; Analysis 5.2). HERS 1998 reported results of borderline statistical significance at 1 year, suggesting increased risk for women with cardiovascular disease taking combined continuous therapy (RR 1.5, 95% CI 1.00 to 2.25; Analysis 2.12), and initial analysis of time trends in HERS 1998 suggested a trend towards increased risk in the HT group that diminished over time. However, subsequent analysis based on the entire 6.8 years of follow‐up (blinded and unblinded) showed no statistically significant variation in risk over time.
4. Stroke and transient ischaemic attack
4.1 Stroke
Relevant comparisons
Six studies (EPAT 2001; EPHT 2006; KEEPS 2012; PEPI 1995; Tierney 2009; WHI 1998) with a total of seven different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, reported this outcome in relatively healthy women; WHI 1998 (oestrogen‐only arm) extended follow‐up to 10.7 years, and Manson 2013 extended follow‐up to 13.2 years in the combined HT arm.
Five studies (ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002; WEST 2001) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), measured this outcome in women with cardiovascular disease.
Results
WHI 1998 (oestrogen‐only HT arm) reported a statistically significant increase in the incidence of stroke at 7.1 years' follow‐up (RR 1.33, 95% CI 1.06 to 1.67; Analysis 1.22). Absolute risk of a stroke increased from 23 per 1000 in the control group to 32 per 1000 (95% CI 25 to 40) in the HT group. Study authors noted that the excess in the intervention arm was due to increased risk of ischaemic rather than haemorrhagic stroke, and that the excess risk became apparent after 4 years' follow‐up (Hendrix 2006). However, increased risk was not maintained during extended follow‐up (overall 10.7 years; RR 1.17, 95% CI 0.97 to 1.40) (LaCroix 2011). Although WHI 1998 (combined HT arm) reported no statistically significant differences between groups in the incidence of stroke during the first 2 years of the study, women taking combined continuous HT were at significantly higher risk of stroke after taking HT for 3 or more years (at 3 years: RR 1.47, 95% CI 1.02 to 2.11; at a mean of 5.6 years: RR 1.39, 95% CI 1.09 to 1.77; at a mean of 7.9 years: RR 1.29, 95% CI 1.06 to 1.56). A statistically significant difference between groups was no longer evident at 13.2 years (RR 1.15, 99% CI 0.99 to 1.33). EPHT 2006 also reported data for this outcome at 3 years; pooling these data with data from WHI 1998 (combined HT arm) resulted in a risk ratio at 3 years of 1.46 (95% CI 1.02 to 2.09; Analysis 1.23). Absolute risk of a stroke increased at 3 years from 6 per 1000 in the control group to 8 per 1000 (95% CI 6 to 12) in the HT group; at 5.6 years from 14 per 1000 in the control group to 19 per 1000 (95% CI 15 to 24) in the HT group; and at 7.9 years from 21 per 1000 in the control group to 28 per 1000 (95% CI 23 to 34) in the HT group.
None of the other studies found any statistically significant differences between HT and placebo for this outcome (Analysis 1.22; Analysis 1.24; Analysis 1.25; Analysis 2.14; Analysis 2.13; Analysis 2.15). As noted above, most of the relevant studies were small.
4.2 Transient ischaemic attack (TIA)
Relevant comparisons
Four studies (ELITE 2014; EPAT 2001; PEPI 1995; Tierney 2009) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for 2 or 3 years, reported this outcome in relatively healthy women.
Three studies (ESPRIT 2002; HERS 1998; WEST 2001) of women with cardiovascular disease with a total of three different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 2 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), also measured this outcome.
Results
Results of analysis show no statistically significant differences between HT and placebo for this outcome (Analysis 1.26; Analysis 1.27; Analysis 2.16; Analysis 2.17; Analysis 2.18).
4.3 Stroke or transient ischaemic attack
Relevant comparisons
One study of relatively healthy women (WISDOM 2007) taking combined continuous HT or placebo for a median of 1 year reported stroke or TIA as a combined outcome. Another study (ERA 2000) of women with known coronary disease taking oestrogen‐only HT, combined continuous therapy or placebo also reported this combined outcome at 3.2 years' mean follow‐up.
Results
Neither study found a statistically significant difference for this outcome between women taking HT and women taking placebo (Analysis 1.29; Analysis 2.20; Analysis 2.19).
5. Venous thromboembolism (pulmonary embolus or deep vein thrombosis)
Relevant comparisons
Six studies (ELITE 2014; EPAT 2001; PEPI 1995; Tierney 2009; WHI 1998; WISDOM 2007) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome in relatively healthy women.
Five studies of women with cardiovascular disease (ERA 2000; ESPRIT 2002; EVTET 2000; HERS 1998; WAVE 2002) with a total of five different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 to 4 years, with unblinded follow‐up to 6.8 years (HERS 1998), also measured venous thromboembolism.
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they needed custodial care.
Results
WHI 1998 (oestrogen‐only HT arm) reported that relatively healthy women taking oestrogen‐only HT (CEE 0.625 mg) were at higher risk of a thromboembolic event than women taking placebo. Risk was highest within the first 2 years and was statistically significant during this time period (RR 2.22, 95% CI 1.12 to 4.39). Absolute risk of an event increased from 2 per 1000 in the control group to 5 per 1000 (95% CI 2 to 10) in the HT group. At a mean follow‐up of 7 years, risk was lower, but the intervention group was still at higher risk bordering on statistical significance (RR 1.32, 95% CI 1.00 to 1.74). The increased risk disappeared during extended follow‐up (overall 10.7 years' follow‐up: RR 1.05, 95% CI 0.84 to 1.31). When deep vein thrombosis was considered as a single outcome (without pulmonary embolism), the rate was significantly lower in the HT group during extended follow‐up (RR 0.63, 95% CI 0.41 to 0.98), although the rate over the entire 10.7 years' intervention and extended follow‐up did not differ significantly between the two groups (RR 1.04, 95% CI 0.84 to 1.29; Analysis 1.30).
WHI 1998 (combined HT arm) reported that relatively healthy women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) were at significantly higher risk of a thromboembolic event than women taking placebo; this applied at 1 to nearly 8 years' follow up (at 1 year: RR 3.59, 95% CI 1.95 to 6.61; at 2 years: RR 2.98, 95% CI 1.88 to 4.71; at 3 years: RR 2.54, 95% CI 1.73 to 3.72; at a mean of 5.6 years: RR 2.03, 95% CI 1.55 to 2.64; at a mean of 7.9 years: RR 1.65, 95% CI 1.32 to 2.05). Analysis of this comparison revealed a statistically significant time trend for diminishing risk of venous thromboembolism over time. WISDOM 2007 also reported data for this outcome at 1 year; pooling these data with data from WHI 1998 (oestrogen‐only HT arm) resulted in a risk ratio at 1 year of 4.28 (95% CI 2.49 to 7.34) (Analysis 1.32). Absolute risk of an event increased at 1 year from 2 per 1000 in the control group to 7 per 1000 (95% CI 4 to 11) in the HT group; at 2 years from 3 per 1000 in the control group to 9 per 1000 (95% CI 6 to 14) in the HT group; and at 5.6 years from 10 per 1000 in the control group to 20 per 1000 (95% CI 15 to 26) in the HT group.
Similarly, in HERS 1998, women with cardiovascular disease who were taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) for 1 to 4 years were significantly more likely to experience a venous thromboembolism than women on placebo (at 1 year: RR 3.26, 95% CI 1.06 to 9.96; at 2 years: RR 3.51, 95% CI 1.42 to 8.66; at 3 years: RR 3.01, 95% CI 1.50 to 6.04; at a mean of 4.1 years: RR 2.62, 95% CI 1.39 to 4.94; Analysis 2.22). Absolute risk of an event increased at 1 year from 3 per 1000 in the control group to 9 per 1000 (95% CI 3 to 29) in the HT group; at 2 years from 4 per 1000 in the control group to 15 per 1000 (95% CI 6 to 38) in the HT group; and at 4.1 years from 9 per 1000 in the control group to 13 per 1000 (95% CI 6 to 28) in the HT group.
None of the other studies found any statistically significant differences between HT and placebo for this outcome (Analysis 1.31; Analysis 2.21; Analysis 5.3).
6. Breast cancer
Relevant comparisons
Nine studies (ELITE 2014; EPAT 2001; EPHT 2006; Greenspan 2005; KEEPS 2012; Notelovitz 2002; PEPI 1995; WHI 1998; WISDOM 2007) with a total of 11 different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, reported this outcome in relatively healthy women. WHI 1998 (combined HT arm) extended follow‐up beyond the planned completion date to achieve a mean 11‐year follow‐up for this outcome in the 85% of women who consented to stay in the study; WHI 1998 (oestrogen‐only arm) extended follow‐up to a total of 10.7 years in the 78% of women who agreed to continue.
Four studies (ERA 2000; ESPRIT 2002; HERS 1998; WAVE 2002) with a total of four different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 2 to 4 years, with unblinded follow‐up to 7.1 years (HERS 1998), measured this outcome in women with cardiovascular disease .
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
WHI 1998 (oestrogen‐only HT arm) reported a non‐statistically significant decrease in risk of breast cancer at 7.1 years' follow‐up among relatively healthy women taking oestrogen‐only HT (CEE 0.625 mg) compared with women taking placebo (RR 0.79, 95% CI 0.61 to 1.01). Follow‐up continued for a median of 5.8 years after the intervention phase. The overall cumulative breast cancer incidence over the 10.7 years' mean follow‐up (median 11.8 years) showed a significantly lower rate in the HT group (RR 0.78, 95% CI 0.63 to 0.96). Absolute risk of breast cancer decreased over 10.7 years' follow‐up from 37 per 1000 in the control group to 29 per 1000 (95% CI 23 to 35) in the HT group. The overall cumulative rate remained lower after a median of 13 years' follow‐up (RR 0.80, 95% CI 0.65 to 0.97). Study authors noted that when event rates in the early and late postintervention periods were compared, hazard ratios (HRs) for breast cancer differed significantly (P = 0.04). The significant difference between groups in HR for breast cancer diminished over time and disappeared at approximately 4.5 years post intervention (Chlebowski 2015a)
WHI 1998 (combined HT arm) reported this outcome at yearly intervals. Results showed no statistically significant differences between groups in the incidence of breast cancer during the first 4 years of follow‐up, but the HT group was at significantly higher risk of breast cancer after taking HT for 5 or more years (at a mean of 5.6 years' follow‐up: RR 1.27, 95% CI 1.03 to 1.56; at a mean of 7.9 years' follow‐up: RR 1.27, 95% CI 1.07 to 1.52). Absolute risk of breast cancer increased at 5.6 years' follow‐up from 19 per 1000 in the control group to 23 per 1000 (95% CI 19 to 29) in the HT group; and at 7.9 years' follow‐up from 26 per 1000 in the control group to 33 per 1000 (95% CI 28 to 40) in the HT group. Analysis in this arm of WHI 1998 revealed a statistically significant trend for increasing breast cancer risk over time in the group taking HT. WISDOM 2007 also reported data for this outcome at a median follow‐up of 1 year. Pooling these data with data from WHI 1998 (combined HT arm) resulted in significantly reduced risk of breast cancer at 1 year in the HT arm (RR 0.53, 95% CI 0.28 to 0.96). However, at a mean of 11 years' follow‐up in WHI 1998 (combined HT arm), the rate of invasive breast cancer was significantly higher in the HT arm (RR 1.25, 95% CI 1.08 to 1.45). Rates remained higher in the intervention arm at a median of 13.2 years' follow‐up (RR 1.28, 95% CI 1.11 to 1.47) (Analysis 6.3). Breast cancers diagnosed in the HT group were of similar histology and stage to those diagnosed among controls but were more likely to be node positive (P = 0.03).
Results of analysis show no statistically significant differences between any other type of HT and placebo for this outcome, although (as noted above) relevant studies were small (Analysis 6.1; Analysis 6.2; Analysis 6.3; Analysis 6.4).
7. Colorectal cancer
Relevant comparisons
Seven studies (ELITE 2014; EPAT 2001; HERS 1998; Greenspan 2005; PEPI 1995; Tierney 2009; WHI 1998, WISDOM 2007) with a total of seven different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 8 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), reported this outcome. Investigators also reported this outcome after extended follow‐up in WHI 1998 (combined HT arm) at 11.6 years (Simon 2012) and at 13.2 years (Manson 2013).
One other small study (Nachtigall 1979) measured this outcome and compared combined sequential HT versus placebo for 10 years in women hospitalised for chronic disease or because they required custodial care.
Results
WHI 1998 (combined HT arm) reported no statistically significant differences in the incidence of colorectal cancer among relatively healthy women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) compared with women taking placebo, at 1 to 4 years' follow‐up. However, women taking combined continuous HT had a significantly lower incidence of colon cancer at a mean follow up of 5.6 years (RR 0.64, 95% CI 0.44 to 0.91). Absolute risk of colorectal cancer decreased from 9 per 1000 in the control group to 6 per 1000 (95% CI 4 to 8) in the HT group. Rates tended to favour the HT group over extended follow‐up: The difference was not statistically significant at 7.9 years (RR 0.76, 95% CI 0.57 to 1.01) nor at 13.2 years (RR 0.80, 95% CI 0.63 to 1.01) but did reach statistical significance at 11.6 years (RR 0.78, 95% CI 0.61 to 0.99).
Results of analysis show no statistically significant differences between any other type of HT and placebo for this outcome (Analysis 6.6; Analysis 6.7; Analysis 6.8; Analysis 6.9).
8. Lung cancer
Relevant comparisons
WHI 1998 (oestrogen‐only arm) reported this outcome in relatively healthy women after a mean follow‐up of 7.1 years; WHI 1998 (combined HT arm) reported this outcome at 5.6 years and after extended follow‐up at 7.9 years and 14 years.
Results
Results of analysis show no statistically significant differences between HT and placebo groups for this outcome (Analysis 6.12; Analysis 6.11; Analysis 6.13).
9. Endometrial cancer
Relevant comparisons
Nine studies (EPAT 2001; ESPRIT 2002; Ferenczy 2002; HERS 1998; KEEPS 2012; Nachtigall 1979; Obel 1993; PEPI 1995; WHI 1998 (combined HT arm)) with a total of 13 different interventions, comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo, for varying durations from 1 year to nearly 10 years, reported this outcome. WHI 1998 (combined HT arm) also reported this outcome after extended follow‐up, at 13.2 years. The study of oestrogen‐only HT versus placebo in women who had undergone surgery for stage I or II endometrial cancer (Barakat 2006) measured recurrent endometrial cancer. In comparisons of oestrogen‐only HT versus placebo (EPAT 2001; ESPRIT 2002; PEPI 1995), all women with a uterus were monitored closely for endometrial hyperplasia, and two studies specified that study medications were withdrawn if atypical hyperplasia was detected (ESPRIT 2002; PEPI 1995).
Results
At 13 years' median follow‐up, rates of endometrial cancer were lower in the combined HT group (RR 0.66, 95% CI 0.48 to 0.90) in WHI 1998.
Results of analysis showed no other statistically significant differences between HT and placebo for this outcome (Analysis 6.14; Analysis 6.15; Analysis 6.14; Analysis 6.16), and no statistically significant differences between groups in rates of recurrent endometrial cancer (Analysis 6.17). One study (Obel 1993) reported no events.
10. Ovarian cancer
Relevant comparisons
WHI 1998 (combined HT arm), which used combined continuous CEE 0.625 mg + MPA 2.5 mg at 5.6 years' mean follow‐up and again after extended follow‐up, at 13.2 years (Manson 2013), reported ovarian cancer incidence, and ELITE 2014, which utilised oestrogen with or without sequential progesterone vaginal gel, reported a single event.
Results
Results of analysis showed no statistically significant differences between groups for this outcome (Analysis 6.18).
11. Gallbladder disease
Relevant comparisons
Four studies (ERA 2000; HERS 1998; PEPI 1995; WHI 1998), which compared oestrogen‐only HT, combined continuous HT and sequential combined HT versus placebo for 3 to over 7 years, reported gallbladder disease requiring surgery. For this outcome, the two largest studies stated that they excluded from analysis women who had had their gallbladder removed (HERS 1998), who reported a history of gallbladder disease, or both (WHI 1998).
Results
Meta‐analysis of the three studies comparing oestrogen‐only HT versus placebo for the outcome of gallbladder disease requiring surgery (ERA 2000; PEPI 1995; WHI 1998) showed a statistically significant increase in risk in the HT group (RR 1.75, 95% CI 1.40 to 2.19); these studies had a mean follow‐up ranging from 3 to 7.1 years. Absolute risk of an event increased from 26 per 1000 in the control group to 45 per 1000 (95% CI 36 to 57) in the HT group. Meta‐analysis of the four studies comparing combined continuous HT versus placebo (ERA 2000; HERS 1998; PEPI 1995; WHI 1998) showed significantly increased risk in the HT group (RR 1.55, 95% CI 1.29 to 1.86); these studies had a mean follow‐up ranging from 3 to 5.6 years. Absolute risk of an event increased from 27 per 1000 in the control group to 47 per 1000 (95% CI 38 to 60) in the HT group. Although these studies had differing lengths of follow‐up, review authors noted no statistical heterogeneity in either meta‐analysis. Similarly, during unblinded follow‐up, HERS 1998 reported an increase in events in the HT group that reached borderline statistical significance (RR 1.63, 95% CI 1.00 to 2.70; Analysis 6.20; Analysis 6.21; Analysis 6.22). WHI 1998 investigators reported that hazard estimates for risk in active and placebo groups started to diverge during the first year of follow‐up, with the oestrogen group separating earlier than the combined continuous HT group.
12. Fractures
12.1 Hip fracture
Relevant comparisons
Five studies, which compared combined continuous HT (HERS 1998; WHI 1998; WISDOM 2007), combined sequential HT (Tierney 2009; WEST 2001) and oestrogen‐only HT (WEST 2001; WHI 1998) versus placebo for between 1 and 7.9 years, with extended follow‐up to 10.7 years in WHI 1998 (oestrogen‐only arm), and with extended follow‐up to 13.2 years in both arms of WHI 1998, reported the incidence of hip fracture.
Results
Both arms of WHI 1998 found a statistically significant reduction in the risk of hip fracture for women taking HT. WHI 1998 (oestrogen‐only HT arm) reported a statistically significant reduction in the risk of hip fracture for women taking HT (CEE 0.625 mg) at 7.1 years' mean follow‐up (RR 0.64, 95% CI 0.45 to 0.93; Analysis 6.23). Absolute risk of a hip fracture decreased from 14 per 1000 in the control group to 9 per 1000 (95% CI 6 to 13) in the HT group. Benefit derived from HT was not maintained during extended follow‐up (to 13.2 years). WHI 1998 (combined HT arm) reported this outcome at yearly intervals and found no statistically significant differences in the incidence of hip fracture during the first 4 years' follow‐up, but at 5.6 years' mean follow‐up, reduction in the risk of hip fracture among women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) was statistically significant (RR 0.68, 95% CI 0.48 to 0.97; Analysis 6.25). Absolute risk of hip fracture decreased from 9 per 1000 in the control group to 6 per 1000 (95% CI 4 to 9) in the HT group. This risk remained significantly lower in the HT group at mean follow‐up of 7.9 years (RR 0.77, 95% CI 0.60 to 0.99) and 13.2 years (RR 0.82, 95% CI 0.69 to 0.97).
However, HERS 1998 found no statistically significant differences between combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) and placebo for this outcome, and the unblinded extension of this study reported a statistically significantly increased risk in the group taking HT from years 4.1 to 6.8 (post randomisation) (RR 2.10, 95% CI 1.06 to 4.16; Analysis 6.25).
Other studies found no statistically significant differences between groups (Analysis 6.24; Analysis 6.26).
12.2 Clinical vertebral fractures
Relevant comparisons
WHI 1998 (oestrogen‐only HT arm) reported the incidence of vertebral fracture at follow‐up of 7.1 years. Two studies of combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) versus placebo (HERS 1998; WHI 1998 (combined HT arm)) also reported the incidence of vertebral fracture at follow‐up from 4 to nearly 8 years.
Results
At a mean of 7.1 years' follow‐up, WHI 1998 (oestrogen‐only HT arm) reported significantly fewer fractures in the oestrogen‐only HT group (CEE 0.625 mg) than in the placebo group (RR 0.64, 95% CI 0.44 to 0.94; Analysis 6.27). Absolute risk of a clinical vertebral fracture decreased from 13 per 1000 in the control group to 8 per 1000 (95% CI 6 to 12) in the HT group. Similarly, at a mean of 5.6 years' follow‐up, WHI 1998 (combined HT arm) reported significantly fewer fractures in the HT group than in the placebo group (RR 0.68, 95% CI 0.48 to 0.97; Analysis 6.28). Absolute risk of a clinical vertebral fracture decreased from 10 per 1000 in the control group to 7 per 1000 (95% CI 5 to 10) in the HT group. At a mean of 7.9 years' follow‐up, WHI 1998 (combined HT arm) no longer observed significant differences between groups. HERS 1998 found no significant differences between groups during follow‐up.
12.3 Any fractures
Relevant comparisons
Nine studies (EPHT 2006; ERA 2000; ESPRIT 2002; Greenspan 2005; HERS 1998; Tierney 2009; WEST 2001; WHI 1998; WISDOM 2007) comprising comparisons of oestrogen‐only HT, combined continuous HT and combined sequential HT versus placebo for 1 to nearly 8 years reported the incidence of any fracture.
Results
Both arms of WHI 1998 showed a statistically significant reduction in the risk of any fracture for women taking HT. Investigators reported this at 5.6 and 7.9 years' mean follow‐up in women taking combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) (at 5.6 years: RR 0.78, 95% CI 0.71 to 0.86; at 7.9 years: RR 0.82, 95% CI 0.76 to 0.89) and at 7.1 years' mean follow‐up in women taking oestrogen‐only HT (CEE 0.625 mg) (RR 0.73, 95% CI 0.65 to 0.80) (Analysis 6.30; Analysis 6.32). At 5.6 years, in WHI 1998 (combined HT arm), absolute risk of any fracture decreased from 111 per 1000 in the control group to 86 per 1000 (95% CI 79 to 94) in the HT group, and in WHI 1998 (oestrogen‐only HT arm) at 7.1 years, absolute risk of any fracture decreased from 140 per 1000 in the control group to 102 per 1000 (95% CI 91 to 112) in the HT group. None of the other studies found any statistically significant differences between HT and placebo for this outcome (Analysis 6.29; Analysis 6.31; Analysis 6.33).
13. Cognitive function
13.1 Global cognitive function
Relevant comparisons
Five studies, which compared low‐dose oestrogen patches versus placebo for 2 years (Yaffe 2006) and combined continuous CEE 0.625 mg with or without MPA 2.5 mg versus placebo for 3 years (Greenspan 2005), oestradiol 1 mg daily with or without intermittent vaginal progesterone gel with follow‐up at 2.5 and 5 years (ELITE 2014), 0.45 mg oral or 0.05 mg transdermal oestrogen with intermittent progesterone 200 mg versus placebo for 4 years (KEEPS 2012), oestrogen‐only HT versus placebo for a mean of 5.6 years (WHI 1998 (WHIMS)) and continuous CEE 0.625 mg + MPA 2.5 mg versus placebo for a mean of 4.2 years (WHI 1998 (WHIMS)), reported this outcome. Researchers measured global cognitive function using a cognitive screening test known as the Modified Mini‐Mental State Examination (3MSE), on which a higher score reflects better cognitive functioning. KEEPS 2012 included women 42 to 58 years of age at randomisation, WHI 1998 (WHIMS) included only women over 65 years of age and Yaffe 2006 included only women over 60 years of age.
Results
Over 2 years' follow‐up in Yaffe 2006, 3 years' in Greenspan 2005, 4 years' in and 2.5 years' or 5 years' in ELITE 2014, investigators noted no significant difference in cognitive function between intervention and placebo groups. Nor did they observe any difference in the effect of treatment when women in Yaffe 2006 were stratified according to cognitive status at baseline (3MSE ≤ 90 or > 90) (Analysis 1.34; Table 2). Inbestigators in ELITE 2014 subgrouped comparisons according to when oestradiol was initiated (within 6 years of menopause vs 10 or more years after menopause). Study results showed no evidence of differences between the two subgroups in the effect of HT on cognition at 2.5 years.
| Study | Comparison | Instrument | Measure | Outcome | Intervention | Effect |
| Oestrogen (CEE or oestradiol) + cyclic oral micronised progesterone 200 mg/d × 12 days per month vs placebo (n = 275) for 48 months | Modified Mini Mental State Examination (MMSE) | Differences between intervention and placebo groups in mean rate of change over time | Global cognition | 0.45 mg/d oral CEE (n = 230) | P = 0.178 | |
| 0.05 mg/d transdermal oestradiol (n = 222) | P = 0.840 |
In both treatment groups and in both placebo groups of WHI 1998 (WHIMS), mean 3MSE scores increased from baseline and continued to increase for 3 to 5 years before they started to decline. Results showed a pattern of higher increases from baseline in 3MSE scores in the placebo groups, which emerged after 1 to 2 years and were maintained throughout the study. The mean difference between groups in 3MSE score changes was of borderline statistical significance in both arms of the study, with results favouring the placebo group; however, in both cases, the lower boundary of the confidence interval was zero (oestrogen‐only HT arm: weighted mean difference (WMD) ‐0.25, 95% CI ‐0.52 to 0.00; combined HT arm: WMD ‐0.18, 95% CI ‐0.35 to 0.00) (Analysis 1.34).
In the WHI 1998 (WHIMS) combined HT arm, a decline of 10 points or more in 3MSE scores (which represents > 2 standard deviations from baseline mean scores) was significantly more likely to occur among women in the active treatment group (RR 1.57, 95% CI 1.10 to 2.24; Analysis 1.31). Study results showed the same trend in the oestrogen‐only HT group, but this finding was not of statistical significance.
13.2 Probable dementia
Relevant comparisons
WHI 1998 (WHIMS), which included only women over 65 years of age and compared oestrogen‐only HT (CEE 0.625 mg) versus placebo for a mean of 5.6 years, and combined continuous HT (CEE 0.625 mg + MPA 2.5 mg) versus placebo for a mean of 4.2 years, reported this outcome.
Results
In the oestrogen‐only HT arm, researchers noted no statistically significant differences between groups. In the combined HT arm, the incidence of probable dementia was significantly higher in the group taking combined continuous HT than in the placebo group (RR 1.97, 95% CI 1.16 to 3.33). At 4.2 years, absolute risk of probable dementia increased from 9 per 1000 in the control group to 18 per 1000 (95% CI 11 to 30) in the HT group (Analysis 1.35).
13.3 Change in dementia status
Relevant comparisons
One small study (Mulnard 2000) included women with mild to moderate Alzheimer's disease. Researchers compared unopposed oestrogen for 1 year versus placebo and examined the primary outcome of change in overall status with relation to Alzheimer's disease, as measured by the Clinical Global Impression of Change Scale.
Results
Results of analysis show no statistically significant differences between groups (Analysis 3.1).
Other analyses
Studies included in any one analysis were insufficient to allow construction of a funnel plot.
Use of a random‐effects model had no material effect on any analyses. Studies in any one analysis were insufficient to permit sensitivity analysis by study risk of bias. Nor did any analyses include studies with marked clinical differences.
Discusión
Resumen de los resultados principales
Enfermedades cardiovasculares
No hay pruebas que indiquen que la terapia hormonal (TH) tiene una función en el tratamiento o la prevención de las enfermedades cardiovasculares. Por el contrario, la TH aumenta significativamente la incidencia de accidente cerebrovascular y tromboembolia venosa y la TH combinada continua también aumenta significativamente el riesgo de eventos coronarios (infarto de miocardio o muerte cardíaca). La TH con estrógeno solo no parece tener efectos estadísticamente significativos (positivos o negativos) sobre la enfermedad coronaria.
En HERS 1998 y WHI 1998 fue evidente un aumento en el riesgo de eventos coronarios y tromboembolia venosa durante el primer año de tratamiento entre las pacientes que recibieron TH combinada continua. Si bien en ambos brazos de WHI 1998 y en la fase cegada de HERS 1998 hubo una tendencia significativa a la disminución del riesgo cardiovascular en el grupo de TH con el transcurso del tiempo, el análisis subsiguiente de los datos de HERS 1998 (que incluían el seguimiento cegado y no cegado) no encontró variaciones estadísticamente significativas del riesgo con el transcurso del tiempo. Los investigadores de WHI 1998 indican que la disminución evidente en el riesgo cardiovascular en años posteriores se puede deber a una aceleración de los eventos durante años anteriores entre las pacientes susceptibles del grupo de TH, y señalan que con la duración más prolongada del tratamiento aumenta el riesgo de cáncer de mama.
WHI 1998 (brazo de TH combinada) realizó análisis de subgrupos predefinidos para evaluar si alguna de las características clínicas de la población de estudio podría haber modulado los efectos coronarios de la TH. Las variables incluyeron: edad, tiempo desde la menopausia, presencia o ausencia de síntomas vasomotores, administración previa de hormonas, estado del factor de riesgo de cardiopatía coronaria y presencia o ausencia de enfermedades cardiovasculares preexistentes. Sin embargo, ninguna de estas variables afectó los resultados de una manera significativa.
Entre las mujeres que recibieron la TH combinada en WHI 1998, las que presentaban una mutación en el factor V de Leiden (un trastorno en la coagulación de la sangre) tuvieron mayor riesgo de tromboembolia venosa (Cushman 2004). El poder estadístico no fue suficiente para permitir que los investigadores determinaran si el exceso de riesgo significativo se asoció con antecedentes de tromboembolia venosa (entre las mujeres que recibieron TH combinada). La incidencia de tromboembolia fue mayor entre las mujeres mayores y con obesidad, aunque estuvo relacionada con un mayor riesgo inicial de un evento, y el cociente de riesgos no fue diferente del de otras mujeres que recibían TH combinada.
Se ha indicado que los efectos vasculares de la TH pueden diferir según la edad de la mujer y el tiempo desde la aparición de la menopausia. Por lo tanto, el estrógeno puede contrarrestar los estadios iniciales de aterosclerosis en las mujeres recientemente menopáusicas al inhibir los depósitos de lípidos dentro del endotelio. Sin embargo, la TH puede tener efectos adversos sobre la enfermedad avanzada al facilitar un aumento de las enzimas que tienden a interrumpir las lesiones ateroescleróticas y al estimular la formación de coágulos (Manson 2013; Reslan 2012). Los hallazgos de los estudios de investigación sobre el efecto de la TH en la menopausia precoz sobre resultados intermedios de la EC son variables (ELITE 2014; KEEPS 2012), y los resultados de investigación sobre este tema continúan (Manson 2015).
Cáncer de mama
En WHI 1998 (brazo de TH combinada), las tasas de cáncer de mama en el grupo de TH fueron inicialmente inferiores que en el grupo placebo, y cuando se combinaron los datos de WHI 1998 y WISDOM 2007, al seguimiento al año, la diferencia alcanzó significación estadística a favor del grupo de intervención. Sin embargo, al cuarto año de administración ocurrieron más eventos en el grupo de TH, y una tendencia estadísticamente significativa mostró un mayor riesgo con el transcurso del tiempo. A una media de seguimiento de 11 años en WHI 1998, las mujeres del grupo de TH combinada tuvieron una tasa significativamente mayor de cáncer de mama invasivo que los controles, y al seguimiento más largo (a una mediana de 13,2 años) no se evidenciaron pruebas de una atenuación del riesgo (Chlebowski 2015a). A los 11 años, la tendencia a una tasa mayor de muerte por cáncer de mama se acercó a la significación estadística (Chlebowski 2010). Este aumento a largo plazo del riesgo fue evidente a pesar de las pruebas de que el riesgo de cáncer de mama asociado con la TH combinada descendió notablemente durante los dos primeros años después de la interrupción de las hormonas (Chlebowski 2009a).
Los investigadores de WHI 1998 señalaron que los cánceres de mama en el grupo de TH combinada se diagnosticaron a un grado similar pero en un estadio más avanzado, e indicaron que la TH combinada puede estimular el crecimiento del cáncer de mama aunque, a la vez que retarda el diagnóstico. Las pruebas indican que la TH combinada aumenta la frecuencia de mamografías anormales e indicaciones para biopsia de mama, pero compromete el rendimiento diagnóstico de ambas pruebas (Chlebowski 2008). Estos factores explicarían la menor incidencia de cáncer de mama entre las pacientes que recibieron el tratamiento combinado durante los dos primeros años en WHI 1998. Los análisis de subgrupos del uso previo de hormonas en WHI 1998 mostraron que la incidencia acumulativa de cáncer de mama con el transcurso del tiempo en las pacientes que recibían la TH combinada aumentó a una mayor tasa que en las pacientes que recibían placebo después de cerca de tres años en las usuarias previas de hormonas y después de cerca de cinco años en las mujeres sin un uso previo. La interferencia con la mamografía impidió la posibilidad de definir con alguna confiabilidad un plazo para la administración segura de la TH combinada (Anderson 2006).
WHI 1998 informó una disminución en el riesgo de cáncer de mama en el brazo de estrógeno sin oposición del ensayo, que alcanzó significación estadística cuando los investigadores tuvieron en cuenta todos los 10,7 años de intervención y el seguimiento prolongado. Las tasas de eventos acumulativos todavía difirieron significativamente entre los grupos durante el seguimiento de 13 años y el riesgo de muerte por cáncer de mama fue inferior en el grupo de TH alrededor de los 12 años. La comparación de los cocientes de riesgos instantáneos durante los períodos tempranos y tardíos posteriores a la intervención indicó que el riesgo menor de cáncer de mama en el brazo de estrógeno persistió por alrededor de 4,5 años después que se proporcionó la intervención, al punto que ya no fue evidente una diferencia significativa entre las intervenciones (Chlebowski 2015a; Chlebowski 2015b). Los análisis de subgrupos mostraron significativamente menos cánceres en estadios iniciales y significativamente menos carcinomas ductales en el grupo de intervención, aunque la incidencia de tumores lobulares no difirió significativamente. Los resultados mostraron que la reducción del riesgo de cáncer de mama se concentró en las mujeres sin enfermedad mamaria benigna (p = 0,01) o con antecedentes familiares de primer grado de cáncer de mama (P = 0,02) (Anderson 2012). La TH con estrógeno solo parece aumentar el número de mujeres que necesitaron mamografía repetida o biopsia de mama, pero (a diferencia de la TH combinada) no parece comprometer de manera significativa la detección del cáncer de mama (Chlebowski 2010a).
Los investigadores de WHI 1998 señalaron que las diferencias entre las participantes entre los dos brazos del estudio no explicaron las diferencias en la incidencia de cáncer de mama e indicaron que el aumento del riesgo en el grupo combinado se podría deber al progestágeno. Tendencias similares de otros estudios (Beral 2003; HERS 1998) apoyan esta teoría. Un estudio de casos y controles anidado que comparó controles pareados de pacientes que desarrollaron cáncer de mama en ambos brazos de WHI 1998 (Zhao 2014) indicó que los cambios después del tratamiento en los estrógenos séricos y las concentraciones de la globulina ligada a las hormonas sexuales, o los cambios en la asociación de dichas concentraciones con el riesgo de enfermedad, podrían explicar el aumento en el riesgo de cáncer de mama observado con la TH combinada y la reducción en el riesgo observada con el estrógeno sin oposición. Se ha observado que la exposición al estrógeno después de un período mantenido de deprivación estrogénica reduce el riesgo de cáncer de mama (Jordan 2015; Obiorah 2013).
Cáncer colorrectal
La reducción significativa en la incidencia de cáncer colorrectal en las pacientes que recibieron TH combinada continua en WHI 1998 se equiparó con el hallazgo de que los cánceres colorrectales diagnosticados en dichas mujeres tendieron a ser más avanzados, con mayor probabilidad de afectación linfática o metastásica. Además, la reducción en la incidencia en el grupo de TH no dio lugar a una reducción de la tasa de mortalidad por cáncer colorrectal durante el seguimiento prolongado (7,1 años), aunque los investigadores señalaron que quizás se requiera un período aún más largo de seguimiento para observar un beneficio en la mortalidad por una reducción en la incidencia de cánceres localizados pequeños. Las mujeres que recibieron TH con estrógeno solo en WHI 1998 no tuvieron una reducción en la tasa de mortalidad por cáncer colorrectal el seguimiento de más de siete años ni durante el seguimiento prolongado a los 10,7 años. En general, no hubo pruebas sólidas que indiquen una reducción clínicamente significativa en las tasas de cáncer colorrectal con estrógeno solo o estrógeno más progestina. Los resultados del estudio observacional WHI apoyaron esta conclusión (Prentice 2009).
Cáncer de pulmón
El análisis post hoc de los datos de WHI 1998 mostró que la TH combinada no aumentó significativamente la incidencia de cáncer de pulmón durante el seguimiento de ocho años, pero aumentó la mortalidad por cáncer de pulmón, independientemente de la situación con respecto al tabaco. Los autores del estudio (Chlebowski 2009) indicaron que lo anterior quizás se deba a que TH combinada estimula el crecimiento de los cánceres pulmonares de células pequeñas preexistentes.
Cánceres ginecológicos
Ninguno de los estudios incluidos encontró un aumento en la incidencia de cáncer endometrial en el grupo de TH. Tres estudios asignaron al azar a las pacientes con útero a la TH con estrógenos solos (EPAT 2001; ESPRIT 2002; PEPI 1995). Como está bien documentado que el cáncer endometrial es un efecto adverso del estrógeno sin oposición (Kurman 1985), en estas mujeres se vigiló estrechamente la hiperplasia atípica de endometrio y recibieron tratamiento (e interrupción del fármaco de estudio) cuando se detectó. PEPI 1995 informó que las pacientes del grupo de TH con estrógeno solo tuvieron mayores probabilidades de desarrollar hiperplasia endometrial atípica que las pacientes del grupo placebo, mientras que en las pacientes de los grupos de TH combinada del mismo estudio no hubo un aumento en el riesgo de hiperplasia. Después del seguimiento prolongado por más de 13 años, las tasas de cáncer endometrial fueron inferiores en el grupo de TH combinada en WHI 1998.
El estudio del tratamiento con estrógeno solo en pacientes sometidas a cirugía por cáncer endometrial estadio I o II tuvo poco poder estadístico debido a la interrupción temprana y a que no pudo refutar o apoyar de manera concluyente la seguridad de este tratamiento con respecto al riesgo de recidiva. Los autores del estudio señalan que las tasas de recidiva fueron bajas, del 1,9% en el grupo placebo y del 2,3% en el grupo de intervención (Barakat 2006).
Los resultados mostraron una tendencia hacia un aumento del riesgo de cáncer de ovario en WHI 1998 (brazo de TH combinada) que no alcanzó significación estadística (Anderson 2003). Como se señaló anteriormente, una revisión sistemática de estudios observacionales (principalmente) (Greiser 2006) indica que la terapia con estrógeno solo y combinada se puede asociar con un aumento en el riesgo de cáncer de ovario.
Un estudio aleatorio con cuatro años de seguimiento en 130 pacientes con antecedentes de cáncer de ovario (Guidozzi 1999) encontró que la terapia hormonal con estrógeno solo no afectó de forma negativa el tiempo de supervivencia libre de enfermedad o general en comparación con ninguna terapia hormonal. Un estudio aleatorio similar (ATH 2015) mostró que las pacientes con síntomas menopáusicos graves después de cáncer de ovario que recibieron TH (de tipos variables, según la preferencia de los especialistas) tuvieron una mejoría en la supervivencia general y libre de recidiva en comparación con controles no que recibieron TH. La presente revisión sistemática no incluyó estos estudios porque carecieron de un grupo control con placebo.
La reducción evidente en el riesgo de cáncer endometrial asociada con la TH combinada se equiparó con la indicación de un aumento en el riesgo de cáncer de ovario (Manson 2013).
Fracturas
Las pruebas sobre la TH y las fracturas no son consistentes. WHI 1998 encontró una reducción significativa en el riesgo de fracturas en las mujeres que recibieron TH combinada continua o TH con estrógeno solo por casi ocho años de seguimiento, pero HERS 1998 no informó efectos beneficiosos en las pacientes que recibieron la TH combinada continua. Además, la continuación no cegada de HERS 1998 encontró un aumento significativo en el riesgo de fractura de cadera en estas mujeres. Los autores del estudio atribuyeron este resultado al azar y señalaron que el efecto fue considerablemente inferior en el análisis de pacientes tratadas, y que dicho resultado carece de plausibilidad biológica (Hulley 2004). Los investigadores de WHI 1998 (brazo de TH combinada) examinaron la hipótesis de que el efecto beneficioso de la TH sobre la incidencia de fracturas difería según los factores de riesgo de fracturas. Encontraron que la reducción del riesgo con la TH no fue mayor en las mujeres con alto riesgo de fractura (Cauley 2003). Sin embargo, WHI 1998 excluyó a las pacientes con osteoporosis grave y no obtuvo de forma sistemática la densidad mineral ósea; por lo tanto, es posible que los efectos beneficiosos de la TH superen los riesgos en algunas pacientes con osteoporosis grave. La reducción en el riesgo de fractura de cadera asociada con la TH no persistió en la fase de seguimiento prolongado de WHI 1998 (TH con estrógeno solo).
Los análisis de la fractura de cadera pueden no haber tenido poder estadístico suficiente para alcanzar resultados concluyentes. El riesgo de fractura de cadera se eleva de manera abrupta a una edad cercana a los 60 años, pero todavía es menor del 0,5% entre las pacientes del Reino Unido de 65 a 69 años de edad (Banks 2009).
La mayoría de las pacientes que necesitan tratamiento por densidad mineral ósea baja requieren tratamiento de por vida, pero el riesgo más alto de eventos cardiovasculares con la TH combinada ocurre durante el primer año de administración. En general, si bien la TH se considera efectiva para la prevención de la osteoporosis posmenopáusica, sólo se suele recomendar como una opción en las pacientes con un riesgo significativo, para las que no son apropiados los tratamientos sin estrógenos (Cranney 2002; NIH 2004).
Resultados cognitivos
WHI 1998 (WHIMS) encontró que la TH combinada y la TH con estrógeno solo no mejoraron la función cognitiva global en las mujeres con más de 65 años de edad. La mejoría en la función cognitiva global (puntuaciones en el Modified Mini‐Mental State Examination [3MSE]) que ocurrió en todos los grupos de participantes durante los primeros años de WHIMS se atribuyó a un efecto de aprendizaje resultante de la administración repetida de pruebas cognitivas (Espeland 2004). La diferencia en las puntuaciones medias entre los grupos de tratamiento activo y placebo tuvo significación estadística marginal y favoreció de manera sistemática a los grupos placebo, aunque esta diferencia fue demasiado pequeña para considerarse clínicamente significativa. Sin embargo, la disminución notoria en las puntuaciones 3MSE (definida como más de dos desviaciones estándar a partir de la media inicial) fue más frecuente en los grupos de tratamiento activo, y esta tendencia alcanzó significación estadística en el grupo de TH combinada. Además, en ambos brazos la TH tuvo un efecto adverso relativamente mayor en las mujeres con puntuaciones 3MSE iniciales más bajas (Espeland 2004).
De modo similar, el resultado de demencia probable presentó una tendencia negativa en ambos grupos de tratamiento activo, lo que alcanzó significación estadística en el grupo de TH combinada. Las pruebas de aumento del riesgo en este grupo comenzaron a aparecer un año después de la asignación al azar y persistieron a los cinco años de seguimiento. El riesgo global de las pacientes que recibieron la TH combinada duplicó el riesgo de las pacientes del grupo placebo correspondiente. Los investigadores señalaron que el riesgo absoluto de demencia permaneció relativamente bajo, y fue de 45 por cada 10 000 mujeres posmenopáusicas mayores de 65 años que habían tomado TH combinada durante año (Shumaker 2004).
Estos resultados fueron inesperados y contrastan claramente con los resultados informados en la investigación anterior. Los investigadores de WHI 1998 (WHIMS) indicaron que lo anterior se podría deber al sesgo de usuario sano observado en los estudios observacionales (por el cual las usuarias de TH tuvieron un mejor pronóstico al inicio que los grupos control), a los efectos diferenciales de la TH en dominios específicos de la cognición no medidos individualmente por 3MSE, o a ambos. Alternativamente, indicaron que es probable que la TH deba iniciarse durante un período crítico, como la menopausia, para proteger la función cognitiva a una edad posterior. La media de edad de la población de WHIMS fue de 71 años, por lo que el estudio no pudo abordar esta teoría, aunque las usuarias previas de TH de WHIMS no obtuvieron puntuaciones mayores (Espeland 2004). Además, los resultados del seguimiento prolongado en WHI 1998 (WHISCA) no muestran pruebas de algún efecto beneficioso en la función cognitiva específica del dominio de la TH combinada o con estrógeno solo.
Una comparación post hoc de la función cognitiva global entre las mujeres más jóvenes en WHI 1998 (Espeland 2013; Vaughan 2013) incluyó mujeres asignadas al azar al brazo activo de WHI 1998 (EEC o TH combinada) versus mujeres en el brazo placebo. Las puntuaciones fueron similares en los dos grupos (P = 0,66). El estudio incluyó a 1376 mujeres de 50 a 55 años de edad cuando se realizó la asignación al azar en WHI 1998. La evaluación cognitiva se realizó a un promedio de 7,2 años después de la finalización del ensayo, cuando las mujeres tenían una edad promedio de 67,2 años y se repitió un año después. Los investigadores del estudio concluyeron que "las terapias con EEC no produjeron efectos beneficiosos o riesgos generales mantenidos en la función cognitiva cuando se administró a mujeres posmenopáusicas con 50 a 55 años de edad". Sin embargo, entre 2880 mujeres que se habían reclutado en WHI 1998 a la edad de 65 a 79 años, las disminuciones a largo plazo en la función cognitiva global se observaron en los grupos de TH (EEC o TH combinada) con respecto al grupo placebo, que consistió en mujeres mayores (P < 0,05). Los efectos fueron pequeños y las disminuciones fueron muy estables. Los resultados no variaron según el tipo de TH, la administración previa o el tiempo desde el último período menstrual (Espeland 2016).
Colecistopatía
Los investigadores observaron una asociación estadísticamente significativa entre la TH y la colecistopatía, con un exceso de riesgo relacionado con la TH con estrógeno solo y con la TH combinada continua. Si bien la mayor parte del poder estadístico de este resultado provino de WHI 1998, los hallazgos relacionados con la TH combinada continua fueron firmemente apoyados por los datos del seguimiento cegado y no cegado de HERS 1998. Los investigadores de WHI 1998 observaron que el riesgo comenzó a aumentar en el grupo activo durante el primer año y pareció aumentar con el transcurso del tiempo. Calcularon que para que se presentara un nuevo caso de colecistopatía era necesario que 323 pacientes tomaran TH con estrógeno solo o que 500 tomaran TH combinada continua durante un año.
Compleción y aplicabilidad general de las pruebas
Tipo de TH
Casi todos los resultados estadísticamente significativos descritos en esta revisión provienen de los dos estudios más grandes HERS 1998 y WHI 1998. Ambos estudios evaluaron 0,625 mg de estrógeno equino conjugado (EEC) oral, con o sin metoxiprogesterona continua (en inglés MPA 2,5 mg). Los estudios más pequeños que utilizaron otros tipos de TH informaron muy pocos o ningún evento clínico grave. En general, no fue posible combinar los resultados de los ensayos individuales porque utilizaron diferentes tipos de TH, que pueden no ser equivalentes en cuanto al efecto, o difirieron con respecto a la población del ensayo, o ambos. Existe polémica acerca de en qué medida los hallazgos de WHI 1998 son aplicables a cualquier tipo de TH que no sea 0,625 mg de EEC combinado continuo oral, con o sin MPA 2,5 mg. Los efectos pueden variar con diferentes estrógenos y progestágenos, diferentes plazos de administración de TRH y diferentes dosis y vías de administración (p.ej. estrógeno sin oposición y progestágeno intrauterino). Pruebas observacionales muestran que el estrógeno transdérmico difiere del estrógeno oral en que no se asocia con un aumento en el riesgo de tromboembolia venosa e indican que algunos tipos de progestágenos son trombogénicos, pero otros son seguros en este sentido (Canonico 2007).
Características de la población
Es importante considerar cualquier aumento del riesgo para la salud en términos absolutos más que relativos. Esta revisión se halla indudablemente dominada por los hallazgos de WHI 1998, que se diseñó para evaluar la eficacia de la TH en la prevención de las principales causas de morbilidad y mortalidad en mujeres mayores (Matthews 1997). No se diseñó para evaluar los riesgos y efectos beneficiosos de la terapia hormonal para el tratamiento de los síntomas menopáusicos, y excluyó expresamente a todas las mujeres con síntomas menopáusicos informados cuya gravedad imposibilitara su asignación al tratamiento con placebo (Anderson 2003). Además, WHI 1998 no incluyó a mujeres menores de 50 años de edad y los resultados del estudio pueden no aplicarse a las mujeres jóvenes con menopausia quirúrgica, por ejemplo, una mujer a la que se le extrajeron ambos ovarios a los cuarenta y tantos años (Kaunitz 2002).
Sin embargo, existen pocas pruebas sobre los efectos a largo plazo de la TH en mujeres más jóvenes y sanas, que muy probablemente la utilicen para tratar los síntomas menopáusicos. Es probable que dichas mujeres se encuentren a principios de los cincuenta años, cuando el riesgo absoluto de un evento potencialmente mortal es bajo; se ha calculado que el riesgo absoluto de muchas enfermedades aproximadamente se duplica con cada década de vida (Hulley 2004). Los análisis de subgrupos de mujeres entre 50 y 59 años de edad en WHI 1998 (brazo de TH combinada) mostraron que entre las mujeres relativamente sanas que recibieron TH combinada continua, el único aumento de riesgo que alcanzó significación estadística fue el de trombosis venosa. El riesgo en el grupo de TH aumentó de ocho trombosis venosas por cada 10 000 mujeres por año a 19 por cada 10 000 mujeres por año. El aumento del riesgo fue mayor durante el primer año de tratamiento, pero continuó durante cinco años más de tratamiento, y fue especialmente alto en las mujeres con obesidad (es decir, las mujeres con un índice de masa corporal superior a 30), que tuvieron un riesgo durante cinco años del 1,4% en comparación con el 0,5% en las mujeres con un peso normal. En el brazo de estrógeno solo de WHI 1998, durante los 10,7 años completos de la intervención y el seguimiento prolongado, las mujeres más jóvenes (edad de 50 a 59 años) asignadas a TH tuvieron significativamente resultados más favorables que las asignadas al azar a placebo. El grupo de TH tuvo cocientes de riesgos instantáneos significativamente inferiores para la cardiopatía coronaria, el infarto de miocardio y la muerte en comparación con el grupo placebo. Los resultados fueron similares para ambos resultados coronarios cuando los datos se estratificaron por tiempo desde la menopausia en lugar de por la edad. Lo anterior contrastó con los hallazgos en las mujeres mayores; las del grupo de TH mostraron una tendencia a tasas mayores de cardiopatía coronaria, infarto de miocardio y muerte y tasas significativamente mayores de cáncer colorrectal y enfermedad crónica. Los autores de WHI 1998 señalaron que las participantes del estudio recibieron estrógeno sin oposición por una duración mediana de menos de seis años, y que los resultados del estudio no se pueden extrapolar a duraciones del tratamiento más prolongadas o más cortas. Sin embargo, es importante destacar que la TH con estrógeno solo está contraindicada en mujeres con útero intacto, ya que se ha determinado que la administración durante uno a cinco años aumenta tres veces el riesgo de cáncer endometrial (a partir de un riesgo de por vida inicial de alrededor del 3% en una mujer de 50 años), con efectos que persisten por varios años tras interrumpir el estrógeno (Grady 1995).
Se ha indicado que los efectos de la TH pueden diferir en dependencia de si se inicia poco después de la menopausia o después de un intervalo prolongado (Barret‐Connor 2007). El análisis de los datos aleatorios (Prentice 2009a) y observacionales (WHI 1998) mostró que en la mayoría de los resultados clínicos, los efectos de la TH no variaron según el momento de administración de la TH; lo anterior se aplicó a la TH con estrógeno solo y a la TH combinada. Una excepción fue el cáncer de mama, en las que el riesgo fue mayor entre las mujeres que comenzaron la TH poco después de la menopausia que en las que tuvieron un intervalo de tiempo más prolongado. El riesgo general de cáncer también fue más alto.
La TH parece conllevar un mayor riesgo de recidiva en las pacientes con antecedentes de cáncer de mama. En Suecia se realizaron dos ensayos no cegados que asignaron a supervivientes de cáncer de mama con síntomas menopáusicos a TH y a tratamiento no hormonal. Ambos estudios se interrumpieron antes de tiempo debido al aumento estadísticamente significativo en la incidencia de cáncer de mama recidivante en el grupo de tratamiento hormonal de uno de los estudios (cociente de riesgos [CR] 3,5; intervalo de confianza [IC] del 95%: 1,5 a 8,1) (Chlebowski 2004; Holmberg 2004). Después de una mediana de seguimiento de cuatro años en este estudio, el riesgo de un nuevo evento de cáncer de mama aumentó significativamente desde el punto de vista clínico y estadístico en el brazo de TH (CR 2,4; IC del 95%: 1,3 a 4,2) (Holmberg 2008). Un estudio similar comenzado en el Reino Unido finalizó el reclutamiento de manera prematura en enero de 2004 (ICR 2001).
En cuanto a los resultados cardiovasculares, los resultados de HERS 1998 apoyan en gran medida los hallazgos de WHI 1998 (brazo de TH combinada), lo que indica que los resultados se pueden generalizar a las mujeres mayores que reciben TH combinada continua, posean o no factores de riesgo cardiovasculares comprobados (aunque los resultados difieren en cuanto al riesgo de fracturas).
Efectos beneficiosos y riesgos para la salud después de interrumpir la TH
WHI 1998 (brazo TH combinada) informó resultados de salud a una media de 2,4 años del seguimiento prolongado después del período de intervención planificado (Heiss 2008). Los datos de seguimiento durante este período estuvieron disponibles para el 95% de las mujeres, y pocas utilizaron la TH durante el seguimiento prolongado (4,3% en el brazo de intervención y 1,2% en el brazo placebo al año después que el estudio se interrumpiera). En el transcurso del seguimiento, el riesgo de eventos coronarios, accidente cerebrovascular y tromboembolia venosa disminuyó en el grupo que se había asignado al azar a TH combinada, y alcanzó un nivel equivalente al del grupo placebo. De manera similar, los resultados no mostraron diferencias significativas entre los grupos en el riesgo de fracturas o de cáncer colorrectal al final del seguimiento posterior a la intervención. Sin embargo, en el grupo que se asignó al azar a TH combinada, el cociente de riesgos instantáneos (CRI) para el resultado "todos los cánceres�? aumentó de 1,03 (IC del 95%: 0,92 a 1,15) durante la fase de intervención a 1,24 (IC del 95%: 1,04 a 1,48) en el período posterior a la intervención. Este aumento del riesgo se atribuyó en parte a la desaparición de la protección evidente anterior contra el cáncer colorrectal, con algún exceso de riesgo continuo de cáncer de mama, junto con el riesgo agregado de cáncer de pulmón en el grupo de TH. Los autores del estudio señalaron que la vigilancia clínica parece estar justificada debido al aumento mantenido del riesgo de neoplasia maligna después de finalizar la TH combinada.
WHI 1998 (brazo de estrógeno solo) informó resultados de salud a una media de 3,9 años del seguimiento prolongado (LaCroix 2011). Los datos del seguimiento en este período estuvieron disponibles para el 78% de las mujeres, de las que sólo una minoría pequeña utilizaba la terapia hormonal (hasta el 4,7% en el brazo de intervención y el 3% en el brazo placebo). En el transcurso del seguimiento prolongado, los resultados fueron consistentes en no mostrar diferencias significativas entre los grupos en las tasas de eventos coronarios. Los aumentos en el riesgo de accidente cerebrovascular y tromboembolia venosa en el brazo de TH desaparecieron rápidamente, y de la misma manera se redujo el riesgo de fractura de cadera en este grupo. Como se señaló anteriormente, la menor incidencia de cáncer de mama persistió y se tornó estadísticamente significativa con el seguimiento prolongado a los 10,7 años (es decir, cuando se incluyeron los períodos de intervención planificada y seguimiento prolongado).
Calidad de la evidencia
La mayoría de los estudios incluidos tuvieron bajo riesgo de sesgo en la mayor parte de los dominios (Figura 1). La calidad general de las pruebas se calificó como moderada, y la limitación principal incluyó las inquietudes acerca de la aplicabilidad de las pruebas porque en su mayoría los datos provinieron de WHI 1998, en el que sólo cerca del 33% de la muestra del estudio tenía de 50 a 59 años de edad al inicio (es decir, la edad a la cual las mujeres tienen grandes probabilidades de considerar la TH para los síntomas vasomotores); la media de edad de las pacientes fue de 63 años.
Un alto índice de mujeres de estos estudios no recibió el tratamiento al cual fueron asignadas. En general, el número de mujeres que interrumpieron la medicación o que tomaron menos del 80% fue desproporcionadamente alto en los grupos de TH, un hecho que quizás se deba a una mayor incidencia de efectos adversos como la hemorragia vaginal. WHI 1998 observó que en los casos en que se interrumpió el tratamiento y se inició un tratamiento diferente al del estudio, independientemente de los factores de riesgo de los resultados clínicos, el correspondiente análisis de intención de tratar subestima los efectos perjudiciales y beneficiosos de la TH en las pacientes con adherencia al tratamiento (WHI 2002). Este estudio incluyó un número desproporcionado de mujeres sin cegamiento en el grupo de TH en comparación con el grupo placebo (40% versus 6%), principalmente para controlar la hemorragia vaginal persistente; se ha indicado que esta falta diferencial de cegamiento pudo haber dado lugar a tasas de detección de infarto de miocardio más elevadas en el grupo de TH, que de otra manera no se hubieran detectado (Shapiro 2003). No obstante, también se ha indicado que es posible que no haya ocurrido un sesgo de detección a un grado tal que justifique las diferencias entre los grupos en relación con la cardiopatía coronaria, y que era más probable que cualquier sesgo actuara en la dirección opuesta, lo que conspiró contra la detección de los efectos (Tucker 2003).
Sesgos potenciales en el proceso de revisión
Esta revisión está sujeta al sesgo de selección de pacientes. En la mayoría de los estudios incluidos la media de la edad de las participantes fue de más de 60 años, y ninguno se centró en las mujeres perimenopáusicas. En todos los 20 estudios, excepto uno, que informaron la media de la edad de las participantes, la media de la edad al reclutamiento fue de más de 50 años. Lo anterior no refleja la práctica clínica habitual con respecto a la prescripción de la TH, que es más probable que se indique para el tratamiento de los síntomas vasomotores en el momento que las mujeres alcanzan la menopausia (Pedersen 2003). Además, las participantes descritas como "relativamente sanas" en esta revisión provinieron en su mayoría de WHI 1998. Los investigadores informaron una alta frecuencia de obesidad y trastornos hipertensivos entre las participantes de WHI 1998; sólo el 30% tenía un peso normal, y el 30% presentaba obesidad mórbida (índice de masa corporal [IMC] > 30 kg/m2); , el 36% recibía tratamiento para la hipertensión o tenía una presión arterial que excedía 140/90 mmHg al reclutamiento.
A pesar de la búsqueda extensiva, puede que no se hayan identificado todos los estudios relevantes. Sin embargo, es poco probable que se haya pasado por alto algún estudio suficientemente grande para influir de manera significativa en los hallazgos generales, por lo que WHI 1998 predominó en la revisión. De manera similar, es concebible que estos datos podrían haberse organizado de una manera diferente (p.ej. para la categorización de las poblaciones de estudio y las dosis de TH), nuevamente el predominio de WHI 1998 hace que sea muy poco probable que lo anterior haya influido en los hallazgos. Se decidió no agrupar los estudios que utilizaron diferentes tipos de TH y este enfoque está apoyado por los hallazgos contradictorios en los dos brazos (TH combinada versus con estrógeno solo) de WHI 1998 en algunos resultados.
La elección de la duración de un año de la TH como valor límite para la inclusión de los estudios fue arbitraria, pero es poco probable que haya introducido sesgo, ya que fue un criterio preespecificado.
Acuerdos y desacuerdos con otros estudios o revisiones
Los actuales hallazgos son consistentes con los de una revisión Cochrane de TH para prevenir las enfermedades cardiovasculares en mujeres posmenopáusicas (Boardman 2015), que concluyó que la administración de TH en las mujeres posmenopáusicas tiene poco o ningún efecto beneficioso para la prevención primaria o secundaria de las enfermedades cardiovasculares y causa un aumento del riesgo de accidente cerebrovascular y eventos tromboembólicos venosos. Boardman 2015 fue diferente de la revisión actual en que los autores agruparon los datos relacionados con el estrógeno sin oposición con los datos de la TH combinada.
Sin embargo, los hallazgos de la presente revisión difieren de los de algunas revisiones sistemáticas anteriores.
En 1997 se realizó una revisión sistemática con las pruebas aleatorias disponibles y se actualizó en el año 2000 con pruebas no publicadas (Hemminki 1997; Hemminki 2000). Los autores de la revisión informaron un odds ratio conservador estimado para los eventos cardiovasculares de 1,34 (IC del 95%: 0,55 a 3,30) entre las mujeres que recibieron TH. Sin embargo, este resultado se basó solamente en 15 eventos secundarios en pacientes asignadas al azar a TH y siete eventos en los grupos control; además, las pruebas proporcionadas no fueron suficientes para excluir un posible efecto beneficioso de la TH.
Beral 2002 agrupó los resultados de cuatro ensayos controlados aleatorios de TH publicados entre 1998 y 2002 (EVTET 2000; HERS 1998; WEST 2001; WHI 1998). No se informó un exceso o una reducción significativos en el riesgo relativo de cardiopatía coronaria, y los resultados negaron el importante efecto beneficioso de la TH sobre los resultados cardiovasculares informado por estudios observacionales previos. Además, describieron un exceso de riesgo de accidente cerebrovascular, embolia pulmonar y cáncer de mama. Los autores de la revisión encontraron que el riesgo de cáncer colorrectal o de fractura del cuello del fémur se redujo significativamente en el grupo de TH y los hallazgos de riesgo de cáncer endometrial no fueron concluyentes. Los autores de la revisión agruparon los resultados de los estudios que utilizaron diferentes tipos de TH durante períodos de tiempo variables. La diferencia más notable entre la revisión actual y Beral 2002 es que la revisión actual encontró un aumento estadísticamente significativo en el riesgo de cardiopatía coronaria entre las mujeres que recibieron TH combinada continua, en particular en el primer año. A diferencia de la revisión actual, Beral 2002 agrupó resultados de estudios de diferentes grupos de participantes y tipos de TH; lo anterior parece explicar por qué los resultados generales difieren.
Salpeter 2004 realizó un metanálisis de 17 ECA de TH que informaron al menos una muerte y concluyó que el riesgo de muerte se redujo significativamente en las mujeres con una media de la edad por debajo de 60 años que recibían TH en comparación con las que recibían placebo, aunque los resultados no mostraron diferencias cuando se compararon las mujeres mayores. Este metanálisis agrupó estudios que difirieron ampliamente en cuanto al tipo de TH utilizada y el estado clínico de las participantes; en varios estudios la muerte no fue un resultado preespecificado. Además, las pacientes con cáncer de ovario con pronóstico deficiente representaron el 60% de los eventos en el metanálisis de los estudios de mujeres jóvenes. En la revisión actual, las pruebas no muestran ventajas en cuanto a la supervivencia en las mujeres que reciben TH, aunque sólo uno de los estudios incluidos (WHI 1998 [TH con estrógeno solo]) analizó a las mujeres más jóvenes como un subgrupo para este resultado. Sin embargo, de los 17 ensayos incluidos por Salpeter y colegas en el metanálisis de mujeres jóvenes, sólo dos cumplieron con los criterios de inclusión de la presente revisión; los otros 15 incluidos en la revisión anterior no fueron cegados, no informaron la mortalidad como resultado primario o secundario, o bien tuvieron una duración inferior a un año.
Una revisión sistemática de los estudios de terapia hormonal y cáncer de ovario (Greiser 2006) incluyó 30 estudios de casos y controles, siete estudios de cohortes, un estudio controlado aleatorio y cuatro estudios de registros de cáncer. Esta revisión encontró un aumento en el riesgo de cáncer de ovario asociado con la administración de TH con estrógeno solo (CR 1,28; IC del 95%: 1,18 a 1,40) o terapia hormonal combinada (CR 1,11; IC del 95%: 1,02 a 1,21). Este riesgo se aplica a varios subtipos histológicos comunes del cáncer de ovario. Los autores de la revisión señalaron que esta revisión se vio limitada por la confianza en los datos observacionales; sin embargo, la heterogeneidad fue baja o moderada en la mayoría de los análisis.
Otra revisión sistemática (Bath 2005) realizó metanálisis a 28 ECA de TH que informaron eventos de accidente cerebrovascular. La TH se asoció con un exceso estadísticamente significativo del riesgo de accidente cerebrovascular, en especial el accidente cerebrovascular isquémico. Más aún, al parecer las participantes del grupo de TH que presentaron un accidente cerebrovascular tuvieron peores respuestas. Esta revisión tuvo criterios de inclusión muy amplios y agrupó una gran variedad de ensayos con diferentes tipos de TH para diversas indicaciones, algunos con participantes masculinos y otros sin control con placebo. No está claro en qué medida los hallazgos se aplican a las mujeres peri y posmenopáusicas.
Un estudio danés (Schierbeck 2012) de 1006 mujeres recientemente posmenopáusicas o perimenopáusicas (edad 45 a 58 años) no fue elegible para esta revisión debido a la falta de cegamiento. Los investigadores informaron que después de diez años de tratamiento, las mujeres que tomaban 17‐B estradiol trifásico con acetato de noretisterona o (en las que se había realizado una histerectomía) estradiol solo, tenían un menor riesgo de presentar el resultado primario, un resultado compuesto de muerte e ingreso hospitalario por insuficiencia cardíaca o infarto de miocardio (cociente de riesgos instantáneos 0,48; IC del 95%: 0,26 a 0,87; P = 0,015). No se evidenció un aumento del riesgo de cáncer, tromboembolia venosa o accidente cerebrovascular. Los autores del estudio atribuyeron la diferencia entre sus resultados y los de WHI 1998 a la menor edad y a la proximidad a la menopausia de sus participantes. Este estudio estuvo limitado por la falta de cegamiento y el uso de un resultado primario compuesto que no se preespecificó en el protocolo de estudio (Marjoribanks 2012; Schroll 2012; Shapiro 2003). Se ha señalado que los investigadores no evaluaron los resultados cardiovasculares, sino que se basaron en datos de médicos individuales introducidos en una base de datos nacional (Abelin 2012).
No se encontraron estudios aleatorios ni revisiones sistemáticas que proporcionaran pruebas acerca de los riesgos de la TH a largo plazo en las mujeres perimenopáusicas o las que tenían menos de 50 años de edad.
Las recomendaciones actuales favorecen la administración de TH a dosis baja para el alivio de los síntomas vasomotores en las mujeres con menos de diez años desde su última menstruación, administrada durante el menor tiempo posible requerido para alcanzar los objetivos terapéuticos, con dosis adaptadas individualmente y revisadas de manera regular (NAMS 2012; NICE 2015; RANZCOG 2012).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 1 Death from any cause: oestrogen‐only HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 2 Death from any cause: combined HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 3 Death from any cause: oestrogen with or without sequential progesterone vaginal gel.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 4 Death from coronary heart disease: oestrogen‐only HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 5 Death from coronary heart disease: combined continuous HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 6 Death from coronary heart disease: combined sequential HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 7 Death from stroke: oestrogen‐only HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 8 Death from stroke: combined sequential HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 9 Death from stroke: combined continuous HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 10 Death from colorectal cancer: oestrogen‐only HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 11 Death from breast cancer: combined continuous HT.
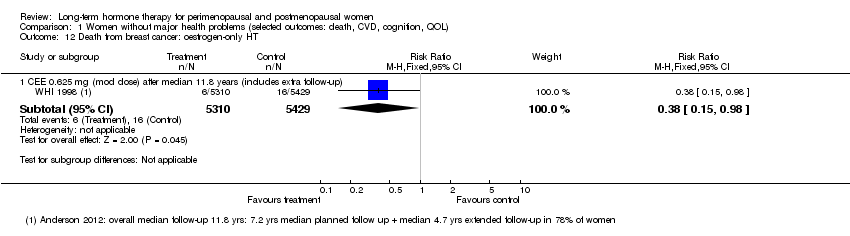
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 12 Death from breast cancer: oestrogen‐only HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 13 Death from colorectal cancer: combined continuous HT.
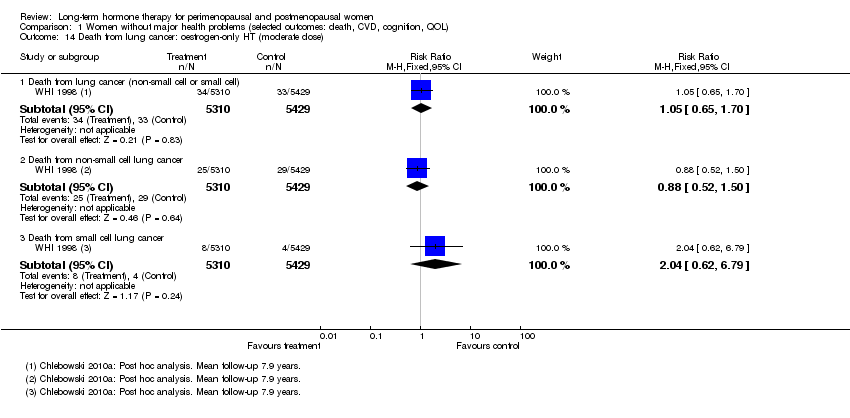
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 14 Death from lung cancer: oestrogen‐only HT (moderate dose).

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 15 Death from lung cancer: combined continuous HT (moderate dose).

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 16 Death from lung cancer: combined sequential HT (low dose oestrogen).
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 17 Death from any cancer: combined continuous HT.
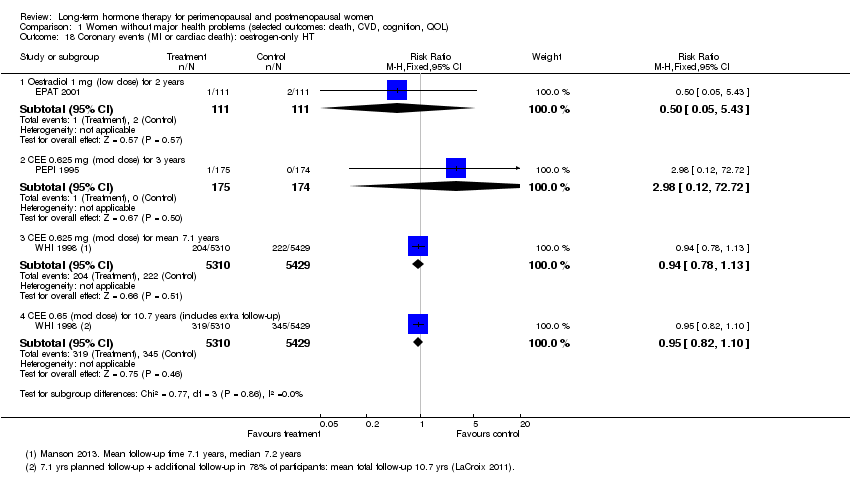
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 18 Coronary events (MI or cardiac death): oestrogen‐only HT.
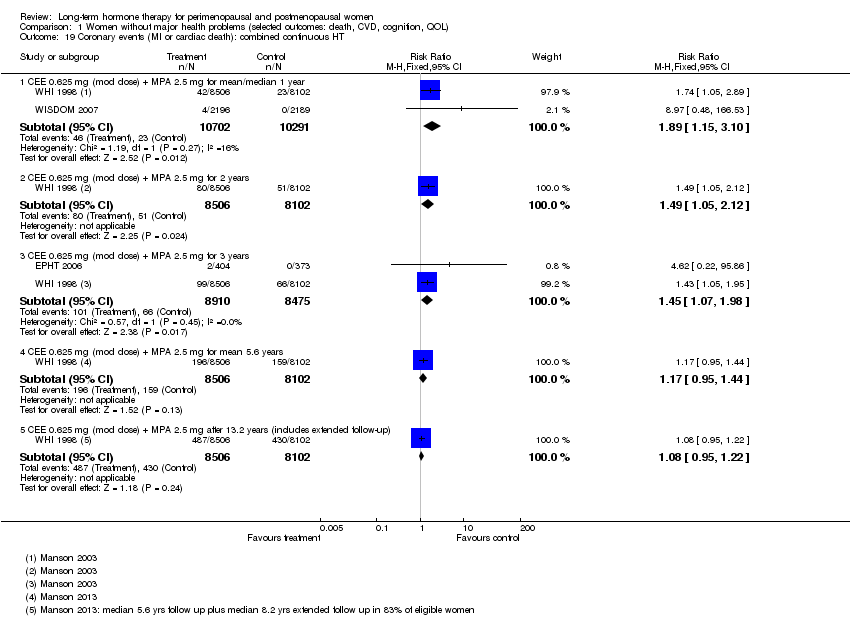
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 19 Coronary events (MI or cardiac death): combined continuous HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 20 Coronary events (MI or cardiac death): combined sequential HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 21 Coronary events (MI or cardiac death): oestrogen with or without sequential progesterone vaginal gel.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 22 Stroke: unopposed oestrogen.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 23 Stroke: combined continuous HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 24 Stroke: combined sequential HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 25 Stroke: combined sequential HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 26 Transient ischaemic attack: oestrogen‐only HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 27 Transient ischaemic attack: combined sequential HT.
Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 28 Transient ischaemic attack: oestrogen with or without sequential progesterone vaginal gel.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 29 Stroke or transient ischaemic attack.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 30 Venous thromboembolism (DVT or PE): oestrogen‐only HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 31 Venous thromboembolism (DVT or PE): combined sequential HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 32 Venous thromboembolism (DVT or PE): combined continuous HT.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 33 Venous thromboembolism (DVT or PE): oestrogen with or without sequential progesterone vaginal gel.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 34 Global cognitive function.

Comparison 1 Women without major health problems (selected outcomes: death, CVD, cognition, QOL), Outcome 35 Probable dementia.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 1 Death from any cause: oestrogen‐only HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 2 Death from any cause: oestrogen‐only or combined sequential HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 3 Death from any cause: combined continuous HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 4 Death from coronary heart disease: oestrogen‐only HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 5 Death from CHD: oestrogen‐only or combined sequential HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 6 Death from CHD: combined continuous HT.
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 7 Coronary event (MI or cardiac death): oestrogen‐only HT.
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 8 Death from stroke: oestrogen‐only or combined sequential HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 9 Death from cancer: combined continuous HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 10 Coronary event (MI or cardiac death): oestrogen‐only HT.
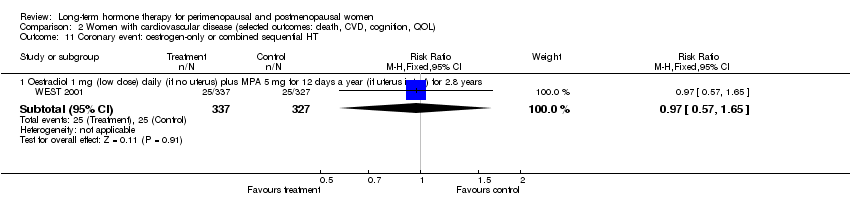
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 11 Coronary event: oestrogen‐only or combined sequential HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 12 Coronary event (MI or cardiac death): combined continuous HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 13 Stroke (first or recurrent): oestrogen‐only HT or combined sequential.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 14 Stroke (first or recurrent): oestrogen‐only HT (mod dose).

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 15 Stroke (first or recurrent): combined continuous HT (mod dose oestrogen).

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 16 Transient ischaemic attack: oestrogen‐only HT (mod dose).

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 17 Transient ischaemic attack: oestrogen‐only or combined sequential HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 18 Transient ischaemic attack: combined continuous HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 19 Stroke or transient ischaemic attack: oestrogen‐only HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 20 Stroke or transient ischaemic attack: combined continuous HT.

Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 21 VTE (first or recurrent PE or DVT): oestrogen‐only HT.
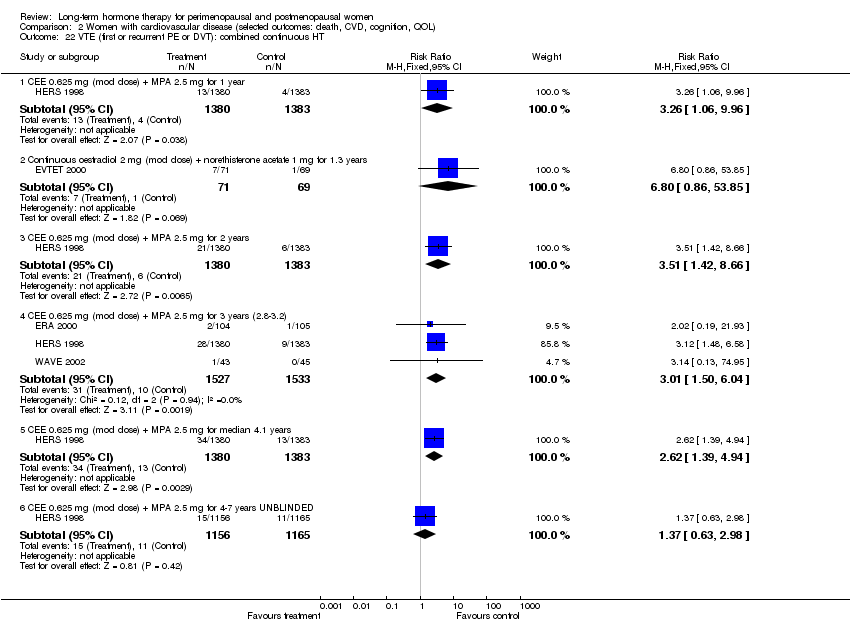
Comparison 2 Women with cardiovascular disease (selected outcomes: death, CVD, cognition, QOL), Outcome 22 VTE (first or recurrent PE or DVT): combined continuous HT.

Comparison 3 Women with dementia, Outcome 1 Worsening of dementia on treatment (by ADCS‐CGIC score): oestrogen‐only HT.
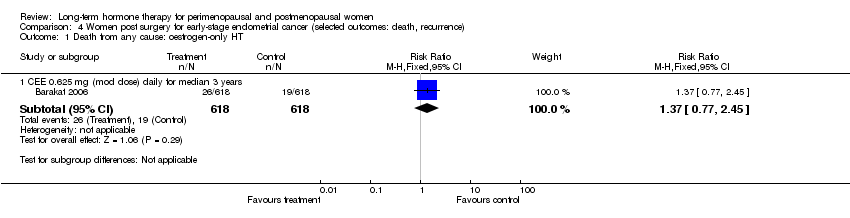
Comparison 4 Women post surgery for early‐stage endometrial cancer (selected outcomes: death, recurrence), Outcome 1 Death from any cause: oestrogen‐only HT.
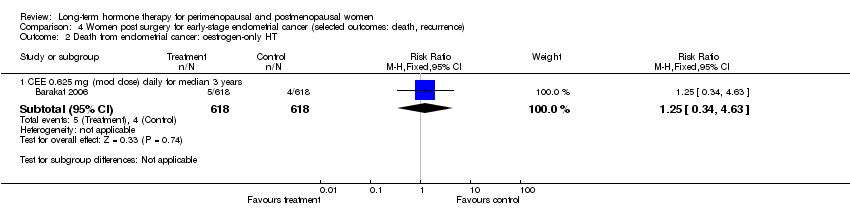
Comparison 4 Women post surgery for early‐stage endometrial cancer (selected outcomes: death, recurrence), Outcome 2 Death from endometrial cancer: oestrogen‐only HT.

Comparison 4 Women post surgery for early‐stage endometrial cancer (selected outcomes: death, recurrence), Outcome 3 Death from CHD: oestrogen‐only HT.

Comparison 5 Women hospitalised with chronic illness (selected outcomes: death, CVD, VTE), Outcome 1 All‐cause death: combined sequential HT.

Comparison 5 Women hospitalised with chronic illness (selected outcomes: death, CVD, VTE), Outcome 2 Myocardial infarction: combined sequential HT.

Comparison 5 Women hospitalised with chronic illness (selected outcomes: death, CVD, VTE), Outcome 3 Venous thromboembolism (DVT or PE): combined sequential HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 1 Breast cancer: oestrogen‐only HT.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 2 Breast cancer: oestrogen‐only or combined HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 3 Breast cancer: combined continuous HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 4 Breast cancer: combined sequential HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 5 Breast cancer: oestrogen with or without sequential progesterone vaginal gel.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 6 Colorectal cancer: oestrogen‐only HT.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 7 Colorectal cancer: oestrogen‐only or combined HT.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 8 Colorectal cancer: combined continuous HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 9 Colorectal cancer: combined sequential HT.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 10 Colorectal cancer: oestrogen with or without sequential progesterone vaginal gel.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 11 Lung cancer: oestrogen‐only HT (moderate dose).

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 12 Lung cancer: combined continuous HT (mod dose oestrogen).
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 13 Lung cancer: combined sequential HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 14 Endometrial cancer: oestrogen‐only HT.
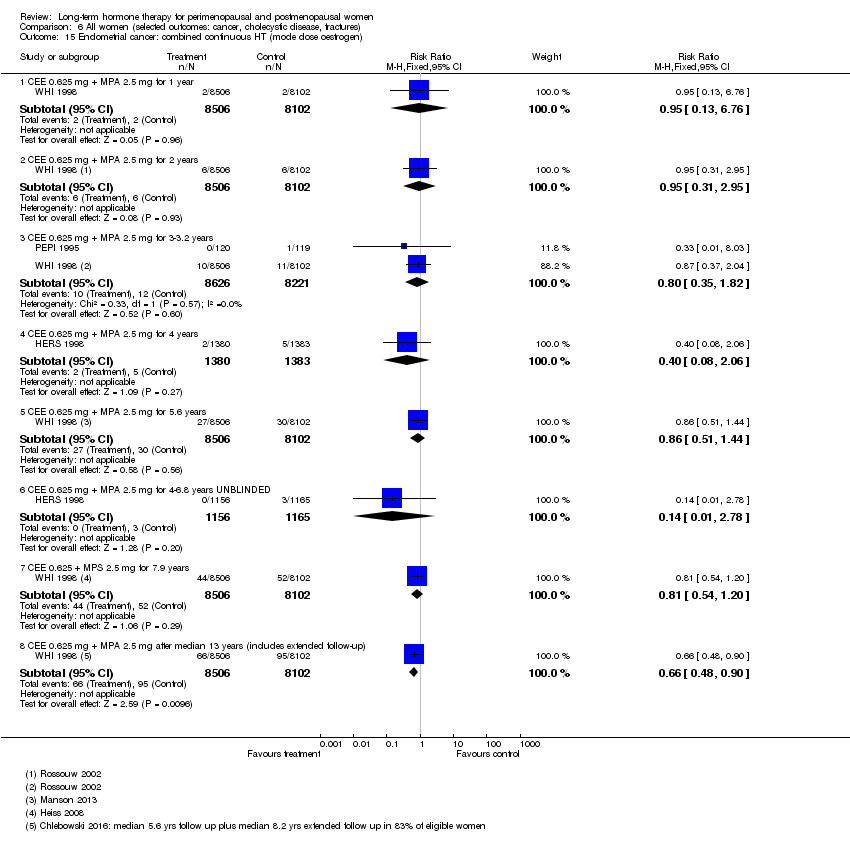
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 15 Endometrial cancer: combined continuous HT (mode dose oestrogen).

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 16 Endometrial cancer: combined sequential HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 17 Recurrent endometrial cancer: oestrogen‐only HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 18 Ovarian cancer: combined continuous HT.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 19 Ovarian cancer: oestrogen with or without sequential progesterone vaginal gel.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 20 Gallbladder disease requiring surgery: oestrogen‐only HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 21 Gallbladder disease requiring surgery: combined continuous HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 22 Gallbladder disease requiring surgery: combined sequential HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 23 Hip fractures: oestrogen‐only HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 24 Hip fractures: oestrogen‐only or combined sequential HT.
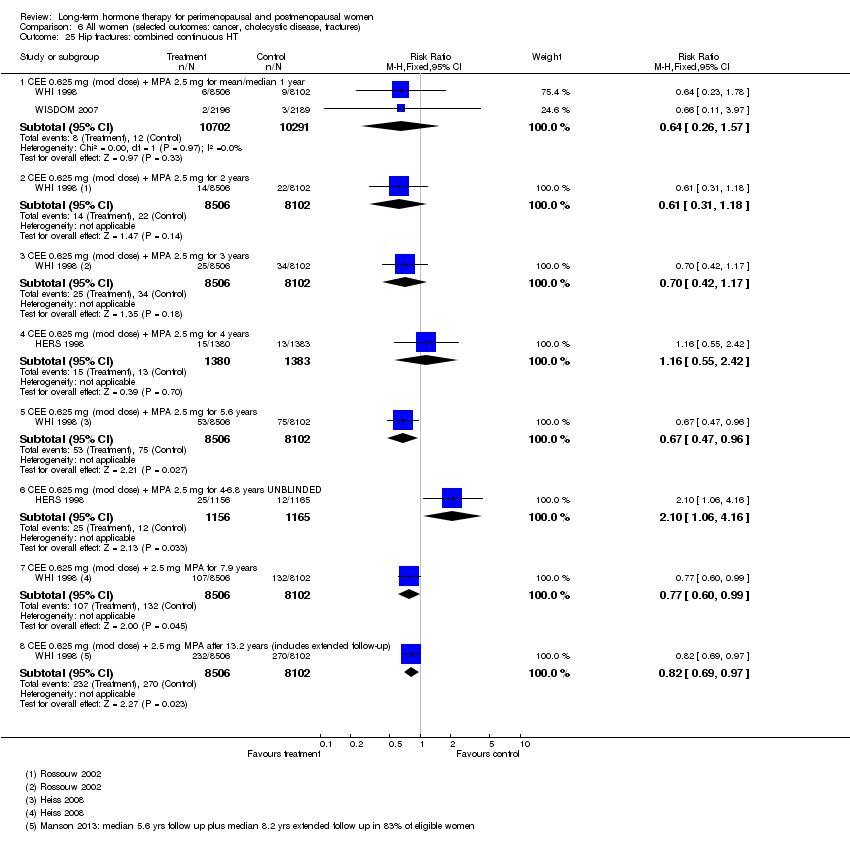
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 25 Hip fractures: combined continuous HT.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 26 Hip fractures: combined sequential HT.
Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 27 Vertebral fractures: oestrogen‐only HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 28 Vertebral fractures: combined continuous HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 29 All clinical fractures: oestrogen‐only or combined sequential HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 30 All clinical fractures: oestrogen‐only HT (moderate dose).

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 31 All clinical fractures: oestrogen‐only or combined HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 32 All clinical fractures: combined continuous HT.

Comparison 6 All women (selected outcomes: cancer, cholecystic disease, fractures), Outcome 33 All clinical fractures: combined sequential HT.
| Combined continuous hormone therapy (HT) compared with placebo for perimenopausal and postmenopausal women | ||||||
| Population: relatively healthy postmenopausal women Setting: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk* | Corresponding risk | |||||
| Placebo | Combined continuous hormone therapy (HT) | |||||
| Coronary events (MI or cardiac death) Follow‐up: mean/median 1 year | 2 per 1000 | 4 per 1000 | RR 1.89 | 20,993 | ⊕⊕⊕⊝ | |
| Stroke | 6 per 1000 | 8 per 1000 | RR 146 | 17,585 | ⊕⊕⊕⊝ | |
| Venous thromboembolism (DVT or PE) Follow‐up: mean/median 1 year | 2 per 1000 | 7 per 1000 | RR 4.28 | 20,993 | ⊕⊕⊕⊝ | |
| Breast cancer | 19 per 1000 | 24 per 1000 | RR 1.27 (1.03 to 1.56) | 16,608 | ⊕⊕⊕⊝ | |
| Death from lung cancer Follow‐up: median 8 yearsb | 5 per 1,000 | 9 per 1000 (6 to 13) | RR 1.74 (1.18 to 2.55) | 16,608 | ⊕⊕⊕⊝ | |
| Gallbladder disease Follow‐up: mean 5.6 years | 16 per 1000 | 27 per 1000 (21 to 34) | RR 1.64 (1.30 to 2.06) | 14,203 (1 study) | ⊕⊕⊕⊝ | |
| All clinical fractures | 111 per 1000 | 87 per 1000 | RR 0.78 | 16,608 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded one level for questionable applicability: Only about 33% of the study sample was 50‐59 years of age at baseline (i.e. the age women are most likely to consider HT for vasomotor symptoms); mean participant age was 63 years. b5.6 years' intervention plus postintervention follow‐up: post hoc analysis. | ||||||
| Oestrogen‐only hormone therapy (HT) compared with placebo for perimenopausal and postmenopausal women | ||||||
| Population: relatively healthy postmenopausal women Setting: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oestrogen‐only hormone therapy (HT) | |||||
| Coronary events (MI or cardiac death) | 41 per 1000 | 38 per 1000 | RR 0.94 | 10,739 | ⊕⊕⊕⊝ | |
| Stroke | 24 per 1000 | 32 per 1000 | RR 1.33 | 10,739 | ⊕⊕⊕⊝ | |
| Venous thromboembolism (DVT or PE) Follow up 1‐2 years | 2 per 1000 | 5 per 1000 (2 to 10) | RR 2.22 (1.12 to 4.39) | 10,739 | ⊕⊕⊕⊝ | |
| Venous thromboembolism (DVT or PE): CEE 0.625 mg (moderate dose) | 16 per 1000 | 21 per 1000 | RR 1.32 | 10,739 | ⊕⊕⊕⊝ | |
| Breast cancer | 25 per 1000 | 20 per 1000 | RR 0.79 | 10,739 | ⊕⊕⊕⊝ | |
| Gallbladder disease Follow‐up: mean 7.1 yearsa | 27 per 1000 | 47 per 1000 (38 to 60) | RR 1.78 (1.42 to 2.24) | 8376 | ⊕⊕⊕⊝ | |
| All clinical fractures | 141 per 1000 | 103 per 1000 | RR 0.73 | 10,739 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aMedian use of CEE 5.9 years (LaCroix 2011). | ||||||
| Study | How defined | Assessment | HT group | Placebo group | Note |
| Discontinuation of therapy for longer than a month (or use of HT in placebo group) | Not stated | 41.1% compliant for whole follow‐up period (median 3 years) | 50.1% compliant for whole follow‐up period (median 3 years) | ||
| > 80% of prescribed treatment taken | Pill counts | Median > 98% over median of 5 years | Median > 98% over median of 5 years | ||
| Percentage of study medication consumed | Pill counts | Level of adherence 95% in the 87% of participants evaluated | Level of adherence 92% in the 92% of participants evaluated | ||
| > 80% of prescribed treatment taken | Number of collected and returned drugs and clinic reports | < 40% compliant at 3 years (estimated from graph) | < 30% compliant at 3 years (estimated from graph) | ||
| Percentage of study medication taken | Pill counts | Level of adherence at 3.2 years: Women on combined HRT, measured in 82% of participants only: 84% | Level of adherence at 3.2 years: | ||
| "Regular tablet use" | Self‐report to family doctor. Self‐report to study nurse at 6 weeks and whenever in contact with trial staff | Number non‐adherent: | Number non‐adherent: | Triallists attribute higher non‐compliance in HRT group to prevalence of vaginal bleeding (reported by 56% in HRT group, 7% in controls) | |
| Adherence not described | |||||
| Adherence not described | |||||
| "Taking at least 80% of medication for at least 80% of entire study period" | Pill counts 6‐monthly | 90% adherent at 3 years | 94% adherent at 3 years | ||
| Taking at least 80% of study medication | Pill counts | 79% adherent at 1 year | 91% adherent at 1 year | Proportion of women who reported taking study medication at 1 year: | |
| Pill or patch counts, percentage used | Pill counts or weights | 94%‐95% in all groups, among women who completed trial at 4 years | |||
| Taking at least 80% of study medication | Plasma oestradiol level evaluation at each visit | No information given in publication | |||
| Adherence not described | |||||
| Adherence not described | |||||
| Adherence not described | |||||
| Taking at least 80% of study medication | Study diary reviewed at clinic visits | Number adherent at 36 months: Women with uterus: | Number adherent at 36 months: Women with uterus: 76% | ||
| Taking at least 80% of study medication | Pill counts weekly | No information given in publication | |||
| Percentage of study medication taken | Pill counts | At 2.8 years: | At 2.8 years: | ||
| Percentage of study medication taken | Self‐report to study nurse 3‐monthly | At 2.8 years: Mean adherence excluding dropouts: 90% | At 2.8 years: Mean adherence excluding dropouts: 90% 24% discontinued medication | ||
| WHI 1998 (unopposed oestrogen arm) | Taking at least 80% of study medication. Temporary discontinuation (e.g. during surgery) permitted | Weighing of returned medication bottles | At 6.8 years, about 53.8% of women were non‐adherent | At 6.8 years, about 53.8% of women were non‐adherent | |
| WHI 1998 (combined arm) | Taking at least 80% of study medication. Temporary discontinuation (e.g. during surgery) permitted | Weighing of returned medication bottles | 42% non‐adherent by 5.2 years | 10.7% crossed to active treatment by 5.2 years | Analyses censoring events 6 months after non‐adherence increased effect sizes |
| Supply of study medication | Time at risk minus temporary interruptions and time after withdrawal from treatment | 73% of time | 86% of time | Women had a 3 month run‐in period on placebo. Only women who took 80% of tablets were randomised | |
| Supply of study medication | Patch counts: 75% use over 2 years counted as compliance | 84% | 84% of time | Women had a 1 week run‐in period. Only compliant women were randomised. |
| Study | Comparison | Instrument | Measure | Outcome | Intervention | Effect |
| Oestrogen (CEE or oestradiol) + cyclic oral micronised progesterone 200 mg/d × 12 days per month vs placebo (n = 275) for 48 months | Modified Mini Mental State Examination (MMSE) | Differences between intervention and placebo groups in mean rate of change over time | Global cognition | 0.45 mg/d oral CEE (n = 230) | P = 0.178 | |
| 0.05 mg/d transdermal oestradiol (n = 222) | P = 0.840 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death from any cause: oestrogen‐only HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 1.2 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 1.3 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.13] |
| 2 Death from any cause: combined HT Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.76, 2.27] |
| 2.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.71, 1.56] |
| 2.3 CEE 0.625 mg (mod dose) + P (as per footnotes) for 3 years | 3 | 18075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.81, 1.46] |
| 2.4 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [0.15, 87.57] |
| 2.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.19] |
| 2.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.20] |
| 2.7 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.08] |
| 3 Death from any cause: oestrogen with or without sequential progesterone vaginal gel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.77] |
| 4 Death from coronary heart disease: oestrogen‐only HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestradiol 1 mg (low dose) daily for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 4.2 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.69, 1.38] |
| 4.3 CEE 0.625 mg (mod dose) after 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.19] |
| 5 Death from coronary heart disease: combined continuous HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
| 5.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.38] |
| 6 Death from coronary heart disease: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.27] |
| 7 Death from stroke: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 CEE 0.625 mg (low dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.58, 2.32] |
| 8 Death from stroke: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 9 Death from stroke: combined continuous HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 1 year | 1 | 4385 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [0.12, 73.37] |
| 9.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.46, 2.35] |
| 10 Death from colorectal cancer: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.66, 2.46] |
| 11 Death from breast cancer: combined continuous HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12 Death from breast cancer: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 CEE 0.625 mg (mod dose) after median 11.8 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.98] |
| 13 Death from colorectal cancer: combined continuous HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.40, 2.29] |
| 13.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 7.1 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.52, 1.96] |
| 13.3 CEE 0.0625 mg (mod dose) + MPA 2.5 mg after 11.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.80, 2.14] |
| 14 Death from lung cancer: oestrogen‐only HT (moderate dose) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Death from lung cancer (non‐small cell or small cell) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.70] |
| 14.2 Death from non‐small cell lung cancer | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.52, 1.50] |
| 14.3 Death from small cell lung cancer | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.62, 6.79] |
| 15 Death from lung cancer: combined continuous HT (moderate dose) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Death from lung cancer (non‐small cell or small cell) at mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.18, 2.55] |
| 15.2 Death from non‐small cell lung cancer at mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.24, 2.93] |
| 15.3 Death from small cell lung cancer at mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.48, 2.81] |
| 15.4 Death from lung cancer (any type) at median 14 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.88, 1.39] |
| 16 Death from lung cancer: combined sequential HT (low dose oestrogen) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 17 Death from any cancer: combined continuous HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 CEE O.625 mg daily (mod dose) + MPA 2.5 mg for 3 years | 1 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.11, 67.80] |
| 17.2 CEE 0.625 mg daily (mod dose) + MPA 2.5 mg for mean 5.2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.87, 1.53] |
| 18 Coronary events (MI or cardiac death): oestrogen‐only HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.43] |
| 18.2 CEE 0.625 mg (mod dose) for 3 years | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.12, 72.72] |
| 18.3 CEE 0.625 mg (mod dose) for mean 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.13] |
| 18.4 CEE 0.65 (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 19 Coronary events (MI or cardiac death): combined continuous HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.15, 3.10] |
| 19.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.05, 2.12] |
| 19.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 2 | 17385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.07, 1.98] |
| 19.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.95, 1.44] |
| 19.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| 20 Coronary events (MI or cardiac death): combined sequential HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.89 [0.24, 101.09] |
| 20.2 1 mg (low dose) 17‐B‐oestradiol daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.27] |
| 20.3 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.71 [0.15, 90.70] |
| 21 Coronary events (MI or cardiac death): oestrogen with or without sequential progesterone vaginal gel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.16] |
| 22 Stroke: unopposed oestrogen Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.86] |
| 22.2 CEE 0.625 mg (mod dose) for mean 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.06, 1.67] |
| 22.3 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.97, 1.40] |
| 23 Stroke: combined continuous HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 1 year | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.86] |
| 23.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.83, 2.06] |
| 23.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 2 | 17385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.02, 2.09] |
| 23.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.09, 1.77] |
| 23.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.06, 1.56] |
| 23.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.99, 1.33] |
| 24 Stroke: combined sequential HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 1 mg (low dose) 17‐B‐oestradiol daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 24.2 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.14] |
| 25 Stroke: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.51] |
| 26 Transient ischaemic attack: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.86] |
| 27 Transient ischaemic attack: combined sequential HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 16.13] |
| 27.2 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.14] |
| 28 Transient ischaemic attack: oestrogen with or without sequential progesterone vaginal gel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Oestradiol 1 mg daily,with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.44] |
| 29 Stroke or transient ischaemic attack Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 1 | 4385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.46] |
| 30 Venous thromboembolism (DVT or PE): oestrogen‐only HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 CEE 0.625 mg (mod dose) for up to 2 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.12, 4.39] |
| 30.2 CEE 0.625 mg (mod dose) for 3 years | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.96 [0.36, 133.75] |
| 30.3 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.00, 1.74] |
| 30.4 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.29] |
| 31 Venous thromboembolism (DVT or PE): combined sequential HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 1 mg 17‐B‐oestradiol (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.46] |
| 31.2 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.14] |
| 31.3 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.73] |
| 31.4 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.08, 19.69] |
| 32 Venous thromboembolism (DVT or PE): combined continuous HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.28 [2.49, 7.34] |
| 32.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [1.88, 4.71] |
| 32.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.73, 3.72] |
| 32.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.55, 2.64] |
| 32.5 CEE 0.625 mg (mod dose) + 2.5 mg MPA for mean 7.9 years | 1 | 16707 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.32, 2.05] |
| 33 Venous thromboembolism (DVT or PE): oestrogen with or without sequential progesterone vaginal gel Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 33.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.25, 8.83] |
| 34 Global cognitive function Show forest plot | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 34.1 Transdermal estradiol 0.014 mg (low dose): MMSE scores (baseline MMSE ≤ 90) | 1 | Mean Difference (Fixed, 95% CI) | ‐1.21 [‐5.05, 2.63] | |
| 34.2 Transdermal estradiol 0.014 mg (low dose): MMSE scores (baseline MMSE > 90) | 1 | Mean Difference (Fixed, 95% CI) | ‐0.3 [‐0.73, 0.13] | |
| 34.3 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years: MMSE scores | 1 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐0.35, 0.15] | |
| 34.4 CEE 0.625 mg (mod dose) for mean 5.2 years: MMSE scores | 1 | Mean Difference (Fixed, 95% CI) | ‐0.26 [‐0.52, 0.00] | |
| 34.5 Combined continuous CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 4.2 years: MMSE scores | 1 | Mean Difference (Fixed, 95% CI) | ‐0.18 [‐0.36, 0.00] | |
| 34.6 Oestrogen with or without sequential progesterone vaginal gel | 1 | Mean Difference (Fixed, 95% CI) | ‐0.03 [‐0.21, 0.15] | |
| 35 Probable dementia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 CEE 0.625 mg (mod dose) for 5.2 years | 1 | 2947 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.89, 2.59] |
| 35.2 Combined continuous CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 4.05 years | 1 | 4532 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.16, 3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death from any cause: oestrogen‐only HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 CEE 0.625 mg (mod dose) daily for 3 years (2.8‐3.2) | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.53, 3.22] |
| 1.2 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.27] |
| 2 Death from any cause: oestrogen‐only or combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.67] |
| 3 Death from any cause: combined continuous HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2.8‐3.2 years | 2 | 297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.28, 2.62] |
| 3.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 3.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐7 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.90, 1.44] |
| 4 Death from coronary heart disease: oestrogen‐only HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 CEE 0.625 mg (mod dose) daily for 2.8‐3.2 years | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.36, 4.77] |
| 4.2 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.18] |
| 5 Death from CHD: oestrogen‐only or combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.81] |
| 6 Death from CHD: combined continuous HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 1 year | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.73, 3.29] |
| 6.2 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 2 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.90, 2.51] |
| 6.3 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 3 years (2.8‐3.2) | 3 | 3060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.88, 1.90] |
| 6.4 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 4+ years (median 4.1) | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.85, 1.67] |
| 6.5 CEE 0.625 mg (mod dose) daily + MPA 2.5 mg for 4‐6.8 years | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.39] |
| 7 Coronary event (MI or cardiac death): oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.39] |
| 8 Death from stroke: oestrogen‐only or combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.95, 8.93] |
| 9 Death from cancer: combined continuous HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 CEE 0.625 mg daily (mod dose) + MPA 2.5 mg for 4+ years (median 4.1) | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.49, 1.57] |
| 9.2 CEE 0.625 mg daily (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.86, 2.65] |
| 10 Coronary event (MI or cardiac death): oestrogen‐only HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 CEE 0.625 (mod dose) daily for 2.8‐3.2 years | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.54, 2.40] |
| 11 Coronary event: oestrogen‐only or combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.57, 1.65] |
| 12 Coronary event (MI or cardiac death): combined continuous HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 1 year | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.00, 2.25] |
| 12.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.91, 1.58] |
| 12.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years (2.8‐3.2) | 3 | 3060 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.33] |
| 12.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 4.1 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.81, 1.19] |
| 12.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.29] |
| 13 Stroke (first or recurrent): oestrogen‐only HT or combined sequential Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Oestradiol 1 mg daily (low dose) (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 14 Stroke (first or recurrent): oestrogen‐only HT (mod dose) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 CEE 0.625 mg (mod dose) daily for 2.8 years | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
| 14.2 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.60, 4.47] |
| 15 Stroke (first or recurrent): combined continuous HT (mod dose oestrogen) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Continuous oestradiol 2 mg (mod dose) + norethisterone acetate 1 mg for 1.3 years | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.82] |
| 15.2 CEE 0.625 mg (mod dose) + MPA for 2.8 years | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.23 [0.26, 105.85] |
| 15.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 4.1 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.90, 1.68] |
| 15.4 CEE 0.625 mg (mod dose) + MPA for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.71, 1.57] |
| 16 Transient ischaemic attack: oestrogen‐only HT (mod dose) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.54, 2.36] |
| 17 Transient ischaemic attack: oestrogen‐only or combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.70, 1.94] |
| 18 Transient ischaemic attack: combined continuous HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.23] |
| 18.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.84] |
| 19 Stroke or transient ischaemic attack: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 CEE 0.625 mg (mod dose) daily for 3.2 years | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.28, 2.78] |
| 20 Stroke or transient ischaemic attack: combined continuous HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3.2 years | 1 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.34, 3.03] |
| 21 VTE (first or recurrent PE or DVT): oestrogen‐only HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.33, 4.55] |
| 21.2 CEE 0.625 mg (mod dose) daily for 2.8‐3.2 years | 2 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.44, 6.17] |
| 22 VTE (first or recurrent PE or DVT): combined continuous HT Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 1 year | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [1.06, 9.96] |
| 22.2 Continuous oestradiol 2 mg (mod dose) + norethisterone acetate 1 mg for 1.3 years | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.80 [0.86, 53.85] |
| 22.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.51 [1.42, 8.66] |
| 22.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years (2.8‐3.2) | 3 | 3060 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [1.50, 6.04] |
| 22.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 4.1 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.39, 4.94] |
| 22.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐7 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.63, 2.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Worsening of dementia on treatment (by ADCS‐CGIC score): oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Unopposed CEE 0.625 mg (mod dose) or 1.25 mg (high dose) daily for 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death from any cause: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 CEE 0.625 mg (mod dose) daily for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.77, 2.45] |
| 2 Death from endometrial cancer: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 CEE 0.625 mg (mod dose) daily for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.63] |
| 3 Death from CHD: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 0.625 mg (mod dose) daily for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause death: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.60] |
| 2 Myocardial infarction: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.14] |
| 3 Venous thromboembolism (DVT or PE): combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breast cancer: oestrogen‐only HT Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen only HRT patch 0.025 (low dose) mg daily for 2 years | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.04] |
| 1.2 Oestradiol 1 mg (low dose) for 2 years | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 1.3 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.25, 3.91] |
| 1.4 Oestradiol patch 0.075 mg (high dose) for 2 years | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.12, 71.04] |
| 1.5 CEE 0.625 mg (mod dose) for 2.8‐3.2 years | 3 | 676 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.38, 11.04] |
| 1.6 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.61, 1.01] |
| 1.7 CEE 0.625 mg (mod dose) after 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.63, 0.96] |
| 1.8 CEE 0.625 mg (mod dose) after 13 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.65, 0.97] |
| 2 Breast cancer: oestrogen‐only or combined HT Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Breast cancer: combined continuous HT Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 23182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.96] |
| 3.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.47, 1.08] |
| 3.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2.8‐3.4 years | 3 | 17733 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.18] |
| 3.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.82, 2.27] |
| 3.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.03, 1.56] |
| 3.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐7 years unblinded | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.52, 2.23] |
| 3.7 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 7.9 years | 1 | 16607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.07, 1.52] |
| 3.8 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 11 years (includes extra follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.08, 1.45] |
| 3.9 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.11, 1.47] |
| 4 Breast cancer: combined sequential HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.85] |
| 4.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.91 [0.44, 34.64] |
| 4.3 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.30, 10.64] |
| 4.4 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.31, 11.02] |
| 4.5 CEE 2.5 mg daily (high dose) + MPA 10 mg for 7 days each cycle for 10 years | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.03] |
| 5 Breast cancer: oestrogen with or without sequential progesterone vaginal gel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.10] |
| 6 Colorectal cancer: oestrogen‐only HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 CEE 0.625 mg (mod dose) for 3 years | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.08] |
| 6.2 CEE 0.625 (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.81, 1.63] |
| 6.3 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.82, 1.49] |
| 7 Colorectal cancer: oestrogen‐only or combined HT Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Colorectal cancer: combined continuous HT Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.32, 1.42] |
| 8.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.50] |
| 8.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 2 | 16956 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.49, 1.34] |
| 8.4 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.32, 1.48] |
| 8.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.91] |
| 8.6 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.46, 1.44] |
| 8.7 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 7.9 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.57, 1.01] |
| 8.8 CEE 0.0625 mg (mod dose) + MPA 2.5 mg after 11.6 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.99] |
| 8.9 CEE 0.0625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.63, 1.01] |
| 9 Colorectal cancer: combined sequential HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.13] |
| 9.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 9.3 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle for 10 years | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.73] |
| 10 Colorectal cancer: oestrogen with or without sequential progesterone vaginal gel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.25, 8.83] |
| 11 Lung cancer: oestrogen‐only HT (moderate dose) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Any lung cancer (non‐small cell or small cell) at 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.48] |
| 12 Lung cancer: combined continuous HT (mod dose oestrogen) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Any lung cancer at 5.6 years (non‐small cell or small cell) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 12.2 Any lung cancer at 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.92, 1.62] |
| 12.3 Any lung cancer after median 14 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.93, 1.38] |
| 13 Lung cancer: combined sequential HT Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 17‐B‐oestradiol 1 mg (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.13, 78.13] |
| 14 Endometrial cancer: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 CEE 0.625 mg (mod dose) for 3‐3.2 years | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 15 Endometrial cancer: combined continuous HT (mode dose oestrogen) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 CEE 0.625 mg + MPA 2.5 mg for 1 year | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.13, 6.76] |
| 15.2 CEE 0.625 mg + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.31, 2.95] |
| 15.3 CEE 0.625 mg + MPA 2.5 mg for 3‐3.2 years | 2 | 16847 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.35, 1.82] |
| 15.4 CEE 0.625 mg + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
| 15.5 CEE 0.625 mg + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.51, 1.44] |
| 15.6 CEE 0.625 mg + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.78] |
| 15.7 CEE 0.625 + MPS 2.5 mg for 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.54, 1.20] |
| 15.8 CEE 0.625 mg + MPA 2.5 mg after median 13 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.48, 0.90] |
| 16 Endometrial cancer: combined sequential HT Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 17‐B‐oestradiol 1 mg (low dose) + dydrogesterone 5 mg days 14‐28 for 2 years | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.08, 45.95] |
| 16.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.03] |
| 16.3 Oestradiol 2 mg (mod dose) + dihydrogesterone 20 mg for 2 years | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [0.16, 67.59] |
| 16.4 CEE 0.045 mg (lowish dose) + 200 mg sequential progesterone for 4 years | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.97 [0.29, 123.81] |
| 16.5 Oestradiol patch 0.05 mg (mod dose) + 200 mg sequential progesterone for 4 years | 1 | 497 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.71 [0.15, 90.70] |
| 16.6 CEE 2.5 mg (high dose) daily + MPA 10 mg for 7 days each cycle for 10 years | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.07] |
| 17 Recurrent endometrial cancer: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Oestrogen (type and dose not stated) for median 3 years | 1 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.54, 2.50] |
| 18 Ovarian cancer: combined continuous HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.76, 2.69] |
| 18.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 19 Ovarian cancer: oestrogen with or without sequential progesterone vaginal gel Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Oestradiol 1 mg daily, with or without cyclic 4% vaginal progesterone gel | 1 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.08] |
| 20 Gallbladder disease requiring surgery: oestrogen‐only HT Show forest plot | 3 | 8930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.40, 2.19] |
| 20.1 CEE 0.625 mg (mod dose) for 3‐3.2 years | 2 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.17, 3.39] |
| 20.2 CEE O.625 mg (mod dose) for 7.1 years | 1 | 8376 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.42, 2.24] |
| 21 Gallbladder disease requiring surgery: combined continuous HT Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 3 years | 2 | 557 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.61, 6.59] |
| 21.2 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 4 years | 1 | 2253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.98, 1.85] |
| 21.3 CEE 0.625 mg (mod dose) + 2.5 mg MPA for mean 5.6 years | 1 | 14203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.30, 2.06] |
| 22 Gallbladder disease requiring surgery: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 CEE 0.625 mg (mod dose) daily + MPA 10 mg days 1‐12 for 3 years | 1 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.37, 10.78] |
| 22.2 CEE 0.625 mg (mod dose) daily + micronised progesterone 200 mg days 1‐12 for 3 years | 1 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.25, 8.67] |
| 23 Hip fractures: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.95] |
| 23.2 CEE 0.625 mg (mod dose) for 10.7 years (includes extra follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 23.3 CEE 0.625 mg (mod dose) after 13.2 years (includes extended follow‐up) | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.74, 1.17] |
| 24 Hip fractures: oestrogen‐only or combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 24.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.42] |
| 25 Hip fractures: combined continuous HT Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean/median 1 year | 2 | 20993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.26, 1.57] |
| 25.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 2 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.18] |
| 25.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.17] |
| 25.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.55, 2.42] |
| 25.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.47, 0.96] |
| 25.6 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.06, 4.16] |
| 25.7 CEE 0.625 mg (mod dose) + 2.5 mg MPA for 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.99] |
| 25.8 CEE 0.625 mg (mod dose) + 2.5 mg MPA after 13.2 years (includes extended follow‐up) | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.97] |
| 26 Hip fractures: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 17‐B‐oestradiol 1 mg (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.27] |
| 27 Vertebral fractures: oestrogen‐only HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.44, 0.94] |
| 28 Vertebral fractures: combined continuous HT Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.37, 1.47] |
| 28.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.96] |
| 28.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.49, 2.48] |
| 28.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.60, 1.01] |
| 29 All clinical fractures: oestrogen‐only or combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 Oestradiol 1 mg (low dose) daily (if no uterus) plus MPA 5 mg for 12 days a year (if uterus intact) for 2.8 years | 1 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.68, 2.19] |
| 30 All clinical fractures: oestrogen‐only HT (moderate dose) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Oestradiol valerate 2 mg (mod dose) for 2 years | 1 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| 30.2 CEE 0.625 mg (mod dose) daily for 3.2 years | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.04] |
| 30.3 CEE 0.625 mg (mod dose) for 7.1 years | 1 | 10739 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.80] |
| 31 All clinical fractures: oestrogen‐only or combined HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 31.1 CEE 0.625 mg (mod dose) with or without 2.5 mg MPA for 3 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 32 All clinical fractures: combined continuous HT Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 CEE 0.625 mg (mod dose) + MPA 2.5 mg for median 1 year | 1 | 4385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.46, 1.02] |
| 32.2 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 3.2‐3.4 years | 2 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.32, 0.87] |
| 32.3 CEE 0.625 mg (mod dose) + MPA 2.5 mg for mean 5.6 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.71, 0.86] |
| 32.4 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4 years | 1 | 2763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 32.5 CEE 0.625 mg (mod dose) + MPA 2.5 mg for 4‐6.8 years UNBLINDED | 1 | 2321 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.91, 1.65] |
| 32.6 CEE 0.0625 mg (mod dose) + MPA 2.5 mg for mean 7.9 years | 1 | 16608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.76, 0.89] |
| 33 All clinical fractures: combined sequential HT Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 17‐B‐oestradiol 1 mg (low dose) daily plus (3 days weekly) 0.35 mg norethindrone for 2 years | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.64] |

