Mefloquina para la prevención del paludismo durante el viaje a zonas endémicas
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Design: retrospective cohort study. Study dates: November 1997 to January 2000 Malaria transmission pattern and local antimalarial drug resistance: various destinations, not specified. Adverse event monitoring: one off telephone interview with parents whose children had previously been prescribed antimalarial prophylaxis. | |
| Participants | Number enrolled: 177 fit inclusion criteria and interviewed, 190 contacted Inclusion criteria: children aged ≤ 13 years who visited the travel clinic at the Children’s Memorial Hospital in Chicago within the study dates. Subjects who were not on other medications. Exclusion criteria: "...data were only included if the child was living with the interviewed parent while taking the antimalarial". "Unwillingness to participate in the study and language barriers". Factors influencing drug allocation: "children... instructed to take mefloquine or chloroquine for malaria prophylaxis". Country of recruitment: USA. Country of malaria exposure: various; Africa 58%, Central or South America 21%, India 12% or Eastern Asia 9%. Duration of exposure to malaria: various, not specified. Type of participants: travellers | |
| Interventions | 1. Mefloquine* 2. Chloroquine* *dosing regimen not specified | |
| Outcomes | 1. Adverse effects; any, nausea, vomiting, diarrhoea, headache, insomnia, abnormal dreams 2. Serious adverse effects 3. Discontinuations of study drug due to adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex and destination of travel were recorded, but were not reported across prophylactic regimens 2. Selection of participants into the study: low Non‐response rate 1.6% 3. Measurement of interventions: moderate The prescription was provided by a travel clinic, but participants were asked to recall if they discontinued their medication 2.8 to 28 months after visiting 4. Departures from intended interventions: serious Information was collected up to 2 years after taking the drug. No information was captured on switches. 5. Missing data: low All information was collected at one time point, there were no losses to follow‐up. 6. Measurement of outcomes: serious The outcome measure was subjective, participants and personnel were not blinded. 7. Selection of the reported results: low All outcomes included in the introduction were reported in the results 8. Other: low "The authors had no financial or other conflicts of interest to disclose" |
| Methods | Design: prospective cohort study Study dates: March 2004 to November 2006 Malaria transmission pattern and local antimalarial drug resistance: malaria attack rate of 44% with P falciparum in another similar study at the time Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 690 soldiers sent questionnaire, 609 respondents Inclusion criteria: all Swedish soldiers deployed to Liberia within the study dates Exclusion criteria: none stated. Factors influencing drug allocation: "...mefloquine was prescribed to almost all soldiers in the first two contingents and to about two‐thirds in the last three contingents. The remaining soldiers were recommended atovaquone/ proguanil. The latter group consisted mainly of those with body weight < 70 kg and those who had already experienced adverse events with mefloquine. No other drug regimes were used". Country of recruitment: Sweden Country of malaria exposure: Liberia Duration of exposure to malaria: 6 months Type of participants: military | |
| Interventions | 1. Mefloquine* 2. Atovaquone‐proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse events; any, nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams nightmares, insomnia sleep disturbance, depression 2. Serious adverse events; serious 3. Adverse events; other (concentration difficulties, mouth ulcers, fever, muscle pain) 4. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 5. Clinical cases of malaria 6. Overall satisfaction with the drug 7. Whether they would take the drug again 8. Measures of adherence to the drug regimen (data provided on aggregate) | |
| Notes | Funding sources: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Information on potential confounders is not provided across prophylactic groups 2. Selection of participants into the study: moderate 609/690 (88%) response rate 3. Measurement of interventions: low All participants were issued with the study drug. 4. Departures from intended interventions: low Switches were recorded and reported 5. Missing data: serious Outcomes were reported from 3 of 5 cohorts. No information was provided for 2 remaining cohorts. 6. Measurement of outcomes: serious The outcome measure was subjective, participants and personnel were not blinded. 7. Selection of the reported results: low All outcomes prespecified in the introduction were reported. 8. Other: moderate Study sponsor not mentioned, but 2 study authors worked for GlaxoSmithKline |
| Methods | Design: RCT Study dates: June to August 1988 Malaria transmission pattern and local drug resistance: local chloroquine resistance Adverse event monitoring: blood taken at induction and at days 57 and 70 of treatment. Interviews regarding side effects when sera taken. Stool sample at induction, at end of exercise and at any time participants sought medical care. | |
| Participants | Number enrolled: 270 Inclusion criteria: soldiers (aged 18 to 40 years), awaiting deployment to Thailand Exclusion criteria: previous history of gastrointestinal illness Country of recruitment: USA Country of malaria exposure: Thailand Duration of exposure to malaria: 5 weeks Type of participants: soldiers, non‐immune | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet) once weekly, starting 1 week before travel and continuing throughout the period of deployment.* 2. Doxycycline (1 capsule containing doxycycline hyclate 100 mg) once daily, starting 1 week before travel and continuing throughout the period of deployment* Co‐interventions: Both groups given doxycycline 100mg daily for suppression of P falciparum and primaquine 45 mg weekly for elimination of liver hypnozoites for 6 weeks on return to the USA. *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Serious adverse event 3. Adverse events; diarrhoea 4. Discontinuation of study drug due to adverse effects 5. Measures of adherence to the drug regimen Outcomes assessed not included in the review: 6. Laboratory tests; enteric pathogens 7. Adverse events; nausea, vomiting, headache, dizziness (data provided on aggregate) | |
| Notes | Funding sources: Pfizer Inc supplied active and placebo doxycycline; Hoffman‐La Roche Inc supplied active and placebo mefloquine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Volunteers were assigned from a computer generated random number list to receive daily doxycycline or weekly mefloquine" |
| Allocation concealment (selection bias) | Unclear risk | Comment: Unclear how the tablets were labelled and whether allocation concealment occurred |
| Blinding of participants and personnel (performance bias) | Low risk | "Soldiers receiving mefloquine also received identical appearing doxycycline placebo capsules daily, and those receiving daily doxycycline received weekly mefloquine placebo tablets" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no explanation of how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | High risk | "Of the 270 volunteers who were deployed, 253 were correctly taking the assigned study malaria prophylaxis on arrival in Korat" Comment: Reasons for not taking medication were not reported. Method of detection for malaria, frequency and duration of follow‐up were not reported |
| Incomplete outcome data (attrition bias); safety | Low risk | Comment: 17 participants (6%) were not "correctly taking the prophylaxis on arrival to Korat" and were excluded from the analysis. Data were not stratified by time point |
| Selective reporting (reporting bias); efficacy | Low risk | "None of the soldiers developed malaria" |
| Selective reporting (reporting bias); safety | Unclear risk | Comment: data for general side effects (e.g. headaches) were presented for the study population but not for each group |
| Other bias | Unclear risk | Comment: study sponsor not mentioned |
| Methods | Design: prospective cohort study Study dates: October 2006 to October 2007 Malaria transmission pattern and local antimalarial drug resistance: various destinations, not specified Adverse event monitoring: not performed | |
| Participants | Number enrolled: 945 Inclusion criteria: People aged ≥ 18 years were eligible if they were planning to travel for 1 to 13 weeks to one or more malaria‐endemic countries. Exclusion criteria: None stated Factors influencing drug allocation: "Dutch national guidelines for travelers’ health advice" Country of recruitment: Netherlands Regions of malaria exposure: various; Asia 48%, Africa 30% and Latin America 22% Duration of exposure to malaria: various; 49% ≤ 13 days, 35% 14 to 28 days and 9% ≥ 29 days Type of participants: travellers | |
| Interventions | 1. Mefloquine: taken 3 weeks prior to arrival, during trip and for 4 weeks after return, dose and frequency of dose not specified 2. Atovaquone‐proguanil: 1 day prior to arrival, during trip and for 7 days after return, dose and frequency of dose not specified 3. Proguanil: On day of arrival, during trip and for 4 weeks after return, dose and frequency of dose not specified | |
| Outcomes | Included in the review: 1. Measures of adherence to the drug regime Outcomes assessed not included in the review: 2. Clinical cases of malaria 3. Predictors of adherence to malaria prophylaxis 4. Use of antimosquito preventive measures | |
| Notes | Funding sources: The Amsterdam Academic Collaborative Center on Public Health is financially supported by the Netherlands Organization for Health Research and Development (ZonMw; grant number 7115 0001, http://www.zonmw.nl/nl/) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Length of stay, travel destination, age and sex were not reported across groups 2. Selection of participants into the study: moderate Non‐response rates were not reported 3. Measurement of interventions: low Participants made daily diary entries during travel 4. Departures from intended interventions: low Participants made daily diary entries during travel 5. Missing data: low Information was collected at one time point 6. Measurement of outcomes: moderate Outcome assessors were not blinded, methods were comparable across groups 7. Selection of the reported results: low Outcomes were reported for 610/620 participants 8. Other: low Government funding |
| Methods | Design: RCT Study dates: July 1983 to March 1984 Malaria transmission pattern and local antimalarial drug resistance: "in this area we believe the efficacy of chloroquine prophylaxis at the time of the study was negligible" Adverse event monitoring: "at each 2 week visit… history of symptoms over the previous fortnight was obtained. Patients were asked about fever, chills, headache, nausea, vomiting, diarrhoea, anorexia, rash, myalgia and dysuria or abnormally coloured urine". Laboratory studies were performed at baseline and at 6 weeks in participants who had not developed malaria | |
| Participants | Number enrolled: 501 Inclusion criteria: "Only males 21 years of age or over were accepted" Exclusion criteria: "All participants were required to have a negative malaria smear (after examination of 200 fields on thick smear) on entry into the study". "...the use of other antimalarials or antibiotics" Country of recruitment: Cambodia Country of malaria exposure: Cambodia Duration of exposure to malaria: ongoing in semi immune population, 14 week study period Type of participants: Thai gem miners with a degree of immunity | |
| Interventions | Included in review comparisons: 1. Mefloquine (2 x 250 mg tablet) fortnightly for 14 weeks* 2. Chloroquine (1 x 300 mg tablet) weekly* Not included in review comparisons: 3. Fansidar (2 x 500 mg sufadoxine and 25 mg pyrimethamine) fortnightly and chloroquine (1 x 300 mg tablet) weekly* *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Adverse events; other (myalgias, rash) Outcomes assessed not included in the review: 3. Laboratory tests; haematocrit, complete blood count, transaminase levels, total and direct bilirubin, alkaline phosphatase, blood urea nitrogen 4. Adverse events; headache, anorexia, fever, chills, nausea, diarrhoea or vomiting (data provided on aggregate) | |
| Notes | Funding sources: Support for this study was from the USA Army Medical Research and Development Command | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assignment… is a 4:3:2 ratio" Comment: method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details of allocation concealment were reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Every two weeks in a double blind fashion one of the investigators administered five tablets to each subject" Comment: not mentioned whether placebo tablets had an identical appearance |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no mention of how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | "Only 194 patients completed the study until positivity or end of the 14 weeks observation period". "Therefore of the original 501 enrollees, 63 were discarded due to positivity at week 0 and 104 were discarded since they never returned beyond week 0". Comment: Losses to follow‐up during the study was not reported across groups |
| Incomplete outcome data (attrition bias); safety | Unclear risk | "Only 194 patients completed the study until positivity or end of the 14 weeks observation period...Any subject missing one appointment was excluded from the study though each subject's records up to the time of exclusion were entered into the survival analysis...After 3 weeks post treatment and a negative malaria smear some patients wishing to continue were reentered under a new study number and were assigned a double blind randomized treatment" |
| Selective reporting (reporting bias); efficacy | Unclear risk | Comment: number of people contracting malaria in each group and person‐weeks in the study were reported |
| Selective reporting (reporting bias); safety | Unclear risk | "There were no significant differences in frequency of complaints among the study groups for headache, anorexia, fever, chills, nausea, diarrhoea, or vomiting". Comment: Data for specific adverse events not reported. Methods section states participants were asked about dysuria and abnormally coloured urine, but this was not reported in the results |
| Other bias | Low risk | Support for this study was from the USA Army Medical Research and Development Command |
| Methods | Design: RCT Study dates: not mentioned Malaria transmission pattern and local antimalarial drug resistance: not applicable Adverse event monitoring: "At each visit, the subject answered two computerised questionnaires (the Environmental Symptoms Questionnaire and the Profile of Mood States) [and] a physician interview was performed" | |
| Participants | Number enrolled: 359 Inclusion criteria: "males at least 18 years old, met military weight standards, were available for weekly administration of medications and monitoring during the 13 week study period, and were willing to give informed consent" Exclusion criteria: "treatment with beta‐blocking agents or other cardiotropic drugs, underlying chronic disease, history of cardiac arrhythmia, medical history of psychiatric or neurological problems within the last 5 years, anaemia or impaired hepatic or renal function. Women were excluded from participation in the study due to the risk of teratogenicity involved when the drug is used in early pregnancy" Country of recruitment: USA Country of malaria exposure: not applicable Duration of exposure to malaria: not applicable Type of participants: military, non‐travellers | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet), larium 228 mg base (F Hoffman La Roche) weekly for 11 weeks 2. Mefloquine (1 x 250 mg tablet), larium 228 mg base (F Hoffman La Roche) weekly for 11 weeks, with loading dose of 1 x 250 mg tablet daily for 3 days during the first week 3. Chloroquine (1 x 300 mg tablet), 300 mg base (F Hoffman La Roche) weekly for 11 weeks | |
| Outcomes | Included in the review: 1. Adverse events; nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams, insomnia 2. Adverse events; other (irritability, poor concentration, anger, moodiness, abdominal distension, anorexia, environmental symptoms questionnaire (ESQ), sleep assessment, Profile of Mood States questionnaire) Outcomes assessed not included in the review: 3. Laboratory tests: haemoglobin, haematocrit, platelets, white blood cell count, alanine aminotransferase, blood urea nitrogen and creatinine 4. Analysis of the dizziness index on the ESQ 5. Spontaneous comments on the ESQ (data provided on aggregate) | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...military personnel were assigned to drug groups in a ratio of approximately 3:3:1…stratification was performed by major subordinate command so that equal proportions of each study group would be represented in each MSC" Comment: not mentioned how the randomisation code was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: method allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) | Low risk | "...the ‘double dummy’ method of blinding was employed with either chloroquine or mefloquine placebos administered with active drug… In addition, during the first week of the study, on days two and three, a single mefloquine tablet or placebo was administered. Both drugs and placebos had an extremely bitter taste... identical placebo tablets" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no description provided of how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | N/A |
| Incomplete outcome data (attrition bias); safety | Unclear risk | Comment: 15 medical withdrawals are reported within the study. It is unclear whether these are the only losses to follow up which occurred, or whether they occurred in the mefloquine loading dose group or weekly administration group. |
| Selective reporting (reporting bias); efficacy | Unclear risk | N/A |
| Selective reporting (reporting bias); safety | High risk | ‘table 5 outlines the percent of the group with symptoms only when significance was demonstrated’ ‘selected haematology and biochemistry tests were performed… no significant differences were noted among the three drugs when comparing the mean values’ Comment: data is not fully reported for ‘other symptoms’; only significant results are reported for the ESQ, and data for spontaneous comments on the ESQ are not reported; data is not fully reported for the POMS. |
| Other bias | Unclear risk | Comment: study sponsor not mentioned, but the lead author is attributed to ‘Pharmaceutical Systems Incorporated’ |
| Methods | Design: RCT Study dates: July 1987 to January 1988 Malaria transmission pattern and local antimalarial drug resistance: "a malaria endemic area". Reports chloroquine, sulfadoxine‐pyrimethamine and quinine resistance within Thailand at the time of the study. Adverse event monitoring: "volunteers asked about adverse events at each visit (weeks 4, 9, 14, 19, 24, 28)...starting week 14, volunteers reporting adverse events were interviewed by members of the hospital team; most of them were also seen by principal investigators" | |
| Participants | Number enrolled: 605 randomized, 3 excluded because of baseline parasitaemia Inclusion criteria: "...healthy male volunteers, aged between 16 and 60, living in this area, were recruited" Exclusion criteria: "persons with a known history of allergy against sulphonamides, with an evidence illness of fever, or which a positive blood film (with or without symptomatic malaria) were excluded" Country of recruitment: Thailand Country of malaria exposure: Thailand Duration of exposure to malaria: trial duration 24 weeks Type of participants: Thai residents in a malaria‐endemic area (presumed semi‐immune) | |
| Interventions | Included in the review: 1. Mefloquine (1 tablet containing 125 mg mefloquine) once weekly, double dose during first 4 weeks* 2. Chloroquine (1 tablet containing 300 mg chloroquine) once weekly* 3. Placebo Not included in the review: 4. Fansifem (1 tablet containing 125 mg mefloquine, 250 mg sulfadoxine, 12.5 mg pyrimethamine) once weekly, double dose during first 2 weeks* 5. Fansidar (1 tablet containing 500 mg sulfadoxine, 25 mg pyrimethamine) once weekly* *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Adverse events; any 3. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 4. Laboratory tests; haematocrit, white blood cell count and neutrophil count | |
| Notes | Funding sources: "The project was jointly organized and conducted by the Malaria Division, Department of Communicable Disease, Ministry of Public Health; the Hoffman‐La Roche company, Basel, Switzerland; and The Faculty of Tropical Medicines, Mahidol University, Bangkok" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible volunteers were randomly assigned to treatment groups" Comment: method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | "The tablets were identical in appearance; they were packed in numbered blister packs and were in addition labelled weeks 1‐24... the coded test drugs for weeks 1‐4 were given to every subject" Comment: no mention of concealed opaque envelopes or central allocation |
| Blinding of participants and personnel (performance bias) | Low risk | "A randomised double blind trial…the tablets were identical in appearance; they were packed in numbered blister packs" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no explanation provided of how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | "Of the 605 subjects originally randomised, 3 were excluded because of baseline parasitaemia... Although some of the volunteers left the study for personal reasons (moving away from the area)" Comment: numbers lost to follow up have not been reported |
| Incomplete outcome data (attrition bias); safety | Unclear risk | "94% (116/123) in the mefloquine group and 98% (119/121) in the placebo group were included for adverse event reporting" "Although some of the volunteers left the study for personal reasons (moving away from the area)" Comment: numbers lost to follow‐up were not reported |
| Selective reporting (reporting bias); efficacy | Low risk | Comment: Malaria cases were fully reported |
| Selective reporting (reporting bias); safety | High risk | Comment: Data were collected but not reported for adherence to drug regimen. Data were provided on aggregate across all time points. The number of adverse events were reported but not types or severity |
| Other bias | High risk | "The project was jointly organized and conducted by the Malaria Division, Department of Communicable Disease, Ministry of Public Health; the Hoffman‐La Roche company, Basel, Switzerland; and The Faculty of Tropical Medicines, Mahidol University, Bangkok" |
| Methods | Design: retrospective cohort study Study dates: June 1992 to July 1994 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 1511 questionnaires distributed, 1054 respondents Inclusion criteria: travellers who visited areas with a risk of malaria infection who were travelling on short trips < 6 weeks duration Exclusion criteria: none mentioned Factors influencing drug allocation: The fact of participating in this study did not change at all the typical prophylaxis when performing, which followed the usual criteria (Google Translate = "El hecho de participar en este estudio no cambio en absoluto el tipico de profilaxis al realizar, que siguio los criterios habituales" Country of recruitment: Spain Country of malaria exposure: various, not specified Duration of exposure to malaria: various, not specified Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine (1 x 250 mg tablet) weekly, starting 1 week prior to travel, during the trip and 4 weeks following return from the malaria‐endemic area 2. Chloroquine (5 mg/kg) weekly, starting 1 week prior to travel, during the trip and 4 weeks following return from the malaria‐endemic area Outcomes assessed not included in the review: 3. Chloroquine and proguanil (chloroquine base 5 mg/kg, once weekly plus proguanil 100 mg daily, if weight < 55 kg and 200 mg daily if weight > 55 kg) starting 1 week prior to travel, during the trip and 4 weeks following return from the malaria‐endemic area | |
| Outcomes | Included in the review: 1. Adverse effects; any, vertigo, visual impairment, nausea, vomiting, abdominal pain, diarrhoea, insomnia, anxiety, depression, pruritis 2. Adverse effects; other (irritability) 3. Discontinuations of study drugs due to adverse effects Outcomes assessed not included in the review: 4. Mean number of symptoms reported per traveller 5. Adverse effects; other, incidence < 1% (amnesia, tremor, paraesthesia, seizures, hyper‐reflexia, drowsiness, asthenia, nervousness, difficulty concentrating, mouth ulcers, acne, cardiac rhythm disturbance) | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Sex was reported across groups. No other confounders were reported 2. Selection of participants into the study: serious 1054/1511 (70%) response rate 3. Measurement of interventions: low The antimalarial prescription was provided by a travel clinic which also performed the study 4. Departures from intended interventions: moderate Discontinuations were reported across groups. It is unclear if information regarding switches was obtained 5. Missing data: low All participants were included in the analysis. All information was included at one time point 6. Measurement of outcomes: serious Comment: the outcome measure was subjective, participants and personnel were not blinded 7. Selection of the reported results: moderate The analysis of the relationship of symptoms by weight was reported only for mefloquine 8. Other: no information No information was provided regarding the study sponsor |
| Methods | Design: cross‐sectional cohort study Study dates: questionnaire emailed July 2012, reminder emails were circulated at 8 and 12 weeks Malaria transmission pattern and local antimalarial drug resistance: various destinations, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 579 questionnaires emailed, 327 responses Inclusion criteria: all Foreign and Commonwealth Office staff posted to a malaria‐endemic area Exclusion criteria: none stated Factors influencing drug allocation: "prophylaxis based on the Advisory Committee on Malaria Prevention in UK Travellers (ACMP) guidelines" Country of recruitment: various, not specified Country of malaria exposure: various, not specified Duration of exposure to malaria: 0 to 3 months N = 16 (4.9%), 4 to 6 months N = 26 (8.0%), 7 to 12 months N = 46 (14.1%), 13 to 36 months N = 75 (22.9%), > 36 months N = 167 (51.1%) Type of participants: UK Foreign and Commonwealth Office staff | |
| Interventions | 1. Mefloquine* 2. Atovaquone‐proguanil* 3. Doxycycline* 4. Chloroquine* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; psychiatric disorders (abnormal dreams) 2. Adverse effects; other (skin sensitivity, indigestion, other psychological) Outcomes assessed not included in the review: 3. Clinical cases of malaria 4. Background knowledge of malaria 5. Attitudes regarding malaria prophylaxis 6. Use of personal protective measures 7. Impact of pregnancy on malaria prevention 8. Measures of adherence to drug regimen (data provided on aggregate) | |
| Notes | Funding sources: not mentioned Communications with study authors: the study authors provided us with access to the full original data set. Thedata set differed from findings in the published version of the paper, and we were unable to determine the cause for differences. The included figures were from the full data set | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate No information on confounders was provided across prophylaxis groups 2. Selection of participants into the study: serious Response rate for the survey was 56.5% 3. Measurement of interventions: moderate Participants were asked to self‐report which medications they were prescribed. Compliance rate was 25% 4. Departures from intended interventions: serious No questions were included in the questionnaire regarding switches between chemoprophylactic regimens 5. Missing data: low All participants were included in the analysis 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: low The entire questionnaire was provided in full, all outcomes included were reported 8. Other: no information Study sponsor not mentioned |
| Methods | Design: RCT Study dates: not mentioned Malaria transmission pattern and local antimalarial drug resistance: not applicable Adverse event monitoring: daily self‐reported diary. Three medical check ups for laboratory and other tests | |
| Participants | Number enrolled: 106 randomized, 95 completed all study procedures Inclusion criteria: "healthy adult staff and students at teaching hospitals in Perth, Western Australia" Exclusion criteria: "Those with a past history of psychiatric conditions, or neurological, cardiac, hepatic or renal disease were excluded, as were pregnant or breastfeeding females and those with a known allergy to, or taking medication known to interact with quinolone drugs. None of the subjects had taken mefloquine in the 3 months before the study" Country of recruitment: Australia Country of malaria exposure: not applicable Duration of follow up: 7 weeks Type of participants: non‐immune non‐travellers | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet), with placebo dose followed 1 week later by 250 mg mefloquine weekly, active treatment duration 4 weeks 2. Placebo, 1 tablet weekly, duration 5 weeks | |
| Outcomes | Included in the review: 1. Measure of adherence to the drug regimen 2. Adverse events: other outcome measures (symbol digit modalities test, digit span forwards and backwards test, ECG, hearing loss at 6kHz) Outcomes assessed not included in the review: 3. Laboratory tests: serum glucose, insulin, ionized calcium, phosphate, magnesium and albumin concentrations 4. Adverse events: headache, lethargy, abdominal pain, diarrhoea, cough, nausea; study reports events occurring in the first week (after both groups had received placebo) and the relative risk of symptoms worsening over time | |
| Notes | Funding sources: "We thank… F. Hoffman La Roche & Co. for financial support" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...allocation… was by a random number code generated by independent Fremantle Hospital Pharmacy staff" |
| Allocation concealment (selection bias) | Low risk | "...who kept the code strictly confidential until the last volunteer had completed the protocol" |
| Blinding of participants and personnel (performance bias) | Low risk | "Tablets were prepared in individually numbered but otherwise unlabelled containers... identical placebo tablets…" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Allocation of active or placebo formulation was by a random number code generated by independent Freemantle hospital staff who kept the code strictly confidential" Comment: not mentioned whether outcomes assessors were blinded |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | N/A |
| Incomplete outcome data (attrition bias); safety | Low risk | "Of 106 randomised volunteers, 95 (90%) completed all study procedures... eight subjects withdrew after initial assessment and three after the second. Follow‐up of these individuals revealed no toxicity in those allocated mefloquine" |
| Selective reporting (reporting bias); efficacy | Unclear risk | N/A |
| Selective reporting (reporting bias); safety | High risk | Comment: not all symptoms were reported, only those occurring in > 10% of participants in both groups. Absolute numbers of participants experiencing each symptom after mefloquine/placebo commenced not provided, only relative risk of symptoms worsening over time |
| Other bias | High risk | "We thank… F. Hoffman La Roche & Co. for financial support" |
| Methods | Design: Retrospective cohort study Study dates: 1 January 2008 to 30 June 2013 Malaria transmission pattern and local antimalarial drug resistance: Various, not specified Adverse event monitoring: Data collected retrospectively from the Defense Medical Surveillance System, the Pharmacy Data Transaction Service and the Theater Medical Data Store | |
| Participants | Number enrolled: 367,840 Inclusion criteria: Active component service members who filled a prescription for mefloquine, doxycycline or atovaquone‐proguanil Exclusion criteria: Doxycycline and atovaquone‐proguanil prescriptions were excluded if the service member previously or concurrently received mefloquine. Doxycycline prescriptions were restricted to 100 mg, once daily, tabular form, minimum 30 day prescription Factors influencing drug allocation: Not specified Country of recruitment: USA Country of malaria exposure: Various, not specified Duration of exposure to malaria: Various, not specified Type of participants: Military | |
| Interventions | 1. Mefloquine (250 mg weekly) 2. Atovaquone‐ proguanil* 3. Doxycycline (100 mg, tabular form, daily dose, 30 day minimum prescription) *dosing regimen not specified | |
| Outcomes | 1. Adverse events (anxiety disorders, depressive disorders, psychoses, insomnia, vertigo) 2. Adverse events; other (adjustment disorders, post‐traumatic stress disorder, tinnitus, suicidal ideation, convulsions, hallucinations, paranoia, confusion) | |
| Notes | Funding source: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Identified confounders were measured and not balanced across groups 2. Selection of participants into the study: low Start of intervention and start of follow‐up coincided for most participants. Retrospective medical records were used, therefore there were no non‐responders 3. Measurement of interventions: moderate Information regarding drug prescriptions were obtained from a medical database, without any verification that users took the prescription 4. Departures from intended interventions: serious Discontinuations and switches between prophylactic regimes were not recorded in the database 5. Missing data: low All records in the research database were included in the analysis 6. Measurement of outcomes: moderate Participants and outcome assessors (physicians) were not blinded. However, information was collected anonymously and on aggregate. Participants were unaware of their participation at the time of seeking healthcare 7. Selection of the reported results: low Outcome data were reported for all outcomes prespecified for analysis 8. Other: no information No information was available regarding the study sponsor. |
| Methods | Design: prospective cohort study Study dates: December 2004 to April 2006 Malaria transmission pattern and local antimalarial drug resistance: various destinations, not specified Adverse event monitoring: "a post travel questionnaire… approximately 1 week after they were due to complete their course of medication" | |
| Participants | Number enrolled: 252 recruited, 185 completed pre‐ and post‐travel questionnaires Inclusion criteria: "...to be eligible, travelers had to be at least 18 years of age and to have been prescribed or supplied... an antimalarial medication as a result of planned travel for a duration of 28 days or less." Exclusion criteria: "travelers participating in other prospective clinical research or observational studies, pregnant travelers or travelers planning to get pregnant during the study were excluded" Factors influencing drug allocation: "Treatment choice was solely at the discretion of the traveler and practitioner" Country of recruitment: UK Country of malaria exposure: various, not reported Duration of exposure to malaria: various, median 14 days (interquartile range 9 to 20) Type of participants: travellers | |
| Interventions | 1. Mefloquine* 2. Atovaquone‐proguanil* 3. Doxycycline* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Any adverse effects 2. Measures of adherence to the drug regimen Outcomes assessed not included in the review: 3. Relative importance of factors in choice of antimalarial drugs, for both healthcare professionals and travellers | |
| Notes | Funding sources: "The study was commissioned and paid for by GlaxoSmithKline" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate "There were statistically significant differences in mean age" Several other confounders were not reported across groups 2. Selection of participants into the study: moderate No information is provided regarding people who did not wish to participate 3. Measurement of interventions: low The antimalarial prescription was provided by a travel clinic which also performed the study 4. Departures from intended interventions: moderate No information was captured regarding switches between interventions of interest 5. Missing data: serious 185/252 participants completed the pre‐ and post‐travel questionnaire. Interim loss to follow up 27% 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate The number of reported side effects was reported, but not the types or severity 8. Other: serious Funded by GlaxoSmithKline; the role of the study sponsor was not made clear |
| Methods | Design: RCT Study dates: not mentioned Malaria transmission pattern and local antimalarial drug resistance: "the 20‐week cumulative incidence of reinfection by P. falciparum to be nearly 100%". No mention of local drug resistance patterns Adverse event monitoring: "...during the prophylaxis and follow‐up phases, health workers visited the subjects 3 times weekly. Subjects with physical complaints were examined by a study physician the next day or on an emergent basis, as needed. Hematologic analysis was done on days 4 and 10 after starting the loading dose phase and during weeks 4, 8, 12, and 15. Biochemical analysis was done during weeks 4, 8, 12, and 15" | |
| Participants | Number enrolled: 530 enrolled and completed radical cure regimen. 509 participants took at least 1 dose of the weekly study drug or placebo and comprised the full intention‐to‐treat data set Inclusion criteria: "Inclusion criteria included the following: age of 18–60 years (men) or 50–60 years (women); lack of significant systemic illness as determined by history, physical examination, and clinical laboratory test results (including negative results of a urine pregnancy test for women); and absence of seizures or other neuropsychiatric illness (past or present)" Exclusion criteria: "The high rate of pregnancy and breast‐feeding in women aged 18–49 years precluded their enrollment... G6PD deficiency accounted for 179 of 338 exclusions" Country of recruitment: Ghana Country of malaria exposure: Ghana Duration of exposure to malaria: trial duration 12 weeks Type of participants: Ghanain residents, semi‐immune | |
| Interventions | Included in the review: 1. Mefloquine (1 x 250 mg tablet, salt), weekly, with supervised 3 day loading dose* 2. Placebo, with supervised 3 day loading dose* Not included in the review: 3. Tafenoquine (1 x 25 mg tablet, base), weekly, with supervised 3 day loading dose* 4. Tafenoquine (1 x 50 mg tablet, base), weekly, with supervised 3 day loading dose* 5. Tafenoquine (1 x 100 mg tablet, base), weekly, with supervised 3 day loading dose* 6. Tafenoquine (1 x 200 mg tablet, base), weekly, with supervised 3 day loading dose* *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1.Clinical cases of malaria 2. Adverse events; any, abdominal pain, diarrhoea, headache 3. Adverse events; other (gastritis, back pain, myalgia, polyarthralgia/arthralgia, respiratory tract infection, sore throat, rash) 4. Discontinuation of study drug due to adverse effects Outcomes assessed not included in the review: 5. Laboratory tests; haematological and biochemical analyses | |
| Notes | Funding sources: USA Army Medical Materiel Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The randomization code was generated in blocks of 11 numbers" Comment: not mentioned how randomization code was produced |
| Allocation concealment (selection bias) | Unclear risk | "Code numbers were assigned according to the chronological order of appearance of the subjects at screening. Study drugs were prepackaged and prelabeled with a unique study number according to the randomization code" Comment: no mention of opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | "A ‘double‐dummy’ design allowed double‐blind administration of tafenoquine and mefloquine active drugs and their corresponding placebos" "A placebo (tafenoquine placebo, GlaxoSmith‐Kline; mefloquine placebo, Hoffmann‐La Roche) served as the negative comparator" Comment: does not report that the tablets were identical |
| Blinding of outcome assessment (detection bias) | Unclear risk | "All slides positive for the presence of malaria causing parasites, and an equal number of randomly selected slides with negative results were reevaluated by a second (blinded) microscopist." Comment: no other mention of outcome assessors being blinded and does not report that the researchers were blinded |
| Incomplete outcome data (attrition bias); efficacy | Low risk | "Data analysis for efficacy used 2 data sets: the 'full, intent‐to‐treat' data set (n=509), comprising all subjects who took at least 1 dose of the weekly study drug or placebo, and the 'per‐protocol' data set (n=428), comprising those subjects who strictly fulfilled the protocol criteria" |
| Incomplete outcome data (attrition bias); safety | Low risk | Comment: The safety and tolerability analyses included data for all participants who received at least 1 dose of the study drug or placebo (N = 513) |
| Selective reporting (reporting bias); efficacy | Low risk | Comment: total number of participants with positive blood smear result at any time during prophylaxis was reported. Clinical cases of malaria were reported |
| Selective reporting (reporting bias); safety | High risk | "There were 9 serious adverse events in the study... No serious adverse events were considered by study physicians to be related to the study drug, and no deaths occurred" Comment: Data for serious adverse events were not attributed to the drug regimen. No information was provided on how causality was assessed |
| Other bias | High risk | Acknowledgement of "Philip Pickford and Rachel Moate (GlaxoSmithKline), for statistical and editorial advisement" |
| Methods | Design: retrospective cohort study Study dates: June 1989 to May 1991 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire. "Any reported illness was followed up by telephone interview about the nature of the illness, during which time more complete information was obtained using standardized questions" | |
| Participants | Number enrolled: 869 participants enrolled, 822 completed follow‐up Inclusion criteria: all individuals attending the International Traveler’s Medical Service at the University of Connecticut Health Center and traveling for ≤ 90 days Exclusion criteria: none mentioned Factors influencing drug allocation: "prior to travel each person was given extensive counseling and written material on the prevention of malaria and traveler’s diarrhea. They were given prescriptions for prophylactic antimalarials" Country of recruitment: USA Country of malaria exposure: Various: Indian subcontinent 21%, central and east Africa 20%, South America 16%, Southeast Asia 14%, West Africa 10%, Central America and Mexico 10%, North Africa 65, East Asia 6%, Carribean 5%, Southern Africa 5%, Middle East 3% Duration of exposure to malaria: median 19 days (up to 90 days) Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Chloroquine* Not included in the review: 2. Chloroquine‐proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Any adverse effects 2. Discontinuations of study drug due to adverse effects 3. Measures of adherence to the drug regime Outcomes assessed not included in the review: 4. Clinical cases of malaria 5. Adverse events (provided for entire cohort, not by type of malaria prophylaxis) 6. Adverse effects; other (all gastrointestinal disorders, all nervous system disorders ‐ no comparative data provided) 7. Illness during and following travel | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex, destination and duration of travel were measured but not reported across groups 2. Selection of participants into the study: moderate Non‐response rate was not reported. 3. Measurement of interventions: low The antimalarial prescription was provided by a travel clinic which also performed the study 4. Departures from intended interventions: moderate Information was provided on discontinuations, but no information was captured on switches between interventions 5. Missing data: low Information on adverse effects was available for all participants who ever filled the prescription for the study drug (571/612, 93%) 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate It is unclear which questions were included in the questionnaire. Information was provided on aggregate 8. Other: no information No information provided on study sponsor |
| Methods | Design: retrospective cohort study Study dates: January to June 1995 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: one‐off telephone interview between 4 and 20 weeks post‐travell | |
| Participants | Number enrolled: 454 eligible travellers, 300 successfully contacted and agreed to participate Inclusion criteria: subjects who visited the travel vaccination service of the regional public health institute in Maastricht if they had returned from their journey to tropical countries between 4 and 20 weeks previously. The group of non‐users was formed by people who travelled either to tropical countries without malaria risk or to cities in malarious areas, and by travellers who were prescribed an antimalarial drug but did not commence use Exclusion criteria: participants who had a serious adverse reaction to mefloquine in the first week Country of recruitment: Netherlands Region of malaria exposure: various; Asia, Africa, South America Duration of exposure to malaria: mean ˜3 weeks (range 1 to 9 weeks) Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine (1 x 250 mg tablet) weekly, taken 1 week prior to leaving, during travel and 4 weeks after departure 2. Non‐users of antimalarials Not included in the review: 3. Proguanil (1 x 100 mg tablet) twice daily, taken during travel and 4 weeks after departure | |
| Outcomes | 1. Adverse events; any, nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams, insomnia, anxiety, depression, pruritis 2. Adverse events; other (palpitations, severity of symptoms, time point of symptoms in relation to drug taking) 3. Discontinuations of study drug due to adverse effects 4. Measure of adherence to the drug regimen | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Travel destination varies significantly between users of mefloquine and non‐users of prophylaxis (6.7% America mefloquine versus 29.0% non‐users) 2. Selection of participants into the study: low 13/454 (2.8%) of travellers successfully contacted refused to participate 3. Measurement of interventions: low Prescription was provided by a travel clinic which also performed the study, and discontinuations were reported 4. Departures from intended interventions: moderate No information regarding switches been interventions of interest was reported 5. Missing data: moderate "If somebody discontinued drug use within a certain period, symptoms that occurred in the following period were not counted" Comment: Mefloquine has a half life of 17 to 21 days 6. Measurement of outcomes: moderate "The participants were specifically asked about symptoms instead of adverse effects...To hide our focus on symptoms as adverse effects of the drugs, participants were informed that the aim of the study was to investigate symptoms during travelling. We structured the questionnaire so that the interviewers asked about symptoms first and drug use last, in order to blind them to the drug used when addressing symptoms" 7. Selection of the reported results: low All prespecified outcomes were reported. 8. Other: no information Funding source was not mentioned |
| Methods | Design: cross‐sectional cohort study Study dates: 2003 Malaria transmission pattern and local antimalarial drug resistance: during the dry season (considered a low risk malaria season). Local chloroquine/proguanil resistance Adverse event monitoring: Patient self‐reported questionnaire | |
| Participants | Number enrolled: 90 questionnaires distributed, 68 responses Inclusion criteria: "all expatriate employees at the mine" Exclusion criteria: non mentioned Country of recruitment: Mali Country of malaria exposure: Mali Duration of exposure to malaria: various, not specified Type of participants: long‐term expatriates | |
| Interventions | Included in the review: 1. Mefloquine 2. Doxycycline 3. Atovaquone‐proguanil Not included in the review: 4. Chloroquine‐proguanil | |
| Outcomes | 1. Adverse effects; any | |
| Notes | Study sponsor not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Sex was recorded but not reported across chemoprophylaxis groups. Duration of travel was not reported. Destination of travel was set by the study design. 2. Selection of participants into the study: serious 68/90 response rate (76%) 3. Measurement of interventions: no information It was unclear whether information on participants chemoprophylaxis was taken from medical records or patient self‐reporting 4. Departures from intended interventions: moderate No information regarding switches between interventions of interest were reported. Discontinuations were reported 5. Missing data: low All information was collected at one time point 6. Measurement of outcomes: serious The outcome measure was subjective. There was no mention of participants or outcome assessors being blinded. 7. Selection of the reported results: no information No information was provided regarding which topics were included within the questionnaire 8. Other: no information Funding source was not mentioned |
| Methods | Design: cross‐sectional cohort study Study dates: June 2009 to June 2011 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 1119 eligible travellers, 316 enrolled Inclusion criteria: "travelers who visited Hibiya Clinic, and requested antimalarial drugs for malaria chemoprophylaxis from June 2009 to June 2011" Exclusion criteria: none mentioned Factors influencing drug allocation: "The choice of anti‐malarial drug was supported by sufficient explanation about the advantages and disadvantages (efficacy, method, duration, side effect, cost and approval) of each drug" Country of recruitment: Japan Region of malaria exposure: various (n): East Africa 76, West Africa 63, South Africa 50, Southeast Asia 36, Central Africa 36, South Pacific 21, South America 16, India 8, North Africa 5, Central America 1 Duration of exposure to malaria: mean 20.0 ± 9.6 days in the atovaquone‐proguanil group and 59.0 ± 15.9 days in the mefloquine group Type of participants: travellers | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet, Mephaquin; Mepha) weekly, starting 1 week prior to arrival, during the stay, and continuing for 4 weeks after leaving the endemic area 2. Atovaquone‐proguanil (1 tablet containing 250 mg atovaquone and 100 mg proguanil, Malarone; GlaxoSmithKline) daily, starting 2 days prior to arrival, during the stay, and for 1 week after leaving the endemic area | |
| Outcomes | 1. Adverse effects (any vertigo/dizziness, nausea, abdominal pain, diarrhoea, headache, insomnia, depression, any cardiovascular, any gastrointestinal, any psychoneurotic, allergic reaction) 2. Discontinuations of study drug due to adverse effects | |
| Notes | Funding sources: not mentioned Communications with the study authors: the study authors provided us with disaggregated study data for the following outcomes: vertigo/dizziness, nausea, abdominal pain, diarrhoea, headache, insomnia, depression. Because we did not get receive the full disaggregated data set, we also retained this study in the analysis of groups of symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate PTravellers in the mefloquine group were significantly younger than travellers in the A/P group (p=0.01)" 2. Selection of participants into the study: serious "316 of 1119 travelers (28.2 %) were enrolled" 3. Measurement of interventions: low The prescription has been provided by travel clinic which also performed the study and discontinuations have been reported 4. Departures from intended interventions: moderate No information was available regarding switches between interventions of interest 5. Missing data: low One participant in the mefloquine group appears to be missing from the adverse events analysis. No reason was given 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: low Study authors provided us with disaggregated study data for individual outcomes 8. Other: serious "The authors wish to acknowledge that Makoto Ono and Tomoko Kawamura of GlaxoSmithKline are highly appreciated for conducting Data Management and Statistics Analysis of this study" |
| Methods | Design: prospective cohort study Study dates: 1 August 2005 to 31 July 2006. Malaria transmission pattern and local antimalarial drug resistance: various, chloroquine resistance specified by country of destination Adverse event monitoring: "Peace Corps medical staff in these countries were provided surveys for distribution during mandatory in‐country volunteer training sessions" | |
| Participants | Number enrolled: 2701 (6216 Peace Corps volunteers during the time period) Inclusion criteria: "all Peace Corps countries with malaria risk" Exclusion criteria: none mentioned Factors influencing drug allocation: "Volunteers are provided chemoprophylaxis (either chloroquine, mefloquine, doxycycline, or atovaquone/proguanil)... medical officers can provide alternative chemoprophylaxis regimens for volunteers when adverse events or other factors require the cessation of any medication" Country of recruitment: various Country of malaria exposure: various Duration of exposure to malaria: "6 months or longer" Type of participants: Peace Corps volunteers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Chloroquine* 3. Doxycycline* 4. Atovaquone‐proguanil* *dosing regimen not specified | |
| Outcomes | 1. Adverse effects; any (mild, moderate, severe, sought medical advice), nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams, insomnia, depression, anxiety, visual disturbance 2. Adverse effects; other (unsteadiness, hair loss, weakness, itchy skin, photosensitivity, yeast infection) 3. Serious adverse effects 4. Discontinuations of study drug due to adverse effects | |
| Notes | Funding sources: "CK and PJ are employed by the Peace Corps, which has a significant number of volunteers taking anti‐malarial medications. There were no other financial disclosures" Communications with study authors: The study authors provided us with access to the disaggregated study data for the specific symptoms mentioned above. The questionnaire in the paper allowed participants to describe side effects from the antimalarial they were currently taking, and any regimen they had previously used. For non‐serious side effects, in line with the original paper, we only included side effects for the subject's original regimen. Where subjects had previously taken more than one regimen, we only include side effects for whichever regimen to which the participant attributed the greater number of side effects; this affected 70/2701 participants. This analysis resulted in a decrease in the effect size for side effects attributed to mefloquine. For serious side effects (hospitalizations) and discontinuations we included all participants entries for all regimens. In addition, our denominator differed from the original paper because we did not exclude participants who had been in post for fewer than six months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate "The questionnaire did not collect demographic information because of privacy concerns" Comment: destination has been reported, but not by type of antimalarial chemoprophylaxis. Duration was set by the study design 2. Selection of participants into the study: serious "A total of 2701 surveys were received yielding a response rate of 43%" 3. Measurement of interventions: moderate Participants were asked to self‐report which prophylaxis they were currently taking and had previously taken 4. Departures from intended interventions: moderate Switches between interventions of interest were reported. Approximately 1/3 of study participants had switched prophylactic regimens 5. Missing data: low We were able to include all participants in the study analysis because we had access to the original data set 6. Measurement of outcomes: serious "If respondents identified any adverse event, the survey instructed them to self‐report which drug they believed caused the adverse event" Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: low We were able to include all results in the analysis because we had access to the original data set 8. Other: low No evidence of pharmaceutical company funding |
| Methods | Design: prospective cohort study Study dates: 2000 to 2003 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: retrospective patient self‐reporting questionnaire | |
| Participants | Number enrolled: 495 enrolled, 284 response rate Inclusion criteria: unclear. Users of the travel medicine department of the lower Saxony regional health office in Hanover, Germany Exclusion criteria: None mentioned Factors influencing drug allocation: "the prescriptions of medications followed individual consultation" Country of recruitment: Germany Country of malaria exposure: various, not specified Duration of exposure to drug: atovaquone‐proguanil mean 2.6 weeks, mefloquine mean 7 weeks Type of participants: short‐term travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Atovaquone‐proguanil* Not included in the review: 3. Chloroquine‐proguanil* 4. Chloroquine (not included in the study analysis) *dosing regimen not specified | |
| Outcomes | 1. Adverse effects; any, nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams, insomnia, pruritis 2. Adverse effects; other (concentration difficulties, palpitations, circulation disorders, rash) 3. Discontinuations of study drug due to adverse effects | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Sex, age and duration of travel were reported but not balanced across groups 2. Selection of participants into the study: serious 284/495 (59.8%) response rate 3. Measurement of interventions: low The prescription was provided by a travel clinic which also performed the study; switches and discontinuations were recorded and reported. 4. Departures from intended interventions: moderate No information was provided regarding switches between prophylactic regimens 5. Missing data: low All information was collected at one time point 6. Measurement of outcomes: serious The outcome measure was subjective. There was no mention of outcome assessors being blinded 7. Selection of the reported results: moderate Insufficient information was provided regarding the questionnaire to know whether all outcomes were reported 8. Other: no information Study sponsor not mentioned |
| Methods | Design: prospective cohort study Study dates: 19 August to 30 September 2013 Malaria transmission pattern and local antimalarial drug resistance: various Adverse event monitoring: participant self‐reported questionnaire | |
| Participants | Number enrolled: 3207 emails sent, 1184 unique, valid responses received Inclusion criteria: "(volunteers in) Peace Corps offices of all 23 countries with active posts in the Africa region to all active Volunteers in‐country" Exclusion criteria: Volunteers serving in Ethiopia, Kenya, Tanzania, Namibia, Botswana, South Africa Region of recruitment: African region except Ethiopia, Kenya, Tanzania, Namibia, Botswana, South Africa Factors influencing drug allocation: "all prophylaxis options (mefloquine, doxycycline, atovaquone‐proguanil) [are] equally available... They are instructed to individualize their choice of agent based on area‐specific recommendations, drug contraindications and precautions, drug tolerance, and dosing schedule" Country of malaria exposure: various: Togo (3.7%), Sierra Leone (6.3%), Uganda (7.8%), Liberia (5.6%), Malawi (2.0%), Cameroon (11.4%), Benin (10.2%), Burkina Faso (1.9%), Zambia (6.0%), Mozambique (4.5%), Ghana (10.8%), Rwanda (5.4%), Gambia (4.4%), Madagascar (11.1%), Swaziland (2.3%) Duration of exposure to malaria: various, not specified Type of participants: Peace Corps volunteers | |
| Interventions | 1. Mefloquine* 2. Atovaquone‐proguanil* 3. Doxycycline* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; any, vertigo, headache, abnormal dreams, insomnia, anxiety, depression, psychosis 2. Adverse effects; other (any neuropsychiatric disorder, any gastrointestinal disorder, any skin or subcutaneous disorder, limb numbness, tinnitus, 'constitutional', genitourinary) 3. Measures of adherence to the drug regimen Outcomes assessed not included in the review: 4. Reasons for non‐adherence (not ascribed to prophylactic regimen, provided on aggregate), 5. Malaria knowledge 6. Health behaviours | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate The age, sex and BMI of included participants was not recorded. The destination and duration of travel was not reported by prophylactic regimen 2. Selection of participants into the study: serious 1184/3248 (36%) response rate 3. Measurement of interventions: moderate Travellers were asked to self‐report which prophylaxis they were taking at various time points during treatment 4. Departures from intended interventions: serious "Two hundred seventy‐six (35%) respondents reported having changed prophylaxis at some point during their service" Comment: this was not provided by prophylactic regimen 5. Missing data: low 703/781 (90%) participants reported data for adherence; 733/781 (94%) participants reported data for adverse events. Data were only included from the 2015 version of the publication 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: low All outcomes prespecified in the methods section were reported 8. Other: no information Study sponsor not mentioned |
| Methods | Design: cross‐sectional cohort study Study dates: February 2000 Malaria transmission pattern and local antimalarial drug resistance: "during February 2000, which was a peak period of malaria transmission in Zimbabwe" Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 660 Inclusion criteria: Passengers in Harare and Victoria Falls international airport during February 2000 Exclusion criteria: "Children under the age of 18 were excluded on the assumption that parents probably influence their health seeking behavior... Excluded, were travelers from the African continent and VIP travelers who exited through special departure lounges" Factors influencing drug allocation: no infromation provided Country of recruitment: Zimbabwe Country of malaria exposure: Zimbabwe Duration of exposure to malaria: various: 1 week or less, N = 317; 8 days to 2 weeks, N = 144; 15 days to 4 weeks, N = 90; > 4 weeks, N = 41 Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Doxycycline* 3. Chloroquine* Not included in the review: 4. Proguanil* 5. Dapsone and pyrimethamine* 6. Chloroquine and proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Measure of adherence to the drug regimen Outcomes assessed not included in the review: 2. Sources of pre‐travel health advice 3. Knowledge about malaria transmission 4. Knowledge about malaria prevention 5. Threat and risk perception | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Sex (P < 0.008), education (P < 0.022), previous episodes of malaria (P < 0.001) and access to pre‐travel advice (P < 0.001) were all significantly associated with reduced compliance at the significance value set by the study. None of these factors were adjusted for in the analysis 2. Selection of participants into the study: moderate "The nonresponse rate was about 10% (n = 65), with the main reason being the short transit time" 3. Measurement of interventions: low Participants were asked to self‐report which prophylactic regimen they were taking while they were still taking it 4. Departures from intended interventions: moderate No information was provided regarding switches between prophylactic regimens 5. Missing data: low Adherence information was not available for 4/595 participants 6. Measurement of outcomes: serious The outcome measure was based on participant self‐reporting; participants and personnel were not blinded. 7. Selection of the reported results: moderate There was insufficient information provided to know what questions were asked regarding adherence 8. Other: low "The authors had no financial or other conflicts of interest to disclose" |
| Methods | Design: retrospective cohort study Study dates: 1 January 2003 to 31 December 2004 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: "An anonymous survey in a post‐travel situation" | |
| Participants | Number enrolled: 1176 agreed to participate, 1237 approached Inclusion criteria: "travellers who had already completed their journey for which they had undergone immunization prophylaxis and who had returned to complete their vaccination schedule" Exclusion criteria: none mentioned Factors influencing drug allocation: "offered health advice following the World Health Organization guidelines for international travel" Country of recruitment: Italy Regions of malaria exposure: 97 countries: 39 states in Africa, 25 in Asia, 16 in North and Central America, 8 in South America, 6 in Europe and 3 in Oceania Duration of exposure to malaria: 1 to 7 days, 8.9%; 8 to 14 days, 30.1%; 15 to 21 days, 34.6%; 22 to 30 days, 16.8%; > 30 days, 8.9%; not available 0.7% Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Atovaquone‐proguanil* 3. Chloroquine* Not included in the review: 4.Chloroquine‐proguanil* 5. Proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; any, visual impairment (blurred vision), nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams (nightmares), insomnia, anxiety (anxiety disorder), depression, psychosis (hallucinations) 2. Adverse effects; other (slight illness, tiredness, restlessness, drowsiness, palpitations, weakness, photosensitization, mental confusion, rash) Outcomes assessed not included in the review: 3. Adverse effects; other, incidence < 1% (liver pain, aerophagy, rise in transaminase levels, gastrointestinal disturbance, epistaxis, fever) 4. Compliance with vaccinations 5. Side effects from vaccinations 6. Occurrence of health problems and unforeseen events during travel in the countries visited 7. Attention to avoiding potentially risky food and drink | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Demographic information was collected, but provided on aggregate for the entire cohort 2. Selection of participants into the study: low 1176 of 1237 (95.1%) response rate 3. Measurement of interventions: serious Participants were asked to self‐report which prophylactic regimen they had used, up to over 12 months since travelling 4. Departures from intended interventions: serious No switches were reported, and this information was not sought in the questionnaire 5. Missing data: low 642/646 (99%) participants were included in the analysis 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: low The questionnaire was provided in full, and all outcomes were reported 8. Other: no information No information was provided regarding the study sponsor |
| Methods | Design: cross‐sectional cohort study Study dates: 13 July to 9 August 1997 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 6633 respondents, 5626 met inclusion criteria Inclusion criteria: "travelers departing Nairobi, or Mombasa, Kenya, from July 13 to August 9, 1997, on flights to Europe, including London, Paris, Frankfurt, Amsterdam, and Rome" Exclusion criteria: residents of African countries, individuals who had remained in Africa for more than 1 year, individuals who visited only non malarious areas, including Nairobi and Lesotho Factors influencing drug allocation: no information available Region of recruitment: Nairobi or Mombasa, Kenya Region of malaria exposure: Nairobi or Mombasa, Kenya Duration of exposure to malaria: < 5 weeks Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Doxycycline* 3. Chloroquine* Not included in the review: 4. Chloroquine‐proguanil* 5. Proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; any, 2. Serious adverse outcomes 3. Adverse effects; other (neuropsychologic, gastrointestinal, respiratory) 4. Measure of adherence to the drug regimen Outcomes assessed not included in the review: 5. Pre‐travel medical advice 6. Compliance with antimosquito measures 7. Self‐treatment of presumed malaria | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate The number of travellers and country of origin was reported, but was not adjusted for in the analysis. Sex, age and duration of stay were reported on aggregate. 2. Selection of participants into the study: serious Response rate 6633/15,487 (43%) 3. Measurement of interventions: low Participants were asked to provide information regarding their prophylactic regimen during their flight home, while they should have still been using it 4. Departures from intended interventions: moderate No information was available regarding switches between alternative prophylactic regimens 5. Missing data: low 4934/4982 (99%) participants included in adverse event reporting 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate There was insufficient information provided regarding the questions included in the questionnaire. Symptoms were grouped together to report outcomes 8. Other: low "The authors had no financial or other conflicts of interest to disclose" |
| Methods | Design: retrospective cohort study Study dates: October to December 2005, with a 2 year follow‐up Malaria transmission pattern and local antimalarial drug resistance: "Malaria endemic area. Local chloroquine/proguanil resistance" Adverse event monitoring: Not clear | |
| Participants | Number enrolled: 33 Inclusion criteria: not explicitly stated. Participants were travellers who took part in a scientific survey and rafting expedition in Ethiopia between October and December 2005 Exclusion criteria: none stated Country of recruitment: various, participants were from "a non‐malarious area, mainly the UK" Country of malaria exposure: Ethiopia Duration of exposure to malaria: 3 months Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine, dose not specified, during travel and 4 weeks after return 2. Atovaquone‐proguanil, dose not specified, during travel and for 1 week after return 3. Doxycycline, dose not specified, during travel and 4 weeks after return Not included in the review: 4. Chloroquine‐proguanil, dose not specified, during travel and 4 weeks after return | |
| Outcomes | Included in the review: 1. Measures of adherence to the drug regimen Outcomes assessed not included in the review: 2. Clinical cases of malaria 3. Adverse effects (information not provided by drug class) 4. Factors influencing choice of prophylaxis | |
| Notes | Funding sources: Work was supported by the Biomedical Research Centre (Grant RG561620 to AMLL) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Demographic information is provided for the entire cohort 2. Selection of participants into the study: low No participants refused to participate in the study. Start of follow‐up began at the start of travel and not at the start of treatment, but this was judged to have a low impact on monitoring self‐reported adherence 3. Measurement of interventions: low Intervention status was determined by one of the participants on the expedition 4. Departures from intended interventions: low There are no documented switches between interventions of interest 5. Missing data: low Two people (6%) were lost to follow‐up in respect to data on efficacy. No participants were lost to follow‐up when monitoring adherence 6. Measurement of outcomes: serious Adherence was monitored by the medical officer on the trip, and reporting may have been influenced by social desirability bias 7. Selection of the reported results: low All prespecified outcomes have been reported 8. Other: low Government funding |
| Methods | Design: retrospective cohort study Study dates: 1 January 1990 and 31 December 1999 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: incident cases of depression, psychoses and panic attacks severe enough to require hospitalisation, referral to a specialist or specific pharmacological treatment within the UK general practice research database | |
| Participants | Number enrolled: 35,370 Inclusion criteria: "men and women aged 17‐79 years who received between one and four prescriptions for mefloquine, proguanil and/or chloroquine, or subjects who received one prescription only for doxycycline... we included only those subjects who medical record contained a code indicating that the person received the drug for malaria prophylaxis within 1 week of the prescription date e.g. ‘travel advice’ or ‘prophylactic drug use’" Exclusion criteria: "participants who received the study drugs on a longer‐term basis...subjects had to be enrolled in the database for at least 12 months before the date of the first prescription for a study drug and had to have had some recorded activity (diagnoses or drug prescriptions) after the prescription(s) for an antimalarial drug... subjects with a history of alcoholism" Country of recruitment: UK Country of malaria exposure: various, not specified Duration of exposure to malaria: various, not specified Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Doxycycline* Not included in the review: 3. Chloroquine‐proguanil* 4. Proguanil* 5. Chloroquine* (data reported combined with proguanil and chloroquine‐proguanil) *dosing regimen not specified | |
| Outcomes | 1. Serious adverse events 2. Adverse events; psychiatric disorders (depression, psychosis) 3. Adverse events; other (panic attacks, suicide) | |
| Notes | Funding sources: "This study was funded by an unconditional grant by F. Hoffmann‐La Roche Ltd, Basel, Switzerland" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Women and those aged 40 to 49 years were at higher risk of depression but this was not adjusted for in the analysis. Risk ratio estimates for psychoses and panic attacks could not be adjusted for because numbers were too small for the multivariate model. Data on destination and duration of travel were not available 2. Selection of participants into the study: low Recruitment onto the General Practice Research Database was unlikely to be related to exposure or outcome 3. Measurement of interventions: moderate "Antimalarial drugs can be used for malaria prophylaxis, for treatment of an acute malaria infection, or as a reserve drug… In order to distinguish these options, we included only those subjects whose medical records contained a code indicating ‘travel advice’ or ‘prophylactic drug use’" 4. Departures from intended interventions: serious Discontinuations and switches between prophylactic regimens were not recorded in this database 5. Missing data: low All participants in the research database were included in the analysis 6. Measurement of outcomes: moderate "...we reviewed all computer records of potential cases and included or excluded cases on the available clinical information, blinded to exposure status" Comment: general practitioners diagnosing patients would have been aware of their exposure status 7. Selection of the reported results: low Information on all outcomes prespecified in the methods section were reported for all participants. 8. Other: serious Funded by Roche pharmaceuticals |
| Methods | Design: retrospective cohort study Study dates: 1 October 2005 to 30 June 2006 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: telephone questionnaire to all travellers to tropical countries for whom antimalarial chemoprophylaxis was prescribed | |
| Participants | Number enrolled: 1906 questionnaires returned Inclusion criteria: participants staying in high risk malarial areas, aged between 18 and 65 years, with no severe underlying disease (e.g. heart disease, diabetes) with an available phone number Exclusion criteria: immigrants (due to potential difficulty in linguistic communication) Country of recruitment: Italy Country of malaria exposure: various: Kenya, Tanzania/Zanzibar, India, Madagascar, Brazil, other countries of South America, South Africa, Senegal, Mali, Myanmar, Ghana, Congo, and others Duration of exposure to malaria: mean stay 2 weeks Type of participants: Travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Chloroquine* 3. Atovaquone + proguanil* 4. Doxycycline* Not included in the review: 5. Chloroquine + proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; any 2. Serious adverse effects 3. Adverse effects; other (any gastrointestinal, any neuropsychiatric) 4. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 5. Clinical cases of malaria 6. Eating habits during travel | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Demographic information was provided on aggregate for the entire cohort 2. Selection of participants into the study: moderate Non‐response rates to the questionnaire were not reported 3. Measurement of interventions: moderate The prescription was provided by several travel clinics which also performed the study. However, it was unclear whether this information was used to determine intervention status or relied on participant self‐reporting 4. Departures from intended interventions: low Discontinuations were reported, with detailed reasons for discontinuations. No switches to alternative regimens were reported 5. Missing data: low All participants were included in the analysis 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: low The methods section makes clear which outcomes were being assessed; all outcomes were reported 8. Other: no information No information was provided regarding the study sponsor |
| Methods | Design: RCT Study dates: January 1987 to November 1990 Malaria transmission pattern and local antimalarial drug resistance: "in an area of seasonal malaria transmission... mefloquine and quinine resistance is increasing in this area, and the proportion of recrudescent infections is rising" Adverse event monitoring: trial occurred over two phases. Phase 1: Weekly basic observations and simple symptom questionnaire. ECG, haematological and biochemical tests were done fortnightly. Children born to women in the trial were assessed at birth and at 3, 6, 12, and 24 months. Phase 2: weekly basic observations and expanded simple symptom questionnaire. ECG and blood tests were performed at baseline, at midstudy and at term. Each delivery was supervised. Additional assessments at 1 week and 2 and 9 months for children born to women in the trial | |
| Participants | Number enrolled: 339 Inclusion criteria: "Women attending the weekly clinic were admitted to the study if they were at > 20 weeks of estimated gestation" Exclusion criteria: Not mentioned Region of recruitment: Thai‐Burmese border Region of malaria exposure: Thai‐Burmese border Duration of exposure to malaria: ongoing exposure in a semi‐immune population, monitored until delivery Type of participants: Pregnant Thai residents in malaria‐endemic area (presumed semi‐immune) | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet, Lariam; Hoffmann‐La Roche) weekly for 4 weeks, then 125 mg weekly until delivery, with 500 mg base loading dose in phase 1 but not phase 2 2. Placebo (1 tablet) weekly until delivery | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Episodes of parasitaemia 3. Serious adverse events (including childhood deaths) 4. Adverse events; vertigo, visual impairment (visual abnormalities), nausea, vomiting, abdominal pain, headache, dizziness, pruritis 5. Adverse events; other (weakness, anorexia, cough, falls, constipation, unsteadiness) 6. Discontinuation of study drug due to adverse effects 7. Adverse pregnancy outcomes (spontaneous abortions, still births, congenital malformations) Outcomes assessed not included in the review: 8. Laboratory tests; haematologic (full blood count, haematocrit) and biochemical (creatinine, blood urea, transaminases, alkaline phosphatase, albumin, globulin) 9. Outcomes related to pregnancy; weight gain during follow‐up, complications of labour, mean duration of labour, maternal anaemia 10. Fetal outcomes; mean birth weight, percent premature, fetal distress 11. Infant follow up; mean age at which children could crawl, sit, walk or talk, Romberg test | |
| Notes | Funding sources: United Nations Development Programme/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases; Wellcome Trust of Great Britain; Praevention Foundation. The Hague (to FLK) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...women were randomized to receive either mefloquine…or placebo" Comment: unclear what method of randomization was used |
| Allocation concealment (selection bias) | Unclear risk | "...the investigators were unaware of the randomisation" Comment: no mention of method used to conceal allocation |
| Blinding of participants and personnel (performance bias) | Low risk | "...double blind...women were randomised to receive either mefloquine…or identical placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | "...the investigators were unaware of the randomisation" |
| Incomplete outcome data (attrition bias); efficacy | Low risk | Comment: total number of participants with positive blood smear result at any time during prophylaxis was reported. Clinical cases of malaria were reported" |
| Incomplete outcome data (attrition bias); safety | High risk | "Ten women (8%) in phase I (3 mefloquine, 7 placebo) and 18 (8%) in phase II (9 in each group) dropped out of the study. The main reason was the discomfort of blood sampling (26 cases) and, in 1 case, pruritus attributed to mefloquine" Comment: 28 women dropped out but reasons were provided for only 27 women; numbers were not provided across groups |
| Selective reporting (reporting bias); efficacy | Low risk | Comment: all episodes of parasitaemia and clinical cases of malaria were reported |
| Selective reporting (reporting bias); safety | High risk | Comment: Data on adverse effects were reported for only participants from phase 2 of the trial (220/339 women). Fifteen symptoms were listed in the comparative table, but the narrative states "twenty questions were asked". Romberg test results were not reported. Biochemical, haematological and ECG parameters were not reported other than "there were no differences" |
| Other bias | Low risk | Funding: United Nations Development Programme/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases; Wellcome Trust of Great Britain; Praevention Foundation. The Hague (to FLK) |
| Methods | Design: RCT Duration of study: May to July 1994 Malaria transmission pattern and local drug resistance: "P. falciparum resistant to sulfadoxine‐pyrimethamine and both P falciparum and P vivax resistant to chloroquine" Adverse event monitoring: symptoms reported in the first week of the study, daily questioning about symptoms, exit questionnaire | |
| Participants | Number enrolled: 204 Inclusion criteria: "All soldiers from military posts that were considered to have high malaria attack rates" Exclusion criteria: history of frequent travel, allergy to one of the study drugs, glucose‐6‐phosphate dehydrogenase deficiency, history of underlying illness Country of recruitment: Indonesia Country of malaria exposure: Indonesia Duration of exposure to malaria: Study duration was approximately 13 weeks Type of participants: military, semi‐immune (60% of participants had prior exposure to malaria) | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet, containing the equivalent of 228 mg mefloquine base) once weekly (after a loading dose of 250 mg per day for 3 days).* 2. Doxycycline hyclate (1 x 100 mg capsule) once daily* 3. Placebo* Co‐interventions: All soldiers were given doxycycline tablets for 4 to 6 weeks to enable clearance of sulfadoxine‐pyrimethamine from the blood before study prophylaxis began. All participants received radical treatment for pre‐existing malaria parasites in the blood and liver prior to beginning study prophylaxis. *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Adverse events; any, nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, insomnia, abnormal dreams 3. Serious adverse events 4. Adverse events; other (all gastrointestinal, all neurologic, constipation, anorexia, fever, malaise, skin related, cough, somnolence, palpitations, sexual dysfunction) 5. Discontinuation of study drug due to adverse effect Outcomes assessed not included in the review: 6. Exit questionnaire (incomplete data reported) | |
| Notes | Funding source: Pfizer Indonesia supplied active and placebo doxycycline; F. Hoffman‐La Roche supplied active and placebo mefloquine, and gave financial support; USA Army Medical Research and Materiel Command gave financial support; USA Naval Medical Research and Development Command gave financial support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Block randomization was used (block size, 15)" Comment: Used a randomization code, but it was not stated how it was generated |
| Allocation concealment (selection bias) | Unclear risk | "The randomization code was stored in individual envelopes in a locked box at the study site...Drugs were packaged into weekly ziplock plastic bags" Comment: Unclear whether the investigators or participants would foresee assignment. There was no mention of central allocation, sequentially numbered drug containers or sequentially numbers opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | "Drugs were packaged into weekly zipper‐lock plastic bags: each bag contained a mefloquine or mefloquine placebo tablet and a blister pack of seven doxycycline or doxycycline placebo capsules (double‐dummy technique)" The placebo medication had an "identical appearance" |
| Blinding of outcome assessment (detection bias) | Low risk | "The randomisation code was stored in individual envelopes in a locked box at the study site. All investigators and study personnel did not have access to or know the randomisation code throughout the study" |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | "Sixteen of the 204 participants did not complete the study" Comment: It was unclear whether the duration of follow up included the post‐prophylaxis period to monitor for relapses |
| Incomplete outcome data (attrition bias); safety | High risk | Exit questionnaire: "Only data from persons who were still receiving the study drug at the time of the questionnaire were included" Comment: numbers not reported |
| Selective reporting (reporting bias); efficacy | Low risk | "The primary end point for efficacy was the first occurrence of malaria, as documented by a positive malaria smear" Comment: all cases of malaria were reported. |
| Selective reporting (reporting bias); safety | High risk | Comment: Not all data were reported from the exit questionnaire; the study reports "...the only statistically significant finding". Data on adverse symptoms were not reported for the placebo group |
| Other bias | Low risk | "Neither of the pharmaceutical companies that provided support played any role in the gathering, analysing or interpreting the data" |
| Methods | Design: RCT Duration of study: April to October 1999 Malaria transmission pattern and local drug resistance: not mentioned Adverse event monitoring: "evaluated 7, 28 and 60 days after return to obtain information about a targeted list of adverse events" | |
| Participants | Number enrolled: 1013 Inclusion criteria: "travellers aged ≥ 3 years and weighing ≥ 11 kg with planned travel of ≤ 28 days to a malaria‐endemic area" Exclusion criteria: "poor general health; drug hypersensitivity (to atovaquone, chloroquine or proguanil); history of alcoholism, seizures or psychiatric or severe neurological disorders; generalized psoriasis; severe blood disorders; pregnancy/lactation; renal, hepatic or cardiac dysfunction; clinical malaria within previous 12 months; travel to malaria endemic area within previous 60 days" Countries of recruitment: Canada, Germany, Netherlands, South Africa, UK Regions of malaria exposure: various malaria‐endemic destinations (79% Africa, 6% South America) Mean duration of exposure to malaria: 2.5 weeks Type of participants: travellers, non‐immune | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet; or alternatively ¼, ½ or ¾ of a tablet, according to body weight) once weekly, starting 1 to 3 weeks before travel and continuing for 4 weeks after travel* 2. Atovaquone‐proguanil (1 combined tablet containing 250 mg atovaquone and 100 mg proguanil hydrochloride; or alternatively 1 to 3 combined tablets for children according to body weight, each tablet containing 62.5 mg atovaquone and 25 mg proguanil hydrochloride) once daily, starting 1 to 2 days before travel and continuing for 1 week after leaving the malaria‐endemic area* *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Clinical cases of malaria (antibody to blood‐stage malaria parasites) 2. Adverse events; any 3. Serious adverse events 4. Adverse effects; any (moderate or severe), visual impairment, nausea, vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams, insomnia, anxiety, depression, pruritis 5. Adverse effects; other (mouth ulcers) 6. Discontinuation of study drug due to adverse effects 7. Measures of adherence to the drug regimen Outcomes assessed not included in the review: 8. Laboratory tests; haematology (haemoglobin level, white blood cell count and platelet count) and chemistry (creatinine and alanine aminotransferase) | |
| Notes | Funding source: GlaxoSmithKline "Subjects were enrolled in study MAL30010"‐ Enrollment criteria and study conduct were described in a separate publication (Høgh 2000) which refers to a different study population (atovaquone‐proguanil versus chloroquine‐proguanil). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated code was used to randomly assign a treatment number" (Høgh 2000) |
| Allocation concealment (selection bias) | Low risk | "Treatment codes were provided to investigators in opaque sealed envelopes, to be opened only if knowledge of study drug assignment was required for management of a medical emergency" (Høgh 2000) |
| Blinding of participants and personnel (performance bias) | Low risk | "For each active drug, capsules or film‐coated tablets were identical in appearance to the matching placebo" |
| Blinding of outcome assessment (detection bias) | Low risk | "All subjects and study personnel remained blinded to treatment assignment with 5 exceptions. Two subjects in the atovaquone‐proguanil group and 3 in the mefloquine group lost their study drug during their return trip from a malaria‐endemic area, and the investigator broke the blind to enable completion of postexposure prophylaxis with active drug" |
| Incomplete outcome data (attrition bias); efficacy | Low risk | "A total of 963 subjects completed the 60‐day follow‐up period and had efficacy information recorded. A total of 915 subjects had paired serum samples available for serological testing" Comment: 963/976 (randomized and received first dose of study drug) = 98.7%. 915/976 = 93.75%. Reasons for leaving the study early were reported and numbers were balanced across groups |
| Incomplete outcome data (attrition bias); safety | Unclear risk | Comment: 96.35% of randomized participants were included in adverse event reporting. Reasons for leaving the study early were reported and numbers were balanced across groups |
| Selective reporting (reporting bias); efficacy | Low risk | Comment: Full clinical details were provided for every episode in which an episode of malaria was considered (4 cases) |
| Selective reporting (reporting bias); safety | High risk | Comment: Data on adverse symptoms were not reported for the placebo group due to a shorter duration of follow‐up. Data were collected 7, 28 and 60 days after travel. However, data were only presented for 7 days after return |
| Other bias | High risk | Funding: GlaxoSmithKline It was not made clear whether the interpretation of the study findings was independent of the study sponsor |
| Methods | Design: RCT Study dates: unclear, during 1977 Malaria transmission pattern and local antimalarial drug resistance: "subjects were resident in an area highly endemic for P. vivax and chloroquine resistant P. falciparum" Adverse event monitoring: "a physician visited the study area each week and conducted a sick call for participating and nonparticipating villagers...Between physician visits, residents were taken to a nearby health centre for serious medical problems" | |
| Participants | Number enrolled: 990 Inclusion criteria: "All eligible and consenting villagers over 10 years of age were included in the study" Exclusion criteria: "Female villagers of childbearing age (15‐44 years) were not considered for inclusion" Country of recruitment: The Bhu Phram Valley, Thailand Country of malaria exposure: The Bhu Phram Valley, Thailand Duration of exposure to malaria: study duration 26 weeks Type of participants: Thai residents, semi‐immune | |
| Interventions | 1. Mefloquine (1 x 180 mg tablet, children 22 to 35 kg ½ dose) weekly 2. Mefloquine (1 x 360 mg tablet, children 22 to 35 kg ¼ dose) weekly 3. Mefloquine (1 x 360 mg tablet, children 22 to 35 kg ¼ dose) every 2 weeks 4. Placebo (1 x tablet) weekly Co‐interventions: "Those who had experienced falciparum parasitemias were given a therapeutic dose of sulfadoxine (1,500 mg)‐pyrimethamine (75 mg), and those with vivax or malariae parasitemias were treated with the standard regimen of chloroquine (1,500 mg over a 3‐day period), followed by primaquine, 15 mg daily for 14 days, for those study subjects known to be G‐6‐PD normal" | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Episodes of parasitaemia 3. Adverse events; any Outcomes assessed not included in the review: 4. Laboratory tests; haematocrit, white cell count, white cell differential, serum glutamic oxaloacetic transaminase, alkaline phosphatase and blood urea nitrogen | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Assignment to one of six treatment groups was made on a stratified random number basis" Comment: no details of how random numbers were generated |
| Allocation concealment (selection bias) | Unclear risk | "In the course of this visit, the technician opened a sealed, numbered envelope, gave the enclosed tablets, and observed the subject swallow them" Comment: no mention of the envelope being opaque |
| Blinding of participants and personnel (performance bias) | Low risk | "Each subject received two tablets each week (medication, placebo or a combination) in order to maintain the double blind nature of the study" "All tablets were identical in appearance" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but not clear how this was achieved |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | "Nine hundred and ninety nine subjects began the 25‐week field trial and 856 completed it (86.5%). 160/189 (85%) of the mefloquine 180 mg weekly group, 169/191 (88%) of the mefloquine 360 mg weekly, 158/184 (86%) of the mefloquine 360 mg fortnightly and 36/44 (82%) of the placebo group completed the trial" Comment: reasons for losses to follow‐up were not reported |
| Incomplete outcome data (attrition bias); safety | Low risk | "There was no clinical evidence of drug toxicity in the 990 study participants, nor were there significant changes in the biochemical parameters" |
| Selective reporting (reporting bias); efficacy | Low risk | "Table 2 shows the number of subjects in each group who completed the study, the number infected with P. falciparum, and the number of episodes of asexual parasitemia" |
| Selective reporting (reporting bias); safety | High risk | "There was no clinical evidence of drug toxicity in the 990 study participants" Comment: it was unclear whether all events that occurred during the 6 month trial period were included |
| Other bias | Unclear risk | Comment: study sponsor not reported |
| Methods | Design: retrospective cohort study Study dates: 1 May 1996 to 30 April 1998 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 5446 questionnaires mailed, 4158 respondents Inclusion criteria: "travellers 18 years old or older, who were not pregnant and had no previous adverse reactions to any of the prescribed drugs" Exclusion criteria: none mentioned Factors influencing drug allocation: "the standard recommendations to Danish travelers were followed" Country of recruitment: Denmark Country of malaria exposure: various, not specified Duration of exposure to malaria: various, not specified Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Chloroquine* Not included in the review: 3. Chloroquine + proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse events; any 2. Serious adverse outcomes 3. Adverse effects; visual impairment (blurred vision), nausea, vomiting, abdominal pain, diarrhoea, dizziness, depression 4. Adverse effects; other (loss of appetite, strange thoughts, tingling, altered spatial perception, mouth ulcers) Outcomes assessed not included in the review: 5. Discontinuation of study drug due to adverse effects (data reported on aggregate) 6. Measure of adherence to the drug regimen (data reported on aggregate) 7. Duration in days of symptoms | |
| Notes | Funding sources: Not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate The questionnaire collected information regarding age, body weight and gender, destination and duration of travel but these were not reported 2. Selection of participants into the study: serious Response rate 4158/5446 (76.3%) 3. Measurement of interventions: low The prescription was provided by a travel clinic which also performed the study, and switches and discontinuations have been recorded and reported 4. Departures from intended interventions: moderate Discontinuations were reported. Although changes in prophylaxis were mentioned, it was unclear whether participants were analysed according to original or subsequent prophylactic grouping 5. Missing data: low 4020/4158 (97%) of participants are included in the analysis for adverse events 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded. It was unclear whether the questionnaire implied causality to the drug regimen 7. Selection of the reported results: moderate The questionnaire included demographic information, but this was not reported. All results were reported according to short‐term or long‐term users of prophylaxis, which was not specified in the methods section 8. Other: no information No information is provided regarding the study sponsor |
| Methods | Design: cross‐sectional cohort study Study dates: November 1993 to October 1994 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient questionnaire sent 2 weeks after travellers return | |
| Participants | Number enrolled: 741 respondents, 918 questionnaires sent Inclusion criteria: "...travelers were asked to participate in the study when they attended TMVC clinics in Adelaide or Melbourne for pretravel consultation. If either doxycycline or mefloquine malaria chemoprophylaxis was recommended for part, or whole, of their itinerary, permission was sought to have them receive a mailed questionnaire" Exclusion criteria: "...under 18 years old, if doxycycline was recommended at doses other than 100mg daily, if other antimalarials were to be used during the intended journey, or if a traveller was not returning home in under 6 months" Factors influencing drug allocation: "Unless a contraindication existed for one or the other drug, the choice of which one to take was left to the traveler, the physician having already discussed, at some length, the different regimens, cost, and commonly reported adverse effects" Country of recruitment: Australia Region of malaria exposure: various (Southeast Asia, Africa, South Asia (India), Pacific) Duration of exposure to malaria: various, not specified Type of participants: travellers | |
| Interventions | 1. Mefloquine* 2. Doxycycline* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse events; any, nausea/vomiting, abdominal pain, diarrhoea, headache, dizziness, abnormal dreams, insomnia, anxiety 2. Serious adverse events 3. Adverse events; other (mood change, palpitations, itching, rash, red skin, vaginal itch) 4. Adverse effects; any 5. Adverse effects; abdominal pain, diarrhoea 6. Discontinuation of study drug due to adverse effects 7. Measure of adherence to the drug regimen Outcomes assessed not included in the review 8. Reasons for choice of antimalarial drug regimen | |
| Notes | Funding sources: "Thanks to Roche and Pfizer pharmaceutical companies for their financial support" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Identified confounders were measured and reported across groups. Mefloquine users were more likely to be female and had longer duration of treatment 2. Selection of participants into the study: serious Response rate 668 of 918 (73%) 3. Measurement of interventions: low The prescription was provided by a travel clinic which also performed the study; discontinuations were recorded and reported 4. Departures from intended interventions: moderate Discontinuations were recorded. It was unclear whether information regarding switches was recorded 5. Missing data: low All information was collected at one time point and all participants were included in the analysis 6. Measurement of outcomes: serious Comment: The outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: serious Information was reported for all adverse events recorded, but participants' assessment of causality to the study drug was only reported for two side effects 8. Other: serious "Sponsored by Roche and Pfizer pharmaceuticals" The role of the study sponsor was not made clear |
| Methods | Design: RCT Study dates: unclear Malaria transmission pattern and local antimalarial drug resistance: not applicable Adverse event monitoring: "Two days after drug ingestion, a second EEG was performed, and a blood sample for mefloquine level was obtained...Travelers were given forms on which to record adverse effects that appeared within 48 hours after drug intake" | |
| Participants | Number enrolled: 90 Inclusion criteria: not explicitly mentioned, included travellers from the Bnia Zion medical centre, Haifa, Israel Exclusion criteria: "Travelers younger than 18 years; with a history of epilepsy or depression, known allergy to mefloquine, cardiac conduction block; using beta‐blockers; or who were pregnant...Travelers with an abnormal baseline EEG (unifocal or repetitive bursts)" Country of recruitment: Israel Country of malaria exposure: not applicable Duration of follow up: 48 hours Type of participants: non‐travellers | |
| Interventions | 1. Mefloquine (1 x Mephaquine 250 mg tablet, Mepha, Aesch, Switzerland) one dose 2. Mefloquine (1 x Larium 250 mg tablet, Roche, Basel, Switzerland) one dose 3. Placebo | |
| Outcomes | 1. Adverse events; any 2. Adverse events; other (neuropsychiatric, abnormal EEG 48 hours after ingestion) | |
| Notes | Funding sources: "Partially funded by Mepha Ltd, Aesch, Switzerland" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible travelers were randomly assigned to one of three groups" "Randomization and statistical tests were carried out using Statmate and InStat" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | "Participants were unaware of their group assignment until they completed their tests" Comment: methods used to blind participants not reported |
| Blinding of outcome assessment (detection bias) | Low risk | "EEG pairs (pre‐ and post‐mefloquine) were examined separately by two senior neurologists who were unaware of group allocation" |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | N/A |
| Incomplete outcome data (attrition bias); safety | Unclear risk | Comment: data were provided for all participants who were not excluded on the basis of abnormal baseline EEG |
| Selective reporting (reporting bias); efficacy | Unclear risk | N/A |
| Selective reporting (reporting bias); safety | Unclear risk | "Adverse effects, mainly gastrointestinal and neuropsychiatric were noted in 26 travellers" Comment: specific nature of each adverse effect is not noted per group |
| Other bias | High risk | Partially funded by Mepha Ltd, Aesch, Switzerland. Comment: the role of the study sponsor was not clear |
| Methods | Design: retrospective cohort study Study dates: July 2003 to June 2004 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 794 Inclusion criteria: Travellers who were visiting five popular tropical regions or countries. Exclusion criteria: aged < 18 years, travelling for more than 2 months, and major acute or chronic diseases Country of recruitment: Germany Country of malaria exposure: Kenya/Tanzania, Senegal/Gambia, India/Nepal, Thailand, Brazil Duration of exposure to malaria: various, mean duration of travel 23.9 days Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Doxycycline* 3. Atovaquone‐proguanil* 4. Chloroquine* Not included in the review: 5. Chloroquine‐proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Narrative description of adverse effects Outcomes assessed not included in the review: 2. Risk behaviours during travel 3. Illness during travel 4. Seeking medical care owing to illness or accident 5. Accidents during travel | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Demographic information was provided for the entire cohort, not by prophylactic regimen 2. Selection of participants into the study: moderate Numbers of participants choosing not to participate in the study were not reported 3. Measurement of interventions: serious Participants were asked to self‐report which prophylaxis they took after return. The time after return was not specified 4. Departures from intended interventions: no information There was insufficient information provided to determine whether the questionnaire contained information regarding discontinuations or switches 5. Missing data: moderate Follow up was obtained for 658 (83%) travellers 6. Measurement of outcomes: serious There was insufficient information on the questionnaire about how adverse effects were sought and if outcome measures were objective. There was no mention of blinding of outcome assessors 7. Selection of the reported results: moderate There was insufficient information provided regarding the questionnaire to determine if all questions were reported. Side effects were grouped to report symptoms 8. Other: no information No information was provided regarding the study sponsor |
| Methods | Design: cohort study Study dates: 1989 Malaria transmission pattern and local antimalarial drug resistance: higher levels of P falciparum than P vivax locally. Local chloroquine and primaquine resistance Adverse event monitoring: unclear | |
| Participants | Number enrolled: 349 Inclusion criteria: Unclear Exclusion criteria: Unclear Country of recruitment: Australia Country of malaria exposure: Papua New Guinea Duration of exposure to malaria: 3 to 13 week training exercises Type of participants: Soldiers | |
| Interventions | Included in the review: 1. Mefloquine (1 x 250 mg weekly) 2. Doxycycline (1 x 100 mg tablet, daily, starting one day before deployment and continuing until 3 days after return) Not included in the review: 3. Doxycycline + primaquine 4. Doxycycline + chloroquine | |
| Outcomes | Included in the review: 1. Narrative description of adverse effects Outcomes assessed not included in the review:: 2. Clinical cases of malaria | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate No demographic information was provided 2. Selection of participants into the study: moderate Numbers of participants choosing not to participate in the study not reported 3. Measurement of interventions: low All participants were soldiers who were issued with medication 4. Departures from intended interventions: moderate No information was provided regarding discontinuations or switches 5. Missing data: moderate No losses to follow‐up or treatment withdrawals were reported, but the paper does not clearly state that none occurred 6. Measurement of outcomes: serious There was insufficient information on how adverse effects were sought and if outcome measures were objective. There was no mention of blinding outcome assessors 7. Selection of the reported results: moderate There was insufficient information provided regarding the questionnaire to determine if all questions were reported. Side effects were grouped to report symptoms. 8. Other: no information No information is provided regarding the study sponsor |
| Methods | Design: cross‐sectional cohort study Study dates: June to December 2000 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 491 Inclusion criteria: "visitors over fifteen who were travelling to South or Central America, Africa, India or South‐East Asia, including China, and who were not suffering from any chronic illness" Exclusion criteria: none mentioned Factors influencing drug allocation: "After talking to the doctor, the doctor wrote whether malaria prophylaxis had been decided on and if so which kind" Country of recruitment: Sweden Region of malaria exposure: various, including South or Central America, Africa, India or Southeast Asia, including China Duration of exposure to malaria: "most were abroad between two to four weeks" Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Chloroquine* 3. Non‐users Not included in the review: 4. Chloroquine‐proguanil* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse events; any, seriously negative effect on the journey 2. Adverse effects; any 3. Adverse effects; other (neuropsychiatric, skin problems) Outcomes assessed not included in the review: 4. Importance attached to prophylaxis 5. Whether travellers had any anxiety about side effects prior to taking prophylaxis | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex, destination and duration of travel data were collected but not reported across groups. BMI was not measured 2. Selection of participants into the study: serious Response rate 62% 3. Measurement of interventions: low The prescription was provided by a travel clinic which also performed the study 4. Departures from intended interventions: moderate Discontinuations were reported, but not across groups. Switches were not recorded 5. Missing data: low All participants who completed both questionnaires were included in the analysis 6. Measurement of outcomes: moderate The outcome measure was subjective; participants and personnel were not blinded. Participants were asked to report all symptoms, and which they felt were due to prophylaxis 7. Selection of the reported results: moderate Symptoms were grouped to report outcomes 8. Other: low Source of funding not mentioned. "competing interests: none declared" |
| Methods | Design: RCT Study dates: July 1987 to June 1988 Malaria transmission pattern and local antimalarial drug resistance: "holoendemic for malaria... at the time of the trial, chloroquine resistance was not a problem" Adverse event monitoring: "study participants were seen weekly up to week 28". Interview with study personnel for events such as "fever, chills, malaise, nausea and vomiting, rashes and other symptoms and signs that could be regarded as adverse events" | |
| Participants | Number enrolled: 567 Inclusion criteria: "...adult males aged 16 to 60 years, judged healthy on clinical grounds (no history of any illness and physical examination revealed no evidence of an acute or chronic illness). The patients were not on any drugs" Exclusion criteria: "...known hypersensitivity to sulphonamides, antimalarial drug treatment in the preceeding four weeks, presence of chronic debilitating disease and inability to attend regularly for follow up" Country of recruitment: Nigeria Country of malaria exposure: Nigeria Duration of exposure to malaria: study duration 24 weeks Type of participants: Nigerian residents, semi‐immune. | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet, Hoffman‐La Roche) weekly for 4 weeks followed by 1 x 125 mg tablet weekly for 20 weeks, total duration 24 weeks* 2. Chloroquine (1 x 300 mg base tablet, Hoffman‐La Roche) weekly, total duration 24 weeks* 3. Placebo, 1 tablet (Hoffman‐La Roche) weekly, total duration 24 weeks* *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Episodes of parasitaemia 3. Adverse events; any, abdominal pain, diarrhoea, headache, dizziness, pruritis, visual impairment (blurred sight) 4. Serious adverse events 5. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 6. Laboratory tests; white blood cell counts, haematocrit, serum glutamic oxaloacetic transaminase and serum glutamic‐pyruvic transaminase 7. Adverse events: rash, muscle stiffness (occurred in < 1% of study participants) | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...subjects were allocated randomly into five groups on the basis of a pre‐determined randomisation list" Comment: no mention of how the list was generated |
| Allocation concealment (selection bias) | Unclear risk | "...blister packs containing a total of 24 tablets were provided for each subject ...The packs and tablets were identical in appearance and were labelled with the appropriate double‐blind number" Comment: no mention of opaque sealed envelopes or central allocation |
| Blinding of participants and personnel (performance bias) | Low risk | "The packs and tablets were identical in appearance" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no description provided of how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | Low risk | Comment: numbers lost to follow up were provided across groups, with reasons provided. 107/113 (95%) mefloquine recipients, 103/115 (90%) chloroquine recipients and 101/114 (89%) placebo recipients completed the trial |
| Incomplete outcome data (attrition bias); safety | Low risk | Comment: reports "number of individuals suffering adverse events during the trial". Numbers lost to follow up were provided across groups, with reasons provided. 107/113 (95%) mefloquine recipients, 103/115 (90%) chloroquine recipients and 101/114 (89%) placebo recipients completed the trial |
| Selective reporting (reporting bias); efficacy | Low risk | Comment: clinical cases of malaria and episodes of parasitaemia are reported for all participants |
| Selective reporting (reporting bias); safety | Unclear risk | "No change of clinical relevance occurred in any of the groups in the above laboratory tests" Comment: there was insufficient information available regarding the collection of adverse events to determine whether the reported list included all events or only a targeted list. Data not fully reported for blood tests |
| Other bias | Unclear risk | Comment: study sponsor not mentioned, but four of the authors are attributed to F Hoffman‐La Roche |
| Methods | Design: RCT Study dates: August 1982 to January 1983 Malaria transmission pattern and local antimalarial drug resistance: region considered hyperendemic. P falciparum resistant to chloroquine and “high prevalence of multiresistant Plasmodium falciparum transmission” Adverse event monitoring: during the initial screening visit, weekly visits, and a final visit at study end, participants were asked about illnesses, mainly about signs and symptoms compatible with malaria, and blood tests were done, including haematocrit and leucocyte count | |
| Participants | Number enrolled: 122 Inclusion criteria: "volunteer soldiers and civilians aggregated to the 5th Battalion of Engineering and Construction in a community in Porto Velho" Exclusion criteria: aged < 12 years and > 55 years, pregnancy, people with debilitating disease, people who took antimalarial drugs in the previous four weeks and people with allergy to sulphonamides Country of recruitment: Brazil Country of malaria exposure: Brazil Duration of exposure to malaria: Mean duration within study (across groups) 16.9 weeks Type of participants: Brazilian soldiers and civilians, semi‐immune | |
| Interventions | Included in review comparisons: 1. Mefloquine (2 x 250 mg tablets, Roche) every 4 weeks* 2. Mefloquine (1 x 250 mg tablet, Roche) every 2 weeks* 3. Placebo Not included in review comparisons: 4. Fansidar* *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Adverse effects; any, anxiety Outcomes assessed not included in the review: 3. Laboratory tests; haematocrit, white blood cell counts, serum glutamic oxaloacetic transaminase and serum glutamic‐pyruvic transaminase | |
| Notes | Funding sources: Laboratory Roche provided mefloquine and “support” for conducting the study. Comando do 5o Batalhão de Engenharia e Construção, Porto Velho, RO, provided laboratory and field installations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as a randomized controlled trial, but no details were given on the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment provided |
| Blinding of participants and personnel (performance bias) | Low risk | "Each week... participants ingested 4 tablets of equal appearance, contained in sealed envelopes" |
| Blinding of outcome assessment (detection bias) | Unclear risk | "Each week... participants ingested 4 tablets of equal appearance, contained in sealed envelopes, with a code pre‐determined for each individual and not opened after the completion of the study" Comment: no mention of blinding of outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | High risk | "120 participants were initially recruited (30 in each group). Six of them were then excluded and were not included in the analysis. 8 participants left the area of study (one after the 10th week and 7 after the 11th week of exposure)" Outcomes were included in the analysis, and were substituted by eight new participants. With these six excluded participants and eight substituted participants, final sample size was 122. Comment: participants were not followed up beyond the active phase of treatment for relapses |
| Incomplete outcome data (attrition bias); safety | Unclear risk | Comment: reasons for losses to follow‐up were not reported |
| Selective reporting (reporting bias); efficacy | Low risk | Comment: all cases of malaria were reported |
| Selective reporting (reporting bias); safety | Unclear risk | Comment: there was insufficient information provided regarding the method of adverse effects monitoring to determine whether all outcomes had been reported |
| Other bias | High risk | Roche provided mefloquine and “support” for conducting the study |
| Methods | Design: retrospective cohort study Study dates: January to June 2007 Malaria transmission pattern and local antimalarial drug resistance: "malaria risk and transmission patterns have been known to shift rapidly in Afghanistan" Adverse event monitoring: "A retrospective, anonymous survey was completed by soldiers returning to Fort Drum, NY from Afghanistan" | |
| Participants | Number enrolled: 2601 surveys distributed, 2351 (90%) returned Inclusion criteria: none mentioned Exclusion criteria: none mentioned Factors influencing drug allocation: "oral mefloquine 250 mg per week was the primary alternative to doxycycline... In some cases, mefloquine was chosen as the first‐line therapy based on either perceived advantages in compliance, unit force protection, and/or operational concerns" Country of recruitment: USA Country of malaria exposure: Afghanistan Duration of exposure to malaria: various, not specified Type of participants: military | |
| Interventions | Included in review comparisons: 1. Mefloquine* 2. Doxycycline* Not included in review comparisons: 3. Atovaquone‐proguanil* (data on adverse events not collected; data on compliance not reported) *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; any, vomiting, diarrhoea 2. Adverse effects; other (heartburn/dyspepsia) 3. Discontinuations of study drug due to adverse effects 4. Measure of adherence to the drug regimen Outcomes assessed not included in the review: 5. Clinical cases of malaria 6. Adverse effects: numbers not reported in both groups (nausea, headache, dizziness, abnormal dreams, insomnia, depression, photosensitivity, rash, loss of appetite, pain and/or difficulty swallowing, vaginitis, lightheadedness, nervousness, ringing in ears, chills) 7. Use of personal protective measures to prevent mosquito bites | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Information was provided on duration of deployment, area of deployment, sex, age group and rank across regimens. Area deployed in Afghanistan and sex were different across groups. No adjustment for confounders was made in the analysis 2. Selection of participants into the study: low Response rate 2351/2601 surveys (90%) 3. Measurement of interventions: moderate Participants were asked to self‐report which prophylaxis was used on return to the USA. It is unclear if participants were still receiving the intervention at this time 4. Departures from intended interventions: serious "There were 520 respondents (25.2%) reporting more than one medication used to prevent malaria over the course of the deployment" 5. Missing data: low Analysis included 1898/2011 (94.4%) respondents for doxycycline, 564/596 (94.6%) respondents for mefloquine 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded. Different criteria were used to assess adverse effects related to mefloquine and doxycycline 7. Selection of the reported results: serious There was insufficient information provided regarding the questionnaire to determine whether all included outcomes were reported. Data for doxycycline were provided by severity gradings but not for mefloquine 8. Other: no information No information is provided regarding the study sponsor |
| Methods | Design: cross‐over RCT Study dates: 1993 to 1994 Malaria transmission pattern and local antimalarial drug resistance: not applicable Adverse event monitoring: "Throughout dosing, the participants were monitored and questioned regarding their general well‐being. The participants were seen 1) prior to taking any medication, 2) at the end of the first week (during which the loading dose was administered, 3) one week before testing, and 4) on the testing day itself when they were asked to report any changes from normal and questioned with regard to any symptoms experienced while taking the drug" | |
| Participants | Number enrolled: 23 Inclusion criteria: "conducted with trainee pilots attending the Swiss Civil Aviation School during the classroom phases of their study" Exclusion criteria: "history of a seizure disorder; psychosis or severe depression; known allergy or sensitivity to mefloquine or related compounds; concurrent use of cardioactive medication; compromised renal or hepatic function; pregnancy or the intention to become pregnant within three months of mefloquine use; use of mefloquine in the preceding two months, and use of hypnotics or tranquillizers during the two weeks prior to testing and alcohol within 12 hr of testing" Country of recruitment: Switzerland Country of malaria exposure: not applicable Duration of follow up: 4 weeks Type of participants: Swissair trainee pilots, did not travel | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet) given daily on 3 consecutive days followed from day 8 by once a week administration of 1 tablet for three consecutive weeks 2. Placebo (1 tablet) given daily on 3 consecutive days followed from day 8 by once a week administration of one tablet for 3 consecutive weeks | |
| Outcomes | Included in the review: 1. Adverse events; any 2. Discontinuations of study drug due to adverse effects 3. Adverse events; other outcomes (instrument co‐ordination analyser, sleep assessment, sway, neurobehavioural evaluation system, profile of mood states) | |
| Notes | Funding sources: This study was sponsored by the F. Hoffmann La Roche Tropical Medicine Unit (Basel, Switzerland) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomization not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details of allocation concealment reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: described as double blind but no mention of whether placebo was identical to the active formulation |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no description of who was blinded and how |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | N/A |
| Incomplete outcome data (attrition bias); safety | Unclear risk | "There was one withdrawal due to dizziness, diarrhea, and flu‐like symptoms and three volunteers spontaneously reported minor sleep‐related AEs (adverse events), including insomnia, unpleasant dreams, superficial sleep, and early awakening. These events all occurred in the mefloquine loading dose phase" Comment: not clear whether this withdrawal was included in the data analysis |
| Selective reporting (reporting bias); efficacy | Unclear risk | N/A |
| Selective reporting (reporting bias); safety | High risk | "The individual Environmental Symptom Questionnaire (ESQ) symptoms were also analyzed and items selected for their relevance to mefloquine administration were assessed by Cochran's Q test for related samples" Comment: intra‐individual changes in scores were obtained during the study, but outcomes were presented as means across groups. Data from the ESQ were not reported, only "no significant differences". Data for the Profile of Mood States questionnaire was presented in a graph with no standard deviations |
| Other bias | High risk | This study was sponsored by the F. Hoffmann La Roche Tropical Medicine Unit (Basel, Switzerland). The role of the study sponsor was not clear |
| Methods | Design: RCT Study dates: 1998 to 2001 Malaria transmission pattern and local drug resistance: not mentioned Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 674 Inclusion criteria: adult travellers aged 18 to 70 years, with planned travel of 1 to 3 weeks to a malaria‐endemic area, and consulting at a travel clinic ≥ 17 days before departure Exclusion criteria: glucose‐6‐phosphate dehydrogenase deficiency, history of severe adverse events with any of the four study drugs or a contra‐indication for their use, pregnancy or unwillingness to adhere to reliable contraception, history of seizures, psychiatric disorders, severely impaired renal or hepatic function, concurrent or recent vaginal infections or bacterial enteric disorders, a history of photosensitivity, or unwillingness to adhere to the study protocol Countries of recruitment: Switzerland, Germany and Israel Region of malaria exposure: sub‐Saharan Africa Duration of exposure to malaria: 1 to 3 weeks Type of participants: travellers | |
| Interventions | 1. Mefloquine (1 capsule containing mefloquine hydrochloride 274.09 mg, equivalent to mefloquine 250 mg base) once weekly, starting 17 days before travel and continuing for 4 weeks after travel* 2. Chloroquine‐proguanil (1 combined capsule containing chloroquine diphosphatase 161.21 mg, equivalent to chloroquine 100 mg base; and 200 mg proguanil hydrochloride) once daily, starting 17 days before travel and continuing for 4 weeks after travel* 3. Doxycycline (1 capsule containing doxycycline monohydrate 100 mg) once daily, starting 17 days before travel and continuing for 4 weeks after travel* 4. Atovaquone‐proguanil (1 combined capsule containing 250 mg atovaquone and 100 mg proguanil hydrochloride) once daily, starting 17 days before travel and continuing for 1 week after travel* *matched placebo for each treatment arm | |
| Outcomes | Included in the review: 1. Adverse events; any 2. Serious adverse events 3. Adverse events; other ('gastrointestinal', 'skin symptoms', 'neuropsychological') ‐ any severity, mild, moderate, severe 4. Discontinuation of study drug due to adverse effects 5. Adverse events; other outcomes (profile of mood states, quality of life score) | |
| Notes | Funding sources: GlaxoSmithKline supplied atovaquone‐proguanil and gave financial support; Zeneca supplied chloroquine‐proguanil; Pfizer supplied doxycycline; Roche supplied mefloquine and gave financial support | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was from a computer generated table of numbers in permuted blocks of five" |
| Allocation concealment (selection bias) | Unclear risk | "Participants were allocated treatment sequentially in order of study numbers. Allocation concealment was by sealed envelope" Comment: not reported whether envelopes were opaque |
| Blinding of participants and personnel (performance bias) | Low risk | "The drugs were provided as identical capsule blister packs in weekly cards" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: Described as double blind but no mention of how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | High risk | Comment: Method of detection for malaria, frequency and duration of follow up were not reported |
| Incomplete outcome data (attrition bias); safety | Unclear risk | "Adverse events were analysed in 623 participants who completed questionnaires at recruitment and at least one of the follow up periods" "Data was collected during recruitment and at follow up 13‐11 days before departure, 6‐4 days before departure and 7‐14 days after departure" Comment: it was unclear how many participants provided data at each time point |
| Selective reporting (reporting bias); efficacy | Low risk | "No cases of malaria were reported for any study arm" |
| Selective reporting (reporting bias); safety | High risk | "Adverse events were analysed in 623 participants who completed questionnaires at recruitment and at least one of the follow up periods" "Data was collected during recruitment and at follow up 13‐11 days before departure, 6‐4 days before departure and 7‐14 days after departure" Comment: Data were presented on aggregate across multiple time points |
| Other bias | High risk | Funding: Pfizer, GlaxoSmithKline, Roche, and Zeneca provided the drugs free of charge. GlaxoSmith Kline and Roche provided research grants. "Competing interests: PS has received speakers’ honorariums and travel expenses from Roche and GlaxoSmithKline. She acted as a consultant to Roche in a drug safety database evaluation. RS has received speakers’ honorariums and travel expenses from GlaxoSmithKline, Roche, and Pfizer. He is also a member of the advisory board of GlaxoSmithKline for malaria prophylaxis related questions. BB has received a speaker’s honorarium and travel expenses from GlaxoSmithKline. HN has received speakers’ honorariums and travel expenses from GlaxoSmithKline on different occasions. He has been principal or coinvestigator in several vaccine trials sponsored by GlaxoSmithKline" |
| Methods | Design: retrospective cohort study Study dates: 1 January 2001 and 1 October 2009 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: Incident cases of a neuropsychiatric disorder including anxiety, stress‐related disorders or psychosis, depression, epilepsy or peripheral neuropathies during or after anti‐malarial drug use within the UK general practice research database | |
| Participants | Number enrolled: Not available Inclusion criteria: "We identified in the general practice research database all patients who had ≥ 1 prescription of mefloquine, chloroquine and/or proguanil or atovaquone/proguanil between January 1, 2001 and October 1, 2009, and who had a pre‐travel consultation within 1 week of the prescription" Exclusion criteria: "We only included subjects who used anti‐malarial drugs for malaria prophylaxis... Furthermore, individuals had at least 12 months of information on prescribed drugs and medical diagnoses before the first prescription date for a study drug. In addition, subjects had recorded activity (diagnoses or drug prescriptions) at any time after the prescription for an anti‐malarial drug to include only subjects who returned to the UK. We excluded all patients with a diagnosis of malaria prior to the start of anti‐malarial drug use, patients with a history of cancer, alcoholism, rheumatoid arthritis; or with an outcome of interest prior to using anti‐malarial drugs. The date of the first neuropsychiatric disorder was the index date for each case" Country of recruitment: UK Country of malaria exposure: various, not specified Duration of exposure to malaria: various, not specified Type of participants: travellers | |
| Interventions | Included in review comparisons: 1. Mefloquine* 2. Atovaquone‐proguanil* Not included in review comparisons: 3. Chloroquine‐proguanil* 4. Unexposed (case‐control design) *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse events; psychiatric disorders (anxiety, depression, psychosis) 2. Adverse events; other ('anxiety or stress related disorders or psychosis', epilepsy, neuropathy, phobia, panic attack) | |
| Notes | Funding sources: F. Hoffmann‐La Roche Ltd., Basel, Switzerland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex and BMI were measured but only reported for people experiencing adverse events 2. Selection of participants into the study: moderate "We excluded all patients with a personal history of recorded neuropsychiatric disorders from the study population, but family history is not consistently recorded in the database" 3. Measurement of interventions: moderate "We only included subjects who used anti‐malarial drugs for malaria prophylaxis. We identified prescriptions for which the GP recorded ‐ within a week of the anti‐malarial drug prescription ‐ specific codes indicating that the person received the prescription for malaria prophylaxis, such as 'travel advice' or “prophylactic drug use” 4. Departures from intended interventions: serious It is possible that participants discontinued or switched medication and this would not have been captured in the study 5. Missing data: moderate The study did not report the total number of participants, only those who experienced adverse events 6. Measurement of outcomes: moderate General practitioners diagnosing patients would have been aware of their exposure status 7. Selection of the reported results: moderate Data for anxiety, stress‐related disorders and psychosis were reported on aggregate 8. Other: serious Study was sponsored by Roche. The role of the funding source was not made clear |
| Methods | Design: cross‐sectional cohort study Study dates: October 1995 to April 1998 Malaria transmission pattern and local antimalarial drug resistance: "both P. falciparum and P. vivax are hyperendemic" Adverse event monitoring: "...we directly contacted all travelers for complete follow‐up and assessment of compliance. Fifty travelers taking primaquine completed a questionnaire regarding side effects" | |
| Participants | Number enrolled: 158 Inclusion criteria: Israelis participating in rafting trips in Southern Ethiopia Exclusion criteria: none mentioned Country of recruitment: Israel Country of malaria exposure: Ethiopia Duration of exposure to malaria: 14 to 20 days Type of participants: travellers | |
| Interventions | Included in review comparisons: 1. Mefloquine (1 x 250 mg tablet) weekly, Starting 1 week prior to departure, during travel and for 4 weeks after return 2. Doxycycline (1 x 100 mg tablet) daily Not included in review comparisons: 3. Primaquine 15 mg daily for travellers with body weight < 70 kg and 30 mg for those weighing > 70 kg, starting 1 day prior to departure and continuing for up to 2 days after departure 4. Hydroxychloroquine* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 2. Clinical cases of malaria 3. Measure of adherence to the drug regimen (not fully reported) 4. Adverse effects; any (methods of detection different for primaquine versus other regimens) | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex and BMI were not reported for any participants. Destination and duration of travel was roughly equivalent across all groups 2. Selection of participants into the study: moderate Subjects were selected on the basis of their travel destination. Start of follow up and start of intervention coincide. No non‐responses were reported 3. Measurement of interventions: moderate "Prior to the trip, participants consulted one of a number of travel clinics in Israel, among them our clinic" Comment: it was unclear how intervention status was ascertained for participants who visited other clinics 4. Departures from intended interventions: low Two discontinuations (158 participants) were reported 5. Missing data: serious "In addition, we directly contacted all travelers for complete follow‐up and assessment of compliance. Fifty travelers taking primaquine completed a questionnaire regarding side effects" It was unclear how information on discontinuations and side effects were obtained for participants who did not take primaquine" 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: serious "In addition, we directly contacted all travelers for complete follow‐up and assessment of compliance. Fifty travelers taking primaquine completed a questionnaire regarding side effects" It was unclear how information on discontinuations and side effects was obtained for participants who did not take primaquine 8. Other: no information No information was provided regarding the study sponsor |
| Methods | Design: cross‐sectional cohort study Study dates: not mentioned Malaria transmission pattern and local antimalarial drug resistance: not applicable Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 45 Inclusion criteria: none mentioned Exclusion criteria: none mentioned Factors influencing drug allocation: "Prior knowledge about the side effect profile of mefloquine forced us to prescribe doxycycline 100 mg daily for aviators and mefloquine 250 mg weekly for non‐aviator crew" Country of recruitment: Israel Country of malaria exposure: Rwanda and Zaire Duration of exposure to malaria: "biweekly flights to and from Rwanda to Zaire with an average of 4 hours stay in the field over a period of 2 months" Type of participants: military | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet) weekly, starting on the day of travel (< 12 hours before the first flight) and continuing until 4 weeks after return 2. Doxycycline (1 x 100 mg tablet) daily, starting on the day of travel (< 12 hours before the first flight) and continuing until 4 weeks after return | |
| Outcomes | Included in the review: 1. Adverse effects; any, nausea, abdominal pain, dizziness 2. Adverse effects; other (fatigue) 3. Discontinuations of study drug due to adverse effects 4. Measure of adherence to the drug regimen Outcomes assessed not included in the review: 5. Clinical cases of malaria | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Sex and BMI were not measured. Destination and duration of travel were set by the study design 2. Selection of participants into the study: low "Prior knowledge about the side effects profile of mefloquine forced us to prescribe doxycycline 100 mg daily for aviators and mefloquine 250 mg weekly for non‐aviator aircrew up to 1 mo after the last return" All participants completed questionnaires. 3. Measurement of interventions: low Type of prophylaxis used was set by the job of the included participants 4. Departures from intended interventions: low "Two non‐aviators were dropped from the study because of receiving the wrong prescription" 5. Missing data: low "Two non‐aviators were dropped from the study because of receiving the wrong prescription" Information was provided for the remaining 43 participants. 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate "...the questionnaire included questions about compliance, side effects attributed to chemoprophylaxis, and any illness after return" No information was provided regarding illness after return. 8. Other: no information No information is provided regarding the study sponsor |
| Methods | Design: retrospective cohort study Study dates: July 2006 to December 2008 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: "Participants… were sent an informative email asking them to complete a web‐based questionnaire" | |
| Participants | Number enrolled: 242 students sent questionnaire, 180 respondents Inclusion criteria: "all medical students who had performed an elective abroad between July 2006 and December 2008, who had visited countries where hepatitis A is endemic, and who had notified the student registrar to obtain study credits" Exclusion criteria: none mentioned Factors influencing drug allocation: "...students are free to visit [our occupational health department] or any other travel clinic including the LUMC in‐hospital travel clinic or their general practitioner" Country of recruitment: Netherlands Country of malaria exposure: none mentioned Duration of exposure to malaria: mean duration of stay = 74 days (range 10 to 224 days ) Type of participants: travellers | |
| Interventions | Included in review comparisons: 1. Mefloquine* 2. Atovaquone‐proguanil* 3. Doxycycline* Not included in review comparisons: 4. Primaquine* 5. Proguanil* 6. Chloroquine* (no data reported) *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; any 2. Serious adverse outcomes 3. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 4. Clinical cases of malaria 5. Risk of infection with bloodborne viruses 6. Health risks while abroad 7. Health problems experienced whilst abroad 8. Health problems experienced on return | |
| Notes | Funding sources: There was no dedicated funding for this project | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex, destination and duration of travel were measured but information not provided across groups. BMI was not measured 2. Selection of participants into the study: serious Response rate 180/242 (74.4%) 3. Measurement of interventions: serious "...six students did not remember which prophylaxis had been prescribed" Students were asked to self‐report which prophylaxis they took an average of 235 days after completing their trip 4. Departures from intended interventions: moderate "Eight students who used mefloquine (20%) stopped the drug prematurely as did ten students on atovaquone‐proguanil (16%) and the student on doxycycline. Only two of these students switched to another prophylaxis" 5. Missing data: low "none of the questionnaires was incomplete" All participants were included in the analysis 6. Measurement of outcomes: serious The outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate Insufficient information was provided on how data on adverse effects were sought 8. Other: low "There was no dedicated funding for this project" |
| Methods | Design: prospective cohort study Study dates: April 2002 to October 2003 Malaria transmission pattern and local antimalarial drug resistance: "20% of recent cases were due to P. falciparum' 'chloroquine resistant P. falciparum" Adverse event monitoring: "common questionnaires were used to investigate the compliance to and side effects of both regimes" | |
| Participants | Number enrolled: 1400 soldiers worked in the region Inclusion criteria: "...all Turkish soldiers were examined in detail and serum samples were taken before heading for the region" Exclusion criteria: "...none of the participants had any chronic disease" Factors influencing drug allocation: "The preference of the preventive regime was related to the availability of the drugs... the prophylaxis was started with doxycycline, which was at hand in March 2002. Then again the soldiers who came after July 2002 were given mefloquine" Country of recruitment: Afghanistan Country of malaria exposure: Afghanistan Duration of exposure to malaria: "The average time of presence for a single soldier in Kabul region was approx. 6 month [sic]" Type of participants: military | |
| Interventions | 1. Mefloquine* 2. Doxycycline* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Serious adverse effects 2. Adverse effects; any, nausea, vomiting, abdominal pain, diarrhoea, headache, insomnia, dyspepsia, anorexia Outcomes assessed not included in the review: 3. Clinical cases of malaria | |
| Notes | Funding sources: Not mentioned Communications with study author: Sonmez 2005 no longer had access to the original study data. However, the study authors confirmed that for table 1: "The comparisons of the number of side effects of both regimes" the number of side effects for specific symptoms e.g. nausea was equivalent to the number of soldiers reporting that side effect. In addition, the authors were able to clarify a discrepancy in the original text: the paper states "27 mefloquine takers (41.2%) reported 43 side effects at the 2nd week of prophylaxis". The total number of mefloquine participants was 228; 41.2% equates to 94 participants. The authors confirmed that the correct figure was 27 mefloquine users (11%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age of participants was balanced across groups. Destination and duration of travel were set by the study design. Sex and BMI were not reported 2. Selection of participants into the study: serious 734 soldiers returned questionnaires (52.2%) 3. Measurement of interventions: low All soldiers were issued with prophylaxis 4. Departures from intended interventions: low Switches between prophylactic regimens were not possible 5. Missing data: low The data were collected at 2 time points. The reported denominator for each time point was the same 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate There was insufficient information provided to be sure that all outcomes included in the questionnaire were reported 8. Other: no information No information was provided regarding the study sponsor |
| Methods | Design: RCT Study dates: January 1989 to June 1989 Malaria transmission pattern and local antimalarial drug resistance: "region endemic for P. falciparum malaria" Adverse event monitoring: "participants had access to a village health center, where they could notify personnel of any malaise or side effects. Clinical examinations and parasitologic tests were performed every 4 weeks. Blood counts were carried out at the end of weeks 4, 19 and 24" | |
| Participants | Number enrolled: 500 Inclusion criteria: "five‐hundred male volunteers, aged 16‐60 years, who were residents of a local village, were randomly assigned" Exclusion criteria: none mentioned Country of recruitment: Adzope region, Ivory Coast Country of malaria exposure: Adzope region, Ivory Coast Duration of exposure to malaria: study duration 20 weeks Type of participants: Ivory Coast residents, semi‐immune | |
| Interventions | Included in review comparisons: 1. Mefloquine (1 x 250 mg tablet) weekly in weeks 1 to 4, (1 x 125 mg tablet) weekly in weeks 5 to 20 2. Chloroquine (1 x 300 mg tablet) weekly for 20 weeks 3. Placebo (1 tablet) weekly for 20 weeks Not included in review comparisons: 4. Fansidar 5. Fansifem | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Episodes of parasitaemia 3. Serious adverse events 4. Adverse events: any, diarrhoea, headache, pruritis Outcomes assessed not included in the review: 5. Laboratory tests; haematocrit and white blood cell count 6. Adverse events: other (leukopenia, malaise; did not occur in any study participants) | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Five‐hundred male volunteers… were randomised" Comment: Method of randomization was not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment was provided |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind". "The medications and placebo were identical in appearance" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no information was provided on how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | Low risk | "Four hundred and ninety‐nine subjects were evaluated for safety (at least one tablet taken and one visit) as well as for efficacy" Comment: 499/500 (99.8%) participants included in the analysis |
| Incomplete outcome data (attrition bias); safety | Low risk | "Four hundred and ninety‐nine subjects were evaluated for safety (at least one tablet taken and one visit) as well as for efficacy" Comment: 499/500 (99.8%) participants included in the analysis |
| Selective reporting (reporting bias); efficacy | Low risk | Comment: all outcomes prespecified in the methods section were reported |
| Selective reporting (reporting bias); safety | Unclear risk | "Blood counts were carried out at the end of weeks 4, 19 and 24" Comment: blood counts were reported only for one participant who developed reversible leukopenia |
| Other bias | Unclear risk | Comment: no information provided regarding the study sponsor |
| Methods | Design: cohort study Study dates: Malpro 1‐ April 1985 to July 1988, Malpro 2‐ July 1988 to December 1991 Malaria transmission pattern and local antimalarial drug resistance: various, not stated Adverse event monitoring: self‐completed questionnaires were distributed and collected by cabin crews to all passengers returning on charter planes | |
| Participants | Number enrolled: 145,003 Inclusion criteria: not explicitly stated. This trial includes two publications, Steffen 1993 states "All passengers returning on charter planes from Mombasa, Kenya, to Europe", whereas Steffen 1990 states "all passengers flying back to Europe from East Africa (Kenya) or West Africa (9 countries)". Data have been included from Steffen 1993 Exclusion criteria: "All travellers who stayed longer than one year in tropical Africa were excluded, as were those who did not spend the main part of their visit in East Africa (Kenya, Tanzania and Uganda)" Country of recruitment: not applicable Region of malaria exposure: East Africa (Kenya, Tanzania, Uganda) Duration of exposure to malaria: various, not stated Type of participants: travellers | |
| Interventions | Included in review comparisons: 1. Mefloquine* 2. Chloroquine (1 x 300 mg tablet) weekly Not included in review comparisons: 3. Chloroquine (1 x 600 mg tablet) weekly 4. Proguanil* 5. Chloroquine + proguanil* 6. Pyrimethamine + sulfadoxine* 7. Non‐users (this population was asked about side effects (adverse effects) and instead answered regarding adverse events *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Serious adverse effects 2. Adverse effects; any (mild, moderate or severe), visual impairment, nausea, headache, dizziness, insomnia, depression, pruritis 3. Adverse effects; other ('other skin', medical consultations due to side effects, incapacitation due to side effects, 'cutaneous', 'redness of the skin', consulted a doctor) 4. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 5. Clinical cases of malaria 6. Measures taken against mosquito bites 7. Sources of pre‐travel health information 8. Places visited in tropical Africa | |
| Notes | Funding sources: "This study was sponsored by F. Hoffman‐La Roche Ltd, Basel, Switzerland" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex and BMI were not reported across different prophylactic groups 2. Selection of participants into the study: moderate "In Malpro 1, 80.1% of all passengers completed the in‐flight questionnaire… in Malpro 2 the response rate [was] 83.9%" 3. Measurement of interventions: low Passengers were asked to self‐report which malaria prophylaxis was used. Data were collected on the journey home, meaning it was likely that passengers were still taking this medication 4. Departures from intended interventions: low Handschin 1997: "2.9% of passengers changed the prophylactic regimen during the observation period" 5. Missing data: moderate Malpro 1 losses to follow‐up 4.1%, Malpro 2 losses to follow‐up 14.1% 6. Measurement of outcomes: moderate The outcome measure was subjective; participants and personnel were not blinded. Serious adverse events were verified independently 7. Selection of the reported results: serious Data on non‐serious side effects were not included from Malpro 1‐ 31% of participants (44,667) were not included 8. Other: serious The study was funded by Roche. The role of the study sponsor was not made clear |
| Methods | Design: quasi‐RCT Study dates: September 1987 to June 1990 Malaria transmission pattern and local antimalarial drug resistance: "primarily P falciparum (> 90%), some P malariae and minimal P ovale... High levels of Plasmodium falciparum resistance to CQ... sensitivity of P. falciparum to mefloquine was documented" Adverse event monitoring: "At the time of each dose, a questionnaire was administered to record symptoms including fever and reported drug side effects since the last visit" | |
| Participants | Number enrolled: 4220 Inclusion criteria: "...consecutive attenders at first antenatal clinic visit were enrolled at three sites… At a fourth side, consecutive attenders in their first and second pregnancy were enrolled" Exclusion criteria: "At this site [fourth site, government district hospital] women with two or more pregnancies were not enrolled because of the large number of patients attending the clinic and the limited number of study staff" Country of recruitment: Malawi Country of malaria exposure: Malawi Duration of exposure to malaria: Ongoing in semi‐immune population ‐ monitored from enrolment for various periods of time Type of participants: pregnant Malawian residents, semi‐immune | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet) weekly, with a single loading dose of 750 mg 2. Chloroquine (1 x 300 mg tablet) weekly, with a loading dose 25 mg of base/kg given as a divided dose over 2 days 3. Chloroquine (1 x 300 mg tablet) weekly | |
| Outcomes | Included in the review: 1. Episodes of parasitaemia 2. Adverse events; any 3. Serious adverse events 4. Discontinuations of study drug due to adverse effects 5. Adverse pregnancy outcomes; still births, abortions Outcomes assessed not included in the review: 6. Frequency of placental malarial infection 7. Frequency of prematurity or intra‐uterine growth retardation 8. Frequency of maternal febrile illness or anaemia 9. Likelihood of infant acquisition of malarial infection | |
| Notes | Funding sources: "This work was supported and made possible by the Africa Bureau, Office of Operations and New Initiatives and the Office of Analysis, Research and Technical Support, the USAID through the Africa Child Survival initiative… The Global Program on AIDS, World Health Organisation provided support for the HIV testing and evaluation portion of this study" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Systematic assignment of regimens was done based on the clinic and day of enrolment… All women making their first antenatal clinic on a given day were assigned to the same regimen; the following day, enrolled women were assigned to the following regimen" |
| Allocation concealment (selection bias) | High risk | "Systematic assignment of regimens was done based on the clinic and day of enrolment… All women making their first antenatal clinic on a given day were assigned to the same regimen; the following day, enrolled women were assigned to the following regimen" |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no mention of participants being blinded to which prophylactic regimen they were taking |
| Blinding of outcome assessment (detection bias) | Unclear risk | "All blood smear examinations were done with the microscopist blinded to the study subject’s antimalarial regimen" Comment: No mention of outcome assessors being blinded to the treatment regimen used when assessing safety outcomes |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | "Among the 4187 enrolled women, 3380 (81%) [were analysed]… 94 did not have an initial blood smear result for comparison, 89 left the study area before follow up, 397 delivered before the follow up visit, 133 missed their appropriate follow up visit, and 94 did not have documented adherence to the drug regimen" Comment: numbers lost to follow up were not reported across groups |
| Incomplete outcome data (attrition bias); safety | High risk | "A total of 4101 women had information available after their first dose and 2976 women had information available after their dose at four weeks" Comment: reasons for missing data were not reported |
| Selective reporting (reporting bias); efficacy | Unclear risk | "Only P falciparum infections were of interest for this study… when P malariae alone was identified these infections were excluded from the analysis" "For the purposes of malaria prevention and infant outcome we analysed the group of women… only if they were enrolled in the study for six or more weeks and had received the appropriate amount of medication during their participation" "A total of 1,790 women delivered in study health facilities had received proper dosing on their antimalarial regimen, and had their peripheral blood examined" Comment: women who had reported fever during pregnancy, and during the 2 weeks prior to delivery was reported, but not reported across antimalarial drug regimens |
| Selective reporting (reporting bias); safety | High risk | "All other complaints e.g. weakness, heart palpitations accounted for less than 15% of reported symptoms" Comment: Data were collected weekly but only reported after the first and the fourth dose |
| Other bias | Low risk | "This work was supported and made possible by the Africa Bureau, Office of Operations and New Initiatives and the Office of Analysis, Research and Technical Support, the USAID through the Africa Child Survival initiative… The Global Program on AIDS, World Health Organisation provided support for the HIV testing and evaluation portion of this study" |
| Methods | Design: Prospective cohort study Study dates: 2009 to 2011 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: "...participants were asked to complete a survey each week during travel and a post‐travel survey within 2–4 weeks after return" | |
| Participants | Number enrolled: 628 participants completed all three surveys, 370 included in the analysis Inclusion criteria: "Travelers were included from among all those enrolled if they received a prescription for chemoprophylaxis, traveled to at least one malaria‐endemic area, and completed pre‐ and post‐travel surveys and at least one during‐travel survey" Exclusion criteria: "To complete the study in a reasonable amount of time, only participants with shorter durations of travel (approximately 2 months) were included" Factors influencing drug allocation: "Several different medications are available for malaria chemoprophylaxis, depending on the traveler’s destination and medical history" Country of recruitment: USA Country of malaria exposure: India (13%), Tanzania (8%), Kenya (7%), South Africa (7%), and Haiti (7%) Duration of exposure to malaria: median travel duration 13 days Type of participants: travellers | |
| Interventions | Included in the review: 1. Mefloquine* 2. Doxycycline* 3. Atovaquone‐proguanil* 4. Chloroquine* Not included in the review: 5. Primaquine* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse effects; any, headache, abnormal dreams 'intense nightmares', any gastrointestinal 2. Discontinuations of study drug due to adverse effects 3. Measure of adherence to the drug regimen Outcomes assessed not included in the review: 4. Clinical cases of malaria 5. Reasons for non‐compliance with chemoprophylaxis (data provided on aggregate), 6. Use of personal protective measures for malaria prevention | |
| Notes | Funding sources: "This work was supported by a cooperative agreement [1 U19CI000508‐01] between the Centers for Disease Control and Prevention and Boston Medical Center" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex, destination and duration of travel were recorded but figures were not reported across prophylactic regimens 2. Selection of participants into the study: moderate No information was provided regarding travellers who did not wish to participate in the study 3. Measurement of interventions: low "The type of chemoprophylaxis prescribed were collected from data entered by clinicians into patients’ medical records" 4. Departures from intended interventions: moderate No switches or discontinuations were reported. It was unclear whether this information was captured in the questionnaire 5. Missing data: low 364/370 (98%) participants were included in the analysis 6. Measurement of outcomes: serious Comment: the outcome measure was subjective, participants and personnel were not blinded 7. Selection of the reported results: moderate Insufficient information provided on how data on adverse effects were obtained to determine whether all outcomes had been reported 8. Other: low Government funding |
| Methods | Design: retrospective cohort study Study dates: 18 July to 16 September 2016 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 8931 Inclusion criteria: Returned Peace Corps volunteers (RPCV) who served between 1995 and 2014 and had an e‐mail address in Peace Corps' RPCV database Exclusion criteria: None mentioned Factors influencing drug allocation: none specified Country of recruitment: USA Country of malaria exposure: various, not specified Duration of exposure to malaria: various, not specified Type of participants: returned Peace Corps volunteers | |
| Interventions | 1. Mefloquine* 2. Doxycycline* 3. Atovaquone‐proguanil* 4. Chloroquine* *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Measure of adherence to the drug regimen Outcomes assessed not included in the review: 2. "Questions about medications before, during, or after Peace Corps, as well as habits such as drinking" | |
| Notes | Funding source: "this research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Important confounders were measured but not been reported across groups. Duration and destination of travel were not measured 2. Selection of participants into the study: serious 8931/47,238 potential respondents included (13% response rate) 3. Measurement of interventions: serious Participants were asked to self‐report which chemoprophylaxis they had taken at least 2 years after they had finished the course 4. Departures from intended interventions: serious Limited information was provided regarding switches between interventions. Participants were asked to self‐report this information at least 2 years after finishing treatment 5. Missing data: low Information on adherence was reported for all participants who answered this question (5026 respondents/5055 who reported taking malaria prophylaxis) 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate There was insufficient information provided to be sure that all outcomes included in the questionnaire were reported 8. Other: low "This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors" |
| Methods | Design: cross‐sectional cohort study Study dates: 2012 and 2013 Malaria transmission pattern and local antimalarial drug resistance: "...high risk of malaria (mainly P. falciparum) in Kenya, although the risk is assessed as very low in Nairobi and in the highlands above 2,500 m... widespread resistance to chloroquine" Adverse event monitoring: "...questionnaire‐based, two‐arm cohort study" | |
| Participants | Number enrolled: 2032 completed questionnaires available, 220 failed to indicate which drug they were taking Inclusion criteria: all military personnel on deployment to Kenya who travelled on one of three main body flights on their return to the UK Exclusion criteria: none mentioned Factors influencing drug allocation: "...the choice of drugs considered in this study was limited to mefloquine or doxycycline... participants were free to use another drug should they experience unacceptable adverse effects or where there was an occupational reason" Country of recruitment: UK Country of malaria exposure: Kenya Duration of exposure to malaria: "The majority of participants spent approximately 6 weeks in Kenya with a small number spending a few weeks longer if they filled an administrative role" Type of participants: military | |
| Interventions | Included in review comparisons: 1. Mefloquine* 2. Doxycycline* Not included in review comparisons: 3. Atovaquone‐proguanil* (results not included in the analysis) *dosing regimen not specified | |
| Outcomes | Included in the review : 1. Adverse effects; any 2. Measure of adherence to the drug regimen Outcomes assessed not included in the review: 3. Clinical cases of malaria 4. Impact of adverse effects on self‐reported ability to work | |
| Notes | Funding sources: "The research was not sponsored by any external body" After we submitted the review for peer referee, the author sent us a spreadsheet containing numbers of events relating to a variety of symptoms after the review had been submitted for publication. These data are not included in the review and will require some clarification over how they were collected to allow us to assess risk of bias. This additional information will be considered in future updates. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate "Although not formally recorded, each unit can be assumed to be composed of similar populations in terms of number, age, gender, occupation, and general health" 2. Selection of participants into the study: serious "Completion rates were consistently poor throughout the study period with only 150 to 250 questionnaires returned per tranche of around 1,000 troops" 3. Measurement of interventions: low Participants were asked to self‐report which medication they were on while still taking the medication" 4. Departures from intended interventions: moderate "...[participants] were invited to complete the questionnaire for whichever drug they took for the longer period" 5. Missing data: moderate "2,032 completed questionnaires available for analysis of which 10.8% (220) failed to indicate which drug they were taking" 6. Measurement of outcomes: serious The outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: serious "In both arms, some participants indicated that they had experienced an adverse effect but did not report how it had impacted upon their ability to work. They were excluded from the final analysis" Mefloquine: 71 participants, doxycycline: 67 participants 8. Other: low "The research was not sponsored by any external body" |
| Methods | Design: cohort study Study dates: 15 to 22 February 2015 Malaria transmission pattern and local antimalarial drug resistance: not specified Adverse event monitoring: patient self‐reported questionnaire | |
| Participants | Number enrolled: 115 (337 eligible) Inclusion criteria: all land‐based members of a UK military expedition to Sierra Leone Exclusion criteria: none specified Country of recruitment: Sierra Leone Country of malaria exposure: Sierra Leone Duration of exposure to malaria: not specified Type of participants: military | |
| Interventions | 1. Mefloquine 2. Doxycycline 3. Atovaquone‐proguanil | |
| Outcomes | Included in the review: 1. Adverse effects: any, nausea, abdominal pain, diarrhoea, dizziness, insomnia 'disturbed sleep', pruritis, indigestion, mouth ulcers, lethargy 2. Measure of adherence to the drug regime | |
| Notes | Funding source: unfunded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Age, sex and BMI were not measured. Demographic information not reported across groups 2. Selection of participants into the study: serious 151 (46.3%) returned survey forms 3. Measurement of interventions: low Participants were asked to self‐report which medication they were taking while taking it 4. Departures from intended interventions: moderate Switches between groups were recorded. 8/151 recipients had medications switched due to unacceptable adverse effects. It was unclear to which drug adverse effects were attributed. 5. Missing data: low Data were reported for all survey respondents. 6. Measurement of outcomes: serious The outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate There was insufficient information provided to be sure that all outcomes included in the questionnaire were reported 8. Other: low "This audit was unfunded" |
| Methods | Design: prospective cohort study Study dates: 24 February to 24 May 1994 Malaria transmission pattern and local antimalarial drug resistance: various, not stated Adverse event monitoring: participant self‐reporting questionnaire | |
| Participants | Number enrolled: 1791 eligible and willing to co‐operate, data obtained from 1501 participants. Inclusion criteria: "...persons who visited the Travel Clinic in the period between 24 February and 24 May, 1994, and who had an anticipated date of return to the Netherlands before the end of the study period, and who had given informed consent" Exclusion criteria: none stated Country of recruitment: Rotterdam, Netherlands Region of malaria exposure: various; Africa, South America, Asia or the Middle East Duration of exposure to malaria: various, not specified Type of participants: travellers | |
| Interventions | Included in review comparisons: 1. Mefloquine (1 x 250 mg tablet) weekly 2. Non‐users of antimalarials Not included in review comparisons: 3. Proguanil (1 x 200 mg tablet) daily | |
| Outcomes | Included in the review: 1. Adverse events; nausea, diarrhoea, dizziness, abnormal dreams, insomnia, anxiety, depression, visual impairment 2. Adverse events; other (agitation, confusion) Outcomes assessed not included in the review: 3. Profile of mood states (only reported in comparison with proguanil) | |
| Notes | Funding sources: Not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Counfounding: low Identified confounders were measured and balanced across groups 2. Selection of participants into the study: moderate 1501/1791 (86% response rate) 3. Measurement of interventions: moderate Comment: the prescription was provided by a travel clinic which also performed the study but no information regarding switches and discontinuations were recorded or reported 4. Departures from intended interventions: moderate No information was provided on discontinuations or switches 5. Missing data: moderate 1227/1449 (85%) participants were included in the analysis; chloroquine‐proguanil users were not included. The number of non‐users decreased from 392 to 340 without explanation 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate It was clear what was asked in the questionnaire. Information was sought on the severity of adverse events but this was not reported 8. Other: no information No information was provided regarding the study sponsor |
| Methods | Design: RCT Malaria transmission pattern and local drug resistance: not mentioned Study dates: unclear Adverse event monitoring: baseline evaluation prior to travel, and follow up date 7 days after the participant left the endemic area and two scheduled telephone conversations | |
| Participants | Number enrolled: 140 Inclusion criteria: travellers aged ≥ 3 years and weighing ≥ 11 kg with planned travel ≤ 28 days to a malaria‐endemic area (Overbosch 2001) Exclusion criteria: In the published report "We excluded those who had risk factors for concentration impairment (e.g. use of opioids, hypnotics, or tranquillizers or use of alcohol 4 hours before testing)" Within Høgh 2000 (unclear if the same exclusion criteria were applied): poor general health; drug hypersensitivity (to atovaquone, chloroquine or proguanil); history of alcoholism, seizures, psychiatric disorders, severe neurological disorders, severe blood disorders; renal, hepatic or cardiac dysfunction; clinical malaria within previous 12 months; travel to malaria‐endemic area within previous 60 days; risk factors for concentration impairment (e.g. use of opioids, hypnotics, or tranquillizers; or use of alcohol 4 hours before testing) Country of recruitment: Rotterdam Travel Clinic, Netherlands Regions of malaria exposure: various malaria endemic destinations (66% in Africa, 13% South America, 24% other) Mean duration of exposure to malaria: 19 days Type of participants: travellers, non‐immune | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet; or ¼, ½ or ¾ of a tablet, according to body weight) once weekly, starting 7 days before travel and continuing for 4 weeks after travel* 2. Atovaquone‐chloroguanil (1 combined tablet containing 250 mg atovaquone and 100 mg proguanil hydrochloride; or alternatively 1 to 3 combined children's tablets according to body weight, each tablet containing 62.5 mg atovaquone and 25 mg proguanil hydrochloride) once daily, starting 1 to 2 days before travel and continuing for 1 week after leaving the malaria‐endemic area* *matched placebo for each treatment arm | |
| Outcomes | 1. Adverse events; other outcomes (profile of mood states, neurobehavioural evaluation system) 2. Measures of adherence to the drug regimen 3. Discontinuations of the study drug due to adverse effects | |
| Notes | Funding source: Netherlands Inspectorate for Healthcare gave financial support 'independently performed in a sample of patients from one center that participated in the MAL30010 multicenter clinical trial'‐ Enrollment criteria and study conduct were described in a separate publication (Høgh 2000) which refers to a different study population (atovaquone‐proguanil versus chloroquine‐proguanil). 'This study was planned and performed independently from the trial by other researchers and without knowledge of its results.' 'Subjects were separately recruited and asked for consent during the initial screening visit of the trial.' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated code was used to randomly assign a treatment number to the three bottles of study drug for every individual. At all sites consecutively enrolled individuals who satisfied all entry criteria received the next treatment number" (Høgh 2000) |
| Allocation concealment (selection bias) | Low risk | "Treatment codes were provided to investigators in opaque sealed envelopes, to be opened only if knowledge of study drug assignment was required for management of a medical emergency" (Høgh 2000) |
| Blinding of participants and personnel (performance bias) | Unclear risk | "To mask differences between the dosing regimes, placebo tablets were used... All placebo treatment regimens were identical to the aforementioned scheme for the active ingredient of mefloquine and atovaquone plus chloroguanide" Comment: did not mention whether the placebo and intervention tablets were identical in appearance |
| Blinding of outcome assessment (detection bias) | Low risk | "The assessments were made by researchers who were unaware of the treatment allocation" |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | N/A |
| Incomplete outcome data (attrition bias); safety | High risk | "We enrolled a total of 140 subjects in the cohort, 119 of whom completed the follow up" Comment: Those who did not complete follow up were not included in the subsequent statistical analysis. The proportion of participants who did not complete the study due to adverse outcomes varied significantly between groups (67% mefloquine and 33% atovaquone plus chloroguanide) |
| Selective reporting (reporting bias); efficacy | Unclear risk | N/A |
| Selective reporting (reporting bias); safety | Low risk | "Data were collected on concurrent medications, as well as subject’s use of coffee, alcohol and illicit drugs" "stratification for sex and adjustment for potential confounders such as smoking and the use of coffee and tea did not affect the result" Comment: these data were not presented |
| Other bias | Low risk | Funding: "For this study came from the Inspectorate for Health Care. Glaxo Wellcome kindly provided us with the treatment allocation codes after completion of the study. No financial support, however, was received from any pharmaceutical company" |
| Methods | Design: RCT Study dates: not mentioned Malaria transmission pattern and local antimalarial drug resistance: not applicable Adverse event monitoring: "After each driving test, subjects [described]... the presence and severity of adverse effects ‐ drowsiness, weakness, headache, fatigue, nervousness, nausea, dizziness and memory disturbance" | |
| Participants | Number enrolled: 42 Inclusion criteria: "...[volunteers] were medically screened by routine blood chemistry and haematology tests, a physical examination including an 12‐lead ECG recording, and urine tests for pregnancy and drugs of abuse" Exclusion criteria: "...clinically relevant abnormalities in any blood test; far‐field, binocular visual acuity that deviated by more than 0.65 dioptres from normal, corrected or uncorrected; known hypersensitivity to any drug; history of any serious gastrointestinal, hepatic, renal neurologic or psychiatric disorder; evidence of drug or alcohol abuse, excessive alcohol or nicotine use; blood donation or participation in a drug trial within the prior 2 months; and for premenopausal females, pregnancy, lactation or failure to exercise reliable birth control" Country of recruitment: Netherlands Country of malaria exposure: not applicable Duration of follow up: 30 days Type of participants: non‐exposed Dutch nationals | |
| Interventions | 1. Mefloquine (1 x 250 mg tablet) weekly, with loading dose of one tablet daily for 3 days in week 1 2. Placebo (1 tablet) weekly, with identical loading regimen of placebo tablets | |
| Outcomes | 1. Adverse events; any, nausea, diarrhoea, headache, dizziness 2. Adverse events; other (fatigue) 3. Discontinuations of study drug due to adverse effects 4. Adverse events; other outcome measures (critical flicker/fusion frequency, critical instability tracking test, standardized stabilimetry method of the International Society of Posturography, tests of driving performance) | |
| Notes | Funding sources: "The study was sponsored by F. Hoffmann‐La Roche Ltd" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study followed a randomised, 2‐arm, double‐blind, parallel group design" Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | "The study followed a randomised, 2‐arm, double‐blind, parallel group design" Comment: method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) | Low risk | "They received mefloquine 250 mg or placebo in identically appearing tablets" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: described as double blind but no description of how this was achieved for researchers and outcome assessors |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | N/A |
| Incomplete outcome data (attrition bias); safety | Low risk | Comment: dropouts were reported. 2/20 participants dropped out of the mefloquine group, one due to adverse effects related to the study drug |
| Selective reporting (reporting bias); efficacy | Unclear risk | N/A |
| Selective reporting (reporting bias); safety | High risk | "...subjects used 10 cm visual‐analogue scales to describe their mood in three dimensions – 'Alertness', 'Contentedness', and 'Calmness'” Comment: outcomes relating to these descriptions were not reported. The study reports "events occurring more than once" in each group |
| Other bias | High risk | "The study was sponsored by F. Hoffmann‐La Roche Ltd" |
| Methods | Design: cross‐sectional cohort study Study dates: April to May 1996 Malaria transmission pattern and local antimalarial drug resistance: "a high risk Malaria area... Chloroquine‐resistant P. falciparum malaria" Adverse event monitoring: "In‐flight self administered questionnaires were distributed and completed by travelers on flights returning to Johannesburg International Airport" | |
| Participants | Number enrolled: 4035 questionnaires distributed, 3051 returned Inclusion criteria: All travelers boarding the only commercial airline serving this area during April and May 1996 were included in the survey Exclusion criteria: None mentioned Country of recruitment: South Africa Country of malaria exposure: South Africa Duration of exposure to malaria: various, not specified Type of participants: travellers | |
| Interventions | Included in review comparisons: 1. Mefloquine* 2. Doxycycline* 3. Chloroquine* Not included in review comparisons: 4. Chloroquine‐proguanil* 5. Proguanil* *dosing regimen not specified | |
| Outcomes | Included in review comparisons: 1. Adverse effects; any Outcomes assessed not included in the review: 2. Sources of information on malaria prior to visit, 3. Use of personal protective measures against mosquitoes, 4. Measures of adherence to the drug regimen (information provided on aggregate), 5. Travellers knowledge of malaria symptoms | |
| Notes | Funding sources: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Confounding: moderate Sex of travellers was not provided by prophylactic regimen. Destination of travel was set by the study design. BMI of travellers and duration of travel were not recorded 2. Selection of participants into the study: serious Response rate 3051/4035 (75%) 3. Measurement of interventions: low Travellers were asked to self‐report which prophylactic regimen they were taking while still using the drug 4. Departures from intended interventions: moderate No discontinuations or switches were reported. This information was not included in the questionnaire 5. Missing data: low Outcome data were available for 973/978 mefloquine recipients and 80/80 doxycycline recipients 6. Measurement of outcomes: serious Comment: the outcome measure was subjective; participants and personnel were not blinded 7. Selection of the reported results: moderate Insufficient information provided on how data on adverse effects were obtained to determine whether all outcomes were reported 8. Other: no information No information was provided regarding the study sponsor. |
| Methods | Design: RCT Study dates: April to July 1993 Malaria transmission pattern and local antimalarial drug resistance: "Incidence of new cases of falciparum malaria during the rainy seasons has been measured at 90% in adults. P. falciparum accounts for > 95% of all malaria in Saradidi" Adverse event monitoring: "Each subject was visited daily at home by an assigned field worker, who asked about symptoms of malaria or drug side effects, obtained malaria smears, or administered drug doses if the subject was not at school" | |
| Participants | Number enrolled: 169 Inclusion criteria: aged 9 to 14 years. "Screening consisted of a physical examination, a urine pregnancy test for girls, and blood tests for complete blood cell count; blood urea nitrogen, serum alanine aminotransferase, and glucose‐6 phosphate dehydrogenase (G6PD) levels; and hemoglobin electrophoresis" Exclusion criteria: none mentioned Country of recruitment: Saradidi Rural Health Project, Nyanza province, Kenya on the shores of Lake Victoria Country of malaria exposure: Saradidi Rural Health Project, Nyanza province, Kenya on the shores of Lake Victoria Duration of exposure to malaria: study duration 4 months Type of participants: Kenyan residents, semi‐immune | |
| Interventions | 1. Melfoquine (1 x 125 mg tablet) weekly, with a second dose given on the third day of the study, equal to their usual weekly medication. 2. Doxycycline (1 x 50 mg tablet) daily 3. Primaquine 4. Multivitamin (1 x tablet containing vitamin A, 2500 IU, thiamine, 1 mg, riboflavin, 0.5 mg, nicotinamide, 7.5 mg, ascorbic acid, 15 mg, vitamin 0 3, 250 IU) daily Co‐interventions: After baseline malaria smears, all subjects received curative therapy for preexisting malaria: 7 days of quinine bisulfate, 300 mg three times daily, and doxycycline, 50 mg twice daily. The first dose of prophylactic drug was given starting the day after curative therapy finished | |
| Outcomes | Included in the review: 1. Clinical cases of malaria 2. Episodes of parasitaemia 3. Discontinuations of study drug due to adverse effects Outcomes assessed not included in the review: 4. Laboratory tests; complete blood cell counts, blood urea nitrogen and serum alanine aminotransferase 5. Mean number of symptoms reported per subject: nausea, abdominal pain, diarrhoea, headache, fever | |
| Notes | Funding sources: Financial support: USA Naval Medical Research and Development Command (work unit no. 623002A.81 0.00 J0 I.HFX. J433). Kenya Medical Research Institute. USA Army Medical Research and Materiel Command Provisional (contract no. DAMDI7‐92‐V‐20J2) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Students from each village school were separately randomized, to control for geographic variation in malaria transmission" Comment: no description of how randomization was performed |
| Allocation concealment (selection bias) | Unclear risk | "All medications were in brown envelopes and were administered 7 days each week by I field worker at each school" Comment: no mention of whether envelopes were sealed or if field workers had access to their content |
| Blinding of participants and personnel (performance bias) | Unclear risk | Comment: no mention of whether participants were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | "None of the malaria slide readers knew which drugs the subjects were taking. None of the field workers visiting the homes daily to ask about symptoms or clinical staff evaluating and treating subjects at the Saradidi Clinic knew which drugs the subjects were taking. If there was concern about a drug side effect, the clinical staff would consult the medical monitor, who would break the code for that subject. This occurred only four times during the studies" |
| Incomplete outcome data (attrition bias); efficacy | Unclear risk | N/A |
| Incomplete outcome data (attrition bias); safety | Unclear risk | Comment: number included in the safety analysis not reported |
| Selective reporting (reporting bias); efficacy | Unclear risk | N/A |
| Selective reporting (reporting bias); safety | Unclear risk | Comment: mean number of symptoms reported per subject during 11 weeks of the study were reported. A targeted list of symptoms was reported, with everything else included in ‘all other’. It was unclear what this list included |
| Other bias | Low risk | Financial support: USA Naval Medical Research and Development Command (work unit no. 623002A.81 0.00 J0 I.HFX. J433). Kenya Medical Research Institute. USA Army Medical Research and Materiel Command Provisional (contract no. DAMDI7‐92‐V‐20J2) |
| Methods | Design: retrospective cohort study Study dates: January 2002 to December 31 2002 Malaria transmission pattern and local antimalarial drug resistance: various, not specified Adverse event monitoring: "The study cohort was electronically linked to the Standardized Inpatient Data Record (SIDR) and the Health Care Service Record (HCSR) to identify hospitalization... We analyzed any‐cause hospitalization (excluding complications of pregnancy, childbirth, and the puerperium, congenital anomalies, and certain conditions originating in the perinatal period)" | |
| Participants | Number enrolled: 397442 Inclusion criteria: "All active‐duty US service members during the period January 1, 2002, and December 31, 2002, as reported by the Defense Manpower Data Center (DMDC), Monterey, CA. The mefloquine prescribed group was defined as service members who had been prescribed a minimum of seven mefloquine tablets beginning in 2002 and who were identified as having been deployed at some point during the same time period. We used two reference groups. The first reference group was comprised of service members who had duty zip codes for either Europe or Japan at some time during 2002 and had no evidence of having been deployed from October 1, 2001 through the individual’s period of observation... The second reference group consisted of US service members who were identified as having been deployed for a minimum of 1 month during 2002" Exclusion criteria: "Both reference groups were restricted to individuals who had no evidence of having received a prescription for mefloquine or chloroquine or a doxycycline prescription for more than 14 tablets.’ ‘Individuals who could not be followed a minimum of 2 months were excluded from the study" Country of recruitment: USA Country of malaria exposure: various, not specified Duration of exposure to malaria: various, not specified Type of participants: military | |
| Interventions | 1. Mefloquine* 2. Non‐users of antimalarials *dosing regimen not specified | |
| Outcomes | Included in the review: 1. Adverse events; serious (any hospitalization, hospitalizations due to vertiginous syndromes, migraine, dizziness and giddiness, anxiety disorders, somatoform disorders, mood disorders, PTSD, substance use disorders, personality disorders, nystagmus or adjustment reaction) Outcomes assessed not included in the review: 2. Hospitalizations coded according to classification system: infectious/parasitic, neoplasms, endocrine, nutritional, metabolic, blood and blood‐forming organs, mental disorders, nervous system, circulatory system, respiratory system, digestive system, genitourinary system, skin and subcutaneous tissues, musculoskeletal and connective tissue, ill‐defined conditions, injury and poisoning | |
| Notes | Funding sources: "This represents report 05–05, supported by the Department of Defense, under work unit no. 60002" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Other bias | Unclear risk | 1. Counfounding: moderate BMI, destination and duration of travel have not been recorded 2. Selection of participants into the study: serious "Follow‐up time began on return from deployment for mefloquine‐prescribed members, and for the deployed reference group, on assignment to Europe or Japan, or January 1, 2002, whichever occurred last for the Europe/Japan reference group" Start of follow up began a long time after start of intervention 3. Measurement of interventions: serious Surrogate measure used for mefloquine exposure. There was a possiblity that some participants in the second deployed reference group took mefloquine 4. Departures from intended interventions: moderate "Both reference groups were restricted to individuals who had no evidence of having received a prescription for mefloquine or chloroquine or a doxycycline prescription for more than 14 tablets" 5. Missing data: moderate "Individuals who could not be followed a minimum of 2 months were excluded from the study" Comment: number of participants in this group not reported 6. Measurement of outcomes: low The outcome measure (hospitalizations) was objective 7. Selection of the reported results: low All prespecified outcomes were reported 8. Other: low Government funding |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Cohort study. C ompared mefloquine with a regimen that is no longer used routinely | |
| Cohort study. R eported on efficacy but no other relevant outcomes | |
| Single arm cohort study | |
| No relevant outcomes reported | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| Cohort study. C ompared mefloquine with a regimen that is no longer used routinely | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| This trial evaluated chemoprophylaxis plus sporozoite immunization | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Cohort study. R eported on efficacy but no other relevant outcomes | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| No relevant outcomes reported | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Cohort study. C ompared mefloquine with a regimen that is no longer used routinely | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Single‐arm cohort study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Cohort study. C ompared mefloquine with a regimen that is no longer used routinely | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| RCT. C ompared mefloquine with a regimen that is no longer used routinely | |
| Cohort study. C ompared mefloquine with a regimen that is no longer used routinely | |
| No relevant outcomes reported | |
| Cohort study. Compare d mefloquine with a regimen that is no longer used routinely | |
| Cohort study. Compare d mefloquine with a regimen that is no longer used routinely | |
| Single arm cohort study | |
| No relevant outcomes reported | |
| Single arm cohort study | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| Single arm cohort study | |
| Single arm cohort study | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Mefloquine not used at a prophylactic dose (e.g. treatment dose or i ntermittent preventive treatment of malaria in pregnancy dose) | |
| Mefloquine was used as a combination regimen with sulph adoxine and pyrimethamine | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| Cohort study. A llocation to study drug was based on the occurrence of adverse effects | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Single arm cohort study | |
| Single arm cohort study | |
| Single arm cohort study | |
| Single arm cohort study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Cohort study. C ompared mefloquine with a regimen that is no longer used routinely. C hloroquine users we re not clearly separated from users of chloroquine‐proguanil | |
| No relevant outcomes reported | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Cohort study. R eported on efficacy but no other relevant outcomes | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| No relevant outcomes reported | |
| Single arm cohort study | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Cohort study. R eported on efficacy but no other relevant outcomes | |
| No relevant outcomes reported | |
| Single arm cohort study | |
| Single arm cohort study | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| Single arm cohort study | |
| Cohort selected on basis of adverse events | |
| No relevant outcomes reported | |
| RCT. C ompared mefloquine with a regimen which is not used routinely | |
| No relevant outcomes reported | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| RCT. Did not include a comparator; compared alternate mefloquine doses | |
| Mefloquine not used at a prophylactic dose (e.g. treatment dose or i ntermittent preventive treatment of malaria in pregnancy dose) | |
| Cohort study. Compared mefloquine with a regimen that is no longer used routinely | |
| Single arm cohort study | |
| Single arm cohort study | |
| Cohort study. Compared mefloquine with a regimen that is no longer used routinely | |
| Cohort study. Compared mefloquine with a regimen that is no longer used routinely | |
| Single arm cohort study | |
| Single arm cohort study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Cohort study. Compared mefloquine with a regimen that is no longer used routinely | |
| No relevant outcomes reported | |
| Mefloquine not used at a prophylactic dose (e.g. treatment dose or i ntermittent preventive treatment of malaria in pregnancy dose) | |
| Cohort selected on basis of adverse events | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| RCT. C ompared mefloquine with a regimen that is no longer use d routinely | |
| Mefloquine not used at a prophylactic dose (e.g. treatment dose or i ntermittent preventive treatment of malaria in pregnancy dose) | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a randomiz ed or cohort study e.g. case report or case control study | |
| Cohort selected on basis of adverse events | |
| RCT. C ompared mefloquine with a regimen that is no longer used routinely | |
| Mefloquine not used at a prophylactic dose (e.g. treatment dose or i ntermittent preventive treatment of malaria in pregnancy dose) | |
| No relevant outcomes reported | |
| Single arm cohort study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Single arm cohort study | |
| Single arm cohort study | |
| Mefloquine not used at a prophylactic dose (e.g. treatment dose or i ntermittent preventive treatment of malaria in pregnancy dose) | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| No relevant outcomes reported | |
| No participants received mefloquine prophylaxis | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Single arm cohort study | |
| Single arm cohort study | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Field study in which troops switched extensively between mefloquine and doxycycline. Unable to attribute side effects to either prophylactic regimen | |
| Cohort selected on basis of adverse events | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Mefloquine not used at a prophylactic dose (e.g. treatment dose or i ntermittent preventive treatment of malaria in pregnancy dose) | |
| Not a research study of malaria prophylaxis e.g. letter to the editor or editorial | |
| Cohort study. C ompared mefloquine with a regimen that is no longer used routinely |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria Show forest plot | 9 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.04, 0.19] |
| Analysis 1.1  Comparison 1 Mefloquine versus placebo/non users, Outcome 1 Clinical cases of malaria. | ||||
| 2 Malaria; episodes of parasitaemia in semi‐immune populations Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Mefloquine versus placebo/non users, Outcome 2 Malaria; episodes of parasitaemia in semi‐immune populations. | ||||
| 2.1 Trials reporting number of participants with parasitaemia | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.06, 0.55] |
| 2.2 Trials reporting number of episodes of parasitaemia | 2 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 5.25] |
| 3 Serious adverse events or effects (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Mefloquine versus placebo/non users, Outcome 3 Serious adverse events or effects (all studies). | ||||
| 3.1 RCTs (adverse events) | 6 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.53] |
| 3.2 Cohort studies (adverse effects) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.39, 24.11] |
| 4 Discontinuations due to adverse effects (all studies) Show forest plot | 7 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.55, 4.88] |
| Analysis 1.4  Comparison 1 Mefloquine versus placebo/non users, Outcome 4 Discontinuations due to adverse effects (all studies). | ||||
| 4.1 RCTs (adverse effects) | 7 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.55, 4.88] |
| 5 Nausea (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Mefloquine versus placebo/non users, Outcome 5 Nausea (all studies). | ||||
| 5.1 RCTs (adverse events) | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.05, 1.73] |
| 5.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.42, 2.43] |
| 6 Vomiting (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Mefloquine versus placebo/non users, Outcome 6 Vomiting (all studies). | ||||
| 6.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.19] |
| 6.2 Cohort studies (adverse events) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.21] |
| 7 Abdominal pain (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Mefloquine versus placebo/non users, Outcome 7 Abdominal pain (all studies). | ||||
| 7.1 RCTs (adverse events) | 3 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.40] |
| 7.2 Cohort studies (adverse events) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.42] |
| 8 Diarrhoea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Mefloquine versus placebo/non users, Outcome 8 Diarrhoea (all studies). | ||||
| 8.1 RCTs (adverse events) | 4 | 589 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.32, 1.62] |
| 8.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.68] |
| 9 Headache (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Mefloquine versus placebo/non users, Outcome 9 Headache (all studies). | ||||
| 9.1 RCTs (adverse events) | 5 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.71, 0.99] |
| 9.2 Cohort studies (adverse events) | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.63, 4.26] |
| 10 Dizziness (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Mefloquine versus placebo/non users, Outcome 10 Dizziness (all studies). | ||||
| 10.1 RCTs (adverse events) | 3 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 10.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.29, 2.49] |
| 11 Abnormal dreams (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Mefloquine versus placebo/non users, Outcome 11 Abnormal dreams (all studies). | ||||
| 11.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.15, 4.80] |
| 12 Insomnia (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Mefloquine versus placebo/non users, Outcome 12 Insomnia (all studies). | ||||
| 12.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.06, 2.02] |
| 13 Anxiety (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 Mefloquine versus placebo/non users, Outcome 13 Anxiety (all studies). | ||||
| 13.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.67, 2.21] |
| 14 Depressed mood (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.14  Comparison 1 Mefloquine versus placebo/non users, Outcome 14 Depressed mood (all studies). | ||||
| 14.1 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [0.65, 9.07] |
| 15 Abnormal thoughts and perceptions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Mefloquine versus placebo/non users, Outcome 15 Abnormal thoughts and perceptions. | ||||
| 15.1 Cohort studies (adverse events) | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.77 [0.79, 42.06] |
| 16 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.16  Comparison 1 Mefloquine versus placebo/non users, Outcome 16 Pruritis (all studies). | ||||
| 16.1 RCTs (adverse events) | 3 | 609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.24] |
| 16.2 Cohort studies (adverse events) | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.71 [1.58, 28.55] |
| 17 Visual impairment (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Mefloquine versus placebo/non users, Outcome 17 Visual impairment (all studies). | ||||
| 17.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.66, 1.46] |
| 17.2 Cohort studies (adverse events) | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.27, 3.19] |
| 18 Vertigo (all studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.18  Comparison 1 Mefloquine versus placebo/non users, Outcome 18 Vertigo (all studies). | ||||
| 18.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.34] |
| 19 Other adverse events (RCTs) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.19  Comparison 1 Mefloquine versus placebo/non users, Outcome 19 Other adverse events (RCTs). | ||||
| 19.1 Arthralgia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.02, 5.48] |
| 19.2 Back pain | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.61] |
| 19.3 Blurred vision | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.89] |
| 19.4 Cough | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.14] |
| 19.5 Constipation | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 19.6 Decreased appetite | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.28] |
| 19.7 Falls | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 19.8 Fatigue | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.14, 5.86] |
| 19.9 Gastritis | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.98] |
| 19.10 Myalgia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.36, 6.57] |
| 19.11 Rash | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 2.30] |
| 19.12 Respiratory tract infection | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.04, 6.61] |
| 19.13 Sore throat | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 2.75] |
| 19.14 Unsteadiness | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 19.15 Weakness | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.17] |
| 20 Other adverse effects (cohort studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.20  Comparison 1 Mefloquine versus placebo/non users, Outcome 20 Other adverse effects (cohort studies). | ||||
| 20.1 Agitation | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.61, 1.82] |
| 20.2 Altered spatial perception | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.4 [0.57, 153.97] |
| 20.3 Confusion | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.78] |
| 20.4 Loss of appetite | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.50] |
| 20.5 Mouth ulcers | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.39, 2.56] |
| 20.6 Palpitations | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.06 [0.44, 147.68] |
| 20.7 Tingling | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.59, 6.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 4 | 744 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.35, 5.19] |
| Analysis 2.1  Comparison 2 Mefloquine versus doxycycline, Outcome 1 Clinical cases of malaria (RCTs). | ||||
| 2 Serious adverse events or effects (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Mefloquine versus doxycycline, Outcome 2 Serious adverse events or effects (all studies). | ||||
| 2.1 RCTs (adverse events) | 3 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.16] |
| 2.2 Cohort studies (adverse effects) | 3 | 3722 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.23, 10.24] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Mefloquine versus doxycycline, Outcome 3 Discontinuations due to adverse effects (all studies). | ||||
| 3.1 RCTs | 4 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.87] |
| 3.2 Cohort studies | 10 | 10165 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.55] |
| 4 Nausea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Mefloquine versus doxycycline, Outcome 4 Nausea (all studies). | ||||
| 4.1 Cohort studies (adverse effects) | 5 | 2683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.30, 0.45] |
| 4.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.74] |
| 4.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.06, 2.43] |
| 5 Vomiting (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Mefloquine versus doxycycline, Outcome 5 Vomiting (all studies). | ||||
| 5.1 Cohort studies (adverse effects) | 4 | 5071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.12, 0.27] |
| 5.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 6 Abdominal pain (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Mefloquine versus doxycycline, Outcome 6 Abdominal pain (all studies). | ||||
| 6.1 Cohort studies (adverse effects) | 4 | 2569 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.07] |
| 6.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.74, 3.70] |
| 6.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.83, 2.18] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Mefloquine versus doxycycline, Outcome 7 Diarrhoea (all studies). | ||||
| 7.1 Cohort studies (adverse effects) | 5 | 5104 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.73] |
| 7.2 RCTs (adverse events) | 2 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.29] |
| 7.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [1.69, 7.59] |
| 8 Dyspepsia (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.8  Comparison 2 Mefloquine versus doxycycline, Outcome 8 Dyspepsia (all studies). | ||||
| 8.1 Cohort studies (adverse effects) | 5 | 5104 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.74] |
| 9 Headache (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Mefloquine versus doxycycline, Outcome 9 Headache (all studies). | ||||
| 9.1 Cohort studies (adverse effects) | 5 | 3322 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.50, 2.92] |
| 9.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.25, 4.27] |
| 9.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.38, 4.34] |
| 10 Dizziness (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.10  Comparison 2 Mefloquine versus doxycycline, Outcome 10 Dizziness (all studies). | ||||
| 10.1 Cohort studies (adverse effects) | 5 | 2633 | Risk Ratio (M‐H, Random, 95% CI) | 3.49 [0.88, 13.75] |
| 10.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.30, 7.16] |
| 10.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.47, 3.90] |
| 10.4 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.62, 0.73] |
| 11 Abnormal dreams (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.11  Comparison 2 Mefloquine versus doxycycline, Outcome 11 Abnormal dreams (all studies). | ||||
| 11.1 Cohort studies (adverse effects) | 4 | 2588 | Risk Ratio (M‐H, Random, 95% CI) | 10.49 [3.79, 29.10] |
| 11.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.07, 15.89] |
| 11.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [2.08, 9.00] |
| 12 Insomnia (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 2.12  Comparison 2 Mefloquine versus doxycycline, Outcome 12 Insomnia (all studies). | ||||
| 12.1 Cohort studies (adverse effects) | 4 | 3212 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [1.19, 14.44] |
| 12.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.65, 6.40] |
| 12.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 4.54 [2.09, 9.83] |
| 12.4 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.43, 0.49] |
| 13 Anxiety (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.13  Comparison 2 Mefloquine versus doxycycline, Outcome 13 Anxiety (all studies). | ||||
| 13.1 Cohort studies (adverse effects) | 3 | 2559 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.04 [9.32, 34.93] |
| 13.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.74 [1.99, 38.40] |
| 13.3 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.47, 0.56] |
| 14 Depressed mood (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.14  Comparison 2 Mefloquine versus doxycycline, Outcome 14 Depressed mood (all studies). | ||||
| 14.1 Cohort studies (adverse effects) | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.43 [5.21, 25.07] |
| 14.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.27 [1.82, 21.62] |
| 14.3 Retrospective healthcare record analysis (adverse events) | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.51, 0.60] |
| 15 Abnormal thoughts and perceptions Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.15  Comparison 2 Mefloquine versus doxycycline, Outcome 15 Abnormal thoughts and perceptions. | ||||
| 15.1 Cohort studies (adverse effects) | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.60 [0.92, 47.20] |
| 15.2 Retrospective healthcare record analyses (adverse events) | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.26, 0.66] |
| 16 Pruritis (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.16  Comparison 2 Mefloquine versus doxycycline, Outcome 16 Pruritis (all studies). | ||||
| 16.1 Cohort studies (adverse effects) | 2 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.91] |
| 16.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.93, 7.78] |
| 17 Photosensitivity (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.17  Comparison 2 Mefloquine versus doxycycline, Outcome 17 Photosensitivity (all studies). | ||||
| 17.1 Cohort studies (adverse effects) | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.05, 0.11] |
| 17.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.49] |
| 18 Yeast infection (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.18  Comparison 2 Mefloquine versus doxycycline, Outcome 18 Yeast infection (all studies). | ||||
| 18.1 Cohort studies (adverse effects) | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.06, 0.16] |
| 18.2 Cohort studies (adverse events) | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.63] |
| 19 Visual impairment (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.19  Comparison 2 Mefloquine versus doxycycline, Outcome 19 Visual impairment (all studies). | ||||
| 19.1 Cohort studies (adverse effects) | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.41, 3.99] |
| 20 Other adverse effects (cohort studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.20  Comparison 2 Mefloquine versus doxycycline, Outcome 20 Other adverse effects (cohort studies). | ||||
| 20.1 Alopecia | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [1.96, 6.03] |
| 20.2 Asthenia | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.89, 3.76] |
| 20.3 Balance disorder | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [1.48, 5.59] |
| 20.4 Decreased appetite | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.42, 3.64] |
| 20.5 Fatigue | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.77] |
| 20.6 Hypoaesthesia | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.48 [3.01, 43.70] |
| 20.7 Malaise | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.71] |
| 20.8 Mouth ulcers | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.02, 11.42] |
| 20.9 Palpitations | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.16, 48.91] |
| 20.10 Tinnitus | 1 | 684 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.20 [0.39, 133.30] |
| 21 Other adverse events (RCTs) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.21  Comparison 2 Mefloquine versus doxycycline, Outcome 21 Other adverse events (RCTs). | ||||
| 21.1 Constipation | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 21.2 Cough | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 1.01] |
| 21.3 Decreased appetite | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [1.24, 10.20] |
| 21.4 Malaise | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.88, 4.69] |
| 21.5 Palpitations | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 21.6 Pyrexia | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.09, 7.42] |
| 21.7 Sexual dysfunction | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.33, 28.51] |
| 21.8 Somnolence | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 22 Other adverse events (cohort studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.22  Comparison 2 Mefloquine versus doxycycline, Outcome 22 Other adverse events (cohort studies). | ||||
| 22.1 Adjustment disorder | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.40, 0.45] |
| 22.2 Confusion | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.24, 19.49] |
| 22.3 Convulsions | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 22.4 Hallucinations | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.08, 0.45] |
| 22.5 Paranoia | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.63] |
| 22.6 Palpitations | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.44 [1.73, 104.38] |
| 22.7 Panic attacks | 1 | 21065 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [0.55, 31.49] |
| 22.8 PTSD | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.53, 0.64] |
| 22.9 Rash | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.50, 2.94] |
| 22.10 Suicidal ideation | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.31, 0.47] |
| 22.11 Suicide | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.32, 4.56] |
| 22.12 Tinnitus | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.61, 0.71] |
| 23 Adherence (cohort studies) Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.23  Comparison 2 Mefloquine versus doxycycline, Outcome 23 Adherence (cohort studies). | ||||
| 23.1 Adherence during travel | 13 | 15583 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.12, 1.18] |
| 23.2 Adherence in the post‐travel period | 4 | 840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 2 | 1293 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 3.1  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 1 Clinical cases of malaria (RCTs). | ||||
| 2 Serious adverse events or effects (all studies) Show forest plot | 3 | 3591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.08, 23.22] |
| Analysis 3.2  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 2 Serious adverse events or effects (all studies). | ||||
| 2.1 Cohort studies | 3 | 3591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.08, 23.22] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 3 Discontinuations due to adverse effects (all studies). | ||||
| 3.1 RCTs | 3 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [1.53, 5.31] |
| 3.2 Cohort studies | 9 | 7785 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [1.83, 4.08] |
| 4 Nausea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 4 Nausea (all studies). | ||||
| 4.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.52, 4.86] |
| 4.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.54, 4.06] |
| 5 Vomiting (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.5  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 5 Vomiting (all studies). | ||||
| 5.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.49, 3.50] |
| 5.2 Cohort studies (adverse effects) | 3 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.08, 4.09] |
| 6 Abdominal pain (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 6 Abdominal pain (all studies). | ||||
| 6.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.56] |
| 6.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.07] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.7  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 7 Diarrhoea (all studies). | ||||
| 7.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.47] |
| 7.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| 8 Mouth ulcers (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.8  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 8 Mouth ulcers (all studies). | ||||
| 8.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.70, 3.00] |
| 8.2 Cohort studies (adverse effects) | 2 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.04, 0.37] |
| 9 Headache (all studies) Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.9  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 9 Headache (all studies). | ||||
| 9.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.99, 2.99] |
| 9.2 Cohort studies (adverse effects) | 8 | 4163 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [1.71, 6.82] |
| 10 Dizziness (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.10  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 10 Dizziness (all studies). | ||||
| 10.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.99 [2.08, 7.64] |
| 10.2 Cohort studies (adverse effects) | 8 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [2.23, 6.58] |
| 10.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.04, 1.46] |
| 11 Abnormal dreams (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.11  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 11 Abnormal dreams (all studies). | ||||
| 11.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.37, 3.04] |
| 11.2 Cohort studies (adverse effects) | 7 | 3848 | Risk Ratio (M‐H, Random, 95% CI) | 6.81 [1.65, 28.15] |
| 12 Insomnia (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.12  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 12 Insomnia (all studies). | ||||
| 12.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [2.56, 7.64] |
| 12.2 Cohort studies (adverse effects) | 8 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.29 [4.37, 12.16] |
| 12.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.44] |
| 13 Anxiety (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.13  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 13 Anxiety (all studies). | ||||
| 13.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.12 [1.82, 20.66] |
| 13.2 Cohort studies (adverse effects) | 4 | 2664 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.10 [3.48, 29.32] |
| 13.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.28, 1.85] |
| 14 Depressed mood (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.14  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 14 Depressed mood (all studies). | ||||
| 14.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.78 [1.71, 19.61] |
| 14.2 Cohort studies (adverse effects) | 6 | 3624 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.02 [3.56, 18.07] |
| 14.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.56, 2.38] |
| 15 Abnormal thoughts and perceptions (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.15  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 15 Abnormal thoughts and perceptions (all studies). | ||||
| 15.1 Cohort studies (adverse effects) | 3 | 2433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.30, 7.42] |
| 15.2 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.69, 12.97] |
| 16 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.16  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 16 Pruritis (all studies). | ||||
| 16.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.60, 2.70] |
| 16.2 Cohort studies (adverse effects) | 3 | 1824 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.40, 10.68] |
| 17 Visual impairment (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.17  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 17 Visual impairment (all studies). | ||||
| 17.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.88, 4.73] |
| 17.2 Cohort studies (adverse effects) | 2 | 1956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.29, 4.72] |
| 18 Other adverse effects (cohort studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.18  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 18 Other adverse effects (cohort studies). | ||||
| 18.1 Allergic reaction | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.04, 14.48] |
| 18.2 Alopecia | 1 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [0.30, 70.01] |
| 18.3 Asthenia | 2 | 1956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.26, 13.12] |
| 18.4 Balance disorder | 1 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.19, 44.19] |
| 18.5 Cough | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.08, 2.92] |
| 18.6 Disturbance in attention | 3 | 1363 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.45 [1.84, 10.77] |
| 18.7 Dyspepsia | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.46] |
| 18.8 Fatigue | 2 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.47, 45.56] |
| 18.9 Hypoaesthesia | 2 | 1946 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.45 [0.93, 21.26] |
| 18.10 Loss of appetite | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.43] |
| 18.11 Muscle pain | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.57 [0.45, 127.80] |
| 18.12 Palpitations | 3 | 2180 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.73, 15.26] |
| 18.13 Photosensitization | 2 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.10, 4.92] |
| 18.14 Pyrexia | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.28 [0.24, 75.57] |
| 18.15 Rash | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.09] |
| 18.16 Restlessness | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.24 [0.32, 84.52] |
| 18.17 Slight illness | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.83 [0.36, 93.84] |
| 18.18 Somnolence | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.21, 11.40] |
| 18.19 Tinnitus | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.13, 42.64] |
| 18.20 Circulatory disorders | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.38 [0.36, 114.01] |
| 19 Other adverse events (cohort studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.19  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 19 Other adverse events (cohort studies). | ||||
| 19.1 Adjustment disorder | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.02] |
| 19.2 Confusion | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.04, 25.96] |
| 19.3 Convulsions | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.79, 2.30] |
| 19.4 Hallucinations | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.79] |
| 19.5 Paranoia | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.08, 36.72] |
| 19.6 PTSD | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.93, 3.26] |
| 19.7 Suicidal ideation | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.03, 2.77] |
| 19.8 Suicide | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.06, 7.78] |
| 19.9 Tinnitus | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.21, 1.68] |
| 20 Adherence (RCTs) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.20  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 20 Adherence (RCTs). | ||||
| 20.1 van Riemsdijk 2002 | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 20.2 Overbosch 2001; during travel | 1 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 20.3 Overbosch 2001; post‐travel | 1 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.74, 0.85] |
| 21 Adherence (cohort studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 3.21  Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 21 Adherence (cohort studies). | ||||
| 21.1 During travel | 6 | 5577 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.86, 1.34] |
| 21.2 Post‐travel | 2 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 4 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.28, 0.52] |
| Analysis 4.1  Comparison 4 Mefloquine versus chloroquine, Outcome 1 Clinical cases of malaria (RCTs). | ||||
| 2 Serious adverse events or effects (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Mefloquine versus chloroquine, Outcome 2 Serious adverse events or effects (all studies). | ||||
| 2.1 RCTs | 4 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.32, 23.85] |
| 2.2 Cohort studies | 6 | 79257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.07] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3  Comparison 4 Mefloquine versus chloroquine, Outcome 3 Discontinuations due to adverse effects (all studies). | ||||
| 3.1 RCTs | 3 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.18] |
| 3.2 Cohort studies in short‐term travellers | 6 | 55397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.26] |
| 3.3 Cohort studies in longer term occupational travellers | 2 | 6085 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [2.41, 3.66] |
| 4 Nausea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Mefloquine versus chloroquine, Outcome 4 Nausea (all studies). | ||||
| 4.1 Cohort studies (adverse effects) | 6 | 58984 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.89, 1.68] |
| 4.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.79] |
| 5 Vomiting (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.5  Comparison 4 Mefloquine versus chloroquine, Outcome 5 Vomiting (all studies). | ||||
| 5.1 Cohort studies (adverse effects) | 5 | 5577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 5.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.36, 3.49] |
| 6 Abdominal pain (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 Mefloquine versus chloroquine, Outcome 6 Abdominal pain (all studies). | ||||
| 6.1 Cohort studies (adverse effects) | 4 | 5440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 6.2 RCTs (adverse events) | 2 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.37, 1.36] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.7  Comparison 4 Mefloquine versus chloroquine, Outcome 7 Diarrhoea (all studies). | ||||
| 7.1 Cohort studies (adverse effects) | 5 | 5577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 7.2 RCTs (adverse events) | 3 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.50] |
| 8 Headache (all studies) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.8  Comparison 4 Mefloquine versus chloroquine, Outcome 8 Headache (all studies). | ||||
| 8.1 Cohort studies (adverse effects) | 6 | 56998 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.34] |
| 8.2 RCTs (adverse events) | 3 | 772 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.31] |
| 9 Dizziness (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.9  Comparison 4 Mefloquine versus chloroquine, Outcome 9 Dizziness (all studies). | ||||
| 9.1 Cohort studies (adverse effects) | 5 | 58847 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.34, 1.70] |
| 9.2 RCTs (adverse events) | 2 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.46] |
| 10 Abnormal dreams (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.10  Comparison 4 Mefloquine versus chloroquine, Outcome 10 Abnormal dreams (all studies). | ||||
| 10.1 Cohort studies (adverse effects) | 4 | 2845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.10, 1.33] |
| 10.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.05, 6.95] |
| 11 Insomnia (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.11  Comparison 4 Mefloquine versus chloroquine, Outcome 11 Insomnia (all studies). | ||||
| 11.1 Cohort studies (adverse effects) | 5 | 56952 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.73, 4.51] |
| 11.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.84] |
| 12 Anxiety (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.12  Comparison 4 Mefloquine versus chloroquine, Outcome 12 Anxiety (all studies). | ||||
| 12.1 Cohort studies (adverse effects) | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.30 [4.37, 9.09] |
| 13 Depressed mood (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.13  Comparison 4 Mefloquine versus chloroquine, Outcome 13 Depressed mood (all studies). | ||||
| 13.1 Cohort studies (adverse effects) | 5 | 58855 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [1.15, 8.57] |
| 14 Abnormal thoughts and perceptions Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.14  Comparison 4 Mefloquine versus chloroquine, Outcome 14 Abnormal thoughts and perceptions. | ||||
| 14.1 Cohort studies (adverse effects) | 4 | 4831 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.49 [2.65, 11.35] |
| 15 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.15  Comparison 4 Mefloquine versus chloroquine, Outcome 15 Pruritis (all studies). | ||||
| 15.1 Cohort studies (adverse effects) | 2 | 55544 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.40] |
| 15.2 RCTs (adverse events) | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.93] |
| 16 Visual impairment (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.16  Comparison 4 Mefloquine versus chloroquine, Outcome 16 Visual impairment (all studies). | ||||
| 16.1 Cohort studies (adverse effects) | 5 | 58847 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.50, 2.44] |
| 16.2 RCTs (adverse events) | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.63] |
| 17 Vertigo (all studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.17  Comparison 4 Mefloquine versus chloroquine, Outcome 17 Vertigo (all studies). | ||||
| 17.1 Cohort studies (adverse effects) | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.05, 23.43] |
| 18 Cohort studies in travellers; prespecified adverse effects Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.18  Comparison 4 Mefloquine versus chloroquine, Outcome 18 Cohort studies in travellers; prespecified adverse effects. | ||||
| 18.1 Vertigo | 1 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.05, 23.43] |
| 18.2 Nausea | 5 | 56847 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.94, 2.13] |
| 18.3 Vomiting | 4 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.55, 1.42] |
| 18.4 Abdominal pain | 3 | 3303 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.30] |
| 18.5 Diarrhoea | 4 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.57, 2.64] |
| 18.6 Headache | 5 | 54861 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.48, 2.65] |
| 18.7 Dizziness | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.10, 2.10] |
| 18.8 Abnormal dreams | 3 | 708 | Risk Ratio (M‐H, Random, 95% CI) | 4.21 [0.57, 31.33] |
| 18.9 Insomnia | 4 | 54815 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.40, 6.10] |
| 18.10 Anxiety | 2 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.53, 29.48] |
| 18.11 Depressed mood | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.75, 8.31] |
| 18.12 Abnormal thoughts or perceptions | 3 | 2694 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [1.58, 12.40] |
| 18.13 Pruritis | 1 | 53407 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 18.14 Visual impairment | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.55, 0.79] |
| 19 Other adverse effects (cohort studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.19  Comparison 4 Mefloquine versus chloroquine, Outcome 19 Other adverse effects (cohort studies). | ||||
| 19.1 Altered spatial perception | 1 | 2032 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.55, 6.45] |
| 19.2 Alopecia | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.27, 2.25] |
| 19.3 Asthenia | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.97, 2.40] |
| 19.4 Balance disorder | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [2.15, 6.00] |
| 19.5 Confusion | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.11, 36.31] |
| 19.6 Decreased appetite | 1 | 2032 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.87, 1.57] |
| 19.7 Fatigue | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.57, 9.80] |
| 19.8 Hypoaesthesia | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 20.26 [1.23, 333.93] |
| 19.9 Irritability | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [0.28, 80.59] |
| 19.10 Mouth ulcers | 2 | 55439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.01, 1.87] |
| 19.11 Paraesthesia | 2 | 2778 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.27, 3.89] |
| 19.12 Palpitations | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.71 [0.91, 24.26] |
| 19.13 Photosensitization | 2 | 2662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.53] |
| 19.14 Restlessness | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.65, 34.46] |
| 19.15 Slight illness | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.64, 10.87] |
| 19.16 Somnolence | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [0.37, 100.36] |
| 19.17 Yeast infection | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.53, 2.49] |
| 20 Other adverse events (RCTs) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.20  Comparison 4 Mefloquine versus chloroquine, Outcome 20 Other adverse events (RCTs). | ||||
| 20.1 Abdominal distension | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.64, 15.27] |
| 20.2 Anger | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 20.3 Disturbance in attention | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.61, 16.47] |
| 20.4 Irritability | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.45, 2.64] |
| 20.5 Loss of appetite | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.35, 3.25] |
| 20.6 Malaise | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.85] |
| 20.7 Mood altered | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.29, 4.34] |
| 21 Pregnancy related outcomes (RCTs) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.21  Comparison 4 Mefloquine versus chloroquine, Outcome 21 Pregnancy related outcomes (RCTs). | ||||
| 21.1 Spontaneous abortions | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.36, 1.79] |
| 21.2 Still births | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.52] |
| 21.3 Congenital malformations | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Adherence (cohort studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.22  Comparison 4 Mefloquine versus chloroquine, Outcome 22 Adherence (cohort studies). | ||||
| 22.1 Short‐term travellers | 3 | 852 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.13] |
| 22.2 Short‐term travellers: after return | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.87] |
| 22.3 Longer‐term occupational travellers | 2 | 5777 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.80, 2.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea; effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 1 Nausea; effects. | ||||
| 1.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.52, 4.86] |
| 1.2 Cohort studies | 11 | 5973 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.78, 3.77] |
| 2 Abdominal pain; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 2 Abdominal pain; effects. | ||||
| 2.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.52, 1.56] |
| 2.2 Cohort studies | 9 | 4494 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.87] |
| 3 Diarrhoea; effects Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 3 Diarrhoea; effects. | ||||
| 3.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.60, 1.47] |
| 3.2 Cohort studies | 10 | 7648 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.28, 1.34] |
| 4 Headache; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 4 Headache; effects. | ||||
| 4.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.99, 2.99] |
| 4.2 Cohort studies | 9 | 5592 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.22, 3.93] |
| 5 Dizziness; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 5 Dizziness; effects. | ||||
| 5.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 3.99 [2.08, 7.64] |
| 5.2 Cohort studies | 9 | 4606 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [1.58, 6.35] |
| 6 Abnormal dreams; effects Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.6  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 6 Abnormal dreams; effects. | ||||
| 6.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.37, 3.04] |
| 6.2 Cohort studies | 7 | 4543 | Risk Ratio (M‐H, Random, 95% CI) | 7.30 [2.51, 21.18] |
| 7 Insomnia; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.7  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 7 Insomnia; effects. | ||||
| 7.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [2.56, 7.64] |
| 7.2 Cohort studies | 9 | 5299 | Risk Ratio (M‐H, Random, 95% CI) | 5.70 [2.83, 11.47] |
| 8 Anxiety; effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.8  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 8 Anxiety; effects. | ||||
| 8.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 6.12 [1.82, 20.66] |
| 8.2 Cohort studies | 4 | 3390 | Risk Ratio (M‐H, Random, 95% CI) | 15.26 [8.66, 26.89] |
| 9 Depressed mood; effects Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.9  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 9 Depressed mood; effects. | ||||
| 9.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 5.78 [1.71, 19.61] |
| 9.2 Cohort studies | 6 | 4236 | Risk Ratio (M‐H, Random, 95% CI) | 7.82 [3.79, 16.12] |
| 10 Abnormal thoughts or perceptions; effects Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.10  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 10 Abnormal thoughts or perceptions; effects. | ||||
| 10.1 Cohort studies | 3 | 3045 | Risk Ratio (M‐H, Random, 95% CI) | 4.20 [0.81, 21.87] |
| 11 Pruritis; effects Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.11  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 11 Pruritis; effects. | ||||
| 11.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.60, 2.70] |
| 11.2 Cohort studies | 3 | 2034 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.16, 4.76] |
| 12 Visual impairment; effects Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.12  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 12 Visual impairment; effects. | ||||
| 12.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.88, 4.73] |
| 12.2 Cohort studies | 3 | 2560 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.05, 4.02] |
| 13 Adherence; during travel Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.13  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 13 Adherence; during travel. | ||||
| 13.1 RCTs | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 13.2 Cohort studies | 11 | 12131 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.03, 1.30] |
| 14 Adherence; after return Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.14  Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 14 Adherence; after return. | ||||
| 14.1 Cohort studies | 4 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |
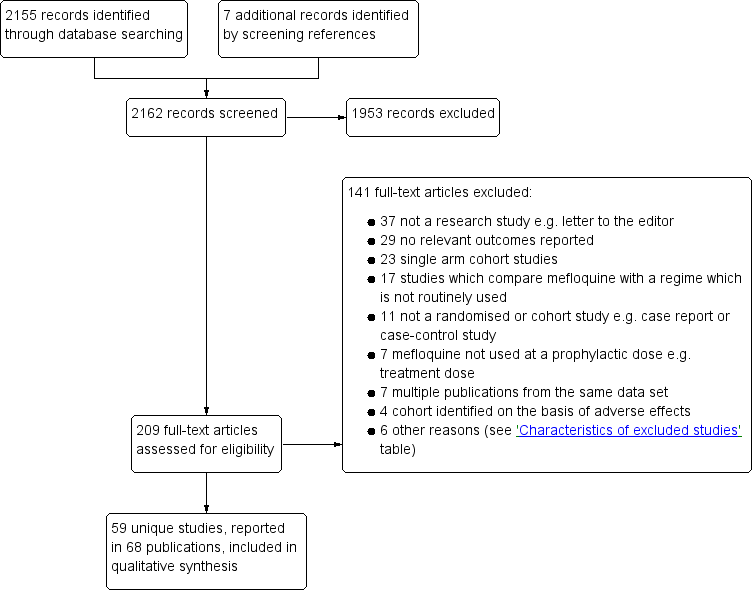
Study flow diagram.
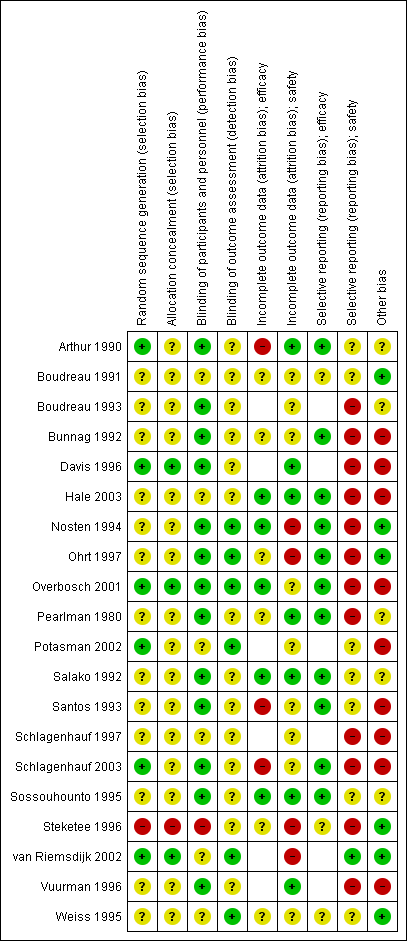
'Risk of bias' summary for RCTs: review authors' judgements about each 'Risk of bias' item for each included study.

'Risk of bias' summary in cohort studies: mefloquine versus placebo/no treatment
1Assesses whether our pre‐defined confounders were measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assess the risk of bias due to influence by a corporate study sponsor.

'Risk of bias' summary in cohort studies: mefloquine versus doxycycline
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.
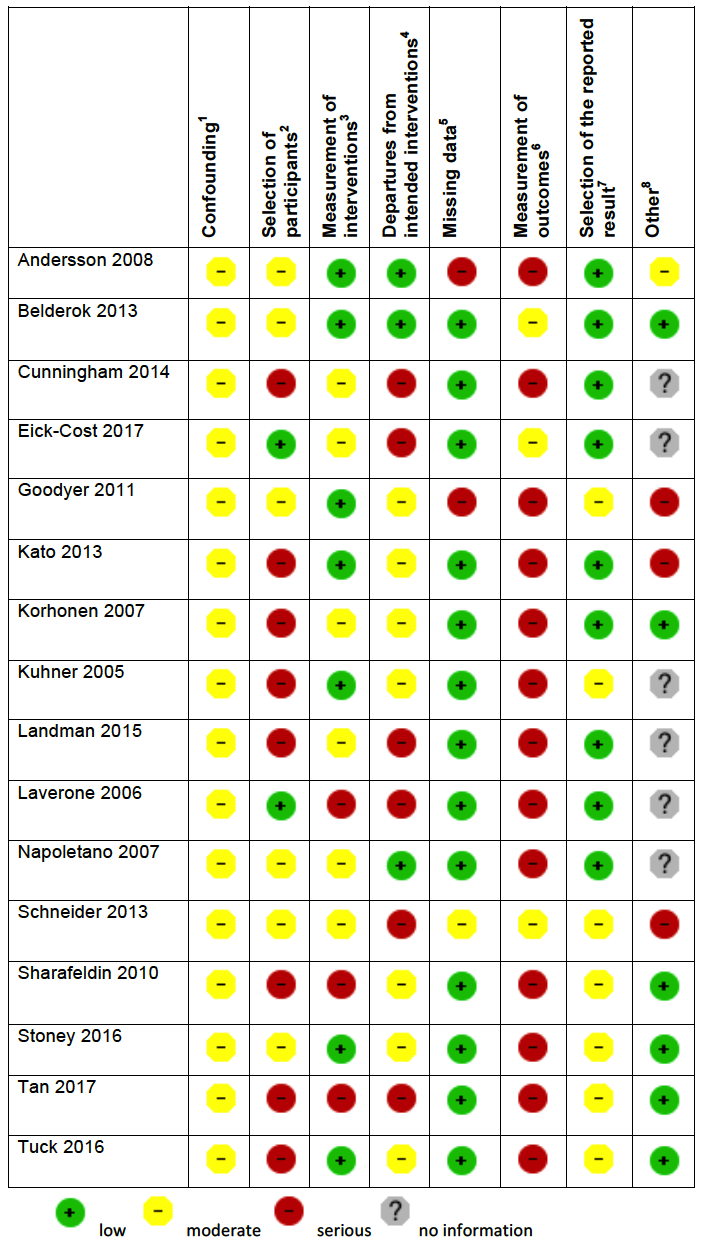
'Risk of bias' summary in cohort studies: mefloquine versus atovaquone‐proguanil
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.

'Risk of bias' summary in cohort studies: mefloquine versus chloroquine
1Assesses whether our pre‐defined confounders are measured and balanced across groups.
2Assesses the non‐response rate of prospective participants.
3Assesses the risk that participants labelled as taking mefloquine (or another antimalarial) actually took something else.
4Assesses the risk that participants whose adverse effects are attributed to mefloquine (or another antimalarial) actually took another drug as well.
5Assesses whether outcome data reasonably complete for most participants and whether intervention status reasonably complete for those in whom it was sought.
6Assesses whether the outcome measure was subjective, and whether participants and outcome assessors were blinded.
7Assesses whether it is clear that all information collected within the study has been reported.
8Assesses the risk of bias due to influence by a corporate study sponsor.
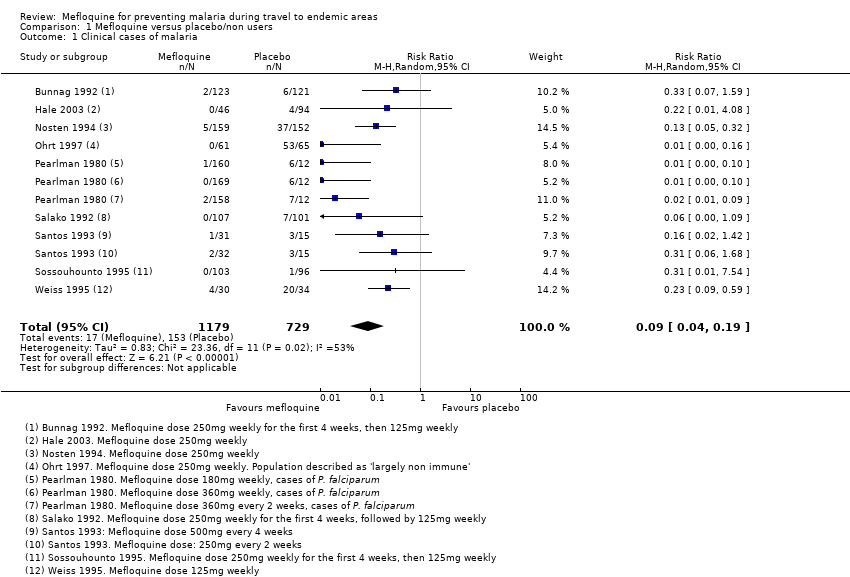
Comparison 1 Mefloquine versus placebo/non users, Outcome 1 Clinical cases of malaria.
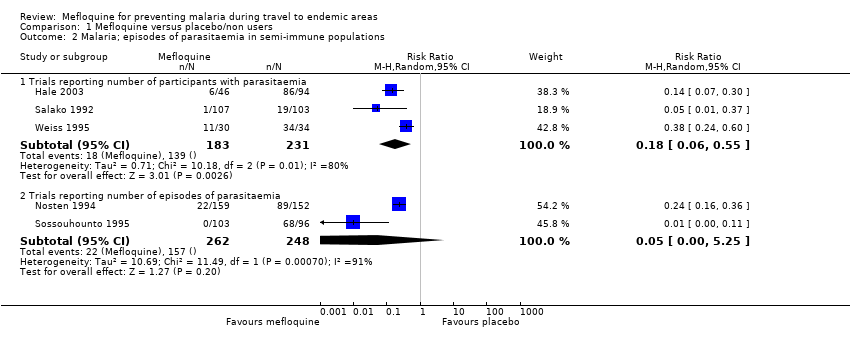
Comparison 1 Mefloquine versus placebo/non users, Outcome 2 Malaria; episodes of parasitaemia in semi‐immune populations.
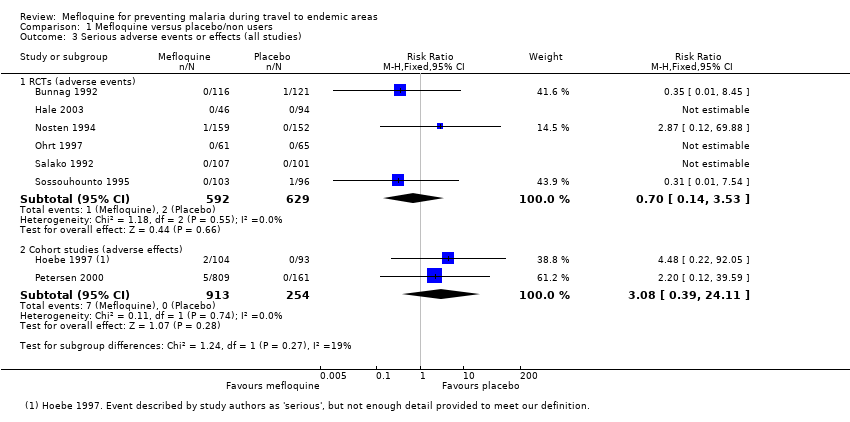
Comparison 1 Mefloquine versus placebo/non users, Outcome 3 Serious adverse events or effects (all studies).
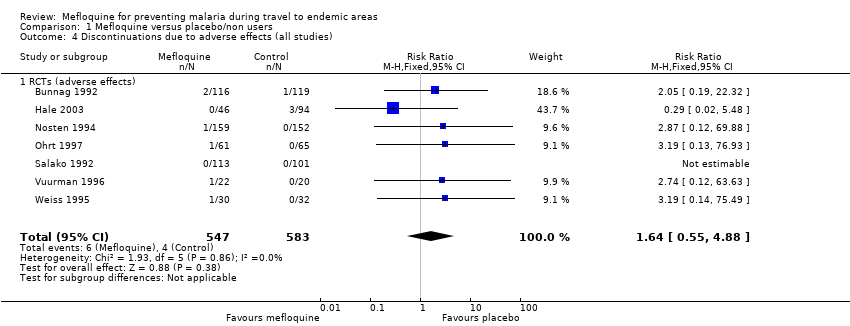
Comparison 1 Mefloquine versus placebo/non users, Outcome 4 Discontinuations due to adverse effects (all studies).
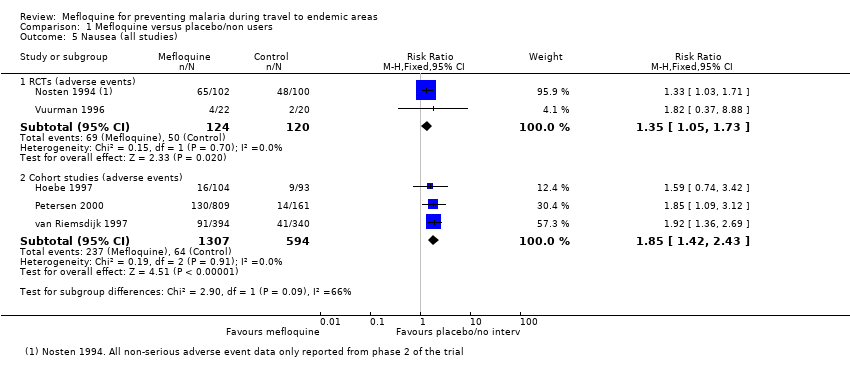
Comparison 1 Mefloquine versus placebo/non users, Outcome 5 Nausea (all studies).
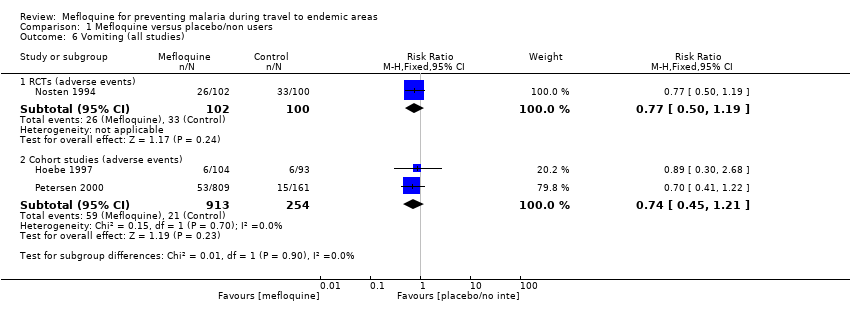
Comparison 1 Mefloquine versus placebo/non users, Outcome 6 Vomiting (all studies).
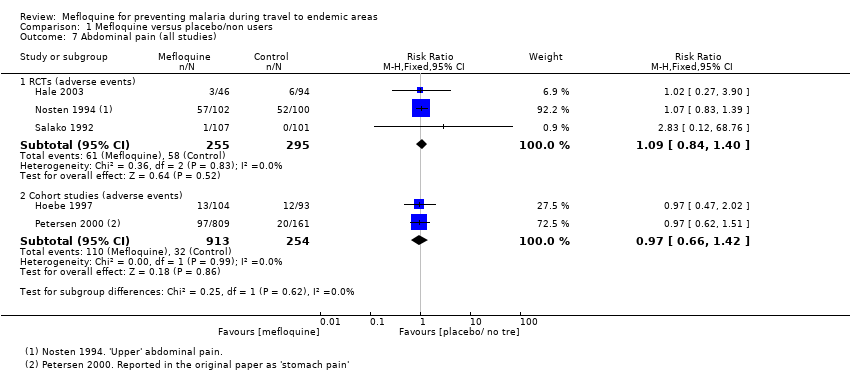
Comparison 1 Mefloquine versus placebo/non users, Outcome 7 Abdominal pain (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 8 Diarrhoea (all studies).
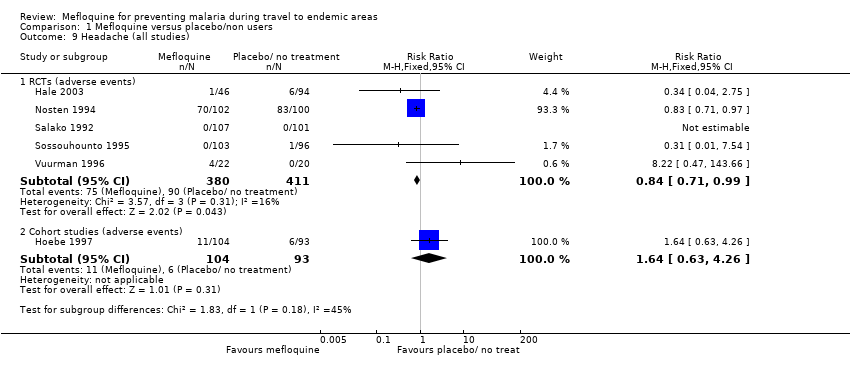
Comparison 1 Mefloquine versus placebo/non users, Outcome 9 Headache (all studies).
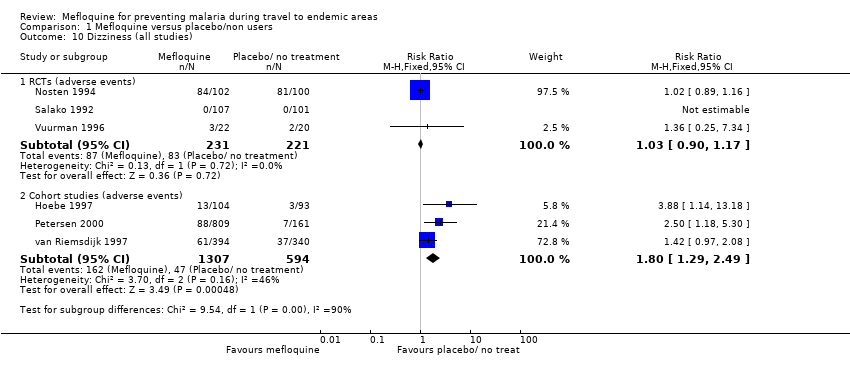
Comparison 1 Mefloquine versus placebo/non users, Outcome 10 Dizziness (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 11 Abnormal dreams (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 12 Insomnia (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 13 Anxiety (all studies).

Comparison 1 Mefloquine versus placebo/non users, Outcome 14 Depressed mood (all studies).
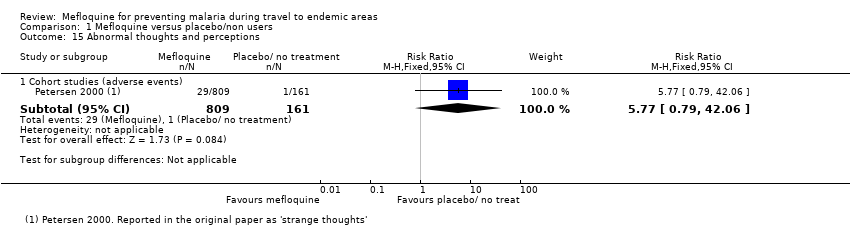
Comparison 1 Mefloquine versus placebo/non users, Outcome 15 Abnormal thoughts and perceptions.
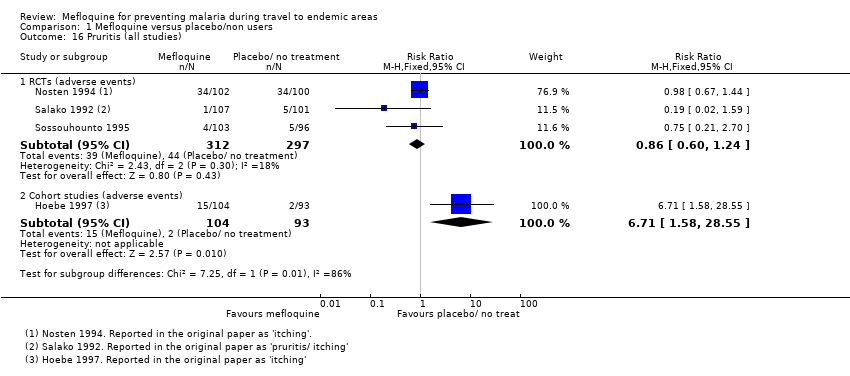
Comparison 1 Mefloquine versus placebo/non users, Outcome 16 Pruritis (all studies).
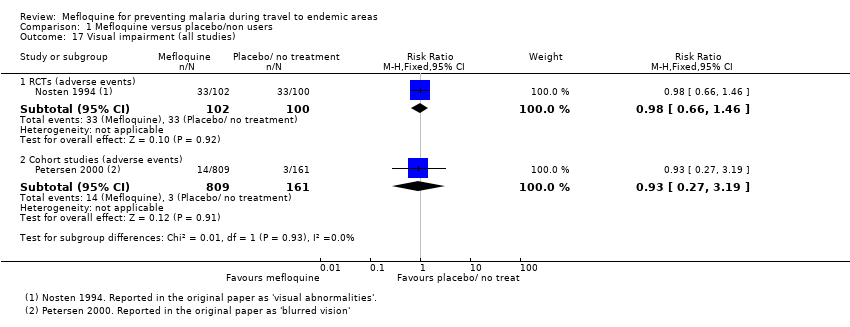
Comparison 1 Mefloquine versus placebo/non users, Outcome 17 Visual impairment (all studies).
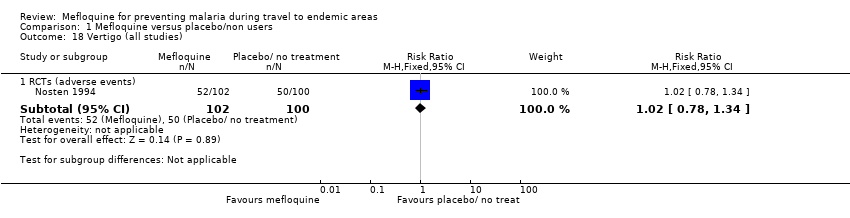
Comparison 1 Mefloquine versus placebo/non users, Outcome 18 Vertigo (all studies).
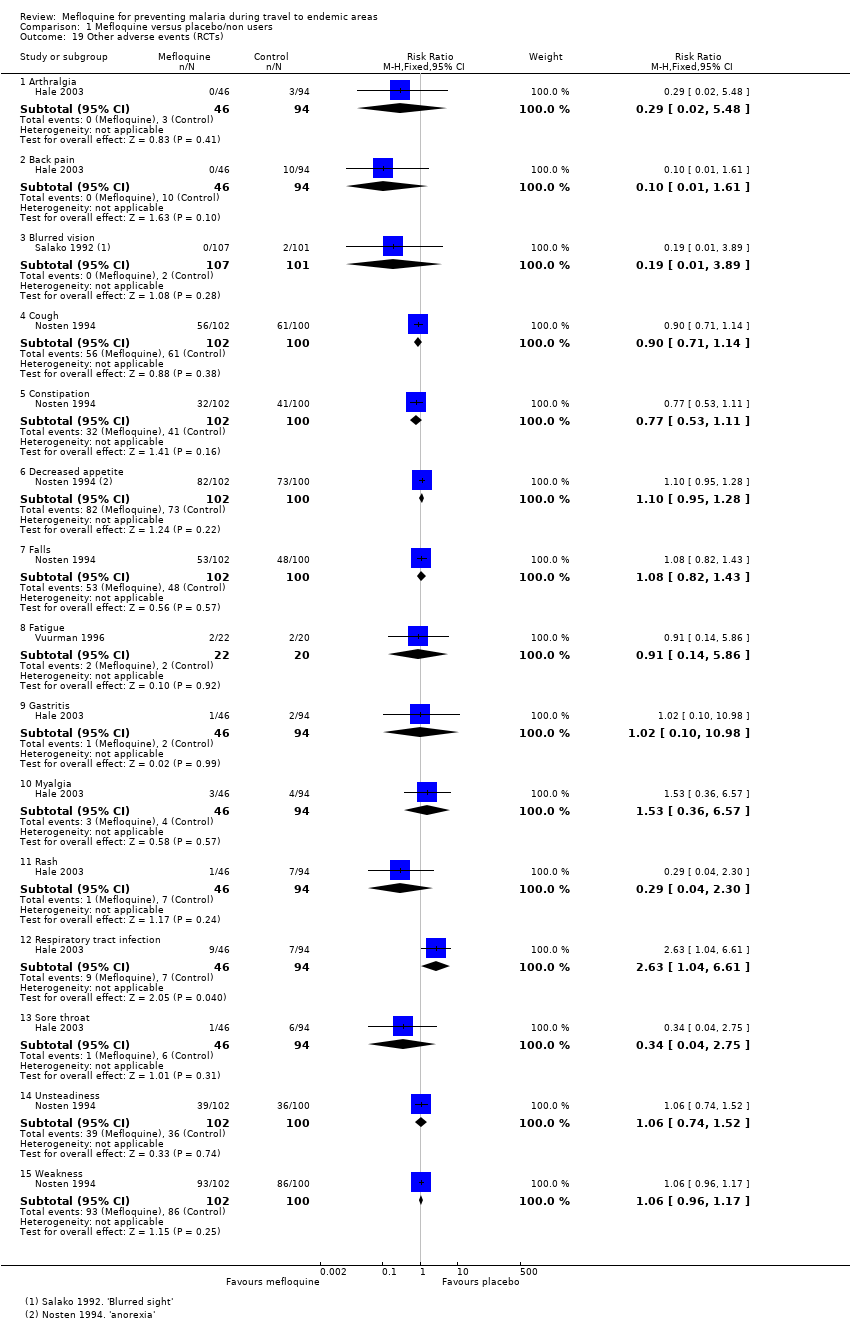
Comparison 1 Mefloquine versus placebo/non users, Outcome 19 Other adverse events (RCTs).
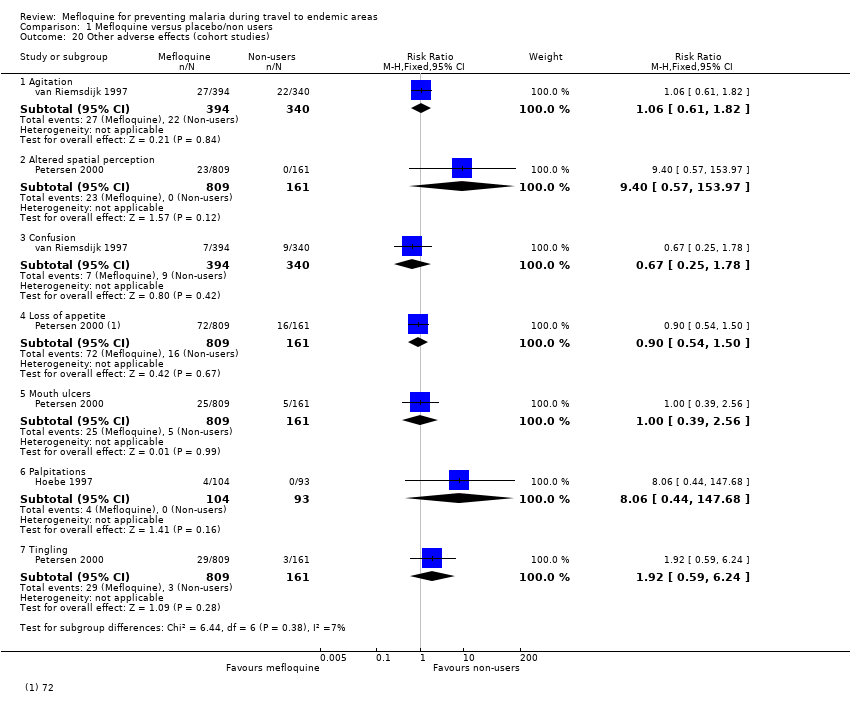
Comparison 1 Mefloquine versus placebo/non users, Outcome 20 Other adverse effects (cohort studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 1 Clinical cases of malaria (RCTs).

Comparison 2 Mefloquine versus doxycycline, Outcome 2 Serious adverse events or effects (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 3 Discontinuations due to adverse effects (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 4 Nausea (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 5 Vomiting (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 6 Abdominal pain (all studies).
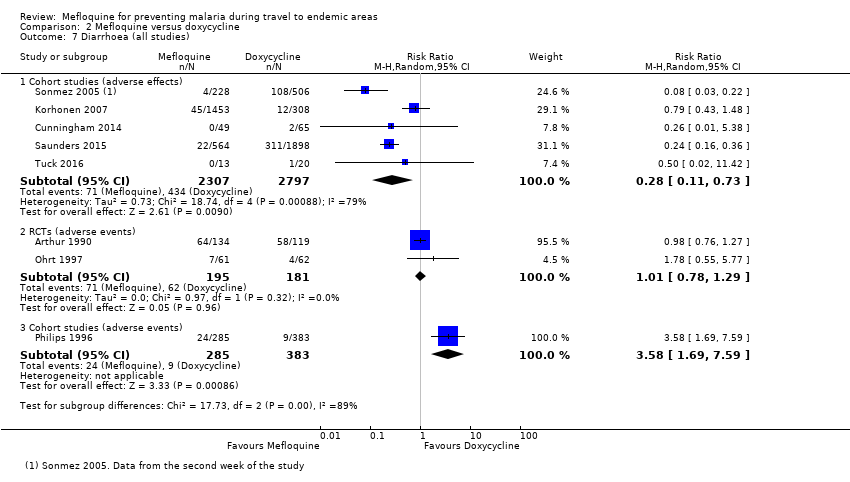
Comparison 2 Mefloquine versus doxycycline, Outcome 7 Diarrhoea (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 8 Dyspepsia (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 9 Headache (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 10 Dizziness (all studies).
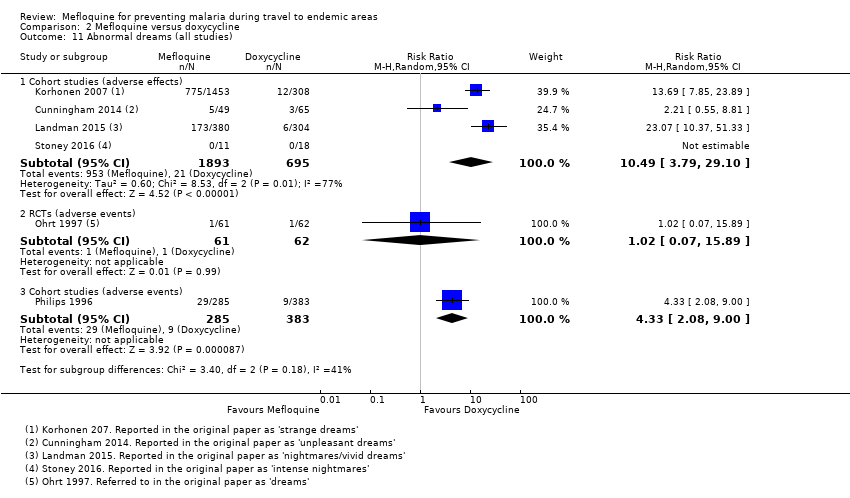
Comparison 2 Mefloquine versus doxycycline, Outcome 11 Abnormal dreams (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 12 Insomnia (all studies).
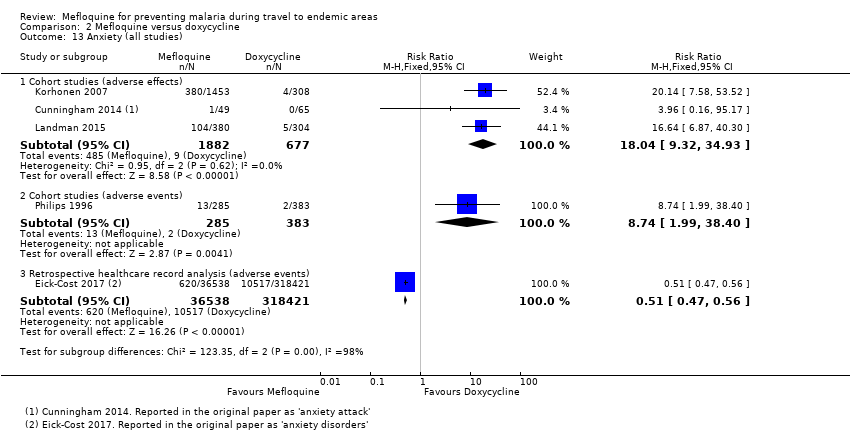
Comparison 2 Mefloquine versus doxycycline, Outcome 13 Anxiety (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 14 Depressed mood (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 15 Abnormal thoughts and perceptions.
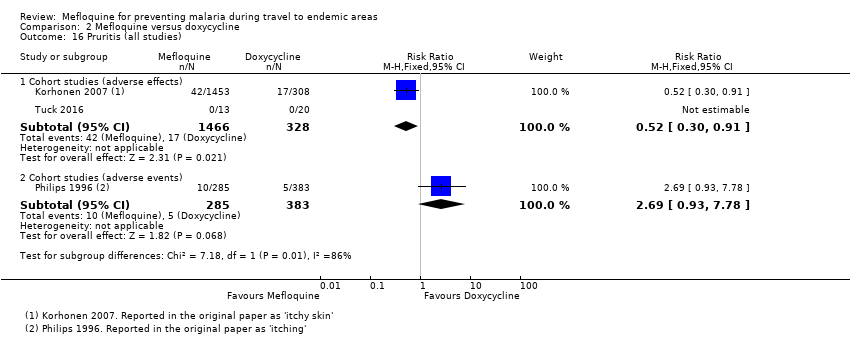
Comparison 2 Mefloquine versus doxycycline, Outcome 16 Pruritis (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 17 Photosensitivity (all studies).
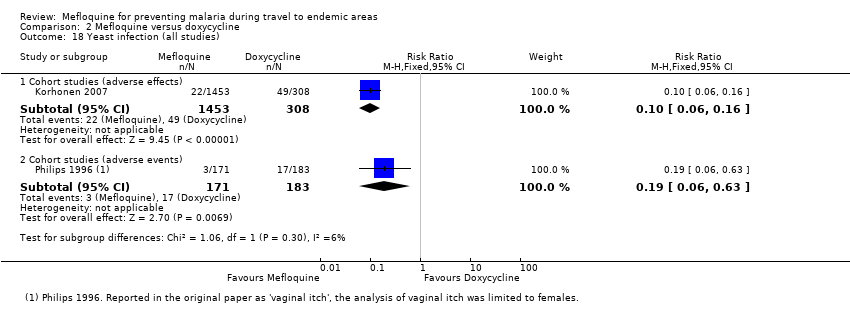
Comparison 2 Mefloquine versus doxycycline, Outcome 18 Yeast infection (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 19 Visual impairment (all studies).

Comparison 2 Mefloquine versus doxycycline, Outcome 20 Other adverse effects (cohort studies).
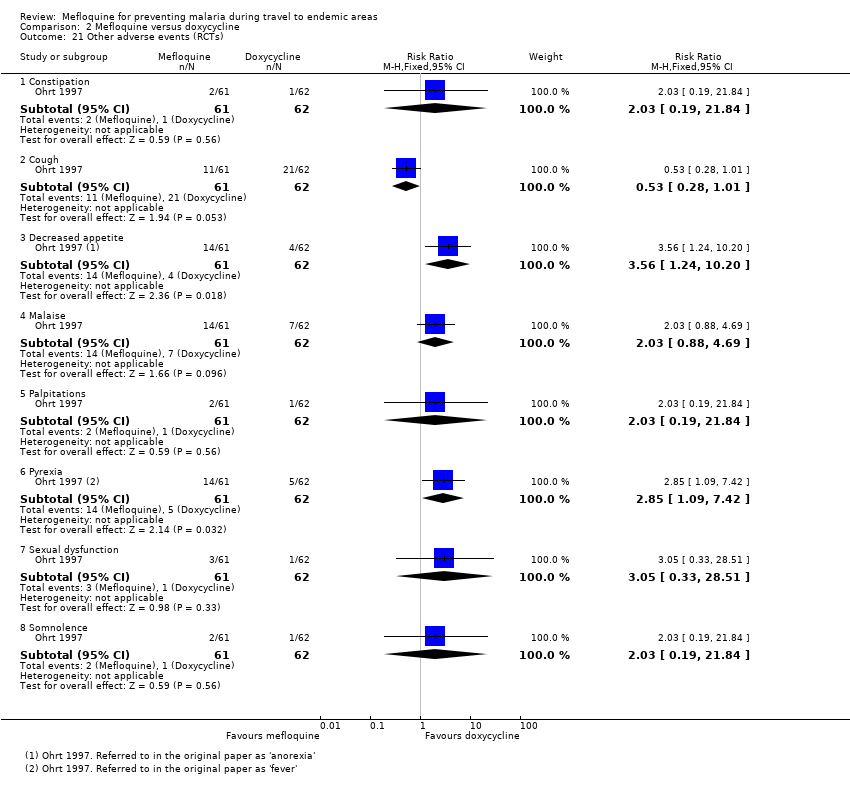
Comparison 2 Mefloquine versus doxycycline, Outcome 21 Other adverse events (RCTs).

Comparison 2 Mefloquine versus doxycycline, Outcome 22 Other adverse events (cohort studies).
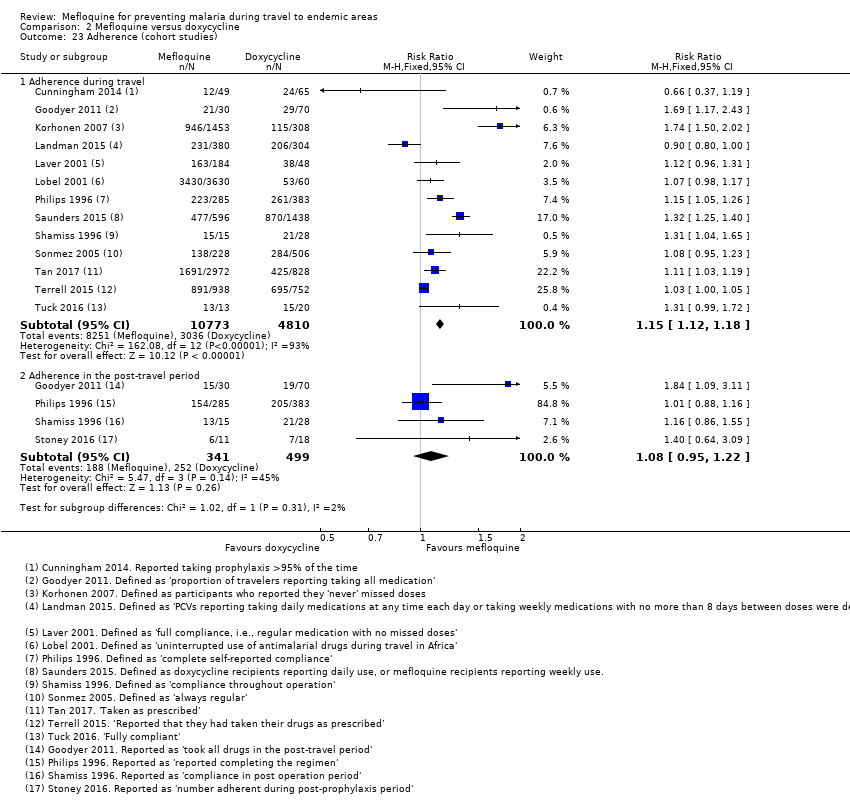
Comparison 2 Mefloquine versus doxycycline, Outcome 23 Adherence (cohort studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 1 Clinical cases of malaria (RCTs).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 2 Serious adverse events or effects (all studies).
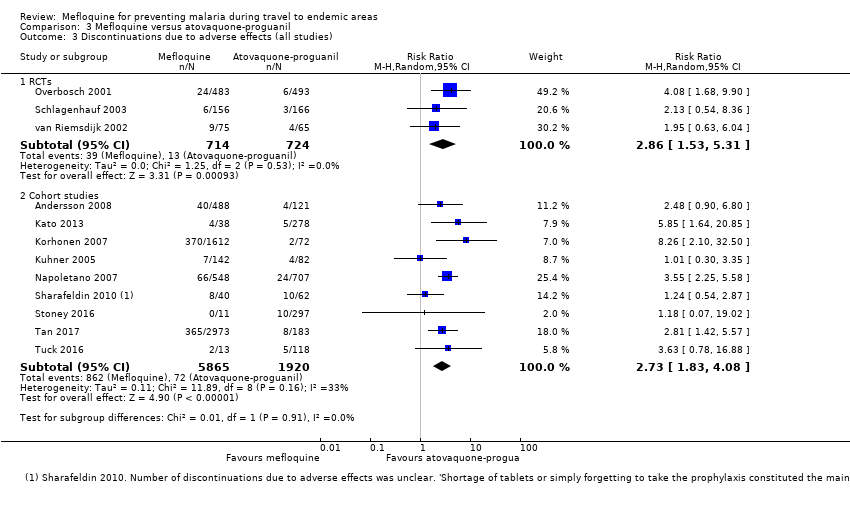
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 3 Discontinuations due to adverse effects (all studies).
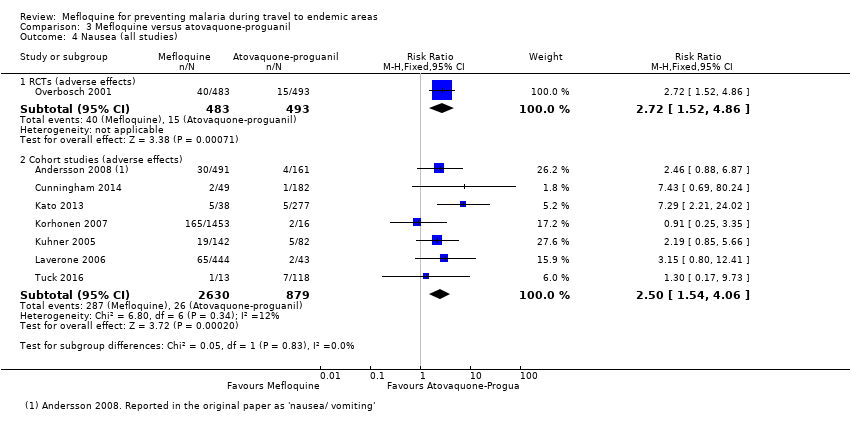
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 4 Nausea (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 5 Vomiting (all studies).
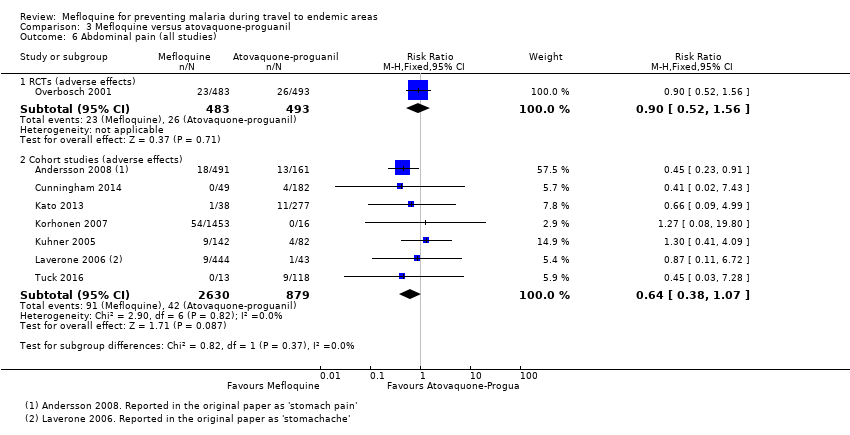
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 6 Abdominal pain (all studies).
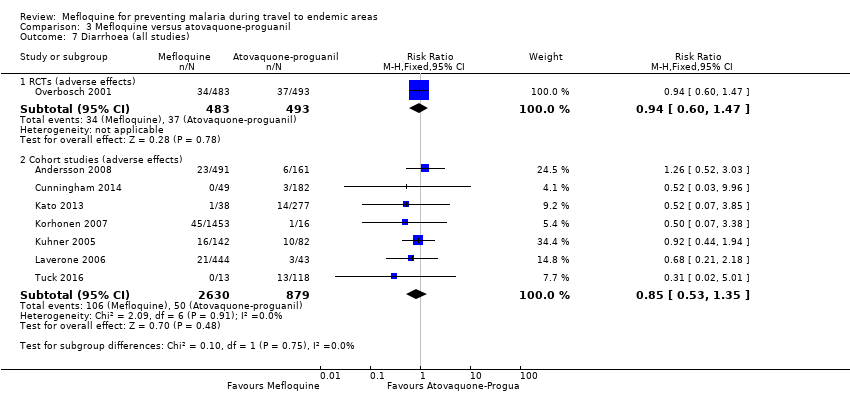
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 7 Diarrhoea (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 8 Mouth ulcers (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 9 Headache (all studies).
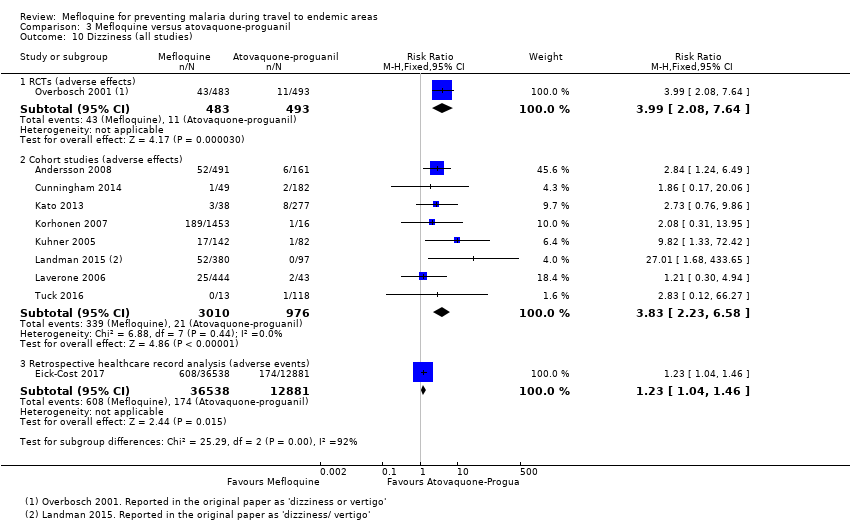
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 10 Dizziness (all studies).
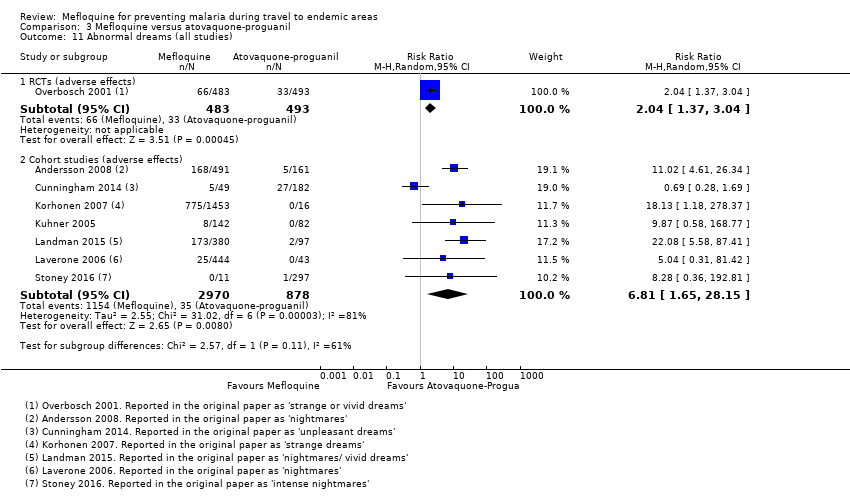
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 11 Abnormal dreams (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 12 Insomnia (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 13 Anxiety (all studies).
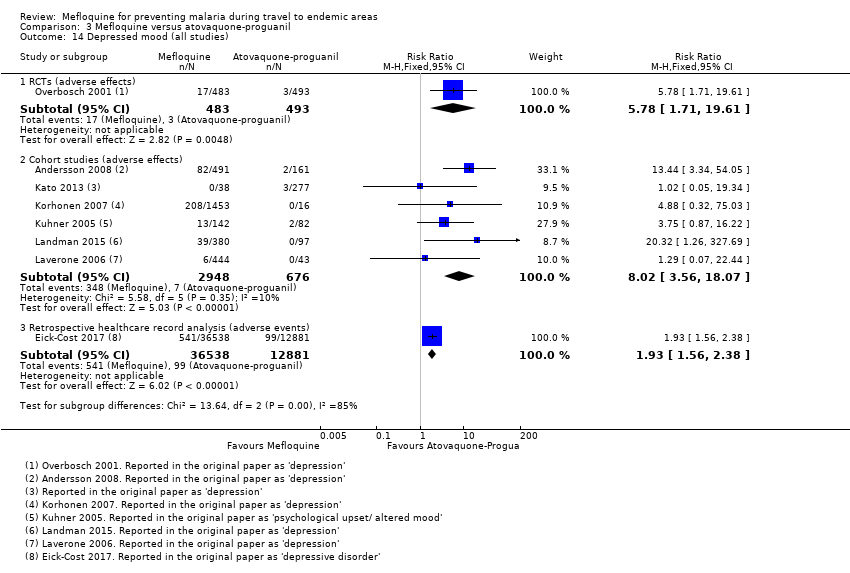
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 14 Depressed mood (all studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 15 Abnormal thoughts and perceptions (all studies).
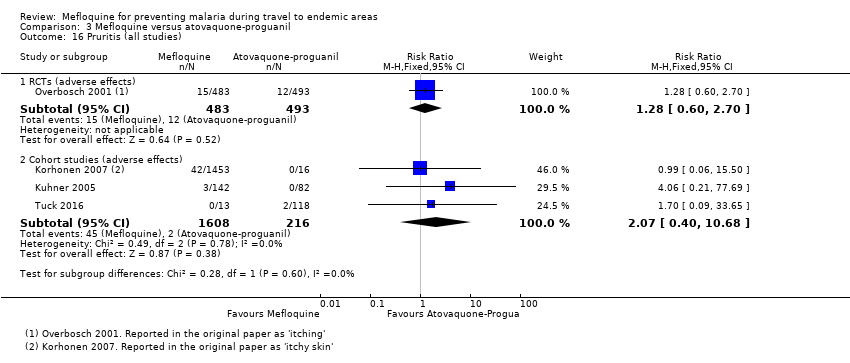
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 16 Pruritis (all studies).
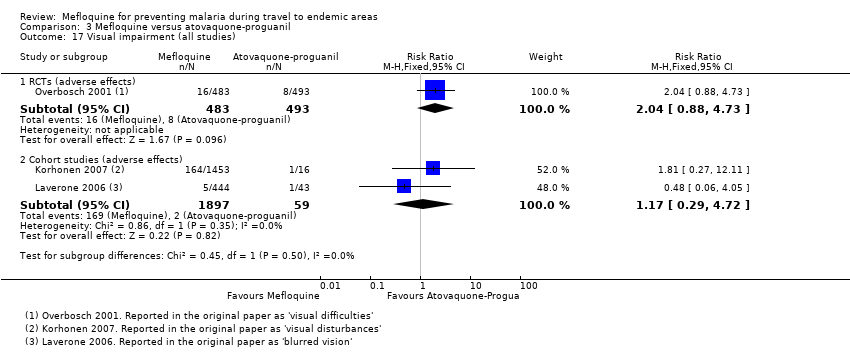
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 17 Visual impairment (all studies).
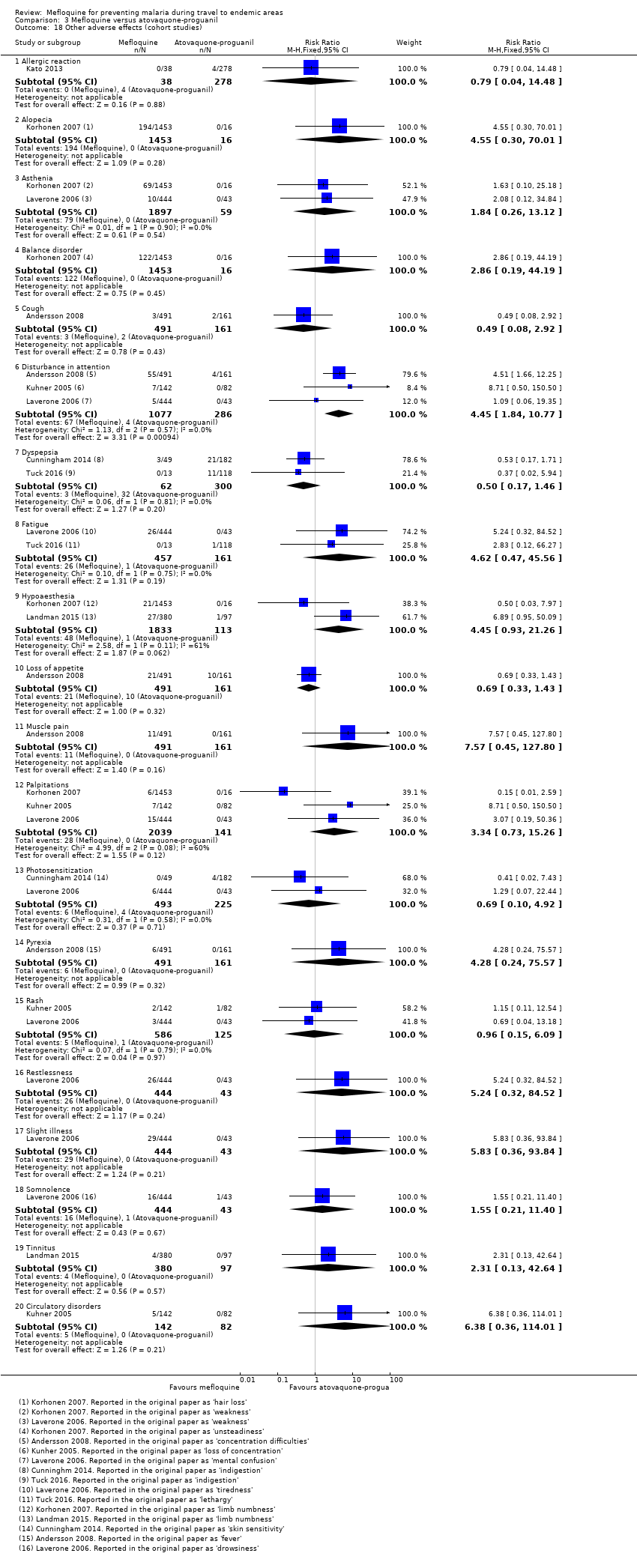
Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 18 Other adverse effects (cohort studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 19 Other adverse events (cohort studies).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 20 Adherence (RCTs).

Comparison 3 Mefloquine versus atovaquone‐proguanil, Outcome 21 Adherence (cohort studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 1 Clinical cases of malaria (RCTs).
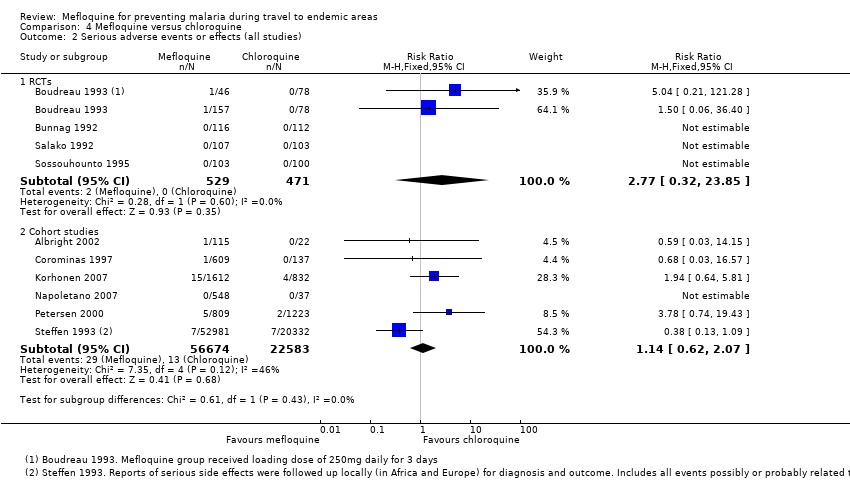
Comparison 4 Mefloquine versus chloroquine, Outcome 2 Serious adverse events or effects (all studies).
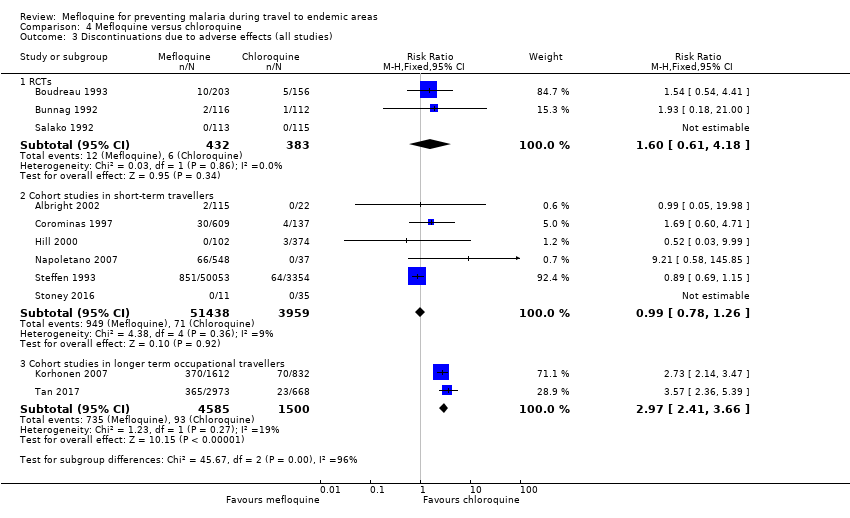
Comparison 4 Mefloquine versus chloroquine, Outcome 3 Discontinuations due to adverse effects (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 4 Nausea (all studies).
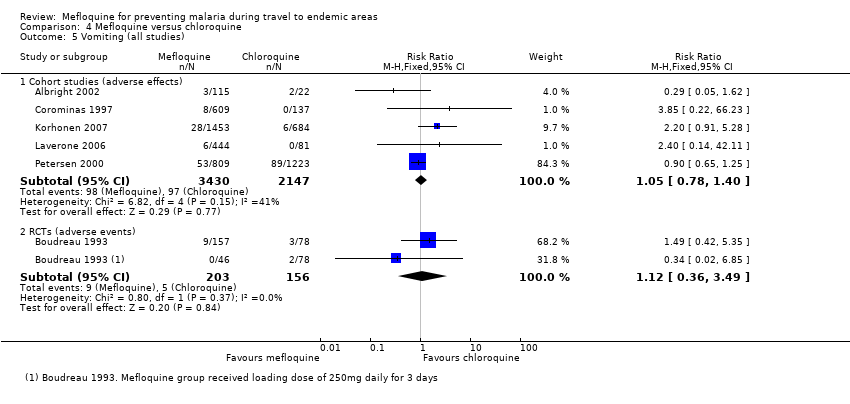
Comparison 4 Mefloquine versus chloroquine, Outcome 5 Vomiting (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 6 Abdominal pain (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 7 Diarrhoea (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 8 Headache (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 9 Dizziness (all studies).
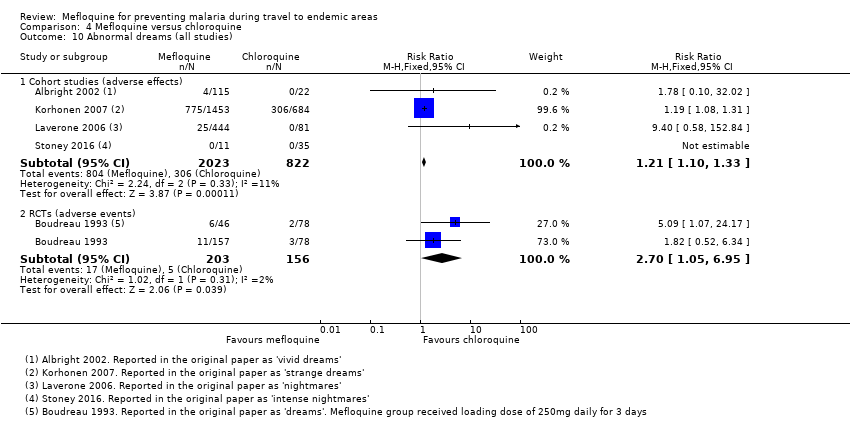
Comparison 4 Mefloquine versus chloroquine, Outcome 10 Abnormal dreams (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 11 Insomnia (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 12 Anxiety (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 13 Depressed mood (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 14 Abnormal thoughts and perceptions.
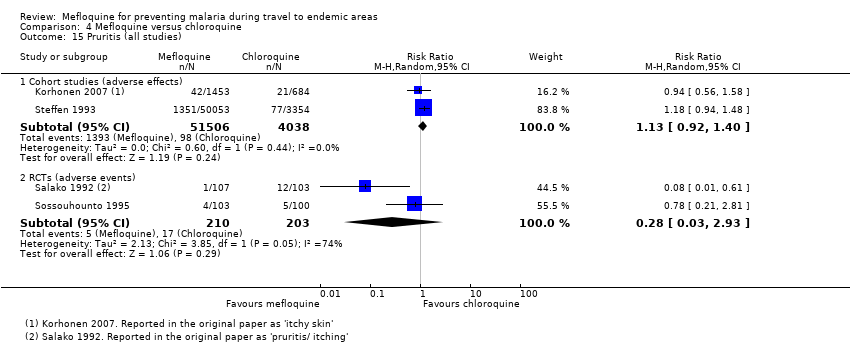
Comparison 4 Mefloquine versus chloroquine, Outcome 15 Pruritis (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 16 Visual impairment (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 17 Vertigo (all studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 18 Cohort studies in travellers; prespecified adverse effects.

Comparison 4 Mefloquine versus chloroquine, Outcome 19 Other adverse effects (cohort studies).

Comparison 4 Mefloquine versus chloroquine, Outcome 20 Other adverse events (RCTs).

Comparison 4 Mefloquine versus chloroquine, Outcome 21 Pregnancy related outcomes (RCTs).

Comparison 4 Mefloquine versus chloroquine, Outcome 22 Adherence (cohort studies).

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 1 Nausea; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 2 Abdominal pain; effects.
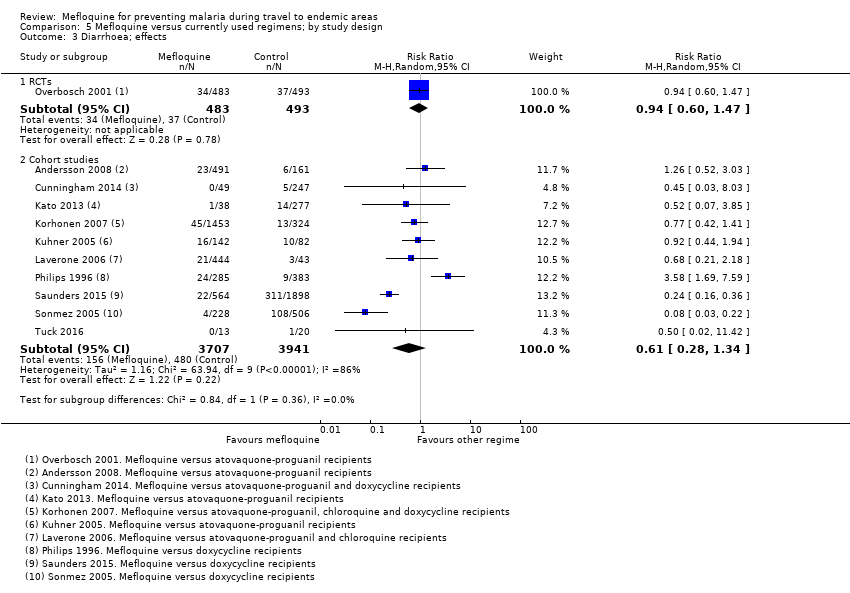
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 3 Diarrhoea; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 4 Headache; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 5 Dizziness; effects.
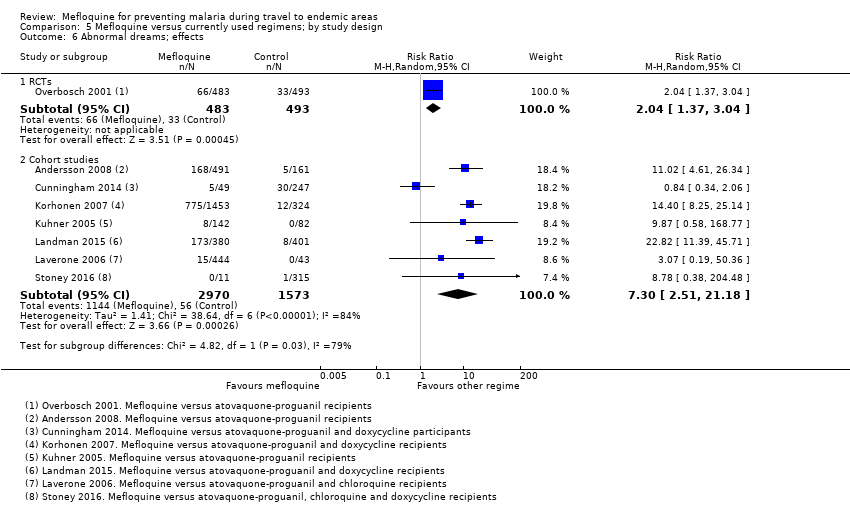
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 6 Abnormal dreams; effects.
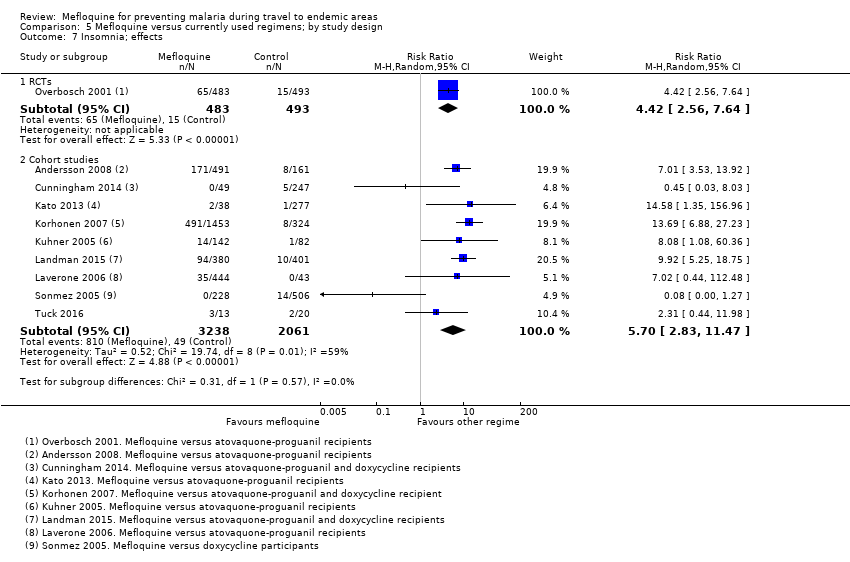
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 7 Insomnia; effects.
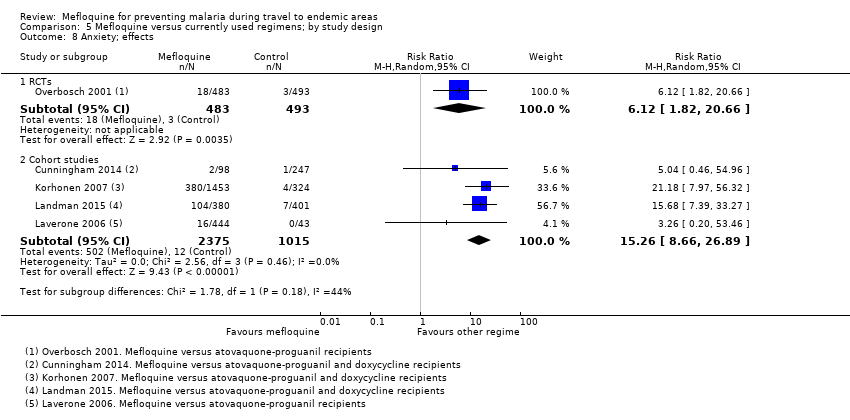
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 8 Anxiety; effects.
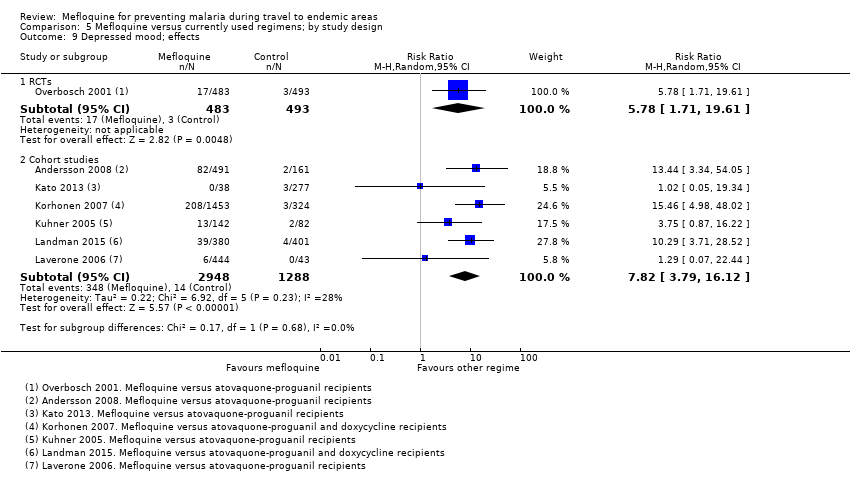
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 9 Depressed mood; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 10 Abnormal thoughts or perceptions; effects.
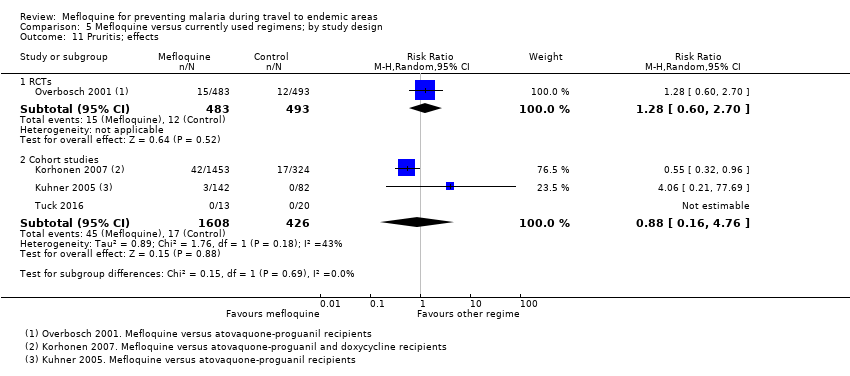
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 11 Pruritis; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 12 Visual impairment; effects.

Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 13 Adherence; during travel.
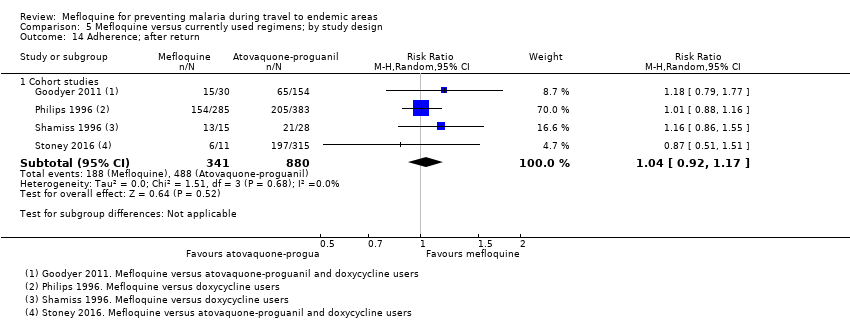
Comparison 5 Mefloquine versus currently used regimens; by study design, Outcome 14 Adherence; after return.
| Mefloquine compared with atovaquone‐proguanil for preventing malaria in travellers | ||||||
| Population: non‐immune adults and children travelling to or living in malaria‐endemic settings Intervention: mefloquine 250 mg weekly Comparison: atovaquone‐proguanil (250 mg atovaquone and 100 mg proguanil hydrochloride) daily Outcome data collection: physicians performed blinded assessment of whether reported symptoms could be related to the study drug | ||||||
| Outcomes | Anticipated absolute effects* | Relative effect | Studies contributing to effect estimate | Additional studies considered in GRADE assessment | Certainty of the evidence | |
| Atovoquone‐proguanil | Mefloquine | |||||
| Clinical malaria | — | — | — | 2 RCTs (1293) | — | ⊕⊕⊝⊝ |
| Serious adverse effects | 0 per 100 | 1 in 100 (0 to 12) | RR 1.40 (0.08 to 23.22) | 4 cohort studies (3693) | 1 RCT (976) | ⊕⊕⊝⊝ |
| Discontinuation of drug due to adverse effects | 2 per 100 | 6 per 100 (3 to 11) | RR 2.86 (1.53 to 5.31) | 3 RCTs (1438) | 7 cohort studies (4498) | ⊕⊕⊕⊕ |
| Abnormal dreams | 7 per 100 | 14 per 100 (10 to 21) | RR 2.04 (1.37 to 3.04) | 1 RCT (976) | 7 cohort studies (3848) | ⊕⊕⊕⊕ |
| Insomnia | 3 per 100 | 13 per 100 (8 to 23) | RR 4.42 (2.56 to 7.64) | 1 RCT (976) | 8 cohort studies (3986) | ⊕⊕⊕⊕ |
| Anxiety | 1 per 100 | 6 per 100 (2 to 21) | RR 6.12 (1.82 to 20.66) | 1 RCT (976) | 4 cohort studies (2664) | ⊕⊕⊕⊝ |
| Depressed mood | 1 per 100 | 6 per 100 (2 to 20) | RR 5.78 (1.71 to 19.61) | 1 RCT (976) | 6 cohort studies (3624) | ⊕⊕⊕⊝ |
| Abnormal thoughts or perceptions | 0 per 100 | 1 per 100 (0 to 4) | RR 1.50 (0.30 to 7.42) | 3 cohort studies (2433) | — | ⊕⊝⊝⊝ |
| Nausea | 3 per 100 | 8 per 100 (5 to 15) | RR 2.72 (1.52 to 4.86) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊕⊕ |
| Vomiting | 1 per 100 | 1 per 100 (0 to 4) | RR 1.31 (0.49 to 3.50) | 1 RCT (976) | 3 cohort studies (2180) | ⊕⊕⊕⊝ |
| Abdominal pain | 5 per 100 | 5 per 100 (3 to 8) | RR 0.90 (0.52 to 1.56) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊝⊝ |
| Diarrhoea | 8 per 100 | 8 per 100 (5 to 12) | RR 0.94 (0.60 to 1.47) | 1 RCT (976) | 7 cohort studies (3509) | ⊕⊕⊕⊝ |
| Headache | 4 per 100 | 7 per 100 (4 to 12) | RR 1.72 (0.99 to 2.99) | 1 RCT (976) | 8 cohort studies (4163) | ⊕⊕⊕⊝ |
| Dizziness | 2 per 100 | 8 per 100 (4 to 15) | RR 3.99 (2.08 to 7.64) | 1 RCT (976) | 8 cohort studies (3986) | ⊕⊕⊕⊕ |
| Pruritis | 2 per 100 | 3 per 100 (1 to 5) | RR 1.28 (0.60 to 2.70) | 1 RCT (976) | 3 cohort studies (1824) | ⊕⊕⊕⊝ |
| Visual impairment | 2 per 100 | 4 per 100 (2 to 9) | RR 2.04 (0.88 to 4.73) | 1 RCT (976) | 2 cohort studies (1956) | ⊕⊕⊕⊝ |
| Mouth ulcers | 2 per 100 | 3 per 100 (1 to 6) | RR 1.45 (0.70 to 3.00) | 1 RCT (976) | 2 cohort studies (783) | ⊕⊕⊕⊝ |
| *The assumed risk is the median control group risk across studies unless stated in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Where the control group risk was 0, we used a value of 0.5 to calculate the corresponding risk in the intervention group. Data from cohort studies were used when data from RCTs were unavailable. 'Summary of findings' tables are usually limited to seven outcomes. For adverse effects this problematic, as there are many, and to include some and not others risks selective reporting. We have therefore included all prespecified outcomes in the table. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: the RCTs were generally at low risk of bias but two of three were sponsored by the manufacturer of one of the study drugs. All cohort studies had methodological problems which could introduce confounding or bias. However, as the GRADE approach automatically downgrades certainty by two levels for non‐randomized studies, we did not downgrade further. | ||||||
| Mefloquine compared with doxycycline for preventing malaria in travellers | ||||||
| Population: Non‐immune adults and children travelling to malaria‐endemic settings Intervention: Mefloquine 250 mg weekly Comparison: Doxycycline 100 mg daily Outcome data collection: Self‐reported symptoms experienced whilst taking prophylaxis (adverse events) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Studies contributing to effect estimate | Additional studies considered in GRADE assessment | Certainty of the evidence | |
| Doxycycline | Mefloquine | |||||
| Clinical malaria | 1 per 100 | 1 per 100 (0 to 5) | RR 1.35 | 4 RCTs (744) | — | ⊕⊕⊝⊝ low1,2,3,4 |
| Serious adverse effects | 6 per 10005 | 9 per 1000 (1 to 61) | RR 1.53 (0.23 to 10.24) | 3 cohort studies (3722) | 3 RCTs, 1 cohort study (682; 3772) | ⊕⊝⊝⊝ |
| Discontinuations due to adverse effects | 2 per 100 | 2 per 100 (1 to 6) | RR 1.08 (0.41 to 2.87) | 4 RCTs (763) | 10 cohort studies (10,165) | ⊕⊕⊝⊝ low1,3,7,8 |
| Abnormal dreams | 3 per 100 | 31 per 100 (11 to 87) | RR 10.49 (3.79 to 29.10) | 4 cohort studies (2588) | 1 RCT, 1 cohort study (123; 688) | ⊕⊝⊝⊝ very low2,6,9,10 |
| Insomnia | 3 per 100 | 12 per 100 (4 to 43) | RR 4.14 (1.19 to 14.44) | 4 cohort studies (3212) | 1 RCT, 2 cohort studies (123; 355,627) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Anxiety | 1 per 100 | 18 per 100 (9 to 35) | RR 18.04 (9.32 to 34.93) | 3 cohort studies (2559) | 2 cohort studies (355,627) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Depressed mood | 1 per 100 | 11 per 100 (5 to 25) | RR 11.43 (5.21 to 25.07) | 2 cohort studies (2445) | 3 cohort studies (430,006) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Abnormal thoughts or perceptions | 0 per 100 | 3 per 100 (0 to 24) | RR 6.60 (0.92 to 47.20) | 2 cohort studies (2445) | 2 cohort studies (376,024) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Nausea | 8 per 100 | 3 per 100 (2 to 4) | RR 0.37 (0.30 to 0.45) | 5 cohort studies (2683) | 1 RCT, 1 cohort study (123; 668) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Vomiting | 5 per 100 | 1 per 100 (1 to 1) | RR 0.18 (0.12 to 0.27) | 4 cohort studies (5071) | 1 RCT (123) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Abdominal pain | 15 per 100 | 5 per 100 (1 to 16) | RR 0.30 (0.09 to 1.07) | 3 cohort studies (2536) | 1 RCT, 1 cohort (123; 668) | ⊕⊝⊝⊝ very low6,7,9,11 |
| Diarrhoea | 5 per 100 | 1 per 100 (1 to 4) | RR 0.28 (0.11 to 0.73) | 5 cohort studies (5104) | 2 RCTs; 1 cohort study (376; 668) | ⊕⊝⊝⊝ very low3,6,10,11 |
| Dyspepsia | 14 per 100 | 4 per 100 (1 to 10) | RR 0.26 (0.09 to 0.74) | 5 cohort studies (5104) | — | ⊕⊝⊝⊝ low2,3,6,10 |
| Headache | 2 per 100 | 2 per 100 (1 to 6) | RR 1.21 (0.50 to 2.92) | 5 cohort studies (3320) | 1 RCT, 1 cohort study (123; 688) | ⊕⊝⊝⊝ very low3,6,7,11 |
| Dizziness | 1 per 100 | 3 per 100 (1 to 14) | RR 3.49 (0.88 to 13.75) | 5 cohort studies (2633) | 1 RCT, 2 cohort studies (123; 355,627) | ⊕⊝⊝⊝ very low3,6,7,11 |
| Visual impairment | 3 per 100 | 7 per 100 (4 to 12) | RR 2.37 (1.41 to 3.99) | 2 cohort studies (1875) | — | ⊕⊝⊝⊝ very low2,6,7,9 |
| Pruritis | 3 per 100 | 2 per 100 (1 to 3) | RR 0.52 (0.30 to 0.91) | 2 cohort studies (1794) | 1 cohort study (688) | ⊕⊝⊝⊝ very low6,9,10,11 |
| Photosensitivity | 19 per 100 | 2 per 100 (1 to 2) | RR 0.08 (0.05 to 0.11) | 2 cohort studies (1875) | 1 cohort study (688) | ⊕⊝⊝⊝ very low2,6,9,10 |
| Vaginal thrush | 16 per 100 | 2 per 100 (1 to 3) | RR 0.10 (0.06 to 0.16) | 1 cohort study (1761) | 1 cohort study (354) | ⊕⊝⊝⊝ very low2,6,9,10 |
| *The assumed risk is the median control group risk across cohort studies unless stated in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Where the control group risk was 0, we used a value of 0.5 to calculate the corresponding risk in the intervention group. Where no RCTs including short‐term travellers reported on our prespecified adverse outcomes, we included information from cohort studies as our primary analysis. 'Summary of findings' tables are usually limited to seven outcomes. For adverse effects this problematic, as there are many, and to include some and not others risks selective reporting. We have therefore included all prespecified outcomes in the table. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No serious risk of bias: none of the RCTs adequately described methods of random sequence generation or allocation concealment, However, given that so few events occurred in these trials, it is unlikely to have introduced bias. | ||||||
| Bias | Authors' judgement | Support for judgement |
| Confounding | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: identified confounders were measured and were balanced across groups (age, sex, destination and duration of travel) Moderate risk: identified confounders were measured and not balanced across groups, or several confounders had not been measured or not reported across groups Serious risk: a critical confounder has been measured and is not balanced across groups |
| Selection of participants into the study | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether selection into the study was unrelated to intervention or unrelated to outcome, and whether start of intervention and start of follow up coincided for most subjects. Non‐responder bias at the point of selection was considered here for cohort studies. We used the following cut offs for non‐response rate: low risk < 10%, moderate risk 10% to 20%, serious risk > 20%. |
| Measurement of interventions | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: the prescription was provided by a travel clinic which also performed the study, and discontinuations were recorded and reported, or all participants were issued with their medication e.g. soldiers or participants were asked to self‐report which medication they took whilst they were taking it. Moderate risk: the prescription was provided by a travel clinic which also performed the study but no information regarding switches and discontinuations was available or patients are asked to self‐report which prophylaxis they took shortly after they finished taking it. Serious risk: Participants were asked to self‐report which prophylaxis they took a long time after they finished taking it. |
| Departures from intended interventions | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether switches between interventions of interest were available. We assessed whether discontinuations and switches between prophylactic regimens had been recorded and reported. |
| Missing data | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether outcome data was reasonably complete for most participants. We recorded missing data for included participants e.g. loss to follow up rates and treatment withdrawals. |
| Measurement of outcomes | Low risk Moderate risk Serious risk Critical risk No information | We assessed whether the outcome measure was objective or subjective. We assessed whether participants or study personnel were blinded to the intervention received. We assessed whether the methods of outcome assessment were comparable across intervention groups. |
| Selection of the reported result | Low risk Moderate risk Serious risk Critical risk No information | We used the following criteria: Low risk: If the questionnaire was provided in full, or it was clear what was asked within it. Moderate risk: If it is unclear which questions are asked, or information was provided on aggregate. Serious risk: If data captured within the questionnaire was clearly missing. |
| Other | Low risk Moderate risk Serious risk Critical risk No information | We reported the study sponsor. We classified the analysis of studies sponsored by pharmaceutical companies as independent of the sponsor when it was clearly stated that the sponsor had no input to the trial analysis. |
| Adapted from Higgins 2011 and ACROBAT‐NSRI tool | ||
| Criterion | Assessment | Explanation |
| On conduct | ||
| Were harms pre‐defined using standardised or precise definitions? | Adequate Inadequate Unclear | We classified as 'adequate' if the study reported explicit definitions for adverse events and effects that allow for reproducible ascertainment e.g. what adverse events were being investigated and what constituted an “event”, what was defined as a serious or severe adverse event. |
| Was ascertainment technique adequately described? | Adequate Inadequate Unclear | We classified as 'adequate' if the study reported methods used to ascertain complications, including who ascertained, timing, and methods used. |
| Was monitoring active or passive? | Active Passive Unclear | We classified monitoring as 'active' when authors reviewed participants at set time points during treatment and enquired about symptoms. |
| Was data collection prospective or retrospective? | Prospective Retrospective Unclear | We classified as ‘prospective’ if data collection occurred during treatment, or ‘retrospective’ if data collection occurred following treatment. |
| For laboratory investigations or other tests | ||
| Was the number and timing of tests adequate? | Adequate Inadequate Unclear | We classified the number and timing of tests as 'adequate', when tests were taken at baseline and at least one time point during prophylaxis. |
| Adapted from Bukiwra 2014 | ||
| Study ID | Participants (immune status) | Number of randomised participants | Mefloquine dose | Drug comparisons of interest | Duration of exposure to malaria | Country of malaria exposure | Local drug resistance |
| Thai male adults (presumed semi‐immune) | 605 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo | 24 weeks (trial duration) | Thailand | Chloroquine, sulphadoxine‐pyrimethamine and quinine resistance | |
| Pregnant women from the Thai‐Burma border (presumed semi‐immune) | 339 | 250 mg weekly for first 4 weeks, then 125 mg weekly until delivery | Placebo | Various in endemic area (monitored until delivery) | Thai‐Burma border | Not mentioned | |
| Thai residents aged 10 to 60 years (semi‐immune) | 990 | 180 mg tablet weekly, 360 mg tablet weekly, 360 mg every 2 weeks with appropriate adjustments for children | Placebo | 26 weeks | Thailand | Chloroquine resistant Plasmodium falciparum | |
| Brazilian civilians and soldiers aged 12 to 55 years (semi‐immune) | 128 | 500 mg every 4 weeks, 250mg every 2 weeks | Placebo | 17 weeks | Brazil | P falciparum resistant to chloroquine and “high prevalence of multiresistant Plasmodium falciparum transmission” | |
| Ivory Coast adult males (semi‐immune) | 500 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo | 20 weeks | Ivory C oast | Not mentioned | |
| Indonesian soldiers ('largely' non‐immune) | 204 | 250 mg weekly | Placebo, doxycycline | 'approximately 13 weeks' | Indonesia | Sulfadoxine‐pyrimethamine and chloroquine resistance | |
| Kenyan children (semi‐immune) | 169 | 125 mg weekly | Placebo (multivitamin), doxycycline, primaquine | 11 weeks | Kenya | Not mentioned | |
| Nigerian adult males (semi‐immune) | 567 | 250 mg weekly for first 4 weeks, then 125 mg weekly | Placebo, chloroquine | 24 weeks (trial duration) | Nigeria | "...at the time of the trial, chloroquine resistance was not a problem" | |
| Ghanain adults (semi‐immune) | 530 | 250 mg weekly | Placebo | 12 weeks | Ghana | Not mentioned | |
| USA soldiers (non‐immune) | 270 | 250 mg weekly | Doxycycline | 8 weeks | Thailand | Local chloroquine resistance | |
| Thai adult males (semi‐immune) | 501 | 500 mg fortnightly | Chloroquine | 14 weeks (trial duration) | Cambodia | Local chloroquine resistance | |
| Pregnant Malawian residents (semi‐immune) | 4220 | 250 mg weekly | Chloroquine | Various in endemic area (monitored until delivery) | Malawi | P falciparum resistant to chloroquine, documented sensitivity of P falciparum to mefloquine |
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Exclusions for psychiatric adverse effects | Trial duration | Source of funding |
| RCTs | ||||||
| Thai male adults | 605 | Interview with study personnel | None | 24 weeks | Roche | |
| Australian adults who did not travel | 106 | Daily self‐reported diary | Past history of psychiatric conditions | 7 weeks | Roche | |
| Ghanain adults | 530 | Interview with study personnel | History of neuropsychiatric illness | 12 weeks | USA Army | |
| Pregnant women, Thai‐Burma border | 339 | Phase 1: weekly symptom questionnaire. Babies were assessed at birth and at 3, 6, 12, and 24 months. Phase 2: weekly symptom questionnaire. Babies were assessed at birth and at 2 and 9 months | None | Various | Government funding | |
| Indonesian soldiers | 204 | Two symptom questionnaires. Daily interview with study personnel | History of underlying illness | 13 weeks | Roche, Pfizer, USA Army | |
| Thai residents aged 10 to 60 years | 990 | Weekly sick call by study personnel | None | 26 weeks | Not mentioned | |
| Israeli adults who did not travel | 90 | Self‐reporting diary | History of depression | 48 hours | Mepha Ltd | |
| Nigerian adult males | 567 | Interview with study personnel | None | 24 weeks | Not mentioned | |
| Brazilian civilians and soldiers aged 12 to 55 | 128 | Interview w ith study personnel | None | 17 weeks | Roche | |
| Swissair trainee pilots who did not travel | 23 | Interview with study personnel | Psychosis or severe depression | 4 weeks | Roche | |
| Ivory C oast adult males | 500 | Access to the village health centre | None | 20 weeks | Not mentioned | |
| Dutch adult who did not travel | 42 | Interview with study personnel | H istory of any serious psychiatric disorder; evidence of drug or alcohol abuse | 30 days | Roche | |
| Kenyan children | 169 | Interview with study personnel | None | 4 months | USA Army | |
| Cohort studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| Danish travellers | 300 | Telephone interview | Allocation based on guidelines and patient preference | Mean 3 weeks, range 1 to 9 weeks | Not mentioned | |
| Danish travellers | 4154 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | Various, not specified | Not mentioned | |
| Swedish travellers | 491 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | " Most", range 2 to 4 weeks | Not mentioned | |
| Danish travellers | 1501 | Participant self‐reported questionnaire | Allocation based on guidelines and patient preference | Mean = 23 days | Not mentioned | |
| USA soldiers | 397,442 | Restrospective analysis of hospital records | No information available | Minimum 1 month | Government funding | |
| Study ID | Description of how adverse outcomes were defined and recorded¹ | Description of ascertainment technique² | Active or passive monitoring? | Prospective or retrospective data collection? |
| Inadequate Comment: No definition of adverse events or effects was provided, it is unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate Comment: ‘serious’ adverse events were not defined, and methods for determining causality not described | Adequate | Active | Prospective | |
| Inadequate Comment: It is unclear what questions were included within the questionnaire and whether and how causality was assessed. ‘Serious’ adverse effects not defined | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Inadequate Comment: Weekly sick call for all villagers | Passive | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No information given in the methods section on definition of adverse outcomes | Inadequate Comment: No description of ascertainment method | Active | Prospective | |
| Inadequate Comment: No definition of adverse events or effects was provided, it was unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it was unclear whether or how causality was assessed | Unclear | Passive | Prospective | |
| Adequate | Unclear | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it was unclear whether or how causality was assessed. | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Unclear 'Filled in after their return' | |
| Adequate | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking their prophylactic regimen | |
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Passive | Retrospective | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? | ||||
| Study ID | Study design | Mefloquine users | Drug comparators | |||
| Events/ participants | Description | Drug | Events/ participants | Description | ||
| Events (not attributed by study authors or participants to the drug regimen) | ||||||
| RCT | 0/116 | ‐ | Placebo | 1/121 | None provided | |
| RCT | 1/159 (women) | One death
Four congenital malformations:
| Placebo | 0/152 (women) | One congenital malformation:
| |
| RCT | 0/103 | ‐ | Placebo | 1/96 | One death (not described) | |
| RCT | 0/61 | ‐ | Placebo | 0/65 | ‐ | |
| Doxycycline | 1/62 | Acute hysteria¹ | ||||
| Cohort study | 8/3703 | 8 hospitalisations
| Doxycycline | 0/69 | ‐ | |
| Chloroquine | 0/119 | ‐ | ||||
| RCT | 10/483 | "...infectious illnesses in 7 subjects and breast cancer, anaphylaxis, or fractured femur in 1 subject each" | Atovaquone‐proguanil | 4/493 | "...infectious illnesses in 3 subjects and cerebral ischemia in 1 subject" | |
| Studies reporting no serious events or effects | ||||||
| RCT | 0/107 | "Adverse events were all mild and there were no deaths" | Placebo Chloroquine | 0/101 0/103 | ‐ ‐ | |
| RCT | 0/134 | "No serious side effects occurred with either drug regimen" | Doxycycline | 0/119 | ‐ | |
| RCT | 0/153 | "Although a large number of adverse events were reported, none were serious" | Doxycycline Atovaquone‐proguanil | 0/153 0/164 | ‐ ‐ | |
| Cohort study | 0/228 | "No drug induced side effects necessitating emergency care were observed" | Doxycycline | 0/506 | ‐ | |
| Cohort study | 0/491 | "No serious adverse events were recorded" | Atovaquone‐proguanil | 0/161 | ‐ | |
| Cohort study | 0/548 | Records hospitalisations, and reports that none occurred in either group of participants | Atovaquone‐proguanil Chloroquine | 0/707 0/37 | ‐ ‐ | |
| RCT | 0/103 | "All side effects were transient (and)... mild" | Chloroquine | 0/100 | ‐ | |
| 1 This trial described a potentially serious adverse event, but did not provide enough detail to meet our definition. | ||||||
| Study ID | Study design | Mefloquine users | Drug comparators | |||
| Events/ participants | Description | Drug | Events/ participants | Description | ||
| Effects (attributed by study authors or participants to the drug regimen) | ||||||
| Cohort study | 2/104 | Two "serious acute adverse reactions"¹
| No treatment | 0/93 | ‐ | |
| Cohort study | 5/809 | 5 hospitalisations:
| Chloroquine | 6/1223 | 2 hospitalisations:
| |
| No treatment | 0/161 | ‐ | ||||
| Cohort study | 15/1612 | 15 hospitalisations:
| Doxycycline | 9/708 | 9 hospitalisations:
| |
| Atovaquone‐proguanil | 0/72 | ‐ | ||||
| Chloroquine | 4/832 | 4 hospitalisations:
| ||||
| Cohortstudy | 4/285 | 3 hospitalisations with "either gastrointestinal or neurologic symptoms" and one seizure | Doxycycline | 1/383 | Severe oesophagitis | |
| RCT | 1/? | One "neuropsychiatric side effect"
| Chloroquine | 0/? | ‐ | |
| Cohort study | 1/115 | One "serious side effect"¹
| Chloroquine | 0/22 | ‐ | |
| Cohort study | 1/609 | One hospitalisation:
| Chloroquine | 0/137 | ‐ | |
| Cohort study | 7/52981 | 7 hospitalisations, including:
| Chloroquine | 7/20332 | 7 hospitalisations. 'Includes':
| |
| Studies reporting no serious events or effects | ||||||
| RCT | 0/46 | Nine serious adverse events in the trial (trial arm not specified) "none of which were considered by study physicians to be related to the study drug" | Placebo | 0/94 | ‐ | |
| RCT | 0/107 | "Adverse events were all mild and there were no deaths" | Placebo Chloroquine | 0/101 0/103 | ‐ ‐ | |
| RCT | 0/134 | "No serious side effects occurred with either drug regimen" | Doxycycline | 0/119 | ‐ | |
| RCT | 0/153 | "Although a large number of adverse events were reported, none were serious" | Doxycycline Atovaquone‐proguanil | 0/153 0/164 | ‐ ‐ | |
| Cohort study | 0/228 | "No drug induced side effects necessitating emergency care were observed" | Doxycycline | 0/506 | ‐ | |
| Cohort study | 0/491 | "No serious adverse events were recorded" | Atovaquone‐proguanil | 0/161 | ‐ | |
| Cohort study | 0/548 | Records hospitalisations, and reports that none occurred in either group of participants | Atovaquone‐proguanil Chloroquine | 0/707 0/37 | ‐ ‐ | |
| RCT | 0/103 | "All side effects were transient (and)... mild" | Chloroquine | 0/100 | ‐ | |
| ¹ This trial described a potentially serious adverse effect, but did not provide enough detail to meet our strict definition. | ||||||
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric adverse effects | Duration of travel | Source of funding |
| Randomized controlled trials | ||||||
| USA soldiers | 270 | Blood tests, stool samples. Interview with study personnel | None | 5 weeks | Not mentioned | |
| Indonesian soldiers | 204 | Interview with study personnel. Exit questionnaire | " History of underlying illness" | 13 weeks | Pfizer and Roche | |
| Non‐immune adult short‐term travellers | 674 | Participant self‐reported questionnaire | History of seizures or psychiatric disorders | 4 to 6 weeks | GlaxoSmithKline and Roche | |
| Kenyan children | 169 | Interview with study personnel | None | 4 months | Government funding | |
| Non‐randomized studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | 0 to 36 months | Not mentioned | |
| USA s oldiers | 367,840 | Data from the Defense Medical Surveillance System, the Pharmacy Data Transaction Service and the Theater Medical Data Store | No information available | Various, not specified | Not mentioned | |
| UK adult short‐term travellers | 185 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | < 28 days | GlaxoSmithKline | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participan t preference | ≥ 6 months | Two staff employed by Peace Corps | |
| Peace Corps volunteers | 1184 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Adult short‐term travellers | 660 | Participant self‐reported questionnaire | No information available | 93% < 4 weeks | " No financial interests to disclose" | |
| Adult short‐term travellers | 5626 | Participant self‐reported questionnaire | No information available | < 5 weeks | " No financial interests to disclose" | |
| UK adults enrolled in UK g eneral p ractice research database | 35,370 | Incident cases of depression, psychoses and panic attacks within the UK general practice research database | No information available | Various, not specified | Roche | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| Australian short‐term travellers | 741 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, mean 3 weeks, maximum 3 months | Roche and Pfizer | |
| USA soldiers | 2351 | Participant self‐reported questionnaire | Primarily doxycycline, soldiers with contra‐indications received mefloquine | > 90% for 10 months or more | Not mentioned | |
| Israeli short‐term travellers | 158 | Participant self‐reported questionnaire | "... daily doxycycline or daily primaquine... was recommended" | 14 to 20 days | Not mentioned | |
| Israeli soldiers | 45 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | "... an average of 4 hours stay in the field over a period of 2 months" | Not mentioned | |
| Dutch medical students | 180 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean 74 days (range 10 to 224 days) | No dedicated funding | |
| Turkish soldiers | 1400 | Participant self‐reported questionnaire | Prior to March 2002: doxycyline After July 2002: mefloquine | A pprox. 6 months | Not mentioned | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| UK soldiers | 2032 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | "... not funded by an external body" | |
| UK soldiers | 151 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | No dedicated funding | |
| Adult short‐term travellers | 3051 | Participant self‐reported questionnaire | No information available | A pprox. 6 weeks | Not mentioned | |
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Inadequate: No definitions provided for serious side effects | Unclear: it is not reported who conducted the interviews | Active | Prospective | |
| Inadequate Comment: No definitions of adverse events or effects were provided, it wa s unclear whether or how causality was assessed | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate " Each subject was visited daily at home by an assigned field worker, who asked about symptoms of malaria or drug side effects" | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Adequate | Adequate | Passive | Prospective | |
| Inadequate " Also included on the questionnaire was a single free‐text question asking travellers to describe any side effects of antimalarial medication" | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: n o information wa s provided regarding the timing of the questionnaire during treatment | |
| Adequate | Adequate | Passive | Unclear Comment: all participants were emailed the questionnaire at one time point, which occurred at varying points during the prophylactic regimen | |
| Inadequate "Travellers… were given a questionnaire that asked for... adverse health events attributed to those drugs" | Adequate | Passive | Unclear Comment: information was collected at the airport, when travellers should still have been taking the prophylactic regimen | |
| Adequate | Adequate | Passive | Retrospective | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Inadequate Comment: it wa s unclear what constituted a serious or severe event and insufficient information on the questions that travellers were asked | Inadequate "... a mailed questionnaire approximately 2 weeks after their anticipated return home date’ ‘if a reply had not been received within 4 weeks an abbreviated questionnaire was sent out." Comment: no details provided regarding abbreviated questionnaire | Active | Retrospective | |
| Inadequate Comment: insufficient information of the questions that travellers were asked | Adequate | Passive | Retrospective | |
| Inadequate "... we directly contacted all travelers for complete follow‐up and assessment of compliance. Fifty travelers taking primaquine completed a questionnaire regarding side effects" | Inadequate Comment: see quote. Different methods of follow up for different forms of prophylaxis | Unclear | Unclear | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate " Questionnaires were distributed and collected by the flight surgeon to 45 aircrew…questionnaires were immediately evaluated and further data collection was done by telephone, if necessary" | Passive | Unclear Comment: it wa s unclear at which time point data collection occurred | |
| Inadequate Comment: n o information wa s provided on how information on adverse effects was sought | Inadequate Comment: n o mention of how adverse events were recorded in the questionnaire | Passive | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Prospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information is reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate " The questionnaire approved by the MODREC included the 19 commonest adverse effects described in the manufacturers’ product documentation" Comment: Adverse events listed in the questionnaire are not reported | Adequate | Passive | Unclear Comment: information obtained during transit through Nairobi back to the UK. It wa s unclear whether participants were still taking prophylaxis at this time point | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Unclear Comment: i t wa s not specified at which point during treatment the questionnaire was administered | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking their prophylactic regimen | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? 3. Monitoring classed as 'active' if it occurred at set time points during treatment. For full description of analysis methods, see Table 2. | ||||
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric adverse effects | Duration of travel | Source of funding |
| Randomized controlled trials | ||||||
| Travellers from Canada, Germany, Netherlands, South Africa, UK | 1013 | Interview with study personnel | "... history of alcoholism, seizures or psychiatric or severe neurological disorders" | Mean 2.5 weeks | GlaxoSmithKline | |
| Non‐immune adult short‐term travellers | 674 | Participant self‐reported questionnaire | " History of seizures or psychiatric disorders" | 4 to 6 weeks | GlaxoSmithKline and Roche | |
| Dutch short‐term travellers | 140 | Interview and testing with study personnel | "H istory of alcoholism, seizures, psychiatric disorders, severe neurological disorders" | Mean 19 days | Government funding | |
| Non‐randomis ed studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| Swedish soldiers | 609 | Participant self‐reported questionnaire | Mainly mefloquine, soldiers with contra‐indications received atovaquone‐proguanil | 6 months | Not mentioned | |
| Dutch short‐term travellers | 945 | Participant self‐reported questionnaire (measured adherence) | Allocation based on guidelines and participant preference | 84% < 29 days | Government funding | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and p articipant preference | 0‐36 months | Not mentioned | |
| USA s oldiers | 367,840 | Data from the Defense Medical Surveillance System, the Pharmacy Data Transaction Service and the Theater Medical Data Store | No information available | Various, not specified | Not mentioned | |
| UK adult short‐term travellers | 185 | Participant self‐reported questionnaire | Allocation based on guidelines and p articipant preference | < 28 days | GlaxoSmithKline | |
| Japanese short‐term travellers | 316 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean 20.0 ± 9.6 days in the atovaquone‐proguanil group and 59.0 ± 15.9 days in the mefloquine group | Not mentioned | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | ≥ 6 months | Two staff employed by Peace Corps | |
| German short‐term travellers | 495 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | A tovaquone‐proguanil mean 2.6 weeks, mefloquine mean 7 weeks | Not mentioned | |
| Peace Corps volunteers | 1184 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Italian short‐term travellers | 1176 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | > 90% 0 to 30 days | Not mentioned | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| UK adults enrolled in UK g eneral p ractice research database | Not available | Incident cases of a neuropsychiatric disorders during or after antimalarial drug use | No information available | Various, not specified | Roche | |
| Dutch medical students | 180 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Mean duration of stay 74 days (range 10 to 224 days) | " N o dedicated funding for this project" | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| UK soldiers | 151 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, not specified | No dedicated funding | |
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Inadequate Comment: insufficient information provided on the questions which soldiers were asked | Inadequate Comment: different ascertainment technique used for one of the three groups, which is inadequately described | Active | Unclear Comment: d ata collection was prospective for 448/609 participants (LA04 and LA05), but retrospective for 161 participants (LA02) | |
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Adequate | Adequate | Passive | Prospective | |
| Inadequate " Also included on the questionnaire was a single free‐text question asking travelers to describe any side effects of antimalarial medication" | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: the timing of this questionnaire has not been made clear | |
| Adequate | Adequate | Passive | Unclear Comment: n o information wa s provided regarding the timing of the questionnaire during treatment | |
| Inadequate Comment: insufficient information provided on the questions that participants were asked | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: all participants were emailed the questionnaire at one time point, which occurred at varying points during the prophylactic regimen | |
| Adequate | Adequate | Passive | Retrospective | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate Comment: n o information is provided on how information on adverse effects was sought | Inadequate Comment: n o mention of how adverse events were recorded in the questionnaire. | Passive | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information is reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Active | Unclear Comment: i t wa s not specified at which point during treatment the questionnaire was administered | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? 3. Monitoring classed as 'active' if it occurred at set time points during treatment. For full description of analysis methods, see Table 2. | ||||
| Study ID | Participants | Number enrolled | Method of adverse event monitoring | Significant exclusions for psychiatric side effects | Trial duration | Source of funding |
| RCT s | ||||||
| Thai gem miners | 501 | Interview with study personnel | None | 14 weeks | USA Army | |
| USA soldiers | 359 | Interview with study personnel and computerised questionnaire | "M edical history of psychiatric or neurological problems within the last 5 years" | 13 weeks | Not mentioned | |
| Thai adult mal es | 605 | Interview with study personnel | None | 24 weeks | Roche | |
| Nigerian adult males | 567 | Interview with study personnel | None | 24 weeks | Not mentioned | |
| Ivory C oast adult males | 500 | " Access to the village health centre. Clinical examination with study personnel" | None | 20 weeks | Not mentioned | |
| Pregnant Malawian women | 4220 | Interview with study personnel | None | Monitored from enrolment to delivery | Government funding | |
| Non‐randomised studies | ||||||
| Participants | Number enrolled | Method of adverse event monitoring | Factors influencing drug allocation | Duration of travel | Source of funding | |
| USA travelling children aged < 13 years | 177 | Interview with study personnel | Allocation based on guidelines and participant preference | Various, not specified | Not mentioned | |
| Spanish short‐term adult travellers | 1054 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Maximum 6 weeks | Not mentioned | |
| UK Foreign and Commonwealth Office staff | 327 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | 0 to 36 months | Not mentioned | |
| USA short‐term travellers | 822 | Interview with study personnel | Allocation based on guidelines and participant preference | Median 19 days, up to 90 days | Not mentioned | |
| Peace Corps volunteers | 2701 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | ≥ 6 months | Two staff employed by Peace Corps | |
| Adult short‐term travellers | 660 | Participant self‐reported questionnaire | No information available | 93% < 4 weeks | " No financial interests to disclose" | |
| Italian short‐term travellers | 1176 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | > 90% 0 to 30 days | Not mentioned | |
| Adult short‐term travellers | 5626 | Participant self‐reported questionnaire | No information available | M ost < 5 weeks | " No financial interests to disclose" | |
| Italian short‐term travellers | 1906 | Telephone interview | Allocation based on guidelines and participant preference | Mean 2 weeks, range 0 to > 35 days | Not mentioned | |
| Danish travellers | 4154 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Various, 65% < 3 weeks | Not mentioned | |
| Swedish short‐term travellers | 491 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | " Most" 2 to 4 weeks | Not mentioned | |
| Adult short‐term travellers | 145,003 | Participant self‐reported questionnaire | No information available | 98% stayed between 1 and 4 weeks | Roche | |
| USA short‐term travellers | 370 | Participant self‐reported questionnaire | Allocation based on guidelines and participant preference | Median duration 13 days | Government funding | |
| Peace Corps volunteers | 8931 | Participant self‐reported questionnaire | No information available | Various, not specified | No dedicated funding | |
| Adult short‐term travellers | 3051 | Participant self‐reported questionnaire | No information available | A pprox. 6 weeks | " not funded by an external body" | |
| Study ID | Harms predefined¹ | Description of ascertainment technique² | Active or passive monitoring?³ | Prospective or retrospective data collection? |
| RCTs | ||||
| Adequate | Adequate | Active | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Inadequate " Adverse events were defined clinically, and starting week 14, volunteers reporting adverse events were interviewed by members of the hospital team" | Adequate | Active | Prospective | |
| Inadequate " Particular attention was paid to complaints such as fever, chills, malaise, nausea and vomiting, rashes and other symptoms and signs that could be regarded as adverse events." Comment: no clear definition of adverse events wa s provided | Adequate | Active | Prospective | |
| Inadequate " Participants had access to a village health center, where they could notify personnel of any malaise or side effects" | Unclear " Clinical examinations and parasitologic tests were performed every 4 weeks" | Passive | Prospective | |
| Adequate | Adequate | Active | Prospective | |
| Cohort studies | ||||
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate Comment: insufficient information wa s provided about the questions that travellers were asked | Adequate | Active | Retrospective | |
| Inadequate Comment: questionnaire included a targeted list of side effects, including " other psychological problems" . What was included within this was not defined | Adequate | Passive | Unclear Comment: questionnaire was performed while participants were still taking chemoprophylaxis medication, although 75% were non‐compliant | |
| Inadequate Comment: insufficient information wa s provided about the questions that travellers were asked | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: No information wa s provided regarding the timing of the questionnaire during treatment | |
| Adequate | Adequate | Passive | Retrospective | |
| Inadequate "Travellers… were given a questionnaire that asked for... adverse health events attributed to those drugs" | Adequate | Passive | Unclear Comment: information was collected at the airport, when travellers should still have been taking the prophylactic regimen | |
| Unclear Comment: adverse events were categorised on a scale of one to four, but it is unclear whether and how causality was assessed | Adequate | Active | Retrospective | |
| Inadequate Comment: i t wa s unclear whether the questionnaire implied causality to the drug regimen | Adequate | Active | Retrospective | |
| Adequate | Adequate | Active | Retrospective | |
| Adequate | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking the prophylactic regimen | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Inadequate Comment: n o information wa s reported on how adverse events were ascertained | Active | Prospective | |
| Adequate | Adequate | Active | Retrospective | |
| Inadequate Comment: insufficient information provided on the questions that travellers were asked | Adequate | Passive | Unclear Comment: information was collected during the flight home, when travellers should still have been taking the prophylactic regimen | |
| 1. Were harms pre‐defined using standardised or precise definitions? 2. Was ascertainment technique adequately described? 3. Monitoring classed as 'active' if it occurred at set time points during treatment. For full description of analysis methods, see Table 2. | ||||
| Mefloquine versus atovaquone‐proguanil and doxycycline | |||
| Outcome | Short‐ term travellers¹ | Longer‐ term travellers² | Test for subgroup |
| Relative effect (RR) | Relative effect (RR) | ||
| Serious adverse effects | RR 5.38 (0.60 to 47.84) 3 cohort studies (2657) | RR 0.93 (0.43 to 2.01) 3 cohort studies (3147) | P = 0.14 |
| Discontinuations due to adverse effects (RCTs) | RR 2.64 (1.51 to 4.62) 5 RCTs (2048) | ‐ | ‐ |
| Discontinuations due to adverse effects (cohort studies) | RR 1.81 (0.86 to 3.80) 7 cohort studies (2907) | RR 1.19 (0.45 to 3.17) 4 cohort studies (5711) | P = 0.50 |
| Nausea | RR 2.02 (0.87 to 4.68) 6 cohort studies (2469) | RR 0.96 (0.22 to 4.18) 3 cohort studies (2725) | P = 0.39 |
| Abdominal pain | RR 0.66 (0.22 to 1.98) 5 cohort studies (1801) | RR 0.30 (0.22 to 0.42) 3 cohort studies (2725) | P = 0.18 |
| Diarrhoea | RR 0.64 (0.15 to 2.71) 5 cohort studies (2428) | RR 0.57 (0.22 to 1.49) 4 cohort studies (5187) | P = 0.89 |
| Headache | RR 2.39 (0.69 to 8.22) 5 cohort studies (2086) | RR 2.09 (1.10 to 3.95) 4 cohort studies (3506) | P = 0.85 |
| Dizziness | RR 3.05 (1.15 to 8.12) 4 cohort studies (1067) | RR 3.84 (1.34 to 11.00) 4 cohort studies (3506) | P = 0.76 |
| Abnormal dreams | RR 6.25 (1.16 to 33.67) 3 cohort studies (1037) | RR 7.62 (2.06 to 28.18) 4 cohort studies (3506) | P = 0.86 |
| Insomnia | RR 3.09 (0.30 to 32.21) 4 cohort studies (1760) | RR 8.67 (4.73 to 15.89) 4 cohort studies (3506) | P = 0.40 |
| Anxiety | RR 3.26 (0.20 to 53.46) 1 cohort study (487) | RR 18.05 (9.75 to 33.42) 3 cohort studies (2854) | P = 0.24 |
| Depressed mood | RR 2.52 (0.76 to 8.29) 3 cohort studies (1026) | RR 12.59 (6.47 to 24.49) 3 cohort studies (3210) | P = 0.02 |
| Abnormal thoughts and behaviours | RR 1.29 (0.07 to 22.44) 1 cohort study (487) | RR 7.78 (1.12 to 54.06) 2 cohort studies (2558) | P = 0.31 |
| Adherence: during travel | RR 1.10 (1.03 to 1.18) 7 cohort studies (7241) | RR 1.20 (0.88 to 1.62) 4 cohort studies (4890) | P = 0.61 |
| Adherence: after return | RR 1.04 (0.92 to 1.17) 4 cohort studies (1221) | ‐ | ‐ |
| 1 Short‐ term travellers: Approximately 3 weeks (range 1 day to 3 months). References: Goodyer 2011; Kato 2013; Kuhner 2005; Napoletano 2007; Laver 2001; Laverone 2006; Lobel 2001; Philips 1996; Schwartz 1999; Shamiss 1996; Sonmez 2005; Stoney 2016; Terrell 2015 | |||
| Mefloquine versus atovaquone‐proguanil and doxycycline | |||
| Outcome | Military¹ | Non‐military² | Test for subgroup |
| Relative effect (RR) | Relative effect (RR) | ||
| Serious adverse effects | 0 events in 1386 participants | RR 1.21 (0.60 to 2.44) 4 cohort studies (4418) | ‐ |
| Discontinuations due to adverse effects (RCTs) | RR 2.08 (0.13 to 32.73) 2 RCTs (441) | RR 2.22 (1.17 to 4.21) 4 RCTs (1669) | P = 0.96 |
| Discontinuations due to adverse effects (cohorts) | RR 1.24 (0.32 to 4.88) 4 cohort studies (3408) | RR 1.89 (1.35 to 2.64) 8 cohort studies (8938) | P = 0.56 |
| Nausea | RR 1.39 (0.36 to 5.36) 4 cohort studies (1578) | RR 1.70 (0.60 to 4.81) 6 cohort studies (3767) | P = 0.26 |
| Abdominal pain | RR 0.43 (0.14 to 1.29) 4 cohort studies (1578) | RR 0.56 (0.23 to 1.35) 5 cohort studies (3099) | P = 0.72 |
| Diarrhoea | RR 0.30 (0.09 to 0.96) 4 cohort studies (3999) | RR 1.05 (0.54 to 2.06) 6 cohort studies (3767) | P = 0.07 |
| Headache | RR 1.19 (0.14 to 9.79) 2 cohort studies (1386) | RR 2.48 (1.40 to 4.40) 7 cohort studies (4206) | P = 0.51 |
| Dizziness | RR 2.95 (1.37 to 6.36) 3 cohort studies (844) | RR 3.58 (1.39 to 9.25) 6 cohort studies (3880) | P = 0.76 |
| Abnormal dreams | RR 11.02 (4.61 to 26.34) 1 cohort study (652) | RR 6.59 (1.74 to 25.00) 6 cohort studies (3891) | P = 0.53 |
| Insomnia | RR 2.34 (0.41 to 13.35) 3 cohort studies (1537) | RR 10.24 (6.26 to 16.76) 6 cohort studies (3880) | P = 0.11 |
| Anxiety | ‐ | RR 16.94 (9.36 to 30.64) 4 cohort studies (3390) | ‐ |
| Depressed mood | RR 13.44 (3.34 to 54.05) 1 cohort study (652) | RR 6.49 (2.66 to 15.85) 5 cohort studies (3584) | P = 0.39 |
| Abnormal thoughts and behaviours | ‐ | RR 5.11 (1.11 to 23.53) 3 cohort studies (3045) | ‐ |
| Adherence: during travel | RR 1.18 (1.00 to 1.40) 5 cohort studies (4652) | RR 1.16 (0.99 to 1.35) 8 cohort studies (10785) | P = 0.85 |
| Adherence: after return | RR 1.16 (0.86 to 1.55) 1 cohort study (43) | RR 1.02 (0.89 to 1.16) 3 cohort studies (1178) | P = 0.44 |
| 1 Military participants: References: RCTs: Arthur 1990; Ohrt 1997. Cohort studies: Andersson 2008, Saunders 2015; Shamiss 1996; Sonmez 2005; Terrell 2015; Tuck 2016 | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria Show forest plot | 9 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.04, 0.19] |
| 2 Malaria; episodes of parasitaemia in semi‐immune populations Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Trials reporting number of participants with parasitaemia | 3 | 414 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.06, 0.55] |
| 2.2 Trials reporting number of episodes of parasitaemia | 2 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 5.25] |
| 3 Serious adverse events or effects (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 RCTs (adverse events) | 6 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.53] |
| 3.2 Cohort studies (adverse effects) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.39, 24.11] |
| 4 Discontinuations due to adverse effects (all studies) Show forest plot | 7 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.55, 4.88] |
| 4.1 RCTs (adverse effects) | 7 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.55, 4.88] |
| 5 Nausea (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 RCTs (adverse events) | 2 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.05, 1.73] |
| 5.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.42, 2.43] |
| 6 Vomiting (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.19] |
| 6.2 Cohort studies (adverse events) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.45, 1.21] |
| 7 Abdominal pain (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 RCTs (adverse events) | 3 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.40] |
| 7.2 Cohort studies (adverse events) | 2 | 1167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.66, 1.42] |
| 8 Diarrhoea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 RCTs (adverse events) | 4 | 589 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.32, 1.62] |
| 8.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.93, 1.68] |
| 9 Headache (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 RCTs (adverse events) | 5 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.71, 0.99] |
| 9.2 Cohort studies (adverse events) | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.63, 4.26] |
| 10 Dizziness (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 RCTs (adverse events) | 3 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.17] |
| 10.2 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.29, 2.49] |
| 11 Abnormal dreams (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.15, 4.80] |
| 12 Insomnia (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.06, 2.02] |
| 13 Anxiety (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Cohort studies (adverse events) | 2 | 931 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.67, 2.21] |
| 14 Depressed mood (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Cohort studies (adverse events) | 3 | 1901 | Risk Ratio (M‐H, Random, 95% CI) | 2.43 [0.65, 9.07] |
| 15 Abnormal thoughts and perceptions Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse events) | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.77 [0.79, 42.06] |
| 16 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 RCTs (adverse events) | 3 | 609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.24] |
| 16.2 Cohort studies (adverse events) | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.71 [1.58, 28.55] |
| 17 Visual impairment (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.66, 1.46] |
| 17.2 Cohort studies (adverse events) | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.27, 3.19] |
| 18 Vertigo (all studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 RCTs (adverse events) | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.34] |
| 19 Other adverse events (RCTs) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Arthralgia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.02, 5.48] |
| 19.2 Back pain | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.61] |
| 19.3 Blurred vision | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.89] |
| 19.4 Cough | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.14] |
| 19.5 Constipation | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 19.6 Decreased appetite | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.95, 1.28] |
| 19.7 Falls | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.82, 1.43] |
| 19.8 Fatigue | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.14, 5.86] |
| 19.9 Gastritis | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.10, 10.98] |
| 19.10 Myalgia | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.36, 6.57] |
| 19.11 Rash | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 2.30] |
| 19.12 Respiratory tract infection | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.04, 6.61] |
| 19.13 Sore throat | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 2.75] |
| 19.14 Unsteadiness | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.74, 1.52] |
| 19.15 Weakness | 1 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.17] |
| 20 Other adverse effects (cohort studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Agitation | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.61, 1.82] |
| 20.2 Altered spatial perception | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.4 [0.57, 153.97] |
| 20.3 Confusion | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.78] |
| 20.4 Loss of appetite | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.50] |
| 20.5 Mouth ulcers | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.39, 2.56] |
| 20.6 Palpitations | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.06 [0.44, 147.68] |
| 20.7 Tingling | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.59, 6.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 4 | 744 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.35, 5.19] |
| 2 Serious adverse events or effects (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RCTs (adverse events) | 3 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.16] |
| 2.2 Cohort studies (adverse effects) | 3 | 3722 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.23, 10.24] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RCTs | 4 | 763 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.41, 2.87] |
| 3.2 Cohort studies | 10 | 10165 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.55] |
| 4 Nausea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Cohort studies (adverse effects) | 5 | 2683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.30, 0.45] |
| 4.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.74] |
| 4.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.06, 2.43] |
| 5 Vomiting (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cohort studies (adverse effects) | 4 | 5071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.12, 0.27] |
| 5.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 6 Abdominal pain (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Cohort studies (adverse effects) | 4 | 2569 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.09, 1.07] |
| 6.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.74, 3.70] |
| 6.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.83, 2.18] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Cohort studies (adverse effects) | 5 | 5104 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.11, 0.73] |
| 7.2 RCTs (adverse events) | 2 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.29] |
| 7.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [1.69, 7.59] |
| 8 Dyspepsia (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Cohort studies (adverse effects) | 5 | 5104 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.74] |
| 9 Headache (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Cohort studies (adverse effects) | 5 | 3322 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.50, 2.92] |
| 9.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 2.31 [1.25, 4.27] |
| 9.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.38, 4.34] |
| 10 Dizziness (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Cohort studies (adverse effects) | 5 | 2633 | Risk Ratio (M‐H, Random, 95% CI) | 3.49 [0.88, 13.75] |
| 10.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.30, 7.16] |
| 10.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [1.47, 3.90] |
| 10.4 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.62, 0.73] |
| 11 Abnormal dreams (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Cohort studies (adverse effects) | 4 | 2588 | Risk Ratio (M‐H, Random, 95% CI) | 10.49 [3.79, 29.10] |
| 11.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.07, 15.89] |
| 11.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [2.08, 9.00] |
| 12 Insomnia (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Cohort studies (adverse effects) | 4 | 3212 | Risk Ratio (M‐H, Random, 95% CI) | 4.14 [1.19, 14.44] |
| 12.2 RCTs (adverse events) | 1 | 123 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.65, 6.40] |
| 12.3 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 4.54 [2.09, 9.83] |
| 12.4 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.43, 0.49] |
| 13 Anxiety (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Cohort studies (adverse effects) | 3 | 2559 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.04 [9.32, 34.93] |
| 13.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.74 [1.99, 38.40] |
| 13.3 Retrospective healthcare record analysis (adverse events) | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.47, 0.56] |
| 14 Depressed mood (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Cohort studies (adverse effects) | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.43 [5.21, 25.07] |
| 14.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.27 [1.82, 21.62] |
| 14.3 Retrospective healthcare record analysis (adverse events) | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.51, 0.60] |
| 15 Abnormal thoughts and perceptions Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse effects) | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.60 [0.92, 47.20] |
| 15.2 Retrospective healthcare record analyses (adverse events) | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.26, 0.66] |
| 16 Pruritis (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Cohort studies (adverse effects) | 2 | 1794 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.91] |
| 16.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [0.93, 7.78] |
| 17 Photosensitivity (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Cohort studies (adverse effects) | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.05, 0.11] |
| 17.2 Cohort studies (adverse events) | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.49] |
| 18 Yeast infection (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Cohort studies (adverse effects) | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.06, 0.16] |
| 18.2 Cohort studies (adverse events) | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.63] |
| 19 Visual impairment (all studies) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Cohort studies (adverse effects) | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.41, 3.99] |
| 20 Other adverse effects (cohort studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Alopecia | 2 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [1.96, 6.03] |
| 20.2 Asthenia | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.89, 3.76] |
| 20.3 Balance disorder | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [1.48, 5.59] |
| 20.4 Decreased appetite | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.42, 3.64] |
| 20.5 Fatigue | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.77] |
| 20.6 Hypoaesthesia | 2 | 2445 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.48 [3.01, 43.70] |
| 20.7 Malaise | 1 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.11, 0.71] |
| 20.8 Mouth ulcers | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.02, 11.42] |
| 20.9 Palpitations | 1 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.16, 48.91] |
| 20.10 Tinnitus | 1 | 684 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.20 [0.39, 133.30] |
| 21 Other adverse events (RCTs) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Constipation | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 21.2 Cough | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.28, 1.01] |
| 21.3 Decreased appetite | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [1.24, 10.20] |
| 21.4 Malaise | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.88, 4.69] |
| 21.5 Palpitations | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 21.6 Pyrexia | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.09, 7.42] |
| 21.7 Sexual dysfunction | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.33, 28.51] |
| 21.8 Somnolence | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.84] |
| 22 Other adverse events (cohort studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Adjustment disorder | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.40, 0.45] |
| 22.2 Confusion | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.24, 19.49] |
| 22.3 Convulsions | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 22.4 Hallucinations | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.08, 0.45] |
| 22.5 Paranoia | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.10, 1.63] |
| 22.6 Palpitations | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.44 [1.73, 104.38] |
| 22.7 Panic attacks | 1 | 21065 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.16 [0.55, 31.49] |
| 22.8 PTSD | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.53, 0.64] |
| 22.9 Rash | 1 | 668 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.50, 2.94] |
| 22.10 Suicidal ideation | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.31, 0.47] |
| 22.11 Suicide | 2 | 376024 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.32, 4.56] |
| 22.12 Tinnitus | 1 | 354959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.61, 0.71] |
| 23 Adherence (cohort studies) Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 Adherence during travel | 13 | 15583 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.12, 1.18] |
| 23.2 Adherence in the post‐travel period | 4 | 840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 2 | 1293 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious adverse events or effects (all studies) Show forest plot | 3 | 3591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.08, 23.22] |
| 2.1 Cohort studies | 3 | 3591 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.08, 23.22] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RCTs | 3 | 1438 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [1.53, 5.31] |
| 3.2 Cohort studies | 9 | 7785 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [1.83, 4.08] |
| 4 Nausea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [1.52, 4.86] |
| 4.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.54, 4.06] |
| 5 Vomiting (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.49, 3.50] |
| 5.2 Cohort studies (adverse effects) | 3 | 2180 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.08, 4.09] |
| 6 Abdominal pain (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.52, 1.56] |
| 6.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.07] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.60, 1.47] |
| 7.2 Cohort studies (adverse effects) | 7 | 3509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.35] |
| 8 Mouth ulcers (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.70, 3.00] |
| 8.2 Cohort studies (adverse effects) | 2 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.04, 0.37] |
| 9 Headache (all studies) Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.99, 2.99] |
| 9.2 Cohort studies (adverse effects) | 8 | 4163 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.42 [1.71, 6.82] |
| 10 Dizziness (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.99 [2.08, 7.64] |
| 10.2 Cohort studies (adverse effects) | 8 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [2.23, 6.58] |
| 10.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.04, 1.46] |
| 11 Abnormal dreams (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.37, 3.04] |
| 11.2 Cohort studies (adverse effects) | 7 | 3848 | Risk Ratio (M‐H, Random, 95% CI) | 6.81 [1.65, 28.15] |
| 12 Insomnia (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.42 [2.56, 7.64] |
| 12.2 Cohort studies (adverse effects) | 8 | 3986 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.29 [4.37, 12.16] |
| 12.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.06, 1.44] |
| 13 Anxiety (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.12 [1.82, 20.66] |
| 13.2 Cohort studies (adverse effects) | 4 | 2664 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.10 [3.48, 29.32] |
| 13.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [1.28, 1.85] |
| 14 Depressed mood (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.78 [1.71, 19.61] |
| 14.2 Cohort studies (adverse effects) | 6 | 3624 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.02 [3.56, 18.07] |
| 14.3 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.56, 2.38] |
| 15 Abnormal thoughts and perceptions (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse effects) | 3 | 2433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.30, 7.42] |
| 15.2 Retrospective healthcare record analysis (adverse events) | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.69, 12.97] |
| 16 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.60, 2.70] |
| 16.2 Cohort studies (adverse effects) | 3 | 1824 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.40, 10.68] |
| 17 Visual impairment (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 RCTs (adverse effects) | 1 | 976 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.88, 4.73] |
| 17.2 Cohort studies (adverse effects) | 2 | 1956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.29, 4.72] |
| 18 Other adverse effects (cohort studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Allergic reaction | 1 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.04, 14.48] |
| 18.2 Alopecia | 1 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.55 [0.30, 70.01] |
| 18.3 Asthenia | 2 | 1956 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.26, 13.12] |
| 18.4 Balance disorder | 1 | 1469 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.19, 44.19] |
| 18.5 Cough | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.08, 2.92] |
| 18.6 Disturbance in attention | 3 | 1363 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.45 [1.84, 10.77] |
| 18.7 Dyspepsia | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.46] |
| 18.8 Fatigue | 2 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [0.47, 45.56] |
| 18.9 Hypoaesthesia | 2 | 1946 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.45 [0.93, 21.26] |
| 18.10 Loss of appetite | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.43] |
| 18.11 Muscle pain | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.57 [0.45, 127.80] |
| 18.12 Palpitations | 3 | 2180 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.73, 15.26] |
| 18.13 Photosensitization | 2 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.10, 4.92] |
| 18.14 Pyrexia | 1 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.28 [0.24, 75.57] |
| 18.15 Rash | 2 | 711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.15, 6.09] |
| 18.16 Restlessness | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.24 [0.32, 84.52] |
| 18.17 Slight illness | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.83 [0.36, 93.84] |
| 18.18 Somnolence | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.21, 11.40] |
| 18.19 Tinnitus | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.13, 42.64] |
| 18.20 Circulatory disorders | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.38 [0.36, 114.01] |
| 19 Other adverse events (cohort studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Adjustment disorder | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.54, 2.02] |
| 19.2 Confusion | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.04, 25.96] |
| 19.3 Convulsions | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.79, 2.30] |
| 19.4 Hallucinations | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.79] |
| 19.5 Paranoia | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.08, 36.72] |
| 19.6 PTSD | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.93, 3.26] |
| 19.7 Suicidal ideation | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.03, 2.77] |
| 19.8 Suicide | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.06, 7.78] |
| 19.9 Tinnitus | 1 | 49419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.21, 1.68] |
| 20 Adherence (RCTs) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 20.1 van Riemsdijk 2002 | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 20.2 Overbosch 2001; during travel | 1 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 20.3 Overbosch 2001; post‐travel | 1 | 966 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.74, 0.85] |
| 21 Adherence (cohort studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 21.1 During travel | 6 | 5577 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.86, 1.34] |
| 21.2 Post‐travel | 2 | 422 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.64, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical cases of malaria (RCTs) Show forest plot | 4 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.28, 0.52] |
| 2 Serious adverse events or effects (all studies) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 RCTs | 4 | 1000 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.32, 23.85] |
| 2.2 Cohort studies | 6 | 79257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.07] |
| 3 Discontinuations due to adverse effects (all studies) Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 RCTs | 3 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.61, 4.18] |
| 3.2 Cohort studies in short‐term travellers | 6 | 55397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.26] |
| 3.3 Cohort studies in longer term occupational travellers | 2 | 6085 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [2.41, 3.66] |
| 4 Nausea (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Cohort studies (adverse effects) | 6 | 58984 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.89, 1.68] |
| 4.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.57, 1.79] |
| 5 Vomiting (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cohort studies (adverse effects) | 5 | 5577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.78, 1.40] |
| 5.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.36, 3.49] |
| 6 Abdominal pain (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Cohort studies (adverse effects) | 4 | 5440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 6.2 RCTs (adverse events) | 2 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.37, 1.36] |
| 7 Diarrhoea (all studies) Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Cohort studies (adverse effects) | 5 | 5577 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 7.2 RCTs (adverse events) | 3 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.50] |
| 8 Headache (all studies) Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Cohort studies (adverse effects) | 6 | 56998 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.34] |
| 8.2 RCTs (adverse events) | 3 | 772 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.31] |
| 9 Dizziness (all studies) Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Cohort studies (adverse effects) | 5 | 58847 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.34, 1.70] |
| 9.2 RCTs (adverse events) | 2 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.46] |
| 10 Abnormal dreams (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Cohort studies (adverse effects) | 4 | 2845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.10, 1.33] |
| 10.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.05, 6.95] |
| 11 Insomnia (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Cohort studies (adverse effects) | 5 | 56952 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.73, 4.51] |
| 11.2 RCTs (adverse events) | 1 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.76, 1.84] |
| 12 Anxiety (all studies) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Cohort studies (adverse effects) | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.30 [4.37, 9.09] |
| 13 Depressed mood (all studies) Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Cohort studies (adverse effects) | 5 | 58855 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [1.15, 8.57] |
| 14 Abnormal thoughts and perceptions Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Cohort studies (adverse effects) | 4 | 4831 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.49 [2.65, 11.35] |
| 15 Pruritis (all studies) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Cohort studies (adverse effects) | 2 | 55544 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.92, 1.40] |
| 15.2 RCTs (adverse events) | 2 | 413 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.93] |
| 16 Visual impairment (all studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 Cohort studies (adverse effects) | 5 | 58847 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.50, 2.44] |
| 16.2 RCTs (adverse events) | 1 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.63] |
| 17 Vertigo (all studies) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Cohort studies (adverse effects) | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.05, 23.43] |
| 18 Cohort studies in travellers; prespecified adverse effects Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Vertigo | 1 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.05, 23.43] |
| 18.2 Nausea | 5 | 56847 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.94, 2.13] |
| 18.3 Vomiting | 4 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.55, 1.42] |
| 18.4 Abdominal pain | 3 | 3303 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.30] |
| 18.5 Diarrhoea | 4 | 3440 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.57, 2.64] |
| 18.6 Headache | 5 | 54861 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.48, 2.65] |
| 18.7 Dizziness | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.10, 2.10] |
| 18.8 Abnormal dreams | 3 | 708 | Risk Ratio (M‐H, Random, 95% CI) | 4.21 [0.57, 31.33] |
| 18.9 Insomnia | 4 | 54815 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.40, 6.10] |
| 18.10 Anxiety | 2 | 1271 | Risk Ratio (M‐H, Random, 95% CI) | 3.94 [0.53, 29.48] |
| 18.11 Depressed mood | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.75, 8.31] |
| 18.12 Abnormal thoughts or perceptions | 3 | 2694 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [1.58, 12.40] |
| 18.13 Pruritis | 1 | 53407 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.94, 1.48] |
| 18.14 Visual impairment | 4 | 56710 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.55, 0.79] |
| 19 Other adverse effects (cohort studies) Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Altered spatial perception | 1 | 2032 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.55, 6.45] |
| 19.2 Alopecia | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.27, 2.25] |
| 19.3 Asthenia | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.97, 2.40] |
| 19.4 Balance disorder | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [2.15, 6.00] |
| 19.5 Confusion | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.11, 36.31] |
| 19.6 Decreased appetite | 1 | 2032 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.87, 1.57] |
| 19.7 Fatigue | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [0.57, 9.80] |
| 19.8 Hypoaesthesia | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 20.26 [1.23, 333.93] |
| 19.9 Irritability | 1 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [0.28, 80.59] |
| 19.10 Mouth ulcers | 2 | 55439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.01, 1.87] |
| 19.11 Paraesthesia | 2 | 2778 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.27, 3.89] |
| 19.12 Palpitations | 3 | 3408 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.71 [0.91, 24.26] |
| 19.13 Photosensitization | 2 | 2662 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.53] |
| 19.14 Restlessness | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.74 [0.65, 34.46] |
| 19.15 Slight illness | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.64, 10.87] |
| 19.16 Somnolence | 1 | 525 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.08 [0.37, 100.36] |
| 19.17 Yeast infection | 1 | 2137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.53, 2.49] |
| 20 Other adverse events (RCTs) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Abdominal distension | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.64, 15.27] |
| 20.2 Anger | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.55] |
| 20.3 Disturbance in attention | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [0.61, 16.47] |
| 20.4 Irritability | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.45, 2.64] |
| 20.5 Loss of appetite | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.35, 3.25] |
| 20.6 Malaise | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.85] |
| 20.7 Mood altered | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.29, 4.34] |
| 21 Pregnancy related outcomes (RCTs) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Spontaneous abortions | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.36, 1.79] |
| 21.2 Still births | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.52] |
| 21.3 Congenital malformations | 1 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Adherence (cohort studies) Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 22.1 Short‐term travellers | 3 | 852 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.90, 1.13] |
| 22.2 Short‐term travellers: after return | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.87] |
| 22.3 Longer‐term occupational travellers | 2 | 5777 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [1.80, 2.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea; effects Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.52, 4.86] |
| 1.2 Cohort studies | 11 | 5973 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.78, 3.77] |
| 2 Abdominal pain; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.52, 1.56] |
| 2.2 Cohort studies | 9 | 4494 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.87] |
| 3 Diarrhoea; effects Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.60, 1.47] |
| 3.2 Cohort studies | 10 | 7648 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.28, 1.34] |
| 4 Headache; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [0.99, 2.99] |
| 4.2 Cohort studies | 9 | 5592 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.22, 3.93] |
| 5 Dizziness; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 3.99 [2.08, 7.64] |
| 5.2 Cohort studies | 9 | 4606 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [1.58, 6.35] |
| 6 Abnormal dreams; effects Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [1.37, 3.04] |
| 6.2 Cohort studies | 7 | 4543 | Risk Ratio (M‐H, Random, 95% CI) | 7.30 [2.51, 21.18] |
| 7 Insomnia; effects Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [2.56, 7.64] |
| 7.2 Cohort studies | 9 | 5299 | Risk Ratio (M‐H, Random, 95% CI) | 5.70 [2.83, 11.47] |
| 8 Anxiety; effects Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 6.12 [1.82, 20.66] |
| 8.2 Cohort studies | 4 | 3390 | Risk Ratio (M‐H, Random, 95% CI) | 15.26 [8.66, 26.89] |
| 9 Depressed mood; effects Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 5.78 [1.71, 19.61] |
| 9.2 Cohort studies | 6 | 4236 | Risk Ratio (M‐H, Random, 95% CI) | 7.82 [3.79, 16.12] |
| 10 Abnormal thoughts or perceptions; effects Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Cohort studies | 3 | 3045 | Risk Ratio (M‐H, Random, 95% CI) | 4.20 [0.81, 21.87] |
| 11 Pruritis; effects Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.60, 2.70] |
| 11.2 Cohort studies | 3 | 2034 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.16, 4.76] |
| 12 Visual impairment; effects Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 RCTs | 1 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.88, 4.73] |
| 12.2 Cohort studies | 3 | 2560 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [1.05, 4.02] |
| 13 Adherence; during travel Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 RCTs | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.88, 1.02] |
| 13.2 Cohort studies | 11 | 12131 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.03, 1.30] |
| 14 Adherence; after return Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Cohort studies | 4 | 1221 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.17] |

