Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator‐associated pneumonia
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Duration: 28 days | |
| Participants | Sample size = 739 | |
| Interventions | Invasive quantitative versus non‐invasive qualitative | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone system, with a variable, undisclosed block size |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | High risk | Physicians were aware of the patient's treatment assignments |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient withdrew consent 2 days after randomisation |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes have been reported in the pre‐specified way and adequately reported |
| Other bias | Low risk | |
| Methods | Duration: 28 days | |
| Participants | Sample size = 413 | |
| Interventions | Invasive quantitative versus non‐invasive qualitative | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables were used to assign patients in blocks of 8, with stratification according to treatment centre |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | High risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | All patients were followed up |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes have been reported in the pre‐specified way and adequately reported |
| Other bias | Low risk | Not detected |
| Methods | Duration: 30 days | |
| Participants | Sample size = 76 Previous use of antibiotic | |
| Interventions | Invasive quantitative versus non‐invasive quantitative | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table into 1 of the 2 groups |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | High risk | Open study |
| Incomplete outcome data (attrition bias) | High risk | All patients were followed up |
| Selective reporting (reporting bias) | Unclear risk | No data to evaluate |
| Other bias | Low risk | Not detected |
| Methods | Duration: not informed | |
| Participants | Sample size = 51 | |
| Interventions | Invasive quantitative versus non‐invasive quantitative | |
| Outcomes | Mortality Intensive care unit (ICU) stay (days) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table into 1 of the 2 groups |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | High risk | Open study |
| Incomplete outcome data (attrition bias) | High risk | All patients were followed up |
| Selective reporting (reporting bias) | Unclear risk | No data to evaluate |
| Other bias | Low risk | Not detected |
| Methods | Duration: not informed | |
| Participants | Sample size = 88 | |
| Interventions | Invasive quantitative versus non‐invasive qualitative | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table into 1 of the 2 groups |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding (performance bias and detection bias) | Unclear risk | It is not stated on the paper |
| Incomplete outcome data (attrition bias) | Low risk | 3 patients were excluded because of transfer to another institution |
| Selective reporting (reporting bias) | Unclear risk | No data to evaluate |
| Other bias | Low risk | Not detected |
APACHE: acute physiology and chronic health evaluation
ICU: intensive care unit
SAPS: simplified acute physiologic score
VAP: ventilator‐associated pneumonia
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| The study is not clearly randomised and is a cross‐over study |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 1240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.11] |
| Analysis 1.1  Comparison 1 Quantitative versus qualitative culture, Outcome 1 Mortality. | ||||
| 2 Antibiotic change Show forest plot | 2 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.54, 4.39] |
| Analysis 1.2  Comparison 1 Quantitative versus qualitative culture, Outcome 2 Antibiotic change. | ||||
| 3 Duration on mechanical ventilation (days) Show forest plot | 2 | 827 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.51, 1.68] |
| Analysis 1.3  Comparison 1 Quantitative versus qualitative culture, Outcome 3 Duration on mechanical ventilation (days). | ||||
| 4 ICU stay (days) Show forest plot | 3 | 1240 | Mean Difference (IV, Fixed, 95% CI) | 0.95 [‐0.14, 2.04] |
| Analysis 1.4  Comparison 1 Quantitative versus qualitative culture, Outcome 4 ICU stay (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 5 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.11] |
| Analysis 2.1  Comparison 2 Invasive versus non‐invasive method, Outcome 1 Mortality. | ||||
| 2 Antibiotic change Show forest plot | 4 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.87, 3.21] |
| Analysis 2.2  Comparison 2 Invasive versus non‐invasive method, Outcome 2 Antibiotic change. | ||||
| 3 Duration on mechanical ventilation (days) Show forest plot | 4 | 954 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.47, 1.68] |
| Analysis 2.3  Comparison 2 Invasive versus non‐invasive method, Outcome 3 Duration on mechanical ventilation (days). | ||||
| 4 ICU stay (days) Show forest plot | 5 | 1367 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [‐0.13, 2.01] |
| Analysis 2.4  Comparison 2 Invasive versus non‐invasive method, Outcome 4 ICU stay (days). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.41] |
| Analysis 3.1  Comparison 3 Invasive quantitative versus non‐invasive quantitative, Outcome 1 Mortality. | ||||
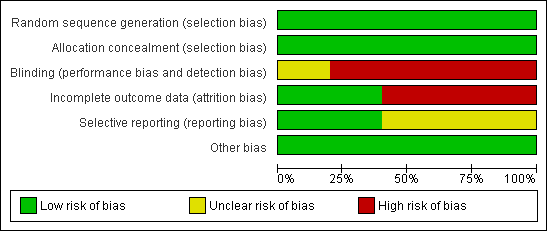
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
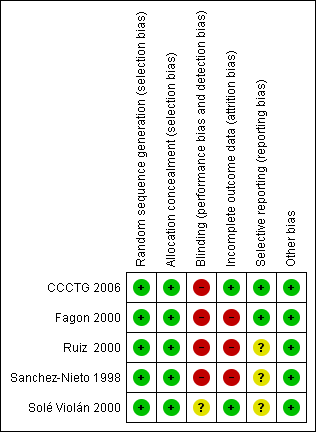
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
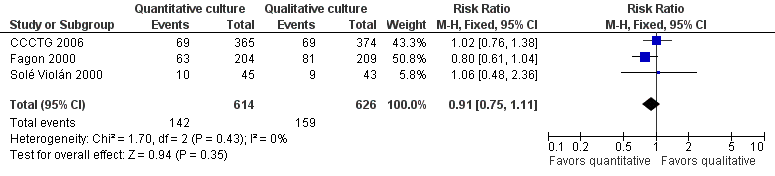
Forest plot of comparison: 1 Quantitative versus qualitative culture, outcome: 1.1 Mortality.

Forest plot of comparison: 1 Quantitative versus qualitative culture, outcome: 1.2 Antibiotic change.

Forest plot of comparison: 1 Quantitative versus qualitative culture, outcome: 1.3 Duration on mechanical ventilation (days).

Forest plot of comparison: 1 Quantitative versus qualitative culture, outcome: 1.4 ICU stay (days).

Comparison 1 Quantitative versus qualitative culture, Outcome 1 Mortality.

Comparison 1 Quantitative versus qualitative culture, Outcome 2 Antibiotic change.
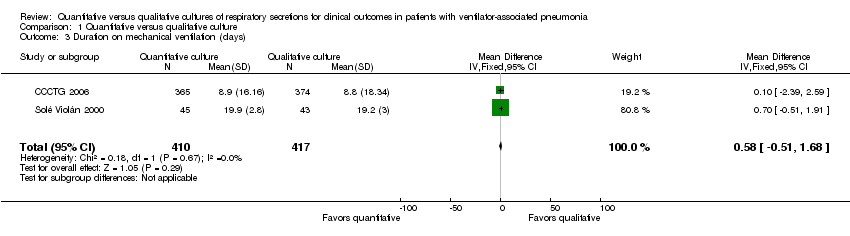
Comparison 1 Quantitative versus qualitative culture, Outcome 3 Duration on mechanical ventilation (days).

Comparison 1 Quantitative versus qualitative culture, Outcome 4 ICU stay (days).
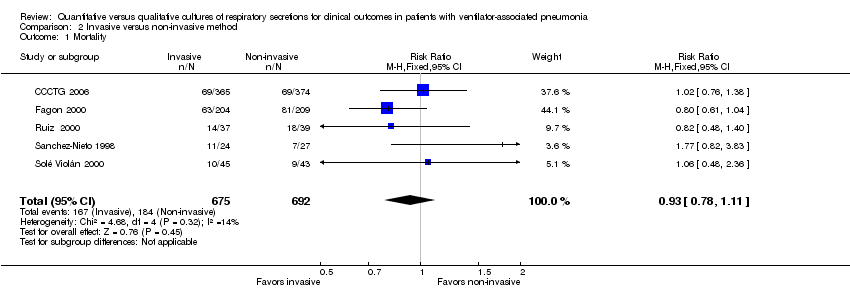
Comparison 2 Invasive versus non‐invasive method, Outcome 1 Mortality.

Comparison 2 Invasive versus non‐invasive method, Outcome 2 Antibiotic change.

Comparison 2 Invasive versus non‐invasive method, Outcome 3 Duration on mechanical ventilation (days).
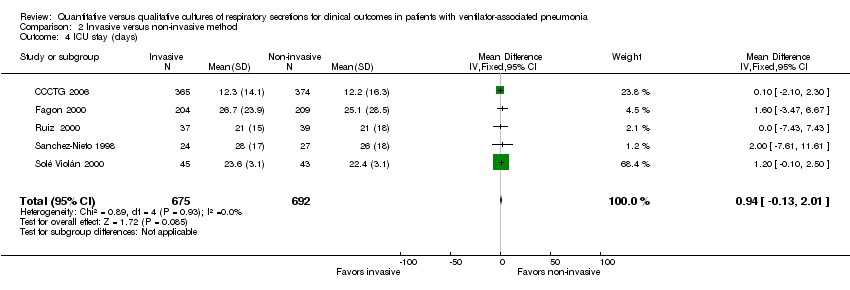
Comparison 2 Invasive versus non‐invasive method, Outcome 4 ICU stay (days).

Comparison 3 Invasive quantitative versus non‐invasive quantitative, Outcome 1 Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 3 | 1240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.11] |
| 2 Antibiotic change Show forest plot | 2 | 827 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.54, 4.39] |
| 3 Duration on mechanical ventilation (days) Show forest plot | 2 | 827 | Mean Difference (IV, Fixed, 95% CI) | 0.58 [‐0.51, 1.68] |
| 4 ICU stay (days) Show forest plot | 3 | 1240 | Mean Difference (IV, Fixed, 95% CI) | 0.95 [‐0.14, 2.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 5 | 1367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.78, 1.11] |
| 2 Antibiotic change Show forest plot | 4 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.87, 3.21] |
| 3 Duration on mechanical ventilation (days) Show forest plot | 4 | 954 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.47, 1.68] |
| 4 ICU stay (days) Show forest plot | 5 | 1367 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [‐0.13, 2.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.41] |

