Planes de acción con educación del paciente breve para las exacerbaciones de la enfermedad pulmonar obstructiva crónica
Resumen
Antecedentes
Las exacerbaciones de la enfermedad pulmonar obstructiva crónica (EPOC) son un causa principal de la disminución del estado de salud e imponen costos elevados en los sistemas de asistencia sanitaria. Los planes de acción ofrecen una forma de autocuidado que puede indicarse en el consultorio externo para ayudar a los pacientes a que reconozcan y comiencen el tratamiento inicial para las exacerbaciones, y así reducir su repercusión.
Objetivos
Comparar los efectos de un plan de acción para las exacerbaciones de la EPOC a través de un único componente breve de educación del paciente y sin un programa integral de autocuidado versus la atención habitual. Los resultados primarios fueron: el uso de recursos de la asistencia sanitaria, la mortalidad y el uso de medicación. Los resultados secundarios fueron: la calidad de vida relacionada con la salud, la morbilidad psicológica, la función pulmonar y la costo‐efectividad.
Métodos de búsqueda
Se hicieron búsquedas en el registro especializado del Grupo Cochrane de Vías Respiratorias (Cochrane Airways Group Specialised Register) y también en CENTRAL, MEDLINE, Embase y en registros de ensayos clínicos. Las búsquedas están actualizadas hasta noviembre 2015. Se hicieron búsquedas manuales en las listas bibliográficas y se estableció contacto con los autores de los estudios para identificar estudios adicionales.
Criterios de selección
Se incluyeron ensayos controlados aleatorios (ECA) y cuasialeatorios que compararon el uso de un plan de acción versus la atención habitual en pacientes con un diagnóstico clínico de EPOC. Se permitió la inclusión de un único componente corto de educación que haría posible la individualización de los planes de acción según las necesidades de tratamiento y los síntomas de los pacientes con EPOC, así como el apoyo continuo dirigido al uso del plan de acción.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos estándar previstos por la Colaboración Cochrane. Para los metanálisis, se dividieron los estudios en subgrupos con seguimiento a través de llamadas telefónicas para facilitar la administración del plan de acción.
Resultados principales
Esta revisión actualizada incluye dos estudios adicionales (y 976 participantes adicionales), para un total de siete ECA de grupos paralelos y 1550 participantes (66% eran hombres). La media de edad de los participantes fue de 68 años y fue similar entre los estudios. La obstrucción al flujo aéreo fue moderadamente grave en tres estudios y grave en cuatro; el volumen espiratorio forzado en un segundo (VEF1) medio posbroncodilatador fue el 54% del valor teórico, y un 27% de los pacientes eran fumadores actuales. Cuatro estudios prepararon planes de acción individualizados; un estudio, un plan oral; y dos estudios, planes de acción escritos estándar. Todos los estudios administraron educación breve sobre la EPOC; y dos, apoyo continuo para el uso de los planes de acción. El seguimiento fue de 12 meses en cuatro estudios y de seis meses en tres estudios.
En comparación con la atención habitual, un plan de acción con seguimiento telefónico redujo significativamente la tasa combinada de hospitalizaciones y visitas al servicio de urgencias por EPOC en el transcurso de 12 meses, en un estudio con 743 participantes (cociente de tasas [CT] 0,59; intervalo de confianza [IC] del 95%: 0,44 a 0,79; pruebas de alta calidad), pero la tasa de hospitalizaciones sola en este estudio no alcanzó significación estadística (CR 0,69; IC del 95%: 0,47 a 1,01; pruebas de calidad moderada). En 12 meses, los planes de acción disminuyeron significativamente la probabilidad del ingreso al hospital (odds ratio [OR] 0,69; IC del 95%: 0,49 a 0,97; n = 897; dos ECA; pruebas de calidad moderada; número necesario a tratar para un lograr un resultado beneficioso adicional (NNTB) 19 [11 a 201]) y probabilidad de una visita al servicio de urgencias (OR 0,55; IC del 95%: 0,38 a 0,78; n = 897; dos ECA; pruebas de calidad moderada; NNTB en 12 meses 12 [9 a 26]) en comparación con la atención habitual.
Los resultados no mostraron ninguna diferencia significativa en la mortalidad por todas las causas por 12 meses (OR 0,88; IC del 95%: 0,59 a 1,31; n = 1134; cuatro ECA; pruebas de calidad moderada debido al intervalo de confianza amplio). Durante 12 meses, la administración de los corticosteroides orales se aumentó con planes de acción comparados con la atención habitual (diferencia de medias [DM] 0,74 ciclos, IC del 95%: 0,12 a 1,35; n = 200; dos ECA; pruebas de calidad moderada) y la dosis de prednisolona acumulativa fue significativamente mayor (DM 779,0 mg, IC del 95%: 533,2 a 10248; n = 743; un ECA; pruebas de alta calidad). El uso de antibióticos en el grupo de intervención fue mayor que en el grupo de atención habitual (subgrupos por seguimiento telefónico) en el transcurso de 12 meses (DM 2,3 ciclos, IC del 95%: 1,8 a 2,7; n = 943; tres ECA; pruebas de calidad moderada).
El análisis de subgrupos por apoyo continuo al uso de planes de acción fue limitado; los autores de la revisión no observaron ninguna diferencia entre los subgrupos en cuanto a la probabilidad del ingreso al hospital, las visitas al servicio de urgencias ni la mortalidad por todas las causas durante 12 meses. El uso de antibióticos en 12 meses mostró una diferencia significativa entre los subgrupos de los estudios con y sin apoyo continuo.
La puntuación general de la calidad de vida en el St. George's Respiratory Questionnaire (SGRQ) mostró una mejoría pequeña, con planes de acción comparados con la la atención habitual en el término de 12 meses (DM ‐2,8; IC del 95%: ‐0,8 a ‐4,8; n = 1009; tres ECA; pruebas de calidad moderada). Las pruebas de baja calidad no mostraron ningún beneficio para la morbilidad psicológica, medida con la escala Hospital Anxiety and Depression Scale (HADS).
Conclusiones de los autores
El uso de los planes de acción para la exacerbación de la EPOC con un único componente educativo breve, junto con el apoyo continuo para el uso del plan de acción, pero sin un programa integral de autocuidado, reduce la utilización de la asistencia sanitaria hospitalaria y aumenta el tratamiento de las exacerbaciones de la EPOC con corticosteroides y antibióticos. El uso de planes de acción de EPOC en este contexto tiene poca probabilidad de aumentar o reducir la mortalidad. De los resultados de esta revisión, no puede determinarse si el beneficio adicional se deriva del apoyo continuo periódico del uso de un plan de acción.
PICOs
Resumen en términos sencillos
Pregunta de la revisión: ¿Son efectivos para la EPOC los planes de acción con educación breve que ayudan a los pacientes a reconocer y responder al empeoramiento de los síntomas?
Se examinaron las pruebas sobre el efecto de los planes de acción para las exacerbaciones en los pacientes con enfermedad pulmonar obstructiva crónica. Se encontraron siete estudios relevantes. La evidencia recogida en esta revisión está actualizada hasta noviembre 2015.
Antecedentes
La enfermedad pulmonar obstructiva crónica (EPOC) es una enfermedad de las vías respiratorias y una causa frecuente es el tabaquismo. Los pacientes con EPOC a menudo presentan un empeoramiento de los síntomas, conocido como una “exacerbación”, para la cual necesitan tratamiento extra y a veces una estancia en el hospital. Un plan de acción es una guía escrita o hablada que se administra, con una educación breve, a los pacientes con EPOC para ayudarlos a reconocer los síntomas de una exacerbación y para que comiencen a tomar el tratamiento extra con mayor anticipación. Los pacientes pueden mantener los fármacos extra en su domicilio o pueden recibir una prescripción para llevarlas a un farmacéutico. Un profesional de la salud hará en ocasiones llamadas telefónicas regulares para ayudar a los pacientes a que usen el plan de acción. Se realizó esta revisión para determinar si contar con un plan de acción para las exacerbaciones de la EPOC mejora la salud y reduce las visitas al hospital.
Características de los estudios
Se encontraron siete estudios relevantes de 1550 pacientes con EPOC. No se incluyeron estudios que administraron otros tratamientos, como un programa de ejercicios o sesiones educativas más largas, junto con un plan de acción. Los pacientes de tres estudios tuvieron apoyo continuo para ayudarles a usar el plan de acción. Los pacientes incluidos en los estudios presentaban síntomas de moderados a severos y tuvieron un seguimiento de seis o 12 meses.
Resultados clave
A los pacientes con EPOC que se les da un plan de acción tienen menos visitas al servicio de urgencias y estancias en el hospital relacionadas con los problemas respiratorios durante un año. Se calcula que por cada 19 pacientes que reciben un plan de acción, un paciente evitaría una estancia hospitalaria para una exacerbación.
Los pacientes con un plan de acción tomaron más corticosteroides y antibióticos para las exacerbaciones (en promedio, apenas algo menos de un ciclo más de corticosteroides y dos ciclos de antibióticos más durante un año).
Algunos estudios demostraron que dar a los pacientes un plan de acción mejoraba la capacidad de reconocer y comenzar por sí solos el tratamiento para los síntomas de empeoramiento de la EPOC.
El plan de acción no logró ningún cambio en las perspectivas de muertes por cualquier causa durante un año, pero hubo cierta variabilidad en este resultado.
No podría precisarse si las llamadas telefónicas de seguimiento agregaron algún beneficio sobre el seguimiento del plan de acción solo.
Calidad de la evidencia
Las pruebas de esta revisión son generalmente independientes y fiables, y los autores están muy o moderadamente seguros de los resultados.
Conclusiones
Se piensa que a los pacientes con EPOC debe dárseles un plan de acción individualizado con un componente educativo corto para que puedan beneficiarse con menos y más breves estancias en el hospital, una mejor comprensión de la necesidad de comenzar el tratamiento por sí solos y el uso apropiado de la medicación para las exacerbaciones.
Conclusiones de los autores
Summary of findings
| Do action plans improve patient outcomes in acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: individuals with exacerbations of chronic obstructive pulmonary disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with action plan | |||||
| Hospitalisations for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.69 | 743 | ⊕⊕⊕⊝ | |||
| Hospitalisations and emergency visits for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.59 | 743 | ⊕⊕⊕⊕ | |||
| At least 1 hospital admission | 209 per 1000 | 154 per 1000 | Odds ratio 0.69 | 897 | ⊕⊕⊕⊝ | |
| Mortality (all‐cause) | 103 per 1000 | 91 per 1000 | Odds ratio 0.88 | 1134 | ⊕⊕⊕⊝ | |
| Courses of oral corticosteroids | Mean courses of oral corticosteroids were 1.05 | Mean courses of oral corticosteroids in the intervention group were 0.74 more (0.12 more to 1.35 more) | ‐ | 200 | ⊕⊕⊕⊝ | |
| Courses of antibiotics | Mean courses of antibiotics ranged from 1.6 to 3.2 | Mean courses of antibiotics in the intervention group were 2.26 more (1.82 more to 2.7 more) | ‐ | 943 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Respiratory‐related quality of life: SGRQ overall score | Mean respiratory‐related quality of life: SGRQ overall score ranged from ‐2 to +6 units | Mean respiratory‐related quality of life: SGRQ overall score in the intervention group was 2.82 units lower (0.83 lower to 4.81 lower) | ‐ | 1009 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Depression score | Mean depression score was ‐0.04 | Mean depression score in the intervention group was 0.25 lower (1.14 lower to 0.64 higher) | ‐ | 154 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWide confidence interval; effect size includes null. bUnclear risk of bias for two studies for allocation and blinding of assessors. cUnclear risk of bias for three studies for allocation and blinding of assessors. dUnclear risk of bias for one study for allocation and blinding of assessors. | ||||||
Antecedentes
Descripción de la afección
La enfermedad pulmonar obstructiva crónica (EPOC) es una enfermedad sistémica, progresiva y heterogénea de significativa importancia para la salud pública global. La EPOC se asocia con una respuesta inflamatoria crónica adquirida, que es el resultado de la exposición continua a la inhalación de partículas nocivas (GOLD 2016; Hogg 2004). Esta respuesta inflamatoria puede inducir la destrucción del parénquima pulmonar y puede interrumpir la reparación normal y los mecanismos de defensa (GOLD 2016). Estas alteraciones anatomopatológicas llevan a la limitación característica al flujo aéreo progresivo, que no es reversible en su totalidad (GOLD 2016).
La EPOC se desarrolla a partir de una combinación de factores genéticos y ambientales y está vinculada más comúnmente al tabaquismo (Halbert 2006). Además del consumo de cigarrillos, la exposición a madera quemada y otros combustibles de biomasa son factores de riesgo importantes en algunas poblaciones (GOLD 2016).
La EPOC es una causa significativa de morbilidad y mortalidad global prevenible. Los cálculos colocan a la EPOC como cuarta causa principal de muerte a nivel global (WHO 2004). Se pronostica un aumento de la prevalencia de la EPOC la incidencia creciente del tabaquismo y al envejecimiento de la población mundial (GOLD 2016). La Organización Mundial de la Salud (OMS) predice que la EPOC se convertirá en la tercera causa principal de muerte para 2030 (WHO 2008). Otros cálculos han previsto que la EPOC se convertirá en la séptima causa principal de vida‐años ajustados por discapacidad para 2030 (Mathers 2006). En 2010, la carga económica de la EPOC en los Estados Unidos se había proyectado en 49 900 000 000 de dólares, con 29 500 000 000 en gastos de asistencia sanitaria directa (American Lung Association 2014). En Australia, se calculó que en 2008, la EPOC le implicó a la economía un costo de AUD 98 000 000 000 (Access 2008).
Según la Global Initiative for Chronic Obstructive Lung Disease (GOLD), se necesita un cociente volumen espiratorio forzado en un segundo posbroncodilatador (VEF1)/capacidad vital forzada (CVF) < 0,70 para el diagnóstico de la EPOC (GOLD 2016). La gravedad de la enfermedad puede clasificarse según el grado de limitación al flujo aéreo, aunque las pruebas indican que se trata de una variable predictiva deficiente de muchas características negativas de la enfermedad. Se ha hallado que los pacientes con una limitación al flujo aéreo similar pertenecen a fenotipos diferentes de la enfermedad, con diferencias en la edad, los síntomas, las comorbilidades y la mortalidad prevista (Agusti 2010; Burgel 2010). Se ha registrado recientemente un aumento del interés en la importancia potencial de la eosinofilia sanguínea y de las vías respiratorias como una variable predictiva de las exacerbaciones y la respuesta a los corticosteroides (Bafadhel 2012; Pascoe 2015), aunque este hecho no se ha tenido en cuenta en la mayoría de los estudios clínicos, como los incluidos en esta revisión.
La presentación, la evolución y las alteraciones patológicas asociadas con la EPOC son variables (Han 2013). La EPOC puede dar lugar a un conjunto de limitaciones funcionales físicas sistémicas, como el déficit de fuerza y función musculoesquelética, menor rendimiento durante el ejercicio y limitaciones funcionales informadas por el paciente (Eisner 2008). Con frecuencia, los pacientes con EPOC presentan comorbilidades múltiples, tanto médicas como psiquiátricas que pueden tener una repercusión significativa en el pronóstico (Barnes 2009; Hanania 2011; Rennard 2006).
Otro factor pronóstico significativo que es un problema importante asociado con la EPOC es la aparición de las exacerbaciones. Las guías GOLD definen la exacerbación de la EPOC como "un evento agudo caracterizado por un empeoramiento de los síntomas respiratorios del paciente que no se corresponde con las variaciones diarias normales y que lleva a un cambio en la medicación” (GOLD 2016). Las exacerbaciones son un causa principal de la disminución del estado de salud y de la calidad de vida relacionada con la salud (Chhabra 2014; Spencer 2004). Por lo general, se tratan con más fármacos broncodilatadores, corticosteroides orales (Walters 2014) y antibióticos (Vollenweider 2012). Los pacientes con exacerbaciones frecuentes de la EPOC presentan un estado más deficiente de la salud, una disminución acelerada en el VEF1, peor calidad de vida y aumento en los ingresos al hospital y mortalidad (Halpin 2012; Vestbo 2011). Las exacerbaciones de la EPOC representan la proporción mayor de la carga total de EPOC para el sistema de asistencia sanitaria (GOLD 2016).
Descripción de la intervención
El tratamiento de la EPOC es complejo y debe incluir un enfoque multidisciplinario y multimodal. Un plan de acción se usa para promover la intervención temprana en las exacerbaciones. Los planes de acción brindan guías que detallan acciones iniciadas por el paciente, como el cambio en los regímenes de medicamentos o la visita a un médico general (MG) u hospital, como respuesta a alteraciones en los síntomas de la EPOC que indican el comienzo de una exacerbación. Un profesional de la salud o un administrador de caso pueden elaborar un plan de acción con el uso de una plantilla y personalizar el plan para los pacientes individuales según los síntomas y el tratamiento regular en curso. Las plantillas para los planes de acción son provistas en línea por algunos grupos de apoyo de las enfermedades pulmonares, y pueden ser accesibles para los pacientes de la atención primaria a bajo costo. En ocasiones un plan de acción va acompañado de prescripciones para la prednisolona y un antibiótico oral.
De qué manera podría funcionar la intervención
Los planes de acción incluyen intervenciones diseñadas para permitirles a los pacientes reconocer e iniciar el tratamiento inicial para las exacerbaciones. Se ha encontrado que los signos precoces de alerta de una exacerbación son bastante consistentes y reconocibles en los pacientes (Kessler 2006). A pesar de este hecho, las pruebas indican que los pacientes no buscan la atención médica para todas las exacerbaciones que presentan (Langsetmo 2008; Walters 2012). Aunque las exacerbaciones no informadas son generalmente menos graves, tienen de todos modos una repercusión en el estado de salud (Langsetmo 2008). Además, algunos pacientes pueden consultar tardíamente para el tratamiento de la exacerbación, y esto se asocia con una recuperación más lenta, una peor calidad de vida y mayor utilización de la asistencia sanitaria (Wilkinson 2004). La naturaleza crónica y progresiva de la EPOC es la base de la importancia del autocuidado.
Con frecuencia se incorporan planes de acción en las intervenciones de autocuidado para la EPOC (Bourbeau 2009). En una revisión sistemática Cochrane se halló que las intervenciones integrales de autocuidado mejoraron la calidad de vida relacionada con la salud y redujeron la utilización de la asistencia sanitaria (Zwerink 2014). En esta revisión, 75% de los estudios incorporaron el uso de un plan de acción, y se formuló la hipótesis de que el número reducido de hospitalizaciones por enfermedades respiratorias que se observó en el grupo de intervención en particular puede haber reflejado dicho uso (Zwerink 2014).
Por qué es importante realizar esta revisión
La falta de consenso sobre una definición operativa de autocuidado de la EPOC ha constituido una barrera a la formulación de recomendaciones claras (Effing 2012). La heterogeneidad entre las intervenciones, las poblaciones de los estudios, el período de seguimiento y las medidas de resultado fue una dificultad para los autores de dos revisiones sistemáticas Cochrane (Kruis 2013; Zwerink 2014) para determinar la forma y el contenido más efectivo del autocuidado para la EPOC. Effing y cols. propusieron una definición conceptual de autocuidado de la EPOC, como sigue: "Aunque es estructurada, una intervención de autocuidado de la EPOC también es personalizada y a menudo incluye componentes múltiples, con objetivos para motivar, alentar y apoyar a los pacientes a que adapten su comportamiento con respecto a la salud de una manera positiva, y a que desarrollen aptitudes para un mejor control de la enfermedad" (Effing 2016). El desarrollo y la evaluación de las intervenciones específicas de autocuidado son importantes para la aplicación de la definición presentada por Effing y colaboradores. Esta revisión es una actualización de una revisión Cochrane publicada por primera vez en 2005 (Turnock 2005). El objetivo de esta revisión es determinar la función y la efectividad de un plan de acción como intervención de autocuidado para los pacientes con EPOC sin un entrenamiento/educación para el autocuidado exhaustivos.
Objetivos
Comparar los efectos de un plan de acción para las exacerbaciones de la EPOC a través de un único componente breve de educación del paciente y sin un programa integral de autocuidado versus la atención habitual. Los resultados primarios fueron: el uso de recursos de la asistencia sanitaria, la mortalidad y el uso de medicación. Los resultados secundarios fueron: la calidad de vida relacionada con la salud, la morbilidad psicológica, la función pulmonar y la costo‐efectividad.
Métodos
Criterios de inclusión de estudios para esta revisión
Tipos de estudios
Ensayos controlados aleatorios (ECA) y cuasialeatorios, con la exclusión de los ensayos cruzados.
Tipos de participantes
Los participantes fueron pacientes con un diagnóstico clínico de EPOC basado en criterios espirométricos, como los de GOLD (GOLD 2016) para la limitación al flujo aéreo persistente (es decir VEF1/CVF posbroncodilatador < 70%) con antecedentes de tabaquismo. Se excluyeron los estudios con pacientes que habían recibido un diagnóstico primario de asma, a menos que se dispusiera de los resultados separados para los pacientes con EPOC.
Tipos de intervenciones
La intervención comprendía un plan de acción con un único componente educativo de corta duración. La parte breve educativa le dio al médico el tiempo necesario para personalizar el plan de acción según las necesidades del tratamiento y los síntomas individuales. El plan de acción se define como una guía oral o escrita con detalles de las intervenciones iniciadas por el paciente (como el cambio del régimen farmacológico o las visitas al MG o al hospital), aplicadas de manera adecuada en respuesta a las alteraciones en los síntomas de la EPOC (p.ej., mayor disnea, mayor cantidad o purulencia del esputo, aumento del uso del inhalador de alivio, disminución del nivel de actividad; es decir, cambios que sugerirían el comienzo de una exacerbación). Los investigadores permitieron el apoyo continuo dirigido al uso del plan de acción telefónico o por contacto directo. No se incluyeron estudios con intervenciones de apoyo al autocuidado más amplias, como la educación individual o grupal en sesiones múltiples durante un período más largo o programas de ejercicio, de forma independiente de si comprendían un plan de acción. Los investigadores compararon la intervención activa versus "la atención habitual" por profesionales de la salud.
Tipos de medida de resultado
Resultados primarios
-
Utilización de la asistencia sanitaria, incluido el ingreso al hospital por una afección respiratoria, el tratamiento en el servicio de urgencias y las visitas al MG para la EPOC.
-
Mortalidad: relacionada con enfermedades respiratorias y por todas las causas.
-
Uso de la medicación: tiempo hasta el comienzo del tratamiento después de la aparición de los síntomas; ciclos/duración de los antibióticos o corticosteroides, o ambos; inicio de la administración de antibióticos o corticosteroides, o ambos, por el paciente.
Resultados secundarios
-
Calidad de vida relacionada con la salud (CVRS) medida en escalas validadas.
-
Morbilidad psicológica: ansiedad y depresión, medidas en escalas validadas.
-
Conocimiento sobre el autocuidado y acciones específicas para la EPOC (basado en la entrevista al paciente).
-
Función pulmonar.
-
Costo‐efectividad.
Results
Description of studies
Results of the search
Review authors identified and screened a total of 574 titles and abstracts since the original review was published in 2005. In 2005, two review authors (AT, JW) assessed the full texts of 11 of 199 identified studies, and included three of these studies in the review (McGeoch 2004; Watson 1997; Wood‐Baker 2006). In 2010, review authors included two (Martin 2004; Rootmensen 2008) of 17 studies identified in the search update. From the updated search conducted during 2014, review authors identified 358 studies for screening, of which they assessed 36 full texts for eligibility (Figure 1). Review authors assessed all previously excluded studies for eligibility if the intervention included ongoing support for action plan use. Review authors excluded 33 citations (representing 26 studies) ‐ four owing to wrong comparator, 24 because of the wrong intervention, three as the result of wrong study design, one because the duration of education exceeded eligibility and one because it was a duplicate citation for a study already excluded. One study was ongoing, and review authors included two studies in the review (Rice 2010; Trappenburg 2011). Searches for this update repeated on 21/11/15, before the review update was submitted, yielded no new studies. Two review authors (JW, MH) conducted screening for the most recent update.
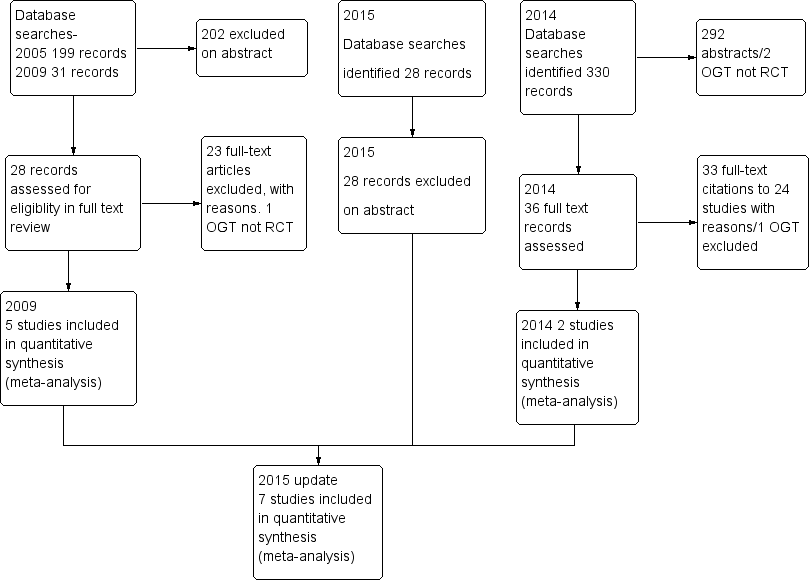
Study flow diagram.
Included studies
See the Characteristics of included studies table.
This review update includes a total of seven parallel‐group RCTs that included 1550 participants with COPD (Table 1). Since the last update appeared in 2010 (Walters 2010), review authors have added two studies (Rice 2010; Trappenburg 2011) with an additional 976 participants. Four trials (Martin 2004; Rice 2010; Rootmensen 2008; Watson 1997) were randomised at patient level, two (McGeoch 2004: Wood‐Baker 2006) were cluster‐randomised at practice level and one (Trappenburg 2011) was randomised by the minimisation technique to control for centre and gender. Four studies recruited participants through GPs. Wood‐Baker 2006 recruited from 54 GPs in 31 practices, and Watson 1997 recruited from 22 GPs in 12 practices. McGeoch 2004 recruited participants attending two groups of general practices but did not specify the number of GPs involved, and Martin 2004 recruited through a consortium of GPs in one region. Rice 2010 recruited participants from a centralised electronic medical record database of a US Veterans Hospital. Trappenburg 2011 recruited participants through scheduled visits to a respiratory nurse at eight regional hospitals and five general practices.
| Study ID | Dates | Recruitment/Randomisation unit | Follow‐up | Length SME (educator) | RAN, n/WD, n | Age*, years/ % male | % current smokers | FEV1 % pred* INT‐CONT | QoL INT‐CONT |
| Not known | Consortium practices, New Zealand/participants | 12 months | Single interview, length not stated (respiratory nurse) | 96/26 | 70/51 | n/a | 35‐34 | 57‐51 | |
| 7/2002‐ 12/2003 | 2 groups of practices, New Zealand/practice | 12 months | 1 hour (practice nurse or respiratory educator) | 159/9 | 71/59 | 28 | 55‐53 | 43‐37 | |
| Rootmensen 2008 (all participants) | Not known | 1 hospital pulmonary outpatient clinic, Netherlands/ participants | 6 months | 45 minutes (pulmonary nurse) | 157 (111 COPD)/17 | 60/55 | 12 | 57‐64 | n/a |
| 07/2004‐ 07/2008 | Centralised electronic medical record database/participants | 12 months | 1 to 1.5‐hour group educational session (case manager) | 743/84 | 70/98 | 22 | 36.1‐38.1 | n/a | |
| 12/2008‐ 12/2010 | 8 regional hospitals and 5 general practices/participants (stratified by gender and centre) | 6 months | Single interview, length not stated (nurse case manager). | 233/41 | 66/57 | 29 | 56.7‐56.5 | n/a | |
| 1993‐ 07/1994 | 12 practices, 22 GPs, New Zealand/participants | 6 months | Single interview, length not stated (practice nurse) | 69/13 | 68/65 | 28 | 37‐36 | 43‐39 | |
| 2002‐2003 | 54 GPs, 31 practices, Australia/practice | 12 months | 1 hour (respiratory research nurse) | 139/27 | 70/76 | 42 | 46‐44 | 47‐47 |
*: mean; AP: action plan; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; GP: general practitioner; INT‐CONT: intervention group‐control group; QoL: % impairment quality of life 0‐100; RAN: randomisation; SME: self‐management education; WD: withdrawal or death.
All participants had received a diagnosis of COPD as a major functionally limiting disease before inclusion. In line with the GOLD criteria for diagnosis of COPD, all participants showed a postbronchodilator FEV1/FVC ratio < 0.70. However, Rootmensen 2008 recruited participants with a diagnosis of COPD or asthma. We included in this review only results for the subgroup of participants with COPD (111 of 191). Participants in Rice 2010 were also required to have one or more of the following during the previous year: hospitalisation or ED visit for COPD, long‐term home oxygen use or course of systemic corticosteroids for COPD. Trappenburg 2011 recruited participants over the age of 40 who were currently using bronchodilator therapy. Participants in Wood‐Baker 2006 were at least 50 years of age. Both Watson 1997 and Wood‐Baker 2006 also required FEV1 < 65% predicted. McGeoch 2004 stated inclusion criteria of symptoms at least weekly and history of one or more exacerbations in the previous 12 months requiring an increase in therapy. Martin 2004 required at least one hospital admission or two acute exacerbations of COPD requiring GP care during the previous 12 months. Entry criteria for Watson 1997 included current use of bronchodilator therapy.
Assessment of baseline characteristics of participants (Table 1) shows that studies involved people of similar age, with mean age from 60 to 71 years and overall mean age of 68 years. All studies included more male participants, ranging from 51% to 98% with overall mean of 66%. The high incidence of male participants in Rice 2010 (98%) reflected recruiting from Veterans Affairs medical centres. The percentage of current smokers in each study group varied from 28% (Wood‐Baker 2006) to 12% (Rootmensen 2008), with overall mean of 27%. Severity of airflow obstruction, as indicated by the overall mean postbronchodilator FEV1 as percentage of predicted value (staged according to the GOLD classification), was moderate in three studies (McGeoch 2004 (54% predicted); Rootmensen 2008 (61% predicted); Trappenburg 2011 (57% predicted)) and severe in four studies (Martin 2004 (54% predicted); Rice 2010 (54% predicted); Watson 1997 (54% predicted); Wood‐Baker 2006 (54% predicted)). At baseline, mean impairment scores for overall quality of life when available (in four studies) (based on St George's Respiratory Questionnaire maximum impairment = 100) ranged from 37 to 57, with mean overall score of 46. Within studies, impairment in quality of life was similar between intervention and control groups.
Three studies specified exclusion of nursing home residents (McGeoch 2004; Watson 1997; Wood‐Baker 2006). Five studies specified exclusion of participants with other primary limiting diseases such as lung cancer and cardiac disease (Martin 2004; McGeoch 2004; Rice 2010; Trappenburg 2011; Watson 1997). Trappenburg 2011 also excluded participants with a primary diagnosis of asthma. Rice 2010 excluded participants without access to a telephone.
Study follow‐up was six months in three studies (Rootmensen 2008; Trappenburg 2011; Watson 1997) and 12 months in four studies (Martin 2004; McGeoch 2004; Rice 2010; Wood‐Baker 2006). Investigators reported a total of 217 withdrawals from the total 1550 participants enrolled and a drop‐out rate ranging from 5% to 27%.
Action plan intervention
Table 2 presents a comparison of action plan interventions. Three studies used a standard written action plan and information booklet (McGeoch 2004; Watson 1997; Wood‐Baker 2006). Martin 2004, Rice 2010 and Trappenburg 2011 used an individualised action plan intervention. Rootmensen 2008 provided an intervention consisting of additional care that included individual instructions for what to do in case of exacerbations.
| Individualised AP | Standard written AP | Support for AP during study period | SME (individual/group) | Prescription /supply OCS | Prescription /supply ABS | Written COPD educational component | Comparison | |
| Written | 3‐Monthly visit regarding use of AP | Individual interview with respiratory nurse, length not stated, individualised action plan according to current treatment and symptoms | All had 7‐day supply | All had 7‐day supply | No | Usual care by own GP | ||
| Yes | No | Individual session by practice nurse or respiratory educator in association with GP 1 hour, covering major points of COPD self‐management plan, and use of validated sputum colour charts | Prescription | Prescription | Educational package | Non‐standard education on COPD according to practice standards | ||
| Written | Monthly phone call from nurse | Group 1‐1.5 hours, individualised action plan with respiratory nurse | Yes | Prescription | Usual care + 1‐page summary of principles of COPD care according to published guidelines. No AP | |||
| Oral | No | Individual protocol‐based educational session covering disease, medications, vaccination, smoking cessation and exacerbation management, 45 minutes in length | Oral medication provided to some, % unknown | Oral medication provided to some, % unknown | No | Usual care | ||
| Written | Standardised phone calls at 1 and 4 months | Individualised action plan education, length of session not stated | 2%' | 22% | ✓ COPD information | Usual care ‐ pharmacological and non‐pharmacological care according to most recent evidence‐based guidelines, specifically AP denied. All included participants seen by respiratory nurse, who systematically checked and discussed aspects of COPD care, including vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. | ||
| Yes | No | Individual session education about use of the action plan with COPD booklet by a senior respiratory outreach nurse; length not stated | Prescription | Prescription | ✓ Guide to living positively with COPD | Usual care by GP, specifically denied access to AP and booklet | ||
| Written | No | Individual educational session with respiratory nurse, covering COPD, smoking cessation, immunisation, nutrition, exercise, sputum clearance, breathing, medication, inhaler use. Individualised action plan developed with GP input. Length not known | 2% | 22% | COPD information booklet | Usual care, COPD information booklet and individual educational session with nurse, but no AP |
ABS: antibiotics; AP: action plan; COPD: chronic obstructive pulmonary disease; GP: general practitioner; OCS: oral corticosteroids; SME: self‐management education.
Wood‐Baker 2006 participants also received an individual educational session with a nurse experienced in managing respiratory disease. Their action plan was a written self‐management plan that was developed in consultation with their treating GP. It listed the participant's maintenance medications and an individualised action plan based on early recognition of symptoms associated with exacerbations of COPD. Seventy‐six per cent received a standard action plan with instructions to self‐initiate a short course of oral corticosteroids and an antibiotic; the other 24% received an action plan with instructions to initiate antibiotics only (N = 10), to double their dose of inhaled corticosteroids and commence an antibiotic (N = 2), to initiate a short course of oral corticosteroids only (N = 1) or to contact their GP (N = 3). Participants following action plans that involved self‐initiation of medication were given prescriptions by their GP. All intervention participants were encouraged to present to their GP early.
Two studies (McGeoch 2004; Watson 1997) used action plans that were identical and provided advice on management of usual care and exacerbations, together with a booklet on self‐management, a prescription from their GP for prednisolone and a broad‐spectrum antibiotic for self‐administration during an exacerbation. Watson 1997 made no attempt to individualise instructions in the action plan, whereas the remaining trials (Martin 2004; McGeoch 2004; Wood‐Baker 2006; Rootmensen 2008) delivered self‐management plan education in an individual session provided by a nurse, a respiratory educator or the participant's GP.
Four trials (Martin 2004; McGeoch 2004; Watson 1997; Wood‐Baker 2006) supplied booklets with action plans that covered topics such as smoking cessation, control of breathlessness, nutrition, exercise, clearance of mucus from the lungs, medications and contact details of community support services. Two trials educated participants on the correct use of inhalers (Rootmensen 2008; Wood‐Baker 2006).
In Rice 2010, participants attended a single 1 to 1.5‐hour group educational session. They received individualised written action plans that included a description of the signs and symptoms of an exacerbation that should prompt initiation of self‐treatment, refillable prescriptions for prednisolone and an oral antibiotic, contact information for a case manager and the telephone number of the 24‐hour VA help line. Participants were instructed to begin action plan medications for symptoms that were substantially worse than usual. A case manager made monthly phone calls to reinforce general principles of COPD management, to review details of the action plan and to answer questions.
In Trappenburg 2011, participants attended an individual educational session with a respiratory nurse, who systematically checked and discussed aspects of COPD care such as vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. Participants received an individualised action plan that included recognition of symptom changes, use of medication/lifestyle prescriptions, additional medication/breathing exercises and energy preservation in case of symptom increase and a contact person/telephone number in case of an exacerbation. For individual participants, it was optional for the case manager (in consultation with the attending physician) to provide self‐treatment medication (course of corticosteroids and/or antibiotics). Two standardised reinforcement sessions were held by telephone at one and four months to evaluate participants' understanding of and adherence to the action plan; when needed, researchers provided additional information.
Control
Investigators provided all control groups with usual care; although this varied between studies, participants were always specifically denied access to the action plan. Wood‐Baker 2006 provided usual care that included provision of a booklet and an individual nurse educational session. McGeoch 2004 provided non‐standardised education based on routine practice at the time. The remaining three trials (Martin 2004; Rootmensen 2008; Watson 1997) supplied no additional education for participants in control groups. Rice 2010 distributed to usual care participants a one‐page handout containing a summary of principles of COPD care based on published guidelines. In Trappenburg 2011, a nurse case manager assessed participants and systematically checked and discussed aspects of COPD care such as vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. Participants had no additional contact with the case manager.
Excluded studies
Ten studies were not RCTs, 11 studies involved comprehensive self‐management programmes in which the action plan component could not be isolated and in nine studies, COPD/self‐management education was delivered in multiple sessions or in a single session of several hours' duration. Fifteen studies included no action plan in the intervention.
Risk of bias in included studies
We have provided full details regarding risk of bias assessment for all included studies in the Characteristics of included studies table, along with a summary of grading in Figure 2 and Figure 3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
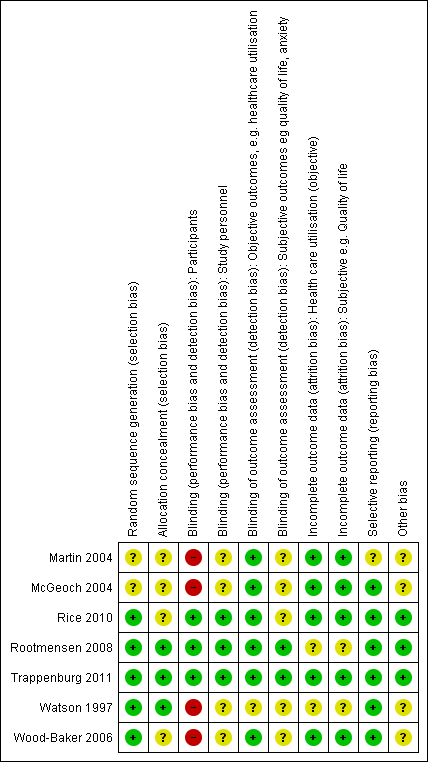
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
With regards to random sequence generation, we assessed five studies as having low risk of bias; two employed permuted block randomisation (Rice 2010; Watson 1997), two the minimisation technique (Rootmensen 2008; Trappenburg 2011) and one a computer‐generated randomised software package (Wood‐Baker 2006). Two studies assessed as having unclear risk reported that they were RCTs but did not describe the method of randomisation used (Martin 2004; McGeoch 2004).
Concerning allocation concealment, we assessed three studies as having low risk of bias (Rootmensen 2008; Trappenburg 2011; Watson 1997). Rootmensen 2008 randomised participants in advance of their clinic attendance and reported these results only to the pulmonary physician just before the visit. Trappenburg 2011 utilised a central web‐based service to conceal the assignment sequence. In Watson 1997, research staff who recruited participants allocated them according to a randomisation list. We assessed four studies as having unclear risk of bias. Three did not report methods of allocation (Martin 2004; Rice 2010; Wood‐Baker 2006), and in McGeoch 2004, researchers allocated participants by practice attendance but did not provide information on allocation of practices.
Blinding
We assessed three studies as having low risk of bias for blinding of participants; two utilised a modified consent procedure by which the major objective of the study was withheld from participants until after the study was completed (Rootmensen 2008; Trappenburg 2011), and in Rice 2010, participants were aware of their allocation, but this awareness was not thought likely to affect primary healthcare utilisation outcomes. Regarding patient‐reported outcomes, we assessed those from Rootmensen 2008 and Trappenburg 2011 as low risk because investigators used a modified consent procedure, and those from Rice 2010 as unclear risk. We assessed four studies as having unclear risk of bias with regards to participants, as they were not blinded (Martin 2004; McGeoch 2004; Watson 1997; Wood‐Baker 2006); this introduced the potential for bias in self‐administered patient assessments, such as quality of life measures and daily diary records. In some practices in McGeoch 2004, GPs may have implemented both intervention and usual care, leading to possible confounding between treatment methods. Martin 2004, McGeoch 2004, Watson 1997 and Wood‐Baker 2006 did not blind outcome assessors, suggesting potential for high bias for subjective outcomes. Rice 2010 adequately blinded assessors.
Incomplete outcome data
Regarding incomplete outcome data for objective healthcare utilisation outcomes, we assessed five studies as having low risk of bias. McGeoch 2004 and Wood‐Baker 2006 reported small numbers lost to follow‐up that were balanced between groups. In Martin 2004, 93 of 96 recruited participants completed follow‐up; three withdrawals occurred for personal reasons, but investigators did not state group allocation. In Rice 2010, the only reason for missing data was death (48 in usual care, 36 in intervention), and study authors were unable to perform intention‐to‐treat analysis. Trappenburg 2011 reported drop‐out rates of 19% in the intervention group and 16% in the control group. For objective healthcare utilisation outcomes, we determined that two studies had unclear risk of bias; Rootmensen 2008 reported on only 90 of 117 participants with COPD, and Watson 1997 noted 13 withdrawals from the 60 participants originally randomised and did not report group allocation for those lost to follow‐up.
Risk of bias assessment concerning incomplete outcome data for subjective outcomes was similar to that for objective outcomes. We assessed five studies as having low risk of bias (Martin 2004; McGeoch 2004; Rice 2010; Trappenburg 2011; Wood‐Baker 2006) and two studies as having unclear risk (Rootmensen 2008; Watson 1997) because researchers did not report withdrawals by group.
Selective reporting
Regarding reporting bias, we assessed six studies as having low risk of bias (McGeoch 2004; Rice 2010; Rootmensen 2008; Trappenburg 2011; Watson 1997; Wood‐Baker 2006). In McGeoch 2004, Rootmensen 2008, Watson 1997 and Wood‐Baker 2006, it was clear that study authors reported all expected outcomes, including those that were prespecified. The protocols for Rice 2010 and Trappenburg 2011 were available, and outcomes reported in these studies were consistent with those prespecified. We assessed Martin 2004 as having unclear risk of bias, as it was not clear that published reports included all expected outcomes and those prespecified.
Other potential sources of bias
We identified no additional sources of bias in Rice 2010, Rootmensen 2008 and Trappenburg 2011. Martin 2004 described a pilot study in which no sample size calculation was performed; study authors did not attempt to examine clustering within practices. McGeoch 2004 reported on statistical analysis to examine the effect of clustering within practices. They analysed the 12‐month change in the outcome variable by using a mixed‐model repeated measures analysis of variance (ANOVA), allowing for cluster‐randomisation of surgeries, and indicated no additional variation from this source beyond that anticipated by between‐subject variation. For this reason, investigators in McGeoch 2004 undertook all analyses by using participants as replicates. In Wood‐Baker 2006, researchers did not perform analyses to examine the effect of clustering within practices. In Watson 1997, baseline analysis showed a statistically significant difference for influenza vaccination in the past year (72% in the intervention group, 44% in the control group).
Effects of interventions
Results: primary outcomes
-
Healthcare utilisation, including respiratory‐related hospital admission, treatment in an emergency department (ED) and general practitioner (GP) visits for chronic obstructive pulmonary disease (COPD).
-
Mortality: respiratory‐related and all‐cause.
-
Use of medications: time to initiation of therapy after symptom onset; course/duration of antibiotic or corticosteroid use, or both; participant initiation of antibiotic or steroid use, or both
Healthcare utilisation
Analysis 1.1 Rate of hospitalisation for COPD/100 patient‐years: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). The difference between action plan with phone follow‐up and control was not statistically significant (rate ratio (RR) 0.69, 95% confidence interval (CI) 0.47 to 1.01).
Analysis 1.2 At least one hospital admission (12‐month follow‐up): For this outcome, we found two relevant trials (n = 897). Results showed a statistically significant difference, with less likelihood for action plan compared with control (subgrouped by phone follow‐up) (odds ratio (OR) 0.69, 95% CI 0.49 to 0.97) and no heterogeneity.
Analysis 1.3 At least one hospital admission (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (OR 0.83, 95% CI 0.30 to 2.31).
Analysis 1.4 Rate of hospital admission for exacerbation (12‐month follow‐up): For this outcome, we found two relevant trials up (n = 205). Results showed no statistically significant difference between action plan and control (mean difference (MD) 0.23, 95% CI ‐0.03 to 0.49) and no heterogeneity (Chi² = 0.30, df = 1 (P = 0.59), I² = 0%).
Analysis 1.5 Rate of hospital admission for exacerbation (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.08 to 0.08).
Analysis 1.6 Rates of hospitalisation and ED visits for COPD/100 patient‐years: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (RR 0.59, 95% CI 0.44 to 0.79).
Analysis 1.7 At least one hospital or ED visit for COPD (12‐month follow‐up): For this outcome, we found one relevant trial (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (OR 0.59, 95% CI 0.43 to 0.80).
Analysis 1.8 Rate of ED visits for COPD/100 patient‐years (12‐month follow‐up): For this outcome, we found one relevant trial (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (RR 0.49, 95% CI 0.33 to 0.73).
Analysis 1.9 Rate of ED visits for COPD (12‐month follow‐up): For this outcome, we found two relevant trials (n = 202). Results showed no statistically significant difference between action plan and control (MD 0.37, 95% CI ‐0.50 to 1.24).
Analysis 1.10 At least one ED visit for COPD (12‐month follow‐up): For this outcome, we found two relevant trials (n = 287). Results showed a statistically significant difference with less likelihood for action plan compared with control (subgrouped by phone follow‐up) (OR 0.55, 95% CI 0.38 to 0.78) and no heterogeneity (Chi² = 0.13, df = 1 (P = 0.72), I² = 0%).
Analysis 1.11 Rate of ED visits for COPD (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.09 to 0.09).
Analysis 1.12 GP visits/phone contacts for COPD (all or urgent): For this outcome, we found one relevant trial with six‐month follow‐up (n = 56), with no statistically significant difference between action plan and control (MD 1.00, 95% CI ‐0.57 to 2.57), and two relevant trials up (n = 200) with 12‐month follow‐up (MD 0.23, 95% CI ‐1.02 to 1.47).
Analysis 1.13 Rate of non‐COPD GP visits or phone contacts: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 200). Results showed no statistically significant difference between action plan and control (MD 1.25, 95% CI ‐1.54 to 4.03) and moderate heterogeneity (Chi² = 2.38, df = 1 (P = 0.12), I² = 58%).
Analysis 1.14 Rate of unscheduled physician visits: For this outcome, we found one relevant trial with six‐month follow‐up (n = 227), with no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.36 to 0.36).
Analysis 1.15 Rate of ambulance calls: For this outcome, we found one relevant trial with six‐month follow‐up (n = 89). Results showed a statistically significant difference between action plan and control, with a higher rate in the action plan group (MD 1.70, 95% CI 0.17 to 3.23).
Analysis 1.16 Total hospital days: For this outcome, we found one relevant trial with12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with fewer days spent in hospital in the action plan group (MD ‐1.10, 95% CI ‐2.00 to ‐0.20).
Analysis 1.17 Total intensive care unit (ICU) days: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with fewer days spent in the ICU in the action plan group (MD ‐0.30, 95% CI ‐0.60 to ‐0.00).
Mortality
Analysis 1.18 All‐cause mortality: For this outcome, we found four relevant trials with 12‐month follow‐up (n = 1134). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (Peto OR 0.88, 95% CI 0.59 to 1.31) and moderate heterogeneity (Chi² = 5.17, df = 3, P = 0.16, I² = 42%) (Figure 4) and imprecision.
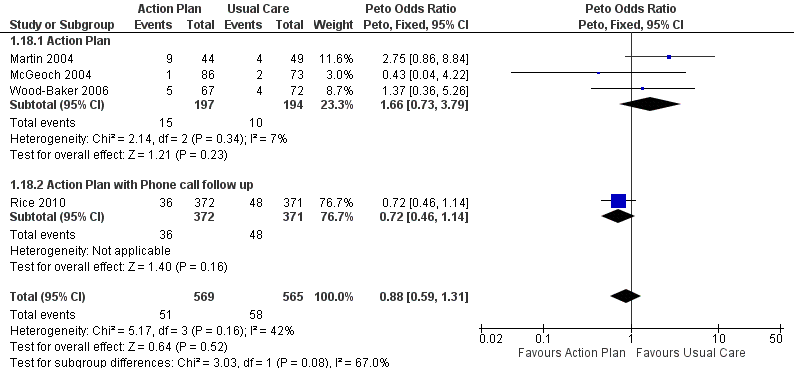
Forest plot of comparison: 1 Action plan versus usual care, outcome: 1.18 Mortality (all cause) 12 months.
Analysis 1.19 Rate of all‐cause mortality per 100 patient‐years: For this outcome, we found one relevant trial with12‐month follow‐up (n = 743). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD ‐3.70, 95% CI ‐8.86 to 1.46), but the result was imprecise.
Analysis 1.20 All‐cause mortality: For this outcome, we found one relevant trial with six‐month follow‐up (n = 229). Results showed no statistically significant difference between action plan with phone follow‐up and control (Peto OR 1.06, 95% CI 0.15 to 7.69), but the result was imprecise.
Use of medication for acute exacerbations of COPD
No data were available on time to initiation of therapy after onset of exacerbation symptoms.
Analysis 1.21 Use of one or more courses of oral steroids for exacerbations: For this outcome, we found one relevant trial with six‐month follow‐up (n = 56), with a statistically significant difference between action plan and control and increased odds of steroid use in the action plan group (OR 6.58, 95% CI 1.29 to 33.62), and one relevant trial with 12‐month follow‐up (n = 154), with no statistically significant difference between action plan and control (OR 1.27, 95% CI 0.34 to 4.69).
Analysis 1.22 The rate of courses of oral steroids for exacerbations in two relevant trials with 12‐month follow‐up (n = 200) showed a statistically significant difference between action plan and control, with an increased rate of steroid use in the action plan group (MD 0.74, 95% CI 0.12 to 1.35) and no heterogeneity (Chi² = 0.37, df = 1, P = 0.54, I² = 0%).
Analysis 1.23 The rate of courses of oral steroids for exacerbations in one relevant trial with six‐month follow‐up (n = 227) showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.23 to 0.23).
Analysis 1.24 The number of days on oral corticosteroids for exacerbations in one relevant trial with six‐month follow‐up (n = 227) showed no statistically significant difference between action plan and control (MD 6.00, 95% CI ‐5.53 to 17.53).
Analysis 1.25 Cumulative dose of prednisolone: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with a greater cumulative dose in the action plan group (MD 779.00 mg, 95% CI 533.23 to 1024.77).
Analysis 1.26 Use of one or more courses of antibiotics for exacerbations: For this outcome, we found one relevant trial with six‐month follow‐up (n = 56) that reported a statistically significant difference between action plan and control (Peto OR 6.51, 95% CI 2.02 to 21.05), and two relevant trials with 12‐month follow‐up (n = 293) that reported a statistically significant difference between action plan and control (Peto OR 1.65, 95% CI 1.01 to 2.69); both outcomes show increased odds of antibiotic use in the action plan group.
Analysis 1.27 Rate of courses of antibiotics for exacerbations over 12 months: For this outcome, we found three relevant trials with 12‐month follow‐up (n = 943). Results showed a statistically significant difference between action plan and control, with a higher rate of antibiotic use in the action plan group (subgrouped by phone follow‐up) (MD 2.26, 95% CI 1.82 to 2.70), and a substantial degree of heterogeneity (Chi² = 10.55, df = 2, P = 0.005, I² = 81%) and a statistically significant test for subgroup difference (Chi² = 10.09, df = 1, P = 0.001, I² = 90.1%). In two studies that compared action plan with control, the MD was 0.78 (95% CI ‐0.24 to 1.79), and in one study that compared action plan with phone follow‐up and control, the MD was 2.60 (95% CI 2.12 to 3.08).
Analysis 1.28 Rate of courses of antibiotics for exacerbations over six months: In one relevant trial with six‐month follow‐up (n = 227), results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.26 to 0.26).
Analysis 1.29 The number of days on antibiotics over six months for exacerbations in one relevant trial with six‐month follow‐up (n = 56) showed a statistically significant difference between action plan and control, with a greater number of days on antibiotics in the action plan group (MD 6.00 days, 95% CI 1.40 to 10.60).
Results: secondary outcomes
Respiratory health‐related quality of life: overall scores: St George’s Respiratory Questionnaire (SGRQ), in which a negative direction for the result indicates improvement
Analysis 1.30 SGRQ overall score at 12 months: For this outcome, we found three relevant trials with 12‐month follow‐up (n = 1009). Results showed a statistically significant difference between action plan and control (subgrouped by phone follow‐up), with better quality of life in the action plan group (MD ‐2.79, 95% CI ‐0.82 to ‐4.77), a substantial degree of heterogeneity (Chi² = 7.98, df = 2, P = 0.02, I² = 75%) and a statistically significant test for subgroup difference (Chi² = 7.11, df = 1, P = 0.008, I² = 85.9%). The MD in two studies (n = 264) that compared action plan with control was not significant (0.32, 95% CI 3.34 to ‐2.70), and one study that compared action plan with phone follow‐up and control (n = 743) noted a significant improvement (MD ‐5.10, 95% CI ‐2.50 to ‐7.70).
Analysis 1.31 SGRQ overall score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐0.83, 95% CI ‐2.93 to 1.27), no heterogeneity and no difference between subgroups at this time point.
Respiratory health‐related quality of life subscales
Analysis 1.32 SGRQ symptom score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD ‐2.55, 95% CI ‐6.92 to 1.83) with no heterogeneity.
Analysis 1.33 SGRQ symptom score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 448). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐2.33, 95% CI ‐6.84 to 2.18), with no heterogeneity.
Analysis 1.34 SGRQ activity limitation score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD 2.87, 95% CI 7.00 to ‐1.26), with no heterogeneity.
Analysis 1.35 SGRQ activity limitation score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD 0.88, 95% CI ‐1.90 to 3.67), with no heterogeneity.
Analysis 1.36 Change in SGRQ impact score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD ‐1.04, 95% CI 2.43 to ‐4.51), with moderate heterogeneity (Chi² = 1.76, df = 1, P = 0.18, I² = 43%).
Analysis 1.37 SGRQ impact score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐1.26, 95% CI ‐3.47 to 0.95), with no heterogeneity.
Generic health‐related quality of life subdomains: measured by Short Form (SF)‐36
For this outcome, we found one relevant trial with six‐month follow‐up (n = 90) that compared action plan and control. Table 3 shows results for eight domains as mean difference (MD) and 95% confidence interval (CI); a positive result indicates improvement.
| Outcome | SF‐36 domain | Mean difference | 95% CI |
| Physical function | 0.30 | ‐7.13 to 7.73 | |
| Role limitation | 9.00 | ‐8.07 to 26.07 | |
| Bodily pain | 18.50 | 6.14 to 30.86 | |
| General health | 2.60 | ‐3.71 to 8.91 | |
| Vitality | 1.60 | ‐4.73 to 7.93 | |
| Social function | 5.30 | ‐4.68 to 15.28 | |
| Role limitation | 7.50 | ‐8.56 to 23.56 | |
| Mental health | 6.30 | 0.64 to 11.96 |
Psychological morbidity: anxiety and depression
Investigators measured these outcomes by using the Hospital Anxiety and Depression Scale (HADS), a 21‐unit scale on which higher score indicates more severe symptoms, in one study that compared action plan with phone follow‐up and control with 12‐month follow‐up (n = 154), and in another study that compared action plan and control with six‐month follow‐up (n = 183). Table 4 shows results for depression and anxiety scores as mean difference (MD) and 95% confidence interval (CI); a negative result indicates fewer symptoms.
| Outcome | Domain | Follow‐up: months | MD | 95% CI | n |
| Depression | 12 | ‐0.25 | ‐1.14 to 0.64 | 154 | |
| Depression | 6 | 0.10 | ‐0.73 to 0.93 | 183 | |
| Anxiety | 12 | 0.14 | ‐1.38 to 1.66 | 154 | |
| Anxiety | 6 | 0.00 | ‐0.83 to 0.83 | 183 |
COPD self‐management for exacerbation and related self‐efficacy
Assessment of these outcomes was based on interviews with participants and use of different questionnaires in three studies that provided relevant data, preventing meta‐analysis of outcomes.
McGeoch 2004 (action plan vs control) used a standardised COPD self‐management questionnaire on which higher score indicates greater self‐efficacy (range 0 to 26), which has been shown to be valid and reliable (Dowson 2004). Rootmensen 2008 (action plan vs control) used a self‐administered self‐management questionnaire that was based on three exacerbation scenarios and included questions adapted from a validated interview‐based questionnaire (Kolbe 1996), on which higher score indicates greater self‐efficacy. Trappenburg 2011 (action plan with phone follow‐up vs control) measured self‐management exacerbation‐related self‐efficacy using a non‐validated questionnaire with 11 items graded on a 5‐point Likert scale. Lower scores indicate greater self‐efficacy for exacerbation‐related self‐management behaviour. Table 5 shows results as mean difference (MD) and 95% confidence interval (CI).
| Outcome | Study | Item | Direction improvement | Months | MD | 95% CI | n |
| Self‐management knowledge when well | + | 12 | 1.10 | 0.46 to 1.74 | 154 | ||
| Self‐management actions when well | + | 12 | 0.50 | ‐0.24 to 1.24 | 154 | ||
| Self‐management knowledge early exacerbation | + | 12 | 1.80 | 0.75 to 2.85 | 154 | ||
| Self‐management actions early exacerbation | + | 12 | 2.30 | 0.96 to 3.64 | 154 | ||
| Self‐management knowledge severe exacerbation | + | 12 | 2.50 | 0.94 to 4.06 | 154 | ||
| Self‐management action severe exacerbation | + | 12 | 1.50 | 0.47 to 2.53 | 154 | ||
| Self‐management exacerbation actions | + | 6 | ‐5.10 | ‐15.26 to 5.06 | 90 | ||
| Self‐efficacy for exacerbation recognition | ‐ | 6 | ‐0.70 | ‐0.98 to ‐0.42 | 183 | ||
| Self‐efficacy for exacerbation prevention/action | ‐ | 6 | ‐0.90 | ‐1.18 to ‐0.62 | 183 |
Lung function: FEV1 % predicted
For this outcome (Analysis 1.59), we found two relevant trials with six‐month follow‐up (n = 179), in which results showed no statistically significant difference between action plan and control (MD 1.83, 95% CI ‐1.05 to 4.71), and one relevant trial with 12‐month follow‐up (n = 293), in which results showed no statistically significant difference between action plan and control (MD 2.00, 95% CI ‐1.89 to 5.89).
Cost‐effectiveness
Analysis 1.60 The cost of hospital admissions (HADM) per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with lower costs in the action plan group (MD ‐1117.00 US$, 95% CI ‐1754.50 to ‐479.50).
Analysis 1.61 The cost of emergency department visits (EDV) per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with lower costs in the action plan group (MD ‐141.00 US$, 95% CI ‐234.31 to ‐47.69).
Analysis 1.62 The cost of pulmonary drug prescriptions per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 15.00 US$, 95% CI ‐6.32 to 36.32).
Sensitivity analysis
We performed a sensitivity analysis to examine changes in SGRQ scores (overall and subscales) (Appendix 4). For Watson 1997, we compared results when we used the standard deviation taken from the largest value in another study of similar duration versus the same outcome when we used the standard deviation calculated from the correlation coefficient of data available for the same outcome in Wood‐Baker 2006. The sample size in Watson 1997 was approximately 50% the size of the other studies. Results showed no change in direction or statistical significance of the pooled difference by either method. We have presented in the text the result obtained with the standard deviation for Watson 1997 based on the value obtained from other studies, and a table in Appendix 4 shows corresponding results with use of an imputed standard deviation.
The small number of included studies limited sensitivity analyses performed by risk of bias grading. The increased likelihood of hospital admission for an acute exacerbation remained significant when we excluded studies with unclear risk of bias for randomisation and allocation concealment.
Discusión
Resumen de los resultados principales
Esta actualización de revisión sistemática resume los efectos de un plan de acción (definido como una guía que detalla acciones iniciadas por el paciente, como el cambio en los regímenes de medicamentos o la visita a un médico general [MG] u hospital, como respuesta a alteraciones en los síntomas de la EPOC que indican el comienzo de una exacerbación) más un componente educativo de corta duración (hasta una hora) solo versus la atención clínica habitual de la EPOC. Siete estudios aleatorios relevantes aportaron datos para la comparación del plan de acción versus la atención habitual para las exacerbaciones de la EPOC. Se incluyeron estudios que prestaron apoyo continuo dirigido al uso del plan de acción, y se excluyeron los estudios con intervenciones de autocuidado más amplias.
Para el resultado primario de utilización de la asistencia sanitaria por las exacerbaciones, las pruebas muestra un beneficio en el término de 12 meses, con menos hospitalizaciones y visitas al servicio de urgencias por la EPOC en un estudio amplio (n = 743) de planes de acción con apoyo telefónico (cociente de tasas [CT] 0,59; intervalo de confianza [IC] del 95%: 0,44 a 0,79; pruebas de calidad moderada [GRADE]) y menor probabilidad de ingreso al hospital en dos estudios (n = 897) (odds ratio [OR] 0,69; IC del 95%: 0,49 a 0,97; pruebas de calidad moderada [GRADE]). Por lo tanto, durante 12 meses en los estudios en que el riesgo inicial de los pacientes era relativamente bajo, el número necesario a tratar para lograr un resultado beneficioso adicional (NNTB) por evitar la hospitalización para una exacerbación fue de 19 (IC del 95%: 11 a 201).
Durante el mismo período de seguimiento, se halló un beneficio para las visitas al servicio de urgencias solas para la EPOC, con menos visitas al servicio de urgencias para la EPOC en un estudio amplio (n = 743) de planes de acción con apoyo telefónico (CR 0,49; IC del 95%: 0,33 a 0,73; (pruebas de alta calidad [GRADE]) y menos probabilidad de una visita al servicio de urgencias para la EPOC en dos estudios (n = 897) (OR 0,55; IC del 95%: 0,38 a 0,78; pruebas de calidad moderada [GRADE]). En 12 meses, el NNTB requerido para evitar una visita al servicio de urgencias para una exacerbación fue de 12 (IC del 95%: 9 a 26). Sin embargo, dos estudios (n = 201) que usaron planes de acción solos no informaron una reducción significativa de la tasa de visitas al servicio de urgencias para la EPOC (DM 0,37; IC del 95%: ‐0,50 a 1,24; pruebas de muy baja calidad [GRADE]). Para los ingresos al hospital solos, un estudio (n = 743) de planes de acción con apoyo telefónico no informó beneficios significativos (CR 0,69; IC del 95%: 0,47 a 1,01; pruebas de calidad moderada [GRADE]). Menos ingresos en el hospital y visitas al servicio de urgencias para la EPOC se tradujeron en costos más bajos para la intervención de planes de acción.
Cuatro estudios (n = 1134) no encontraron cambios significativos en la mortalidad por todas las causas en el término de 12 meses para el uso de planes de acción, con o sin apoyo telefónico (OR 0,88; IC del 95%: 0,59 a 1,31; pruebas de calidad moderada [GRADE]), pero los intervalos de confianza no descartan un beneficio ni un daño importante asociado con la intervención.
Pruebas claras indican que los planes de acción aumentaron el tratamiento para las exacerbaciones de la EPOC en el seguimiento de 12 meses. Dos estudios (n = 200) informaron un aumento de los ciclos de corticosteroides orales (DM 0,74; IC del 95%: 0,12 a 1,35; pruebas de calidad moderada [GRADE]) , y un estudio (n = 743) informó un aumento de la dosis acumulativa de corticosteroides orales con apoyo telefónico al uso de planes de acción (779,0 mg de prednisolona, IC del 95%: 533,2 a 1024,8; pruebas de alta calidad [GRADE]). Tres estudios (n = 943) informaron un aumento significativo de los ciclos de antibióticos (DM 2,26; IC del 95%: 1,82 a 2,70; pruebas de calidad moderada [GRADE]).
Los estudios mostraron un beneficio estadísticamente significativo para la calidad de vida relacionada con la enfermedad respiratoria a partir del uso de planes de acción en 12 meses. Con la puntuación general del St. George Respiratory Questionnaire (SGRQ), tres estudios (n = 1009) informaron que la puntuación fue 2,8 unidades más baja (de 0,8 más baja a 4,8 más baja (pruebas de calidad moderada [GRADE]). El intervalo de confianza incluye la diferencia mínima clínicamente importante de 4 unidades. La revisión no encontró pruebas claras de un beneficio para la morbilidad psicológica en la depresión o la ansiedad, medida con la Hospital Anxiety and Depression Scale (HADS) en un único estudio de 12 meses (pruebas de baja calidad [GRADE]).
Las pruebas también indican un efecto positivo sobre el conocimiento del autocuidado adecuado para las exacerbaciones en tres estudios que usaron diferentes instrumentos de medición. Se encontraron pruebas claras de que los planes de acción con educación limitada mejoraron el reconocimiento y las acciones para un autocuidado adecuado durante los estadios iniciales y en las exacerbaciones severas, y resultaron en un aumento de la autoeficacia para la prevención de la exacerbación y las acciones a tomar.
Análisis de subgrupos: efecto del apoyo continuo dirigido al uso del plan de acción telefónico o por contacto directo
La variación en las mediciones de los estudios limitó las formas en que podrían agruparse los metanálisis según el apoyo continuo al uso de un plan de acción. Dos resultados de utilización de la asistencia sanitaria aportaron datos para los análisis de subgrupos. Para la probabilidad de al menos un ingreso al hospital en 12 meses (Análisis 1.2), en un estudio sin apoyo continuo (OR 0,97; IC del 95%: 0,31 a 3,03) y en un estudio con apoyo telefónico continuo (OR 0,66; IC del 95%: 0,46 a 0,95), los resultados no mostraron ninguna heterogeneidad entre los resultados de los subgrupos (Chi² = 0,39; gl = 1, P = 0,53, I² = 0%). En los mismos estudios, para la probabilidad de al menos una visita al servicio de urgencias en 12 meses (Análisis 1.10), el estudio sin apoyo continuo (OR 0,64; IC del 95%: 0,25 a 1,66) y el estudio con apoyo telefónico (OR 0,53; IC del 95%: 0,36 a 0,78) no reveló ninguna heterogeneidad entre los subgrupos (Chi² = 0,13; gl = 1, P = 0,72, I² = 0%).
Para la mortalidad por todas las causas en el término de 12 meses (Análisis 1.18), tres estudios sin apoyo continuo (OR 1,66; IC del 95%: 0,73 a 3,79) y un estudio con apoyo telefónico continuo (OR 0,72; IC del 95%: 0,46 a 1,14) mostraron una heterogeneidad moderada entre los resultados de los subgrupos (Chi² = 5,17; gl = 3, P = 0,16, I² = 42%); sin embargo, los resultados del análisis de las diferencias de subgrupos no fueron estadísticamente significativos (Chi² = 3,03; gl = 1, P = 0,08, I² = 67,0%).
Para el uso de la medicación para las exacerbaciones, sólo un resultado permitió el análisis de subgrupos. Para los ciclos de antibióticos por 12 meses (Análisis 1.27), en dos estudios sin apoyo continuo (DM 0,78; IC del 95%: ‐0,24 a 1,79) y en un estudio con apoyo telefónico (DM 2,60; IC del 95%: 2,12 a 3,08), el análisis agrupado mostró una heterogeneidad significativa (Chi² = 10,55; gl = 2; p = 0,005, I² = 81%), y los resultados del análisis de las diferencias de los subgrupos fueron significativos (Chi² = 10,09; gl = 1; P = 0,001; I² = 90,1%)
Compleción y aplicabilidad general de las pruebas
Las búsquedas de esta revisión son actuales a noviembre de 2015, y los resultados de la revisión se basan en siete estudios con 1550 pacientes con EPOC sintomáticos, con características bastante típicas de la población con EPOC en mayor riesgo de exacerbaciones. En cuatro estudios, el volumen espiratorio forzado a un segundo (VEF1) medio de los pacientes fue < 50% del previsto, y en tres estudios < 60% del previsto. Tres estudios requirieron antecedentes de exacerbaciones en el último año (Martin 2004; McGeoch 2004; Rice 2010). Dos estudios excluyeron a los participantes que recibían oxigenoterapia domiciliaria (Martin 2004; McGeoch 2004), y un estudio (Watson 1997) excluyó a los que recibían tratamiento corticosteroide oral a largo plazo. El reclutamiento de los estudios fue comunitario, y los participantes presentaban un número variable de enfermedades concomitantes. Los resultados de los metanálisis, incluidos los de la utilización de la asistencia sanitaria, los ingresos en hospitales y las consultas al servicio de urgencias, se basan en un reducido número de estudios con períodos de seguimiento similares, y el riesgo inicial de ingreso al hospital de los estudios incluidos es relativamente bajo. Se desconoce la aplicabilidad de los resultados a las poblaciones con riesgo inicial alto.
Según se muestra en la Tabla adicional 2, el formato de la intervención incluía elementos comunes en el plan de acción de autocuidado: el reconocimiento temprano de las exacerbaciones a partir de los síntomas, las intervenciones iniciadas por el paciente y las instrucciones para buscar la atención médica. La educación de autocuidado se limitó a una única sesión breve, y así la intervención excluyó los programas de apoyo de autocuidado multifacéticos. La información disponible de tres estudios indicó que la duración de la sesión educativa fue de 45 minutos (Rootmensen 2008), 60 minutos (McGeoch 2004) y 60 a 90 minutos (Rice 2010). Esta actualización incluyó el apoyo para poner en práctica el plan de acción brindado hasta por un mes mediante contacto directo o por teléfono. Se hizo este cambio para representar la práctica clínica en que se usan planes de acción en ámbitos ambulatorios con alguna forma de apoyo continuo. Rice 2010 y Trappenburg 2011 incluyeron el apoyo para el uso de planes de acción. Para los ingresos en hospitales o las visitas al servicio de urgencias combinadas, Rice 2010 informó un cociente de tasas de 0,78 (IC del 95%: 0,35 a 1,74; P = 0,53) para los pacientes que recibían cuatro a ocho llamadas versus cero a tres llamadas. La tasa para los participantes con nueve o más llamadas versus cero a tres llamadas mostró una reducción significativa con un aumento de los contactos telefónicos (CR 0,46; IC del 95%: 0,22 a 0,96; P = 0,04).
Con el agregado de los estudios nuevos, esta actualización ha mostrado un mayor beneficio para los resultados del paciente, además de una mejoría en el conocimiento y la comprensión de las acciones adecuadas frente a una exacerbación. El análisis de los datos de mortalidad fue posible solamente para la mortalidad por todas las causas, ya que no se dispuso de datos para la mortalidad relacionada con la enfermedad respiratoria. Los estudios informaron pocos datos de efectos adversos a pesar del mayor uso de corticosteroides orales en el grupo de plan de acción. En Walters 2014, una reacción medicamentosa adversa fue significativamente más probable con el tratamiento con corticosteroides de las exacerbaciones agudas que con placebo (OR 2,33; IC del 95%: 1,60 a 3,40) y el número necesario a tratar para que se produzca un resultado perjudicial adicional (NNTH) fue de 6 (IC del 95%: 4 a 10). La hiperglucemia fue el evento adverso más frecuente (OR 4,95; IC del 95%: 2,47 a 9,91). Los datos sobre los resultados informados por el paciente son escasos; esta revisión muestra el beneficio para la calidad de vida general relacionada con la enfermedad respiratoria, aunque el uso de diferentes instrumentos o períodos de seguimiento impidió los metanálisis para la calidad de vida genérica y la morbilidad psicológica.
Calidad de la evidencia
Entre los resultados primarios, esta actualización de revisión, que incorpora dos estudios nuevos realizados desde 2009, presenta pruebas de un beneficio para las medidas de utilización de la asistencia sanitaria de importancia variable; la calidad de los efectos en los ingresos hospitalarios y las visitas al servicio de urgencias generalmente alta o moderada, cuando se basó en un número mayor de estudios o en estudios con un tamaño grande de la muestra, y una menor calidad cuando se basó en estudios más pequeños realizados con anterioridad (antes de 2004). Debido a la imprecisión, los autores de la revisión disminuyeron la calidad a moderada para el resultado de mortalidad. Se calificó el beneficio claro derivado de un mayor uso del tratamiento para las exacerbaciones agudas como moderado (ciclos de corticosteroides orales) o alto (dosis acumulativas de corticosteroides orales), y como moderado para los ciclos de antibióticos. Para los resultados informados por el paciente, se calificó la mejoría en la calidad de vida relacionada con la enfermedad respiratoria como de calidad moderada; aunque mejoró la calidad de vida relacionada con la salud, el tamaño del efecto medio fue pequeño, por lo que este hecho puede no ser clínicamente significativo para la mayoría de los pacientes. Se calificó como baja la calidad de las pruebas para el dominio psicológico de la depresión, y se no observó ninguna diferencia significativa.
Sesgos potenciales en el proceso de revisión
Puede haber factores de confusión en los estudios basados en la atención primaria mediante la autoselección de los médicos generales (MG), con un interés en la EPOC (Watson 1997), en quienes sería más probable tratar las exacerbaciones de la EPOC con antibióticos o prednisolona ante la falta de un plan de acción. Sin embargo, esto fue menos probable en los estudios que usaron asignación al azar en grupos (McGeoch 2004; Wood‐Baker 2006). Wood‐Baker 2006 no explicaron el efecto del agrupamiento. En el metanálisis, los autores de la revisión incluyeron resultados de la media de los grupos disponibles para los pacientes individuales, lo que podría llevar a la sobrestimación del efecto. Los autores de la revisión (excepto el autor del estudio) extrajeron e introdujeron todos los datos de Wood‐Baker 2006. Los autores de la revisión que no eran investigadores en un estudio tomaron decisiones sobre disminución de calidad en las tablas de Resumen de los resultados. McGeoch 2004 informó métodos de análisis que dieron cuenta del agrupamiento.
Acuerdos y desacuerdos con otros estudios o revisiones
Como el objetivo primario de esta revisión es examinar los efectos específicos de un plan de acción de autocuidado para las exacerbaciones de la EPOC, se restringieron los estudios incluidos a los que excluyeron el entrenamiento y la educación en autocuidado amplios, a menudo en formato grupal, y a veces como parte de un programa de rehabilitación pulmonar. En la práctica clínica en algunos ámbitos, un plan de acción puede incluir alguna forma de apoyo continuo al uso de planes de acción, a menudo durante el tratamiento del caso para los pacientes ambulatorios o en los que reciben atención primaria. Por este motivo, se decidió que esta actualización debía incluir estudios con apoyo continuo por hasta un mes limitado al uso del plan de acción. Se predefinió este cambio en los criterios de inclusión de la revisión anterior (Walters 2010) en el protocolo actualizado. El análisis de subgrupos posiblemente indica un mayor efecto para el apoyo telefónico regular.
En comparación con la versión anterior de esta revisión, las pruebas de calidad moderada indican ahora un beneficio en la utilización de la asistencia sanitaria para los planes de acción para la EPOC. En gran parte, este desarrollo se debe a la inclusión de dos estudios nuevos (Rice 2010; Trappenburg 2011) que trataron el apoyo continuo al uso de planes de acción y aportaron un adicional de 976 pacientes al análisis agrupado.
Otras revisiones sistemáticas consideraron los efectos de las intervenciones de autocuidado con o sin planes de acción (protocolo publicado [Zwerink 2014]), y examinaron los planes de acción que forman parte de un programa educativo de autocuidado amplio (Lenferink 2015) o el programa integral de rehabilitación pulmonar (McCarthy 2015). En Zwerink 2014, 74% de los estudios (n = 23) incluyeron un plan de acción como parte de la intervención de autocuidado. De modo similar a la revisión, Zwerink 2014 encontró una reducción de la probabilidad de hospitalización relacionada con la enfermedad respiratoria (OR 0,57; IC del 95%: 0,43 a 0,75) en nueve estudios que informaron un beneficio similar en el número necesario a tratar para lograr un resultado beneficioso adicional (NNTB) en una población con riesgo inicial bajo de evitar el ingreso al hospital (20; IC del 95%: 15 a 35). No fue posible crear subgrupos de al menos tres estudios que no usaran un plan de acción en la intervención; por lo tanto, los autores de la revisión no realizaron análisis de subgrupos para Zwerink 2014. Por lo tanto, el beneficio logrado por un plan de acción con un componente corto de educación del paciente equivale al logrado por programas educativos y programas de autocuidado más integrales. Una revisión de los programas de rehabilitación pulmonar, en que los subgrupos compararon ensayos de "ejercicio solo" (n = 31 ensayos) y ensayos de "ejercicio más componentes más integrales" (n = 34), no observaron diferencias significativas en el efecto sobre la calidad de vida relacionada con la enfermedad respiratoria (McCarthy 2015). La ausencia de efecto sobre la mortalidad informada en esta revisión es similar al resultado de ningún efecto sobre la mortalidad en la revisión de la educación en autocuidado (Zwerink 2014), que no incluyó datos de Fan 2012 sobre un programa integral de manejo de la atención que comprendía planes de acción. Los investigadores de Fan 2012 interrumpieron el estudio antes de tiempo porque los autores no pudieron explicar un número mayor de muertes en el grupo de intervención versus grupo de control de manera satisfactoria.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Action plan versus usual care, outcome: 1.18 Mortality (all cause) 12 months.

Comparison 1 Action plan versus usual care, Outcome 1 Hospitalizations for COPD /100 patient years.

Comparison 1 Action plan versus usual care, Outcome 2 At least 1 hospital admission (12 months).

Comparison 1 Action plan versus usual care, Outcome 3 at least 1 Hospital Admission (6 months).

Comparison 1 Action plan versus usual care, Outcome 4 Hospital admission (12 months).
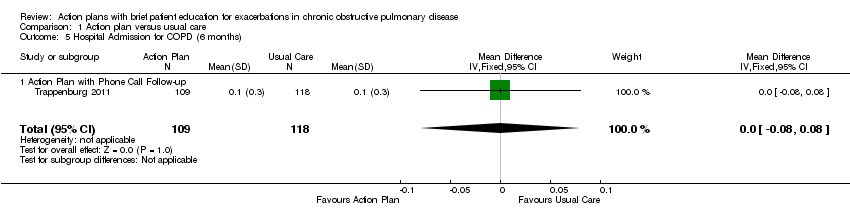
Comparison 1 Action plan versus usual care, Outcome 5 Hospital Admission for COPD (6 months).
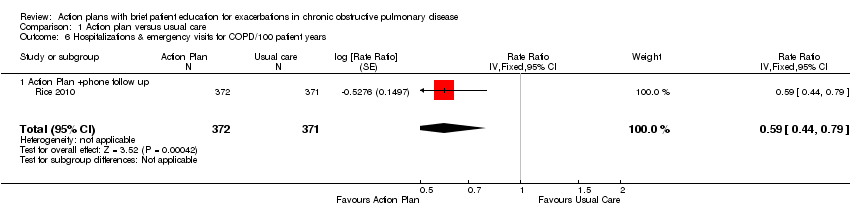
Comparison 1 Action plan versus usual care, Outcome 6 Hospitalizations & emergency visits for COPD/100 patient years.

Comparison 1 Action plan versus usual care, Outcome 7 At Least 1 Hospital or Emergency Department Visit for COPD.

Comparison 1 Action plan versus usual care, Outcome 8 Emergency department visits for COPD /100 patient years.

Comparison 1 Action plan versus usual care, Outcome 9 Emergency department visit for COPD (12 months).

Comparison 1 Action plan versus usual care, Outcome 10 At least 1 emergency department visit (12 months).

Comparison 1 Action plan versus usual care, Outcome 11 Emergency Department Visits for COPD (6 months).
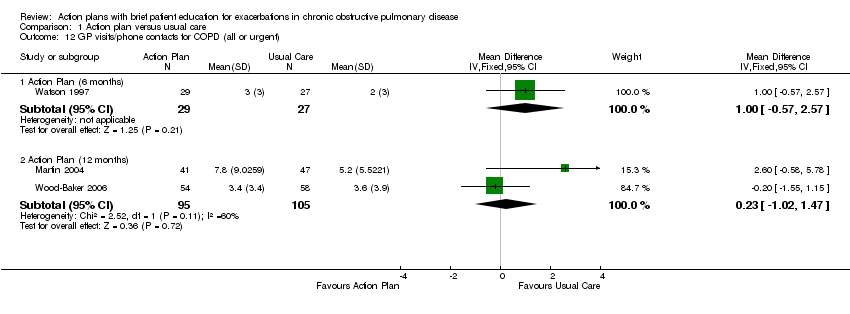
Comparison 1 Action plan versus usual care, Outcome 12 GP visits/phone contacts for COPD (all or urgent).
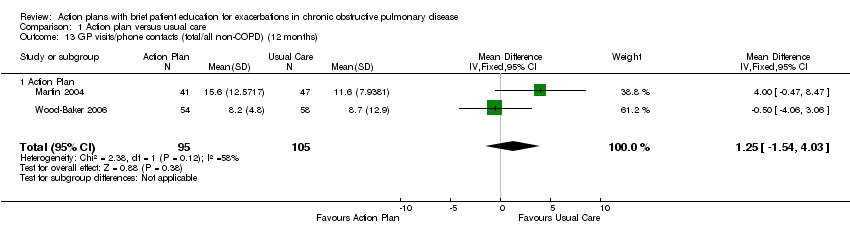
Comparison 1 Action plan versus usual care, Outcome 13 GP visits/phone contacts (total/all non‐COPD) (12 months).

Comparison 1 Action plan versus usual care, Outcome 14 Unscheduled Physician Visits (6 months).

Comparison 1 Action plan versus usual care, Outcome 15 Ambulance calls (total).

Comparison 1 Action plan versus usual care, Outcome 16 Total Hospital Days (12 months).

Comparison 1 Action plan versus usual care, Outcome 17 Total ICU Days (12 months).
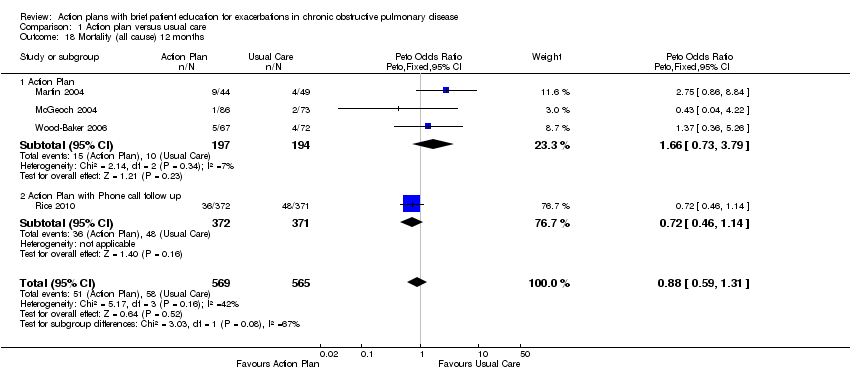
Comparison 1 Action plan versus usual care, Outcome 18 Mortality (all cause) 12 months.

Comparison 1 Action plan versus usual care, Outcome 19 Mortality (all cause) per 100 Patient‐Years (12 months).
Comparison 1 Action plan versus usual care, Outcome 20 Mortality (all cause) 6 months.

Comparison 1 Action plan versus usual care, Outcome 21 At least 1 course oral steroids for exacerbation.

Comparison 1 Action plan versus usual care, Outcome 22 Courses of oral corticosteroids (12 months).

Comparison 1 Action plan versus usual care, Outcome 23 Courses of Corticosteroids (6 months).

Comparison 1 Action plan versus usual care, Outcome 24 Days on corticosteroids (6 months).

Comparison 1 Action plan versus usual care, Outcome 25 Prednisolone mg (12 months).

Comparison 1 Action plan versus usual care, Outcome 26 At least 1 course antibiotics for exacerbation.

Comparison 1 Action plan versus usual care, Outcome 27 Courses of antibiotics (12 months).

Comparison 1 Action plan versus usual care, Outcome 28 Courses of Antibiotics (6 months).

Comparison 1 Action plan versus usual care, Outcome 29 Days on antibiotics (6 months).

Comparison 1 Action plan versus usual care, Outcome 30 SGRQ overall score (12 months).

Comparison 1 Action plan versus usual care, Outcome 31 SGRQ overall score (6 months).
Comparison 1 Action plan versus usual care, Outcome 32 SGRQ symptoms (12 months).

Comparison 1 Action plan versus usual care, Outcome 33 SGRQ symptoms (6 months).

Comparison 1 Action plan versus usual care, Outcome 34 SGRQ activity limitation (12 months).

Comparison 1 Action plan versus usual care, Outcome 35 SGRQ activity limitation (6 months).
Comparison 1 Action plan versus usual care, Outcome 36 SGRQ impact (12 months).

Comparison 1 Action plan versus usual care, Outcome 37 SGRQ impact score (6 months).

Comparison 1 Action plan versus usual care, Outcome 38 SF36 physical function (6 months).

Comparison 1 Action plan versus usual care, Outcome 39 SF36 role limitation physical (6 months).
Comparison 1 Action plan versus usual care, Outcome 40 SF36 bodily pain (6 months).

Comparison 1 Action plan versus usual care, Outcome 41 SF36 general health (6 months).

Comparison 1 Action plan versus usual care, Outcome 42 SF36 vitality (6 months).

Comparison 1 Action plan versus usual care, Outcome 43 SF36 mental health (6 months).

Comparison 1 Action plan versus usual care, Outcome 44 SF36 role limitation emotional (6 months).
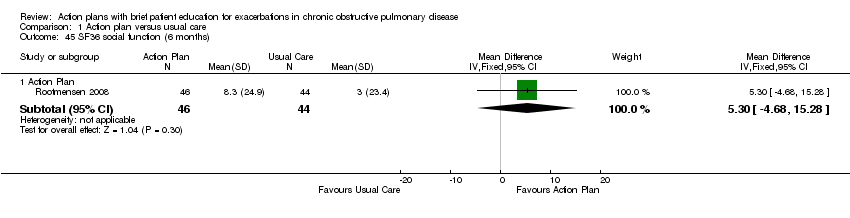
Comparison 1 Action plan versus usual care, Outcome 45 SF36 social function (6 months).

Comparison 1 Action plan versus usual care, Outcome 46 HADS ‐ depression score (12 months).
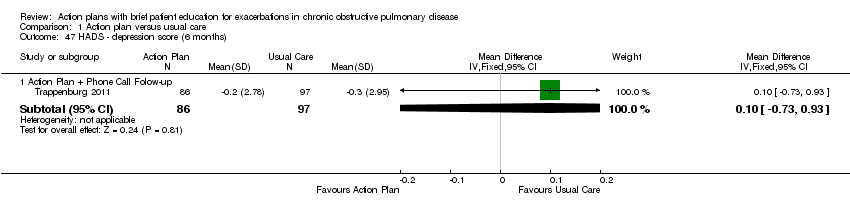
Comparison 1 Action plan versus usual care, Outcome 47 HADS ‐ depression score (6 months).

Comparison 1 Action plan versus usual care, Outcome 48 HADS ‐ anxiety score (12 months).

Comparison 1 Action plan versus usual care, Outcome 49 HADS ‐ anxiety score (6 months).

Comparison 1 Action plan versus usual care, Outcome 50 Exacerbation knowledge when well (12 months).

Comparison 1 Action plan versus usual care, Outcome 51 Exacerbation actions when well (12 months).

Comparison 1 Action plan versus usual care, Outcome 52 Early exacerbation knowledge (12 months).
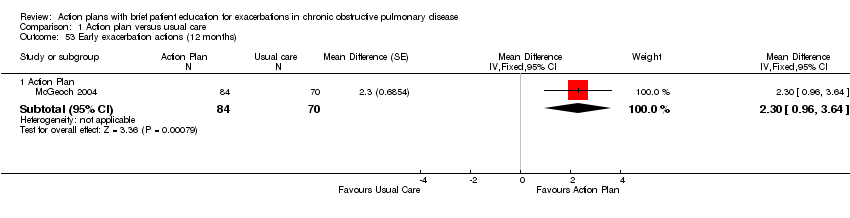
Comparison 1 Action plan versus usual care, Outcome 53 Early exacerbation actions (12 months).
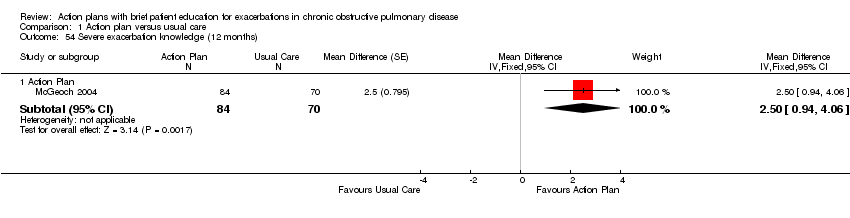
Comparison 1 Action plan versus usual care, Outcome 54 Severe exacerbation knowledge (12 months).

Comparison 1 Action plan versus usual care, Outcome 55 Severe exacerbation actions (12 months).

Comparison 1 Action plan versus usual care, Outcome 56 Self‐management exacerbation actions (6 months).

Comparison 1 Action plan versus usual care, Outcome 57 Self‐efficacy for Exacerbation Recognition (6 months).

Comparison 1 Action plan versus usual care, Outcome 58 Self‐efficacy for Exacerbation Prevention/Action (6 months).

Comparison 1 Action plan versus usual care, Outcome 59 FEV1 % predicted.
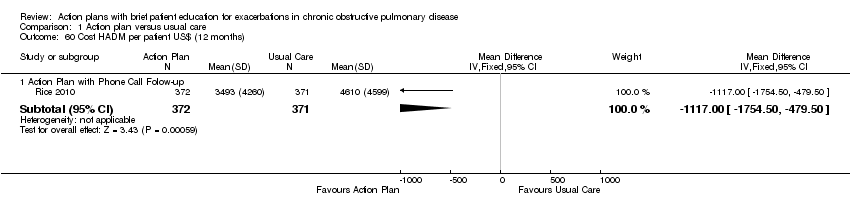
Comparison 1 Action plan versus usual care, Outcome 60 Cost HADM per patient US$ (12 months).

Comparison 1 Action plan versus usual care, Outcome 61 Cost EDV Per Patient US$ (12 months).

Comparison 1 Action plan versus usual care, Outcome 62 Cost Pulmonary Drug Prescriptions per Patient US$ (12 months).
| Do action plans improve patient outcomes in acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: individuals with exacerbations of chronic obstructive pulmonary disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with action plan | |||||
| Hospitalisations for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.69 | 743 | ⊕⊕⊕⊝ | |||
| Hospitalisations and emergency visits for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.59 | 743 | ⊕⊕⊕⊕ | |||
| At least 1 hospital admission | 209 per 1000 | 154 per 1000 | Odds ratio 0.69 | 897 | ⊕⊕⊕⊝ | |
| Mortality (all‐cause) | 103 per 1000 | 91 per 1000 | Odds ratio 0.88 | 1134 | ⊕⊕⊕⊝ | |
| Courses of oral corticosteroids | Mean courses of oral corticosteroids were 1.05 | Mean courses of oral corticosteroids in the intervention group were 0.74 more (0.12 more to 1.35 more) | ‐ | 200 | ⊕⊕⊕⊝ | |
| Courses of antibiotics | Mean courses of antibiotics ranged from 1.6 to 3.2 | Mean courses of antibiotics in the intervention group were 2.26 more (1.82 more to 2.7 more) | ‐ | 943 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Respiratory‐related quality of life: SGRQ overall score | Mean respiratory‐related quality of life: SGRQ overall score ranged from ‐2 to +6 units | Mean respiratory‐related quality of life: SGRQ overall score in the intervention group was 2.82 units lower (0.83 lower to 4.81 lower) | ‐ | 1009 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Depression score | Mean depression score was ‐0.04 | Mean depression score in the intervention group was 0.25 lower (1.14 lower to 0.64 higher) | ‐ | 154 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWide confidence interval; effect size includes null. bUnclear risk of bias for two studies for allocation and blinding of assessors. cUnclear risk of bias for three studies for allocation and blinding of assessors. dUnclear risk of bias for one study for allocation and blinding of assessors. | ||||||
| Study ID | Dates | Recruitment/Randomisation unit | Follow‐up | Length SME (educator) | RAN, n/WD, n | Age*, years/ % male | % current smokers | FEV1 % pred* INT‐CONT | QoL INT‐CONT |
| Not known | Consortium practices, New Zealand/participants | 12 months | Single interview, length not stated (respiratory nurse) | 96/26 | 70/51 | n/a | 35‐34 | 57‐51 | |
| 7/2002‐ 12/2003 | 2 groups of practices, New Zealand/practice | 12 months | 1 hour (practice nurse or respiratory educator) | 159/9 | 71/59 | 28 | 55‐53 | 43‐37 | |
| Rootmensen 2008 (all participants) | Not known | 1 hospital pulmonary outpatient clinic, Netherlands/ participants | 6 months | 45 minutes (pulmonary nurse) | 157 (111 COPD)/17 | 60/55 | 12 | 57‐64 | n/a |
| 07/2004‐ 07/2008 | Centralised electronic medical record database/participants | 12 months | 1 to 1.5‐hour group educational session (case manager) | 743/84 | 70/98 | 22 | 36.1‐38.1 | n/a | |
| 12/2008‐ 12/2010 | 8 regional hospitals and 5 general practices/participants (stratified by gender and centre) | 6 months | Single interview, length not stated (nurse case manager). | 233/41 | 66/57 | 29 | 56.7‐56.5 | n/a | |
| 1993‐ 07/1994 | 12 practices, 22 GPs, New Zealand/participants | 6 months | Single interview, length not stated (practice nurse) | 69/13 | 68/65 | 28 | 37‐36 | 43‐39 | |
| 2002‐2003 | 54 GPs, 31 practices, Australia/practice | 12 months | 1 hour (respiratory research nurse) | 139/27 | 70/76 | 42 | 46‐44 | 47‐47 | |
| *: mean; AP: action plan; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; GP: general practitioner; INT‐CONT: intervention group‐control group; QoL: % impairment quality of life 0‐100; RAN: randomisation; SME: self‐management education; WD: withdrawal or death. | |||||||||
| Individualised AP | Standard written AP | Support for AP during study period | SME (individual/group) | Prescription /supply OCS | Prescription /supply ABS | Written COPD educational component | Comparison | |
| Written | 3‐Monthly visit regarding use of AP | Individual interview with respiratory nurse, length not stated, individualised action plan according to current treatment and symptoms | All had 7‐day supply | All had 7‐day supply | No | Usual care by own GP | ||
| Yes | No | Individual session by practice nurse or respiratory educator in association with GP 1 hour, covering major points of COPD self‐management plan, and use of validated sputum colour charts | Prescription | Prescription | Educational package | Non‐standard education on COPD according to practice standards | ||
| Written | Monthly phone call from nurse | Group 1‐1.5 hours, individualised action plan with respiratory nurse | Yes | Prescription | Usual care + 1‐page summary of principles of COPD care according to published guidelines. No AP | |||
| Oral | No | Individual protocol‐based educational session covering disease, medications, vaccination, smoking cessation and exacerbation management, 45 minutes in length | Oral medication provided to some, % unknown | Oral medication provided to some, % unknown | No | Usual care | ||
| Written | Standardised phone calls at 1 and 4 months | Individualised action plan education, length of session not stated | 2%' | 22% | ✓ COPD information | Usual care ‐ pharmacological and non‐pharmacological care according to most recent evidence‐based guidelines, specifically AP denied. All included participants seen by respiratory nurse, who systematically checked and discussed aspects of COPD care, including vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. | ||
| Yes | No | Individual session education about use of the action plan with COPD booklet by a senior respiratory outreach nurse; length not stated | Prescription | Prescription | ✓ Guide to living positively with COPD | Usual care by GP, specifically denied access to AP and booklet | ||
| Written | No | Individual educational session with respiratory nurse, covering COPD, smoking cessation, immunisation, nutrition, exercise, sputum clearance, breathing, medication, inhaler use. Individualised action plan developed with GP input. Length not known | 2% | 22% | COPD information booklet | Usual care, COPD information booklet and individual educational session with nurse, but no AP | ||
| ABS: antibiotics; AP: action plan; COPD: chronic obstructive pulmonary disease; GP: general practitioner; OCS: oral corticosteroids; SME: self‐management education. | ||||||||
| Outcome | SF‐36 domain | Mean difference | 95% CI |
| Physical function | 0.30 | ‐7.13 to 7.73 | |
| Role limitation | 9.00 | ‐8.07 to 26.07 | |
| Bodily pain | 18.50 | 6.14 to 30.86 | |
| General health | 2.60 | ‐3.71 to 8.91 | |
| Vitality | 1.60 | ‐4.73 to 7.93 | |
| Social function | 5.30 | ‐4.68 to 15.28 | |
| Role limitation | 7.50 | ‐8.56 to 23.56 | |
| Mental health | 6.30 | 0.64 to 11.96 |
| Outcome | Domain | Follow‐up: months | MD | 95% CI | n |
| Depression | 12 | ‐0.25 | ‐1.14 to 0.64 | 154 | |
| Depression | 6 | 0.10 | ‐0.73 to 0.93 | 183 | |
| Anxiety | 12 | 0.14 | ‐1.38 to 1.66 | 154 | |
| Anxiety | 6 | 0.00 | ‐0.83 to 0.83 | 183 |
| Outcome | Study | Item | Direction improvement | Months | MD | 95% CI | n |
| Self‐management knowledge when well | + | 12 | 1.10 | 0.46 to 1.74 | 154 | ||
| Self‐management actions when well | + | 12 | 0.50 | ‐0.24 to 1.24 | 154 | ||
| Self‐management knowledge early exacerbation | + | 12 | 1.80 | 0.75 to 2.85 | 154 | ||
| Self‐management actions early exacerbation | + | 12 | 2.30 | 0.96 to 3.64 | 154 | ||
| Self‐management knowledge severe exacerbation | + | 12 | 2.50 | 0.94 to 4.06 | 154 | ||
| Self‐management action severe exacerbation | + | 12 | 1.50 | 0.47 to 2.53 | 154 | ||
| Self‐management exacerbation actions | + | 6 | ‐5.10 | ‐15.26 to 5.06 | 90 | ||
| Self‐efficacy for exacerbation recognition | ‐ | 6 | ‐0.70 | ‐0.98 to ‐0.42 | 183 | ||
| Self‐efficacy for exacerbation prevention/action | ‐ | 6 | ‐0.90 | ‐1.18 to ‐0.62 | 183 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospitalizations for COPD /100 patient years Show forest plot | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 1.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 2 At least 1 hospital admission (12 months) Show forest plot | 2 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.97] |
| 2.1 Action Plan | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.31, 3.03] |
| 2.2 Action Plan + Phonecall Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.95] |
| 3 at least 1 Hospital Admission (6 months) Show forest plot | 1 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.30, 2.31] |
| 3.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.30, 2.31] |
| 4 Hospital admission (12 months) Show forest plot | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.03, 0.49] |
| 4.1 Action Plan | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.03, 0.49] |
| 5 Hospital Admission for COPD (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 5.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 6 Hospitalizations & emergency visits for COPD/100 patient years Show forest plot | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.44, 0.79] |
| 6.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.44, 0.79] |
| 7 At Least 1 Hospital or Emergency Department Visit for COPD Show forest plot | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 7.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 8 Emergency department visits for COPD /100 patient years Show forest plot | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 8.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 9 Emergency department visit for COPD (12 months) Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 9.1 Action Plan | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 10 At least 1 emergency department visit (12 months) Show forest plot | 2 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.78] |
| 10.1 Action Plan | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.25, 1.66] |
| 10.2 Action Plan + Phone Call Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.78] |
| 11 Emergency Department Visits for COPD (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 11.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 12 GP visits/phone contacts for COPD (all or urgent) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Action Plan (6 months) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.57, 2.57] |
| 12.2 Action Plan (12 months) | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.02, 1.47] |
| 13 GP visits/phone contacts (total/all non‐COPD) (12 months) Show forest plot | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.54, 4.03] |
| 13.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.54, 4.03] |
| 14 Unscheduled Physician Visits (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 14.1 Action Plan with Phonecall Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 15 Ambulance calls (total) Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.17, 3.23] |
| 15.1 Action Plan | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.17, 3.23] |
| 16 Total Hospital Days (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [0.00, ‐0.20] |
| 16.1 Action Plan + Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [0.00, ‐0.20] |
| 17 Total ICU Days (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| 17.1 Action Plan + Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| 18 Mortality (all cause) 12 months Show forest plot | 4 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 18.1 Action Plan | 3 | 391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [0.73, 3.79] |
| 18.2 Action Plan with Phone call follow up | 1 | 743 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.46, 1.14] |
| 19 Mortality (all cause) per 100 Patient‐Years (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐8.86, 1.46] |
| 19.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐8.86, 1.46] |
| 20 Mortality (all cause) 6 months Show forest plot | 1 | 229 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.15, 7.66] |
| 20.1 Action Plan with Phone Call Follow‐up | 1 | 229 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.15, 7.66] |
| 21 At least 1 course oral steroids for exacerbation Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Action Plan (6 months) | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.58 [1.29, 33.62] |
| 21.2 Action Plan (12 months) | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.34, 4.69] |
| 22 Courses of oral corticosteroids (12 months) Show forest plot | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.12, 1.35] |
| 22.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.12, 1.35] |
| 23 Courses of Corticosteroids (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.23, 0.23] |
| 23.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.23, 0.23] |
| 24 Days on corticosteroids (6 months) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.53, 17.53] |
| 24.1 Action Plan | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.53, 17.53] |
| 25 Prednisolone mg (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 779.0 [533.23, 1024.77] |
| 25.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 779.0 [533.23, 1024.77] |
| 26 At least 1 course antibiotics for exacerbation Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 26.1 Action Plan (6 months) | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.51 [2.02, 21.05] |
| 26.2 Action Plan (12 months) | 2 | 293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.01, 2.69] |
| 27 Courses of antibiotics (12 months) Show forest plot | 3 | 943 | Mean Difference (IV, Fixed, 95% CI) | 2.26 [1.82, 2.70] |
| 27.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [‐0.24, 1.79] |
| 27.2 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [2.12, 3.08] |
| 28 Courses of Antibiotics (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 28.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 29 Days on antibiotics (6 months) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [1.40, 10.60] |
| 29.1 Action Plan | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [1.40, 10.60] |
| 30 SGRQ overall score (12 months) Show forest plot | 3 | 1009 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.77, ‐0.82] |
| 30.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐2.70, 3.34] |
| 30.2 Action Plan + Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐7.70, ‐2.50] |
| 31 SGRQ overall score (6 months) Show forest plot | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐2.93, 1.27] |
| 31.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐3.03, 2.37] |
| 31.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐4.94, 1.74] |
| 32 SGRQ symptoms (12 months) Show forest plot | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐7.14, 3.47] |
| 32.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐7.14, 3.47] |
| 33 SGRQ symptoms (6 months) Show forest plot | 4 | 448 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐6.92, 1.83] |
| 33.1 Action Plan | 3 | 265 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐8.34, 4.20] |
| 33.2 Action Plan + Phone Call Follow‐up (change from baseline) | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐9.10, 3.10] |
| 34 SGRQ activity limitation (12 months) Show forest plot | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 2.87 [‐1.26, 7.00] |
| 34.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 2.87 [‐1.26, 7.00] |
| 35 SGRQ activity limitation (6 months) Show forest plot | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.90, 3.67] |
| 35.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | 1.41 [‐1.99, 4.82] |
| 35.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐5.05, 4.65] |
| 36 SGRQ impact (12 months) Show forest plot | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.51, 2.43] |
| 36.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.51, 2.43] |
| 37 SGRQ impact score (6 months) Show forest plot | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐3.47, 0.95] |
| 37.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐4.45, 1.39] |
| 37.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.9 [‐4.27, 2.47] |
| 38 SF36 physical function (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 38.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐7.13, 7.73] |
| 39 SF36 role limitation physical (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 39.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐8.07, 26.07] |
| 40 SF36 bodily pain (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 40.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 18.5 [6.14, 30.86] |
| 41 SF36 general health (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 41.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐3.71, 8.91] |
| 42 SF36 vitality (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 42.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [‐4.73, 7.93] |
| 43 SF36 mental health (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 6.3 [0.64, 11.96] |
| 44 SF36 role limitation emotional (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 44.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 7.5 [‐8.56, 23.56] |
| 45 SF36 social function (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 45.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐4.68, 15.28] |
| 46 HADS ‐ depression score (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 46.1 Action Plan | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.14, 0.64] |
| 47 HADS ‐ depression score (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 47.1 Action Plan + Phone Call Folow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.73, 0.93] |
| 48 HADS ‐ anxiety score (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 Action Plan | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.38, 1.66] |
| 49 HADS ‐ anxiety score (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 Action Plan + Phone Call Follow‐up (change from baseline) | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.83, 0.83] |
| 50 Exacerbation knowledge when well (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 50.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.1 [0.46, 1.74] |
| 51 Exacerbation actions when well (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 51.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 0.5 [‐0.24, 1.24] |
| 52 Early exacerbation knowledge (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 52.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.80 [0.75, 2.85] |
| 53 Early exacerbation actions (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 53.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 2.3 [0.96, 3.64] |
| 54 Severe exacerbation knowledge (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 54.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 2.5 [0.94, 4.06] |
| 55 Severe exacerbation actions (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 55.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.5 [0.47, 2.53] |
| 56 Self‐management exacerbation actions (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 56.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐5.1 [‐15.26, 5.06] |
| 57 Self‐efficacy for Exacerbation Recognition (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 57.1 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.98, ‐0.42] |
| 58 Self‐efficacy for Exacerbation Prevention/Action (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 58.1 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.18, ‐0.62] |
| 59 FEV1 % predicted Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 59.1 6 months | 2 | 179 | Mean Difference (IV, Fixed, 95% CI) | 1.83 [‐1.05, 4.71] |
| 59.2 12 months | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐1.89, 5.89] |
| 60 Cost HADM per patient US$ (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 60.1 Action Plan with Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1117.0 [‐1754.50, ‐479.50] |
| 61 Cost EDV Per Patient US$ (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 61.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐141.0 [‐234.31, ‐47.69] |
| 62 Cost Pulmonary Drug Prescriptions per Patient US$ (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 62.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 15.00 [‐6.32, 36.32] |

