Irradiación parcial de la mama versus radioterapia en toda la mama para el cáncer de mama temprano
Resumen
Antecedentes
El tratamiento conservador de la mama en las mujeres con cáncer de mama consiste en la escisión local del tumor (para lograr márgenes libres) seguida de radioterapia (RT). La mayoría de las recidivas reales ocurre en el mismo cuadrante del tumor original. Es posible que la radioterapia en toda la mama (RTTM) no proteja contra la aparición de un nuevo cáncer primario que se desarrolle en otros cuadrantes de la mama. En esta revisión Cochrane, se investigó la administración de radiación a un volumen limitado de la mama alrededor del lecho tumoral (irradiación parcial de la mama [IPM]) a veces con una duración acortada del tratamiento (irradiación parcial acelerada de la mama [IPAM]).
Objetivos
Determinar si la IPM/IPAM es equivalente o mejor que el tratamiento convencional o la RTTM hipofraccionada después del tratamiento conservador de la mama para el cáncer de mama en estadio temprano.
Métodos de búsqueda
El 27 de agosto de 2020, se realizaron búsquedas en el Registro especializado del Grupo Cochrane de Cáncer de mama (Cochrane Breast Cancer Group), CENTRAL, MEDLINE, Embase, CINAHL y en tres bases de datos de ensayos. Se realizaron búsquedas de literatura gris: OpenGrey (septiembre de 2020), listas de referencias de artículos, resúmenes de congresos y resúmenes publicados, y no se aplicaron restricciones de idioma.
Criterios de selección
Ensayos controlados aleatorizados (ECA) sin factores de confusión, que evaluaran la cirugía conservadora más IPM/IPAM versus la cirugía conservadora más RTTM. Fueron elegibles ensayos publicados y no publicados.
Obtención y análisis de los datos
Dos autores de la revisión (BH y ML) realizaron la extracción de los datos, utilizaron la herramienta de riesgo de sesgo de Cochrane y resolvieron cualquier desacuerdo mediante debate, además evaluaron la certeza de la evidencia para los desenlaces principales mediante el método GRADE. Los desenlaces principales fueron la supervivencia sin recidiva local, la apariencia estética, la supervivencia general, la toxicidad (fibrosis subcutánea), la supervivencia específica de la causa, la supervivencia sin metástasis a distancia y la mastectomía posterior. Se introdujeron los datos en Review Manager 5 para su análisis.
Resultados principales
Se incluyeron nueve ECA que reclutaron a 15 187 mujeres con cáncer de mama invasivo o carcinoma ductal in situ (6,3%) con tumores unifocales de grado I o II T1‐2N0‐1M0 (menos de 2 cm o 3 cm o menos) que recibieron tratamiento conservador de la mama con márgenes negativos. Esta es la segunda actualización de la revisión e incluye dos nuevos estudios y 4432 participantes más.
La supervivencia sin recidiva local probablemente se reduce ligeramente (en 3/1000; IC del 95%: 6 menos a 0 menos) con el uso de IPM/IPAM en comparación con la RTTM (cociente de riesgos instantáneos [CRI] 1,21; intervalo de confianza [IC] del 95%: 1,03 a 1,42; ocho estudios, 13 168 participantes; evidencia de certeza moderada).
La apariencia estética (informada por personal médico o de enfermería) es probablemente peor (en 63/1000; IC del 95%: 35 más a 92 más) con el uso de IPM/IPAM (odds ratio [OR] 1,57; IC del 95%: 1,31 a 1,87; seis estudios, 3652 participantes; evidencia de certeza moderada).
La supervivencia general es similar (0/1000 menos; IC del 95%: 6 menos a 6 más) con IPM/IPAM y RTTM (CRI 0,99; IC del 95%: 0,88 a 1,12; ocho estudios, 13 175 participantes; evidencia de certeza alta).
La toxicidad tardía de la radiación (fibrosis subcutánea) probablemente aumenta (en 14/1000 más; IC del 95%: 102 más a 188 más) con IPM/IPAM (OR 5,07; IC del 95%: 3,81 a 6,74; dos estudios, 3011 participantes; evidencia de certeza moderada).
El uso de IPM/IPAM probablemente da lugar a poca diferencia (1/1000 menos; IC del 95%: 6 menos a 3 más) en la supervivencia específica de la causa (CRI 1,06; IC del 95%: 0,83 a 1,36; siete estudios, 9865 participantes; evidencia de certeza moderada).
Se encontró que el uso de IPM/IPAM en comparación con la RTTM probablemente da lugar a poca o ninguna diferencia (1/1000 menos [IC del 95%: 4 menos a 6 más]) en la supervivencia sin metástasis a distancia (CRI 0,95; IC del 95%: 0,80 a 1,13; siete estudios, 11 033 participantes; evidencia de certeza moderada).
Se encontró que el uso de IPM/IPAM en comparación con la RTTM da lugar a poca o ninguna diferencia (2/1000 menos; IC del 95%: 20 menos a 20 más) en las tasas de mastectomía (OR 0,98; IC del 95%: 0,78 a 1,23; tres estudios, 3740 participantes, evidencia de certeza alta).
Conclusiones de los autores
Al parecer la supervivencia sin recidiva local es probablemente peor con IPM/IPAM; sin embargo, la diferencia fue pequeña y casi todas las mujeres permanecen sin recidiva local. La supervivencia general es similar con la IPM/IPAM y la RTTM, y no se encontró apenas diferencias en otros desenlaces oncológicos. Algunos efectos tardíos (fibrosis subcutánea) podrían ser peores con IPM/IPAM y su uso probablemente se asocia con peores desenlaces estéticos. Las limitaciones de los datos actualmente disponibles hacen que no sea posible establecer conclusiones definitivas acerca de la eficacia y la seguridad o las formas de proporcionar la IPM/IPAM. Se está a la espera de la finalización de los ensayos en curso.
PICO
Resumen en términos sencillos
Irradiación parcial de la mama para el cáncer de mama temprano
¿Cuál es el problema?
El cáncer de mama es el cáncer más común que presentan las mujeres.
Las mujeres con cáncer de mama en estadio temprano que deciden conservar la mama necesitan recibir radioterapia (RT), así como cirugía para extirpar el cáncer y asegurarse de que no vuelva a crecer. La RT es un tratamiento con rayos X de alta energía. Recibir RT para el cáncer de mama suele suponer entre 15 a 30 visitas al servicio de RT, cinco veces por semana.
Si el cáncer de mama vuelve a crecer en la misma mama (lo que se denomina recidiva local), tiende a reaparecer en la zona en la que se extrajo. Las mujeres también pueden desarrollar un nuevo cáncer (nuevo "primario en otro lugar") en otra parte de la misma mama. No está claro si la RT, administrada para detener la reaparición del cáncer en el lugar donde se encontraba el primer cáncer, detiene el crecimiento de "otros primarios en otro lugar".
¿Por qué es importante este tema?
Siempre se quiere tratar el área más pequeña que se pueda con la RT porque implica menos efectos secundarios. Tratar sólo una parte de la mama podría significar que la RT se podría utilizar de nuevo en otra parte de la misma mama si fuera necesario. Las nuevas formas de administrar la RT hacen que el tratamiento de una parte de la mama se pueda hacer con menos tratamientos. Es probable que esto sea más fácil para las mujeres y cueste menos dinero.
¿Qué se comparó?
La pregunta de la revisión fue si la administración de RT a una parte de la mama (denominada irradiación parcial de la mama [IPM]) es tan buena como la administración de RT en toda la mama. La IPM se puede administrar con una duración más corta del tratamiento (denominada irradiación parcial acelerada de la mama [IPAM]).
Para que este tratamiento sea aceptable, tendría que controlar el cáncer como lo hace la administración de RT en toda la mama. También sería importante que la IPM provocara los mismos efectos secundarios y proporcionara la misma apariencia a la mama que el tratamiento de toda la mama.
¿Qué se encontró?
Se encontraron nueve estudios en los que participaron 15 187 mujeres. La evidencia está actualizada hasta el 27 de agosto de 2020. La recidiva local es probablemente algo más frecuente con IPAM/IPM (evidencia de calidad moderada) y la apariencia de la mama (calificada por médicos y enfermeras) fue probablemente peor con IPAM/IPM (evidencia de calidad moderada). Probablemente haya poca diferencia en la supervivencia (evidencia de calidad alta). La fibrosis tardía por radiación (cambio en la apariencia y la sensación de la mama) probablemente aumenta con IPAM/IPM. Probablemente haya pocas diferencias en las muertes relacionadas con el cáncer de mama y en la diseminación del cáncer de mama por el cuerpo con el uso de IPAM/IPM. El uso de IPAM/IPM apenas influye en el número de mujeres que necesitan una mastectomía (extirpación de toda la mama) debido a los efectos secundarios tardíos inaceptables o a una recidiva local.
¿Qué significan los resultados?
Esto significa que, por el momento, la IPM no proporciona el mismo control del cáncer en la mama que el tratamiento de toda la mama, pero la diferencia es pequeña. Podría causar efectos secundarios peores. Hay siete grandes estudios en curso que serán importantes para responder esta pregunta. Se espera tener una respuesta más clara en la próxima actualización de esta revisión.
Authors' conclusions
Summary of findings
| PBI/APBI compared to WBRT for early breast cancer | ||||||
| Patient or population: health problem or population Setting: Academic, tertiary and community practice Intervention: PBI/APBI Comparison: WBRT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with WBRT | Risk with PBI/APBI | |||||
| Local recurrence‐free survival ‐ total (LR‐FS) | Low | HR 1.21 | 13168 | ⊕⊕⊕⊝ | ||
| 985 per 1000 | 982 per 1000 | |||||
| Cosmesis, physician/nurse‐reported | 138 per 1000 | 201 per 1000 | OR 1.57 | 3652 | ⊕⊕⊕⊝ | |
| Overall survival | Low | HR 0.99 | 13175 | ⊕⊕⊕⊕ | ||
| 949 per 1000 | 949 per 1000 | |||||
| Subcutaneous fibrosis (late RT toxicity) | 43 per 1000 | 184 per 1000 | OR 5.07 | 3011 | ⊕⊕⊕⊝ | |
| Cause‐specific survival (C‐SS) | Low | HR 1.06 | 9865 | ⊕⊕⊕⊝ | ||
| 983 per 1000 | 982 per 1000 | |||||
| Distant metastasis‐free survival (DM‐FS) | Low | HR 0.95 | 11033 | ⊕⊕⊕⊝ | ||
| 971 per 1000 | 972 per 1000 | |||||
| Subsequent mastectomy | 97 per 1000 | 95 per 1000 | OR 0.98 | 3740 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_416473753410884319. | ||||||
| a. There was considerable clinical heterogeneity with respect to radiotherapy dose, technique and use of quality assurance procedures. However, the techniques employed delivered a dose that was the same or higher in the APBI/PBI arm than the WBRT arm, which should mean the local recurrence‐free survival is better or at least the same. | ||||||
Background
Description of the condition
Breast cancer is the most common cancer occurring in women. One in eight women living in the US, Australia and the UK has a lifetime risk of being diagnosed with breast cancer (Cancer 2020; Howlader 2009; ONS 2010). Breast cancer is the second most common cause of cancer death in women.
Historically, mastectomy was the recommended therapeutic option for all stages of breast cancer. However, large randomised controlled trials (RCTs) demonstrated equivalent survival for women with early‐stage disease (Stages I, II) whether they were treated with breast‐conserving therapy or mastectomy (EBCTCG 1995; Fisher 1995; Fisher 2002; Jacobson 1995; Poggi 2003; van Dongen 2000; Veronesi 1995; Veronesi 2002). Consequently, breast conservation has become the preferred management option for these women.
Breast‐conserving therapy consists of local excision of the tumour (achieving clear margins) followed by radiotherapy (RT). RT is given to sterilise tumour cells that may remain after surgery. This practice is supported by data from detailed pathological examination of mastectomy specimens where residual tumour was found more than 2 cm from the original tumour in 41% of participants (Holland 1985). Conventional RT delivers 45 Gray (Gy) to 50 Gy to the whole breast over five weeks frequently followed by a boost to the tumour bed (the most likely site of residual tumour cells) of 10 Gy to 16 Gy over one to two weeks. This prolonged duration of treatment negatively impacts on quality of life (Whelan 2000), and contributes to the higher mastectomy rates observed in women residing in rural and remote areas who wish to avoid being away from home and family for extended periods (Schroen 2005).
Hypofractionated whole breast radiotherapy (WBRT) regimens, in which 40 Gy to 42.5 Gy is delivered to the whole breast over three to four weeks using a larger radiation dose with each treatment have been investigated. Compared to conventional WBRT, hypofractionated WBRT results in no difference in breast recurrence rates at five and 10 years, no difference in overall survival (OS) and an improvement in cosmetic outcomes (START 2008; START B 2008; Whelan 2002).
RCTs have shown that the addition of conventional or hypofractionated WBRT to local excision decreases ipsilateral breast (same breast) recurrence rates from 30% to 40% (Fisher 1995; Fisher 2002; Freeman 1981; Lagios 1983; Montgomery 1978) to 10% to 20% with 10 to 15 years of follow‐up (Fisher 1995; Fisher 2002). Modern breast‐conserving therapy with postoperative WBRT achieves local control rates of 3.3% to 3.4% at five years and 5.2% to 6.7% at 10 years (Haviland 2013). With careful patient selection (aged 65 years or greater, T1‐2 tumours, tumour not greater than 3 cm, margin negative, oestrogen receptor positive and all receiving tamoxifen), surgery alone can achieve local recurrence rates of 4.1% at five years (Kunkler 2015). An ipsilateral recurrence can either be a true recurrence of the original cancer (typically arising in the same quadrant as the original tumour and known as local recurrence) or a second primary tumour developing elsewhere in that same breast. Studies evaluating ipsilateral breast tumour recurrence patterns showed that new primaries increasingly contribute to the rate of recurrence after five to eight years while true recurrence rates stabilise (Krauss 2004; Smith 2000). If WBRT was successful in preventing the recurrence of new primary cancers, the rate of such cancers in the treated breast should be lower than the rate of development of cancers in the other breast (contralateral breast cancer). Studies of ipsilateral breast tumour recurrence patterns have not found this (Krauss 2004; Smith 2000). Furthermore, studies examining primary and re‐excision pathological specimens removed at the time of breast‐conserving surgery revealed residual tumour 15 mm or less from the primary tumour in 91% of the specimens (Wallner 2004).
Thus, as most true recurrences occur in the same quadrant as the original tumour and as WBRT does not appear to protect against the development of new primary cancer, investigators are examining the role of partial breast irradiation (PBI).
Description of the intervention
PBI (also known as less than WBRT) refers to irradiation of a limited volume of breast tissue around the tumour bed. It may be achieved by any of the following techniques.
-
Intracavitary brachytherapy or MammoSite (applying radioactive sources directly into the cavity left after surgical removal of the tumour either at the time of surgery or at a later date, the latter requiring a second procedure).
-
Interstitial brachytherapy (inserting catheters into the surgical cavity and surrounding tissue to temporarily deliver radioactive sources).
-
Intraoperative techniques using electrons or x‐rays at 50 kilovoltage peak (kVp) (using a dedicated machine to deliver a very localised radiation dose to the surgical cavity in the operating room or by moving the person with an open wound to the radiation machine, which may be in a different part of the hospital).
-
External beam radiotherapy (EBRT) using either three‐dimensional conformal radiotherapy (3D‐CRT) (EBRT delivered in the postoperative setting to a volume of breast tissue around the tumour cavity using a standard linear accelerator in a radiation oncology department) or other methods.
Conventional RT typically delivers a radiation dose of 2 Gy with each treatment. Some PBI techniques deliver a larger than standard dose of radiation with each treatment, allowing the overall duration of treatment to be shortened. This is termed accelerated partial breast irradiation (APBI).
How the intervention might work
PBI/APBI will only be of benefit if it confers the same local control benefit as standard WBRT with acceptable toxicity and cosmesis. Currently, some authorities consider PBI/APBI to be an experimental therapy (Clinical Evidence). The National Comprehensive Cancer Network (NCCN) state, "Preliminary studies of APBI suggest rates of local control in patients with early‐stage breast cancer may be comparable to those treated with WBRT. Follow‐up, however is limited and studies are ongoing" (NCCN). Because this technique has been widely adopted outside the context of clinical trials, published guidelines exist that identify women with early breast cancer for whom this technique may be safe. For those women ineligible for a trial, carefully selected women with early breast cancer may be offered PBI/APBI (Bellon 2011; Polgár 2010; Smith 2009).
PBI/APBI has several potential advantages including:
-
a reduction in treatment‐related toxicities, as a smaller volume of breast tissue is irradiated;
-
increased utilisation of breast conservation;
-
a reduction in RT waiting times; a reduction in the overall treatment duration of a common malignancy has the potential to substantially impact on RT waiting times in countries with strained resources (including the UK, Canada, Australia and New Zealand);
-
a greater chance of preserving the breast should a recurrence occur elsewhere in the breast;
-
easier integration with chemotherapy schedules because RT time will be shorter, thus avoiding delays.
PBI/APBI has several potential disadvantages including:
-
an increased risk of local recurrence due to geographic miss. This is either because treatment was delivered before full pathological examination was obtained or because of difficulty in reproducing the target volume (the tissue that needed to be treated with RT) daily;
-
increased late toxicity. The late effects of radiation are dependent on the dose of radiation given at each treatment and, as PBI/APBI delivers a large radiation dose per fraction, late toxicity may be increased with resultant poor cosmetic outcome or breast appearance (cosmesis);
-
more patient inconvenience as some techniques may require a second anaesthetic or a further invasive procedure;
-
several techniques (e.g. interstitial and intracavitary brachytherapy) require operator expertise and specialised equipment that may not be available in all centres;
-
invasive techniques of delivering PBI/APBI (e.g. interstitial and intracavitary brachytherapy) may be associated with toxicity such as infection and delays in wound healing. Scarring following insertion of interstitial brachytherapy catheters can negatively impact on cosmetic appearance.
Why it is important to do this review
PBI/APBI has the potential to change the pattern of practice for a common malignancy and thereby impact on resource utilisation, patient satisfaction and quality of life. However, as PBI/APBI is currently an experimental therapy, it must be thoroughly evaluated before being adopted as the new standard of care for early‐stage breast cancer. PBI/APBI can be recommended if it is as effective or better than conventional or hypofractionated WBRT for cancer‐related outcomes (local relapse‐free survival (LR‐FS), survival, breast cancer‐specific survival and metastasis‐free survival) as well as patient‐orientated outcomes (cosmesis, quality of life and consumer preference). We found one systematic review that concluded, "The data on PBI/APBI compared to whole‐breast irradiation are insufficient to draw any conclusions about the relative effectiveness of these modalities" (BlueCross BlueShield). The fact that the benefit versus risk profile of PBI/APBI is currently unknown makes it an ideal topic for a systematic review.
Objectives
To determine whether PBI/APBI is equivalent to or better than conventional or hypofractionated WBRT after breast‐conserving therapy for early‐stage breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
RCTs evaluating conservative surgery plus PBI/APBI versus conservative surgery plus WBRT. The comparisons had to be unconfounded (i.e. treatments given to the randomised groups had to differ only in relation to the volume of the breast irradiated). Trials incorporating adjuvant treatments, such as chemotherapy, monoclonal antibodies or hormonal therapy, were eligible if the RCT applied these other treatments in exactly the same way to both groups. Published and unpublished studies were eligible.
We did not consider studies in which PBI was used as a boost following conventional EBRT for inclusion.
Types of participants
Women with histologically confirmed early‐stage breast cancer who had conservative surgery. Early breast cancer included tumours classified as American Joint Committee on Cancer (AJCC) Stage T1‐2N0‐1M0 (Fleming 1997). Surgery could include lumpectomy and wide local excision or quadrantectomy, with or without axillary dissection, axillary sampling or sentinel node biopsy. Women with a previous diagnosis of breast cancer were not eligible for inclusion.
Types of interventions
Radiation delivered to the partial breast (PBI) and PBI using larger than standard radiation dose per fraction such that the overall treatment time was reduced (APBI). We considered any method of PBI/APBI delivery consistent with contemporary practice, including, but not limited to, intracavitary brachytherapy or MammoSite, interstitial brachytherapy, intraoperative techniques such as electrons or x‐rays at 50 kVp or EBRT using 3D‐CRT or other methods. Conventional breast RT is delivered to the whole breast with or without the supraclavicular fossa and axilla, using standard fractionation (1.8 Gy to 3.0 Gy per fraction) to deliver a total of 40 Gy to 61 Gy at the reference point. Treatment could include a boost (using electrons, interstitial therapy, EBRT or new techniques).
Types of outcome measures
Primary outcomes
1.1 Local recurrence‐free survival (LR‐FS) in the ipsilateral breast. We defined local recurrence as a recurrence of the same histological type of cancer within the same quadrant of the breast as the primary cancer.
1.2 Cosmesis (cosmetic outcome or breast appearance).
Secondary outcomes
2.1 Overall survival (OS, time from date of randomisation to death from any cause, or number of deaths from any cause).
2.2 Toxicity (including acute and late effects of RT, chemotherapy‐related toxicity and surgical toxicity; individual protocol‐based definitions).
2.3 New primary tumours in ipsilateral breast, 'elsewhere primaries.' We defined a new primary as a lesion arising in a quadrant of the breast that was different from the original cancer or a tumour of a different histological subtype occurring anywhere within the breast.
2.4 Cause‐specific survival (C‐SS, deaths due to breast cancer at five years).
2.5 Distant metastasis‐free survival (DM‐FS, in isolation or at the same time as local recurrence (the occurrence of metastases at five years)).
2.6 Relapse‐free survival (R‐FS, length of time after treatment during which there was no recurrence). Recurrence referred to breast cancer in the ipsilateral breast or elsewhere in the body, excluding a new breast cancer in the contralateral breast.
2.7 Locoregional recurrence‐free survival (L‐RR‐FS, comprised local recurrence, 'elsewhere' ipsilateral breast primaries (a new primary cancer in the same breast) and regional nodal relapse).
2.8 Subsequent mastectomy (ipsilateral partial mastectomy, modified radical mastectomy or radical mastectomy).
2.9 Compliance, defined as the number of women who commenced treatment with PBI/APBI or conventional EBRT and completed the treatment course.
2.10 Costs (monetary costs of PBI versus EBRT) to women, government and insurance companies.
2.11 Quality of life (using trial‐specific instruments). The effects of PBI/APBI and EBRT on global quality of life and the physical, emotional and psychological domains.
2.12 Consumer preference, that is, did women prefer PBI/APBI or WBRT given the advantages and disadvantages of each approach.
Search methods for identification of studies
Electronic searches
Electronic databases
We searched the following databases:
-
Cochrane Breast Cancer Group Specialised Register (27 August 2020). Details of search strategies used to identify studies and the procedure used to code references are outlined in the group's module (Cochrane Breast Cancer Group). We extracted studies on the specialised register with keywords 'early breast cancer', 'radiotherapy', 'partial breast irradiation', 'whole breast irradiation', 'whole breast radiotherapy', 'brachytherapy', 'high‐dose‐rate brachytherapy', 'accelerated partial breast irradiation', 'tumour bed boost', 'sole tumour bed irradiation', 'MammoSite', 'radiotherapy', 'PBI', 'APBI' and 'interstitial brachytherapy' for consideration;
-
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Library (2020, Issue 8; Appendix 1);
-
MEDLINE via Ovid SP (January 1966 to 27 August 2020; Appendix 2);
-
Embase via Ovid SP (1980 to 27 August 2020; Appendix 3);
-
CINAHL via EBSCO (1981 to 27 August 2020; Appendix 4);
We modified the MEDLINE search strategy to search the other databases, without language restrictions (Appendix 2).
Unpublished literature
We searched following registers for ongoing clinical trials:
-
International Standard Randomised Controlled Trial Number Register (www.controlled-trials.com/isrctn) (7 September 2020);
-
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) (27 August 2020; Appendix 5);
-
US clinical trials registry (www.clinicaltrials.gov) (27 August 2020; Appendix 6).
Grey literature
We checked OpenGrey (www.opengrey.eu/) (7 September 2020; Appendix 7).
Searching other resources
We contacted researchers located from the grey literature and unpublished literature to ask if they were aware of any other trials on this topic. We contacted the Rodríguez authors on 15 October 2012 for more information. We contacted the IRMA authors on 2 October 2012 and they supplied us with the trial protocol. We contacted the authors of Polgár 2007 on 24 August 2013; we gratefully received and incorporated data from the authors of Polgár 2007 in November 2013.
Handsearching
We handsearched several conference proceedings and published abstracts including:
-
Adjuvant Therapy for Primary Breast Cancer International Conference (2001);
-
Primary Therapy of Early Breast Cancer (2001 and 2003);
-
6th and 7th Nottingham International Breast Cancer Meeting Conference Reports;
-
23rd and 24th Congress of the International Association for Breast Cancer Research;
-
3rd and 4th Perspectives in Breast Cancer Conference Reports;
-
26th and 27th Annual San Antonio Breast Cancer Symposium;
-
4th European Breast Cancer Conference;
-
94th and 95th American Association of Cancer Research;
-
American Society for Clinical Oncology (Anon 1995 to 2005; 2012 to 2016);
-
European Society for Therapeutic and Radiation Oncology (1990, 1993, 2000 to 2010, 2012 to 2018);
-
5th and 6th Milan Breast Cancer Conference;
-
Australian Breast Cancer Conference (2004);
-
27th and 28th Annual Symposium of the American Society of Breast Disease;
-
Centers for Disease Control and Prevention (CDC) Cancer Conference (2003);
-
Radiotherapy and Oncology: Proceedings of World Congress of Brachytherapy 2012.
Data collection and analysis
Selection of studies
Two review authors (ML and BH) checked the titles and abstracts retrieved by the searches, and removed duplicate records. Each review author independently assessed the full text of the studies thought relevant to the review and we resolved any differences in assessment by discussion. We performed trial assessments with the results masked. In cases where data were limited or information on trial methods was limited, we requested further information from the trial authors.
Data extraction and management
Two review authors (ML and BH) performed data extraction and resolved disagreements through discussion. We entered data into Review Manager Web for analysis (RevMan Web 2019). Where possible, we extracted data on tumour stage, nodal status, margin status, receptor status, hormonal manipulation, treatment allocation and surgery performed. The information extracted on RT included overall treatment time, radiation dose, dose per fraction and method of PBI. We extracted outcome data on local recurrence, deaths (all‐cause and breast cancer deaths), new ipsilateral primaries, mastectomy rate, distant metastases, treatment‐related toxicity (including that related to acute and late effects of RT and to surgery), cosmesis, costs of treatment, consumer preference and quality of life.
We derived data for OS, LR‐FS, DM‐FS, disease‐free survival and C‐SS from information in the text (ELIOT; Livi 2015; RAPID; Rodríguez; TARGIT), and data received from trial authors (Polgár 2007). Trial authors of Polgár 2007 provided the hazard ratios (HR), 95% confidence intervals (CI) and P values, together with the number of events with further follow‐up. We used the spreadsheet developed by Matthew Sydes (Tierney 2007) to derive O‐E (observed minus expected events) and variance using the number of events, HRs, P values where available or calculated using the Review Manager Web calculator (RevMan Web 2019). Although there were data for L‐RR‐FS for ELIOT, we did not include them in the analysis because some women were treated with regional nodal RT.
We converted the radiation doses to the equivalent dose in 2 Gy fractions (EQD2) (Maciejewski 1986; Withers 1983), using the formula: EQD2 = D [d + (alpha/beta/2 + alpha/beta)], where D = total dose, d = dose per fraction and alpha/beta = 4 Gy (Owen 2006). This was to facilitate comparison of radiation doses given at differing dose per fraction.
We plan to convert brachytherapy (radiation sources applied directly to the body) to the biological equivalent dose (BED) using the method of Stitt 1992 should it be necessary to pool brachytherapy data in updates of this review.
Studies reported global cosmetic outcome using the Harvard Cosmetic Score (Polgár 2007; Livi 2015; Rodríguez), the European Organisation for Research and Treatment of Cancer (EORTC) Cosmetic rating System for Breast Cancer) (Aaronson 1998; GEC‐ESTRO; RAPID), and a software program (TARGIT). These were all four‐point scales; the results were dichotomised into good/excellent and fair/poor, with occurrence of fair/poor being counted as 'events' (Table 1).
| Cosmetic score |
|---|
| Excellent |
| Good |
| Fair |
| Poor |
One study reported telangiectasia (using NCI CTCAE Version 3.0 (National Cancer Institute Common Terminology Criteria for Adverse Events); also a three‐point scale)(NCI): we recorded any women with Grade 2 or higher toxicity as having events (RAPID). For acute and late RT toxicity, we considered the Grade 2 or greater toxicity in NCI CTCAE Version 3.0, 4.0 and EORTC/RTOG CTC (European Organisation for Research and Treatment of Cancer/Research and Treatment of Cancer Common Toxicity Criteria); all included the same clinical events, so summated these data (Table 2; Table 3; Table 4; Table 5).
| RTOG CTC | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|
| Acute skin | Follicular, faint or dull erythema/epilation/dry desquamation/decreased sweating | Tender or bright erythema, patchy moist desquamation/moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | Ulceration, haemorrhage, necrosis |
| Late skin toxicity | Slight atrophy, pigmentation change, some hair loss | Patchy atrophy, moderate telangiectasia, total hair loss | Marked atrophy, gross telangiectasia | Ulceration |
| Late subcutaneous fibrosis | Slight induration, loss of subcutaneous fat | Moderate fibrosis (asymptomatic) < 10% linear field contraction | Severe induration, loss of subcutaneous tissue, linear contraction > 10% | Ulceration |
CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria.
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Induration (subcutaneous fibrosis) | Increased density on palpation | Moderate increase in density, not interfering with ADL; marked increase in density and firmness on palpation with or without minimal retraction | Dysfunction interfering with ADL; very marked density, retraction or fixation | — |
| Telangiectasia | Few | Moderate | Many and confluent | — |
| Pain | Pain mild, not interfering with function | Moderate pain; pain or analgesics interfering with function, but not with ADL | Severe pain; pain or analgesics interfering with ADL | Disability |
ADL: activities of daily living; NCI CTC: National Cancer Institute Common Toxicity Criteria.
| Adverse effect | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|---|---|---|---|---|
| Pain | Mild, not interfering with function | Moderate/analgesics, interferes with function, but not ADL | Severe or interferes with ADL, or both | — | — |
| Telangiectasia | Few | Moderate | Many or confluent, or both | — | — |
| Acute skin toxicity | Faint erythema or dry desquamation | Moderate‐to‐brisk erythema, patchy moist desquamation | Moist desquamation not limited to creases or skin folds, bleeding subsequent to minor trauma or abrasion | Skin necrosis or full dermal thickness ulceration | Death |
| Induration/fibrosis | Increased density on palpation | Moderate functional impairment, not interfering with ADL, increased density and firmness on palpation with or without minor retraction | Interferes with ADL, very marked increased density, retraction or fixation | — | — |
ADL: activities of daily living; CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; RTOG: Radiation Therapy Oncology Group.
| Adverse effect | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|---|---|---|---|---|
| Subcutaneous fibrosis | — | Mild induration, can move skin parallel to the plane (sliding) and perpendicular to the plane (pinching up) | Moderate induration, can slide, cannot pinch up skin | Severe induration cannot slide or pinch skin | Death |
| Telangiectasia | < 10 % of body surface area | ≥ 10% of body surface area, psychosocial impact | — | — | — |
| Pain | mild | Moderate, not limiting instrumental ADL | Severe, limiting self‐care ADL | — | — |
ADL: activities of daily living; CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; RTOG: Radiation Therapy Oncology Group.
CTCAE 2009.
Three studies reported radiological or asymptomatic fat necrosis (ELIOT; Polgár 2007; RAPID); we were able to report Grade II or greater fat necrosis, as this had the same definition in all three scales (Table 6).
| Grade | Findings |
|---|---|
| 0 | No fat necrosis |
| 1 | Asymptomatic fat necrosis (only radiological or cytological findings, or both) |
| 2 | Symptomatic fat necrosis not requiring medication (palpable mass with or without mild pain) |
| 3 | Symptomatic fat necrosis requiring medication (palpable mass with significant pain) |
| 4 | Symptomatic fat necrosis requiring surgical intervention |
Assessment of risk of bias in included studies
Two review authors (BH and ML) assessed trials to check that they met the inclusion criteria and independently assessed methodological quality. Two review authors (BH and ML) independently undertook risk of bias table assessments (EF resolved disagreements) and reported them in the text and as a figure.
We described the risk of bias for each included trial. Two review authors (BH and ML) judged risk of bias in eight specific domains, and resolved any differences through discussion. The eight domains were:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessors for objective outcomes;
-
blinding of outcome assessors for subjective outcomes;
-
incomplete outcome data;
-
selective outcome reporting;
-
other sources of bias (i.e. early stopping and differences in follow‐up examinations).
We will perform sensitivity analyses on the basis of trial quality when additional trials are available. We plan to perform the analysis both with and without trials at low risk of bias to assess the effect of bias on the results when more trials are available to examine.
Measures of treatment effect
We presented results as HR for time‐to‐event data and odds ratios (OR) with 95% CI where this was not possible (Higgins 2021).
For future updates, if we find results for continuous variables (such as quality of life), we will summarise them using the mean difference (when studies use the same measurement scales) or the standardised mean difference (when studies use different measurement scales) (Higgins 2021).
Unit of analysis issues
Because the unit of analysis was the individual participant, we did not anticipate any unit of analysis issues. Where studies had multiple groups (IMPORT), the participants were analysed in the group they were randomised into, so no relevant groups were omitted and double counting was avoided. If cluster RCTs are identified in future updates, we will account for clustering to avoid overestimation of precision which would make CIs too narrow and give the study too much weight. This intervention would not allow crossover studies to be done.
Dealing with missing data
If data are missing in future updates, we will contact the original investigators (by written correspondence).
We will specify what assumptions we make, for example, if we presume the missing data were missing at random, or that missing data were assumed to have a particular value such as a poor outcome. We will, if necessary, perform a sensitivity analysis to see how sensitive results are to the assumptions we have made. We will address the potential implications of this in the 'Discussion' section.
Assessment of heterogeneity
We assessed heterogeneity both visually and statistically using the Chi2 test of heterogeneity (Altman 1992; Walker 1988), and I2 statistic (Higgins 2002; Higgins 2003). The criteria for identification of heterogeneity are a P less than 0.10 for the Chi2 test (acknowledging the limitations of this process) and an I2 statistic greater than 50%. Where we identified significant heterogeneity, we explored the reasons for it and made a cautious attempt to explain the heterogeneity.
Assessment of reporting biases
We acknowledge that there are multiple potential sources of reporting biases, including, but not limited to, publication bias, time‐lag bias, duplicate publication bias and selective outcome reporting. By searching multiple sources including trial registries, we hope to minimise publication bias. We noted the early reporting for the TARGIT was an example of time‐lag bias. We planned to use funnel plots to evaluate funnel plot asymmetry, but considered that visual interpretation is subjective and that statistical methods to evaluate funnel plot asymmetry are unlikely to be valid if there are fewer than 10 included trials.
Data synthesis
We applied the intention‐to‐treat principle in analysing data from the trials and determined a weighted mean treatment effect using the fixed‐effect model to combine results (Mantel 1959) with Review Manager Web (RevMan Web 2019).
We used the Mantel‐Haenszel methods to calculate pooled results when there was no significant heterogeneity (Greenland 1985; Mantel 1959), or if otherwise, the random‐effects model of Der Simonian and Laird (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
In future updates if data are available, we may perform subgroup analyses to investigate whether the effects of using PBI/APBI or conventional breast RT differ depending on nodal status, margin status, receptor status, hormonal manipulation or tumour stage.
If heterogeneity is identified in future updates, we will assess it both statistically and visually using the Chi2 test of heterogeneity (Altman 1992; Walker 1988), and I2 statistic (Higgins 2002; Higgins 2003). If we do identify significant heterogeneity, we will explore the reasons for it and attempt to explain it.
Sensitivity analysis
We planned to perform a sensitivity analysis by excluding the trials at high risk of bias for subjective outcomes (ELIOT; Livi 2015; Polgár 2007; Rodríguez), but because all studies were at high risk of bias for lack of blinding, this was not done. We performed a sensitivity analysis by excluding the study at high risk of 'other bias' (TARGIT); this was a post‐hoc decision. In future updates, if adequate data are available, we will perform a sensitivity analysis to assess the robustness of the results by excluding studies at high risk of bias for subjective outcomes and unpublished trials.
Summary of findings and assessment of the certainty of the evidence
We graded the quality of the evidence and created a summary of findings table using the following outcomes.
-
LR‐FS.
-
Cosmesis.
-
OS (follow‐up: 5‐year survival).
-
Late toxicity: late subcutaneous breast fibrosis.
-
C‐SS.
-
DM‐FS.
-
Subsequent mastectomy.
The population included women with early breast cancer and the intervention was PBI/APBI versus WBRT. We used GRADEpro GDT and the GRADE approach to evaluate the strength of the evidence (GRADE 2013). To calculate the absolute risk for the control group for time‐to‐event outcomes, we estimated the event‐free rate at a specific time point (five years for LR‐FS, BC‐SS, DM‐FS, R‐FS and OS) from the Kaplan‐Meier curves or reported event rates. We entered these estimated values in GRADEpro GDT, which automatically calculated the corresponding absolute risks for the intervention group at five years.
Results
Description of studies
See: Characteristics of included studies table.
Results of the search
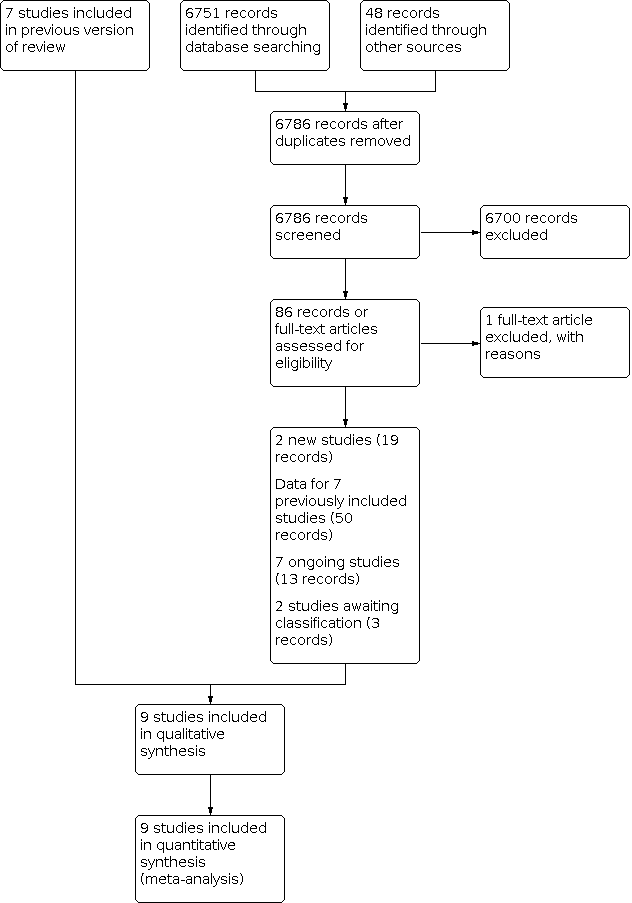
For this review update, based on our search strategy, we identified and screened 6751 references from the major medical databases and identified 48 additional references from other sources. After exclusion of duplicates and screening of references (by title or abstract), we evaluated 86 full‐text references and excluded one reference (see Characteristics of excluded studies table). Of the remaining 85 references, 19 records related to two new included studies (IMPORT; NSABP‐B39/RTOG), 54 records provided data for seven previously included studies (ELIOT; GEC‐ESTRO; Livi 2015; Polgár 2007; RAPID; Rodríguez; TARGIT) , 13 records for seven ongoing studies (NCT00892814; NCT03553797; NCT03583619; NCT03616626; NCT03637738; IRMA; SHARE), and three records for two studies awaiting classification (NCT02375048; Yadav 2019). See Figure 1.

Overall, when combining the original (Hickey 2016) and review update, there were nine included studies, seven excluded studies, two studies await classification and seven ongoing studies. In summary, this review update included nine studies and the qualitative analysis included nine studies (Figure 1).
Included studies
Design
Nine RCTs enrolled 15,187 women (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; NSABP‐B39/RTOG; Polgár 2007; RAPID; Rodríguez; TARGIT); we studied 14,245 women included in these studies. These studies enrolled women from July 1998 to May 2004 (Polgár 2007), February 2006 to July 2011 (RAPID), November 2000 to December 2007 (ELIOT), March 2000 to June 2011 (TARGIT), March 2005 to June 2013 (Livi 2015), April 2004 to July 2009 (GEC‐ESTRO), March 2005 to April 2016 (NSABP‐B39/RTOG), and 2007 to 2010 (IMPORT). Rodríguez did not state accrual dates.
Sample size
Polgár 2007 enrolled 258 women of a planned sample size of 570 participants, RAPID enrolled 2135 women, Rodríguez enrolled 102 women, ELIOT enrolled 1305 women, TARGIT enrolled 2298 women, Livi 2015 enrolled 520 women, IMPORT enrolled 2016 women, and GEC‐ESTRO enrolled 1184 women, and NSABP‐B39/RTOG enrolled 4216 women.
Setting
Four studies were single institution trials from tertiary institutions: one in Hungary (Polgár 2007), two in Italy (ELIOT; Livi 2015), and one in Spain (Rodríguez). GEC‐ESTRO, TARGIT, NSABP‐B39/RTOG, and RAPID were multicentred, international studies. IMPORT was a multicentre study from the UK.
Participants
A total of 15,187 women with invasive breast cancer or ductal carcinoma in situ (DCIS (6.3%) with T1‐2N0‐1M0 Grade I or II unifocal tumours (less than 2cm or 3 cm or less) treated with breast‐conserving surgery who had negative margins (see Characteristics of included studies table and Table 7).
| Study | Age | Stage | Margins | Tumour size | Nodal status | Surgery |
|---|---|---|---|---|---|---|
| After 2000, < 40 years excluded | Unifocal tumour, pT1N0‐1miM0, Grade I or II | Negative | < 2.0 cm (< 2 mm: 1/258, ≥ 2 mm: 246/258 or had no tumour at ink: 11/258) | — | WLE | |
| 48–75 years | "Early breast cancer," "suitable for breast conservation" | Not described | ≤ 2.5 cm | AD if SNBx positive | BCS | |
| > 40 years | — | Negative, ≥ 5 mm | ≤ 2.5 cm | — | WLE or quadrantectomy | |
| ≥ 45 years | T1 and small T2N0‐1M0 invasive breast cancer, suitable for BCS, available for 10 years' follow‐up | ≥ 1 mm Re‐excision strongly advised for close or positive margins | — | — | BCS | |
| ≥ 40 years | DCISa or invasive breast cancer | Negative | ≤ 3 cm | Negative axillary nodal involvement including micrometastasis (> 0.2 mm or positive cells only identified on IHC as determined by sentinel node biopsy; axillary node dissection or clinical examination for DCIS only. | BCS | |
| ≥ 60 years | Invasive ductal carcinoma (pT1‐2cNO MO), unifocal tumour, Grade I or II | < 3 mm | ≤ 3 cm | — | — | |
| ≥ 50 years | Invasive breast cancer pT1‐2pN0 who have < 1% annual risk of local recurrence | ≥ 2 mm | ≤ 3 cm | 0–3 nodes positive | BCS | |
| > 40 years | Stage 0, I or II pN0/pNmi breast cancer (including DCIS) no vascular invasion, unifocal or unicentric disease only, no LVINote: 60/1184 (5%) participants had DCIS | ≥ 2 mm in invasive disease, 5 mm in DCIS | Lesions < 3 cm in diameter | Node negative, for DCIS alone: sentinel node biopsy optional | WLE or quadrantectomy, level I–II axillary dissection, removing ≥ 6 (preferably 10 lymph nodes) | |
| > 18 years | T0‐2N0‐1M0 DCIS or invasive breast cancer Note: 531/4216 (12%) participants had DCIS | Negative: "free of cancer, including DCSI" | Lesions < 3 cm | ≤ 3 involved nodes permitted | Lumpectomy |
AD: axillary dissection; BCS: breast‐conserving surgery; DCIS: ductal carcinoma in situ; IHC: immunohistochemistry; LVI: lymphovascular invasion; SNBx: sentinel node biopsy; WLE: wide local excision.
a366/2135 participants had DCIS.
Interventions
Experimental arms
PBI using:
-
APBI (ELIOT; GEC‐ESTRO; Polgár 2007; RAPID; TARGIT; NSABP‐B39/RTOG), conventional fractionation (Livi 2015; Rodríguez), or hypofractionation (IMPORT);
-
brachytherapy:
-
high‐dose rate (HDR) brachytherapy;
-
pulsed‐dose rate (PDR) brachytherapy;
-
-
EBRT delivered:
| Study | RT quality assurance | RT technique | PIB/APBI target volume definition |
|---|---|---|---|
| Postimplant CT scans were performed | Interstitial brachytherapy (88/128) EBRT using photons (40/128) 2‐dimensional CT‐based | PTV: excision cavity delineated by the surgical clips + 2 cm isotropic margin. For interstitial therapy: if electrons were used, 6–15 MeV were used to treat the cavity with a 2 cm margin | |
| Not stated | Intraoperative electrons 6–9 MeV | CTV: quote: "decided according to the site and size of the tumour. The energy of the electron beams was selected according to the thickness of the gland measured by a graduated needle" | |
| Not stated | EBRT (IMRT) | CTV = +1 cm isotropic margin around surgical clips PTV = CTV +10 mm isotropic margina | |
| Nob | Intraoperative kV RT 3D‐CRT for WBRT | The target volume was the tumour cavityc | |
| Yesd | EBRT (3D‐CRT) APBI: 3–5 non‐coplanar fields | CTV = tumour bed on CT (surgical clips plus a 1‐cm margin inside breast PTV = CTV +1 cm isotropic margin | |
| Not stated | EBRT (3D‐CRT) | PTV was defined by contouring the same quadrant as the primary tumor sitee | |
| Yesf | Field‐in‐field IMRT | Tumour bed, surgical clipsg recommended | |
| Yesh | HDR or PDR multicatheter brachytherapy | PBI: tumour bedi +2 cm isotropic margin | |
| Quote: "Every institution's RT facilities were quality assessed and each case of APBI was centrally reviewed for RT quality." Benchmarking performed with "dummy run" | HDR brachytherapyj APBI EBRT: 3D‐CRTk (IMRT not permitted). WBRT: 3D‐CRT (IMRT not permitted) | No RNI permitted WBRT: entire ipsilateral breast APBI: CTV = cavity = PTV | |
| Study | RT quality assurance | RT technique | Target volume definition |
3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; CRT: conformal radiotherapy; CT: computer tomography; CTV: clinical target volume; EBRT: external beam radiotherapy; HDR: high‐dose rate; IMRT: intensity‐modulated radiotherapy; PDR: pulsed‐dose rate; PIB: partial breast irradiation; PTV: planning target volume; RNI: regional nodal irradiation; RT: radiotherapy; WBRT: whole breast radiotherapy.
aThe surgeons were requested to place clips at the borders of the surgical bed, using a minimum of four clips. CTV was drawn on a planning CT (0.3 mm slices) with a uniform 1 cm margin around the surgical clips, then a 1 cm margin added to construct the PTV.
bFor the WBRT component of TARGIT, as long as treating centres conformed to a formal quality management system issued by the International Standards Organisation, no additional quality assurance was required. For the APBI, quality assurance was performed according to the manufacturer's instructions and the resulting data to be made available to the trials centre. Data were submitted either annually or after every 50th participant treated with Intrabeam.
cQuote: "The appropriately sized (1.5–5.0 cm diameter) applicator is placed in the tumour bed using a meticulous surgical technique, including a carefully inserted purse‐string suture that ensures that breast tissues at risk of local recurrence receive the prescribed dose while skin and deeper structures are protected. Radiation is delivered over 20–45 min to the tumour bed. The surface of the tumour bed typically receives 20 Gy that attenuates to 5–7 Gy at 1 cm depth."
dBefore study opening, physicians' tumour bed contouring and centres' APBI planning were credentialed. Centres completed real‐time review of at least 10 APBI patient cases before treatment and final review of all patient cases.
eTo avoid interobserver variability, this was performed by the same radiation oncologist. Surgical clips were not available at the institution at the time.
fBaseline questionnaire completed by centre, UK Radiotherapy Trial Quality Assurance (RTQA) validated the treatment technique, a phantom was used, all plans and data sets collected and stored at RTQA, every 10th enrolled participant was selected at random to have thermo‐luminescent dosimetry measurements, which were sent to RTQA.
gIf no clips had been inserted, ultrasound, magnetic resonance imaging or CT was used. If no localisation procedure had been done, study entry was permitted if the clinician was confident that clinical localisation was accurate.
hBoth pre‐ and postimplant assessment of geometry using CT, dose prescription and calculations were in accordance with International Commission of Radiation Units and Measurements (ICRU) 58 and strict dose volume histogram and dose maximums were mandated, the post‐hoc quality assurance requirements were clearly detailed in the study protocol (GEC‐ESTRO).
iTumour bed localised using clips, preoperative mammographic and ultrasound imaging and planning scan.
jHDR multicatheter, HDR single entry (MammoSite single‐lumen, MammoSite multi‐lumen, Contura multi‐lumen balloon).
k10 fractions, given with six‐hour gap on five treatment days within an eight‐day period.
Control arm
WBRT using conventional fractionation: dose ranged from 42.5 Gy to 56 Gy, the use of a boost was variable (see Table 8 for details).
Co‐interventions
Chemotherapy and hormonal manipulation: in GEC‐ESTRO, Livi 2015, NSABP‐B39/RTOG, Polgár 2007, RAPID, and TARGIT, women received adjuvant systemic therapy according to institutional protocol, and Rodríguez treated oestrogen receptor‐positive women with hormonal manipulation. Systemic therapy in ELIOT was "administered according to the European Institute of Oncology policy" at the time (see Characteristics of included studies table for further details). In total, 6372/6820 (93%) women received hormonal manipulation and 977/6820 (14.3%) women received chemotherapy.
ELIOT treated women with four or more involved nodes with regional nodal RT. No women in any of the other included studies received regional nodal RT (Table 8).
Outcomes
1.0 Primary outcomes
1.1 Local recurrence‐free survival
Nine studies reported local recurrence in the ipsilateral breast as a discrete outcome (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; NSABP‐B39/RTOG; Polgár 2007; RAPID;Rodríguez; TARGIT), but because in Rodríguez there were zero events on both study arms, it was not included in the analysis.
1.2 Cosmesis
Six studies reported cosmesis (cosmetic outcome). Polgár 2007, Livi 2015, and Rodríguez reported a global cosmetic result (used the Harvard Cosmetic Score (see Table 1)). GEC‐ESTRO and RAPID used the EORTC Cosmetic Rating System for Breast Cancer (Aaronson 1998); both were four‐point scales. GEC‐ESTRO evaluated cosmesis using digital photos, with both participant‐reported outcomes and physician‐reported outcomes using a four‐point scale (Wazer 1992; see Table 9). GEC‐ESTRO, RAPID, and Rodríguez included participant‐reported outcomes. Trained nurses also assessed cosmetic outcomes in RAPID. Assessment of cosmetic outcome was blinded in RAPID. TARGIT assessed cosmetic outcome at a single centre in a substudy that included 105 women. A software program, blinded to treatment arm, assessed digital photos at a median 23 months using a four‐point scale.
| Score | Definition |
|---|---|
| Excellent | Perfect symmetry, no visible distortion or skin changes and no visible catheter entry/exit sequelae |
| Good | Slight skin distortion, retraction or oedema, any visible telangiectasia, any visible catheter entry/exit scar or mild hyperpigmentation |
| Fair | Moderate distortion of the nipple or breast symmetry, moderate hyperpigmentation, or prominent skin retraction, oedema or telangiectasia |
| Poor | Marked distortion, oedema, fibrosis or severe hyperpigmentation |
2.0 Secondary outcomes
2.1 Overall survival
Nine studies reported OS (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; NSABP‐B39/RTOG; Polgár 2007; RAPID;Rodríguez ; TARGIT) but because in Rodríguez there were zero events on both study arms, it was not included in the analysis.
2.2 Toxicity
Five studies reported acute skin toxicity. Rodríguez, Livi 2015, and TARGIT used the Radiation Therapy Oncology Group Common Toxicity Criteria (RTOG CTC) (Cox 1995; Table 2), RAPID used CTCAE Version 4.0 (CTCAE 2009) and ELIOT used a five‐point scale (which was not referenced).
Six studies reported late toxicity (telangiectasia (small blood vessels visible on the treated skin), breast pain, fat necrosis and subcutaneous fibrosis). Polgár 2007 reported fat necrosis at four years according to a five‐point institutional scale (Table 6); RAPID reported late RT toxicity (telangiectasia, induration (subcutaneous fibrosis), breast pain and fat necrosis) using the National Cancer Institute Common Toxicity Criteria (NCI CTC 3.0; NCI; three‐point scales; Table 3). GEC‐ESTRO reported late toxicity (breast pain, late subcutaneous toxicity and late RT skin toxicity) using the RTOG/EORTC CTC (Cox 1995). ELIOT reported fat necrosis and radiological radiation pneumonitis using late effects of normal tissue – subjective objective management analytic criteria (LENT‐SOMA; Pavy 1995). Livi 2015 scored late RT toxicity using the RTOG/EORTC CTC (Cox 1995). TARGIT reported "complications arising six months after randomisation" and reported RTOG/EORTC CTC (Cox 1995) Grade III or IV RT‐related skin complications, haematomas/seromas requiring greater than three aspirations or surgery, wound infections requiring intravenous antibiotics or surgery, and skin breakdown/delayed wound healing.
2.3 New primary tumours in ipsilateral breast ('elsewhere primaries')
Four studies reported new primary tumours in ipsilateral breast (ELIOT; GEC‐ESTRO; Livi 2015; RAPID).
2.4 Cause‐specific survival
Seven studies reported C‐SS (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; Polgár 2007; TARGIT; RAPID).
2.5 Distant metastasis‐free survival
Eight studies reported DM‐FS (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; Polgár 2007; NSABP‐B39/RTOG; TARGIT; Rodríguez) but because in Rodríguez there were zero events on both study arms, it was not included in the analysis.
2.6 Relapse‐free survival
Six studies reported R‐FS: IMPORT; Livi 2015;GEC‐ESTRO; Polgár 2007; TARGIT; NSABP‐B39/RTOG; RAPID). The data reported in GEC‐ESTRO were not in a usable form.
2.7 Locoregional recurrence‐free survival
Six studies reported L‐RR‐FS (ELIOT; GEC‐ESTRO; Livi 2015; Polgár 2007; Rodríguez; TARGIT), but because in Rodríguez there were zero events on both study arms, it was not included in the analysis.
2.8 Subsequent mastectomy
Three studies reported subsequent mastectomy (GEC‐ESTRO; Polgár 2007; TARGIT).
2.9 Compliance
All nine studies reported compliance (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; NSABP‐B39/RTOG; Polgár 2007; RAPID; Rodríguez; TARGIT).
2.10 Costs
We found no trials reporting costs.
2.11 Quality of life
Three studies reported quality of life. RAPID used the Breast Cancer Questionnaire (a validated modification of the Breast Cancer Chemotherapy Quality of Life Questionnaire; Levine 1998) and GEC‐ESTRO and Livi 2015 used the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30; Aaronson 1998) and Quality of Life Questionnaire – Breast Specific Module (QLQ‐BR23) (Sprangers 1996).
2.12 Consumer preference
We found no trials reporting consumer preference.
The included RCTs differed in several ways.
-
Population included.
-
Margin size.
-
Target volume definition for the treated PBI/APBI volume varied between the trials.
-
Radiation dose prescribed differed greatly between the trials (see Table 10).
-
RT technique for PBI/APBI delivery differed.
-
RT fractionation varied across studies.
-
Quality assurance of RT.
-
One study did not accrue the full sample size: Polgár 2007 stopped early because a competing multicentred RCT was started (for details see Table 7, Table 8, Table 11).
| Trial | PBI/APBI dose | Fraction size (Gy) | EQD2 PBI/APBI alpha/beta = 4 | Control dose | Fraction size (Gy) | EQD2 Control alpha/beta = 4 |
|---|---|---|---|---|---|---|
| 20 Gy at surface of the applicator (attenuated to 5–7 Gy at 1 cm) (APBI) | 80 at cavity surface 12.8 at 1 cm | 80 Gy at cavity surface 12.8 Gy at 1 cm | 40–56 Gy/20–28 fractions ± 10–16 Gy boost | 2 | 40–56 Gy ± 10–16 Gy | |
| 30 Gy/5 daily fractions EBRT IMRT. 100% of the PTV was covered by 95% of the prescribed dose | 6 | 50 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost | 2 | 50 + 10 = 60 Gy | |
| 38.5 Gy/10 fractions twice daily (with 6‐hour gap) Dose‐evaluation volume (that part of PTV within the breast) received 95–107% of prescription dose | 3.85 | 49.4 Gy | 50 Gy/25 fractions or 42.5 Gy/16 fractions ± boost (10 Gy/4–5 fractions) based on criteria such as young age or close margins, prespecified by centre | 2 or 2.65 | 50 Gy or 47.1 Gy | |
| 37.5 Gy/10 fractions twice daily (with 6‐hour gap) (APBI). PTV covered by ≥ 95% of prescribed dose, with < 105% hot spot | 3.75 | 47.48 Gy | 48 Gy/24 fractions ± 10 Gy/5 fractions boost | 2 | 48 ± 10 = 48–58 Gy | |
| 7 × 5.2 Gy HDR (APBI) or 50 Gy/25 fractions (PBI) | 5.2 or 2 | 57.5 Gy or 50 Gy | 50 Gy/25 fractions (3D‐CRT was not used) | 2 | 50 Gy | |
| 30.3 Gy/7 fractions or 32 Gy/8 fractions HDR twice daily or 50 Gy at 0.6–0.8 Gy/hour pulses (1 pulse per hour, 24 hours per day) PDR | 7–8 | 41.64–42.67 Gy | 50.0–50.4 Gy to a reference point + 10 Gy/5 fractions boost. Electron dose was prescribed to the point of maximum dose on the beam axis (Dmax), ensuring the 85% isodose encompassed the tumour bed | 1.8–2.0 | 48.72–50 + 10 = 58.72–60 Gy | |
| 21 Gy/1 fraction at 90% using 6–9 MeV | 21 | 84 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost (using electrons) | 2.0 | 50 + 10 Gy | |
| 40 Gy/15 fractions (EBRT) | 2.72 | 45.23 | 40 Gy/15 fractions 36 Gy/15 fractions + boost 40 Gy/15 fractions | 2.72 2.4 | 45.23 38.4 + 45.23 |
3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; CT: computer tomography; EBRT: external beam radiotherapy; EQD2: equivalent dose in 2 Gy fractions; Gy: Gray; HDR: high‐dose‐rate; IMRT: intensity‐modulated radiotherapy; MeV: mega electron volt; PBI: partial breast irradiation; PDR: pulsed‐dose rate; PTV: planning target volume.
| Study | Mammography | Radiotherapy toxicity | Patient‐reported outcomes (PRO) | Cosmesis |
|---|---|---|---|---|
| At 6 months, then annually | Acute: CTCAEa Late: EORTC/RTOGb and LENT‐SOMAc | Not assessed | Harvard Cosmetic Scored | |
| Annually | LENT‐SOMAc | Not assessed | Not assessed | |
| Annually | Acute and late: EORTC/RTOGb | EORTC QLQ‐C30e QLQ‐BR23 breast cancer modulef | Harvard Cosmetic Scored | |
| Annually | Acute: nil Late: EORTC/RTOG,b LENT‐SOMA,c CTCAEa | EORTC QLQ‐C30e QLQ‐BR23 breast cancer module Body‐image scale | Clinician and nurse assessed | |
| Annually | Acute and late: NCI version 3.0 | EORTC QLQ‐C30e QLQ‐BR23 breast cancer module Body‐image scale | EORTC/RTOG Rating Systemg | |
| Baseline 6 months after RT than annually | Late: EORTC/RTOGb | Not assessed | Harvard Cosmetic Scored | |
| Annually 1–5 years, 3 yearly to 10 years | Symptomatic rib fracture and lung fibrosis Ischaemic heart disease recorded at 1, 2, 5 and 10 years' follow‐up | EORTC QLQ‐C30e QLQ‐BR23 breast cancer module Body‐image scale protocol‐specific questionsh HADS scale EuroQol EQ‐5D‐3L health status questionnaire at baseline; 6 months; 1, 2 and 5 years | Patient‐ and clinician‐assessedi Photosj | |
| At 6, 12, 18, 24 months after radiotherapy then annually for 10 years | Fat necrosis measured using Lövey scoring systemk Physician scored late toxicity Acute radiotherapy toxicity: CTCAE version 3.0a LENT‐SOMAc Late RT toxicity: EORTC/RTOGb | Breast pain and arm lymphoedema measured by CTCAE version 3.0a EORTC QLQ‐C30e and QLQ‐BR23 at baseline and during follow‐up | Harvard Cosmetic Scored Physician‐ and patient‐reported Digital photosl | |
| Annually | Acute radiotherapy toxicity: CTCAE version 4.0 Late RT toxicity: CTCAE version 4.0 | Not assessed | Physician reported |
CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Breast Specific Module; EORTC/RTOG: European Organisation for Research and Treatment of Cancer/Radiation Therapy Oncology Group; HADS: Hospital Anxiety and Depression Scale; LENT‐SOMA: late effects in normal tissues – subjective objective, management and analytic; RT: radiotherapy.
aTrotti 2013.
bRubin 1995.
cAnon 1995.
dHarvard Cosmetic Score uses a four‐point scale: excellent, good, fair or poor (Harris 1979).
eThe EORTC QLQ‐C30 includes nine multi‐item scales: five functional (physical, role, emotional, cognitive and social), three symptom scales (fatigue, pain and nausea‐vomiting) and a global health status (GHS) health‐related quality of life scale. There are six single‐item symptom measures: insomnia, appetite loss, constipation, diarrhoea and financial difficulties). The symptom measures are scored on a four‐point scale, with high scores representing a higher symptom burden. The GHS scale is scored using a visual analogue scale: one (very bad) to seven (excellent), so a higher score on GHS or the functional scale is good (Aaronson 1998).
fThe BR23 module uses 23 questions to assess symptoms, treatment adverse effects, body image, sexual function and future perspective using five multi‐item scales. The symptom measures are scored on a four‐point scale, so high scores represent a higher symptom burden. The other aspects are scored so a higher score is better (Sprangers 1996).
gThe EORTC/RTOG Rating System is a four‐point scale, assessed by trained nurses, physicians and participant‐reported outcomes (Aaronson 1998).
hHas skin appearance changed, overall breast appearance changed, breast become smaller, breast become harder or firmer to touch, is shoulder stiffness present?
iBreast shrinkage, distortion, induration, breast oedema, telangiectasia assessed using four‐point scale (not at all, a little, quite a bit and very much). Photos taken at baseline and 2 and 5 years.
jPhotos scored as showing no change, mild or marked change in breast appearance at 2 and 5 years compared with baseline by three observers masked to treatment allocation using a validated consensus method (Haviland 2008).
kLövey 2007.
lDigital photos assessed using a validated consensus method by three observers masked to treatment allocation.
Excluded studies
We excluded seven studies. Two studies did not use contemporary surgical or RT techniques, one study was not an RCT, three studies used PBI in both study arms and one study used WBRT in both study arms (for details, see Characteristics of excluded studies table).
Risk of bias in included studies
See Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were at low risk of bias for random sequence generation. Three were at unclear risk of allocation concealment (ELIOT; Polgár 2007; Rodríguez), and the remaining six studies were at low risk of bias for allocation concealment (GEC‐ESTRO; IMPORT; Livi 2015; NSABP‐B39/RTOG; RAPID; TARGIT).
Blinding
Objective outcomes
All studies were at low risk of performance bias for lack of blinding for objective outcomes. All studies were at low risk of detection bias for objective outcomes.
Subjective outcomes
Seven studies were at high risk of performance bias for lack of blinding for subjective outcomes (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; NSABP‐B39/RTOG; Polgár 2007; TARGIT), one study was at unclear risk of bias (Rodríguez), and one study was at low risk of bias for this domain (RAPID). One study was at low risk of detection bias for subjective outcomes (RAPID), and eight studies were at high risk of bias (ELIOT; GEC‐ESTRO; IMPORT; Livi 2015; NSABP‐B39/RTOG; Polgár 2007; Rodríguez; TARGIT).
Incomplete outcome data
Nine studies were at low risk of attrition bias.
Selective reporting
Three studies were at low risk of reporting bias (GEC‐ESTRO; NSABP‐B39/RTOG; TARGIT), while the remaining studies were at unclear risk.
Other potential sources of bias
Polgár 2007 had low risk of bias as they stopped the trial early because a competing trial started recruiting (GEC‐ESTRO). We identified no other sources of bias for ELIOT, GEC‐ESTRO, IMPORT, Livi 2015, and RAPID so deemed them at low risk of bias. Rodríguez was an interim report and, therefore, at unclear risk of other bias.
Risk of bias by outcome
1.1 Local recurrence‐free survival
Seven of eight studies contributing data to this outcome were at low risk of bias. One study was at high risk of bias for inadequate follow‐up duration to detect the outcome (TARGIT).
1.2 Cosmesis
One of six studies contributing data with respect to physician or nurse‐evaluated cosmesis was at low risk of bias, and four of six at high risk of bias for lack of blinding for outcome assessors.
2.1 Overall survival
All eight studies contributing data to this outcome were at low risk of bias for sequence generation; four of eight studies were at unclear risk of bias for allocation concealment.
2.2 Toxicity
2.2.1 Acute radiotherapy toxicity
All studies contributing data to this outcome were at high risk of bias for lack of blinding for outcome assessors.
2.2.2 Late radiotherapy toxicity
All studies contributing data to this outcome were at high risk of bias for lack of blinding for outcome assessors, with the exception of radiological fat necrosis, where two of two studies were at low risk of bias.
2.3 New primary tumours in ipsilateral breast 'elsewhere primary'
Three of four studies contributing data to this outcome were at low risk of detection bias.
2.4 Cause‐specific survival
Six of seven studies contributing data to C‐SS were at high risk of bias for lack of blinding for outcome assessors.
2.5 Distant metastasis‐free survival
Five of seven studies contributing data to DM‐FS were at high risk of bias for lack of blinding for outcome assessors.
2.6 Relapse‐free survival
Four of six studies contributing data to R‐FS were at high risk of bias for lack of blinding for outcome assessors.
2.7 Locoregional recurrence‐free survival
All studies contributing data to this outcome were at high risk of bias for lack of blinding for outcome assessors.
2.8 Subsequent mastectomy
All studies contributing data to this outcome were at low risk of bias for detection bias.
2.9 Compliance
This outcome was deemed at low risk of bias.
2.10 Costs
Deemed at low risk of bias.
2.11 Quality of life (using trial‐specific instruments)
All studies contributing data to this outcome were at high risk of bias for lack of blinding for outcome assessors.
2.12 Consumer preference
None of the studies assessed consumer preference.
Effects of interventions
See: Summary of findings 1 Summary of Findings Table ‐ PBI?APBI compared to WBRT for early breast cancer
Primary outcomes
1.1 Local recurrence‐free survival in the ipsilateral breast
There were 630 local recurrences in the 13,168 women in eight studies.
The use of PBI/APBI probably slightly reduces LR‐RFS (HR 1.21, 95% CI 1.03 to 1.42; I2 = 27%; P = 0.21; moderate‐certainty evidence, downgraded for imprecision; Analysis 1.1; Figure 3). There was little evidence of heterogeneity on visual inspection and statistical testing. The mean effect of the use of PBI is associated with three more local recurrences per 1000 participants at five years (the true value lies between zero and six more).

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.1 Local recurrence‐free survival.
All studies were at low risk of bias for this outcome, so a sensitivity analysis was not performed.
1.2 Cosmesis
Cosmetic outcome is probably worse with PBI/APBI versus WBRT.
-
Participant‐reported (OR 2.08, 95% CI 1.68 to 2.57; I2 = 91%, P = 0.0008; moderate‐certainty evidence, downgraded for risk of detection bias and heterogeneity; Analysis 1.2). We identified 453 events in 2775 participants from two studies (GEC‐ESTRO; RAPID).
-
Physician or trained nurse observer‐reported (OR 1.57, 95% CI 1.31 to 1.87; 6 studies, 3652 participants; I2 = 90%; P < 0.0008; moderate‐certainty evidence, downgraded for lack of blinding and heterogeneity; Analysis 1.3). There was evidence of heterogeneity. We studied 616 events in 3652 participants in six studies. The mean effect of the use of PBI is associated with 63 more adverse cosmetic outcomes per 1000 participants (the true value lies between 35 and 92 more).
We performed a sensitivity analysis by excluding studies at high risk of bias for blinding of outcome assessors for subjective outcomes for physician‐reported cosmesis (Livi 2015; Polgár 2007; Rodríguez). We found cosmesis was probably worse with PBI/APBI versus WBRT (OR 1.83, 95% CI 1.51 to 2.24; I2 = 93%; P = 0.00001). There may be some heterogeneity.
The planned subgroup analyses were not possible.
Secondary outcomes
2.1 Overall survival
The use of PBI/APBI versus WBRT probably makes little or no difference to survival with 1052 deaths in eight studies with 13,175 participants (HR 0.99, 95% CI 0.88 to 1.12; I2 = 0%; P = 0.27; high‐certainty evidence; Analysis 1.4; Figure 4). The mean effect of the use of PBI is associated with 0 fewer deaths per 1000 participants at five years (the true value lies between six fewer and six more). There was no heterogeneity on either visual inspection or statistical testing.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.3 Overall survival.
2.2 Toxicity
2.2.1 Acute radiotherapy toxicity
PBI/APBI probably reduces acute skin toxicity compared with WBRT (OR 0.76, 95% CI 0.66 to 0.88; I2 = 99%; P < 0.00001; moderate‐certainty evidence, downgraded for lack of blinding and heterogeneity; Analysis 1.5). We studied 834 events in 3925 participants in four studies. The mean effect of the use of PBI is associated with 46 fewer Grade II or greater toxicity events per 1000 participants (the true value lies between 67 fewer and 22 fewer). There was evidence of heterogeneity.
There was no difference in haematomas needing surgical aspiration between APBI and WBRT (P = 0.338; figure from text; TARGIT).
Seromas needing greater than three aspirations were more frequent with APBI compared with WBRT (P = 0.012; figure from text; TARGIT).
There was no difference in infection requiring intravenous antibiotics or surgical intervention between APBI and WBRT (P = 0.292; figure from text; TARGIT).
There was no difference in skin breakdown or delayed healing between APBI and WBRT (P = 0.155; figure from text; TARGIT). The mean effect of the use of PBI is associated with 15 fewer late RT toxicity episodes per 1000 participants (the true value lies between 25 fewer and three more).
2.2.2 Late radiotherapy toxicity
Late skin toxicity may increase with PBI/APBI versus WBRT (OR 2.27, 95% CI 1.63 to 3.15; 3 studies, 3465 participants; I² = 93%; P < 0.00001; low‐certainty evidence, downgraded for risk of detection bias, heterogeneity and imprecision; Analysis 1.6).
Telangiectasia may be worse with PBI/APBI compared with PBI (OR 4.40, 95% CI 3.34 to 5.80; 2 studies, 3010 participants; low‐certainty evidence, downgraded for risk of detection bias and heterogeneity; Analysis 1.7). The mean effect of the use of PBI is associated with 129 more telangiectasia episodes per 1000 participants (the true value lies between 93 more and 317 more).
PBI/APBI may increase fat necrosis compared with WBRT (OR 2.76, 95% CI 1.74 to 4.38; 4 studies, 3565 participants; I2 = 50%; moderate‐certainty evidence, downgraded for lack of blinding and imprecision; Analysis 1.8). The mean effect of the use of PBI is associated with 25 more fat necrosis events per 1000 participants (the true value lies between 11 more and 48 more). There was some heterogeneity.
PBI/APBI may increase subcutaneous fibrosis compared with WBRT (OR 5.07, 95% CI 3.81 to 6.74; 2 studies, 3011 participants; moderate‐certainty evidence, downgraded for risk of detection bias and heterogeneity; Analysis 1.9). The mean effect of the use of PBI is associated with 141 more subcutaneous fibrosis events per 1000 participants (the true value lies between 102 and 188 more).
Breast pain may increase with PBI/APBI versus WBRT (OR 1.81, 95% CI 1.15 to 2.86; 2 studies, 3012 participants; low‐certainty evidence, downgraded for lack of blinding, heterogeneity and imprecision; Analysis 1.10). ELIOT reported no difference in breast pain (data not shown). Breast pain was reduced with PBI compared with WBRT in GEC‐ESTRO (P = 0.04; figures from text).
2.3 New primary tumours in ipsilateral breast, 'elsewhere primaries'
New primaries in the treated breast may increase with PBI/APBI compared with WBRT (OR 2.82, 95% CI 1.55 to 5.12; I2 = 76%; P = 0.005; low‐certainty evidence, downgraded for possible inconsistency and imprecision; Analysis 1.11). There were 55 events in 5144 participants in four studies. The mean effect of the use of PBI is associated with 9 more "elsewhere primaries" per 1000 participants (the true value lies between 3 more and 19 more). There was evidence of heterogeneity.
2.4 Cause‐specific survival
We found that C‐SS is probably similar with PBI/APBI versus WBRT (HR 1.06, 95% CI 0.83 to 1.36; 7 studies, 9865 participants; I2 = 0%; P = 0.93; moderate‐certainty evidence, downgraded for lack of blinding and imprecision; Analysis 1.12; Figure 5). The mean effect of the use of PBI is associated with 1 more breast cancer death per 1000 participants at five years (the true value lies between 3 fewer and 6 more). We found no evidence of heterogeneity on either visual inspection or statistical testing.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.8 Cause‐specific survival.
2.5 Distant metastasis‐free survival
The use of PBI/APBI versus WBRT probably makes little or no difference to DM‐FS (HR 0.95, 95% CI 0.80 to 1.13; 7 studies, 11,033 participants; I2 = 25%; P = 0.24; moderate‐certainty evidence, downgraded for high risk of detection bias for lack of blinding and imprecision; Analysis 1.13; Figure 6). The mean effect of the use of PBI is associated with 1 fewer metastatic event per 1000 participants at five years (the true value lies between 6 fewer and 4 more). There was no heterogeneity with either visual inspection or statistical testing.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.9 Distant metastasis‐free survival.
2.6 Relapse‐free survival
The use of PBI/APBI versus WBRT probably decreases RF‐S (HR 1.25, 95% CI 1.05 to 1.48; 6 studies; 11,756 participants; I2 = 0%; P = 0.61; moderate‐certainty evidence, downgraded for high risk of detection bias for lack of blinding and imprecision.; Analysis 1.14). The mean effect of the use of PBI is associated with 29 more breast cancer relapses per 1000 participants at five years (the true value lies between 8 more and 43 more). We found little evidence of heterogeneity.
2.7 Locoregional recurrence‐free survival
L‐RR‐FS may be decreased with PBI/APBI compared with WBRT (HR 1.36, 95% CI 1.06 to 1.74; 5 studies; 6718 participants; I2 = 69%; P = 0.01; low‐certainty evidence, downgraded for lack of blinding, heterogeneity and imprecision; Analysis 1.15). The mean effect of the use of PBI is associated with 9 more locoregional relapses per 1000 participants at five years (the true value lies between 1 more and 12 more). We found evidence of heterogeneity.
2.8 Subsequent mastectomy
Subsequent mastectomy rate is little or no different with PBI/APBI compared with WBRT (OR 0.98, 95% CI 0.78 to 1.23; 3 studies, 3740 participants; I2 = 0%; P = 0.54; high‐certainty evidence; Analysis 1.16). The mean effect of the use of PBI is associated with 2 fewer mastectomies per 1000 participants (the true value lies between 20 fewer and 20 more). There were 350 events in 3740 participants in three studies. There was no evidence of heterogeneity.
2.9 Compliance
Nine studies found that 94.9% of women randomised to PBI/APBI received treatment. For those women randomised to receive WBRT, 95.6% of women received the planned RT.
2.10 Costs
Secondary analysis of the TARGIT data reports conflicting findings: in the UK setting, a modelling‐based study using participant level data found for the pre‐pathology stratum, APBI (using TARGIT intra‐operative technique) is less costly, with similar quality adjusted life years (QALYs) than whole breast irradiation (Vaidya 2016a). In the United States setting, APBI (using TARGIT intra‐operative technique) is less costly (Alvarado 2013; Shah 2014) and associated with more (Alvarado 2013) or fewer (Shah 2014) QALYs.
2.11 Quality of life
At baseline, both PBI/APBI and WBRT participants (GEC‐ESTRO; Livi 2015) had similar EORTC QLQ‐C30 and QLQ‐BR23 (Livi 2015), and Global Health score (GHS) (GEC‐ESTRO) (see Table 11).
Assessment at two years' follow‐up revealed that participants in the PBI/APBI arms had better QLQ‐C30 in most domains (except dyspnoea, constipation and diarrhoea) and better QLQ‐BR23 in most domains (except sexual functioning, sexual enjoyment and hair loss) in the PBI/APBI arm (Livi 2015) (Table 11). Breast symptoms were increased (in a clinically relevant amount) for participants in the WBRT arm (GEC‐ESTRO; Livi 2015).
At five years follow‐up, GHS scores did not differ between women receiving APBI/PBI and WBRT (P = 0.95; GEC‐ESTRO). Breast symptoms were increased (in a clinically relevant amount) for participants in the WBRT arm (IMPORT). There was no difference between study arms for body image at five years' follow‐up (IMPORT). Quality of life was assessed in RAPID but has not been reported yet.
2.12 Consumer preference
There were no data on consumer preference.
Discussion
Summary of main results
LR‐FS is probably slightly worse with APBI/PBI, but there appears to be little detriment or difference to other oncological outcomes including survival. R‐FS probably decreases and L‐RR‐FS may be decreased with APBI/PBI. The cosmetic outcome is probably worse with APBI/PBI. APBI appears to make little or no difference to mastectomy rate, and primaries in the ipsilateral breast 'elsewhere primaries' may be increased. The economic and psychological impact of the increase in 'elsewhere primaries' is uncertain.
Overall completeness and applicability of evidence
There were limitations in completeness and applicability of evidence due to clinical heterogeneity between the trials, duration of follow‐up and little or no useful information available for some outcome measures.
Clinical heterogeneity between the trials
There was evident clinical heterogeneity in both the study participants and the interventions.
The PBI/APBI delivered was heterogeneous in dose, technique, fractionation and target delineation.
-
The RT doses varied between the trials. In six of eight studies, the EQD2 delivered in the APBI arm was equivalent or within 5% of the WBRT dose (except for ELIOT and TARGIT; see Table 10). Despite the dose equivalence in the APBI arm, we found that LR‐FS was probably slightly worse with APBI, which suggests there may be technical issues with RT delivery (e.g. target volume definition or target coverage).
-
The RT techniques used to deliver PBI/APBI varied (see Table 8).
-
The trials differed in fractionation used; all the studies used greater than 2 Gy per fraction in the experimental arm, except for Polgár 2007, where some women in the PBI/APBI arm received 2 Gy per fraction (see Table 10).
-
The techniques used to define the target volume in GEC‐ESTRO, Livi 2015, Polgár 2007, RAPID, and TARGIT were consistent with current practice, but ELIOT provided inadequate details for the technique used in to be certain of this (see Table 8).
Avoiding geographic miss is imperative in RT. Delivery of RT is a technical exercise. Peters 2010 demonstrated the extremely important role that quality assurance plays in the delivery of high‐quality RT. Poor‐quality RT (a non‐compliant plan) for locally advanced head and neck cancer was associated with a 20% detriment in survival and a 29% detriment to locoregional control (Peters 2010). Four studies did not mention quality assurance (ELIOT; Livi 2015; Rodríguez; TARGIT). Although Polgár 2007 assessed implant quality, less than 20% of the implants had postimplant CT to document planning target volume (PTV) coverage and quality assurance for APBI using electrons or WBRT was not mentioned (see Characteristics of included studies table). RAPID and IMPORT included an extensive quality assurance programme with credentialing, real‐time and post‐hoc review of radiation quality. GEC‐ESTRO had rigorous pre‐ and postimplant quality assurance procedures.
The lack of precision in target definition, the variety of methods used to deliver PBI/APBI (even within the experimental arm of the trials; see Table 8) and the omission of robust quality assurance mean that the RT delivered in some trials was not reproducible. We cannot be certain of the dose delivered and the volume of breast treated.
We found there was heterogeneity for the outcome of cosmesis (physician‐reported). There were some possible sources of clinical heterogeneity that may have contributed to this: in Polgár 2007, 40/128 (30%) women in the PBI/APBI arm received 50 Gy in 25 fractions to the tumour bed using EBRT; these women who had conventionally fractionated RT at the same dose as the WBRT arm were likely to have very similar cosmetic outcome. Livi 2015 delivered PBI/APBI using IMRT, with careful dose constraints applied: we know that use of IMRT for WBRT is associated with improvement in cosmetic outcome (Donovan 2007; Pignol 2008).
Modern breast‐conserving therapy with postoperative WBRT achieves local control rates of 3.3% to 3.4% at five years and 5.2% to 6.7% at 10 years (Haviland 2013). With careful participant selection (aged 65 years or greater, T1‐2 tumours, 3 cm or less, margin negative, oestrogen receptor‐positive and all receiving tamoxifen), surgery alone can achieve local recurrence rates of 4.1% at five years (Kunkler 2015). The risk of local recurrence continues with longer follow‐up, in modern series where women with good prognosis were treated with surgery alone, the 10‐year risk of locoregional or distant recurrence was 19.9% (reduced to 6.3% with RT; Blamey 2013; Fisher 2002; Fyles 2004; Hughes 2004; Potter 2007; Prescott 2007; Winzer 2010). Even if PBI/APBI is poorly targeted, in a low‐risk population being treated with systemic therapy (which greatly improves local control) (Burskin 2019), low local recurrence rates can be expected.
The paradigm for PBI/APBI relies on the basis that WBRT does not reduce the number of 'elsewhere primaries' within the treated breast, but we found that PBI/APBI may cause an increase in 'elsewhere primaries' (OR 1.77, 95% CI 1.07 to 2.92; Analysis 1.11).
These data do not apply to women who have had oncoplastic surgery.
Duration of follow‐up
The length of follow‐up in the included trials was adequate to detect local recurrences (10 years (Polgár 2007), 69 months (ELIOT), 10.7 years (Livi 2015), 60 months (Rodríguez), 72.2 months (IMPORT), 79.2 months (GEC‐ESTRO), 103.2 months (TARGIT)). Local recurrences continue to increase over time (at about 1% per year), as can be seen in the START data: 3.3% to 3.4% at five years and 5.2% to 6.7% at 10 years (Haviland 2013). Any effect on breast C‐SS related to local control requires much longer follow‐up (EBCTCG 2011). None of the included trials had sufficient follow‐up to report meaningful OS results.
We know that RT reduces the risk of any first recurrence, and although the proportional reduction is most evident in the first year after treatment, it is still present in years five to nine (RR 0.59, 95% CI 0.5 to 0.7; EBCTCG 2011). Even in modern studies that included low‐risk women treated with lumpectomy (enrolled after 1989), the 10‐year risk of locoregional and distant recurrence was 19.9% (reduced to 6.3% with RT; Blamey 2013; Fisher 2002; Fyles 2004; Hughes 2004; Potter 2007; Prescott 2007; Winzer 2010). Even women at low risk of recurrence (if they are at risk for another 30 years) are likely to have increases in recurrence with longer follow‐up. The use of PBI/APBI may mean they are at risk of 'elsewhere primaries' for this time period.
Outcomes measures with little or no useful information
The authors of TARGIT (completed) indicated that cosmesis, participant satisfaction, health economics and consumer preference will be the subject of a subprotocol and information with respect to these outcomes will be available in the future. The seven ongoing trials that we found are likely to address these outcomes (see Characteristics of ongoing studies table), and more data will be reported for included studies relating to these outcomes in future.
Quality of the evidence
The available evidence does allow for the drawing of robust conclusions in respect of the objective of the review: to determine whether PBI/APBI is equivalent to or better than conventional or hypofractionated WBRT after breast‐conserving therapy for early‐stage breast cancer. We found nine studies and analysed 14,245 of the 15,187 women enrolled. See summary of findings Table 1.
LR‐FS: we did not downgrade for indirectness because the included studies used contemporary RT techniques. We did not downgrade for inconsistency (I2 = 27%). We downgraded for imprecision; there were more than 300 events (322), and although the optimum information size (OIS) had been met, the CIs did not exclude either clinically unimportant harms or clinically important harms. We did not downgrade for publication bias, given the systematic literature search we had performed. The overall certainty of evidence for LR‐FS was moderate.
Cosmesis (physician or nurse reported): we downgraded for risk of bias for lack of blinding of outcome assessors and inconsistency (I2 = 90%): although all studies used the same four‐point scale. We did not downgrade for indirectness. We did not downgrade for imprecision; OIS was met (there were greater than 300 events (591)) and the CIs excluded the possibility of clinically unimportant effects. We did not downgrade for publication bias given the systematic literature search we had performed. The overall certainty of evidence for cosmesis was moderate.
OS: we did not downgrade for risk of bias, indirectness, inconsistency (I2 = 0%; P = 0.53) or imprecision: OIS was met (the CIs crossed one, but excluded clinically unimportant harms or meaningful benefits). We did not downgrade for publication bias (based on examination of a Funnel plot). The overall certainty of evidence for OS was high.
Late RT toxicity (subcutaneous fibrosis): we did not downgrade for indirectness. We downgraded for lack of blinding and inconsistency (I2 = 89%). We did not downgrade for imprecision (as we reported on more than 300 events). We did not downgrade for publication bias. The overall certainty of evidence for subcutaneous fibrosis was moderate.
C‐SS: we did not downgrade for indirectness or inconsistency (I2 = 0%; P = 0.93). We downgraded for lack of blinding and imprecision, although OIS was met, there were fewer than 300 events (116 events) and the CIs did not include the possibility of clinically significant benefits or harms from PBI/APBI. We did not downgrade for publication bias for this outcome. The overall certainty of evidence for C‐SS was moderate.
DM‐FS: we did not downgrade for risk of indirectness or inconsistency (I2 = 25%; P = 0.24). We downgraded for lack of blinding and imprecision: although OIS was met, there were fewer than 300 events (123 events) and the CIs included clinical meaningful benefits and clinically insignificant harms. We did not downgrade for publication bias. The overall certainty of evidence for DM‐FS was moderate.
Subsequent mastectomy: we did not downgrade for risk of bias. We did not downgrade for indirectness or inconsistency (I2 = 0%; P = 0.54). We downgraded for imprecision: although OIS was met, and there were more than 300 events (350) and the CIs did not exclude clinically meaningful benefits or insignificant harms. The overall certainty of evidence for subsequent mastectomy was low.
Potential biases in the review process
We consider that we have identified all the relevant completed RCTs. For reporting of adverse effects, the precision was low because the OIS was not met. This may improve in future updates, as more information with respect to these outcomes is reported.
We included 1031/14,319 (7%) women who had DCIS in this study population.
Agreements and disagreements with other studies or reviews
We found nine systematic reviews comparing PBI/APBI with WBRT.
We found one meta‐analysis (Valachis 2010), which included one study (Polgár 2007) in common with our systematic review and recruited 1140 women (Dodwell 2005; Polgár 2007; Ribeiro 1993). They found no difference for mortality (OR 0.91, 95% CI 0.67 to 1.23; P = 0.55) and distant metastasis (OR 0.74, 95% CI 0.51 to 1.08; P = 0.12) for the comparison of PBI/APBI versus WBRT. PBI/APBI was associated with increased local recurrence (OR 2.15, 95% CI 1.40 to 3.31; P = 0.001) and axillary recurrence (OR 3.43, 95% CI 2.06 to 5.71; P < 0.0001) when compared with WBRT. They concluded that PBI does not seem to jeopardise survival and may be used as an alternative to WBRT (Valachis 2010). The authors pooled the data from the trials (see Data synthesis section).
We found one systematic review (search date 2010; BlueCross BlueShield). They included two RCTs (Polgár 2007; Ribeiro 1993 (which we excluded)) and seven non‐RCTs. They concluded that "the body of evidence on interstitial PBI/APBI compared to conventional whole‐breast irradiation is weak, and it is extremely weak (i.e. no comparative studies) for balloon brachytherapy, intraoperative PBI/APBI, and external‐beam PBI/APBI. The data on PBI/APBI compared to whole‐breast irradiation are insufficient to draw any conclusions about the relative effectiveness of these modalities. Furthermore, it is becoming increasingly clear that each type of PBI/APBI should be judged on its own merits, and studies comparing different PBI/APBI techniques to each other as well as to whole‐breast irradiation are needed" (BlueCross BlueShield).
We found one review that used a systematic search (search date February 2014; Marta 2015). Our review differed in that we included updated data for TARGIT and they included Dodwell 2005 and Ribeiro 1993 (which we excluded). They reported decreased local control with APBI versus WBRT (HR 4.54, 95% CI 1.78 to 11.61; P = 0.002). They concluded that APBI was associated with an increase in local recurrence, but found no difference in other outcomes.
Kong 2014 performed a systematic search (search date June 2012) including 10 studies, and included two retrospective cohorts, two matched pair analyses and three prospective cohort studies. They found that PBI/APBI was associated with increased local recurrence (OR 1.54, 95% CI 1.15 to 2.06; P = 0.004). They found no difference for the comparison of PBI/APBI for the outcomes of distant metastases, OS and disease‐free survival.
One review by Ye 2013 included 919 women in four studies; their search was not systematic (search date June 2013). They included a matched pair analysis, and the fourth study was not referenced. They reported no difference in local control, but appeared to have double counted the data for Polgár 2007 at two time points. They concluded that other outcomes did not differ, although they reported more women who were treated with APBI had good/excellent cosmesis.
Vaidya 2016 (search date November 2015, non‐systematic) included studies in English. For OS, they included four studies and reported 1.3% (95% CI −2.5% to 0.0%) difference in deaths when comparing WBRT and PBI (ELIOT; GEC‐ESTRO; Livi 2015; TARGIT). For C‐SS they included four studies (ELIOT; GEC‐ESTRO; Livi 2015;TARGIT). They concluded that "the use of PBI does not compromise breast cancer or overall mortality."
The systematic review by Korzets 2019 (search date January 2018) included data from nine studies. Their search was not systematic and limited to English language studies, and evidence was not assessed for risk of bias or quality. They found similar findings for: LR‐FS (HR 1.56, 95% CI 0.97 to 2.52), cosmesis (OR 1.23, 95% CI 0.99 to 1.52), relapse (OR 1.06, 95% CI 0.91 to 1.20) and survival (OR 0.84, 95% CI 0.71 to 1.01).
Viani 2020 reported a systematic review and meta‐analysis (search date December 2019). They included 11 RCTs, the nine we reported in our review and Dodwell 2005 and Ribeiro 1993 (which we excluded because the RT techniques used do not reflect current practice). They report on 14,436 participants and for local recurrence (LR) at five years (OR 1.46, 95% CI 1.15 to 2.00) and OS (OR 0.96, 95% CI 0.82 to 1.13). Their conclusions agreed with ours: "the LR with partial breast irradiation is low and similar to WBRT in selected early breast cancer patients."
Several guidelines have been published on PBI/APBI. The American Society of Therapeutic Radiology and Oncology (ASTRO) consensus evolved because it was clear that many women are treated in the US with PBI/APBI outside clinical trials. They indicated a patient population "suitable" for PBI/APBI outside the context of a clinical trial: women aged 60 years and over, with pT1N0(i‐, i+)M0, margins greater than 2 mm, unifocal disease, oestrogen receptor‐positive, who have had no neoadjuvant therapy. They also described women deemed unsuitable for PBI/APBI. They state that, "patients who choose treatment with PBI/APBI should be informed that whole‐breast irradiation (WBI) is an established treatment with a much longer track record that has documented long‐term effectiveness and safety" (Smith 2009). This statement provides guidance in selecting participants who may be appropriate for PBI/APBI outside the context of a clinical trial, but the Task Force strongly endorsed enrolment of all eligible participants considering PBI/APBI onto NSABP‐B39/RTOG and encouraged enrolment of other participants considering PBI/APBI, particularly those not in the "suitable" group, into prospective clinical studies to address many of the unanswered questions in PBI/APBI (Smith 2009). NCCN stated, "patients are encouraged to enter clinical trials, and endorses the ASTRO guidelines."
In the absence of an available trial, the American College of Radiology (ACR) Appropriateness Criteria panel recommends following the consensus guidelines of the ASTRO (Bellon 2011).
The GEC‐ESTRO Breast Cancer Working Group recommends three categories guiding patient selection for PBI/APBI:
-
a low‐risk group for whom PBI/APBI outside the context of a clinical trial is an acceptable treatment option; including participants aged at least 50 years with unicentric, unifocal, pT1‐2 (less than 30 mm) pN0, non‐lobular invasive breast cancer without the presence of an extensive intraductal component (EIC) and lymphovascular invasion (LVI) and with negative surgical margins of at least 2 mm;
-
a high‐risk group, for whom PBI/APBI is considered contraindicated; including participants aged 40 years or less; having positive margins, with or without any of the following pathological features (multicentric or large (greater than 30 mm) tumours, EIC‐positive or LVI‐positive tumours, four or more positive lymph nodes, unknown axillary status (pNx)); and
-
an intermediate‐risk group, for whom PBI/APBI is considered acceptable only in the context of prospective clinical trials (Polgár 2010).
The NICE 2018 guidelines recommend that PBI can be considered for women treated with breast‐conserving surgery (excluding lobular histology) who have clear margins, are 50 years or older, with tumours 3 cm or less, which are N0, Grade I‐II, oestrogen receptor positive, HER2 negative and who have been advised to have endocrine therapy for five years. NICE recommends using EBRT to deliver PBI and advising women that while the local control (LC) at five years is equivalent to that with WBRT, LC rates at greater than five years are unknown and that the use of PBI may be associated with less late toxicity.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.1 Local recurrence‐free survival.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.3 Overall survival.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.8 Cause‐specific survival.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.9 Distant metastasis‐free survival.

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 1: Local recurrence‐free survival

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 2: Cosmesis (participant‐reported)

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 3: Cosmesis, physician/nurse‐reported

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 4: Overall survival

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 5: Acute radiotherapy (RT) skin toxicity

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 6: Late RT skin toxicity

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 7: Telangiectasia (late RT toxicity)

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 8: Fat necrosis

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 9: Subcutaneous fibrosis (late RT toxicity)

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 10: Breast pain (late RT toxicity)

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 11: New primary tumours in ipsilateral breast, 'elsewhere primary'

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 12: Cause‐specific survival

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 13: Distant metastasis‐free survival

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 14: Relapse‐free survival

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 15: Locoregional recurrence‐free survival

Comparison 1: Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 16: Subsequent mastectomy
| PBI/APBI compared to WBRT for early breast cancer | ||||||
| Patient or population: health problem or population Setting: Academic, tertiary and community practice Intervention: PBI/APBI Comparison: WBRT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with WBRT | Risk with PBI/APBI | |||||
| Local recurrence‐free survival ‐ total (LR‐FS) | Low | HR 1.21 | 13168 | ⊕⊕⊕⊝ | ||
| 985 per 1000 | 982 per 1000 | |||||
| Cosmesis, physician/nurse‐reported | 138 per 1000 | 201 per 1000 | OR 1.57 | 3652 | ⊕⊕⊕⊝ | |
| Overall survival | Low | HR 0.99 | 13175 | ⊕⊕⊕⊕ | ||
| 949 per 1000 | 949 per 1000 | |||||
| Subcutaneous fibrosis (late RT toxicity) | 43 per 1000 | 184 per 1000 | OR 5.07 | 3011 | ⊕⊕⊕⊝ | |
| Cause‐specific survival (C‐SS) | Low | HR 1.06 | 9865 | ⊕⊕⊕⊝ | ||
| 983 per 1000 | 982 per 1000 | |||||
| Distant metastasis‐free survival (DM‐FS) | Low | HR 0.95 | 11033 | ⊕⊕⊕⊝ | ||
| 971 per 1000 | 972 per 1000 | |||||
| Subsequent mastectomy | 97 per 1000 | 95 per 1000 | OR 0.98 | 3740 | ⊕⊕⊕⊕ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_416473753410884319. | ||||||
| a. There was considerable clinical heterogeneity with respect to radiotherapy dose, technique and use of quality assurance procedures. However, the techniques employed delivered a dose that was the same or higher in the APBI/PBI arm than the WBRT arm, which should mean the local recurrence‐free survival is better or at least the same. | ||||||
| Cosmetic score |
|---|
| Excellent |
| Good |
| Fair |
| Poor |
| RTOG CTC | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|
| Acute skin | Follicular, faint or dull erythema/epilation/dry desquamation/decreased sweating | Tender or bright erythema, patchy moist desquamation/moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | Ulceration, haemorrhage, necrosis |
| Late skin toxicity | Slight atrophy, pigmentation change, some hair loss | Patchy atrophy, moderate telangiectasia, total hair loss | Marked atrophy, gross telangiectasia | Ulceration |
| Late subcutaneous fibrosis | Slight induration, loss of subcutaneous fat | Moderate fibrosis (asymptomatic) < 10% linear field contraction | Severe induration, loss of subcutaneous tissue, linear contraction > 10% | Ulceration |
| CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria. | ||||
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Induration (subcutaneous fibrosis) | Increased density on palpation | Moderate increase in density, not interfering with ADL; marked increase in density and firmness on palpation with or without minimal retraction | Dysfunction interfering with ADL; very marked density, retraction or fixation | — |
| Telangiectasia | Few | Moderate | Many and confluent | — |
| Pain | Pain mild, not interfering with function | Moderate pain; pain or analgesics interfering with function, but not with ADL | Severe pain; pain or analgesics interfering with ADL | Disability |
| ADL: activities of daily living; NCI CTC: National Cancer Institute Common Toxicity Criteria. | ||||
| Adverse effect | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|---|---|---|---|---|
| Pain | Mild, not interfering with function | Moderate/analgesics, interferes with function, but not ADL | Severe or interferes with ADL, or both | — | — |
| Telangiectasia | Few | Moderate | Many or confluent, or both | — | — |
| Acute skin toxicity | Faint erythema or dry desquamation | Moderate‐to‐brisk erythema, patchy moist desquamation | Moist desquamation not limited to creases or skin folds, bleeding subsequent to minor trauma or abrasion | Skin necrosis or full dermal thickness ulceration | Death |
| Induration/fibrosis | Increased density on palpation | Moderate functional impairment, not interfering with ADL, increased density and firmness on palpation with or without minor retraction | Interferes with ADL, very marked increased density, retraction or fixation | — | — |
| ADL: activities of daily living; CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; RTOG: Radiation Therapy Oncology Group. | |||||
| Adverse effect | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|---|---|---|---|---|
| Subcutaneous fibrosis | — | Mild induration, can move skin parallel to the plane (sliding) and perpendicular to the plane (pinching up) | Moderate induration, can slide, cannot pinch up skin | Severe induration cannot slide or pinch skin | Death |
| Telangiectasia | < 10 % of body surface area | ≥ 10% of body surface area, psychosocial impact | — | — | — |
| Pain | mild | Moderate, not limiting instrumental ADL | Severe, limiting self‐care ADL | — | — |
| ADL: activities of daily living; CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; RTOG: Radiation Therapy Oncology Group. | |||||
| Grade | Findings |
|---|---|
| 0 | No fat necrosis |
| 1 | Asymptomatic fat necrosis (only radiological or cytological findings, or both) |
| 2 | Symptomatic fat necrosis not requiring medication (palpable mass with or without mild pain) |
| 3 | Symptomatic fat necrosis requiring medication (palpable mass with significant pain) |
| 4 | Symptomatic fat necrosis requiring surgical intervention |
| Study | Age | Stage | Margins | Tumour size | Nodal status | Surgery |
|---|---|---|---|---|---|---|
| After 2000, < 40 years excluded | Unifocal tumour, pT1N0‐1miM0, Grade I or II | Negative | < 2.0 cm (< 2 mm: 1/258, ≥ 2 mm: 246/258 or had no tumour at ink: 11/258) | — | WLE | |
| 48–75 years | "Early breast cancer," "suitable for breast conservation" | Not described | ≤ 2.5 cm | AD if SNBx positive | BCS | |
| > 40 years | — | Negative, ≥ 5 mm | ≤ 2.5 cm | — | WLE or quadrantectomy | |
| ≥ 45 years | T1 and small T2N0‐1M0 invasive breast cancer, suitable for BCS, available for 10 years' follow‐up | ≥ 1 mm Re‐excision strongly advised for close or positive margins | — | — | BCS | |
| ≥ 40 years | DCISa or invasive breast cancer | Negative | ≤ 3 cm | Negative axillary nodal involvement including micrometastasis (> 0.2 mm or positive cells only identified on IHC as determined by sentinel node biopsy; axillary node dissection or clinical examination for DCIS only. | BCS | |
| ≥ 60 years | Invasive ductal carcinoma (pT1‐2cNO MO), unifocal tumour, Grade I or II | < 3 mm | ≤ 3 cm | — | — | |
| ≥ 50 years | Invasive breast cancer pT1‐2pN0 who have < 1% annual risk of local recurrence | ≥ 2 mm | ≤ 3 cm | 0–3 nodes positive | BCS | |
| > 40 years | Stage 0, I or II pN0/pNmi breast cancer (including DCIS) no vascular invasion, unifocal or unicentric disease only, no LVINote: 60/1184 (5%) participants had DCIS | ≥ 2 mm in invasive disease, 5 mm in DCIS | Lesions < 3 cm in diameter | Node negative, for DCIS alone: sentinel node biopsy optional | WLE or quadrantectomy, level I–II axillary dissection, removing ≥ 6 (preferably 10 lymph nodes) | |
| > 18 years | T0‐2N0‐1M0 DCIS or invasive breast cancer Note: 531/4216 (12%) participants had DCIS | Negative: "free of cancer, including DCSI" | Lesions < 3 cm | ≤ 3 involved nodes permitted | Lumpectomy | |
| AD: axillary dissection; BCS: breast‐conserving surgery; DCIS: ductal carcinoma in situ; IHC: immunohistochemistry; LVI: lymphovascular invasion; SNBx: sentinel node biopsy; WLE: wide local excision. | ||||||
| Study | RT quality assurance | RT technique | PIB/APBI target volume definition |
|---|---|---|---|
| Postimplant CT scans were performed | Interstitial brachytherapy (88/128) EBRT using photons (40/128) 2‐dimensional CT‐based | PTV: excision cavity delineated by the surgical clips + 2 cm isotropic margin. For interstitial therapy: if electrons were used, 6–15 MeV were used to treat the cavity with a 2 cm margin | |
| Not stated | Intraoperative electrons 6–9 MeV | CTV: quote: "decided according to the site and size of the tumour. The energy of the electron beams was selected according to the thickness of the gland measured by a graduated needle" | |
| Not stated | EBRT (IMRT) | CTV = +1 cm isotropic margin around surgical clips PTV = CTV +10 mm isotropic margina | |
| Nob | Intraoperative kV RT 3D‐CRT for WBRT | The target volume was the tumour cavityc | |
| Yesd | EBRT (3D‐CRT) APBI: 3–5 non‐coplanar fields | CTV = tumour bed on CT (surgical clips plus a 1‐cm margin inside breast PTV = CTV +1 cm isotropic margin | |
| Not stated | EBRT (3D‐CRT) | PTV was defined by contouring the same quadrant as the primary tumor sitee | |
| Yesf | Field‐in‐field IMRT | Tumour bed, surgical clipsg recommended | |
| Yesh | HDR or PDR multicatheter brachytherapy | PBI: tumour bedi +2 cm isotropic margin | |
| Quote: "Every institution's RT facilities were quality assessed and each case of APBI was centrally reviewed for RT quality." Benchmarking performed with "dummy run" | HDR brachytherapyj APBI EBRT: 3D‐CRTk (IMRT not permitted). WBRT: 3D‐CRT (IMRT not permitted) | No RNI permitted WBRT: entire ipsilateral breast APBI: CTV = cavity = PTV | |
| Study | RT quality assurance | RT technique | Target volume definition |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; CRT: conformal radiotherapy; CT: computer tomography; CTV: clinical target volume; EBRT: external beam radiotherapy; HDR: high‐dose rate; IMRT: intensity‐modulated radiotherapy; PDR: pulsed‐dose rate; PIB: partial breast irradiation; PTV: planning target volume; RNI: regional nodal irradiation; RT: radiotherapy; WBRT: whole breast radiotherapy. aThe surgeons were requested to place clips at the borders of the surgical bed, using a minimum of four clips. CTV was drawn on a planning CT (0.3 mm slices) with a uniform 1 cm margin around the surgical clips, then a 1 cm margin added to construct the PTV. | |||
| Score | Definition |
|---|---|
| Excellent | Perfect symmetry, no visible distortion or skin changes and no visible catheter entry/exit sequelae |
| Good | Slight skin distortion, retraction or oedema, any visible telangiectasia, any visible catheter entry/exit scar or mild hyperpigmentation |
| Fair | Moderate distortion of the nipple or breast symmetry, moderate hyperpigmentation, or prominent skin retraction, oedema or telangiectasia |
| Poor | Marked distortion, oedema, fibrosis or severe hyperpigmentation |
| Trial | PBI/APBI dose | Fraction size (Gy) | EQD2 PBI/APBI alpha/beta = 4 | Control dose | Fraction size (Gy) | EQD2 Control alpha/beta = 4 |
|---|---|---|---|---|---|---|
| 20 Gy at surface of the applicator (attenuated to 5–7 Gy at 1 cm) (APBI) | 80 at cavity surface 12.8 at 1 cm | 80 Gy at cavity surface 12.8 Gy at 1 cm | 40–56 Gy/20–28 fractions ± 10–16 Gy boost | 2 | 40–56 Gy ± 10–16 Gy | |
| 30 Gy/5 daily fractions EBRT IMRT. 100% of the PTV was covered by 95% of the prescribed dose | 6 | 50 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost | 2 | 50 + 10 = 60 Gy | |
| 38.5 Gy/10 fractions twice daily (with 6‐hour gap) Dose‐evaluation volume (that part of PTV within the breast) received 95–107% of prescription dose | 3.85 | 49.4 Gy | 50 Gy/25 fractions or 42.5 Gy/16 fractions ± boost (10 Gy/4–5 fractions) based on criteria such as young age or close margins, prespecified by centre | 2 or 2.65 | 50 Gy or 47.1 Gy | |
| 37.5 Gy/10 fractions twice daily (with 6‐hour gap) (APBI). PTV covered by ≥ 95% of prescribed dose, with < 105% hot spot | 3.75 | 47.48 Gy | 48 Gy/24 fractions ± 10 Gy/5 fractions boost | 2 | 48 ± 10 = 48–58 Gy | |
| 7 × 5.2 Gy HDR (APBI) or 50 Gy/25 fractions (PBI) | 5.2 or 2 | 57.5 Gy or 50 Gy | 50 Gy/25 fractions (3D‐CRT was not used) | 2 | 50 Gy | |
| 30.3 Gy/7 fractions or 32 Gy/8 fractions HDR twice daily or 50 Gy at 0.6–0.8 Gy/hour pulses (1 pulse per hour, 24 hours per day) PDR | 7–8 | 41.64–42.67 Gy | 50.0–50.4 Gy to a reference point + 10 Gy/5 fractions boost. Electron dose was prescribed to the point of maximum dose on the beam axis (Dmax), ensuring the 85% isodose encompassed the tumour bed | 1.8–2.0 | 48.72–50 + 10 = 58.72–60 Gy | |
| 21 Gy/1 fraction at 90% using 6–9 MeV | 21 | 84 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost (using electrons) | 2.0 | 50 + 10 Gy | |
| 40 Gy/15 fractions (EBRT) | 2.72 | 45.23 | 40 Gy/15 fractions 36 Gy/15 fractions + boost 40 Gy/15 fractions | 2.72 2.4 | 45.23 38.4 + 45.23 | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; CT: computer tomography; EBRT: external beam radiotherapy; EQD2: equivalent dose in 2 Gy fractions; Gy: Gray; HDR: high‐dose‐rate; IMRT: intensity‐modulated radiotherapy; MeV: mega electron volt; PBI: partial breast irradiation; PDR: pulsed‐dose rate; PTV: planning target volume. | ||||||
| Study | Mammography | Radiotherapy toxicity | Patient‐reported outcomes (PRO) | Cosmesis |
|---|---|---|---|---|
| At 6 months, then annually | Acute: CTCAEa Late: EORTC/RTOGb and LENT‐SOMAc | Not assessed | Harvard Cosmetic Scored | |
| Annually | LENT‐SOMAc | Not assessed | Not assessed | |
| Annually | Acute and late: EORTC/RTOGb | EORTC QLQ‐C30e QLQ‐BR23 breast cancer modulef | Harvard Cosmetic Scored | |
| Annually | Acute: nil Late: EORTC/RTOG,b LENT‐SOMA,c CTCAEa | EORTC QLQ‐C30e QLQ‐BR23 breast cancer module Body‐image scale | Clinician and nurse assessed | |
| Annually | Acute and late: NCI version 3.0 | EORTC QLQ‐C30e QLQ‐BR23 breast cancer module Body‐image scale | EORTC/RTOG Rating Systemg | |
| Baseline 6 months after RT than annually | Late: EORTC/RTOGb | Not assessed | Harvard Cosmetic Scored | |
| Annually 1–5 years, 3 yearly to 10 years | Symptomatic rib fracture and lung fibrosis Ischaemic heart disease recorded at 1, 2, 5 and 10 years' follow‐up | EORTC QLQ‐C30e QLQ‐BR23 breast cancer module Body‐image scale protocol‐specific questionsh HADS scale EuroQol EQ‐5D‐3L health status questionnaire at baseline; 6 months; 1, 2 and 5 years | Patient‐ and clinician‐assessedi Photosj | |
| At 6, 12, 18, 24 months after radiotherapy then annually for 10 years | Fat necrosis measured using Lövey scoring systemk Physician scored late toxicity Acute radiotherapy toxicity: CTCAE version 3.0a LENT‐SOMAc Late RT toxicity: EORTC/RTOGb | Breast pain and arm lymphoedema measured by CTCAE version 3.0a EORTC QLQ‐C30e and QLQ‐BR23 at baseline and during follow‐up | Harvard Cosmetic Scored Physician‐ and patient‐reported Digital photosl | |
| Annually | Acute radiotherapy toxicity: CTCAE version 4.0 Late RT toxicity: CTCAE version 4.0 | Not assessed | Physician reported | |
| CTCAE: Common Terminology Criteria for Adverse Events; EORTC: European Organisation for Research and Treatment of Cancer; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Breast Specific Module; EORTC/RTOG: European Organisation for Research and Treatment of Cancer/Radiation Therapy Oncology Group; HADS: Hospital Anxiety and Depression Scale; LENT‐SOMA: late effects in normal tissues – subjective objective, management and analytic; RT: radiotherapy. aTrotti 2013. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Local recurrence‐free survival Show forest plot | 8 | 13168 | (Exp[(O‐E) / V], Fixed, 95% CI) | 1.21 [1.03, 1.42] |
| 1.1.1 5 years' follow‐up | 5 | 8265 | (Exp[(O‐E) / V], Fixed, 95% CI) | 1.20 [0.99, 1.45] |
| 1.1.2 10 years' follow‐up | 3 | 4903 | (Exp[(O‐E) / V], Fixed, 95% CI) | 1.22 [0.92, 1.64] |
| 1.2 Cosmesis (participant‐reported) Show forest plot | 2 | 2775 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.68, 2.57] |
| 1.3 Cosmesis, physician/nurse‐reported Show forest plot | 6 | 3652 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.31, 1.87] |
| 1.4 Overall survival Show forest plot | 8 | 13175 | Hazard Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.99 [0.88, 1.12] |
| 1.5 Acute radiotherapy (RT) skin toxicity Show forest plot | 4 | 3925 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.66, 0.88] |
| 1.6 Late RT skin toxicity Show forest plot | 3 | 3465 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.27 [1.63, 3.15] |
| 1.7 Telangiectasia (late RT toxicity) Show forest plot | 2 | 3010 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.40 [3.34, 5.80] |
| 1.8 Fat necrosis Show forest plot | 4 | 3565 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.76 [1.74, 4.38] |
| 1.9 Subcutaneous fibrosis (late RT toxicity) Show forest plot | 2 | 3011 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.07 [3.81, 6.74] |
| 1.10 Breast pain (late RT toxicity) Show forest plot | 2 | 3012 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.15, 2.86] |
| 1.11 New primary tumours in ipsilateral breast, 'elsewhere primary' Show forest plot | 4 | 5144 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.82 [1.55, 5.12] |
| 1.12 Cause‐specific survival Show forest plot | 7 | 9865 | Hazard Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.06 [0.83, 1.36] |
| 1.13 Distant metastasis‐free survival Show forest plot | 7 | 11033 | Hazard Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 0.95 [0.80, 1.13] |
| 1.14 Relapse‐free survival Show forest plot | 6 | 11756 | Hazard Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.25 [1.05, 1.48] |
| 1.15 Locoregional recurrence‐free survival Show forest plot | 5 | 6718 | Hazard Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.36 [1.06, 1.74] |
| 1.16 Subsequent mastectomy Show forest plot | 3 | 3740 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |