Частичное облучение молочной железы на ранней стадии рака
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Phase III RCT Single‐centred, tertiary institution Country: Italy Median follow‐up: 68 months | |
| Participants | Women aged 48‐75 years with early breast cancer, maximum tumour diameter 2.5 cm, "suitable for breast conservation". All women with positive sentinel node biopsy had axillary dissection | |
| Interventions | Experimental arm: intraoperative electron therapy to deliver 21 Gy at the 90% isodose delivered at the time of surgery after tumour excision using 6‐9 MeV Control arm: postoperative EBRT (50 Gy/25 fractions + 10 Gy/5 fraction boost using electrons) | |
| Outcomes |
| |
| Notes | Target volume: 4‐12 MeV to 90% isodose 10‐30 mm around sutured surgical breach NB: women with ≥ 4 involved nodes were treated with RNI (50 Gy/25 fractions). Adjuvant therapies were administered according to the European Institute of Oncology policy at the time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the data centre, allocation was done by telephone with a computer‐generated list using a randomly permuted block design, stratified by tumour size (<1.0 cm vs 1.0‐1.4 cm vs ≥1.5 cm)", page 1270, paragraph 3 This method represented an adequate randomization method, therefore, we judged this domain at low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Immediately before the intervention, the surgeon contacted the data centre by telephone to receive the allocation group. At the data centre, allocation was done by telephone", page 1270, paragraph 3 Because the details of how this was done were not reported, we judged this domain at unclear risk of bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Study coordinators, clinicians who verified eligibility criteria after pathological assessment of the surgical specimen, clinicians who followed up patients, investigators who did the statistical analyses, and the patients themselves were aware of the assignment", page 1270, paragraph 3 We judged this domain at low risk of bias |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "Study coordinators... clinicians who followed up patients... and the patients themselves were aware of the assignment", page 1270, paragraph 3 We judged this domain at low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "We defined local recurrence as the reappearance of the carcinoma at the site of the surgical intervention. We defined second ipsilateral breast tumours as any new carcinoma appearing in other quadrants of the same breast. IBTR was defined as the sum of local recurrence plus second ipsilateral tumours. A regional nodal failure included any recurrence in the ipsilateral axillary, supraclavicular, or internal mammary nodal regions. Distant metastases were defined as any recurrence to distant organs. Overall survival was defined as the time from diagnosis to last follow‐up or time of death" Quote: "Patients were followed up with a clinical examination every 3 months, an ultrasound mammary scan every 6 months, and a mammogram every year; examinations of the lung, liver, and bone were modulated according to a personalised assessment of risk" Quote: "investigators who did the statistical analyses", page 1270, paragraph 3 Although the outcome assessors for objective outcomes were not blinded, the clear pre‐specified definitions of what constituted outcomes and the pre‐specified follow‐up protocol reduced the risk of bias for this domain |
| Blinding of outcome assessment (detection bias) | High risk | Quote: ''Study coordinators, clinicians who verified eligibility criteria after pathological assessment of the surgical specimen, clinicians who followed up patients, investigators who did the statistical analyses, and the patients themselves were aware of the assignment", page 1270, paragraph 3 Quote: "Side‐effects were scored using the Late Effect of Normal Tissue‐ Subjective Objective Management Analytic criteria" Because the assessment of subjective outcomes was not blinded, we judged this domain at high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions were reported and there was no post‐randomization attrition (see Figure 1) so we judged this domain at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Outcomes specified in paper:
Outcomes reported:
We did not have access to the study protocol, so judged this domain at unclear risk of bias |
| Other bias | Low risk | No other sources of bias identified |
| Methods | Phase III RCT Open‐label trial Country: Germany Median follow‐up: 79.2 months | |
| Participants | Women aged > 40 years with Stage 0, I or II breast cancer (including DCIS), no lymph or vascular invasion, lesions < 3 cm in diameter, pN0/pNmi, DCIS alone, sentinel node biopsy optional. Clear margin (≥ 2 mm in invasive disease, 5 mm in DCIS), unifocal or unicentric disease only | |
| Interventions | Experimental arm: APBI Interstitial brachytherapy HDR 32 Gy/8 fractions or 30.3 Gy/7 fractions PDR 50 Gy at 0.6‐0.8 Gy/fractions given hourly Control arm: external beam WBRT 50.0‐50.4 Gy/1.8‐2.0 Gy fractions (5‐28) plus 10 Gy/5 fraction boost | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Target volume: tumour bed plus 20‐ to 30‐mm radial margin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was stratified by study centre, menopausal status, and tumour type (e.g., invasive carcinoma vs DCIS), with a block size of ten, according to an automated dynamic algorithm", page 3, randomization and masking, paragraph 1 We judged this at low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised centrally at the Department of Medical Informatics, Biometry and Epidemiology, University Erlangen‐Nuremberg, Germany, via an online interface", page 3, randomization and masking, paragraph 1 This process was described as concealed and remote, so we judged this at low risk of bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Neither patients nor investigators were masked to treatment allocation", page 3, randomization and masking, paragraph 1 Although participants and personnel were not blinded, it is unlikely to have introduced bias, so we judged this domain at low risk of bias |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "Neither patients nor investigators were masked to treatment allocation", page 3, randomization and masking, paragraph 1 Although participants and personnel were not blinded, it is unlikely to have introduced bias, so we judged this domain at low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Follow up mammography was scheduled at 6, 12, 18 and 24 months after radiation therapy", page 3, paragraph 8 Although outcome assessors were not blinded, we considered the pre‐specified follow‐up protocol meant this domain was at low risk of bias |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Clinical examination included documentation of late side‐effects with Common Terminology Criteria for Adverse Events and with the Radiation Therapy Oncology Group (RTOG)/European Organisation for Research and Treatment of Cancer (EORTC) Late Radiation Morbidity Scoring Schema 14", page 3 Blinding of outcome assessors was not mentioned, we thought despite the pre‐specified follow‐up schema and the use of a Grading system for documenting late effects, meant this domain was at high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "after randomisation, 98 patients...administrative error", page 4, paragraph 1 Post‐randomization exclusions are detailed by arm, with reasons, so we deemed this outcome at low risk of bias |
| Selective reporting (reporting bias) | Low risk | Quote: "Detailed analyses of early and late side‐effects, quality of life, and cosmetic results are not presented here", page 4, paragraph 1 Although detailed reporting of acute and late adverse effects and quality of life were not in this publication, the authors made it clear there will be further publications, so we judged this at low risk of bias. We had access to the study protocol |
| Other bias | Low risk | We did not consider there was other bias, so judged this domain at low risk of bias. |
| Methods | RCT Single centre Setting: cancer centre Country: Italy Median follow‐up: 60 months | |
| Participants | Women aged > 40 years, wide local excision or quadrantectomy for invasive breast cancer, negative margins, tumour size ≤ 25 mm | |
| Interventions | PBI/APBI (using IMRT) vs. WBRT (conventional RT) | |
| Outcomes | Not specified in report | |
| Notes | Study has not completed accrual (target 520 women) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to receive either WBI or APBI using IMRT in a 1:1 ratio. Allocation was performed with a computer‐generated sequence using a randomly permuted block design, without any stratification of main prognostic factors", page 453, paragraph 1 We judged this at low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "The random sequence was kept by an external centre (local Oncological Centre for Departmental Reference, CORD). The clinicians were required to query it every time an eligible patient had provided written informed consent to determine the allocation arm", page 453, paragraph 1 We judged this at low risk of bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "Clinicians, investigators and the patients themselves were aware of the arm assignment", page 453, paragraph 1 Blinding would have been difficult in view of the 2 very obviously different treatments, we judged this domain at low risk of bias |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: "Clinicians, investigators and the patients themselves were aware of the arm assignment", page 453, paragraph 1 Blinding would have been difficult in view of the 2 very obviously different treatments, we judged this domain at low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Clinicians and investigators were not blinded to treatment arm, but the pre‐specified mammographic follow‐up would have reduced the risk of bias related to the primary outcome |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "mammography was annually programmed", page 454, paragraph 13 The clinicians and investigators were not blinded to treatment arm, which makes assessment of subjective outcomes at high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No exclusions or attrition reported, so we judged this domain at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | We did not review the protocol, so judged this domain at unclear risk of bias |
| Other bias | Low risk | We did not identify any other sources of bias |
| Methods | RCT Single‐centre trial Country: Hungary Accrual dates July 1998 to May 2004 Median follow‐up: 10.2 years | |
| Participants | 258 women with invasive breast cancer Inclusion criteria: wide excision with negative margins, unifocal tumour, tumour size < 20 mm, clinically or pathologically N0, or single microscopic nodal metastasis (> 0.2 mm and < 2.0 mm), i.e. pT1N0‐1miM0, Grade I or II Exclusion criteria: bilateral breast cancer, prior unilateral or contralateral breast cancer, concomitant or previous other malignancies, invasive lobular cancer, pure ductal or lobular cancer in situ (pTis). After 2001, women aged < 40 years excluded Mean age 58‐59 years (given for each arm) | |
| Interventions | Experimental arm: PBI; 88/128 women had 7 × 5.2 Gy HDR multi‐catheter brachytherapy and 40/128 women unsuitable for HDR had 50 Gy/25 fractions electron beam RT to partial breast Control arm: 50 Gy/25 fractions WBRT (130 women) Surgery: wide excision (resection of tumour with ≥ 1 cm macroscopic free margin). Cavity marked with titanium clips Central pathology review performed Systemic therapy given according to institutional protocol Baseline mammography was performed at 6 months after RT then annually. Women were seen every 3 months in the first year, then once every 6 months | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Early stopping at 258 women enrolled because another multicentred trial commenced LR defined as any detection of cancer in the treated breast, confirmed histologically. An "elsewhere breast failure" defined as ipsilateral (LR) ≥ 2 cm from the clips. All other LR classified as true recurrence or marginal miss Cosmetic score Harvard criteria, scored by treating radiation oncologist and chief investigator at analysis date (June to August 2006). In case of discrepancy, worst score used for analysis Event‐free intervals defined as time between date of surgery and date of event or last follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomised". Polgár 2007, page 694, paragraph 3 Quote: "randomly allocated to treatment options by a sealed envelope system in blocks of 10" Randomization was done by the main investigator (C.P.) Polgár 2007, page 695, paragraph 2 The trial was likely to have been randomized |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly allocated to treatment options by a sealed envelope system in blocks of 10". Polgár 2007, page 695, paragraph 2 Allocation concealment appears to have been done, although the description was incomplete, which contributed to the judgement of unclear bias risk |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants: not mentioned, unlikely to have been done Quote: "Blinding of physicians performing treatments and follow‐up of patients was not possible for technical reasons". Polgár 2007, page 695, paragraph 2 Physicians: not done Quote: "Blinding of physicians performing treatments and follow‐up of patients was not possible for technical reasons". Polgár 2007, page 695, paragraph 2 We judged this domain at low risk of bias |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants: not mentioned, unlikely to have been done Quote: "Blinding of physicians performing treatments and follow‐up of patients was not possible for technical reasons". Polgár 2007, page 695, paragraph 2 Physicians: not done Quote: "Blinding of physicians performing treatments and follow‐up of patients was not possible for technical reasons". Polgár 2007, page 695, paragraph 2 We judged this domain at low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Participants: not mentioned, unlikely to have been done Quote: "Blinding of physicians performing treatments and follow‐up of patients was not possible for technical reasons". Polgár 2007, page 695, paragraph 2 Physicians: not done, but in view of pre‐specified follow‐up protocol, with regular mammography, unlikely to have introduced bias Quote: "Patients were seen every three months in the first two years after RT and every six months thereafter. Baseline mammography was performed six months after completion of RT and yearly thereafter". Polgár 2007, page 697, paragraph 6 Quote: "Blinding of physicians performing treatments and follow‐up of patients was not possible for technical reasons". Polgár 2007, page 695, paragraph 2 Quote: "Local recurrence...proved by histological confirmation in every case". Polgár 2007, page 697, paragraph 6 Assessors: not done Unlikely to be a source of bias in view of the pre‐specified schedule for follow‐up visits and investigations. Local recurrence required biopsy confirmation, which would reduce the risk of bias in evaluation of this outcome |
| Blinding of outcome assessment (detection bias) | High risk | Participants: not mentioned, unlikely to have introduced bias Physicians: not mentioned, may be a source of bias Assessors: not mentioned, unlikely to have been done, this is potentially a source of bias Quote: "Cosmetic outcome scored independently by treating radiation oncologist and the main investigator…in the case of discrepancy, the worse cosmetic score was used for analysis". Polgár 2007, page 697, paragraph 5 |
| Incomplete outcome data (attrition bias) | Low risk | Exclusions: none Attrition: 0 in experimental group, 2 in control group (declined follow‐up at 18 and 22 months postoperatively) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes in methods section:
Outcomes reported in paper:
Outcomes in methods and protocol: protocol not reviewed |
| Other bias | Low risk | Trial stopped early (the trial enrolled 258 women of a planned sample size of 570 participants) because of a competing trial, GEC‐ESTRO, started recruiting |
| Methods | Phase III RCT; stratified for age, tumour histology, tumour size, adjuvant hormonal therapy and clinical centre Country: Canada, Australia, New Zealand Median follow‐up: 36 months | |
| Participants | Women aged ≥ 40 years with new diagnosis of DCIS or with microscopically clear margins after BCS of non‐invasive or invasive disease (or no residual disease on re‐excision). Negative axillary nodal involvement including micrometastasis (> 0.2 mm or positive cells only identified on IHC as determined by sentinel node biopsy; axillary node dissection; or clinical examination for DCIS only. Tumour size ≤ 3 cm | |
| Interventions | Experimental arm: APBI (3D‐CRT: 38.5 Gy in 10 fractions, bd over 5‐8 days. 6‐8 hour gap between doses required) Control arm: WBRT (42.5 Gy in 16 fractions daily over 22 days). Women with large breast size: 50 Gy in 25 fractions over 25 days. Boost 10 Gy in 4 or 5 fractions over 4‐7 days is permitted for those women deemed at moderate to high risk of LR as per local cancer centre guidelines | |
| Outcomes | Primary:
Secondary:
| |
| Notes | QA: extensive QA processes (credentialling, real‐time and post‐hoc plan review) NCT00282035 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomly assigned using a telephone‐based central minimization procedure" This was deemed an adequate method of sequence generation and the domain judged at low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | The sequence generation was described as "telephone‐based central minimisation procedure", inadequate details were provided, so we deemed this at unclear risk of bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | For technical reasons, blinding of participants and personnel was not possible, but is unlikely to have introduced bias |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | For technical reasons, blinding of participants and personnel was not possible, but is unlikely to have introduced bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Bilateral mammograms were performed annually" We judged this domain at low risk of bias, because the mammography interval was pre‐specified and this ensures the primary objective outcome (IBTR) was at low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "..addition to nurse and patient assessments, cosmesis was assessed by two panels of three radiation oncologists using the digital photographs. The physicians had breast cancer expertise and were trained to use the EORTC Cosmetic Rating System. After demonstrating good agreement in the ability to identify adverse cosmesis (0.71; Appendix, online only), each panel reviewed half of the available 3‐year post‐RT photo‐ graphs. The panels, blinded to treatment allocation, provided one consensus global cosmetic score for each patient (Appendix, online only)" It was not stated whether the trained nurses evaluating the cosmetic outcome were blinded to treatment arm; however, the physician reviewers were blinded to treatment arm, so we judged this at low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Because this was an interim report, we were unable to assess the number of exclusions or attrition, so judged the domain at unclear risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Because this was an interim report, we judged this domain at unclear risk of bias |
| Other bias | Unclear risk | No other sources of bias noted |
| Methods | Phase III RCT (relative non‐inferiority) Country: Spain Accrual dates: not stated, started accrual 2004 Median follow‐up: 60 months | |
| Participants | 102 women with invasive ductal carcinoma (pT1‐2cNO MO), aged ≥ 60 years old, unifocal tumour, ≤ 3 cm, Grade I or II | |
| Interventions | Experimental arm: PBI/APBI delivered by 3D‐CRT at 48 Gy/24 fractions ± 10 Gy boost (depending on risk factors for local recurrence). 51 women Control arm: conventional WBRT at 48 Gy/24 fractions ± 10 Gy boost (51 women) | |
| Outcomes |
| |
| Notes | QA: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a computer‐generated, randomized list", page 1052, paragraph 5 This was an adequate method of sequence generation, so we judge this domain at low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not clearly described, so we judged this domain at unclear risk of bias |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Binding of participants and personnel was not mentioned, and probably not done, as it would have seen difficult in view of the technical aspects of the 2 intervention arms. We judged this domain at low risk of bias |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Binding of participants and personnel was not mentioned, and probably not done, as it would have seen difficult in view of the technical aspects of the 2 intervention arms. We judged this domain at low risk of bias |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Baseline mammography was performed 6 months after the completion of radiation therapy and yearly thereafter. Abdominal ultrasonography, chest radiography, and blood tests were performed at least annually. Local recurrence was defined as any histologically confirmed cancer tissue in the treated breast", page 1053, paragraph 2 Because the mammography intervals were pre‐specified and local recurrence required histological confirmation, the lack of blinding on the part of the outcome assessors was not judged at high risk of bias |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "Cosmetic results were evaluated according to the Harvard criteria at baseline and at each follow‐up visit by the treating radiation oncologist", page 1053, paragraph 5 All participants who had a minimum of 1 year follow‐up were asked to rate cosmetic results on a 10‐point scale, as follows: excellent (10‐9), good (8‐6), fair (5‐4), or poor (3‐1) Acute, late RT toxicity and cosmesis were evaluated by the treating physician (not blinded, so at risk of bias). However, participants also rated the cosmetic outcome as well. Despite this participant‐reported outcome, we judged this outcome at high risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Because this was an interim report, we judged it at unclear risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Because this was an interim report, we judged it at unclear risk of bias |
| Other bias | Unclear risk | Because this was an interim report, we judged it at unclear risk of bias |
| Methods | Multicentre international randomized non‐inferiority Phase III trial Accrual: March 2000 ‐ data lock 2 May 2010 Country: 11 countries (across Europe, UK, US and Australia) Median follow‐up: 29 months (1222/3451 (35%)) had 60 months' follow‐up | |
| Participants | 1113 women aged ≥ 45 years, with T1 and small T2N0‐1M0 invasive breast cancer, suitable for BCS, available for 10 years' follow‐up | |
| Interventions | Experimental arm: 1 fraction of RT given intraoperatively (using Intrabeam); 50 kV 20 Gy/fraction at 2 mm beyond surface of 1.5‐5.0 cm spherical applicator placed in excision cavity Control arm: standard postoperative RT (40‐56 Gy ± 10‐16 Gy boost) | |
| Outcomes | Primary:
Secondary:
| |
| Notes | Intrabeam uses low kilovolt x‐rays to deliver 20 Gy at the surface of the tumour bed, attenuating to 5‐7 Gy at 1 cm. QA: training and auditing by member of International Standards Organisation (ISO) required before centre could join NCT00983684, ISCTN 34086741, ISRCTN 34086741, REC No. 99/0307, UKCRN | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedules were generated centrally by computer (securely kept in trial centres in Perth for Australian centres and London, UK, for all other centres)", page 94, paragraph 1 |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned in a 1:1 ratio…with blocks stratified by centre". Abstract, page 1, paragraph 2 Quote: "The randomisation schedules were generated centrally by computer (securely kept in trial centres in Perth for Australian centres, and London, UK for all other centres). Requests for randomisation were via telephone or fax to the trials office (Perth or London), where a trained member of staff checked patient eligibility. Treatment was allocated from a pre‐printed randomisation schedule available to authorised staff only. Written confirmation of randomisation was sent by fax to the site". Methods, page, 94, paragraph 1 This trial was likely to have had adequate allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants: "Neither patients nor investigators or their teams were masked to treatment assignment". Abstract, page 1, paragraph 2 Physicians: "Neither patients nor investigators or their teams were masked to treatment assignment". Abstract, page 1, paragraph 2 Quote: "Individual centres were unblinded to treatment given in their own centres, but they were not given access to these data for other sites". Methods, page 1, paragraph 3 Quote: "Patient assessments were scheduled at entry" Because of the nature of the intervention, it was not possible to blind the women participating or the personnel involved in their care, this is not likely to have resulted in bias. Because the patient assessments were scheduled at trial entry with pre‐specified times for follow‐up visits this is likely to have reduced the risk of bias from the lack of blinding of personnel |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants: "Neither patients nor investigators or their teams were masked to treatment assignment". Abstract, page 1, paragraph 2 Physicians: "Neither patients nor investigators or their teams were masked to treatment assignment". Abstract, page 1, paragraph 2 Quote: "Individual centres were unblinded to treatment given in their own centres, but they were not given access to these data for other sites". Methods, page 4, paragraph 3 Quote: "Patient assessments were scheduled at entry" Because of the nature of the intervention, it was not possible to blind the women participating or the personnel involved in their care, this is not likely to have resulted in bias. Because the participant assessments were scheduled at trial entry with pre‐specified times for follow‐up visits this is likely to have reduced the risk of bias from the lack of blinding of personnel |
| Blinding of outcome assessment (detection bias) | Low risk | Participants: not relevant Physicians: not relevant Assessors: "Patient's assessments were scheduled at entry, 3 months and 6 months", page 94, paragraph 4 This means the risk of lead time bias was reduced Quote: "We recommend that mammography of the ipsilateral breast occurs annually and of the contralateral breast at least every three years". TARGIT protocol, 7.1 page 25 Quote: "Confidential unblinded reports for the DMC, and blinded reports for the ISO were produced by the trial statistician. Unblinded analyses were done according to a prespecified statistical analysis plan". Methods, page 4, paragraph 3 If there were pre‐specified time intervals for mammography, this would have reduced the risk of bias for detection of the primary endpoint: local relapse |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The secondary outcome measure of local toxicity, or morbidity was assessed from data recorded on the complications form which contained a prespecified checklist", page 94, paragraph 3 It was not stated who assessed the subjective outcomes, however, we know: "Neither patients nor investigators or their teams were masked to treatment assignment". Abstract, page 1, paragraph 2 The blinding of outcome assessors was not reported, this does mean that there was risk of bias with assessment of toxicity; however, the use of a pre‐specified form would help to reduce bias because the data would be collected for all women Quote: "digital photographs...were assessed, blinded to treatment arm" (Keshtgar et al. Journal of Clinical Oncology Vol. 28. 2010:7S, abstract 570) |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All randomised patients were included in the intention‐to‐treat analysis". Abstract, page 1, paragraph 2 Quote: "When displaying the results, we restricted the duration of follow up to four years...since fewer than 420 (< 20%) patients had follow up beyond this point", page 95, paragraph 4 Because the outcomes were reported with a follow‐up duration of 4 years, this does mean there is a high risk of bias because they reported on < 20% of the participants |
| Selective reporting (reporting bias) | Low risk | Outcomes specified in the protocol:
Outcomes specified in methods:
Outcomes reported in paper:
The authors stated that, "no changes were made to trial outcomes after commencement of the trial" The outcomes pre‐specified in the protocol were not all reported, this probably reflects the short follow‐up duration, but we consider these outcomes are likely to be reported in future publications |
| Other bias | High risk | Short duration of follow‐up puts the outcomes reported at high risk of bias |
3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; BCS: breast‐conserving surgery; bd: twice a day; DCIS: ductal carcinoma in situ; DMC: data monitoring committee; EBRT: external beam radiotherapy; Gy: Gray; HDR: high‐dose‐rate; IBRT: ipsilateral breast tumour recurrence; IHC: immunohistochemistry; IMRT: intensity‐modulated radiotherapy; LENT‐SOMA: late effects in normal tissues ‐ subjective, objective, management and analytic; LR: local recurrence; M: metastases; MeV: mega electron volts; N: lymph node; PBI: partial breast irradiation; PDR: pulsed‐dose‐rate; QA: quality assurance; RCT: randomized controlled trial; RNI: regional nodal irradiation; RT: radiotherapy; RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria; WBRT: whole breast radiotherapy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| RCT, but used surgical, systemic management and RT techniques that were not consistent with contemporary practice | |
| Phase II RCT | |
| PBI used in both study arms | |
| PBI used in both study arms | |
| PBI used in both study arms | |
| RCT, but used surgical, systemic management and RT techniques that were not consistent with contemporary practice | |
| WBRT used in both study arms | |
| Feasibility study and not an RCT |
PBI: partial breast irradiation; RCT: randomized controlled trial; RT: radiation treatment; WBRT: whole breast radiotherapy.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized Phase II trial |
| Participants | Women aged 55‐70 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental arm: APBI APBI was delivered at a dose of 30 Gy in 5‐6 Gy/day fractions over 10 days (every other day) with IGRT at each treatment Control arm: hypofractionated WBRT Dose prescription was 40.5 Gy to planning target volume whole breast (PTV WB) and 48.0 Gy to PTV boost in 15 fractions over 3 weeks, with simultaneous integrated boost delivering 2.7 and 3.2 Gy/fraction for each PTV, respectively. Daily IGRT were generated before each treatment session in each participant to verify the set‐up |
| Outcomes | Primary:
Secondary:
|
| Notes | Randomized study on postmenopausal women with early‐stage breast cancer: adjuvant hypofractionated WBI vs. APBI. Starts 2015, 700 women Email: marta.scorsetti%40humanitas.it |
ALND: axillary lymph node dissection; APBI: accelerated partial breast irradiation; ER: oestrogen receptor; GTV: gross tumour volume; Gy: Gray; IGRT: image‐guided radiotherapy; N: lymph node; PgR: progesterone receptor; PTV: planning target volume; SN: sentinel node biopsy; WBI: whole breast irradiation.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomized Trial Testing Intensity Modulated and Partial Organ Radiotherapy After Breast Conservation Surgery for Early Breast Cancer |
| Methods | Randomized, Phase III multicentre trial |
| Participants | Women aged > 50 years, with invasive breast cancer pT1‐2pN0, who have had BCS with negative margins (≥ 2 mm) who have < 1% annual risk of local recurrence |
| Interventions | Experimental arm 1: reduced WBRT and standard PBI once daily on days 1‐5 for 3 weeks IMRT Arm 1: 40 Gy/15 fractions Arm 2: 40 Gy/15 fractions + integrated boost to WB 36 Gy/15 fractions Experimental arm 2: PBI daily on days 1‐5 for 3 weeks Control: standard WBRT day 1‐5 for 3 weeks |
| Outcomes | Primary: local tumour control in the ipsilateral breast Secondary: location of tumour relapse, contralateral primary breast cancer, regional or distant metastases, late adverse effects in normal tissues (photographic, physician and participant assessments), quality of life, cost‐effectiveness |
| Starting date | October 2006 |
| Contact information | J Yarnold, Royal Marsden, London |
| Notes | NCT00814567 CSDR0000629765, ICR‐IMPORT‐LOW, ICR‐CTSU/2006/10001, ISCTN12852634, EU‐20896 Target volume: 6 pairs of clips in cavity = CTV + 10 mm = PTV |
| Trial name or title | Breast Cancer with Low Risk of Local Recurrence: Partial and Accelerated Radiation with Three‐Dimensional Conformal Radiotherapy (3DCRT) Vs. Standard Radiotherapy After Conserving Surgery (Phase III Study) |
| Methods | Multicentre Phase III controlled randomized, unblinded study of non‐inferiority |
| Participants | Women aged ≥ 49 years, ECOG 0‐2, undergoing conservative breast surgery for invasive breast cancer, pT1‐2 (< 3 cm in diameter) pN0‐N1 M0, unifocal, resection margins histologically negative (2 mm) at first intervention or after subsequent widening |
| Interventions | Experimental arm: 38.5 Gy total in 10 fractions (3.85 Gy per fraction), twice a day with an interval of at least 6 hours between the 2 fractions, for 5 consecutive working days of the sole cavity Control arm: 50.0 Gy in 25 fractions (2 Gy per fraction), once a day for 5 days in the week RT of the entire breast |
| Outcomes | Primary: survival free of local ipsilateral recurrence as prime event Secondary: global survival, loco‐regional recurrence‐free, distant recurrence‐free, acute and late toxicity (RTOG) and cosmetic result |
| Starting date | ‐ |
| Contact information | Data Center Office Clinical Trials Office, Integrated Department of Oncology and Hematology Polyclinic Hospital, University of Modena and Reggio Emilia R. D'Amico, G. Jovic, R. Vicini Tel. 059 4223865 Email: [email protected] |
| Notes | Study currently accruing participants. Target volume: GTV + 15 mm = CTV + 5 mm = PTV |
| Trial name or title | A Randomised Phase II Study of Conventional Whole Breast Irradiation (WBI) Versus Partial Breast Irradiation (PBI) for Women with Stage 0, I, or II Breast Cancer |
| Methods | Randomized, multicentre Phase III trial |
| Participants | Women aged > 18 years with histologically confirmed DCIS or invasive adenocarcinoma of the breast, negative histological margins, no more than 3 axillary nodes involved, must have had BCS |
| Interventions | Experimental arm: PBI 5 days per week for 5‐7 weeks Control arm: WBRT bd on 5 days over 5‐10 days (50 Gy in 25 fractions at 1.8‐2 Gy per fraction, optional boost to 60‐66 Gy) Brachytherapy 34 Gy/10 fractions MammoSite 34 Gy/10 fractions 3DCRT 38.5 Gy/10 fractions |
| Outcomes | Primary: in‐breast tumour recurrence Secondary: survival, event‐free survival, distant disease‐free survival, quality of life and participant‐reported cosmesis, physician‐reported cosmesis and toxicity |
| Starting date | March 2005 |
| Contact information | Study chair: F Vicini, William Beaumont Hospital‐ Royal Oak Campus J White, Medical College of Wisconsin |
| Notes | NCT00103181 NSABP B‐39/RTOG 0413, SWOG‐NSABP‐B‐39 Collaborators: Southwest Oncology Group, National Cancer Institute, RTOG, National Surgical Adjuvant Breast and Bowel Project For brachytherapy and 3DCRT: cavity plus 15 mm = CTV + 10 mm = PTV MammoSite: PTV = 10 mm expansion on balloon minus balloon volume |
| Trial name or title | Standard or Hypofractionated Radiotherapy Versus Accelerated Partial Breast Irradiation (PBI/APBI) for Breast Cancer (SHARE) Phase III Multicentric Trial Comparing Accelerated Partial Breast Irradiation (PBI/APBI) Versus Standard or Hypofractionated Whole Breast Irradiation in Low Risk of Local Recurrence of Breast Cancer |
| Methods | Multicentre RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental arm: APBI 3D‐CRT 40 Gy/10 fractions Control arm: standard or hypofractionated radiotherapy |
| Outcomes | Primary:
Secondary:
|
| Starting date | October 2010 |
| Contact information | Jerome Lemonnier, PhD; Tel: +33 1 7193 6702; Email: j‐[email protected] |
| Notes |
3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; BCS: breast‐conserving surgery; bd: twice a day; CTV: clinical target volume; DCIS: ductal carcinoma in situ; ECOG: Eastern Cooperative Oncology Group; ER: oestrogen receptor; GTV: gross tumour volume; Gy: Gray; IGRT: image‐guided radiotherapy; IMRT: intensity‐modulated radiotherapy; M: metastases; N: lymph node; PBI: partial breast irradiation; PgR: progesterone receptor; PTV: planning target volume; RCT: randomized controlled trial; RT: radiotherapy; RTOG: Radiation Therapy Oncology Group; SN: sentinel node biopsy; WB: whole breast; T: tumour; WBRT: whole breast radiotherapy.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
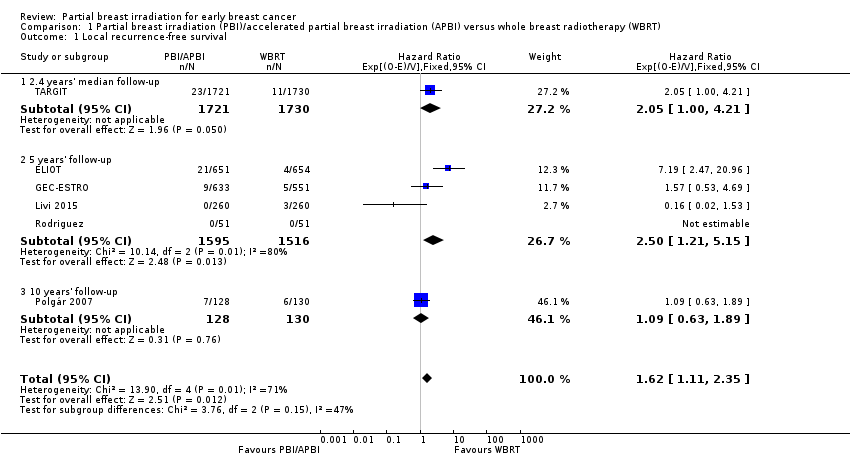
| 1 Local recurrence‐free survival Show forest plot | 6 | 6820 | Hazard Ratio (95% CI) | 1.62 [1.11, 2.35] |
| Analysis 1.1 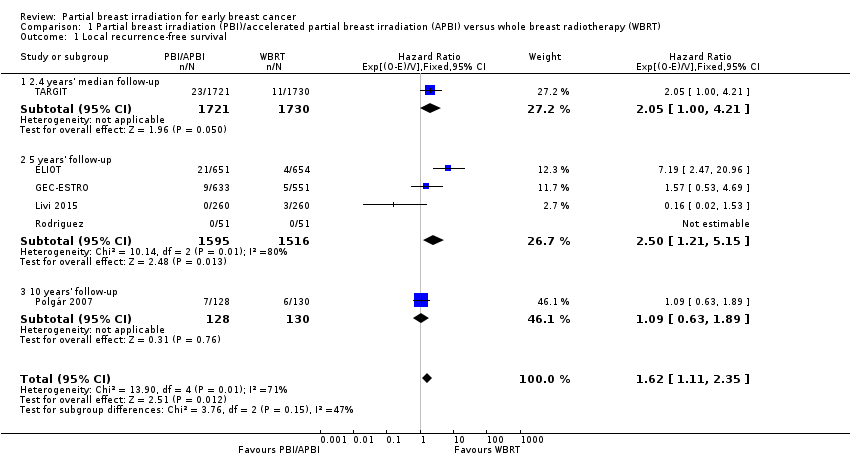 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 1 Local recurrence‐free survival. | ||||
| 1.1 2.4 years' median follow‐up | 1 | 3451 | Hazard Ratio (95% CI) | 2.05 [1.00, 4.21] |
| 1.2 5 years' follow‐up | 4 | 3111 | Hazard Ratio (95% CI) | 2.50 [1.21, 5.15] |
| 1.3 10 years' follow‐up | 1 | 258 | Hazard Ratio (95% CI) | 1.09 [0.63, 1.89] |
| 2 Cosmesis, physician‐reported Show forest plot | 5 | 1720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| Analysis 1.2 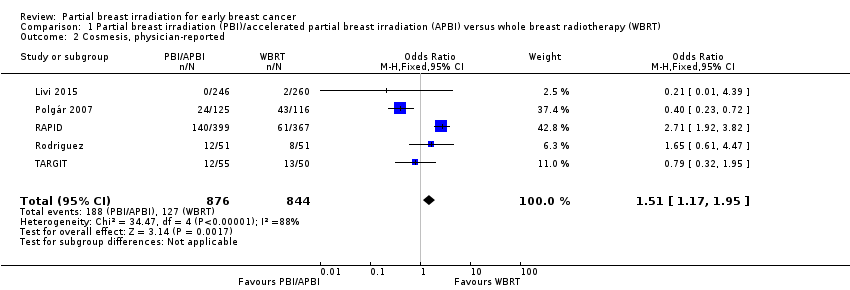 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 2 Cosmesis, physician‐reported. | ||||
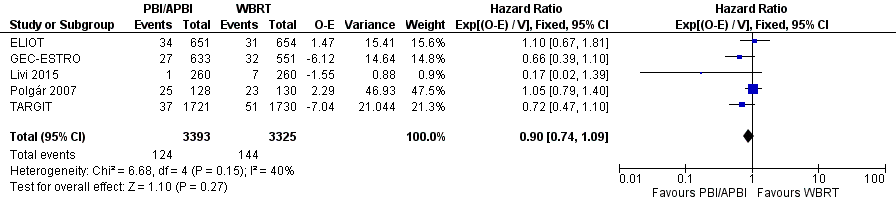
| 3 Overall survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 0.90 [0.74, 1.09] |
| Analysis 1.3 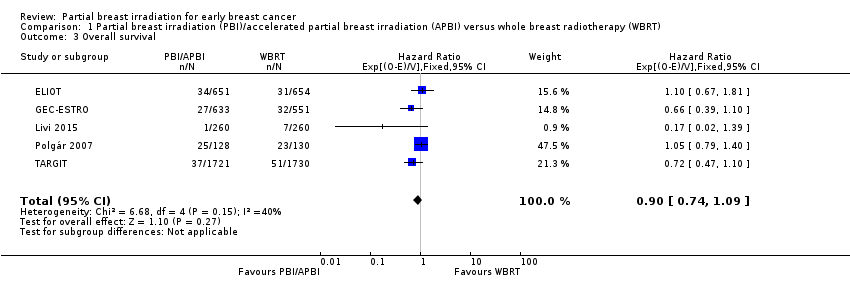 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 3 Overall survival. | ||||
| 4 Acute radiotherapy (RT) skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.09] |
| Analysis 1.4  Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 4 Acute radiotherapy (RT) skin toxicity. | ||||
| 5 Late RT skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.39] |
| Analysis 1.5  Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 5 Late RT skin toxicity. | ||||
| 6 Fat necrosis Show forest plot | 3 | 1319 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.02, 2.43] |
| Analysis 1.6  Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 6 Fat necrosis. | ||||
| 7 'Elsewhere primary' Show forest plot | 3 | 3009 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.51, 10.41] |
| Analysis 1.7 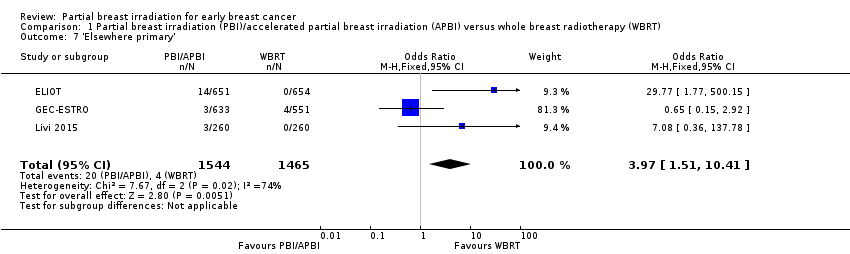 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 7 'Elsewhere primary'. | ||||
| 8 Cause‐specific survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 1.08 [0.73, 1.58] |
| Analysis 1.8 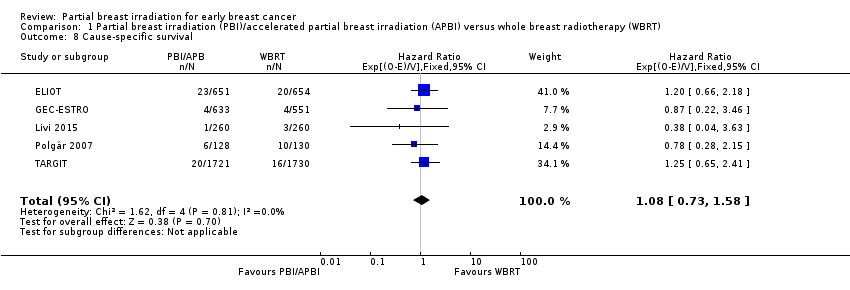 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 8 Cause‐specific survival. | ||||
| 9 Distant metastasis‐free survival Show forest plot | 4 | 3267 | Hazard Ratio (95% CI) | 0.94 [0.65, 1.37] |
| Analysis 1.9 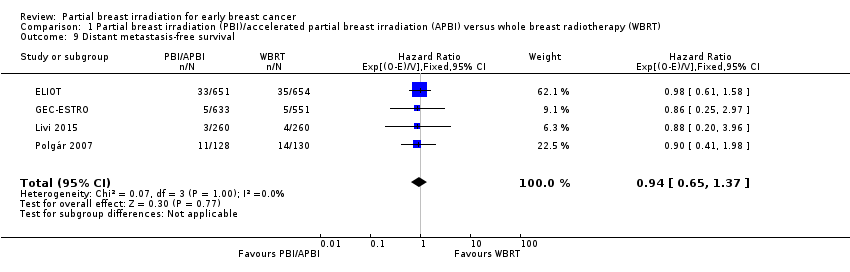 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 9 Distant metastasis‐free survival. | ||||
| 10 Relapse‐free survival Show forest plot | 3 | 3811 | Hazard Ratio (95% CI) | 1.36 [0.88, 2.09] |
| Analysis 1.10 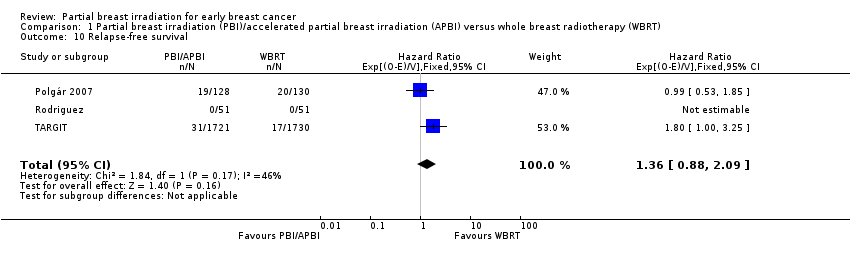 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 10 Relapse‐free survival. | ||||
| 11 Loco‐regional recurrence‐free survival Show forest plot | 2 | 3553 | Hazard Ratio (95% CI) | 1.80 [1.00, 3.25] |
| Analysis 1.11 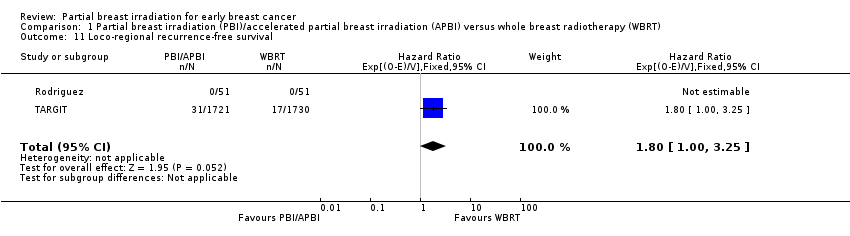 Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 11 Loco‐regional recurrence‐free survival. | ||||
| 12 Mastectomy Show forest plot | 3 | 4817 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.77, 1.87] |
| Analysis 1.12  Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 12 Mastectomy. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.1 Local recurrence‐free survival.
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.3 Overall survival.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.8 Cause‐specific survival.
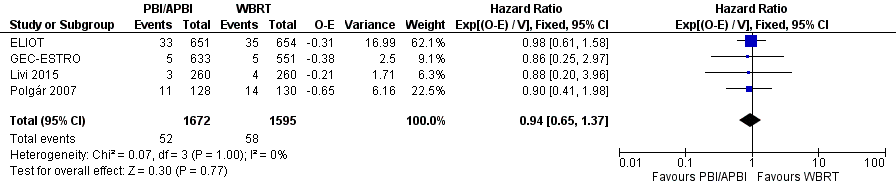
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.9 Distant metastasis‐free survival.
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 1 Local recurrence‐free survival.
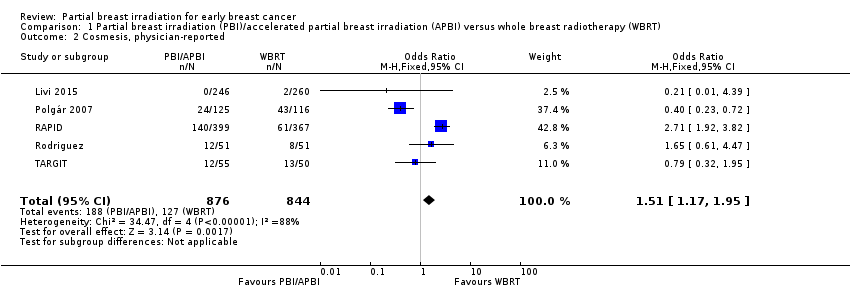
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 2 Cosmesis, physician‐reported.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 3 Overall survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 4 Acute radiotherapy (RT) skin toxicity.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 5 Late RT skin toxicity.
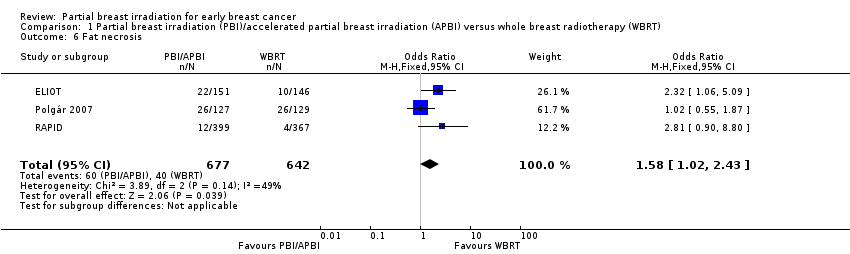
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 6 Fat necrosis.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 7 'Elsewhere primary'.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 8 Cause‐specific survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 9 Distant metastasis‐free survival.
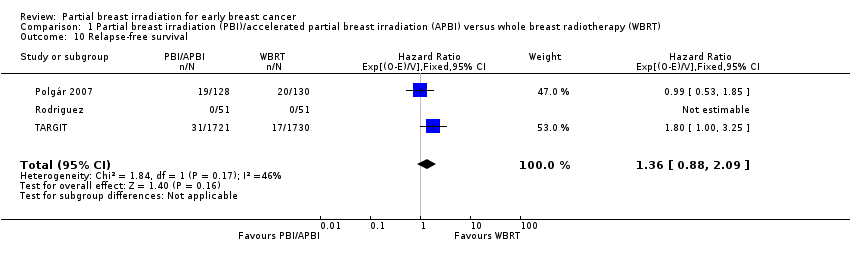
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 10 Relapse‐free survival.
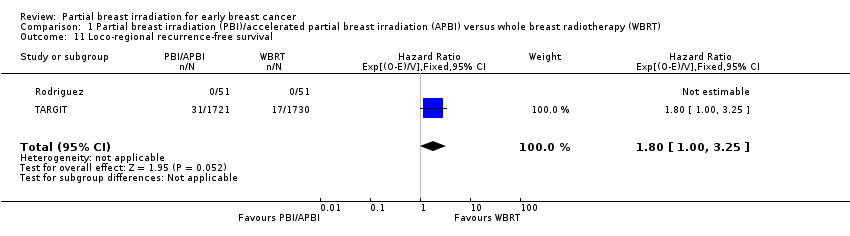
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 11 Loco‐regional recurrence‐free survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 12 Mastectomy.
| PBI/APBI for women with early breast cancer | ||||||
| Patient or population: women with early breast cancer Setting: radiotherapy centres Intervention: PBI/APBI Comparison: whole breast radiotherapy (WBRT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with WBRT | Risk with PBI/APBI | |||||
| Local recurrence‐free survival at 5 years | Study population | HR 1.62 | 6820 | ⊕⊕⊝⊝ | ‐ | |
| 10 per 10001 | 16 per 1000 | |||||
| Cosmesis assessed with 4‐point scale Follow‐up: range 29‐122 months | Study population | OR 1.51 | 1720 | ⊕⊕⊝⊝ | Cosmesis was assessed using a 4‐point scale. We reported those women with poor/fair cosmesis at final review | |
| 150 per 1000 | 218 per 1000 | |||||
| Late radiotherapy toxicity (subcutaneous fibrosis) Follow‐up: median 36 months | Study population | OR 6.58 | 766 | ⊕⊕⊕⊝ | Assessed using National Cancer Institute 3‐point scale, events were defined as: Grade II or higher toxicity Physician assessors, at 3 years' follow‐up | |
| 22 per 1000 | 128 per 1000 | |||||
| Cause‐specific survival at 5 years | Study population | HR 1.08 | 6718 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 10002 | 22 per 1000 | |||||
| Distant metastasis‐free survival at 5 years | Study population | HR 0.94 | 3267 | ⊕⊕⊕⊝ | ‐ | |
| 33 per 10002 | 31 per 1000 | |||||
| Mastectomy rate Follow‐up: range 29‐122 months | Study population | OR 1.20 | 4817 | ⊕⊕⊝⊝ | Mastectomy rate reflected both local recurrence and adverse cosmetic outcome | |
| 15 per 1000 | 18 per 1000 | |||||
| Mortality | Study population | HR 0.90 | 6718 | ⊕⊕⊕⊕ | Survival advantage from radiotherapy for breast cancer is not apparent before 15 years' follow‐up (EBCTCG 2011) | |
| 51 per 10002 | 46 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The baseline risk for the control group was calculated at the 5‐year time point from 5 studies. | ||||||
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
| Score | Definition |
| Excellent | Perfect symmetry, no visible distortion or skin changes and no visible catheter entry/exit sequelae |
| Good | Slight skin distortion, retraction or oedema, any visible telangiectasia, any visible catheter entry/exit scar or mild hyperpigmentation |
| Fair | Moderate distortion of the nipple or breast symmetry, moderate hyperpigmentation, or prominent skin retraction, oedema or telangiectasia |
| Poor | Marked distortion, oedema, fibrosis or severe hyperpigmentation |
| RTOG CTC | Grade I | Grade II | Grade III | Grade IV |
| Description | Follicular, faint or dull erythema / epilation / dry desquamation / decreased sweating | Tender or bright erythema, patchy moist desquamation / moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | Ulceration, haemorrhage, necrosis |
| RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria. | ||||
| Grade | Findings |
| 0 | No fat necrosis |
| 1 | Asymptomatic fat necrosis (only radiological or |
| 2 | Symptomatic fat necrosis not requiring medication |
| 3 | Symptomatic fat necrosis requiring medication |
| 4 | Symptomatic fat necrosis requiring surgical |
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Induration (subcutaneous fibrosis) | Increased density on palpation | Moderate increase in density, not interfering with ADL; marked increase in density and firmness on palpation with or without minimal retraction | Dysfunction interfering with ADL; very marked density, retraction or fixation | ‐ |
| Telangiectasia | Few | Moderate | Many and confluent | ‐ |
| Pain | Pain mild, not interfering with function | Moderate pain; pain or analgesics interfering with function, but not with ADL | Severe pain; pain or analgesics interfering with ADL | Disability |
| ADL: activities of daily living; NCI CTC: National Cancer Institute Common Toxicity Criteria. | ||||
| Trial | PBI/APBI dose | Fraction size (Gy) | EQD2 PBI/APBI | Control dose | Fraction size (Gy) | EQD2 Control |
| 20 Gy at surface of the applicator (attenuated to 5‐7 Gy at 1 cm) (APBI) | 80 at cavity surface 12.8 at 1 cm | 80 Gy at cavity surface 12.8 Gy at 1 cm | 40‐56 Gy/20‐28 fractions ± 10‐16 Gy boost | 2 | 40‐56 Gy ± 10‐16 Gy | |
| 30 Gy/5 daily fractions EBRT IMRT. 100% of the PTV was covered by 95% of the prescribed dose | 6 | 75 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost | 2 | 50 + 10 = 60 Gy | |
| 38.5 Gy/10 fractions bd (with 6 hour gap) Dose‐evaluation volume (that part of PTV within the breast) received 95‐107% of prescription dose | 3.85 | 74.1 Gy | 50 Gy/25 fractions or 42.5 Gy/16 fractions ± boost (10 Gy/4‐5 fractions) based on criteria such as young age or close margins, pre‐specified by centre | 2 or 2.65 | 50 or 47.1 Gy | |
| 37.5 Gy/10 fractions bd (with 6 hour gap) (APBI). PTV covered by ≥ 95% of prescribed dose, with < 105% hot spot | 3.75 | 71.22 Gy | 48 Gy/24 fractions ± 10 Gy/5 fractions boost | 2 | 48 ± 10 = 48‐58 Gy | |
| 7 × 5.2 Gy HDR (APBI) or 50 Gy/25 fractions (PBI). | 5.2 or 2 | 53.6 Gy or 50 Gy | 50 Gy/25 fractions (3D‐CRT was not used) | 2 | 50 Gy | |
| 30.3 Gy/7 fractions or 32 Gy/8 fractions HDR twice daily or 50 Gy at 0.6‐0.8 Gy/hour pulses (1 pulse per hour, 24 hours per day) PDR | 7‐8 | 41.64‐42.67 Gy | 50.0‐50.4 Gy to a reference point + 10 Gy/5 fractions boost. Electron dose was prescribed to the point of maximum dose on the beam axis (Dmax), ensuring the 85% isodose encompassed the tumour bed | 1.8‐2.0 | 48.72‐50 + 10 = 58.72‐60 Gy | |
| 21 Gy/1 fraction at 90% using 6‐9 MeV | 21 | 131.2 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost (using electrons) | 2.0 | 50 + 10 Gy | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; bd: twice daily; CT: computer tomography; EBRT: external beam radiotherapy; EQD2: equivalent dose in 2 Gy fractions; Gy: Gray; HDR: high‐dose‐rate; IMRT: intensity‐modulated radiotherapy; MeV: mega electron volt; PBI: partial breast irradiation; PDR: pulsed‐dose‐rate; PTV: planning target volume. | ||||||
| Trial | RT technique |
| Interstitial brachytherapy (88/128) EBRT using photons (40/128) | |
| intra‐operative electrons | |
| EBRT (IMRT) | |
| intra‐operative kV RT | |
| EBRT | |
| EBRT (3D‐CRT) | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; EBRT: external beam radiotherapy; IMRT: intensity‐modulated radiotherapy; RT: radiotherapy. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local recurrence‐free survival Show forest plot | 6 | 6820 | Hazard Ratio (95% CI) | 1.62 [1.11, 2.35] |
| 1.1 2.4 years' median follow‐up | 1 | 3451 | Hazard Ratio (95% CI) | 2.05 [1.00, 4.21] |
| 1.2 5 years' follow‐up | 4 | 3111 | Hazard Ratio (95% CI) | 2.50 [1.21, 5.15] |
| 1.3 10 years' follow‐up | 1 | 258 | Hazard Ratio (95% CI) | 1.09 [0.63, 1.89] |
| 2 Cosmesis, physician‐reported Show forest plot | 5 | 1720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| 3 Overall survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 0.90 [0.74, 1.09] |
| 4 Acute radiotherapy (RT) skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.09] |
| 5 Late RT skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.39] |
| 6 Fat necrosis Show forest plot | 3 | 1319 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.02, 2.43] |
| 7 'Elsewhere primary' Show forest plot | 3 | 3009 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.51, 10.41] |
| 8 Cause‐specific survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 1.08 [0.73, 1.58] |
| 9 Distant metastasis‐free survival Show forest plot | 4 | 3267 | Hazard Ratio (95% CI) | 0.94 [0.65, 1.37] |
| 10 Relapse‐free survival Show forest plot | 3 | 3811 | Hazard Ratio (95% CI) | 1.36 [0.88, 2.09] |
| 11 Loco‐regional recurrence‐free survival Show forest plot | 2 | 3553 | Hazard Ratio (95% CI) | 1.80 [1.00, 3.25] |
| 12 Mastectomy Show forest plot | 3 | 4817 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.77, 1.87] |

