Tratamiento con inhibidores de la bomba de protones iniciado antes del diagnóstico endoscópico en la hemorragia digestiva alta
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Study characteristics | ||
| Methods | Large multicentre (two centres) double‐blind RCT | |
| Participants | Country: UK. Included 1147 participants (578 on PPI; 569 on placebo). PU 43.9%; oesophageal varices 2.5% of total participants. Excluded people with "bleeding of such severity that immediate surgery was indicated", bleeding that developed in inpatients, and people on warfarin | |
| Interventions | 1. Omeprazole 80 mg IV immediately, then 3 doses of 40 mg IV at 8‐hour intervals, then 40 mg oral every 12 hours for 101 hours or until surgery, discharge or death 2. Identical regimen with IV and oral placebo (the IV placebo was mannitol) Post‐intervention drug treatment at discretion of physician. Initial endoscopic haemostatic treatment at discretion of endoscopist (administered only to a minority of high‐risk participants) | |
| Outcomes | 40‐day mortality; rebleeding; surgery; stigmata of recent haemorrhage at index endoscopy; time to discharge; number of participants requiring blood transfusion 40‐day mortality, rebleeding and surgery were also reported by peptic ulcer site. | |
| Notes | Only a few of the high‐risk participants with PU received initial endoscopic treatment. Timing of assessment of rebleeding and surgery not clear. There is a typo causing uncertainty about the results for initial endoscopic haemostatic treatment (page 145): “Thirty nine patients received endoscopic treatment (15 in omeprazole group, 17 in placebo group)". 39 is different that the sum of 15 plus 17; therefore, one of the three numbers is a typo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No description of sequence generation was provided by the authors, but given that block randomisation was used ("treatments were randomised in blocks of 10") and the overall conduct quality of the study, we judged that the sequence generation method was probably adequate. |
| Allocation concealment (selection bias) | Unclear risk | No description of the methods applied to ensure allocation concealment |
| Blinding of participants and personnel (performance bias) | Low risk | The authors stated that the study was "double blind" and that mortality assessors were blinded, although they did not specify who else was blinded. The authors also reported that the appearances of study treatment and placebo were identical. We judged that participants and personnel were probably blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | The authors stated that the study was "double blind" and that mortality assessors were blinded (mortality was the predefined main outcome), although they did not specify who else was blinded. The authors also reported that the appearances of study treatment and placebo were identical. Although there is no clear statement for assessors for outcomes other than mortality, we judged that probably all outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Initially 1154 participants were randomised. The authors reported that 4 participants were not given the study treatment (1 in the omeprazole group and 3 in the placebo group), and in 3 participants, the treatment given could not subsequently be clearly identified. Hence, 1147 were "successfully randomised" (578 in the omeprazole group and 569 in the placebo group). The authors provided the reasons for protocol violations in 98 participants (62 participants were prescribed concomitant H2RA, 18 entered the trial for second time, 15 started treatment more than 12 hours after admission, 3 were under age or possibly pregnant). The reasons for protocol violations were not separately reported for each study arm. However, both intention‐to‐treat and per‐protocol analyses were reported, and for each outcome, the results of those two analyses did not differ substantially. Overall, we judged that it is unlikely that incomplete outcome data would have biased the results. |
| Selective reporting (reporting bias) | Low risk | A preregistered protocol is not available, but the authors have reported all the key outcomes (mortality, rebleeding and surgery) that are expected to have been recorded in an upper GI bleeding study. |
| Other bias | Low risk | No evidence of other bias |
| Study characteristics | ||
| Methods | Multicentre (two centres) double‐blind RCT | |
| Participants | Country: UK. 414 participants in total (102 on PPI; 103 on placebo; 103 on tranexamic acid; 106 on tranexamic acid plus PPI). In the PPI and placebo groups combined: peptic ulcer bleeding 37.2%; bleeding from oesophageal varices 7.1%. Excluded "bleeding so severe as to require immediate surgical intervention" | |
| Interventions | 1. Lansoprazole 60 mg orally (stat), followed by 30 mg orally plus dummy medication four times daily for four days or discharge or until a clinical endpoint occurred 2. Placebo ‐ double dummy technique 3. Tranexamic acid 2 g orally (stat), followed by 1 g orally plus dummy medication four times daily for four days or discharge or until a clinical endpoint occurred 4. Tranexamic acid and lansoprazole ‐ both active drugs as above for four days or discharge or until a clinical endpoint occurred. Post‐intervention drug treatment not mentioned. Initial endoscopic haemostatic treatment (adrenaline injection) offered for participants with active bleeding | |
| Outcomes | 30‐day mortality; 30‐day surgery; rebleeding (timing unclear); stigmata of recent haemorrhage at index endoscopy; number of participants requiring blood transfusion | |
| Notes | In meta‐analysis, we included group 1 (lansoprazole alone) as active treatment group and group 2 (placebo) as control group. Participants who received tranexamic acid or the combination of PPI and tranexamic acid were not included in meta‐analysis. Timing of assessment of rebleeding not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No description of sequence generation was provided by the authors, but given that block randomisation was used ("randomised in blocks of 4") and the overall conduct quality of the study, we judged that the sequence generation method was probably adequate. |
| Allocation concealment (selection bias) | Unclear risk | The authors did not report methods of allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | The authors described this study as "double blind, double dummy". Although they did not specifically state who was blinded, it is reasonable to presume that participants and personnel were blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | The authors described this study as "double blind, double dummy". Although they did not specifically state who was blinded, it is reasonable to presume that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Authors provide a clear disposition of participants at each stage after randomisation, with separate results for each of the 4 study arms. Of the 414 participants enrolled, 55 not endoscoped. Of the 298 endoscoped, 61 were not GI bleed. 248 participants were eligible and 50 not eligible for further evaluation due to protocol violations (39 more than 72 hours after start of bleeding, 9 more than 8 hours from 1st dose to endoscopy, 2 participants with no trial data). 20 further participants were not evaluable (14 participants missed 2 or more doses, 4 endoscopy received before trial treatment, 3 prohibited drugs during trial and 1 previously in trial). At each stage after randomisation, the reasons for withdrawal were adequately balanced amongst the 4 treatment arms. Clinical outcomes were analysed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | A preregistered protocol is not available, but the authors have reported all the key outcomes (mortality, rebleeding and surgery) that are expected to have been recorded in an upper GI bleeding study. |
| Other bias | Low risk | No evidence of other bias |
| Study characteristics | ||
| Methods | Single centre, open RCT | |
| Participants | Country: Turkey. Included 58 participants (30 on PPI, 28 on H2RA). Peptic ulcer bleeding 77.6%. Excluded people with "massive bleeding requiring immediate surgery". | |
| Interventions | 1. Omeprazole 80 mg IV bolus (over 5 min) as soon as possible after admission, followed by 40 mg IV bolus once a day for 6 days; then, omeprazole 20 mg orally once a day for 3 weeks | |
| Outcomes | Mortality, rebleeding, stigmata of recent haemorrhage at index endoscopy, number of participants requiring blood transfusion | |
| Notes | This study was published as an abstract in English, and as full text in Turkish. The authors kindly provided a complete translation of the full text in English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors reported no details on sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The authors reported no details on allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | No mention of blinding status or measures to ensure blinding in the full‐text publication. The primary author stated by email that "patiens [sic] were randomised double‐blindedly [sic]" but no specific measures to ensure blinding were mentioned. The PPI and the H2RA were distinctly different with regards to preparation (omeprazole was available as dried powder flacon (small bottle with a cap) with a 10 mL solvent, while ranitidine was available as 50 mg/2 ml ampoules) and administration frequency (omeprazole was administered once daily, while ranitidine was administered 3 times a day). Therefore, without specific measures to ensure blinding (i.e. double dummy methods), it is unlikely that participants and personnel would have been or remained blinded. Thus, we considered the study to be at high risk of performance bias for all outcomes. |
| Blinding of outcome assessment (detection bias) | High risk | The authors did not mention blinding status or measures to ensure blinding. Given that the PPI and the H2RA were distinctly different with regards to preparation and administration frequency, without specific measures to ensure blinding (i.e. double dummy methods), the outcome assessors would have been unblinded.The risk of detection bias is low for the outcome of mortality, but high for all other outcomes (including the outcome of rebleeding, given that the criteria for rebleeding required some subjective judgements). |
| Incomplete outcome data (attrition bias) | Low risk | The authors provided clear disposition for all randomised participants. No participant was lost within the first 6 days (all participants underwent scheduled endoscopy on day 1 and day 6). 20% of the participants in the PPI group were not endoscoped in the 1st month (one participant died of massive upper GI bleeding due to end‐stage liver disease, two participants moved to another city, one participant was operated on for appendicitis, two participants refused repeat endoscopy). 29% of the participants in the H2RA group were not endoscoped in the 1st month (one participant died of pancreatic carcinoma with massive upper GI bleeding, one participant had coronary bypass surgery, one participant had unstable heart disease, 3 participants refused the endoscopy procedure and 2 participants with unknown reasons could not be re‐examined). Athough the authors omitted these participants "from the rest of the study", their detailed reporting allowed us to include them in our intention‐to‐treat analysis (30‐day endoscopy was not expected to influence any of the outcomes of interest of this review). |
| Selective reporting (reporting bias) | Low risk | A preregistered protocol is not available, but the authors have reported all the key outcomes (mortality, rebleeding and surgery) that are expected to have been recorded in an upper GI bleeding study. |
| Other bias | Low risk | No evidence of other bias |
| Study characteristics | ||
| Methods | Single centre, double‐blind RCT | |
| Participants | Setting: Hong Kong. 631 participants randomised (314 in the PPI group and 317 in the placebo group). Peptic ulcer bleeding 59.7%; variceal bleed 3.8%. Excluded long‐term aspirin users (these participants were enrolled in another concurrent trial) and those with refractory shock requiring emergency endoscopy | |
| Interventions | 1. Intravenous omeprazole 80 mg bolus at randomisation and continuous intravenous infusion at 8 mg/hour until endoscopy 2. Intravenous placebo bolus followed by continuous intravenous infusion until endoscopy Participants with high‐risk stigmata requiring endoscopic treatment were transferred to a gastroenterology ward and treated with PPI 8 mg/hour infusion for 72 hours followed by 8 weeks of oral omeprazole 40 mg once daily; (if they were H pylori positive, they were given one‐week triple eradication therapy, followed by 7 weeks of oral omeprazole 40 mg once daily). Participants who did not require endoscopic therapy returned to the general medical ward; their medical treatment after endoscopy was not described clearly, but most likely it was oral omeprazole 40 mg once daily. It was not stated if their treatment after discharge was the same as for the participants discharged from the gastroenterology ward. | |
| Outcomes | 30‐day mortality, rebleeding, surgery, proportion of participants requiring endoscopic therapy, length of hospital stay and mean units of blood transfusion reported according to treatment group. Stigmata of haemorrhage reported only in peptic ulcer participants | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A "computer‐generated list of random numbers in blocks of 20" was used |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered sealed packages were prepared centrally in the pharmacy and sent to the wards with lowest numbered pack to be opened by the resident treating the participant. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and all investigators were blinded to the treatment groups. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants and all investigators were blinded to the treatment groups. |
| Incomplete outcome data (attrition bias) | Low risk | Clear reporting of disposition of participants per study arm at each stage of the study. Only 5/319 (PPI arm) and 2/319 (placebo arm) participants were excluded from the analyses (3 received the wrong diagnosis, 1 had small‐bowel obstruction, 1 had a history of total gastrectomy, 1 had cholangitis, 2 withdrew voluntarily). 2 of the 314 participants did not undergo endoscopy. Of the 319 participants in the placebo group, 2 were excluded from the analysis, 1 because of wrong diagnosis and the other withdrew voluntarily. |
| Selective reporting (reporting bias) | Low risk | The authors have measured and reported all outcomes listed in the pre‐registered protocol (NCT00164866). |
| Other bias | Low risk | No evidence of other bias |
| Study characteristics | ||
| Methods | Single centre, open RCT | |
| Participants | Country: Croatia. Participants with acute upper GI bleeding admitted to intensive care unit. | |
| Interventions | 1. IV pantoprazole 80 mg bolus after randomisation and IV 40 mg three times per day IV for 5 days. 2. The control group received no treatment until endoscopy and thereafter was treated with pantoprazole 40 mg IV bolus followed by IV 40 mg three times per day for 5 days. | |
| Outcomes | Mortality, rebleeding, surgery, length of stay in ICU, mean number of blood units transfused and lesion stabilisation (on repeat endoscopy at 5 days). No mention of follow‐up duration and time of outcome measurement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract publication. The authors did not provide details of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Abstract publication. The authors did not provide details of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Abstract publication. The authors did not mention blinding status or measures to ensure blinding. Given that the control group did not receive placebo treatment, the study was probably non‐blinded. |
| Blinding of outcome assessment (detection bias) | High risk | Abstract publication. The authors did not mention blinding status or measures to ensure blinding. Given that the control group did not receive placebo treatment, the study was probably non‐blinded. The risk of detection bias is low for the outcome of mortality, but high for all other outcomes (including the outcome of rebleeding, given that the criteria for rebleeding required some subjective judgements). |
| Incomplete outcome data (attrition bias) | Unclear risk | Abstract publication. Authors do not provide disposition of participants at each stage of study. Hence it is difficult to assess the completeness of the data. The authors do not report withdrawal or dropout data. |
| Selective reporting (reporting bias) | High risk | Abstract publication. A preregistered protocol is not available. Results for mortality were not reported numerically; the authors simply state that "a difference in mortality rates has not yet been demonstrated". (Selective under‐reporting of data on mortality: reported but with inadequate detail for the data to be included in a meta‐analysis) |
| Other bias | Low risk | No evidence of other bias |
| Study characteristics | ||
| Methods | Single centre, open RCT | |
| Participants | Country: Poland. Included 102 participants (50 on PPI and 52 on placebo). Peptic ulcer bleeding 75.5% of total; no participants with oesophageal varices (hepatic insufficiency was an exclusion criterion). | |
| Interventions | 1. Omeprazole delivered via IV bolus; the dosing regimen is unclear: stated as "40 mg" or "80 mg" or "120 mg" (presumably representing total daily doses) 2. Ranitidine delivered via IV bolus; the dosing regimen is unclear: stated as "150 mg" or "200 mg" or "300‐400 mg" (presumably representing total daily doses) Unclear if participants within each treatment arm were allocated to each dosing group by a random method or not. Duration of treatment depending on continuation of bleeding. Initial endoscopic haemostatic treatment not mentioned. | |
| Outcomes | Mortality; surgery; stigmata of recent haemorrhage at index endoscopy; number of participants requiring blood transfusion. Timing of outcome assessment not clear | |
| Notes | Timing of assessment of rebleeding not clear. Initial endoscopic haemostatic treatment not mentioned. Dosing of pharmacological treatments not clear. Rebleeding rates could not be extracted because the study was designed to assess time needed for bleeding cessation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Series of random odd and even numbers generated by computer |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were reported |
| Blinding of participants and personnel (performance bias) | High risk | The authors described the study as "open". It was not a blinded study. |
| Blinding of outcome assessment (detection bias) | High risk | The authors described the study as "open". It was not a blinded study. The risk of detection bias is low for the outcome of mortality, but high for all other outcomes (including the outcome of rebleeding, given that the criteria for rebleeding required some subjective judgements). |
| Incomplete outcome data (attrition bias) | Low risk | Reported disposition of all randomised participants and outcomes for all participants randomised. No withdrawals or dropouts were observed during the study period. |
| Selective reporting (reporting bias) | High risk | A preregistered protocol is not available. Rebleeding not reported as an outcome (the study was designed to assess cessation of bleeding at day 5 with clinical and laboratory criteria). |
| Other bias | Low risk | No evidence of other bias |
GI: gastrointestinal; H2RA: histamine‐2 receptor antagonist; ICU: intensive care unit; IV: intravenous(ly); min: minute(s); PPI: proton pump inhibitor; PU: peptic ulcer; RCT: randomised controlled trial; stat: 'statim', meaning immediately
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Cost‐effectiveness study. Not an RCT | |
| Not an RCT. Retrospective observation study | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomised controlled trial in participants with peptic ulcer bleeding with outcome being serial gastric pH measurements over 24 hours, in response to treatment with somatostatin, PPI and placebo. | |
| Restricted to participants with bleeding from peptic ulcer and acute gastric mucosal lesions. Randomised after endoscopy | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| RCT comparing oral and IV PPI in non‐variceal upper GI bleeding. Does not satisfy the inclusion criteria of this review | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only. Preliminary data for Sung 2009b | |
| Randomisation post endoscopy. One of the study groups received second look endoscopy with additional endoscopic hemostatic treatment | |
| Limited to peptic ulcer bleeding. Randomisation after endoscopy | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Chen 2012 | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation after endoscopy. Comparison between low‐ and high‐dose IV PPI. Participants with peptic ulcer bleeding and comorbid illness | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Cheng 2009a | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Cheng 2009a | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Cheng 2014 | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Restricted to peptic ulcer bleeding participants only and randomisation after endoscopy | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Chwiesko 2016 | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of upper GI bleeding | |
| Not a randomised controlled trial | |
| Randomised after endoscopy | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of stress ulcer | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Unable to obtain copy of publication | |
| Not an RCT. Letter to the editor | |
| Restricted to endoscopically verified participants and only gastric ulcers, duodenal ulcers and erosions were included | |
| Restricted to peptic ulcer bleeding participants only | |
| Randomised after endoscopy. Restricted to peptic ulcer bleeding participants only | |
| Unclear when randomisation took place | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Control group was not either placebo or H2RA alone; compared omeprazole alone versus the combination of ranitidine and endoscopic haemostatic therapy. Restricted to participants with ulcers or haemorrhagic gastritis. Randomisation post endoscopic diagnosis | |
| Intragastric pH study. Limited to peptic ulcer bleeding | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy in peptic ulcer bleeding | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| RCT on bleeding peptic ulcer participants. Randomised post endoscopic haemostasis | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Not an RCT. Retrospective observation study | |
| Randomisation post endoscopy. RCT comparing oral PPI to endoscopic haemoclipping in peptic ulcer bleeding. Does not satisfy the inclusion criteria of this review | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Post hoc analysis of the 2 PPI versus placebo studies from Hong Kong (Lau and Sung) | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Cost‐effectiveness study | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Lee 2012 | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Not an RCT. Compared PPI regimens | |
| Not an RCT. Letter to the editor | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Restricted to duodenal ulcer participants | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared original versus generic esomeprazole (same regimen) in people with peptic ulcer bleeding | |
| Not an RCT. Compared PPI regimens | |
| Designed to assess healing rates. Did not report any of the prespecified outcomes of this systematic review. Randomisation post endoscopy. | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Not an RCT. Compared different PPIs in people with peptic ulcer bleeding | |
| Restricted to peptic ulcer bleeding participants only. Randomisation post endoscopy | |
| Not an RCT. Retrospective review of oral PPI versus IV PPI in peptic ulcer bleeding post endoscopic haemostasis | |
| Randomisation after endoscopy | |
| The main population of interest was excluded post hoc: people with upper GI bleeding of “peptic origin” were randomised to ranitidine or omeprazole, and all underwent endoscopy within 24 hours, but those with active spurting bleeding and those who underwent endoscopic haemostatic treatment were excluded from the study. | |
| Randomisation post endoscopy; restricted to participants with peptic ulcer bleeding | |
| This paper has been retracted, due to plagiarism (copy of another study) | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Not an RCT. Compared different PPIs in people with peptic ulcer bleeding | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Randomisation after endoscopy; restricted to peptic ulcer bleeding | |
| Not an RCT. Letter to the editor | |
| Restricted to peptic ulcer bleeding participants only. Not an RCT | |
| Restricted to peptic ulcer bleeding participants only; control group not either placebo or H2RA alone; compared two different regimens of PPI | |
| Not an RCT. Compared different PPIs in people with peptic ulcer bleeding | |
| Not an RCT. This article is a comment on another RCT (Khuroo 1997). | |
| Randomisation post endoscopy. Participants with visible vessel and adherent clot | |
| Randomisation post endoscopy. Participants with peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Post hoc analysis for Sung 2009a. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Sung 2014 | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Post hoc analysis of Theyventhiran 2013a, a study that compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Not an RCT. Letter to the editor | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Ucbilek 2015 | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Control group was not either placebo or H2RA alone; compared to intravenous regimens of omeprazole at different doses | |
| Selected participants with bleeding from gastric ulcer, duodenal ulcer, erosions and peptic oesophagitis only. Participants with bleeding from non‐peptic sources were excluded from the analysis. | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared PPI with control in treatment of stress‐induced gastrointestinal bleeding in people with high‐energy multiple fractures. | |
| Randomisation post endoscopic haemostasis. Participants with peptic ulcer bleeding | |
| Unable to gain copy of publication | |
| Randomisation after endoscopy | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only. Preliminary data for Yen 2012 | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Participants with peptic ulcer bleeding with low‐risk stigmata | |
| Randomisation post endoscopy. Compared PPI with control in treatment of peptic ulcer only | |
| Randomisation post endoscopy. Compared different PPI regimens in treatment of peptic ulcer bleeding only | |
| Randomisation post endoscopy. Participants with peptic ulcer bleeding |
GI: gastrointestinal; IV: intravenous(ly); PPI: proton pump inhibitor; PU: peptic ulcer; RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality Show forest plot | 5 | 2143 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.77, 1.66] |
| Analysis 1.1  Comparison 1: Main analysis, Outcome 1: Mortality | ||||
| 1.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| Analysis 1.2  Comparison 1: Main analysis, Outcome 2: Rebleeding | ||||
| 1.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| Analysis 1.3  Comparison 1: Main analysis, Outcome 3: Surgery | ||||
| 1.4 Participants requiring blood transfusion Show forest plot | 4 | 1512 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.20] |
| Analysis 1.4  Comparison 1: Main analysis, Outcome 4: Participants requiring blood transfusion | ||||
| 1.5 Proportion of participants with stigmata of recent haemorrhage Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.21] |
| Analysis 1.5  Comparison 1: Main analysis, Outcome 5: Proportion of participants with stigmata of recent haemorrhage | ||||
| 1.6 Proportion of participants with stigmata of recent haemorrhage plus Lau 2007 Show forest plot | 5 | 1709 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Analysis 1.6  Comparison 1: Main analysis, Outcome 6: Proportion of participants with stigmata of recent haemorrhage plus Lau 2007 | ||||
| 1.7 Proportion of participants with blood in stomach Show forest plot | 3 | 1230 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.30] |
| Analysis 1.7  Comparison 1: Main analysis, Outcome 7: Proportion of participants with blood in stomach | ||||
| 1.8 Proportion of participants with active bleeding Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
| Analysis 1.8  Comparison 1: Main analysis, Outcome 8: Proportion of participants with active bleeding | ||||
| 1.9 Proportion of participants with active bleeding plus Lau 2007 Show forest plot | 5 | 1709 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.95] |
| Analysis 1.9  Comparison 1: Main analysis, Outcome 9: Proportion of participants with active bleeding plus Lau 2007 | ||||
| 1.10 Endoscopic haemostatic treatment at index endoscopy Show forest plot | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Analysis 1.10  Comparison 1: Main analysis, Outcome 10: Endoscopic haemostatic treatment at index endoscopy | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| Analysis 2.1  Comparison 2: Subgroup analysis according to risk of bias, Outcome 1: Mortality | ||||
| 2.1.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.84] |
| 2.1.2 high risk of bias | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.18, 2.46] |
| 2.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| Analysis 2.2  Comparison 2: Subgroup analysis according to risk of bias, Outcome 2: Rebleeding | ||||
| 2.2.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.16] |
| 2.2.2 high risk of bias | 2 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.03] |
| 2.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| Analysis 2.3  Comparison 2: Subgroup analysis according to risk of bias, Outcome 3: Surgery | ||||
| 2.3.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 2.3.2 high risk of bias | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.22, 3.37] |
| 2.4 Proportion of participants with stigmata of recent haemorrhage Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.21] |
| Analysis 2.4  Comparison 2: Subgroup analysis according to risk of bias, Outcome 4: Proportion of participants with stigmata of recent haemorrhage | ||||
| 2.4.1 low or unclear risk of bias | 2 | 1172 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.55] |
| 2.4.2 high risk of bias | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.46, 2.19] |
| 2.5 Endoscopic haemostatic treatment at index endoscopy Show forest plot | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Analysis 2.5  Comparison 2: Subgroup analysis according to risk of bias, Outcome 5: Endoscopic haemostatic treatment at index endoscopy | ||||
| 2.5.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| Analysis 3.1  Comparison 3: Subgroup analysis according to geographic location, Outcome 1: Mortality | ||||
| 3.1.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.43, 2.96] |
| 3.1.2 Non‐Asian | 3 | 1454 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.48, 1.94] |
| 3.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| Analysis 3.2  Comparison 3: Subgroup analysis according to geographic location, Outcome 2: Rebleeding | ||||
| 3.2.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.45, 2.40] |
| 3.2.2 Non‐Asian | 3 | 1432 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.59, 1.04] |
| 3.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| Analysis 3.3  Comparison 3: Subgroup analysis according to geographic location, Outcome 3: Surgery | ||||
| 3.3.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.39] |
| 3.3.2 Non‐Asian | 4 | 1534 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.66, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| Analysis 4.1  Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 1: Mortality | ||||
| 4.1.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.07, 2.07] |
| 4.1.2 Intravenous PPI | 4 | 1938 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.80, 1.85] |
| 4.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| Analysis 4.2  Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 2: Rebleeding | ||||
| 4.2.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.40, 2.54] |
| 4.2.2 Intravenous PPI | 4 | 1916 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.13] |
| 4.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| Analysis 4.3  Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 3: Surgery | ||||
| 4.3.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 2.01] |
| 4.3.2 Intravenous PPI | 5 | 2018 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| Analysis 5.1  Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 1: Mortality | ||||
| 5.1.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.41, 3.23] |
| 5.1.2 Non‐high dose PPI | 4 | 1512 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.73, 1.76] |
| 5.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| Analysis 5.2  Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 2: Rebleeding | ||||
| 5.2.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.56, 3.53] |
| 5.2.2 Non‐high dose PPI | 4 | 1490 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 5.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| Analysis 5.3  Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 3: Surgery | ||||
| 5.3.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.39] |
| 5.3.2 Non‐high dose PPI | 5 | 1592 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.66, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| Analysis 6.1  Comparison 6: Subgroup analysis according to control treatment, Outcome 1: Mortality | ||||
| 6.1.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.84] |
| 6.1.2 PPI versus H2RA | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.18, 2.46] |
| 6.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| Analysis 6.2  Comparison 6: Subgroup analysis according to control treatment, Outcome 2: Rebleeding | ||||
| 6.2.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.16] |
| 6.2.2 PPI versus H2RA | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.14, 2.26] |
| 6.2.3 PPI versus no treatment | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.12] |
| 6.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| Analysis 6.3  Comparison 6: Subgroup analysis according to control treatment, Outcome 3: Surgery | ||||
| 6.3.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 6.3.2 PPI versus H2RA | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.45, 5.18] |
| 6.3.3 PPI versus no treatment | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.07, 2.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| Analysis 7.1  Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 1: Mortality | ||||
| 7.1.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.82] |
| 7.1.2 No mention of endoscopic haemostatic treatment at index endoscopy | 1 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.14, 2.66] |
| 7.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| Analysis 7.2  Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 2: Rebleeding | ||||
| 7.2.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.13] |
| 7.2.2 No mention of endoscopic haemostatic treatment at index endoscopy | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.12] |
| 7.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| Analysis 7.3  Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 3: Surgery | ||||
| 7.3.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 7.3.2 No mention of endoscopic haemostatic treatment at index endoscopy | 2 | 182 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.22, 3.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Mortality Show forest plot | 2 | 580 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.52, 3.70] |
| Analysis 8.1  Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 1: Mortality | ||||
| 8.2 Rebleeding Show forest plot | 2 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| Analysis 8.2  Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 2: Rebleeding | ||||
| 8.3 Surgery Show forest plot | 2 | 880 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.40] |
| Analysis 8.3  Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 3: Surgery | ||||
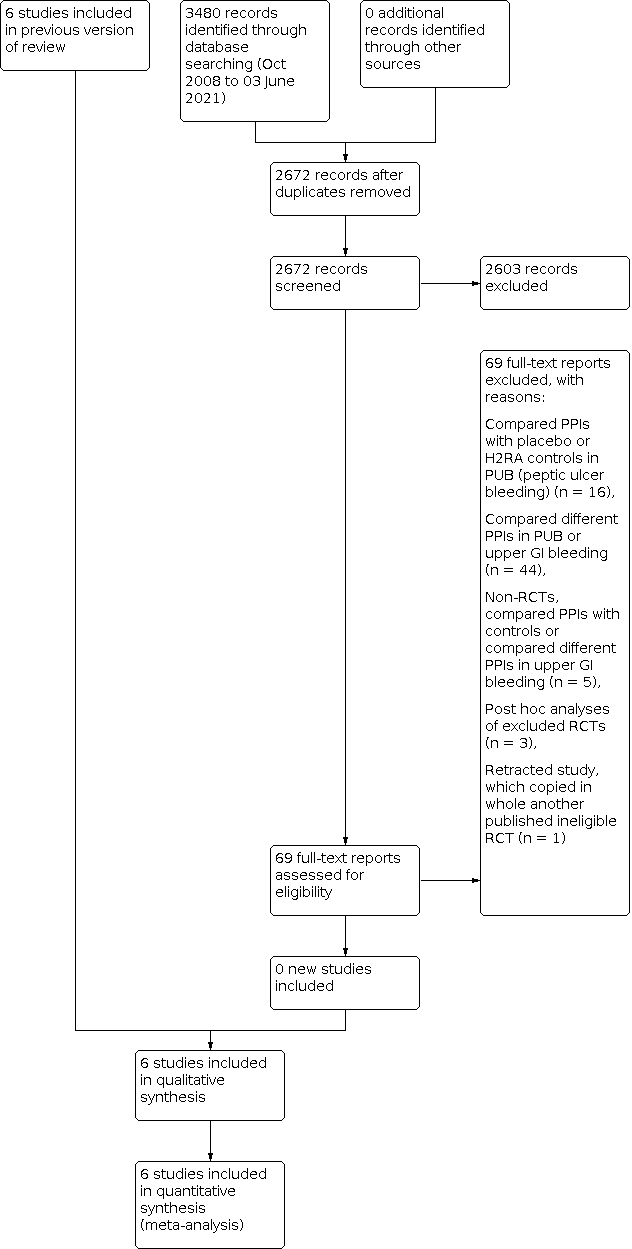
Study flow diagram: review update 03 June 2021

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Trial sequential analysis (TSA) for outcome mortality

Trial sequential analysis (TSA) for outcome rebleeding

Comparison 1: Main analysis, Outcome 1: Mortality

Comparison 1: Main analysis, Outcome 2: Rebleeding

Comparison 1: Main analysis, Outcome 3: Surgery

Comparison 1: Main analysis, Outcome 4: Participants requiring blood transfusion

Comparison 1: Main analysis, Outcome 5: Proportion of participants with stigmata of recent haemorrhage

Comparison 1: Main analysis, Outcome 6: Proportion of participants with stigmata of recent haemorrhage plus Lau 2007

Comparison 1: Main analysis, Outcome 7: Proportion of participants with blood in stomach

Comparison 1: Main analysis, Outcome 8: Proportion of participants with active bleeding

Comparison 1: Main analysis, Outcome 9: Proportion of participants with active bleeding plus Lau 2007

Comparison 1: Main analysis, Outcome 10: Endoscopic haemostatic treatment at index endoscopy

Comparison 2: Subgroup analysis according to risk of bias, Outcome 1: Mortality

Comparison 2: Subgroup analysis according to risk of bias, Outcome 2: Rebleeding

Comparison 2: Subgroup analysis according to risk of bias, Outcome 3: Surgery

Comparison 2: Subgroup analysis according to risk of bias, Outcome 4: Proportion of participants with stigmata of recent haemorrhage

Comparison 2: Subgroup analysis according to risk of bias, Outcome 5: Endoscopic haemostatic treatment at index endoscopy

Comparison 3: Subgroup analysis according to geographic location, Outcome 1: Mortality

Comparison 3: Subgroup analysis according to geographic location, Outcome 2: Rebleeding

Comparison 3: Subgroup analysis according to geographic location, Outcome 3: Surgery

Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 1: Mortality

Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 2: Rebleeding

Comparison 4: Subgroup analysis according to route of PPI administration, Outcome 3: Surgery

Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 1: Mortality

Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 2: Rebleeding

Comparison 5: Subgroup analysis according to PPI dose (high dose versus non‐high dose), Outcome 3: Surgery

Comparison 6: Subgroup analysis according to control treatment, Outcome 1: Mortality

Comparison 6: Subgroup analysis according to control treatment, Outcome 2: Rebleeding

Comparison 6: Subgroup analysis according to control treatment, Outcome 3: Surgery

Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 1: Mortality

Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 2: Rebleeding

Comparison 7: Subgroup analysis according to use of endoscopic haemostatic treatment at index endoscopy, Outcome 3: Surgery

Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 1: Mortality

Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 2: Rebleeding

Comparison 8: Sensitivity analysis restricted to peptic ulcer participants, Outcome 3: Surgery
| Main analysis: proton pump inhibitor treatment compared to H2RA, placebo or no treatment in people with upper gastrointestinal bleeding prior to endoscopic diagnosis | |||||
| Patient or population: people with upper gastrointestinal bleeding prior to endoscopic diagnosis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | |
|---|---|---|---|---|---|
| With control treatment | With PPI treatment | ||||
| Mortality ‐ within 30 days | Study population | OR 1.14 | 2143 | ⊕⊕⊝⊝ | |
| 45 per 1000 | 6 more per 1000 | ||||
| Rebleeding ‐ within 30 days | Study population | OR 0.81 | 2121 | ⊕⊕⊝⊝ | |
| 131 per 1000 | 23 less per 1000 | ||||
| Surgery ‐ within 30 days | Study population | OR 0.91 | 2223 | ⊕⊕⊝⊝ | |
| 77 per 1000 | 6 less per 1000 | ||||
| Proportion of participants with stigmata of recent haemorrhage at index endoscopy | Study population | OR 0.80 | 1332 | ⊕⊕⊝⊝ | |
| 465 per 1000 | 42 less per 1000 | ||||
| Endoscopic haemostatic treatment at index endoscopy | Study population | OR 0.68 | 1983 | ⊕⊕⊕⊝ | |
| 118 per 1000 | 33 less per 1000 | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aThe certainty of evidence for mortality was rated down one level due to serious study limitations. Two of the five studies had high risk of bias (high risk of performance bias due to lack of blinding; unclear risk of selection bias due to unclear random sequence generation and unclear allocation concealment), and two other studies had unclear risk of bias (unclear risk of selection bias due to unclear allocation concealment). Of note, if by a 'sensitivity approach' the above‐mentioned four studies were excluded, then all the evidence would be derived from one RCT at low risk of bias (Lau 2007): the effect estimate would remain similar, and there would be no study limitations, but the imprecision would further worsen and would be considered very serious (due to very wide 95% CI and only 15 events in total) requiring rating down by two levels for imprecision; therefore, the certainty of evidence would be low with either approach. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Mortality Show forest plot | 5 | 2143 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.77, 1.66] |
| 1.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 1.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 1.4 Participants requiring blood transfusion Show forest plot | 4 | 1512 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.75, 1.20] |
| 1.5 Proportion of participants with stigmata of recent haemorrhage Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.21] |
| 1.6 Proportion of participants with stigmata of recent haemorrhage plus Lau 2007 Show forest plot | 5 | 1709 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 1.7 Proportion of participants with blood in stomach Show forest plot | 3 | 1230 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.30] |
| 1.8 Proportion of participants with active bleeding Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.74 [0.53, 1.02] |
| 1.9 Proportion of participants with active bleeding plus Lau 2007 Show forest plot | 5 | 1709 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.47, 0.95] |
| 1.10 Endoscopic haemostatic treatment at index endoscopy Show forest plot | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 2.1.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.84] |
| 2.1.2 high risk of bias | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.18, 2.46] |
| 2.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 2.2.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.16] |
| 2.2.2 high risk of bias | 2 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.03] |
| 2.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 2.3.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 2.3.2 high risk of bias | 3 | 240 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.22, 3.37] |
| 2.4 Proportion of participants with stigmata of recent haemorrhage Show forest plot | 4 | 1332 | Odds Ratio (M‐H, Random, 95% CI) | 0.80 [0.52, 1.21] |
| 2.4.1 low or unclear risk of bias | 2 | 1172 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.55] |
| 2.4.2 high risk of bias | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.46, 2.19] |
| 2.5 Endoscopic haemostatic treatment at index endoscopy Show forest plot | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 2.5.1 low or unclear risk of bias | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 3.1.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.43, 2.96] |
| 3.1.2 Non‐Asian | 3 | 1454 | Odds Ratio (M‐H, Random, 95% CI) | 0.96 [0.48, 1.94] |
| 3.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 3.2.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 1.04 [0.45, 2.40] |
| 3.2.2 Non‐Asian | 3 | 1432 | Odds Ratio (M‐H, Random, 95% CI) | 0.78 [0.59, 1.04] |
| 3.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 3.3.1 Asian | 2 | 689 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.39] |
| 3.3.2 Non‐Asian | 4 | 1534 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.66, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 4.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 4.1.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.07, 2.07] |
| 4.1.2 Intravenous PPI | 4 | 1938 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.80, 1.85] |
| 4.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 4.2.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.40, 2.54] |
| 4.2.2 Intravenous PPI | 4 | 1916 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.13] |
| 4.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 4.3.1 Oral PPI | 1 | 205 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.12, 2.01] |
| 4.3.2 Intravenous PPI | 5 | 2018 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.67, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 5.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 5.1.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.16 [0.41, 3.23] |
| 5.1.2 Non‐high dose PPI | 4 | 1512 | Odds Ratio (M‐H, Random, 95% CI) | 1.13 [0.73, 1.76] |
| 5.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 5.2.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 1.40 [0.56, 3.53] |
| 5.2.2 Non‐high dose PPI | 4 | 1490 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.02] |
| 5.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 5.3.1 High dose PPI | 1 | 631 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.19, 2.39] |
| 5.3.2 Non‐high dose PPI | 5 | 1592 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.66, 1.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 6.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 6.1.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.84] |
| 6.1.2 PPI versus H2RA | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.18, 2.46] |
| 6.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 6.2.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.16] |
| 6.2.2 PPI versus H2RA | 1 | 58 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.14, 2.26] |
| 6.2.3 PPI versus no treatment | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.12] |
| 6.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 6.3.1 PPI versus placebo | 3 | 1983 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 6.3.2 PPI versus H2RA | 2 | 160 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.45, 5.18] |
| 6.3.3 PPI versus no treatment | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.07, 2.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 7.1 Mortality Show forest plot | 5 | 2143 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.76, 1.70] |
| 7.1.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.78, 1.82] |
| 7.1.2 No mention of endoscopic haemostatic treatment at index endoscopy | 1 | 102 | Odds Ratio (M‐H, Random, 95% CI) | 0.60 [0.14, 2.66] |
| 7.2 Rebleeding Show forest plot | 5 | 2121 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 7.2.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.13] |
| 7.2.2 No mention of endoscopic haemostatic treatment at index endoscopy | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 0.39 [0.14, 1.12] |
| 7.3 Surgery Show forest plot | 6 | 2223 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.65, 1.26] |
| 7.3.1 Reported use of endoscopic haemostatic treatment at index endoscopy | 4 | 2041 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.27] |
| 7.3.2 No mention of endoscopic haemostatic treatment at index endoscopy | 2 | 182 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.22, 3.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 8.1 Mortality Show forest plot | 2 | 580 | Odds Ratio (M‐H, Random, 95% CI) | 1.39 [0.52, 3.70] |
| 8.2 Rebleeding Show forest plot | 2 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| 8.3 Surgery Show forest plot | 2 | 880 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.40] |

