Tratamiento con inhibidores de la bomba de protones iniciado antes del diagnóstico endoscópico en la hemorragia digestiva alta
Resumen
Antecedentes
La hemorragia digestiva alta es una razón frecuente para el ingreso hospitalario de urgencia. Los inhibidores de la bomba de protones (IBP) reducen el ácido gástrico y se utilizan para controlar la hemorragia digestiva alta. Sin embargo, hay evidencia contradictoria en cuanto a la eficacia clínica de los inhibidores de la bomba de protones iniciados antes de la endoscopia en personas con hemorragia digestiva alta.
Objetivos
Evaluar los efectos del tratamiento con IBP iniciado antes de la endoscopia en personas con hemorragia digestiva alta.
Métodos de búsqueda
Se realizaron búsquedas en las bases de datos CENTRAL, MEDLINE, Embase y CINAHL y en los resúmenes de congresos importantes hasta octubre de 2008 para las versiones anteriores de esta revisión, y en abril de 2018, octubre de 2019 y el 3 de junio de 2021 para esta actualización. También se estableció contacto con expertos en el área y se realizaron búsquedas de ensayos adicionales en registros de ensayos y en las listas de referencias de ensayos.
Criterios de selección
Se seleccionaron ensayos controlados aleatorizados (ECA) que compararon el tratamiento con un IBP (oral o intravenoso) versus un tratamiento de control con placebo, antagonistas de los receptores de histamina‐2 (ARH2) o ningún tratamiento, antes de la endoscopia en personas hospitalizadas con hemorragia digestiva alta no investigada.
Obtención y análisis de los datos
Al menos dos autores de la revisión evaluaron de forma independiente la elegibilidad de los estudios, extrajeron los datos y evaluaron el riesgo de sesgo. Los desenlaces evaluados a los 30 días fueron: mortalidad (desenlace principal de esta revisión), nuevas hemorragias, estigmas de alto riesgo de hemorragias recientes (hemorragia activa, vasos visibles sin hemorragia o coágulos adheridos) en la endoscopia de referencia, tratamiento hemostático endoscópico en la endoscopia de referencia, tiempo hasta el alta, necesidad de transfusión de sangre y efectos adversos. Se utilizaron los procedimientos metodológicos estándar previstos por Cochrane.
Resultados principales
Se incluyeron seis ECA con 2223 participantes. No se han publicado nuevos estudios desde la búsqueda en la literatura realizada en 2008 para la versión anterior de esta revisión. De los estudios incluidos, se consideró que uno tuvo bajo riesgo de sesgo, dos tuvieron riesgo de sesgo incierto y tres alto riesgo de sesgo.
Los metanálisis indican que el uso de los IBP antes de la endoscopia podría no reducir la mortalidad (odds ratio [OR] 1,14; intervalo de confianza [IC] del 95%: 0,76 a 1,70; cinco estudios; evidencia de certeza baja) y podría reducir las nuevas hemorragias (OR 0,81; IC del 95%: 0,62 a 1,06; cinco estudios; evidencia de certeza baja). Además, el uso de IBP antes de la endoscopia podría no reducir la necesidad de cirugía (OR 0,91; IC del 95%: 0,65 a 1,26; seis estudios; evidencia de certeza baja) y podría no reducir la proporción de participantes con estigmas de alto riesgo de hemorragia reciente en la endoscopia de referencia (OR 0,80; IC del 95%: 0,52 a 1,21; cuatro estudios; evidencia de certeza baja). El uso de IBP antes de la endoscopia posiblemente reduce la necesidad de realizar un tratamiento hemostático endoscópico en el momento de la endoscopia de referencia (OR 0,68; IC del 95%: 0,50 a 0,93; tres estudios; evidencia de certeza moderada).
No hubo datos suficientes para determinar el efecto del uso de IBP antes de la endoscopia sobre las transfusiones de sangre (dos estudios; no fue posible el metanálisis; evidencia de certeza muy baja) ni el tiempo hasta el alta (un estudio; evidencia de certeza muy baja).
No hubo heterogeneidad sustancial entre los ensayos en ningún análisis.
Conclusiones de los autores
Existe evidencia de certeza moderada de que el tratamiento con IBP iniciado antes de la endoscopia para la hemorragia digestiva alta posiblemente reduzca la necesidad de realizar un tratamiento hemostático endoscópico en el momento de la endoscopia de referencia. Sin embargo, no hay evidencia suficiente para concluir si el tratamiento con IBP antes de la endoscopia aumenta, reduce o no tiene efecto sobre otros desenlaces clínicos, como la mortalidad, las nuevas hemorragias y la necesidad de operar. Se justifica la realización de otros ECA bien diseñados que se ajusten a las normas actuales para el tratamiento hemostático endoscópico y las cointervenciones adecuadas, y que garanticen que los IBP en dosis altas solo se administren a las personas que recibieron tratamiento hemostático endoscópico, independientemente de la asignación al azar inicial. Sin embargo, dado que podría ser poco realista alcanzar el tamaño de información óptimo, los ensayos multicéntricos pragmáticos pueden aportar evidencia valiosa sobre este tema.
PICO
Resumen en términos sencillos
Tratamiento con inhibidores de la bomba de protones iniciado antes de la endoscopia en la hemorragia digestiva alta
Antecedentes
Las hemorragias del esófago (el canal que conecta la garganta con el estómago), el estómago o el duodeno (la primera parte del intestino delgado) son una urgencia médica frecuente. Los estudios de investigación han sugerido que reducir la cantidad de ácido del estómago podría ayudar a controlar la hemorragia, pero se desconoce si es beneficioso comenzar ese tratamiento pronto; es decir, antes de la endoscopia (la exploración del esófago, estómago y duodeno con una cámara de fibra óptica).
Pregunta de la revisión
Se revisó la evidencia sobre el efecto de un tipo de antiácido (inhibidores de la bomba de protones) comparado con ningún tratamiento (placebo) u otro tipo de antiácido (antagonista de los receptores de histamina‐2) comenzado antes de la endoscopia en personas con hemorragia digestiva alta.
Características de los estudios
La evidencia está actualizada hasta junio de 2021. Se incluyeron seis estudios con 2223 participantes. Todos los estudios se llevaron a cabo en un entorno hospitalario e incluyeron participantes con signos clínicos de hemorragia digestiva alta.
Estos estudios proporcionaron datos para los siguientes desenlaces: muerte (cinco estudios, 2143 participantes); nuevas hemorragias digestivas altas (cinco estudios, 2121 participantes); cirugía (seis estudios, 2223 participantes); proporción de participantes con sangrado activo o signos de hemorragia grave reciente en la primera endoscopia (cuatro estudios, 1332 participantes); y necesidad de tratamiento endoscópico (como la inyección de medicamentos o la cauterización de vasos sanguíneos) para la hemorragia (tres estudios, 1983 participantes). Un estudio proporcionó datos sobre el tiempo hasta el alta, y dos estudios comunicaron datos sobre la necesidad de transfusión de sangre.
Resultados clave
Sigue sin saberse si el tratamiento con un inhibidor de la bomba de protones antes de la endoscopia afectó el riesgo de muerte, de nuevas hemorragias, la necesidad de cirugía, la proporción de participantes con hallazgos de hemorragia grave activa o reciente en la primera endoscopia, el tiempo hasta el alta o la necesidad de transfusiones de sangre. Sin embargo, el tratamiento con un inhibidor de la bomba de protones antes de la endoscopia probablemente redujo la necesidad de tratamiento endoscópico de la hemorragia.
Certeza de la evidencia
La certeza (calidad) de la evidencia fue baja a moderada, debido principalmente a las limitaciones en el diseño y la realización de algunos estudios, y a la incapacidad de obtener una estimación precisa del efecto (debido a un número insuficiente de participantes y episodios en los estudios incluidos).
Authors' conclusions
Summary of findings
| Main analysis: proton pump inhibitor treatment compared to H2RA, placebo or no treatment in people with upper gastrointestinal bleeding prior to endoscopic diagnosis | |||||
| Patient or population: people with upper gastrointestinal bleeding prior to endoscopic diagnosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| With control treatment | With PPI treatment | ||||
| Mortality ‐ within 30 days | Study population | OR 1.14 | 2143 | ⊕⊕⊝⊝ | |
| 45 per 1000 | 6 more per 1000 | ||||
| Rebleeding ‐ within 30 days | Study population | OR 0.81 | 2121 | ⊕⊕⊝⊝ | |
| 131 per 1000 | 23 less per 1000 | ||||
| Surgery ‐ within 30 days | Study population | OR 0.91 | 2223 | ⊕⊕⊝⊝ | |
| 77 per 1000 | 6 less per 1000 | ||||
| Proportion of participants with stigmata of recent haemorrhage at index endoscopy | Study population | OR 0.80 | 1332 | ⊕⊕⊝⊝ | |
| 465 per 1000 | 42 less per 1000 | ||||
| Endoscopic haemostatic treatment at index endoscopy | Study population | OR 0.68 | 1983 | ⊕⊕⊕⊝ | |
| 118 per 1000 | 33 less per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aThe certainty of evidence for mortality was rated down one level due to serious study limitations. Two of the five studies had high risk of bias (high risk of performance bias due to lack of blinding; unclear risk of selection bias due to unclear random sequence generation and unclear allocation concealment), and two other studies had unclear risk of bias (unclear risk of selection bias due to unclear allocation concealment). Of note, if by a 'sensitivity approach' the above‐mentioned four studies were excluded, then all the evidence would be derived from one RCT at low risk of bias (Lau 2007): the effect estimate would remain similar, and there would be no study limitations, but the imprecision would further worsen and would be considered very serious (due to very wide 95% CI and only 15 events in total) requiring rating down by two levels for imprecision; therefore, the certainty of evidence would be low with either approach. | |||||
Background
Description of the condition
Upper gastrointestinal (GI) bleeding remains a common reason for emergency hospital admission. It is a major cause of morbidity and mortality and is responsible for considerable medical care costs (Abougergi 2015; Gilbert 1990; Longstreth 1997; Wuerth 2018). The incidence of upper GI bleeding seems to have been decreasing over time. For example, its incidence in the USA decreased from 108 per 100,000 adults per year in 1994 to 78 per 100,000 in 2009 (Abougergi 2015). Still, the direct in‐hospital economic burden of upper GI bleeding in the USA increased from 3300 million US dollars in 1989 to 7600 million US dollars in 2009 (Abougergi 2015). Short‐term mortality (in‐hospital and 30‐day mortality) from upper GI bleeding is currently around 2% (Higham 2002; Paimela 2002; Van Leerdam 2003; Wuerth 2018).
Peptic ulcers are the most frequent cause of acute upper GI bleeding. Western countries accounted for about 50% of all causes of upper GI bleeding in the 1990s and about 30% in 2012 (Laine 1994; Silverstein 1981; Wuerth 2018). Bleeding from oesophageal or gastric varices in people with portal hypertension accounts for about 7% of all cases, although there are large regional differences in incidence (Wuerth 2018).
Most peptic ulcers are due to chronic Helicobacter pylori (H pylori) infection or nonsteroidal anti‐inflammatory drugs (NSAIDs), including aspirin (even in low doses), or both (Lanas 2017). A substantial proportion of peptic ulcers are idiopathic (Ciociola 1999; Wong 2012), while rare causes include such entities as Crohn's disease, cytomegalovirus infection and Zollinger‐Ellison syndrome (Leontiadis 2001; McColl 2009). Interestingly, exposure to large‐scale natural disasters and accommodation in evacuation shelters has been shown to be a risk factor for peptic ulcer bleeding (Kanno 2013; Kanno 2015).
Description of the intervention
Proton pump inhibitors (PPIs) reduce the production of gastric acid. They are prodrugs that are activated by acid and bind covalently to the H+/K+‐ATPase (hydrogen potassium ATPase, the parietal cell enzyme that acts as the gastric proton pump, acidifying the gastric content), thus inhibiting it. PPIs reduce both basal and stimulated gastric acid secretion, resulting in substantially greater inhibition of gastric acid secretion than histamine‐2 receptor antagonists (H2RAs). PPIs are metabolised and inactivated by the hepatic cytochrome P450 system (Huang 2001).
PPIs available as oral formulations include omeprazole, lansoprazole, pantoprazole, rabeprazole, esomeprazole and dexlansoprazole. Depending on the country, some PPIs (such as omeprazole, pantoprazole, lansoprazole and esomeprazole) are also available as intravenous formulations.
PPIs are used in several upper GI disorders, including non‐bleeding peptic ulcers, bleeding peptic ulcers, gastroesophageal reflux disease, Barrett oesophagus, eosinophilic oesophagitis, dyspepsia and (along with antibiotics) H pylori infection. They are also used for the prevention of upper GI ulcers and bleeding for people on antiplatelet drugs or NSAIDs (Ali 2018).
How the intervention might work
Current management of upper GI bleeding includes resuscitation with fluid replacement and possible blood transfusion, management of comorbidities and specific treatments that aim to stop the bleeding, reduce the risk of rebleeding or both. These latter treatments include medications, endoscopic haemostasis, and surgical and radiological interventions, and differ depending on the cause of the upper GI bleeding. With regards to pharmacotherapy, PPIs are recommended for non‐variceal upper GI bleeding, especially for peptic ulcer bleeding (Barkun 2019; NICE 2012), while somatostatin, somatostatin analogues and terlipressin are recommended for variceal bleeding (Garcia‐Tsao 2007; NICE 2012).
The cessation of bleeding from a peptic ulcer or erosion is thought to be impeded by gastric acid by two mechanisms: firstly, through inhibition of clot formation and promotion of clot lysis; and secondly, by ongoing tissue damage (Kolkman 1996). Since PPI therapy inhibits gastric acid secretion and increases intragastric pH, this could facilitate clot formation, stabilise the clot and hasten healing (Barkun 2006). This rationale is based on results of in vitro studies that observed that an increase in intragastric pH to above 6 is required to inactivate pepsin and inhibit fibrinolysis (Green 1978; Low 1980).
All recent guidelines on the management of non‐variceal upper GI bleeding, including those from the Canadian Association of Gastroenterology (Barkun 2019), the American College of Gastroenterology (Laine 2012), and NICE (National Institute for Health and Care Excellence, NICE 2012), have recommended the use of PPIs after endoscopy in people with peptic ulcer bleeding with high‐risk stigmata on endoscopy.
The rationale for using PPIs prior to endoscopy in people with upper GI bleeding is less compelling, given that this is a mixed population that includes people bleeding from causes other than peptic ulcers. PPIs are expected to benefit mainly people with bleeding from peptic ulcers; that is, about half of people presenting with upper GI bleeding. Individuals bleeding from other causes, such as erosive oesophagitis, or gastric or duodenal erosions, may be less likely to benefit from such treatment. People bleeding from varices would be unlikely to benefit. Furthermore, the few RCTs that have addressed this research question have produced uncertain results regarding mortality and rebleeding (Sreedharan 2010). Not surprisingly, recent guidelines have interpreted the evidence variably and have issued diverging recommendations regarding the use of PPIs prior to endoscopy in people with upper GI bleeding, either conditionally recommending it (Barkun 2019; Laine 2012), or recommending against it (NICE 2012).
Why it is important to do this review
This is an update of a systematic review previously published in 2006 (Dorward 2006), and 2010 (Sreedharan 2010), which aims to ascertain the role of PPI therapy initiated prior to endoscopic diagnosis in otherwise uninvestigated upper GI bleeding. Given that the evidence from the previous versions of this review was inconclusive and has been interpreted differently in recent guidelines, we aimed to update the literature search to identify any new studies that could potentially change or strengthen the conclusions of this review. (The previous version of this review was based on a literature search conducted 10 years ago.) Furthermore, this update allowed us to apply the newer Cochrane risk of bias tool and assess the certainty of the evidence with the GRADE approach, which was not a standard feature of older Cochrane reviews.
Objectives
To assess the effects of PPI treatment initiated prior to endoscopy in people with acute upper GI bleeding.
Methods
Criteria for considering studies for this review
Types of studies
RCTs that compared the efficacy of pre‐endoscopic PPI versus placebo or an H2RA were eligible for inclusion in this review. We considered for inclusion published and unpublished studies, full articles and abstracts. We included only studies that evaluated PPI initiated prior to endoscopy upon presentation with upper GI bleeding. We included only RCTs with a parallel design (i.e. we did not include cross‐over trials). Cluster‐randomised trials were not eligible for inclusion.
Types of participants
Trials were eligible for inclusion in the review if they recruited participants admitted to hospital with upper GI bleeding, or inpatients who developed upper GI bleeding after having been admitted for other reasons, or both. The only studies included were those that randomised people with upper GI bleeding before the cause of bleeding was ascertained by endoscopy. The studies could have recruited either all people with upper GI bleeding or people with upper GI bleeding presumed to be of non‐variceal aetiology (i.e. people with suspected variceal bleeding could have been excluded). Within each study, treatment groups had to be treated similarly other than the therapies being compared.
Types of interventions
To be included in the review, the tested regimen had to meet specific criteria. The treatment group had to have received a PPI prior to endoscopy, and the control group had to have received a placebo, an H2RA or no treatment prior to endoscopy. Otherwise, the control group had to have been managed similarly to the active treatment group. The method of delivery of PPI and control treatment included both intravenous and oral administration.
Types of outcome measures
Primary outcomes
The primary outcome measure in this review was all‐cause mortality, defined as any death occurring within 30 days or at the time point closest to 30 days after randomisation.
Secondary outcomes
The secondary outcome measures were:
-
rebleeding within 30 days or at the time point closest to 30 days after randomisation;
-
composite outcome of surgery or transcatheter arterial embolisation (TAE) for continued or recurrent bleeding within 30 days or at the time point closest to 30 days after randomisation;
-
time to discharge, expressed as mean or median with a measure of variability;
-
blood transfusions, expressed as mean or median number of units transfused per participant and standard deviation;
-
proportion of participants with high‐risk stigmata at index (initial) endoscopy (i.e. active spurting, oozing blood, non‐bleeding visible vessel or adherent clot);
-
proportion of participants who received endoscopic haemostatic treatment at index endoscopy;
-
adverse effects.
Of note, in previous versions of the review, we included 'length of hospital stay' as one of the secondary outcomes. We intended to capture length of stay for participants who were discharged. However, we found that some studies did not distinguish between hospital stay ended by death and hospital stay ended by discharge. For this review update, we have used the term 'time to discharge' because it expresses more accurately the outcome we intend to capture.
Search methods for identification of studies
Electronic searches
We searched the following databases in October 2008 for the previous version of this review (Sreedharan 2010), and in April 2018, October 2019, and 03 June 2021 for this update.
-
CENTRAL (via Ovid Evidence‐Based Medicine Reviews Database (EBMR), Issue May, 2021) (Appendix 1).
-
MEDLINE (via Ovid, from 1946 to 03 June 2021) (Appendix 2).
-
Embase (via Ovid, from 1974 to 03 June 2021) (Appendix 3).
We placed no restrictions on the language of publication.
Searching other resources
For the previous versions of this review, we handsearched published abstracts from Digestive Disease Week, United European Gastroenterology Week, American College of Gastroenterology annual meeting, World Congress of Gastroenterology and British Society of Gastroenterology annual meetings (1997 to October 2008). For this update, because Embase now includes proceedings from these conferences (2000 onwards), a handsearch of conference proceedings was unnecessary, and we searched these abstracts through our main electronic search. We contacted authors of trial reports published only as abstracts and asked them to contribute full data sets or completed papers. We handsearched the reference lists of identified articles for further relevant trials.
For the previous version of this review, we searched CINAHL (Cumulative Index to Nursing and Allied Health Literature) (via EBSCO) from 1984 to October 2008. For this update, we did not search CINAHL. Since 2019, CINAHL references for RCTs and quasi‐RCTs have been identified and added to CENTRAL through Cochrane's Centralised Search Service project. CINAHL trial records identified in the backlog were added to CENTRAL in July 2020. Considering all previous searches did not identify any eligible RCTs in CINAHL, searching CINAHL after searching CENTRAL was not deemed to be necessary for this review.
We also searched ClinicalTrials.gov (www.clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/) on 3 June 2021.
The following resources were also searched for the two previous versions of this review.
-
www.controlledtrials.com (no longer active; assessed on Jan 5, 2022)
-
National Cancer Institute (www.cancer.gov/about-cancer).
-
European Organisation for Research and Treatment of Cancer (www.eortc.org/).
-
SWOG Cancer Research Network (www.swog.org/).
-
Canadian Cancer Trials Group (www.ctg.queensu.ca/).
-
CenterWatch (www.centerwatch.com/).
-
National Research Register (NRR).
-
The trials register of the Cochrane Upper GI and Pancreatic Diseases (UGPD) Group (former name of Cochrane Gut).
Members of the Cochrane UGPD Group and experts in the field were contacted and asked to provide details of outstanding clinical trials and any relevant unpublished material.
Data collection and analysis
Selection of studies
Three review authors (TK, YY and GL) independently checked titles and abstracts identified from the search for fulfilment of predefined inclusion criteria. If we could not confidently excluded a study after reading the abstract, we obtained and assessed the full text. We documented the reasons for exclusion, and compiled a list of excluded studies with reasons for exclusion for those studies that: (a) were only excluded after assessing the full text; (b) caused disagreement or uncertainty amongst the assessors; or (c) some readers might have considered to be eligible for inclusion. We discussed any discrepancies about eligibility until we reached consensus. We sought further information from trial authors if it was not clear from the information presented whether the trial met the inclusion criteria.
Data extraction and management
For the 2006 version of this review, three review authors independently extracted data (Aravamuthan Sreedharan (AS), Stephanie Dorward and Grigorios Leontiadis). For the 2010 review update, two review authors independently extracted data (AS and Janet Martin). Review authors collected data using a data extraction form designed in advance. Review authors resolved any discrepancies in data extraction through discussion to reach consensus. In the event of unpublished or missing data from included studies, review authors contacted the study authors to try to obtain the relevant information.
For this review update, two review authors (TK and YY) independently extracted data (from all studies, including studies included in previous versions of the review) in data extraction forms designed in advance. A third review author (GL) checked the data and acted as adjudicator.
In addition to data relating to the predefined outcomes, we also recorded information about the following study characteristics.
-
Setting: single centre versus multicentre.
-
Geographical location.
-
Inclusion and exclusion criteria.
-
Participant characteristics.
-
Baseline comparability of treatment groups.
-
Randomisation process.
-
Blinding of outcome assessors, participants and carers.
-
Details of the PPI and the control treatment: which medication was used, route of administration (oral, intravenous (IV) bolus, IV continuous infusion), dose and duration of treatment.
-
What concomitant treatments were used, especially whether haemostatic endoscopic treatment was applied (which haemostatic modalities were used; for which stigmata was haemostatic endoscopic treatment applied).
-
Adverse effects (were these actively sought or not?).
-
Proportion of participants eventually found to be bleeding from peptic ulcers at index endoscopy.
-
Dropouts with reasons per treatment group.
Assessment of risk of bias in included studies
We assessed the risk of bias of each included study with regards to sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias, using the standard approach described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, hereafter referred to as the Cochrane Handbook). Where a domain was judged to be at unclear risk of bias, we contacted authors of the primary study to provide relevant unpublished or missing data.
It is important to elaborate on our approach to selective outcome reporting. We considered a study to be at low risk of bias for selective outcome reporting if: (a) a preregistered protocol was available, and all outcomes mentioned in the protocol were reported in adequate detail in the final publication; or (b) a preregistered protocol was not available, but the final publication reported all key outcomes expected to have been recorded in any upper GI bleeding study (i.e. mortality, rebleeding and surgery or transcatheter arterial embolisation (TAE)).
For a study that reported all outcomes outlined in a preregistered protocol (or a study without a preregistered protocol that reported all key outcomes), if one of the non‐key outcomes was not reported, the study was still considered to be at overall low risk of bias for selective outcome reporting. However, the fact that this (non‐key) outcome was not reported by one or more studies could potentially undermine our confidence in the results calculated from the remaining studies. Therefore, we carefully assessed if the risk of selective reporting bias for that specific outcome was high enough to undermine our confidence in the results for that outcome.
Measures of treatment effect
Primary outcome
The primary outcome was mortality rate, defined as death from any cause within 30 days of randomisation. We expected dichotomous mortality data, expressed as odds ratios (OR) with 95% confidence intervals (CI).
Secondary outcomes
We expected dichotomous data for secondary outcomes, including rebleeding rate, requirement for surgery or TAE within 30 days and proportion of participants with high‐risk stigmata of recent haemorrhage (active bleeding, non‐bleeding visible vessel or adherent clot). These were also expressed as 'OR' with 95% CI.
We expected continuous data for blood transfusion requirements and intended to extract these as means with standard deviation for each intervention group.
We expected continuous data for time to discharge. However, since these data were expected to be skewed, we decided a priori to present the results narratively and not to perform a meta‐analysis for this outcome. The same approach was used for the outcome of length of stay in previous versions of the review.
Unit of analysis issues
Studies with multiple treatment groups
We decided a priori to include studies with multiple treatment groups in the review, provided the studies satisfied the inclusion criteria. We only included the study arms that received eligible interventions (i.e. PPI for the treatment group and either H2RA, placebo or no treatment for the control group). Any other study arms were excluded from this review.
Other study designs
We did not include cluster‐randomised trials. We also excluded cross‐over trials (this design would have not been meaningful for an acute condition such as upper GI bleeding with outcomes such as mortality that can occur only once).
Dealing with missing data
We requested missing data from study authors. We performed analyses using an intention‐to‐treat approach.
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test and the I2 statistic, along with visual inspection of the forest plots. Heterogeneity was considered statistically significant when the P value was less than 0.10 from the Chi2 test. Heterogeneity was considered substantial when the I2 statistic was greater than 50%. We looked for an explanation for heterogeneity and reported this in the review. We performed prespecified subgroup analyses to assess potential sources of heterogeneity.
Assessment of reporting biases
Published and unpublished studies in all languages were eligible for inclusion in this review. We arranged for non‐English language studies to be translated. We explored publication bias using funnel plots. We intended to assess publication bias statistically using Egger's test (Egger 1997) if 10 or more studies were included per analysis. (With fewer than 10 studies, as in this review, the power of the test is too low to distinguish chance from real asymmetry (Sterne 2011).)
Data synthesis
We performed meta‐analysis using Review Manager 5.3 (Review Manager 2020) with the Mantel‐Haenszel method for dichotomous outcomes and the inverse variance method for continuous outcomes. We used a random‐effects model for all analyses, independent of the assessment of heterogeneity. We used an intention‐to‐treat approach in all analyses.
Subgroup analysis and investigation of heterogeneity
We hypothesised potential reasons for heterogeneity a priori, and assessed heterogeneity for three outcomes (mortality, rebleeding and surgery or TAE), by conducting subgroup analyses for:
-
risk of bias: low risk of bias versus unclear and high risk of bias;
-
geographical location: Asian versus non‐Asian location (Leontiadis 2005a; Leontiadis 2005b);
-
PPI regimen: intravenous versus oral; high‐dose regimen versus non‐high‐dose regimen (high‐dose regimen defined as equivalent to a dose of omeprazole or pantoprazole 80 mg bolus intravenously followed by an intravenous infusion of 8 mg/hour for at least 72 hours);
-
control treatment: H2RA versus placebo or no treatment;
-
haemostatic endoscopic treatment at index endoscopy being reported to have been applied versus no mention or reporting that such treatment was not applied at index endoscopy.
Sensitivity analysis
We performed the following pre‐planned sensitivity analyses.
-
Fixed‐effect model.
-
Risk ratio (RR) as the summary statistic.
-
Restricting the analyses (for mortality, rebleeding and surgery or TAE) to participants who were subsequently (at index endoscopy) found to have peptic ulcer bleeding.
-
Measuring transfusion requirements as a dichotomous outcome: OR for participants requiring transfusion of at least one unit of blood.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach (study limitations (risk of bias), inconsistency, imprecision, indirectness, and publication bias or other concerns) to assess the certainty of the evidence (Schünemann 2020). Two review authors (TK and YY) independently made judgements regarding the certainty of evidence. A third review author (GL) checked these judgements, and, if necessary, we resolved any disagreements by consulting a fourth review author (FT).
We prepared a summary of findings (SoF) table for the main comparison (i.e. PPI versus H2RA, placebo or no treatment), using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the certainty of evidence in footnotes. We included the following six outcomes in the GRADE assessment: mortality, rebleeding within 30 days, surgery/TAE, proportion of participants with stigmata of recent haemorrhage, proportion of participants with active bleeding, and endoscopic haemostatic therapy at index endoscopy.
Results
Description of studies
Results of the search
Search results for previous versions of this review
The initial search strategy used in September 2005 for the CENTRAL, MEDLINE, Embase and CINAHL databases identified 94 articles. When the search was re‐run in October 2008, 33 further articles were identified. Handsearching reference lists from these articles and searching major conference proceedings identified no further trials. Review authors identified no further trials through contacting members of the former Cochrane UGPD Group (now Cochrane Gut), experts in the field of gastroenterology and pharmaceutical companies marketing PPIs. After reviewing the abstracts of the above articles, review authors excluded 69 as clearly not relevant, and three due to inadequate data. The main reason for exclusion was the study was not an RCT. Review authors retrieved the full articles for the remaining 54 trials, and obtained translations for those published in languages other than English. One trial was published only in abstract form (Naumovski 2005). Of these trials, 49 did not meet the eligibility criteria and were excluded because randomisation had taken place after endoscopy, or they had been restricted to peptic ulcer bleeding only.
Search results for the current version of this review
Our updated search strategy was run in April 2018 and yielded 1856 articles. In addition to the six trials included in the previous versions of the review, we reviewed an additional 69 articles as full text. Of these, 16 compared a PPI with control treatment in people with endoscopically‐confirmed peptic ulcer bleeding. We excluded one study which had been retracted due to plagiarism (Phulpoto 2013; it copied another published study that randomised to PPI versus control after endoscopy). We excluded 44 studies which compared different PPI regimens in people with peptic ulcer bleeding or unselected upper GI bleeding. We excluded five that were not RCTs (Liang 2012; Lu 2012; Motiei 2017; Robinson 2017; Songur 2011). Three were post hoc analyses of RCTs that had already been excluded (Lau 2014; Sung 2009b; Theyventhiran 2013b). Therefore, we added no new trials to the 2010 version of this review (Sreedharan 2010). The results of the updated search are shown in Figure 1.
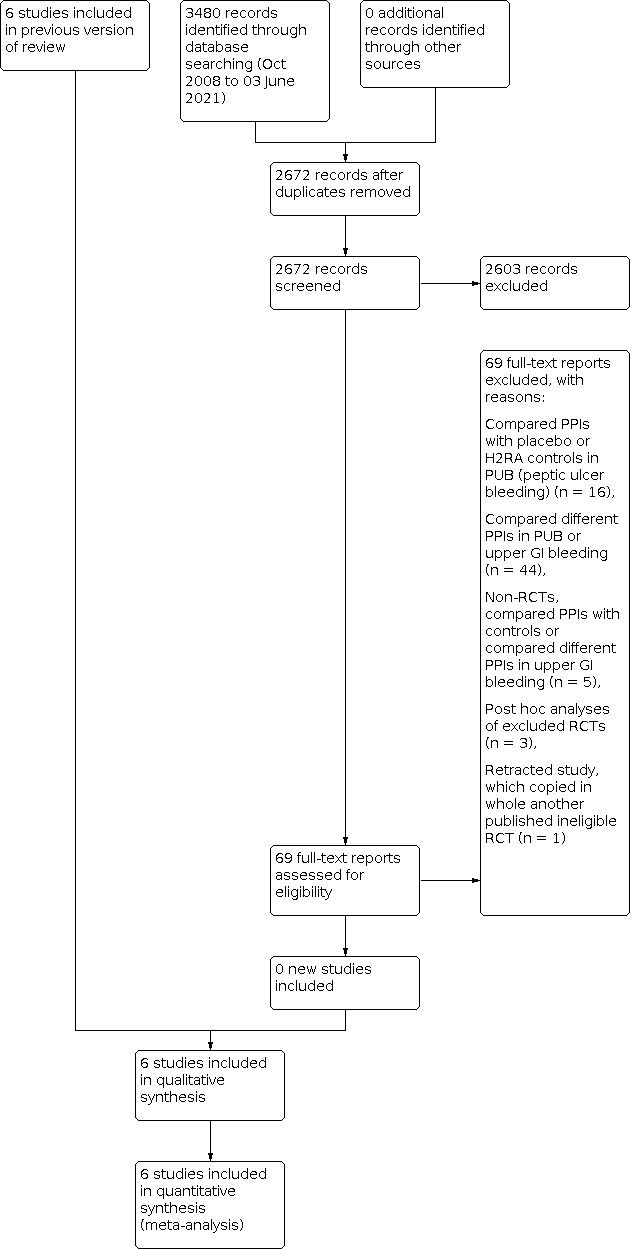
Study flow diagram: review update 03 June 2021
We performed a final update of the search on 23 October 2019 and 3 June 2021. We found no additional eligible trials. In total, we have screened 2672 citations since 2010.
Six trials are included in our systematic review (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Naumovski 2005; Wallner 1996) (see Characteristics of included studies table). Of these, five were full, peer‐reviewed publications (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Wallner 1996), and one was published only as an abstract (Naumovski 2005). Five of the trials were published in English and one in Turkish (Hulagu 1995). The authors of two of the trials provided us with additional information (Hulagu 1995; Lau 2007).
We identified no ongoing studies or studies awaiting classification.
Included studies
Design
All six included studies were RCTs with a parallel group design. We discuss other aspects of trial design in 'Risk of bias in included studies'.
Setting
Four of the studies were conducted in single centres (Hulagu 1995; Lau 2007; Naumovski 2005; Wallner 1996;) and two studies were conducted in two centres (Daneshmend 1992; Hawkey 2001). All studies took place in a hospital setting.
Four trials were conducted in Europe (Daneshmend 1992; Hawkey 2001; Naumovski 2005; Wallner 1996), and two in Asia (Hulagu 1995; Lau 2007) (the Hulagu 1995 trial was conducted in the Asian part of Turkey).
Participants
All trials included participants with clinical signs of upper GI bleeding. Three studies defined these as haematemesis or melaena, or both (Daneshmend 1992; Hulagu 1995; Lau 2007).
The number of participants per trial ranged from 58 (Hulagu 1995), to 1147 (Daneshmend 1992). In one trial (Hawkey 2001), we included only two of the four treatment groups in our analysis: the PPI‐only group and the placebo group. One trial reported stigmata of haemorrhage only for the subgroup of participants with peptic ulcer disease (Lau 2007); hence, these data were not included in the main analysis for this outcome.
The six trials included in the main analyses comprised 2223 participants (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Naumovski 2005; Wallner 1996). Of these, 1114 were randomised to PPI treatment and 1109 to control treatment.
The mean age of participants was reported in three trials: 58.4 years with a standard deviation (SD) of 19.9 years (Hawkey 2001); 59.5 years with SD 19 years (Daneshmend 1992); and 54 years (Naumovski 2005). One trial reported mean age with SD per treatment group (Lau 2007): 61.7 (SD 17.9) in the PPI group and 62.3 (SD 17.5) in the control group. Two trials reported only median age per treatment group: 54 years for the PPI group and 56 years for the control group in one trial (Wallner 1996), and 58.1 years for the PPI group and 49.5 years for the control group in the other trial (Hulagu 1995).
Five studies reported the gender of participants; in all five, there was a male predominance. The male to female ratio was 74/28 (Wallner 1996), 729/418 (Daneshmend 1992), 251/163 (Hawkey 2001), 409/222 (Lau 2007) and 52/28 (Naumovski 2005). We obtained additional information from Hulagu and colleagues: the male‐to‐female ratio was 37/21 (Hulagu 1995).
Daneshmend 1992 specifically excluded participants who developed bleeding after being admitted to hospital for other reasons. It was not clear whether such participants had been excluded from the other studies.
None of the studies was confined to participants with peptic ulcer bleeding, although one study reported outcomes of stigmata of haemorrhage only for participants with peptic ulcer bleeding (Lau 2007). The percentage of participants with peptic ulcer bleeding per trial was as follows: 43.9% (Daneshmend 1992); 37.2% (Hawkey 2001; PPI‐alone and placebo groups combined); 75.5% (Wallner 1996); 59.7% (Lau 2007); and 77.6% (Hulagu 1995). One conference abstract did not mention the percentage of participants with peptic ulcer bleeding (Naumovski 2005).
Three of the studies did not exclude participants with bleeding from oesophageal varices. Such participants comprised: 2.5% of total participants in Daneshmend 1992; 7.1% of total participants in Hawkey 2001 (PPI‐alone and placebo groups combined); and 3.8% in Lau 2007. One other trial aimed to limit the inclusion of such individuals by excluding people with existing hepatic insufficiency (Wallner 1996). In the full report we obtained from Hulagu and colleagues, no participants with oesophageal varices were included (Hulagu 1995). Naumovski 2005 did not state any exclusion of variceal bleeding but mentioned that the only lesions considered were erosive gastritis, and gastric and duodenal ulcers.
Comorbidity was reported in detail only in Lau 2007. Lau and colleagues reported comorbid medical illness per treatment group. Of the 314 participants in the PPI group, 17 had cirrhosis, 32 had cancer, 18 had cardiovascular disease and two had chronic renal failure. In the control group, out of 317 participants, 19 had cirrhosis, 23 had cancer, 25 had cardiovascular disease and three had chronic renal failure. From information stated in the exclusion criteria, we presume that three of the trials did not include severely ill participants (Daneshmend 1992; Hawkey 2001; Hulagu 1995), with terminal illness and malignancy stated as examples. Naumovski 2005 included 11 participants with renal dysfunction and six with hepatic dysfunction (all participants were admitted to the intensive care unit). Wallner 1996 did not include participants with hepatic insufficiency or malignancy. It was not possible to make comparisons of comorbidity amongst the trials.
Two trials reported data on the haemodynamic status of participants on admission (Lau 2007; Wallner 1996). Lau 2007 reported a mean systolic blood pressure of 116.2 (SD 20.4) in the PPI group and 117.3 (SD 21.9) in the placebo group. The number of participants with a systolic blood pressure of less than 90 mm Hg was 30 (9.6%) in the PPI group and 28 (8.8%) in the control group. In Wallner 1996, 8.8% of total participants had haemodynamic shock, defined as systolic blood pressure of less than 80 mm Hg; 29.5% of participants had systolic blood pressure below 100 mm Hg or a heart rate above 100 beats per minute. Two other studies stated the mean and standard deviation of systolic blood pressure and pulse rate per treatment group but did not report the percentage of participants with shock (Daneshmend 1992; Hulagu 1995).
Two trials reported the proportion of participants on NSAIDs per treatment arm (Hawkey 2001; Lau 2007). In Lau 2007, 70 (22.3%) participants in the PPI group and 74 (23.3%) in the placebo group were on NSAIDs. In Hawkey 2001, 35% of all randomised participants used NSAIDs (29% when only the PPI‐alone and placebo arms were considered). Hulagu 1995 reported that 62% of participants used such medications. Lau 2007 specifically excluded long‐term aspirin users with co‐existing cardiovascular comorbidity. (These individuals were included in another concurrent trial.)
None of the trials reported Hpylori status of all randomised participants. Lau 2007 reported 95/178 (53.4%) participants with peptic ulcer disease in the PPI group and 109/182 (59.9%) participants in the placebo group to be positive for H pylori, either by biopsy urease testing or histology.
One study reported the proportion of participants using a PPI or H2RA in the four weeks before admission (Lau 2007). In the PPI group, 38 (12.1%) had taken acid suppressant treatment compared to 40 (12.6%) in the placebo group. One study reported the proportion of participants receiving "ulcer‐healing medication" on admission: 14/55 in the control group and 8/58 in the PPI group (Hawkey 2001).
Lau 2007 reported that 25.5% of participants had previous peptic ulcer disease in the PPI group compared to 25.2% in the placebo group. In the same study, 21.7% of participants in the PPI group had a previous GI bleed compared to 20.8% in the placebo group. Daneshmend 1992 reported that the proportion of participants with previous peptic ulcer disease and previous upper GI bleeding was 26% and 23%, respectively, in the PPI group, compared to 26% and 21%, respectively, in the placebo group. Hulagu 1995 reported that 53% in the PPI group and 46% in the H2RA group had previous peptic ulcer disease.
Baseline comparability of treatment groups
We assessed baseline comparability of the two treatment groups and whether the researchers reported analyses adjusted for prognostic variables in addition to the standard unadjusted analyses. The CONSORT (Consolidated Standards of Reporting Trials) 2010 Statement discourages significance testing for baseline differences in RCTs: "Such hypothesis testing is superfluous and can mislead investigators and their readers. Rather, comparisons at baseline should be based on consideration of the prognostic strength of the variables measured and the size of any chance imbalances that have occurred" (Moher 2010). So as to not to perpetuate the practice of significance testing for baseline differences in RCTs, we did not report such P values in this Cochrane Review even when the original trials had done so.
In the Hulagu 1995 trial, the two treatment groups appeared comparable at baseline with regards to known prognostic factors, except for age, which was higher in the H2RA group (mean 58.1 years) than the PPI group (mean 49.5 years).
Similarly, in the Daneshmend 1992 trial, the treatment groups appeared comparable at baseline with regards to known prognostic factors, except for age: the PPI group "were slightly older" than the placebo group (mean age 60 versus 59 years). Logistic regression analyses were conducted to assess the combined effects of age, initial systolic blood pressure, bleeding site, centre and treatment group on the rates of rebleeding, surgery and death, and authors reported that "relations between treatment groups and rebleeding (p=0.10), operation (p=0.64), or mortality (p=0.33) were not significant". Since adjusted metrics of relative efficacy (such as 'OR' or 'RR') were not reported, we were unable to use adjusted metrics in the meta‐analysis or compare adjusted versus unadjusted metrics.
In the Hawkey 2001 trial, the treatment groups appeared comparable at baseline with regards to known prognostic factors, except for "ulcer healing" medications on admission (more participants had been receiving such medications on admission in the placebo group compared to the PPI group; 14/55 versus 8/58) and the number of participants who were "high risk" as categorised in a non‐standardised way by the admitting team (fewer in the control group compared to the PPI group: 7/55 versus 11/58). Logistic regression analysis was the principal analytical method, with a large number of prognostic factors being included. For the outcome of blood in the stomach (which was the primary outcome of the trial), the adjusted OR was reported numerically (OR 0.22, 95% CI 0.07 to 0.63) and was of slightly larger magnitude in favour of PPI treatment than the unadjusted OR (0.31, 95% CI 01.4 to 0.69). The adjusted ORs for the clinical outcomes were only reported graphically but appeared very similar to the unadjusted results.
In the Lau 2007 trial, the two groups appeared to be comparable for known prognostic factors. No adjustment was performed for prognostic factors.
In the Wallner 1996 trial, the two groups appeared to be comparable for known prognostic factors except for age, which was slightly higher in the H2RA group than the PPI group (median 56 years versus 54 years). No adjustment was performed for prognostic factors.
The Naumovski 2005 trial, which was only available as an abstract, did not report baseline characteristics of the two treatment groups. No adjustment was performed for prognostic factors.
Presence of inclusion and exclusion criteria
All trials had well‐defined inclusion criteria. Five trials also reported exclusion criteria in detail. The sixth trial, published as an abstract only (Naumovski 2005), did not specify any exclusion criteria.
Intervention described in detail
Detailed descriptions of the type, route and method of administration, dose and duration of medication used in both study groups were available for five trials. For the sixth trial (Wallner 1996), the dosing of PPI and control treatment dosing was unclear: three different dosage regimens were used for each group, but it was not clear how participants were allocated to each dosing regimen.
See also Characteristics of included studies tables for details of the interventions.
Interventions
Active treatment
Four trials used intravenous omeprazole as an active treatment (Daneshmend 1992; Hulagu 1995; Lau 2007; Wallner 1996). One study used oral lansoprazole (Hawkey 2001). The final study used intravenous pantoprazole (Naumovski 2005). We describe PPI doses in detail in the Characteristics of included studies table. In summary, the dose during the first few days after study entry was as follows.
-
In Daneshmend 1992: omeprazole 80 mg intravenously (IV) immediately, then 3 doses of 40 mg IV at 8‐hour intervals, then 40 mg orally every 12 hours for 101 hours or until surgery, discharge or death.
-
In Hawkey 2001: lansoprazole 60 mg orally immediately, followed by 30 mg orally plus dummy medication 4 times daily for 4 days or until discharge or until a clinical endpoint occurred.
-
In Hulagu 1995: omeprazole 80 mg IV bolus (over 5 minutes) as soon as possible after admission, followed by 40 mg IV bolus once a day for 6 days.
-
In Lau 2007: intravenous omeprazole 80 mg bolus at randomisation and continuous intravenous infusion at 8 mg/hour until endoscopy. Participants with high‐risk stigmata requiring endoscopic treatment were treated with omeprazole 8 mg/hour infusion for 72 hours. The remaining participants were most likely treated with oral omeprazole 40 mg once daily.
-
In Naumovski 2005: intravenous pantoprazole 80 mg bolus after randomisation and 40 mg intravenously three times per day for 5 days.
-
In Wallner 1996: omeprazole delivered via intravenous bolus; the dosing regimen was unclear: stated as "40 mg" or "80 mg" or "120 mg" (presumably representing total daily doses).
Therefore, only the Lau 2007 trial used the 'high‐dose regimen' (80 mg bolus IV followed by 8 mg/hour) as predefined in the methods of this review (see Subgroup analysis and investigation of heterogeneity). In the Wallner 1996 study, the dose was unclear. In the remaining four studies (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Naumovski 2005), the doses were lower than the predefined 'high‐dose regimen'. However, these were still substantially higher than the 20 mg to 40 mg per day doses that are approved for the treatment of gastro‐oesophageal reflux disease or non‐bleeding peptic ulcer.
The duration of PPI treatment prior to endoscopy was reported for one trial only (Lau 2007): the mean (± SD) duration of infusion before endoscopy was 14.7 ± 6.3 hours in the omeprazole group and 15.2 ± 6.2 hours in the placebo group.
It should be noted that, amongst the six included trials, three different study designs were used regarding who received PPI treatment after the index endoscopy (prior to endoscopy, all six trials had a similar design, comparing PPI versus control). The three study designs were as follows.
Design #1
After endoscopy, regardless of the initial randomisation, PPI was only given to those who received endoscopic haemostatic treatment for peptic ulcers. The Lau 2007 trial followed this design. It compared high‐dose IV PPI (80 mg bolus IV followed by 8 mg/hour) and placebo prior to endoscopy in participants with upper GI bleeding. After endoscopy, PPI infusion (8 mg/hour) was given only to those who received endoscopic haemostatic treatment for peptic ulcer bleeding, regardless of their initial randomisation (i.e. those who had been receiving PPI prior to endoscopy remained on PPI infusion after the endoscopic haemostatic treatment, while those who had been receiving placebo prior to endoscopy were started on PPI infusion after endoscopic haemostatic treatment). It is unclear if participants with peptic ulcers who did not require endoscopic treatment and participants with lesions such as “oesophagitis, gastropathy, duodenitis or erosions” (about 36% of the study population) received 40 mg of PPI orally or no PPI.
Design #2
After endoscopy, all participants remained in their assigned treatment arm for a predetermined period regardless of the timing of endoscopy, the endoscopic findings or the application of endoscopic treatment. Four trials used this design (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Wallner 1996).
-
Daneshmend 1992 and Hawkey 2001 provided the assigned treatments for four days or until discharge or the occurrence of a clinical outcome.
-
Hulagu 1995 provided the assigned treatments for six days.
-
Wallner 1996 provided the assigned treatments for an unspecified period.
Design #3
After endoscopy, all participants received PPI. The Naumovski 2005 trial used this design. Prior to endoscopy, participants were randomised to (a) start treatment immediately (prior to endoscopy) with IV PPI and continue IV PPI treatment after endoscopy for five days, or (b) to have no treatment prior to endoscopy and start IV PPI treatment after endoscopy and continue for five days.
Obviously, each of the three designs answers a separate clinical question. Future guideline panels will need to carefully compare their questions in PICO (Population, Intervention, Comparator, Outcome) format against the designs of the included studies.
Control treatment
Three of the trials used placebo as control treatment (Daneshmend 1992; Hawkey 2001; Lau 2007). Of these, one stated that the placebo was intravenous mannitol (Daneshmend 1992). Two trials compared active treatment to an H2RA: Hulagu 1995 used intravenous ranitidine followed by oral famotidine, while Wallner 1996 used intravenous ranitidine. The remaining study, Naumovski 2005, used no treatment in the control group as the study was designed to compare PPI treatment initiated prior to endoscopy versus PPI treatment initiated after endoscopy. In Naumovski 2005, participants in the control group were treated with IV pantoprazole 40 mg IV bolus after endoscopy followed by 40 mg three times per day for five days. Of note, the Hawkey 2001 trial also randomised participants to two additional treatment arms (four arms in total): tranexamic acid alone and tranexamic acid plus lansoprazole. As mentioned previously, these two treatment arms were not included in our analyses.
See Characteristics of included studies table for details of interventions, including dose and duration of use.
Co‐interventions
Endoscopic haemostatic treatment was both an outcome and a co‐intervention in this review. Endoscopic haemostatic treatment was offered in selected participants in three of the trials (Daneshmend 1992; Hawkey 2001; Lau 2007).
Lau 2007 reported the mean duration between admission and index endoscopy to be 14.7 (SD 6.3) hours in the PPI group and 15.2 (SD 6.2) hours in the placebo group. They administered endoscopic haemostatic treatment with adrenaline injection plus heater probe thermocoagulation to peptic ulcers with active bleeding or non‐bleeding visible vessels. (Adherent clots were irrigated or cheese‐wired, and underlying lesions treated as above.) Variceal bleeding was treated with cyanoacrylate glue (for gastric varices) or variceal ligation (for oesophageal varices). This was the only trial that applied a clearly defined protocol and criteria for endoscopic haemostatic treatment and also the only trial that used a protocol for endoscopic haemostatic treatment that simulates modern (2020) practice for endoscopic therapy. In this study, 60/314 (19.1%) participants underwent endoscopic therapy in the PPI group compared to 90 /317 (28.4%) in the control group.
Daneshmend 1992 performed the index endoscopy "generally within 24 hours of admission", "but gastroenterologists at both hospitals were available on a 24‐hour basis for earlier endoscopy in patients whose clinical condition required it". They noted: "Endoscopic treatment of bleeding such as injection of varices was allowed at the discretion of the endoscopist". In the Results section, Daneshmend and colleagues reported that endoscopic haemostatic treatment included variceal sclerotherapy or "treatment of ulcer bleeding by diathermy, heater probe, or sclerotherapy". Of note, there is some uncertainty about the results of this study with regards to the need for initial endoscopic haemostatic treatment: “Thirty nine patients received endoscopic treatment (15 in omeprazole group, 17 in placebo group)" (page 145). Since 39 is different from the sum of 15 plus 17, one of the three numbers is a typographical or transcription error.
Hawkey 2001 planned to perform the index endoscopy on the morning following admission or earlier if clinically indicated. They reported that endoscopy was performed at a median of 19 hours (interquartile range 11 to 24 hours) after admission. They planned to provide endoscopic haemostatic treatment (adrenaline injection) to participants with active bleeding. However, the final results show that the number of participants with active bleeding and the number of participants who had endoscopic haemostatic treatment were not identical (12 and 10 in the placebo group; 8 and 10 in the PPI group, respectively).
Hulagu 1995 did not mention endoscopic haemostatic treatment in the full‐text publication. However, on 15 October 2005, they provided additional information via email at our request and stated that adrenaline injection was applied in participants "with active bleeding and visible vessel". The number of participants who underwent endoscopic haemostatic treatment was not reported. Hulagu 1995 performed the index endoscopy in all participants within 24 hours of admission (additional information via email communication, 15 October 2005).
The published reports of two trials did not mention endoscopic haemostatic treatment (Naumovski 2005; Wallner 1996); it is unlikely that endoscopic haemostatic treatment had been consistently offered to participants. Wallner 1996 performed the index endoscopy on the first or second day of admission. Naumovski 2005 did not report the timing of endoscopy.
Excluded studies
In the previous version of this review, 49 studies did not meet the eligibility criteria and were excluded. The main reasons for exclusion were randomisation after endoscopy or being restricted to peptic ulcer bleeding.
In the current version of this review, we excluded an additional 69 full‐text studies. The main reasons these studies were ineligible is that they compared different PPI regimens or they performed randomisation after index endoscopy (see Characteristics of excluded studies table).
Risk of bias in included studies
Our risk of bias assessments are summarised in Figure 2 and Figure 3, and described in detail in the risk of bias tables in the Characteristics of included studies. Where we judged the risk of bias to be unclear, we contacted authors of the primary studies to obtain further information.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
Four trials adequately described an acceptable method of sequence generation (Daneshmend 1992; Hawkey 2001; Lau 2007; Wallner 1996). The remaining two trials did not describe the method of sequence generation (Naumovski 2005; Wallner 1996).
Allocation concealment
One trial had adequate concealment of allocation (Lau 2007). The remaining five trials had unclear concealment of allocation (Daneshmend 1992; Hawkey 2001; Hulagu 1994a; Naumovski 2005; Wallner 1996).
Blinding
Three trials were 'doubled‐blinded'; that is, participants, carers and outcome assessors were blinded (Daneshmend 1992; Hawkey 2001; Lau 2007).
One trial was unblinded (Wallner 1996).
One trial did not mention blinding status or measures to ensure blinding in the full‐text publication (Hulagu 1995). In an email, the primary author stated that "patiens [sic] were randomised double‐blindedly [sic]" but mentioned no specific measures to ensure blinding (Hulagu 1995). The PPI and the H2RA groups were distinctly different with regards to preparation (omeprazole was available as dried powder flacon (small bottle with a cap) with a 10 mL solvent, while ranitidine was available as 50 mg/2 ml ampoules) and administration frequency (omeprazole was administered once daily, while ranitidine was administered three times a day). Therefore, without specific measures to ensure blinding (i.e. double dummy methods), it is unlikely that participants and personnel would have been blinded, and we considered the study to be at high risk of performance bias for all outcomes.
The sixth trial (Naumovski 2005), which was only available as a conference abstract, did not report any information about blinding but was a comparison between PPI and no treatment until endoscopy. Given that the control group did not receive placebo treatment, the study was not blinded.
Incomplete outcome data
Five trials described withdrawals and dropouts in detail (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Wallner 1996). We considered these to be at low risk of attrition bias. Of these, Wallner 1996 reported that there were no dropouts. Three trials clearly described the reasons for dropout in each treatment group at each stage of the study (Daneshmend 1992; Hawkey 2001; Lau 2007). All dropouts were assumed to have failed treatment, and the clinical outcomes were analysed on an intention‐to‐treat basis. Hulagu 1995 reported that two participants in the H2RA group did not receive endoscopy at 30 days and were "excluded". However, these two participants were included in the final analysis. The authors did not specify whether the outcomes were imputed or if last observation carried forward (LOCF) was used at 30 days for these two participants.
The remaining trial was an abstract publication (Naumovski 2005). The authors did not provide the disposition of participants at each stage of the study. Therefore, it is difficult to assess the completeness of the data. We considered this study to be at unclear risk of attrition bias.
We have described the withdrawals and dropouts for each study in detail in the risk of bias table under Characteristics of included studies.
Selective reporting
We considered four trials to be at low risk of bias for selective reporting of the key outcomes (mortality, rebleeding and surgery/TAE). Three of these reported all key outcomes (mortality, rebleeding and surgery/TAE) that are expected to have been recorded in all upper GI bleeding studies (Daneshmend 1992; Hawkey 2001; Hulagu 1995). The fourth trial, Lau 2007, measured and reported all outcomes listed in the preregistered protocol (NCT00164866). However, the outcome 'stigmata of recent haemorrhage' was reported only for the subgroup of participants with peptic ulcer bleeding and not for all randomised participants. The absence of these data for all randomised participants precluded the inclusion of this study in the main analysis, which might have influenced the overall results for this outcome.
We considered the two remaining studies to be at high risk of bias for selective reporting. The Naumovski 2005 study was a conference abstract, and a preregistered protocol was not available. Results for mortality were not reported numerically; the authors simply stated that "a difference in mortality rates has not yet been demonstrated". We judged this to be selective under‐reporting (results reported, but not in adequate detail to be included in meta‐analysis). A preregistered protocol was not available for the Wallner 1996 trial. Rebleeding was not reported as an outcome. (The study was designed to assess a different outcome; namely, "cessation of bleeding at day 5" with clinical and laboratory criteria.)
Other potential sources of bias
We considered all studies to be at low risk of bias for other potential sources of bias.
Effects of interventions
Main analysis: all studies
Primary outcome
Mortality ‐ 30 days or at point closest to 30 days
Five trials reported mortality rates per treatment group (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Wallner 1996). One trial did not state the timing of assessment (Wallner 1996). The other trials reported mortality at 40 days (Daneshmend 1992), 30 days (Hawkey 2001; Lau 2007), and at both 6 and 30 days (Hulagu 1995). The sixth trial did not report numerical results on mortality but stated only that "a difference has not been demonstrated" (Naumovski 2005).
The five trials that reported mortality rates per treatment group comprised 1074 participants in the PPI group and 1069 in the control group (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Wallner 1996). Meta‐analysis of these five trials did not rule out a clinically important reduction or a clinically important increase in mortality with PPI treatment (OR 1.14, 95% CI 0.76 to 1.70; Analysis 1.1). Changing to a fixed‐effect model or changing the summary statistic to risk ratio (RR) did not substantially alter the direction, precision or size of the pooled effect. There was no substantial heterogeneity (P = 0.60, I2 = 0%). Unweighted pooled mortality rates were 5.0% for PPI treatment and 4.5% for control treatment.
Of note, in the Daneshmend 1992 study, follow‐up was for a period of 40 days, and 40‐day mortality was reported, although all deaths had occurred within 30 days.
Secondary outcomes
Rebleeding
Rebleeding data per group for all randomised participants could be extracted for five trials (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Naumovski 2005), comprising 1064 participants in the PPI group and 1057 in the control group.
One trial assessed and reported rebleeding at 6 and 30 days (Hulagu 1995); the 30‐day results were included in this analysis. The Lau 2007 study reported rebleeding rates at 30 days. The other three trials that reported rebleeding rates did not clarify the timeframe of rebleeding assessment (Daneshmend 1992; Hawkey 2001; Naumovski 2005). However, in Daneshmend 1992, it was probably 40 days, and in Hawkey 2001, it was probably 30 days.
Rebleeding rates could not be extracted for the Wallner 1996 trial because it was designed to assess the time needed for bleeding cessation. (This trial reported that the time required for bleeding cessation was shorter on omeprazole than control treatment, the difference being "statistically significant".)
Meta‐analysis of the five trials could not rule out a clinically important reduction or a slight increase in rebleeding with PPI treatment (OR 0.81, 95% CI 0.62 to 1.06; Analysis 1.2) (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Naumovski 2005). The results were consistent in sensitivity analyses that used a fixed‐effect model or RR. There was no substantial heterogeneity (P = 0.45, I2 = 0%).
Composite outcome of surgery or transcatheter arterial embolisation (TAE)
All six trials reported surgical intervention rates per group for all randomised participants (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007; Naumovski 2005; Wallner 1996), comprising 1114 in the PPI group and 1109 in the control group. Since no study mentioned if any participants underwent TAE, this composite outcome was probably equal to one of its components, namely surgery. For simplicity, we refer to this outcome as "surgery" in the remainder of the review and the figures.
Two trials reported timing of assessment for surgery (Hawkey 2001; Lau 2007); namely, at 30 days.
Meta‐analysis of all six trials could not exclude a clinically important reduction or a clinically important increase in surgery with PPI treatment (OR 0.91, 95% CI 0.65 to 1.26; Analysis 1.3). Heterogeneity amongst trials was not substantial (P = 0.59, I2 = 0%). The results were consistent in sensitivity analyses that used a fixed‐effect model or RR.
Time to discharge
Four trials reported results on "length of stay" (Daneshmend 1992; Lau 2007; Naumovski 2005; Wallner 1996). However, three of these did not distinguish between hospital stay ended by discharge (= time to discharge) and hospital stay ended by death (i.e. it is unclear if hospital stay ended by death was excluded or not) (Lau 2007; Naumovski 2005; Wallner 1996). Daneshmend 1992 was the only trial that specifically reported time to discharge.
-
In Daneshmend 1992, median time to discharge was five days in the PPI group and six days in the placebo group; no comparative statistics were reported for this result.
-
Lau 2007 reported that PPI treatment reduced "length of hospital stay" (unclear if length of stay ended by death was included in this analysis). Median (range) stay was 3 (1 to 43) days in the PPI group and 3 (1 to 54) days in the placebo group (reported P = 0.003). Mean (SD) stay was 4.5 (5.3) days and 4.9 (5.1) days, respectively (reported P = 0.24). This trial also reported that the proportion of participants with "hospital stay < 3 days" was 60% in the PPI group and 49.2% in the placebo group (RR 1.23, 95% CI 1.07 to 1.42).
-
Wallner 1996 reported "length of hospital stay" (unclear if length of stay ended by death was included in these results). Median (range; SD) stay was 8.0 (3 to 26; 4.8) days in the PPI group and 7.6 (3 to 21; 4.5) days in the H2RA group; no comparative statistics were reported for this result.
-
Naumovski 2005 reported "length of stay in intensive care unit" (unclear if length of stay ended by death was included in these results): 4.6 days in the PPI group versus 7.9 days in the no‐PPI group. It is unclear if the results were reported as mean or median values. No comparative statistics were reported for this result.
We made an a priori decision not to pool the above results by meta‐analysis, since we expected these data to be skewed (i.e. not having a normal distribution). An overall conclusion on the effect of PPI treatment on time to discharge could not be reached, especially since the only study that specifically reported time to discharge did not assess statistically differences between the two groups (Daneshmend 1992).
Blood transfusion
Two trials reported blood transfusions as a continuous outcome; that is, as units of blood transfused (Lau 2007; Naumovski 2005). Lau 2007 reported mean (SD) units of blood transfused in the PPI group to be 1.54 (2.41) compared to 1.88 (3.44) in the placebo group (P = 0.12). Naumovski 2005 reported mean units of blood transfused in the PPI group to be 2.5 compared to 4.2 in the placebo group (P = 0.001). However, SD or other measures of variability were not reported. Therefore, these data could not be pooled by meta‐analysis with the results of the other trials.
Overall, there is very low‐certainty evidence for the effects of pre‐endoscopic PPI treatment on blood transfusions, with four of the six trials not reporting continuous outcomes; one trial at low risk of bias with 631 participants not finding evidence of a difference (Lau 2007); and one trial at high risk of bias with 80 participants (which has not been published in full) reporting a reduction of blood transfusions with PPI treatment (Naumovski 2005).
Sensitivity (alternative) analysis
The other four trials reported the proportions of participants who received blood transfusion in the PPI and control groups as a dichotomous outcome (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Wallner 1996). Each of these trials concluded that there was no evidence of a difference with PPI treatment. In a post hoc analysis, we pooled these four trials, comprising 760 participants in the PPI group and 752 in the control group. Meta‐analysis of these four trials could not exclude a clinically important reduction or a clinically important increase in the proportion of participants who received a blood transfusion with PPI treatment (OR 0.95, 95% CI 0.75 to 1.20; Analysis 1.4). There was no substantial heterogeneity amongst the trials (P = 0.36, I2 = 6.1%). The results were consistent in sensitivity analyses using a fixed‐effect model or RR.
Proportion of participants with stigmata of recent haemorrhage at index endoscopy
Four trials reported the proportion of participants with stigmata of recent haemorrhage (active spurting or oozing, non‐bleeding visible vessel or adherent clot) at index endoscopy per treatment group for all participants (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Wallner 1996). A fifth trial reported this outcome only for the subpopulation of participants with bleeding ulcers (377 participants, i.e. 59.7% of the total study population) (Lau 2007); we included this trial only in a post hoc sensitivity analysis.
Timing of index endoscopy is worth mentioning because it could have influenced this outcome. Of the five above‐mentioned trials, index endoscopy was performed within 24 hours of admission in three (Daneshmend 1992; Hawkey 2001; Hulagu 1995). In one trial (Wallner 1996), index endoscopy was performed within the first 24 to 48 hours of admission. In the final trial (Lau 2007), the mean time between admission and index endoscopy was 14.7 (SD 6.3) hours in the PPI group and 15.2 (SD 6.2) hours in the placebo group.
Naumovski 2005 did not report timing of endoscopy or details on stigmata of recent haemorrhage.
The four trials comprised 672 participants in the PPI arm and 660 in the control arm (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Wallner 1996). Meta‐analysis of these four trials could not exclude a clinically important reduction or a clinically important increase in the proportion of participants with stigmata of recent haemorrhage at index endoscopy (OR 0.80, 95% CI 0.52 to 1.21; Analysis 1.5). There was no substantial heterogeneity amongst the trials (P = 0.20, I2 = 35%). This result was not robust to one of the sensitivity analyses. It was consistent in a sensitivity analysis using RR as the summary statistic (RR 0.91, 95% CI 0.71 to 1.16). However, when we applied a fixed‐effect model, the meta‐analysis suggested that PPI treatment may reduce the proportion of participants with stigmata of recent haemorrhage at index endoscopy (OR 0.67, 95% CI 0.54 to 0.84).
When, by post hoc sensitivity analysis, we included the fifth trial by Lau 2007, which reported index endoscopy stigmata only for participants with peptic ulcer, the meta‐analysis of five trials showed that PPI treatment may reduce the proportion of participants with stigmata of recent haemorrhage at index endoscopy (OR 0.68, 95% CI 0.50 to 0.93; Analysis 1.6). Again, this result was not robust to one of the sensitivity analyses. It was consistent in a sensitivity analysis using a fixed‐effect model. However, when RR was used as the summary statistic, the meta‐analysis could not rule out a clinically important reduction or a slight increase in the proportion of participants with stigmata of recent haemorrhage at index endoscopy with PPI treatment (RR 0.84, 95% CI 0.67 to 1.06).
Proportion of participants with blood in the stomach
Three trials reported the proportion of participants with blood in the stomach at index endoscopy per treatment group (Daneshmend 1992; Hawkey 2001; Hulagu 1995). This was a post hoc analysis in the previous version of this review (Sreedharan 2010), and we retained it in this update. These three trials comprised 622 participants in the PPI group and 608 in the control group. There was substantial heterogeneity amongst the three trials (P = 0.07, I2 = 63%). Meta‐analysis of these three trials could not rule out a clinically important reduction or a clinically important increase in the proportion of participants with blood in the stomach at index endoscopy with PPI treatment (OR 0.64, 95% CI 0.32 to 1.30; Analysis 1.7).
Again, this result was not robust to one of the sensitivity analyses. It was consistent in a sensitivity analysis using RR as the summary statistic. However, when a fixed‐effect model was used, the meta‐analysis suggested that the proportion of participants with blood in the stomach at index endoscopy may be reduced with PPI treatment (OR 0.70, 95% CI 0.54 to 0.91).
Proportion of participants with active bleeding
Four trials reported the proportion of participants with active bleeding (spurting or oozing bleeding) at index endoscopy per treatment group (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Wallner 1996). The trials comprised 672 participants in the PPI arm and 660 in the control arm. Meta‐analysis of these four trials showed that PPI treatment probably reduced the proportion of participants with active bleeding at index endoscopy (i.e. the results probably excluded a clinically important harm and were compatible with no effect or a clinically important benefit: OR 0.74, 95% CI 0.53 to 1.02; Analysis 1.8). There was no substantial heterogeneity amongst the trials (P = 0.52, I2 = 0%). The results were consistent in sensitivity analyses using a fixed‐effect model or RR.
Lau 2007 reported the proportion of participants with active bleeding at index endoscopy only for participants with peptic ulcer disease (377 participants, i.e. 59.7% of the total study population). Therefore, we included it only included in a post hoc sensitivity analysis. This study reported that PPI treatment reduced the proportion of participants with actively bleeding ulcer at index endoscopy (RR 0.44, 95% CI 0.23 to 0.83). When we added this study to the meta‐analysis, the results showed that PPI treatment probably reduced the proportion of participants with active bleeding at index endoscopy (OR 0.67, 95% CI 0.47 to 0.95; without substantial heterogeneity; Analysis 1.9). This result was consistent in sensitivity analyses using a fixed‐effect model or RR.
Need for endoscopic haemostatic treatment at index endoscopy
Three trials reported the proportion of participants who received endoscopic haemostatic treatment at index endoscopy per treatment group (Daneshmend 1992; Hawkey 2001; Lau 2007). These three trials comprised 985 participants in the PPI group and 998 in the control group. Meta‐analysis of these trials showed that the rate of endoscopic haemostatic treatment was probably lower in the PPI group (OR 0.68, 95% CI 0.50 to 0.93; Analysis 1.10). There was no substantial heterogeneity (P = 0.41, I2 = 0%). The results were consistent in sensitivity analyses using a fixed‐effect model or RR.
Lau 2007 also reported the proportion of participants with peptic ulcer bleeding who received endoscopic haemostatic treatment at index endoscopy: 42/187 (22.5%) in the PPI group and 70/190 (36.8%) in the placebo group (RR 0.61, 95% CI 0.44 to 0.84).
Adverse effects
Four trials reported adverse effects related to PPI or control (placebo or H2RA) treatment (Hawkey 2001; Hulagu 1995; Lau 2007; Wallner 1996). Daneshmend 1992 reported adverse effects only for the PPI arm but not for the placebo arm. Naumovski 2005, published in abstract form only, included no mention of adverse effects.
Overall, it was not possible to perform any meaningful meta‐analyses on the adverse effects of PPIs. Below we report the results of the individual studies.
-
Hulagu 1995 reported adverse effects in detail. Adverse effects on omeprazole (active treatment) were fatigue (3%), diarrhoea (7%), vertigo (3%), nausea (7%) and constipation (3%). Adverse effects on ranitidine (control) were dyspepsia (4%), epigastric pain (4%), nausea (4%), constipation (4%) and fatigue (4%).
-
Wallner 1996 stated that no adverse effects were observed in the omeprazole group (active treatment) or ranitidine group (control).
-
Hawkey 2001 stated that “there were no significant differences in the number or pattern of adverse events, of severe adverse events, or of adverse events leading to withdrawal among the four treatment groups”, including lansoprazole (active treatment) and placebo (control), but did not report numerical results.
-
Lau 2007 reported the incidence of serious adverse effects per treatment arm in detail (no differences were found between the two arms) and stated that none of the serious adverse effects was judged by investigators to be related to omeprazole (active treatment) or placebo (control).
-
Daneshmend 1992 reported that there was “no evidence of any toxic effect of omeprazole”.
Overall, these results are suggestive that short‐term pre‐endoscopic PPI treatment is unlikely to cause serious adverse effects. However, given the relatively small overall number of included participants, this systematic review cannot confidently rule out rare serious adverse effects. With regards to non‐serious adverse effects of short‐term pre‐endoscopic PPI treatment, there was no consistent signal found in this systematic review. However, none of the trials reported having used a systematic approach to collect information from the participants about non‐serious adverse effects.
Trial sequential analysis
We performed trial sequential analysis (TSA) for the outcomes of mortality and rebleeding to quantify the statistical reliability of data in the cumulative meta‐analysis, whilst adjusting significance levels for sparse data and repetitive testing on accumulating data (Thorlund 2011). We calculated the required information size (RIS) using the reported outcome rates in the PPI and control arms for a type I error of 0.05 and a type II error of 0.20. TSA showed that the RIS was not reached. The RIS for mortality was 39,852 participants; this review included 2143 participants in the mortality analysis. The RIS for rebleeding was 6,245 participants; this review included 2121 participants in the rebleeding analysis). Furthermore, the TSA monitoring boundaries were not crossed (Figure 4; Figure 5). Therefore, according to the TSA results, the statistical reliability of the data is not sufficient for a firm conclusion about either mortality or rebleeding.
Trial sequential analysis (TSA) for outcome mortality

Trial sequential analysis (TSA) for outcome rebleeding
Subgroup Analyses
Subgroup analysis according to risk of bias
Mortality
Of the five trials that reported mortality rates, three were at low or unclear risk of bias (Daneshmend 1992; Hawkey 2001; Lau 2007). The pooled OR was 1.20 (95% CI 0.79 to 1.84; test for heterogeneity P = 0.38, I2 = 0%). The remaining two trials were at high risk of bias (Hulagu 1995; Wallner 1996). The pooled OR was 0.66 (95% CI 0.18 to 2.46; test for heterogeneity P = 0.60, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.40, I2 = 0%; Analysis 2.1).
Rebleeding
Of the five trials that reported rebleeding rates, three were at low or unclear risk of bias (Daneshmend 1992; Hawkey 2001; Lau 2007). The pooled OR was 0.87 (95% CI 0.65 to 1.16; test for heterogeneity P = 0.51, I2 = 0%). The remaining two trials were at high risk of bias (Hulagu 1995; Naumovski 2005). The pooled OR was 0.45 (95% CI 0.19 to 1.03; test for heterogeneity P = 0.69, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.14, I2 = 48.8%; Analysis 2.2).
Surgery
All six trials reported surgical intervention rates. Three were at low or unclear risk of bias (Daneshmend 1992; Hawkey 2001; Lau 2007). The pooled OR was 0.90 (95% CI 0.64 to 1.27; test for heterogeneity P = 0.59, I2 = 0%). Three trials were at high risk of bias (Hulagu 1995; Naumovski 2005; Wallner 1996). The pooled OR was 0.85 (95% CI 0.22 to 3.37; test for heterogeneity P = 0.18, I2 = 44%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.94, I2 = 0%; Analysis 2.3).
Subgroup analysis according to geographic location
Mortality
Two trials were conducted in Asia (Turkey (Hulagu 1995), and Hong Kong (Lau 2007)). The pooled OR was 1.13 (95% CI 0.43 to 2.96; test for heterogeneity P = 0.89, I2 = 0%). The other three trials that reported mortality rates were conducted in Europe (UK (Daneshmend 1992; Hawkey 2001) and Poland (Wallner 1996)). The pooled OR was 0.96 (95% CI 0.48 to 1.94; test for heterogeneity P = 0.26, I2 = 26%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.79, I2 = 0%; Analysis 3.1).
Rebleeding
For the two trials that were conducted in Asia (Hulagu 1995; Lau 2007), the pooled OR was 1.05 (95% CI 0.45 to 2.40; test for heterogeneity P = 0.28, I2= 13%). For the three European trials (Daneshmend 1992; Hawkey 2001; Wallner 1996), the pooled OR was 0.78 (95% CI 0.59 to 1.04; test for heterogeneity P = 0.37, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.54, I2 = 0%; Analysis 3.2).
Surgery
For the two trials that were conducted in Asia (Hulagu 1995; Lau 2007), the pooled OR was 0.67 (95% CI 0.19 to 2.39). Hulagu 1995 had no events in either treatment arm. Therefore, it did not contribute to the meta‐analysis. The test for heterogeneity was not applicable. For the four European trials (Daneshmend 1992; Hawkey 2001; Naumovski 2005; Wallner 1996), the pooled OR was 1.05 (95% CI 0.45 to 2.40; test for heterogeneity P = 0.43, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.63, I2 = 0%; Analysis 3.3).
Subgroup analysis according to route of PPI administration (oral versus intervenous)
Mortality
Four trials used intravenous PPI treatment (Daneshmend 1992; Hulagu 1995; Lau 2007; Wallner 1996). The pooled OR was 1.21 (95% CI 0.80 to 1.85; test for heterogeneity P = 0.79, I2 = 0%). Hawkey 2001 used oral PPI treatment (OR 0.39, 95% CI 0.07 to 2.07). There was no definitive evidence of a difference between the two subgroups, although there was moderate heterogeneity (test for subgroup differences P = 0.20, I2 = 40%; Analysis 4.1).
Rebleeding
Four trials used intravenous PPI (Daneshmend 1992; Hulagu 1995; Lau 2007; Naumovski 2005). The pooled OR was 0.79 (95% CI 0.55 to 1.13; test for heterogeneity P = 0.33, I2 = 13%). Hawkey 2001 used oral PPI treatment (OR 1.01, 95% CI 0.40 to 2.54). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.62, I2 = 0%; Analysis 4.2).
Surgery
Five trials used intravenous PPI treatment (Daneshmend 1992; Hulagu 1995; Lau 2007; Naumovski 2005; Wallner 1996). The pooled OR was 0.94 (95% CI 0.67 to 1.31; test for heterogeneity P = 0.56, I2 = 0%). Hawkey 2001 used oral PPI treatment (OR 0.49, 95% CI 0.12 to 2.01). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.38, I2 = 0%; Analysis 4.3).
Subgroup analysis according to PPI dose (high‐dose versus non‐high‐dose)
Mortality
One trial, Lau 2007, used high‐dose PPI (predefined as 80 mg bolus followed by 8 mg/hour infusion) while awaiting endoscopy (OR 1.16, 95% CI 0.41 to 3.23). The other four trials that reported mortality rates used non‐high doses of PPI (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Wallner 1996). The pooled OR was 1.13 (95% CI 0.73 to 1.76; test for heterogeneity P = 0.44, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.97, I2 = 0%; Analysis 5.1).
Rebleeding
Lau 2007 used high‐dose PPI prior to endoscopy (OR 1.40, 95% CI 0.56 to 3.53). The other four trials that reported rebleeding rates used non‐high doses of PPI (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Naumovski 2005). The pooled OR was 0.77 (95% CI 0.58 to 1.02; test for heterogeneity P = 0.53, I2 = 0%). There was no definitive evidence of a difference between the two subgroups, although the heterogeneity was moderate (test for subgroup differences P = 0.23, I2 = 31.8%; Analysis 5.2).
Surgery
Lau 2007 used high‐dose PPI prior to endoscopy (OR 0.67, 95% CI 0.19 to 2.39). The other four trials that reported rebleeding rates used non‐high doses of PPI (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Naumovski 2005). The pooled OR was 0.93 (95% CI 0.66 to 1.30; test for heterogeneity P = 0.46, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.63, I2 = 0%; Analysis 5.3).
Subgroup analysis according to control treatment
Mortality
Three trials compared PPI to placebo (Daneshmend 1992; Hawkey 2001; Lau 2007). The pooled OR was 1.20 (95% CI 0.79 to 1.84; test for heterogeneity P = 0.38, I2 = 0%). Two trials compared PPI to an H2RA (Wallner 1996; Hulagu 1995). The pooled OR was 0.66 (95% CI 0.18 to 2.46; test for heterogeneity P = 0.79, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.40, I2 = 0%; Analysis 6.1).
Rebleeding
Three trials compared PPI to placebo (Daneshmend 1992; Hawkey 2001; Lau 2007). The pooled OR was 0.87 (95% CI 0.65 to 1.16; test for heterogeneity P = 0.51, I2 = 0%). Hulagu 1995 compared PPI to an H2RA (OR 0.56, 95% CI 0.14 to 2.26), and Naumovski 2005 compared PPI treatment with no treatment (OR 0.39, 95% CI 0.14 to 1.12). There was no evidence of a difference between the three subgroups (test for subgroup differences P = 0.31, I2 = 14.5%; Analysis 6.2).
Surgery
Three trials compared PPI to placebo (Daneshmend 1992; Hawkey 2001; Lau 2007). The pooled OR was 0.90 (95% CI 0.64 to 1.27; test for heterogeneity P = 0.59, I2 = 0%). Two trials compared PPI to an H2RA (Hulagu 1995; Wallner 1996). The pooled OR was 1.53 (95% CI 0.45 to 5.18). Hulagu 1995 did not contribute to this analysis because it was a zero event trial for both arms. Naumovski 2005 compared PPI treatment with no treatment (OR 0.37, 95% CI 0.07 to 2.02). There was no evidence of a difference between the three subgroups (test for subgroup differences P = 0.41, I2 = 0%; Analysis 6.3).
Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy
The rationale for conducting this subgroup analysis is that, in studies where endoscopic haemostatic treatment is applied during the index endoscopy, the main clinical outcomes (mortality, rebleeding and surgery) are expected to be reduced in both groups (PPI and control group). Therefore, the relative efficacy of pre‐endoscopic PPI treatment would be more difficult to detect. Furthermore, it would provide an estimate of the efficacy of pre‐endoscopic PPI treatment in a subgroup of studies that would not suffer from indirectness (lack of applicability) with regards to their protocol for endoscopic haemostatic treatment.
Mortality
Of the five trials that were included in the analysis of mortality, one did not mention the use of endoscopic haemostatic treatment at index endoscopy (Wallner 1996). This trial showed an OR of 0.60 (95% CI 0.14 to 2.66). In the remaining four trials, endoscopic haemostatic treatment was offered at index endoscopy (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007). The pooled OR was 1.20 (95% CI 0.78 to 1.82; test for heterogeneity P = 0.58, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.38, I2 = 0%; Analysis 7.1).
It is worth reporting the results of the Lau 2007 trial separately because, amongst the four trails that offered endoscopic haemostatic treatment at index endoscopy, it was the only one that applied endoscopic haemostatic treatment that simulates modern (2020) practice for endoscopic therapy (see detailed description in the Included studies section 'Co‐interventions ‐ Endoscopic haemostatic treatment'). Lau 2007 showed an OR of 1.16 (95% CI 0.41 to 3.23). There was no evidence of a subgroup difference between the estimate from Lau 2007 and the pooled estimate from the other three studies (Daneshmend 1992; Hawkey 2001; Hulagu 1995) or four studies (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Wallner 1996).
Rebleeding
Of the five trials included in the analysis of rebleeding, one did not mention the use of endoscopic haemostatic treatment at index endoscopy (Naumovski 2005). This trial showed an OR of 0.39 (95% CI 0.14 to 1.12). In the other four trials, endoscopic haemostatic treatment was offered at index endoscopy (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007). The pooled OR was 0.85 (95% CI 0.65 to 1.13; test for heterogeneity P = 0.64, I2 = 0%). There was no definitive evidence of a difference between the two subgroups, although the heterogeneity was moderate to substantial (test for subgroup differences P = 0.16, I2 = 49.5%; Analysis 7.2).
It is worth reporting the results of the Lau 2007 trial separately because, amongst the four trails that offered endoscopic haemostatic treatment at index endoscopy, it was the only one that applied endoscopic haemostatic treatment that simulates modern (2020) practice for endoscopic therapy (see detailed description in the Included studies section 'Co‐interventions ‐ Endoscopic haemostatic treatment'). Lau 2007 showed an OR of 1.40 (95% CI 0.56 to 3.53). There was no evidence of a subgroup difference between the estimate from Lau 2007 and the pooled estimate from the other three studies (Daneshmend 1992; Hawkey 2001; Hulagu 1995) or four studies (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Naumovski 2005).
Surgery
Two of the six trials did not mention the use of endoscopic haemostatic treatment at index endoscopy (Wallner 1996; Naumovski 2005). The pooled OR was 0.85 (95% CI 0.22 to 3.37; test for heterogeneity P = 0.18, I2 = 44%). In the other four trials, endoscopic haemostatic treatment was offered at index endoscopy (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Lau 2007). The pooled OR was 0.90 (95% CI 0.64 to 1.27; test for heterogeneity P = 0.59, I2 = 0%). There was no evidence of a difference between the two subgroups (test for subgroup differences P = 0.94, I2 = 0%; Analysis 7.3).
It is worth reporting the results of the Lau 2007 trial separately because, amongst the four trails that offered endoscopic haemostatic treatment at index endoscopy, it was the only one that applied endoscopic haemostatic treatment that simulates modern (2020) practice for endoscopic therapy (see detailed description in the Included studies section 'Co‐interventions ‐ Endoscopic haemostatic treatment'). Lau 2007 showed an OR 0.67 (95% CI 0.19 to 2.39). There was no evidence of a subgroup difference between the estimate from Lau 2007 and the pooled estimate from the other three (Daneshmend 1992; Hawkey 2001; Hulagu 1995) or five studies (Daneshmend 1992; Hawkey 2001; Hulagu 1995; Naumovski 2005; Wallner 1996).
Sensitivity analyses
We performed sensitivity analyses using a fixed‐effect model and risk ratio (RR) as the summary statistic in all main analyses. The results are reported in the main results section, along with each main analysis. Analysis of the proportion of participants that received at least one unit of blood transfusion in the PPI and control groups is also shown along with the main analysis for blood transfusion requirements; see Analysis 1.4).
Outcomes for participants with peptic ulcer bleeding
Mortality
Two trials reported mortality rates separately for participants who were found to have peptic ulcer bleeding at index endoscopy (Daneshmend 1992; Wallner 1996). These trials comprised 282 participants in the PPI arm and 298 in the control arm. The pooled OR was 1.39 (95% CI 0.52 to 3.70; test for heterogeneity P = 0.20, I2 = 39%; Analysis 8.1).
The Daneshmend 1992 trial also reported outcomes separately for participants bleeding from gastric and duodenal ulcers. In participants with gastric ulcer bleeding, mortality was 7% in the PPI group and 5% in the control group (OR 0.96, 95% CI 0.32 to 2.84). In participants with bleeding duodenal ulcer, mortality was 11% in the PPI group compared to 5% in the control group (OR 2.35, 95% CI 0.97 to 5.65).
Rebleeding
Two trials reported rebleeding rates separately for participants who were found to have peptic ulcer bleeding at index endoscopy (Daneshmend 1992; Lau 2007). These trials comprised 433 participants in the PPI arm and 447 in the control arm. The pooled OR was 0.86 (95% CI 0.59 to 1.26; test for heterogeneity P = 0.54, I2 = 0%; Analysis 8.2).
The Daneshmend 1992 trial also reported rebleeding rates separately for participants bleeding from gastric and duodenal ulcers. The rebleeding rates were 27% in the PPI group and 25% in the placebo group in participants with bleeding gastric ulcer (OR 0.68, 95% CI 0.48 to 1.14) and 21% in the PPI group and 29% in the placebo group for those with bleeding duodenal ulcer (OR 1.11, 95% CI 0.58 to 2.14).
Surgery
Two trials reported surgical intervention rates separately for participants who were found to have peptic ulcer bleeding at index endoscopy (Daneshmend 1992; Lau 2007). These trials comprised 433 participants in the PPI arm and 447 in the control arm. The pooled OR was 0.91 (95% CI 0.59 to 1.40; test for heterogeneity P = 0.80, I2 = 0%; Analysis 8.3).
The Daneshmend 1992 trial also reported this outcome separately for gastric and duodenal ulcer participants. Amongst participants with bleeding gastric ulcer, 19% in the PPI group underwent surgery versus 17% in the placebo group (OR 0.85, 95% CI 0.48 to 1.48). Amongst participants with bleeding duodenal ulcer, 18% in the PPI group underwent surgery compared to 21% in the placebo group (OR 1.10, 95% CI 0.52 to 2.31).
The Hawkey 2001 trial provided outcomes for participants with peptic ulcer (42.4% of the total study population) but not per treatment group. The rates of rebleeding (13.9%), surgery (6.6%) and death (4.4%) in peptic ulcer participants did not differ substantially from the overall outcomes of this study.
Discussion
Summary of main results
This updated Cochrane Review included six RCTs that assessed the efficacy and safety of PPI treatment initiated before endoscopy in people with upper GI bleeding.
The primary outcome of this review was 30‐day all‐cause mortality. We found low‐certainty evidence that could not rule out either a moderate reduction or a moderate increase in mortality with pre‐endoscopic PPI use (OR 1.14, 95% CI 0.76 to 1.70).
With regards to rebleeding, we found low‐certainty evidence that could not rule out either a moderate reduction or a small increase in rebleeding with pre‐endoscopic PPI (OR 0.81, 95% CI 0.62 to 1.06).
We also found low‐certainty evidence that could not rule out either a moderate reduction or a clinically important increase in surgery with pre‐endoscopic PPI (OR 0.91, 95% CI 0.65 to 1.26).
The effect of pre‐endoscopic PPI treatment on the stigmata of haemorrhage at index endoscopy was first described by Daneshmend and colleagues (Daneshmend 1992). We found low‐certainty evidence that could not rule out either a moderate reduction or a moderate increase in the proportion of participants with high‐risk stigmata of recent haemorrhage at index endoscopy with pre‐endoscopic PPI use (OR 0.80, 95% CI 0.52 to 1.21). However, this was the only main analysis and was not robust to sensitivity analyses. When we applied the fixed‐effect model, or when we included the Lau 2007 trial which reported index endoscopy stigmata only for participants with peptic ulcer (but not for all study participants, as the other trials did), we found low‐certainty evidence that pre‐endoscopic PPIs caused a moderate reduction in the proportion of participants with high‐risk stigmata of recent haemorrhage at index endoscopy.
Finally, we found moderate‐certainty evidence that pre‐endoscopic PPI treatment probably caused a moderate reduction in the need for endoscopic haemostatic treatment at index endoscopy (OR 0.68, 95% CI 0.50 to 0.93).
None of the planned subgroup analyses (according to risk of bias, geographical location, oral versus intravenous administration of PPI, high‐dose versus non‐high‐dose PPI, control treatment being H2RA versus placebo or no treatment, haemostatic endoscopic treatment at index endoscopy) for the outcomes of mortality, rebleeding or surgery showed evidence of a difference between subgroups.
Overall completeness and applicability of evidence
The applicability (internal validity) of the evidence that this review has generated relates inversely to the concept of indirectness, which is one of the domains that determine the certainty of evidence according to the GRADE approach (see Quality of the evidence). Overall, the evidence has addressed the review question well, and the results are generalisable to the intended population. We judged indirectness as not serious for all important outcomes, with the exception of the outcome 'endoscopic haemostatic treatment at index endoscopy' for which the indirectness was moderate (because two of the three included studies used indications and techniques for endoscopic haemostatic treatment that were different from modern practice). Regarding endoscopic haemostatic treatment, the Lau 2007 trial was the only one that used a protocol that simulates modern (2020) practice for endoscopic treatment.
Finally, we described the three different study designs used by the included trials with regards to who received PPI treatment after endoscopy:
-
after endoscopy, regardless of the initial randomisation, PPI was only given to those who received endoscopic haemostatic treatment for peptic ulcers;
-
after endoscopy, all participants remained in their assigned treatment arm for a predetermined period regardless of the timing of endoscopy, the endoscopic findings or the application of endoscopic treatment; or
-
after endoscopy, all participants received PPI.
Each of these three designs answers a different clinical question. Clinicians and future guideline panels will need to carefully compare their questions in PICO (Population, Intervention, Comparator, Outcome) format against the designs of the included studies. Some guideline panels may decide to include all three designs, while others may prefer to include only one or two of these designs to generate evidence.
Quality of the evidence
Using the GRADE approach, we assessed the certainty (quality) of the evidence for the outcomes of mortality, rebleeding, surgery, stigmata of recent haemorrhage at index endoscopy and endoscopic haemostatic treatment at index endoscopy. The summary of findings Table 1 provides detailed descriptions and justifications for downgrading the certainty of the evidence.
In brief, there was low‐certainty evidence for the main outcome (mortality) and the other two important clinical outcomes (rebleeding and surgery). We rated the evidence down due to serious study limitations (risk of bias) and serious imprecision (wide 95% confidence intervals of the pooled effect compatible with no effect as well as clinically important benefit and clinically important harm).
For the (surrogate) outcome of stigmata of recent haemorrhage at index endoscopy, the certainty of evidence was low for similar reasons (serious study limitations and serious imprecision).
For the need for endoscopic haemostatic treatment at index endoscopy (a clinical outcome, the relative importance of which has been rated variably by guideline panels), the certainty of evidence was moderate, due to the combination of moderate study limitations, moderate indirectness and moderate imprecision.
Potential biases in the review process
Before updating this review, we made some changes to the methods that we had previously implemented (see Differences between protocol and review). All changes were aimed at increasing the stringency of the methods in accordance with current methodological standards for Cochrane Reviews. None of the changes had a predictable influence on the magnitude or direction of the results.
Our decision to select mortality as the primary outcome of this review, although none of the included studies had used mortality as their primary outcome, led to analyses that were inadequately powered to rule out a clinically important difference between PPI and control treatment. However, most patients and clinicians would consider mortality to be the most important outcome. Therefore, future guideline panels should anchor their decisions accordingly.
There is potential bias associated with multiple testing, but all analyses were preplanned and correspond to clinically important questions that are repeatedly raised in clinical encounters as well as lectures, workshops and guideline panels. Furthermore, if we had omitted selected preplanned analyses (in an attempt to attenuate multiple testing), we would have also introduced bias. Currently, there is no specific guidance on how to adjust meta‐analyses for multiple testing.
We used a comprehensive search strategy that erred on the side of sensitivity and took the unusual step of having three authors independently assessing titles, abstracts and full‐text articles. We included trials regardless of publication status or language of publication. Therefore, it is unlikely that any other published RCTs were missed. However, we cannot rule out the possibility of publication bias due to trials that remained unpublished, especially since the small number of included trials precluded the application of funnel plots and statistical tests that would look for evidence of publication bias and small‐study effects.
Agreements and disagreements with other studies or reviews
Our literature search did not identify any systematic reviews on this topic published as independent full‐text articles. This was a rather surprising observation, given the large number of narrative (non‐systematic) reviews and editorials published on this topic and the large number of full‐text systematic reviews that have assessed closely‐related questions on post‐endoscopic PPI use.
We identified only one systematic review published as a conference abstract (Healy 2015), and one systematic review included in the publication of a clinical practice guideline (NICE 2012). Both systematic reviews included four RCTs each. The systematic review in the NICE 2012 guideline included the Daneshmend 1992, Hawkey 2001, Wallner 1996 and Lau 2007 trials ‐ four of the six studies included in this Cochrane Review. The Healy 2015 systematic review did not report which four studies it included, but judging from the total number of participants, it is likely it included the three larger of these studies (Daneshmend 1992; Hawkey 2001; Lau 2007).
Both of the previous systematic reviews reached numerical results very similar to those of this Cochrane Review. Healy 2015 reported that "pre endoscopic use of a PPI was not associated with any statistically significant reduction in rates of re‐bleeding (OR 0.87, 95% CI 0.66‐1.15), mortality (OR 1.13, 95% CI 0.75‐16.9) or rates of surgery (OR 0.98, 95% CI 0.70‐1.36) when compared to placebo or H2RA". The systematic review in the NICE 2012 guideline reported a RR of 1.18 (95% CI 0.79 to 1.76) for mortality, 0.89 (95% CI 0.7 to 1.13) for rebleeding, and 0.91 (95% CI 0.67 to 1.24) for surgery.
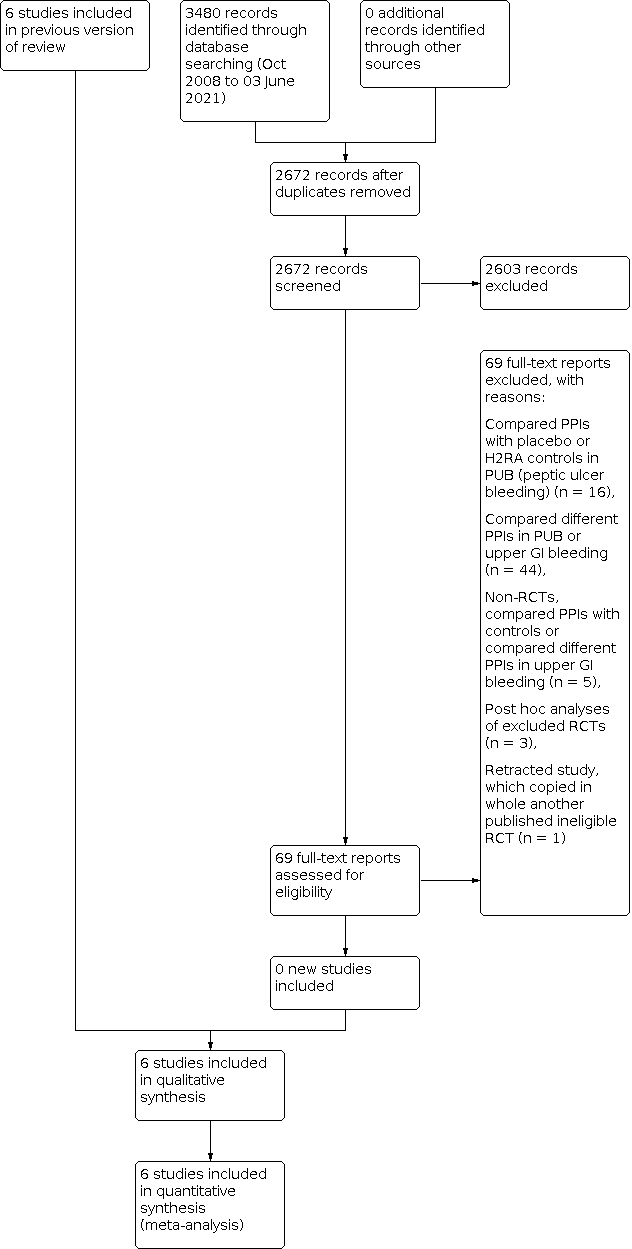
Study flow diagram: review update 03 June 2021

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Trial sequential analysis (TSA) for outcome mortality

Trial sequential analysis (TSA) for outcome rebleeding

Comparison 1: Main analysis, Outcome 1: Mortality

Comparison 1: Main analysis, Outcome 2: Rebleeding

Comparison 1: Main analysis, Outcome 3: Surgery

Comparison 1: Main analysis, Outcome 4: Participants requiring blood transfusion

Comparison 1: Main analysis, Outcome 5: Proportion of participants with stigmata of recent haemorrhage

Comparison 1: Main analysis, Outcome 6: Proportion of participants with stigmata of recent haemorrhage plus Lau 2007

Comparison 1: Main analysis, Outcome 7: Proportion of participants with blood in stomach

Comparison 1: Main analysis, Outcome 8: Proportion of participants with active bleeding

Comparison 1: Main analysis, Outcome 9: Proportion of participants with active bleeding plus Lau 2007

Comparison 1: Main analysis, Outcome 10: Endoscopic haemostatic treatment at index endoscopy

Comparison 2: Subgroup analysis according to risk of bias, Outcome 1: Mortality

Comparison 2: Subgroup analysis according to risk of bias, Outcome 2: Rebleeding

Comparison 2: Subgroup analysis according to risk of bias, Outcome 3: Surgery

Comparison 2: Subgroup analysis according to risk of bias, Outcome 4: Proportion of participants with stigmata of recent haemorrhage

Comparison 2: Subgroup analysis according to risk of bias, Outcome 5: Endoscopic haemostatic treatment at index endoscopy

Comparison 3: Subgroup analysis according to geographic location, Outcome 1: Mortality

Comparison 3: Subgroup analysis according to geographic location, Outcome 2: Rebleeding

Comparison 3: Subgroup analysis according to geographic location, Outcome 3: Surgery

Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 1: Mortality

Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 2: Rebleeding

Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 3: Surgery

Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 1: Mortality

Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 2: Rebleeding

Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 3: Surgery

Comparison 6: Subgroup analysis according to control treatment, Outcome 1: Mortality

Comparison 6: Subgroup analysis according to control treatment, Outcome 2: Rebleeding

Comparison 6: Subgroup analysis according to control treatment, Outcome 3: Surgery

Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 1: Mortality

Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 2: Rebleeding

Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 3: Surgery

Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 1: Mortality

Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 2: Rebleeding

Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 3: Surgery
| Main analysis: proton pump inhibitor treatment compared to H2RA, placebo or no treatment in people with upper gastrointestinal bleeding prior to endoscopic diagnosis | |||||
| Patient or population: people with upper gastrointestinal bleeding prior to endoscopic diagnosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| With control treatment | With PPI treatment | ||||
| Mortality ‐ within 30 days | Study population | OR 1.14 | 2143 | ⊕⊕⊝⊝ | |
| 45 per 1000 | 6 more per 1000 | ||||
| Rebleeding ‐ within 30 days | Study population | OR 0.81 | 2121 | ⊕⊕⊝⊝ | |
| 131 per 1000 | 23 less per 1000 | ||||
| Surgery ‐ within 30 days | Study population | OR 0.91 | 2223 | ⊕⊕⊝⊝ | |
| 77 per 1000 | 6 less per 1000 | ||||
| Proportion of participants with stigmata of recent haemorrhage at index endoscopy | Study population | OR 0.80 | 1332 | ⊕⊕⊝⊝ | |
| 465 per 1000 | 42 less per 1000 | ||||
| Endoscopic haemostatic treatment at index endoscopy | Study population | OR 0.68 | 1983 | ⊕⊕⊕⊝ | |
| 118 per 1000 | 33 less per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aThe certainty of evidence for mortality was rated down one level due to serious study limitations. Two of the five studies had high risk of bias (high risk of performance bias due to lack of blinding; unclear risk of selection bias due to unclear random sequence generation and unclear allocation concealment), and two other studies had unclear risk of bias (unclear risk of selection bias due to unclear allocation concealment). Of note, if by a 'sensitivity approach' the above‐mentioned four studies were excluded, then all the evidence would be derived from one RCT at low risk of bias (Lau 2007): the effect estimate would remain similar, and there would be no study limitations, but the imprecision would further worsen and would be considered very serious (due to very wide 95% CI and only 15 events in total) requiring rating down by two levels for imprecision; therefore, the certainty of evidence would be low with either approach. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality Show forest plot | 5 | 2143 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.77, 1.66] |
| 1.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 1.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 1.4 Participants requiring blood transfusion Show forest plot | 4 | 1512 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.20] |
| 1.5 Proportion of participants with stigmata of recent haemorrhage Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.21] |
| 1.6 Proportion of participants with stigmata of recent haemorrhage plus Lau 2007 Show forest plot | 5 | 1709 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 1.7 Proportion of participants with blood in stomach Show forest plot | 3 | 1230 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.30] |
| 1.8 Proportion of participants with active bleeding Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
| 1.9 Proportion of participants with active bleeding plus Lau 2007 Show forest plot | 5 | 1709 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.95] |
| 1.10 Endoscopic haemostatic treatment at index endoscopy Show forest plot | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 2.1.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.84] |
| 2.1.2 high risk of bias | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.18, 2.46] |
| 2.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 2.2.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.16] |
| 2.2.2 high risk of bias | 2 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.03] |
| 2.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 2.3.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 2.3.2 high risk of bias | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.22, 3.37] |
| 2.4 Proportion of participants with stigmata of recent haemorrhage Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.21] |
| 2.4.1 low or unclear risk of bias | 2 | 1172 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.55] |
| 2.4.2 high risk of bias | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.46, 2.19] |
| 2.5 Endoscopic haemostatic treatment at index endoscopy Show forest plot | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 2.5.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 3.1.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.43, 2.96] |
| 3.1.2 Non‐Asian | 3 | 1454 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.48, 1.94] |
| 3.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 3.2.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.45, 2.40] |
| 3.2.2 Non‐Asian | 3 | 1432 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.59, 1.04] |
| 3.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 3.3.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.39] |
| 3.3.2 Non‐Asian | 4 | 1534 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.66, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 4.1.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.07, 2.07] |
| 4.1.2 Intravenous PPI | 4 | 1938 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.80, 1.85] |
| 4.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 4.2.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.40, 2.54] |
| 4.2.2 Intravenous PPI | 4 | 1916 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.13] |
| 4.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 4.3.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 2.01] |
| 4.3.2 Intravenous PPI | 5 | 2018 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 5.1.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.41, 3.23] |
| 5.1.2 Non‐high dose PPI | 4 | 1512 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.73, 1.76] |
| 5.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 5.2.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.56, 3.53] |
| 5.2.2 Non‐high dose PPI | 4 | 1490 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 5.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 5.3.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.39] |
| 5.3.2 Non‐high dose PPI | 5 | 1592 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.66, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 6.1.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.84] |
| 6.1.2 PPI versus H2RA | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.18, 2.46] |
| 6.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 6.2.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.16] |
| 6.2.2 PPI versus H2RA | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.14, 2.26] |
| 6.2.3 PPI versus no treatment | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.12] |
| 6.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 6.3.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 6.3.2 PPI versus H2RA | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.45, 5.18] |
| 6.3.3 PPI versus no treatment | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.07, 2.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 7.1.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.82] |
| 7.1.2 No mention of endoscopic haemostatic treatment at index endoscopy | 1 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.14, 2.66] |
| 7.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 7.2.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.13] |
| 7.2.2 No mention of endoscopic haemostatic treatment at index endoscopy | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.12] |
| 7.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 7.3.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 7.3.2 No mention of endoscopic haemostatic treatment at index endoscopy | 2 | 182 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.22, 3.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Mortality Show forest plot | 2 | 580 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.52, 3.70] |
| 8.2 Rebleeding Show forest plot | 2 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| 8.3 Surgery Show forest plot | 2 | 880 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.40] |

