Частичное облучение молочной железы на ранней стадии рака
Appendices
Appendix 1. The Cochrane Central Register of Controlled Trials (CENTRAL)
1. MESH DESCRIPTOR Breast Neoplasms EXPLODE ALL TREES
2. breast AND (cancer* OR tumour* OR tumor* OR neoplas*)
3. #1 OR #2
4. brachytherapy:MH
5. brachytherapy
6. partial breast
7. partial breast irradiation
8. whole breast irradiation
9. whole breast radiotherapy
10. less than whole breast rad$
11. high‐dose‐rate brachytherapy
12. acceleration partial breast irradiation
13. acceleration irradiation
14. tumour bed boost
15. sole tumour boost
16. tumour bed boost
17. tumor bed boost
18. sole tumour bed irradiation
19. mammosite
20. MESH DESCRIPTOR Radiotherapy, Conformal EXPLODE ALL TREES WITH QUALIFIERS AE
21. MESH DESCRIPTOR Radiotherapy, Conformal EXPLODE ALL TREES WITH QUALIFIERS MT
22. (balloon dilation):MH
23. MESH DESCRIPTOR Radiotherapy
24. OR/4‐23
25. #3 AND #24
Appendix 2. MEDLINE (Ovid) (1966 to present)
1.RANDOMIZED CONTROLLED TRIAL.pt
2. CONTROLLED CLINICAL TRIAL.pt
3. RANDOMIZED CONTROLLED TRIALS.sh
4. RANDOM ALLOCATION.sh
5. DOUBLE BLIND METHOD.sh
6. SINGLE BLIND METHOD.sh
7. or/1‐6
8. (ANIMALS not HUMANS).sh
9. 7 not 8
10. CLINICAL TRIAL.pt
11. exp CLINICAL TRIALS/
12. (clin$ adj25 trial$).ti,ab
13. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab
14. PLACEBOS.sh
15. placebo$.ti,ab
16. random$.ti,ab
17. RESEARCH DESIGN.sh
18. or/10‐16
19. 18 not 8
20. 19 not 9
21. 9 or 20
22. exp breast neoplasms/
23. exp "neoplasms, ductal, lobular, and medullary"/
24. exp breast/
25. exp neoplasms/
26. 24 AND 25
27 (breast$ adj5 (neoplasm$ or cancer$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or dcis or ductal or infiltrat$ or intraductal$ or lobular or medullary)).mp.
28 exp mammary neoplasms/
29 (mammar$ adj5 (neoplasm$ or cancer$ or tumo?r$ or carcinoma$ or adenocarcinoma$ or sarcoma$ or dcis or ductal or infiltrat$ or intraductal$ or lobular or medullary)).mp
30. or/22, 23,26‐29
31. partial breast irradiation.sh,kw,ti,ab
32. partial breast.sh,kw,ti,ab
33. whole breast irradiation.sh,kw,ti,ab
34. whole breast radiotherapy.mp
35. less than whole breast rad$.mp
36. brachytherapy.sh,kw,ti,ab
37. high‐dose‐rate brachytherapy.sh,kw,ti,ab
38. accelerated partial breast irradiation.sh,kw,ti,ab
39. tumour bed boost.sh.kw.ti.ab
40. sole tumour bed irradiation.sh,kw,ti,ab
41. MammoSite.sh.kw.ti.ab
42. Breast Neoplasms/ rt.sh
43. Radiotherapy, Conformal/adverse events.sh
44. Radiotherapy, Conformal/methods.sh
45. Brachytherapy.sh
46. Balloon dilation.sh
47. Radiotherapy/.sh
48. or/31‐47
49. 48 AND 30
50. 49 AND 21
Appendix 3. EMBASE
1. randomised AND controlled AND trial
2. controlled AND clinical AND trial
3. randomi*ed:ab
4. placebo:ab
5. randomly:ab
6. trial:ab
7. groups:ab
8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
9. 'breast'/exp AND 'neoplasm'/exp
10. locally AND advance* NEAR/6 breast AND cancer*
11. locally AND advance* NEAR/6 breast AND neoplas*
12. locally AND advance* NEAR/6 breast AND carcinoma*
13. locally AND advance* NEAR/6 breast AND tumour*
14. locally AND advance* NEAR/6 breast AND tumor*
15. early NEAR/6 breast AND cancer*
16. early NEAR/6 breast AND neoplas*
17. early NEAR/6 breast AND carcinoma*
18. early NEAR/6 breast AND tumour*
19. early NEAR/6 breast AND tumor*
20. #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR $18 OR $19
21. ‘radiotherapy’/exp OR radiotherapy
22. ‘adjuvant’/exp OR adjuvant AND (‘radiotherapy’/exp OR radiotherapy)
23. ‘radiation/exp OR radiation AND (‘therapy’/exp OR therapy)
24. #21 OR #22 OR #23
25. #8 AND #20 AND #24
Appendix 4. CINAHL
1. MH Clinical Trials
2. PT Clinical Trial
3. TX clini* n1 trial*
4. TX ((singl* n1 blind*) or (singl* n1 mask**))
5. TX randomi* control* trial*
6. MH random assignment
7. TX random* allocat*
8. TX placebo*
9. MH "Placebos"
10. MH Quantitative Studies
11. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
12. MH breast neoplasms
13. TI breast cancer OR SU breast cancer OR AB breast cancer
14. TI breast tumour OR SU breast tumour OR AB breast tumour
15. MM "Carcinoma, Ductal, Breast"
16. #12 OR #13 OR #14 OR #15
17. MM "radiotherapy, Conformal/AE"
18. MM "radiotherapy, Conformal/methods"
19. SU partial breast OR TI partial breast OR AB partial breast
20. SU whole breast irradiation OR TI whole breast irradiation OR AU whole breast irradiation
21. SU whole breast radiotherapy
22. SU whole breast rad$ OR TI whole breast rad$ OR AB whole breast rad$
23. SU brachytherapy OR TI brachytherapy OR AB brachytherapy
24. MH brachytherapy
25. MH radiotherapy
26. SU whole breast radiotherapy OR TI whole breast radiotherapy OR AB whole breast radiotherapy
27. SU high‐dose‐rate brachytherapy OR AB high‐dose‐rate brachytherapy OR TI high‐dose‐rate brachytherapy
28. SU accelerated partial breast irradiation OR TI accelerated partial breast irradiation OR AB accelerated partial breast irradiation
29. SU tumour bed boost OR TI tumour bed boost OR AB tumour bed boost
30. SU MammoSite OR TI MammoSite OR AB MammoSite
31. MH "Breast Neoplasms+/RT"
32. #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31
33. #11 AND #16 AND #32
Appendix 5. Current Contents
1. TS = clinical trial*
2. TS = research design
3. TS = comparative stud*
4. TS = evaluation stud*
5. TS = controlled trial*
6. TS = follow‐up study
7. TS = prospective stud*
8. TS = random*
9. TS = placebo
10. TS = single blind*
11. TS = double blind*
12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11
13. TS = breast cancer
14. TS = breast neoplasms
15. #12 OR #13
16. TS = partial breast irradiation
17. TS = whole breast irradiation
18. TS = less than whole breast
19. TS = brachytherapy
20. TS = tumour bed boost
21. TS = Mammosite
22. #15 OR #16 OR #17 OR #18 OR #19 OR #20
23. TS = radiotherapy
24. TS = radiation therapy
25. #22 OR #23
26. #12 AND #21 AND #24
Appendix 6. WHO ICTRP search portal
Basic search:
-
Partial irradiation for early breast cancer
-
Early breast cancer AND partial irradiation
-
Early breast cancer AND partial breast irradiation
-
Early breast cancer AND Mammosite
-
Early breast cancer AND Intracavitary brachytherapy
-
Early breast cancer AND Interstitial brachytherapy
-
Early breast cancer AND accelerated partial breast irradiation
-
Early breast cancer and less than whole breast radiotherapy
Advanced search:
1. Title: Partial irradiation for early breast cancer
Recruitment status: all
2. Condition: early breast cancer
Intervention: partial breast irradiation OR Mammosite OR intracavitary brachytherapy OR interstitial brachytherapy OR accelerated partial breast irradiation OR less than whole breast radiotherapy
Recruitment status: all
Appendix 7. ClinicalTrials.gov
Basic searches:
-
Partial irradiation for early breast cancer
-
Early breast cancer AND partial irradiation
-
Early breast cancer AND partial breast irradiation
-
Early breast cancer AND Mammosite
-
Early breast cancer AND Intracavitary brachytherapy
-
Early breast cancer AND Interstitial brachytherapy
-
Early breast cancer AND accelerated partial breast irradiation
-
Early breast cancer and less than whole breast radiotherapy
Advanced searches:
1. Search terms: Partial irradiation for early breast cancer
Recruitment: All studies
Study type: All studies
Gender: All studies
2. Condition: Early breast cancer
Intervention: Partial breast irradiation OR Mammosite OR intracavitary brachytherapy OR interstitial brachytherapy OR accelerated partial breast irradiation OR less than whole breast radiotherapy
Recruitment: All studies
Study type: All studies
Gender: All studies
Appendix 8. OpenGrey
1. (breast cancer OR breast neoplasm* OR breast adenocarcinoma) AND (radiation OR irradiation OR radiotherapy OR radio‐therapy))

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.1 Local recurrence‐free survival.
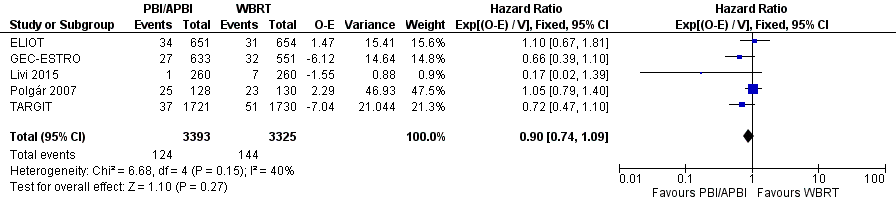
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.3 Overall survival.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.8 Cause‐specific survival.
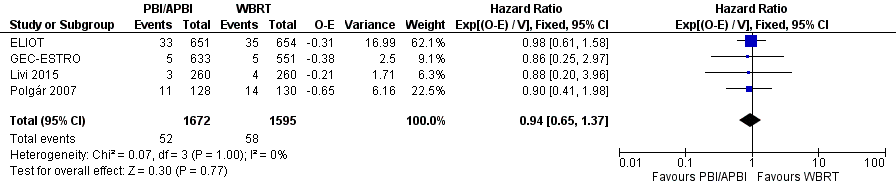
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.9 Distant metastasis‐free survival.
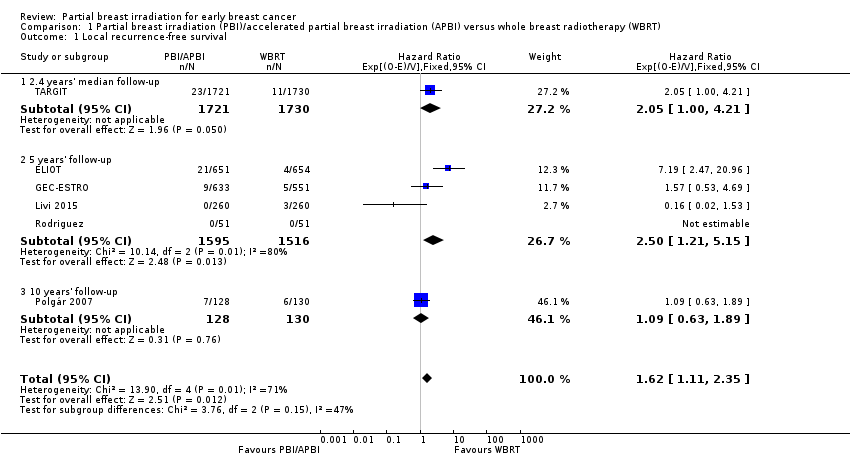
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 1 Local recurrence‐free survival.
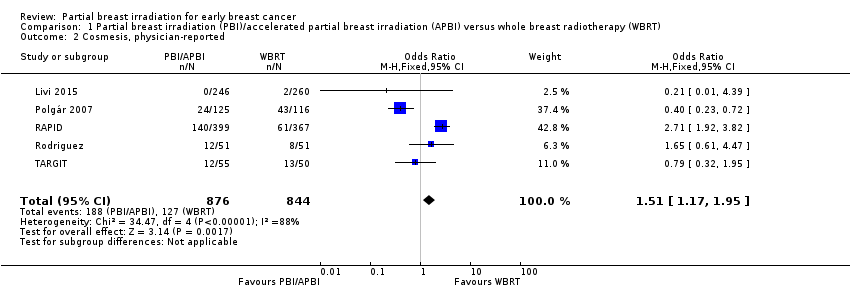
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 2 Cosmesis, physician‐reported.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 3 Overall survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 4 Acute radiotherapy (RT) skin toxicity.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 5 Late RT skin toxicity.
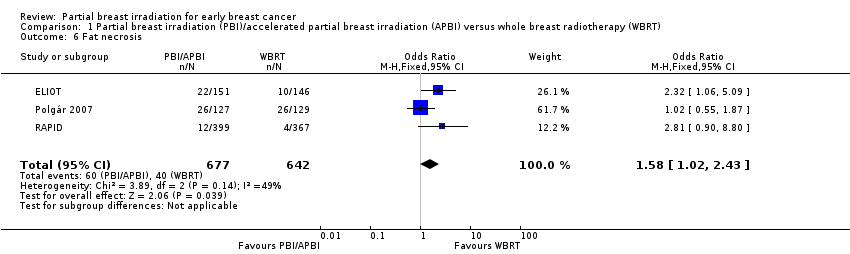
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 6 Fat necrosis.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 7 'Elsewhere primary'.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 8 Cause‐specific survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 9 Distant metastasis‐free survival.
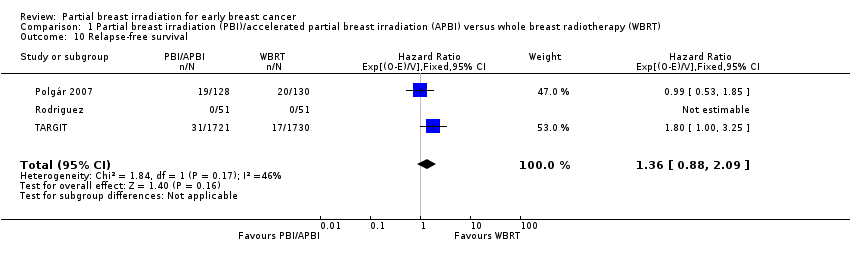
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 10 Relapse‐free survival.
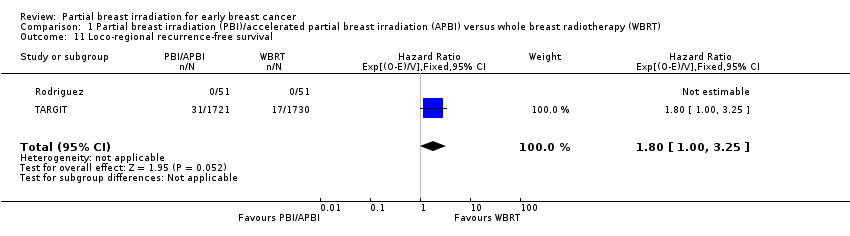
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 11 Loco‐regional recurrence‐free survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 12 Mastectomy.
| PBI/APBI for women with early breast cancer | ||||||
| Patient or population: women with early breast cancer Setting: radiotherapy centres Intervention: PBI/APBI Comparison: whole breast radiotherapy (WBRT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with WBRT | Risk with PBI/APBI | |||||
| Local recurrence‐free survival at 5 years | Study population | HR 1.62 | 6820 | ⊕⊕⊝⊝ | ‐ | |
| 10 per 10001 | 16 per 1000 | |||||
| Cosmesis assessed with 4‐point scale Follow‐up: range 29‐122 months | Study population | OR 1.51 | 1720 | ⊕⊕⊝⊝ | Cosmesis was assessed using a 4‐point scale. We reported those women with poor/fair cosmesis at final review | |
| 150 per 1000 | 218 per 1000 | |||||
| Late radiotherapy toxicity (subcutaneous fibrosis) Follow‐up: median 36 months | Study population | OR 6.58 | 766 | ⊕⊕⊕⊝ | Assessed using National Cancer Institute 3‐point scale, events were defined as: Grade II or higher toxicity Physician assessors, at 3 years' follow‐up | |
| 22 per 1000 | 128 per 1000 | |||||
| Cause‐specific survival at 5 years | Study population | HR 1.08 | 6718 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 10002 | 22 per 1000 | |||||
| Distant metastasis‐free survival at 5 years | Study population | HR 0.94 | 3267 | ⊕⊕⊕⊝ | ‐ | |
| 33 per 10002 | 31 per 1000 | |||||
| Mastectomy rate Follow‐up: range 29‐122 months | Study population | OR 1.20 | 4817 | ⊕⊕⊝⊝ | Mastectomy rate reflected both local recurrence and adverse cosmetic outcome | |
| 15 per 1000 | 18 per 1000 | |||||
| Mortality | Study population | HR 0.90 | 6718 | ⊕⊕⊕⊕ | Survival advantage from radiotherapy for breast cancer is not apparent before 15 years' follow‐up (EBCTCG 2011) | |
| 51 per 10002 | 46 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The baseline risk for the control group was calculated at the 5‐year time point from 5 studies. | ||||||
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
| Score | Definition |
| Excellent | Perfect symmetry, no visible distortion or skin changes and no visible catheter entry/exit sequelae |
| Good | Slight skin distortion, retraction or oedema, any visible telangiectasia, any visible catheter entry/exit scar or mild hyperpigmentation |
| Fair | Moderate distortion of the nipple or breast symmetry, moderate hyperpigmentation, or prominent skin retraction, oedema or telangiectasia |
| Poor | Marked distortion, oedema, fibrosis or severe hyperpigmentation |
| RTOG CTC | Grade I | Grade II | Grade III | Grade IV |
| Description | Follicular, faint or dull erythema / epilation / dry desquamation / decreased sweating | Tender or bright erythema, patchy moist desquamation / moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | Ulceration, haemorrhage, necrosis |
| RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria. | ||||
| Grade | Findings |
| 0 | No fat necrosis |
| 1 | Asymptomatic fat necrosis (only radiological or |
| 2 | Symptomatic fat necrosis not requiring medication |
| 3 | Symptomatic fat necrosis requiring medication |
| 4 | Symptomatic fat necrosis requiring surgical |
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Induration (subcutaneous fibrosis) | Increased density on palpation | Moderate increase in density, not interfering with ADL; marked increase in density and firmness on palpation with or without minimal retraction | Dysfunction interfering with ADL; very marked density, retraction or fixation | ‐ |
| Telangiectasia | Few | Moderate | Many and confluent | ‐ |
| Pain | Pain mild, not interfering with function | Moderate pain; pain or analgesics interfering with function, but not with ADL | Severe pain; pain or analgesics interfering with ADL | Disability |
| ADL: activities of daily living; NCI CTC: National Cancer Institute Common Toxicity Criteria. | ||||
| Trial | PBI/APBI dose | Fraction size (Gy) | EQD2 PBI/APBI | Control dose | Fraction size (Gy) | EQD2 Control |
| 20 Gy at surface of the applicator (attenuated to 5‐7 Gy at 1 cm) (APBI) | 80 at cavity surface 12.8 at 1 cm | 80 Gy at cavity surface 12.8 Gy at 1 cm | 40‐56 Gy/20‐28 fractions ± 10‐16 Gy boost | 2 | 40‐56 Gy ± 10‐16 Gy | |
| 30 Gy/5 daily fractions EBRT IMRT. 100% of the PTV was covered by 95% of the prescribed dose | 6 | 75 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost | 2 | 50 + 10 = 60 Gy | |
| 38.5 Gy/10 fractions bd (with 6 hour gap) Dose‐evaluation volume (that part of PTV within the breast) received 95‐107% of prescription dose | 3.85 | 74.1 Gy | 50 Gy/25 fractions or 42.5 Gy/16 fractions ± boost (10 Gy/4‐5 fractions) based on criteria such as young age or close margins, pre‐specified by centre | 2 or 2.65 | 50 or 47.1 Gy | |
| 37.5 Gy/10 fractions bd (with 6 hour gap) (APBI). PTV covered by ≥ 95% of prescribed dose, with < 105% hot spot | 3.75 | 71.22 Gy | 48 Gy/24 fractions ± 10 Gy/5 fractions boost | 2 | 48 ± 10 = 48‐58 Gy | |
| 7 × 5.2 Gy HDR (APBI) or 50 Gy/25 fractions (PBI). | 5.2 or 2 | 53.6 Gy or 50 Gy | 50 Gy/25 fractions (3D‐CRT was not used) | 2 | 50 Gy | |
| 30.3 Gy/7 fractions or 32 Gy/8 fractions HDR twice daily or 50 Gy at 0.6‐0.8 Gy/hour pulses (1 pulse per hour, 24 hours per day) PDR | 7‐8 | 41.64‐42.67 Gy | 50.0‐50.4 Gy to a reference point + 10 Gy/5 fractions boost. Electron dose was prescribed to the point of maximum dose on the beam axis (Dmax), ensuring the 85% isodose encompassed the tumour bed | 1.8‐2.0 | 48.72‐50 + 10 = 58.72‐60 Gy | |
| 21 Gy/1 fraction at 90% using 6‐9 MeV | 21 | 131.2 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost (using electrons) | 2.0 | 50 + 10 Gy | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; bd: twice daily; CT: computer tomography; EBRT: external beam radiotherapy; EQD2: equivalent dose in 2 Gy fractions; Gy: Gray; HDR: high‐dose‐rate; IMRT: intensity‐modulated radiotherapy; MeV: mega electron volt; PBI: partial breast irradiation; PDR: pulsed‐dose‐rate; PTV: planning target volume. | ||||||
| Trial | RT technique |
| Interstitial brachytherapy (88/128) EBRT using photons (40/128) | |
| intra‐operative electrons | |
| EBRT (IMRT) | |
| intra‐operative kV RT | |
| EBRT | |
| EBRT (3D‐CRT) | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; EBRT: external beam radiotherapy; IMRT: intensity‐modulated radiotherapy; RT: radiotherapy. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local recurrence‐free survival Show forest plot | 6 | 6820 | Hazard Ratio (95% CI) | 1.62 [1.11, 2.35] |
| 1.1 2.4 years' median follow‐up | 1 | 3451 | Hazard Ratio (95% CI) | 2.05 [1.00, 4.21] |
| 1.2 5 years' follow‐up | 4 | 3111 | Hazard Ratio (95% CI) | 2.50 [1.21, 5.15] |
| 1.3 10 years' follow‐up | 1 | 258 | Hazard Ratio (95% CI) | 1.09 [0.63, 1.89] |
| 2 Cosmesis, physician‐reported Show forest plot | 5 | 1720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| 3 Overall survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 0.90 [0.74, 1.09] |
| 4 Acute radiotherapy (RT) skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.09] |
| 5 Late RT skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.39] |
| 6 Fat necrosis Show forest plot | 3 | 1319 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.02, 2.43] |
| 7 'Elsewhere primary' Show forest plot | 3 | 3009 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.51, 10.41] |
| 8 Cause‐specific survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 1.08 [0.73, 1.58] |
| 9 Distant metastasis‐free survival Show forest plot | 4 | 3267 | Hazard Ratio (95% CI) | 0.94 [0.65, 1.37] |
| 10 Relapse‐free survival Show forest plot | 3 | 3811 | Hazard Ratio (95% CI) | 1.36 [0.88, 2.09] |
| 11 Loco‐regional recurrence‐free survival Show forest plot | 2 | 3553 | Hazard Ratio (95% CI) | 1.80 [1.00, 3.25] |
| 12 Mastectomy Show forest plot | 3 | 4817 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.77, 1.87] |

