Entrenamiento de los músculos respiratorios para la fibrosis quística
Información
- DOI:
- https://doi.org/10.1002/14651858.CD006112.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 24 mayo 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Fibrosis quística y enfermedades genéticas
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Up to 2018
Brian Houston drafted the protocol with comments from Helen Handoll and Cees van der Schans.
Nicola Mills, Arturo Solis‐Moya and Brian Houston extracted data and assessed study quality. Brian Houston drafted the full review with comments from Nicola Mills and Arturo Solis‐Moya.
Brian Houston acts as guarantor of the review.
From 2018
Nathan Hilton drafted the update with comments from Arturo Solis‐Moya.
Nathan Hilton and Arturo Solis‐Moya extracted data and assessed study quality.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
None known.
Acknowledgements
We acknowledge the valuable contribution to the protocol and review stage of this review from previous review authors (Professor Cees van der Schans, Brian Houston, Helen Handoll, Nicola Mills).
We thank Nikki Jahnke for her help and advice at both the protocol and review stage.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Dec 17 | Respiratory muscle training for cystic fibrosis | Review | Gemma Stanford, Harrigan Ryan, Arturo Solis-Moya | |
| 2018 May 24 | Respiratory muscle training for cystic fibrosis | Review | Nathan Hilton, Arturo Solis‐Moya | |
| 2013 Nov 21 | Inspiratory muscle training for cystic fibrosis | Review | Brian W Houston, Nicola Mills, Arturo Solis‐Moya | |
| 2008 Oct 08 | Inspiratory muscle training for cystic fibrosis | Review | Brian W Houston, Nicola Mills, Arturo Solis‐Moya | |
| 2006 Jul 19 | Inspiratory muscle training for cystic fibrosis | Protocol | Brian Houston, Cees P van der Schans | |
Differences between protocol and review
Update 2011
Due to Teesside University (location of the lead author) changing its search engine, some of the search strategies have been re‐written. The search strategies in Appendix 5 have superseded those in Appendix 1.
Update 2018
In line with current Cochrane guidance, summary of findings tables have been added. The title has been changed from 'Inspiratory muscle training for cystic fibrosis' to 'Respiratory muscle training for cystic fibrosis'.
The new author team has re‐ordered the outcomes to prioritise outcomes that are deemed more clinically meaningful to people with CF.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 1 RMT (80% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
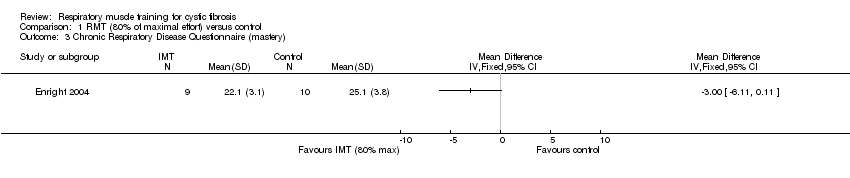
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 3 Chronic Respiratory Disease Questionnaire (mastery).
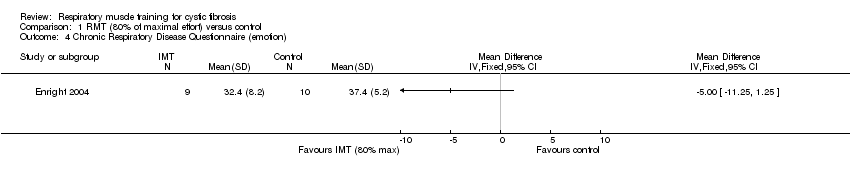
Comparison 1 RMT (80% of maximal effort) versus control, Outcome 4 Chronic Respiratory Disease Questionnaire (emotion).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 2 RMT (60% of maximal effort) versus control, Outcome 2 PImax (cm H₂O).
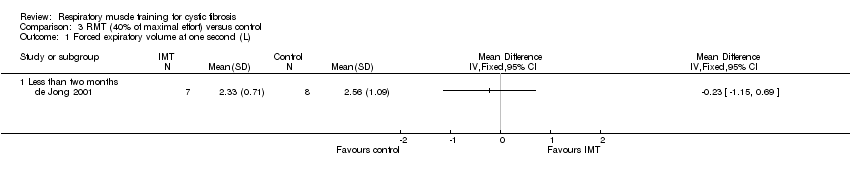
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 2 Forced expiratory volume at one second (% predicted).

Comparison 3 RMT (40% of maximal effort) versus control, Outcome 3 Forced vital capacity (L).
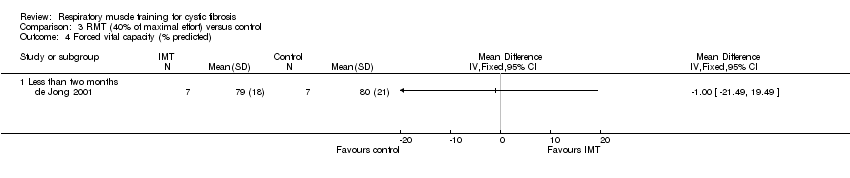
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 4 Forced vital capacity (% predicted).
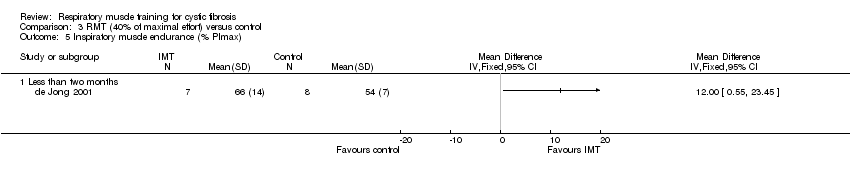
Comparison 3 RMT (40% of maximal effort) versus control, Outcome 5 Inspiratory muscle endurance (% PImax).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 1 Forced expiratory volume at one second (L).

Comparison 4 RMT (20% of maximal effort) versus control, Outcome 2 Forced vital capacity (L).
| Respiratory muscle training compared with control for cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: respiratory muscle trainingₑ Comparison: controlₑ | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Controlₑ | Respiratory muscle trainingₑ | |||||
| FEV1: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 145 (7 studies including 2 cross‐over studies) | ⊕⊝⊝⊝ | Studies reported FEV1 as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| FVC: % predicted Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 114 (5 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Studies reported FVC as % predicted, litres or z score. One study with respiratory muscle training level 30% of maximal effort reported a significant improvement within the training group. | |
| Exercise capacity: VO2max (mL/kg/min) Follow‐up: 6‐12 weeks | No significant differences between the respiratory muscle training group and the control group were reported in any study. | NA | 54 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | One study with an unspecified level of resistance reported a significant improvement within the respiratory muscle training group. | |
| HRQoL: total score Follow‐up: 8 weeks | Two studies reported no significant differences between the respiratory muscle training group and the control group. One study reported significant improvements in the parameters of mastery and emotion in the respiratory muscle training group compared to the control group. | NA | 69 (3 studies including 1 cross‐over study) | ⊕⊝⊝⊝ | Two studies used the Chronic Respiratory Disease Questionnaire (CRDQ) and one study used the cystic fibrosis questionnaire (CFQ). | |
| Respiratory muscle function: maximal inspiratory pressure (PImax) Follow‐up: 6‐10 weeks | Significant improvements were observed in all respiratory muscle training groups. Two studies reported no significant differences between the respiratory muscle training group and the control group. | NA | 51 (3 studies including 1 cross‐over study) | ⊕⊕⊝⊝ | ||
| Respiratory muscle function: inspiratory capacity Follow‐up: NA | NA | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. The resistance level of the respiratory muscle training intervention was variable; three studies used 80% of maximal effort, one study used 60% of maximal effort, one study used 40% of maximal effort, one study used 30% of maximal effort and three studies did not specify the level of resistance. Control groups were also variable; cycle ergometer, H20, treatment as usual, standard chest physiotherapy, low resistance threshold loading device, no training or sham training. 2. Downgraded twice due to serious risk of bias: the included studies lacked methodological detail relating to methods of randomisation, allocation concealment and blinding. Most of the studies were at high risk of bias due to lack of blinding, incomplete outcome data or selective reporting, or both. 3. Downgraded due to imprecision: studies included a small number of participants and numerical results were not available for some of the studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Chronic Respiratory Disease Questionnaire (mastery) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Chronic Respiratory Disease Questionnaire (emotion) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 PImax (cm H₂O) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced expiratory volume at one second (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Forced vital capacity (% predicted) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Inspiratory muscle endurance (% PImax) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Less than two months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Forced expiratory volume at one second (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Forced vital capacity (L) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Two to six months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

