Mefloquine for preventing malaria during travel to endemic areas
Abstract
Background
Mefloquine is one of four antimalarial agents commonly recommended for preventing malaria in travellers to malaria‐endemic areas. Despite its high efficacy, there is controversy about its psychological side effects.
Objectives
To summarize the efficacy and safety of mefloquine used as prophylaxis for malaria in travellers.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published on the Cochrane Library; MEDLINE; Embase (OVID); TOXLINE (https://toxnet.nlm.nih.gov/newtoxnet/toxline.htm); and LILACS. We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; http://www.who.int/ictrp/en/) and ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home) for trials in progress, using 'mefloquine', 'Lariam', and 'malaria' as search terms. The search date was 22 June 2017.
Selection criteria
We included randomized controlled trials (for efficacy and safety) and non‐randomized cohort studies (for safety). We compared prophylactic mefloquine with placebo, no treatment, or an alternative recommended antimalarial agent. Our study populations included all adults and children, including pregnant women.
Data collection and analysis
Two review authors independently assessed the eligibility and risk of bias of trials, extracted and analysed data. We compared dichotomous outcomes using risk ratios (RR) with 95% confidence intervals (CI). Prespecified adverse outcomes are included in 'Summary of findings' tables, with the best available estimate of the absolute frequency of each outcome in short‐term international travellers. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 20 RCTs (11,470 participants); 35 cohort studies (198,493 participants); and four large retrospective analyses of health records (800,652 participants). Nine RCTs explicitly excluded participants with a psychiatric history, and 25 cohort studies stated that the choice of antimalarial agent was based on medical history and personal preference. Most RCTs and cohort studies collected data on self‐reported or clinician‐assessed symptoms, rather than formal medical diagnoses.
Mefloquine efficacy
Of 12 trials comparing mefloquine and placebo, none were performed in short‐term international travellers, and most populations had a degree of immunity to malaria. The percentage of people developing a malaria episode in the control arm varied from 1% to 82% (median 22%) and 0% to 13% in the mefloquine group (median 1%).
In four RCTs that directly compared mefloquine, atovaquone‐proguanil and doxycycline in non‐immune, short‐term international travellers, only one clinical case of malaria occurred (4 trials, 1822 participants).
Mefloquine safety versus atovaquone‐proguanil
Participants receiving mefloquine were more likely to discontinue their medication due to adverse effects than atovaquone‐proguanil users (RR 2.86, 95% CI 1.53 to 5.31; 3 RCTs, 1438 participants; high‐certainty evidence). There were few serious adverse effects reported with mefloquine (15/2651 travellers) and none with atovaquone‐proguanil (940 travellers).
One RCT and six cohort studies reported on our prespecified adverse effects. In the RCT with short‐term travellers, mefloquine users were more likely to report abnormal dreams (RR 2.04, 95% CI 1.37 to 3.04, moderate‐certainty evidence), insomnia (RR 4.42, 95% CI 2.56 to 7.64, moderate‐certainty evidence), anxiety (RR 6.12, 95% CI 1.82 to 20.66, moderate‐certainty evidence), and depressed mood during travel (RR 5.78, 95% CI 1.71 to 19.61, moderate‐certainty evidence). The cohort studies in longer‐term travellers were consistent with this finding but most had larger effect sizes. Mefloquine users were also more likely to report nausea (high‐certainty evidence) and dizziness (high‐certainty evidence).
Based on the available evidence, our best estimates of absolute effect sizes for mefloquine versus atovaquone‐proguanil are 6% versus 2% for discontinuation of the drug, 13% versus 3% for insomnia, 14% versus 7% for abnormal dreams, 6% versus 1% for anxiety, and 6% versus 1% for depressed mood.
Mefloquine safety versus doxycycline
No difference was found in numbers of serious adverse effects with mefloquine and doxycycline (low‐certainty evidence) or numbers of discontinuations due to adverse effects (RR 1.08, 95% CI 0.41 to 2.87; 4 RCTs, 763 participants; low‐certainty evidence).
Six cohort studies in longer‐term occupational travellers reported our prespecified adverse effects; one RCT in military personnel and one cohort study in short‐term travellers reported adverse events. Mefloquine users were more likely to report abnormal dreams (RR 10.49, 95% CI 3.79 to 29.10; 4 cohort studies, 2588 participants, very low‐certainty evidence), insomnia (RR 4.14, 95% CI 1.19 to 14.44; 4 cohort studies, 3212 participants, very low‐certainty evidence), anxiety (RR 18.04, 95% CI 9.32 to 34.93; 3 cohort studies, 2559 participants, very low‐certainty evidence), and depressed mood (RR 11.43, 95% CI 5.21 to 25.07; 2 cohort studies, 2445 participants, very low‐certainty evidence). The findings of the single cohort study reporting adverse events in short‐term international travellers were consistent with this finding but the single RCT in military personnel did not demonstrate a difference between groups in frequencies of abnormal dreams or insomnia.
Mefloquine users were less likely to report dyspepsia (RR 0.26, 95% CI 0.09 to 0.74; 5 cohort studies, 5104 participants, low certainty‐evidence), photosensitivity (RR 0.08, 95% CI 0.05 to 0.11; 2 cohort studies, 1875 participants, very low‐certainty evidence), vomiting (RR 0.18, 95% CI 0.12 to 0.27; 4 cohort studies, 5071 participants, very low‐certainty evidence), and vaginal thrush (RR 0.10, 95% CI 0.06 to 0.16; 1 cohort study, 1761 participants, very low‐certainty evidence).
Based on the available evidence, our best estimates of absolute effect for mefloquine versus doxycyline were: 2% versus 2% for discontinuation, 12% versus 3% for insomnia, 31% versus 3% for abnormal dreams, 18% versus 1% for anxiety, 11% versus 1% for depressed mood, 4% versus 14% for dyspepsia, 2% versus 19% for photosensitivity, 1% versus 5% for vomiting, and 2% versus 16% for vaginal thrush.
Additional analyses, including comparisons of mefloquine with chloroquine, added no new information. Subgroup analysis by study design, duration of travel, and military versus non‐military participants, provided no conclusive findings.
Authors' conclusions
The absolute risk of malaria during short‐term travel appears low with all three established antimalarial agents (mefloquine, doxycycline, and atovaquone‐proguanil).
The choice of antimalarial agent depends on how individual travellers assess the importance of specific adverse effects, pill burden, and cost. Some travellers will prefer mefloquine for its once‐weekly regimen, but this should be balanced against the increased frequency of abnormal dreams, anxiety, insomnia, and depressed mood.
PICO
Plain language summary
Can mefloquine prevent malaria during travel to areas where the disease is widespread?
We summarized trials that evaluated the effectiveness and safety of mefloquine when used to prevent malaria in people travelling to areas where the disease is widespread. We searched for relevant studies up to 22 June 2017 and included 20 randomized trials that involved 11,470 participants, 35 cohort studies (198,493 participants) and four large retrospective analyses of health records (800,652 participants).
What are the concerns about mefloquine and what are the alternatives?
Mefloquine is often prescribed to prevent malaria during travel to areas where the disease is widespread. However, there is controversy about the safety of mefloquine, especially when prescribed for military personnel in stressful situations, and there have been reports of depression and suicide.
The only commonly‐used alternative drugs are doxycycline (which can cause skin problems and indigestion) and atovaquone‐proguanil (which is often more expensive).
What the research says
Mefloquine appears to be a highly effective drug to reduce the risk of malaria (low‐certainty evidence), however, evidence did not come from short‐term international travellers.
Mefloquine has not been shown to have more frequent serious side effects than either atovaquone‐proguanil (low‐certainty evidence) or doxycycline (very low‐certainty evidence).
People who take mefloquine are more likely to stop taking the drug due to side effects than people who take atovaquone‐proguanil (high‐certainty evidence), but may be equally as likely to stop as people who take doxycyline (low‐certainty evidence).
People taking mefloquine are more likely to have abnormal dreams, insomnia, anxiety and depressed mood during travel than people who take atovaquone‐proguanil (moderate‐certainty evidence) or doxycyline (very low‐certainty evidence). Doxycycline users are more likely to have dyspepsia, photosensitivity, vomiting, and vaginal thrush (very low‐certainty evidence).
Authors' conclusions
Summary of findings
| Mefloquine compared with atovaquone‐proguanil for preventing malaria in travellers | ||||||
| Population: non‐immune adults and children travelling to or living in malaria‐endemic settings Intervention: mefloquine 250 mg weekly Comparison: atovaquone‐proguanil (250 mg atovaquone and 100 mg proguanil hydrochloride) daily Outcome data collection: physicians performed blinded assessment of whether reported symptoms could be related to the study drug | ||||||
| Outcomes | Anticipated absolute effects* | Relative effect | Studies contributing to effect estimate | Additional studies considered in GRADE assessment | Certainty of the evidence | |
| Atovoquone‐proguanil | Mefloquine | |||||
| Clinical malaria | — | — | — | 2 RCTs (1293) | — | ⊕⊕⊝⊝ |
| Serious adverse effects | 0 per 100 | 1 in 100 (0 to 12) | RR 1.40 (0.08 to 23.22) | 4 cohort studies (3693) | 1 RCT (976) | ⊕⊕⊝⊝ |
| Discontinuation of drug due to adverse effects | 2 per 100 | 6 per 100 (3 to 11) | RR 2.86 (1.53 to 5.31) | 3 RCTs (1438) | 7 cohort studies (4498) | ⊕⊕⊕⊕ |
| Abnormal dreams | 7 per 100 | 14 per 100 (10 to 21) | RR 2.04 (1.37 to 3.04) | 1 RCT (976) | 7 cohort studies (3848) | ⊕⊕⊕⊕ |
| Insomnia | 3 per 100 | 13 per 100 (8 to 23) | RR 4.42 (2.56 to 7.64) | 1 RCT (976) | 8 cohort studies (3986) | ⊕⊕⊕⊕ |
| Anxiety | 1 per 100 | 6 per 100 (2 to 21) | RR 6.12 (1.82 to 20.66) | 1 RCT (976) | 4 cohort studies (2664) | ⊕⊕⊕⊝ |
| Depressed mood | 1 per 100 | 6 per 100 (2 to 20) | RR 5.78 (1.71 to 19.61) | 1 RCT (976) | 6 cohort studies (3624) | ⊕⊕⊕⊝ |
| Abnormal thoughts or perceptions | 0 per 100 | 1 per 100 (0 to 4) | RR 1.50 (0.30 to 7.42) | 3 cohort studies (2433) | — | ⊕⊝⊝⊝ |
| Nausea | 3 per 100 | 8 per 100 (5 to 15) | RR 2.72 (1.52 to 4.86) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊕⊕ |
| Vomiting | 1 per 100 | 1 per 100 (0 to 4) | RR 1.31 (0.49 to 3.50) | 1 RCT (976) | 3 cohort studies (2180) | ⊕⊕⊕⊝ |
| Abdominal pain | 5 per 100 | 5 per 100 (3 to 8) | RR 0.90 (0.52 to 1.56) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊝⊝ |
| Diarrhoea | 8 per 100 | 8 per 100 (5 to 12) | RR 0.94 (0.60 to 1.47) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊕⊝ |
| Headache | 4 per 100 | 7 per 100 (4 to 12) | RR 1.72 (0.99 to 2.99) | 1 RCT (976) | 8 cohort studies (4163) | ⊕⊕⊕⊝ |
| Dizziness | 2 per 100 | 8 per 100 (4 to 15) | RR 3.99 (2.08 to 7.64) | 1 RCT (976) | 8 cohort studies (3986) | ⊕⊕⊕⊕ |
| Pruritis | 2 per 100 | 3 per 100 (1 to 5) | RR 1.28 (0.60 to 2.70) | 1 RCT (976) | 3 cohort studies (1824) | ⊕⊕⊕⊝ |
| Visual impairment | 2 per 100 | 4 per 100 (2 to 9) | RR 2.04 (0.88 to 4.73) | 1 RCT (976) | 2 cohort studies (1956) | ⊕⊕⊕⊝ |
| Mouth ulcers | 2 per 100 | 3 per 100 (1 to 6) | RR 1.45 (0.70 to 3.00) | 1 RCT (976) | 2 cohort studies (783) | ⊕⊕⊕⊝ |
| *The assumed risk is the median control group risk across studies unless stated in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Where the control group risk was 0, we used a value of 0.5 to calculate the corresponding risk in the intervention group. Data from cohort studies were used when data from RCTs were unavailable. 'Summary of findings' tables are usually limited to seven outcomes. For adverse effects this problematic, as there are many, and to include some and not others risks selective reporting. We have therefore included all prespecified outcomes in the table. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: the RCTs were generally at low risk of bias but two of three were sponsored by the manufacturer of one of the study drugs. All cohort studies had methodological problems which could introduce confounding or bias. However, as the GRADE approach automatically downgrades certainty by two levels for non‐randomized studies, we did not downgrade further. | ||||||
| Mefloquine compared with doxycycline for preventing malaria in travellers | ||||||
| Population: Non‐immune adults and children travelling to malaria‐endemic settings Intervention: Mefloquine 250 mg weekly Comparison: Doxycycline 100 mg daily Outcome data collection: Self‐reported symptoms experienced whilst taking prophylaxis (adverse events) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Studies contributing to effect estimate | Additional studies considered in GRADE assessment | Certainty of the evidence | |
| Doxycycline | Mefloquine | |||||
| Clinical malaria | 1 per 100 | 1 per 100 (0 to 5) | RR 1.35 | 4 RCTs (744) | — | ⊕⊕⊝⊝ low1,2,3,4 |
| Serious adverse effects | 6 per 10005 | 9 per 1000 (1 to 61) | RR 1.53 (0.23 to 10.24) | 3 cohort studies (3722) | 3 RCTs, 1 cohort study (682; 3772) | ⊕⊝⊝⊝ |
| Discontinuations due to adverse effects | 2 per 100 | 2 per 100 (1 to 6) | RR 1.08 (0.41 to 2.87) | 4 RCTs (763) | 10 cohort studies (10,165) | ⊕⊕⊝⊝ low1,3,7,8 |
| Abnormal dreams | 3 per 100 | 31 per 100 (11 to 87) | RR 10.49 (3.79 to 29.10) | 4 cohort studies (2588) | 1 RCT, 1 cohort study (123; 688) | ⊕⊝⊝⊝ very low2,6,9,10 |
| Insomnia | 3 per 100 | 12 per 100 (4 to 43) | RR 4.14 (1.19 to 14.44) | 4 cohort studies (3212) | 1 RCT, 2 cohort studies (123; 355,627) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Anxiety | 1 per 100 | 18 per 100 (9 to 35) | RR 18.04 (9.32 to 34.93) | 3 cohort studies (2559) | 2 cohort studies (355,627) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Depressed mood | 1 per 100 | 11 per 100 (5 to 25) | RR 11.43 (5.21 to 25.07) | 2 cohort studies (2445) | 3 cohort studies (430,006) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Abnormal thoughts or perceptions | 0 per 100 | 3 per 100 (0 to 24) | RR 6.60 (0.92 to 47.20) | 2 cohort studies (2445) | 2 cohort studies (376,024) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Nausea | 8 per 100 | 3 per 100 (2 to 4) | RR 0.37 (0.30 to 0.45) | 5 cohort studies (2683) | 1 RCT, 1 cohort study (123; 668) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Vomiting | 5 per 100 | 1 per 100 (1 to 1) | RR 0.18 (0.12 to 0.27) | 4 cohort studies (5071) | 1 RCT (123) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Abdominal pain | 15 per 100 | 5 per 100 (1 to 16) | RR 0.30 (0.09 to 1.07) | 3 cohort studies (2536) | 1 RCT, 1 cohort (123; 668) | ⊕⊝⊝⊝ very low6,7,9,11 |
| Diarrhoea | 5 per 100 | 1 per 100 (1 to 4) | RR 0.28 (0.11 to 0.73) | 5 cohort studies (5104) | 2 RCTs; 1 cohort study (376; 668) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Dyspepsia | 14 per 100 | 4 per 100 (1 to 10) | RR 0.26 (0.09 to 0.74) | 5 cohort studies (5104) | — | ⊕⊝⊝⊝ low2,3,6,10 |
| Headache | 2 per 100 | 2 per 100 (1 to 6) | RR 1.21 (0.50 to 2.92) | 5 cohort studies (3320) | 1 RCT, 1 cohort study (123; 688) | ⊕⊝⊝⊝ very low3,6,7,11 |
| Dizziness | 1 per 100 | 3 per 100 (1 to 14) | RR 3.49 (0.88 to 13.75) | 5 cohort studies (2633) | 1 RCT, 2 cohort studies (123; 355,627) | ⊕⊝⊝⊝ very low3,6,7,11 |
| Visual impairment | 3 per 100 | 7 per 100 (4 to 12) | RR 2.37 (1.41 to 3.99) | 2 cohort studies (1875) | — | ⊕⊝⊝⊝ very low2,6,7,9 |
| Pruritis | 3 per 100 | 2 per 100 (1 to 3) | RR 0.52 (0.30 to 0.91) | 2 cohort studies (1794) | 1 cohort study (688) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Photosensitivity | 19 per 100 | 2 per 100 (1 to 2) | RR 0.08 (0.05 to 0.11) | 2 cohort studies (1875) | 1 cohort study (688) | ⊕⊝⊝⊝ very low2,6,9,10 |
| Vaginal thrush | 16 per 100 | 2 per 100 (1 to 3) | RR 0.10 (0.06 to 0.16) | 1 cohort study (1761) | 1 cohort study (354) | ⊕⊝⊝⊝ very low2,6,9,10 |
| *The assumed risk is the median control group risk across cohort studies unless stated in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Where the control group risk was 0, we used a value of 0.5 to calculate the corresponding risk in the intervention group. Where no RCTs including short‐term travellers reported on our prespecified adverse outcomes, we included information from cohort studies as our primary analysis. 'Summary of findings' tables are usually limited to seven outcomes. For adverse effects this problematic, as there are many, and to include some and not others risks selective reporting. We have therefore included all prespecified outcomes in the table. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: none of the RCTs adequately described methods of random sequence generation or allocation concealment, However, given that so few events occurred in these trials, it is unlikely to have introduced bias. | ||||||
Background
Description of the condition
Malaria is a parasitic protozoal infection which is usually transmitted through the bite of female Anopheles mosquitoes (Warrell 2002). It is most common in tropical and subtropical regions. Clinical disease is caused by infection of red blood cells by one of four Plasmodium species: P. falciparum, P. vivax,P. ovale, andP. malariae (WHO 2017). Humans can also become infected by forms of malaria that usually infect animals, such asP. knowlesi (WHO 2017). Clinical presentation is nonspecific and varied; symptoms include fever, chills, headache, diarrhoea, muscle cramps, and abdominal pain (WHO 2015). Severe disease is usually caused by infection with P. falciparum, but can also occur following infection with P. vivax and P. knowlesi. Host factors determining severity include genetics, host immune status, and age (WHO 2015).
The true global incidence and prevalence of malaria is difficult to determine; the highest disease burden occurs in sub‐Saharan Africa where vital registration and disease notification systems are weak (Murray 2014). However, the latest World Health Organization (WHO) figures estimate 212 million new cases of malaria in 2015 leading to 429,000 deaths (WHO 2016). Around 125 million travellers visit malaria‐endemic areas annually, and all need to take steps to prevent infection with malaria (Croft 2005). Each year there are between 10,000 and 30,000 known cases of malaria in returned travellers, but the real figure is likely to be higher due to under‐reporting (WHO 2017).
The individual risk of acquiring malaria is determined by the host immune status, the area travelled to, the duration of travel and season, and the use of prevention measures. Pregnant women, young children and non‐immune travellers are particularly vulnerable to severe disease if they become infected (WHO 2015). In Europe, the incidence of malaria is higher in people who travel to their country of origin to visit friends and relatives than in tourists (Behrens 2015). However, mortality is higher in tourists (Behrens 2015).
The natural life cycle of malaria involves the consecutive infection of two hosts: female Anopheles mosquitoes and humans (CDC 2015a). The female mosquito acquires the disease when taking a blood meal from an infected human host. It will then become infectious over a period of 10 to 14 days depending on the region. Sporozoites are injected into the human host the next time the mosquito feeds. These travel via the blood stream to the liver and develop into schizonts which then rupture releasing merozoites. Merozoites invade erythrocytes and undergo asexual replication. Some of these develop through ring stage trophozoites into schizonts which rupture releasing further merozoites and thus perpetuate the infection. Others will develop into female and male gametocytes which are ingested by Anopheles mosquitoes during a blood meal leading to the spread of disease.
Description of the intervention
Mefloquine has been available for use in Europe since 1985 and the USA since 1990 (Schlagenhauf 1999). Alongside atovaquone‐proguanil and doxycycline, it is considered standard chemoprophylaxis by many international health guidelines (CDC 2015b; PHAC 2014; PHE 2015; WHO 2017).
Mefloquine belongs to the aryl amino acid group of antimalarial agents. Mefloquine has a long half life and is given as a weekly dose of 250 mg when used for prophylaxis in adults (Schlagenhauf 2010). Mefloquine is effective against all five strains of malaria known to affect humans. Although guidelines vary, many state that mefloquine should be taken for two to three weeks before travel and continued for four weeks following return (WHO 2017).
There are several situations in which mefloquine is potentially advantageous. All guidelines recommend that where avoidable pregnant women should not travel to areas where malaria is endemic (WHO 2017). However, where travel is essential, mefloquine is often the preferred option. Mefloquine is widely considered to be safe within the second and third trimesters of pregnancy and guidelines increasingly recommend its use in the first trimester (CDC 2015b; Schlagenhauf 2010). Mefloquine is suitable for both children who weigh more than 5 kg and breastfeeding mothers (Schlagenhauf 2010).
Doxycycline has restrictions on its use during pregnancy due to effects on skeletal development found in animal studies. The use of atovaquone‐proguanil is limited by a lack of evidence for safety (PHE 2015). Chloroquine‐proguanil is considered safe for pregnant women, but its use is limited by widespread resistance (PHAC 2014).
The main side effects of mefloquine are gastrointestinal, neurological and psychological. Psychological side effects vary from those considered to be very common (including insomnia and abnormal dreaming) to those with unknown frequency (including psychosis and suicidal ideation) (eMC 2015a). Existing drug labels suggest that these side effects are both prodromal and dose related (eMC 2015a).
How the intervention might work
Malaria chemoprophylaxis is defined as the use of antimalarial medication to prevent the clinical symptoms of malaria (Schlagenhauf 2010). This is because no drugs are able to prevent the introduction of infection by destroying the sporozoites injected by the female Anopheles mosquito. Chemoprophylaxis is one of several tools used to prevent malaria; other recommended measures include sleeping under insecticide‐treated bed nets, wearing insecticide‐treated clothing, and applying chemical repellent sprays to the skin surface (WHO 2017). None of these methods provide complete protection and a combination of approaches is advised.
Chemoprophylaxis works by blocking the development or reproduction of the malaria parasite at various stages in its life cycle:
-
doxycycline and mefloquine are examples of suppressive prophylactics and act in the blood stream as the schizonts invade erythrocytes. Doxycycline therefore needs to be taken for at least one month after returning from endemic areas (Shanks 2005);
-
atovaquone‐proguanil and primaquine have effects on the early liver stages of Plasmodium spp and prevent the progression to blood stage parasites which cause clinical illness. These agents therefore only need to be taken for one week after leaving the malaria‐endemic area (Shanks 2005).
Currently, the baseline efficacy of doxycycline, atovaquone‐proguanil and mefloquine when used as prophylaxis to prevent malaria is thought to be similar. Most guidelines therefore recommend selecting appropriate antimalarial prophylaxis based on individual choice, pre‐existing conditions, side effect profile, and drug resistance patterns in the destination country (CDC 2015b; PHE 2015; WHO 2017). Drug resistance to all antimalarial agents is a growing concern, and mefloquine resistance has been reported in some areas of north‐western Thailand (Treiber 2010; Treiber 2011).
In addition, the efficacy of all forms of malaria prevention is impeded by adherence. Nearly all cases of fatal malaria in travellers occur due to non‐adherence with prophylactic measures (Schlagenhauf 2010). However, this needs to be balanced against the tolerability and safety of chemoprophylaxis; the frequency of mild to moderate adverse drug reactions varies from 32% to 45% (Schlagenhauf 2003). Both policy makers and individual travellers need to balance carefully the risk benefit profile of contracting malaria against using chemoprophylaxis.
Why it is important to do this review
Mefloquine has long been associated with neurological and psychological side effects which range from mild headaches and dizziness to reports of suicide and psychosis. The frequency and severity of these outcomes has been debated. In 2013 the USA Food and Drug Administration (FDA) released a safety communication regarding potential long‐term and significant neurological and psychiatric side effects of mefloquine (FDA 2013). This included the addition of a boxed warning to the drug label, the most serious form of warning that can be issued. Similarly in Europe in 2014 the European Medicine Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) required a change to the summary of product characteristics noting that "...in a small number of patients it has been reported that neuropsychiatric reactions (for example, depression, dizziness or vertigo and loss of balance) may persist for months or longer, even after discontinuation of the drug" (EMA 2014). This has been incorporated into summaries of product characteristics throughout Europe. Most recently the UK Defence Committee has suggested mefloquine should only be used as a drug of last resort (UK Parliament 2016).
Previous reviews on this topic have limited analyses to randomized controlled trials (RCTs) (Jacquerioz 2009; Jacquerioz 2015). However, RCTs are not always the optimal study design to determine the type, prevalence or nature of adverse events and adverse effects, and many set inclusion criteria which exclude groups of people who are likely to be affected (Loke 2007). In addition, adverse effects are often the primary outcome measure of non‐randomized trials, meaning that researchers may attempt to capture and define adverse events in a more rigorous manner than when they are a tertiary measure (Loke 2011).
This Cochrane Review update broadened study inclusion criteria to include non‐randomized studies that provide useful information regarding the side effect profile of mefloquine.
This review did not address:
-
the efficacy or safety of alternative forms of malaria chemoprophylaxis;
-
the use by pregnant women of mefloquine as intermittent presumptive treatment of malaria, or;
-
the use by travellers of emergency standby malaria treatment.
This new edition replaces the Cochrane Review on mefloquine for preventing malaria in non‐immune adult travellers (Jacquerioz 2015). Malaria prophylaxis in children living in endemic areas, chemoprophylaxis in pregnant women, and malaria prevention in people with sickle cell disease have been assessed in other Cochrane Reviews (Meremikwu 2008; Oniyangi 2006; Radeva‐Petrova 2014).
Objectives
To summarize the efficacy and safety of mefloquine used as prophylaxis for malaria in travellers.
Methods
Criteria for considering studies for this review
Types of studies
For efficacy we included randomized and quasi‐randomized controlled trials, including cluster‐randomized trials.
For safety we also included non‐randomized controlled trials/cohort studies. We included both prospective and retrospective cohort studies, but excluded studies where recruitment was linked to the occurrence of specific adverse events.
A list of study design features for all included studies is included in Appendix 1.
Types of participants
Adults and children, including pregnant women.
Types of interventions
Intervention
Mefloquine at a prophylactic dose (for example, 250 mg once weekly in adults and equivalent dosing for children).
Control
Placebo, no intervention or an alternative malaria chemoprophylaxis agent in current use.
Types of outcome measures
Efficacy
Clinical cases of malaria.
Safety
-
Adverse effects of any severity: defined as "an adverse event for which the causal relation between the intervention and the event is at least a reasonable possibility" (Loke 2011);
-
serious adverse effects are those "leading to death, [which] are life threatening, require inpatient hospitalization or prolongation of existing hospitalization, or result in persistent or significant disability or incapacity, or is a congenital anomaly/birth defect" (ICH 1994);
-
adverse events of any severity: defined as “any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment” (WHO‐ART 2008);
-
serious adverse events are those "leading to death, [which] are life threatening, require inpatient hospitalization or prolongation of existing hospitalization, or result in persistent or significant disability or incapacity, or is a congenital anomaly/birth defect." (ICH 1994);
-
discontinuations of study drug due to adverse effects;
-
measures of adherence to the drug regimen.
Pregnancy‐related outcomes:
-
adverse pregnancy outcomes: spontaneous abortions, stillbirths, congenital malformations.
Study authors often use the terms 'adverse event', 'adverse effect' or 'side effect' interchangeably and loosely. Where possible, we used the definitions described above to distinguish adverse events and adverse effects. Adverse effects encompasses reporting by study authors of 'adverse effects', 'side effects', 'adverse events attributed to the study drug', 'adverse reactions', and 'symptoms related to the study drugs'.
Search methods for identification of studies
We attempted to find all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 2:
-
Cochrane Infectious Diseases Group Specialized Register to 22 June 2017;
-
Central Register of Controlled Trials (CENTRAL), published on the Cochrane Library to 22 June 2017;
-
MEDLINE (PubMed) from 1966 to 22 June 2017;
-
Embase (Ovid) from 1974 to 22 June 2017; and
-
LILACS (Bireme) from 1982 to 22 June 2017.
We also searched the WHO International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov (https://clinicaltrials.gov/) for trials in progress, using 'mefloquine', 'Lariam', and 'malaria' as search terms (22 June 2017).
For the safety analysis we also searched MEDLINE (PubMed) (1966 to 22 June 2017), Embase (Ovid) (1974 to 22 June 2017), and TOXLINE (1980 to 22 June 2017) (https://toxnet.nlm.nih.gov/newtoxnet/toxline.htm). The following MEDLINE terms were adapted as needed: ("Mefloquine/adverse effects"[Mesh] OR "Mefloquine/poisoning"[Mesh] OR "Mefloquine/toxicity"[Mesh] ); Mefloquine ti, ab AND (safety OR tolerability OR death*OR suicid* OR adverse OR reaction* OR “side effect*”) ti, ab.
Searching other resources
We checked the reference lists of included studies for any references not identified by our searches.
Data collection and analysis
Selection of studies
Two review authors independently screened the results of the literature search for potentially relevant trials using Covidence software (Covidence 2017), and looked for multiple publications from the same data set. Full text copies were retrieved for all trials deemed potentially relevant for inclusion.
Two review authors then independently assessed all identified trials for inclusion in the review using the prespecified inclusion criteria. Any disagreements were resolved through discussion.
Data extraction and management
Two review authors independently extracted data using a standardized and pre‐piloted data collection form. When available we extracted data on:
-
details of study: start and end dates, setting (country of recruitment and country of malaria exposure), study design, method of participant recruitment and selection, number of participants enrolled, number of participants for whom data was available, mean duration of exposure to malaria, antimalarial resistance pattern of mefloquine and the comparator;
-
study participants: inclusion and exclusion criteria, age, gender, body mass index (BMI), pregnancy status, risk factors (for malaria and for adverse outcomes), immune or non‐immune participants, military or non military;
-
details of the intervention: drug dose during prophylaxis, use of a loading dose, duration of drug therapy before and after travel, frequency of drug administration and use of any co‐interventions;
-
outcomes measured and reported including definition, method of detection, timing in relation to treatment, duration and frequency of monitoring.
We resolved any disagreements through discussion, and where necessary we consulted a third review author. If clarification was necessary, we attempted to contact the trial authors for further information.
For dichotomous data, we recorded the number of participants experiencing the event and the number analysed in each group. For continuous outcome data, we extracted arithmetic means and standard deviations for each group together with the numbers analysed in each group. We also extract medians and ranges where provided.
We extracted details of all serious adverse events and effects. For non‐serious adverse events and effects we sought information on the following specific symptoms and groups of symptoms which are frequently associated with mefloquine, doxycycline or atovaquone‐proguanil:
-
ear and labyrinth disorders: vertigo;
-
eye disorders: visual impairment;
-
gastrointestinal disorders: nausea, vomiting, abdominal pain, diarrhoea, dyspepsia;
-
nervous system disorders: dizziness and headaches;
-
psychiatric disorders: abnormal dreams, insomnia, anxiety, depression, psychosis; and
-
skin and subcutaneous tissue disorders: pruritis, photosensitivity, vaginal candida.
We also reported data on all other very common (> 1/10) and common (> 1/100 to < 1/10) adverse events and adverse effects, as defined by the electronic Medicines Compendium (eMC 2015b).
Where possible we attempted to derive absolute estimates of adverse outcomes (events or effects). For all adverse outcomes, we included only the denominator trials that actively reported the presence or absence of each specific adverse event or effect.
Most RCTs and cohort studies collected data on self‐reported or clinician‐assessed symptoms rather than formal medical diagnoses. Therefore, we reported outcomes as symptoms. For example, we reported on 'depressed mood' rather than 'depression'.
When deciding which relative effect measure to present in 'Summary of findings' tables, we considered which meta‐analysis most closely answered our PICO (population, intervention, comparator, outcome/s) question. We created a decision tree in advance to assess the directness of a group of studies in relation to: the population studied (short‐term international travellers versus other populations), outcomes measured (adverse effects versus adverse events), and study design (RCTs versus cohort studies). The intervention and comparator were fixed in each drug‐pair comparison. Other less direct meta‐analyses were used in our appraisal of the certainty of the evidence. The decision tree used is provided in Appendix 3.
Conventionally, 'Summary of findings' tables include up to seven outcomes. However, the key questions for clinical decision making relate to adverse effects, and therefore limiting the number of outcomes a priori was problematic, as we could not know in advance which adverse effects mefloquine would have. To constrain the number of outcomes in the 'Summary of findings' tables to seven would mean only reporting outcomes where effects were shown, which would lead to selective reporting.
We included 'Summary of findings' tables for comparisons of mefloquine with doxycycline and atovaquone‐proguanil. This decision was made because chloroquine is used less frequently than mefloquine, doxycyline and atovaquone‐proguanil. As reported in Results, the adverse effect profile of mefloquine in comparison to chloroquine was consistent with comparisons with doxycycline and atovaquone‐proguanil.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study. For randomized and quasi‐randomized controlled trials we used Cochrane's 'Risk of bias' tool (Higgins 2011). We followed the guidance for making judgements on the risk of bias in five domains: sequence generation; allocation concealment; blinding (of participants, personnel and outcome assessors); incomplete outcome data; selective outcome reporting and other risk of bias. We categorized these judgements as low risk of bias, high risk of bias, or unclear risk of bias.
For non‐randomized (cohort) studies we assessed the risk of bias using the Cochrane Risk of Bias Assessment Tool for Non‐Randomized Studies of Interventions (now referred to as ROBINS‐I) (ACROBAT‐NSRI tool). We followed the guidance for making judgements on the risk of bias in eight domains: confounding, selection of participants into the study, measurement of interventions, departures from intended interventions, missing data, selection of the reported result and other risk of bias. We categorized these judgements as low risk of bias, moderate risk of bias, serious risk of bias and critical risk of bias. Where no information was provided on a category, this was stated. The criteria we used to make specific judgements are provided in Table 1.
| Bias | Authors' judgement | Support for judgement |
| Confounding | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: identified confounders were measured and were balanced across groups (age, sex, destination and duration of travel) Moderate risk: identified confounders were measured and not balanced across groups, or several confounders had not been measured or not reported across groups Serious risk: a critical confounder has been measured and is not balanced across groups |
| Selection of participants into the study | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether selection into the study was unrelated to intervention or unrelated to outcome, and whether start of intervention and start of follow up coincided for most subjects. Non‐responder bias at the point of selection was considered here for cohort studies. We used the following cut offs for non‐response rate: low risk < 10%, moderate risk 10% to 20%, serious risk > 20%. |
| Measurement of interventions | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: the prescription was provided by a travel clinic which also performed the study, and discontinuations were recorded and reported, or all participants were issued with their medication e.g. soldiers or participants were asked to self‐report which medication they took whilst they were taking it. Moderate risk: the prescription was provided by a travel clinic which also performed the study but no information regarding switches and discontinuations was available or patients are asked to self‐report which prophylaxis they took shortly after they finished taking it. Serious risk: Participants were asked to self‐report which prophylaxis they took a long time after they finished taking it. |
| Departures from intended interventions | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether switches between interventions of interest were available. We assessed whether discontinuations and switches between prophylactic regimens had been recorded and reported. |
| Missing data | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether outcome data was reasonably complete for most participants. We recorded missing data for included participants e.g. loss to follow up rates and treatment withdrawals. |
| Measurement of outcomes | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether the outcome measure was objective or subjective. We assessed whether participants or study personnel were blinded to the intervention received. We assessed whether the methods of outcome assessment were comparable across intervention groups. |
| Selection of the reported result | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: If the questionnaire was provided in full, or it was clear what was asked within it. Moderate risk: If it is unclear which questions are asked, or information was provided on aggregate. Serious risk: If data captured within the questionnaire was clearly missing. |
| Other | Low risk Moderate risk Serious risk Critical risk No information | We reported the study sponsor. We classified the analysis of studies sponsored by pharmaceutical companies as independent of the sponsor when it was clearly stated that the sponsor had no input to the trial analysis. |
Adapted from Higgins 2011 and ACROBAT‐NSRI tool
For adverse events and adverse effects, we assessed the risk of bias in the conduct of the study by examining whether harms were predefined using standardized or precise definitions, ascertainment methods were adequately described, monitoring was active or passive and data collection was prospective or retrospective (Table 2). For laboratory tests and other investigations we assessed whether the number and timing of the tests was adequate.
| Criterion | Assessment | Explanation |
| On conduct | ||
| Were harms pre‐defined using standardised or precise definitions? | Adequate Inadequate Unclear | We classified as 'adequate' if the study reported explicit definitions for adverse events and effects that allow for reproducible ascertainment e.g. what adverse events were being investigated and what constituted an “event”, what was defined as a serious or severe adverse event. |
| Was ascertainment technique adequately described? | Adequate Inadequate Unclear | We classified as 'adequate' if the study reported methods used to ascertain complications, including who ascertained, timing, and methods used. |
| Was monitoring active or passive? | Active Passive Unclear | We classified monitoring as 'active' when authors reviewed participants at set time points during treatment and enquired about symptoms. |
| Was data collection prospective or retrospective? | Prospective Retrospective Unclear | We classified as ‘prospective’ if data collection occurred during treatment, or ‘retrospective’ if data collection occurred following treatment. |
| For laboratory investigations or other tests | ||
| Was the number and timing of tests adequate? | Adequate Inadequate Unclear | We classified the number and timing of tests as 'adequate', when tests were taken at baseline and at least one time point during prophylaxis. |
Adapted from Bukiwra 2014
We resolved any disagreement through discussion, and where necessary, we consulted a third review author.
Measures of treatment effect
We analysed data using Review Manager 5 (RevMan 5) (RevMan 2014) and combined dichotomous data using risk ratios (RR). For continuous data summarized by arithmetic means and standard deviations, we combined data using mean differences (MD). We present RRs and MD with 95% confidence intervals (CI) and report medians and ranges in tables for non‐RCTs.
Unit of analysis issues
When trials included more than two comparison groups, we split the trial for analysis as individual pair‐wise comparisons. If more than one comparison group was included in a meta‐analysis, we ensured that participants were only counted once by dividing the cases and participants evenly between the comparisons.
For clinical cases of malaria, we included participants as the unit of analysis, such that each participant was counted once in the intervention or placebo arm. Where study reporting was unclear regarding the unit of analysis (that is, total clinical cases of malaria rather than clinical cases in each participant) we noted this in footnotes and performed a sensitivity analysis excluding these results.
Dealing with missing data
If data from trial reports were insufficient, unclear, or missing, we attempted to contact the trial authors for additional information.
Our primary analysis was a complete‐case analysis which excluded all participants without treatment outcomes. No imputation measures for missing data were applied.
Where studies had grouped symptoms together by body system when reporting safety outcomes, we contacted authors to obtain disaggregated data. We obtained two additional full data sets (Cunningham 2014; Korhonen 2007) and received further clarification from two study authors (Kato 2013; Sonmez 2005). The full details of subsequent analyses are provided in the characteristics of included studies tables.
Assessment of heterogeneity
We assessed heterogeneity among trials by inspecting forest plots for overlapping CIs, applying the Chi² test with a 10% level of statistical significance, and using the I² statistic with a value of 50% to denote moderate levels of heterogeneity.
Assessment of reporting biases
We were unable to assess publication bias using funnel plots because there were too few trials reporting the same outcomes.
Data synthesis
We carried out statistical analyses using RevMan 5 (RevMan 2014). We analysed randomized controlled trials (RCTs) and non‐RCTs separately, and compared interventions as individual pair‐wise comparisons.
In the absence of heterogeneity, we used a fixed‐effect model. Where we identified moderate heterogeneity, and it was appropriate to combine data, we used the random‐effects model. When it was not appropriate to combine data in a meta‐analysis, we tabulated data and reported outcomes as a narrative.
We report the term used for each adverse event in each trial. Where trials used different terminology for similar adverse events and adverse effects, we coded them using the preferred term based on Medical Dictionary for Regulatory Activities (MedDRA) terminology (for example, sleepiness, somnolence) and analysed these together (MedDRA 2016).
Subgroup analysis and investigation of heterogeneity
We explored possible sources of heterogeneity using subgroup analyses (study design, military versus non‐military participants, short‐ versus long‐duration of travel).
Sensitivity analysis
We conducted sensitivity analyses to evaluate the robustness of the results to the risk of bias components, by excluding studies at high or unclear risk of bias.
Results
Description of studies
Results of the search
Searches (conducted 22 June 2017) identified 2155 records; we screened seven additional studies after reviewing reference lists. Of these, we excluded 1953 after assessing titles and abstracts. We retrieved 209 full text publications to assess for inclusion.
Included studies
We included 20 randomized controlled trials (RCTs) (11,470 participants), 35 cohort studies (190,286 participants) and four large retrospective analyses of health records (800,652 participants).
Efficacy outcomes were reported in 14 RCTs conducted between 1977 and 2003 in Thailand (four trials), Brazil, Cambodia, Ghana, Indonesia, Ivory Coast, Malawi, Nigeria, Kenya and two studies which included travellers to various destinations (10,710 participants). Two were conducted in short‐term international travellers (Overbosch 2001; Schlagenhauf 2003); nine involved general populations living in endemic areas who are likely to have some immunity to malaria (Boudreau 1991; Bunnag 1992; Hale 2003; Nosten 1994; Pearlman 1980; Salako 1992; Sossouhounto 1995; Steketee 1996; Weiss 1995), two recruited non‐immune military personnel (Arthur 1990; Ohrt 1997), and one recruited a mixed military and civilian semi‐immune population (Santos 1993).
All 20 included RCTs and 35 cohort studies reported safety outcomes. Nine RCTs explicitly excluded participants with a psychiatric history, and 25 cohort studies stated that the choice of antimalarial agent was based on medical history and personal preference. Most RCTs and cohort studies collected data on self‐reported or clinician‐assessed 'symptoms', rather than formal medical diagnoses. Consequently, when describing these data we used non‐medical descriptions such as 'depressed mood' rather than 'depression', even where the trial authors described the symptom as depression. However, four retrospective cohort studies analysed healthcare records (Eick‐Cost 2017; Meier 2004; Schneider 2013; Wells 2006) and looked for people with formal mental health diagnoses. Where outcomes were presented grouped by organ system, we approached study authors for additional data and received full data sets for two studies (Cunningham 2014; Korhonen 2007) and additional information from another two (Kato 2013; Sonmez 2005).
Three RCTs (1827 participants) and 24 cohort studies (170,487 participants) included short‐term international travellers. Five cohort studies included long‐term occupational travellers (UK Foreign and Commonwealth Office Staff and Peace Corps volunteers) (13,211 participants); four RCTs (961 participants) and six cohort studies (6588 participants) included military personnel (including 1 study with a mixed military and civilian population). Thirteen RCTs included local residents who did not travel outside their home countries: Australia (Davis 1996), Ghana (Hale 2003), Israel (Potasman 2002), Ivory Coast (Sossouhounto 1995), Kenya (Weiss 1995), Malawi (Steketee 1996), the Netherlands (Vuurman 1996), Nigeria (Salako 1992), Switzerland (Schlagenhauf 1997) and Thailand (Boudreau 1991, Bunnag 1992, Nosten 1994, Pearlman 1980).
Seven RCTs and three cohort studies were sponsored by Roche (manufacturer of mefloquine), three RCTs and one cohort study were sponsored by GlaxoSmithKline (manufacturer of atovaquone‐proguanil), one RCT was sponsored by Pfizer (manufacturer of doxycycline), and one by Mepha Ltd (manufacturer of a film‐coated form of mefloquine). Only one RCT and one cohort study reported whether the study sponsor had any influence over collecting, analysis or interpretation of study results or the decision to publish.
Excluded studies
We excluded 141 studies after full‐text screening (Figure 1). We excluded 37 studies because they were not research studies; 29 studies reported no relevant outcomes; 23 studies were single arm cohort studies and did not meet our inclusion criteria; 17 studies compared mefloquine with a regime which is not routinely used; 11 studies were not a randomized or cohort study (for example, case report or case‐control study); in seven studies mefloquine was not used at a prophylactic dose, for example, treatment dose; seven studies were multiple publications from the same data set as included studies; four cohort studies the population was identified on the basis of having experienced adverse effects and we excluded 6 studies for other reasons. We have provided full details in the 'Characteristics of excluded studies' tables.

Study flow diagram.
Risk of bias in included studies
We performed 'Risk of bias' assessments for the included RCTs using the Cochrane 'Risk of bias' assessment tool. We assessed the risk of bias in the cohort studies using the ACROBAT‐NSRI tool (now referred to as ROBINS‐I). For a summary of the 'Risk of bias' assessments for RCTs see Figure 2.
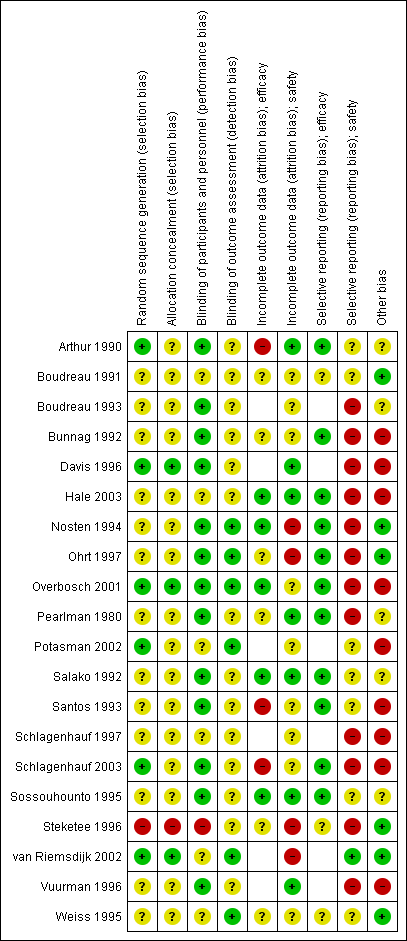
'Risk of bias' summary for RCTs: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Three trials were at low risk of selection bias, with adequate descriptions of generation of the random sequence and allocation concealment (Davis 1996; Overbosch 2001; van Riemsdijk 2002). A further 16 trials were at unclear risk of selection bias due to providing insufficient information regarding their methodology. One trial described sequential allocation of unblinded participants (Steketee 1996).
Blinding
Seven trials adequately described blinding of study personnel, including blinding of pathology technicians when detecting malaria, and blinding of outcome assessors when assessing safety outcomes (Nosten 1994; Ohrt 1997; Overbosch 2001; Potasman 2002; Schlagenhauf 2003; van Riemsdijk 2002; Weiss 1995). The remaining 13 trials did not adequately describe how outcome assessors were blinded.
Incomplete outcome data
Six trials had low and balanced losses to follow‐up rates for efficacy outcomes (Hale 2003; Nosten 1994; Overbosch 2001; Salako 1992; Sossouhounto 1995; Weiss 1995). One trial was at high risk of bias because investigators did not follow up participants beyond the active phase of treatment for relapses (Santos 1993). Two studies did not make the method of detection of malaria, frequency or duration of follow up clear (Arthur 1990; Schlagenhauf 2003).
Seven trials had low losses to follow‐up rates for adverse outcomes (Arthur 1990; Davis 1996; Hale 2003; Pearlman 1980; Salako 1992; Sossouhounto 1995; Weiss 1995). We judged four of the trials to be at high risk of bias because investigators did not provide numbers of participants lost to follow up across groups (Nosten 1994; Steketee 1996); did not assess all participants who received the study drug in the final analysis (Ohrt 1997); and because the proportion of participants who did not complete the study due to adverse outcomes varied significantly between groups (van Riemsdijk 2002).
Selective reporting
Fourteen trials reported on efficacy outcomes, and twelve of these appropriately reported all outcomes.
However, 21 trials reported on our safety outcomes and only nine of these appropriately reported on all pre‐specified outcomes. Three of these trials only reported on statistically significant differences between groups (Boudreau 1993; Pearlman 1980; Schlagenhauf 1997), and another four did not report data from all time points (Bunnag 1992; Nosten 1994; Ohrt 1997; Overbosch 2001). Two trials reported aggregate data across multiple time points (Schlagenhauf 2003; Steketee 1996), one trial only reports symptoms which occurred in > 10% of participants in each study arm (Davis 1996). Vuurman 1996 only reported events which occurred more than once and Hale 2003 reports the total number of serious adverse events does not allocate them to a drug regimen.
Other potential sources of bias
Seven trials were sponsored by Roche (manufacturer of mefloquine) (Bunnag 1992; Davis 1996; Ohrt 1997; Santos 1993; Schlagenhauf 1997; Schlagenhauf 2003; Vuurman 1996), three were sponsored by GlaxoSmithKline (manufacturer of atovaquone‐proguanil) (Hale 2003; Overbosch 2001; Schlagenhauf 2003), one by Pfizer (manufacturer of doxycycline) (Ohrt 1997), and one by Mepha Ltd (manufacturer of a film‐coated form of mefloquine) (Potasman 2002). Only one made the role of the study sponsor clear (Ohrt 1997).
We have presented details of the risk of bias of cohort studies in the 'Effects of interventions' section.
Effects of interventions
See: Summary of findings for the main comparison Mefloquine versus atovaquone‐proguanil for preventing malaria in travellers; Summary of findings 2 Mefloquine versus doxycycline for preventing malaria in travellers
Comparison 1: Mefloquine versus placebo or no treatment
Description of studies
RCTs
Nine RCTs comparing prophylactic mefloquine with placebo reported efficacy (4032 participants, Table 3), and 13 reported safety outcomes (4293 participants, Table 4). The trials were conducted between 1977 and 2003, and none included participants travelling outside their home country. One trial conducted among soldiers in Indonesia described participants as non‐immune (Ohrt 1997), but immunity is likely to be low in other trials from Asia (Bunnag 1992; Nosten 1994; Pearlman 1980). The participants in four trials from Africa were described as semi‐immune (Hale 2003; Salako 1992; Sossouhounto 1995; Weiss 1995). Santos 1993 was conducted in an area of Brazil in which endemic transmission occurs.
| Study ID | Participants (immune status) | Number of randomised participants | Mefloquine dose | Drug comparisons of interest | Duration of exposure to malaria | Country of malaria exposure | Local drug resistance |
| Thai male adults (presumed semi‐immune) | 605 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo | 24 weeks (trial duration) | Thailand | Chloroquine, sulphadoxine‐pyrimethamine and quinine resistance | |
| Pregnant women from the Thai‐Burma border (presumed semi‐immune) | 339 | 250 mg weekly for first 4 weeks, then 125 mg weekly until delivery | Placebo | Various in endemic area (monitored until delivery) | Thai‐Burma border | Not mentioned | |
| Thai residents aged 10 to 60 years (semi‐immune) | 990 | 180 mg tablet weekly, 360 mg tablet weekly, 360 mg every 2 weeks with appropriate adjustments for children | Placebo | 26 weeks | Thailand | Chloroquine resistant Plasmodium falciparum | |
| Brazilian civilians and soldiers aged 12 to 55 years (semi‐immune) | 128 | 500 mg every 4 weeks, 250mg every 2 weeks | Placebo | 17 weeks | Brazil | P falciparum resistant to chloroquine and “high prevalence of multiresistant Plasmodium falciparum transmission” | |
| Ivory Coast adult males (semi‐immune) | 500 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo | 20 weeks | Ivory C oast | Not mentioned | |
| Indonesian soldiers ('largely' non‐immune) | 204 | 250 mg weekly | Placebo, doxycycline | 'approximately 13 weeks' | Indonesia | Sulfadoxine‐pyrimethamine and chloroquine resistance | |
| Kenyan children (semi‐immune) | 169 | 125 mg weekly | Placebo (multivitamin), doxycycline, primaquine | 11 weeks | Kenya | Not mentioned | |
| Nigerian adult males (semi‐immune) | 567 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo, chloroquine | 24 weeks (trial duration) | Nigeria | "...at the time of the trial, chloroquine resistance was not a problem" | |
| Ghanain adults (semi‐immune) | 530 | 250 mg weekly | Placebo | 12 weeks | Ghana | Not mentioned | |
| USA soldiers (non‐immune) | 270 | 250 mg weekly | Doxycycline | 8 weeks | Thailand | Local chloroquine resistance | |
| Thai adult males (semi‐immune) | 501 | 500 mg fortnightly | Chloroquine | 14 weeks (trial duration) | Cambodia | Local chloroquine resistance | |
| Pregnant Malawian residents (semi‐immune) | 4220 | 250 mg weekly | Chloroquine | Various in endemic area (monitored until delivery) | Malawi | P falciparum resistant to chloroquine, documented sensitivity of P falciparum to mefloquine |
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Exclusions for psychiatric adverse effects | Trial duration | Source of funding |
| RCTs | ||||||
| Thai male adults | 605 | Interview with study personnel | None | 24 weeks | Roche | |
| Australian adults who did not travel | 106 | Daily self‐reported diary | Past history of psychiatric conditions | 7 weeks | Roche | |
| Ghanain adults | 530 | Interview with study personnel | History of neuropsychiatric illness | 12 weeks | USA Army | |
| Pregnant women, Thai‐Burma border | 339 | Phase 1: weekly symptom questionnaire. Babies were assessed at birth and at 3, 6, 12, and 24 months. Phase 2: weekly symptom questionnaire. Babies were assessed at birth and at 2 and 9 months | None | Various | Government funding | |
| Indonesian soldiers | 204 | Two symptom questionnaires. Daily interview with study personnel | History of underlying illness | 13 weeks | Roche, Pfizer, USA Army | |
| Thai residents aged 10 to 60 years | 990 | Weekly sick call by study personnel | None | 26 weeks | Not mentioned | |
| Israeli adults who did not travel | 90 | Self‐reporting diary | History of depression | 48 hours | Mepha Ltd | |
| Nigerian adult males | 567 | Interview with study personnel | None | 24 weeks | Not mentioned | |
| Brazilian civilians and soldiers aged 12 to 55 | 128 | Interview w ith study personnel | None | 17 weeks | Roche | |
| Swissair trainee pilots who did not travel | 23 | Interview with study personnel | Psychosis or severe depression | 4 weeks | Roche | |
| Ivory C oast adult males | 500 | Access to the village health centre | None | 20 weeks | Not mentioned | |
| Dutch adult who did not travel | 42 | Interview with study personnel | H istory of any serious psychiatric disorder; evidence of drug or alcohol abuse | 30 days | Roche | |
| Kenyan children | 169 | Interview with study personnel | None | 4 months | USA Army | |
| Cohort studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| Danish travellers | 300 | Telephone interview | Allocation based on guidelines and patient preference | Mean 3 weeks, range 1 to 9 weeks | Not mentioned | |
| Danish travellers | 4154 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | Various, not specified | Not mentioned | |
| Swedish travellers | 491 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | " Most", range 2 to 4 weeks | Not mentioned | |
| Danish travellers | 1501 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | Mean = 23 days | Not mentioned | |
| USA soldiers | 397,442 | Restrospective analysis of hospital records | No information available | Minimum 1 month | Government funding | |
Seven trials used mefloquine at a dose of 250 mg weekly (or equivalent doses for children), four at 250 mg weekly for the first four weeks and then 125 mg weekly for the remainder of the study, and one trial used mefloquine doses of 500 mg every four weeks and 250 mg every two weeks (Santos 1993). Pearlman 1980 used mefloquine doses of 180 mg weekly, 360 mg weekly and 360 mg fortnightly. Trial duration varied from 48 hours to 26 weeks.
For safety, nine trials used interviews with study personnel to elicit adverse events (Bunnag 1992; Hale 2003; Nosten 1994; Ohrt 1997; Salako 1992; Santos 1993; Schlagenhauf 1997; Vuurman 1996; Weiss 1995). Of these, six trials questioned participants about symptoms at least weekly (Hale 2003; Nosten 1994; Ohrt 1997; Salako 1992; Vuurman 1996; Weiss 1995). Two trials used participant self‐reported diaries to record any adverse events (Davis 1996, Potasman 2002). Pearlman 1980 used a weekly 'sick call' by study personnel and Sossouhounto 1995 provided 'access to the village health centre'. Only two trials used explicit definitions for adverse events and effects that allow for reproducible ascertainment (Davis 1996, Vuurman 1996). For safety outcomes, nine of the 13 trials adequately described how adverse events were ascertained. Eleven trials actively sought adverse events, and all 13 collected data prospectively (Table 5).
| Study ID | Description of how adverse outcomes were defined and recorded¹ | Description of ascertainment technique² | Active or passive monitoring? | Prospective or retrospective data collection? |
| Inadequate Comment: No definition of adverse events or effects was provided, it is unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate Comment: ‘serious’ adverse events were not defined, and methods for determining causality not described | Adequate | Active | Prospective | |
| Inadequate Comment: It is unclear what questions were included within the questionnaire and whether and how causality was assessed. ‘Serious’ adverse effects not defined | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Inadequate Comment: Weekly sick call for all villagers | Passive | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No information given in the methods section on definition of adverse outcomes | Inadequate Comment: No description of ascertainment method | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it was unclear whether or how causality was assessed | Unclear | Passive | Prospective | |
| Adequate | Unclear | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it was unclear whether or how causality was assessed. | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Unclear 'Filled in after their return' | |
| Adequate | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking their prophylactic regimen | |
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Passive | Retrospective | |
1. Were harms pre‐defined using standardised or precise definitions?
2. Was ascertainment technique adequately described?
Eleven of thirteen which assessed safety outcomes trials did not adequately describe random sequence generation or allocation concealment, and eight did not adequately describe how outcome assessors and study personnel were blinded. We judged eight trials to be at high risk of selective outcome reporting with regard to safety outcomes. In two trials, this was because the overall number of adverse events in each study arm was reported, but not the type or severity (Bunnag 1992; Potasman 2002). Davis 1996 reported only adverse events that occurred in more than 10% of participants in both study arms; Vuurman 1996 reported only adverse events that occurred more than once; and Nosten 1994 only reported on adverse events in the second phase of the trial.
Five trials were funded by Roche (manufacturer of mefloquine) (Bunnag 1992; Davis 1996; Santos 1993; Schlagenhauf 1997; Vuurman 1996) and one by GlaxoSmithKline (manufacturer of atovaquone‐proguanil) (Hale 2003) and one by Mepha Ltd (manufacturer of a film‐coated form of mefloquine) (Potasman 2002).
Cohort studies
Five cohort studies compared mefloquine users with participants who travelled but did not take antimalarial prophylaxis at all (Hoebe 1997; Petersen 2000; Rietz 2002; van Riemsdijk 1997; Wells 2006). Four of these were conducted in travellers, and one in military personnel (Table 4).
Two cohort studies included travellers who were prescribed an antimalarial agent but did not commence using (Hoebe 1997; Petersen 2000) and two asked travellers about an extensive list of general complaints which could have occurred during their journey (Rietz 2002; van Riemsdijk 1997). Wells 2006 was a retrospective healthcare record analysis looking at hospitalizations in active‐duty USA military personnel (397, 442 participants).
Two cohort studies had non‐response rates of over 20%. Wells 2006 was at serious risk for selection of participants and measurement of outcomes because start of follow up began after participants had finished taking mefloquine, authors used surrogate measures for mefloquine exposure and there was a possibility that some participants in the reference groups took mefloquine. Four cohort studies actively sought information from participants about adverse events and only one (van Riemsdijk 1997) obtained information prospectively (see Figure 3).

'Risk of bias' summary in cohort studies: mefloquine versus placebo/no treatment
1Assesses whether our pre‐defined confounders were measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assess the risk of bias due to influence by a corporate study sponsor.
Efficacy
Mefloquine is highly efficacious in reducing clinical cases of malaria compared to placebo, although there were important differences among trials, particularly regarding the dose of mefloquine used, populations studied and the risk of malaria in the control group (Analysis 1.1). The risk of malaria was highest in the trial in military personnel travelling to Indonesia, described as "largely non‐immune", where 53/65 (81%) of those in the placebo group had an episode of malaria compared to 0/67 (0%) with mefloquine (RR 0.01, 95% CI 0.00 to 0.16; Ohrt 1997, 126 participants). In the remaining trials the risk of malaria with placebo ranged from 1% to 59% (Bunnag 1992; Hale 2003; Nosten 1994; Pearlman 1980; Salako 1992; Santos 1993; Sossouhounto 1995; Weiss 1995).
Although quantitative heterogeneity was high, the direction of the effect was consistent across all trials. We performed a series of subgroup analyses by dose and immune status of participants, but this did not explain the heterogeneity or provide a reliable point estimate of efficacy with subgroups.
Five trials also reported the effect on parasitaemia (which was much more common than clinical malaria) (Hale 2003; Nosten 1994; Salako 1992; Sossouhounto 1995; Weiss 1995). Overall, mefloquine reduced numbers of participants who developed parasitaemia by around 80% (RR 0.18, 95% CI 0.06 to 0.55; 3 trials, 414 participants, Analysis 1.2), and substantially reduced the number of episodes of parasitaemia (RR 0.05, 95% CI 0.00 to 5.25; 2 trials, 510 participants, Analysis 1.2).
Safety
Serious adverse events or effects
Only three serious adverse events were reported from six RCTs, none of which were attributed to the drug regimen (1/592 mefloquine users versus 2/629 placebo; 6 trials; 1221 participants, Analysis 1.3). The serious event in the mefloquine user was the death of a pregnant woman who received mefloquine (septic shock after an emergency caesarean section for obstructed labour) (Nosten 1994). For serious pregnancy‐related outcomes, Nosten 1994 reported four congenital malformations in the mefloquine group: limb dysplasia (1 case), ventricular septal defect (2 cases), amniotic bands (1 case) and one in the placebo group: anencephaly. All were considered unrelated to the drug regimen (Table 6).
| Study ID | Study design | Mefloquine users | Drug comparators | |||
| Events/ participants | Description | Drug | Events/ participants | Description | ||
| Events (not attributed by study authors or participants to the drug regimen) | ||||||
| RCT | 0/116 | ‐ | Placebo | 1/121 | None provided | |
| RCT | 1/159 (women) | One death
Four congenital malformations:
| Placebo | 0/152 (women) | One congenital malformation:
| |
| RCT | 0/103 | ‐ | Placebo | 1/96 | One death (not described) | |
| RCT | 0/61 | ‐ | Placebo | 0/65 | ‐ | |
| Doxycycline | 1/62 | Acute hysteria¹ | ||||
| Cohort study | 8/3703 | 8 hospitalisations
| Doxycycline | 0/69 | ‐ | |
| Chloroquine | 0/119 | ‐ | ||||
| RCT | 10/483 | "...infectious illnesses in 7 subjects and breast cancer, anaphylaxis, or fractured femur in 1 subject each" | Atovaquone‐proguanil | 4/493 | "...infectious illnesses in 3 subjects and cerebral ischemia in 1 subject" | |
| Studies reporting no serious events or effects | ||||||
| RCT | 0/107 | "Adverse events were all mild and there were no deaths" | Placebo Chloroquine | 0/101 0/103 | ‐ ‐ | |
| RCT | 0/134 | "No serious side effects occurred with either drug regimen" | Doxycycline | 0/119 | ‐ | |
| RCT | 0/153 | "Although a large number of adverse events were reported, none were serious" | Doxycycline Atovaquone‐proguanil | 0/153 0/164 | ‐ ‐ | |
| Cohort study | 0/228 | "No drug induced side effects necessitating emergency care were observed" | Doxycycline | 0/506 | ‐ | |
| Cohort study | 0/491 | "No serious adverse events were recorded" | Atovaquone‐proguanil | 0/161 | ‐ | |
| Cohort study | 0/548 | Records hospitalisations, and reports that none occurred in either group of participants | Atovaquone‐proguanil Chloroquine | 0/707 0/37 | ‐ ‐ | |
| RCT | 0/103 | "All side effects were transient (and)... mild" | Chloroquine | 0/100 | ‐ | |
1 This trial described a potentially serious adverse event, but did not provide enough detail to meet our definition.
By comparison in cohort studies, seven serious adverse effects (all attributed by study authors to the drug regimen) were reported among 913 mefloquine users, compared to none in 254 travellers who did not use antimalarials (RR 3.08, 95% CI 0.39 to 24.11; 2 studies, 1167 participants; Analysis 1.3; Table 7). Five of these were psychological (depression) and two were neurological adverse effects (dizziness).
| Study ID | Study design | Mefloquine users | Drug comparators | |||
| Events/ participants | Description | Drug | Events/ participants | Description | ||
| Effects (attributed by study authors or participants to the drug regimen) | ||||||
| Cohort study | 2/104 | Two "serious acute adverse reactions"¹
| No treatment | 0/93 | ‐ | |
| Cohort study | 5/809 | 5 hospitalisations:
| Chloroquine | 6/1223 | 2 hospitalisations:
| |
| No treatment | 0/161 | ‐ | ||||
| Cohort study | 15/1612 | 15 hospitalisations:
| Doxycycline | 9/708 | 9 hospitalisations:
| |
| Atovaquone‐proguanil | 0/72 | ‐ | ||||
| Chloroquine | 4/832 | 4 hospitalisations:
| ||||
| Cohortstudy | 4/285 | 3 hospitalisations with "either gastrointestinal or neurologic symptoms" and one seizure | Doxycycline | 1/383 | Severe oesophagitis | |
| RCT | 1/? | One "neuropsychiatric side effect"
| Chloroquine | 0/? | ‐ | |
| Cohort study | 1/115 | One "serious side effect"¹
| Chloroquine | 0/22 | ‐ | |
| Cohort study | 1/609 | One hospitalisation:
| Chloroquine | 0/137 | ‐ | |
| Cohort study | 7/52981 | 7 hospitalisations, including:
| Chloroquine | 7/20332 | 7 hospitalisations. 'Includes':
| |
| Studies reporting no serious events or effects | ||||||
| RCT | 0/46 | Nine serious adverse events in the trial (trial arm not specified) "none of which were considered by study physicians to be related to the study drug" | Placebo | 0/94 | ‐ | |
| RCT | 0/107 | "Adverse events were all mild and there were no deaths" | Placebo Chloroquine | 0/101 0/103 | ‐ ‐ | |
| RCT | 0/134 | "No serious side effects occurred with either drug regimen" | Doxycycline | 0/119 | ‐ | |
| RCT | 0/153 | "Although a large number of adverse events were reported, none were serious" | Doxycycline Atovaquone‐proguanil | 0/153 0/164 | ‐ ‐ | |
| Cohort study | 0/228 | "No drug induced side effects necessitating emergency care were observed" | Doxycycline | 0/506 | ‐ | |
| Cohort study | 0/491 | "No serious adverse events were recorded" | Atovaquone‐proguanil | 0/161 | ‐ | |
| Cohort study | 0/548 | Records hospitalisations, and reports that none occurred in either group of participants | Atovaquone‐proguanil Chloroquine | 0/707 0/37 | ‐ ‐ | |
| RCT | 0/103 | "All side effects were transient (and)... mild" | Chloroquine | 0/100 | ‐ | |
¹ This trial described a potentially serious adverse effect, but did not provide enough detail to meet our strict definition.
Wells 2006 was a retrospective healthcare record analysis that reported adverse events. It compared numbers of hospitalizations in military personnel who had been prescribed mefloquine and were deployed to active duty in malarial areas, with those who had been deployed to non‐malarial areas, and with military personnel with duty zip codes for Europe or Japan, who had not been deployed to active duty. Mefloquine users were less likely to be hospitalized (after deployment) with mood disorders (RR 0.38, 95% CI 0.17 to 0.86; 241,239 participants) or for any cause (RR 0.60, 95% CI 0.51 to 0.71; 241,239 participants) than military personnel who did not receive any antimalarial agents (but who were deployed to a war zone).
Discontinuations due to adverse effects
Within RCTs the number of people who discontinued the study drug due to adverse effects was low in both groups: 6/541 (1.1%) with mefloquine versus 4/583 (0.7%) with placebo (RR 1.64, 95% CI 0.55 to 4.88; 7 trials, 1124 participants, Analysis 1.4). No comparative data were available on this outcome from cohort studies because the comparison was with no treatment.
Prespecified adverse events or effects
None of the RCTs or cohort studies for this comparison reported on adverse effects (symptoms attributed by researchers or participants to the drug regimen). All comparisons were for adverse events (all symptoms that occurred while taking the study drug).
Gastrointestinal symptoms
Within RCTs, participants who received mefloquine were more likely to experience nausea than those who took placebo (RR 1.35, 95% CI 1.05 to 1.73; 2 trials, 244 participants, Analysis 1.5), but there was no difference between groups for vomiting, abdominal pain or diarrhoea (Analysis 1.6; Analysis 1.7; Analysis 1.8). The results from cohort studies were consistent with this finding, with more mefloquine users experiencing nausea (RR 1.85, 95% CI 1.42 to 2.43; 3 studies, 1901 participants, Analysis 1.5).
One RCT in pregnant women (Nosten 1994) reported on both upper and lower abdominal pain. Inclusion of both groups of results in sensitivity analyses had no impact on the results.
Neurological symptoms
Mefloquine users in RCTs were no more likely that recipients who took placebo to experience headache (RR 0.84, 95% CI 0.71 to 0.99; 5 trials, 791 participants, Analysis 1.9) or dizziness (RR 1.03, 95% CI 0.90 to 1.17; 3 trials, 452 participants, Analysis 1.10). This is in contrast to cohort studies, in which participants who took mefloquine were significantly more likely to experience dizziness than participants who travelled but took no prophylaxis (RR 1.80, 95% CI 1.29 to 2.49; 3 studies, 1901 participants, Analysis 1.10).
Psychological symptoms
None of the RCTs included in the analysis reported on any of our prespecified psychological symptoms. Participants in cohort studies who received mefloquine were more likely than participants who did not take prophylaxis to experience abnormal dreams (RR 2.35, 95% CI 1.15 to 4.80; 2 cohort studies, 931 participants, Analysis 1.11), and insomnia (RR 1.46, 95% CI 1.06 to 2.02; 2 cohort studies, 931 participants, Analysis 1.12). Effects on anxiety (RR 1.21, 95% CI 0.67 to 2.21; 2 cohort studies, 931 participants; I² statistic = 48%; Analysis 1.13), depressed mood (RR 2.43, 95% CI 0.65 to 9.07; 3 cohort studies, 1901 participants, I² statistic = 72%, Analysis 1.14) and abnormal thoughts or perceptions (RR 5.77, 95% CI 0.79 to 42.06; 1 cohort study, 970 participants, Analysis 1.15), were not consistent across studies, and overall, did not reach standard levels of statistical significance.
Other symptoms
Mefloquine users in cohort studies were more likely to experience pruritis (RR 6.71, 95% CI 1.58 to 28.55; 1 cohort study, 197 participants, Analysis 1.16). However, this finding was not replicated in RCTs (RR 0.86, 95% CI 0.60 to 1.24; 3 RCTs, 609 participants, Analysis 1.16). There was no difference between groups for visual impairment and vertigo in either RCTs nor cohort studies (Analysis 1.17; Analysis 1.18).
Other adverse events reported in more than 1% of study participants (in either study arm) in RCTs and cohort studies are presented in Analysis 1.19 and Analysis 1.20. Only respiratory tract infection reached statistical significance between groups; data were from a single trial with few events (RR 2.63, 95% CI 1.04 to 6.61; 1 trial, 140 participants).
Studies reporting groups of symptoms or other outcomes which could be used as proxy markers of psychological or neurological adverse effects are reported in Appendix 4.
Pregnancy outcomes
Nosten 1994 conducted an RCT in pregnant women over 20 weeks gestation. There was no reported difference between mefloquine and placebo for spontaneous abortions (RR 0.48, 95% CI 0.04 to 5.22; 311 participants), still births (RR 2.63, 95% CI 0.86 to 8.08; 311 participants) or congenital malformations (RR 3.82, 95% CI 0.43 to 33.83; 311 pregnant women). However, the trial was significantly underpowered to evaluate these outcomes.
Adherence
In their RCT, Davis 1996 reported on any measure of adherence to the drug regimen assessed by pill count and direct questioning. Reported adherence was 100% in both arms.
Comparison 2: Mefloquine versus doxycycline
Description of studies
RCTs
Four RCTs, enrolling 1317 participants, reported on both efficacy and safety (Table 8). One was conducted in short‐term travellers (Schlagenhauf 2003), two in military personnel (Arthur 1990; Ohrt 1997) and one in Kenyan children (Weiss 1995). The populations were described as non‐immune (Arthur 1990; Schlagenhauf 2003), "largely" non‐immune (Ohrt 1997) and semi‐immune (Weiss 1995). Trial duration varied from four weeks to four months. The method for detecting malaria was unclear in two trials (Arthur 1990; Schlagenhauf 2003). Three studies conducted daily interviews with participants to monitor for adverse events (Arthur 1990; Ohrt 1997; Weiss 1995) and one used a participant self‐reporting questionnaire (Schlagenhauf 2003).
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric adverse effects | Duration of travel | Source of funding |
| Randomized controlled trials | ||||||
| USA soldiers | 270 | Blood tests, stool samples. Interview with study personnel | None | 5 weeks | Not mentioned | |
| Indonesian soldiers | 204 | Interview with study personnel. Exit questionnaire | " History of underlying illness" | 13 weeks | Pfizer and Roche | |
| Non‐immune adult short‐term travellers | 674 | Participant self‐reported questionnaire | History of seizures or psychiatric disorders | 4 to 6 weeks | GlaxoSmithKline and Roche | |
| Kenyan children | 169 | Interview with study personnel | None | 4 months | Government funding | |
| Non‐randomized studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | 0 to 36 months | Not mentioned | |
| USA s oldiers | 367,840 | Data from the Defense Medical Surveillance System, the Pharmacy Data Transaction Service and the Theater Medical Data Store | No information available | Various, not specified | Not mentioned | |
| UK adult short‐term travellers | 185 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | < 28 days | GlaxoSmithKline | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participan t preference | ≥ 6 months | Two staff employed by Peace Corps | |
| Peace Corps volunteers | 1184 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Adult short‐term travellers | 660 | Participant self‐reported questionnaire | No information available | 93% < 4 weeks | " No financial interests to disclose" | |
| Adult short‐term travellers | 5626 | Participant self‐reported questionnaire | No information available | < 5 weeks | " No financial interests to disclose" | |
| UK adults enrolled in UK g eneral p ractice research database | 35,370 | Incident cases of depression, psychoses and panic attacks within the UK general practice research database | No information available | Various, not specified | Roche | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| Australian short‐term travellers | 741 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, mean 3 weeks, maximum 3 months | Roche and Pfizer | |
| USA soldiers | 2351 | Participant self‐reported questionnaire | Primarily doxycycline, soldiers with contra‐indications received mefloquine | > 90% for 10 months or more | Not mentioned | |
| Israeli short‐term travellers | 158 | Participant self‐reported questionnaire | "... daily doxycycline or daily primaquine... was recommended" | 14 to 20 days | Not mentioned | |
| Israeli soldiers | 45 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | "... an average of 4 hours stay in the field over a period of 2 months" | Not mentioned | |
| Dutch medical students | 180 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean 74 days (range 10 to 224 days) | No dedicated funding | |
| Turkish soldiers | 1400 | Participant self‐reported questionnaire | Prior to March 2002: doxycyline After July 2002: mefloquine | A pprox. 6 months | Not mentioned | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| UK soldiers | 2032 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | "... not funded by an external body" | |
| UK soldiers | 151 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | No dedicated funding | |
| Adult short‐term travellers | 3051 | Participant self‐reported questionnaire | No information available | A pprox. 6 weeks | Not mentioned | |
None of the RCTs adequately described allocation concealment. Blinding of participants was adequately described in all but Weiss 1995; two trials did not adequately describe how outcome assessors were blinded (Arthur 1990; Schlagenhauf 2003). We also considered Ohrt 1997 and Schlagenhauf 2003 to be at high risk of selective outcome reporting because they did not report all collected data: Ohrt 1997 completed an exit questionnaire within the last month of the study, but did not report all results; Schlagenhauf 2003 collected data at baseline, twice before travel and once on return, but only presented data for participants "who completed questionnaires at recruitment and at least one of the follow up periods". All four studies collected information on adverse events actively and prospectively (Table 9). Schlagenhauf 2003 was funded by GlaxoSmithKline (manufacturer of atovaquone‐proguanil) and Roche (manufacturer of mefloquine) and Ohrt 1997 was funded by Roche and Pfizer (manufacturers of doxycycline) but specified that "neither of the pharmaceutical companies that provided support played any role in the gathering, analysing or interpreting the data".
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Inadequate: No definitions provided for serious side effects | Unclear: it is not reported who conducted the interviews | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it wa s unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate " Each subject was visited daily at home by an assigned field worker, who asked about symptoms of malaria or drug side effects" | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Adequate | Adequate | Passive | Prospective | |
| Inadequate " Also included on the questionnaire was a single free‐text question asking travellers to describe any side effects of antimalarial medication" | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: n o information wa s provided regarding the timing of the questionnaire during treatment | |
| Adequate | Adequate | Passive | Unclear Comment: all participants were emailed the questionnaire at one time point, which occurred at varying points during the prophylactic regimen | |
| Inadequate "Travellers… were given a questionnaire that asked for... adverse health events attributed to those drugs" | Adequate | Passive | Unclear Comment: information was collected at the airport, when travellers should still have been taking the prophylactic regimen | |
| Adequate | Adequate | Passive | Retrospective | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Inadequate Comment: it wa s unclear what constituted a serious or severe event and insufficient information on the questions that travellers were asked | Inadequate "... a mailed questionnaire approximately 2 weeks after their anticipated return home date’ ‘if a reply had not been received within 4 weeks an abbreviated questionnaire was sent out." Comment: no details provided regarding abbreviated questionnaire | Active | Retrospective | |
| Inadequate Comment: insufficient information of the questions that travellers were asked | Adequate | Passive | Retrospective | |
| Inadequate "... we directly contacted all travelers for complete follow‐up and assessment of compliance. Fifty travelers taking primaquine completed a questionnaire regarding side effects" | Inadequate Comment: see quote. Different methods of follow up for different forms of prophylaxis | Unclear | Unclear | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate " Questionnaires were distributed and collected by the flight surgeon to 45 aircrew…questionnaires were immediately evaluated and further data collection was done by telephone, if necessary" | Passive | Unclear Comment: it wa s unclear at which time point data collection occurred | |
| Inadequate Comment: n o information wa s provided on how information on adverse effects was sought | Inadequate Comment: n o mention of how adverse events were recorded in the questionnaire | Passive | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Prospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information is reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate " The questionnaire approved by the MODREC included the 19 commonest adverse effects described in the manufacturers’ product documentation" Comment: Adverse events listed in the questionnaire are not reported | Adequate | Passive | Unclear Comment: information obtained during transit through Nairobi back to the UK. It wa s unclear whether participants were still taking prophylaxis at this time point | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Unclear Comment: i t wa s not specified at which point during treatment the questionnaire was administered | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking their prophylactic regimen | |
1. Were harms pre‐defined using standardised or precise definitions?
2. Was ascertainment technique adequately described?
3. Monitoring classed as 'active' if it occurred at set time points during treatment.
For full description of analysis methods, see Table 2.
Cohort studies
We included 20 cohort studies that assessed and reported safety outcomes, in a total of 435,209 participants. Of these, 10 were conducted in short‐term travellers (Goodyer 2011; Laver 2001; Lobel 2001; Meier 2004; Napoletano 2007; Philips 1996; Schwartz 1999; Sharafeldin 2010; Stoney 2016; Waner 1999), four in longer‐term occupational travellers (Cunningham 2014; Korhonen 2007; Landman 2015; Tan 2017) and six in military personnel (Eick‐Cost 2017; Saunders 2015; Shamiss 1996; Sonmez 2005; Terrell 2015; Tuck 2016); none included pregnant women. Most (17 cohort studies) used participant self‐reported questionnaires to monitor adverse events.
Ten cohort studies had non‐response rates of over 20% (Cunningham 2014; Korhonen 2007; Landman 2015; Lobel 2001; Philips 1996; Sharafeldin 2010; Tan 2017; Terrell 2015; Tuck 2016; Waner 1999), (Figure 4). We judged two to be at high risk of missing data; Goodyer 2011 included pre‐ and post‐travel questionnaires, with an interim loss to follow‐up rate of 27%, and Terrell 2015 excluded participants from the analysis if they reported an adverse effect but did not record its impact on their ability to work. None of these studies blinded participants or mentioned outcome assessors being blinded to intervention status. Seven studies collected data retrospectively, and eight collected information at an unclear or variable time point during treatment (Table 9). One study (Goodyer 2011) was funded by GlaxoSmithKline (manufacturer of atovaquone‐proguanil), one (Meier 2004) by Roche (manufacturer of mefloquine), and one (Philips 1996) by Roche and Pfizer (manufacturers of doxycycline) (see Figure 4).
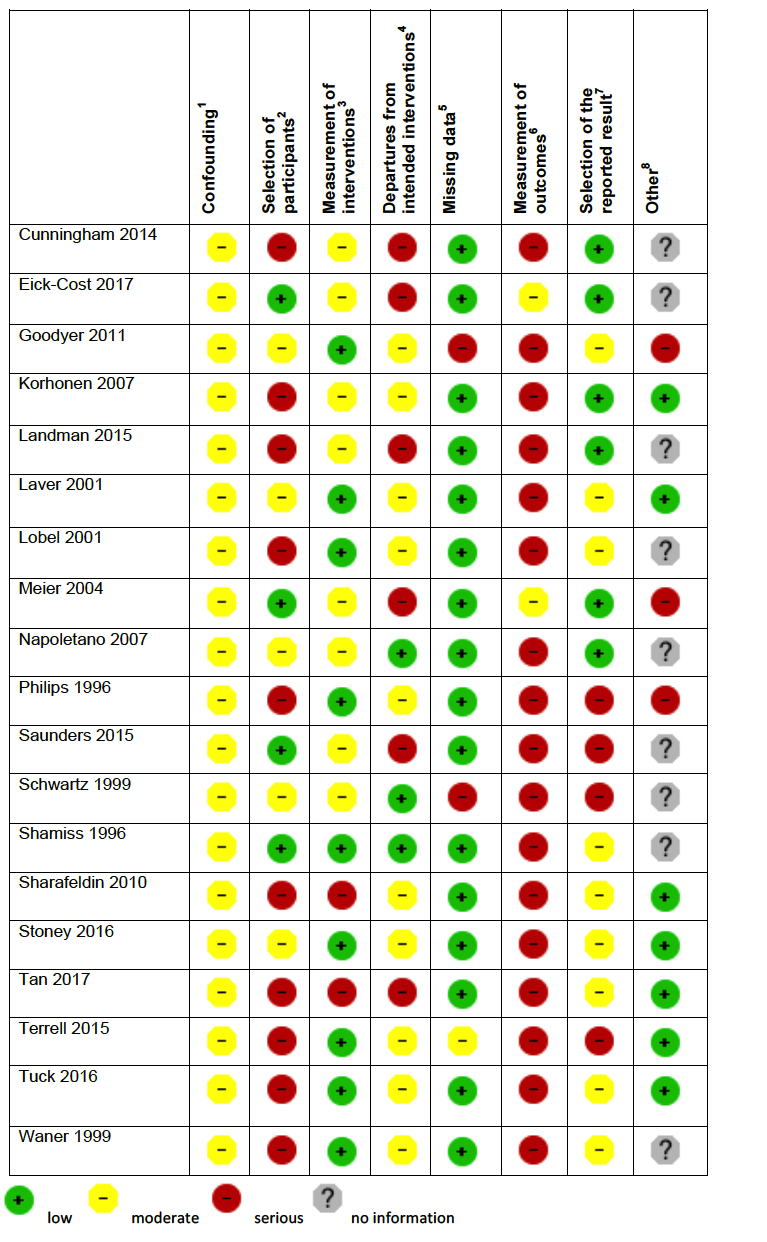
'Risk of bias' summary in cohort studies: mefloquine versus doxycycline
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.
Efficacy
Only seven episodes of malaria were reported while participants were receiving prophylaxis; similar numbers of participants were infected in both arms (4 episodes in 378 mefloquine users versus 3 episodes in 366 doxycycline users: RR 1.35, 95% CI 0.35 to 5.19; 4 trials, 744 participants, Analysis 2.1).
Weiss 1995 reported on episodes of parasitaemia in the semi‐immune population. There was no clear difference between groups (RR 1.47, 95% CI 0.68 to 3.14; 62 participants).
Safety
Serious adverse events or effects
Only Ohrt 1997 described an adverse event as "serious" (acute hysteria) in a doxycycline user, but did not provide sufficient detail to meet our definition. No other serious adverse outcomes were described in RCTs including 348 mefloquine users and 334 doxycycline users (Analysis 2.2; Table 6).
In comparison, three cohort studies reported a total of 29 serious adverse effects (attributed to the study drug by users): 19 in 2125 mefloquine users, and 10 in 1597 doxycycline users (RR 1.53, 95% CI 0.23 to 10.24; 3 cohort studies, 3722 participants; Analysis 2.2, Table 7).
Serious adverse effects in mefloquine users were psychological (4 cases) or due to dizziness (3), heart palpitations (2), limb numbness (1), abdominal pain (1), visual disturbance (1), yeast infection (1), passing out (2), seizure (1) and three hospitalizations with "either gastrointestinal or neurologic symptoms". In contrast, serious adverse effects in doxycycline users were due to gastrointestinal disturbance (6), anaemia (1), photosensitivity (1), oesophagitis (1) and cough (1).
In addition, a cohort study (Lobel 2001) reported on hospitalizations in users of mefloquine and doxycycline which were not necessarily attributed to the drug regimen (adverse events). There were eight hospitalizations in 3703 mefloquine users, and none in 69 doxycycline users, with no statistically significant difference between groups (RR 0.32, 95% CI 0.02 to 5.51; 3772 participants, Table 6).
Discontinuations due to adverse effects
There were no overall differences between groups in numbers of discontinuations due to adverse effects in the RCTs (8/391 mefloquine users, 8/382 doxycycline users, RR 1.08, 95% CI 0.41 to 2.87; 4 RCTs, 773 participants, Analysis 2.3) or cohort studies (852/6116 mefloquine users, 378/4049 doxycycline users, RR 0.92, 95% CI 0.54 to 1.55; 10 cohort studies, 10,165 participants, Analysis 2.3). However, heterogeneity among cohort studies was high (I² statistic = 85%).
Prespecified adverse outcomes
Prespecified adverse effects (attributed to the study drug) were only reported by cohort studies conducted in long‐term occupational travellers (3 studies) and military personnel (3 studies). These form our primary analysis (see Appendix 3 for decision tree).
One RCT in military personnel (Ohrt 1997) and one cohort study in short‐term international travellers (Philips 1996) reported on all symptoms experienced by participants while taking the study drug (adverse events). Two large retrospective analyses of health records in general practice (Meier 2004) and USA military personnel (Eick‐Cost 2017) databases compared rates of incident neurological or psychological diagnoses in participants who had received a prescription for mefloquine or doxycycline (adverse events).
Gastrointestinal symptoms
Across the cohort studies reporting adverse effects, mefloquine users were less likely to report nausea (RR 0.37, 95% CI 0.30 to 0.45; 5 cohort studies, 2683 participants, Analysis 2.4), vomiting (RR 0.18, 95% CI 0.12 to 0.27; 4 cohort studies, 5071 participants, Analysis 2.5), abdominal pain (RR 0.30, 95% CI 0.09 to 1.07; 4 cohort studies, 2569 participants, Analysis 2.6) and diarrhoea (RR 0.28, 95% CI 0.11 to 0.73; 5 cohort studies, 5104 participants, Analysis 2.7).
However, this finding was not consistent across study types. In the single RCT in military personnel that reported adverse events, no differences were demonstrated for nausea, vomiting, abdominal pain or diarrhoea. In the single cohort study in short‐term international travellers reporting adverse events, mefloquine users were more likely to report nausea and diarrhoea; there was no difference between groups for abdominal pain (Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7).
Dyspepsia was consistently more common in doxycycline users but there was substantial heterogeneity in the size of this effect (RR 0.26, 95% CI 0.09 to 0.74; 5 cohort studies, 5104 participants, I² statistic = 77%, Analysis 2.8)
Neurological symptoms
In the cohort studies reporting adverse effects, no difference was demonstrated for headache (RR 1.21, 95% CI 0.50 to 2.92; 5 cohort studies, 3322 participants, Analysis 2.9) or dizziness (RR 3.49, 95% CI 0.88 to 13.75; 5 cohort studies, 2633 participants, Analysis 2.10).
In the RCT in military personnel (Ohrt 1997) and a cohort study in short‐term international travellers (Philips 1996) both headache and dizziness were more common in mefloquine users. However, a large retrospective analysis of health records in military personnel (Eick‐Cost 2017) found higher rates of dizziness in doxycycline users (Analysis 2.9; Analysis 2.10).
Psychological symptoms
In the cohort studies reporting adverse effects, mefloquine users were more likely to report abnormal dreams (RR 10.49, 95% CI 3.79 to 29.10; 4 cohort studies, 2588 participants, Analysis 2.11), insomnia (RR 4.14, 95% CI 1.19 to 14.44; 4 cohort studies, 3212 participants, Analysis 2.12), anxiety (RR 18.04, 95% CI 9.32 to 34.93; 3 cohort studies, 2559 participants, Analysis 2.13) and depressed mood (RR 11.43, 95% CI 5.21 to 25.07; 2 cohort studies, 2445 participants, Analysis 2.14). There were 15 episodes of abnormal thoughts and perceptions with mefloquine and none with doxycyline in cohort studies reporting adverse effects (RR 6.60, 95% CI 0.92 to 47.20; 2 cohort studies, 2445 participants, Analysis 2.15).
The findings of the single cohort study in short‐term international travellers reporting adverse events (Philips 1996) were consistent with this. However in the single RCT (Ohrt 1997) and the large retrospective healthcare record analyses, there were either no differences between groups, or doxycycline users were more likely to experience psychological symptoms (Analysis 2.11; Analysis 2.12; Analysis 2.13; Analysis 2.14; Analysis 2.15).
Other prespecified symptoms
Pruritis was more common in doxycycline users in cohort studies reporting adverse effects (RR 0.52, 95% CI 0.30 to 0.91; 2 cohort studies, 1794 participants, Analysis 2.16), but more common with mefloquine in the single cohort in short‐term travellers reporting adverse events (RR 2.69, 95% CI 0.93 to 7.78; 1 cohort study, 668 participants).
In cohort studies reporting adverse effects, photosensitivity was more common in doxycycline users (RR 0.08, 95% CI 0.05 to 0.11; 2 cohort studies, 1875 participants, Analysis 2.17), as was vaginal yeast infection in female participants (RR 0.10, 95% CI 0.06 to 0.16; 1 cohort study, 1761 participants, Analysis 2.18). The findings of the single cohort study in short‐term travellers reporting adverse events were consistent with this finding (Analysis 2.17; Analysis 2.18).
Visual impairment was more commonly reported among mefloquine users (RR 2.37, 95% CI 1.41 to 3.99; 2 cohort studies, 1875 participants; Analysis 2.19).
Other adverse events and effects
A range of other adverse effects were reported by the cohort studies. These included alopecia (hair loss), asthenia (physical weakness), balance disorder, decreased appetite, fatigue, hypoaesthesia (numbness), malaise, mouth ulcers, palpitations and tinnitus (Analysis 2.20). Mefloquine users were more likely to report alopecia (RR 3.44, 95% CI 1.96 to 6.03; 2 cohort studies, 1875 participants), unsteadiness (RR 2.87, 95% CI 1.48 to 5.59; 1 cohort study, 1761 participants) and limb numbness (RR 11.48, 95% CI 3.01 to 43.70; 2 cohort studies, 2445 participants), but were less likely to report malaise (RR 0.28, 95% CI 0.11 to 0.71; 1 cohort study, 734 participants).
Additional adverse events reported in the RCT and cohort studies are presented in Analysis 2.21 and Analysis 2.22 respectively. In Eick‐Cost 2017, a large retrospective healthcare record analysis in USA military personnel that reported adverse events, mefloquine users were less likely than doxycycline users to receive formal medical diagnoses of adjustment disorder (RR 0.43, 95% CI 0.40 to 0.45; 354,959 participants), convulsions (RR 0.58, 95% CI 0.45 to 0.75), hallucinations (RR 0.18, 95% CI 0.08 to 0.45), post‐traumatic stress disorder (PTSD) (RR 0.58, 95% CI 0.53 to 0.64), suicidal ideation (RR 0.38, 95% CI 0.31 to 0.47), and tinnitus (RR 0.65, 95% CI 0.61 to 0.71). There were no differences in overall rates of suicide in the large retrospective healthcare record analyses (4/53,029 mefloquine users and 15/322,995 doxycycline users; RR 1.21, 95% CI 0.32 to 4.56, Analysis 2.22).
Studies reporting groups of symptoms or other outcomes that could be used as proxy markers of psychological or neurological adverse effects are reported in Appendix 5.
Adherence
Arthur 1990, an RCT, performed serological assays to assess adherence. Arthur 1990 reported measurable serum drug levels at the end of the trial in 87% of 119 military personnel prescribed doxycycline and 92% of 134 who were prescribed mefloquine. However, medication was administered under the supervision of each participant's squad leader.
Thirteen cohort studies compared the proportion of participants with 100% self‐reported adherence and found higher rates of adherence during travel in mefloquine users (RR 1.15, 95% CI 1.12 to 1.18; 13 cohort studies, 15,583 participants, Analysis 2.23), but no differences between groups in the post‐travel period (RR 1.08, 95% CI 0.95 to 1.22; 4 cohort studies, 840 participants, Analysis 2.23). Most (77%) mefloquine users described themselves as adherent during travel (range 24% to 100%), compared to 63% of doxycycline users (range 37% to 92%). In the post‐travel period this dropped to 55% of mefloquine users (range 50% to 87%) and 51% of doxycycline users (range 27% to 75%). There was no difference in the results when the analysis was limited to short‐term international travellers (RR 1.11, 95% CI 1.06 to 1.17; 4 cohort studies; 8390 participants).
Comparison 3: Mefloquine versus atovaquone‐proguanil
Description of studies
RCTs
Two RCTs in non‐immune travellers reported efficacy, with most participants visiting sub‐Saharan Africa for fewer than three weeks (Overbosch 2001; Schlagenhauf 2003). Efficacy was assessed by testing for antibodies to a circumsporozoite protein four weeks after travel in the study by Overbosch 2001, and the method was unclear in Schlagenhauf 2003.
Three RCTs (Overbosch 2001; Schlagenhauf 2003; van Riemsdijk 2002), and 16 cohort studies (Andersson 2008; Belderok 2013; Cunningham 2014; Eick‐Cost 2017; Goodyer 2011; Kato 2013; Korhonen 2007; Kuhner 2005; Landman 2015; Laverone 2006; Napoletano 2007; Schneider 2013; Sharafeldin 2010; Stoney 2016; Tan 2017; Tuck 2016) assessed and reported safety outcomes (Table 10).
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric adverse effects | Duration of travel | Source of funding |
| Randomized controlled trials | ||||||
| Travellers from Canada, Germany, Netherlands, South Africa, UK | 1013 | Interview with study personnel | "... history of alcoholism, seizures or psychiatric or severe neurological disorders" | Mean 2.5 weeks | GlaxoSmithKline | |
| Non‐immune adult short‐term travellers | 674 | Participant self‐reported questionnaire | " History of seizures or psychiatric disorders" | 4 to 6 weeks | GlaxoSmithKline and Roche | |
| Dutch short‐term travellers | 140 | Interview and testing with study personnel | "H istory of alcoholism, seizures, psychiatric disorders, severe neurological disorders" | Mean 19 days | Government funding | |
| Non‐randomis ed studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| Swedish soldiers | 609 | Participant self‐reported questionnaire | Mainly mefloquine, soldiers with contra‐indications received atovaquone‐proguanil | 6 months | Not mentioned | |
| Dutch short‐term travellers | 945 | Participant self‐reported questionnaire (measured adherence) | Allocation based on guidelines and participant preference | 84% < 29 days | Government funding | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and p articipant preference | 0‐36 months | Not mentioned | |
| USA s oldiers | 367,840 | Data from the Defense Medical Surveillance System, the Pharmacy Data Transaction Service and the Theater Medical Data Store | No information available | Various, not specified | Not mentioned | |
| UK adult short‐term travellers | 185 | Participant self‐reported questionnaire | Allocation based on guidelines and p articipant preference | < 28 days | GlaxoSmithKline | |
| Japanese short‐term travellers | 316 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean 20.0 ± 9.6 days in the atovaquone‐proguanil group and 59.0 ± 15.9 days in the mefloquine group | Not mentioned | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | ≥ 6 months | Two staff employed by Peace Corps | |
| German short‐term travellers | 495 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | A tovaquone‐proguanil mean 2.6 weeks, mefloquine mean 7 weeks | Not mentioned | |
| Peace Corps volunteers | 1184 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Italian short‐term travellers | 1176 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | > 90% 0 to 30 days | Not mentioned | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| UK adults enrolled in UK g eneral p ractice research database | Not available | Incident cases of a neuropsychiatric disorders during or after antimalarial drug use | No information available | Various, not specified | Roche | |
| Dutch medical students | 180 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean duration of stay 74 days (range 10 to 224 days) | " N o dedicated funding for this project" | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| UK soldiers | 151 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | No dedicated funding | |
Two RCTs included adults and children aged ≥ 3 years (Overbosch 2001; van Riemsdijk 2002); all other studies were restricted to adults. The RCTs described participants as non‐immune travellers, and most participants visited sub‐Saharan Africa for fewer than three weeks. The cohort studies included short‐term travellers (Belderok 2013; Goodyer 2011; Kato 2013; Kuhner 2005; Laverone 2006; Napoletano 2007; Schneider 2013; Sharafeldin 2010; Stoney 2016), longer‐term occupational travellers (Cunningham 2014; Korhonen 2007; Landman 2015; Tan 2017) and military personnel (Andersson 2008; Eick‐Cost 2017; Tuck 2016).
All three RCTs that assessed and reported safety outcomes collected information on adverse events actively and prospectively, and predefined harms using standardized and precise definitions (Overbosch 2001; Schlagenhauf 2003; van Riemsdijk 2002; Table 11). Only Overbosch 2001 performed a blinded assessment of whether there was a reasonable possibility that each adverse event was caused by the study drug (adverse effects). Overbosch 2001 was funded by GlaxoSmithKline (manufacturer of atovaquone‐proguanil) and Schlagenhauf 2003 received funding from both GlaxoSmithKline and Roche (manufacturers of mefloquine).
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Inadequate Comment: insufficient information provided on the questions which soldiers were asked | Inadequate Comment: different ascertainment technique used for one of the three groups, which is inadequately described | Active | Unclear Comment: d ata collection was prospective for 448/609 participants (LA04 and LA05), but retrospective for 161 participants (LA02) | |
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Adequate | Adequate | Passive | Prospective | |
| Inadequate " Also included on the questionnaire was a single free‐text question asking travelers to describe any side effects of antimalarial medication" | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: the timing of this questionnaire has not been made clear | |
| Adequate | Adequate | Passive | Unclear Comment: n o information wa s provided regarding the timing of the questionnaire during treatment | |
| Inadequate Comment: insufficient information provided on the questions that participants were asked | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: all participants were emailed the questionnaire at one time point, which occurred at varying points during the prophylactic regimen | |
| Adequate | Adequate | Passive | Retrospective | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate Comment: n o information is provided on how information on adverse effects was sought | Inadequate Comment: n o mention of how adverse events were recorded in the questionnaire. | Passive | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information is reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Unclear Comment: i t wa s not specified at which point during treatment the questionnaire was administered | |
1. Were harms pre‐defined using standardised or precise definitions?
2. Was ascertainment technique adequately described?
3. Monitoring classed as 'active' if it occurred at set time points during treatment.
For full description of analysis methods, see Table 2.
Cohort studies
In the cohort studies, safety was assessed by self‐reported questionnaires (Andersson 2008; Belderok 2013; Cunningham 2014; Goodyer 2011; Kato 2013; Korhonen 2007; Kuhner 2005; Landman 2015; Laverone 2006; Sharafeldin 2010; Stoney 2016; Tan 2017; Tuck 2016), telephone interview (Napoletano 2007), and retrospective analysis of a healthcare records (Eick‐Cost 2017; Schneider 2013). Seven studies collected adverse event data retrospectively and six collected these data at an unclear or variable time point during treatment (Table 11). One study (Goodyer 2011) was funded by GlaxoSmithKline (manufacturer of atovaquone‐proguanil) and one (Schneider 2013) was funded by Roche (manufacturer of mefloquine) (Figure 5).
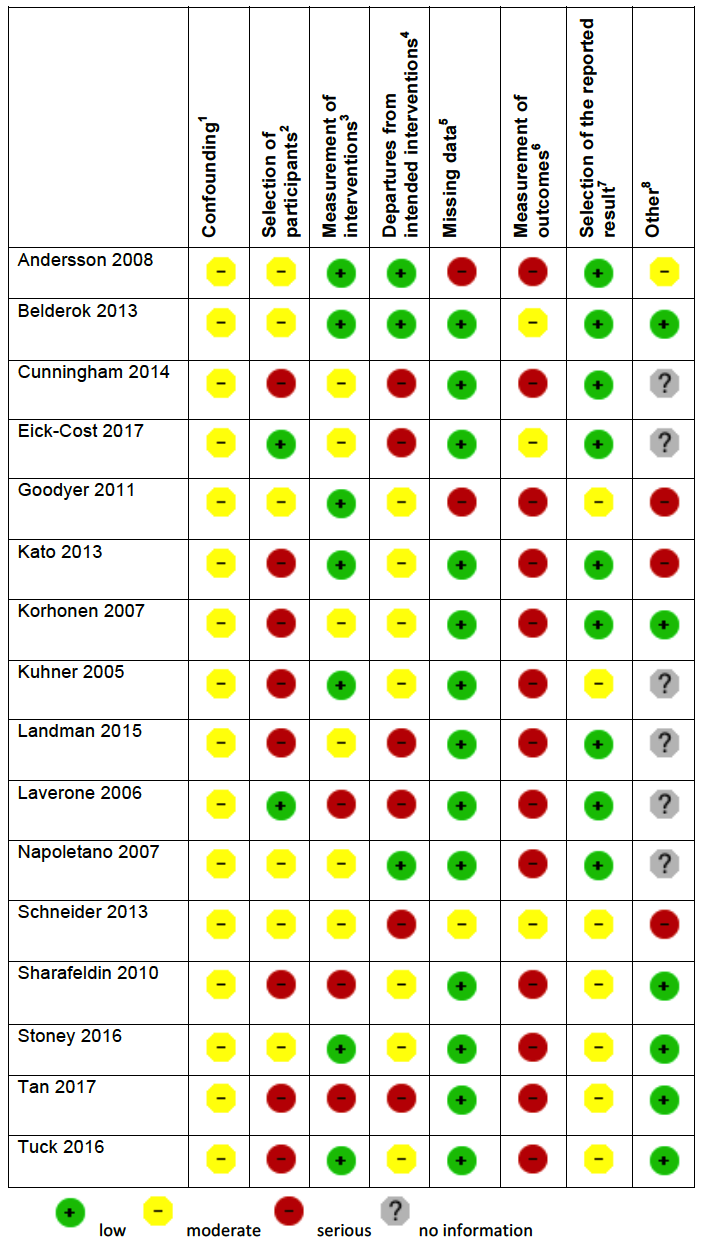
'Risk of bias' summary in cohort studies: mefloquine versus atovaquone‐proguanil
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.
Efficacy
No clinical cases of malaria were recorded (2 RCTs, 636 mefloquine users; 657 atovaquone‐proguanil users).
Safety
Serious adverse events or effects
Overbosch 2001, an RCT, reported 10 serious adverse events in 483 participants who received mefloquine and four in 493 participants who received atovaquone‐proguanil. None were considered attributable to the drug regimen (Table 6).
Three cohort studies reported a total of 15 serious adverse effects (attributed by participants to the study drug) in 2651 mefloquine users (Table 7). There were no serious adverse effects reported in participants who received atovaquone‐proguanil (940 users). The difference between groups was not statistically significant (RR 1.40, 95% CI 0.08 to 23.22; 3 cohort studies, 3591 participants, Analysis 3.2).
The serious adverse effects in mefloquine users were: psychological (4 cases), dizziness (3), heart palpitations (2), limb numbness (1), abdominal pain (1), visual disturbance (1), yeast infection (1), and passing out (2).
Discontinuations due to adverse effects
In the RCTs, participants who received mefloquine were more likely to discontinue their medication due to adverse effects than participants who took atovaquone‐proguanil (39/714 mefloquine versus 13/724 atovaquone‐proguanil; RR 2.86, 95% CI 1.53 to 5.31; 3 RCTs, 1438 participants, Analysis 3.3).
The overall effect size was similar in the cohort studies (RR 2.73, 95% CI 1.83 to 4.08; 9 cohort studies, 7785 participants, Analysis 3.3).
Prespecified adverse effects
Gastrointestinal symptoms
Mefloquine users were more likely to report nausea than atovaquone‐proguanil users with similar effect sizes in the RCT (RR 2.72, 95% CI 1.52 to 4.86; 976 participants) and overall in the cohort studies (RR 2.50, 95% CI 1.54 to 4.06; 7 cohort studies, 3509 participants, Analysis 3.4). There were no consistent differences in the frequency of reported vomiting (Analysis 3.5), abdominal pain (Analysis 3.6) or diarrhoea (Analysis 3.7). Mouth ulcers were less commonly reported with mefloquine in cohort studies (RR 0.12, 95% CI 0.04 to 0.37; 2 cohort studies, 783 participants), but not in the RCT (RR 1.45, 95% CI 0.70 to 3.00; 976 participants; Analysis 3.8).
Neurological symptoms
Mefloquine users were more likely to report headache although this did not reach standard levels of statistical significance in the RCT (RR 1.72, 95% CI 0.99 to 2.99; 976 participants). The effect was larger and consistent across the cohort studies (RR 3.42, 95% CI 1.71 to 6.82; 8 cohort studies, 4163 participants, I² statistic = 0%, Analysis 3.9). Similarly, dizziness was more common in mefloquine users in the RCT (RR 3.99, 95% CI 2.08 to 7.64) and consistently more common in the cohort studies (RR 3.83, 95% CI 2.23 to 6.58; 8 cohort studies, 3986 participants, Analysis 3.10). The same trend was seen in the retrospective healthcare record analyses, although the effect size was smaller (RR 1.23, 95% CI 1.04 to 1.46; 49,419 participants).
Psychological symptoms
In the RCT, mefloquine users were more likely than atovaquone‐proguanil users to report abnormal dreams (RR 2.04, 95% CI 1.37 to 3.04), insomnia (RR 4.42, 95% CI 2.56 to 7.64), anxiety (RR 6.12, 95% CI 1.82 to 20.66) and depressed mood (RR 5.78, 95% CI 1.71 to 19.61; 976 participants) (Overbosch 2001). Consistent, larger effects were seen in the cohort studies: abnormal dreams (RR 6.81, 95% CI 1.65 to 28.15; 7 cohort studies, 3848 participants, Analysis 3.11), insomnia (RR 7.29, 95% CI 4.37 to 12.16; 8 cohort studies, 3986 participants, Analysis 3.12), anxiety (RR 10.10, 95% CI 3.48 to 29.32; 4 cohort studies, 2664 participants, Analysis 3.13) and depressed mood (RR 8.02, 95% CI 3.56 to 18.07; 6 cohort studies, 3624 participants, Analysis 3.14). In addition, 21 mefloquine users and no atovaquone‐proguanil users reported abnormal thoughts or perceptions, but the difference between groups was not statistically significant (RR 1.50, 95% CI 0.30 to 7.42; 3 cohort studies, 2441 participants, Analysis 3.15).
Consistent effects were seen in the retrospective healthcare record analysis (adverse events, Eick‐Cost 2017) although the effect size was smaller.
Other prespecified adverse symptoms
No differences were demonstrated for pruritis (1 RCT, 3 cohort studies; Analysis 3.16); or visual impairment (1 RCT, 2 cohort studies; Analysis 3.17).
Other adverse outcomes
Other adverse effects reported in more than 1% of study participants in cohort studies (in either study arm) included: allergic reaction, alopecia (hair loss), asthenia (weakness), balance disorder, cough, disturbance in attention, dyspepsia, fatigue, hypoaesthesia, loss of appetite, muscle pain, palpitation, photosensitization, pyrexia, rash, restlessness, slight illness, somnolence, tinnitus and circulatory disorders (Analysis 3.18). Mefloquine users were more likely to report concentration difficulties (RR 4.45, 95% CI 1.84 to 10.77; 3 cohort studies, 1363 participants).
In the large retrospective healthcare record analyses which reported adverse events, mefloquine users were more likely to receive formal medical diagnoses of adjustment disorder (RR 1.76, 95% CI 1.54 to 2.02; 49,419 participants, Analysis 3.19), PTSD (RR 2.51, 95% CI 1.93 to 3.26; Analysis 3.19), suicidal ideation (RR 1.69, 95% CI 1.03 to 2.77; Analysis 3.19) and tinnitus (RR 1.42, 95% CI 1.21 to 1.68; Analysis 3.19). However, users were less likely to experience hallucinations (RR 0.25, 95% CI 0.08 to 0.79; Analysis 3.19).
Studies reporting groups of symptoms, or other outcomes which could be used as proxy markers of psychological or neurological adverse effects, are reported in Appendix 6.
Adherence
van Riemsdijk 2002 monitored adherence through reference to the participants' diary cards and counts of returned study medication. It was found that 93% of mefloquine users were completely adherent, compared to 98.3% of atovaquone‐proguanil users (RR 0.95, 95% CI 0.88 to 1.02; 1 RCT, 119 participants, Analysis 3.20).
Overbosch 2001 defined participants as adherent if they took at least 80% of prescribed doses. Overbosch 2001 also found no difference between the groups during travel (RR 0.98, 95% CI 0.95 to 1.01; 966 participants; Analysis 3.20). However, analysis in the post‐travel period found that mefloquine users were less likely to complete the regimen (RR 0.80, 95% CI 0.74 to 0.85; 966 participants); 93% of mefloquine users were adherent during travel, dropping to 70% in the post‐travel period, compared to 95% and 88% for atovaquone‐proguanil.
Six cohort studies compared the proportion of participants with 100% self‐reported adherence and found no difference during travel (RR 1.08, 95% CI 0.86 to 1.34; 6 cohort studies, 5577 participants, Analysis 3.21) or in the post‐travel period (RR 0.89, 95% CI 0.64 to 1.23; 2 cohort studies, 422 participants, Analysis 3.21). In these studies, 60% of mefloquine users described themselves as adherent during travel, dropping to 51% in the post‐travel period, compared to 53% and 62% respectively for people who took atovaquone‐proguanil.
Belderok 2013 categorized travellers as adherent if they took at least 75% of prescribed doses. Belderok 2013 reported higher rates of adherence in participants who took mefloquine both during and after travel. Meta‐analysis of these results did not result in a significant difference (during travel: RR 1.04, 95% CI 0.77 to 1.40; 5 cohort studies, 2810 participants, post‐travel: RR 1.07, 95% CI 0.72 to 1.59; 3 cohort studies, 941 participants).
Pregnancy outcomes
One cohort study included respondents who were pregnant (Cunningham 2014) but did not report which prophylaxis the women took or on any outcomes related to pregnancy.
Mefloquine versus chloroquine
Description
RCTs
We included five RCTs comparing mefloquine with chloroquine that reported on efficacy and six on safety (Table 12). Trials were conducted in immune or semi‐immune adult populations in the Ivory Coast (Sossouhounto 1995), Malawi (Steketee 1996), Nigeria (Salako 1992) Thailand (Boudreau 1991; Bunnag 1992) and the USA. (Boudreau 1993). The Malawi trial by Steketee 1996 was limited to pregnant women. None included non‐immune travellers or children. All six trials used interview with study personnel to obtain information about adverse events. Boudreau 1993 excluded participants with a history of psychiatric or neurological problems.
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric side effects | Trial duration | Source of funding |
| RCT s | ||||||
| Thai gem miners | 501 | Interview with study personnel | None | 14 weeks | USA Army | |
| USA soldiers | 359 | Interview with study personnel and computerised questionnaire | "M edical history of psychiatric or neurological problems within the last 5 years" | 13 weeks | Not mentioned | |
| Thai adult mal es | 605 | Interview with study personnel | None | 24 weeks | Roche | |
| Nigerian adult males | 567 | Interview with study personnel | None | 24 weeks | Not mentioned | |
| Ivory C oast adult males | 500 | " Access to the village health centre. Clinical examination with study personnel" | None | 20 weeks | Not mentioned | |
| Pregnant Malawian women | 4220 | Interview with study personnel | None | Monitored from enrolment to delivery | Government funding | |
| Non‐randomised studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| USA travelling children aged < 13 years | 177 | Interview with study personnel | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Spanish short‐term adult travellers | 1054 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Maximum 6 weeks | Not mentioned | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | 0 to 36 months | Not mentioned | |
| USA short‐term travellers | 822 | Interview with study personnel | Allocation based on guidelines and participant preference | Median 19 days, up to 90 days | Not mentioned | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | ≥ 6 months | Two staff employed by Peace Corps | |
| Adult short‐term travellers | 660 | Participant self‐reported questionnaire | No information available | 93% < 4 weeks | " No financial interests to disclose" | |
| Italian short‐term travellers | 1176 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | > 90% 0 to 30 days | Not mentioned | |
| Adult short‐term travellers | 5626 | Participant self‐reported questionnaire | No information available | M ost < 5 weeks | " No financial interests to disclose" | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| Danish travellers | 4154 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, 65% < 3 weeks | Not mentioned | |
| Swedish short‐term travellers | 491 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | " Most" 2 to 4 weeks | Not mentioned | |
| Adult short‐term travellers | 145,003 | Participant self‐reported questionnaire | No information available | 98% stayed between 1 and 4 weeks | Roche | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| Adult short‐term travellers | 3051 | Participant self‐reported questionnaire | No information available | A pprox. 6 weeks | " not funded by an external body" | |
None of the trials adequately described random sequence generation or allocation concealment. Participants were adequately blinded in four trials (Boudreau 1993; Bunnag 1992; Salako 1992; Sossouhounto 1995), the trial in pregnant women did not blind participants or outcome assessors (Steketee 1996). We judged three of the trials to be at high risk of selective reporting of safety outcomes. Bunnag 1992 was funded by Roche (manufacturer of mefloquine). Five trials actively sought information on adverse events (Boudreau 1991; Boudreau 1993; Bunnag 1992; Salako 1992; Steketee 1996) and all collected information prospectively (Table 13).
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate " Adverse events were defined clinically, and starting week 14, volunteers reporting adverse events were interviewed by members of the hospital team" | Adequate | Active | Prospective | |
| Inadequate " Particular attention was paid to complaints such as fever, chills, malaise, nausea and vomiting, rashes and other symptoms and signs that could be regarded as adverse events." Comment: no clear definition of adverse events wa s provided | Adequate | Active | Prospective | |
| Inadequate " Participants had access to a village health center, where they could notify personnel of any malaise or side effects" | Unclear " Clinical examinations and parasitologic tests were performed every 4 weeks" | Passive | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate Comment: insufficient information wa s provided about the questions that travellers were asked | Adequate | Active | Retrospective | |
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Inadequate Comment: insufficient information wa s provided about the questions that travellers were asked | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: No information wa s provided regarding the timing of the questionnaire during treatment | |
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate "Travellers… were given a questionnaire that asked for... adverse health events attributed to those drugs" | Adequate | Passive | Unclear Comment: information was collected at the airport, when travellers should still have been taking the prophylactic regimen | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Inadequate Comment: i t wa s unclear whether the questionnaire implied causality to the drug regimen | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking the prophylactic regimen | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information wa s reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking the prophylactic regimen | |
1. Were harms pre‐defined using standardised or precise definitions?
2. Was ascertainment technique adequately described?
3. Monitoring classed as 'active' if it occurred at set time points during treatment.
For full description of analysis methods, see Table 2.
Cohort studies
We included 15 cohort studies in this comparison; 12 included short‐term travellers (Albright 2002; Corominas 1997; Hill 2000; Laver 2001; Laverone 2006; Lobel 2001; Napoletano 2007; Petersen 2000; Rietz 2002; Steffen 1993; Stoney 2016; Waner 1999) and three longer‐term occupational travellers (Cunningham 2014; Korhonen 2007; Tan 2017) (Table 12). Albright 2002 included only children. Twelve studies used participant‐self reported questionnaires to collect information about adverse events; three of these, including the largest study (Steffen 1993, 145,003 participants), collected information from travellers flying back to Europe from Africa. The remaining three studies collected information through interviews with study personnel (Albright 2002; Hill 2000; Napoletano 2007)
Eight of the cohort studies had non‐response rates of over 20% (Figure 6). We judged 14 cohort studies to be at low risk of missing data, the largest study (Steffen 1993) was at moderate risk due to a 15% loss to follow‐up between the first and second questionnaire in the second phase of the study. Steffen 1993 did not report on non‐serious adverse effects from the first phase of the study (44,677 participants) and was funded by Roche (manufacturer of mefloquine). Six studies collected information about adverse events at set time points (Corominas 1997; Hill 2000; Napoletano 2007; Petersen 2000; Rietz 2002; Stoney 2016; Tan 2017), and one collected information prospectively (Stoney 2016) (Table 13; Figure 6).

'Risk of bias' summary in cohort studies: mefloquine versus chloroquine
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.
Efficacy
Participants who took mefloquine were less likely to experience malaria than participants who took chloroquine (RR 0.38, 95% CI 0.28 to 0.52; 4 RCTs, 877 participants, Analysis 4.1). However, two RCTs were conducted in settings with known chloroquine resistance at the study sites, and the other two reported no episodes of malaria in either study arm. All RCTs included semi‐immune populations, and were conducted over 20 years ago.
Safety
Serious adverse events or effects
Across four RCTs, two serious adverse events were reported in 529 mefloquine users and none in 471 chloroquine users; the difference between groups was not significant (RR 2.77, 95% CI 0.32 to 23.85; 5 RCTs, 1000 participants, Analysis 4.2, Table 6). Both events were psychiatric admissions due to depression and suicidal thoughts; both study participants had previous psychiatric histories. In one case, the participant's psychiatrist did not think the event was drug‐related, and in the other "felt this individual's current depression was not drug related, unless it was aggravated by inability to sleep". Additionally, Steketee 1996 described one withdrawal due to a "neuropsychiatric side effect" (disorientation to time and place) but did not provide enough detail to meet our definition of serious adverse event or effect.
Four cohort studies reported a total of 29 serious adverse effects (attributed by users to the study drug) in 56,674 mefloquine users, and 13 serious adverse effects in 22,583 chloroquine users. The difference between groups was not statistically significant (RR 1.14, 95% CI 0.62 to 2.07; 6 cohort studies; 79,257 participants; Analysis 4.2). Serious side effects in mefloquine users were psychological (11 cases), dizziness (5), seizures (3), heart palpitations (2), abdominal pain (1), blackout (2), visual disturbance (1), limb numbness (1), yeast infection (1), and two which were not described (Table 7). Those in chloroquine users were psychological (4 cases), seizures (3), abdominal pain (1) and visual disturbance (1).
Discontinuations of the study drug due to adverse effects
There was no differences between groups in the number of discontinuations due to adverse effects in the RCTs (RR 1.60, 95% CI 0.61 to 4.18; 3 RCTs, 815 participants, Analysis 4.3) or cohort studies in short‐term international travellers (RR 0.99, 95% CI 0.78 to 1.26; 6 cohort studies, 55,397 participants, Analysis 4.3). However, in the two cohort studies in longer‐term occupational travellers, mefloquine users were significantly more likely to stop taking medication (RR 2.97, 95% CI 2.41 to 3.66; 2 cohort studies; 6085 participants; Analysis 4.3).
Prespecified adverse effects
The RCTs only reported adverse events (all symptoms without assessing whether they might be related to the study drug). Our primary analysis was therefore taken from the six cohort studies reporting adverse effects.
Gastrointestinal symptoms
There were no consistent differences between groups for nausea (RR 1.23, 95% CI 0.89 to 1.68; I² statistic = 78%, 6 cohort studies, 58,984 participants, Analysis 4.4), vomiting (RR 1.05, 95% CI 0.78 to 1.40; 5 cohort studies, 5577 participants, Analysis 4.5) or abdominal pain (RR 0.99, 95% CI 0.80 to 1.22; 4 cohort studies, 5440 participants; Analysis 4.6). This was consistent with adverse events reported by RCTs (Analysis 4.4; Analysis 4.5; Analysis 4.6)
Overall, mefloquine users were less likely to report diarrhoea but this finding was from a single cohort study with over 90% of the weight in the meta‐analysis (RR 0.84, 95% CI 0.74 to 0.95; 5 cohort studies, 5577 participants; Analysis 4.7). No difference was seen in the RCTs (Analysis 4.7).
Neurological symptoms
In the cohort studies, there was no substantial difference between groups in the proportion of participants reporting headache (RR 0.84, 95% CI 0.53 to 1.34; 6 cohort studies, 56,998 participants, Analysis 4.8), but mefloquine users reported more dizziness (RR 1.51, 95% CI 1.34 to 1.70; 5 cohort studies, 56,710 participants; Analysis 4.9). The RCTs reporting adverse events did not demonstrate a difference between groups (Analysis 4.8; Analysis 4.9).
Psychological symptoms
Across the cohort studies, mefloquine users were more likely to report abnormal dreams (RR 1.21, 95% CI 1.10 to 1.33; 4 cohort studies, 2845 participants, Analysis 4.10), anxiety (RR 6.30, 95% CI 4.37 to 9.09; 3 cohort studies, 3408 participants, Analysis 4.12), depressed mood (RR 3.14, 95% CI 1.15 to 8.57; I² statistic = 90%; 5 cohort studies, 58,855 participants, Analysis 4.13) and abnormal thoughts or behaviour (RR 5.49, 95% CI 2.65 to 11.35; 4 cohort studies, 4831 participants, Analysis 4.14). Of these outcomes only abnormal dreams was reported by RCTs and the result was consistent with the cohort studies (Analysis 4.10). Insomnia was reported by five cohort studies (RR 1.81, 95% CI 0.73 to 4.51; 5 cohort studies, 56952 participants) and two RCTs (RR 1.19, 95% CI 0.76 to 1.84; 2 RCTs, 359 participants), and no consistent differences were seen between groups (Analysis 4.11).
Other prespecified adverse symptoms
There were no consistent differences demonstrated in reported pruritis between groups in cohort studies (RR 1.13, 95% CI 0.92 to 1.40; 2 cohort studies; 55,544 participants) or RCTs (RR 0.28, 95% CI 0.03 to 2.93; 2 RCTs, 413 participants; Analysis 4.15). There were no differences in visual impairment in cohort studies (RR 1.10, 95% CI 0.50 to 2.44; I² statistic = 90%, 5 cohort studies, 58,847 participants), or in the single RCT (RR 0.14, 95% CI 0.01 to 2.63; 210 participants, Analysis 4.16).
Prespecified adverse symptoms restricted to cohort studies in short‐term travellers
Analysis 4.18 presents the pre‐specified adverse symptoms restricted to the cohort studies in short‐term travellers.
Other adverse outcomes
Other adverse effects reported by cohort studies were alopecia (hair loss), asthenia, altered spatial perception, balance disorder, confusion, decreased appetite, fatigue, hypoaesthesia, irritability, mouth ulcers, paraesthesia, palpitation, photosensitization, restlessness, slight illness, somnolence and yeast infection (Analysis 4.19). Of note, single cohort studies found that mefloquine users were more likely to report altered spatial perception (RR 3.16, 95% CI 1.55 to 6.45; 2032 participants), unsteadiness (RR 3.59, 95% CI 2.15 to 6.00; 2137 participants), alopecia (RR 1.69, 95% CI 1.27 to 2.25; 2137 participants), limb numbness (RR 20.26, 95% CI 1.23 to 333.93; 2137 participants) and tingling (RR 2.22, 95% CI 1.27 to 3.89; 2 cohort studies, 2778 participants).
Other adverse events reported by RCTs were abdominal distension, anger, disturbance in attention, irritability, loss of appetite, malaise and altered mood (Analysis 4.20). No statistically significant differences were noted.
Pregnancy‐related outcomes
One quasi‐randomized trial (Steketee 1996) was conducted in pregnant Malawian women and reported no difference between mefloquine and chloroquine for spontaneous abortions (RR 0.80, 95% CI 0.36 to 1.79; 2334 participants), still births (RR 1.01, 95% CI 0.67 to 1.52; 2334 participants) or congenital malformations (0 events in either study arm, 2334 participants, Analysis 4.21). Steketee 1996 sequentially allocated participants to each drug regimen, and did not blind participants or study personnel.
Adherence
Three cohort studies in short‐term travellers (Hill 2000; Laver 2001; Rietz 2002) compared the proportion of participants with 100% self‐reported adherence and found no difference overall (RR 1.00, 95% CI 0.90 to 1.13; 3 cohort studies, 852 participants, Analysis 4.22). Among participants in these studies, 84% of mefloquine users described themselves as adherent during travel (range 71% to 88%) compared to 82% of chloroquine users (range 82% to 85%). In the two studies in longer‐term occupational travellers, self‐reported adherence was higher in mefloquine users (RR 2.02, 95% CI 1.80 to 2.26; 2 cohort studies, 5777 participants).
One study (Stoney 2016) measured adherence in the post‐travel period and found no difference (RR 1.00, 95% CI 0.54 to 1.87; 46 participants, Analysis 4.22). However, rates of completion were low in both groups (56% in mefloquine users and 54% in chloroquine users).
Subgroup analyses
Given the similarity in adverse effect profiles for mefloquine compared to the two main alternatives (doxycycline and atovaquone‐proguanil), we combined findings from the two comparisons and performed a series of subgroup analyses to explore the effects of study design, duration of travel, and military versus non‐military participants.
Prespecified adverse effects
Study design
Only one RCT performed a blinded assessment of whether there was a reasonable possibility that any reported symptoms could be related to the study drug (Overbosch 2001). We compared this with participants self‐reporting of adverse effects in cohort studies. The findings were largely consistent across study designs with mefloquine users experiencing higher rates of headache (Analysis 5.4), dizziness (Analysis 5.5), abnormal dreams (Analysis 5.6), insomnia (Analysis 5.7), anxiety (Analysis 5.8) and depressed mood (Analysis 5.9). Although the relative risk of psychiatric side effects was consistently slightly higher in cohort studies, in only one case was the test for subgroup differences statistically significant (abnormal dreams: RCT: RR 2.04, 95% CI 1.37 to 3.04; 976 participants, cohort studies: RR 7.30, 95% CI 2.51 to 21.18; 7 cohort studies, 4543 participants, test for subgroup differences P = 0.03).
Duration of travel
The relative risk of all psychological adverse effects was higher with longer‐term travel than in short‐term travel; insomnia (short‐term RR 3.09 versus longer‐term RR 8.67), anxiety (short‐term RR 3.26 versus longer‐term RR 18.05), depressed mood (short‐term RR 2.52 versus longer‐term RR 12.59) and abnormal thoughts and perceptions (short‐term RR 1.29 versus longer‐term RR 7.78) (Table 14). However, in only one case was the test for subgroup differences statistically significant (P range 0.02 to 0.40). This same effect was not observed with gastrointestinal symptoms (nausea, abdominal pain, diarrhoea) or neurological symptoms (headache, dizziness).
| Mefloquine versus atovaquone‐proguanil and doxycycline | |||
| Outcome | Short‐ term travellers¹ | Longer‐ term travellers² | Test for subgroup |
| Relative effect (RR) | Relative effect (RR) | ||
| Serious adverse effects | RR 5.38 (0.60 to 47.84) 3 cohort studies (2657) | RR 0.93 (0.43 to 2.01) 3 cohort studies (3147) | P = 0.14 |
| Discontinuations due to adverse effects (RCTs) | RR 2.64 (1.51 to 4.62) 5 RCTs (2048) | ‐ | ‐ |
| Discontinuations due to adverse effects (cohort studies) | RR 1.81 (0.86 to 3.80) 7 cohort studies (2907) | RR 1.19 (0.45 to 3.17) 4 cohort studies (5711) | P = 0.50 |
| Nausea | RR 2.02 (0.87 to 4.68) 6 cohort studies (2469) | RR 0.96 (0.22 to 4.18) 3 cohort studies (2725) | P = 0.39 |
| Abdominal pain | RR 0.66 (0.22 to 1.98) 5 cohort studies (1801) | RR 0.30 (0.22 to 0.42) 3 cohort studies (2725) | P = 0.18 |
| Diarrhoea | RR 0.64 (0.15 to 2.71) 5 cohort studies (2428) | RR 0.57 (0.22 to 1.49) 4 cohort studies (5187) | P = 0.89 |
| Headache | RR 2.39 (0.69 to 8.22) 5 cohort studies (2086) | RR 2.09 (1.10 to 3.95) 4 cohort studies (3506) | P = 0.85 |
| Dizziness | RR 3.05 (1.15 to 8.12) 4 cohort studies (1067) | RR 3.84 (1.34 to 11.00) 4 cohort studies (3506) | P = 0.76 |
| Abnormal dreams | RR 6.25 (1.16 to 33.67) 3 cohort studies (1037) | RR 7.62 (2.06 to 28.18) 4 cohort studies (3506) | P = 0.86 |
| Insomnia | RR 3.09 (0.30 to 32.21) 4 cohort studies (1760) | RR 8.67 (4.73 to 15.89) 4 cohort studies (3506) | P = 0.40 |
| Anxiety | RR 3.26 (0.20 to 53.46) 1 cohort study (487) | RR 18.05 (9.75 to 33.42) 3 cohort studies (2854) | P = 0.24 |
| Depressed mood | RR 2.52 (0.76 to 8.29) 3 cohort studies (1026) | RR 12.59 (6.47 to 24.49) 3 cohort studies (3210) | P = 0.02 |
| Abnormal thoughts and behaviours | RR 1.29 (0.07 to 22.44) 1 cohort study (487) | RR 7.78 (1.12 to 54.06) 2 cohort studies (2558) | P = 0.31 |
| Adherence: during travel | RR 1.10 (1.03 to 1.18) 7 cohort studies (7241) | RR 1.20 (0.88 to 1.62) 4 cohort studies (4890) | P = 0.61 |
| Adherence: after return | RR 1.04 (0.92 to 1.17) 4 cohort studies (1221) | ‐ | ‐ |
1 Short‐ term travellers: Approximately 3 weeks (range 1 day to 3 months). References: Goodyer 2011; Kato 2013; Kuhner 2005; Napoletano 2007; Laver 2001; Laverone 2006; Lobel 2001; Philips 1996; Schwartz 1999; Shamiss 1996; Sonmez 2005; Stoney 2016; Terrell 2015
2 Longer‐ term travellers: Approximately 6 months (range 0 to 36 months in Cunningham 2014 . Otherwise 3 months or longer). References Andersson 2008; Cunningham 2014; Korhonen 2007; Landman 2015; Saunders 2015; Sharafeldin 2010
Military versus non‐military participants
There were no significant differences in the relative risk of adverse effects between military and non‐military participants (Table 15). Very few cohort studies in military personnel reported on our prespecified symptoms. In one of these in which military personnel who took mefloquine for 6 months or longer (Andersson 2008), the rates of psychological side effects were significantly higher than in short‐term travellers, but not significantly different from other trials in longer‐term travellers.
| Mefloquine versus atovaquone‐proguanil and doxycycline | |||
| Outcome | Military¹ | Non‐military² | Test for subgroup |
| Relative effect (RR) | Relative effect (RR) | ||
| Serious adverse effects | 0 events in 1386 participants | RR 1.21 (0.60 to 2.44) 4 cohort studies (4418) | ‐ |
| Discontinuations due to adverse effects (RCTs) | RR 2.08 (0.13 to 32.73) 2 RCTs (441) | RR 2.22 (1.17 to 4.21) 4 RCTs (1669) | P = 0.96 |
| Discontinuations due to adverse effects (cohorts) | RR 1.24 (0.32 to 4.88) 4 cohort studies (3408) | RR 1.89 (1.35 to 2.64) 8 cohort studies (8938) | P = 0.56 |
| Nausea | RR 1.39 (0.36 to 5.36) 4 cohort studies (1578) | RR 1.70 (0.60 to 4.81) 6 cohort studies (3767) | P = 0.26 |
| Abdominal pain | RR 0.43 (0.14 to 1.29) 4 cohort studies (1578) | RR 0.56 (0.23 to 1.35) 5 cohort studies (3099) | P = 0.72 |
| Diarrhoea | RR 0.30 (0.09 to 0.96) 4 cohort studies (3999) | RR 1.05 (0.54 to 2.06) 6 cohort studies (3767) | P = 0.07 |
| Headache | RR 1.19 (0.14 to 9.79) 2 cohort studies (1386) | RR 2.48 (1.40 to 4.40) 7 cohort studies (4206) | P = 0.51 |
| Dizziness | RR 2.95 (1.37 to 6.36) 3 cohort studies (844) | RR 3.58 (1.39 to 9.25) 6 cohort studies (3880) | P = 0.76 |
| Abnormal dreams | RR 11.02 (4.61 to 26.34) 1 cohort study (652) | RR 6.59 (1.74 to 25.00) 6 cohort studies (3891) | P = 0.53 |
| Insomnia | RR 2.34 (0.41 to 13.35) 3 cohort studies (1537) | RR 10.24 (6.26 to 16.76) 6 cohort studies (3880) | P = 0.11 |
| Anxiety | ‐ | RR 16.94 (9.36 to 30.64) 4 cohort studies (3390) | ‐ |
| Depressed mood | RR 13.44 (3.34 to 54.05) 1 cohort study (652) | RR 6.49 (2.66 to 15.85) 5 cohort studies (3584) | P = 0.39 |
| Abnormal thoughts and behaviours | ‐ | RR 5.11 (1.11 to 23.53) 3 cohort studies (3045) | ‐ |
| Adherence: during travel | RR 1.18 (1.00 to 1.40) 5 cohort studies (4652) | RR 1.16 (0.99 to 1.35) 8 cohort studies (10785) | P = 0.85 |
| Adherence: after return | RR 1.16 (0.86 to 1.55) 1 cohort study (43) | RR 1.02 (0.89 to 1.16) 3 cohort studies (1178) | P = 0.44 |
1 Military participants: References: RCTs: Arthur 1990; Ohrt 1997. Cohort studies: Andersson 2008, Saunders 2015; Shamiss 1996; Sonmez 2005; Terrell 2015; Tuck 2016
2 Non‐military participants: References: RCTs: Overbosch 2001; Schlagenhauf 2003; van Riemsdijk 2002; Weiss 1995. Cohort studies: Cunningham 2014; Goodyer 2011; Kato 2013; Kuhner 2005; Korhonen 2007; Landman 2015; Laver 2001; Laverone 2006; Lobel 2001; Napoletano 2007; Philips 1996; Schwartz 1999; Sharafeldin 2010; Stoney 2016
Adherence
Study design
Across cohort studies, self‐reported complete adherence was slightly higher in participants who took mefloquine than in users of other antimalarial agents (RR 1.16, 95% CI 1.03 to 1.30; 11 cohort studies, 12131 participants, Analysis 5.13). However, there was no difference in self‐reported completion of the treatment after return (RR 1.04, 95% CI 0.92 to 1.17; 4 cohort studies, 1221 participants, Analysis 5.14).
Duration of travel
Self‐reported complete adherence was slightly higher in short‐term travellers who took mefloquine than users of other antimalarial agents (RR 1.10, 95% CI 1.03 to 1.18; 7 cohort studies, 7241 participants). However, the same effect was not seen in longer‐term travellers (RR 1.20, 95% CI 0.88 to 1.62; 4 cohort studies, 4890 participants, test for subgroup differences P = 0.61, Table 14).
There was no overall difference in rates of completing the treatment regimen after return in short‐term travellers who took mefloquine than in those who received other antimalarial agents (RR 1.04, 95% CI 0.92 to 1.17; 4 cohort studies, 1221 participants). No studies in longer‐term travellers monitored adherence after return.
Military versus non‐military participants
There were no differences in self‐reported complete adherence when comparing military versus non‐military participants, either during travel or after return (Table 15).
Discussion
Summary of main results
Mefloquine efficacy
We included 12 randomized controlled trials (RCTs) that compared mefloquine with placebo; none were performed in short‐term international travellers, and most populations had a degree of immunity to malaria. The percentage of people developing a malaria episode in the control arm varied from 1% to 82% (median 22%) and in the mefloquine group 0% to 13% (median 1%).
In four other RCTs that directly compared mefloquine, atovaquone‐proguanil and doxycycline in non‐immune, short‐term international travellers, only one clinical case of malaria occurred (low certainty evidence).
Mefloquine safety versus currently used alternatives
Serious adverse effects have been reported for mefloquine and doxycyline, but not for atovaquone‐proguanil. Serious adverse effects are uncommon, and on statistical testing, no difference was detected between mefloquine and atovaquone‐proguanil (low‐certainty evidence), or between mefloquine and doxycycline (very low‐certainty evidence).
Participants who received mefloquine were more likely to discontinue their medication due to adverse effects than participants who received atovaquone‐proguanil (high‐certainty evidence), but there was no difference in comparisons with doxycycline (low‐certainty evidence).
We included one RCT and six cohort studies that reported our prespecified adverse effects that compared mefloquine and atovaquone‐proguanil. In the RCT in short‐term travellers, mefloquine users were more likely to report abnormal dreams (moderate‐certainty evidence), insomnia (moderate‐certainty evidence), anxiety (moderate‐certainty evidence), and depressed mood during travel (moderate‐certainty evidence). The cohort studies in longer‐term travellers were consistent with these findings but most had larger effect sizes. Mefloquine users were also more likely to report nausea (high‐certainty evidence) and dizziness (high‐certainty evidence).
We included six cohort studies in longer‐term occupational travellers that compared mefloquine with doxycycline which reported our prespecified adverse effects. We also included one RCT in military personnel and one cohort in short‐term travellers that reported adverse events. Mefloquine users were more likely to report abnormal dreams (very low‐certainty evidence), insomnia (very low‐certainty evidence), anxiety (very low‐certainty evidence) and depressed mood (very low‐certainty evidence). The findings of the single cohort study reporting adverse events in short‐term international travellers were consistent with these findings but the single RCT in military personnel did not demonstrate a difference between groups in the frequency of abnormal dreams or insomnia. Doxycycline users were more likely to report dyspepsia (very low‐certainty evidence), photosensitivity (very low‐certainty evidence), vomiting (very low‐certainty evidence) and vaginal thrush (very low‐certainty evidence).
Comparisons with chloroquine showed a broadly consistent pattern with these results.
Overall completeness and applicability of evidence
Mefloquine has been licensed for prevention of malaria in travellers since the late 1980s, and as such, it is perhaps surprising how few well‐conducted RCTs were available. However, because we were mainly interested in the adverse effect profiles of different antimalarial agents, cohort studies (of which there are many) are probably the most appropriate study design despite their inherent limitations. Most RCTs excluded people with a previous history of mental health problems, precluding an analysis of whether psychological side effects are more common in this group. Conversely, many of the cohort studies explicitly stated that the choice of antimalarial agent was influenced by both past medical history and personal preference. While this undoubtedly introduces some confounding between study groups, we consider this confounding to be appropriate and directly applicable to clinical practice. Similarly, we would normally be cautious about interpreting unblinded self‐reported assessments of adverse effects and causality. In this scenario, self‐reported adverse effects provide useful and relevant information for travellers, who would also be unblinded. It should be noted that the reported adverse effects are largely self‐reported psychiatric symptoms and not formal psychiatric diagnoses.
Given the heterogeneity in trial design, mefloquine doses used, and the study population, we were unable to derive a reliable estimate for mefloquine efficacy. However, the evidence suggests that mefloquine is likely to be highly effective in reducing clinical episodes of malaria. Comparative trials found no difference in efficacy between mefloquine and atovaquone‐proguanil or doxycycline for preventing clinical malaria, but the number of malaria episodes was very low, and consequently, much larger trials would be needed to exclude clinically important differences. As a consequence, knowledge about antimalarial resistance patterns in the country of travel seems an appropriate approach to decision making rather than further RCTs.
The choice between antimalarial agents will therefore depend on how individual travellers rate the relative importance of specific adverse effects, pill burden and cost. Prophylactic mefloquine is widely acknowledged to cause abnormal dreams and psychological adverse effects and we found consistent evidence for these effects across comparisons with atovaquone‐proguanil, doxycycline and chloroquine (the most commonly used alternatives). Doxycycline does not have the same risk of psychological adverse effects, but is associated with increased risk of photosensitivity, dyspepsia, and vaginal thrush, which some travellers will undoubtedly consider important. In line with this, participants who received mefloquine were more likely to discontinue treatment due to adverse effects than participants who received atovaquone‐proguanil, but there was no difference in comparisons with doxycycline.
We found estimating the risk of serious psychological adverse effects from the studies was not straightforward. Study authors used the term 'serious' loosely, and often did not provide us with the detail required to determine whether these events met standardized definitions. Furthermore, the estimates of the absolute risk in both mefloquine and comparator arm varied considerably between trials, which may be related to data collection methods and the cut‐offs used rather than true differences among populations. Overall, we did not identify large differences in the risk of serious adverse effects among antimalarial agents; but what we did find was that the nature of these serious adverse effects corresponded with the known side effect profile of each drug.
The findings of our related systematic review which analysed deaths and parasuicides associated with mefloquine prophylaxis, and included case reports, had findings consistent with this (Tickell‐Painter 2017). This systematic review reports that there were no suicides we could reliably attribute to mefloquine prophylaxis, and one para‐suicide with a possible causal association. In the analysed reports, we identified two deaths with a probable association that appeared to be idiosyncratic drug reactions; the remaining eight deaths we categorised as “unlikely” to be related to mefloquine, or “unclassifiable”.
We believe it is important that the large retrospective healthcare record analyses did not demonstrate a clear quantitative association between mefloquine use and formal mental health disorders. This may reflect the inadequacy of the study methods to detect this outcome, but may also reflect the transient nature of the mood disturbance, with resolution once mefloquine is discontinued. We were unable to comment on the severity or duration of the reported adverse effects based on the available data.
The data on mefloquine at a prophylactic dose during pregnancy were limited (2 RCTs; no comparative cohort studies). Both RCTs included semi‐immune populations who did not travel.
Mefloquine has an advantage as the only malaria prophylaxis with a once weekly regimen. Many have cited this as a mechanism to improve adherence, which is notoriously low in all users of antimalarial prophylaxis. However, the evidence base for this assertion is weak, with almost all data originating from cohort studies which reported a variety of measures of self‐reported complete adherence.
We were unable to perform some prespecified subgroup analyses including children versus adults, female versus male travellers and pregnant versus non‐pregnant women. This meant we were unable to test whether women were more likely to experience adverse effects from mefloquine use (which has been widely reported in the literature).
We appreciate that the distinction between adverse events (all events regardless of relationship to the study drug) and adverse effects (events attributed by study authors or participants to the study drug) can seem arbitrary and cause confusion. However, we consulted extensively with methodologists who advised that both outcomes are useful to decision makers, and there is no overall gold standard. For example, reporting only the adverse effects (for example, hospitalizations, psychiatric side effects) thought to be attributed to the drug regimen can introduce selective bias by the study authors. For controversial or pharmaceutical company‐funded studies this can distort the outcomes. By comparing all events across both groups any difference in the relative risk can be compared without the potential for selective bias. However, this does have its own limitations, such as if the two groups were not comparable at baseline or if the sample size is not big enough to exclude differences due to chance. We therefore chose to include both options (events and effects) to give readers and decision makers the complete picture.
Quality of the evidence
In the 'Summary of findings' tables we present what we consider to be the best estimate of effect for each outcome, within each comparison. Where possible we chose the estimate from RCTs reporting adverse effects, but where this was not available we used estimates from cohort studies. However, when making judgements about the certainty of evidence we considered all the evidence available, as well as the consistency of the effect across different population groups and study designs.
For the comparison of mefloquine with atovaquone‐proguanil, the best estimates of effect came from a single, well‐conducted RCT in short‐term travellers, recording participant‐reported adverse effects. The findings of this study were supported by seven cohort studies in long‐term occupational travellers and military personnel. We considered the evidence of increased risk of abnormal dreams and insomnia to be high certainty because the effects were consistent across all population groups. However, we downgraded the effect estimate on anxiety and depressed mood for inconsistency to moderate certainty because there was substantial variation in the effect size across populations, with much larger effects in long‐term travellers and military personnel.
For the comparison of mefloquine with doxycycline, the only available RCT was very small, and reported adverse events rather than adverse effects. Consequently, we considered the effect estimates from cohort studies to be more reliable. Evidence from cohort studies was automatically downgraded to low based on the inherent bias in the study design. We further downgraded almost all estimates of effect for indirectness, because most data were from long‐term travellers and military personnel, and may therefore over estimate the effect in short‐term travel. The evidence is therefore considered to be very low‐certainty with little confidence in the size of the effect. It is important to note however, that the pattern of adverse effects with mefloquine in these cohort studies is entirely consistent with the pattern seen in comparisons of mefloquine with atovaquone‐proguanil and chloroquine.
Potential biases in the review process
During the course of this review we made changes to the protocol. Two changes were made to shorten the overall length of the review:
-
we excluded comparisons of mefloquine with primaquine and tafenoquine because these are planned for assessment in another Cochrane Review (Rodrigo 2016);
-
we excluded single‐arm cohort studies because there were sufficient data from comparative studies to reach reasonable conclusions. These studies have been analysed for the very rare outcomes of death or attempted suicide in another systematic review (Tickell‐Painter 2017).
We do not think these decisions biased the review.
Agreements and disagreements with other studies or reviews
Several recently published reviews regarding the safety of mefloquine have been narrative, and included little or no description of methods applied and a lack of clearly defined and prespecified outcomes (McCarthy 2015; Nevin 2015; Schlagenhauf 2010). McCarthy 2015 and Nevin 2015 discuss the policy implications of mefloquine use by the military which was beyond the scope of this Cochrane Review.
Schlagenhauf 2010 highlighted several areas in which mefloquine prophylaxis may be considered advantageous (during pregnancy and while breastfeeding, in long‐term travellers, travellers who are visiting friends and relatives and families with small children). The main disagreement with our review was in regard to safety in long‐term travellers, in whom the review authors refer to mefloquine as "a good option if well tolerated". This is based on a narrative analysis of a single cohort study which compared mefloquine users with users of chloroquine‐proguanil, which was not included in this review (Lobel 1993).
Our review added data from several additional studies evaluating longer‐term use (Andersson 2008; Cunningham 2014; Korhonen 2007; Landman 2015), and we found some observational evidence that risk of adverse effects was higher than with short‐term travel.
Our findings are broadly consistent with the previous version of this Cochrane Review, which was withdrawn (Jacquerioz 2015). Jacquerioz 2015 found higher rates of neuropsychiatric adverse events in mefloquine users compared with users of both atovaquone‐proguanil and doxycycline. We expanded on this finding by providing estimated risks for specific neurological and psychiatric symptoms, and by including additional data from cohort studies. Jacquerioz 2015 included a brief analysis of case reports of deaths associated with mefloquine in the Discussion. We excluded this analysis from this update, but this aspect has been addressed in a separate review of single‐arm cohort studies and case reports (Tickell‐Painter 2017).
Two recent reviews included evaluations of mefloquine efficacy and safety during pregnancy. González 2014 concluded there were no indications that mefloquine use during pregnancy carries an increased risk for the foetus. González 2014 included additional studies to those we included in this Cochrane Review, including mefloquine when used at treatment dose, or as intermittent presumptive treatment in pregnancy. Muanda 2015 also included mefloquine when used as intermittent presumptive treatment in pregnancy. Muanda 2015 reported findings from two trials in which the number of adverse events (Briand 2009), and number of serious adverse events (González 2014a) was higher in participants who received mefloquine as intermittent presumptive treatment in pregnancy than in those who received sulphadoxine‐pyrimethamine.
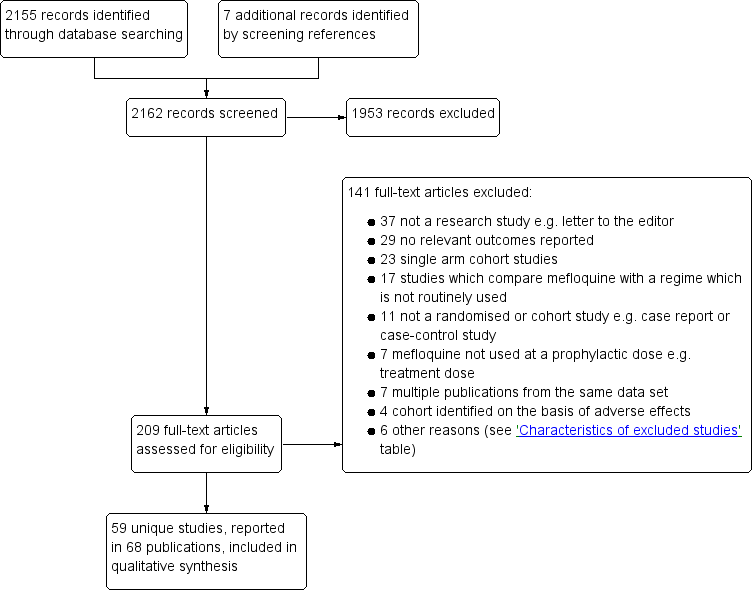
Study flow diagram.
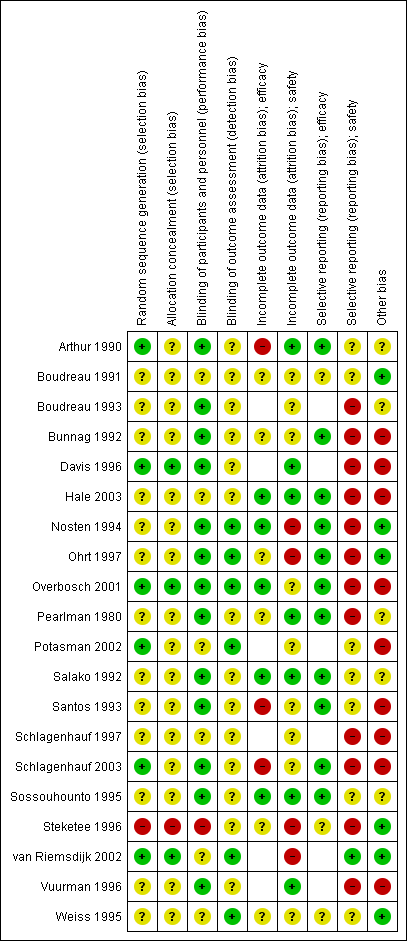
'Risk of bias' summary for RCTs: review authors' judgements about each 'Risk of bias' item for each included study.

'Risk of bias' summary in cohort studies: mefloquine versus placebo/no treatment
1Assesses whether our pre‐defined confounders were measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assess the risk of bias due to influence by a corporate study sponsor.

'Risk of bias' summary in cohort studies: mefloquine versus doxycycline
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.
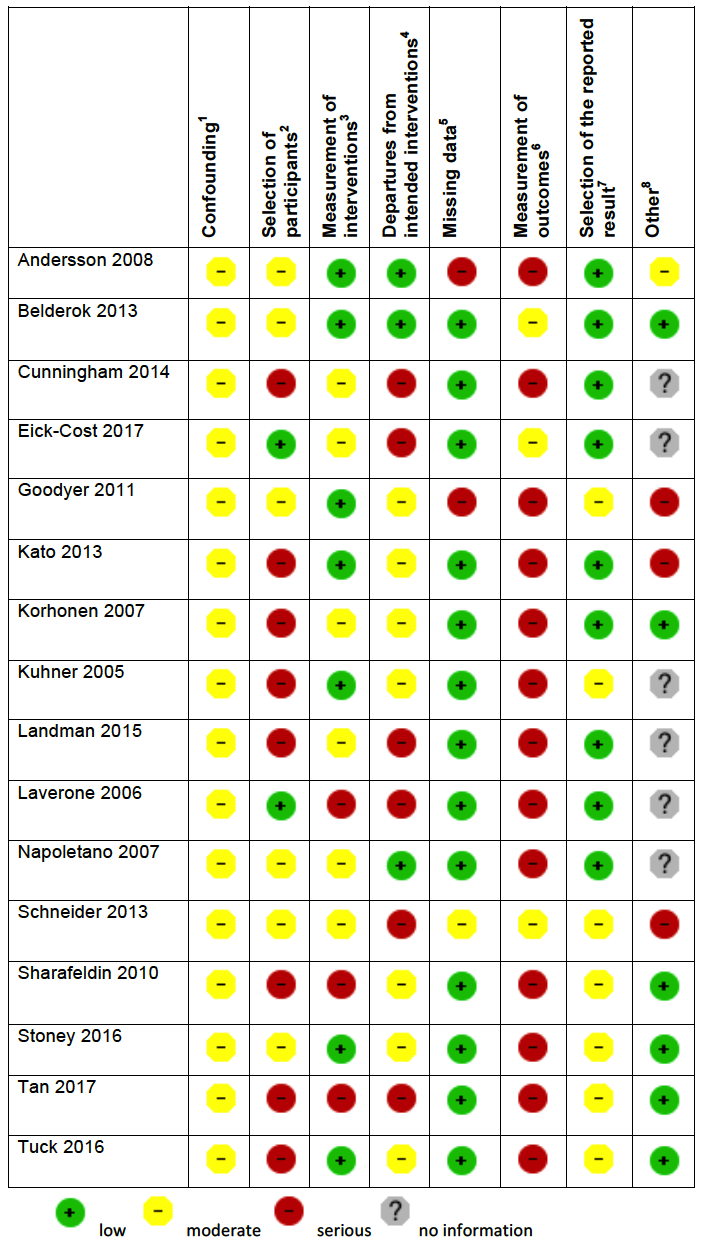
'Risk of bias' summary in cohort studies: mefloquine versus atovaquone‐proguanil
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.

'Risk of bias' summary in cohort studies: mefloquine versus chloroquine
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.
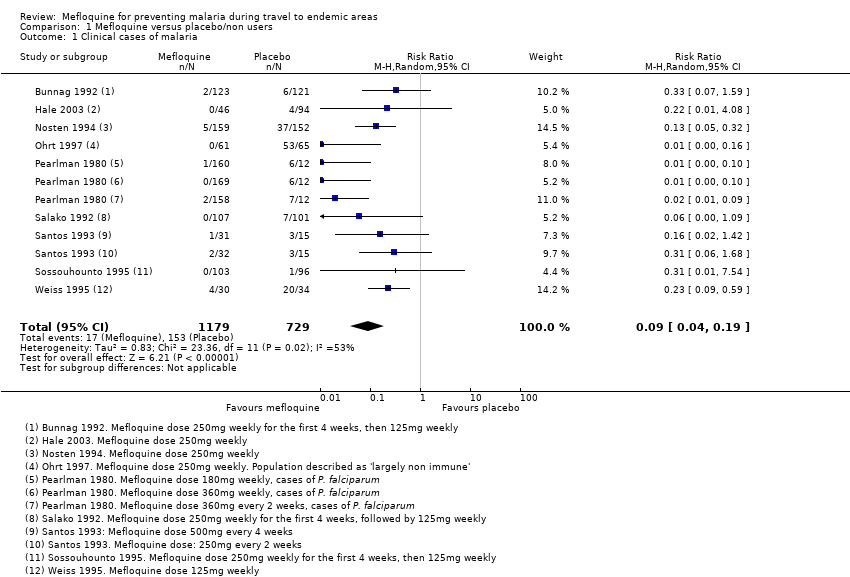
Comparison 1 Mefloquine versus placebo/non users, Outcome 1 Clinical cases of malaria.
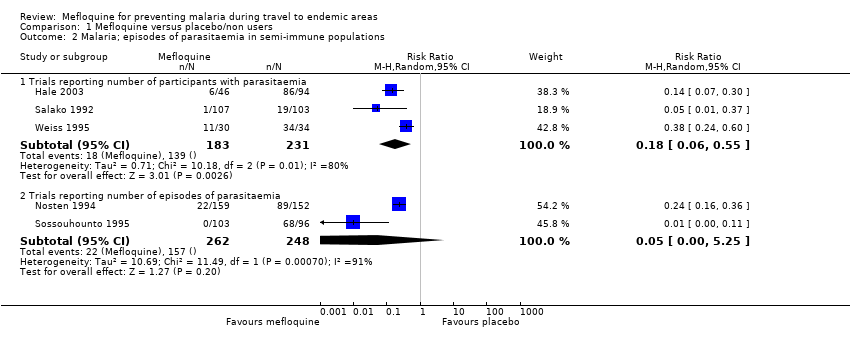
Comparison 1 Mefloquine versus placebo/non users, Outcome 2 Malaria; episodes of parasitaemia in semi‐immune populations.
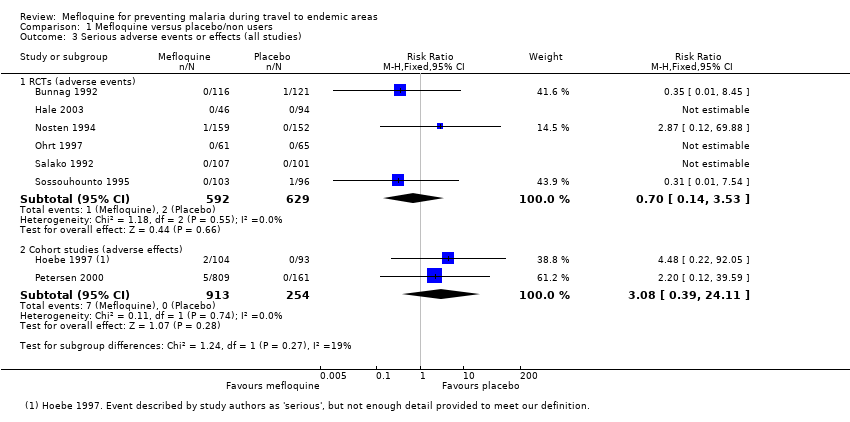
Comparison 1 Mefloquine versus placebo/non users, Outcome 3 Serious adverse events or effects (all studies).
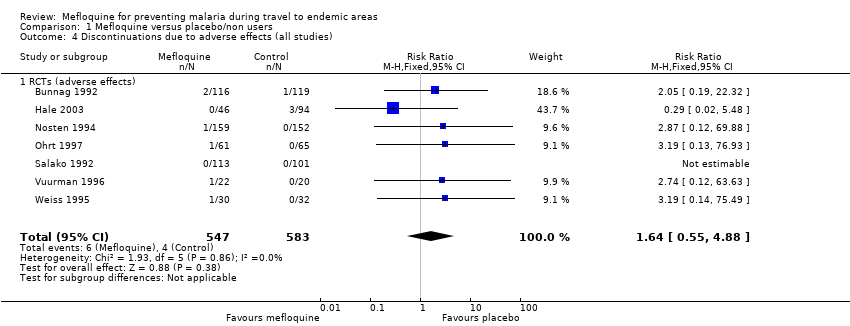
Comparison 1 Mefloquine versus placebo/non users, Outcome 4 Discontinuations due to adverse effects (all studies).
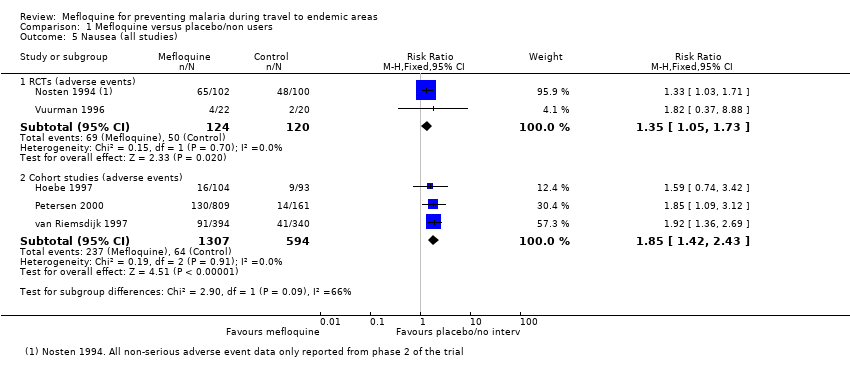
Comparison 1 Mefloquine versus placebo/non users, Outcome 5 Nausea (all studies).
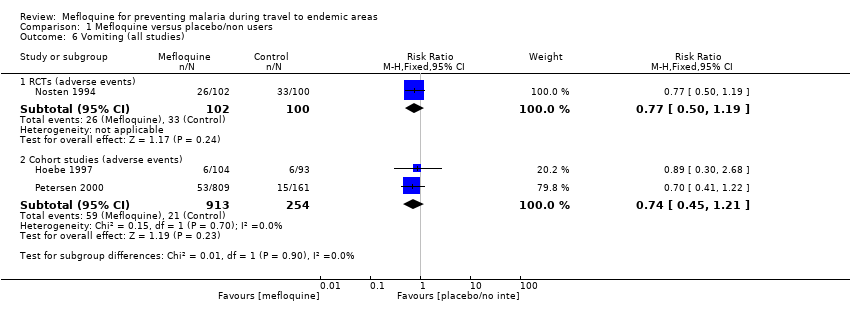
Comparison 1 Mefloquine versus placebo/non users, Outcome 6 Vomiting (all studies).
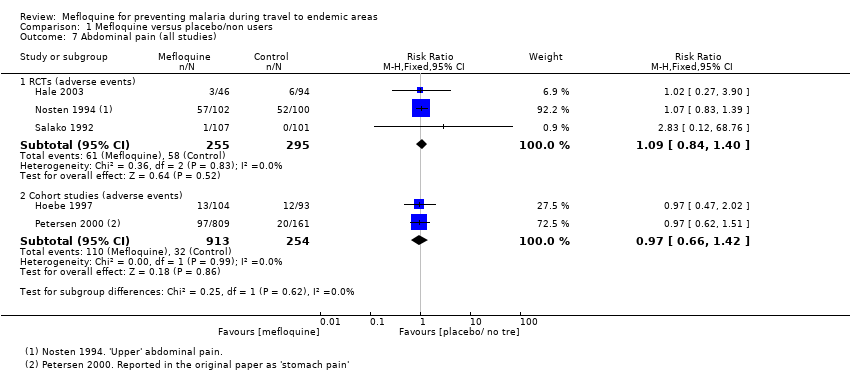
Comparison 1 Mefloquine versus placebo/non users, Outcome 7 Abdominal pain (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 8 Diarrhoea (all studies).
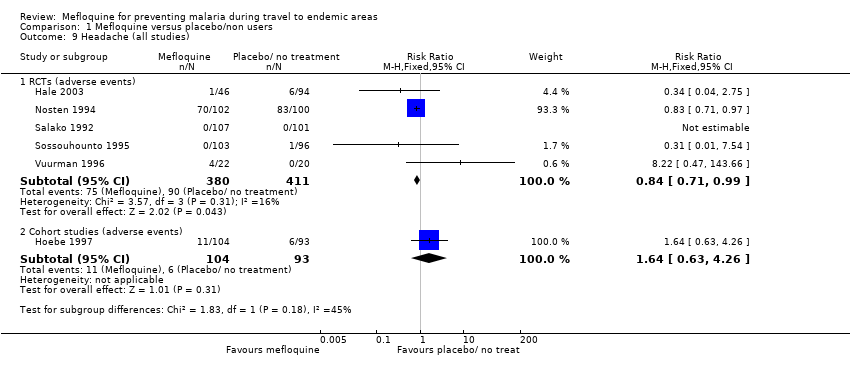
Comparison 1 Mefloquine versus placebo/non users, Outcome 9 Headache (all studies).
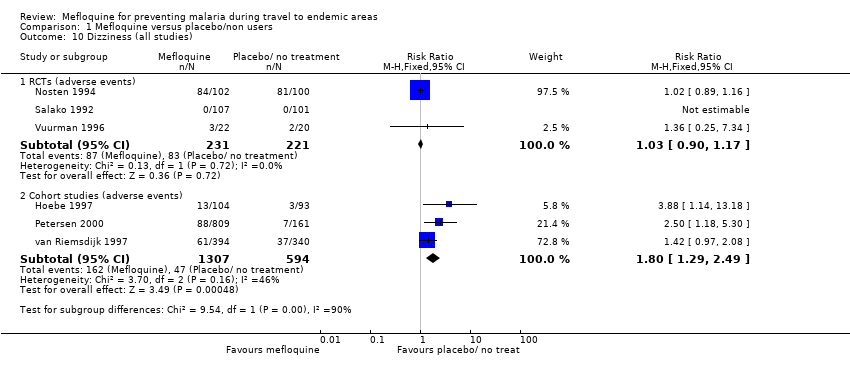
Comparison 1 Mefloquine versus placebo/non users, Outcome 10 Dizziness (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 11 Abnormal dreams (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 12 Insomnia (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 13 Anxiety (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 14 Depressed mood (all studies).
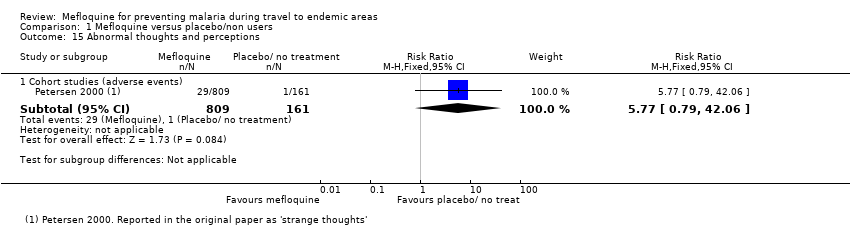
Comparison 1 Mefloquine versus placebo/non users, Outcome 15 Abnormal thoughts and perceptions.
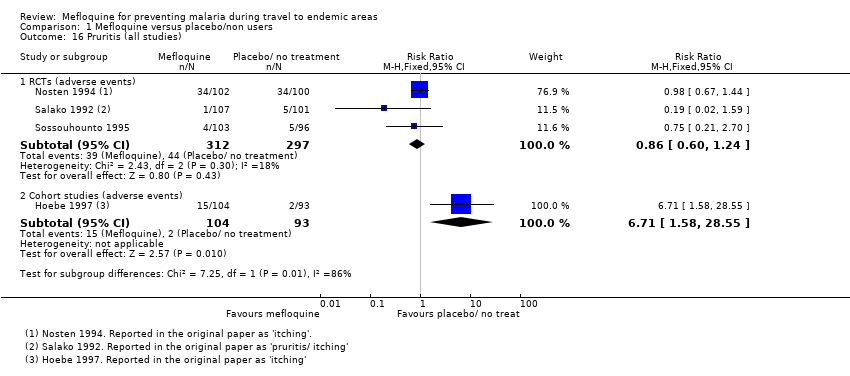
Comparison 1 Mefloquine versus placebo/non users, Outcome 16 Pruritis (all studies).
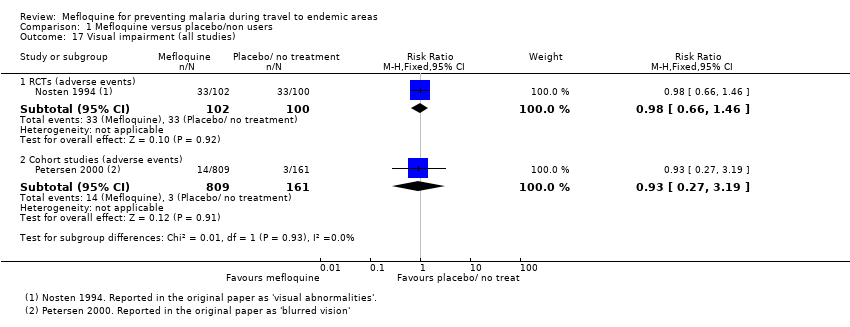
Comparison 1 Mefloquine versus placebo/non users, Outcome 17 Visual impairment (all studies).
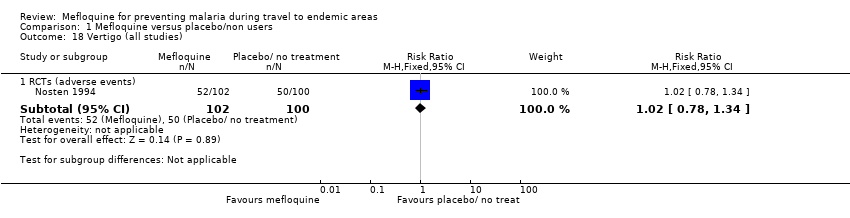
Comparison 1 Mefloquine versus placebo/non users, Outcome 18 Vertigo (all studies).
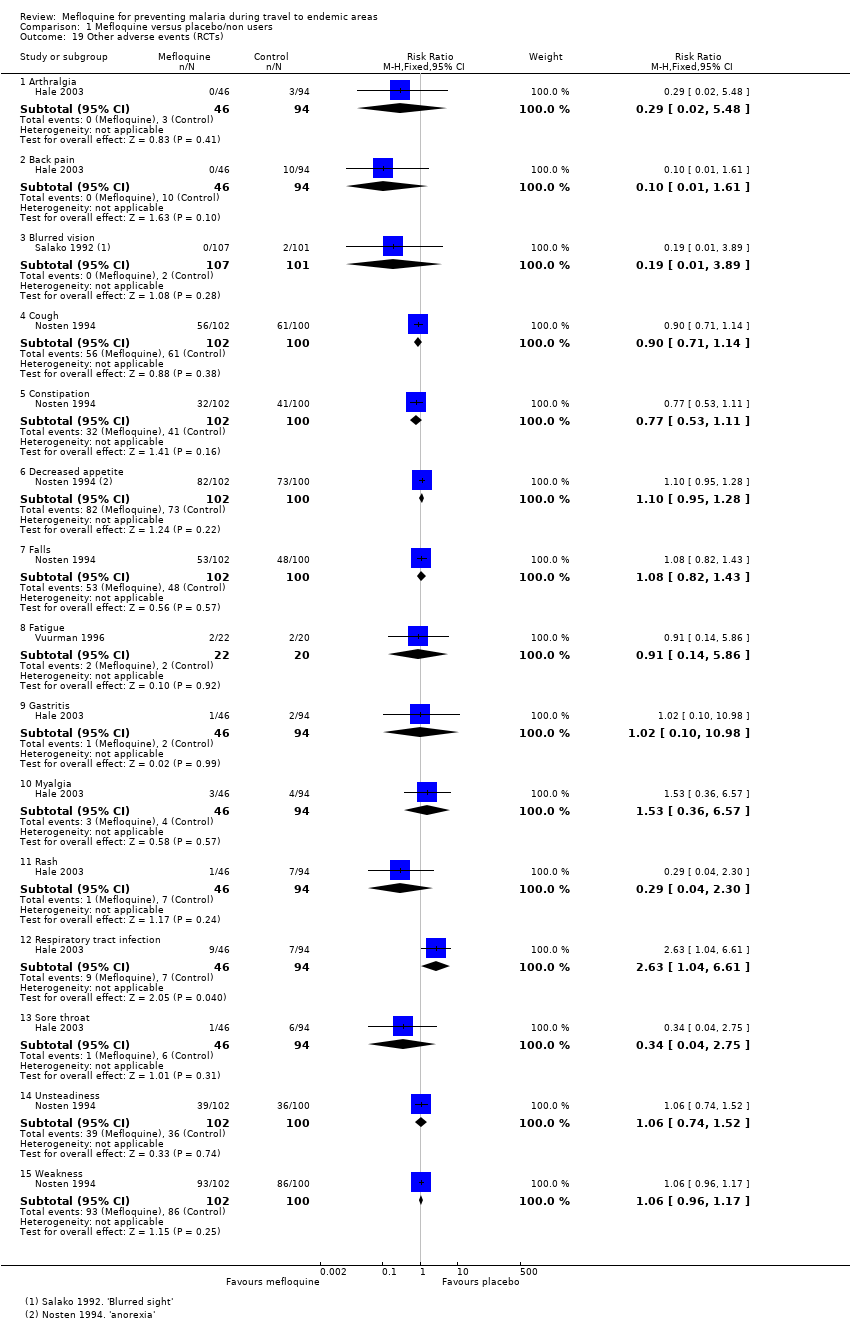
Comparison 1 Mefloquine versus placebo/non users, Outcome 19 Other adverse events (RCTs).
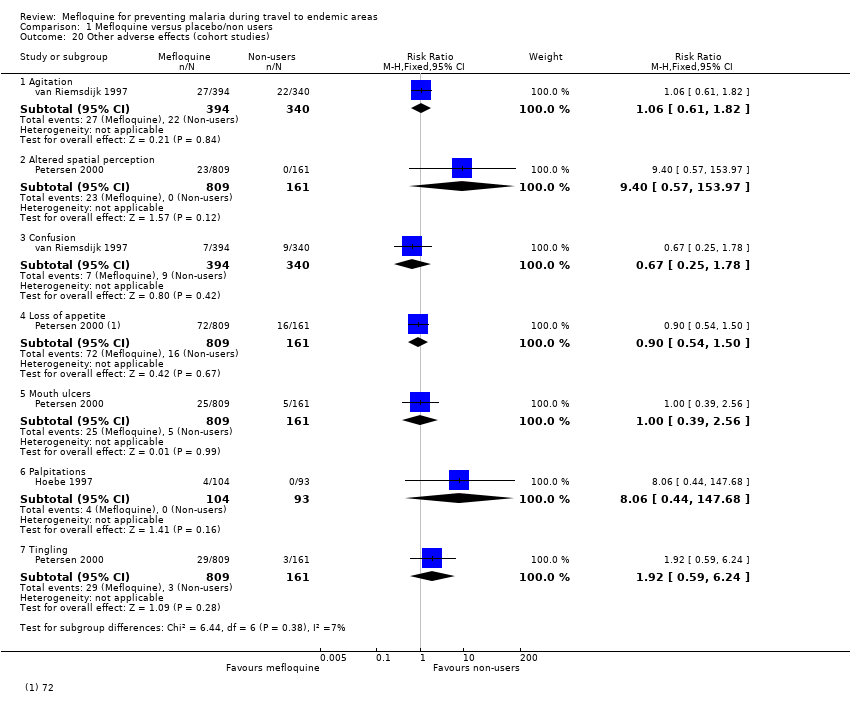
Comparison 1 Mefloquine versus placebo/non users, Outcome 20 Other adverse effects (cohort studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 1 Clinical cases of malaria (RCTs).

Comparison 2 Mefloquine versus doxycycline, Outcome 2 Serious adverse events or effects (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 3 Discontinuations due to adverse effects (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 4 Nausea (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 5 Vomiting (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 6 Abdominal pain (all studies).
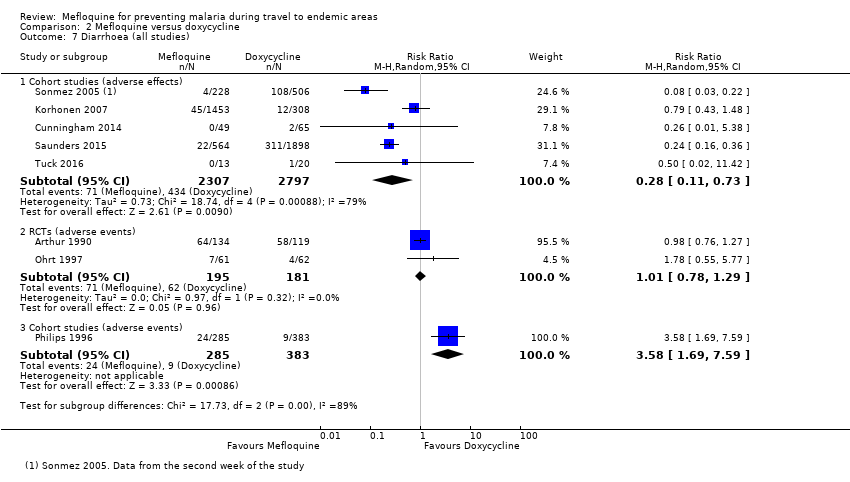
Comparison 2 Mefloquine versus doxycycline, Outcome 7 Diarrhoea (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 8 Dyspepsia (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 9 Headache (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 10 Dizziness (all studies).
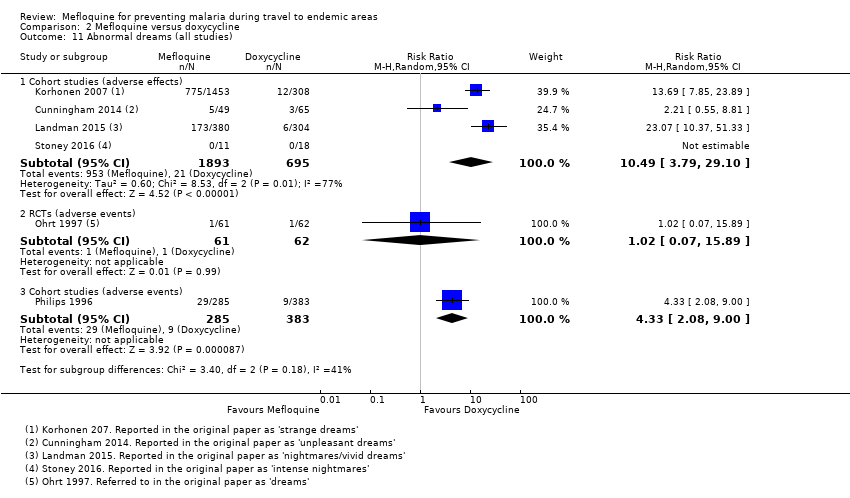
Comparison 2 Mefloquine versus doxycycline, Outcome 11 Abnormal dreams (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 12 Insomnia (all studies).
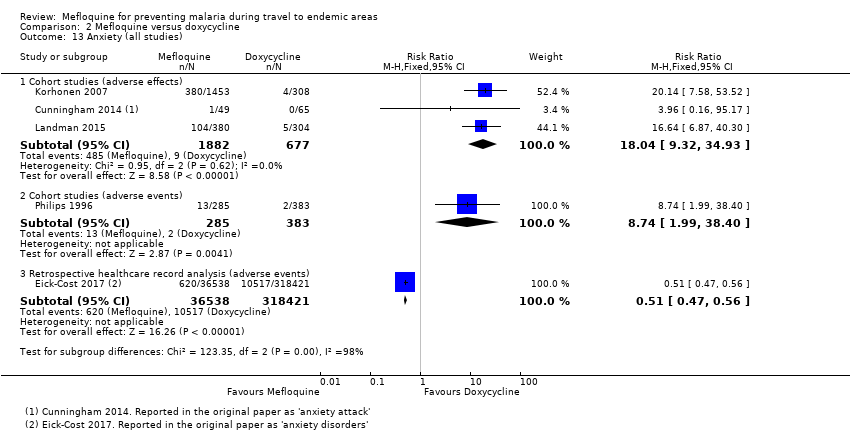
Comparison 2 Mefloquine versus doxycycline, Outcome 13 Anxiety (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 14 Depressed mood (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 15 Abnormal thoughts and perceptions.
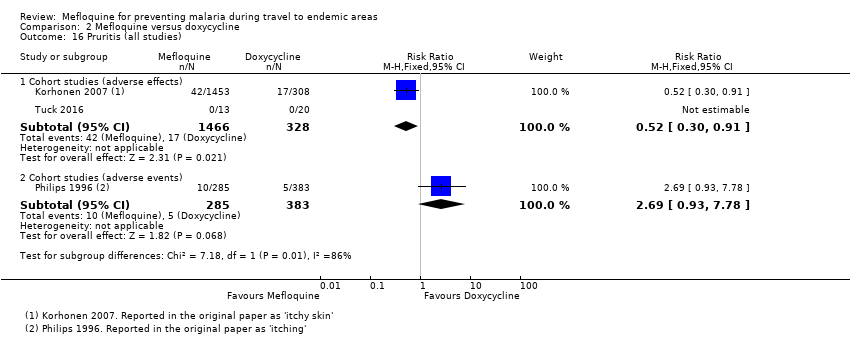
Comparison 2 Mefloquine versus doxycycline, Outcome 16 Pruritis (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 17 Photosensitivity (all studies).
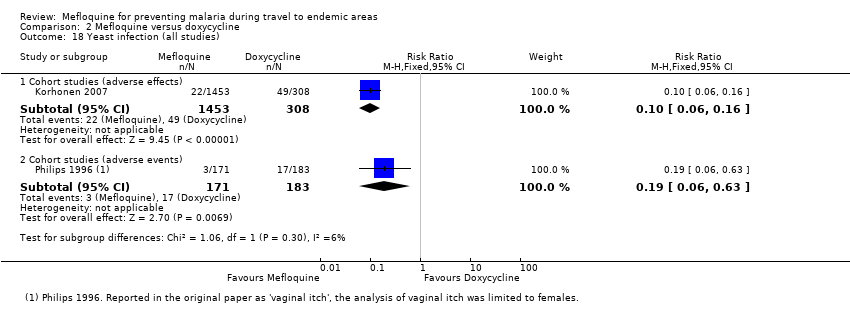
Comparison 2 Mefloquine versus doxycycline, Outcome 18 Yeast infection (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 19 Visual impairment (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 20 Other adverse effects (cohort studies).
Comparison 2 Mefloquine versus doxycycline, Outcome 21 Other adverse events (RCTs).

Comparison 2 Mefloquine versus doxycycline, Outcome 22 Other adverse events (cohort studies).
Comparison 2 Mefloquine versus doxycycline, Outcome 23 Adherence (cohort studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 1 Clinical cases of malaria (RCTs).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 2 Serious adverse events or effects (all studies).
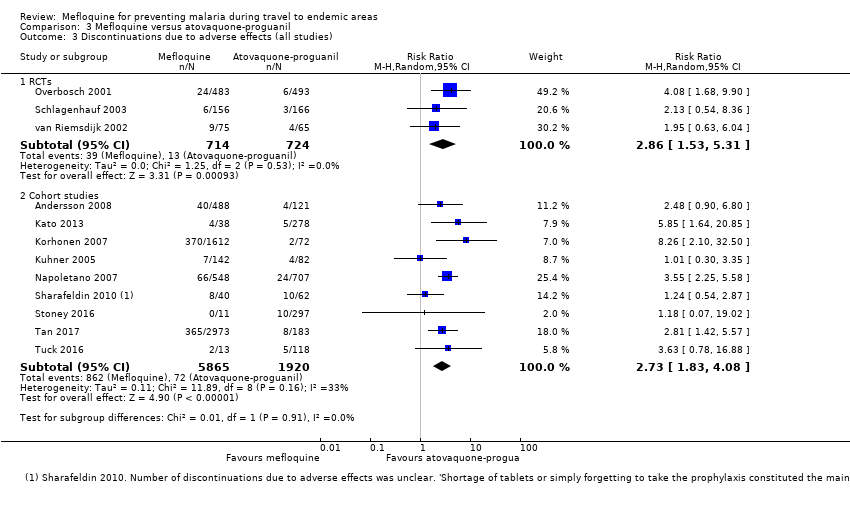
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 3 Discontinuations due to adverse effects (all studies).
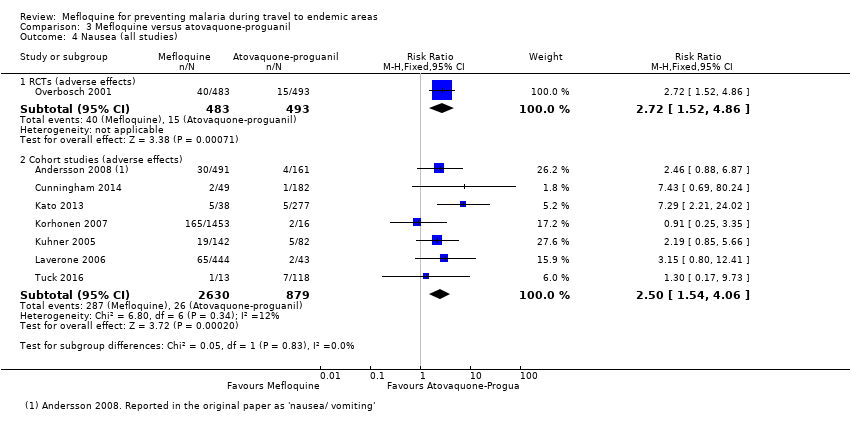
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 4 Nausea (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 5 Vomiting (all studies).
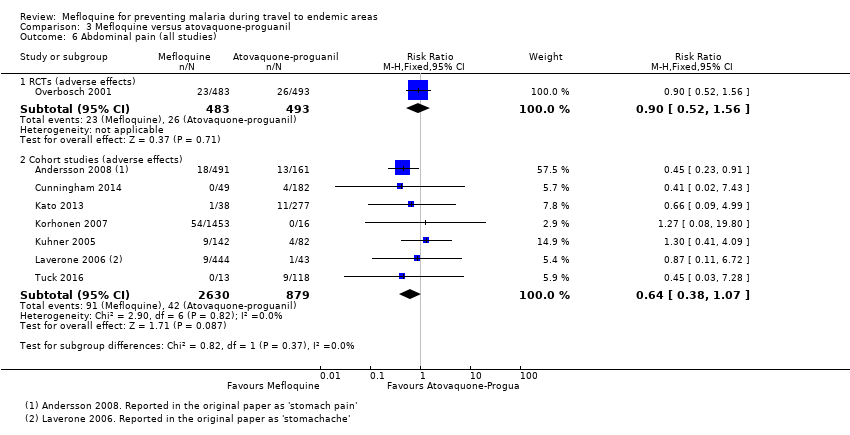
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 6 Abdominal pain (all studies).
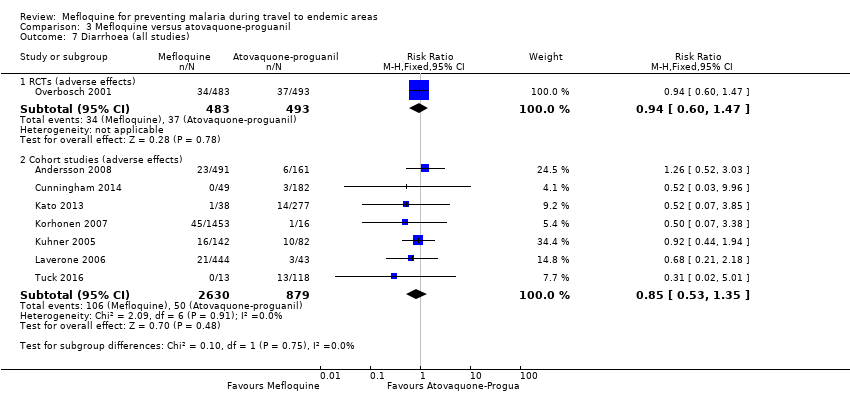
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 7 Diarrhoea (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 8 Mouth ulcers (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 9 Headache (all studies).
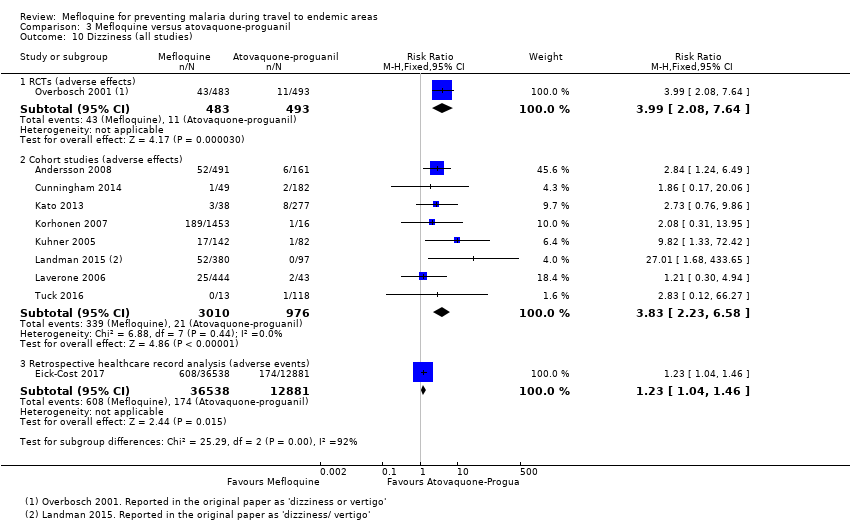
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 10 Dizziness (all studies).
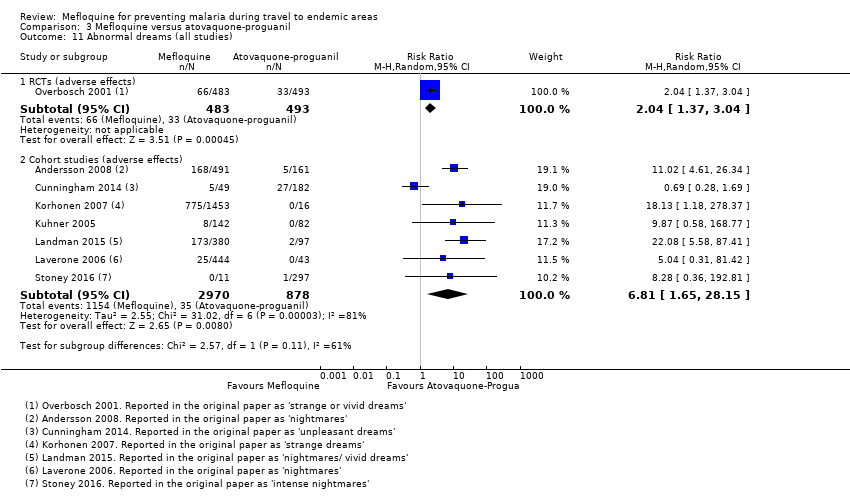
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 11 Abnormal dreams (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 12 Insomnia (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 13 Anxiety (all studies).
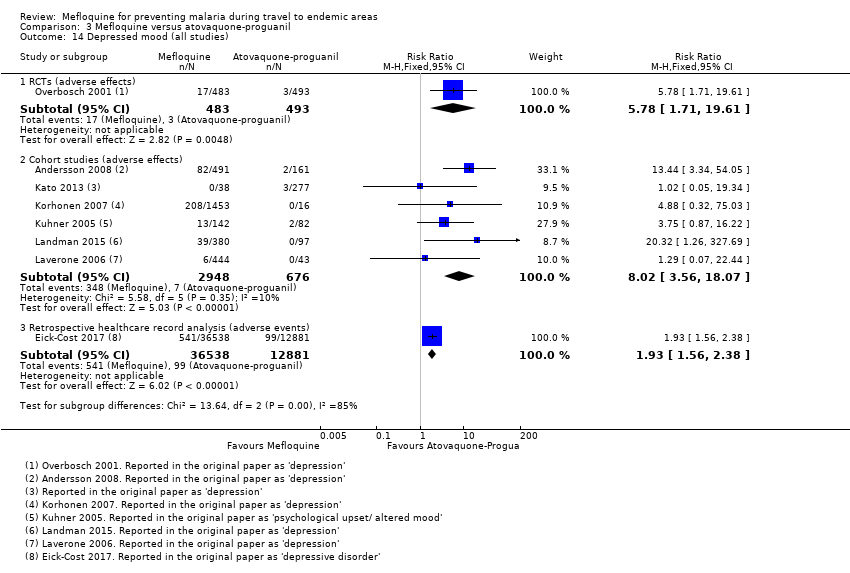
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 14 Depressed mood (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 15 Abnormal thoughts and perceptions (all studies).
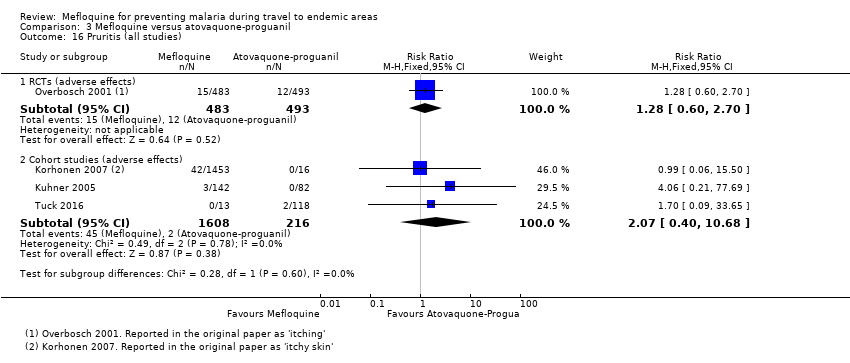
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 16 Pruritis (all studies).
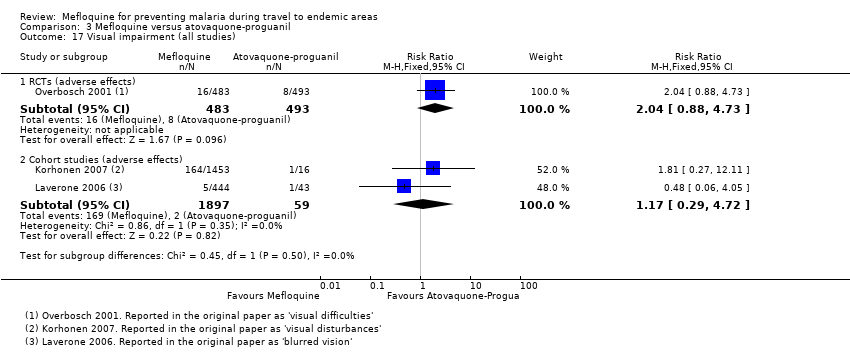
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 17 Visual impairment (all studies).
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 18 Other adverse effects (cohort studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 19 Other adverse events (cohort studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 20 Adherence (RCTs).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 21 Adherence (cohort studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 1 Clinical cases of malaria (RCTs).
Comparison 4 Mefloquine versus chloroquine, Outcome 2 Serious adverse events or effects (all studies).
Comparison 4 Mefloquine versus chloroquine, Outcome 3 Discontinuations due to adverse effects (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 4 Nausea (all studies).
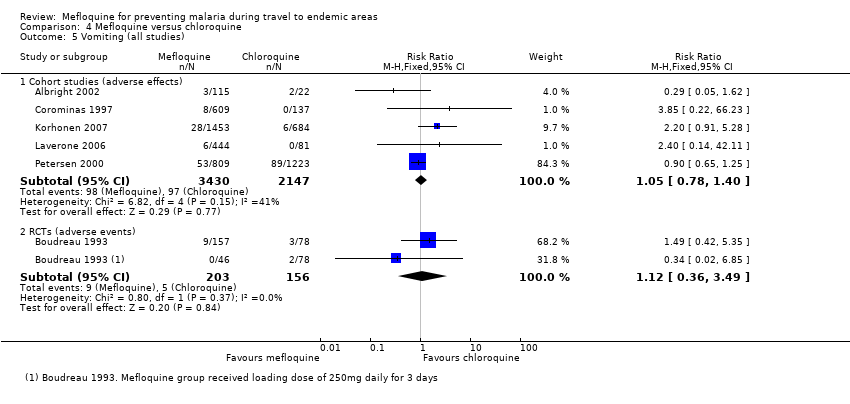
Comparison 4 Mefloquine versus chloroquine, Outcome 5 Vomiting (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 6 Abdominal pain (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 7 Diarrhoea (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 8 Headache (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 9 Dizziness (all studies).
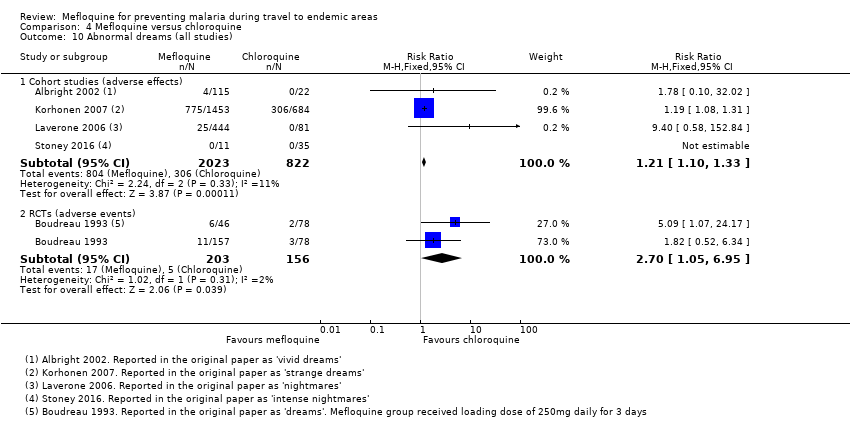
Comparison 4 Mefloquine versus chloroquine, Outcome 10 Abnormal dreams (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 11 Insomnia (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 12 Anxiety (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 13 Depressed mood (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 14 Abnormal thoughts and perceptions.
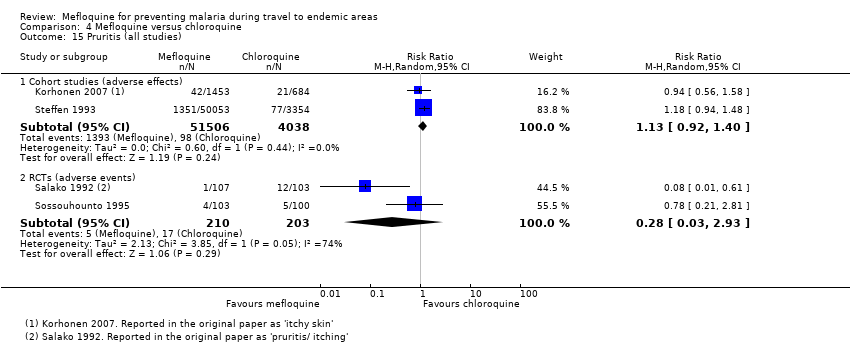
Comparison 4 Mefloquine versus chloroquine, Outcome 15 Pruritis (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 16 Visual impairment (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 17 Vertigo (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 18 Cohort studies in travellers; prespecified adverse effects.

Comparison 4 Mefloquine versus chloroquine, Outcome 19 Other adverse effects (cohort studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 20 Other adverse events (RCTs).

Comparison 4 Mefloquine versus chloroquine, Outcome 21 Pregnancy related outcomes (RCTs).

Comparison 4 Mefloquine versus chloroquine, Outcome 22 Adherence (cohort studies).

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 1 Nausea; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 2 Abdominal pain; effects.
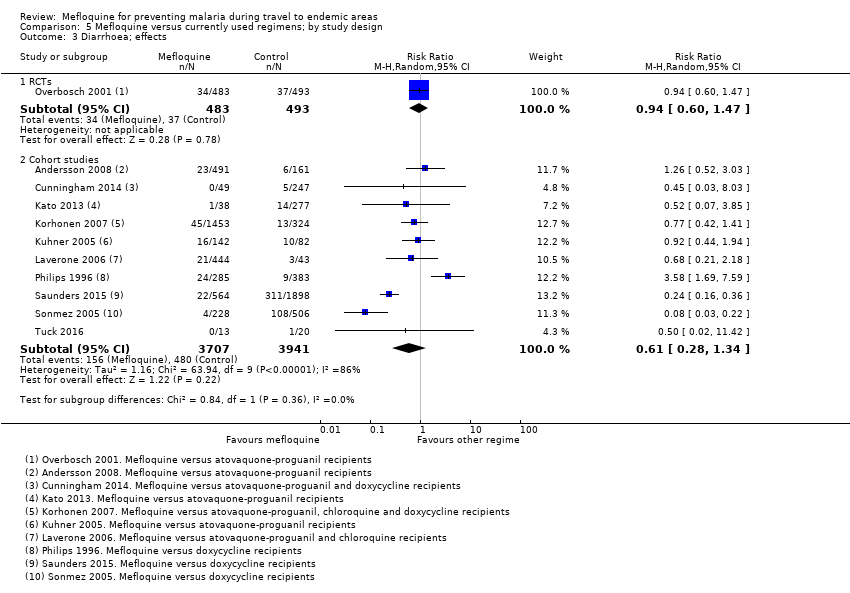
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 3 Diarrhoea; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 4 Headache; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 5 Dizziness; effects.
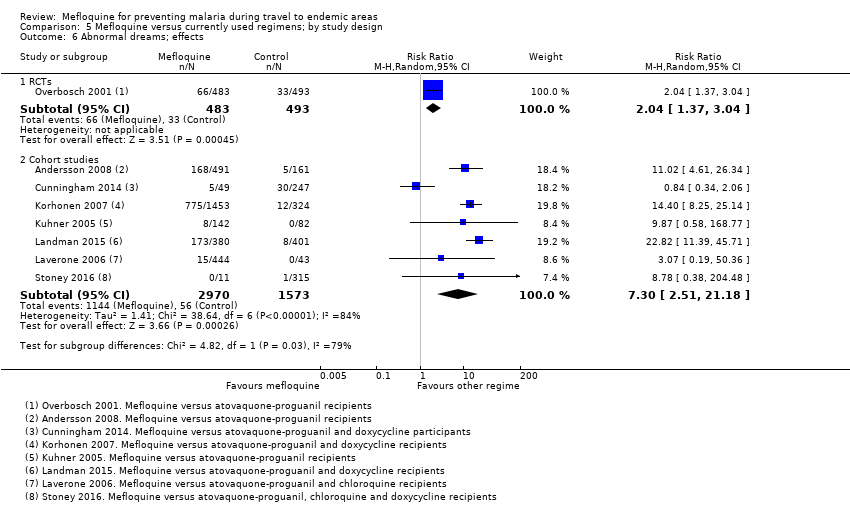
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 6 Abnormal dreams; effects.
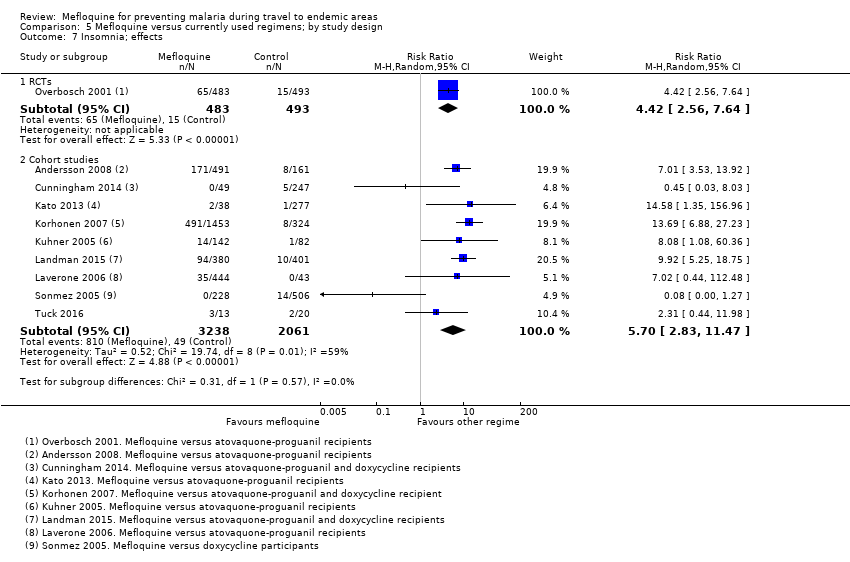
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 7 Insomnia; effects.
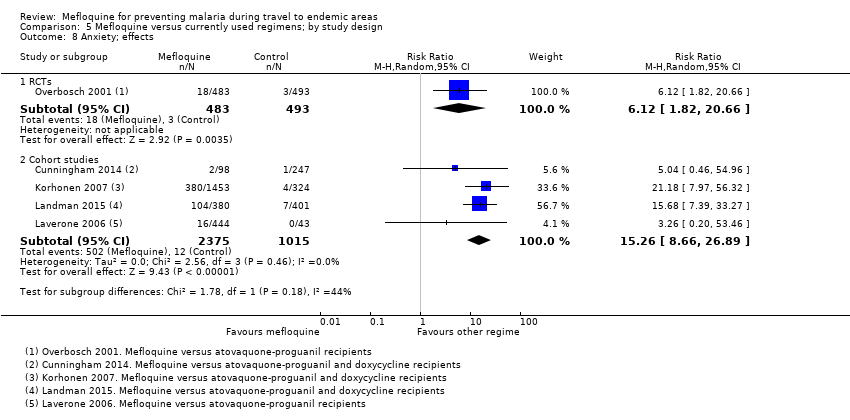
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 8 Anxiety; effects.
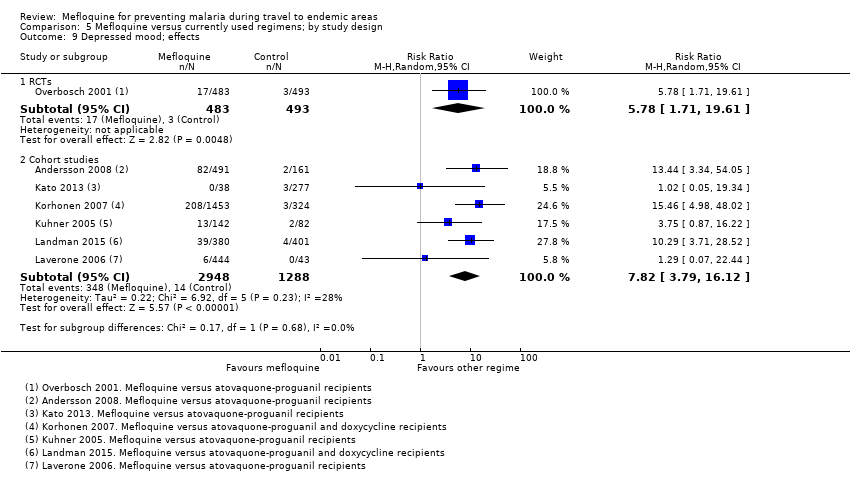
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 9 Depressed mood; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 10 Abnormal thoughts or perceptions; effects.
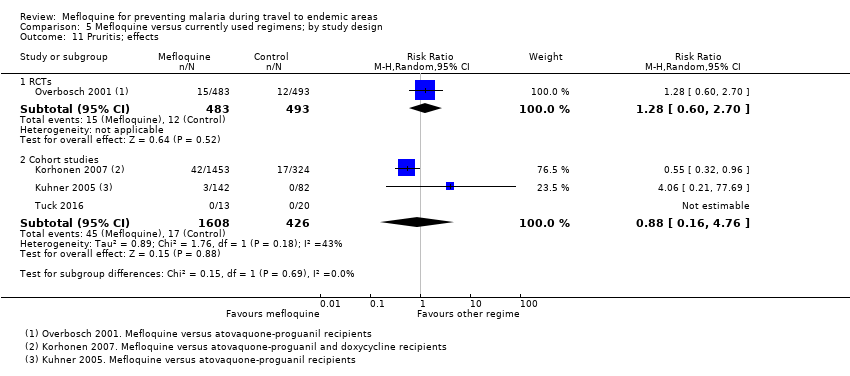
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 11 Pruritis; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 12 Visual impairment; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 13 Adherence; during travel.
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 14 Adherence; after return.
| Mefloquine compared with atovaquone‐proguanil for preventing malaria in travellers | ||||||
| Population: non‐immune adults and children travelling to or living in malaria‐endemic settings Intervention: mefloquine 250 mg weekly Comparison: atovaquone‐proguanil (250 mg atovaquone and 100 mg proguanil hydrochloride) daily Outcome data collection: physicians performed blinded assessment of whether reported symptoms could be related to the study drug | ||||||
| Outcomes | Anticipated absolute effects* | Relative effect | Studies contributing to effect estimate | Additional studies considered in GRADE assessment | Certainty of the evidence | |
| Atovoquone‐proguanil | Mefloquine | |||||
| Clinical malaria | — | — | — | 2 RCTs (1293) | — | ⊕⊕⊝⊝ |
| Serious adverse effects | 0 per 100 | 1 in 100 (0 to 12) | RR 1.40 (0.08 to 23.22) | 4 cohort studies (3693) | 1 RCT (976) | ⊕⊕⊝⊝ |
| Discontinuation of drug due to adverse effects | 2 per 100 | 6 per 100 (3 to 11) | RR 2.86 (1.53 to 5.31) | 3 RCTs (1438) | 7 cohort studies (4498) | ⊕⊕⊕⊕ |
| Abnormal dreams | 7 per 100 | 14 per 100 (10 to 21) | RR 2.04 (1.37 to 3.04) | 1 RCT (976) | 7 cohort studies (3848) | ⊕⊕⊕⊕ |
| Insomnia | 3 per 100 | 13 per 100 (8 to 23) | RR 4.42 (2.56 to 7.64) | 1 RCT (976) | 8 cohort studies (3986) | ⊕⊕⊕⊕ |
| Anxiety | 1 per 100 | 6 per 100 (2 to 21) | RR 6.12 (1.82 to 20.66) | 1 RCT (976) | 4 cohort studies (2664) | ⊕⊕⊕⊝ |
| Depressed mood | 1 per 100 | 6 per 100 (2 to 20) | RR 5.78 (1.71 to 19.61) | 1 RCT (976) | 6 cohort studies (3624) | ⊕⊕⊕⊝ |
| Abnormal thoughts or perceptions | 0 per 100 | 1 per 100 (0 to 4) | RR 1.50 (0.30 to 7.42) | 3 cohort studies (2433) | — | ⊕⊝⊝⊝ |
| Nausea | 3 per 100 | 8 per 100 (5 to 15) | RR 2.72 (1.52 to 4.86) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊕⊕ |
| Vomiting | 1 per 100 | 1 per 100 (0 to 4) | RR 1.31 (0.49 to 3.50) | 1 RCT (976) | 3 cohort studies (2180) | ⊕⊕⊕⊝ |
| Abdominal pain | 5 per 100 | 5 per 100 (3 to 8) | RR 0.90 (0.52 to 1.56) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊝⊝ |
| Diarrhoea | 8 per 100 | 8 per 100 (5 to 12) | RR 0.94 (0.60 to 1.47) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊕⊝ |
| Headache | 4 per 100 | 7 per 100 (4 to 12) | RR 1.72 (0.99 to 2.99) | 1 RCT (976) | 8 cohort studies (4163) | ⊕⊕⊕⊝ |
| Dizziness | 2 per 100 | 8 per 100 (4 to 15) | RR 3.99 (2.08 to 7.64) | 1 RCT (976) | 8 cohort studies (3986) | ⊕⊕⊕⊕ |
| Pruritis | 2 per 100 | 3 per 100 (1 to 5) | RR 1.28 (0.60 to 2.70) | 1 RCT (976) | 3 cohort studies (1824) | ⊕⊕⊕⊝ |
| Visual impairment | 2 per 100 | 4 per 100 (2 to 9) | RR 2.04 (0.88 to 4.73) | 1 RCT (976) | 2 cohort studies (1956) | ⊕⊕⊕⊝ |
| Mouth ulcers | 2 per 100 | 3 per 100 (1 to 6) | RR 1.45 (0.70 to 3.00) | 1 RCT (976) | 2 cohort studies (783) | ⊕⊕⊕⊝ |
| *The assumed risk is the median control group risk across studies unless stated in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Where the control group risk was 0, we used a value of 0.5 to calculate the corresponding risk in the intervention group. Data from cohort studies were used when data from RCTs were unavailable. 'Summary of findings' tables are usually limited to seven outcomes. For adverse effects this problematic, as there are many, and to include some and not others risks selective reporting. We have therefore included all prespecified outcomes in the table. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: the RCTs were generally at low risk of bias but two of three were sponsored by the manufacturer of one of the study drugs. All cohort studies had methodological problems which could introduce confounding or bias. However, as the GRADE approach automatically downgrades certainty by two levels for non‐randomized studies, we did not downgrade further. | ||||||
| Mefloquine compared with doxycycline for preventing malaria in travellers | ||||||
| Population: Non‐immune adults and children travelling to malaria‐endemic settings Intervention: Mefloquine 250 mg weekly Comparison: Doxycycline 100 mg daily Outcome data collection: Self‐reported symptoms experienced whilst taking prophylaxis (adverse events) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Studies contributing to effect estimate | Additional studies considered in GRADE assessment | Certainty of the evidence | |
| Doxycycline | Mefloquine | |||||
| Clinical malaria | 1 per 100 | 1 per 100 (0 to 5) | RR 1.35 | 4 RCTs (744) | — | ⊕⊕⊝⊝ low1,2,3,4 |
| Serious adverse effects | 6 per 10005 | 9 per 1000 (1 to 61) | RR 1.53 (0.23 to 10.24) | 3 cohort studies (3722) | 3 RCTs, 1 cohort study (682; 3772) | ⊕⊝⊝⊝ |
| Discontinuations due to adverse effects | 2 per 100 | 2 per 100 (1 to 6) | RR 1.08 (0.41 to 2.87) | 4 RCTs (763) | 10 cohort studies (10,165) | ⊕⊕⊝⊝ low1,3,7,8 |
| Abnormal dreams | 3 per 100 | 31 per 100 (11 to 87) | RR 10.49 (3.79 to 29.10) | 4 cohort studies (2588) | 1 RCT, 1 cohort study (123; 688) | ⊕⊝⊝⊝ very low2,6,9,10 |
| Insomnia | 3 per 100 | 12 per 100 (4 to 43) | RR 4.14 (1.19 to 14.44) | 4 cohort studies (3212) | 1 RCT, 2 cohort studies (123; 355,627) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Anxiety | 1 per 100 | 18 per 100 (9 to 35) | RR 18.04 (9.32 to 34.93) | 3 cohort studies (2559) | 2 cohort studies (355,627) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Depressed mood | 1 per 100 | 11 per 100 (5 to 25) | RR 11.43 (5.21 to 25.07) | 2 cohort studies (2445) | 3 cohort studies (430,006) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Abnormal thoughts or perceptions | 0 per 100 | 3 per 100 (0 to 24) | RR 6.60 (0.92 to 47.20) | 2 cohort studies (2445) | 2 cohort studies (376,024) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Nausea | 8 per 100 | 3 per 100 (2 to 4) | RR 0.37 (0.30 to 0.45) | 5 cohort studies (2683) | 1 RCT, 1 cohort study (123; 668) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Vomiting | 5 per 100 | 1 per 100 (1 to 1) | RR 0.18 (0.12 to 0.27) | 4 cohort studies (5071) | 1 RCT (123) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Abdominal pain | 15 per 100 | 5 per 100 (1 to 16) | RR 0.30 (0.09 to 1.07) | 3 cohort studies (2536) | 1 RCT, 1 cohort (123; 668) | ⊕⊝⊝⊝ very low6,7,9,11 |
| Diarrhoea | 5 per 100 | 1 per 100 (1 to 4) | RR 0.28 (0.11 to 0.73) | 5 cohort studies (5104) | 2 RCTs; 1 cohort study (376; 668) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Dyspepsia | 14 per 100 | 4 per 100 (1 to 10) | RR 0.26 (0.09 to 0.74) | 5 cohort studies (5104) | — | ⊕⊝⊝⊝ low2,3,6,10 |
| Headache | 2 per 100 | 2 per 100 (1 to 6) | RR 1.21 (0.50 to 2.92) | 5 cohort studies (3320) | 1 RCT, 1 cohort study (123; 688) | ⊕⊝⊝⊝ very low3,6,7,11 |
| Dizziness | 1 per 100 | 3 per 100 (1 to 14) | RR 3.49 (0.88 to 13.75) | 5 cohort studies (2633) | 1 RCT, 2 cohort studies (123; 355,627) | ⊕⊝⊝⊝ very low3,6,7,11 |
| Visual impairment | 3 per 100 | 7 per 100 (4 to 12) | RR 2.37 (1.41 to 3.99) | 2 cohort studies (1875) | — | ⊕⊝⊝⊝ very low2,6,7,9 |
| Pruritis | 3 per 100 | 2 per 100 (1 to 3) | RR 0.52 (0.30 to 0.91) | 2 cohort studies (1794) | 1 cohort study (688) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Photosensitivity | 19 per 100 | 2 per 100 (1 to 2) | RR 0.08 (0.05 to 0.11) | 2 cohort studies (1875) | 1 cohort study (688) | ⊕⊝⊝⊝ very low2,6,9,10 |
| Vaginal thrush | 16 per 100 | 2 per 100 (1 to 3) | RR 0.10 (0.06 to 0.16) | 1 cohort study (1761) | 1 cohort study (354) | ⊕⊝⊝⊝ very low2,6,9,10 |
| *The assumed risk is the median control group risk across cohort studies unless stated in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Where the control group risk was 0, we used a value of 0.5 to calculate the corresponding risk in the intervention group. Where no RCTs including short‐term travellers reported on our prespecified adverse outcomes, we included information from cohort studies as our primary analysis. 'Summary of findings' tables are usually limited to seven outcomes. For adverse effects this problematic, as there are many, and to include some and not others risks selective reporting. We have therefore included all prespecified outcomes in the table. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: none of the RCTs adequately described methods of random sequence generation or allocation concealment, However, given that so few events occurred in these trials, it is unlikely to have introduced bias. | ||||||
| Bias | Authors' judgement | Support for judgement |
| Confounding | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: identified confounders were measured and were balanced across groups (age, sex, destination and duration of travel) Moderate risk: identified confounders were measured and not balanced across groups, or several confounders had not been measured or not reported across groups Serious risk: a critical confounder has been measured and is not balanced across groups |
| Selection of participants into the study | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether selection into the study was unrelated to intervention or unrelated to outcome, and whether start of intervention and start of follow up coincided for most subjects. Non‐responder bias at the point of selection was considered here for cohort studies. We used the following cut offs for non‐response rate: low risk < 10%, moderate risk 10% to 20%, serious risk > 20%. |
| Measurement of interventions | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: the prescription was provided by a travel clinic which also performed the study, and discontinuations were recorded and reported, or all participants were issued with their medication e.g. soldiers or participants were asked to self‐report which medication they took whilst they were taking it. Moderate risk: the prescription was provided by a travel clinic which also performed the study but no information regarding switches and discontinuations was available or patients are asked to self‐report which prophylaxis they took shortly after they finished taking it. Serious risk: Participants were asked to self‐report which prophylaxis they took a long time after they finished taking it. |
| Departures from intended interventions | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether switches between interventions of interest were available. We assessed whether discontinuations and switches between prophylactic regimens had been recorded and reported. |
| Missing data | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether outcome data was reasonably complete for most participants. We recorded missing data for included participants e.g. loss to follow up rates and treatment withdrawals. |
| Measurement of outcomes | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether the outcome measure was objective or subjective. We assessed whether participants or study personnel were blinded to the intervention received. We assessed whether the methods of outcome assessment were comparable across intervention groups. |
| Selection of the reported result | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: If the questionnaire was provided in full, or it was clear what was asked within it. Moderate risk: If it is unclear which questions are asked, or information was provided on aggregate. Serious risk: If data captured within the questionnaire was clearly missing. |
| Other | Low risk Moderate risk Serious risk Critical risk No information | We reported the study sponsor. We classified the analysis of studies sponsored by pharmaceutical companies as independent of the sponsor when it was clearly stated that the sponsor had no input to the trial analysis. |
| Adapted from Higgins 2011 and ACROBAT‐NSRI tool | ||
| Criterion | Assessment | Explanation |
| On conduct | ||
| Were harms pre‐defined using standardised or precise definitions? | Adequate Inadequate Unclear | We classified as 'adequate' if the study reported explicit definitions for adverse events and effects that allow for reproducible ascertainment e.g. what adverse events were being investigated and what constituted an “event”, what was defined as a serious or severe adverse event. |
| Was ascertainment technique adequately described? | Adequate Inadequate Unclear | We classified as 'adequate' if the study reported methods used to ascertain complications, including who ascertained, timing, and methods used. |
| Was monitoring active or passive? | Active Passive Unclear | We classified monitoring as 'active' when authors reviewed participants at set time points during treatment and enquired about symptoms. |
| Was data collection prospective or retrospective? | Prospective Retrospective Unclear | We classified as ‘prospective’ if data collection occurred during treatment, or ‘retrospective’ if data collection occurred following treatment. |
| For laboratory investigations or other tests | ||
| Was the number and timing of tests adequate? | Adequate Inadequate Unclear | We classified the number and timing of tests as 'adequate', when tests were taken at baseline and at least one time point during prophylaxis. |
| Adapted from Bukiwra 2014 | ||
| Study ID | Participants (immune status) | Number of randomised participants | Mefloquine dose | Drug comparisons of interest | Duration of exposure to malaria | Country of malaria exposure | Local drug resistance |
| Thai male adults (presumed semi‐immune) | 605 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo | 24 weeks (trial duration) | Thailand | Chloroquine, sulphadoxine‐pyrimethamine and quinine resistance | |
| Pregnant women from the Thai‐Burma border (presumed semi‐immune) | 339 | 250 mg weekly for first 4 weeks, then 125 mg weekly until delivery | Placebo | Various in endemic area (monitored until delivery) | Thai‐Burma border | Not mentioned | |
| Thai residents aged 10 to 60 years (semi‐immune) | 990 | 180 mg tablet weekly, 360 mg tablet weekly, 360 mg every 2 weeks with appropriate adjustments for children | Placebo | 26 weeks | Thailand | Chloroquine resistant Plasmodium falciparum | |
| Brazilian civilians and soldiers aged 12 to 55 years (semi‐immune) | 128 | 500 mg every 4 weeks, 250mg every 2 weeks | Placebo | 17 weeks | Brazil | P falciparum resistant to chloroquine and “high prevalence of multiresistant Plasmodium falciparum transmission” | |
| Ivory Coast adult males (semi‐immune) | 500 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo | 20 weeks | Ivory C oast | Not mentioned | |
| Indonesian soldiers ('largely' non‐immune) | 204 | 250 mg weekly | Placebo, doxycycline | 'approximately 13 weeks' | Indonesia | Sulfadoxine‐pyrimethamine and chloroquine resistance | |
| Kenyan children (semi‐immune) | 169 | 125 mg weekly | Placebo (multivitamin), doxycycline, primaquine | 11 weeks | Kenya | Not mentioned | |
| Nigerian adult males (semi‐immune) | 567 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo, chloroquine | 24 weeks (trial duration) | Nigeria | "...at the time of the trial, chloroquine resistance was not a problem" | |
| Ghanain adults (semi‐immune) | 530 | 250 mg weekly | Placebo | 12 weeks | Ghana | Not mentioned | |
| USA soldiers (non‐immune) | 270 | 250 mg weekly | Doxycycline | 8 weeks | Thailand | Local chloroquine resistance | |
| Thai adult males (semi‐immune) | 501 | 500 mg fortnightly | Chloroquine | 14 weeks (trial duration) | Cambodia | Local chloroquine resistance | |
| Pregnant Malawian residents (semi‐immune) | 4220 | 250 mg weekly | Chloroquine | Various in endemic area (monitored until delivery) | Malawi | P falciparum resistant to chloroquine, documented sensitivity of P falciparum to mefloquine |
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Exclusions for psychiatric adverse effects | Trial duration | Source of funding |
| RCTs | ||||||
| Thai male adults | 605 | Interview with study personnel | None | 24 weeks | Roche | |
| Australian adults who did not travel | 106 | Daily self‐reported diary | Past history of psychiatric conditions | 7 weeks | Roche | |
| Ghanain adults | 530 | Interview with study personnel | History of neuropsychiatric illness | 12 weeks | USA Army | |
| Pregnant women, Thai‐Burma border | 339 | Phase 1: weekly symptom questionnaire. Babies were assessed at birth and at 3, 6, 12, and 24 months. Phase 2: weekly symptom questionnaire. Babies were assessed at birth and at 2 and 9 months | None | Various | Government funding | |
| Indonesian soldiers | 204 | Two symptom questionnaires. Daily interview with study personnel | History of underlying illness | 13 weeks | Roche, Pfizer, USA Army | |
| Thai residents aged 10 to 60 years | 990 | Weekly sick call by study personnel | None | 26 weeks | Not mentioned | |
| Israeli adults who did not travel | 90 | Self‐reporting diary | History of depression | 48 hours | Mepha Ltd | |
| Nigerian adult males | 567 | Interview with study personnel | None | 24 weeks | Not mentioned | |
| Brazilian civilians and soldiers aged 12 to 55 | 128 | Interview w ith study personnel | None | 17 weeks | Roche | |
| Swissair trainee pilots who did not travel | 23 | Interview with study personnel | Psychosis or severe depression | 4 weeks | Roche | |
| Ivory C oast adult males | 500 | Access to the village health centre | None | 20 weeks | Not mentioned | |
| Dutch adult who did not travel | 42 | Interview with study personnel | H istory of any serious psychiatric disorder; evidence of drug or alcohol abuse | 30 days | Roche | |
| Kenyan children | 169 | Interview with study personnel | None | 4 months | USA Army | |
| Cohort studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| Danish travellers | 300 | Telephone interview | Allocation based on guidelines and patient preference | Mean 3 weeks, range 1 to 9 weeks | Not mentioned | |
| Danish travellers | 4154 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | Various, not specified | Not mentioned | |
| Swedish travellers | 491 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | " Most", range 2 to 4 weeks | Not mentioned | |
| Danish travellers | 1501 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | Mean = 23 days | Not mentioned | |
| USA soldiers | 397,442 | Restrospective analysis of hospital records | No information available | Minimum 1 month | Government funding | |
| Study ID | Description of how adverse outcomes were defined and recorded¹ | Description of ascertainment technique² | Active or passive monitoring? | Prospective or retrospective data collection? |
| Inadequate Comment: No definition of adverse events or effects was provided, it is unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate Comment: ‘serious’ adverse events were not defined, and methods for determining causality not described | Adequate | Active | Prospective | |
| Inadequate Comment: It is unclear what questions were included within the questionnaire and whether and how causality was assessed. ‘Serious’ adverse effects not defined | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Inadequate Comment: Weekly sick call for all villagers | Passive | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No information given in the methods section on definition of adverse outcomes | Inadequate Comment: No description of ascertainment method | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it was unclear whether or how causality was assessed | Unclear | Passive | Prospective | |
| Adequate | Unclear | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it was unclear whether or how causality was assessed. | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Unclear 'Filled in after their return' | |
| Adequate | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking their prophylactic regimen | |
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Passive | Retrospective | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? | ||||
| Study ID | Study design | Mefloquine users | Drug comparators | |||
| Events/ participants | Description | Drug | Events/ participants | Description | ||
| Events (not attributed by study authors or participants to the drug regimen) | ||||||
| RCT | 0/116 | ‐ | Placebo | 1/121 | None provided | |
| RCT | 1/159 (women) | One death
Four congenital malformations:
| Placebo | 0/152 (women) | One congenital malformation:
| |
| RCT | 0/103 | ‐ | Placebo | 1/96 | One death (not described) | |
| RCT | 0/61 | ‐ | Placebo | 0/65 | ‐ | |
| Doxycycline | 1/62 | Acute hysteria¹ | ||||
| Cohort study | 8/3703 | 8 hospitalisations
| Doxycycline | 0/69 | ‐ | |
| Chloroquine | 0/119 | ‐ | ||||
| RCT | 10/483 | "...infectious illnesses in 7 subjects and breast cancer, anaphylaxis, or fractured femur in 1 subject each" | Atovaquone‐proguanil | 4/493 | "...infectious illnesses in 3 subjects and cerebral ischemia in 1 subject" | |
| Studies reporting no serious events or effects | ||||||
| RCT | 0/107 | "Adverse events were all mild and there were no deaths" | Placebo Chloroquine | 0/101 0/103 | ‐ ‐ | |
| RCT | 0/134 | "No serious side effects occurred with either drug regimen" | Doxycycline | 0/119 | ‐ | |
| RCT | 0/153 | "Although a large number of adverse events were reported, none were serious" | Doxycycline Atovaquone‐proguanil | 0/153 0/164 | ‐ ‐ | |
| Cohort study | 0/228 | "No drug induced side effects necessitating emergency care were observed" | Doxycycline | 0/506 | ‐ | |
| Cohort study | 0/491 | "No serious adverse events were recorded" | Atovaquone‐proguanil | 0/161 | ‐ | |
| Cohort study | 0/548 | Records hospitalisations, and reports that none occurred in either group of participants | Atovaquone‐proguanil Chloroquine | 0/707 0/37 | ‐ ‐ | |
| RCT | 0/103 | "All side effects were transient (and)... mild" | Chloroquine | 0/100 | ‐ | |
| 1 This trial described a potentially serious adverse event, but did not provide enough detail to meet our definition. | ||||||
| Study ID | Study design | Mefloquine users | Drug comparators | |||
| Events/ participants | Description | Drug | Events/ participants | Description | ||
| Effects (attributed by study authors or participants to the drug regimen) | ||||||
| Cohort study | 2/104 | Two "serious acute adverse reactions"¹
| No treatment | 0/93 | ‐ | |
| Cohort study | 5/809 | 5 hospitalisations:
| Chloroquine | 6/1223 | 2 hospitalisations:
| |
| No treatment | 0/161 | ‐ | ||||
| Cohort study | 15/1612 | 15 hospitalisations:
| Doxycycline | 9/708 | 9 hospitalisations:
| |
| Atovaquone‐proguanil | 0/72 | ‐ | ||||
| Chloroquine | 4/832 | 4 hospitalisations:
| ||||
| Cohortstudy | 4/285 | 3 hospitalisations with "either gastrointestinal or neurologic symptoms" and one seizure | Doxycycline | 1/383 | Severe oesophagitis | |
| RCT | 1/? | One "neuropsychiatric side effect"
| Chloroquine | 0/? | ‐ | |
| Cohort study | 1/115 | One "serious side effect"¹
| Chloroquine | 0/22 | ‐ | |
| Cohort study | 1/609 | One hospitalisation:
| Chloroquine | 0/137 | ‐ | |
| Cohort study | 7/52981 | 7 hospitalisations, including:
| Chloroquine | 7/20332 | 7 hospitalisations. 'Includes':
| |
| Studies reporting no serious events or effects | ||||||
| RCT | 0/46 | Nine serious adverse events in the trial (trial arm not specified) "none of which were considered by study physicians to be related to the study drug" | Placebo | 0/94 | ‐ | |
| RCT | 0/107 | "Adverse events were all mild and there were no deaths" | Placebo Chloroquine | 0/101 0/103 | ‐ ‐ | |
| RCT | 0/134 | "No serious side effects occurred with either drug regimen" | Doxycycline | 0/119 | ‐ | |
| RCT | 0/153 | "Although a large number of adverse events were reported, none were serious" | Doxycycline Atovaquone‐proguanil | 0/153 0/164 | ‐ ‐ | |
| Cohort study | 0/228 | "No drug induced side effects necessitating emergency care were observed" | Doxycycline | 0/506 | ‐ | |
| Cohort study | 0/491 | "No serious adverse events were recorded" | Atovaquone‐proguanil | 0/161 | ‐ | |
| Cohort study | 0/548 | Records hospitalisations, and reports that none occurred in either group of participants | Atovaquone‐proguanil Chloroquine | 0/707 0/37 | ‐ ‐ | |
| RCT | 0/103 | "All side effects were transient (and)... mild" | Chloroquine | 0/100 | ‐ | |
| ¹ This trial described a potentially serious adverse effect, but did not provide enough detail to meet our strict definition. | ||||||
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric adverse effects | Duration of travel | Source of funding |
| Randomized controlled trials | ||||||
| USA soldiers | 270 | Blood tests, stool samples. Interview with study personnel | None | 5 weeks | Not mentioned | |
| Indonesian soldiers | 204 | Interview with study personnel. Exit questionnaire | " History of underlying illness" | 13 weeks | Pfizer and Roche | |
| Non‐immune adult short‐term travellers | 674 | Participant self‐reported questionnaire | History of seizures or psychiatric disorders | 4 to 6 weeks | GlaxoSmithKline and Roche | |
| Kenyan children | 169 | Interview with study personnel | None | 4 months | Government funding | |
| Non‐randomized studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | 0 to 36 months | Not mentioned | |
| USA s oldiers | 367,840 | Data from the Defense Medical Surveillance System, the Pharmacy Data Transaction Service and the Theater Medical Data Store | No information available | Various, not specified | Not mentioned | |
| UK adult short‐term travellers | 185 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | < 28 days | GlaxoSmithKline | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participan t preference | ≥ 6 months | Two staff employed by Peace Corps | |
| Peace Corps volunteers | 1184 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Adult short‐term travellers | 660 | Participant self‐reported questionnaire | No information available | 93% < 4 weeks | " No financial interests to disclose" | |
| Adult short‐term travellers | 5626 | Participant self‐reported questionnaire | No information available | < 5 weeks | " No financial interests to disclose" | |
| UK adults enrolled in UK g eneral p ractice research database | 35,370 | Incident cases of depression, psychoses and panic attacks within the UK general practice research database | No information available | Various, not specified | Roche | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| Australian short‐term travellers | 741 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, mean 3 weeks, maximum 3 months | Roche and Pfizer | |
| USA soldiers | 2351 | Participant self‐reported questionnaire | Primarily doxycycline, soldiers with contra‐indications received mefloquine | > 90% for 10 months or more | Not mentioned | |
| Israeli short‐term travellers | 158 | Participant self‐reported questionnaire | "... daily doxycycline or daily primaquine... was recommended" | 14 to 20 days | Not mentioned | |
| Israeli soldiers | 45 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | "... an average of 4 hours stay in the field over a period of 2 months" | Not mentioned | |
| Dutch medical students | 180 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean 74 days (range 10 to 224 days) | No dedicated funding | |
| Turkish soldiers | 1400 | Participant self‐reported questionnaire | Prior to March 2002: doxycyline After July 2002: mefloquine | A pprox. 6 months | Not mentioned | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| UK soldiers | 2032 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | "... not funded by an external body" | |
| UK soldiers | 151 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | No dedicated funding | |
| Adult short‐term travellers | 3051 | Participant self‐reported questionnaire | No information available | A pprox. 6 weeks | Not mentioned | |
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Inadequate: No definitions provided for serious side effects | Unclear: it is not reported who conducted the interviews | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it wa s unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate " Each subject was visited daily at home by an assigned field worker, who asked about symptoms of malaria or drug side effects" | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Adequate | Adequate | Passive | Prospective | |
| Inadequate " Also included on the questionnaire was a single free‐text question asking travellers to describe any side effects of antimalarial medication" | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: n o information wa s provided regarding the timing of the questionnaire during treatment | |
| Adequate | Adequate | Passive | Unclear Comment: all participants were emailed the questionnaire at one time point, which occurred at varying points during the prophylactic regimen | |
| Inadequate "Travellers… were given a questionnaire that asked for... adverse health events attributed to those drugs" | Adequate | Passive | Unclear Comment: information was collected at the airport, when travellers should still have been taking the prophylactic regimen | |
| Adequate | Adequate | Passive | Retrospective | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Inadequate Comment: it wa s unclear what constituted a serious or severe event and insufficient information on the questions that travellers were asked | Inadequate "... a mailed questionnaire approximately 2 weeks after their anticipated return home date’ ‘if a reply had not been received within 4 weeks an abbreviated questionnaire was sent out." Comment: no details provided regarding abbreviated questionnaire | Active | Retrospective | |
| Inadequate Comment: insufficient information of the questions that travellers were asked | Adequate | Passive | Retrospective | |
| Inadequate "... we directly contacted all travelers for complete follow‐up and assessment of compliance. Fifty travelers taking primaquine completed a questionnaire regarding side effects" | Inadequate Comment: see quote. Different methods of follow up for different forms of prophylaxis | Unclear | Unclear | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate " Questionnaires were distributed and collected by the flight surgeon to 45 aircrew…questionnaires were immediately evaluated and further data collection was done by telephone, if necessary" | Passive | Unclear Comment: it wa s unclear at which time point data collection occurred | |
| Inadequate Comment: n o information wa s provided on how information on adverse effects was sought | Inadequate Comment: n o mention of how adverse events were recorded in the questionnaire | Passive | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Prospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information is reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate " The questionnaire approved by the MODREC included the 19 commonest adverse effects described in the manufacturers’ product documentation" Comment: Adverse events listed in the questionnaire are not reported | Adequate | Passive | Unclear Comment: information obtained during transit through Nairobi back to the UK. It wa s unclear whether participants were still taking prophylaxis at this time point | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Unclear Comment: i t wa s not specified at which point during treatment the questionnaire was administered | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking their prophylactic regimen | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? 3. Monitoring classed as 'active' if it occurred at set time points during treatment. For full description of analysis methods, see Table 2. | ||||
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric adverse effects | Duration of travel | Source of funding |
| Randomized controlled trials | ||||||
| Travellers from Canada, Germany, Netherlands, South Africa, UK | 1013 | Interview with study personnel | "... history of alcoholism, seizures or psychiatric or severe neurological disorders" | Mean 2.5 weeks | GlaxoSmithKline | |
| Non‐immune adult short‐term travellers | 674 | Participant self‐reported questionnaire | " History of seizures or psychiatric disorders" | 4 to 6 weeks | GlaxoSmithKline and Roche | |
| Dutch short‐term travellers | 140 | Interview and testing with study personnel | "H istory of alcoholism, seizures, psychiatric disorders, severe neurological disorders" | Mean 19 days | Government funding | |
| Non‐randomis ed studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| Swedish soldiers | 609 | Participant self‐reported questionnaire | Mainly mefloquine, soldiers with contra‐indications received atovaquone‐proguanil | 6 months | Not mentioned | |
| Dutch short‐term travellers | 945 | Participant self‐reported questionnaire (measured adherence) | Allocation based on guidelines and participant preference | 84% < 29 days | Government funding | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and p articipant preference | 0‐36 months | Not mentioned | |
| USA s oldiers | 367,840 | Data from the Defense Medical Surveillance System, the Pharmacy Data Transaction Service and the Theater Medical Data Store | No information available | Various, not specified | Not mentioned | |
| UK adult short‐term travellers | 185 | Participant self‐reported questionnaire | Allocation based on guidelines and p articipant preference | < 28 days | GlaxoSmithKline | |
| Japanese short‐term travellers | 316 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean 20.0 ± 9.6 days in the atovaquone‐proguanil group and 59.0 ± 15.9 days in the mefloquine group | Not mentioned | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | ≥ 6 months | Two staff employed by Peace Corps | |
| German short‐term travellers | 495 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | A tovaquone‐proguanil mean 2.6 weeks, mefloquine mean 7 weeks | Not mentioned | |
| Peace Corps volunteers | 1184 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Italian short‐term travellers | 1176 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | > 90% 0 to 30 days | Not mentioned | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| UK adults enrolled in UK g eneral p ractice research database | Not available | Incident cases of a neuropsychiatric disorders during or after antimalarial drug use | No information available | Various, not specified | Roche | |
| Dutch medical students | 180 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean duration of stay 74 days (range 10 to 224 days) | " N o dedicated funding for this project" | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| UK soldiers | 151 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | No dedicated funding | |
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Inadequate Comment: insufficient information provided on the questions which soldiers were asked | Inadequate Comment: different ascertainment technique used for one of the three groups, which is inadequately described | Active | Unclear Comment: d ata collection was prospective for 448/609 participants (LA04 and LA05), but retrospective for 161 participants (LA02) | |
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Adequate | Adequate | Passive | Prospective | |
| Inadequate " Also included on the questionnaire was a single free‐text question asking travelers to describe any side effects of antimalarial medication" | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: the timing of this questionnaire has not been made clear | |
| Adequate | Adequate | Passive | Unclear Comment: n o information wa s provided regarding the timing of the questionnaire during treatment | |
| Inadequate Comment: insufficient information provided on the questions that participants were asked | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: all participants were emailed the questionnaire at one time point, which occurred at varying points during the prophylactic regimen | |
| Adequate | Adequate | Passive | Retrospective | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate Comment: n o information is provided on how information on adverse effects was sought | Inadequate Comment: n o mention of how adverse events were recorded in the questionnaire. | Passive | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information is reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Unclear Comment: i t wa s not specified at which point during treatment the questionnaire was administered | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? 3. Monitoring classed as 'active' if it occurred at set time points during treatment. For full description of analysis methods, see Table 2. | ||||
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric side effects | Trial duration | Source of funding |
| RCT s | ||||||
| Thai gem miners | 501 | Interview with study personnel | None | 14 weeks | USA Army | |
| USA soldiers | 359 | Interview with study personnel and computerised questionnaire | "M edical history of psychiatric or neurological problems within the last 5 years" | 13 weeks | Not mentioned | |
| Thai adult mal es | 605 | Interview with study personnel | None | 24 weeks | Roche | |
| Nigerian adult males | 567 | Interview with study personnel | None | 24 weeks | Not mentioned | |
| Ivory C oast adult males | 500 | " Access to the village health centre. Clinical examination with study personnel" | None | 20 weeks | Not mentioned | |
| Pregnant Malawian women | 4220 | Interview with study personnel | None | Monitored from enrolment to delivery | Government funding | |
| Non‐randomised studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| USA travelling children aged < 13 years | 177 | Interview with study personnel | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Spanish short‐term adult travellers | 1054 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Maximum 6 weeks | Not mentioned | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | 0 to 36 months | Not mentioned | |
| USA short‐term travellers | 822 | Interview with study personnel | Allocation based on guidelines and participant preference | Median 19 days, up to 90 days | Not mentioned | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | ≥ 6 months | Two staff employed by Peace Corps | |
| Adult short‐term travellers | 660 | Participant self‐reported questionnaire | No information available | 93% < 4 weeks | " No financial interests to disclose" | |
| Italian short‐term travellers | 1176 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | > 90% 0 to 30 days | Not mentioned | |
| Adult short‐term travellers | 5626 | Participant self‐reported questionnaire | No information available | M ost < 5 weeks | " No financial interests to disclose" | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| Danish travellers | 4154 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, 65% < 3 weeks | Not mentioned | |
| Swedish short‐term travellers | 491 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | " Most" 2 to 4 weeks | Not mentioned | |
| Adult short‐term travellers | 145,003 | Participant self‐reported questionnaire | No information available | 98% stayed between 1 and 4 weeks | Roche | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| Adult short‐term travellers | 3051 | Participant self‐reported questionnaire | No information available | A pprox. 6 weeks | " not funded by an external body" | |
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate " Adverse events were defined clinically, and starting week 14, volunteers reporting adverse events were interviewed by members of the hospital team" | Adequate | Active | Prospective | |
| Inadequate " Particular attention was paid to complaints such as fever, chills, malaise, nausea and vomiting, rashes and other symptoms and signs that could be regarded as adverse events." Comment: no clear definition of adverse events wa s provided | Adequate | Active | Prospective | |
| Inadequate " Participants had access to a village health center, where they could notify personnel of any malaise or side effects" | Unclear " Clinical examinations and parasitologic tests were performed every 4 weeks" | Passive | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate Comment: insufficient information wa s provided about the questions that travellers were asked | Adequate | Active | Retrospective | |
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Inadequate Comment: insufficient information wa s provided about the questions that travellers were asked | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: No information wa s provided regarding the timing of the questionnaire during treatment | |
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate "Travellers… were given a questionnaire that asked for... adverse health events attributed to those drugs" | Adequate | Passive | Unclear Comment: information was collected at the airport, when travellers should still have been taking the prophylactic regimen | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Inadequate Comment: i t wa s unclear whether the questionnaire implied causality to the drug regimen | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking the prophylactic regimen | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information wa s reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking the prophylactic regimen | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? 3. Monitoring classed as 'active' if it occurred at set time points during treatment. For full description of analysis methods, see Table 2. | ||||
| Mefloquine versus atovaquone‐proguanil and doxycycline | |||
| Outcome | Short‐ term travellers¹ | Longer‐ term travellers² | Test for subgroup |
| Relative effect (RR) | Relative effect (RR) | ||
| Serious adverse effects | RR 5.38 (0.60 to 47.84) 3 cohort studies (2657) | RR 0.93 (0.43 to 2.01) 3 cohort studies (3147) | P = 0.14 |
| Discontinuations due to adverse effects (RCTs) | RR 2.64 (1.51 to 4.62) 5 RCTs (2048) | ‐ | ‐ |
| Discontinuations due to adverse effects (cohort studies) | RR 1.81 (0.86 to 3.80) 7 cohort studies (2907) | RR 1.19 (0.45 to 3.17) 4 cohort studies (5711) | P = 0.50 |
| Nausea | RR 2.02 (0.87 to 4.68) 6 cohort studies (2469) | RR 0.96 (0.22 to 4.18) 3 cohort studies (2725) | P = 0.39 |
| Abdominal pain | RR 0.66 (0.22 to 1.98) 5 cohort studies (1801) | RR 0.30 (0.22 to 0.42) 3 cohort studies (2725) | P = 0.18 |
| Diarrhoea | RR 0.64 (0.15 to 2.71) 5 cohort studies (2428) | RR 0.57 (0.22 to 1.49) 4 cohort studies (5187) | P = 0.89 |
| Headache | RR 2.39 (0.69 to 8.22) 5 cohort studies (2086) | RR 2.09 (1.10 to 3.95) 4 cohort studies (3506) | P = 0.85 |
| Dizziness | RR 3.05 (1.15 to 8.12) 4 cohort studies (1067) | RR 3.84 (1.34 to 11.00) 4 cohort studies (3506) | P = 0.76 |
| Abnormal dreams | RR 6.25 (1.16 to 33.67) 3 cohort studies (1037) | RR 7.62 (2.06 to 28.18) 4 cohort studies (3506) | P = 0.86 |
| Insomnia | RR 3.09 (0.30 to 32.21) 4 cohort studies (1760) | RR 8.67 (4.73 to 15.89) 4 cohort studies (3506) | P = 0.40 |
| Anxiety | RR 3.26 (0.20 to 53.46) 1 cohort study (487) | RR 18.05 (9.75 to 33.42) 3 cohort studies (2854) | P = 0.24 |
| Depressed mood | RR 2.52 (0.76 to 8.29) 3 cohort studies (1026) | RR 12.59 (6.47 to 24.49) 3 cohort studies (3210) | P = 0.02 |
| Abnormal thoughts and behaviours | RR 1.29 (0.07 to 22.44) 1 cohort study (487) | RR 7.78 (1.12 to 54.06) 2 cohort studies (2558) | P = 0.31 |
| Adherence: during travel | RR 1.10 (1.03 to 1.18) 7 cohort studies (7241) | RR 1.20 (0.88 to 1.62) 4 cohort studies (4890) | P = 0.61 |
| Adherence: after return | RR 1.04 (0.92 to 1.17) 4 cohort studies (1221) | ‐ | ‐ |
| 1 Short‐ term travellers: Approximately 3 weeks (range 1 day to 3 months). References: Goodyer 2011; Kato 2013; Kuhner 2005; Napoletano 2007; Laver 2001; Laverone 2006; Lobel 2001; Philips 1996; Schwartz 1999; Shamiss 1996; Sonmez 2005; Stoney 2016; Terrell 2015 | |||
| Mefloquine versus atovaquone‐proguanil and doxycycline | |||
| Outcome | Military¹ | Non‐military² | Test for subgroup |
| Relative effect (RR) | Relative effect (RR) | ||
| Serious adverse effects | 0 events in 1386 participants | RR 1.21 (0.60 to 2.44) 4 cohort studies (4418) | ‐ |
| Discontinuations due to adverse effects (RCTs) | RR 2.08 (0.13 to 32.73) 2 RCTs (441) | RR 2.22 (1.17 to 4.21) 4 RCTs (1669) | P = 0.96 |
| Discontinuations due to adverse effects (cohorts) | RR 1.24 (0.32 to 4.88) 4 cohort studies (3408) | RR 1.89 (1.35 to 2.64) 8 cohort studies (8938) | P = 0.56 |
| Nausea | RR 1.39 (0.36 to 5.36) 4 cohort studies (1578) | RR 1.70 (0.60 to 4.81) 6 cohort studies (3767) | P = 0.26 |
| Abdominal pain | RR 0.43 (0.14 to 1.29) 4 cohort studies (1578) | RR 0.56 (0.23 to 1.35) 5 cohort studies (3099) | P = 0.72 |
| Diarrhoea | RR 0.30 (0.09 to 0.96) 4 cohort studies (3999) | RR 1.05 (0.54 to 2.06) 6 cohort studies (3767) | P = 0.07 |
| Headache | RR 1.19 (0.14 to 9.79) 2 cohort studies (1386) | RR 2.48 (1.40 to 4.40) 7 cohort studies (4206) | P = 0.51 |
| Dizziness | RR 2.95 (1.37 to 6.36) 3 cohort studies (844) | RR 3.58 (1.39 to 9.25) 6 cohort studies (3880) | P = 0.76 |
| Abnormal dreams | RR 11.02 (4.61 to 26.34) 1 cohort study (652) | RR 6.59 (1.74 to 25.00) 6 cohort studies (3891) | P = 0.53 |
| Insomnia | RR 2.34 (0.41 to 13.35) 3 cohort studies (1537) | RR 10.24 (6.26 to 16.76) 6 cohort studies (3880) | P = 0.11 |
| Anxiety | ‐ | RR 16.94 (9.36 to 30.64) 4 cohort studies (3390) | ‐ |
| Depressed mood | RR 13.44 (3.34 to 54.05) 1 cohort study (652) | RR 6.49 (2.66 to 15.85) 5 cohort studies (3584) | P = 0.39 |
| Abnormal thoughts and behaviours | ‐ | RR 5.11 (1.11 to 23.53) 3 cohort studies (3045) | ‐ |
| Adherence: during travel | RR 1.18 (1.00 to 1.40) 5 cohort studies (4652) | RR 1.16 (0.99 to 1.35) 8 cohort studies (10785) | P = 0.85 |
| Adherence: after return | RR 1.16 (0.86 to 1.55) 1 cohort study (43) | RR 1.02 (0.89 to 1.16) 3 cohort studies (1178) | P = 0.44 |
| 1 Military participants: References: RCTs: Arthur 1990; Ohrt 1997. Cohort studies: Andersson 2008, Saunders 2015; Shamiss 1996; Sonmez 2005; Terrell 2015; Tuck 2016 | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria Show forest plot | 9 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.04, 0.19] |
| 2 Malaria; episodes of parasitaemia in semi‐immune populations Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Trials reporting number of participants with parasitaemia | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.06, 0.55] |
| 2.2 Trials reporting number of episodes of parasitaemia | 2 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 5.25] |
| 3 Serious adverse events or effects (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 RCTs (adverse events) | 6 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.53] |
| 3.2 Cohort studies (adverse effects) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.39, 24.11] |
| 4 Discontinuations due to adverse effects (all studies) Show forest plot | 7 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.55, 4.88] |
| 4.1 RCTs (adverse effects) | 7 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.55, 4.88] |
| 5 Nausea (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 RCTs (adverse events) | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.05, 1.73] |
| 5.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.42, 2.43] |
| 6 Vomiting (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.19] |
| 6.2 Cohort studies (adverse events) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.21] |
| 7 Abdominal pain (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 RCTs (adverse events) | 3 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.40] |
| 7.2 Cohort studies (adverse events) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.42] |
| 8 Diarrhoea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 RCTs (adverse events) | 4 | 589 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.32, 1.62] |
| 8.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.68] |
| 9 Headache (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 RCTs (adverse events) | 5 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.71, 0.99] |
| 9.2 Cohort studies (adverse events) | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.63, 4.26] |
| 10 Dizziness (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 RCTs (adverse events) | 3 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 10.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.29, 2.49] |
| 11 Abnormal dreams (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.15, 4.80] |
| 12 Insomnia (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.06, 2.02] |
| 13 Anxiety (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.67, 2.21] |
| 14 Depressed mood (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [0.65, 9.07] |
| 15 Abnormal thoughts and perceptions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse events) | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.77 [0.79, 42.06] |
| 16 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 RCTs (adverse events) | 3 | 609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.24] |
| 16.2 Cohort studies (adverse events) | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.71 [1.58, 28.55] |
| 17 Visual impairment (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.66, 1.46] |
| 17.2 Cohort studies (adverse events) | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.27, 3.19] |
| 18 Vertigo (all studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.34] |
| 19 Other adverse events (RCTs) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Arthralgia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.02, 5.48] |
| 19.2 Back pain | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.61] |
| 19.3 Blurred vision | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.89] |
| 19.4 Cough | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.14] |
| 19.5 Constipation | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 19.6 Decreased appetite | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.28] |
| 19.7 Falls | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 19.8 Fatigue | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.14, 5.86] |
| 19.9 Gastritis | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.98] |
| 19.10 Myalgia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.36, 6.57] |
| 19.11 Rash | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 2.30] |
| 19.12 Respiratory tract infection | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.04, 6.61] |
| 19.13 Sore throat | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 2.75] |
| 19.14 Unsteadiness | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 19.15 Weakness | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.17] |
| 20 Other adverse effects (cohort studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Agitation | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.61, 1.82] |
| 20.2 Altered spatial perception | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.4 [0.57, 153.97] |
| 20.3 Confusion | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.78] |
| 20.4 Loss of appetite | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.50] |
| 20.5 Mouth ulcers | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.39, 2.56] |
| 20.6 Palpitations | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.06 [0.44, 147.68] |
| 20.7 Tingling | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.59, 6.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 4 | 744 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.35, 5.19] |
| 2 Serious adverse events or effects (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RCTs (adverse events) | 3 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.16] |
| 2.2 Cohort studies (adverse effects) | 3 | 3722 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.23, 10.24] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RCTs | 4 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.87] |
| 3.2 Cohort studies | 10 | 10165 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.55] |
| 4 Nausea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cohort studies (adverse effects) | 5 | 2683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.30, 0.45] |
| 4.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.74] |
| 4.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.06, 2.43] |
| 5 Vomiting (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cohort studies (adverse effects) | 4 | 5071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.12, 0.27] |
| 5.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 6 Abdominal pain (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Cohort studies (adverse effects) | 4 | 2569 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.07] |
| 6.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.74, 3.70] |
| 6.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.83, 2.18] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Cohort studies (adverse effects) | 5 | 5104 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.73] |
| 7.2 RCTs (adverse events) | 2 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.29] |
| 7.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [1.69, 7.59] |
| 8 Dyspepsia (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Cohort studies (adverse effects) | 5 | 5104 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.74] |
| 9 Headache (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Cohort studies (adverse effects) | 5 | 3322 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.50, 2.92] |
| 9.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.25, 4.27] |
| 9.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.38, 4.34] |
| 10 Dizziness (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Cohort studies (adverse effects) | 5 | 2633 | Risk Ratio (M‐H, Random, 95% CI) | 3.49 [0.88, 13.75] |
| 10.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.30, 7.16] |
| 10.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.47, 3.90] |
| 10.4 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.62, 0.73] |
| 11 Abnormal dreams (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Cohort studies (adverse effects) | 4 | 2588 | Risk Ratio (M‐H, Random, 95% CI) | 10.49 [3.79, 29.10] |
| 11.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.07, 15.89] |
| 11.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [2.08, 9.00] |
| 12 Insomnia (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Cohort studies (adverse effects) | 4 | 3212 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [1.19, 14.44] |
| 12.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.65, 6.40] |
| 12.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 4.54 [2.09, 9.83] |
| 12.4 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.43, 0.49] |
| 13 Anxiety (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Cohort studies (adverse effects) | 3 | 2559 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.04 [9.32, 34.93] |
| 13.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.74 [1.99, 38.40] |
| 13.3 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.47, 0.56] |
| 14 Depressed mood (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Cohort studies (adverse effects) | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.43 [5.21, 25.07] |
| 14.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.27 [1.82, 21.62] |
| 14.3 Retrospective healthcare record analysis (adverse events) | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.51, 0.60] |
| 15 Abnormal thoughts and perceptions Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse effects) | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.60 [0.92, 47.20] |
| 15.2 Retrospective healthcare record analyses (adverse events) | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.26, 0.66] |
| 16 Pruritis (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Cohort studies (adverse effects) | 2 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.91] |
| 16.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.93, 7.78] |
| 17 Photosensitivity (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Cohort studies (adverse effects) | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.05, 0.11] |
| 17.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.49] |
| 18 Yeast infection (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Cohort studies (adverse effects) | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.06, 0.16] |
| 18.2 Cohort studies (adverse events) | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.63] |
| 19 Visual impairment (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Cohort studies (adverse effects) | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.41, 3.99] |
| 20 Other adverse effects (cohort studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Alopecia | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [1.96, 6.03] |
| 20.2 Asthenia | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.89, 3.76] |
| 20.3 Balance disorder | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [1.48, 5.59] |
| 20.4 Decreased appetite | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.42, 3.64] |
| 20.5 Fatigue | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.77] |
| 20.6 Hypoaesthesia | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.48 [3.01, 43.70] |
| 20.7 Malaise | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.71] |
| 20.8 Mouth ulcers | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.02, 11.42] |
| 20.9 Palpitations | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.16, 48.91] |
| 20.10 Tinnitus | 1 | 684 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.20 [0.39, 133.30] |
| 21 Other adverse events (RCTs) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Constipation | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 21.2 Cough | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 1.01] |
| 21.3 Decreased appetite | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [1.24, 10.20] |
| 21.4 Malaise | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.88, 4.69] |
| 21.5 Palpitations | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 21.6 Pyrexia | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.09, 7.42] |
| 21.7 Sexual dysfunction | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.33, 28.51] |
| 21.8 Somnolence | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 22 Other adverse events (cohort studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Adjustment disorder | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.40, 0.45] |
| 22.2 Confusion | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.24, 19.49] |
| 22.3 Convulsions | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 22.4 Hallucinations | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.08, 0.45] |
| 22.5 Paranoia | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.63] |
| 22.6 Palpitations | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.44 [1.73, 104.38] |
| 22.7 Panic attacks | 1 | 21065 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [0.55, 31.49] |
| 22.8 PTSD | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.53, 0.64] |
| 22.9 Rash | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.50, 2.94] |
| 22.10 Suicidal ideation | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.31, 0.47] |
| 22.11 Suicide | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.32, 4.56] |
| 22.12 Tinnitus | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.61, 0.71] |
| 23 Adherence (cohort studies) Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 Adherence during travel | 13 | 15583 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.12, 1.18] |
| 23.2 Adherence in the post‐travel period | 4 | 840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 2 | 1293 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events or effects (all studies) Show forest plot | 3 | 3591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.08, 23.22] |
| 2.1 Cohort studies | 3 | 3591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.08, 23.22] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RCTs | 3 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [1.53, 5.31] |
| 3.2 Cohort studies | 9 | 7785 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [1.83, 4.08] |
| 4 Nausea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.52, 4.86] |
| 4.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.54, 4.06] |
| 5 Vomiting (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.49, 3.50] |
| 5.2 Cohort studies (adverse effects) | 3 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.08, 4.09] |
| 6 Abdominal pain (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.56] |
| 6.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.07] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.47] |
| 7.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| 8 Mouth ulcers (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.70, 3.00] |
| 8.2 Cohort studies (adverse effects) | 2 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.04, 0.37] |
| 9 Headache (all studies) Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.99, 2.99] |
| 9.2 Cohort studies (adverse effects) | 8 | 4163 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [1.71, 6.82] |
| 10 Dizziness (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.99 [2.08, 7.64] |
| 10.2 Cohort studies (adverse effects) | 8 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [2.23, 6.58] |
| 10.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.04, 1.46] |
| 11 Abnormal dreams (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.37, 3.04] |
| 11.2 Cohort studies (adverse effects) | 7 | 3848 | Risk Ratio (M‐H, Random, 95% CI) | 6.81 [1.65, 28.15] |
| 12 Insomnia (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [2.56, 7.64] |
| 12.2 Cohort studies (adverse effects) | 8 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.29 [4.37, 12.16] |
| 12.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.44] |
| 13 Anxiety (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.12 [1.82, 20.66] |
| 13.2 Cohort studies (adverse effects) | 4 | 2664 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.10 [3.48, 29.32] |
| 13.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.28, 1.85] |
| 14 Depressed mood (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.78 [1.71, 19.61] |
| 14.2 Cohort studies (adverse effects) | 6 | 3624 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.02 [3.56, 18.07] |
| 14.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.56, 2.38] |
| 15 Abnormal thoughts and perceptions (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse effects) | 3 | 2433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.30, 7.42] |
| 15.2 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.69, 12.97] |
| 16 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.60, 2.70] |
| 16.2 Cohort studies (adverse effects) | 3 | 1824 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.40, 10.68] |
| 17 Visual impairment (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.88, 4.73] |
| 17.2 Cohort studies (adverse effects) | 2 | 1956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.29, 4.72] |
| 18 Other adverse effects (cohort studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Allergic reaction | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.04, 14.48] |
| 18.2 Alopecia | 1 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [0.30, 70.01] |
| 18.3 Asthenia | 2 | 1956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.26, 13.12] |
| 18.4 Balance disorder | 1 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.19, 44.19] |
| 18.5 Cough | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.08, 2.92] |
| 18.6 Disturbance in attention | 3 | 1363 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.45 [1.84, 10.77] |
| 18.7 Dyspepsia | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.46] |
| 18.8 Fatigue | 2 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.47, 45.56] |
| 18.9 Hypoaesthesia | 2 | 1946 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.45 [0.93, 21.26] |
| 18.10 Loss of appetite | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.43] |
| 18.11 Muscle pain | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.57 [0.45, 127.80] |
| 18.12 Palpitations | 3 | 2180 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.73, 15.26] |
| 18.13 Photosensitization | 2 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.10, 4.92] |
| 18.14 Pyrexia | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.28 [0.24, 75.57] |
| 18.15 Rash | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.09] |
| 18.16 Restlessness | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.24 [0.32, 84.52] |
| 18.17 Slight illness | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.83 [0.36, 93.84] |
| 18.18 Somnolence | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.21, 11.40] |
| 18.19 Tinnitus | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.13, 42.64] |
| 18.20 Circulatory disorders | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.38 [0.36, 114.01] |
| 19 Other adverse events (cohort studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Adjustment disorder | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.02] |
| 19.2 Confusion | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.04, 25.96] |
| 19.3 Convulsions | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.79, 2.30] |
| 19.4 Hallucinations | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.79] |
| 19.5 Paranoia | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.08, 36.72] |
| 19.6 PTSD | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.93, 3.26] |
| 19.7 Suicidal ideation | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.03, 2.77] |
| 19.8 Suicide | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.06, 7.78] |
| 19.9 Tinnitus | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.21, 1.68] |
| 20 Adherence (RCTs) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 van Riemsdijk 2002 | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 20.2 Overbosch 2001; during travel | 1 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 20.3 Overbosch 2001; post‐travel | 1 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.74, 0.85] |
| 21 Adherence (cohort studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 During travel | 6 | 5577 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.86, 1.34] |
| 21.2 Post‐travel | 2 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 4 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.28, 0.52] |
| 2 Serious adverse events or effects (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 RCTs | 4 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.32, 23.85] |
| 2.2 Cohort studies | 6 | 79257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.07] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 RCTs | 3 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.18] |
| 3.2 Cohort studies in short‐term travellers | 6 | 55397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.26] |
| 3.3 Cohort studies in longer term occupational travellers | 2 | 6085 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [2.41, 3.66] |
| 4 Nausea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Cohort studies (adverse effects) | 6 | 58984 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.89, 1.68] |
| 4.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.79] |
| 5 Vomiting (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cohort studies (adverse effects) | 5 | 5577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 5.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.36, 3.49] |
| 6 Abdominal pain (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cohort studies (adverse effects) | 4 | 5440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 6.2 RCTs (adverse events) | 2 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.37, 1.36] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Cohort studies (adverse effects) | 5 | 5577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 7.2 RCTs (adverse events) | 3 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.50] |
| 8 Headache (all studies) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Cohort studies (adverse effects) | 6 | 56998 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.34] |
| 8.2 RCTs (adverse events) | 3 | 772 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.31] |
| 9 Dizziness (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Cohort studies (adverse effects) | 5 | 58847 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.34, 1.70] |
| 9.2 RCTs (adverse events) | 2 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.46] |
| 10 Abnormal dreams (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Cohort studies (adverse effects) | 4 | 2845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.10, 1.33] |
| 10.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.05, 6.95] |
| 11 Insomnia (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Cohort studies (adverse effects) | 5 | 56952 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.73, 4.51] |
| 11.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.84] |
| 12 Anxiety (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Cohort studies (adverse effects) | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.30 [4.37, 9.09] |
| 13 Depressed mood (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Cohort studies (adverse effects) | 5 | 58855 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [1.15, 8.57] |
| 14 Abnormal thoughts and perceptions Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Cohort studies (adverse effects) | 4 | 4831 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.49 [2.65, 11.35] |
| 15 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse effects) | 2 | 55544 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.40] |
| 15.2 RCTs (adverse events) | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.93] |
| 16 Visual impairment (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Cohort studies (adverse effects) | 5 | 58847 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.50, 2.44] |
| 16.2 RCTs (adverse events) | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.63] |
| 17 Vertigo (all studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Cohort studies (adverse effects) | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.05, 23.43] |
| 18 Cohort studies in travellers; prespecified adverse effects Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Vertigo | 1 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.05, 23.43] |
| 18.2 Nausea | 5 | 56847 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.94, 2.13] |
| 18.3 Vomiting | 4 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.55, 1.42] |
| 18.4 Abdominal pain | 3 | 3303 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.30] |
| 18.5 Diarrhoea | 4 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.57, 2.64] |
| 18.6 Headache | 5 | 54861 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.48, 2.65] |
| 18.7 Dizziness | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.10, 2.10] |
| 18.8 Abnormal dreams | 3 | 708 | Risk Ratio (M‐H, Random, 95% CI) | 4.21 [0.57, 31.33] |
| 18.9 Insomnia | 4 | 54815 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.40, 6.10] |
| 18.10 Anxiety | 2 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.53, 29.48] |
| 18.11 Depressed mood | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.75, 8.31] |
| 18.12 Abnormal thoughts or perceptions | 3 | 2694 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [1.58, 12.40] |
| 18.13 Pruritis | 1 | 53407 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 18.14 Visual impairment | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.55, 0.79] |
| 19 Other adverse effects (cohort studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Altered spatial perception | 1 | 2032 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.55, 6.45] |
| 19.2 Alopecia | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.27, 2.25] |
| 19.3 Asthenia | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.97, 2.40] |
| 19.4 Balance disorder | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [2.15, 6.00] |
| 19.5 Confusion | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.11, 36.31] |
| 19.6 Decreased appetite | 1 | 2032 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.87, 1.57] |
| 19.7 Fatigue | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.57, 9.80] |
| 19.8 Hypoaesthesia | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 20.26 [1.23, 333.93] |
| 19.9 Irritability | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [0.28, 80.59] |
| 19.10 Mouth ulcers | 2 | 55439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.01, 1.87] |
| 19.11 Paraesthesia | 2 | 2778 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.27, 3.89] |
| 19.12 Palpitations | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.71 [0.91, 24.26] |
| 19.13 Photosensitization | 2 | 2662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.53] |
| 19.14 Restlessness | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.65, 34.46] |
| 19.15 Slight illness | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.64, 10.87] |
| 19.16 Somnolence | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [0.37, 100.36] |
| 19.17 Yeast infection | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.53, 2.49] |
| 20 Other adverse events (RCTs) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Abdominal distension | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.64, 15.27] |
| 20.2 Anger | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 20.3 Disturbance in attention | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.61, 16.47] |
| 20.4 Irritability | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.45, 2.64] |
| 20.5 Loss of appetite | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.35, 3.25] |
| 20.6 Malaise | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.85] |
| 20.7 Mood altered | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.29, 4.34] |
| 21 Pregnancy related outcomes (RCTs) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Spontaneous abortions | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.36, 1.79] |
| 21.2 Still births | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.52] |
| 21.3 Congenital malformations | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Adherence (cohort studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 Short‐term travellers | 3 | 852 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.13] |
| 22.2 Short‐term travellers: after return | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.87] |
| 22.3 Longer‐term occupational travellers | 2 | 5777 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.80, 2.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea; effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.52, 4.86] |
| 1.2 Cohort studies | 11 | 5973 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.78, 3.77] |
| 2 Abdominal pain; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.52, 1.56] |
| 2.2 Cohort studies | 9 | 4494 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.87] |
| 3 Diarrhoea; effects Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.60, 1.47] |
| 3.2 Cohort studies | 10 | 7648 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.28, 1.34] |
| 4 Headache; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.99, 2.99] |
| 4.2 Cohort studies | 9 | 5592 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.22, 3.93] |
| 5 Dizziness; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 3.99 [2.08, 7.64] |
| 5.2 Cohort studies | 9 | 4606 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [1.58, 6.35] |
| 6 Abnormal dreams; effects Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.37, 3.04] |
| 6.2 Cohort studies | 7 | 4543 | Risk Ratio (M‐H, Random, 95% CI) | 7.30 [2.51, 21.18] |
| 7 Insomnia; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [2.56, 7.64] |
| 7.2 Cohort studies | 9 | 5299 | Risk Ratio (M‐H, Random, 95% CI) | 5.70 [2.83, 11.47] |
| 8 Anxiety; effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 6.12 [1.82, 20.66] |
| 8.2 Cohort studies | 4 | 3390 | Risk Ratio (M‐H, Random, 95% CI) | 15.26 [8.66, 26.89] |
| 9 Depressed mood; effects Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 5.78 [1.71, 19.61] |
| 9.2 Cohort studies | 6 | 4236 | Risk Ratio (M‐H, Random, 95% CI) | 7.82 [3.79, 16.12] |
| 10 Abnormal thoughts or perceptions; effects Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Cohort studies | 3 | 3045 | Risk Ratio (M‐H, Random, 95% CI) | 4.20 [0.81, 21.87] |
| 11 Pruritis; effects Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.60, 2.70] |
| 11.2 Cohort studies | 3 | 2034 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.16, 4.76] |
| 12 Visual impairment; effects Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.88, 4.73] |
| 12.2 Cohort studies | 3 | 2560 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.05, 4.02] |
| 13 Adherence; during travel Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 RCTs | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 13.2 Cohort studies | 11 | 12131 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.03, 1.30] |
| 14 Adherence; after return Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Cohort studies | 4 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |

