Antagonistas opiáceos Mu para la disfunción intestinal inducida por opiáceos en pacientes con cáncer y en pacientes que reciben cuidados paliativos
Appendices
Appendix 1. Search strategies for searches ran 2017
| 157 ‐Mu‐opioid antagonists for opioid‐induced bowel dysfunction August 2017 |
| Database searched | Date of last search | April 2016 | August 2017 | Total |
| CENTRAL (the Cochrane Library) Issue 7 of 12, 2017 | 30 August 2017 | 67 | 17 | 84 |
| MEDLINE and MEDLINE in Process (Ovid) 2007 to 28 August 2017 | 29 August 2017 | 171 | 36 | 207 |
| EMBASE (Ovid) 2007 to 2017 week 35 | 29 August 2017 | 264 | 36 | 300 |
| CINAHL (EBSCO) 1982 to August 2017 | 29 August 2017 | 37 | 0 | 37 |
| Web of Science ISI (SCI‐EXPANDED & CPCI‐S) 1945 to 28 August 2017 | 29 August 2017 | 251 | 32 | 283 |
| Total | 790 | 121 | 911 | |
| After deduplication | 557 | 76 | 633 | |
CENTRAL
#1 MESH DESCRIPTOR Constipation 920
#2 (constipat* or laxation or (bowel near2 dysfunction*)):TI,AB,KY 5843
#3 MESH DESCRIPTOR Ileus EXPLODE ALL TREES 143
#4 MESH DESCRIPTOR Gastrointestinal Motility EXPLODE ALL TREES 2592
#5 MESH DESCRIPTOR Gastrointestinal Tract EXPLODE ALL TREES 9982
#6 #1 OR #2 OR #3 OR #4 OR #5 16865
#7 MESH DESCRIPTOR Narcotic Antagonists EXPLODE ALL TREES 2776
#8 ((Naltrexone or Naloxone or Methylnaltrexone or nalmefene or Alvimopan or ADL 8‐2698 or LY246736)) or ((mu‐opioid near2 (receptor* or antagonist*)) or (pentazocine or nalbuphine or buprenorphine or dezocine or butorphanol or loperamide or PAMORA or movantik or naloxegol)):TI,AB,KY 5872
#9 MESH DESCRIPTOR Receptors, Opioid EXPLODE ALL TREES 348
#10 ((neoplasm* or cancer* or carcinoma* or neoplasia* or adenocarcinoma* or tumor or malignan* or tumour*)):TI,AB,KY 94907
#11 MESH DESCRIPTOR neoplasms EXPLODE ALL TREES 45998
#12 ((palliat* or terminal* or endstage or hospice* or (end near3 life) or (care near3 dying) or ((advanced or late or last or end or final) near3 (stage* or phase*)))):TI,AB,KY 15402
#13 MESH DESCRIPTOR Palliative Care 1214
#14 MESH DESCRIPTOR Terminal Care EXPLODE ALL TREES 314
#15 #12 OR #13 OR #14 15418
#16 #10 OR #11 99655
#17 #7 OR #8 OR #9 6102
#18 #15 OR #16 110040
#19 #6 AND #17 AND #18 95
#20 2007 TO 2016:YR 360460
#21 #19 AND #20 67
MEDLINE
1 Constipation/ (11563)
2 (constipat* or laxation or (bowel adj2 dysfunction*)).tw. (16978)
3 exp Ileus/ (4645)
4 exp Gastrointestinal Motility/ (34225)
5 exp Gastrointestinal Tract/ (578429)
6 1 or 2 or 3 or 4 or 5 (610305)
7 exp Narcotic Antagonists/ (34192)
8 (Naltrexone or Naloxone or Methylnaltrexone or nalmefene or Alvimopan or ADL 8‐2698 or LY246736 or MNTX or oxycodone or targinact).mp. (32527)
9 ((mu‐opioid adj2 (receptor* or antagonist*)) or (pentazocine or nalbuphine or buprenorphine or dezocine or butorphanol or loperamide or PAMORA or movantik or naloxegol )).mp. (16793)
10 exp Receptors, Opioid/ (23399)
11 or/7‐10 (58741)
12 (neoplasm* or cancer* or carcinoma* or neoplasia* or adenocarcinoma* or tumor or malignan* or tumour*).tw. (2224162)
13 exp Neoplasms/ (2813163)
14 12 or 13 (3230772)
15 (palliat* or terminal* or endstage or hospice* or (end adj3 life) or (care adj3 dying) or ((advanced or late or last or end or final) adj3 (stage* or phase*))).tw. (571727)
16 Palliative Care/ (44067)
17 exp Terminal Care/ (43534)
18 15 or 16 or 17 (610189)
19 14 or 18 (3708789)
20 6 and 11 and 19 (435)
21 randomized controlled trial.pt. (411978)
22 controlled clinical trial.pt. (90457)
23 randomized.ab. (308871)
24 placebo.ab. (157136)
25 drug therapy.fs. (1841827)
26 randomly.ab. (218163)
27 trial.ab. (319135)
28 groups.ab. (1379535)
29 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 (3495620)
30 exp animals/ not humans.sh. (4221321)
31 29 not 30 (2977237)
32 20 and 31 (239)
33 (2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016*).ed. (7189774)
34 32 and 33 (171)
Embase
1 Constipation/ (66990)
2 (constipat* or laxation or (bowel adj2 dysfunction*)).tw. (31395)
3 exp Ileus/ (10689)
4 exp Gastrointestinal Motility/ (29756)
5 exp Gastrointestinal Tract/ (36303)
6 1 or 2 or 3 or 4 or 5 (144623)
7 exp Narcotic Antagonist/ (56632)
8 (Naltrexone or Naloxone or Methylnaltrexone or nalmefene or Alvimopan or ADL 8‐2698 or LY246736 or MNTX or oxycodone or targinact).mp. (64688)
9 ((mu‐opioid adj2 (receptor* or antagonist*)) or (pentazocine or nalbuphine or buprenorphine or dezocine or butorphanol or loperamide or PAMORA or movantik or naloxegol)).mp. (40254)
10 exp Opiate receptor/ (33249)
11 or/7‐10 (111413)
12 exp neoplasm/ (3639889)
13 (neoplasm* or cancer* or carcinoma* or neoplasia* or adenocarcinoma* or tumor or malignan* or tumour*).tw. (3148234)
14 12 or 13 (4328511)
15 (palliat* or terminal* or endstage or hospice* or (end adj3 life) or (care adj3 dying) or ((advanced or late or last or end or final) adj3 (stage* or phase*))).tw. (734729)
16 exp palliative therapy/ (77974)
17 terminal care/ or hospice care/ (33098)
18 15 or 16 or 17 (770267)
19 14 or 18 (4905872)
20 6 and 11 and 19 (1858)
21 random$.tw. (1070907)
22 factorial$.tw. (27377)
23 crossover$.tw. (56887)
24 cross over$.tw. (25402)
25 cross‐over$.tw. (25402)
26 placebo$.tw. (235531)
27 (doubl$ adj blind$).tw. (166956)
28 (singl$ adj blind$).tw. (17421)
29 assign$.tw. (283715)
30 allocat$.tw. (102682)
31 volunteer$.tw. (205346)
32 Crossover Procedure/ (46656)
33 double‐blind procedure.tw. (234)
34 Randomized Controlled Trial/ (400175)
35 Single Blind Procedure/ (21855)
36 or/21‐35 (1679747)
37 (animal/ or nonhuman/) not human/ (5008260)
38 36 not 37 (1491043)
39 20 and 38 (352)
40 (2007* or 2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015* or 2016*).dd. (13117895)
41 39 and 40 (264)
CINAHL
S29 S28 AND S20
S28 S23 or S27
S27 S24 OR S25 OR S26
S26 (MH "Terminal Care+")
S25 (MH "Palliative Care")
S24 (palliat* or terminal* or endstage or hospice* or (end N3 life) or (care N3 dying) or ((advanced or late or last or end or final) N3 (stage* or phase*)))
S23 S21 or S22
S22 (neoplasm* or cancer* or carcinoma* or neoplasia* or adenocarcinoma* or tumor or malignan* or tumour*)
S21 (MH "Neoplasms+")
S20 S19 and S10
S19 S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18
S18 (allocat* random*)
S17 (MH "Quantitative Studies")
S16 (MH "Placebos")
S15 placebo*
S14 (random* allocat*)
S13 (MH "Random Assignment")
S12 (Randomi?ed control* trial*)
S11 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S10 S5 AND S19
S9 S6 or S7 or S8
S8 ((mu‐opioid N2 (receptor* or antagonist*))or (pentazocine or nalbuphine or buprenorphine or dezocine or butorphanol or loperamide or PAMORA or movantik or naloxegol )
S7 (Naltrexone or Naloxone or Methylnaltrexone or nalmefene or Alvimopan or ADL 8‐2698 or LY246736 or MNTX or oxycodone or targinact)
S6 (MH "Narcotic Antagonists+")
S5 S1 OR S2 OR S3 OR S4
S4 (MH "Gastrointestinal Motility+")
S3 (MH "Intestinal Obstruction+")
S2 (constipat* or laxation or (bowel N2 dysfunction*))
S1 (MH "Constipation")
Web of Science
#19 #15 and #18
#18 #17 or #16
#17 ((palliat* or terminal* or endstage or hospice* or (end near/3 life) or (care near/3 dying) or ((advanced or late or last or end or final) near/3 (stage* or phase*))))
#16 ((neoplasm* or cancer* or carcinoma* or neoplasia* or adenocarcinoma* or tumor or malignan* or tumour*))
#15 #14 AND #9
#14 #13 OR #12 OR #11 OR #10
#13 TS=trial* OR TI=trial*
#12 TI=clin* OR TS=clin*
#11 TI=randomi* OR TS=randomi*
#10 TS=Randomized clinical trial* OR TI=Randomized clinical trial*
#9 #8 AND #5
#8 #7 OR #6
#7 TOPIC: (((mu‐opioid near/2 (receptor* or antagonist*)) or ((pentazocine or nalbuphine or
buprenorphine or dezocine or butorphanol or loperamide or PAMORA or movantik or naloxegol))
#6 TOPIC: ((Naltrexone or Naloxone or Methylnaltrexone or nalmefene or Alvimopan or ADL 8‐2698 or LY246736 or MNTX or oxycodone or targinact))
#5 #4 OR #3 OR #2 OR #1
#4 TOPIC: ("Gastrointestinal Tract")
#3 TOPIC: ("Gastrointestinal Motility")
# 2 TOPIC: (Ileus)
# 1 TOPIC: ((constipat* or laxation or (bowel near/2 dysfunction*)))
Appendix 2. Letter to pharmaceutical companies
Example, as was sent to AstraZeneca, of letter sent to pharmaceutical companies
Research and Communications
Manager (or equivalent)
AstraZeneca (Global HQ)
Floors 7‐9,
2 Kingdom Street,
Paddington Central,
London, W2 6BD, UK
Email: [email protected]
Phone: +44 020767997
March 31st 2016
Dear Sir or Madam
Mu‐opioid antagonists for opioid‐induced bowel dysfunction in cancer and palliative care patients ‐ a Cochrane systematic review
We address you in order to request your assistance. We are conducting a systematic review on the effect of mu‐opioid antagonists for opioid‐induced bowel dysfunction in cancer and palliative care patients. We are working with the Cochrane Pain, Palliative and Supportive Care Review Group (www.papas.cochrane.org).
Our systematic review intends to include all relevant literature empirically describing both the positive and possibly negative effects of mu‐opioid antagonists. We believe that conducting this review is in the common interest of patients, doctors and pharmaceutical manufacturers. Furthermore, it is an important ethical issue. The results from this review will, in the future, guide authorities, clinicians and researchers when it comes to considering the use of a mu‐opioid antagonist in the treatment of opioid‐induced bowel dysfunction for cancer and palliative care patients.
Our Cochrane review will be comprehensive. The currently included studies come from our search for literature through international scientific databases. However, the published literature only provides us with limited and possibly selective knowledge, since it is unlikely that all studies and data are available through these databases. By contacting authors of significant publications, experts in the field and pharmaceutical companies, we hope to be informed of additional studies, published as well as unpublished. This approach has been used in other Cochrane systematic reviews investigating medical preparations for common illnesses such as Attention Deficit Hyperactivity Disorder (http://www.bmj.com/content/351/bmj.h5203).
We hope you will assist us with providing studies and data that are relevant for our review. We are aware from searches of electronic citation databases including PubMed and clinicaltrials.gov of one trial for which AstraZeneca are the responsible party/study sponsors (NCT01384292). As previously noted, we are interested in data regarding both positive and negative effects of mu‐opioid antagonists for opioid‐induced bowel dysfunction in cancer and palliative care patients, from randomised clinical trials, regardless of the year the data were recorded or published.
We will state which companies we have been in contact with, and acknowledge those who have assisted us with provision of data. We would be happy to meet a representative from your company if you would like to speak in person. If you have any questions, please contact us.
Enclosed below in this letter is a list of the currently included studies in our review.
We look forward to your response.
Yours faithfully
Bridget Candy
PhD, Senior Research Fellow
University College London,
6th Floor, Maple House,
149 Tottenham Court Road,
London W1T 7NF, UK
Phone: +44 02076799713
E‐mail: [email protected]
Louise Jones
Senior Clinical Lecturer
University College London,
6th Floor, Maple House,
149 Tottenham Court Road,
London W1T 7NF, UK
Patrick Stone
Professor of Palliative and End of Life Care,
Marie Curie Palliative Care Research Department
University College London,
6th Floor, Maple House,
149 Tottenham Court Road,
London W1T 7NF, UK
Phil Larkin
Professor of Clinical Nursing
School of Nursing, Midwifery and Health Systems,
University College Dublin, Belfield, Dublin 4, Republic of Ireland
Vicky Vickerstaff
Statistician
University College London,
6th Floor, Maple House,
149 Tottenham Court Road,
London W1T 7NF, UK
(copy sent via [email protected])
List of the currently included studies in our review
Ahmedzai SH, Nauck F, Bar‐Sela G, Bosse B, Leyendecker P, Hopp M. A randomized, double‐blind, active‐ controlled, double‐dummy, parallel‐group study to determine the safety and efficacy of oxycodone/naloxone prolonged‐release tablets in patients with moderate/severe, chronic cancer pain. Palliative Medicine 2012; 26: 50‐60.
Bull J, Wellman CV, Israel RJ, Barrett AC, Paterson C, Forbes WP. Fixed‐Dose Subcutaneous Methylnaltrexone in Patients with Advanced Illness and Opioid‐Induced Constipation: Results of a Randomized, Placebo‐Controlled Study and Open‐Label Extension. Journal of Palliative Medicine 2015;18:593‐600.
Chamberlain BH, Cross K, Winston JL , Thomas J, Wang W, Su C, Israel RJ. Methylnaltrexone Treatment of Opioid‐Induced Constipation in Patients with Advanced Illness. Journal of Pain and Symptom Management 2009;38: 683‐90.
Portenoy RK, Thomas J, Moehl Boatwright ML, Galasso FL, Stambler N, Von Gunten CF, et al. Subcutaneous methylnaltrexone for the treatment of opioid‐induced constipation in patients with advanced illness: a double‐ blind, randomised, parallel group, dose‐ranging study. Journal of Pain and Symptom Management 2008;35: 458‐68.
Slatkin N, Thomas J, Lipman AG, Wilson G, Boatwright ML, Wellman C, et al. Methylnaltrexone for treatment of opioid‐induced constipation in advanced illness patients. Journal of Supportive Oncology 2009;7: 39‐46.
Sykes NP. An investigation of the ability of oral naloxone to correct opioid‐related constipation in patients with advanced cancer. Palliative Medicine 1996;10:135‐44.
Thomas J, Karver S, Cooney GA, et al. A randomized, placebo‐controlled trial of subcutaneous methylnaltrexone for the treatment of opioid‐ induced constipation in patients with advanced illness. New England Journal of Medicine 2008;358: 2332‐4.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
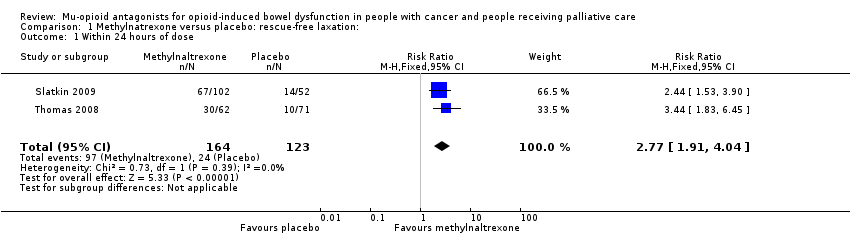
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 1 Within 24 hours of dose.

Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 2 Within 4 hours after 4 of the 7 doses.
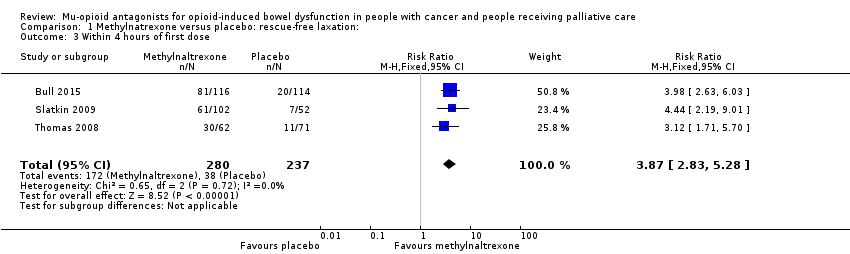
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 3 Within 4 hours of first dose.

Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 4 Within 4 hours after 1 or 2 doses of the first 4 doses.

Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 5 Improvement in constipation distress at day 1.
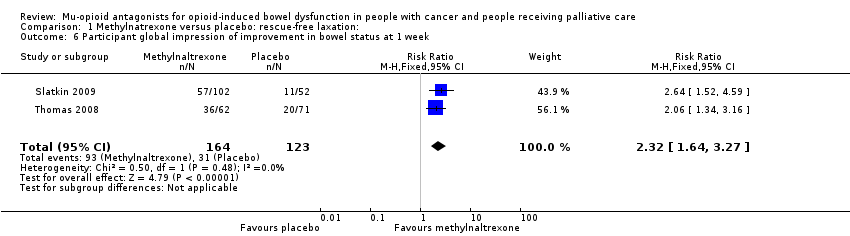
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 6 Participant global impression of improvement in bowel status at 1 week.
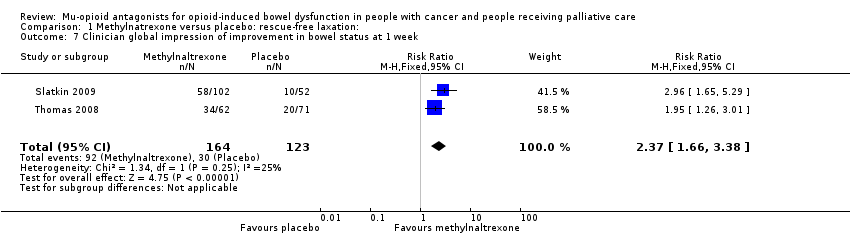
Comparison 1 Methylnatrexone versus placebo: rescue‐free laxation:, Outcome 7 Clinician global impression of improvement in bowel status at 1 week.

Comparison 2 Methylnaltrexone versus placebo: serious adverse event, Outcome 1 Serious adverse event.

Comparison 3 Methylnaltrexone versus placebo: adverse event, Outcome 1 Adverse events.

Comparison 3 Methylnaltrexone versus placebo: adverse event, Outcome 2 Dropouts due to adverse event.

Comparison 4 Oxycodone/naloxone prolonged‐release tablets versus oxycodone prolonged‐release: adverse event, Outcome 1 Adverse events.
| Naldemedine compared to placebo for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Settings: cancer care Intervention: naldemedine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Naldemedine | |||||
| Laxation response within 24 hours of dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14a | 375 per 1000 | 724 per 1000 | RR 1.93 (1.36 to 2.74) NNTB 2.88 (2.04 to 4.92) | 225 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: opioid withdrawalc | — | — | 0.1 mg: MD ‐0.13 (‐0.57 to 0.31); 0.2 mg: MD ‐0.40 (‐0.87 to 0.07); 0.4 mg: MD ‐0.02 (‐0.45 to 0.41) | 225 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: pain intensity | — | — | — | — | — | Not reported |
| Serious adverse eventsa | — | — | 5 SAEs occurred, all in naldemedine group. | 225 (1 study) | ⊕⊕⊝⊝ | — |
| Adverse eventsa | 518 per 1000 | 704 per 1000 (539 to 927) | RR 1.36 (1.04 to 1.79) | 225 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio; SAE: serious adverse events. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by clinician or self‐report and in the case of adverse events using severity grades according to the Common Terminology Criteria for Adverse Events. | ||||||
| Lower‐dose naldemedine compared to higher‐dose naldemedine for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Setting: cancer care Intervention: lower dose naldemedine 0.1 mg daily Comparison: higher dose naldemedine 0.2 mg or 0.4 mg daily | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Higher dose 0.2 mg/0.4 mg daily | Lower dose 0.1 mg daily | |||||
| Laxation response within 24 hours of dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14a | 0.1 mg vs 0.2 mg: 776 per 1000 0.1 mg vs 0.4 mg: 821 per 1000 | 0.1 mg vs 0.2 mg: 564 per 1000 (430 to 739) 0.1 mg vs 0.4 mg: 564 per 1000 (433 to 733) | 0.1 mg vs 0.2 mg: RR 0.73 (0.55 to 0.95) 0.1 mg vs 0.4 mg: RR 0.69 (0.53 to 0.89) | 226 (1 study) 0.1 mg vs 0.2 mg: n = 113 0.1 mg vs 0.4 mg: n = 111 | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: opioid withdrawal | — | — | — | — | — | Not reported |
| Effect on analgesia: pain intensity | — | — | — | — | — | Not reported |
| Serious adverse events | — | — | — | — | — | Not reported |
| Adverse events | — | — | — | — | — | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by self‐report. | ||||||
| Naloxone compared with placebo for cancer and people receiving palliative care with opioid‐induced bowel dysfunction | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Settings: cancer care Intervention: naloxone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Naloxone | |||||
| Laxation response within 24 hours of a dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14 | — | — | — | — | — | Not reported |
| Effect on analgesia: opioid withdrawal | — | — | — | — | — | Not reported |
| Effect on analgesia: pain intensitya | — | — | No statistical difference in pain experienced when taking placebo or naloxone. Full data, including pre‐cross‐over results, were not provided. | 17 (1 study) | ⊕⊝⊝⊝ | — |
| Serious adverse events | — | — | — | — | — | Not reported |
| Adverse events | — | — | — | — | — | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured using 4‐point scale (0 = no pain, 3 = severe pain). | ||||||
| Oxycodone/naloxone prolonged release tablets compared with oxycodone prolonged‐released tablets for opioid‐induced bowel dysfunction | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Settings: cancer care Intervention: oxycodone/naloxone prolonged‐release tablets Comparison: oxycodone prolonged‐released tablets | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oxycodone | Oxycodone/naloxone | |||||
| Laxation response within 24 hours of dose | — | — | — | — | — | Not reported |
| Laxation response between day 1 and day 14 | — | — | — | — | — | Not reported |
| Effect on analgesia: opioid withdrawalc | — | — | Intervention group: mean 6.64 (SD 5.97) comparison group: mean 7.29 (SD 4.59) at 7 days | 184 (1 study) | ⊕⊕⊕⊝ Moderateb | — |
| Effect on analgesia: pain intensitya | — | — | Intervention group: mean 3.50 (SD 1.88) and comparison group: mean 3.52 (SD 1.80) at 4 weeks | 184 (1 study) | ⊕⊕⊕⊝ Moderateb | Another study, Dupoiron 2017 also found outcome to be similar between trial arms, but did not provide any data. |
| Serious adverse events | 43 per 1000 | 87 per 1000 (27 to 279) | RR 2.00 (95% CI 0.62 to 6.41) | 184 (1 study) | ⊕⊕⊝⊝ Lowb,d | — |
| Adverse events | 754 per 1000 | 815 per 1000 (709 to 935) | RR 1.08 (95% CI 0.94 to 1.24) | 234 (2 studies) | ⊕⊕⊕⊝ Moderateb | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured using the Brief Pain Inventory‐Short Form. | ||||||
| Methylnaltrexone compared to placebo for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Setting: palliative care Intervention: methylnaltrexone Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with methylnaltrexone | |||||
| Laxation response within 24 hours of dosea | 195 per 1000 | 568 per 1000 | RR 2.77 (1.91 to 4.04) | 287 | ⊕⊕⊕⊝ Moderateb | — |
| Laxation response between day 1 and day 14 (specifically within 4 hours after 4 or more of the 7 doses)a | 52 per 1000 | 517 per 1000 | RR 9.98 (4.96 to 20.09) | 305 | ⊕⊕⊕⊝ Moderate b,c | — |
| Effect on analgesia: opioid withdrawald | Study 1: day 1: MD 0.00 (‐0.46 to 0.46); day 14: MD 0.10 (‐0.63 to 0.83) Study 2: median change to day 2 = 0 in both trials arms | 236 (2 studies) | ⊕⊕⊕⊝ Moderateb | ‐ | ||
| Effect on analgesia: pain intensitye | Study 1: at 4 hours (methylnaltrexone 0.15 mg/kg: MD ‐0.76 (‐1.47 to 0.05); methylnaltrexone 0.3 mg/kg: MD ‐0.25 (‐0.91 to 0.41) Study 2: at day 1 and 14 (day 1: MD 0.20 (‐0.62 to 1.02); day 14: MD ‐0.70 (‐1.52 to 0.12) | 287 (2 studies) | ⊕⊕⊝⊝ Lowb,f | Another study, Bull 2015, found similar pain intensity experienced in trial arms, full data not provided. | ||
| Serious adverse events | 238 per 1000 | 142 per 1000 | RR 0.59 (0.38 to 0.93) | 364 | ⊕⊕⊕⊝ Moderateb | — |
| Adverse events | 700 per 1000 | 815 per 1000 | RR 1.17 (CI 0.94 to 1.45) | 518 | ⊕⊕⊝⊝ Lowb,g | Heterogeneity was substantial (74%). It was explained in sensitivity analysis by omitting the trial at a high risk of bias because of small sizes. The effect estimate was reduced. The direction of effect not changed. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by self‐report or clinician report. dMeasured using the modified Himmelsbach Opioid Withdrawal Scale. | ||||||
| Lower dose methylnaltrexone compared to higher dose for opioid‐induced bowel dysfunction in cancer and people receiving palliative care | ||||||
| Patient or population: people with cancer and people receiving palliative care with opioid‐induced bowel dysfunction Setting: palliative care Intervention 1: lower‐dose methylnaltrexone (study 1: 3 doses, 1 week, 1 mg; study 2: 1 dose, 0.15 mg/kg) Intervention 2: higher‐dose methylnaltrexone (study 1: 3 doses, 1 week, 5‐12.5 mg; study 2: 1 dose, 0.30 mg/kg) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Higher dose | Lower dose | |||||
| Laxation response within 24 hours of first dosea | Study 1: 609 per 1000 Study 2: 639 per 1000 | Study 1: 499 per 1000 (250 to 100) Study 2: 681 per 1000 (515 to 904) | Study 1: RR 0.82 (0.41 to 1.66) Study 2: RR 1.07 (0.81 to 1.42) | 135 (2 studies) Study 1: n = 33 Study 2: n = 102 | ⊕⊕⊝⊝ Lowb | Unable to combine study data as methylnaltrexone low and higher doses differed per trial |
| Laxation responsea | At 3 days: 706 per 1000 | At 3 days: 332 per 1000 | At 3 days: RR 0.47 (0.18 to 1.25) | 33 participants (1 study) | ⊕⊕⊝⊝ Lowb | Unable to combine study data as methylnaltrexone low and higher doses differed per trial |
| At 5 days: 688 per 1000 | At 5 days: 144 per 1000 | At 3 days: RR 0.21 (0.03 to 1.31) | ||||
| Effect on analgesia: opioid withdrawalc | — | — | MD ‐0.04 (‐0.73 to 0.65) | 102 participants (1 study) | ⊕⊕⊝⊝ Lowb | Another study,Portenoy 2008, also found outcome to be similar between trial arms, but did not provide any data |
| Effect on analgesia: pain intensityd | — | — | MD ‐0.51 (‐1.49 to 0.47) | 102 participants (1 study) | ⊕⊕⊝⊝ Lowb | Another study, Portenoy 2008, also found outcome to be similar between trial arms, but did not provide any data |
| Serious adverse event | — | — | — | — | Not reported | |
| Adverse event | Study 1: 1000 per 1000 Study 2: 800 per 1000 | Study 1: 1000 per 1000 (1000 to 1000) Study 2: 723 per 1000 (580 to 902) | Study 1: RR 1.00 (1.00 to 1.00) Study 2: RR 0.90 (0.73 to 1.13) | 135 (2 studies) Study 1: n = 33 Study 2: n = 102 | ⊕⊕⊝⊝ Lowb | Unable to combine study data as methylnaltrexone low and higher doses differed per trial |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aMeasured by clinician or self‐report. bDowngraded by two levels for study limitations: one for unclear risk of bias (reporting bias) and one for small sample size (high risk of bias). cMeasured using the modified Himmelsbach Opioid Withdrawal Scale. dMeasured by participant‐rated scale 0‐10. | ||||||
| Adverse event | Naldemedine (%) | Placebo (%) |
| Diarrhoea | 67 (39) | 14 (25) |
| Decreased WBC count | 9 (5) | 3 (5) |
| Abdominal pain | 6 (4) | 0 (0) |
| Vomiting | 5 (3) | 0 (0) |
| Bone marrow failure | 3 (2) | 2 (4) |
| Decreased appetite | 6 (4) | 1 (2) |
| Nasopharyngitis | 4 (2) | 1 (2) |
| Nausea | 4 (2) | 4 (7) |
| Rash | 3 (2) | 2 (4) |
| Decreased platelet count | 3 (2) | 0 (0) |
| Decreased total protein | 7 (4) | 1 (2) |
| Glucose in urine | 4 (2) | 1 (2) |
| Abnormal haematology test | 2 (1) | 0 (0) |
| Decreased RBC count | 4 (2) | 0 (0) |
| Hypertension | 2 (1) | 0 (0) |
| Increased blood alkaline phosphatase | 4 (2) | 1 (2) |
| Increased blood lactate dehydrogenase | 2 (1) | 1 (2) |
| Increased blood pressure | 2 (1) | 0 (0) |
| Increased blood urea | 4 (2) | 1 (2) |
| Increased WBC count | 1 (2) | 2 (4) |
| Protein present in urine | 5 (3) | 0 (0) |
| Upper abdominal pain | 3 (2) | 1 (2) |
| RBC: red blood cell; WBC: white blood cell. All comparisons were not statistically significant. | ||
| Methylnaltrexone vs placeboa | |
| AEs | RR 1.07, 95% CI 0.96 to 1.19 |
| AE of abdominal pain | RR 2.15, 95% CI 1.28 to 3.62 |
| AE of nausea | RR 0.87, 95% CI 0.46 to 1.65 |
| AE of vomiting | RR 0.70, 95% CI 0.33 to 1.47 |
| aomitting trial of high risk of bias. AE: adverse event; CI: confidence intervals; RR: risk ratio. | |
| Adverse event | RR (95% CI) | I²statistic on heterogeneity |
| Abdominal pain | 2.39 (1.07 to 5.34) | 65% |
| Diarrhoea | 1.02 (0.93 to 1.11) | 51% |
| Dizziness | 4.09 (0.99 to 16.83) | 0% |
| Falls | 1.02 (0.89 to 1.16) | 84% |
| Flatulence | 2.09 (1.07 to 4.08) | 0% |
| Nausea | 0.97 (0.89 to 1.06) | 63% |
| Peripheral oedema | 1.01 (0.50 to 2.03) | 0% |
| Restlessness | 0.83 (0.32 to 2.12) | 0% |
| Somnolence | 1.00 (0.93 to 1.08) | 73% |
| Vomiting | 0.99 (0.92 to 1.08) | 67% |
| CI: confidence interval; RR: risk ratio. | ||
| Adverse event | Methylnaltrexone (%) | Placebo (%) |
| Abdominal distensiona | 1 (2) | 6 (8) |
| Abdominal tendernessa | 1 (2) | 4 (6) |
| Astheniaa | 4 (6) | 4 (6) |
| Anxietyb | 5 (4.9) | 0 (0) |
| Arthralgiab | 3 (2.9) | 1 (1.9) |
| Back painc | 9 (7.8) | 3 (2.9) |
| Confusional statec | 7 (6.0) | 9 (7.9) |
| Dehydrationa | 2 (3) | 4 (6) |
| Fatigueb | 4 (3.9) | 1 (1.9) |
| Hypotensiona | 0 (0) | 4 (6) |
| Increased body temperaturea | 5 (8) | 2 (3) |
| Lethergya | 4 (6) | 4 (6) |
| Malignant‐neoplasm progressiona | 7 (11) | 9 (13) |
| Pain exacerbationb | 8 (8) | 2 (4) |
| Rhinorrhoeab | 6 (5.9) | 1 (1) |
| Sweating increasedb | 8 (7.8) | 4 (7.7) |
| Tachycardiaa | 1 (1) | 4 (6) |
| aReported in trial by Thomas 2008. bReported in trial by Slatkin 2009. cReported in trial by Bull 2015. | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Within 24 hours of dose Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.91, 4.04] |
| 2 Within 4 hours after 4 of the 7 doses Show forest plot | 2 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.98 [4.96, 20.09] |
| 3 Within 4 hours of first dose Show forest plot | 3 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.87 [2.83, 5.28] |
| 4 Within 4 hours after 1 or 2 doses of the first 4 doses Show forest plot | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.89 [4.46, 10.66] |
| 5 Improvement in constipation distress at day 1 Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.34, 2.59] |
| 6 Participant global impression of improvement in bowel status at 1 week Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [1.64, 3.27] |
| 7 Clinician global impression of improvement in bowel status at 1 week Show forest plot | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.66, 3.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serious adverse event Show forest plot | 2 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 3 | 518 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.94, 1.45] |
| 2 Dropouts due to adverse event Show forest plot | 2 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.54, 2.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse events Show forest plot | 2 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.94, 1.24] |

