Entrenamiento de los músculos respiratorios para la fibrosis quística
Resumen
Antecedentes
La fibrosis quística es la enfermedad autosómica recesiva más frecuente en las poblaciones blancas y causa disfunción respiratoria en la mayoría de personas. Se han descrito en la literatura numerosos tipos de entrenamiento muscular respiratorio para mejorar la función respiratoria y la calidad de vida relacionada con la salud en personas con fibrosis quística. Por tanto, se necesita una revisión sistemática de esta bibliografía para establecer la efectividad del entrenamiento muscular espiratorio (entrenamiento muscular inspiratorio o espiratorio) en los resultados clínicos en la fibrosis quística. Esta es una actualización de una revisión publicada anteriormente.
Objetivos
Determinar la efectividad del entrenamiento de los músculos respiratorios en los resultados clínicos de las personas con fibrosis quística.
Métodos de búsqueda
Se realizaron búsquedas en el registro de ensayos del Grupo Cochrane de Fibrosis Quística (Cochrane Cystic Fibrosis and Genetic Disorders Group), que comprenden referencias identificadas por búsquedas exhaustivas en bases de datos electrónicas y por búsqueda manual en revistas pertinentes y en libros de resúmenes de congresos.
Fecha de la búsqueda más reciente: 17 de abril de 2018.
Se realizó una búsqueda manual en el Journal of Cystic Fibrosis and Pediatric Pulmonology, junto con una búsqueda electrónica en las bases de datos de ensayos en línea hasta el 7 de mayo de 2018.
Criterios de selección
Ensayos controlados aleatorizados que compararan entrenamiento muscular respiratorio con un grupo control en los pacientes con fibrosis quística.
Obtención y análisis de los datos
Los autores de la revisión seleccionaron de forma independiente artículos para su inclusión, evaluaron la calidad metodológica de los estudios y extrajeron los datos. Cuando fue necesario, se buscó información adicional de los autores del ensayo. La calidad de la evidencia se evaluó mediante el sistema GRADE.
Resultados principales
Los autores identificaron 19 estudios, de los cuales nueve con 202 participantes cumplieron los criterios de inclusión de la revisión. Hubo bastantes diferencias en la calidad metodológica y narrativa entre los estudios incluidos. Cuatro de los nueve estudios incluidos estaban publicados sólo como resúmenes y les faltaban detalles concisos, lo cual limitaba la información disponible. Siete estudios fueron estudios de grupos paralelos y dos tuvieron un diseño cruzado. Las intervenciones de entrenamiento de los músculos respiratorios variaron drásticamente, con una frecuencia, intensidad y duración que oscilaba entre tres veces por semana y dos veces al día, entre el 20% y el 80% del esfuerzo máximo, y entre 10 y 30 minutos, respectivamente. El número de participantes osciló entre 11 y 39 en los estudios incluidos; cinco estudios se realizaron en adultos solamente y cuatro en una combinación de niños y adultos.
No se informó ninguna mejora significativa en el resultado primario de la función pulmonar (volumen espiratorio forzado en un segundo y capacidad vital forzada) (evidencia de calidad muy baja). Aunque no se informó de ningún cambio en la capacidad de ejercicio evaluada por la tasa máxima de uso de oxígeno, se encontró una mejora del 10% en la duración del ejercicio cuando se trabajaba al 60% del esfuerzo máximo en un estudio (n = 20) (evidencia de muy baja calidad). En un estudio posterior (n = 18), al trabajar al 80% del esfuerzo máximo, la calidad de vida relacionada con la salud mejoró en los dominios de la maestría y las emociones (evidencia de muy baja calidad). En cuanto a los resultados secundarios de la revisión, un estudio (n = 11) encontró un cambio significativo en la presión intramural, la capacidad residual funcional y la presión inspiratoria máxima después del entrenamiento (evidencia de baja calidad). Un estudio adicional (n = 22) informó que la resistencia de los músculos respiratorios fue significativamente mayor en el grupo de entrenamiento (P < 0,01). Ningún estudio incluido informó ningún otro resultado secundario. No se pudieron realizar metanálisis debido a la falta de consistencia y detalles en las medidas de resultados informadas.
Conclusiones de los autores
No existe evidencia suficiente que indique si esta intervención es beneficiosa o no. Los profesionales de la salud deben considerar el uso del entrenamiento de los músculos respiratorios caso por caso. Se necesitan más estudios de calidad metodológica de confianza para determinar la efectividad del entrenamiento de los músculos respiratorios en las personas con fibrosis quística. Los investigadores deben tener en cuenta los siguientes resultados clínicos en futuros estudios: función muscular respiratoria, función pulmonar, capacidad de ejercicio, ingresos hospitalarios y calidad de vida relacionada con la salud. Los cambios sensorial‐perceptivos, como la sensación de esfuerzo respiratorio (por ejemplo, la calificación de la disnea percibida) y la sensación de esfuerzo periférico (por ejemplo, la calificación del esfuerzo percibido) también pueden ayudar a dilucidar los mecanismos que sustentan la eficacia del entrenamiento de los músculos respiratorios.
PICO
Resumen en términos sencillos
Entrenamiento de los músculos que provocan la expansión y contracción del tórax para las personas con fibrosis quística
Pregunta de la revisión
¿Cuáles son los efectos del entrenamiento de los músculos que provocan la expansión y contracción del tórax en las personas con fibrosis quística?
Antecedentes
La fibrosis quística es la enfermedad genética más frecuente en las poblaciones blancas y causa problemas con los pulmones en la mayoría de personas con esta enfermedad. El entrenamiento de los músculos que provocan la expansión y contracción del tórax podría ayudar a mejorar la función pulmonar y la calidad de vida de las personas con fibrosis quísticas.
Fecha de la búsqueda
La evidencia está actualizada hasta: 17 de abril de 2018.
Características de los estudios
Se buscaron estudios en los que las personas con fibrosis quística fueron asignadas al azar a un grupo de entrenamiento de los músculos respiratorios o a un grupo de control. Se incluyeron nueve estudios con 202 personas que utilizaron una amplia variedad de métodos y niveles de entrenamiento. En siete de los estudios, el grupo de tratamiento y el grupo de control sólo recibieron entrenamiento de los músculos respiratorios o un tratamiento de control (un estudio tenía tres grupos en total: uno que recibía tratamiento de control y dos que recibían diferentes niveles de entrenamiento). En un estudio los participantes recibieron ambos tipos de tratamientos, pero en un orden aleatoria. Por último, un estudio comparó el entrenamiento con la atención habitual. Los estudios duraron un máximo de 12 semanas y todos fueron bastante pequeños; el más grande sólo contó con la participación de 29 personas. Los estudios incluyeron a pacientes con un abanico de edades a partir de seis años pero la mayoría parecieron ser adultos. Los estudios informaron varios resultados. Todos informaron sobre alguna medida de fuerza muscular respiratoria, y la mayoría informó sobre al menos una medida de función pulmonar, sin embargo sólo tres estudios informaron sobre la calidad de vida.
Resultados clave
No se pudieron combinar los resultados para responder a la pregunta de la revisión, porque los estudios o no publicaron suficientes detalles o no usaron las mismas mediciones estándar. Ningún estudio encontró diferencias en la función pulmonar después del entrenamiento, pero uno de los estudios informó una mejoría en la duración del ejercicio cuando se entrenaba al 60% del esfuerzo máximo y un estudio adicional que entrenó a los participantes al 80% del esfuerzo máximo informó algunas mejoras en los juicios sobre la calidad de vida. Hubo cierta evidencia de una mejoría en la función muscular respiratoria en un estudio.
Dada esta falta de información, no se puede hacer una recomendación a favor o en contra del entrenamiento de los músculos respiratorios. Los estudios futuros deben tratar de mejorar los métodos de los realizados anteriormente, y deben informar utilizando mediciones estandarizadas.
Calidad de la evidencia
En general, no estuvo claro cómo se dividía a las personas en grupos para el tratamiento y si esto habría afectado a los resultados. En dos estudios se afirmó que las personas que evaluaban los resultados no sabían qué tratamiento habían recibido los participantes, pero esto no estaba claro en otros estudios. Hubo personas que abandonaron tres de los estudios por razones que pueden estar directamente relacionadas con el tratamiento y, por lo tanto, pueden introducir un riesgo de sesgo en los resultados. Otros no especificaron cuántas personas abandonaron los estudios. Se evaluó la calidad de la evidencia y se consideró que la evidencia de la función pulmonar, la capacidad de ejercicio y la calidad de vida relacionada con la salud eran de muy baja calidad, pero la evidencia de la función muscular respiratoria de baja calidad.
Authors' conclusions
Summary of findings
| Respiratory muscle training compared with control for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: respiratory muscle trainingₑ Comparison: controlₑ | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Controlₑ | Respiratory muscle trainingₑ | |||||
| FEV1: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 145 (7 studies including 2 cross‐over studies) | ⊕⊝⊝⊝ | Studies reported FEV1 as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| FVC: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 114 (5 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Studies reported FVC as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| Exercise capacity: VO2max (mL/kg/min) Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 54 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | One study with an unspecified level of resistance reported a significant improvement within the respiratory muscle training group. | |
| HRQoL: total score Follow‐up: 8 weeks | Two studies reported no significant differences between the respiratory muscle training group and the control group. One study reported significant improvements in the parameters of mastery and emotion in the respiratory muscle training group compared to the control group. | NA | 69 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Two studies used the Chronic Respiratory Disease Questionnaire (CRDQ) and one study used the cystic fibrosis questionnaire (CFQ). | |
| Respiratory muscle function: maximal inspiratory pressure (PImax) Follow‐up: 6‐10 weeks | Significant improvements were observed in all respiratory muscle training groups. Two studies reported no significant differences between the respiratory muscle training group and the control group. | NA | 51 (3 studies including 1 cross‐over study) | ⊕⊕⊝⊝ | ||
| Respiratory muscle function: inspiratory capacity Follow‐up: NA | NA | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The resistance level of the respiratory muscle training intervention was variable; three studies used 80% of maximal effort, one study used 60% of maximal effort, one study used 40% of maximal effort, one study used 30% of maximal effort and three studies did not specify the level of resistance. Control groups were also variable; cycle ergometer, H20, treatment as usual, standard chest physiotherapy, low resistance threshold loading device, no training or sham training. 2. Downgraded twice due to serious risk of bias: the included studies lacked methodological detail relating to methods of randomisation, allocation concealment and blinding. Most of the studies were at high risk of bias due to lack of blinding, incomplete outcome data or selective reporting, or both. 3. Downgraded due to imprecision: studies included a small number of participants and numerical results were not available for some of the studies. | ||||||
Background
Description of the condition
Cystic fibrosis (CF) is caused by a genetic mutation that disturbs the function of the CF transmembrane conductance regulator (CFTR) channel (Elborn 2016). Dysfunction of the CFTR channel reduces chloride secretion resulting in dehydrated and viscous secretions that become difficult to expectorate (Wine 1999). The pathological consequences of this mutation can impact function of the respiratory, reproductive, and digestive systems, as well as affecting temperature regulation and fluid balance (Wheatley 2011). Although the median predicted survival currently stands at 45.1 years (Carr 2016), the care and management of CF has improved dramatically over the last decade (De Boeck 2016).
Respiratory dysfunction accounts for approximately 80% to 95% of mortality (Lyczak 2002) and is a major cause of morbidity in CF. This is the manifestation of bacterial colonisation and chronic pulmonary infection that imposes structural lung damage, mostly bronchiectasis and small airway obstruction (Cantin 2015). Recurrent pulmonary infection results in the progressive deterioration of pulmonary function and eventually respiratory failure (Grasemann 2013). Other physiological factors such as inflammation, gas trapping and mucous plugging contribute towards further airway obstruction, thus reducing forced expiratory volumes and causing excessive dyspnoea (Kozlowska 2008). Co‐morbidities such as thoracic kyphosis can exacerbate this, restricting lung expansion, decreasing lung volumes, and leading to an increased work of breathing (Aris 1998; Denton 1981).
Therapy in CF has evolved around preventing deterioration in pulmonary function which has led to a substantial increase in the life expectancy of those living with the condition. Thus, any intervention that improves pulmonary function and other associated health outcomes would be of great benefit in those with CF.
Description of the intervention
Respiratory muscle training (RMT) is a form of exercise training specifically targeting the muscles that drive expansion or contraction of the chest, or both. Training can stress the musculature of inspiration or expiration, or both, depending upon the type of training performed and the specification of the individual device. There are two main types of RMT, namely resistive training and normocapnic hyperpnoea. Resistive training can be performed using either flow‐resistive loading or pressure‐threshold loading, and requires the use of a portable hand‐held device. Both devices typically involve a one‐way valve mechanism, such that only the inspiratory or expiratory musculature can be trained at one given time. Flow‐resistive loading involves breathing through a hole of small diameter (resistor), thus limiting airflow, increasing the work of breathing and challenging the respiratory musculature. The resistance (load) applied to the respiratory musculature can be adjusted according to the diameter of the hole, whereby reducing the diameter increases airflow limitation. Pressure‐threshold training creates a similar physiological challenge, and involves breathing with sufficient force to overcome a spring‐loaded valve to enable airflow. The resistance (load) is set at a proportion of maximal static inspiratory mouth pressure (PImax). Resistive training regimens vary in terms of the intensity (load), duration (sets, repetitions and time) and frequency (sessions per week), depending upon the desired physiological outcome i.e. muscular strength or endurance. Overall training volume can be altered using a combination of intensity, duration and frequency, and training can be either continuous or interval in nature. While resistive training can be performed under the supervision of a suitably qualified therapist, users may perform this independently.
Normocapnic hyperpnoea is a type of RMT that requires the individual to ventilate at a high proportion of their maximum voluntary ventilation (MVV) for a predetermined period of time. Complex rebreathing circuitry is required to perform this form of training and to minimise the risk of inducing hypercapnia. Unlike resistive training, normocapnic hyperpnoea trains both the inspiratory and expiratory musculature simultaneously. As the physiological challenge is derived from high ventilation, as opposed to high resistive load, the training load is determined by the rate of minute ventilation. For the same reason, normocapnic hyperpnoea targets muscular endurance as opposed to muscular strength.
How the intervention might work
It has been suggested that RMT may improve health‐related quality of life, pulmonary function and exercise capacity in people with CF. Some studies have postulated that RMT may enhance the clearance of mucous from the lungs (Chatham 2004), which is considered to be a fundamental aspect in preventing pulmonary infection. Although RMT may be thought to improve pulmonary function, exercise tolerance and quality of life, the causal mechanisms remain unclear.
In those without CF, several potential mechanisms have been postulated that may also be comparable in those with CF. Perceptual changes have been found to occur following RMT, whereby a significant reduction in the perception of respiratory effort has been observed (Romer 2002). Numerous mechanisms have been hypothesised, including an improvement in the contractile properties of the inspiratory musculature, and a desensitising effect on the sensory input from the inspiratory muscles to the brain (El‐Manshawi 1986; Revelette 1987; Wilson 1990). Significant reductions in the perception of peripheral effort have also been observed, attributed to a changes in acid‐base balance and respiratory muscle blood flow (Caine 2000).
In CF, these mechanisms may also account for improvements in health‐related quality of life, pulmonary function and exercise capacity that have been observed. As a combination of structural lung damage and physical deconditioning can increase the perception of respiratory (dyspnoea) and peripheral effort in people with CF, the need for this review is apparent.
Why it is important to do this review
Prior to the original version of this review (Houston 2008), there was no systematic review of the currently available evidence from randomised controlled trials (RCTs) or quasi‐randomised controlled trials as to whether RMT is beneficial, nor on the optimal regime (i.e. the nature of the training load and specifics of the training protocol), for people with CF. The effect of RMT on improving health outcomes in CF remains unclear. Previous versions of this Cochrane Review were unable to conclude if this treatment is either beneficial or not, due to the apparent lack of clinical trials, particularly those of a high‐quality; this version of the review is the latest update of the original review (Houston 2009; Houston 2013).
Objectives
To determine the effectiveness of respiratory muscle training on clinical outcomes in people with cystic fibrosis.
Methods
Criteria for considering studies for this review
Types of studies
Any parallel or cross‐over randomised controlled trial (RCT) comparing RMT with a control group.
Types of participants
Any person with CF who has been diagnosed by sweat testing, genotyping or both. Participants were included irrespective of gender, age, or the presence of co‐morbidities.
Types of interventions
Trials were considered for inclusion if the author(s) had compared RMT with a control group, such as a placebo (e.g. sham‐training) or no intervention. RMT included either inspiratory or expiratory muscle training, or both, including resistive loading (flow‐resistive or pressure threshold, or both) and normocapnic hyperpnoea training. Singing training interventions were excluded from this review. Studies that performed RMT in combination with any other form of physical exercise training were excluded from the review. Combining these interventions is the subject of another Cochrane Review (Radtke 2015).
Types of outcome measures
Primary outcomes
-
Pulmonary function
-
forced expiratory volume at one second (FEV1)
-
forced vital capacity (FVC)
-
-
Exercise capacity (measured by e.g. maximal oxygen uptake (VO2max), exercise duration, etc.)
-
Health‐related quality of life (measured by e.g. Chronic Respiratory Disease Questionnaire (CRDQ) (Chauvin 2008), Cystic Fibrosis Questionnaire (CFQ) (Wenninger 2003), etc.)
Secondary outcomes
-
Respiratory muscle function
-
maximal inspiratory pressure (PImax)
-
inspiratory capacity (IC)
-
-
Respiratory muscle strength and endurance (RME)
-
Frequency and duration of respiratory infections, hospitalisations
-
Adherence
-
Death or survival
-
Adverse effects (pneumothorax, musculoskeletal pains or injuries, others)
-
Costs
Search methods for identification of studies
Trial searches were not restricted by date, language, or publication status.
Electronic searches
We identified relevant studies from the Group's Cystic Fibrosis Trials Register using the term: inspiratory muscle training.
The Group's Cystic Fibrosis Trials Register, which is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of four major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference, the North American Cystic Fibrosis Conference and the Australia and New Zealand Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website.
Date of the last search of the Group's Cystic Fibrosis Trials Register: 17 April 2018.
We also performed separate searches of clinicaltrials.gov and the WHO ICTRP databases (Appendix 1; Appendix 2). Date of the last search: 07 May 2017.
For versions of the review up to and including 2014 the former author team also performed separate searches of the following databases: MEDLINE, Embase, CINAHL, AMED (Allied and Complementary Medicine), PEDro (The Physiotherapy Evidence Database), BIOSIS Previews, Science Direct and SCOPUS to 2013, using both the Cochrane RCT and Cystic Fibrosis search filters; and terms specific to the intervention (Appendix 3; Appendix 4; Appendix 5; Appendix 6). We also searched Current Controlled Trials and the UK National Research Register for ongoing and recently completed studies. These searches last run on the 01 August 2013 (Appendix 5; Appendix 6; Appendix 7).
Searching other resources
For the original review, we also contacted manufacturers and study investigators and checked reference lists of relevant literature.
Data collection and analysis
Where the text below describes "the authors", up to the 2018 update the original three authors (BH, NM and ASM) are referred to; thereafter "the authors" refers to NH and ASM.
Selection of studies
To identify potentially eligible studies, authors screened the titles and abstracts of each record retrieved from the search. They then obtained the full‐text articles and inspected these independently for the review, including titles and abstracts that could not be rejected with certainty. If authors could not reach an agreement they would have resolved these issues by discussion and the involvement of another person if necessary.
Data extraction and management
The review authors assessed the methodological quality of the selected studies and independently extracted data using a standardised data collection form based upon the recommendation in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Review authors entered all eligible studies into the Review Manager software (RevMan 2014). The authors did not need to resolve any disagreements.
Assessment of risk of bias in included studies
The authors used the Cochrane risk of bias tool to independently assess the study quality according to the following criteria: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and any other identified sources of bias (Higgins 2011). The external validity of each study was also considered, particularly the description of study participants (e.g. mean FEV1) and the study intervention (e.g. controlling inspiratory flow rate), as well as the reliability of reported outcomes (e.g. familiarity).
Measures of treatment effect
The authors graphically presented quantitative data for the outcomes listed in the inclusion criteria. For continuous outcomes (pulmonary function, exercise capacity, quality of life, respiratory muscle function, frequency and duration of respiratory infections, hospitalisations and costs) where they were able to obtain data, they calculated the mean differences (MD) and presented these with 95% confidence intervals (CIs). No analysable data were available for dichotomous outcomes; for future updates, if we are able to analyse dichotomous outcomes (adherence, survival and adverse effects), we plan to calculate the risk ratio (RR) and 95% CIs for dichotomous outcomes.
Unit of analysis issues
Ideally when conducting a meta‐analysis combining results from cross‐over studies, the authors will use the inverse variance methods that are recommended by Elbourne (Elbourne 2002). However, if there are limited data available they plan to either use first‐arm data only or treat the cross‐over study as if it was of parallel design (assuming a correlation of zero as the most conservative estimate). Elbourne says that this approach produces conservative results as it does not take into account within‐patient correlation (Elbourne 2002). Also each participant appears in both the treatment and control group, so the two groups are not independent. There are two cross‐over studies included in the review (Asher 1983; Bieli 2017). Currently the authors have reported results from these studies narratively due to data limitations.
Where studies measured data longitudinally, the authors based the analysis on the results from the final time point. Methods are not yet available to carry out a meta‐analysis of aggregate longitudinal data, where individual patient data (IPD) is not available.
Dealing with missing data
Where data were missing, the review authors attempted to contact the author(s) of the study to obtain the missing data. Only the available data were analysed (i.e. without imputing missing data).
Assessment of heterogeneity
The review authors would have conducted a meta‐analysis and assessed heterogeneity if sufficient studies had been included and combined in the review. The authors planned to examine heterogeneity between comparable studies using a standard Chi² test with the alpha level of significance set at P < 0.05. Levels of heterogeneity would have been determined using the I² statistic, whereby I² greater than 50% was considered to be substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
If sufficient data were available, the authors planned to explore potential publication bias by preparing a funnel plot. Where possible the authors compared the 'Methods' section of the full study report to the outcomes reported in the 'Results' section to identify any selective outcome reporting.
Data synthesis
The authors planned to pool the results of comparable groups of studies using the fixed‐effect model and to calculate the relevant 95% CIs if sufficient data were available.
Subgroup analysis and investigation of heterogeneity
The authors did not plan to perform any meta‐analyses if there had been substantial and statistically significant heterogeneity. If the authors had been able to combine a sufficient number of included studies and had identified substantial heterogeneity (as defined above), the authors planned to explore this using the following subgroup analyses:
-
type of RMT (e.g. resistive loading or normocapnic hypopnea)
-
regimen of RMT (e.g. daily versus three times per week)
-
characteristics of study participants
-
age (up to 16 years versus older than 16 years)
-
gender
-
participants with poor respiratory muscle strength compared to those with preserved strength (e.g. determined by PImax)
-
participants with mild airflow obstruction compared to those with severe hyperinflation airflow obstruction (e.g. determined by FEV1)
-
-
definition of outcome measures
Sensitivity analysis
If authors had been able to include and combine a sufficient number of studies, they would have performed sensitivity analyses based upon the methodological quality of the included studies, including allocation concealment, blinding of outcome assessments, and analyses performed on an intention‐to‐treat basis.
Summary of findings tables
In a post hoc change at the 2018 update, and in accordance with current Cochrane guidance, we have included a summary of findings table presenting results comparing respiratory muscle training (all intensities) versus control comparison. We have included the six outcomes which we consider to be the most clinically relevant.
-
FEV1 % predicted
-
FVC % predicted
-
VO2max (mL/kg/min)
-
health‐related quality of life (overall score)
-
PImax
-
IC
We assess the quality of the evidence for each outcome using the GRADE approach. This approach is based on the risk of bias within the studies, relevance to our population of interest (indirectness), unexplained heterogeneity or inconsistency, imprecision of the results or high risk of publication bias. We downgraded the evidence once if the risk was serious and twice if the risk was deemed to be very serious.
Results
Description of studies
Results of the search
The database searches identified 275 potentially eligible studies. The authors reviewed the abstracts of the studies and identified nine studies with a total of 202 participants for inclusion (Albinni 2004; Amelina 2006; Asher 1983; Bieli 2017; Chatham 1997; de Jong 2001; Enright 2004; Heward 2000; Sawyer 1993). Of these nine studies, four were published as abstracts only (Albinni 2004; Amelina 2006; Chatham 1997; Heward 2000). A further seven studies were excluded (Howard 2000; Irons 2012; Keens 1977; Patterson 2004; Santana‐Sosa 2013; Sartori 2008; Vivodtzev 2013). Two studies are currently listed as 'Awaiting classification' until further details are available.
Included studies
A full comparison of the included studies can be found in the table (Characteristics of included studies).
Study design
All studies were RCTs. Of these, seven were of a parallel design (Albinni 2004; Amelina 2006; Chatham 1997; de Jong 2001; Enright 2004; Heward 2000; Sawyer 1993) and two studies had a cross‐over design (Asher 1983; Bieli 2017). One study utilised a three‐way comparison between two different RMT interventions and control condition (Enright 2004). The duration of the intervention ranged from four (Asher 1983) to 12 weeks (Albinni 2004) and all outcomes were recorded at the end of the study period. All studies were relatively small with the number of participants ranging from 11 (Asher 1983) to 39 (Heward 2000). The studies were run in a number of different countries: USA (Heward 2000; Sawyer 1993); UK (Chatham 1997; Enright 2004); Netherlands (de Jong 2001); Switzerland (Bieli 2017); Canada (Asher 1983); Russia (Amelina 2006); and Austria (Albinni 2004). While most were single‐centre studies (Albinni 2004; Amelina 2006; Bieli 2017; de Jong 2001; Enright 2004; Sawyer 1993), it was unclear if three studies were single or multicentre (Asher 1983; Chatham 1997; Heward 2000).
Participants
Participant characteristics were not consistently reported across studies. Two studies did not report the exact mean age or age range of participants, but indicated that they were adults (Amelina 2006; Chatham 1997). One further study explicitly stated that participants were adults and gave a mean age of 22.5 years (Heward 2000). Two studies reported age ranges that included children, adolescents and young adults; 6 to 18 years (Albinni 2004) and 9 to 24 years (Asher 1983). The remaining four studies gave more detailed information on participant age and indeed split according to treatment group (Bieli 2017; de Jong 2001; Enright 2004; Sawyer 1993). In each study, ages were similar between groups, but while the mean age for participants in two groups was in the early 20s (de Jong 2001; Enright 2004), in one study it indicated that participants were children and adolescents (Sawyer 1993).
Six of the studies did not report on the gender split in participants (Albinni 2004; Amelina 2006; Asher 1983; Chatham 1997; Heward 2000; Sawyer 1993). Three studies reported on gender split by treatment group; in one study there were equal numbers of males and females (de Jong 2001) and in the remaining studies there were slightly more males than females (Bieli 2017; Enright 2004).
Interventions
There was great variation as to the method and level of training employed by the included studies. In the intervention group, three studies used 80% of maximal effort (Chatham 1997; Enright 2004; Heward 2000); one study used 60% of maximal effort (Sawyer 1993); one study used 40% of maximal effort (de Jong 2001); one study used 30% of maximal effort (Amelina 2006) and three studies did not specify the level of resistance (Albinni 2004; Asher 1983; Bieli 2017). The frequency of training also varied. In two studies the frequency and duration of training was unclear (Chatham 1997; Heward 2000). Four studies used similar training regimens (Amelina 2006; Asher 1983; Bieli 2017; Sawyer 1993). Of these, two studies used a duration for each session of 10 to 15 minutes twice daily (Amelina 2006; Bieli 2017); one study used 15 minutes twice daily (Asher 1983) and one study used 30 minutes in total daily (Sawyer 1993). The remaining two studies used a regimen of training three times per week, but the duration of the sessions either varied (Enright 2004) or was not stated (Albinni 2004).
Outcomes
The outcome measures selected by the studies also varied greatly. All studies reported some measure of respiratory or inspiratory muscle strength. Most studies reported at least one measure of pulmonary function, principally FEV1 (Albinni 2004; Amelina 2006; Asher 1983; Bieli 2017; de Jong 2001; Enright 2004; Sawyer 1993) and FVC (Albinni 2004; Amelina 2006; Bieli 2017; de Jong 2001; Enright 2004). Six studies reported on exercise capacity (Albinni 2004; Amelina 2006; Asher 1983; Bieli 2017; de Jong 2001; Sawyer 1993), specifically maximal oxygen uptake (VO2max) (Albinni 2004; Asher 1983; de Jong 2001) and exercise duration (Bieli 2017; Sawyer 1993). One study reported on exercise capacity but did not specify the outcome measure (Amelina 2006).
Three studies reported a measure of health‐related quality of life using either the Chronic Respiratory Disease Questionnaire (CRDQ) (Chatham 1997; Enright 2004) or a combination of the Cystic Fibrosis Questionnaire (CFQ) and the Cystic Fibrosis Clinical Score (CFCS) (Bieli 2017). Other studies also reported on related outcomes such as perceived breathlessness (Albinni 2004; de Jong 2001) and fatigue (de Jong 2001).
Excluded studies
A total of seven studies were excluded (seeCharacteristics of excluded studies). One study was excluded as the allocation was not randomised (Keens 1977), whereas two studies were excluded as they were not interventional trials (Patterson 2004; Sartori 2008). A further three studies were excluded as the intervention was not appropriate and could not be deemed a form of RMT (Howard 2000; Irons 2012; Vivodtzev 2013). Although one study used a form of RMT, it was combined with another form of exercise training, and was excluded as changes could not be attributed to RMT alone (Santana‐Sosa 2013).
Studies awaiting classification
There are two studies currently listed as 'awaiting classification', both of which are currently published as abstracts only (Giacomodonato 2015; Ozaydin 2010).
Study design
Two studies stated that participants were randomised to either the control or intervention group, but gave no further details regarding study design (Giacomodonato 2015; Ozaydin 2010).
Participants
One study included 10 participants with CF aged between 21 and 40 years; there were six males and four females (Giacomodonato 2015). The second study included 28 participants with CF with a mean (SD) age 13.18 (3.65) years and a mean (SD) baseline value for FEV1 % predicted of 89.51% (19.47) (Ozaydin 2010).
Interventions
The Giacomodonato study allocated participants to either RME training with normocapnic hyperpnoea or standard chest physiotherapy (Giacomodonato 2015). Participants in the intervention group were asked to maintain 70% of 12s maximum voluntary ventilation (MVV) until this could not be sustained, for 15 minutes daily over eight weeks.
Ozaydin allocated 14 participants to IMT training using a threshold loading device at 30% to 80% maximal inspiratory pressure (MIP) and 14 participants to sham training at 10% MIP, for 20 minutes on five days per week (Ozaydin 2010).
Outcomes
One study stated that it had measured RME, six minute walk test (6MWT) distance, health‐related quality of life (using the CFQ) and lung function (FVC, FEV1, MIP, MEP) pre‐ and post‐intervention. However, the abstract only reported 6MWT distance between the groups following the intervention and we await the full publication for further results (Giacomodonato 2015).
The second study also measured the 6MWT distance and pulmonary function; in addition the investigators measured peripheral muscle strength (hand grip, shoulder abductors, elbow flexors) (Ozaydin 2010).
Risk of bias in included studies
Allocation
Generation of allocation sequence
Although all nine of the studies state that they randomised their participants to the treatment groups, only one study indicated the method of allocation by stating that they employed the minimisation method (de Jong 2001). This study was graded as having a low risk of bias, whereas the remaining eight studies were graded as having an unclear risk of bias.
Concealment of allocation sequence
None of the included studies made specific reference as to how, or even whether, this was addressed. All studies were assessed as having an unclear risk of bias in relation to this criteria.
Blinding
Performance bias
All the included studies were graded as having a high risk of performance bias since in all studies there was a clear difference between the experimental and control training. This ranged from no details being provided for the control group (Asher 1983) and 'no training' (Albinni 2004; Enright 2004; Heward 2000), through to minimal training and "sham" training (Amelina 2006; Chatham 1997; de Jong 2001; Sawyer 1993) and standard chest physiotherapy (Bieli 2017). Although the methodological difficulties of blinding the participants to this type of intervention are acknowledged, this can be addressed in some cases (e.g. RMT versus sham) but not all (e.g. training versus no training).
Dectection bias
Two studies blinded the outcome assessors at the final data collection session, although they did not state whether this was the case at the initial assessment or if the same assessors were used (Enright 2004; Sawyer 1993). A third study reports that the observers were blinded, although it does not expand on the level of this (Asher 1983). The remaining six studies make no overt reference to any blinding of outcome assessors and are deemed a high risk of bias (Albinni 2004; Amelina 2006; Bieli 2017; Chatham 1997; de Jong 2001; Heward 2000).
Incomplete outcome data
Only one study referred to the intention‐to‐treat principle (Bieli 2017). The trial reported that six out of 22 participants withdrew from the study, of these four participants discontinued the study in the control period, suggesting that withdrawal was not directly linked to the intervention in these cases. However, participants that withdrew did have a tendency to be older with characteristics of more advanced lung disease (Bieli 2017). This study was judged to have a low risk of bias.
Three studies were judged to have a high risk of bias (Asher 1983; de Jong 2001; Sawyer 1993). In the Asher study, two participants did not perform one of the post‐treatment outcome measures, due to expiration up to residual volume resulting in coughing and there are no details on the regimen of the control group (Asher 1983). In the de Jong study, one participant in the intervention group withdrew after experiencing earache at a training intensity equating to 40% PImax (de Jong 2001). In the Sawyer study, two participants did not complete their pulmonary function tests: one was due to an oversight on the part of the researchers; and the other did not complete the test. There is no indication as to which group these participants are from (Sawyer 1993).
The remaining five studies were judged to have an unclear risk of bias (Albinni 2004; Amelina 2006; Chatham 1997; Enright 2004; Heward 2000). Two studies gave limited information on withdrawals; in the Amelina study, one participant in the intervention group did not complete the trial (Amelina 2006) and in the Chatham study, three participants in the control group did not complete the trial (Chatham 1997). Neither of these two studies offered any explanation for these withdrawals; however, as they are both abstracts published in conference proceedings and that there are likely to be editorial constraints responsible for this. Furthermore, both trials failed to provide statistical data on their control groups, merely stating that there was no change in their outcomes; therefore they have been graded as having an unclear risk of bias. The remaining three trials do not provide any information with regards to participant withdrawals (Albinni 2004; Enright 2004; Heward 2000).
Selective reporting
One study reported on all outcome measures selected, although some health‐related quality of life domains were unreported; nevertheless the study was deemed to have a low risk of selective reporting bias (Bieli 2017). One study reported that the investigators carried out post‐training measures of pulmonary function but do not report the results (Heward 2000). It is acknowledged that this study is only published as an abstract; however, there is a potential risk of bias due to the limited reporting of their outcomes. Likewise for the study by Amelina, two outcomes (respiratory muscle strength and dyspnoea) are reported to have been analysed, but no data are provided for them (Amelina 2006). There was insufficient information provided by the other publications to make a judgement on the risk of bias due to selective reporting from six trials and have been judged to have an unclear risk of bias (Albinni 2004; Asher 1983; Chatham 1997; de Jong 2001; Enright 2004; Sawyer 1993).
Other potential sources of bias
All the included studies have been graded an unclear risk of bias as none provided sufficient information to arrive at a definitive conclusion.
Effects of interventions
See: Summary of findings for the main comparison
Due to the lack of studies using comparable intensities of RMT or outcome measures, or both, we are unable to conduct meta‐analyses at this time. All outcome measures were recorded at the end of the study period in each study.
Primary outcomes
1. Pulmonary function
Seven of the nine included studies reported some measure of pulmonary function; two included studies did not report on this outcome (Chatham 1997; Heward 2000). The quality of the evidence was judged to be very low (summary of findings Table for the main comparison).
a. FEV1
Seven studies reported FEV1 in either litres (L) (Albinni 2004; de Jong 2001; Enright 2004; Sawyer 1993), % predicted (Amelina 2006; Asher 1983; de Jong 2001) or using the z score (Bieli 2017). We judged the quality of the evidence to be very low (summary of findings Table for the main comparison).
One study reported FEV1 (L) at less than two months (de Jong 2001) and three studies reported at two to six months (Amelina 2006; Enright 2004; Sawyer 1993). There was no significant difference (P > 0.05) between groups in terms of FEV1 measured in L in any study irrespective of working at 80% (Enright 2004), 60% (Sawyer 1993), 40% (de Jong 2001) or 20% (Enright 2004) of maximal effort (Analysis 1.1; Analysis 2.1; Analysis 3.1; Analysis 4.1). One study reported data for analysis for FEV1 % predicted at 40% of maximal capacity (de Jong 2001); again no statistically significant differences were found between treatment groups (Analysis 3.2). A further study comparing 30% of maximal capacity to control reported on FEV1 % predicted but only presented within‐group changes (Amelina 2006). One cross‐over study (unspecified level of resistance) reported the FEV1 z score and found no significant difference (P = 0.436) between the treatment groups (Bieli 2017). Two studies (unspecified level of resistance) did not report any data, but state that there was no change in FEV1 in either the RMT or the control group (Albinni 2004; Asher 1983).
b. FVC
Six studies reported FVC in either L (Albinni 2004; de Jong 2001; Enright 2004), % predicted (Amelina 2006; de Jong 2001) or using the z score (Bieli 2017). We judged the quality of the evidence to be very low (summary of findings Table for the main comparison).
Between‐group comparisons did not reveal a significant difference (P > 0.05) in FVC (L) irrespective of working at 80% (Enright 2004), 40% (de Jong 2001) or 20% (Enright 2004) of maximal effort (Analysis 1.2; Analysis 3.2; Analysis 4.2). This was also true for FVC (% predicted) as reported by de Jong (de Jong 2001) (Analysis 3.4). One study reported data for analysis for FVC (% predicted) at 40% of maximal capacity (de Jong 2001) and again no statistically significant differences were found between treatment groups (Analysis 3.4). A further study comparing 30% of maximal capacity to control only reported within‐group improvement in FVC (% predicted) (Amelina 2006). One study did not report any data, but stated that there was no change in FVC in either the RMT or the control group (Albinni 2004). This was also the case for FVC (z score) as reported by Bieli (Bieli 2017).
2. Exercise capacity
Six studies reported some measure of exercise capacity (Albinni 2004; Amelina 2006; Asher 1983; Bieli 2017; de Jong 2001; Sawyer 1993); however, Amelina did not provide any units of measurement nor was there any explanation as to the method of assessment, but investigators did state that there was no improvement (Amelina 2006). The quality of the evidence was judged to be very low (summary of findings Table for the main comparison).
a. VO2max
Three studies reported VO2max as mL/kg/min‐1, but not in a form that we could analyse (Albinni 2004; Asher 1983; de Jong 2001). One study reported no significant difference (P = 0.99) between groups when working at 40% of maximal effort (de Jong 2001). A further study reported mean (SD) VO2max (mL/kg/min‐1) at 31.6 (5.0) mL/kg/min‐1 and 29.9 (6.4) mL/kg/min‐1 pre and post‐intervention, with no significant difference (Asher 1983). A third study only reported within‐group improvements, with no data to allow inclusion in our analysis (Albinni 2004).
b. Exercise duration
Two studies reported this outcome (Bieli 2017; Sawyer 1993). The cross‐over study (unspecified level of resistance) did not provide data we could analyse, but reported no significant difference in exercise duration, measured using a constant workload cycling test, between groups (P = 0.169) (Bieli 2017). In the second study between‐group comparisons found a 10% significant (P < 0.03) improvement when working at 60% of maximal effort (Sawyer 1993).
3. Health‐related quality of life
Three studies reported a measure of health‐related quality of life; two studies used the CRDQ (Enright 2004; Chatham 1997) and one study used the CFQ and the CFCS (Bieli 2017). The quality of the evidence was judged to be very low (summary of findings Table for the main comparison).
The CRDQ evaluates four domains considered important to individuals with chronic airflow obstruction; dyspnoea, mastery, fatigue and emotion (Chauvin 2008). Chatham found a significant improvement (P < 0.01) between groups in the two parameters of mastery and emotion when working at 80% of maximal effort (Chatham 1997); however at the same level of effort, Enright found no significant differences between groups (Analysis 1.3; Analysis 1.4). Enright also found no significant difference between groups working at 20% of maximal effort (Enright 2004). Using the CFQ, Bieli found no difference in health‐related quality of life between treatment groups (no specified level of resistance) (Bieli 2017).
Bieli also measured symptom severity using the CF clinical score (CFCS) which indicates overall symptom severity, but no significant difference was reported between groups at baseline or post‐intervention (Bieli 2017).
Secondary outcomes
1. Respiratory muscle function
Three studies reported PImax (Amelina 2006; Asher 1983; Sawyer 1993), but only one study provided data for analysis (Sawyer 1993). The quality of the evidence was judged to be low (summary of findings Table for the main comparison).
a. maximal inspiratory pressure (PImax)
After data analysis, Sawyer reported a significant difference in favour of the treatment group (60% of maximal effort), MD 26.00 (95% CI 8.63 to 43.47) (Analysis 2.2). One study compared 30% resistance to control, but only reported within‐group changes in PImax (Amelina 2006). Asher (unspecified level of resistance) utilised two inspiratory measures (prevailing intramural pressure‐functional residual capacity (Pim‐FRC) and PImax) suggesting that one measurement technique was used but at two different lung volumes (Asher 1983). The study reported significant changes in both measures in the RMT group (P < 0.025 and P < 0.05 respectively). The investigators also reported that, for the Pim‐FRC measure, only three participants registered an increase that was more than two standard deviations (2SD) from the control group; and for the PImax measure, two participants had an increase greater than two SD from the control values.
b. inspiratory capacity (IC)
No studies reported this outcome.
2. Respiratory muscle strength and RME
Five studies reported RME (Albinni 2004; Amelina 2006; Bieli 2017; Chatham 1997; de Jong 2001), but only three studies reported between‐group comparisons (Albinni 2004; Bieli 2017; de Jong 2001).
One study presented values for mean (SD) and a P value which we were able to include in our analysis (de Jong 2001); results showed a statistically significant difference in favour of the RMT group, MD 12.00 (95% CI 0.55 to 23.45) (Analysis 3.5). The remaining two studies did not provide data we could analyse. Albinni reported a significant improvement (P = 0.002) in RME in the training group (unspecified resistance) (Albinni 2004). Bieli also reported that RME was significantly longer in the training group (P < 0.01) (Bieli 2017).
One study reported a within‐group improvement in the training group when working at 80% of maximal effort, but no data from the control group were reported (Chatham 1997). Likewise the final study reported a within‐group improvement in the training group when working at 30% of maximal effort, but no data from the control group were reported (Amelina 2006).
3. Frequency and duration of respiratory infections, hospitalisations
No studies reported this outcome.
4. Adherence to the IMT regimen
No studies reported this outcome.
5. Death or survival
No studies reported this outcome.
6. Adverse effects (pneumothorax, musculoskeletal pains or injuries, others)
One study reported that one participant out of 16 experienced earache whilst performing IMT at 40% of maximal effort (de Jong 2001).
7. Costs
No studies reported this outcome.
Discussion
Summary of main results
The main finding of this systematic review is that there is insufficient evidence to conclude if RMT has a positive effect on health outcomes in people with CF. This finding is based upon the small number of included studies (n = 9) and the small sample sizes used across the studies (n = 11 to 39). Of the nine included studies, only five (comprising 98 participants) were fully published studies (Asher 1983; Bieli 2017; de Jong 2001; Enright 2004; Sawyer 1993) highlighting the need for further research. Abstracts from conference proceedings limit the amount of detailed data that are presented and thus the data that can be extracted.
Pulmonary function is routinely measured in clinical practice to monitor chest disease severity, specifically FEV1 and FVC. No differences were reported between groups in pulmonary function, including both FEV1 and FVC, in any study. Only three of the studies included in the review reported that they assessed health‐related quality of life (Bieli 2017; Chatham 1997; Enright 2004). Although no statistically significant difference was reported in two of the studies (Bieli 2017; Enright 2004), one study reported a significantly greater improvement in the two parameters of mastery and emotion in the treatment group (P < 0.01) (Chatham 1997). Five of the nine studies reported some measure of exercise capacity (Albinni 2004; Asher 1983; Bieli 2017; de Jong 2001; Sawyer 1993). Of these, only one study reported a significant improvement with a 10% increase in exercise duration (P < 0.03) when working at 60% of maximal effort (Sawyer 1993).
Descriptive analysis of considered studies suggests that PImax and RME time are the measures that can best detect the effects of a RMT intervention. Although it is acknowledged that this is not supported by a meta‐analysis of studies, they are the measures the included studies report as showing significant improvement within the RMT groups. Adverse events were consistently not reported by the studies, with only one making specific reference to this (de Jong 2001).
Overall completeness and applicability of evidence
Due to the life‐limiting nature of CF, the clinical status of the participants recruited to studies in this population is of particular importance. The participants from the included studies have a mean age of approximately 18.5 years. With average life expectancy around 45.1 years (Carr 2016), the participants in these studies are effectively in middle age. It is possible that due to the progressive nature of the condition, the pulmonary function of the participants limited the effectiveness of RMT in comparison to a healthy population. There is an apparent under‐use of assessing dyspnoea and exercise capacity following RMT which may limit the external validity of the research base. In healthy individuals, both an individual’s perception of breathlessness and their maximal exercise capacity has found to improve following RMT (El‐Manshawi 1986; Romer 2002).
Quality of the evidence
Overall, the methodological quality of the included studies was inconsistent and none addressed all aspects completely. A systematic review requires homogeneity between the included studies to allow firm conclusions to be drawn. Despite finding nine studies which met the inclusion criteria of this review, the variation in their methodologies and outcomes was such that no combined analyses could be made. These differences occurred in all the major aspects of the studies including the outcomes employed, the units of measurement for certain outcomes and the method and extent to which the clinical status of the participants was established or reported.
The execution of the included studies (randomisation and blinding) ranged from being fully acknowledged, considered and reported to either being merely stated as "randomised to the two groups" or not being mentioned. The external validity (with particular regard to the participant demographics) was explicit in only four of the studies (Bieli 2017; de Jong 2001; Enright 2004; Sawyer 1993). This aspect is of particular significance given that people who have CF are the target population. The nature of the disease means that two people of similar age, height and weight may have been affected by the condition differently and therefore may not "match" with regards to clinical status.
Although all studies state that they randomised their participants to the treatment groups, only one study indicated the method of allocation by stating that they employed the minimisation method (de Jong 2001). This study was graded as having a low risk of bias, whereas the remaining eight studies were graded as having an unclear risk of bias.
All the included studies were graded as having a high risk of performance bias, however studies are encouraged to specify more detail with regards to the training group. Although it may be difficult to blind the participants to which treatment arm they are randomised to, five studies made use of "sham" training for the control (Amelina 2006; Chatham 1997; de Jong 2001; Enright 2004; Sawyer 1993). Two studies blinded the outcome assessors at the final data collection session, whereas the majority of studies make no overt reference to any blinding. Out of the nine studies, none reported on all outcome measures thus suggesting a potential risk of bias. Two studies report withdrawals, but offer no explanation for these (Amelina 2006; Chatham 1997). Overall, all of the included studies have been graded an unclear risk of bias as none provided sufficient information to arrive at a definitive conclusion.
Following the GRADE assessments, pulmonary function, exercise capacity and health‐related quality of life were deemed to be have very low‐quality evidence, whereas evidence for respiratory muscle function was deemed to be of low quality (summary of findings Table for the main comparison).
Potential biases in the review process
We are not aware of any potential biases in the review process.
Agreements and disagreements with other studies or reviews
Overally, the current systematic review supports previous versions of this review and continues to highlight the need for more research in this area, specifically studies of high methodological quality.

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
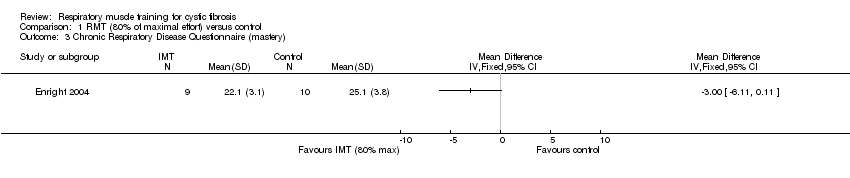
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery).
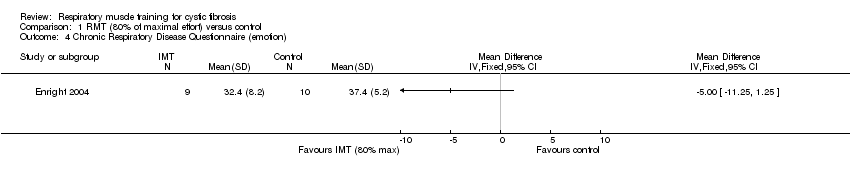
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 2 PImax (cm H₂O).
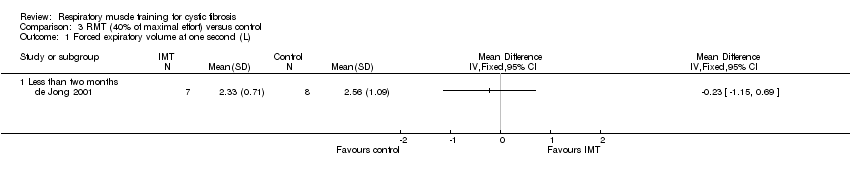
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (L).
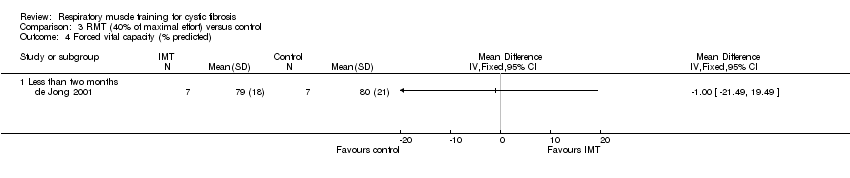
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted).
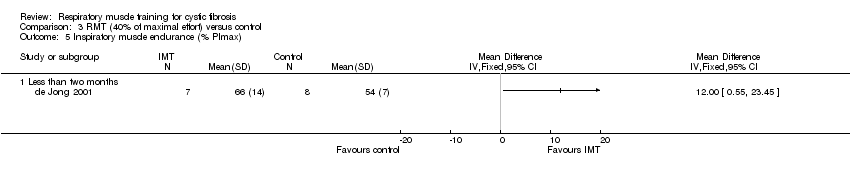
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (% PImax).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
| Respiratory muscle training compared with control for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: respiratory muscle trainingₑ Comparison: controlₑ | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Controlₑ | Respiratory muscle trainingₑ | |||||
| FEV1: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 145 (7 studies including 2 cross‐over studies) | ⊕⊝⊝⊝ | Studies reported FEV1 as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| FVC: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 114 (5 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Studies reported FVC as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| Exercise capacity: VO2max (mL/kg/min) Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 54 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | One study with an unspecified level of resistance reported a significant improvement within the respiratory muscle training group. | |
| HRQoL: total score Follow‐up: 8 weeks | Two studies reported no significant differences between the respiratory muscle training group and the control group. One study reported significant improvements in the parameters of mastery and emotion in the respiratory muscle training group compared to the control group. | NA | 69 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Two studies used the Chronic Respiratory Disease Questionnaire (CRDQ) and one study used the cystic fibrosis questionnaire (CFQ). | |
| Respiratory muscle function: maximal inspiratory pressure (PImax) Follow‐up: 6‐10 weeks | Significant improvements were observed in all respiratory muscle training groups. Two studies reported no significant differences between the respiratory muscle training group and the control group. | NA | 51 (3 studies including 1 cross‐over study) | ⊕⊕⊝⊝ | ||
| Respiratory muscle function: inspiratory capacity Follow‐up: NA | NA | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The resistance level of the respiratory muscle training intervention was variable; three studies used 80% of maximal effort, one study used 60% of maximal effort, one study used 40% of maximal effort, one study used 30% of maximal effort and three studies did not specify the level of resistance. Control groups were also variable; cycle ergometer, H20, treatment as usual, standard chest physiotherapy, low resistance threshold loading device, no training or sham training. 2. Downgraded twice due to serious risk of bias: the included studies lacked methodological detail relating to methods of randomisation, allocation concealment and blinding. Most of the studies were at high risk of bias due to lack of blinding, incomplete outcome data or selective reporting, or both. 3. Downgraded due to imprecision: studies included a small number of participants and numerical results were not available for some of the studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PImax (cm H₂O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (% PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

