Corticosteroide y agonista beta 2 de acción prolongada combinados en un inhalador versus agonistas beta2 de acción prolongada para la enfermedad pulmonar obstructiva crónica
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, double‐blind parallel study. Duration: 52 weeks. | |
| Participants |
| |
| Interventions | Run‐in 4 weeks with open‐label fluticasone/salmeterol 250/50 Diskus BID. Following run‐in, subjects were randomised to FPS 250/50 or salmeterol twice‐daily via DISKUS for 52 weeks. Clinic visits were conducted at screening, day 1 (randomisation), and after 4, 8, 12, 20, 28, 36, 44, and 52 weeks. Treatments were assigned in blocks using a centre‐based randomisation schedule. Assignment to blinded study medication was stratified based on subjects' FEV1 response to albuterol at screen visit. Oral corticosteroids and antibiotics were allowed for the acute treatment of a COPD exacerbation. | |
| Outcomes | The primary efficacy endpoint was the annual rate of moderate/severe exacerbations. Secondary endpoints were the time to first moderate/severe exacerbation, the annual rate of exacerbations requiring oral corticosteroids, and pre‐dose FEV1. Related endpoints included the annual rate of all exacerbations, time to onset of each moderate/severe exacerbation, and diary records of dyspnoea scores, nighttime awakenings due to COPD, and use of supplemental albuterol. | |
| Notes | The run‐in with combined therapy precluded exacerbations due to ICS withdrawal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned in block centre‐based randomisation schedule and stratified according to salbutamol response in FEV1 at baseline. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Identical Diskus inhaler device. |
| Incomplete outcome data (attrition bias) | High risk | 39% discontinued on salmeterol and 32% on FPS. |
| Incomplete outcome data (attrition bias) | High risk | 39% discontinued on salmeterol and 32% on FPS. |
| Selective reporting (reporting bias) | Low risk | Contributes data to all primary outcomes. |
| Methods | Parallel group study. Randomisation: unclear. Blinding: double‐blind (identical inhaler devices). Trial duration: 12 months with two week run‐in of treatment optimisation. Allocation concealment: unclear. Withdrawals: stated. Intention‐to‐treat analysis: stated. | |
| Participants |
| |
| Interventions | Run‐in phase: All participants received 30 mg oral prednisolone BiD and 2 x 4.5 mcg formoterol BiD (2 weeks).
Additional treatment groups not covered in this review:
Inhaler device: Turbuhaler. | |
| Outcomes | Time to first exacerbation; change in post‐medication FEV1; number of exacerbations; time to and number of OCS‐treated episodes; am and pm PEF, slow VC, HRQL, symptoms, use of reliever medication, AEs. | |
| Notes | Classified as 'poorly reversible population'. P values used to calculate pooled SEMs for the following outcomes: Health related quality of life; FEV1; rescue medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information reported. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | High risk | 44% withdrew on formoterol and 29% on BDF. |
| Incomplete outcome data (attrition bias) | High risk | 44% withdrew on formoterol and 29% on BDF. |
| Selective reporting (reporting bias) | High risk | No data available for analysis of exacerbations as dichotomous data or hospitalisations. |
| Methods | Parallel group study. Trial duration: 12 months. Baseline characteristics: comparable. Intention‐to‐treat analysis: Yes. | |
| Participants |
| |
| Interventions | Run‐in: 2 weeks treatment with theophylline and prn SABA.
Additional treatment groups not covered in this review
Participants were on concomitant therapy: SABA prn and theophylline 400ug/day, for 12 months. Inhaler device: Diskus. | |
| Outcomes | FEV1, Delta FEV1, PEF am, symptom scores, rescue medication use, exacerbations (event rate and mean number per year). | |
| Notes | Classified as 'poorly reversible population'. Mild exacerbation: requirement for increase in SABA prn by >2 occasions/24hrs on two or more consecutive days compared with baseline mean of last seven days of run‐in Moderate exacerbation: condition requiring treatment with antibiotics and/or oral corticosteroids Severe exacerbation: Condition requiring emergency hospital treatment and/or hospitalisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; no other information reported. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | Unclear risk | Only 12 participants. |
| Incomplete outcome data (attrition bias) | Unclear risk | Only 12 participants. |
| Selective reporting (reporting bias) | High risk | No data available for the primary outcomes. |
| Methods | Randomised, double‐blind, parallel group study (study code SCO40043). Treatments were assigned in blocks using a centre based randomisation schedule. Patients were stratified based on FEV1 response to albuterol at screening. Study duration: 52 weeks. | |
| Participants |
| |
| Interventions | 4‐week run‐in period with open‐label FPS 250/50 via DISKUS. FPS 250/50 or salmeterol 50 mg twice daily via DISKUS for 12 months. As‐needed albuterol was provided for use throughout the study. Nine visits after screening. | |
| Outcomes | Annual rate of moderate to severe exacerbations. Time to first moderate to severe exacerbation, the annual rate of exacerbations requiring oral corticosteroids, and pre‐dose FEV1. Related endpoints were the annual rate of all exacerbations (mild and moderate to severe), duration of moderate to severe exacerbations, time to onset of each moderate to severe exacerbation. Exacerbation recovery time (determined by length of oral corticosteroid and antibiotic courses and hospital stays) and diary records of dyspnoea, nighttime awakenings due to COPD, and use of supplemental albuterol. | |
| Notes | In 782 patients with COPD (mean FEV1 Z0.94 0.36 L, 33% predicted normal), treatment with fluticasone propionate/salmeterol 250/50 significantly reduced (1) the annual rate of moderate to severe exacerbations by 30.5% compared with salmeterol (1.06 and 1.53 per subject per year, respectively, P < 0.001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatments were assigned in blocks using a centre based randomisation schedule. Patients were stratified based on FEV1 response to albuterol at screening. |
| Allocation concealment (selection bias) | Unclear risk | No details in the paper. |
| Blinding (performance bias and detection bias) | Unclear risk | Following run in, patients were randomised to FPS 250/50 or salmeterol 50 mg (Serevent; GlaxoSmithKline) twice daily via DISKUS. |
| Incomplete outcome data (attrition bias) | High risk | 38% discontinued on salmeterol and 30% on FPS. |
| Incomplete outcome data (attrition bias) | High risk | 38% discontinued on salmeterol and 30% on FPS. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to the analysis of hospitalisations. |
| Methods | Parallel group study. Trial duration: 24 weeks with 2‐week run‐in period. Baseline characteristics: comparable. Intention‐to‐treat analysis: not stated. | |
| Participants |
| |
| Interventions | Run‐in: 2 weeks treatment with placebo inhaler and prn SABA.
Additional treatment groups not covered in this review
Inhaler device: Diskus. | |
| Outcomes | Lung function: Change in FEV1 from baseline to end of study (M). PEF data not stratified by reversibility. Quality of life: CRDQ, CBSQ not stratified by reversibility. Dyspnoea and symptoms: Transitional Dyspnoea index, Baseline dyspnoea index not stratified by reversibility. Exacerbations. Rescue salbutamol use. | |
| Notes | FEV1 reversibility < 12% or 200 mL (of baseline FEV1) Reversibility stratified data. Mean % increase non‐reversible patients = 8.8. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | High risk | 32% withdrew on salmeterol and 30% on FPS. |
| Incomplete outcome data (attrition bias) | High risk | 32% withdrew on salmeterol and 30% on FPS. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to analysis of exacerbations as rate ratios, mortality or hospitalisation. |
| Methods | Randomised controlled trial, parallel group design. | |
| Participants |
| |
| Interventions | Run‐in: 4 weeks on stable medication.
Inhaler device: DPI. | |
| Outcomes | Withdrawals; Exacerbations (defined according to Rodriguez Roisin Chest article at stages II and III); FEV1; Rescue medication; symptoms; SGRQ; adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation schedule. |
| Allocation concealment (selection bias) | Low risk | Centrally generated schedule. |
| Blinding (performance bias and detection bias) | Low risk | Identicial inhaler devices. |
| Incomplete outcome data (attrition bias) | High risk | 21% discontinued on salmeterol and 20% on FPS. |
| Incomplete outcome data (attrition bias) | High risk | 21% discontinued on salmeterol and 20% on FPS. |
| Selective reporting (reporting bias) | Low risk | Contributes data to all primary outcomes. |
| Methods | Randomised controlled parallel group study. Trial duration: 24 weeks. Baseline characteristics: comparable. Intention‐to‐treat analysis: stated. | |
| Participants |
| |
| Interventions | Run‐in: 2 weeks treatment with placebo inhaler and prn SABA.
Additional treatment groups not covered in this review
Inhaler device: Diskus. | |
| Outcomes | Lung function: Change in FEV1 from baseline to end of study (M). Quality of life: CRDQ, CBSQ not stratified by reversibility. Dyspnoea and symptoms: End of study dyspnoea (TDI). Exacerbations. Rescue salbutamol use. | |
| Notes | COPD subjects reversible and non‐reversible, < 15% (baseline) improvement in FEV1 to salbutamol. Reversibility stratified data. Mean FEV1 reversibility 11.0%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; stratified by reversibility and investigative site. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | High risk | 28% withdrew on salmeterol and 32% on FPS. |
| Incomplete outcome data (attrition bias) | High risk | 28% withdrew on salmeterol and 32% on FPS. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to analysis of exacerbations as rate ratios, mortality or hospitalisations. |
| Methods | Randomised controlled parallel group design. | |
| Participants |
| |
| Interventions | Run‐in: 2 weeks, single‐blind placebo.
Additional treatment groups not covered in this review
Inhaler device: DPI. | |
| Outcomes | Withdrawals; exercise time; FEV1; adverse events. | |
| Notes | Study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | Low risk | One patient withdrew on salmeterol and three on FPS. |
| Incomplete outcome data (attrition bias) | Low risk | One patient withdrew on salmeterol and three on FPS. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to the analysis of exacerbations as rate ratios, mortality or hospitalisations. |
| Methods | Randomised, double‐blind, double dummy, parallel group, active and placebo‐controlled, multi‐centre trial. | |
| Participants |
| |
| Interventions | 4 weeks run‐in where ICS was permitted. Four groups: Budesonide/formoterol HFA pressurized metered‐dose inhaler 160/4.5 BID. Budesonide/formoterol HFA pressurized metered‐dose inhaler 80/4.5 BID. Formoterol dry powder inhaler 4.5 BID. Placebo. Duration: 12 months. | |
| Outcomes | 1) pre‐dose and 1‐hour post‐dose FEV1 2) FVC measured at all clinic visits, and 3) morning and evening peak expiratory flow(PEF) recorded daily. 4)exacerbations. 5) dyspnoea. 6) health status. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in one of four treatments. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Patients received both a pressurized metered‐dose inhaler (pMDI) and a dry powder inhaler (DPI) containing either active treatment or double‐dummy placebo as appropriate. |
| Incomplete outcome data (attrition bias) | High risk | 32% discontinued on formoterol and 28% on BDF. |
| Incomplete outcome data (attrition bias) | High risk | 32% discontinued on formoterol and 28% on BDF. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to analysis of exacerbations as dichotomous data or hospitalisations. |
| Methods | RCT, parallel group design. Trial duration: 24 weeks. | |
| Participants |
| |
| Interventions |
Inhaler device: DPI. | |
| Outcomes | Withdrawals; FEV1; morning PEF; quality of life (SQRG); symptoms (TDI) adverse events. | |
| Notes | Unpublished study downloaded from ctr.gsk.co.uk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | Unclear risk | 14% withdrew on salmeterol and 11% on FPS. |
| Incomplete outcome data (attrition bias) | Unclear risk | 14% withdrew on salmeterol and 11% on FPS. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to analysis of exacerbations as rate ratio, dichotomous data or hospitalisations. |
| Methods | Parallel group study. Intention‐to‐treat analysis: stated. | |
| Participants |
| |
| Interventions | Run‐in: 2 weeks. Treatment with prn SABA only.
Additional treatment groups not covered in this review
Inhaler device: Turbuhaler. | |
| Outcomes | Symptoms, adverse events, exacerbations, lung function. | |
| Notes | Classified as 'poorly reversible' subgroup. Exacerbation defined as requirement of oral steroids and/or antibiotics and/or hospitalisation for respiratory symptoms. Mild exacerbation defined as requirement of >/= 4 inhalations per day. P values used to calculate pooled SEMs for following outcomes: Symptoms; rescue medication usage. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme. |
| Allocation concealment (selection bias) | Low risk | At each centre, eligible patients received an enrolment code and then after run‐in, participants were allocated the next consecutive patient number. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | High risk | 32% withdrew on formoterol and 28% on BDF. |
| Incomplete outcome data (attrition bias) | High risk | 32% withdrew on formoterol and 28% on BDF. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to analysis of exacerbations as dichotomous data, hospitalisations or pneumonia. |
| Methods | 6‐month, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel group, multi‐centre study. | |
| Participants |
| |
| Interventions | After 2 weeks of treatment based on previous therapy (ICSs and short‐acting bronchodilators allowed during the run‐in period), patients received one of the following treatments | |
| Outcomes | Pre‐dose and 1 hour post‐dose FEV1. 12‐hour spirometry, pre‐dose and 1‐hour post‐dose Inspiratory Capacity. Pre‐dose morning and evening PEF. Dyspnoea, health‐related quality of life. COPD exacerbations. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced blocks according to a computer‐generated randomisation scheme at each site. |
| Allocation concealment (selection bias) | Unclear risk | Information not available. |
| Blinding (performance bias and detection bias) | Low risk | To maintain blinding, patients received both a pressurized metered‐dose inhaler (pMDI) and a dry powder inhaler (DPI) containing either active treatment or placebo, or combinations of active treatment and placebo, as appropriate. (Double blind, double dummy design) |
| Incomplete outcome data (attrition bias) | High risk | 21% discontinued on LABA and 14% on combination therapy. |
| Incomplete outcome data (attrition bias) | High risk | 21% discontinued on LABA and 14% on combination therapy. |
| Selective reporting (reporting bias) | Unclear risk | Does not contribute data to analysis of exacerbations as dichotomous data or hospitalisations. |
| Methods | RCT, parallel group design. Trial duration: 156 weeks. Baseline characteristics: comparable. Intention‐to‐treat analysis: stated. | |
| Participants |
| |
| Interventions | Run‐in: 2 weeks. All maintenance treatment with ICS and LABA ceased.
Additional treatment groups not covered in this review
Inhaler device: DPI. | |
| Outcomes | All cause mortality; change in SGRQ; exacerbations (requiring antibiotics, steroids, hospitalisation or combination of these); lung function; withdrawals; adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated scheme. Permuted block randomisation with stratification for smoking status and country. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation scheme. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | Low risk | Mortality was the primary outcome and vital status was checked in those who withdrew. |
| Incomplete outcome data (attrition bias) | High risk | 36.9% withdrew on salmeterol and 34.1% on FPS. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to analysis of exacerbations as dichotomous data. |
| Methods | RCT, parallel group design. Trial duration: 2 week run‐in period, 52 weeks treatment, 2‐week follow‐up. Baseline characteristics: comparable. Intention‐to‐treat analysis: stated. | |
| Participants |
| |
| Interventions | Run‐in: 2 weeks. All maintenance treatment with ICS and LABA ceased.
Additional treatment groups not covered in this review
Inhaler device: DPI. | |
| Outcomes | FEV1; PEF; exercise tolerance; quality of life: SGRQ; dyspnoea and symptoms (symptom score for shortness of breath, cough and sputum production); exacerbations (defined as requirement for antibiotics, oral steroids or both); rescue salbutamol use. | |
| Notes | FEV1 reversibility (% predicted normal). Mean Reversibility (% predicted) = 3.8. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | Centralised, third party randomisation. |
| Blinding (performance bias and detection bias) | Low risk | Identical inhaler devices. |
| Incomplete outcome data (attrition bias) | High risk | 32% withdrew on salmeterol and 25% on FPS. |
| Incomplete outcome data (attrition bias) | High risk | 32% withdrew on salmeterol and 25% on FPS. |
| Selective reporting (reporting bias) | High risk | Does not contribute data to analysis of exacerbations as dichotomous data or hospitalisations. |
DPI: Dry powder inhaler
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| All the arms had tiotropium | |
| Inflammatory outcomes for FPS | |
| Combination of oxygen and bronchodilators | |
| Combined with tiotropium and different outcomes | |
| Bench research of glucocorticoids receptors | |
| ARM with LAMA (tiotropium) | |
| Short term. Outcome was onset of action. No LABA alone arm | |
| Only one arm with FPS | |
| Outcome was bacterial colonization for FPS | |
| Genetic study of FPS | |
| FPS with no comparison | |
| FPS with no comparison |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation rates (combined inhaler versus LABA alone) Show forest plot | 9 | Rate ratio (Random, 95% CI) | 0.76 [0.68, 0.84] | |
| Analysis 1.1  Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 1 Exacerbation rates (combined inhaler versus LABA alone). | ||||
| 1.1 Fluticasone/salmeterol | 5 | Rate ratio (Random, 95% CI) | 0.77 [0.66, 0.89] | |
| 1.2 Budesonide/formoterol | 4 | Rate ratio (Random, 95% CI) | 0.73 [0.64, 0.83] | |
| 2 Number of participants with one or more exacerbation Show forest plot | 6 | 3357 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| Analysis 1.2  Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 2 Number of participants with one or more exacerbation. | ||||
| 2.1 Fluticasone/salmeterol | 6 | 3357 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| 2.2 Budesonide/formoterol | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Hospitalisations Show forest plot | 3 | Rate ratio (Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 3 Hospitalisations. | ||||
| 3.1 Fluticasone/salmeterol | 3 | Rate ratio (Random, 95% CI) | 0.79 [0.55, 1.13] | |
| 4 Mortality Show forest plot | 10 | 10681 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.11] |
| Analysis 1.4  Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 4 Mortality. | ||||
| 4.1 Fluticasone/salmeterol | 6 | 7408 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
| 4.2 Budesonide/formoterol | 4 | 3273 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.40, 2.67] |
| 5 Pneumonia Show forest plot | 12 | 11076 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [1.20, 2.01] |
| Analysis 1.5  Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 5 Pneumonia. | ||||
| 5.1 Fluticasone/salmeterol | 9 | 8242 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [1.25, 2.45] |
| 5.2 Budesonide/formoterol | 3 | 2834 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.73] |
| 6 Pneumonia subgrouped by dose Show forest plot | 12 | 11076 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [1.26, 1.93] |
| Analysis 1.6  Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 6 Pneumonia subgrouped by dose. | ||||
| 6.1 Lower dose FPS (250/50 bid) | 3 | 1934 | Odds Ratio (M‐H, Random, 95% CI) | 2.19 [1.35, 3.53] |
| 6.2 Higher dose FPS (500/50 bid) | 6 | 6308 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.92, 2.61] |
| 6.3 Lower dose BDF (160/9 bid) | 2 | 1164 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.53, 2.26] |
| 6.4 Higher dose BDF (320/9 bid) | 3 | 1670 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.60, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations by type Show forest plot | 5 | Rate ratio (Random, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 1 Exacerbations by type. | ||||
| 1.1 Requirement for oral steroids | 4 | Rate ratio (Random, 95% CI) | 0.71 [0.62, 0.81] | |
| 1.2 Hospitalisation | 3 | Rate ratio (Random, 95% CI) | 0.79 [0.55, 1.13] | |
| 2 Mortality by duration Show forest plot | 6 | 7408 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
| Analysis 2.2  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 2 Mortality by duration. | ||||
| 2.1 Mortality: three year data | 1 | 3054 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2.2 Mortality: >one and <three year data | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mortality: one year data | 5 | 4354 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.70] |
| 2.4 Mortality: 6 month data | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change from baseline in St George's Respiratory Questionnaire (total score) Show forest plot | 6 | SGRQ units (Random, 95% CI) | ‐1.58 [‐2.15, ‐1.01] | |
| Analysis 2.3  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 3 Change from baseline in St George's Respiratory Questionnaire (total score). | ||||
| 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms) Show forest plot | 4 | St George's RQ score (Random, 95% CI) | ‐2.78 [‐3.88, ‐1.68] | |
| Analysis 2.4  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms). | ||||
| 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity) Show forest plot | 4 | SGRQ units (Random, 95% CI) | ‐1.31 [‐2.38, ‐0.24] | |
| Analysis 2.5  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity). | ||||
| 6 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact) Show forest plot | 4 | SGRQ units (Random, 95% CI) | ‐1.41 [‐2.33, ‐0.50] | |
| Analysis 2.6  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 6 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact). | ||||
| 7 End of treatment St George's Respiratory Questionnaire scores (total score) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.7  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 7 End of treatment St George's Respiratory Questionnaire scores (total score). | ||||
| 8 End of treatment St George's Respiratory Questionnaire scores (domain ‐ symptoms) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 8 End of treatment St George's Respiratory Questionnaire scores (domain ‐ symptoms). | ||||
| 9 Change from baseline in Chronic Respiratory Disease Questionnaire scores Show forest plot | 2 | 680 | Mean Difference (IV, Random, 95% CI) | 2.83 [0.25, 5.41] |
| Analysis 2.9  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 9 Change from baseline in Chronic Respiratory Disease Questionnaire scores. | ||||
| 10 End of treatment Transitional dyspnea index (TDI) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.10  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 10 End of treatment Transitional dyspnea index (TDI). | ||||
| 11 End of treatment symptom scores Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.11  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 11 End of treatment symptom scores. | ||||
| 12 Change from baseline in Transitional Dyspnoea Index (TDI) Show forest plot | 2 | 677 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.47, 1.68] |
| Analysis 2.12  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 12 Change from baseline in Transitional Dyspnoea Index (TDI). | ||||
| 13 Change in MRC rated dyspnoea Show forest plot | 1 | symptoms (Random, 95% CI) | Totals not selected | |
| Analysis 2.13  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 13 Change in MRC rated dyspnoea. | ||||
| 14 Change from baseline in dyspnoea score Show forest plot | 2 | Mean Difference (Random, 95% CI) | ‐0.09 [‐0.13, ‐0.05] | |
| Analysis 2.14  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 14 Change from baseline in dyspnoea score. | ||||
| 15 Mean Change nighttime awakenings Show forest plot | 2 | 1554 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.07, ‐0.61] |
| Analysis 2.15  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 15 Mean Change nighttime awakenings. | ||||
| 16 Change from baseline in predose FEV1 Show forest plot | 5 | Litres (Random, 95% CI) | 0.07 [0.05, 0.10] | |
| Analysis 2.16  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 16 Change from baseline in predose FEV1. | ||||
| 16.1 Reversible population | 3 | Litres (Random, 95% CI) | 0.07 [0.02, 0.12] | |
| 16.2 Partially reversible population (mixed population) | 2 | Litres (Random, 95% CI) | 0.08 [0.04, 0.12] | |
| 16.3 Poorly reversible population | 2 | Litres (Random, 95% CI) | 0.06 [0.01, 0.12] | |
| 17 Change from baseline in postdose FEV1 Show forest plot | 3 | Litres (Random, 95% CI) | 0.05 [0.03, 0.06] | |
| Analysis 2.17  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 17 Change from baseline in postdose FEV1. | ||||
| 18 End of treatment FEV1 (Litres) Show forest plot | 2 | 1780 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| Analysis 2.18  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 18 End of treatment FEV1 (Litres). | ||||
| 19 FEV1 (% predicted ‐ absolute scores) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.19  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 19 FEV1 (% predicted ‐ absolute scores). | ||||
| 20 Change from baseline in am PEF (L/min) Show forest plot | 2 | L/min (Random, 95% CI) | 11.61 [7.91, 15.30] | |
| Analysis 2.20  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 20 Change from baseline in am PEF (L/min). | ||||
| 21 Change from baseline in rescue medication usage (puffs/day) Show forest plot | 4 | 2435 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.61, ‐0.16] |
| Analysis 2.21  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 21 Change from baseline in rescue medication usage (puffs/day). | ||||
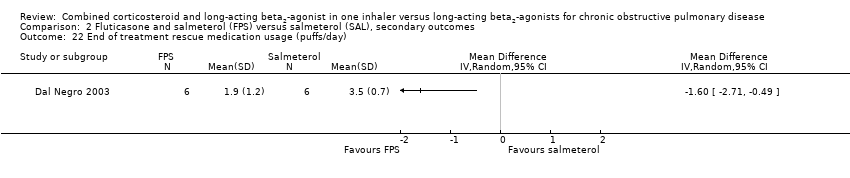
| 22 End of treatment rescue medication usage (puffs/day) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 2.22  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 22 End of treatment rescue medication usage (puffs/day). | ||||
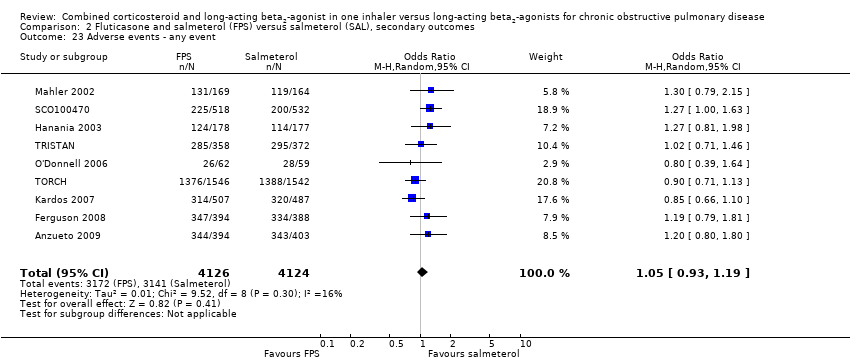
| 23 Adverse events ‐ any event Show forest plot | 9 | 8250 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.93, 1.19] |
| Analysis 2.23  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 23 Adverse events ‐ any event. | ||||
| 24 Adverse events ‐ candidiasis Show forest plot | 6 | 3118 | Odds Ratio (M‐H, Random, 95% CI) | 3.75 [2.33, 6.04] |
| Analysis 2.24  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 24 Adverse events ‐ candidiasis. | ||||
| 25 Adverse events ‐ pneumonia Show forest plot | 9 | 8242 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [1.25, 2.45] |
| Analysis 2.25  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 25 Adverse events ‐ pneumonia. | ||||
| 26 Adverse events ‐ headache Show forest plot | 8 | 7237 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.90, 1.26] |
| Analysis 2.26  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 26 Adverse events ‐ headache. | ||||
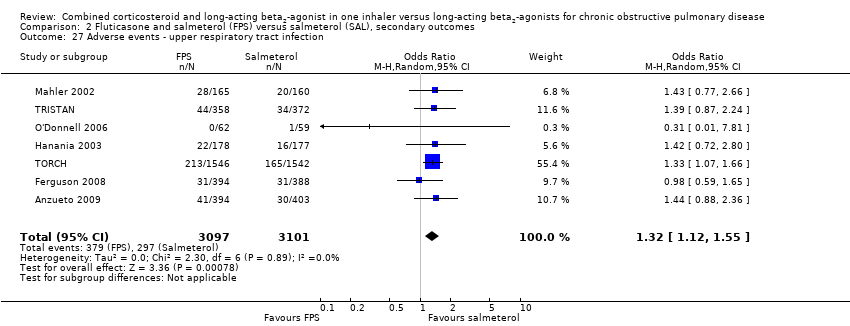
| 27 Adverse events ‐ upper respiratory tract infection Show forest plot | 7 | 6198 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [1.12, 1.55] |
| Analysis 2.27  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 27 Adverse events ‐ upper respiratory tract infection. | ||||
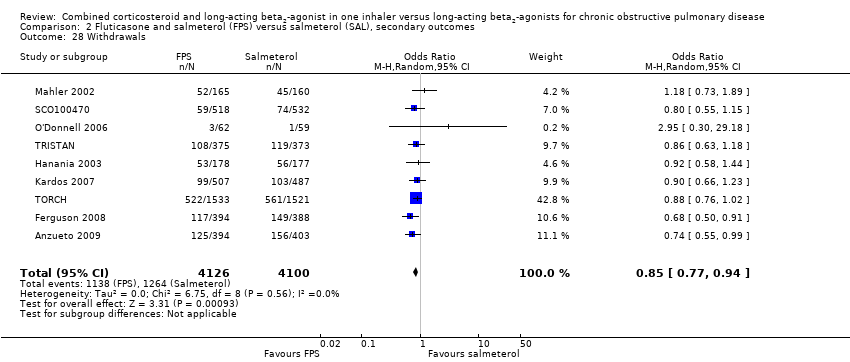
| 28 Withdrawals Show forest plot | 9 | 8226 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.94] |
| Analysis 2.28  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 28 Withdrawals. | ||||
| 29 Withdrawals due to lack of efficacy Show forest plot | 6 | 6739 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.72] |
| Analysis 2.29  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 29 Withdrawals due to lack of efficacy. | ||||
| 30 Withdrawals due to adverse events Show forest plot | 8 | 7895 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.79, 1.02] |
| Analysis 2.30  Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 30 Withdrawals due to adverse events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life ‐ SGRQ (change scores) Show forest plot | 4 | 3442 | SGRQ (Random, 95% CI) | ‐2.69 [‐3.82, ‐1.55] |
| Analysis 3.1  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 1 Quality of life ‐ SGRQ (change scores). | ||||
| 2 Quality of life ‐ SGRQ (change scores) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 2 Quality of life ‐ SGRQ (change scores). | ||||
| 3 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms) Show forest plot | 2 | 2233 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐5.13, ‐1.72] |
| Analysis 3.3  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 3 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms). | ||||
| 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity) Show forest plot | 2 | 2215 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐2.87, ‐0.27] |
| Analysis 3.4  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity). | ||||
| 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact) Show forest plot | 2 | 2222 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐3.60, ‐0.95] |
| Analysis 3.5  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact). | ||||
| 6 Mean FEV1 (% increase from baseline) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.6  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 6 Mean FEV1 (% increase from baseline). | ||||
| 7 Mean change from baseline in pre dose FEV1 to the average over the randomised treatment period. Show forest plot | 2 | 1203 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.00, 0.09] |
| Analysis 3.7  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 7 Mean change from baseline in pre dose FEV1 to the average over the randomised treatment period.. | ||||
| 8 Symptoms ‐ breathlessness (change scores) Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.12, ‐0.01] |
| Analysis 3.8  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 8 Symptoms ‐ breathlessness (change scores). | ||||
| 9 Change from baseline in cough score Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.11, ‐0.00] |
| Analysis 3.9  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 9 Change from baseline in cough score. | ||||
| 10 Change from baseline in rescue medication usage (puffs/day) Show forest plot | 2 | 2310 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.57, ‐0.09] |
| Analysis 3.10  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 10 Change from baseline in rescue medication usage (puffs/day). | ||||
| 11 Adverse events ‐ 'serious' events Show forest plot | 4 | 3243 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.25] |
| Analysis 3.11  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 11 Adverse events ‐ 'serious' events. | ||||
| 12 Adverse events ‐ candidiasis Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.12  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 12 Adverse events ‐ candidiasis. | ||||
| 13 Withdrawals due to adverse events Show forest plot | 4 | 3243 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| Analysis 3.13  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 13 Withdrawals due to adverse events. | ||||
| 14 Withdrawals due to worsening COPD symptoms Show forest plot | 2 | 918 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.74] |
| Analysis 3.14  Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 14 Withdrawals due to worsening COPD symptoms. | ||||
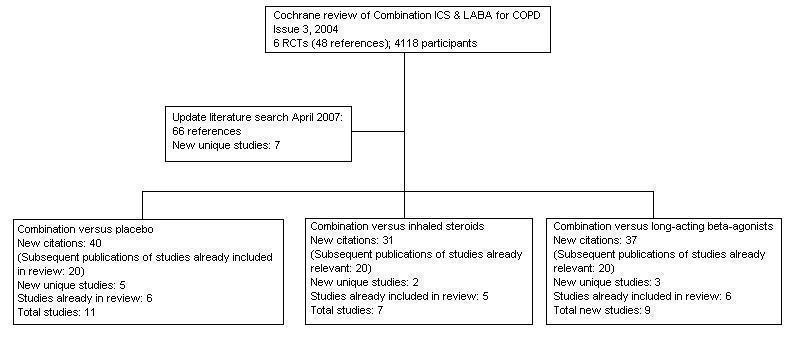
Flow chart to illustrate separation of review between three comparisons. Six RCTs met the original entry criteria of the review. All of these had a placebo and long‐acting beta2‐agonist arm, and five assessed combination against steroids. Seven new studies with one or more control comparisons were identified: five had a placebo arm, three had a long‐acting beta2agonist arm, and two had an inhaled steroid treatment arm.

Study flow diagram for 2007‐2011 literature searches.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
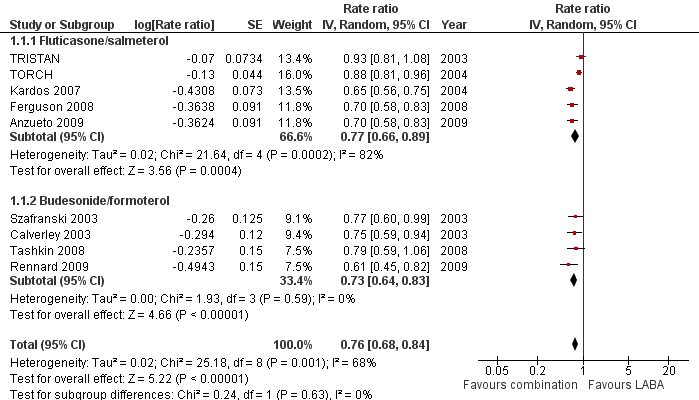
Forest plot of comparison: 1 Combined inhalers versus long‐acting beta2‐agonists (primary outcomes), outcome: 1.1 Exacerbation rates (combined treatment versus beta2‐agonist).

Forest plot of comparison: 1 Combined inhalers versus Long‐acting beta2‐agonists (Primary Outcomes), outcome: 1.2 Mortality.
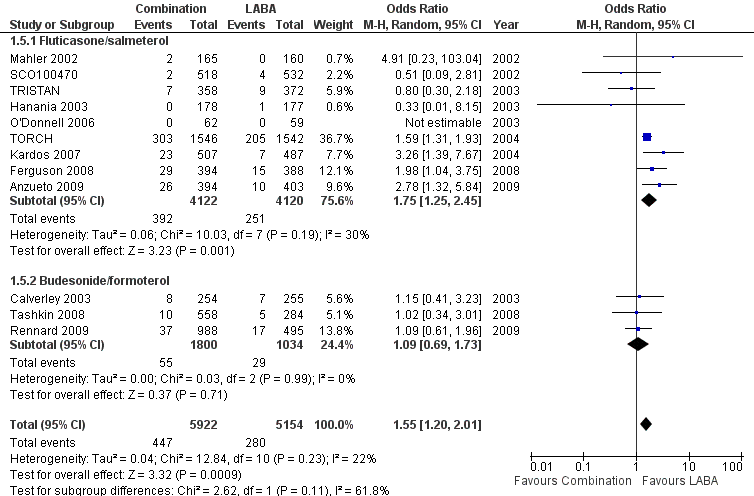
Forest plot of comparison: 1 Combined inhalers versus long‐acting beta2‐agonists (primary outcomes), outcome: 1.3 Pneumonia.
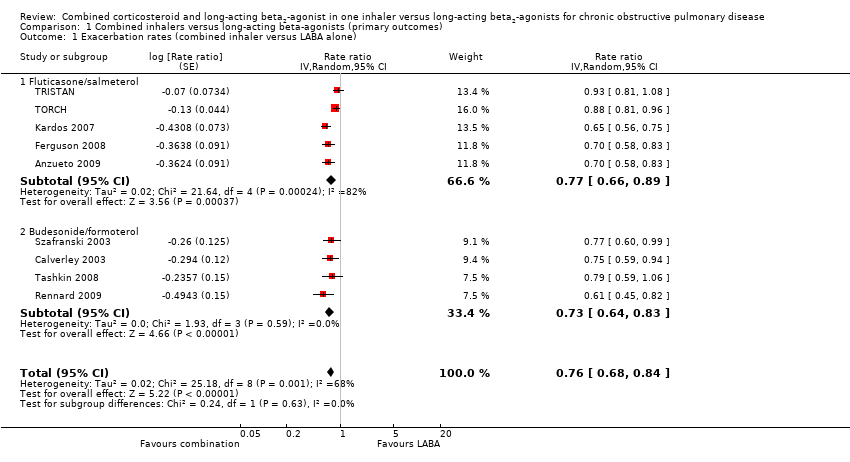
Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 1 Exacerbation rates (combined inhaler versus LABA alone).

Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 2 Number of participants with one or more exacerbation.

Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 3 Hospitalisations.

Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 4 Mortality.

Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 5 Pneumonia.

Comparison 1 Combined inhalers versus long‐acting beta‐agonists (primary outcomes), Outcome 6 Pneumonia subgrouped by dose.
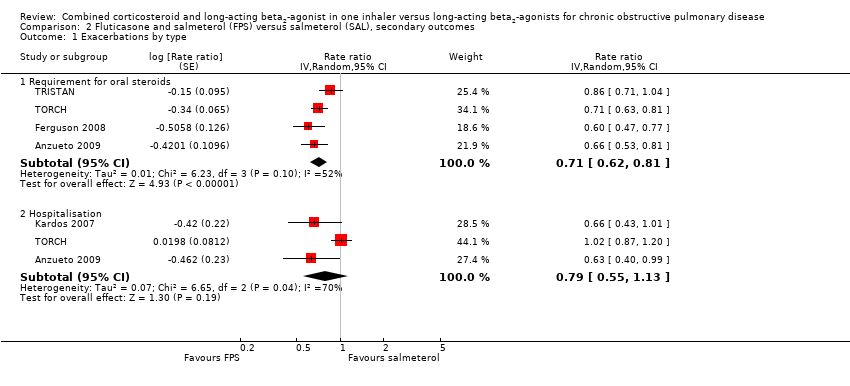
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 1 Exacerbations by type.
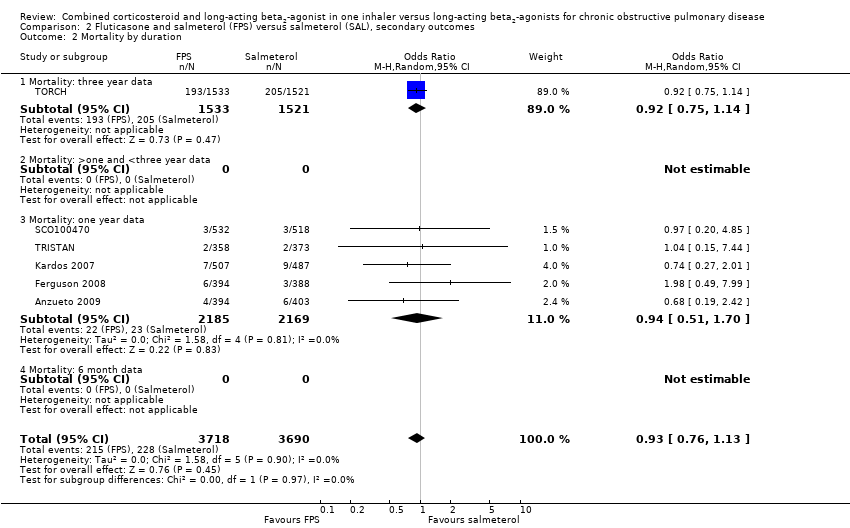
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 2 Mortality by duration.
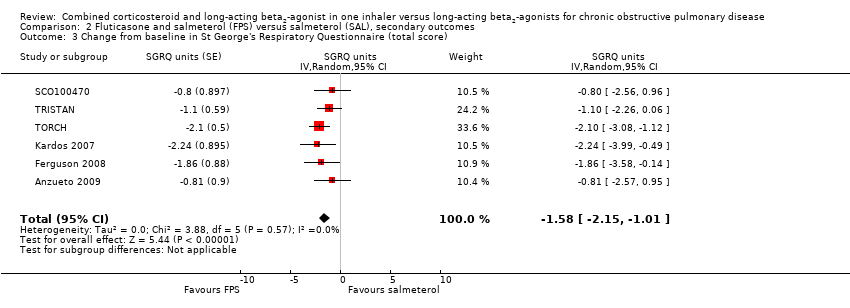
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 3 Change from baseline in St George's Respiratory Questionnaire (total score).

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms).
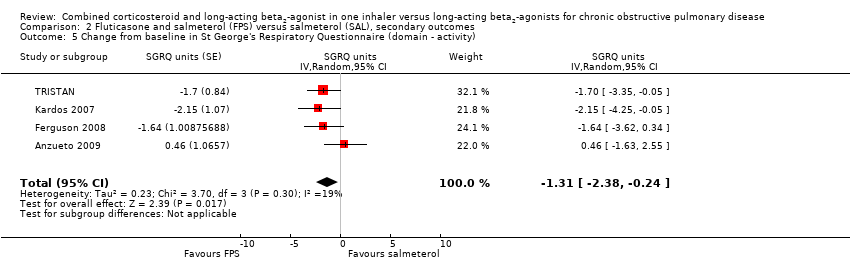
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity).

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 6 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact).
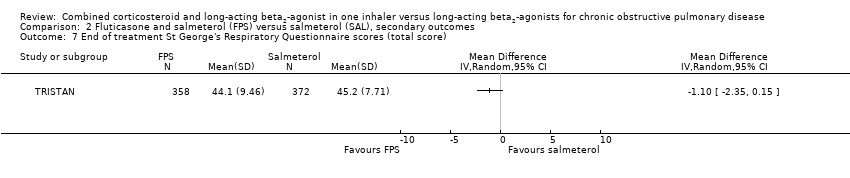
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 7 End of treatment St George's Respiratory Questionnaire scores (total score).

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 8 End of treatment St George's Respiratory Questionnaire scores (domain ‐ symptoms).

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 9 Change from baseline in Chronic Respiratory Disease Questionnaire scores.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 10 End of treatment Transitional dyspnea index (TDI).

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 11 End of treatment symptom scores.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 12 Change from baseline in Transitional Dyspnoea Index (TDI).

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 13 Change in MRC rated dyspnoea.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 14 Change from baseline in dyspnoea score.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 15 Mean Change nighttime awakenings.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 16 Change from baseline in predose FEV1.
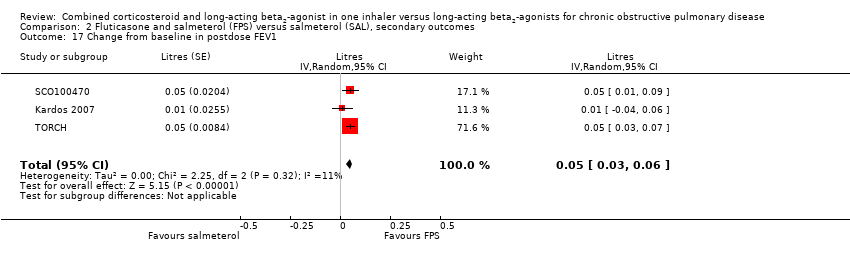
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 17 Change from baseline in postdose FEV1.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 18 End of treatment FEV1 (Litres).
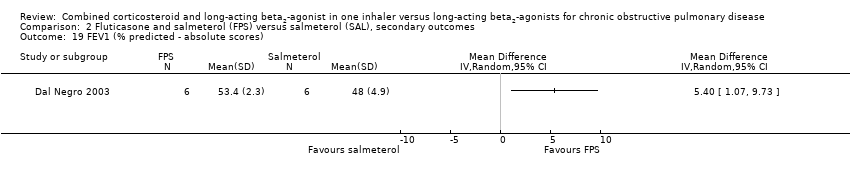
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 19 FEV1 (% predicted ‐ absolute scores).
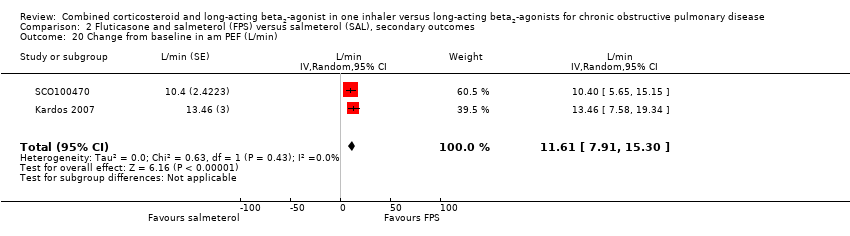
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 20 Change from baseline in am PEF (L/min).

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 21 Change from baseline in rescue medication usage (puffs/day).
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 22 End of treatment rescue medication usage (puffs/day).
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 23 Adverse events ‐ any event.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 24 Adverse events ‐ candidiasis.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 25 Adverse events ‐ pneumonia.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 26 Adverse events ‐ headache.
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 27 Adverse events ‐ upper respiratory tract infection.
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 28 Withdrawals.

Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 29 Withdrawals due to lack of efficacy.
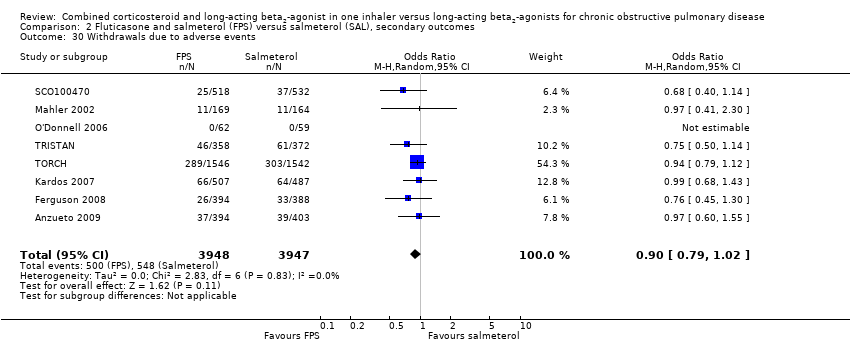
Comparison 2 Fluticasone and salmeterol (FPS) versus salmeterol (SAL), secondary outcomes, Outcome 30 Withdrawals due to adverse events.
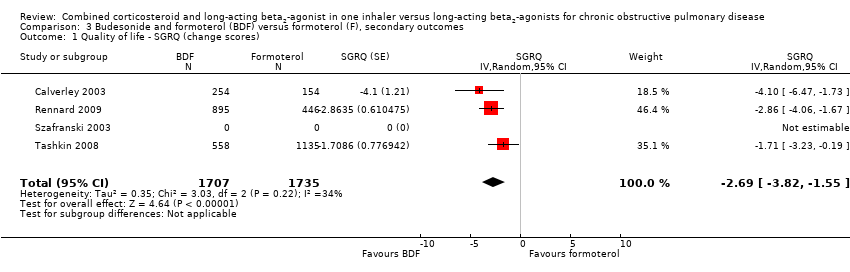
Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 1 Quality of life ‐ SGRQ (change scores).

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 2 Quality of life ‐ SGRQ (change scores).
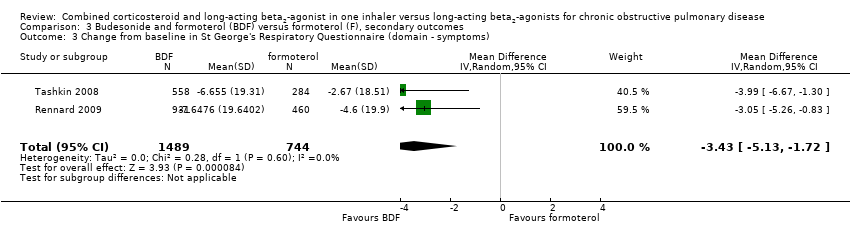
Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 3 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms).

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity).

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact).

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 6 Mean FEV1 (% increase from baseline).
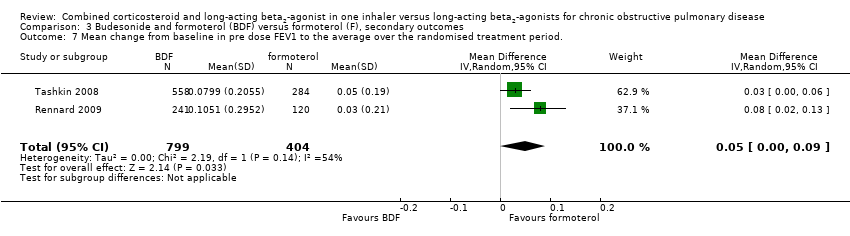
Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 7 Mean change from baseline in pre dose FEV1 to the average over the randomised treatment period..

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 8 Symptoms ‐ breathlessness (change scores).

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 9 Change from baseline in cough score.
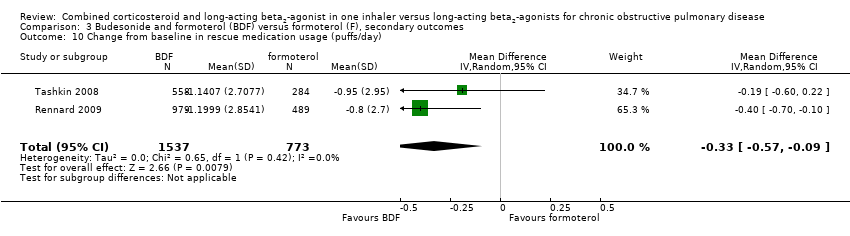
Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 10 Change from baseline in rescue medication usage (puffs/day).

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 11 Adverse events ‐ 'serious' events.
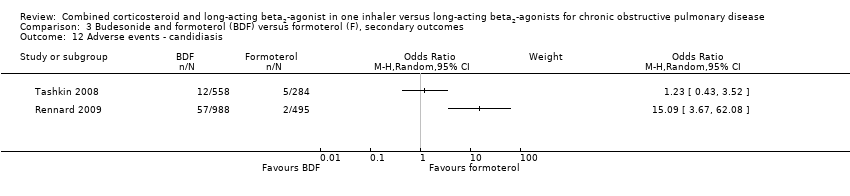
Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 12 Adverse events ‐ candidiasis.
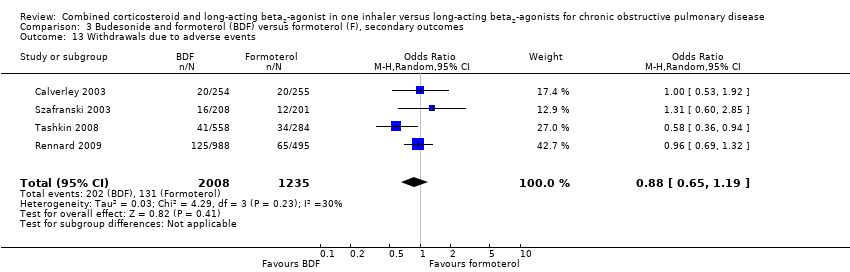
Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 13 Withdrawals due to adverse events.

Comparison 3 Budesonide and formoterol (BDF) versus formoterol (F), secondary outcomes, Outcome 14 Withdrawals due to worsening COPD symptoms.
| Combined inhalers compared to LABAs for COPD | ||||||
| Patient or population: COPD Setting: community | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| LABA inhalers | Combined inhalers | |||||
| Annual Exacerbation Rates | 1 per person per year | 0.76 per person per year (0.32 exacerbations fewer to 0.16 exacerbations fewer) | Rate ratio 0.76 (0.68 to 0.84) | 99211 | ⊕⊕⊝⊝ | The control arm exacerbation rates ranged from 0.9 to 1.5 per year in the studies of at least one year duration that included participants with severe COPD who had at least one exacerbation in the previous year.4 |
| Number of people experiencing one or more exacerbations Follow up: median 1 year | 47 per 100 | 42 per 100 (38 to 46) | OR 0.83 (0.70 to 0.98) | 3357 (6 studies) | ⊕⊕⊕⊝ | The evidence summarised for this outcome comes only from studies evaluating fluticasone/salmeterol. As such evidence for this outcome does not apply to budesonide/formoterol. |
| Annual hospitalisation rates Follow‐up: 1 to 3 years | 0.16 per person per year6 | 0.15 per person per year (0.1 to 0.21) | Rate ratio 0.79 (0.55 to 1.13) | 48791 (3 studies) | ⊕⊕⊝⊝ very low2,3,7 | |
| Mortality | 8 per 1000 | 7 per 1000 | OR 0.92 | 10681 | ⊕⊕⊕⊝ | The majority of data on mortality was derived from TORCH (vital status was ascertained in the patients who withdrew from treatment in this study). Control arm event rates varied from 0.4% to 5% in the one year studies. |
| Pneumonia | 27 per 1000 | 41 per 1000 | OR 1.55 | 11076 | ⊕⊕⊕⊝ | See Table 2 for control arm event rates in all studies |
| *The basis for the assumed risk (was the median control group risk across studies of one year duration). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 This is the number of participants randomised in the studies. The analysis took account of the amount of time the participants were enrolled for prior to withdrawal. 2 Risk of bias (‐1): High withdrawal rates in all the longer trials. 5 Publication bias (‐1): Data from a key study of fluticasone and salmeterol (TORCH) and from studies evaluating budesonide/formoterol were not available as binary data and did not contribute to this measurement of exacerbations. Whilst the analysis of the data as rates in these studies is likely to reflect the individual trial protocols, we cannot be sure that their absence from this outcome measure has little or no impact on the pooled odds ratio. 6 Annualised rates of hospitalisation have been estimated from the three year study TORCH. 7 Imprecision (‐1): Confidence interval includes possible harm and benefit. 8 See Table 2 for a range of NNT(H) results for risk of pneumonia across the trials of different durations. | ||||||
| Version | Detail |
| 1st published version ‐ Issue 4, 2003 (All years to April 2002) | References identified: 34 |
| Update: Issue 3, 2004 (April 2003‐April 2004) | References identified: 12 |
| Update: Issue 3, 2005 (April 2004‐April 2005) | References identified: 52 |
| Update: April 2005 ‐ April 2007 | References identified: 66 |
| Update: April 2007‐ November 2011 | References identified: 207 |
| Study ID | Study duration | Rate on LABA alone % | NNT (calculated from pooled OR using Visual Rx) |
| 8 weeks | 0 | NA | |
| 24 weeks | 0.6 | 291 (192 to 525) | |
| 24 weeks | 0 | NA | |
| 24 weeks | 0.8 | 219 (145 to 395) | |
| 26 weeks | 1.76 | 107 (59 to 291) | |
| 48 weeks | 1.4 | 126 (84 to 228) | |
| 52 weeks | 2.4 | 75 (50 to 135) | |
| 52 weeks | 2.7 | 67 (45 to 120) | |
| 52 weeks | 2.5 | 76 (42 to 207) | |
| 52 weeks | 3.43 | 56 (31 to 152) | |
| 52 weeks | 3.9 | 50 (28 to 135) | |
| 156 weeks | 13.3 | 17 (12 to 29) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbation rates (combined inhaler versus LABA alone) Show forest plot | 9 | Rate ratio (Random, 95% CI) | 0.76 [0.68, 0.84] | |
| 1.1 Fluticasone/salmeterol | 5 | Rate ratio (Random, 95% CI) | 0.77 [0.66, 0.89] | |
| 1.2 Budesonide/formoterol | 4 | Rate ratio (Random, 95% CI) | 0.73 [0.64, 0.83] | |
| 2 Number of participants with one or more exacerbation Show forest plot | 6 | 3357 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| 2.1 Fluticasone/salmeterol | 6 | 3357 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| 2.2 Budesonide/formoterol | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Hospitalisations Show forest plot | 3 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Fluticasone/salmeterol | 3 | Rate ratio (Random, 95% CI) | 0.79 [0.55, 1.13] | |
| 4 Mortality Show forest plot | 10 | 10681 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.11] |
| 4.1 Fluticasone/salmeterol | 6 | 7408 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
| 4.2 Budesonide/formoterol | 4 | 3273 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.40, 2.67] |
| 5 Pneumonia Show forest plot | 12 | 11076 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [1.20, 2.01] |
| 5.1 Fluticasone/salmeterol | 9 | 8242 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [1.25, 2.45] |
| 5.2 Budesonide/formoterol | 3 | 2834 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.69, 1.73] |
| 6 Pneumonia subgrouped by dose Show forest plot | 12 | 11076 | Odds Ratio (M‐H, Random, 95% CI) | 1.56 [1.26, 1.93] |
| 6.1 Lower dose FPS (250/50 bid) | 3 | 1934 | Odds Ratio (M‐H, Random, 95% CI) | 2.19 [1.35, 3.53] |
| 6.2 Higher dose FPS (500/50 bid) | 6 | 6308 | Odds Ratio (M‐H, Random, 95% CI) | 1.55 [0.92, 2.61] |
| 6.3 Lower dose BDF (160/9 bid) | 2 | 1164 | Odds Ratio (M‐H, Random, 95% CI) | 1.10 [0.53, 2.26] |
| 6.4 Higher dose BDF (320/9 bid) | 3 | 1670 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.60, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Exacerbations by type Show forest plot | 5 | Rate ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Requirement for oral steroids | 4 | Rate ratio (Random, 95% CI) | 0.71 [0.62, 0.81] | |
| 1.2 Hospitalisation | 3 | Rate ratio (Random, 95% CI) | 0.79 [0.55, 1.13] | |
| 2 Mortality by duration Show forest plot | 6 | 7408 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.13] |
| 2.1 Mortality: three year data | 1 | 3054 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.75, 1.14] |
| 2.2 Mortality: >one and <three year data | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Mortality: one year data | 5 | 4354 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.70] |
| 2.4 Mortality: 6 month data | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Change from baseline in St George's Respiratory Questionnaire (total score) Show forest plot | 6 | SGRQ units (Random, 95% CI) | ‐1.58 [‐2.15, ‐1.01] | |
| 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms) Show forest plot | 4 | St George's RQ score (Random, 95% CI) | ‐2.78 [‐3.88, ‐1.68] | |
| 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity) Show forest plot | 4 | SGRQ units (Random, 95% CI) | ‐1.31 [‐2.38, ‐0.24] | |
| 6 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact) Show forest plot | 4 | SGRQ units (Random, 95% CI) | ‐1.41 [‐2.33, ‐0.50] | |
| 7 End of treatment St George's Respiratory Questionnaire scores (total score) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 End of treatment St George's Respiratory Questionnaire scores (domain ‐ symptoms) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Change from baseline in Chronic Respiratory Disease Questionnaire scores Show forest plot | 2 | 680 | Mean Difference (IV, Random, 95% CI) | 2.83 [0.25, 5.41] |
| 10 End of treatment Transitional dyspnea index (TDI) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 End of treatment symptom scores Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Change from baseline in Transitional Dyspnoea Index (TDI) Show forest plot | 2 | 677 | Mean Difference (IV, Random, 95% CI) | 0.61 [‐0.47, 1.68] |
| 13 Change in MRC rated dyspnoea Show forest plot | 1 | symptoms (Random, 95% CI) | Totals not selected | |
| 14 Change from baseline in dyspnoea score Show forest plot | 2 | Mean Difference (Random, 95% CI) | ‐0.09 [‐0.13, ‐0.05] | |
| 15 Mean Change nighttime awakenings Show forest plot | 2 | 1554 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.07, ‐0.61] |
| 16 Change from baseline in predose FEV1 Show forest plot | 5 | Litres (Random, 95% CI) | 0.07 [0.05, 0.10] | |
| 16.1 Reversible population | 3 | Litres (Random, 95% CI) | 0.07 [0.02, 0.12] | |
| 16.2 Partially reversible population (mixed population) | 2 | Litres (Random, 95% CI) | 0.08 [0.04, 0.12] | |
| 16.3 Poorly reversible population | 2 | Litres (Random, 95% CI) | 0.06 [0.01, 0.12] | |
| 17 Change from baseline in postdose FEV1 Show forest plot | 3 | Litres (Random, 95% CI) | 0.05 [0.03, 0.06] | |
| 18 End of treatment FEV1 (Litres) Show forest plot | 2 | 1780 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 19 FEV1 (% predicted ‐ absolute scores) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Change from baseline in am PEF (L/min) Show forest plot | 2 | L/min (Random, 95% CI) | 11.61 [7.91, 15.30] | |
| 21 Change from baseline in rescue medication usage (puffs/day) Show forest plot | 4 | 2435 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.61, ‐0.16] |
| 22 End of treatment rescue medication usage (puffs/day) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23 Adverse events ‐ any event Show forest plot | 9 | 8250 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.93, 1.19] |
| 24 Adverse events ‐ candidiasis Show forest plot | 6 | 3118 | Odds Ratio (M‐H, Random, 95% CI) | 3.75 [2.33, 6.04] |
| 25 Adverse events ‐ pneumonia Show forest plot | 9 | 8242 | Odds Ratio (M‐H, Random, 95% CI) | 1.75 [1.25, 2.45] |
| 26 Adverse events ‐ headache Show forest plot | 8 | 7237 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.90, 1.26] |
| 27 Adverse events ‐ upper respiratory tract infection Show forest plot | 7 | 6198 | Odds Ratio (M‐H, Random, 95% CI) | 1.32 [1.12, 1.55] |
| 28 Withdrawals Show forest plot | 9 | 8226 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.77, 0.94] |
| 29 Withdrawals due to lack of efficacy Show forest plot | 6 | 6739 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.39, 0.72] |
| 30 Withdrawals due to adverse events Show forest plot | 8 | 7895 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.79, 1.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Quality of life ‐ SGRQ (change scores) Show forest plot | 4 | 3442 | SGRQ (Random, 95% CI) | ‐2.69 [‐3.82, ‐1.55] |
| 2 Quality of life ‐ SGRQ (change scores) Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Change from baseline in St George's Respiratory Questionnaire (domain ‐ symptoms) Show forest plot | 2 | 2233 | Mean Difference (IV, Random, 95% CI) | ‐3.43 [‐5.13, ‐1.72] |
| 4 Change from baseline in St George's Respiratory Questionnaire (domain ‐ activity) Show forest plot | 2 | 2215 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐2.87, ‐0.27] |
| 5 Change from baseline in St George's Respiratory Questionnaire (domain ‐ impact) Show forest plot | 2 | 2222 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐3.60, ‐0.95] |
| 6 Mean FEV1 (% increase from baseline) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Mean change from baseline in pre dose FEV1 to the average over the randomised treatment period. Show forest plot | 2 | 1203 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.00, 0.09] |
| 8 Symptoms ‐ breathlessness (change scores) Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.12, ‐0.01] |
| 9 Change from baseline in cough score Show forest plot | 2 | 2308 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.11, ‐0.00] |
| 10 Change from baseline in rescue medication usage (puffs/day) Show forest plot | 2 | 2310 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.57, ‐0.09] |
| 11 Adverse events ‐ 'serious' events Show forest plot | 4 | 3243 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.25] |
| 12 Adverse events ‐ candidiasis Show forest plot | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Withdrawals due to adverse events Show forest plot | 4 | 3243 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.65, 1.19] |
| 14 Withdrawals due to worsening COPD symptoms Show forest plot | 2 | 918 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.74] |

