Household interventions for secondary prevention of domestic lead exposure in children
Abstract
Background
Lead exposure is a serious health hazard, especially for children. It is associated with physical, cognitive and neurobehavioural impairment in children. There are many potential sources of lead in the environment, therefore trials have tested many household interventions to prevent or reduce lead exposure. This is an update of a previously published review.
Objectives
To assess the effects of household interventions intended to prevent or reduce further lead exposure in children on improvements in cognitive and neurobehavioural development, reductions in blood lead levels and reductions in household dust lead levels.
Search methods
In March 2020, we updated our searches of CENTRAL, MEDLINE, Embase, 10 other databases and ClinicalTrials.gov. We also searched Google Scholar, checked the reference lists of relevant studies and contacted experts to identify unpublished studies.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of household educational or environmental interventions, or combinations of interventions to prevent lead exposure in children (from birth to 18 years of age), where investigators reported at least one standardised outcome measure.
Data collection and analysis
Two authors independently reviewed all eligible studies for inclusion, assessed risk of bias and extracted data. We contacted trialists to obtain missing information. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 17 studies (three new to this update), involving 3282 children: 16 RCTs (involving 3204 children) and one quasi‐RCT (involving 78 children). Children in all studies were under six years of age. Fifteen studies took place in urban areas of North America, one in Australia and one in China. Most studies were in areas with low socioeconomic status. Girls and boys were equally represented in those studies reporting this information. The duration of the intervention ranged from three months to 24 months in 15 studies, while two studies performed interventions on a single occasion. Follow‐up periods ranged from three months to eight years. Three RCTs were at low risk of bias in all assessed domains. The other 14 studies were at unclear or high risk of bias; for example, we considered two RCTs and one quasi‐RCT at high risk of selection bias and six RCTs at high risk of attrition bias. National or international research grants or governments funded 15 studies, while the other two did not report their funding sources.
Education interventions versus no intervention
None of the included studies in this comparison assessed effects on cognitive or neurobehavioural outcomes, or adverse events. All studies reported data on blood lead level outcomes.
Educational interventions showed there was probably no evidence of a difference in reducing blood lead levels (continuous: mean difference (MD) –0.03, 95% confidence interval (CI) –0.13 to 0.07; I² = 0%; 5 studies, 815 participants; moderate‐certainty evidence; log‐transformed data), or in reducing floor dust levels (MD –0.07, 95% CI –0.37 to 0.24; I² = 0%; 2 studies, 318 participants; moderate‐certainty evidence).
Environmental interventions versus no intervention
Dust control: one study in this comparison reported data on cognitive and neurobehavioural outcomes, and on adverse events in children. The study showed numerically there may be better neurobehavioural outcomes in children of the intervention group. However, differences were small and the CI included both a beneficial and non‐beneficial effect of the environmental intervention (e.g. mental development (Bayley Scales of Infant Development‐II): MD 0.1, 95% CI –2.1 to 2.4; 1 study, 302 participants; low‐certainty evidence). The same study did not observe any adverse events related to the intervention during the eight‐year follow‐up, but observed two children with adverse events in the control group (1 study, 355 participants; very low‐certainty evidence).
Meta‐analysis also found no evidence of effectiveness on blood lead levels (continuous: MD –0.02, 95% CI –0.09 to 0.06; I² = 0%; 4 studies, 565 participants; moderate‐certainty evidence; log‐transformed data). We could not pool the data regarding floor dust levels, but studies reported that there may be no evidence of a difference between the groups (very low‐certainty evidence).
Soil abatement: the two studies assessing this environmental intervention only reported on the outcome of 'blood lead level'. One study showed a small effect on blood lead level reduction, while the other study showed no effect. Therefore, we deem the current evidence insufficient to draw conclusions about the effectiveness of soil abatement (very low‐certainty evidence).
Combination of educational and environmental interventions versus standard education
Studies in this comparison only reported on blood lead levels and dust lead levels. We could not pool the studies in a meta‐analysis due to substantial differences between the studies. Since the studies reported inconsistent results, the evidence is currently insufficient to clarify whether a combination of interventions reduces blood lead levels and floor dust levels (very low‐certainty evidence).
Authors' conclusions
Based on available evidence, household educational interventions and environmental interventions (namely dust control measures) show no evidence of a difference in reducing blood lead levels in children as a population health measure. The evidence of the effects of environmental interventions on cognitive and neurobehavioural outcomes and adverse events is uncertain too.
Further trials are required to establish the most effective intervention for reducing or even preventing further lead exposure. Key elements of these trials should include strategies to reduce multiple sources of lead exposure simultaneously using empirical dust clearance levels. It is also necessary for trials to be carried out in low‐ and middle‐income countries and in differing socioeconomic groups in high‐income countries.
PICO
Plain language summary
Household interventions for preventing domestic lead exposure in children
Why is this review important?
Lead exposure is a serious health risk, especially for children. Lead poisoning at high levels can cause anaemia, multi‐organ damage, seizures, coma and death in children. At chronic low levels, it can lead to cognitive (thought processes), psychological (mental and emotional states) and neurobehavioural impairment (e.g. aggression, hyperactivity). There are many potential sources of lead in the environment, therefore researchers have studied different educational and environmental household interventions to reduce lead exposure in children, such as parental education, removal of lead dust or home remediation work. However, it is not clear if and to what extent these interventions work in reducing or preventing further lead exposure in children.
Who will be interested in this review?
‐ Parents and carers who want to prevent domestic lead exposure in children.
‐ Health professionals and decision‐makers who are interested in methods to prevent domestic lead exposure in children.
What questions does this review aim to answer?
We wanted to find out if educational or environmental household interventions, or combinations of both, are effective in preventing or reducing further domestic lead exposure in children up to 18 years of age. We were interested in looking at improvements in cognitive and neurobehavioural development, potential harms, reductions in blood lead levels and household lead dust levels.
Which studies were included in the review?
We searched databases up to March 2020 for randomised controlled trials (RCTs; where participants are randomly assigned, in this case, to one or more groups to receive the treatment and one group that does not) and quasi‐RCTs (where children are assigned to groups using methods that are not strictly random). We found 17 studies (three new to this update), involving 3282 children from birth to six years of age. The studies investigated educational or environmental interventions, or a combination of both, to reduce domestic lead exposure in children. Children in all studies were under six years of age. Fifteen studies took place in urban areas of North America, one in Australia and one in China. Most studies were performed in areas with low socioeconomic status. Boys and girls were equally represented in the studies. The duration of the intervention ranged from three months to 24 months in 15 studies, and two studies performed an intervention on a single occasion. Fourteen studies used flawed methods that could distort their results, making them less trustworthy.
Follow‐up periods ranged from three months to eight years. National or international research grants or governments funded 15 studies; two studies did not report their funding sources.
What does the evidence from the review reveal?
Educational interventions: none of the included studies in this comparison assessed effects on cognitive or neurobehavioural outcomes, or harms. Compared to no intervention, educational interventions probably result in no differences in blood lead levels of young children or floor dust levels (moderate‐quality evidence).
Environmental interventions: one study comparing dust control measures with no intervention showed little to no difference in cognitive and neurobehavioural outcomes between the groups after three years to eight years. The same study assessed harms and found none associated with the intervention, but observed two children with side effects in the control group. All included studies in this comparison found that dust control did not lead to more or less reduced blood lead levels of young children (moderate‐quality evidence) or floor dust levels than no intervention (very low‐quality evidence). Two studies assessed the effect of soil abatement and did not allow any conclusions about its effectiveness (very low‐quality evidence).
Combination of educational and environmental interventions versus standard education: there is insufficient evidence that combination interventions reduce blood lead levels or floor dust lead levels (very low‐quality evidence), and further studies need to address this research gap.
What should happen next?
More research is needed to find out what is effective for preventing children's exposure to lead. Studies should be carried out in different socioeconomic groups in high‐, middle‐ and low‐income countries to consider how interventions work in contexts shaped by different levels of industrialisation or environmental and occupational health safety regulations.
Authors' conclusions
Summary of findings
| Education interventions versus no intervention for preventing domestic lead exposure in children | ||||||
| Patient or population: children (aged 0–2 years) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants (studies) | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| No intervention | Educational interventions | |||||
| Cognitive and neurobehavioural outcomes | None of the included studies assessed effects on cognitive or neurobehavioural outcomes | — | — | — | — | |
| Adverse events | None of the included studies assessed adverse event outcomes | — | — | — | — | |
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 3–18 months | The mean blood lead level (continuous, log transformed) ranged across control groups from 1.24 to 2.51a,b | The mean blood lead level (continuous, log transformed) in the intervention groups was 0.03 lower (0.13 lower to 0.07 higher) a | — | 815 | ⊕⊕⊕⊝ | Included studies: Lanphear 1996a; Lanphear 1999; Wasserman 2002; Jordan 2003; Brown 2006 |
| Household dust: hard floor dust lead levels (continuous) Scale: 0–40 Follow‐up: 6 months | The mean floor dust level – hard floor – ranged across control groups from 1.65 to 2.28a,b | The mean floor dust level – hard floor – in the intervention groups was 0.07 lower (0.37 lower to 0.24 higher) b | — | 318 | ⊕⊕⊕⊝ | Included studies: Lanphear 1996a; Lanphear 1999 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aPost‐treatment value. | ||||||
| Environmental interventions versus no intervention for preventing domestic lead exposure in children | ||||||
| Patient or population: children (aged 0–6 years) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| No intervention | Environmental interventions | |||||
| Dust control interventions | ||||||
| Cognitive and neurobehavioural outcomes Scale Wechsler IQ: BRIEF: Follow‐up: 3–8 years | Children in the intervention group had numerically better cognitive and neurobehavioural outcomes, but differences were small and 95% CI included beneficial and non‐beneficial effects. Difference of mean scores after 8 years of selected scales:
For detailed results of subscales and additional scales reported see Effects of interventions. | — | 224–302 (1 study) | ⊕⊕⊝⊝ | Included study: Braun 2018 | |
| Adverse events Follow‐up: 3–8 years | 1 study reported that after 8 years they did not observe any adverse events in the intervention group. In the control group, 1 child had an injury because of a stair gateway installed and another child had elevated blood lead concentrations (28 µg/dL). | — | 355 (1 study) | ⊕⊝⊝⊝ | ||
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 6–24 months | The mean blood lead level (continuous, log transformed) ranged across control groups from 0.53to 2.9e | The mean blood lead level (continuous, log transformed) in the intervention groups was 0.02 lower (0.09 lower to 0.06 higher) e | — | 565 (4 studies) | ⊕⊕⊕⊝ | Included studies: Hilts 1995; Rhoads 1999; Boreland 2009; Braun 2018 |
| Household dust: floor dust lead levels | None of the included studies assessed floor dust lead levels. | — | — | — | — | |
| Soil abatement interventions | ||||||
| Cognitive and neurobehavioural outcomes | None of the included studies assessed cognitive and neurobehavioural outcomes. | — | — | — | — | |
| Adverse events | None of the included studies assessed adverse events. | — | — | — | — | |
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 11–24 months | 2 studies performed soil abatement interventions (Weitzman 1993; Farrell 1998). Farrell 1998 reported results as a "total effect" showing no statistical significance, and no data were available for our analyses. Weitzman 1993 reported a statistically significant effect in favour of the intervention. The difference in mean change scores between the intervention group and control group A (loose interior dust abatement and paint removal) was –1.5 µg/dL (SD 4.9), and between the intervention group and control group B (loose interior paint removal only) was –1.9 µg/dL (SD 5.0). No measure of variance was available for post‐treatment means or mean change scores, so further analysis was not possible in this review. | — | 378 (2 studies) | ⊕⊝⊝⊝ Very lowf,g | Included studies Weitzman 1993; Farrell 1998 | |
| Household dust: floor dust lead levels | None of the included studies reported floor dust lead levels. | — | — | — | — | |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IQ: intelligence quotient; MD: mean difference; n: number of study participants with a measurement; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because of imprecision: total population size was fewer than 400 and the 95% confidence interval included both a benefit and no benefit of the intervention. | ||||||
| Combination interventions versus no intervention for preventing domestic lead exposure in children | ||||||
| Patient or population: children (aged 0–4 years) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Standard education | Combination interventions | |||||
| Cognitive and neurobehavioural outcomes | None of the included studies assessed cognitive and neurobehavioural outcomes. | — | — | — | — | |
| Adverse events | None of the included studies assessed adverse events. | — | — | — | — | |
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 6–24 months | The 4 studies that used a combination of interventions compared to standard education showed inconclusive results. While Charney 1983 reported a significant effect favouring treatment with arithmetic means for post‐treatment blood lead levels of 31.7 µg/dL (SD 2.6) in the intervention group and 37.8 µg/dL (SD 7.9) in the control group, Aschengrau 1998, Campbell 2011, and Sterling 2004 showed little to no difference between combination interventions and standard education on blood lead levels. | — | 426 (4 studies) | ⊕⊝⊝⊝ Very lowa,b | Included studies Charney 1983; Aschengrau 1998; Sterling 2004; Campbell 2011 | |
| Household dust: floor dust lead levels Follow‐up: 6–12 months | Aschengrau 1998 found no evidence for an effect on floor dust lead levels, with median changes for floor dust lead level being –0.002 mg/m² (–0.2 µg/feet², SD 0.8 µg/feet²) in the intervention group and 0.001 mg/m² (0.0 µg/feet², SD 0.2 µg/feet²) in the control group. A second study also found no evidence for an effect on floor dust lead levels (Campbell 2011). | — | 336 (2 studies) | ⊕⊝⊝⊝ Very lowa,b | ||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because of imprecision; inconclusive and contradicting results of studies. | ||||||
Background
Description of the condition
Lead is a metal that has been used since prehistoric times. Over the years, its expansive distribution and mobilisation in the environment has resulted in increasing human exposure and uptake (Tong 2000). Lead poisoning is a serious and recognised health hazard with major socioeconomic implications (UNEP‐UNICEF 1997; Attina 2013). At high levels, lead poisoning in children can cause anaemia, multi‐organ damage, renal damage, seizures, coma and death. At chronic low levels, lead toxicity causes significant cognitive, psychological and neurobehavioural impairment (UNEP‐UNICEF 1997; Tong 2000; Mason 2014).
Global lead exposure is responsible for 2.5 million disability‐adjusted life years (DALYs) from intellectual disability, 1.3 million DALYs from chronic kidney disease and 20.6 million DALYs from chronic vascular diseases (GBD 2018). In Europe, lead produced 3% of total DALYs in children (Rojas‐Rueda 2019). There are many potential sources of lead in the environment, including lead industries, mining and smelting; leaded petrol; lead‐based paint; water piping, fixtures and solder; and consumer products and hobbies that use lead. Lead from these sources is most commonly found in paint, dust, soil or water. Risk factors for lead exposure include socioeconomic disadvantage, residence in an area with lead industry, renovation or deterioration of older houses containing lead‐based paint, and residence in countries where leaded petrol or aviation fuel is still used (Tong 2000; Miranda 2011).
Blood lead levels in the general population in the USA have fallen significantly since the late 1970s with the phasing out of lead petrol and bans on the use of lead in paints and lead solder used in canned foods and other consumer products (Jacobs 2006). However, concern has now grown regarding chronic low‐level exposure within the environment (Tong 2000). The major source of environmental lead dust exposure in children in low‐ and middle‐income countries is lead‐based paints and other lead hazards in housing. Older housing with peeling or flaking paint or current renovations can result in increased lead dust levels (EHU 2002).
Occupational and environmental exposures continue to be a serious global problem, especially in low‐ and middle‐income countries, which may be rapidly industrialising (Tong 2000). People in these settings, especially children, may have higher levels of lead exposure due to unregulated industrial emission, weak environmental and occupational health safety regulations, and cottage (domestic) industries such as metal polishing and smelting (UNEP‐UNICEF 1997). Many countries have implemented or proposed legally binding restrictions on lead in paints for domestic use, and consequently this will become a less important source of exposure over time. Nevertheless, lead‐based paints for household use are still available for purchase in several low‐ and middle‐income countries such as Argentina, China, Ethiopia, Ghana, India, Malaysia and Tunisia (Kessler 2014). In view of rapid industrialisation and the persistence of lead in the environment, this is likely to remain a significant public health issue in these countries for many years (Tong 2000).
Children are at higher risk of lead exposure and lead toxicity. This is due to their increased intake of lead per unit of bodyweight compared with adults and their higher rate of physiological uptake (up to 50% compared with 10% to 15% in adults; UNEP‐UNICEF 1997). Young children often place objects in their mouths resulting in lead‐contaminated dust and soil ingestion. Furthermore, a young child's developing body, and in particular the central nervous system, is more vulnerable to the effects of lead (Bellinger 2008; Mason 2014; Hauptman 2017).
Dust is an important residential media for lead exposure and the strongest predictor of blood lead levels. Floor dust exceeding 0.108 mg/m² (10.0 µg/feet²) is currently recognised as hazardous (EPA 2020).
In 1991, the Centers for Disease Control and Prevention (CDC) defined blood lead levels of 10.0 µg/dL or more as a "blood lead level of concern" for children aged one year to five years (CDC 1991). However, studies have shown that adverse effects on cognitive function in children are proportional at even lower blood lead levels (Canfield 2003; Lanphear 2005a; Kordas 2006; Evens 2015), suggesting that there is no safe level of blood lead for children (CDC 2005; Grandjean 2010; CDC 2012). Therefore, in 2012 the CDC followed the advice of the Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP) to replace the term 'level of concern' with an upper reference interval value, which they defined as the 97.5th percentile of blood lead levels (currently 5.0 μg/dL) from the National Health and Nutrition Examination Survey (NHANES), which includes US children aged one year to five years (Wheeler 2013).
Of further concern, the effects of lead are thought to be largely irreversible, so reducing or eliminating lead from the body does not significantly improve the neuropsychological manifestations (Tong 2000). Chelation agents, currently the mainstay of treatment in children with blood lead concentrations greater than 45.0 µg/dL, reduce the mortality of severe acute lead encephalopathy, but they do little to remove the lead sequestered in bone (greater than 94% of the body burden in adults, 70% of the body burden in children (O'Flaherty 1995)), neither do they reverse neuropsychological effects (Chisolm 2001; Rogan 2001; Dietrich 2004). Due to the higher rate of bone turnover in young children, the mean half‐life of lead in blood is significantly longer (eight months to 11 months with acute exposure and 20 months to 38 months with prolonged exposure) than that of adults (15 days), and bone can be a prolonged source of lead in the blood (Manton 2000; Chisolm 2001). However, one study in 157 lead‐exposed children showed a shorter half‐life of lead in blood of children under the age of three years (mean 6.9, standard deviation (SD) 4.0 days) and children three years or older (mean 19.3, SD 14.1 days), indicating a faster turnover of lead in children's blood than earlier studies showed (Specht 2019).
Chisolm 2001 estimated that the cost of chelation therapy in children who were previously exposed to lead is higher than environmental interventions and is unlikely to have significant long‐term benefit. Therefore, the ultimate goal for the management of this public health issue should be to prevent toxicity by controlling lead hazards in the environment (Chisolm 2001).
Description of the intervention
This review focuses on interventions for secondary prevention in children who are already exposed to lead sources. It includes interventions that aim to reduce existing lead exposure or prevent further lead exposure in children with low or relatively modest blood lead levels. Most research has focused on environmental and educational preventive interventions. Educational interventions address parental awareness by imparting knowledge of lead exposure pathways, hygiene, and household dust control measures to prevent ingestion of dust and soil (Campbell 2000). Several papers have studied the effectiveness of educational interventions to encourage home cleaning, and these studies varied in the extent of cleaning activities and educational programmes. The results have not supported the effectiveness of education alone (Campbell 2000).
Environmental prevention focuses on improvement in risk assessment, development of housing‐based standards for lead‐based paint hazards, as well as safe and cost‐effective lead hazard reduction techniques (Campbell 2000). Several studies have been published regarding various lead reduction techniques and their relative effectiveness and safety. These have studied both abatement (permanent elimination of lead sources through removal of paint and dust, replacement of lead containing structures and covering of lead‐contaminated soil), and interim controls pending abatement (specialised cleaning, repairs, maintenance, painting and temporary containment). Different randomised controlled trials (RCTs) have tested a variety of environmental lead hazard control interventions to decrease children's blood lead level and home dust lead levels, with most follow‐up extending from six months to two years post‐intervention. Comparison of environmental interventions has been difficult due to variations in intervention types, blood collection techniques, adjustments for age and season, dust lead‐loading quantification and statistical analyses (Campbell 2000).
How the intervention might work
Removal of sources of lead, specialised cleanings, repairs and maintenance around the house (environmental interventions) aim to reduce exposure to domestic lead and lead dust. Educational interventions focused on parents aim to raise parental awareness of lead hazards and motivate them to reduce lead hazards for their children. Through education, parents should also learn about lead exposure pathways and how to clean their home to keep it in a lead‐safe condition. Enabling parents by educational means aims to reduce exposure to domestic lead and lead dust, thereby decreasing the risk of lead ingestion and ultimately lead poisoning. As a short effect of the intervention, one should be able to measure decreased lead dust in the household. Ultimately, the removal or reduction of lead exposure should result in decreased blood lead levels and better cognitive and neurobehavioural outcomes. However, once blood lead levels in children are elevated, internal lead stores mobilised from bone and soft tissue can keep the blood lead level high for months to years depending on the initial blood value.
Why it is important to do this review
Lead poisoning has long been linked with physical, cognitive and neurobehavioural impairment in children. Despite efforts to reduce environmental, occupational and industrial lead exposure worldwide, children living in areas with older housing and in low‐ and middle‐income countries with weak industrial regulations continue to show evidence of lead exposure. There has been research on many household interventions, and it is important to examine their effectiveness.
This is an update of the review by Nussbaumer‐Streit 2016, which found no evidence of effectiveness for household interventions for education or dust control measures in reducing blood lead levels in children as a population health measure. The 2016 review concluded there was insufficient evidence for soil abatement or combination interventions, and that further trials were required to establish the most effective intervention for the prevention of lead exposure. Hence, it is important to update this review looking for any advances in the area.
Objectives
To assess the effects of household interventions intended to prevent or reduce further lead exposure in children on improvements in cognitive and neurobehavioural development, reductions in blood lead levels and reductions in household dust lead levels.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs or quasi‐RCTs (which use a method of allocation that is not truly random, for example, by date of birth, medical record number, or order in which participants are included in the study such as alternation).
The main reason to focus on such study designs is to account for secular trends in blood lead levels. Children's blood lead levels have declined since the late 1970s (Jacobs 2006) and studies that attempt to test the effect of interventions in the absence of a control group may overestimate their effect because of the downward trend in blood lead concentrations. Furthermore, children's blood lead levels, which peak at about two years of age, typically decline as they mature primarily because they no longer exhibit as many mouthing behaviours (Hornung 2009). Thus, any observational study that enrolls children aged less than two years may erroneously conclude that the intervention led to a reduction in blood lead levels even though children's blood lead levels would have declined anyway. Finally, children's blood lead levels rise during summer months, partly due to increased contact with soil and increased exposure to lead paint in window sills during this season (Haley 2004). If the intervention does not account for seasonal variation it may under‐ or overestimate the effect of an intervention.
Types of participants
Children (from birth to 18 years of age) and their parents or carers.
Types of interventions
Interventions that aim to reduce domestic lead exposure compared to no intervention or standard measures/recommendations. In this review, we classified interventions as follows.
-
Educational interventions. One or more educational sessions for parents that aim to raise parental awareness of lead exposure pathways and the dangers lead can have on their children as well as teaching them how to keep their home in lead‐safe condition and how to prevent ingestion of dust and soil. Eligible educational interventions had to provide more than standard information via, for example, a brochure.
-
Environmental (household) interventions. These include specialised cleaning, repairs, maintenance, soil abatement (removal and replacement), painting and temporary containment of lead hazards.
-
Combinations of the above interventions.
We excluded interventions involving nutritional supplementation.
Types of outcome measures
We included studies reporting any of the outcomes described below.
Primary outcomes
-
Cognitive and neurobehavioural outcomes in children, assessed with standardised measures of outcome such as assessment of a child's intelligence quotient (IQ), development or behaviour. Suitable IQ measures were the Stanford Binet Intelligence Scale (Smith 1989), the Wechsler Intelligence Scale for Children (Wechsler 1991), and the Wechsler Preschool and Primary Scale of Intelligence (Wechsler 1989). An example of a suitable development measure is the Griffiths Mental Development Scales (Griffiths 1954; Griffiths 1970), and for behaviour, the Child Behavior Checklist (Achenbach 1991).
-
Adverse events of the intervention in children (e.g. injuries or poisoning through cleansing agents).
Secondary outcomes
-
Blood lead levels in children (venous blood sample or capillary blood sample; AAP 1998).
-
Household dust measures of lead exposure (e.g. lead loading of household floor dust).
-
Cost of intervention (e.g. cost of cleaning supplies, soil abatement or education).
Instruments were confined to those with at least one standardised outcome measure (such as blood lead level) used for intervention and control groups. We considered outcomes for any follow‐up duration (short term: six months to 18 months; long‐term: longer than 18 months).
Blood lead levels in children from venous and capillary blood samples were assessed together as one outcome of blood lead levels.
We used cognitive and neurobehavioural outcomes, adverse events, blood lead levels in children and household dust measures to complete summary of findings Table 1, summary of findings Table 2 and summary of findings Table 3.
Search methods for identification of studies
The Cochrane Information Specialist for the Developmental, Psychosocial and Learning Problems Group updated the searches of the following databases to March 2020 using the search strategies in Appendix 1. We made some changes to the databases (see Differences between protocol and review).
Details of the previous search strategies are available in Nussbaumer‐Streit 2016.
Electronic searches
We searched the following databases and trials registers in March 2020.
-
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 3) in the Cochrane Library, which includes the Specialised Register of the Cochrane Developmental Psychosocial and Learning Problems Group. Searched 25 March 2020.
-
MEDLINE Ovid (1946 to March week 2 2020).
-
MEDLINE In‐Process & Other Non‐Indexed Citations Ovid (searched 24 March 2020).
-
MEDLINE Epub Ahead of Print Ovid (searched 24 March 2020).
-
Embase Ovid (1980 to 24 March 2020).
-
APA PsycINFO Ovid (1806 to March week 3 2020).
-
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1937 to 25 March 2020).
-
Sociological Abstracts ProQuest (1952 to 25 March 2020).
-
ERIC EBSCOhost (Education Resources Information Center; 1966 to 25 March 2020).
-
Science Citation Index Web of Science (SCI; 1970 to 24 March 2020).
-
Conference Proceedings Citation Index‐Science Web of Science (CPCI‐S; 1990 to 24 March 2020).
-
Cochrane Database of Systematic Reviews (2020, Issue 3) part of the Cochrane Library.
-
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; searched 25 March 2020).
-
ClinicalTrials.gov (clinicaltrials.gov; searched 27 March 2020).
-
World Health Organization (WHO) ICTRP (www.who.int/ictrp/en); access attempted 27 March 2020 but was limited to users within WHO).
Searching other resources
We examined the reference list of relevant studies and contacted experts to determine whether any unpublished or ongoing trials existed. In May 2020, we also searched Google Scholar and found no further studies. In July 2020, the Information Specialist ran searches in MEDLINE and Embase to identify any corrections or retractions of included studies; we found none.
Data collection and analysis
We described the methods used in the following sections. For additional methods archived for updates of this review, see Yeoh 2006 and Appendix 2.
Selection of studies
We used Cochrane's Screen4Me workflow to help assess the search results. Screen4Me comprises three components: 1. known assessments – a service that matches records in the search results to records that have already been screened in Cochrane Crowd and have been labelled as an RCT or as not an RCT; 2. the RCT classifier – a machine learning model that distinguishes RCTs from non‐RCTs; and 3. if appropriate, Cochrane Crowd – Cochrane's citizen science platform where the Crowd help to identify and describe health evidence. For more information about Screen4Me and the evaluations that have been done, see the Screen4Me web page on the Cochrane Information Specialist's portal (community.cochrane.org/organizational-info/resources/resources-groups/information-specialists-portal). More detailed information regarding evaluations of the Screen4Me components can be found in: McDonald 2017; Thomas 2017; Marshall 2018; and Noel‐Storr 2018.
In this update, Cochrane's Screen4Me prescreened all records identified by the search and grouped them into 'possibly an RCT' and 'unlikely to be an RCT'. The author team then uploaded all records to the screening software Covidence (www.covidence.org), and dually and independently screened records based on titles and abstracts (VM, AD, GW, AC, LKB, LMP, SL, SKL, BNS). We retrieved potentially relevant records as full‐text reports and screened them dually and independently in Covidence (VM, AD, GW, AC, LKB, LMP, SL, SKL, BNS). We resolved conflicts regarding inclusion/exclusion of an article by discussion and consensus.
Data extraction and management
We stored records yielded by the electronic searches in reference management software (EndNote 2012). We recorded and managed the results of abstract and full‐text screening, including information on the reasons for exclusion at full‐text assessment, in the Endnote database. We organised data using Review Manager 5 (Review Manager 2020). We developed and piloted data extraction forms a priori, extracting the information described below.
-
Methods: study design, study location or setting, recruitment, follow‐up, intention‐to‐treat, power calculation.
-
Participants: eligibility criteria, participation rate, reason for non‐participation, numbers analysed, number of dropouts/withdrawals, reasons for dropouts/withdrawals, baseline characteristics (sex, mean age, mean blood lead levels for each treatment group).
-
Interventions: brief descriptions of intervention (including frequency and duration of intervention events) and usual care provided.
-
Outcomes: timing of follow‐up events, outcomes assessed and scales used.
-
Notes: information on funding, conflicts of interests and further information to aid understanding of the study.
Two authors (BY and SW prior to 2016; BNS and SL in 2016; GW and VM in 2020) independently completed the data extraction forms for each study. They resolved disagreements by discussion. One author (BNS) transferred the data to Review Manager 5 (Review Manager 2020).
Assessment of risk of bias in included studies
In the previous version of this review (Yeoh 2014), two authors (of BY, SW, GR and NL) assessed the risk of bias of included studies. In the 2016 version (Nussbaumer‐Streit 2016), two authors (BNS and UG) assessed the 'blinding' domain in accordance with the updated methodological criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), as 'blinding of participants and personnel' and 'blinding of outcome assessment'. In 2020, two authors (VM and GW) assessed the risk of bias of the three newly identified studies.
For each included study, we rated the following domains at high, low or unclear risk of bias.
-
Sequence generation describes the method used to generate the allocation sequence to allow an assessment of whether it should produce comparable groups.
-
Allocation concealment describes the method used to conceal the allocation sequence in sufficient detail to determine whether investigators or participants could have foreseen intervention allocations before or during enrolment.
-
Blinding of participants and personnel describes all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received.
-
Blinding of outcome assessment describes all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
-
Incomplete outcome data describes the completeness of outcome data, including attrition and exclusions from the analysis.
-
Selective outcome reporting considers whether trialists reported on all relevant and prespecified outcomes.
-
Other sources of bias considers any important concerns about bias not addressed by the other domains (listed above) in the tool.
For cluster‐RCTs, we additionally looked at the risk of recruitment bias, baseline imbalance, loss to follow‐up of clusters and unit of analysis bias.
Where there was insufficient information in the published study regarding methodology or results in an extractable form, one author (BNS) contacted trial authors via email. If we did not receive a reply after the first contact, we sent one reminder. We did not score risk of bias on an additive basis.
Measures of treatment effect
Dichotomous data
Where outcomes from either standardised instruments or diagnostic evaluations were expressed as proportions, we calculated the risk ratio (RR) with 95% confidence intervals (CIs). We chose to calculate the RR over the odds ratio (OR), because the OR is more difficult to interpret correctly and potentially misleading to the reader.
For dichotomous data, we performed the analysis on the number of children with blood lead levels at or above two thresholds: 10.0 µg/dL (0.48 µmol/L) and 15.0 µg/dL (0.72 µmol/L). We chose these cut‐off points because most primary research to date has used them, although the current CDC reference value of 5.0 µg/dL suggests that a lower threshold is indicated (CDC 2012). We did not calculate risk differences because they strongly depend on baseline risks and are not as stable as RRs (Higgins 2019).
Continuous data
Where standardised assessment tools generated a score as the outcome measure, we conducted comparisons between the means of these scores. We used post‐treatment means and SD in all meta‐analyses. We used the mean difference (MD) of post‐treatment means as the outcome measure of choice because all studies reported outcomes on the same scale. As blood lead level data are typically positively skewed, included studies often provided log transformation of lead data (presented as geometric means). To prepare data for meta‐analysis, we performed a natural log transformation of all geometric means. We calculated SDs from geometric CIs, where necessary, using the calculation for small sample size (Higgins 2011) to integrate it in the meta‐analysis. If trials provided arithmetic means and SDs, we contacted trial authors to clarify that the data were normally distributed, and if no clarification was available, we assumed that this was the case. We then converted arithmetic means and SDs to approximate means and SDs on the log‐transformed scale according to Higgins 2008 before including them in the meta‐analysis. Where raw data were available, we calculated post‐treatment means and SDs on the log‐transformed data. We also performed exponentiation of the results.
Unit of analysis issues
Cluster‐randomised trials
To determine the impact of possible unit of analysis errors arising from inadequate adjustment for cluster randomisation in published results by Hilts 1995, we used a range of intraclass correlation coefficients (ICCs) to calculate a design effect to reduce the size of each trial to its 'effective sample size' (Higgins 2019). We then used data generated from this approach in the meta‐analysis. We used a range of ICCs (0.001, 0.01, 0.1, 0.2) due to no reliable ICCs being available from cluster trial authors, similar studies or resources that provided examples of ICCs (Ukoumunne 1999). We calculated design effects according to the equation: 1 + (M – 1) ICC, where M = 6, the mean cluster size of households used in the study (Hilts 1995). We calculated design effects using an ICC of 0.001 or less, resulting in no change in the sample sizes for intervention and control groups, so we did not use these data in further analyses.
Studies with multiple treatment groups
We reported the results of each treatment group narratively for the only two studies consisting of multiple treatment groups (Sterling 2004; Nicholson 2018).
Dealing with missing data
Where some data on trial methods or results were not available in the study reports, we contacted trial authors. Where no reply was forthcoming or full data were not made available, we included available data only in the meta‐analysis, where possible. We reported results of studies with missing data narratively.
For each study, we assessed the participation rate (enrolled/eligible). We also stated the number of participants who were in the final analysis as a proportion of all randomised participants in each study and presented reasons for missing data (see Characteristics of included studies table for more information).
Assessment of heterogeneity
We assessed consistency of results visually and by examining the I² statistic (Higgins 2002), a quantity that describes the approximate proportion of variation in point estimates that is due to heterogeneity rather than sampling error. In addition, we used the Chi² test to assess the statistical significance of the heterogeneity. We considered a P value less than 0.10 as statistically significant. We reported Tau², an estimate of the between‐study variance in a random‐effects meta‐analysis.
We examined clinical heterogeneity by comparing PICO (patient/population/problem, intervention, comparator, outcome) definitions of included studies. We assessed methodological heterogeneity by comparing study designs.
Assessment of reporting biases
We were unable to draw funnel plots to assess reporting biases due to the small number of studies included in the meta‐analyses.
Data synthesis
When two or more studies reported data that could be combined, we performed a meta‐analysis. For any given outcome, we calculated the MD for continuous data and RR for dichotomous data with 95% CIs, using both the random‐effects and fixed‐effect models (Mantel Haenszel for dichotomous outcomes, inverse variance for continuous outcomes). We reported the results of the random‐effects models because we assumed that the effects of secondary prevention are not identical across different populations and settings. The results of the random‐effects and fixed‐effect models, in general, were similar. We analysed data from RCTs separately from quasi‐RCTs.
Subgroup analysis and investigation of heterogeneity
We organised studies into subgroups for clinically different interventions as described below.
-
Educational interventions.
-
Environmental (household) – dust control and soil abatement – interventions.
-
Combination – educational and dust control – interventions.
Sensitivity analysis
We conducted a sensitivity analysis to assess the impact of Brown 2006 on the results of the meta‐analysis, as it had higher baseline blood lead levels than the other studies within the educational intervention subgroup. We also assessed the impact of Wasserman 2002 in a sensitivity analysis, because they measured blood lead levels after three months, while all other studies included in the meta‐analysis measured the outcome after six months (or later) after baseline.
'Summary of findings' tables
With the exception of cost of intervention, we assessed the certainty of evidence for each outcome deemed critical for decision‐making using the GRADE approach (Guyatt 2011). As recommended by GRADE, we constructed a 'Summary of findings' table for the main interventions: education strategies for preventing domestic lead exposure in children; environmental strategies (dust control, soil abatement) for preventing domestic lead exposure in children, and combinations interventions for preventing lead exposure in children. We presented the results from the meta‐analyses (or the narrative results if no meta‐analysis was possible) in the 'Summary of findings' tables (summary of findings Table 1; summary of findings Table 2; summary of findings Table 3). For blood lead levels, we calculated meta‐analyses with dichotomous and continuous data, but we only reported the results of the continuous outcome measures in the 'Summary of findings' tables, because more studies contributed to these meta‐analyses. To judge the certainty of evidence, we assessed the risk of bias, inconsistency, indirectness, imprecision and publication bias of the evidence base for each outcome. The judgement of certainty of evidence was based on GRADE's four categories: high certainty, when further research is very unlikely to change our confidence in the estimate of effect; moderate certainty, when further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low certainty, when further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; or very low certainty, when we are very uncertain about the estimate of the effect (Balshem 2011; Guyatt 2011). Two authors (BN, VM) independently judged the certainty of evidence and resolved conflicts by discussion.
Results
Description of studies
Results of the search
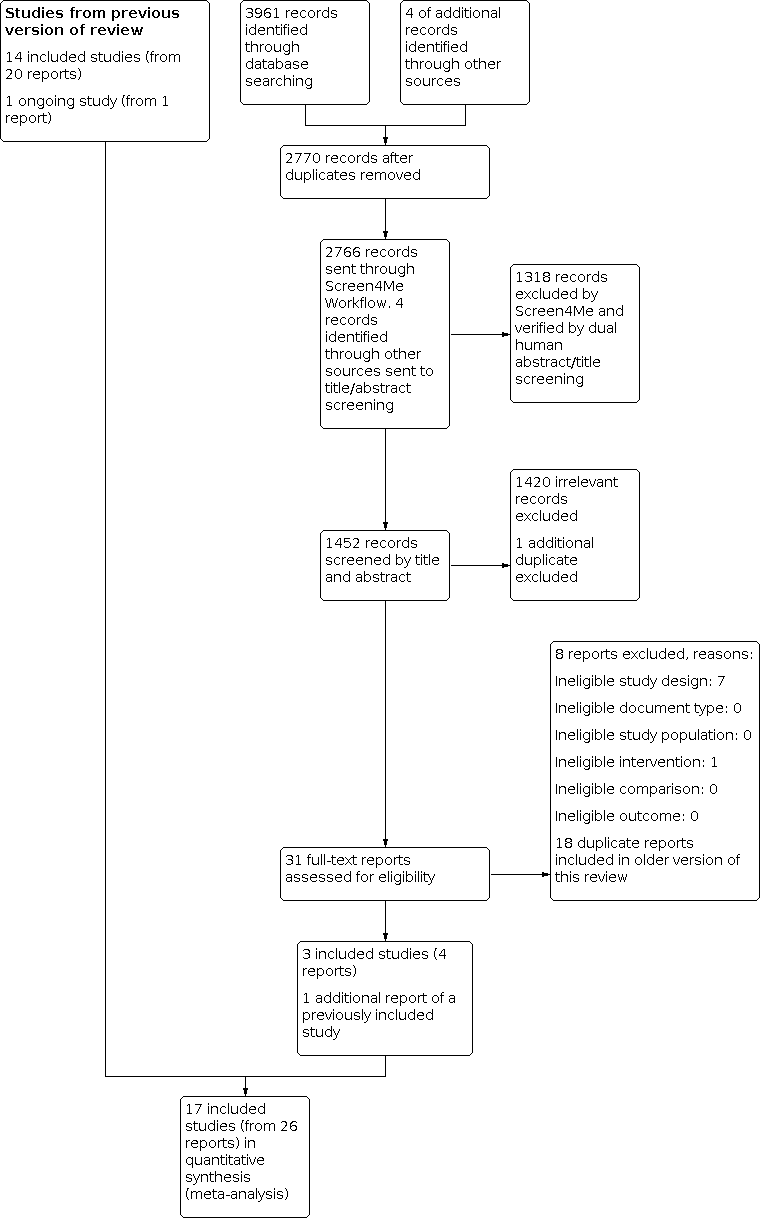
The electronic searches identified 2766 records, after removing duplicates. In assessing the studies for eligibility, we used Cochrane's Screen4Me workflow, to help identify potential records of RCTs and quasi‐RCTs. The results of the Screen4Me assessment process shown in Figure 1. Because Screen4Me is a new tool, two authors (from LP, LB, SL, SL, BN) independently verified that the rejected records were not RCTs or quasi‐RCTs, and thus ineligible for the review. We then assessed the remaining 1448 records remaining after Screen4Me, plus four additional abstracts identified by searching reference lists. Two authors (from VM, AC, AD, GW, BN) independently screened titles and abstracts. We retrieved the full‐text publications of 31 records and two authors (from VM, AC, AD, GW, BN) independently screened them. Our updated search identified one additional report for a previously included study (Weitzman 1993), and four records of three new included studies ; Shen 2004; Braun 2018; Nicholson 2018 (see Figure 2). Shen 2004 must have been overlooked in former updates. Braun 2018 was identified in 2016 as an ongoing study (Nussbaumer‐Streit 2016); the report found at that time was brought forward for inclusion in this update.

Screen4Me summary diagram. RCT: randomised controlled trial.
Study flow diagram.
Included studies
This review has 17 included studies of which 16 are RCTs (Weitzman 1993; Hilts 1995; Lanphear 1996a; Aschengrau 1998; Farrell 1998; Lanphear 1999; Rhoads 1999; Wasserman 2002; Jordan 2003; Shen 2004; Sterling 2004; Brown 2006; Boreland 2009; Campbell 2011; Braun 2018; Nicholson 2018), and one is a quasi‐RCT (Charney 1983). The trials correspond to 26 records and involved 3282 children under six years of age. For details on all included studies, see Characteristics of included studies table.
Design
All studies used a parallel‐group design, with one study also performing the intervention on volunteers from the control group at a later date (Weitzman 1993). As there was no parallel control group in this second phase, we did not include these results in our review. Another study by Campbell 2011 included a matched control group in addition to the two randomised arms at the analysis stage. The study methods had prespecified this group, but it was not part of the randomisation process so we could not include the results of this review. As a consequence, we only included data from the two randomised study arms (maintenance education group = treatment arm, and standard education group = control arm). One study, Nicholson 2018, randomised participants to the intervention group and to an active control group (education on lead poisoning through brochures). In addition, they also analysed a "passive control group". Therefore, they analysed retrospectively routinely collected data from chart reviews of children who received no intervention. Data from this 'passive control group' was not included in this review because this group was not randomised to the study. Fifteen studies used individuals or households (Charney 1983; Weitzman 1993; Lanphear 1996a; Aschengrau 1998; Lanphear 1999; Rhoads 1999; Wasserman 2002; Jordan 2003; Shen 2004; Sterling 2004; Brown 2006; Boreland 2009; Campbell 2011; Braun 2018; Nicholson 2018), and two studies used clusters (neighbourhoods (Hilts 1995), and blocks of six households (Farrell 1998)), as the unit of allocation for randomisation.
Sample sizes
Five studies had fewer than 100 participants (Charney 1983; Aschengrau 1998; Wasserman 2002; Boreland 2009; Nicholson 2018). Seven studies had 100 to 200 participants (Weitzman 1993; Hilts 1995; Lanphear 1996a; Rhoads 1999; Shen 2004; Sterling 2004; Brown 2006), and five studies had more than 200 participants (Farrell 1998; Lanphear 1999; Jordan 2003; Campbell 2011; Braun 2018).
Participants and setting
Fifteen studies were in urban areas of North America; one study was performed in Australia (Boreland 2009); and one in China (Shen 2004). Most studies were performed in areas of low socioeconomic status, with a significant proportion of participants living in rental accommodation with below average household income levels. More than half the included studies involved significant proportions of people identifying themselves as African‐American or Hispanic. Boys and girls were equally represented in those studies reporting this information. Nine studies did not report the proportion of boys and girls included in the studies (Hilts 1995; Lanphear 1996a; Farrell 1998; Lanphear 1999; Rhoads 1999; Wasserman 2002; Jordan 2003; Brown 2006; Nicholson 2018).
Sixteen studies recruited participants from routine screening programmes, medical clinics, previous lead studies or community volunteers, and they excluded children who had clinical symptoms, were receiving treatment for lead toxicity (e.g. chelation) or had high blood lead levels requiring intervention (greater than 20.0 µg/dL to 24.0 µg/dL; 0.97 µmol/L to 1.16 µmol/L) (Weitzman 1993; Hilts 1995; Lanphear 1996a; Aschengrau 1998; Farrell 1998; Lanphear 1999; Rhoads 1999; Wasserman 2002; Jordan 2003; Shen 2004; Sterling 2004; Brown 2006; Boreland 2009; Campbell 2011; Braun 2018; Nicholson 2018). Charney 1983 recruited participants from a lead poisoning clinic, and 15% of children were reported to have had previous treatment for lead toxicity.
Baseline mean blood lead levels varied across studies, with seven studies reporting levels below 10.0 µg/dL (0.48 µmol/L) (Lanphear 1996a; Lanphear 1999; Wasserman 2002; Jordan 2003; Campbell 2011; Braun 2018; Nicholson 2018), five reporting levels between 10.0 µg/dL and 14.0 µg/dL (0.48 µmol/L and 0.68 µmol/L) (Weitzman 1993; Hilts 1995; Farrell 1998; Rhoads 1999; Sterling 2004), four reporting levels between 15.0 µg/dL and 19.0 µg/dL (0.72 µmol/L and 0.92 µmol/L) (Aschengrau 1998; Shen 2004; Brown 2006; Boreland 2009), and one reporting levels above 20.0 µg/dL (0.97 µmol/L) (Charney 1983). See Table 1 for more information.
| Study ID | Mean blood lead level at baseline (µg/dL) | Age at baseline (months) |
|---|---|---|
| 15.0–19.0 | 24–36 | |
| 15.0–19.0 | 42 | |
| 0.7 | 0 | |
| 15.0–19.0 | 12–24 | |
| 2.6–2.7 | 8–14 | |
| 38 | 43–45 | |
| 10.0–14.0 | 6–72 | |
| 10.0–14.0 | 24–36 | |
| < 10.0 | < 12 | |
| 6.6–6.8 | 12–24 | |
| 2.8–2.9 | 6.7 | |
| 5.28 | 47 | |
| 10.0–14.0 | 12–24 | |
| 15.0–19.0 | 49 | |
| 10.0–14.0 | 34–43 | |
| 2.6–4.5 | 22–24 | |
| 10.0–14.0 | 4–36 |
With regards to age at baseline, the children had a mean age of less than 12 months in four studies (Lanphear 1999; Jordan 2003; Campbell 2011; Braun 2018), 12 months to 24 months in four studies (Lanphear 1996a; Rhoads 1999; Wasserman 2002; Brown 2006), 24 months to 36 months in three studies (Weitzman 1993; Hilts 1995; Aschengrau 1998), and more than 36 months in five studies (Charney 1983; Shen 2004; Sterling 2004; Boreland 2009; Nicholson 2018). One study did not report the mean age; the age range was six months to six years (Farrell 1998). See Characteristics of included studies for more information.
Interventions
The interventions used in the studies were educational, environmental or a combination of both (see Table 2; for detailed information on the interventions used see Characteristics of included studies tables). In studies using educational interventions, four studies used education alone (Wasserman 2002; Jordan 2003; Shen 2004; Brown 2006), and two studies used education plus provision of cleaning products (Lanphear 1996a; Lanphear 1999). Of the studies using environmental interventions, two studies used soil abatement (Weitzman 1993; Farrell 1998), and five studies used dust control interventions (Hilts 1995; Rhoads 1999; Boreland 2009; Braun 2018; Nicholson 2018). Four studies used a combination of lead dust control or education or hazard reduction interventions (Charney 1983; Aschengrau 1998; Sterling 2004; Campbell 2011). See Table 2 for more information.
| Study ID | Education | Dust control | Soil abatement | Combination |
|---|---|---|---|---|
| — | — | — | Yes | |
| — | Yes | — | — | |
| — | Yes | — | — | |
| Yes | — | — | — | |
| — | — | — | Yes | |
| — | — | — | Yes | |
| — | — | Yes | — | |
| — | Yes | — | — | |
| Yes | — | — | — | |
| Yes | — | — | — | |
| Yes | — | — | — | |
| — | Yes | — | — | |
| — | Yes | — | — | |
| Yes | — | — | — | |
| — | — | — | Yes | |
| Yes | — | — | — | |
| — | — | Yes | — |
Intervention integrity
We contacted trial authors with requests to provide additional information about intervention integrity. Authors reported general difficulties in providing consistent environmental and educational interventions in a community setting and inconsistent adherence to recommended housekeeping practices. Investigators did not measure adherence.
Control
Two studies used a placebo control group in which participants received household safety items but no special education on lead prevention or any assistance with household cleaning (Rhoads 1999), or participated in an injury hazard control intervention (Braun 2018). Fourteen studies did not use any placebo intervention (Charney 1983; Weitzman 1993; Hilts 1995; Lanphear 1996a; Aschengrau 1998; Farrell 1998; Lanphear 1999; Wasserman 2002; Jordan 2003; Shen 2004; Sterling 2004; Brown 2006; Boreland 2009; Campbell 2011). Eight studies gave the control groups educational information on lead and methods on dust control or hazard reduction (or both) that were available to the general community with no additional input from the researchers (Charney 1983; Aschengrau 1998; Farrell 1998; Lanphear 1999; Wasserman 2002; Brown 2006; Campbell 2011; Nicholson 2018). In three studies, both intervention and control groups received basic educational brochures or information about reduction of lead hazards separate to the intervention (Hilts 1995; Lanphear 1996a; Boreland 2009). In two studies, both groups received home lead assessment and feedback (Jordan 2003; Sterling 2004), and in one study, both groups received internal lead hazard reduction, with the intervention group also receiving the intervention of interest – soil abatement (Weitzman 1993).
Intervention duration
For 15 studies, the duration of the intervention ranged between three months and 24 months. In the two studies that used soil abatement intervention, the intervention was performed on one occasion during the study (Weitzman 1993; Farrell 1998).
Outcomes
One study used standardised measures to assess cognitive and neurobehavioural outcomes and actively gathered information on adverse events in children (Braun 2018).
Blood lead level was the standardised outcome reported in all studies. Six studies reported household floor dust (Hilts 1995; Lanphear 1996a; Aschengrau 1998; Lanphear 1999; Campbell 2011; Braun 2018; see Table 3).
| Study ID | Neurobehavioural and cognitive outcomes | Adverse events | Blood lead (continuous) | Blood lead (dichotomous) | Household dust lead levels: floors | Household dust lead levels: windows | Cost | Other |
|---|---|---|---|---|---|---|---|---|
| — | — | Yes | — | Yes | Yes | — | — | |
| — | — | Yes | — | — | — | Yes | — | |
| Yes | Yes | Yes | — | Yes | Yes | — | ||
| — | — | Yes | Yes | Yes | — | Yes | Parent‐Child Interaction scale | |
| — | — | Yes | — | Yes | Yes | — | Chicago Parents Knowledge Test | |
| — | — | Yes | Yes | — | — | — | — | |
| — | — | — | — | — | — | Yes | Total effect (blood lead levels) | |
| — | — | Yes | Yes | Yes | — | Yes | — | |
| — | — | Yes | — | — | — | — | — | |
| — | — | Yes | Yes | Yes | Yes | — | — | |
| — | — | Yes | Yes | Yes | Yes | — | — | |
| — | — | Yes | — | — | — | — | Lead exposure risk, brochure effectiveness, cleaning home repair behaviour, lead knowledge | |
| — | — | Yes | Yes | Yes | Yes | — | Maternal knowledge lead poisoning | |
| — | — | Yes | — | — | — | — | ||
| — | — | — | Yes | — | — | Yes | — | |
| — | — | Yes | Yes | — | — | Yes | Chicago Parents Knowledge Test | |
| — | — | Yes | — | — | — | — | — |
Seven studies provided both continuous and dichotomous blood lead level data (Charney 1983; Hilts 1995; Lanphear 1996a; Lanphear 1999; Rhoads 1999; Wasserman 2002; Brown 2006). Eight studies provided only continuous data (Weitzman 1993; Aschengrau 1998; Jordan 2003; Shen 2004; Boreland 2009; Campbell 2011; Braun 2018; Nicholson 2018), one study provided only dichotomous data (Sterling 2004), and one study reported results in terms of 'total effect' (Farrell 1998). Additionally, three studies provided raw data (Lanphear 1996a; Lanphear 1999; Wasserman 2002).
For continuous data, eight of the 15 studies reported geometric means (Hilts 1995; Lanphear 1996a; Lanphear 1999; Jordan 2003; Brown 2006; Boreland 2009; Campbell 2011; Braun 2018), and six studies reported arithmetic means (Charney 1983; Weitzman 1993; Aschengrau 1998; Rhoads 1999; Wasserman 2002; Nicholson 2018). Aschengrau 1998 reported data as having a normal distribution. As no clarification was available for the remaining studies providing arithmetic means, we assumed that the data were normally distributed. Shen 2004 reported values without classifying whether they were arithmetic means or medians and SDs or standard errors (SEs). We contacted the first trial author for clarification, but received no response.
Six studies provided limited data detailing study costs (Hilts 1995; Farrell 1998; Wasserman 2002; Sterling 2004; Brown 2006; Boreland 2009).
Follow‐up duration
The period of follow‐up from baseline ranged from three months to eight years, with most studies reporting blood lead levels at three months to 12 months postintervention. Four studies provided data at longer time points (Lanphear 1999; Jordan 2003; Campbell 2011; Braun 2018). Lanphear 1999 collected data up to 18 months postintervention with a follow‐up publication at 48 months (Lanphear 2000). Jordan 2003 had follow‐up data reported at four‐month intervals up to three years postintervention. Campbell 2011 reported the blood lead levels after 24 months' follow‐up. Braun 2018 reported the blood lead levels when the children were one, two, three, four, five and eight years of age.
We used short‐term postintervention data from the three long‐term studies in our meta‐analysis (six months for Lanphear 1999, 18 months for Jordan 2003, and 24 months for Braun 2018), to enable a more comparable follow‐up period to the other included studies. With regard to household dust level outcomes, we used six‐month follow‐up data for the two studies with data on educational interventions (Lanphear 1996a; Lanphear 1999). Because Braun 2018 was the only study assessing cognitive and neurobehavioural outcomes, we used the data from the longest follow‐up period for these outcomes, as it was not necessary to make it comparable with other included studies for these outcomes. For all other outcomes, we used the latest available follow‐up data.
Funding
Of the 17 included studies, two studies did not mention their funding source (Wasserman 2002; Sterling 2004), the others were funded by national or international research grants or governments.
Excluded studies
See Characteristics of excluded studies table.
Across all versions of the review (Yeoh 2008; Yeoh 2012; Yeoh 2014; Nussbaumer‐Streit 2016), including this update, we assessed 58 full‐text reports for eligibility. Of these, we included 26 reports (17 studies) in the review and excluded 31 records (31 studies; reasons for exclusion are provided in the Characteristics of excluded studies table).
For this update, we excluded eight studies. Seven studies used ineligible study designs: Ettinger 2002 and Greene 2015 only reported before‐after analyses; Haynes 2002 was a systematic review; NCT00011661 was a non‐randomised trial; NCT03640143 and Schultz 1999 were observational studies; and Beck‐Sagué 2019 was a commentary on a study on lead prevention. We also excluded one study because it did not assess the intervention we were interested in: Adubato 2003 assessed ways to improve retention of families in lead prevention programmes but not effectiveness and safety of lead prevention programmes.
We excluded 24 studies in the previous versions of this review (Yeoh 2014; Nussbaumer‐Streit 2016). Five were observational studies (NCT00000104; NCT00011674; Farfel 1990; Malcoe 2004; Dixon 2012), two used qualitative research methods (Thomas 2013; Feit 2014), one was a discussion paper about the link between lead and asthma (Maharaj 2007), three used a before‐after design without a comparison group (Boreland 2006; Phoenix 2013; Wilson 2015), one was a systematic review (a former version of this Cochrane Review; Yeoh 2014), and one was a cross‐sectional study (Whitehead 2014). Additionally, we excluded four studies because they used an ineligible, historical control without randomisation (EPA 1996; EPA 1997; Taha 1999; Pollak 2002), and one study because it compared two groups from different study bases (Omidpanah 1998). We excluded three studies because they did not measure an outcome that was relevant for this Cochrane Review: Marlowe 2001 measured hair lead levels, Dugbatey 2005 measured maternal blood levels, and Butterfield 2011 measured parent's self‐efficacy and precaution adoption. We also excluded Zimmermann 2006 because it investigated iron fortification as the intervention. Finally, we excluded one study because it did not answer a key question of the review: Untimanon 2012 did not focus on preventing lead exposure but on contamination modes. One previously excluded study, Aschengrau 1994, is included in this version of the review as it is an additional report of Weitzman 1993.
Risk of bias in included studies
This review includes 16 RCTs and one quasi‐RCT in which alternate clinic numbers determined allocation to groups (Charney 1983). We received responses from the corresponding authors of 16 included studies when we contacted them to provide missing information on methodology or results; however, in many instances, some of the requested information was not available. Shen 2004 did not respond.
Figure 3 and Figure 4 show the risk of bias of each domain for all included studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Of the 17 RCTs, the method of randomisation was adequate for 14 studies with available information. Nine studies used random number generators, tables or lists (Weitzman 1993; Lanphear 1996a; Aschengrau 1998; Lanphear 1999; Wasserman 2002; Jordan 2003; Brown 2006; Campbell 2011; Braun 2018), two studies used coin toss (Farrell 1998; Boreland 2009), one study used numbered slips of paper (Hilts 1995), one study shuffled sealed envelopes (Nicholson 2018), and one study used permutated blocks of varying length (Rhoads 1999). Methods of randomisation were unclear in two studies (Shen 2004; Sterling 2004).
We rated the quasi‐RCT, in which alternate clinic numbers determined allocation to groups, as high risk of bias (Charney 1983).
Allocation concealment
Of the 17 RCTs, nine used adequate methods of allocation concealment with either sealed envelopes or a central office (Hilts 1995; Lanphear 1996a; Lanphear 1999; Rhoads 1999; Wasserman 2002; Jordan 2003; Brown 2006; Braun 2018; Nicholson 2018). Allocation concealment remained unclear in five studies (Farrell 1998; Shen 2004; Sterling 2004; Boreland 2009; Campbell 2011). Two RCTs did not report adequate concealment (Weitzman 1993; Aschengrau 1998).
We assessed the quasi‐RCT at high risk of bias because it had no allocation concealment (Charney 1983).
Blinding
Blinding participants and personnel (performance bias)
Although not every study blinded participants and personnel, we rated the risk for performance bias for 'objective outcomes' as low, because the participants' knowledge on treatment allocation probably had no influence on outcomes such as blood lead level and household dust levels. One study reported neurobehavioural outcomes and did not state clearly who was blinded (Braun 2018). Because this 'subjective outcome' could be influenced by blinding, we rated the risk of bias to be unclear for neurobehavioural outcomes reported in that study.
Blinding outcome assessment (detection bias)
All but three studies blinded outcome assessors; the personnel collecting dust samples in Campbell 2011 and in Nicholson 2018 knew the household assignment. Since it is unclear whether this knowledge could have biased 'objective outcomes' such as blood lead levels or dust lead levels, we rated the risk of detection bias unclear. Braun 2018 did not specify whether outcome assessors were blinded, so we also rated risk of detection bias unclear.
Incomplete outcome data
We rated risk for attrition bias separately for the outcomes of blood lead levels and household dust measures of lead exposure. Braun 2018 was the only study that also reported neurobehavioural outcomes. As it had the same attrition rate for all outcomes, we added the judgement to the 'blood lead level' rating.
For neurobehavioural outcomes, we rated the risk of attrition bias as low in one study because the attrition rate was acceptable and similar in both groups (Braun 2018).
For blood lead levels, we rated the risk of attrition bias as low in 11 studies because the attrition rate was acceptable and similar in both the intervention and control groups (Weitzman 1993; Hilts 1995; Lanphear 1996a; Lanphear 1999; Rhoads 1999; Shen 2004; Brown 2006; Boreland 2009; Campbell 2011; Braun 2018; Nicholson 2018). In one study, we rated the risk of attrition as unclear because the overall attrition rate was quite high, but it was the same in both groups (Charney 1983). In the other studies, we rated the risk of attrition bias as high. In Aschengrau 1998, the overall attrition rate was 41%; it was 18% points higher in the intervention group than in the control group. In Jordan 2003, the attrition rate was 38%, with no information on the attrition rate in the treatment arms. In Wasserman 2002, the overall attrition rate was acceptable (21%); however, it was much higher in the control group (30%) than in the intervention group (12%). In Farrell 1998, the attrition rate was 55%, and in Sterling 2004 it was 61%.
Of the 10 studies that reported on household dust measures of lead exposure, we rated six at low risk of attrition bias because the attrition rate was acceptable and similar in both the intervention and control groups (Weitzman 1993; Hilts 1995; Lanphear 1996a; Lanphear 1999; Brown 2006; Braun 2018). We assessed one study at unclear risk because numbers and reasons for missing data were not available (Rhoads 1999). In Aschengrau 1998, Campbell 2011, and Sterling 2004, we rated the risk of attrition bias for household dust measures of lead exposure as high, because overall attrition rates were 46% (Aschengrau 1998), 64% (Campbell 2011), and 66% (Sterling 2004).
The most common reasons reported for withdrawal were that families had moved out of the area or were no longer reachable.
We contacted trial authors to determine if they had analysed participants in the groups to which they were randomised (intention‐to‐treat). One study reported no dropouts (Shen 2004). None of the other studies performed complete measures of all participants' outcomes (full intention‐to‐treat analysis). Nine studies analysed data based on available participants' outcomes (available‐case analysis; Weitzman 1993; Hilts 1995; Lanphear 1996a; Lanphear 1999; Brown 2006; Boreland 2009; Campbell 2011; Braun 2018; Nicholson 2018). We were unable to determine if five studies used data from all available participants (Charney 1983; Rhoads 1999; Wasserman 2002; Jordan 2003; Sterling 2004). In two studies, participants were excluded from analyses if non‐study interventions (such as any lead hazard reduction measures performed independently of study intervention) occurred during the study (Aschengrau 1998; Farrell 1998).
Selective reporting
Information from trial authors suggest that published records of eight studies included all expected outcomes, including those they had prespecified (Hilts 1995; Lanphear 1996a; Lanphear 1999; Wasserman 2002; Brown 2006; Boreland 2009; Campbell 2011; Braun 2018). For eight studies there was insufficient information, so we rated the risk of bias for this domain as unclear (Charney 1983; Weitzman 1993; Aschengrau 1998; Farrell 1998; Rhoads 1999; Shen 2004; Sterling 2004; Nicholson 2018). One study described measuring household dust lead outcomes but did not report these data in the article, and we could not obtain any information on these outcomes from the author (Jordan 2003).
Other potential sources of bias
Two of the 17 included studies were cluster‐RCTs, a design that can be affected by additional sources of bias. Risk of recruitment bias and risk of bias due to baseline imbalance was low in Hilts 1995 and Farrell 1998, as baseline characteristics were comparable and randomisation was achieved by coin toss. Risk of bias due to unit of analysis was high in Hilts 1995, since Hilts 1995 randomised clusters of households but used individuals as the unit of analysis; and unclear in Farrell 1998, who used neighbourhood clusters, but it was unclear how they performed the analysis, as data were not available.
We identified no other potential sources of bias in the remaining 15 studies, so rated these at low risk of bias on this domain.
Effects of interventions
See: Summary of findings 1 Education interventions versus no intervention for preventing domestic lead exposure in children; Summary of findings 2 Environmental interventions versus no intervention for preventing domestic lead exposure in children; Summary of findings 3 Combination interventions versus standard education for preventing domestic lead exposure in children
We present results sequentially by intervention type, by outcome measure and by type of data (continuous and dichotomous). Cost data are presented at the end of this section for all intervention types combined.
We classified the 17 studies into subgroups based on type of intervention, as combining these significantly different types of intervention would not be clinically appropriate.
-
Education (Lanphear 1996a; Lanphear 1999; Wasserman 2002; Jordan 2003; Shen 2004; Brown 2006).
-
Environmental:
-
dust control (Hilts 1995; Rhoads 1999; Boreland 2009; Braun 2018; Nicholson 2018);
-
soil abatement (Weitzman 1993; Farrell 1998).
-
-
Combination of education and dust control (Charney 1983; Aschengrau 1998; Sterling 2004; Campbell 2011).
Comparison 1. Education interventions versus no intervention or standard education
Primary outcomes
Cognitive and neurobehavioural outcomes in children
None of the studies included in this comparison assessed cognitive and neurobehavioural outcomes
Adverse events of the intervention in children
None of the studies included in this comparison assessed adverse events.
Secondary outcomes
Blood lead levels in children
Six studies of educational interventions reported continuous blood lead levels (Lanphear 1996a; Lanphear 1999; Wasserman 2002; Jordan 2003; Shen 2004; Brown 2006), and all but one (Shen 2004) were included in meta‐analysis. Geometric means were readily available from four studies. Wasserman 2002 provided raw data. Shen 2004 reported data without clarifying what exactly these values were (mean, median, SDs, or SEs). Therefore, we did not include these data in the meta‐analysis and provided a narrative description of the results below.
See summary of findings Table 1.
Continuous data
Meta‐analysis of log‐transformed summary data showed no evidence of a treatment effect (MD –0.03, 95% CI –0.13 to 0.07; 5 studies, 815 participants; Tau² = 0.00; Chi² =1.95, degrees of freedom (df) = 4 (P = 0.74); I² = 0%; moderate‐certainty evidence; Analysis 1.1). Exponentiation of the result produced a treatment effect of 1.0 µg/dL (95% CI 0.9 to 1.1; analysis not shown). The mean age for participants in these studies was less than two years. Baseline blood level was less than 10.0 µg/dL (0.48 µmol/L) in all studies except for Brown 2006. As the baseline blood lead level for Brown 2006 was 15.0 µg/dL to 19.0 µg/dL (0.72 µmol/L to 0.92 µmol/L), we performed a sensitivity analysis to assess the effect of clinical heterogeneity. Excluding Brown 2006 also resulted in no evidence of a treatment effect (MD –0.01, 95% CI –0.13 to 0.11; I² = 0%; analysis not shown). Exponentiation of the result produced a treatment effect of 1.0 µg/dL (95% CI 0.9 to 1.1; analysis not shown). All but one study measured the blood lead levels at six months or later. Wasserman 2002 measured blood lead levels at three months. Since it is possible that no effects can be observed after three months, due to the long half‐life of lead in children, we excluded Wasserman 2002 in a sensitivity analysis. However, this did not change the result of the meta‐analysis markedly (MD –0.05, 95% CI –0.16 to 0.06; 4 studies, 765 participants; I² = 0%; analysis not shown).
In the study by Shen 2004, the blood lead levels decreased by 35% (from 15.9 to 10.4 µg/dL; 107 participants) in the educational intervention group and by 20% (from 16.6 to 13.3 µg/dL; 93 participants) in the control group after three months. This change was statistically significant (P < 0.001; not presented more precisely in the study). However, the study conducted more than 20 tests for statistical significance on several outcomes and did not adjust for multiple testing, so this needs to be interpreted with caution.
Dichotomous data
We performed meta‐analysis of dichotomous data for four studies, as dichotomous outcomes were not available for Jordan 2003. Meta‐analysis for numbers of children with blood lead level of 10.0 µg/dL (0.48 µmol/L) or more showed no evidence of a treatment effect (RR 1.02, 95% CI 0.79 to 1.30; Tau² = 0.00; Chi² = 1.87, df = 3 (P = 0.60); I² = 0%; 520 participants; moderate‐certainty evidence; Analysis 1.2). Meta‐analysis of data reported as numbers of children with blood lead levels of 15.0 µg/dL (0.72 µmol/L) or more also showed no evidence of a treatment effect (RR 0.60, 95% CI 0.33 to 1.09; Tau² = 0.00, Chi² = 0.65, df = 2 (P = 0.72); I² = 0%; 520 participants; moderate‐certainty evidence; Analysis 1.3).
Household dust measures of lead exposure
Continuous data
Two of the five studies had log‐transformed summary data available on hard floor dust lead levels for this intervention (Lanphear 1996a; Lanphear 1999). The mean hard floor dust level was below the 0.431 mg/m² (40.0 μg/feet²) dust lead standard established by the US Department of Housing and Urban Development (HUD) and the US Environmental Protection Agency (EPA) for the home environment. The meta‐analysis of the log‐transformed summary data showed no evidence of treatment effect (MD –0.07, 95% CI –0.37 to 0.24; Tau² = 0.00; Chi² = 0.04, df = 1 (P = 0.85); I² = 0%; 318 participants; moderate‐certainty evidence; Analysis 1.4). Exponentiation of the result produced a treatment effect of 0.010 mg/m² (95% CI 0.008 to 0.014; analysis not shown; 0.9 µg/feet², 95% CI 0.7 to 1.3). Brown 2006 reported post‐intervention floor dust lead levels as geometric means, but the type of floor was not specified (hard floor or carpet), so we did not include the data in the meta‐analysis. After one year, the dust lead level was 0.095 mg/m² (8.8 µg/feet²) in the control group and 0.059 mg/m² (5.5 µg/feet²) in the intervention group, and the difference was statistically significant (P < 0.05, not presented more precisely in the study). One study had data on carpet floor showing 0.038 mg/m² (95% CI 0.017 to 0.081; 3.5 µg/feet², 95% CI 1.6 to 7.6) in the intervention group and 0.044 mg/m² (95% CI 0.014 to 0.138; 4.1 µg/feet², 95% CI 1.3 to 12.8) in the control group after seven months (P = 0.72) (Lanphear 1996a).
Three of the five studies reported outcomes on window dust lead levels. We did not pool these studies in a meta‐analysis because investigators used different surfaces to collect the dust lead samples (window sill, window troughs, window wells or sills in general). Brown 2006 reported dust lead levels in "other sills", not specifying where they were. One year after the intervention the levels in the intervention group were lower than in the control group: 0.273 mg/m² (25.4 µg/feet²) in the intervention group compared to 0.563 mg/m² (52.3 µg/feet²) in the control group (P < 0.05, not presented more precisely in the study). Lanphear 1996a measured dust lead levels in interior window sills and window wells. At the end of the study in interior window sills, the level was 0.961 mg/m² (95% CI 0.260 to 3.539; 89.3 µg/feet², 95% CI 24.2 to 328.8) in the intervention group compared to 0.972 mg/m² (95% CI 0.203 to 4.648; 90.3 µg/feet², 95% CI 18.9 to 431.9) in the control group. At the end of the study in window wells, the level was 32.421 mg/m² (95% CI 2.723 to 379.212; 3012.0 µg/feet², 95% CI 253.0 to 35,230.0) in the intervention group compared to 40.989 mg/m² (95% CI 4.434 to 379.212; 3808.0 µg/feet², 95% CI 412.0 to 35,230.0) in the control group. Lanphear 1999 showed similar lead levels in the control and intervention group at the end of the study in window sills (intervention: 1.153 mg/m² (107.1 µg/feet²); control: 1.547 mg/m² (143.7 µg/feet²); P = 0.19) and in window troughs (intervention: 23.397 mg/m² (2173.7 µg/feet²); control 28.516 mg/m² (2649.2 µg/feet²); P = 0.54).
Comparison 2. Environmental interventions versus no intervention or another intervention not aiming to influence domestic lead exposure
Primary outcomes
Cognitive and neurobehavioural outcomes in children
Dust control
Braun 2018 was the only study to measure cognitive and neurobehavioural outcomes. They assessed MDs in neurobehavioural tests between children in the intervention and control groups. The dust control intervention started before the children were born (at 32 weeks' gestation). The study team reduced sources of lead in the participants homes, for example, by installing tap‐water filters, repairing and repainting peeling or deteriorating lead‐based paint, or replacing windows. If necessary, additional cleaning was applied. Mothers in the control group received injury prevention training. Children in the intervention group had slightly better cognitive and neurobehavioural outcomes, but differences were small and 95% CIs included both beneficial and non‐beneficial effects. We summarise the results of all five standardised scales used in the study below. Values were derived from linear regression, which accounted for repeated outcomes measures. To aid interpretation of the results, we also provide the mean (SD) from the normalisation sample for each scale. A negative coefficient favours treatment.
-
Behaviour Assessment System for Children, Second Edition, a parent‐reported questionnaire used to assess behavioural problems after eight years (measured at two, three, four, five and eight years; mean in normalisation sample: 50 (SD 10)):
-
externalised: MD –0.7, 95% CI –2.3 to 0.9;
-
internalised: MD –1.2, 95% CI –2.8 to 0.5;
-
Behaviour Symptom Index: MD –0.7, 95% CI –2.3 to 0.8;
-
aggression: MD –0.9, 95% CI –2.4 to 0.7;
-
anxiety: MD –1.6, 95% CI –3.2 to –0.1;
-
attention: MD –0.4, 95% CI –2.0 to 1.2;
-
atypicality: MD 0.0, 95% CI –1.7 to 1.7;
-
hyperactivity: MD –0.3, 95% CI –2.0 to 1.4;
-
somatisation: MD 0.0, 95% CI –1.6 to 1.5; and
-
withdrawal: MD –0.5, 95% –2.1 to 1.1.
-
-
Bayley Scales of Infant Development, Second Edition, used to assess children's mental and psychomotor development after three years (measured at one, two and three years of age; mean in normalisation sample: 100 (SD 15)):
-
mental development: MD 0.1, 95% CI –2.1 to 2.4; and
-
psychomotor development: MD 0.4, 95% CI –2.2 to 3.0.
-
-
Behaviour Rating Inventory of Executive Function, a parent‐reported questionnaire used to measure children's executive functions after eight years (measured at three, four, five and eight years of age; mean in normalisation sample: 50 (SD 10)):
-
global executive composite: MD –0.7, 95% CI –3.0 to 1.6;
-
inhibition: MD –0.7, 95% CI –2.8 to 1.5;
-
ability to shift: MD 0.3, 95% CI –1.7 to 2.2;
-
emotional control: MD 0.0, 95% CI –1.9 to 2.0;
-
working memory: MD –0.8, 95% CI –3.1 to 1.5; and
-
planning/organisation: MD –1.0, 95% CI –3.0 to 1.0.
-
-
Conners' Continuous Performance Test, used to measure children's execution functions after eight years (measured at five and eight years of age; mean in normalisation sample: 50 (SD 10)):
-
commission: MD –1.7, 95% CI –3.5 to 0.1;
-
omission: MD 3.9, 95% CI –0.8 to 8.7; and
-
reaction time: MD 0.9, 95% CI –1.9 to 3.7.
-
-
Wechsler Preschool and Primary Scale of Intelligence, Third Edition, used at five years of age, and the Wechsler Intelligence Scale for Children, Fourth Edition, used at eight years of age, to evaluate children's cognitive abilities (mean in normalisation sample: 100 (SD 15)):
-
full‐scale IQ: MD 0.5, 95% CI –3.3 to 4.2;
-
performance IQ: MD 0.8, 95% CI –3.2 to 4.8;
-
processing speed: MD –0.2, 95% CI –3.7 to 3.3; and
-
verbal IQ: MD 0.6, 95% CI –3.2 to 4.3.
-
We rated the certainty of evidence for cognitive and neurobehavioural outcomes as low, because of high risk of bias and imprecision.
Soil abatement
None of the studies included in this comparison assessed cognitive and neurobehavioural outcomes regarding soil abatement.
Adverse events of the intervention in children
Dust control
Braun 2018 reported that after eight years they did not observe any adverse events in the intervention group. In the control group, one child had an injury because of a stair gateway installed and another child had elevated blood lead concentrations (28 µg/dL). We rated the certainty of evidence as very low because of high risk of bias and imprecision (we downgraded by two levels for high imprecision).
Soil abatement
None of the studies included in this comparison assessed adverse events.
Secondary outcomes
Blood lead levels in children
Dust control
Continuous data
Five studies used dust control interventions (Hilts 1995; Rhoads 1999; Boreland 2009; Braun 2018; Nicholson 2018). Hilts 1995, Boreland 2009, and Braun 2018 reported log‐transformed summary data while Rhoads 1999 and Nicholson 2018 reported arithmetic means and SDs for blood lead levels. The meta‐analysis of log‐transformed summary data from Hilts 1995, Boreland 2009, Rhoads 1999, and Braun 2018 showed no evidence of a treatment effect (MD –0.02, 95% CI –0.09 to 0.06; Tau² = 0%; I² = 0%, 565 participants; moderate‐certainty evidence; Analysis 2.1). Nicholson 2018 was not included in the meta‐analysis because it was a multiple treatment study. It compared three interventions and a control. The post‐treatment arithmetic means in blood lead level effects were similar in all groups (blood lead level post‐treatment arithmetic mean: "cleaning kit" = 2.72 (SD 2.31), 20 participants; "risk assessment" = 2.70 (SD 1.64), 20 participants; "cleaning kit + risk assessment" = 2.76 (SD 1.92), 24 participants; control (educational brochures) = 2.87 (SD 1.77), 22 participants). Exponentiation of the result produced a treatment effect of 1 µg/dL (95% CI 0.9 to 1.1; analysis not shown).
Dichotomous data
We performed a meta‐analysis of dichotomous data for two studies (Hilts 1995; Rhoads 1999). The meta‐analysis for numbers of children with a blood lead level of 10.0 µg/dL (0.48 µmol/L) or more showed no evidence of a treatment effect (RR 0.93, 95% CI 0.73 to 1.18; Tau² = 0.00; Chi² = 0.00, df = 1 (P = 0.96); I² = 0%; 210 participants; moderate‐certainty evidence; Analysis 2.2), and this was also the case for children with blood lead levels at or above 15.0 µg/dL (0.72 µmol/L; RR 0.86, 95% CI 0.35 to 2.07; Tau² = 0.23; Chi² = 2.25, df = 1 (P = 0.13); I² = 56%; 210 participants; low‐certainty evidence; Analysis 2.3).
Impact of clustering and unit of analysis errors
We calculated effective sample sizes for the cluster‐RCT for a range of ICCs before incorporating this study into the meta‐analysis (Hilts 1995). For blood lead levels of 10.0 µg/L (0.48 µmol/L) or more, the result was inconclusive because the 95% CI included both an increase and a possible decrease of blood lead levels when we adjusted the meta‐analysis for clustering: ICC 0.01 (RR 0.93, 95% CI 0.73 to 1.18; Tau² = 0.00; Chi² = 0.02, df = 1 (P = 0.94); I² = 0%; 2 studies, 204 participants; Analysis 2.4); ICC 0.1 (RR 0.95, 95% CI 0.72 to 1.24; Tau² = 0.00; Chi² = 0.00, df = 1 (P = 0.95); I² = 0%; 2 studies, 173 participants; Analysis 2.5); or ICC 0.2 (RR 0.97, 95% CI 0.72 to 1.29; Tau² 0.00; Chi² = 0.05, df = 1 (P = 0.83); I² = 0%; 2 studies, 155 participants; Analysis 2.6). For blood lead levels of 15.0 µg/dL (0.72 µmol/L) or more, the results were similar when the meta‐analysis was adjusted for clustering: ICC 0.01 (RR 0.82, 95% CI 0.37 to 1.81; Tau² = 0.15; Chi² = 1.81, df = 1 (P = 0.18); I² = 45%; two studies, 204 participants; Analysis 2.7); ICC 0.1 (RR 0.83, 95% CI 0.34 to 2.03; Tau² = 0.20; Chi² = 1.92, df = 1 (P = 0.17); I² = 48%; 2 studies, 173 participants; Analysis 2.8); or ICC 0.2 (RR 0.75, 95% CI 0.34 to 1.66; Tau² = 0.08; Chi² = 1.33, df = 1 (P = 0.25); I² = 25%; 2 studies, 155 participants; Analysis 2.9). Thus, correcting for unit of analysis errors did not alter the overall outcome.
Soil abatement
Two studies performed soil abatement interventions (Weitzman 1993; Farrell 1998). As no blood lead level data were available in a usable form from Farrell 1998 and follow‐up was less than 60%, comparison was not possible. Farrell 1998 reported results as a "total effect" showing no statistical significance, and no data were available for our analyses. Weitzman 1993 reported a statistically significant effect in favour of the intervention. The difference in mean change scores between the intervention group and control group A (loose interior dust abatement and paint removal) was –1.5 µg/dL (SD 4.9), and between the intervention group and control group B (loose interior paint removal only) was –1.9 µg/dL (SD 5.0). No measure of variance was available for post‐treatment means or mean change scores, so further analysis was not possible in this review.
Household dust measures of lead exposure
Dust control
Braun 2018 compared geometric means for post‐treatment dust lead loadings on different surfaces. After two years, dust lead loadings were lower in the intervention group than in the control group (floor: –24%, 95% CI –43 to 1 (intervention: 0.012 mg/m² (1.1 µg/feet²), control: 0.015 mg/m² (1.4 µg/feet²)); window sill: –40%, 95% CI –60 to –11 (intervention: 0.108 mg/m² (10 µg/feet²), control: 0.183 mg/m² (17 µg/feet²)); and window trough: –47%, 95% CI –68 to –10 (intervention: 1.173 mg/m² (109 µg/feet²), control: 2.196 mg/m² (204 µg/feet²))).
Another study provided household carpet lead measures for dust control interventions (Hilts 1995), reporting a reduction in geometric means for post‐treatment dust lead level, which was 0.360 mg/m² (33.5 µg/feet²) in the intervention group and 0.230 mg/m² (21.4 µg/feet²) in the control group but classified the reduction as not clinically significant. Rhoads 1999 provided geometric means of lead loading from floor wipes, sill wipes and vacuum cleaner after one year. Investigators reported comparable results in the groups for floor wipes (intervention: 0.163 mg/m² (15.1 µg/feet²), control: 0.207 mg/m² (19.2 µg/feet²)) or vacuum (intervention: 1.618 mg/m² (150.3 µg/feet²), control: 2.307 mg/m² (214.3 µg/feet²)). For sill wipes, the difference was statistically significant (P < 0.05, not presented more precisely in the study), and lead levels were 0.263 mg/m² (24.4 µg/feet²) in the intervention group and 0.522 mg/m² (48.5 µg/feet²) in the control group.
Soil abatement
No studies reported household dust lead levels for this intervention.
Comparison 3. Combination interventions versus standard education
Primary outcomes
Cognitive and neurobehavioural outcomes in children
None of the studies included in this comparison assessed cognitive and neurobehavioural outcomes
Adverse events of the intervention in children
None of the studies included in this comparison assessed adverse events.
Secondary outcomes
Blood lead levels in children
Of the four studies that used a combination of interventions, two reported continuous data (Aschengrau 1998; Campbell 2011), one arithmetic means (Aschengrau 1998), and one geometric means (Campbell 2011). One study reported dichotomous data (Sterling 2004), and the fourth was clinically very different because it was a quasi‐RCT (Charney 1983), with high mean baseline blood lead levels (greater than 30.0 µg/dL, or 1.44 µmol/L) and older participants (mean age 3.5 years). Therefore, it was not possible or appropriate to combine any of these studies in a meta‐analysis.
Aschengrau 1998 reported arithmetic means for post‐treatment blood lead levels as 11.5 µg/dL (SD 3.2) in the intervention group and 10.4 µg/dL (SD 3.1) in the control group. An analysis of these post‐treatment scores performed in our review allowed no conclusion about efficacy because the 95% CIs included both a possible increase as well as a possible decrease of blood lead levels (MD 1.1 µg/dL, 95% CI –1.5 to 3.7; analysis not shown). Sterling 2004 reported dichotomous data with 4/10 (40%) in intervention group 1; 6/14 (43%) in intervention group 2, and 6/15 (40%) in the control group having blood lead levels less than 10.0 µg/dL (0.48 µmol/L) post‐treatment, but this study had small numbers and less than 40% follow‐up. An analysis of these data performed in our review, reported as numbers of children with blood lead levels at or above 10.0 µg/dL (0.48 µmol/L), showed no evidence of treatment effect (intervention group one – newsletters and education: RR 1.00, 95% CI 0.52 to 1.92; intervention group two – newsletters, education and specialised cleaning: RR 0.95, 95% CI 0.52 to 1.76; analyses not shown). Charney 1983 reported a significant effect favouring treatment with arithmetic means for post‐treatment blood lead levels of 31.7 µg/dL (SD 2.6) in the intervention group and 37.8 µg/dL (SD 7.9) in the control group. Campbell 2011 reported similar geometric means for blood lead levels after 12 months for the intervention group (2.6 µg/dL) and control group (2.7 µg/dL) (P = 0.68). Likewise, blood lead levels for the intervention group (3.5 µg/dL) and the control group (3.9 µg/dL) were not significantly different after two years (P = 0.20).
Household dust measures of lead exposure
One study provided continuous data of hard floor and window dust lead levels for this intervention subgroup (Aschengrau 1998). We found no treatment effect, with median changes for floor dust lead level being –0.002 mg/m² (–0.2 µg/feet², SD 0.8 µg/feet²) in the intervention group and 0.001 mg/m² (0.0 µg/feet², SD 0.2 µg/feet²) in the control group. For window sills, the mean change in the intervention group was –0.006 mg/m² (–0.5 µg/feet², SD 1.3 µg/feet²) in the intervention group and –0.005 mg/m² (–0.5 µg/feet², SD 1.0 µg/feet²) in the control group; for window wells, it was –0.007 mg/m² (–0.7 µg/feet², SD 0.9 µg/feet²) in the intervention group and 0.000 mg/m² (0.0 µg/feet², SD 1.6 µg/feet²) in the control group. A second study provided dichotomous data with no significant difference observed in the number of households with positive dust lead levels (floor greater than 0.431 mg/m² (40.0 µg/feet²); window greater than 2.691 mg/m² (250.0 µg/feet²)) between the intervention (17/59) and control (11/51) groups at 12 months post‐treatment (Campbell 2011).
Cost of intervention
Six studies provided cost data for their intervention or study, reporting large variations in costs depending on the types of interventions and types of cost data collected. The calculations often omitted the costs of researchers and educators. With regard to educational interventions, Brown 2006 noted that, on average, comparison families spent USD 108.78 and intervention families spent USD 43.01 on cleaning supplies. Wasserman 2002 reported that Medicaid paid for medical check‐ups, and researchers spent USD 11 per blood test. With dust control interventions, Hilts 1995 reported that the entire study cost approximately USD 200,000, but no detailed costs for the intervention were available. Boreland 2009 reported that the mean cost per household was AUD 5000 (in 1994), but ranged from AUD 1000 to AUD 20,000. For soil abatement, Farrell 1998 estimated that the mean cost per household was USD 1700, with the entire study costing USD 5 million. For a combination of interventions, Sterling 2004 reported a mean cost per quarterly cleaning of USD 500 per household, and Campbell 2011 reported median costs of lead hazard control or remediation work over a 12‐month period of USD 4656 for 42 control households and USD 5512 for 36 intervention households. No cost data were available for 10 studies (Charney 1983; Weitzman 1993; Lanphear 1996a; Aschengrau 1998; Lanphear 1999; Rhoads 1999; Jordan 2003; Braun 2018; Nicholson 2018; Shen 2004).
Discussion
Summary of main results
Overall, 17 studies met our eligibility criteria for inclusion in this review. The only study assessing cognitive and neurobehavioural outcomes compared environmental interventions (dust control) with no intervention and found slightly better outcomes in the dust control group, but differences were small and 95% CIs included both a possible beneficial and non‐beneficial effect allowing no valid conclusions about the effect. The same study was also the only one reporting on adverse events and stated that there were no adverse events in the intervention group, and two adverse events in the control group within eight years of follow‐up (Braun 2018). The results of this systematic review also indicated that there was probably no evidence of a difference in reducing blood lead levels in children by educational interventions and dust control interventions. Another environmental measure is soil abatement (removal and replacement). The only two studies on soil abatement assessed only the effect on blood lead levels in children and showed inconclusive results.
We could not pool studies assessing the effect of combination intervention groups in a meta‐analysis due to substantial differences between studies. Since studies reported inconsistent results, the evidence is currently insufficient to clarify whether a combination of interventions reduces blood lead levels and floor dust levels (very low‐certainty evidence). One study showed a treatment effect with a combined (education and dust control) intervention (Charney 1983). As this was a quasi‐RCT and had participants with high baseline blood lead levels (more than 30.0 µg/dL), it was clinically distinct from other included studies. The significant blood lead level reduction after intervention is consistent with previous findings that interventions are likely to be more beneficial in children who have higher baseline blood lead levels (Charney 1983; Haynes 2002). This finding requires further research to assess whether interventions are better aimed at particular populations of children.
Overall completeness and applicability of evidence
Only one of the included studies used standardised measures to assess cognitive and neurobehavioural outcomes (Braun 2018), despite this being one of the main adverse outcomes of elevated lead exposure. However, in view of the magnitude of blood lead level reductions reported in the studies with significant treatment effect and the known correlation between blood lead level and cognition, we do not expect that we would have found any significant improvement in cognitive outcomes even if investigators had measured them.
Only one study reported on adverse events in children (Braun 2018). Future trials need to better examine and report adverse effects and ensure that sample sizes are sufficiently large to allow this.
Some experts point out that blood lead levels as primary outcomes are not suitable across all levels or durations of exposure. One review described blood lead levels as the most suitable biomarkers when assessing recent or current exposures to lead (weeks or months) at low or moderate levels (Barbosa 2005), but at high levels of exposure, a curvilinear relationship between blood lead levels and exposure makes its use as a biomarker more difficult (Bergdahl 2008). Alternative measures have not been shown to be superior to blood lead levels when monitoring lead exposure (Barbosa 2005; Bergdahl 2008). Lead is, moreover, a bone‐seeking element, a characteristic that is especially relevant for constant lead exposure (Rust 1999). From the bone, it can be released into the blood (Rabinowitz 1991; Gulson 2003), which is especially relevant for pregnant women and children (Manton 2000; Gulson 2003). This release of lead from bone into blood adversely affects the reduction of blood lead levels (Rust 1999; Gwiazda 2005). Therefore, it is necessary to assess remediation efforts over prolonged periods of time (i.e. at least six months to 12 months). While we analysed data measured at least six months after baseline for all but one study, Wasserman 2002 (measured after three months), where effects can be expected, it is possible that small effects of the intervention might have needed a longer follow‐up period to be measurable.
The participants in the included studies were all children younger than six years of age when the studies started. Although we looked for studies in children from birth to 18 years of age, we did not identify any studies of older children or adolescents. Future studies need to focus also on preventing lead exposure in these groups. All but two of the studies were conducted in North America (Shen 2004; Boreland 2009), and we cannot rule out that the treatment effects would be different if the interventions were transferred to other contexts.
Meta‐analysis was not possible for all interventions or outcomes due to the clinical diversity of trials, use of different outcome measures and different forms of data reported. No more than seven studies used a similar intervention and even within these intervention subgroups, the reported intervention varied significantly; for example, type of education, duration of intervention, study setting and whether supplies were provided. In addition, there were variations in baseline lead levels and mean age. However, due to the limited number of studies within each intervention type, there were insufficient data for subgroup analyses according to baseline age, blood lead levels or household settings. One study reported different effects of the intervention depending on ethnicity (Braun 2018), observing significant increases in verbal IQ in white children, as well as significant decreases in blood lead levels and dust lead levels in black children. Subgroups according to ethnicity should be included in updates of this review.
The issues of clinical diversity, inconsistent participant compliance with household cleaning practices, variability in interventions, suboptimal recruitment numbers and losses to follow‐up that reduce study power may all contribute to the lack of clear effect demonstrated in a meta‐analysis of study results. The effectiveness of other more intensive interventions, or interventions performed over a longer duration than those available to date, is not yet known. Also, the trials in this review largely focus on participants with low socioeconomic status in the USA, in rental housing and, as such, results may not be generalisable to different populations.
We lack studies of full remediation, as interventions evaluated were unable to eliminate all ongoing environmental lead sources and were limited to household interventions. Therefore, it is possible that recontamination occurred during the trial period. Thus, while reduction in lead‐contaminated household dust may be needed to reduce or prevent childhood lead exposure, it is not sufficient. It may be necessary to eliminate the ongoing source of lead exposure by removing or eliminating ongoing contamination from lead‐based paint and other residential lead hazards. Furthermore, other sources of lead contamination, such as passive smoking, water, diet or sources outside the home, may have limited the possible benefit of interventions. Another reason for lack of treatment effect may be that most included studies had a follow‐up period of 12 months or less, and the long half‐life of lead in children may contaminate short‐term outcomes. However, the study with the longest follow‐up also showed inconclusive results in treatment effects on cognitive and neurobehavioural outcomes and blood lead levels after eight years because the corresponding 95% CIs included both possible beneficial and non‐beneficial effects (Braun 2018). However, this study started very early with interventions (before birth) and children had very low baseline blood lead levels which might also be an explanation for finding no difference.
Quality of the evidence
For educational interventions, we assessed the certainty of evidence from continuous blood lead level outcomes as moderate, so further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. We assessed the certainty of evidence for dichotomous blood lead level outcomes as moderate because the 95% CIs around the pooled estimate included no effect and appreciable harm or benefit, and because the total number of events was fewer than 300. We assessed the certainty of evidence for household dust measures of lead exposure as moderate; we downgraded one level for imprecision because the total number of participants was fewer than 400. This means that for the latter two outcomes, further research is likely to have an important impact in our confidence in the estimate of effect and may change it.
For environmental interventions, we rated the certainty of evidence from cognitive and neurobehavioural outcomes as low, because of an increased risk of bias and imprecision. Low‐certainty evidence indicates that it is very likely that further research will have an important impact on our confidence in the estimate of effect and is likely to change the estimate. For adverse events, we judged the certainty of evidence as very low, because we downgraded one level for risk of bias and two levels for imprecision. This means that for adverse events we are very uncertain about the estimate of the effect. We judged the certainty of evidence from continuous and dichotomous blood lead level outcomes as moderate, so further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. We downgraded the evidence certainty by one level because of imprecision.
Potential biases in the review process
We judged the risk of potential biases in the review process to be low. Some of the authors of this update were new. To avoid any biases caused by changing authors we set a high value on intense communication and exchange of information with the original team. Publication bias and selective outcome reporting are potential limitations of any systematic review. Although we searched for grey and unpublished literature, the extent and impact of reporting biases of this body of evidence is impossible to determine. We cannot rule out that any bias was introduced by combining capillary and venous blood samples for analysis; however, we assume that any changes between groups would have been detected with either method, because the same methods were used within the studies.
Agreements and disagreements with other studies or reviews
A previous review limited to low‐cost, lead hazard control interventions, which included only four trials, reported no substantial effect on mean blood lead concentration but noted treatment effect with dichotomous data for reducing the number of participants with blood lead levels of 15.0 µg/dL or more (Haynes 2002). Haynes 2002 differed from our review in that it combined the results of different types of interventions in a meta‐analysis. Our review showed no conclusive results on the efficacy of lead prevention programmes for participants with blood lead levels of 15.0 µg/dL or more.
Another systematic review assessed the effectiveness of interventions to reduce lead exposure through consumer products and drinking water (Pfadenhauer 2016). This review reported on blood lead levels and, similarly to this review, could also not confirm the effectiveness of educational interventions.
On the surface, these results may appear to contradict observational studies that report a reduction in dust lead loadings and, on average, a decrease in children's blood lead levels (Clark 2004). However, the key question is whether the interim lead hazard controls or partial abatement led to a significant reduction or increase among at‐risk (i.e. younger) children who exhibit mouthing behaviours. The observational data show that household interventions lead to a significant increase in blood lead concentration for young children, especially six‐month old infants. Compared with children over 40 months of age, the odds of having an increase in blood lead levels of 5.0 μg/dL or higher following abatement were high (OR 11.18, 95% CI 2.80 to 44.16 for six‐month‐old infants; OR 3.69, 95% CI 1.68 to 8.09 for 12‐month‐old children; OR 1.79, 95% CI 1.07 to 2.99 for 18‐month‐old children; and OR 1.18, 95% CI 0.79 to 1.76 for 24‐month‐old children). However, these findings need to be interpreted with caution since the lead paint hazard reduction measures and clean‐up practices used in this study are no longer allowed. Nevertheless, these results indicate that the floor clearance levels used by the HUD grantees (less than 1.076 mg/m² or 2.153 mg/m² (100.0 μg/feet² or 200.0 μg/feet²)) are insufficient to protect children. This is not surprising; there is considerable evidence that dust lead levels under 0.108 mg/m² (10.0 μg/feet²) are associated with a large increase in the risk of children having a blood lead level of more than 10.0 µg/dL (Lanphear 1996b; Lanphear 1998; Lanphear 2005b; Dixon 2009). Thus, even if lead hazard controls or renovation activities can be safely implemented, if we rely on empirically derived but obsolete dust clearance standards, the measures may actually increase young children's blood lead concentrations.
Although we could not show a beneficial effect of household interventions on blood lead levels, on a population level, blood lead levels have fallen significantly since the late 1970s (Jacobs 2006). Maybe interventions in single households are not enough, because there are multiple other lead sources outside of households. Eventually more comprehensive interventions implemented on a population level are needed to further reduce lead exposure in children.
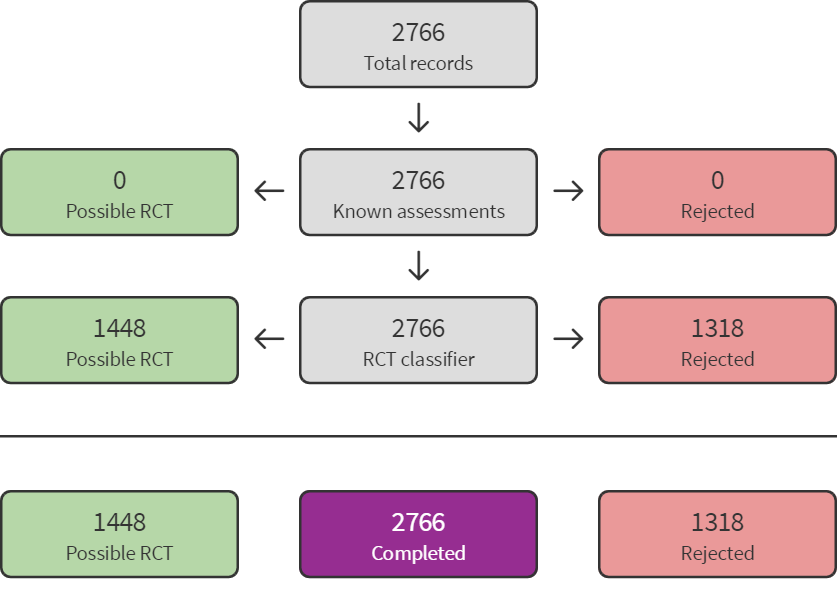
Screen4Me summary diagram. RCT: randomised controlled trial.
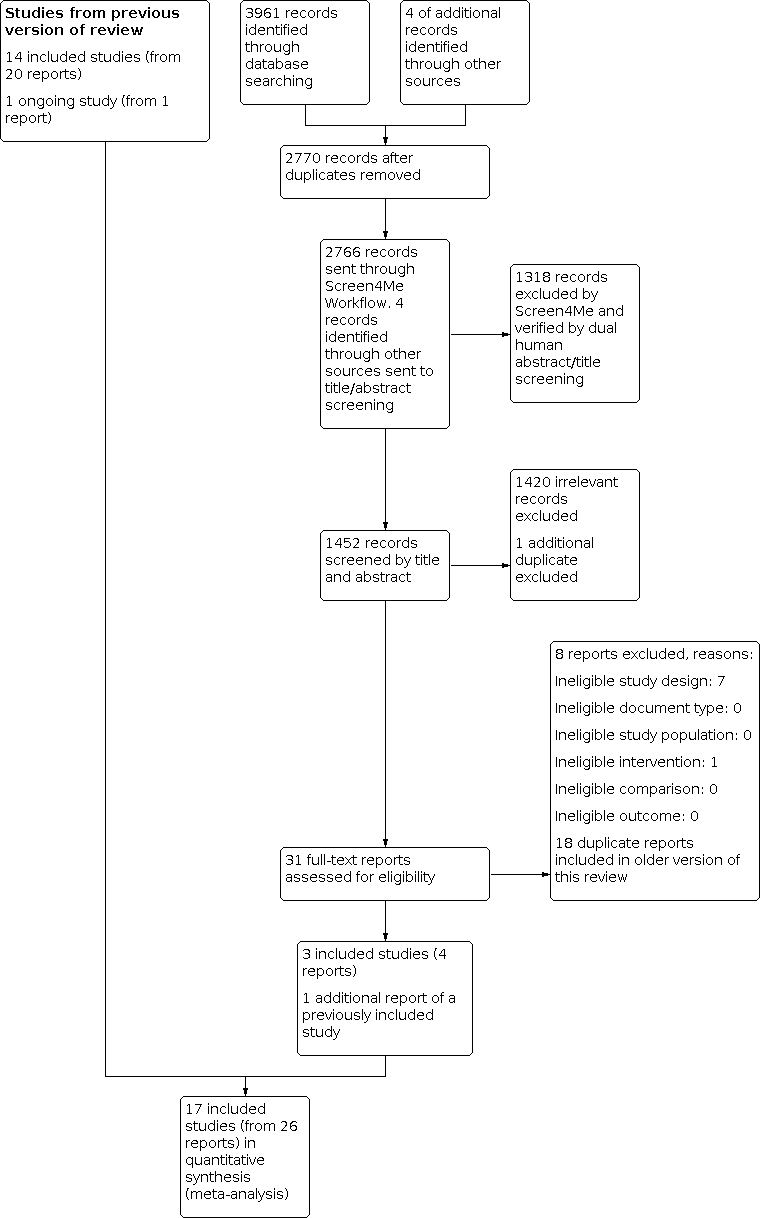
Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Education interventions compared to no intervention or standard education, Outcome 1: Blood lead level (continuous)

Comparison 1: Education interventions compared to no intervention or standard education, Outcome 2: Blood lead level ≥ 10.0 µg/dL (dichotomous)

Comparison 1: Education interventions compared to no intervention or standard education, Outcome 3: Blood lead level ≥ 15.0 µg/dL (dichotomous)

Comparison 1: Education interventions compared to no intervention or standard education, Outcome 4: Floor dust – hard floor

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 1: Blood lead level (continuous)

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 2: Blood lead level ≥ 10.0 µg/dL (dichotomous)

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 3: Blood lead level ≥ 15.0 µg/dL (dichotomous)

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 4: Blood lead level ≥ 10.0 µg/dL (dichotomous): intraclass correlation coefficient (ICC) 0.01

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 5: Blood lead level ≥ 10.0 µg/dL (dichotomous): ICC 0.1

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 6: Blood lead level ≥ 10.0 µg/dL (dichotomous): ICC 0.2

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 7: Blood lead level ≥ 15.0 µg/dL (dichotomous): ICC 0.01

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 8: Blood lead level ≥ 15.0 µg/dL (dichotomous): ICC 0.1

Comparison 2: Environmental interventions (dust control) compared to no intervention or another intervention not aimed to influence domestic lead exposure, Outcome 9: Blood lead level ≥ 15.0 µg/dL (dichotomous): ICC 0.2
| Education interventions versus no intervention for preventing domestic lead exposure in children | ||||||
| Patient or population: children (aged 0–2 years) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants (studies) | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| No intervention | Educational interventions | |||||
| Cognitive and neurobehavioural outcomes | None of the included studies assessed effects on cognitive or neurobehavioural outcomes | — | — | — | — | |
| Adverse events | None of the included studies assessed adverse event outcomes | — | — | — | — | |
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 3–18 months | The mean blood lead level (continuous, log transformed) ranged across control groups from 1.24 to 2.51a,b | The mean blood lead level (continuous, log transformed) in the intervention groups was 0.03 lower (0.13 lower to 0.07 higher) a | — | 815 | ⊕⊕⊕⊝ | Included studies: Lanphear 1996a; Lanphear 1999; Wasserman 2002; Jordan 2003; Brown 2006 |
| Household dust: hard floor dust lead levels (continuous) Scale: 0–40 Follow‐up: 6 months | The mean floor dust level – hard floor – ranged across control groups from 1.65 to 2.28a,b | The mean floor dust level – hard floor – in the intervention groups was 0.07 lower (0.37 lower to 0.24 higher) b | — | 318 | ⊕⊕⊕⊝ | Included studies: Lanphear 1996a; Lanphear 1999 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aPost‐treatment value. | ||||||
| Environmental interventions versus no intervention for preventing domestic lead exposure in children | ||||||
| Patient or population: children (aged 0–6 years) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| No intervention | Environmental interventions | |||||
| Dust control interventions | ||||||
| Cognitive and neurobehavioural outcomes Scale Wechsler IQ: BRIEF: Follow‐up: 3–8 years | Children in the intervention group had numerically better cognitive and neurobehavioural outcomes, but differences were small and 95% CI included beneficial and non‐beneficial effects. Difference of mean scores after 8 years of selected scales:
For detailed results of subscales and additional scales reported see Effects of interventions. | — | 224–302 (1 study) | ⊕⊕⊝⊝ | Included study: Braun 2018 | |
| Adverse events Follow‐up: 3–8 years | 1 study reported that after 8 years they did not observe any adverse events in the intervention group. In the control group, 1 child had an injury because of a stair gateway installed and another child had elevated blood lead concentrations (28 µg/dL). | — | 355 (1 study) | ⊕⊝⊝⊝ | ||
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 6–24 months | The mean blood lead level (continuous, log transformed) ranged across control groups from 0.53to 2.9e | The mean blood lead level (continuous, log transformed) in the intervention groups was 0.02 lower (0.09 lower to 0.06 higher) e | — | 565 (4 studies) | ⊕⊕⊕⊝ | Included studies: Hilts 1995; Rhoads 1999; Boreland 2009; Braun 2018 |
| Household dust: floor dust lead levels | None of the included studies assessed floor dust lead levels. | — | — | — | — | |
| Soil abatement interventions | ||||||
| Cognitive and neurobehavioural outcomes | None of the included studies assessed cognitive and neurobehavioural outcomes. | — | — | — | — | |
| Adverse events | None of the included studies assessed adverse events. | — | — | — | — | |
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 11–24 months | 2 studies performed soil abatement interventions (Weitzman 1993; Farrell 1998). Farrell 1998 reported results as a "total effect" showing no statistical significance, and no data were available for our analyses. Weitzman 1993 reported a statistically significant effect in favour of the intervention. The difference in mean change scores between the intervention group and control group A (loose interior dust abatement and paint removal) was –1.5 µg/dL (SD 4.9), and between the intervention group and control group B (loose interior paint removal only) was –1.9 µg/dL (SD 5.0). No measure of variance was available for post‐treatment means or mean change scores, so further analysis was not possible in this review. | — | 378 (2 studies) | ⊕⊝⊝⊝ Very lowf,g | Included studies Weitzman 1993; Farrell 1998 | |
| Household dust: floor dust lead levels | None of the included studies reported floor dust lead levels. | — | — | — | — | |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IQ: intelligence quotient; MD: mean difference; n: number of study participants with a measurement; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because of imprecision: total population size was fewer than 400 and the 95% confidence interval included both a benefit and no benefit of the intervention. | ||||||
| Combination interventions versus no intervention for preventing domestic lead exposure in children | ||||||
| Patient or population: children (aged 0–4 years) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Standard education | Combination interventions | |||||
| Cognitive and neurobehavioural outcomes | None of the included studies assessed cognitive and neurobehavioural outcomes. | — | — | — | — | |
| Adverse events | None of the included studies assessed adverse events. | — | — | — | — | |
| Blood lead levels (continuous) Scale: 0–30 Follow‐up: 6–24 months | The 4 studies that used a combination of interventions compared to standard education showed inconclusive results. While Charney 1983 reported a significant effect favouring treatment with arithmetic means for post‐treatment blood lead levels of 31.7 µg/dL (SD 2.6) in the intervention group and 37.8 µg/dL (SD 7.9) in the control group, Aschengrau 1998, Campbell 2011, and Sterling 2004 showed little to no difference between combination interventions and standard education on blood lead levels. | — | 426 (4 studies) | ⊕⊝⊝⊝ Very lowa,b | Included studies Charney 1983; Aschengrau 1998; Sterling 2004; Campbell 2011 | |
| Household dust: floor dust lead levels Follow‐up: 6–12 months | Aschengrau 1998 found no evidence for an effect on floor dust lead levels, with median changes for floor dust lead level being –0.002 mg/m² (–0.2 µg/feet², SD 0.8 µg/feet²) in the intervention group and 0.001 mg/m² (0.0 µg/feet², SD 0.2 µg/feet²) in the control group. A second study also found no evidence for an effect on floor dust lead levels (Campbell 2011). | — | 336 (2 studies) | ⊕⊝⊝⊝ Very lowa,b | ||
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level because of imprecision; inconclusive and contradicting results of studies. | ||||||
| Study ID | Mean blood lead level at baseline (µg/dL) | Age at baseline (months) |
|---|---|---|
| 15.0–19.0 | 24–36 | |
| 15.0–19.0 | 42 | |
| 0.7 | 0 | |
| 15.0–19.0 | 12–24 | |
| 2.6–2.7 | 8–14 | |
| 38 | 43–45 | |
| 10.0–14.0 | 6–72 | |
| 10.0–14.0 | 24–36 | |
| < 10.0 | < 12 | |
| 6.6–6.8 | 12–24 | |
| 2.8–2.9 | 6.7 | |
| 5.28 | 47 | |
| 10.0–14.0 | 12–24 | |
| 15.0–19.0 | 49 | |
| 10.0–14.0 | 34–43 | |
| 2.6–4.5 | 22–24 | |
| 10.0–14.0 | 4–36 |
| Study ID | Education | Dust control | Soil abatement | Combination |
|---|---|---|---|---|
| — | — | — | Yes | |
| — | Yes | — | — | |
| — | Yes | — | — | |
| Yes | — | — | — | |
| — | — | — | Yes | |
| — | — | — | Yes | |
| — | — | Yes | — | |
| — | Yes | — | — | |
| Yes | — | — | — | |
| Yes | — | — | — | |
| Yes | — | — | — | |
| — | Yes | — | — | |
| — | Yes | — | — | |
| Yes | — | — | — | |
| — | — | — | Yes | |
| Yes | — | — | — | |
| — | — | Yes | — |
| Study ID | Neurobehavioural and cognitive outcomes | Adverse events | Blood lead (continuous) | Blood lead (dichotomous) | Household dust lead levels: floors | Household dust lead levels: windows | Cost | Other |
|---|---|---|---|---|---|---|---|---|
| — | — | Yes | — | Yes | Yes | — | — | |
| — | — | Yes | — | — | — | Yes | — | |
| Yes | Yes | Yes | — | Yes | Yes | — | ||
| — | — | Yes | Yes | Yes | — | Yes | Parent‐Child Interaction scale | |
| — | — | Yes | — | Yes | Yes | — | Chicago Parents Knowledge Test | |
| — | — | Yes | Yes | — | — | — | — | |
| — | — | — | — | — | — | Yes | Total effect (blood lead levels) | |
| — | — | Yes | Yes | Yes | — | Yes | — | |
| — | — | Yes | — | — | — | — | — | |
| — | — | Yes | Yes | Yes | Yes | — | — | |
| — | — | Yes | Yes | Yes | Yes | — | — | |
| — | — | Yes | — | — | — | — | Lead exposure risk, brochure effectiveness, cleaning home repair behaviour, lead knowledge | |
| — | — | Yes | Yes | Yes | Yes | — | Maternal knowledge lead poisoning | |
| — | — | Yes | — | — | — | — | ||
| — | — | — | Yes | — | — | Yes | — | |
| — | — | Yes | Yes | — | — | Yes | Chicago Parents Knowledge Test | |
| — | — | Yes | — | — | — | — | — |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Blood lead level (continuous) Show forest plot | 5 | 815 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 1.2 Blood lead level ≥ 10.0 µg/dL (dichotomous) Show forest plot | 4 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.79, 1.30] |
| 1.3 Blood lead level ≥ 15.0 µg/dL (dichotomous) Show forest plot | 4 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.33, 1.09] |
| 1.4 Floor dust – hard floor Show forest plot | 2 | 318 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.37, 0.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Blood lead level (continuous) Show forest plot | 4 | 565 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.09, 0.06] |
| 2.2 Blood lead level ≥ 10.0 µg/dL (dichotomous) Show forest plot | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.18] |
| 2.3 Blood lead level ≥ 15.0 µg/dL (dichotomous) Show forest plot | 2 | 210 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.35, 2.07] |
| 2.4 Blood lead level ≥ 10.0 µg/dL (dichotomous): intraclass correlation coefficient (ICC) 0.01 Show forest plot | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.18] |
| 2.5 Blood lead level ≥ 10.0 µg/dL (dichotomous): ICC 0.1 Show forest plot | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.72, 1.24] |
| 2.6 Blood lead level ≥ 10.0 µg/dL (dichotomous): ICC 0.2 Show forest plot | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.72, 1.29] |
| 2.7 Blood lead level ≥ 15.0 µg/dL (dichotomous): ICC 0.01 Show forest plot | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.37, 1.81] |
| 2.8 Blood lead level ≥ 15.0 µg/dL (dichotomous): ICC 0.1 Show forest plot | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.34, 2.03] |
| 2.9 Blood lead level ≥ 15.0 µg/dL (dichotomous): ICC 0.2 Show forest plot | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.34, 1.66] |

