Profilaxis antimicrobiana sistémica para la gastrostomía endoscópica percutánea
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised controlled trial. | |
| Participants | Male and female patients. | |
| Interventions | 'Pull' technique | |
| Outcomes | Peristomal infection. | |
| Notes | Group 3 excluded from analysis as not randomised.Used Jain et al criteria for wound assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined computer‐generated randomisation scheme. |
| Allocation concealment (selection bias) | Unclear risk | Review authors unable to contact study authors for clarification. |
| Blinding (performance bias and detection bias) | Low risk | Patients received intravenous antibiotics or saline. |
| Blinding (performance bias and detection bias) | Low risk | Procedure by doctor or nurse not involved in the study. |
| Blinding (performance bias and detection bias) | Low risk | Blinded to treatment group. |
| Incomplete outcome data (attrition bias) | Unclear risk | 8 (5.6%) of 141 patients excluded because of incomplete data. Comment: A 5.6% drop out rate makes the study at unclear risk of attrition bias. However the study reports 133 patients "were analysable on an intention to treat analysis". |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Balanced for gender, age, reason for PEG, underlying conditions. |
| Timing of outcome assessment similar in all groups? | Low risk | In all groups outcomes were assessed immediately after procedure plus three, five and seven days. |
| Methods | Randomised controlled trial. | |
| Participants | Consecutive male and female patients. PEG tube inserted for oropharyngeal cancer (n= 56), neurological (n= 32) and other (n= 12). | |
| Interventions | 'Pull' and 'push' techniques. | |
| Outcomes | Peristomal infection. | |
| Notes | Criteria for wound assessment: minor infection present if redness with or without purulent discharge, major infection judged by physician as those requiring antibiotics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated; no placebo given. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | PEG placement failed in four patients (4%). |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | All groups were balanced for age, reason for PEG, underlying conditions. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes assessed in all groups twice weekly for one month. |
| Methods | Randomised controlled trial | |
| Participants | Male and female patients. PEG tube inserted for ear, nose or throat cancer (127), neurological disease (42), oesophageal cancer (n= 30), stroke (n= 11), dementia (n= 1), gastric cancer (n= 1), and other (n= 22). Included if able to consent to participation in the study after receiving oral and written information Excluded if ongoing antibiotic treatment, illness too severe to allow the patient to participate, allergy to any of the antibiotic alternatives. Total number of patients randomised; n = 234 | |
| Interventions | Pull technique. | |
| Outcomes | Primary outcome ‐ wound infection Clinically evident peristomal infection at follow up appointment infection within 7‐ 14 days after insertion of the PEG catheter. | |
| Notes | Criteria for wound assessment: a clinically identifiable wound infection, as judged by a red zone around the catheter or occurrence of pus, subcutaneous swelling, and pain on palpation in the area around the catheter. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation process was conducted by personnel at a hospital department not engaged in the care of the included patients" |
| Allocation concealment (selection bias) | Low risk | "The randomisation process was conducted by personnel at a hospital department not engaged in the care of the included patients" |
| Blinding (performance bias and detection bias) | Low risk | "the blinding of the patients was accomplished by using intravenous fluid and manipulating the newly inserted PEG catheter in all patients. This sham manoeuvre was facilitated by the use of sedation" |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Low risk | "The nurses who evaluated the patients at follow‐up visit were not involved in insertion of the PEG catheter, including the administration of antibiotics" |
| Incomplete outcome data (attrition bias) | High risk | Out of 34 dropouts (14.5%) or withdrawals, a total of twelve participants in each group did not undergo PEG placement for anatomical reasons. Other attrition included five deaths, one patient pulled out PEG, three lost to follow‐up and one received co‐trimoxazole after being randomised to cefuroxime. Comment: 15% drop out rate makes the study at high risk of attrition bias, but ITT analysis undertaken. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Both groups were balanced for age,smoking, diabetes, indications for PEG.Slight gender imbalance 42 females in group 1 versus 31 females in group 2. |
| Timing of outcome assessment similar in all groups? | Low risk | 7‐14 days for both groups. |
| Methods | Randomised controlled trial. | |
| Participants | Male and female patients aged over 18 years requiring enteral feeding via PEG tube > six weeks. PEG tube inserted for neurological disease (n= 145), tumour (n= 63) and other (n= 8). (Taken from Table 1 ‐ 216 patients evaluable as 21 dropouts). | |
| Interventions | 'Pull' technique: Numbers originally randomised into groups not given in trial report. | |
| Outcomes | Peristomal infection. | |
| Notes | Used modified Jain et al criteria for wound assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in blocks of four using Rancode 3.1. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | 'Study monitors' involved but specific role not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 21 drop outs (8.9%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes addressed. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Both groups balanced for gender, age, reasons for PEG tube, BMI,indications for PEG, underlying conditions. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in both groups were assessed at one, two, four and ten days (mean 8.7). |
| Methods | Randomised controlled trial. | |
| Participants | Male and female patients with proportionately more males (n = 243) than females (n = 93). PEG tube inserted for malignant disease (n= 210), neurological disorders (n= 97). (Taken from Table 1 ‐ 307 patients evaluable as 40 dropouts). Included if had functional resorption and digestive capacity with temporary, or permanent, dysphagia. | |
| Interventions | Modified 'pull' technique. Numbers originally randomised into groups not given in trial report | |
| Outcomes | Peristomal infection. | |
| Notes | Used Jain et al and Shapiro et al criteria for wound assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | High risk | Randomly assigned to group after consent taken, and at least one day before procedure. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | 40 drop outs (11.5%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | All groups were balanced for age, gender, weight, Karnofksy index, reasons for PEG. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in all groups were assessed daily for seven days. Those discharged were assessed over the telephone or via the outpatient department, but the group was not specified. |
| Methods | Randomised controlled trial. | |
| Participants | Male and female patients (distribution not stated) who had given consent. PEG tube inserted for CVA (n= 53), oropharyngeal cancer (n= 27), CNS trauma (n= 8), CNS infection (n= 3), CNS degenerative disease (n= 10) and miscellaneous (n= 6). | |
| Interventions | Technique not stated, but presumed to be 'pull' as the authors state 'the feeding tube traverses the mouth and pharynx'. Group 2 not receiving antibiotics were randomly assigned to: | |
| Outcomes | Peristomal infection. | |
| Notes | Criteria for wound assessment scoring devised by Jain et al which used indicators previously used by Shapiro et al (1982). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule generated by Hewlitt‐Packard HP‐67 pocket calculator. |
| Allocation concealment (selection bias) | Low risk | One author (KPC) was responsible for (and the only one aware of) assignment and did not evaluate the wounds. |
| Blinding (performance bias and detection bias) | Low risk | Stated as 'double blind'. |
| Blinding (performance bias and detection bias) | Low risk | Stated as 'double blind'. |
| Blinding (performance bias and detection bias) | Low risk | Outcomes assessed by team members blind to placebo allocation. |
| Incomplete outcome data (attrition bias) | Low risk | No drop outs. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes addressed. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Unclear risk | All groups were balanced for gender, underlying conditions, reasons for PEG. In group 1 the mean age was slightly but significantly less than that of patients who did not receive prophylaxis. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in all groups were assessed daily for seven days. |
| Methods | Randomised controlled trial. | |
| Participants | All male patients with dysphagia, consent, and functionally‐intact gastrointestinal tract. PEG tube inserted for underlying malignancy (n= 18) and neurological (n= 15). (Taken from Table 4 ‐ 33 patients evaluable as 4 dropouts). | |
| Interventions | 'Pull' technique: Numbers originally randomised into groups not given in trial report | |
| Outcomes | Peristomal infection. | |
| Notes | Criteria for wound assessment: red, tender, indurated area at exit site with pain ± systemic signs of leukocytosis and fever. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by pharmacist. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment as 'foil‐covered vials of identical appearance and equivalent volume' by pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | Stated: 'foil‐covered vials of identical appearance and equivalent volume'. |
| Blinding (performance bias and detection bias) | Low risk | Stated: 'foil‐covered vials of identical appearance and equivalent volume'. |
| Blinding (performance bias and detection bias) | Low risk | Known to pharmacist only. |
| Incomplete outcome data (attrition bias) | Low risk | Four drop outs (10.8%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Both groups were balanced for malignancy, neurological disease. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in both groups were assessed at more than three days (endpoint not stated). |
| Methods | Randomised controlled trial. | |
| Participants | Consenting male and female patients, who had not received antibiotics in the preceding three days. PEG tube inserted for neurological (n= 49), Cancer (n= 4) and miscellaneous (n= 5). (Taken from Table 1 ‐ 58 patients evaluable as 17 dropouts). | |
| Interventions | Technique not stated. Numbers originally randomised into groups not given in trial report | |
| Outcomes | Peristomal infection. | |
| Notes | Criteria for wound assessment: modified ASEPSIS scoring system used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Provided by the hospital pharmacy'. |
| Allocation concealment (selection bias) | Low risk | Administered by 'endoscopist 15‐30 min prior to endoscopy with no prior knowledge of the sequence in the randomisation'. |
| Blinding (performance bias and detection bias) | Low risk | Stated as 'double blind'. |
| Blinding (performance bias and detection bias) | Low risk | Stated as 'double blind'. |
| Blinding (performance bias and detection bias) | Low risk | 'The infection control team was blinded to the randomisation'. |
| Incomplete outcome data (attrition bias) | High risk | 17 in total, seven died, eight drop outs, two lost to follow‐up (22.6%). Comment: A 15% drop out rate makes the study at high risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Both groups were balanced for age, gender, reasons for PEG. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in both groups were assessed daily for seven days, and then day 28. |
| Methods | Randomised controlled trial. | |
| Participants | Consenting male and female patients with dysphagia, 18 years and over. PEG tube inserted for malignancy (n= 55) and neurological disease (n= 29) (Taken from Table 1 ‐ 84 patients evaluable on a per protocol analysis). | |
| Interventions | Thread 'pull' technique. Numbers originally randomised into groups not given in trial report | |
| Outcomes | Peristomal infection. | |
| Notes | Included data from a previous study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block design with separate sequences of random numbers for each centre to ensure equal numbers. |
| Allocation concealment (selection bias) | Low risk | Prepared by pharmacy. |
| Blinding (performance bias and detection bias) | Low risk | Stated as 'double blind'. |
| Blinding (performance bias and detection bias) | Low risk | Blinding of investigators, study nurses, reviewers and data managers. |
| Blinding (performance bias and detection bias) | Low risk | Blinding of investigators, study nurses, reviewers and data managers. |
| Incomplete outcome data (attrition bias) | Unclear risk | 13 drop outs (12.3%). Report stated that ITT analysis was performed on 93 patients who received PEG but 106 patients were randomised and 13 people dropped out and there are no details on how this missing data were handled. The judgement is therefore unclear. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes stated. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Both groups were balanced for age, gender, previous method of feeding, underlying conditions, performance status reason for PEG, Karnofsky index. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in both groups were assessed daily for seven days and mortality at 30 days. |
| Methods | Randomised controlled trial. | |
| Participants | Consenting male and female patients who had not received antibiotics in the preceding 2 days. PEG tube inserted for CVA (n= 57), dementia (n= 10), neurogenic dysphagia (n= 11) and miscellaneous (n= 18). | |
| Interventions | 'Pull' technique. | |
| Outcomes | Peristomal infection. | |
| Notes | Used Jain et al wound assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Closed envelopes opened at random by an endoscopy nurse. |
| Allocation concealment (selection bias) | Low risk | 'Closed envelopes that were shuffled and opened at random by an endoscopy nurse after the patient had given consent for the study'. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Low risk | Stomal site inspected by the investigator who was blinded to regime. |
| Incomplete outcome data (attrition bias) | Low risk | No drop outs, five patient deaths in 1 week, 1 from gastric leakage on day 6 (5.2%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes addressed. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | All groups were balanced for age, gender, steroids, diabetes, size of PEG tube, reasons for PEG. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in both groups were assessed on day three or four and day seven. |
| Methods | Randomised controlled trial. | |
| Participants | Male and female patients over 16 years of age. PEG tube inserted for CVA (n= 61), neurological (n= 35) and miscellaneous (n= 5). (Taken from Table 2 Error in paper as these total 101. The number quoted as 'analysable' for each outcome is 83 for peristomal infection, 97 for systemic infection, and 99 for seven‐day mortality). | |
| Interventions | Pull technique. Numbers originally randomised into groups not given in trial report | |
| Outcomes | Peristomal infection. | |
| Notes | Used Jain et al criteria for wound assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Done in advance using a random number generator'. |
| Allocation concealment (selection bias) | Low risk | 'Study assignment cards kept with medication packs in pharmacy'. |
| Blinding (performance bias and detection bias) | Low risk | 'Patient blinded'. Syringe covered with opaque sleeve prepared by endoscopy nurse. |
| Blinding (performance bias and detection bias) | Low risk | Syringe covered with opaque sleeve prepared by endoscopy nurse. |
| Blinding (performance bias and detection bias) | Low risk | 'Study investigator (outcome assessor) blinded'. |
| Incomplete outcome data (attrition bias) | Low risk | 11 withdrawals (10%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes addressed. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Both groups were balanced for age, gender, weight, underlying conditions. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in both groups were assessed daily for seven days. |
| Methods | Randomised controlled trial. | |
| Participants | Male and female patients 16‐89 years. PEG tube inserted for cancer (n= 93). (Taken from Figure 1 ‐ 93 patients evaluable as 4 dropouts). | |
| Interventions | Push technique gastropexy PEG. | |
| Outcomes | Peristomal infection. | |
| Notes | Used Jain et al and Gossner criteria for wound assessment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Block randomisation and computer‐generated random numbers'. |
| Allocation concealment (selection bias) | Low risk | 'Sequentially numbered, opaque, sealed envelopes'. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) | Low risk | Injections prepared by non‐study staff. |
| Blinding (performance bias and detection bias) | Low risk | Wound assessment by members of nutrition support team. |
| Incomplete outcome data (attrition bias) | Low risk | Four drop outs (4.1%). |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | Both groups were balanced for age, gender, BMI, location of malignancy, reason for PEG. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in both groups were assessed daily for seven days. |
| Methods | Randomised controlled trial. | |
| Participants | Characteristics of patients unclear. PEG tube inserted for CVA and head and neck cancer (no numbers provided). | |
| Interventions | Technique not stated. | |
| Outcomes | Peristomal infection. | |
| Notes | Group 3 excluded from analysis, as not randomised. Power calculation not performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Generated random sequence known only to those in the pharmacy'. |
| Allocation concealment (selection bias) | Low risk | Pharmacy dispensed 'Identical vials with equivalent volume'. |
| Blinding (performance bias and detection bias) | Low risk | Stated as 'double blind'. |
| Blinding (performance bias and detection bias) | Low risk | Stated as 'double blind'. |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | No drop outs. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Baseline imbalance? (Was the study free of baseline imbalance?) | Low risk | All groups were balanced for age, reasons for PEG, underlying conditions. |
| Timing of outcome assessment similar in all groups? | Low risk | Outcomes in all groups were assessed daily for one week. |
Abbreviations
> = more than
± = with or without
ITT = intention‐to‐treat analysis
IV = intravenous
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not an RCT. | |
| Not an RCT but an audit. | |
| A critique of two RCTs. | |
| Not an RCT. | |
| Not an RCT but a cohort study that did not investigate the use of antibiotics. | |
| Abstract only: unable to obtain study data via trial authors or full‐text article via British Library. | |
| Not an RCT. | |
| All patients received prophylactic and concomitant antibiotics. | |
| Not an RCT: did not investigate the use of antibiotics. | |
| A systematic review. The patient data included were a duplicate of those in the included studies. | |
| Examined PEG feeding not placement. | |
| Not an RCT. | |
| Not an RCT. | |
| A comparison between push and pull methods of placement. Antibiotics were given to all patients. | |
| A comparison of the use of an over tube to reduce infection in PEG tube placement. Antibiotics were given to all patients. | |
| Not an RCT. | |
| A systematic review. The patient data included were a duplicate of those in the included studies. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 8 | 586 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.22, 0.53] |
| Analysis 1.1  Comparison 1 Systemic antibiotic (IV) compared with placebo, Outcome 1 Peristomal infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 3 | 623 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.17, 0.53] |
| Analysis 2.1  Comparison 2 Systemic antibiotic (IV) compared with no intervention, Outcome 1 Peristomal infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 12 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| Analysis 3.1  Comparison 3 Systemic antibiotic (IV) compared with placebo/no intervention/skin antiseptic, Outcome 1 Peristomal infection. | ||||
| 2 Allocation concealment Show forest plot | 12 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.34, 0.65] |
| Analysis 3.2  Comparison 3 Systemic antibiotic (IV) compared with placebo/no intervention/skin antiseptic, Outcome 2 Allocation concealment. | ||||
| 2.1 adequate allocation concealment | 8 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.88] |
| 2.2 unclear/inadequate concealment | 4 | 717 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.21, 0.58] |
| 3 Sponsorship Show forest plot | 12 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| Analysis 3.3  Comparison 3 Systemic antibiotic (IV) compared with placebo/no intervention/skin antiseptic, Outcome 3 Sponsorship. | ||||
| 3.1 Trials with no sponsorship | 10 | 962 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.25, 0.56] |
| 3.2 Trials with sponsorship | 2 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systemic antibiotic (IV) compared with systemic antibiotic (IV) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Systemic antibiotic compared with systemic antibiotic, Outcome 1 Systemic antibiotic (IV) compared with systemic antibiotic (IV). | ||||
| 1.1 Peristomal infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Systemic antibiotic (PEG) compared with systemic antibiotic (IV) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2  Comparison 4 Systemic antibiotic compared with systemic antibiotic, Outcome 2 Systemic antibiotic (PEG) compared with systemic antibiotic (IV). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 15.78 [1.90, 130.86] |
| Analysis 5.1  Comparison 5 Systemic antibiotic (IV) compared with systemic antibiotic (IV) and skin antiseptic, Outcome 1 Peristomal infection. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 15.63 [1.84, 133.09] |
| Analysis 6.1  Comparison 6 Skin antiseptic compared with systemic antibiotic (IV) and skin antiseptic, Outcome 1 Peristomal infection. | ||||

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
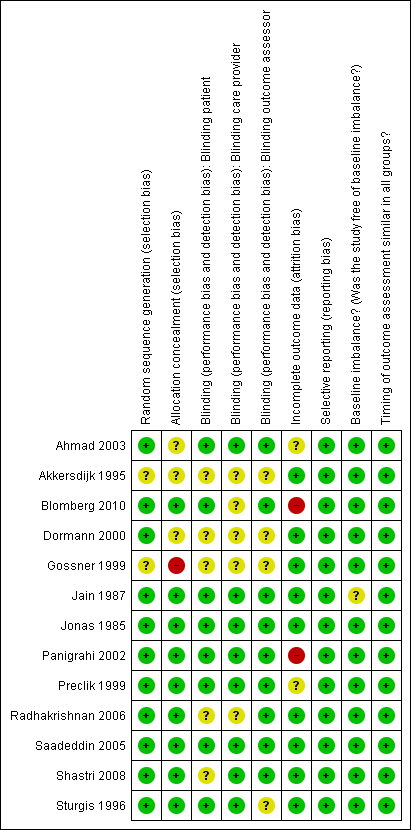
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Systemic antibiotic (IV) compared with placebo, Outcome 1 Peristomal infection.

Comparison 2 Systemic antibiotic (IV) compared with no intervention, Outcome 1 Peristomal infection.
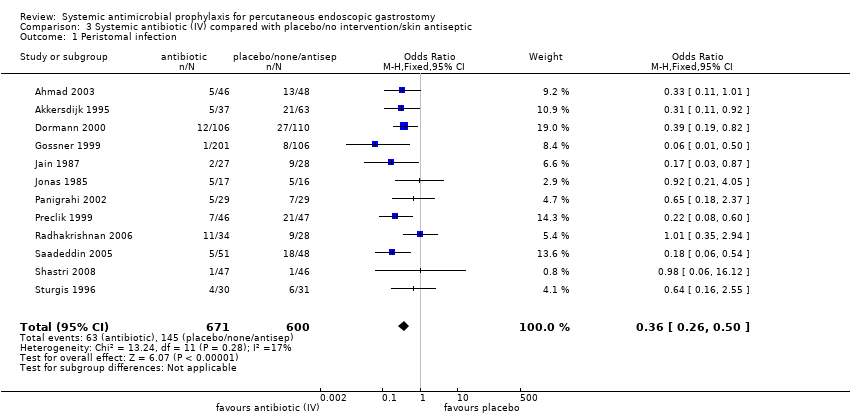
Comparison 3 Systemic antibiotic (IV) compared with placebo/no intervention/skin antiseptic, Outcome 1 Peristomal infection.
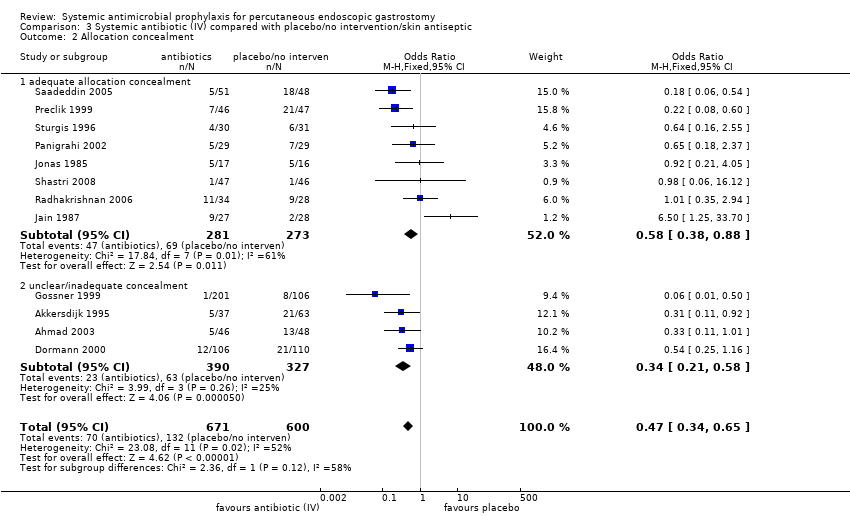
Comparison 3 Systemic antibiotic (IV) compared with placebo/no intervention/skin antiseptic, Outcome 2 Allocation concealment.

Comparison 3 Systemic antibiotic (IV) compared with placebo/no intervention/skin antiseptic, Outcome 3 Sponsorship.

Comparison 4 Systemic antibiotic compared with systemic antibiotic, Outcome 1 Systemic antibiotic (IV) compared with systemic antibiotic (IV).
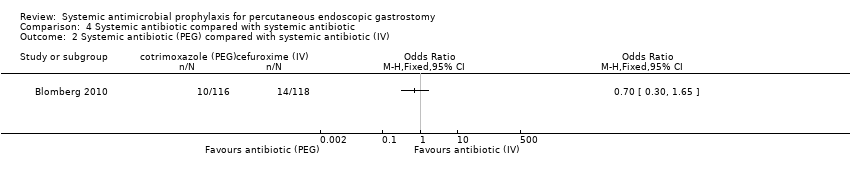
Comparison 4 Systemic antibiotic compared with systemic antibiotic, Outcome 2 Systemic antibiotic (PEG) compared with systemic antibiotic (IV).

Comparison 5 Systemic antibiotic (IV) compared with systemic antibiotic (IV) and skin antiseptic, Outcome 1 Peristomal infection.
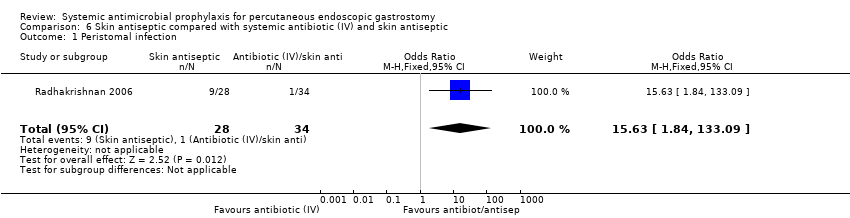
Comparison 6 Skin antiseptic compared with systemic antibiotic (IV) and skin antiseptic, Outcome 1 Peristomal infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 8 | 586 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.22, 0.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 3 | 623 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.17, 0.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 12 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| 2 Allocation concealment Show forest plot | 12 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.34, 0.65] |
| 2.1 adequate allocation concealment | 8 | 554 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.38, 0.88] |
| 2.2 unclear/inadequate concealment | 4 | 717 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.21, 0.58] |
| 3 Sponsorship Show forest plot | 12 | 1271 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.26, 0.50] |
| 3.1 Trials with no sponsorship | 10 | 962 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.25, 0.56] |
| 3.2 Trials with sponsorship | 2 | 309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Systemic antibiotic (IV) compared with systemic antibiotic (IV) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Peristomal infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Systemic antibiotic (PEG) compared with systemic antibiotic (IV) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 15.78 [1.90, 130.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Peristomal infection Show forest plot | 1 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 15.63 [1.84, 133.09] |

