Erradicación del Helicobacter pylori para la prevención del cáncer gástrico
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | RCT | |
| Participants | Country: Colombia, two communities in Narino Province. 976 participants with confirmed histologic diagnoses of multifocal non‐metaplastic atrophy and/or intestinal metaplasia, 2 precancerous lesions. Mean age 51.1 years (range 29‐69 years), 46.1% male. Method to confirm presence of H. pylori: histological examination of gastric biopsies obtained at upper GI endoscopy. Only 852 out of 976 participants were eligible and treated. Study period: started in 1991 | |
| Interventions | 1. Bismuth subsalicylate 262 mg, amoxicillin 500 mg, and metronidazole 375 mg 3 times daily for 2 weeks, including regimens with or without dietary supplements (n = 437). 2. Placebo, including regimens with or without dietary supplements (n = 415). Participants assigned to anti‐H. pylori treatment who tested positive for H. pylori at 36 months were treated again for 14 days with amoxicillin (1 g twice a day), clarithromycin (500 mg twice a day), and either omeprazole (20 mg twice a day) or lansoprazole (30 mg twice a day). Factorial design: 8 different regimens: H. pylori eradication with or without one of the 4 dietary supplements of beta‐carotene (30 mg once per day) and/or ascorbic acid (1 g twice a day) or placebo: A) placebo only; B) anti‐H. pylori; C) beta‐carotene (BC); D) ascorbic acid (AA); E) H. pylori eradication + BC; F) H. pylori eradication + AA; G) BC + AA; and H) H. pylori eradication + BC + AA. Last point of follow‐up 6 years. | |
| Outcomes | Histological examination of gastric biopsies obtained at upper GI endoscopy at 6 years. Primary outcome was "progression of preneoplastic lesions". Other outcomes: relative risks of progression, no change, and regression from multifocal non‐metaplastic atrophy and intestinal metaplasia. | |
| Notes | 1. High‐risk participants (all with confirmed histologic diagnoses of multifocal non‐metaplastic atrophy and/or intestinal metaplasia, 2 precancerous lesions), primary outcome was "progression of preneoplastic lesions". 2. Randomised to anti‐H. pylori triple therapy and/or dietary supplementation with AA, BC, or their corresponding placebos. 3. 976 were randomised, but only 852 were eligible and treated after randomisation (7 refused and 117 ineligible). 852 were included in the ITT analyses, and all randomised participants were included in the sensitivity analyses. 631 participants completed the trials and were included in the complete case analyses. 4. Details of gastric cancer data were reported in Correa 2001(a letter). 5. H. pylori eradication rate 58.0% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated lists produced in New Orleans and applied in the field in Pasto, three strata: atrophy (without metaplasia), intestinal metaplasia, or dysplasia. |
| Allocation concealment (selection bias) | Low risk | Central randomisation, therefore allocation was concealed. |
| Blinding of participants and personnel (performance bias) | High risk | No double‐blinding for eradication vs non‐eradication (because an appropriate placebo was not available for bismuth subsalicylate), double‐blinding only applied to supplements vs placebo: "After a factorial design, a double‐blind approach—i.e., study investigators and subjects were unaware of treatment assignments, supplements and placebos were provided in identical coded tablets by Hoffmann‐La Roche Inc" |
| Blinding of outcome assessment (detection bias) | Low risk | At the end of the study, a single experienced pathologist, blinded to treatment assignment and all other study variables, examined all biopsy specimens collected at baseline and after 72 months of follow‐up. |
| Incomplete outcome data (attrition bias) | Unclear risk | 976 were randomised but only 852 were eligible and treated after randomisation (7 refused and 117 ineligible). 852 were included in the ITT analyses, and all randomised participants were included in the sensitivity analyses. 631 participants completed the trial and were included in the complete case analysis. 221 participants withdrew before their 72‐month evaluation: 102 quit treatment, 59 were lost to follow‐up, 34 dropped out of the study because of pregnancy and other medical conditions, 18 died of causes unrelated to gastric cancer, and 8 developed cancer other than gastric cancer. The average rate of loss was 4.3% per year over the 6‐year trial. Withdrew in 72 months = 117 (26.8%) vs 104 (25%) in all H. pylori eradication arms vs control arms. However, it is likely those who had cancer would have come back for treatment although these individuals did not complete the follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No data were reported for deaths from gastric cancer or adverse events. |
| Other bias | Low risk | No other risk of bias is noted. |
| Methods | RCT | |
| Participants | Country: China. 11 villages in Yantai County, Shandong Province. 587 volunteers underwent upper endoscopy with biopsy specimens obtained from the antrum and corpus. H. pylori–infected volunteers with or without symptoms of dyspepsia were randomised. Mean (range) age 52.0 (35‐75) years, 47.8% men. Method to confirm H. pylori infection: histological examination and rapid urease testing. 33.7% participants with preneoplastic lesions at baseline. Study period: screened participants in 1996 | |
| Interventions | 1. Omeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg twice daily for 1 week (n = 295 in Leung 2004 and 276 in Zhou 2008). 2. Placebo twice daily for 1 week (n = 292 in Leung 2004 and 276 in Zhou 2008). Sample size 587 in Leung 2004; 552 in Zhou 2008. Follow‐up: 5 years in Leung 2004; 10 years in Zhou 2008. | |
| Outcomes | Histological examination at 2, 5, 8, and 10 years. | |
| Notes | 1. Data from Zhou 2008 (276 vs 276) rather than Leung 2004a (295 vs 292) were entered in the main analysis, because Zhou 2008 abstract had 10 years of follow‐up. Inconsistent data were noted between Zhou 2008 and Leung 2004a. Zhou et al. had a series of publications but with smaller sample size and fewer gastric cancers than those reported by Leung 2004a. It is likely Zhou 2008 excluded some participants and then regrouped them based on H. pylori status. 2. In Zhou 2005a and Zhou 2005b, participants were regrouped as eradicated group and H.pylori‐negative group (included those failing eradication or controls) as 246 vs 306 (276 + 30 vs 276 ‐ 30). 3. According to Leung 2004, 152 (75 vs 77) were lost to follow‐up; these participants were considered as no gastric cancer in the ITT analysis. 4. Mortality data were available in Leung 2004a but not in Zhou 2008, therefore a larger sample size and larger number of participants with gastric cancer in Leung 2004a. 5. H. pylori eradication rate 55.6% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by instructions in sealed envelopes. The instructions were constructed according to a random‐number list generated by computer. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used, central randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo was used. Medications had identical appearances. Both participants and physicians were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Slides were coded in a random manner such that the pathologist was blinded to the identity of participants, treatment assignment, and year at which biopsies were obtained. |
| Incomplete outcome data (attrition bias) | High risk | Data from Zhou 2008 were used for the main analyses due to the longer follow‐up period. However, Zhou 2008 is a conference proceeding with a smaller sample size and fewer gastric cancer cases than those in the full publication (Leung 2004a). According to Leung 2004, 152 (75 vs 77) were lost to follow‐up; these participants were considered as no gastric cancer in the ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Reported prespecified outcomes. Mortality data were available in Leung 2004 but not in Zhou 2008, leading therefore to different sample sizes in these analyses. |
| Other bias | High risk | Inconsistent data were noted between Zhou 2008 and Leung 2004a. Zhou et al. had a series of publications but with smaller sample size and fewer gastric cancers than reported by Leung 2004a (inconsistencies in data reporting at the 2 follow‐up points, with 10 gastric cancers reported at 5 years, compared with 9 at 10 years). |
| Methods | RCT | |
| Participants | Country: Japan, 145 centres 692 healthy volunteers between 20 to 59 years with H. pylori infection. Mean age not reported (range 20‐59 years), proportion men not reported. Number of participants with preneoplastic lesions at baseline was not reported, although the aim was "the regression/progression of atrophy by one grade or more". Study period: not clear. | |
| Interventions | 1. Lansoprazole 30 mg, amoxicillin 1.5 g, clarithromycin 400 mg once daily for 1 week (n = 379) 2. Non‐eradication group Method to confirm H. pylori infection was not reported. Follow‐up: ≥ 4 years | |
| Outcomes | Histological examination ≥ 4 years; regression/progression of atrophy by 1 grade or more in the eradicated and non‐eradicated groups in participants followed ≥ 4 years. | |
| Notes | 1. Updated searched on Dec 2013: still no follow‐up full publication. 2. Original study planned 2 outcomes, but finally decided to only evaluate "the prevention of the onset and progression of atrophy of gastric mucosa by H. pylori elimination" and did not evaluate "comparative study on the frequency of stomach cancer". 3. H. pylori eradication rate 74.4% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Conference proceeding, participants were "randomised", but the sample size is imbalanced (379 vs 313). |
| Allocation concealment (selection bias) | Unclear risk | Conference proceeding, no detailed information was provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Conference proceeding, no detailed information was provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Conference proceeding, no detailed information was provided. |
| Incomplete outcome data (attrition bias) | Unclear risk | Conference proceeding, no detailed information for losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Conference proceeding, no detailed information was provided. |
| Other bias | Unclear risk | Conference proceeding, no detailed information was provided. |
| Methods | RCT | |
| Participants | Country: China. 7 villages in Changle County, Fujian Province. 2423 healthy participants recruited for a screening endoscopic study. Participants without endoscopic lesions and positive for H. pylori infection (n = 1628) were randomised. Those with peptic ulcer were excluded. Mean age 42.2 (range 35‐65) years, 54.0% men. Method to confirm H. pylori infection: histological examination and rapid urease testing. 37.7% participants with preneoplastic lesions at baseline (gastric atrophy, intestinal metaplasia, dysplasia). Study period: 1994 to Jan 2002 | |
| Interventions | 1. Omeprazole 20 mg, amoxicillin/clavulanic acid 750 mg, metronidazole 400 mg twice daily for 2 weeks (n = 817) 2. Placebo (n = 813) Follow‐up: 7.5 years | |
| Outcomes | Incidence of gastric cancer: gastric cancer in participants with or without precancerous lesions at baseline was the secondary outcome. Histological examination at 7.5 years or, if diagnosed before 7.5 years, review of clinical records and pathology specimens by 3 blinded clinicians. | |
| Notes | This was the first study targeted at a general population (less than 40% with precancerous lesions); a few discrepancies were seen between Wong 2002 abstract and Wong 2004 full publication. Inconsistent sample size between the full publication followed up at 7.5 years (817 vs 813) (Wong 2004) and the conference abstract followed up at 7 years (819 vs 809) (Wong 2002). We used data from the full publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation generated by computer. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by drawing a sealed envelope that contained a pre‐assigned random treatment generated by computer. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled study, endoscopists were blinded and participants were followed by blinded clinical team, likely participants were blinded as well. |
| Blinding of outcome assessment (detection bias) | Low risk | Endoscopists and histologists were blinded, clinical team in Hong Kong who reviewed the gastric cancer cases were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 735/817 vs 703/813 followed at 7.5 years, losses to follow‐up with reasons were balanced between 2 groups (7.7% vs 11.4%); 62% had follow‐up endoscopy, but those who refused endoscopy were followed up in clinics. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes. |
| Other bias | Unclear risk | Inconsistent sample size between the full publication followed up at 7.5 years (817 vs 813) (Wong 2004) and the conference abstract followed up at 7 years (819 vs 809) (Wong 2002). |
| Methods | RCT | |
| Participants | Country: China. 12 villages in Linqu County, Shandong Province. 1024 participants with H. pylori infection and advanced gastric lesions (severe chronic atrophic gastritis, intestinal metaplasia, indefinite dysplasia, or dysplasia); mean age 53.0 (range 35 to 64) years, 46.4% men. Method to confirm H. pylori infection:13Carbon‐urea breath testing. Histology was also performed. 100% participants with preneoplastic lesions at baseline Study period: 2002‐2009 | |
| Interventions | Anti‐H. pylori treatment and/or COX‐2 inhibitor or placebo in a 2x2 factorial design: 1. Omeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg + placebo twice daily for 1 week (n = 255) 2. Placebo (n = 258) 3. Omeprazole 20 mg, amoxicillin 1 g, clarithromycin 500 mg twice daily for 1 week + celecoxib (n = 255) 4. Celecoxib + placebo (n = 256) Follow‐up: 5 years | |
| Outcomes | Gastric cancer: histological examination at 5 years. Regression or progression of advanced gastric lesions. | |
| Notes | 2x2 factorial design, in the main analysis we did not include data from the 2 arms that used celecoxib, only data for H. pylori eradication only vs placebo only (n = 513). The celecoxib arms were included in a sensitivity analysis. Eradication rate: 63.5% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised treatment assignments were generated blindly by Westat Inc, (Rockville, MD, USA) after eligibility was determined. |
| Allocation concealment (selection bias) | Low risk | Central randomisation was used. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind, placebo‐controlled trial. All placebos were identical in number, size, and colour to the original medications. Both participants and investigators were blinded to treatment assignments. |
| Blinding of outcome assessment (detection bias) | Low risk | Endoscopists and pathologists were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 234/258 (90.7%) vs 233/255 (91.4%) participants had follow‐up gastric biopsy data and the authors reported outcomes for these, losses to follow‐up with reasons were balanced and provided, 89.7% completed the repeat upper endoscopy and histology. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes. |
| Other bias | Low risk | No other risk of bias was noted. |
| Methods | RCT | |
| Participants | Country: China. 13 villages in Linqu County, Shandong Province. 2258 participants were randomly selected in villages and given baseline endoscopy. Mean age 46.8 (range 35 to 64) years, 50.0% men. Excluded participants who were too ill or who refused. Method to confirm H. pylori infection: serological testing 64% participants with preneoplastic lesions at baseline Study period: 1994‐2010 | |
| Interventions | 1. Omeprazole 20 mg and amoxicillin 1 g twice daily for 2 weeks, with or without vitamin or garlic supplements (n = 1130). Participants who had continued evidence of infection after 3 months received a repeat course of treatment for 2 weeks unless they had previously developed rashes or other evidence of allergy to the initial treatment. 2. Placebo, with or without vitamin or garlic supplements (n = 1128)
2x2x2 factorial design: H. pylori eradication; dietary supplementation with capsules containing vitamin C, vitamin E, and selenium; dietary supplementation with capsules containing steam‐distilled garlic oil and Kyolic aged garlic extract. Garlic and vitamin supplements were not given in June and July 1999, and garlic supplements were not given in September 2002 because of interruptions in the availability of the supplement. Follow up: 14.7 years. | |
| Outcomes | Gastric cancer: Histological examination, clinical, laboratory, or pathological data and cause‐specific mortality. Prevalence of dysplasia and other precancerous gastric lesions: prevalence of dysplasia or gastric cancer (score > 6); prevalence of severe chronic atrophic gastritis, intestinal metaplasia, dysplasia, or gastric cancer; and average severity score, effects of one‐time H. pylori treatment and long‐term vitamin or garlic supplements in reducing the prevalence of advanced precancerous gastric lesions. Secondary endpoints: rates of transition from baseline to final histopathologic states and the effects of treatments on these rates of transition; evidence of the effectiveness of amoxicillin and omeprazole in eradicating H. pylori; and blood pressure at the time of the final examination. | |
| Notes | 1. You 2006a: no gastric cancer data for those who did not receive dietary supplement; some participants were ineligible after randomisation and were excluded in the main analyses, these were included in the sensitivity analyses. 2. Some data were obtained from the authors. 3. Inconsistent sample size between Gail 1998 protocol (3411 randomised = 2285 H. pylori‐positive and 1126 H. pylori‐negative); and 3365 randomised (2258 H. pylori‐positive vs 1107 H. pylori‐negative) in You 2006,. 4. Eradication rate: 73.2%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We masked both the subjects and the researchers to treatment assignment. After confirming the eligibility of subjects, we assigned treatments randomly at Westat, Inc. in the United States and used this assignment to distribute coded bottles of capsules from the pharmacy in the city of Weifang in Shandong Province" |
| Allocation concealment (selection bias) | Low risk | Central randomisation, with distribution of coded bottles of capsules from the pharmacy. Pill bottles bearing codes corresponding to those assignments were then distributed to the study participants. |
| Blinding of participants and personnel (performance bias) | Low risk | Look‐alike placebo capsules containing lactose and starch for amoxicillin and sucrose and starch for omeprazole were given to serologically positive controls and to all seronegative participants. To protect blinding, the investigators randomly selected an equal number of participants of the placebo arm from the same village and 10‐year age range for retreatment with placebo. Look‐alike placebo capsules were also used for supplements. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors were blinded, only 1 person had the authority to break the code when necessary (e.g. toxicity). |
| Incomplete outcome data (attrition bias) | Low risk | 2285 H. pylori‐positive participants randomised in Gail 1998, 2258 H. pylori‐positive participants were analysed in You 2006. Overall, only 13% of participants did not have final gastric biopsy data. |
| Selective reporting (reporting bias) | Low risk | Reported prespecified outcomes, some data were obtained from the authors. |
| Other bias | Low risk | No other risk of bias was noted. |
GI: gastrointestinal
ITT: intention‐to‐treat
RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Trial of people undergoing endoscopic mucosal resection of gastric cancer | |
| No incident gastric cancers detected during follow‐up | |
| Did not compare the interventions of interest | |
| No gastric cancer data, follow‐up study of Moayyedi 2000 whose primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community | |
| Trial of people undergoing endoscopic mucosal resection of gastric cancer | |
| Not a RCT | |
| No gastric cancer data. Primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community | |
| Prospective follow‐up study, not RCT | |
| No gastric cancer data | |
| Not a RCT | |
| Case control study, not RCT | |
| Cohort study, not RCT | |
| Cohort study, not RCT | |
| No gastric cancer data, duplicate publication of Harvey 2004, whose primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community | |
| Cohort study, not RCT | |
| No gastric cancer data, cost‐effectiveness analysis of Moayyedi 2000, whose primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community | |
| No incident gastric cancers detected during follow‐up | |
| No gastric cancer data. Primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community | |
| Cohort study, not RCT | |
| Not RCT, reported data for glandular atrophy and intestinal metaplasia | |
| Cohort study, not RCT | |
| Cohort study, not RCT | |
| Cohort study, not RCT | |
| Cohort study, not RCT | |
| Cohort study, not RCT | |
| No gastric cancer data. Primary objective was to study the effect of H. pylori eradication therapy on dyspepsia in the community | |
| Cohort study, not RCT |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer ‐ modified ITT analysis Show forest plot | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| Analysis 1.1 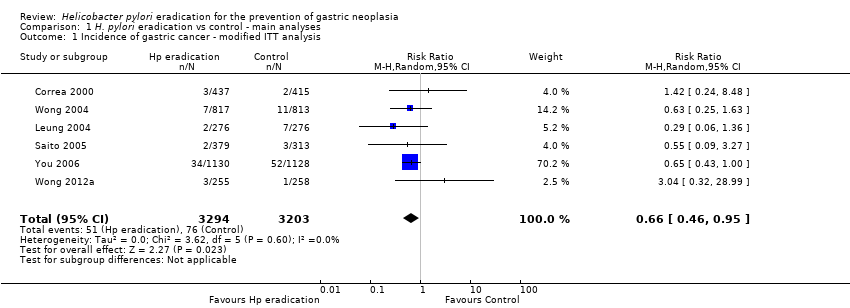 Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 1 Incidence of gastric cancer ‐ modified ITT analysis. | ||||
| 2 Incidence of gastric cancer ‐ complete case analysis Show forest plot | 6 | 6038 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.94] |
| Analysis 1.2 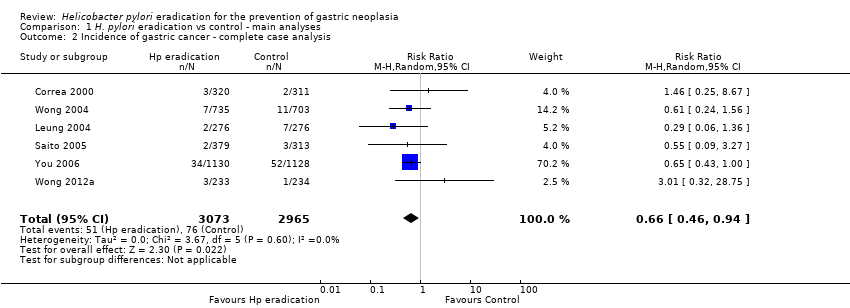 Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 2 Incidence of gastric cancer ‐ complete case analysis. | ||||
| 3 Death from gastric cancer ‐ modified ITT analysis Show forest plot | 3 | 4475 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.40, 1.11] |
| Analysis 1.3  Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 3 Death from gastric cancer ‐ modified ITT analysis. | ||||
| 4 Death from all causes ‐ modified ITT analysis Show forest plot | 4 | 5253 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.38] |
| Analysis 1.4  Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 4 Death from all causes ‐ modified ITT analysis. | ||||
| 5 Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis Show forest plot | 1 | 1630 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.18, 21.91] |
| Analysis 1.5 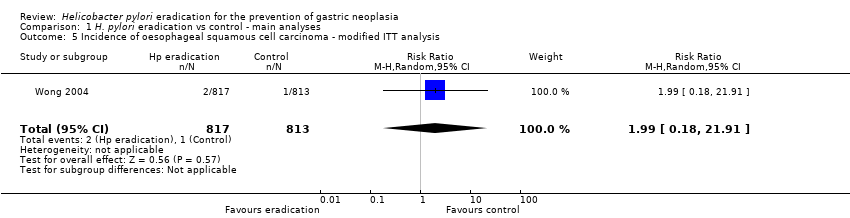 Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 5 Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer according to presence or absence of pre‐neoplastic lesions at baseline Show forest plot | 6 | 6481 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.45, 1.17] |
| Analysis 2.1 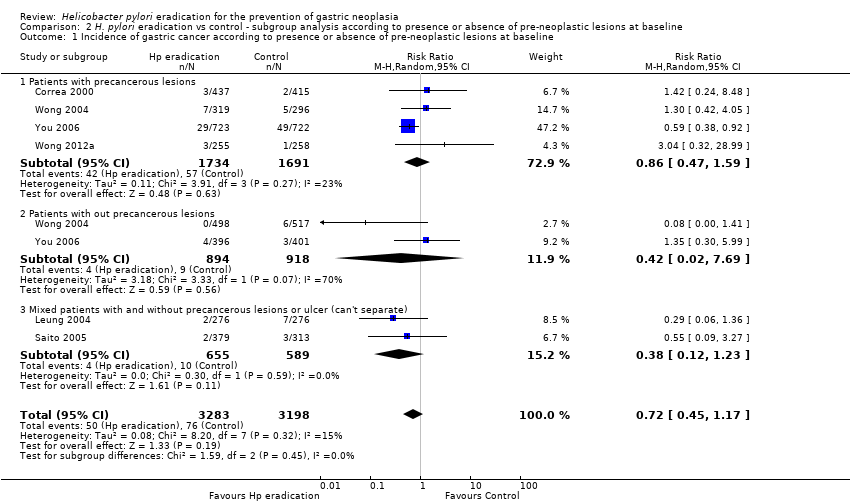 Comparison 2 H. pylori eradication vs control ‐ subgroup analysis according to presence or absence of pre‐neoplastic lesions at baseline, Outcome 1 Incidence of gastric cancer according to presence or absence of pre‐neoplastic lesions at baseline. | ||||
| 1.1 Patients with precancerous lesions | 4 | 3425 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 1.2 Patients with out precancerous lesions | 2 | 1812 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.02, 7.69] |
| 1.3 Mixed patients with and without precancerous lesions or ulcer (can't separate) | 2 | 1244 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.12, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer according to use of vitamins or anti‐oxidants Show forest plot | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.01] |
| Analysis 3.1  Comparison 3 H. pylori eradication vs control ‐ subgroup analysis according to use of vitamins or antioxidants, Outcome 1 Incidence of gastric cancer according to use of vitamins or anti‐oxidants. | ||||
| 1.1 Without antioxidants | 6 | 4160 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.46, 1.45] |
| 1.2 With antioxidants | 2 | 2337 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer ‐ modified ITT analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004 Show forest plot | 6 | 6532 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.49, 0.98] |
| Analysis 4.1  Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 1 Incidence of gastric cancer ‐ modified ITT analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004. | ||||
| 2 Incidence of gastric cancer ‐ complete case analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004 Show forest plot | 6 | 5921 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.98] |
| Analysis 4.2  Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 2 Incidence of gastric cancer ‐ complete case analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004. | ||||
| 3 Incidence of gastric cancer‐ modified ITT analysis including the two arms of celecoxib from Wong 2012 Show forest plot | 6 | 7008 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.97] |
| Analysis 4.3 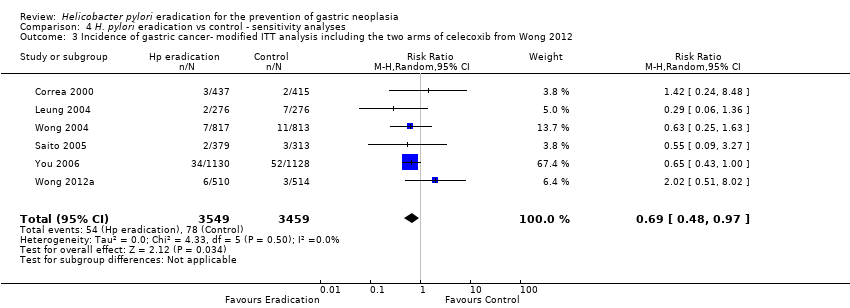 Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 3 Incidence of gastric cancer‐ modified ITT analysis including the two arms of celecoxib from Wong 2012. | ||||
| 4 Incidence of gastric cancer ‐ modified ITT analysis including all randomised patients from Correa 2000 and You 2006 who were found subsequently to be ineligible or did not receive treatment Show forest plot | 6 | 6648 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.95] |
| Analysis 4.4 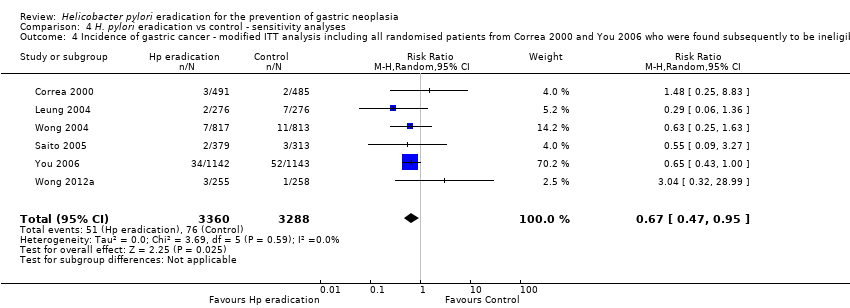 Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 4 Incidence of gastric cancer ‐ modified ITT analysis including all randomised patients from Correa 2000 and You 2006 who were found subsequently to be ineligible or did not receive treatment. | ||||
| 5 Incidence of gastric cancer ‐ missing data imputation based on various assumptions Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.5 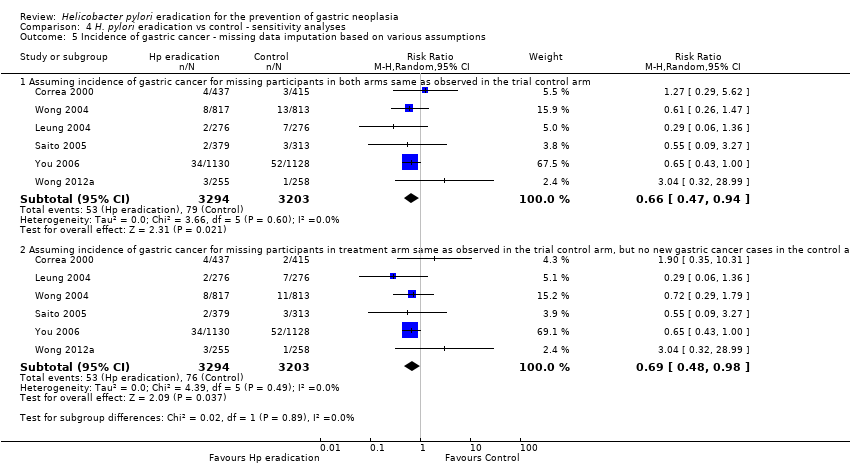 Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 5 Incidence of gastric cancer ‐ missing data imputation based on various assumptions. | ||||
| 5.1 Assuming incidence of gastric cancer for missing participants in both arms same as observed in the trial control arm | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.47, 0.94] |
| 5.2 Assuming incidence of gastric cancer for missing participants in treatment arm same as observed in the trial control arm, but no new gastric cancer cases in the control arm among those with missing data | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.98] |

Study flow diagram for RCTs

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 1 Incidence of gastric cancer ‐ modified ITT analysis.
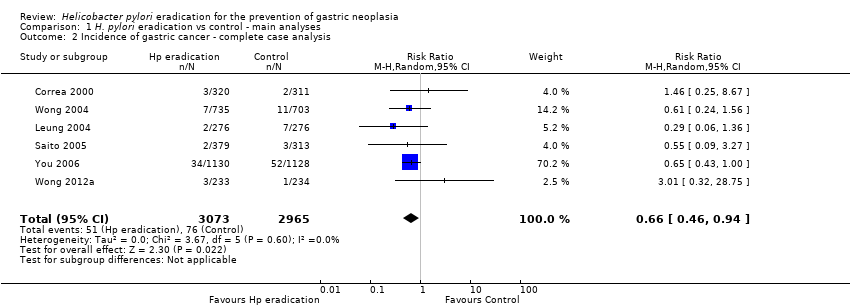
Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 2 Incidence of gastric cancer ‐ complete case analysis.

Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 3 Death from gastric cancer ‐ modified ITT analysis.

Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 4 Death from all causes ‐ modified ITT analysis.

Comparison 1 H. pylori eradication vs control ‐ main analyses, Outcome 5 Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis.

Comparison 2 H. pylori eradication vs control ‐ subgroup analysis according to presence or absence of pre‐neoplastic lesions at baseline, Outcome 1 Incidence of gastric cancer according to presence or absence of pre‐neoplastic lesions at baseline.

Comparison 3 H. pylori eradication vs control ‐ subgroup analysis according to use of vitamins or antioxidants, Outcome 1 Incidence of gastric cancer according to use of vitamins or anti‐oxidants.

Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 1 Incidence of gastric cancer ‐ modified ITT analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004.
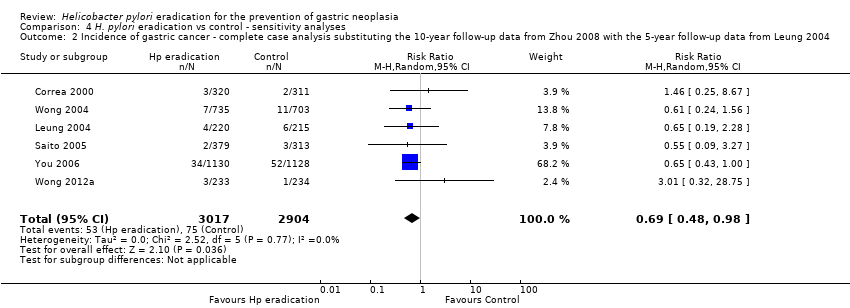
Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 2 Incidence of gastric cancer ‐ complete case analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004.

Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 3 Incidence of gastric cancer‐ modified ITT analysis including the two arms of celecoxib from Wong 2012.
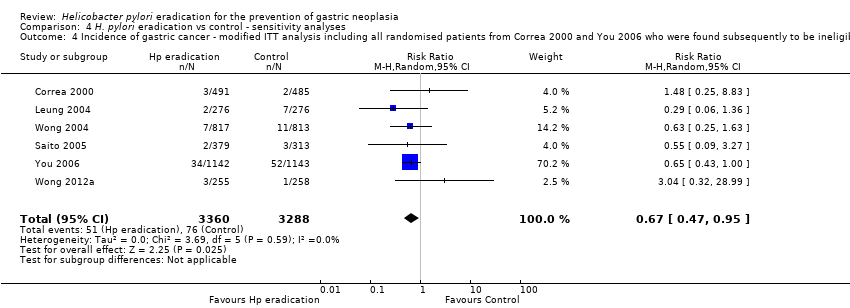
Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 4 Incidence of gastric cancer ‐ modified ITT analysis including all randomised patients from Correa 2000 and You 2006 who were found subsequently to be ineligible or did not receive treatment.

Comparison 4 H. pylori eradication vs control ‐ sensitivity analyses, Outcome 5 Incidence of gastric cancer ‐ missing data imputation based on various assumptions.
| H. pylori eradication therapy compared to control for the prevention of gastric neoplasia in healthy asymptomatic infected individuals | ||||||
| Patient or population: healthy asymptomatic H. pylori‐infected individuals | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | H. pylori eradication therapy to prevent subsequent gastric cancer | |||||
| Incidence of gastric cancer ‐ modified ITT analysis | 24 per 1000 | 16 per 1000 | RR 0.66 | 6497 | ⊕⊕⊕⊝ | Number needed to treat to benefit was 124 (95% CI 78 to 843) |
| Death from gastric cancer ‐ modified ITT analysis | 16 per 1000 | 11 per 1000 | RR 0.67 | 4475 | ⊕⊕⊕⊝ | |
| Death from all causes ‐ modified ITT analysis | 67 per 1000 | 73 per 1000 | RR 1.09 | 5253 | ⊕⊕⊕⊝ | |
| Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis | 1 per 1000 | 2 per 1000 | RR 1.99 | 1630 | ⊕⊕⊕⊝ | |
| Adverse events | See comment | See comment | Not estimable | 0 | See comment | Adverse events were poorly reported across the studies and could not be summarised. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 As all but one study was conducted in East Asia, it is not possible to assess the effect of searching for and eradicating H. pylori in Western populations. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer ‐ modified ITT analysis Show forest plot | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| 2 Incidence of gastric cancer ‐ complete case analysis Show forest plot | 6 | 6038 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.94] |
| 3 Death from gastric cancer ‐ modified ITT analysis Show forest plot | 3 | 4475 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.40, 1.11] |
| 4 Death from all causes ‐ modified ITT analysis Show forest plot | 4 | 5253 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.38] |
| 5 Incidence of oesophageal squamous cell carcinoma ‐ modified ITT analysis Show forest plot | 1 | 1630 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.18, 21.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer according to presence or absence of pre‐neoplastic lesions at baseline Show forest plot | 6 | 6481 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.45, 1.17] |
| 1.1 Patients with precancerous lesions | 4 | 3425 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.59] |
| 1.2 Patients with out precancerous lesions | 2 | 1812 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.02, 7.69] |
| 1.3 Mixed patients with and without precancerous lesions or ulcer (can't separate) | 2 | 1244 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.12, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer according to use of vitamins or anti‐oxidants Show forest plot | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 1.01] |
| 1.1 Without antioxidants | 6 | 4160 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.46, 1.45] |
| 1.2 With antioxidants | 2 | 2337 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of gastric cancer ‐ modified ITT analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004 Show forest plot | 6 | 6532 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.49, 0.98] |
| 2 Incidence of gastric cancer ‐ complete case analysis substituting the 10‐year follow‐up data from Zhou 2008 with the 5‐year follow‐up data from Leung 2004 Show forest plot | 6 | 5921 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.98] |
| 3 Incidence of gastric cancer‐ modified ITT analysis including the two arms of celecoxib from Wong 2012 Show forest plot | 6 | 7008 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.97] |
| 4 Incidence of gastric cancer ‐ modified ITT analysis including all randomised patients from Correa 2000 and You 2006 who were found subsequently to be ineligible or did not receive treatment Show forest plot | 6 | 6648 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.95] |
| 5 Incidence of gastric cancer ‐ missing data imputation based on various assumptions Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Assuming incidence of gastric cancer for missing participants in both arms same as observed in the trial control arm | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.47, 0.94] |
| 5.2 Assuming incidence of gastric cancer for missing participants in treatment arm same as observed in the trial control arm, but no new gastric cancer cases in the control arm among those with missing data | 6 | 6497 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.48, 0.98] |

