Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department
Abstract
Background
Pediatric acute respiratory infections (ARIs) represent a significant burden on pediatric Emergency Departments (EDs) and families. Most of these illnesses are due to viruses. However, investigations (radiography, blood, and urine testing) to rule out bacterial infections and antibiotics are often ordered because of diagnostic uncertainties. This results in prolonged ED visits and unnecessary antibiotic use. The risk of concurrent bacterial infection has been reported to be negligible in children over three months of age with a confirmed viral infection. Rapid viral testing in the ED may alleviate the need for precautionary testing and antibiotic use.
Objectives
To determine if the use of a rapid viral detection test for children with an acute respiratory infection (ARI) in Emergency Departments (EDs) changes patient management and resource use in the ED, compared to not using a rapid viral detection test. We hypothesized that rapid viral testing reduces antibiotic use in the ED as well as reduces the rate of ancillary testing and length of ED visits.
Search methods
We searched CENTRAL (2014, Issue 6), MEDLINE (1950 to July week 1, 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (15 July 2014), EMBASE.com (1988 to July 2014), HealthStar (1966 to 2009), BIOSIS Previews (1969 to July 2014), CAB Abstracts (1973 to July 2014), CBCA Reference (1970 to 2007) and ProQuest Dissertations and Theses (1861 to 2009).
Selection criteria
Randomized controlled trials (RCTs) of rapid viral testing for children with ARIs in the ED.
Data collection and analysis
Two review authors used the inclusion criteria to select trials, evaluate their quality, and extract data. We obtained missing data from trial authors. We expressed differences in rate of investigations and antibiotic use as risk ratios (RRs), and expressed difference in ED length of visits as mean differences (MDs), with 95% confidence intervals (CIs).
Main results
No new trials were identified in this 2014 update. We included four trials (three RCTs and one quazi‐RCT), with 759 children in the rapid viral testing group and 829 in the control group. Three out of the four studies were comparable in terms of young age of participants, with one study increasing the age of inclusion up to five years of age. All studies included either fever or respiratory symptoms as inclusion criteria (two required both, one required fever or respiratory symptoms, and one required only fever). All studies were comparable in terms of exclusion criteria, intervention, and outcome data. In terms of risk of bias, one study failed to utilize a random sequence generator, one study did not comment on completeness of outcome data, and only one of four studies included allocation concealment as part of the study design. None of the studies definitively blinded participants.
Rapid viral testing resulted in a trend toward decreased antibiotic use in the ED, but this was not statistically significant. We found lower rates of chest radiography (RR 0.77, 95% CI 0.65 to 0.91) in the rapid viral testing group, but no effect on length of ED visits, or blood or urine testing in the ED. No study made mention of any adverse effects related to viral testing.
Authors' conclusions
There is insufficient evidence to support routine rapid viral testing to reduce antibiotic use in pediatric EDs. Rapid viral testing may or may not reduce rates of antibiotic use, and other investigations (urine and blood testing); these studies do not provide enough power to resolve this question. However, rapid viral testing does reduce the rate of chest X‐rays in the ED. An adequately powered trial with antibiotic use as an outcome is needed.
PICO
Plain language summary
Rapid viral testing for children in the Emergency Department with fever and respiratory symptoms
Review question
Does rapid viral testing in the Emergency Department influence the treatment of children with fever and breathing symptoms?
Background
Otherwise healthy children, aged 0 to 18 years, admitted to Emergency Departments (EDs) with fever and respiratory symptoms represent a major burden to the healthcare system, as well as significant anxiety and expense to parents and caregivers. Physicians often order diagnostic tests and may prescribe antibiotics when they are unsure of the cause of the illness and are concerned about the possibility of serious bacterial infection. However, in most cases, fever and respiratory symptoms are caused by viruses. In addition, in children in whom a virus is found to be the cause of their illness, the risk of serious bacterial infection is very low. We conducted this review to assess whether a rapid viral test, done in the ED, changes what physicians do when treating these children.
Study characteristics
We reviewed studies retrievable as of July 2014. We included four prospective controlled studies of previously healthy children under 18 years of age who attended an ED of an urgent care clinic because of fever and respiratory symptoms.
Key results
Based on these four studies, involving 759 study participants, we found that in previously healthy children coming to the ED with fever and respiratory symptoms, a rapid viral test showed a trend towards fewer antibiotic prescriptions, but this finding was not statistically significant. However, we found that rapid viral testing reduces the use of chest X‐rays. There are also blood and urine investigations that can be undertaken. The true impact of this intervention on the frequency of blood and urine testing, as well as the length of the ED visit, requires trials with larger numbers of children. None of the included studies reported harm or adverse events related to the intervention tested.
Quality of the evidence
The quality of the evidence was considered moderate with regard to risk of bias, indirectness, imprecision, publication bias and inconsistency. While none of the studies used blinding, the impact of the use of rapid viral testing is in its ability to provide diagnostic information. Blinding of this interventions to the clinician would be impossible and make the intervention useless.
Authors' conclusions
Background
Description of the condition
Acute respiratory infections (ARIs) are a serious public health issue and rank among the top five causes of illness and hospitalization in children. During influenza seasons, fever and respiratory infection symptoms make up to 25% of all reasons for a visit to an Emergency Department (ED) (Silka 2003). Although ARIs can be caused by bacteria, they are most commonly caused by viral infections. A rapid diagnosis of a viral infection may lead to a reduction in the use of antibiotics, additional testing, and possibly admissions. The most commonly implicated causal viruses are influenza (A and B), respiratory syncytial virus (RSV), human parainfluenza (1, 2 and 3), rhinovirus, and adenovirus. These viruses account for 35% to 87% of children with an ARI. The variability in the range of positive viral diagnosis may be affected by the choice of viral tests used, and their scope of viral detection (Jennings 2004; Weigl 2000). There is a risk of concurrent bacterial infection in children with a confirmed viral ARI. A study of children aged 3 to 36 months with recognizable viral infections showed a concurrent rate of bacteremia of 0.01% to 0.8% (Greens 1999). A prospective multicenter study of infants less than 60 days old with an ARI showed a significant difference in the rate of urinary tract infection between RSV positive (5.4%) and negative infants (10.1%), a non‐significant difference in the rate of bacteremia (1.1% and 2.3%) and no cases of bacterial meningitis among the 251 RSV positive infants and eight cases out of 938 RSV negative infants (not statistically significant) (Levine 2004).
However, symptoms of viral ARI overlap with those of bacterial infections (such as pneumonia, bacteremia, and meningitis) and, in some cases, are difficult to distinguish. Without a confirmed viral diagnosis, medical assessment and diagnostic tests are often used before a decision on patient management, parental advice, and/or hospital admission are made. These precautionary tests lead to intense use of human health resources (nursing, laboratory, and radiology staff) and hospital facilities. Furthermore, these tests are often invasive, sometimes unnecessarily prolonging a child's visit to the ED, resulting in suboptimal ED service provision and contributing to lengthy ED wait times and overcrowding.
ARIs impose large costs on the health system, from a high number of physician visits, ED visits, hospitalization, and antibiotic prescriptions. Studies comparing health care utilization for ARIs in children 0 to 15 years old during an influenza season and the rest of the year showed significant excess in physician visits (28,000 to 51,000/100,000 age‐specific population annually), ED visits (˜1600/100,000 age‐specific population annually), hospital admission (300 to 9500/100,000 age‐specific population annually), and antibiotic prescription (31,000/100,000 age‐specific population annually). Most of this burden came from children below three years of age (Menec 2003; Neuzil 2000).
A study comparing the costs associated with a visit to the ED versus a primary care provider showed that the average cost for assessing a patient for an ARI in the ED (excluding antibiotics cost) is USD 206 to USD 221, and in comparison is USD 101 to USD 106 in a primary care provider's office. Up to 60% of patients with a common cold are treated with antimicrobials, which cost USD 37.5 million annually (Rosenstein 1998), despite most ARIs being caused by viruses. The physician and nursing costs only contributed to 17.5% of ARI management costs (Martin 2000). This suggests that extra investigations and antibiotic prescribing in the ED may be responsible for much of any unnecessary costs.
During the severe acute respiratory syndrome (SARS) outbreak in 2003, there was access to rapid respiratory viral diagnosis in acute care settings (that is, provision of same‐day identification of influenza virus A and B, and parainfluenza virus 1, 2 and 3), RSV, and adenovirus. This enabled rapid, informed patient management decisions and helped with triaging. This suggests a role for rapid viral diagnosis in alleviating the burden on EDs and improving health service delivery and health resource allocation in the situation of increased use of EDs for ARI symptoms. A prompt viral diagnosis might improve decision‐making and reduces unnecessary hospital admittance, prescription of antibiotics, and further diagnostic investigations.
This is supported by observational data from retrospective chart reviews of children admitted to hospital, with subsequent confirmed diagnosis of adenovirus infection revealing a change in management for 36% of the children, including revision of antibiotic treatment and use of antiviral therapy (Rocholl 2004). Similarly, chart reviews of children testing positive via a rapid influenza diagnostic test were less likely to be prescribed antibiotics in the ED (20% versus 53%; P value = 0.04) and when admitted were on antibiotics for fewer days (3.5 days versus 5.4 days; P value = 0.03) (Noyola 2000). Children with an early diagnosis of influenza also had fewer blood tests (17% versus 44%; P value = 0.02) and urine tests performed (2% versus 24%; P value = 0.006), compared to those children with a late diagnosis (Sharma 2002).
Description of the intervention
Advances in virology testing now allow for viral detection within 30 to 120 minutes by direct immunofluorescent antibody detection. These have been reported to have high sensitivity (up to 90%) and specificity (up to 99%) (Vega 2005). Confirmation of specific diagnosis of viral respiratory infection is now accessible and reliable.
How the intervention might work
A better diagnosis of children presenting to the ED with fever and respiratory symptoms may improve their management by allowing more rational decisions about other investigations and treatment.
Why it is important to do this review
This literature has yet to be systematically reviewed. There may be evidence of substantial reductions in unnecessary investigation costs and antibiotic prescribing for children with ARIs in the ED, by positively identifying a viral illness rather than attempting to exclude a more serious bacterial cause.
Objectives
To determine if the use of a rapid viral detection test for children with an acute respiratory infection (ARI) in Emergency Departments (EDs) changes patient management and resource use in the ED, compared to not using a rapid viral detection test. We hypothesized that rapid viral testing reduces antibiotic use in the ED as well as reduces the rate of ancillary testing and length of ED visits.
Methods
Criteria for considering studies for this review
Types of studies
-
Randomized controlled trials (RCTs) evaluating the use of rapid viral diagnosis in children admitted to the ED with an ARI.
Types of participants
We included:
-
studies of otherwise healthy children aged 0 to 18 years old; or
-
studies which reported separately on subgroups of children under 18 years of age, admitted to an ED with a clinical presentation consistent with an ARI (fever and respiratory symptoms such as cough, runny nose, sore throat, or congested nose).
We did not consider:
-
studies including participants who are immunocompromised;
-
studies including participants who have underlying chronic severe respiratory conditions (cystic fibrosis or bronchopulmonary dysplasia); or
-
studies including participants with chronic heart conditions (such as uncorrected cyanotic heart lesions or prosthetic valves).
Types of interventions
Rapid viral diagnosis from nasal pharyngeal aspirates or swabs by direct or indirect immunofluorescent antibody test, enzyme immunoassays, optical immunoassay, or molecular testing such as multiplex polymerase chain reaction. Rapid viral diagnosis implies that results are made available during the participants' stay in the ED. The intervention group will include participants who have rapid viral diagnostic testing, while participants in the control group will have had no rapid viral diagnostic test performed, or the treating physician will have had no knowledge about the test results.
Types of outcome measures
Primary outcomes
-
Antimicrobial prescription rate in the ED: we considered a reduction of antibiotic use by 25% (risk ratio (RR) 0.75) as clinically important.
Secondary outcomes
-
Length of hospital (ED) stay: we considered a reduction of 30 minutes as clinically important.
-
Rate of ancillary tests (any blood tests or chest imaging or urine investigations) requested: we considered a reduction in ancillary testing of 25% (RR 0.75) as clinically important.
-
Rate of physician visit (ED or office) within two weeks after discharge from ED: we considered a relative increase in physician visit within two weeks of discharge from an ED of 10% (RR 1.10) as clinically important.
-
Hospital admission rate: we considered a reduction in admission rate of 25% (RR 0.75) as clinically important.
-
Acceptability of nasal specimen collection sampling for rapid viral testing (discomfort level with invasiveness of the procedure).
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 6), which contains the Cochrane ARI Group's Specialized Register, MEDLINE (December 2011 to July week 1, 2014), EMBASE, (December 2011 to July 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (15 July 2014), BIOSIS Previews (December 2011 to July 2014) and CAB Abstracts (December 2011 to July 2014). Details of previous searches are in Appendix 1.
We used the search strategy in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008) revision; Ovid format (Lefebvre 2011). We also used a filter to identify 'child' studies based on the work of Boluyt 2008. We adapted this search strategy to search EMBASE (see Appendix 3), MEDLINE In‐Process & Other Non‐Indexed Citations (see Appendix 4), BIOSIS (see Appendix 5), and CAB Abstracts (see Appendix 6). We did not apply any language or publication restrictions.
Searching other resources
We included articles derived from the original search, which were provided by the principal researcher and were tracked forward using the Cited Reference Search feature in Web of Science and the Scopus Citation Tracker. We searched ClinicalTrials.gov (www.clinicaltrials.gov) and WHO ICTRP (www.who.int/ictrp) for completed and ongoing trials (latest search 17 July 2014). We searched the Pediatric Academic Society and Society for Pediatric Research joint conference abstracts databases from 2000 to 2010 for identification of meeting abstracts.
Data collection and analysis
Two review authors (QD, PE) independently extracted and verified data entry for accuracy. We used the Review Manager statistical package to conduct the analyses (RevMan 2014).
Selection of studies
Two review authors (QD, PE) screened titles and abstracts of identified citations to exclude trials which were clearly not relevant or did not meet the inclusion criteria for the review. We retrieved the full article for further examination for all abstracts or titles deemed relevant or potentially meeting the criteria by either review author. The two review authors assessed these articles to confirm that they met the inclusion criteria for the review.
Data extraction and management
Two review authors (QD, PE) independently extracted data from the published studies using standardized data extraction forms. We contacted trial authors to obtain unpublished information, including outcome data that were not explicitly stated in the published papers. We resolved disagreements in data extraction by discussion and consensus.
Assessment of risk of bias in included studies
The review authors evaluated the methodological quality of each trial using the 'Risk of bias' tool (Higgins 2011).
Measures of treatment effect
We expressed dichotomous data, such as antibiotic prescription in the ED (primary objective), ancillary tests performed in the ED, admission to the hospital and physician visits or re‐visits to the ED within two weeks of discharge from the original ED visit, as risk ratios (RRs). We expressed continuous data, such as mean length of stay in the ED, as mean differences (MDs).
Unit of analysis issues
All included studies used individual participants as the unit of randomization and analysis.
Dealing with missing data
We still included studies with missing data, and included discussions of the implications of the missing data in the 'Risk of bias' tables. Where data were incomplete, we contacted the original investigators. We explored the context surrounding missing data with the study authors, but only performed analyses on available data.
Assessment of heterogeneity
We tested heterogeneity using the Chi2 test as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of reporting biases
We intended to use visual inspection of funnel plots to assess for publication bias and small study effects, but the small number of studies included in this review would make the interpretation of these plots difficult and of questionable meaning.
Data synthesis
We analyzed pooled differences for the rate of investigations and antibiotic use using the Mantel‐Haenszel test and expressed these as RRs with 95% confidence interval (CIs). We analyzed pooled difference in ED length of visits using the inverse variance method and expressed these as MDs with 95% CIs. We applied the random‐effects model to all statistical analyses.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analyses as data were not consistently available by age groups.
Sensitivity analysis
We performed a sensitivity analysis comparing studies where we deemed the risk of bias adequate for inclusion. Given the invasive nature of specimen acquisition for rapid respiratory viral testing, the intervention cannot be blinded and, therefore, we deemed no study free of bias.
Results
Description of studies
In this 2014 update, in addition to the previous studies described in the review, we attempted to retrieve information on a trial reported as completed on ClinicalTrials.gov, which seemed to meet our inclusion criteria. However, we were unable to find a report of its results or to make contact with its principal investigator. In total, we are still left with nine prospective controlled trials on the impact of rapid viral testing in children in the ED. We included four studies in this review: three RCTs (Bonner 2003; Doan 2009; Poehling 2006) and one quazi‐RCT (Iyer 2006). We excluded five studies (Abanses 2006; Cohen 2007; Esposito 2003; Ozkaya 2009; Ozkaya 2010). See Characteristics of excluded studies table for descriptions and reasons for exclusion.
Results of the search
Electronic database searches resulted in 1568 references (after we eliminated duplicates), of which eight were prospective studies of rapid viral testing in children. A search of the Pediatric Academic Society conference proceedings only yielded references which were already recovered from the electronic search. We found one additional potential study from the ClinicalTrials.gov registry, but many attempts at contacting the trial authors to enquire about the status of this study were unsuccessful. Snowballing, using Scopus and Web of Sciences, and handsearching through references of included studies yielded one additional study of rapid viral testing in children (Cohen 2007). We carefully reviewed a total of nine studies, of which four met all of the inclusion criteria.
Included studies
Bonner 2003 was a single‐center RCT assessing participants presenting to a large United States tertiary center pediatric ED with fever and symptoms of an acute respiratory illness for less than 72 hours. The goal of the study was to assess whether prior knowledge of a positive influenza test changed physician decision‐making and management of these participants. A total of 418 participants were enrolled with 391 completing the study.
Details pertaining to participants, outcome measures, and limitations are found in the Characteristics of included studies tables.
Poehling 2006 was a RCT assessing participants presenting to a pediatric ED or acute care clinic, with signs and symptoms of respiratory tract infections. Data were analyzed and reported separately for these two populations. We only considered the study population enrolled from the ED. The goal of the study was to assess whether a rapid diagnosis of influenza affects the evaluation and treatment of children with acute respiratory illnesses. A total of 468 participants were enrolled in the study.
Approximately 20% of their study population were deemed high‐risk medical participants (as defined in the publication Red Book (CID 2003)) and influenza vaccination was recommended for these patients (i.e. 1) children with chronic disorders of the pulmonary or cardiovascular systems, including asthma; 2) children who have required regular medical follow‐up or hospitalization during the preceding year because of chronic metabolic diseases, including diabetes mellitus, renal dysfunction, hemoglobinopathies, or immunosuppression including immunosuppression caused by medications or by human immunodeficiency (HIV) virus; and 3) children and adolescents who are receiving long‐term aspirin therapy and, therefore, might be at risk for developing Reye's syndrome after an influenza infection). We contacted the primary trial author for further clarification.
Of these 20%, only five participants had a condition that may have met our exclusion criteria (congenital heart disease, bronchopulmonary dysplasia of unknown severity, and possible immune defect, reported by parents but unrelated to chemotherapy). The rest had asthma, which is not an exclusion criteria for our review. We obtained the raw data, excluding these five participants, from the primary author and used the data for this meta‐analysis.
Details pertaining to participants, outcome measures, and limitations are found in the Characteristics of included studies tables.
Iyer 2006 was a prospective, quazi‐RCT assessing participants presenting to a large, urban, tertiary care pediatric ED. The goal of this study was to assess the effect of rapid influenza diagnosis on physician management of previously healthy febrile participants, aged 2 to 24 months, at risk for serious bacterial infection. Despite the fact that this study only mentioned fever as an inclusion criteria, close to 90% of the children enrolled in the study also had symptoms of an acute respiratory illness. A total of 700 participants were included in the study.
Details pertaining to participants, outcome measures, and limitations are found in the Characteristics of included studies tables.
Doan 2009 was a single‐center, open‐label, RCT assessing participants presenting to a large Canadian tertiary center pediatric ED. The goal of the study was to measure the effect of a multi‐viral rapid diagnostic test on the clinical management and resource utilization pertaining to healthy children who presented to the ED with signs and symptoms of a febrile ARI. A total of 204 participants were enrolled with 200 completing the study.
Details pertaining to participants, outcome measures, and limitations are found in the Characteristics of included studies tables.
Excluded studies
Esposito 2003 was a single‐center RCT assessing children presenting to a pediatric ED with fever and signs/symptoms of a respiratory illness. The goal of the study was to assess the effect of a rapid diagnosis of influenza on the management of children with influenza‐like illnesses.
Children meeting the inclusion criteria were randomized to undergo tonsillar/pharyngeal swabs for rapid influenza testing or standard care. Results of influenza testing were made available to the treating physician within approximately 10 minutes, who then decided on further testing and management.
Endpoints analyzed in this study included: rates of routine blood examinations, chest X‐rays, antibiotic prescription and days on antibiotics, admission to hospital, and antiviral drug use.
In this study, participants with a positive influenza diagnosis were significantly less likely to receive routine blood examinations or be prescribed antibiotics when compared with those not receiving rapid viral testing. No significant differences were found between the two groups with respect to rates of chest X‐rays or admission to hospital. If children were prescribed antibiotics, there was no difference in length of antibiotic use. No children were prescribed antivirals.
We excluded this study due to the fact that children with underlying illnesses were included; the study included children with congenital heart disease, asthma, malignancy, neurological deficits, and cystic fibrosis.
Abanses 2006 was a large, single‐center RCT assessing healthy participants aged 3 to 36 months presenting to a large urban pediatric ED (64,000 patient visits per year) with fever. The goal of the study was to assess how rapid influenza testing of febrile infants and children affected physician decision‐making with respect to diagnostic testing as well as ED charges and patient time in the ED. Although the inclusion criteria was based on fever, we analyzed this paper as a large proportion of children (> 60%) were found to have respiratory symptoms in the form of tachypnea.
Children meeting the inclusion criteria were randomized into two groups. One group had rapid influenza test results available to the treating physician prior to assessment, while the other group had influenza testing done only at the discretion of the treating physician after initial assessment. Study endpoints, as stated above, were: rates of diagnostic testing, ED charges, and length of ED visit.
Although block randomizations are mentioned, what is described is actually cluster‐randomization by 24‐hour periods. Despite the initial intent to conduct a RCT, non‐adherence to the protocol led to a significant number of participants not receiving the treatment they were randomly allocated to receive. A decision was made to analyze data as per actual treatment received, hence a convenience sample. Although there is mention of intention‐to‐treat analysis yielding no significant difference in the outcome measures between the two study groups, the results were not reported. Due to the failed randomization, this study did not meet this review's inclusion criteria.
Cohen 2007 was a multicenter, cluster‐RCT of 30 community pediatric offices in France; 16 offices were randomized to use Quickvue rapid influenza test and 14 were not. A total of 602 participants, aged 1 to 17 years, with influenza‐like illnesses (chills, upper respiratory symptoms, headaches, or myalgia) and without focal infections, were enrolled. The primary objective was to compare oseltamivir use, and secondary objectives included comparisons of clinical presentation, ancillary testing, and antibiotic use between the two study groups.
This study found that with participants enrolled in pediatric offices where rapid influenza testing was used, oseltamivir was used more frequently (37.9% versus 13.7%, P value < 0.0001). Antibiotics (9.5% versus 3.9%, P value = 0.008) and chest radiography (4.0% versus 1.2%, P value = 0.035) were also more frequently used in the rapid influenza testing group. Statistically and clinically significant differences in clinical features between the two study groups included a younger mean age (4.7 versus 5.7 years old, P value = 0.0001), and a larger proportion of asthmatic participants (15.9% versus 10.2%, P value = 0.04).
This is the first RCT of rapid influenza testing in community pediatric practices. We did not include this study in this review because the setting was not in the ED. One particular concern with this study is the large number of analytical comparisons (well over 30) without corrections surrounding the statistical significance level.
Ozkaya 2009 was a study of children aged between 3 and 14 years, treated at a pediatric ED with influenza‐like illnesses, to evaluate the impact of rapid viral testing on the rate of antibiotic prescription. The intervention group underwent rapid influenza testing and results were made available to the treating clinician prior to making a decision regarding antibiotic prescription. Not only did the control group undergo rapid influenza testing after the child had already been prescribed antibiotics, but children were only enrolled into the control group if an antibiotic had been prescribed. The rate of antibiotic prescription in the group of children with a known influenza status was not compared to the incidence of antibiotic prescription in a group of children with an unknown influenza status; it was compared to a pre‐established rate of antibiotic prescription (100%), as dictated by the investigators, through their selection criteria. We excluded this study from this review as the significance of such a comparison and the interpretation of this study are arguable.
Ozkaya 2010 was a study involving children aged 8 months to 11 years, treated at a pediatric ED with signs and symptoms consistent with an influenza‐like illness, to evaluate the impact of rapid influenza testing on the rate of ancillary testing, ED length of stay, and rate of admission to an observation unit. One hundred and fifty screened children were eligible to participate in the study, but only 75 were enrolled. The reason for this was not explained. The intervention group had nasopharyngeal swabs collected for testing with influenza A/B rapid test kits, and results were made available to the treating clinician prior to their initial clinical assessment. The other group was managed without knowledge of their influenza status. Rapid influenza testing was ordered by the investigator (not involved in the participants' clinical management) at the time of other ancillary testing being ordered by the treating clinician. The timing of patient selection and enrollment into this control group is not explicitly described. Allocation concealment and blindness to the outcome measures at the time of enrollment cannot be assured. Furthermore, treatment allocation was not randomized, nor described. We excluded this study from this review due to these significant methodological issues.
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 1 and summarized in Figure 2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Bonner 2003 and Doan 2009 used computer randomization programs to block randomize participants to their study groups. Poehling 2006 used a random number generator to randomize study days in blocks of four and six. Iyer 2006 allocated participants to study groups using alternating days.
Blinding
This intervention does not lend itself to blinding.
Incomplete outcome data
We successfully retrieved all incomplete outcome data by contacting the individual study authors.
Selective reporting
We found no selective reporting in the included trials.
Other potential sources of bias
In the two trials where participants are individually randomized (Bonner 2003; Doan 2009), as opposed to using randomizing days, as in the Poehling 2006 trial, there is potential for contamination. If many children are rapidly diagnosed with influenza on a given day (in the intervention group), it is possible that children without rapid viral testing (in the control group) would be assumed by the treating physician to have influenza due to the commonality in their presentation with children in the intervention group. This would introduce a conservative bias, reducing the difference in effect between the two study groups and increasing a type II error.
Effects of interventions
Primary outcome
1. Antimicrobial prescription rate in the Emergency Department (ED)
All four studies reported the proportion of participants receiving or being prescribed antibiotics in the ED by study groups. Three did not find a statistically significant effect despite a trend favoring rapid viral testing. Bonner 2003 was the only trial to report a statistically significant effect for rapid influenza testing on antibiotic prescription (risk ratio (RR) 0.66, 95% confidence interval (CI) 0.45 to 0.96). Pooled results showed a non‐statistically significant trend for reduced antibiotic prescription in the ED favoring the treatment group (RR 0.89, 95% CI 0.71 to 1.12) (Analysis 1.1). Sensitivity analysis using the three trials deemed adequate (higher quality) by the method of Higgins 2011 (Bonner 2003; Doan 2009; Poehling 2006), did not find a statistically significant effect either (RR 0.86, 95% CI 0.61 to 1.22) (Analysis 1.2).
Secondary outcomes
1. Length of hospital (ED) stay: we considered a reduction of 30 minutes as clinically important
Three studies reported on this outcome. Only Bonner 2003 showed a statistically significant effect, while Doan 2009 and Iyer 2006 only showed a trend favoring rapid viral testing. Pooled results showed no statistically significant reduction in mean ED length of visit (mean difference (MD) ‐10.6 minutes, 95% CI ‐22.47 to 1.25) (Analysis 2.1). Sensitivity analysis using only the two trials deemed adequate by the method of Higgins 2011 (Bonner 2003; Doan 2009), did not find a statistically significant effect either (MD ‐19.47, 95% CI ‐51.38 to 12.44) (Analysis 2.2).
2. Rate of ancillary tests (any blood tests, urine investigations or chest radiography) requested
Blood tests
All four studies reported proportions of participants undergoing blood investigations. Bonner 2003 and Iyer 2006 reported complete blood count (CBC) and blood cultures separately. We anticipated substantial overlap between participants receiving CBC and blood cultures and so contacted study authors who provided the data for us to analyze them as one outcome. Pooled results showed a lower rate of blood investigations in the treatment group, which was not statistically significant (RR 0.79, 95% CI 0.62 to 1.0) (Analysis 3.1). Sensitivity analysis of the three trials deemed adequate by the method of Higgins 2011 (Bonner 2003; Doan 2009; Poehling 2006), however, found a significant effect (RR 0.61, 95% CI 0.42 to 0.89) (Analysis 3.3).
Urine investigations
All four studies reported the proportion of participants undergoing urine investigations. Bonner 2003 and Iyer 2006 reported urine analyses and urine cultures separately. We anticipated some overlap between participants undergoing urine analysis and urine cultures and so contacted study authors, who provided the data for us to analyze them as one outcome. None of the studies found a statistically significant difference in rate of urine investigations between the study groups. Pooled results showed no meaningful nor statistically significant effect of rapid viral testing on urine investigations in the ED (RR 0.97, 95% CI 0.79 to 1.19) (Analysis 3.2). Sensitivity analysis using the three trials deemed adequate by the method of Higgins 2011 (Bonner 2003; Doan 2009; Poehling 2006), found similar results (RR 0.93, 95% CI 0.70 to 1.25) (Analysis 3.4).
Chest radiography
All four studies reported on this outcome. Three did not find a statistically significant effect despite a trend favoring rapid viral testing. Bonner 2003 was the only one to report a statistically significant effect for rapid influenza testing on chest radiography (RR 0.61, 95% CI 0.40 to 0.92). Pooled results showed a statistically significant effect of rapid viral testing on chest radiography in the ED favoring the treatment group (RR 0.77, 95% CI 0.65 to 0.91) (Analysis 4.1). Sensitivity analysis of the three trials deemed adequate by the method of Higgins 2011 found a similar but stronger effect (RR 0.59, 95% CI 0.43 to 0.81) (Analysis 4.2).
3. Rate of physician visit (ED or office) within two weeks after discharge from ED
Only two studies reported on this outcome (Doan 2009; Iyer 2006). Neither found a statistically significant effect, and pooled results did not find a significant effect either (RR 1.00, 95% CI 0.77 to 1.29) (Analysis 5.1). Only Doan 2009 was deemed adequate by the method of Higgins 2011, hence we did not perform a sensitivity analysis for this outcome.
4. Hospital admission rate
Only one study reported this outcome (Iyer 2006). Point of care testing for influenza increased rates of admission compared with standard influenza testing, with an admission rate of 11.6% (95% CI 8.2 to 15.0) for point of care testing and an admission rate of 10.4% (95% CI 7.2 to 13.6) for standard influenza testing.
5. Acceptability of nasal specimen collection sampling for rapid viral testing
None of the included studies provided data on this outcome.
Heterogeneity
We performed tests of heterogeneity for all outcome measures. There was no suggestion of significant heterogeneity, but the small number of trials included in this review may have contributed to the lack of significance on these statistical tests.
Discussion
Summary of main results
This meta‐analysis demonstrated that the use of a rapid viral diagnostic test did not dramatically affect physician decision‐making. The only exception to this was the fact that a rapid diagnosis of a viral infection reduced the rate of chest radiography use in the Emergency Department (ED). A weak trend toward reduction in antibiotics and ED length of visit was seen, but these were not statistically significant.
Overall completeness and applicability of evidence
The results of this meta‐analysis suggest a benefit in using rapid respiratory viral testing, mainly for reducing the rate of chest radiography and blood investigations, but the evidence surrounding antibiotics is still incomplete. Although a weak trend for a reduction in antibiotic prescription rate was shown, this was not statistically significant and the results of individual trials on this outcome were conflicting, making the current evidence not yet applicable.
Most studies of rapid viral testing have been aimed at detecting influenza virus only, except for one, which used a multi‐respiratory viral panel. While a multi‐viral panel can capture a larger number of viruses, the test used by Doan 2009 was laboratory bound and not as freely accessible to clinicians as the rapid influenza test, which can be performed at the bedside, and therefore offers a much more rapid result for the treating physician. Although point‐of‐care testing for respiratory syncytial virus (RSV) is available and has been shown to have high sensitivity (90%) and specificity (92%) (Mackie 2001), we have not found any trials using rapid RSV testing meeting the criteria for our review. Considering that RSV and influenza formed 73% to 95% of the positive viral tests in the study by Doan 2009, perhaps using point‐of‐care testing for influenza and RSV in the ED through future studies would provide more evidence to support the practice of rapid viral testing in the ED.
The evidence we gathered through this review is still lacking information on key issues surrounding the implementation of rapid viral testing in the ED. We have found no information on safety and side effects of this intervention, nor cost comparisons between the rapid viral test and the averted ancillary testing by using this intervention. It will be difficult to evaluate the value caregivers assign to averting blood sampling, radiography exposure, and unnecessary antibiotics, as well as shortening their ED visit, and may require a different approach from randomized controlled trials (RCTs).
Quality of the evidence
Three out of four of the included studies are high‐quality RCTs. The one quazi‐RCT was clearly stated as such and the methodology was well described (Iyer 2006). We have therefore presented the results for individual outcomes (where possible) with and without the contribution of the quazi‐RCT.
The bias from contamination, which may have been introduced with the two trials of individual subject randomization (Bonner 2003; Doan 2009), would be a conservative one and strengthens the validity of the significant findings in this meta‐analysis.
Potential biases in the review process
To the best of our knowledge, no bias was introduced during the review process.
Agreements and disagreements with other studies or reviews
Reasons for lack of effect on antibiotic prescription rates and urine investigations are unclear. Although Levine 2004 and Byington 2004 reported lower rates of bacterial infections in febrile infants less then three months old who tested positive for viral infection, the rate of bacterial urinary tract infection in that group was not negligible (up to 7%). It is possible that physicians may still be apprehensive in dismissing the potential for a concurrent urinary tract infection, despite the presence of a virus and persist in ordering urine tests and prescribing precautionary antibiotics. Urinary testing is dependent on obtaining a urine sample, which in young children may take a long time, hence prolong the ED length of visits.
However, Purcell 2002 reports the rate of bacterial infection (all were urinary tract infections) in febrile RSV positive children up to two years old (one‐third were less than three months old), to be much lower, at less than 1%, which calls into question this precautionary practice, at least in children over three months old.
A number of rapid viral testing studies report subgroup analyses of participants with positive rapid viral results versus those with negative results. These demonstrated that a positive rapid viral diagnosis reduced the number of ancillary tests, antibiotics prescribed, and ED length of visits. While it is interesting to see that a positive result can reduce the number of additional tests, the question is whether the rapid viral test is worth doing before one knows its result. As rapid viral diagnosis requires an invasive and uncomfortable test for the children (either through nasopharyngeal swabs or washings), it is important to determine how it may affect the outcome of the tested population as a whole. In the four studies mentioned above, the rate of positive viral diagnosis ranged from as low as 19% to 66% (19% to 52% for influenza testing alone and 66% for multi‐viral testing). Therefore, at least one‐third of the children received an invasive test that may not have altered the course of their work‐up or management. As one cannot definitively predict whether a child will have a positive test prior to doing it, this represents a large number of unhelpful tests, which will actually add to the burden presented by children with acute febrile respiratory illnesses.
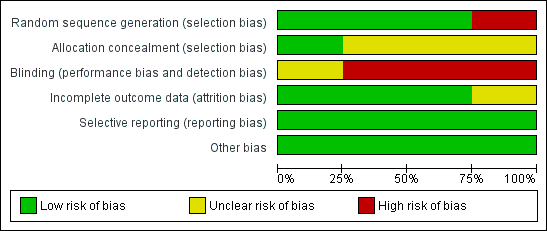
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotics use, Outcome 1 Antibiotics prescribed in ED.

Comparison 1 Antibiotics use, Outcome 2 Sensitivity analysis per risk of bias.

Comparison 2 ED length of visit, Outcome 1 Mean ED length of visit in minutes.
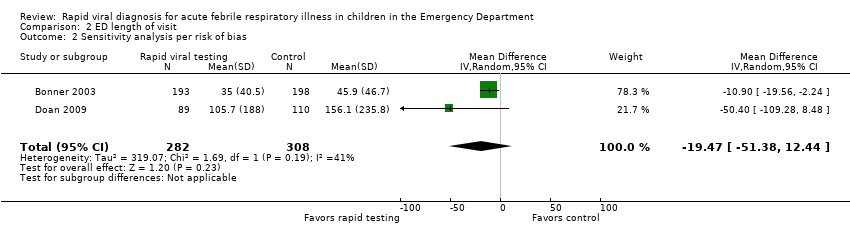
Comparison 2 ED length of visit, Outcome 2 Sensitivity analysis per risk of bias.
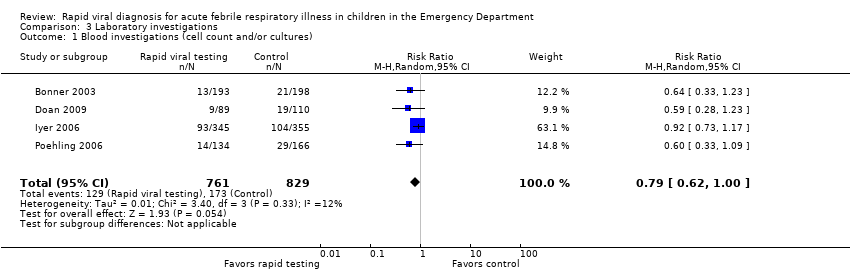
Comparison 3 Laboratory investigations, Outcome 1 Blood investigations (cell count and/or cultures).

Comparison 3 Laboratory investigations, Outcome 2 Urine testing.

Comparison 3 Laboratory investigations, Outcome 3 Blood investigation: sensitivity analysis per risk of bias.
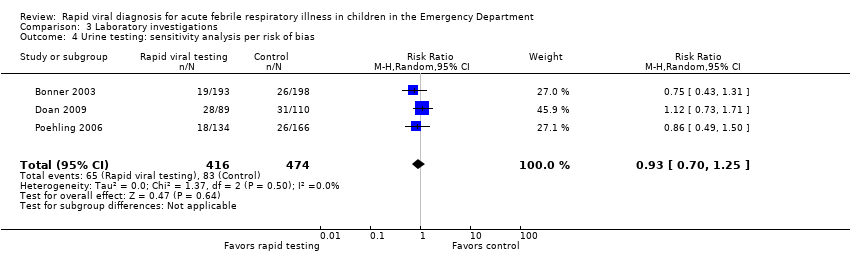
Comparison 3 Laboratory investigations, Outcome 4 Urine testing: sensitivity analysis per risk of bias.

Comparison 4 Chest radiography, Outcome 1 Chest radiography.
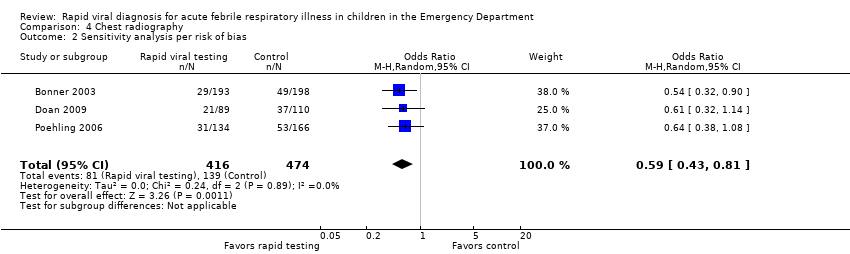
Comparison 4 Chest radiography, Outcome 2 Sensitivity analysis per risk of bias.

Comparison 5 Visits to physician or ED post ED discharge, Outcome 1 Post ED discharge visit to MD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Antibiotics prescribed in ED Show forest plot | 4 | 1590 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.71, 1.12] |
| 2 Sensitivity analysis per risk of bias Show forest plot | 3 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.61, 1.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean ED length of visit in minutes Show forest plot | 3 | 1290 | Mean Difference (IV, Random, 95% CI) | ‐10.61 [‐22.47, 1.25] |
| 2 Sensitivity analysis per risk of bias Show forest plot | 2 | 590 | Mean Difference (IV, Random, 95% CI) | ‐19.47 [‐51.38, 12.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Blood investigations (cell count and/or cultures) Show forest plot | 4 | 1590 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.00] |
| 2 Urine testing Show forest plot | 4 | 1588 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.19] |
| 3 Blood investigation: sensitivity analysis per risk of bias Show forest plot | 3 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.89] |
| 4 Urine testing: sensitivity analysis per risk of bias Show forest plot | 3 | 890 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.70, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Chest radiography Show forest plot | 4 | 1590 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.65, 0.91] |
| 2 Sensitivity analysis per risk of bias Show forest plot | 3 | 890 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post ED discharge visit to MD Show forest plot | 2 | 899 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.77, 1.29] |

