برنامههای عملیاتی با آموزش مختصر بیمار برای تشدید بیماری در بیماری مزمن انسدادی ریه
چکیده
پیشینه
تشدیدهای بیماری مزمن انسدادی ریه (chronic obstructive pulmonary disease; COPD) یک عامل مهم در کاهش وضعیت سلامت فرد است و باعث میشود هزینههای زیادی بر سیستم مراقبت سلامت وارد شود. برنامههای عملیاتی فرمهایی از خود‐مدیریتی هستند که میتوانند در وضعیتهای خارج بیمارستان اعمال شوند تا به افراد در شناسایی و شروع زودرس درمان تشدیدهای بیماری کمک کنند و تاثیرات ناگوار آنها کاهش یابد.
اهداف
بررسی تاثیرات یک برنامه عملیاتی برای تشدیدهای COPD با یک بخش آموزشی کوتاه برای بیماران بدون برنامه جامع خود‐مدیریتی در برابر مراقبت معمول. پیامدهای اولیه، بهرهوری مراقبت سلامت، مورتالیتی و کاربرد دارویی است. پیامدهای ثانویه کیفیت زندگی مرتبط با سلامت، موربیدیتی روانشناختی، کارکرد ریه و هزینه‐اثربخشی بود.
روشهای جستوجو
ما پایگاه ثبت تخصصی گروه راههای هوایی در کاکرین را همراه با CENTRAL؛ MEDLINE؛ Embase و پایگاههای ثبت کارآزماییهای بالینی جستوجو کردیم. جستوجوها تا نوامبر 2015 بهروز هستند. ما فهرست کتابشناختی مقالات مربوط را جستوجو کرده و با نویسندگان مطالعه برای شناسایی مطالعات بیشتر تماس گرفتیم.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) و شبه‐RCTهایی را وارد کردیم که در آنها استفاده از یک برنامه عملیاتی را در برابر مراقبت معمول در بیماران با تشخیص بالینی COPD مقایسه شده بود. ما اجازه دادیم که یک بخش آموزشی کوتاه به برنامه وارد شود چون این بخش باعث میشود برنامه عملیاتی برای هر فردی (individualisation) بر طبق نیازهای مدیریتی و نشانههای COPD به کار رود، همچنین حمایت مداوم به استفاده از برنامه عملیاتی معطوف شده است.
گردآوری و تجزیهوتحلیل دادهها
از روشهای استاندارد روششناسی مورد نظر کاکرین استفاده کردیم. ما به منظور انجام متاآنالیز (meta‐analysis)، مطالعات را از طریق پیگیری تلفنی به زیر‐گروههایی تقسیم کردیم که باعث تسهیل استفاده از برنامه عملیاتی میشود.
نتایج اصلی
این مرور بهروز شده شامل دو مطالعه بیشتر است (یعنی 976 شرکتکننده اضافی)، در مجموع هفت گروه موازی از RCTها با 1550 شرکتکننده وجود داشت که 66% آنها مرد بودند. میانگین سنی شرکتکنندگان، 68 سال بود که میان مطالعات مشابه بود. انسداد جریان هوا در سه مطالعه نسبتا شدید و در چهار مطالعه شدید بود، ميانگين حجم بازدمی اجباری در یک ثانیه (FEV1) بعد از استفاده از برونکودیلاتور، 54% پیشبینی شده بود، 27% از شرکتکنندگان در زمان انجام مرور سیگاری بودند. در چهار مطالعه برنامههای عملیاتی فردی مهیا شده بود، در یک مطالعه یک برنامه شفاهی و در دو مطالعه برنامههای عملیاتی نوشته شده استاندارد استفاده شده بود. همه مطالعات بخش آموزشی کوتاه را برای COPD فراهم کرده بودند و در دو مطالعه نیز حمایت مداوم برای استفاده از برنامه عملیاتی وجود داشت. در چهار مطالعه، پیگیری 12 ماه و در سه مطالعه شش ماه بود.
یک برنامه عملیاتی با پیگیری تلفنی در مقایسه با مراقبت معمول، نرخ ترکیب بستری شدن و ویزیت اورژانس را برای COPD طی 12 ماه در یک مطالعه با 743 شرکتکننده به طور معناداری کاهش داد (نسبت میزان (RR): 0.59؛ 95% فاصله اطمینان (CI): 0.44 تا 0.79؛ شواهد با کیفیت بالا)، اما نرخ بستری شدن به تنهایی در این مطالعه اهمیت آماری نداشت (RR: 0.69؛ 95% CI؛ 0.47 تا 1.01؛ شواهد با کیفیت متوسط). برنامه عملیاتی، احتمال پذیرش بیمارستانی را طی 12 ماه در مقایسه با مراقبت معمول به طور معناداری کاهش داد (نسبت شانس (OR): 0.69؛ 95% CI؛ 0.49 تا 0.97؛ 897 شرکتکننده؛ دو RCT؛ شواهد با کیفیت متوسط؛ تعداد افراد مورد نیاز جهت درمان تا حصول یک پیامد مثبت بیشتر (number needed to treat for an additional beneficial outcome; NNTB): 19 (11 تا 201)) و همچنین برنامه عملیاتی، احتمال ویزیت اورژانس را در مقایسه با مراقبت معمول به طور معناداری کاهش داد (OR: 0.55؛ 95% CI؛ 0.38 تا 0.78؛ 897 = n؛ دو RCT؛ شواهد با کیفیت متوسط؛ NNTB طی 12 ماه: 12 (9 تا 26)).
نتایج نشان میدهد که هیچ تفاوت معناداری در مورتالیتی به هر علتی طی 12 ماه وجود ندارد (OR: 0.88؛ 95% CI؛ 0.59 تا 1.31؛ 1134 = n؛ چهار RCT؛ شواهد با کیفیت متوسط به دلیل فاصله اطمینان زیاد). استفاده از کورتیکواستروئیدهای خوراکی طی 12 ماه در مقایسه با مراقبت معمول با برنامه عملیاتی افزایش یافته بود (تفاوت میانگین (MD): 0.74 دوره؛ 95% CI؛ 0.12 تا 1.35؛ 200 = n؛ دو RCT؛ شواهد با کیفیت متوسط)، همچنین دوز پردنیزولون تجمعی (cumulative) در گروه برنامه عملیاتی به طور معناداری بالا بود (MD: 779.0 میلیگرم؛ 95% CI؛ 533.2 تا 10248؛ 743 = n؛ یک RCT؛ شواهد با کیفیت بالا). استفاده از آنتیبیوتیک در گروه مداخله نسبت به گروه مراقبت معمول طی 12 ماه بالاتر بود (با پیگیری تلفنی به زیر‐گروههایی تقسیم شده بودند) (MD؛ 2.3 دوره؛ 95% CI؛ 1.8 تا 2.7؛ 943 = n؛ سه RCT؛ شواهد با کیفیت متوسط).
تجزیهوتحلیل زیر‐گروه با حمایت مداوم برای برنامه عملیاتی محدود بود، نویسندگان مرور توجه کرده بودند که هیچ تفاوت زیر‐گروهی در احتمال بستری در بیمارستان، ویزیت اورژانس یا مورتالیتی به هر علتی طی 12 ماه وجود ندارد. استفاده از آنتیبیوتیک طی 12 ماه نشان داد که یک تفاوت معنادار بین زیر‐گروهها در مطالعات وجود دارد چه حمایت مداوم وجود داشته باشد چه وجود نداشته باشد.
نمره کلی کیفیت زندگی بر اساس پرسشنامه تنفسی سنتجورج (St George’s Respiratory Questionnaire; SGRQ) نشان داد که یک بهبودی اندک با برنامه عملیاتی در مقایسه با مراقبت معمول طی 12 ماه وجود دارد (MD: ‐2.8؛ 95% CI؛ 0.8‐ تا 4.8‐؛ 1009 = n؛ سه RCT؛ شواهد با کیفیت متوسط). شواهد با کیفیت پائین نشان میدهد که هیچ مزیتی برای موربیدیتی روانی اندازهگیری شده در مقیاس افسردگی و اضطراب در بیمارستان (Hospital Anxiety and Depression Scale; HADS) وجود ندارد.
نتیجهگیریهای نویسندگان
استفاده از برنامه عملیاتی برای تشدید COPD همراه با یک بخش آموزشی کوتاه با حمایت مداوم در جهت استفاده از برنامه عملیاتی، بدون برنامه جامع خود‐مدیریتی باعث کاهش هزینههای خدمات مراقبت سلامت برای بستری شدن در بیمارستان و افزایش درمان تشدید COPD با کورتیکواستروئیدها و آنتیبیوتیکها میشود. استفاده از برنامه عملیاتی COPD احتمال ندارد در این زمینه مورتالیتی را افزایش یا کاهش دهد. اینکه مزیت بیشتری از حمایتهای مدام دورهای در جهت استفاده از برنامه عملیاتی، به دست میآید یا خیر، از نتایج این مرور قابل تعیین نیست.
PICOs
خلاصه به زبان ساده
سوال مطالعه مروری: آیا برنامههای عملیاتی با آموزش مختصر برای کمک به بیماران در شناسایی نشانههای بدتر شدن COPD و پاسخ و درمان به این نشانهها موثر است یا خیر؟
ما شواهد مربوط به تاثیرات برنامههای عملیاتی را در افراد مبتلا به تشدید بیماری مزمن انسدادی ریه مرور کردیم. هفت مطالعه مرتبط را پیدا کردیم. شواهد گردآوری شده در این مرور تا نوامبر 2015 بهروز است.
پیشینه
بیماری مزمن انسدادی ریه (chronic obstructive pulmonary disease; COPD) یک بیماری راههای هوایی است که به طور شایعی با سیگار کشیدن مرتبط است. افراد مبتلا به COPD غالبا دچار بدتر شدن نشانهها میشوند، این حالت «تشدید» (exacerbation) بیماری نامیده میشود، در این حالت بیماران نیازمند درمانهای اضافیتر هستند و گاهی باید در بیمارستان بستری شوند. یک برنامه عملیاتی یک راهنمای گفتاری یا نوشتاری است که با آموزش مختصری به بیماران مبتلا به COPD داده میشود تا به آنها در شناسایی نشانههای تشدید بیماری و شروع سریع درمانهای اضافی کمک کند. افراد ممکن است داروهای اضافی را در خانه دریافت کنند یا ممکن است نسخهای برای دریافت دارو بگیرند. برخی اوقات یک متخصص سلامت تماسهای تلفنی منظمی با بیماران دارد تا به آنها در استفاده از برنامه عملیاتی کمک کند. ما این مرور را برای یافتن این نکته انجام دادیم که یک برنامه عملیاتی برای افراد مبتلا به تشدید COPD باعث بهبودی سلامت آنها و کاهش بستری در بیمارستان میشود یا خیر.
ویژگیهای مطالعه
هفت مطالعه مرتبط را با 1550 فرد مبتلا به COPD یافتیم. مطالعاتی را که شامل یک برنامه عملیاتی همراه با درمانهای دیگر مانند برنامه ورزشی یا جلسات آموزشی بلندمدت بودند، در این مرور وارد نکردیم. افراد در سه مطالعه حمایت مداوم داشتند تا به آنها در استفاده از برنامه عملیاتی کمک کند. افراد مطالعات وارد شده نشانههای متوسط تا شدید داشتند و برای شش یا 12 ماه پیگیری شدند.
نتایج کلیدی
افراد مبتلا به COPD که برنامه عملیاتی را دریافت کردهاند، طی یک سال به میزان کمتری ویزیت اورژانس و بستری در بیمارستان را به دلیل مشکلات تنفسی داشتهاند. بر اساس محاسبه ما به ازای هر 19 نفری که برنامه عملیاتی را دریافت کردهاند، یک نفر به دلیل تشدید بیماری نیازی به بستری در بیمارستان نداشته است.
افرادی که برنامه عملیاتی را دریافت کرده بودند، از کورتیکواستروئید و آنتیبیوتیک بیشتری برای تشدید COPD در یک سال استفاده کردند، به طور میانگین یک دوره بیشتر از کورتیکواستروئیدها و دو دوره بیشتر از آنتیبیوتیکها استفاده کردند.
برخی مطالعات نشان دادند افرادی که برنامه عملیاتی را دریافت کرده بودند، در توانایی آنها برای شناسایی و خود‐مدیریتی درمان در هنگام بدتر شدن نشانهها در تشدید COPD بهبودی ایجاد شده است.
افرادی که برنامه عملیاتی دریافت کردهاند، هیچ تفاوتی در احتمال مرگ به هر علتی طی یک سال در آنها وجو نداشت، با این حال این یافته تنوع زیادی دارد.
ما نمیتوانیم بگوییم که پیگیری تلفنی مزایای بیشتری نسبت به پیگیری برنامه عملیاتی به تنهایی دارد یا خیر.
کیفیت شواهد
شواهد در این مرور به طور کلی مستقل و قابل اعتماد هستند، ما درباره نتایج بسیار یا نسبتا مطمئن هستیم.
نتیجهگیریها
ما باور داریم که افراد مبتلا به COPD باید یک برنامه عملیاتی فردی شده را با یک بخش آموزشی کوتاه دریافت کنند، چون باعث بستری شدن کوتاهتر و کمتر در بیمارستان میشود، همچنین موجب فهم بهتری از نیازها برای شروع درمان توسط خود فرد و کاربرد درست داروها در هنگام تشدید COPD میشود.
Authors' conclusions
Summary of findings
| Do action plans improve patient outcomes in acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: individuals with exacerbations of chronic obstructive pulmonary disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with action plan | |||||
| Hospitalisations for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.69 | 743 | ⊕⊕⊕⊝ | |||
| Hospitalisations and emergency visits for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.59 | 743 | ⊕⊕⊕⊕ | |||
| At least 1 hospital admission | 209 per 1000 | 154 per 1000 | Odds ratio 0.69 | 897 | ⊕⊕⊕⊝ | |
| Mortality (all‐cause) | 103 per 1000 | 91 per 1000 | Odds ratio 0.88 | 1134 | ⊕⊕⊕⊝ | |
| Courses of oral corticosteroids | Mean courses of oral corticosteroids were 1.05 | Mean courses of oral corticosteroids in the intervention group were 0.74 more (0.12 more to 1.35 more) | ‐ | 200 | ⊕⊕⊕⊝ | |
| Courses of antibiotics | Mean courses of antibiotics ranged from 1.6 to 3.2 | Mean courses of antibiotics in the intervention group were 2.26 more (1.82 more to 2.7 more) | ‐ | 943 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Respiratory‐related quality of life: SGRQ overall score | Mean respiratory‐related quality of life: SGRQ overall score ranged from ‐2 to +6 units | Mean respiratory‐related quality of life: SGRQ overall score in the intervention group was 2.82 units lower (0.83 lower to 4.81 lower) | ‐ | 1009 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Depression score | Mean depression score was ‐0.04 | Mean depression score in the intervention group was 0.25 lower (1.14 lower to 0.64 higher) | ‐ | 154 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWide confidence interval; effect size includes null. bUnclear risk of bias for two studies for allocation and blinding of assessors. cUnclear risk of bias for three studies for allocation and blinding of assessors. dUnclear risk of bias for one study for allocation and blinding of assessors. | ||||||
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a systemic, progressive, heterogeneous disease with significant worldwide public health importance. COPD is associated with a chronic innate inflammatory response that results from continuous exposure to inhaled noxious particles (GOLD 2016; Hogg 2004). This inflammatory response may induce destruction of lung parenchyma and may disrupt normal repair and defence mechanisms (GOLD 2016). These pathological changes lead to characteristic progressive airflow limitation that is not fully reversible (GOLD 2016).
COPD develops from a combination of genetic and environmental factors and is most commonly linked to cigarette smoking (Halbert 2006). In addition to cigarette smoking, exposures such as burning of wood and other biomass fuels are important risk factors for some populations (GOLD 2016).
COPD is a significant cause of preventable worldwide morbidity and mortality. Estimates have placed COPD as the fourth leading cause of death globally (WHO 2004). The prevalence of COPD is predicted to increase owing to the persisting incidence of smoking and ageing of the global population (GOLD 2016). The World Health Organization (WHO) predicts that COPD will become the third leading cause of death by 2030 (WHO 2008). Other estimates have predicted that COPD will become the seventh leading cause of disability‐adjusted life‐years (DALYs) by 2030 (Mathers 2006). In 2010, the economic burden of COPD in the United States was projected to be $49.9 billion, including $29.5 billion in direct healthcare expenditures (American Lung Association 2014). In Australia, it was estimated that in 2008, COPD cost the economy $98 billion AUD (Access 2008).
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) advises that a postbronchodilator forced expiratory volume of one second (FEV1)/forced vital capacity (FVC) ratio < 0.70 is needed for a diagnosis of COPD (GOLD 2016). Disease severity can be classified by the degree of airflow limitation, although evidence suggests that this is a poor predictor of many negative features of the disease. Patients with similar airflow limitations have been found to belong to different disease phenotypes and to have marked differences in age, symptoms, comorbidities and predicted mortality (Agusti 2010; Burgel 2010). Interest in the potential importance of airway and blood eosinophilia as a predictor of exacerbations and their response to corticosteroids has recently increased (Bafadhel 2012; Pascoe 2015), but this has not been taken into account in most clinical studies, such as those included in this review.
The presentation, progression and pathological abnormalities associated with COPD are variable (Han 2013). COPD can result in an array of systemic physical functional limitations including poor musculoskeletal strength and function, poor exercise performance and self‐reported functional limitations (Eisner 2008). Patients with COPD often have multiple comorbidities spanning both medical and psychiatric illnesses that can have a significant impact on prognosis (Barnes 2009; Hanania 2011; Rennard 2006).
Another important prognostic factor that is a major problem associated with COPD is the occurrence of exacerbations. The GOLD guidelines define a COPD exacerbation as 'an acute event characterised by a worsening of the patient’s respiratory symptoms that is beyond normal day‐to‐day variations and leads to a change in medication' (GOLD 2016). Exacerbations are a major driver of decline in health status and health‐related quality of life (Chhabra 2014; Spencer 2004). They are usually managed with increased bronchodilator medication, oral corticosteroids (Walters 2014) and antibiotics (Vollenweider 2012). People with frequent exacerbations of COPD experience poorer health status, accelerated decline in FEV1, worsened quality of life and increased hospital admissions and mortality (Halpin 2012; Vestbo 2011). COPD exacerbations account for the greatest proportion of the total COPD burden on the healthcare system (GOLD 2016).
Description of the intervention
Management of COPD is complex and should involve a multi‐disciplinary and multi‐modality approach. An action plan is used to encourage early intervention for exacerbations. Action plans provide guidelines detailing self‐initiated actions, such as changing medication regimens or visiting a general practitioner (GP) or hospital, to be undertaken in response to alterations in symptoms of COPD suggesting the start of an exacerbation. A healthcare provider or case manager can develop an action plan by using a template and can personalise the plan for individual patients according to their symptoms and ongoing regular management. Templates for action plans are provided online by some lung support groups, and they can be given to patients in primary care at low cost. Sometimes an action plan is accompanied by prescriptions for prednisolone and an oral antibiotic.
How the intervention might work
Action plans include interventions designed to allow patients to recognise and initiate early treatment for exacerbations. The early warning signs of an exacerbation have been found to be fairly consistent and recognisable within individuals (Kessler 2006). Despite this fact, evidence suggests that patients do not seek medical care for all of the exacerbations that they experience (Langsetmo 2008; Walters 2012). Unreported exacerbations are usually less severe but still impact health status (Langsetmo 2008). Furthermore, some patients may present late for treatment of their exacerbation, and this is associated with slower recovery, worse quality of life and increased healthcare utilisation (Wilkinson 2004). The chronic and progressive nature of COPD underlies the importance of self‐management.
Action plans are frequently incorporated into self‐management interventions for COPD (Bourbeau 2009). A Cochrane systematic review found that comprehensive self‐management interventions improved health‐related quality of life and decreased healthcare utilisation (Zwerink 2014). In this review, 75% of studies incorporated the use of an action plan, and it was hypothesised that the decreased number of respiratory‐related hospitalisations observed in the intervention group may particularly have reflected this (Zwerink 2014).
Why it is important to do this review
Lack of consensus on an operational definition of COPD self‐management has been a barrier to the formulation of clear recommendations (Effing 2012). Heterogeneity among interventions, study populations, follow‐up time and outcome measures made it difficult for review authors in two Cochrane systematic reviews (Kruis 2013; Zwerink 2014) to determine the most effective form and content of self‐management for COPD. Effing et al proposed a conceptual definition of COPD self‐management, stated as follows: "A COPD self‐management intervention is structured but personalised and often multi‐component, with goals of motivating, engaging and supporting the patients to positively adapt their health behaviours and develop skills to better manage their disease" (Effing 2016). Development and evaluation of specific self‐management interventions is important for application of the definition presented by Effing et al. This review is an update of a Cochrane Review first published in 2005 (Turnock 2005). The aim of this review is to determine the role and effectiveness of an action plan as a self‐management intervention provided for patients with COPD without comprehensive self‐management education/training.
Objectives
To compare effects of an action plan for COPD exacerbations provided with a single short patient education component and without a comprehensive self‐management programme versus usual care. Primary outcomes were healthcare utilisation, mortality and medication use. Secondary outcomes were health‐related quality of life, psychological morbidity, lung function and cost‐effectiveness.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs, excluding cross‐over trials.
Types of participants
Participants were patients with a clinical diagnosis of COPD based on spirometric criteria such as those of GOLD (GOLD 2016) for persistent airflow limitation (i.e. postbronchodilator FEV1/FVC < 70%) with a history of smoking. We excluded studies with participants who had received a primary diagnosis of asthma, unless separate results were available for participants with COPD.
Types of interventions
The intervention consisted of an action plan with a single educational component of short duration. The short educational portion allowed time the clinician needed to personalise the action plan according to individual management needs and symptoms. An action plan is defined as a written or oral guideline that details self‐initiated interventions (such as changing medication regimens or visiting a GP or hospital) undertaken in response to alterations in symptoms of COPD (e.g. increased breathlessness, increased amount or purulence of sputum, increased use of a relief inhaler, decreased activity level) (i.e. changes that would suggest commencement of an exacerbation). Investigators permitted ongoing support directed at use of the action plan delivered by telephone or direct contact. We deliberately did not include studies with broader self‐management support interventions, such as individual or group education delivered in multiple sessions over a longer period or exercise programmes, irrespective of whether they included an action plan. Researchers compared the active intervention versus 'usual care' delivered by healthcare providers.
Types of outcome measures
Primary outcomes
-
Healthcare utilisation, including respiratory‐related hospital admission, treatment in an emergency department (ED) and GP visits for COPD.
-
Mortality: respiratory‐related and all‐cause.
-
Use of medication: time to initiation of therapy after symptom onset; courses/duration of antibiotic or corticosteroid use, or both; participant initiation of antibiotic or steroid use, or both.
Secondary outcomes
-
Health‐related quality of life (HRQoL) measured on validated scales.
-
Psychological morbidity: anxiety and depression, measured on validated scales.
-
COPD self‐management knowledge and intended actions (based on participant interview).
-
Lung function.
-
Cost‐effectiveness.
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 1 for details). We searched all records in the CAGR using the search strategy presented in Appendix 2.
We carried out additional searches of CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, ClinicalTrials.gov, the WHO trials portal and the Australian New Zealand Clinical Trials Registry (ANZCTR). We have listed in Appendix 3 the search strategies used for these databases. We searched all databases from their inception to November 2015, and we imposed no restrictions on language of publication.
Searching other resources
From full‐text papers obtained, we handsearched bibliographic lists for additional articles. We contacted researchers for information about their ongoing trials and conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO trials portal (www.who.int/ictrp/en/).
Data collection and analysis
Selection of studies
At least two review authors (MH, JW) assessed potentially relevant trials by screening full texts to independently select trials for inclusion and to identify and record reasons for exclusion of ineligible studies. We resolved disagreements through discussion or, if required, we consulted a third review author (RWB). We identified and excluded duplicates and collated multiple reports of the same study, so that each study (rather than each report) was the unit of interest in the review. We recorded the selection process as a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram.
Data extraction and management
We used a data collection form to record study characteristics and outcome data. Two review authors (MH, JW) independently extracted the following characteristics from reports of included studies.
-
Methods: study design, total duration of study, number of study centres and locations, study setting and duration and date of study.
-
Participants: N, mean age, age range, gender, withdrawals, inclusion criteria and exclusion criteria.
-
Interventions: study treatment, comparisons and cointerventions.
-
Outcomes: primary and secondary outcomes specified and collected and time points reported.
-
Notes: funding for trial, trial registration and notable conflicts of interest of trial authors.
Two review authors (MH, JW) independently extracted outcome data from reports of included studies. MH entered the data into Review Manager, and JW double‐checked the data. We checked that data were entered correctly by comparing data presented in the systematic review against the study reports.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study (MH, JW) using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by consultation with another review author (RWB). We assessed risk of bias according to the following domains.
-
Random sequence generation.
-
Allocation concealment.
-
Blinding of participants and personnel.
-
Blinding of outcome assessment.
-
Incomplete outcome data.
-
Selective outcome reporting.
-
Other bias(es).
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across studies for each of the domains listed. When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to those outcomes.
Measures of treatment effect
We analysed dichotomous outcomes using Mantel‐Haenszel odds ratios with a 95% confidence interval (CI). When events were rare, we employed the Peto odds ratio. We entered scale data with a consistent direction of effect.
For continuous variables, we analysed data as mean differences (MDs), with 95% CIs. We used standardised mean difference (SMDs) with 95% CIs when different scales of measurement had been used for a particular outcome. The SMD expresses the difference in means between treatment groups in units of the pooled standard deviation.
We undertook meta‐analyses only when this was meaningful, that is, when treatments, participants and the underlying clinical question were similar.
When skewed data were available (reported as medians and interquartile ranges), we described them narratively.
For 'time‐to‐event' outcomes such as log hazard ratios, we used the fixed‐effect generic inverse variance outcome to combine results. This method gives a weighted average of the effect estimates of separate studies (Deeks 2001). We calculated the number needed to treat for an additional beneficial outcome from the pooled odds ratio and confidence interval, using baseline risk in the control group.
Unit of analysis issues
We analysed dichotomous data by using participants as the unit of analysis.
Dealing with missing data
We contacted investigators to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only), or to clarify details of methods.
When this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
If no information on the variability of an effect estimate (confidence interval or P value) was available, we imputed standard deviations. We used one of two methods: borrowing the standard deviation (SD) from another study of similar duration (using the largest value when more than one study provided results), or calculating a correlation coefficient (R value) using data from another study according to methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We carried out an assessment of possible heterogeneity for pooled effects, when the null hypothesis was that all studies were evaluating the same effect, by using a Breslow‐Day test of heterogeneity; a P value < 0.05 was considered to indicate significant differences between studies.
In addition, we used the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2011). We interpreted statistical heterogeneity as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity and 50% to 90% may represent substantial heterogeneity (Higgins 2011).
We assessed clinical and methodological heterogeneity by recording differences in study design and participant characteristics between individual studies. When we found substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analysis.
Assessment of reporting biases
We tried to minimise reporting bias resulting from non‐publication of studies or selective outcome reporting by using a broad search strategy, checking references of included studies and relevant systematic reviews and contacting study authors for additional outcome data. We planned to visually inspect funnel plots if 10 or more studies contributed to outcome analysis.
Data synthesis
We used a fixed‐effect model and performed a sensitivity analysis with a random‐effects model if we noted unexplained heterogeneity. We presented the findings of our primary outcomes and other important outcomes in a 'Summary of findings' table according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (generated with the use of GradePro software) (seven specified a priori in the update).
-
Hospital admission ‐ respiratory‐related.
-
Emergency department attendance ‐ respiratory‐related.
-
Mortality.
-
Quality of life.
-
Use of oral corticosteroids.
-
Use of antibiotics.
-
Psychological morbidity.
Subgroup analysis and investigation of heterogeneity
In this review update, we planned a priori subgroup analysis based on:
-
comparison of studies with ongoing support directed at use of an action plan versus those conducted without such support;
-
severity of COPD: participants with mild to moderate CODP versus those with severe to very severe COPD; and
-
design of the action plan.
Sensitivity analysis
In assessing heterogeneity, we considered possible causes arising from details of study design. We performed sensitivity analyses by using a random‐effects model versus a fixed‐effect model in assessing risk of bias and in identifying other potential confounders; for studies published only as abstracts, we used various methods to impute a missing standard deviation.
Results
Description of studies
Results of the search
Review authors identified and screened a total of 574 titles and abstracts since the original review was published in 2005. In 2005, two review authors (AT, JW) assessed the full texts of 11 of 199 identified studies, and included three of these studies in the review (McGeoch 2004; Watson 1997; Wood‐Baker 2006). In 2010, review authors included two (Martin 2004; Rootmensen 2008) of 17 studies identified in the search update. From the updated search conducted during 2014, review authors identified 358 studies for screening, of which they assessed 36 full texts for eligibility (Figure 1). Review authors assessed all previously excluded studies for eligibility if the intervention included ongoing support for action plan use. Review authors excluded 33 citations (representing 26 studies) ‐ four owing to wrong comparator, 24 because of the wrong intervention, three as the result of wrong study design, one because the duration of education exceeded eligibility and one because it was a duplicate citation for a study already excluded. One study was ongoing, and review authors included two studies in the review (Rice 2010; Trappenburg 2011). Searches for this update repeated on 21/11/15, before the review update was submitted, yielded no new studies. Two review authors (JW, MH) conducted screening for the most recent update.
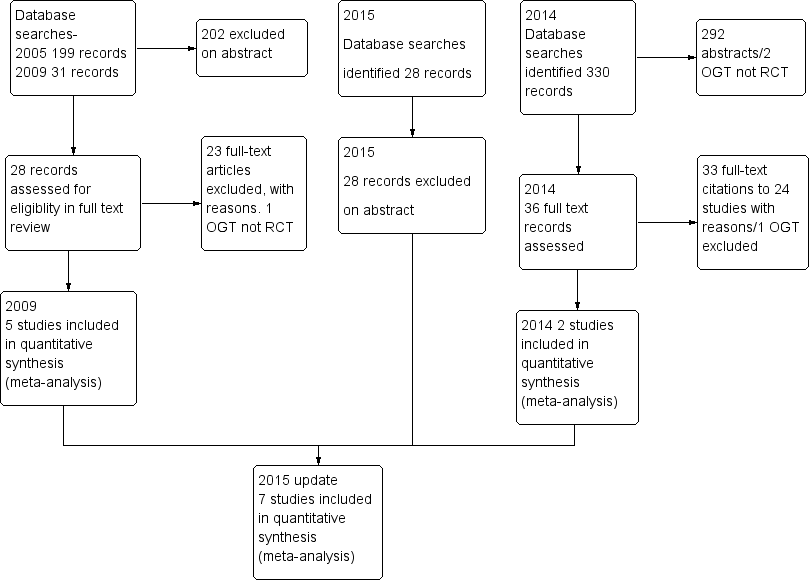
Study flow diagram.
Included studies
See the Characteristics of included studies table.
This review update includes a total of seven parallel‐group RCTs that included 1550 participants with COPD (Table 1). Since the last update appeared in 2010 (Walters 2010), review authors have added two studies (Rice 2010; Trappenburg 2011) with an additional 976 participants. Four trials (Martin 2004; Rice 2010; Rootmensen 2008; Watson 1997) were randomised at patient level, two (McGeoch 2004: Wood‐Baker 2006) were cluster‐randomised at practice level and one (Trappenburg 2011) was randomised by the minimisation technique to control for centre and gender. Four studies recruited participants through GPs. Wood‐Baker 2006 recruited from 54 GPs in 31 practices, and Watson 1997 recruited from 22 GPs in 12 practices. McGeoch 2004 recruited participants attending two groups of general practices but did not specify the number of GPs involved, and Martin 2004 recruited through a consortium of GPs in one region. Rice 2010 recruited participants from a centralised electronic medical record database of a US Veterans Hospital. Trappenburg 2011 recruited participants through scheduled visits to a respiratory nurse at eight regional hospitals and five general practices.
| Study ID | Dates | Recruitment/Randomisation unit | Follow‐up | Length SME (educator) | RAN, n/WD, n | Age*, years/ % male | % current smokers | FEV1 % pred* INT‐CONT | QoL INT‐CONT |
| Not known | Consortium practices, New Zealand/participants | 12 months | Single interview, length not stated (respiratory nurse) | 96/26 | 70/51 | n/a | 35‐34 | 57‐51 | |
| 7/2002‐ 12/2003 | 2 groups of practices, New Zealand/practice | 12 months | 1 hour (practice nurse or respiratory educator) | 159/9 | 71/59 | 28 | 55‐53 | 43‐37 | |
| Rootmensen 2008 (all participants) | Not known | 1 hospital pulmonary outpatient clinic, Netherlands/ participants | 6 months | 45 minutes (pulmonary nurse) | 157 (111 COPD)/17 | 60/55 | 12 | 57‐64 | n/a |
| 07/2004‐ 07/2008 | Centralised electronic medical record database/participants | 12 months | 1 to 1.5‐hour group educational session (case manager) | 743/84 | 70/98 | 22 | 36.1‐38.1 | n/a | |
| 12/2008‐ 12/2010 | 8 regional hospitals and 5 general practices/participants (stratified by gender and centre) | 6 months | Single interview, length not stated (nurse case manager). | 233/41 | 66/57 | 29 | 56.7‐56.5 | n/a | |
| 1993‐ 07/1994 | 12 practices, 22 GPs, New Zealand/participants | 6 months | Single interview, length not stated (practice nurse) | 69/13 | 68/65 | 28 | 37‐36 | 43‐39 | |
| 2002‐2003 | 54 GPs, 31 practices, Australia/practice | 12 months | 1 hour (respiratory research nurse) | 139/27 | 70/76 | 42 | 46‐44 | 47‐47 |
*: mean; AP: action plan; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; GP: general practitioner; INT‐CONT: intervention group‐control group; QoL: % impairment quality of life 0‐100; RAN: randomisation; SME: self‐management education; WD: withdrawal or death.
All participants had received a diagnosis of COPD as a major functionally limiting disease before inclusion. In line with the GOLD criteria for diagnosis of COPD, all participants showed a postbronchodilator FEV1/FVC ratio < 0.70. However, Rootmensen 2008 recruited participants with a diagnosis of COPD or asthma. We included in this review only results for the subgroup of participants with COPD (111 of 191). Participants in Rice 2010 were also required to have one or more of the following during the previous year: hospitalisation or ED visit for COPD, long‐term home oxygen use or course of systemic corticosteroids for COPD. Trappenburg 2011 recruited participants over the age of 40 who were currently using bronchodilator therapy. Participants in Wood‐Baker 2006 were at least 50 years of age. Both Watson 1997 and Wood‐Baker 2006 also required FEV1 < 65% predicted. McGeoch 2004 stated inclusion criteria of symptoms at least weekly and history of one or more exacerbations in the previous 12 months requiring an increase in therapy. Martin 2004 required at least one hospital admission or two acute exacerbations of COPD requiring GP care during the previous 12 months. Entry criteria for Watson 1997 included current use of bronchodilator therapy.
Assessment of baseline characteristics of participants (Table 1) shows that studies involved people of similar age, with mean age from 60 to 71 years and overall mean age of 68 years. All studies included more male participants, ranging from 51% to 98% with overall mean of 66%. The high incidence of male participants in Rice 2010 (98%) reflected recruiting from Veterans Affairs medical centres. The percentage of current smokers in each study group varied from 28% (Wood‐Baker 2006) to 12% (Rootmensen 2008), with overall mean of 27%. Severity of airflow obstruction, as indicated by the overall mean postbronchodilator FEV1 as percentage of predicted value (staged according to the GOLD classification), was moderate in three studies (McGeoch 2004 (54% predicted); Rootmensen 2008 (61% predicted); Trappenburg 2011 (57% predicted)) and severe in four studies (Martin 2004 (54% predicted); Rice 2010 (54% predicted); Watson 1997 (54% predicted); Wood‐Baker 2006 (54% predicted)). At baseline, mean impairment scores for overall quality of life when available (in four studies) (based on St George's Respiratory Questionnaire maximum impairment = 100) ranged from 37 to 57, with mean overall score of 46. Within studies, impairment in quality of life was similar between intervention and control groups.
Three studies specified exclusion of nursing home residents (McGeoch 2004; Watson 1997; Wood‐Baker 2006). Five studies specified exclusion of participants with other primary limiting diseases such as lung cancer and cardiac disease (Martin 2004; McGeoch 2004; Rice 2010; Trappenburg 2011; Watson 1997). Trappenburg 2011 also excluded participants with a primary diagnosis of asthma. Rice 2010 excluded participants without access to a telephone.
Study follow‐up was six months in three studies (Rootmensen 2008; Trappenburg 2011; Watson 1997) and 12 months in four studies (Martin 2004; McGeoch 2004; Rice 2010; Wood‐Baker 2006). Investigators reported a total of 217 withdrawals from the total 1550 participants enrolled and a drop‐out rate ranging from 5% to 27%.
Action plan intervention
Table 2 presents a comparison of action plan interventions. Three studies used a standard written action plan and information booklet (McGeoch 2004; Watson 1997; Wood‐Baker 2006). Martin 2004, Rice 2010 and Trappenburg 2011 used an individualised action plan intervention. Rootmensen 2008 provided an intervention consisting of additional care that included individual instructions for what to do in case of exacerbations.
| Individualised AP | Standard written AP | Support for AP during study period | SME (individual/group) | Prescription /supply OCS | Prescription /supply ABS | Written COPD educational component | Comparison | |
| Written | 3‐Monthly visit regarding use of AP | Individual interview with respiratory nurse, length not stated, individualised action plan according to current treatment and symptoms | All had 7‐day supply | All had 7‐day supply | No | Usual care by own GP | ||
| Yes | No | Individual session by practice nurse or respiratory educator in association with GP 1 hour, covering major points of COPD self‐management plan, and use of validated sputum colour charts | Prescription | Prescription | Educational package | Non‐standard education on COPD according to practice standards | ||
| Written | Monthly phone call from nurse | Group 1‐1.5 hours, individualised action plan with respiratory nurse | Yes | Prescription | Usual care + 1‐page summary of principles of COPD care according to published guidelines. No AP | |||
| Oral | No | Individual protocol‐based educational session covering disease, medications, vaccination, smoking cessation and exacerbation management, 45 minutes in length | Oral medication provided to some, % unknown | Oral medication provided to some, % unknown | No | Usual care | ||
| Written | Standardised phone calls at 1 and 4 months | Individualised action plan education, length of session not stated | 2%' | 22% | ✓ COPD information | Usual care ‐ pharmacological and non‐pharmacological care according to most recent evidence‐based guidelines, specifically AP denied. All included participants seen by respiratory nurse, who systematically checked and discussed aspects of COPD care, including vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. | ||
| Yes | No | Individual session education about use of the action plan with COPD booklet by a senior respiratory outreach nurse; length not stated | Prescription | Prescription | ✓ Guide to living positively with COPD | Usual care by GP, specifically denied access to AP and booklet | ||
| Written | No | Individual educational session with respiratory nurse, covering COPD, smoking cessation, immunisation, nutrition, exercise, sputum clearance, breathing, medication, inhaler use. Individualised action plan developed with GP input. Length not known | 2% | 22% | COPD information booklet | Usual care, COPD information booklet and individual educational session with nurse, but no AP |
ABS: antibiotics; AP: action plan; COPD: chronic obstructive pulmonary disease; GP: general practitioner; OCS: oral corticosteroids; SME: self‐management education.
Wood‐Baker 2006 participants also received an individual educational session with a nurse experienced in managing respiratory disease. Their action plan was a written self‐management plan that was developed in consultation with their treating GP. It listed the participant's maintenance medications and an individualised action plan based on early recognition of symptoms associated with exacerbations of COPD. Seventy‐six per cent received a standard action plan with instructions to self‐initiate a short course of oral corticosteroids and an antibiotic; the other 24% received an action plan with instructions to initiate antibiotics only (N = 10), to double their dose of inhaled corticosteroids and commence an antibiotic (N = 2), to initiate a short course of oral corticosteroids only (N = 1) or to contact their GP (N = 3). Participants following action plans that involved self‐initiation of medication were given prescriptions by their GP. All intervention participants were encouraged to present to their GP early.
Two studies (McGeoch 2004; Watson 1997) used action plans that were identical and provided advice on management of usual care and exacerbations, together with a booklet on self‐management, a prescription from their GP for prednisolone and a broad‐spectrum antibiotic for self‐administration during an exacerbation. Watson 1997 made no attempt to individualise instructions in the action plan, whereas the remaining trials (Martin 2004; McGeoch 2004; Wood‐Baker 2006; Rootmensen 2008) delivered self‐management plan education in an individual session provided by a nurse, a respiratory educator or the participant's GP.
Four trials (Martin 2004; McGeoch 2004; Watson 1997; Wood‐Baker 2006) supplied booklets with action plans that covered topics such as smoking cessation, control of breathlessness, nutrition, exercise, clearance of mucus from the lungs, medications and contact details of community support services. Two trials educated participants on the correct use of inhalers (Rootmensen 2008; Wood‐Baker 2006).
In Rice 2010, participants attended a single 1 to 1.5‐hour group educational session. They received individualised written action plans that included a description of the signs and symptoms of an exacerbation that should prompt initiation of self‐treatment, refillable prescriptions for prednisolone and an oral antibiotic, contact information for a case manager and the telephone number of the 24‐hour VA help line. Participants were instructed to begin action plan medications for symptoms that were substantially worse than usual. A case manager made monthly phone calls to reinforce general principles of COPD management, to review details of the action plan and to answer questions.
In Trappenburg 2011, participants attended an individual educational session with a respiratory nurse, who systematically checked and discussed aspects of COPD care such as vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. Participants received an individualised action plan that included recognition of symptom changes, use of medication/lifestyle prescriptions, additional medication/breathing exercises and energy preservation in case of symptom increase and a contact person/telephone number in case of an exacerbation. For individual participants, it was optional for the case manager (in consultation with the attending physician) to provide self‐treatment medication (course of corticosteroids and/or antibiotics). Two standardised reinforcement sessions were held by telephone at one and four months to evaluate participants' understanding of and adherence to the action plan; when needed, researchers provided additional information.
Control
Investigators provided all control groups with usual care; although this varied between studies, participants were always specifically denied access to the action plan. Wood‐Baker 2006 provided usual care that included provision of a booklet and an individual nurse educational session. McGeoch 2004 provided non‐standardised education based on routine practice at the time. The remaining three trials (Martin 2004; Rootmensen 2008; Watson 1997) supplied no additional education for participants in control groups. Rice 2010 distributed to usual care participants a one‐page handout containing a summary of principles of COPD care based on published guidelines. In Trappenburg 2011, a nurse case manager assessed participants and systematically checked and discussed aspects of COPD care such as vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. Participants had no additional contact with the case manager.
Excluded studies
Ten studies were not RCTs, 11 studies involved comprehensive self‐management programmes in which the action plan component could not be isolated and in nine studies, COPD/self‐management education was delivered in multiple sessions or in a single session of several hours' duration. Fifteen studies included no action plan in the intervention.
Risk of bias in included studies
We have provided full details regarding risk of bias assessment for all included studies in the Characteristics of included studies table, along with a summary of grading in Figure 2 and Figure 3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
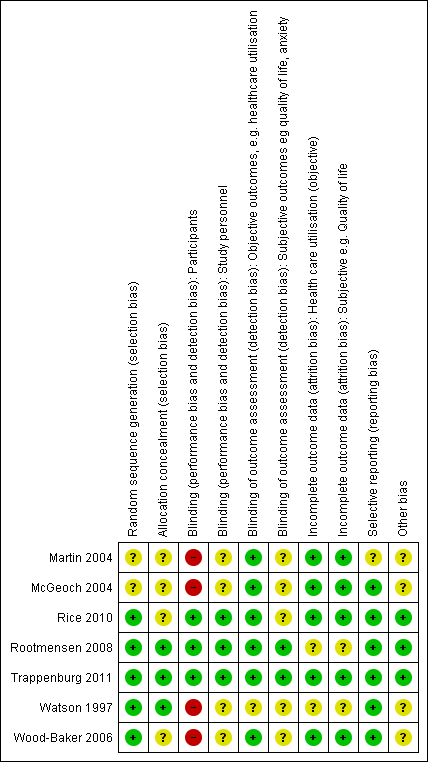
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
With regards to random sequence generation, we assessed five studies as having low risk of bias; two employed permuted block randomisation (Rice 2010; Watson 1997), two the minimisation technique (Rootmensen 2008; Trappenburg 2011) and one a computer‐generated randomised software package (Wood‐Baker 2006). Two studies assessed as having unclear risk reported that they were RCTs but did not describe the method of randomisation used (Martin 2004; McGeoch 2004).
Concerning allocation concealment, we assessed three studies as having low risk of bias (Rootmensen 2008; Trappenburg 2011; Watson 1997). Rootmensen 2008 randomised participants in advance of their clinic attendance and reported these results only to the pulmonary physician just before the visit. Trappenburg 2011 utilised a central web‐based service to conceal the assignment sequence. In Watson 1997, research staff who recruited participants allocated them according to a randomisation list. We assessed four studies as having unclear risk of bias. Three did not report methods of allocation (Martin 2004; Rice 2010; Wood‐Baker 2006), and in McGeoch 2004, researchers allocated participants by practice attendance but did not provide information on allocation of practices.
Blinding
We assessed three studies as having low risk of bias for blinding of participants; two utilised a modified consent procedure by which the major objective of the study was withheld from participants until after the study was completed (Rootmensen 2008; Trappenburg 2011), and in Rice 2010, participants were aware of their allocation, but this awareness was not thought likely to affect primary healthcare utilisation outcomes. Regarding patient‐reported outcomes, we assessed those from Rootmensen 2008 and Trappenburg 2011 as low risk because investigators used a modified consent procedure, and those from Rice 2010 as unclear risk. We assessed four studies as having unclear risk of bias with regards to participants, as they were not blinded (Martin 2004; McGeoch 2004; Watson 1997; Wood‐Baker 2006); this introduced the potential for bias in self‐administered patient assessments, such as quality of life measures and daily diary records. In some practices in McGeoch 2004, GPs may have implemented both intervention and usual care, leading to possible confounding between treatment methods. Martin 2004, McGeoch 2004, Watson 1997 and Wood‐Baker 2006 did not blind outcome assessors, suggesting potential for high bias for subjective outcomes. Rice 2010 adequately blinded assessors.
Incomplete outcome data
Regarding incomplete outcome data for objective healthcare utilisation outcomes, we assessed five studies as having low risk of bias. McGeoch 2004 and Wood‐Baker 2006 reported small numbers lost to follow‐up that were balanced between groups. In Martin 2004, 93 of 96 recruited participants completed follow‐up; three withdrawals occurred for personal reasons, but investigators did not state group allocation. In Rice 2010, the only reason for missing data was death (48 in usual care, 36 in intervention), and study authors were unable to perform intention‐to‐treat analysis. Trappenburg 2011 reported drop‐out rates of 19% in the intervention group and 16% in the control group. For objective healthcare utilisation outcomes, we determined that two studies had unclear risk of bias; Rootmensen 2008 reported on only 90 of 117 participants with COPD, and Watson 1997 noted 13 withdrawals from the 60 participants originally randomised and did not report group allocation for those lost to follow‐up.
Risk of bias assessment concerning incomplete outcome data for subjective outcomes was similar to that for objective outcomes. We assessed five studies as having low risk of bias (Martin 2004; McGeoch 2004; Rice 2010; Trappenburg 2011; Wood‐Baker 2006) and two studies as having unclear risk (Rootmensen 2008; Watson 1997) because researchers did not report withdrawals by group.
Selective reporting
Regarding reporting bias, we assessed six studies as having low risk of bias (McGeoch 2004; Rice 2010; Rootmensen 2008; Trappenburg 2011; Watson 1997; Wood‐Baker 2006). In McGeoch 2004, Rootmensen 2008, Watson 1997 and Wood‐Baker 2006, it was clear that study authors reported all expected outcomes, including those that were prespecified. The protocols for Rice 2010 and Trappenburg 2011 were available, and outcomes reported in these studies were consistent with those prespecified. We assessed Martin 2004 as having unclear risk of bias, as it was not clear that published reports included all expected outcomes and those prespecified.
Other potential sources of bias
We identified no additional sources of bias in Rice 2010, Rootmensen 2008 and Trappenburg 2011. Martin 2004 described a pilot study in which no sample size calculation was performed; study authors did not attempt to examine clustering within practices. McGeoch 2004 reported on statistical analysis to examine the effect of clustering within practices. They analysed the 12‐month change in the outcome variable by using a mixed‐model repeated measures analysis of variance (ANOVA), allowing for cluster‐randomisation of surgeries, and indicated no additional variation from this source beyond that anticipated by between‐subject variation. For this reason, investigators in McGeoch 2004 undertook all analyses by using participants as replicates. In Wood‐Baker 2006, researchers did not perform analyses to examine the effect of clustering within practices. In Watson 1997, baseline analysis showed a statistically significant difference for influenza vaccination in the past year (72% in the intervention group, 44% in the control group).
Effects of interventions
Results: primary outcomes
-
Healthcare utilisation, including respiratory‐related hospital admission, treatment in an emergency department (ED) and general practitioner (GP) visits for chronic obstructive pulmonary disease (COPD).
-
Mortality: respiratory‐related and all‐cause.
-
Use of medications: time to initiation of therapy after symptom onset; course/duration of antibiotic or corticosteroid use, or both; participant initiation of antibiotic or steroid use, or both
Healthcare utilisation
Analysis 1.1 Rate of hospitalisation for COPD/100 patient‐years: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). The difference between action plan with phone follow‐up and control was not statistically significant (rate ratio (RR) 0.69, 95% confidence interval (CI) 0.47 to 1.01).
Analysis 1.2 At least one hospital admission (12‐month follow‐up): For this outcome, we found two relevant trials (n = 897). Results showed a statistically significant difference, with less likelihood for action plan compared with control (subgrouped by phone follow‐up) (odds ratio (OR) 0.69, 95% CI 0.49 to 0.97) and no heterogeneity.
Analysis 1.3 At least one hospital admission (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (OR 0.83, 95% CI 0.30 to 2.31).
Analysis 1.4 Rate of hospital admission for exacerbation (12‐month follow‐up): For this outcome, we found two relevant trials up (n = 205). Results showed no statistically significant difference between action plan and control (mean difference (MD) 0.23, 95% CI ‐0.03 to 0.49) and no heterogeneity (Chi² = 0.30, df = 1 (P = 0.59), I² = 0%).
Analysis 1.5 Rate of hospital admission for exacerbation (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.08 to 0.08).
Analysis 1.6 Rates of hospitalisation and ED visits for COPD/100 patient‐years: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (RR 0.59, 95% CI 0.44 to 0.79).
Analysis 1.7 At least one hospital or ED visit for COPD (12‐month follow‐up): For this outcome, we found one relevant trial (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (OR 0.59, 95% CI 0.43 to 0.80).
Analysis 1.8 Rate of ED visits for COPD/100 patient‐years (12‐month follow‐up): For this outcome, we found one relevant trial (n = 743). Results showed a statistically significant difference with less likelihood for action plan with phone follow‐up compared with control (RR 0.49, 95% CI 0.33 to 0.73).
Analysis 1.9 Rate of ED visits for COPD (12‐month follow‐up): For this outcome, we found two relevant trials (n = 202). Results showed no statistically significant difference between action plan and control (MD 0.37, 95% CI ‐0.50 to 1.24).
Analysis 1.10 At least one ED visit for COPD (12‐month follow‐up): For this outcome, we found two relevant trials (n = 287). Results showed a statistically significant difference with less likelihood for action plan compared with control (subgrouped by phone follow‐up) (OR 0.55, 95% CI 0.38 to 0.78) and no heterogeneity (Chi² = 0.13, df = 1 (P = 0.72), I² = 0%).
Analysis 1.11 Rate of ED visits for COPD (six‐month follow‐up): For this outcome, we found one relevant trial (n = 227). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.09 to 0.09).
Analysis 1.12 GP visits/phone contacts for COPD (all or urgent): For this outcome, we found one relevant trial with six‐month follow‐up (n = 56), with no statistically significant difference between action plan and control (MD 1.00, 95% CI ‐0.57 to 2.57), and two relevant trials up (n = 200) with 12‐month follow‐up (MD 0.23, 95% CI ‐1.02 to 1.47).
Analysis 1.13 Rate of non‐COPD GP visits or phone contacts: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 200). Results showed no statistically significant difference between action plan and control (MD 1.25, 95% CI ‐1.54 to 4.03) and moderate heterogeneity (Chi² = 2.38, df = 1 (P = 0.12), I² = 58%).
Analysis 1.14 Rate of unscheduled physician visits: For this outcome, we found one relevant trial with six‐month follow‐up (n = 227), with no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.36 to 0.36).
Analysis 1.15 Rate of ambulance calls: For this outcome, we found one relevant trial with six‐month follow‐up (n = 89). Results showed a statistically significant difference between action plan and control, with a higher rate in the action plan group (MD 1.70, 95% CI 0.17 to 3.23).
Analysis 1.16 Total hospital days: For this outcome, we found one relevant trial with12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with fewer days spent in hospital in the action plan group (MD ‐1.10, 95% CI ‐2.00 to ‐0.20).
Analysis 1.17 Total intensive care unit (ICU) days: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with fewer days spent in the ICU in the action plan group (MD ‐0.30, 95% CI ‐0.60 to ‐0.00).
Mortality
Analysis 1.18 All‐cause mortality: For this outcome, we found four relevant trials with 12‐month follow‐up (n = 1134). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (Peto OR 0.88, 95% CI 0.59 to 1.31) and moderate heterogeneity (Chi² = 5.17, df = 3, P = 0.16, I² = 42%) (Figure 4) and imprecision.
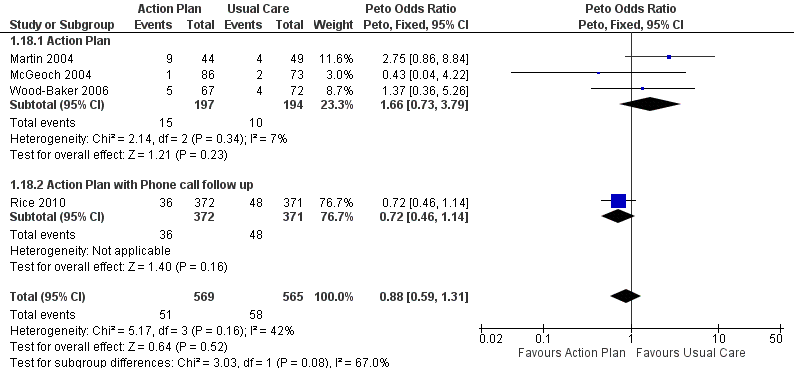
Forest plot of comparison: 1 Action plan versus usual care, outcome: 1.18 Mortality (all cause) 12 months.
Analysis 1.19 Rate of all‐cause mortality per 100 patient‐years: For this outcome, we found one relevant trial with12‐month follow‐up (n = 743). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD ‐3.70, 95% CI ‐8.86 to 1.46), but the result was imprecise.
Analysis 1.20 All‐cause mortality: For this outcome, we found one relevant trial with six‐month follow‐up (n = 229). Results showed no statistically significant difference between action plan with phone follow‐up and control (Peto OR 1.06, 95% CI 0.15 to 7.69), but the result was imprecise.
Use of medication for acute exacerbations of COPD
No data were available on time to initiation of therapy after onset of exacerbation symptoms.
Analysis 1.21 Use of one or more courses of oral steroids for exacerbations: For this outcome, we found one relevant trial with six‐month follow‐up (n = 56), with a statistically significant difference between action plan and control and increased odds of steroid use in the action plan group (OR 6.58, 95% CI 1.29 to 33.62), and one relevant trial with 12‐month follow‐up (n = 154), with no statistically significant difference between action plan and control (OR 1.27, 95% CI 0.34 to 4.69).
Analysis 1.22 The rate of courses of oral steroids for exacerbations in two relevant trials with 12‐month follow‐up (n = 200) showed a statistically significant difference between action plan and control, with an increased rate of steroid use in the action plan group (MD 0.74, 95% CI 0.12 to 1.35) and no heterogeneity (Chi² = 0.37, df = 1, P = 0.54, I² = 0%).
Analysis 1.23 The rate of courses of oral steroids for exacerbations in one relevant trial with six‐month follow‐up (n = 227) showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.23 to 0.23).
Analysis 1.24 The number of days on oral corticosteroids for exacerbations in one relevant trial with six‐month follow‐up (n = 227) showed no statistically significant difference between action plan and control (MD 6.00, 95% CI ‐5.53 to 17.53).
Analysis 1.25 Cumulative dose of prednisolone: For this outcome, we found one relevant trial with 12‐month follow‐up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with a greater cumulative dose in the action plan group (MD 779.00 mg, 95% CI 533.23 to 1024.77).
Analysis 1.26 Use of one or more courses of antibiotics for exacerbations: For this outcome, we found one relevant trial with six‐month follow‐up (n = 56) that reported a statistically significant difference between action plan and control (Peto OR 6.51, 95% CI 2.02 to 21.05), and two relevant trials with 12‐month follow‐up (n = 293) that reported a statistically significant difference between action plan and control (Peto OR 1.65, 95% CI 1.01 to 2.69); both outcomes show increased odds of antibiotic use in the action plan group.
Analysis 1.27 Rate of courses of antibiotics for exacerbations over 12 months: For this outcome, we found three relevant trials with 12‐month follow‐up (n = 943). Results showed a statistically significant difference between action plan and control, with a higher rate of antibiotic use in the action plan group (subgrouped by phone follow‐up) (MD 2.26, 95% CI 1.82 to 2.70), and a substantial degree of heterogeneity (Chi² = 10.55, df = 2, P = 0.005, I² = 81%) and a statistically significant test for subgroup difference (Chi² = 10.09, df = 1, P = 0.001, I² = 90.1%). In two studies that compared action plan with control, the MD was 0.78 (95% CI ‐0.24 to 1.79), and in one study that compared action plan with phone follow‐up and control, the MD was 2.60 (95% CI 2.12 to 3.08).
Analysis 1.28 Rate of courses of antibiotics for exacerbations over six months: In one relevant trial with six‐month follow‐up (n = 227), results showed no statistically significant difference between action plan with phone follow‐up and control (MD 0.00, 95% CI ‐0.26 to 0.26).
Analysis 1.29 The number of days on antibiotics over six months for exacerbations in one relevant trial with six‐month follow‐up (n = 56) showed a statistically significant difference between action plan and control, with a greater number of days on antibiotics in the action plan group (MD 6.00 days, 95% CI 1.40 to 10.60).
Results: secondary outcomes
Respiratory health‐related quality of life: overall scores: St George’s Respiratory Questionnaire (SGRQ), in which a negative direction for the result indicates improvement
Analysis 1.30 SGRQ overall score at 12 months: For this outcome, we found three relevant trials with 12‐month follow‐up (n = 1009). Results showed a statistically significant difference between action plan and control (subgrouped by phone follow‐up), with better quality of life in the action plan group (MD ‐2.79, 95% CI ‐0.82 to ‐4.77), a substantial degree of heterogeneity (Chi² = 7.98, df = 2, P = 0.02, I² = 75%) and a statistically significant test for subgroup difference (Chi² = 7.11, df = 1, P = 0.008, I² = 85.9%). The MD in two studies (n = 264) that compared action plan with control was not significant (0.32, 95% CI 3.34 to ‐2.70), and one study that compared action plan with phone follow‐up and control (n = 743) noted a significant improvement (MD ‐5.10, 95% CI ‐2.50 to ‐7.70).
Analysis 1.31 SGRQ overall score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐0.83, 95% CI ‐2.93 to 1.27), no heterogeneity and no difference between subgroups at this time point.
Respiratory health‐related quality of life subscales
Analysis 1.32 SGRQ symptom score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD ‐2.55, 95% CI ‐6.92 to 1.83) with no heterogeneity.
Analysis 1.33 SGRQ symptom score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 448). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐2.33, 95% CI ‐6.84 to 2.18), with no heterogeneity.
Analysis 1.34 SGRQ activity limitation score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD 2.87, 95% CI 7.00 to ‐1.26), with no heterogeneity.
Analysis 1.35 SGRQ activity limitation score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD 0.88, 95% CI ‐1.90 to 3.67), with no heterogeneity.
Analysis 1.36 Change in SGRQ impact score at 12 months: For this outcome, we found two relevant trials with 12‐month follow‐up (n = 266). Results showed no statistically significant difference between action plan and control (MD ‐1.04, 95% CI 2.43 to ‐4.51), with moderate heterogeneity (Chi² = 1.76, df = 1, P = 0.18, I² = 43%).
Analysis 1.37 SGRQ impact score at six months: For this outcome, we found four relevant trials with six‐month follow‐up (n = 452). Results showed no statistically significant difference between action plan and control (subgrouped by phone follow‐up) (MD ‐1.26, 95% CI ‐3.47 to 0.95), with no heterogeneity.
Generic health‐related quality of life subdomains: measured by Short Form (SF)‐36
For this outcome, we found one relevant trial with six‐month follow‐up (n = 90) that compared action plan and control. Table 3 shows results for eight domains as mean difference (MD) and 95% confidence interval (CI); a positive result indicates improvement.
| Outcome | SF‐36 domain | Mean difference | 95% CI |
| Physical function | 0.30 | ‐7.13 to 7.73 | |
| Role limitation | 9.00 | ‐8.07 to 26.07 | |
| Bodily pain | 18.50 | 6.14 to 30.86 | |
| General health | 2.60 | ‐3.71 to 8.91 | |
| Vitality | 1.60 | ‐4.73 to 7.93 | |
| Social function | 5.30 | ‐4.68 to 15.28 | |
| Role limitation | 7.50 | ‐8.56 to 23.56 | |
| Mental health | 6.30 | 0.64 to 11.96 |
Psychological morbidity: anxiety and depression
Investigators measured these outcomes by using the Hospital Anxiety and Depression Scale (HADS), a 21‐unit scale on which higher score indicates more severe symptoms, in one study that compared action plan with phone follow‐up and control with 12‐month follow‐up (n = 154), and in another study that compared action plan and control with six‐month follow‐up (n = 183). Table 4 shows results for depression and anxiety scores as mean difference (MD) and 95% confidence interval (CI); a negative result indicates fewer symptoms.
| Outcome | Domain | Follow‐up: months | MD | 95% CI | n |
| Depression | 12 | ‐0.25 | ‐1.14 to 0.64 | 154 | |
| Depression | 6 | 0.10 | ‐0.73 to 0.93 | 183 | |
| Anxiety | 12 | 0.14 | ‐1.38 to 1.66 | 154 | |
| Anxiety | 6 | 0.00 | ‐0.83 to 0.83 | 183 |
COPD self‐management for exacerbation and related self‐efficacy
Assessment of these outcomes was based on interviews with participants and use of different questionnaires in three studies that provided relevant data, preventing meta‐analysis of outcomes.
McGeoch 2004 (action plan vs control) used a standardised COPD self‐management questionnaire on which higher score indicates greater self‐efficacy (range 0 to 26), which has been shown to be valid and reliable (Dowson 2004). Rootmensen 2008 (action plan vs control) used a self‐administered self‐management questionnaire that was based on three exacerbation scenarios and included questions adapted from a validated interview‐based questionnaire (Kolbe 1996), on which higher score indicates greater self‐efficacy. Trappenburg 2011 (action plan with phone follow‐up vs control) measured self‐management exacerbation‐related self‐efficacy using a non‐validated questionnaire with 11 items graded on a 5‐point Likert scale. Lower scores indicate greater self‐efficacy for exacerbation‐related self‐management behaviour. Table 5 shows results as mean difference (MD) and 95% confidence interval (CI).
| Outcome | Study | Item | Direction improvement | Months | MD | 95% CI | n |
| Self‐management knowledge when well | + | 12 | 1.10 | 0.46 to 1.74 | 154 | ||
| Self‐management actions when well | + | 12 | 0.50 | ‐0.24 to 1.24 | 154 | ||
| Self‐management knowledge early exacerbation | + | 12 | 1.80 | 0.75 to 2.85 | 154 | ||
| Self‐management actions early exacerbation | + | 12 | 2.30 | 0.96 to 3.64 | 154 | ||
| Self‐management knowledge severe exacerbation | + | 12 | 2.50 | 0.94 to 4.06 | 154 | ||
| Self‐management action severe exacerbation | + | 12 | 1.50 | 0.47 to 2.53 | 154 | ||
| Self‐management exacerbation actions | + | 6 | ‐5.10 | ‐15.26 to 5.06 | 90 | ||
| Self‐efficacy for exacerbation recognition | ‐ | 6 | ‐0.70 | ‐0.98 to ‐0.42 | 183 | ||
| Self‐efficacy for exacerbation prevention/action | ‐ | 6 | ‐0.90 | ‐1.18 to ‐0.62 | 183 |
Lung function: FEV1 % predicted
For this outcome (Analysis 1.59), we found two relevant trials with six‐month follow‐up (n = 179), in which results showed no statistically significant difference between action plan and control (MD 1.83, 95% CI ‐1.05 to 4.71), and one relevant trial with 12‐month follow‐up (n = 293), in which results showed no statistically significant difference between action plan and control (MD 2.00, 95% CI ‐1.89 to 5.89).
Cost‐effectiveness
Analysis 1.60 The cost of hospital admissions (HADM) per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with lower costs in the action plan group (MD ‐1117.00 US$, 95% CI ‐1754.50 to ‐479.50).
Analysis 1.61 The cost of emergency department visits (EDV) per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed a statistically significant difference between action plan with phone follow‐up and control, with lower costs in the action plan group (MD ‐141.00 US$, 95% CI ‐234.31 to ‐47.69).
Analysis 1.62 The cost of pulmonary drug prescriptions per participant over 12 months: For this outcome, we found one relevant trial with 12‐month follow up (n = 743). Results showed no statistically significant difference between action plan with phone follow‐up and control (MD 15.00 US$, 95% CI ‐6.32 to 36.32).
Sensitivity analysis
We performed a sensitivity analysis to examine changes in SGRQ scores (overall and subscales) (Appendix 4). For Watson 1997, we compared results when we used the standard deviation taken from the largest value in another study of similar duration versus the same outcome when we used the standard deviation calculated from the correlation coefficient of data available for the same outcome in Wood‐Baker 2006. The sample size in Watson 1997 was approximately 50% the size of the other studies. Results showed no change in direction or statistical significance of the pooled difference by either method. We have presented in the text the result obtained with the standard deviation for Watson 1997 based on the value obtained from other studies, and a table in Appendix 4 shows corresponding results with use of an imputed standard deviation.
The small number of included studies limited sensitivity analyses performed by risk of bias grading. The increased likelihood of hospital admission for an acute exacerbation remained significant when we excluded studies with unclear risk of bias for randomisation and allocation concealment.
Discussion
Summary of main results
This systematic review update summarises the effects of an action plan (defined as a guideline detailing self‐initiated actions such as changing medication regimens or visiting a general practitioner (GP) or hospital, to be undertaken in response to alterations in symptoms of chronic obstructive pulmonary disease (COPD) suggesting the start of an exacerbation) with an accompanying educational component of short duration only (up to one hour) versus usual clinical care in COPD. Seven relevant randomised studies contributed to the comparison of action plan versus usual care for exacerbations of COPD. We included studies that provided ongoing support directed at use of the action plan, and we excluded studies with broader self‐management interventions.
For the primary outcome of healthcare utilisation for exacerbations, evidence shows benefit over 12 months, with fewer hospitalisations and emergency department (ED) visits for COPD in a large study (n = 743) of action plans with phone support (rate ratio (RR) 0.59, 95% confidence interval (CI) 0.44 to 0.79, moderate‐quality evidence (GRADE)) and decreased likelihood of hospital admission in two studies (n = 897) (odds ratio (OR) 0.69, 95% CI 0.49 to 0.97, moderate‐quality evidence (GRADE)). Thus over 12 months in studies in which participants had relatively low baseline risk, the number needed to treat for an additional beneficial outcome (NNTB) derived by avoiding hospitalisation for an exacerbation was 19 (95% CI 11 to 201).
Over the same follow‐up period, we found benefit for ED visits alone for COPD, with fewer ED visits for COPD in a large study (n = 743) of action plans with phone support (RR 0.49, 95% CI 0.33 to 0.73, high‐quality evidence (GRADE)) and less likelihood of an ED visit for COPD in two studies (n = 897) (OR 0.55, 95% CI 0.38 to 0.78, moderate‐quality evidence (GRADE)). Over 12 months, the NNTB required to avoid an ED visit for an exacerbation was 12 (95% CI 9 to 26). However, two studies (n = 201) that used action plans alone reported no significant reduction in the rate of ED visits for COPD (MD 0.37, 95% CI ‐0.50 to 1.24, very low‐quality evidence (GRADE)). For hospital admissions alone, one study (n = 743) of action plans with phone support reported no significant benefit (RR 0.69, 95% CI 0.47 to 1.01, moderate‐quality evidence (GRADE)). Fewer hospital admissions and ED visits for COPD translated into lower costs for the action plan intervention.
Four studies (n = 1134) found no significant change in all‐cause mortality over 12 months for action plan use, with or without phone support (OR 0.88, 95% CI 0.59 to 1.31, moderate‐quality evidence (GRADE)), but confidence intervals do not rule out important benefit or harm associated with the intervention.
Clear evidence indicates that action plans increased treatment for exacerbations of COPD over 12‐month follow‐up. Two studies (n = 200) reported an increase in courses of oral corticosteroids (MD 0.74, 95% CI 0.12 to 1.35, moderate‐quality evidence (GRADE)), and one study (n = 743) reported an increase in the cumulative dose of oral corticosteroids with phone support for action plan use (779.0 mg prednisolone, 95% CI 533.2 to 1024.8, high‐quality evidence (GRADE)). Three studies (n = 943) reported a significant increase in courses of antibiotics (MD 2.26, 95% CI 1.82 to 2.70, moderate‐quality evidence (GRADE)).
Studies have shown statistically significant benefit for respiratory‐related quality of life with action plan use over 12 months. Using St George's Respiratory Questionnaire (SGRQ) overall score, three studies (n = 1009) reported that the score was 2.8 units lower ‐ from 0.8 lower to 4.8 lower (moderate‐quality evidence (GRADE)). The confidence interval includes the minimum clinically important difference of 4 units. The review found no clear evidence of benefit for psychological morbidity in depression or anxiety as measured by the Hospital Anxiety and Depression Scale (HADS) in a single study over 12 months (low‐quality evidence (GRADE)).
Evidence also shows a positive effect on knowledge of appropriate self‐management for exacerbations in three studies that used different measurement instruments. We found clear evidence that action plans with limited education improved recognition and actions for appropriate self‐management during early stages and in severe exacerbations and led to increased self‐efficacy for exacerbation prevention and actions.
Subgroup analysis: effect of ongoing support directed at use of the action plan delivered by telephone or direct contact
Variation in study measurements limited the ways meta‐analyses could be grouped according to ongoing support for use of an action plan. Two healthcare utilisation outcomes contributed to subgroup analyses. For the likelihood of at least one hospital admission in 12 months (Analysis 1.2), in one study without ongoing support (OR 0.97, 95%CI 0.31 to 3.03) and in one study with ongoing phone support (OR 0.66, 95%CI 0.46 to 0.95), results showed no heterogeneity between subgroup results (Chi² = 0.39, df = 1, P = 0.53, I² = 0%). In the same studies, for the likelihood of at least one ED visit in 12 months (Analysis 1.10), the study without ongoing support (OR 0.64, 95%CI 0.25 to 1.66) and the study with phone support (OR 0.53, 95%CI 0.36 to 0.78) showed no heterogeneity between subgroups (Chi² = 0.13, df = 1, P = 0.72, I² = 0%).
For all‐cause mortality over 12 months (Analysis 1.18), three studies without ongoing support (OR 1.66, 95% CI 0.73 to 3.79) and one study with ongoing phone support (OR 0.72, 95% CI 0.46 to 1.14) showed moderate heterogeneity between subgroup results (Chi² = 5.17, df = 3, P = 0.16, I² = 42%); however, results of the test for subgroup differences were not statistically significant (Chi² = 3.03, df = 1, P = 0.08, I² = 67.0%).
For use of medication for exacerbations, only one outcome permitted subgroup analysis. For courses of antibiotics over 12 months (Analysis 1.27), in two studies with no ongoing support (MD 0.78, 95% CI ‐0.24 to 1.79) and in one study with phone support (MD 2.60, 95% CI 2.12 to 3.08), pooled analysis showed substantial heterogeneity (Chi² = 10.55, df = 2, P = 0.005, I² = 81%), and results of the test for subgroup differences were significant (Chi² = 10.09, df = 1, P = 0.001, I² = 90.1%)
Overall completeness and applicability of evidence
Our searches for this review are current to November 2015, and review results are based on seven studies that included 1550 symptomatic participants with COPD, with fairly typical characteristics of the COPD population at higher risk of exacerbation. In four studies, participants' mean forced expiratory volume at one second (FEV1) was < 50% predicted, and in three studies < 60% predicted. Three studies required a history of exacerbations in the past year (Martin 2004; McGeoch 2004; Rice 2010). Two studies excluded participants receiving home oxygen therapy (Martin 2004; McGeoch 2004), and one study (Watson 1997) excluded those receiving long‐term oral steroid therapy. Study recruitment was community based, and participants had varying numbers of comorbidities. The results of meta‐analyses, including those for healthcare utilisation, hospital admissions and ED presentations, are based on small numbers of studies with similar follow‐up periods, and the included studies have a relatively low baseline risk for hospital admissions. The applicability of findings to populations with high baseline risk is not known.
As shown in Additional Table 2, the format of the intervention had common elements in the self‐management action plan: early recognition of exacerbations based on symptoms, appropriate self‐initiated interventions and directions to seek medical care. Self‐management education was limited to a single short session, and so the intervention excluded multi‐faceted self‐management support programmes. Available information from three studies showed that the length of educational input was 45 minutes (Rootmensen 2008), 60 minutes (McGeoch 2004) and 60 to 90 minutes (Rice 2010). This update included support for implementing the action plan provided up to monthly by direct contact or by phone call. We made this change to reflect clinical practice in which action plans may be delivered in the outpatient setting with some form of ongoing support. Rice 2010 and Trappenburg 2011 included support for action plan use. For combined hospital admissions or ED visits, Rice 2010 reported a rate ratio of 0.78 (95% CI 0.35 to 1.74; P = 0.53) for participants receiving four to eight calls versus zero to three calls. The rate for participants with nine or more calls versus zero to three calls showed a significant reduction with increased phone contacts (RR 0.46, 95% CI 0.22 to 0.96; P = 0.04).
With the addition of new studies, this update has shown greater benefit for patient outcomes in addition to improvement in knowledge and understanding of appropriate actions in the event of an exacerbation. Analysis of mortality data was possible only for all‐cause mortality, as no data were available for respiratory‐related mortality. Studies reported few adverse effects data despite increased use of oral corticosteroids in the action plan group. In Walters 2014, an adverse drug reaction was significantly more likely with corticosteroid treatment of acute exacerbations than with placebo (OR 2.33, 95% CI 1.60 to 3.40), and the number needed to treat for an additional harmful outcome (NNTH) was 6 (95% CI 4 to 10). Hyperglycaemia was the most common adverse event (OR 4.95, 95% CI 2.47 to 9.91). Data on patient‐reported outcomes are few; this review shows benefit for overall respiratory‐related quality of life, but use of different instruments or follow‐up periods precluded meta‐analyses for generic quality of life and psychological morbidity.
Quality of the evidence
Among primary outcomes, this review update, which incorporates two new studies conducted since 2009, shows evidence of benefit for measures of healthcare utilisation of varying strength, generally high or moderate quality of effects on hospital admissions and ED visits when based on greater numbers of studies or on studies with large sample sizes and lower quality when based on smaller studies conducted earlier (before 2004). Review authors downgraded the result for mortality to moderate quality owing to imprecision. We graded the clear benefit derived from greater use of treatment for acute exacerbations as moderate (courses of oral corticosteroids) or high (cumulative doses of oral corticosteroids), and as moderate for courses of antibiotics. For patient‐reported outcomes, we graded respiratory‐related quality of life improvement as showing moderate quality; although health‐related quality of life was improved, the mean effect size was small, so this may not be clinically important for most patients. We graded the quality of evidence for the psychological domain of depression as low and noted no significant differences.
Potential biases in the review process
Confounding may be present in studies based in primary care through self‐selection of general practitioners (GPs) with an interest in COPD (Watson 1997), who might be more likely to treat COPD exacerbations with antibiotics or prednisolone in the absence of an action plan. However, in studies that used cluster‐randomisation (McGeoch 2004; Wood‐Baker 2006) this was less likely. Wood‐Baker 2006 did not account for the effect of clustering. In the meta‐analysis, review authors included available group mean results for individual participants, potentially leading to overestimation of effect. Review authors other than the study author extracted and entered all data from Wood‐Baker 2006. Review authors who were not trialists in a study made decisions on downgrades for Summary of findings tables. McGeoch 2004 reported methods of analysis that accounted for clustering.
Agreements and disagreements with other studies or reviews
As the primary objective of this review is to examine the specific effects of a self‐management action plan for exacerbations of COPD, we restricted included studies to those that excluded broad self‐management training and education, often delivered in a group format, and sometimes as part of a pulmonary rehabilitation programme. In clinical practice in some settings, an action plan may include some form of ongoing support for action plan use, often provided during case management for outpatients or those receiving primary care. For this reason, we decided that this update should include studies with up to monthly ongoing support limited to use of the action plan. We prespecified this change to the inclusion criteria of the old review (Walters 2010) in the updated protocol. Subgroup analysis indicates possibly greater effect for such regular phone support.
In comparison with the previous version of this review, moderate‐quality evidence now suggests benefit in healthcare utilisation for COPD action plans. This development is largely due to inclusion of two new studies (Rice 2010; Trappenburg 2011) that featured ongoing support for action plan use and contributed an additional 976 participants to the pooled analysis.
Other systematic reviews looked at effects of self‐management interventions provided with or without action plans (protocol published (Zwerink 2014)), and examined action plans that form part of a broad self‐management educational programme (Lenferink 2015) or comprehensive pulmonary rehabilitation programme (McCarthy 2015). In Zwerink 2014, 74% of studies (n = 23) included an action plan as part of the self‐management intervention. Similar to our review, Zwerink 2014 found a reduction in the likelihood of respiratory‐related hospitalisation (OR 0.57, 95% CI 0.43 to 0.75) in nine studies reported similar benefit in number needed to treat for an additional beneficial outcome (NNTB) in a population at low baseline risk of avoiding hospital admission (20, 95% CI 15 to 35). It was not possible to create subgroups of at least three studies that did not use an action plan in the intervention; thus review authors for Zwerink 2014 performed no subgroup analyses. The benefit achieved by an action plan with a short patient education component is thus comparable with that achieved by more comprehensive educational programmes and self‐management programmes. A review of pulmonary rehabilitation programmes, in which subgroups compared 'exercise only’ trials (n = 31 trials) and ’exercise plus more comprehensive components’ trials (n = 34), noted no significant differences in effects on respiratory‐related quality of life (McCarthy 2015). The lack of effect on mortality reported in this review is similar to the finding of no effect on mortality in the review of self‐management education (Zwerink 2014), which did not include data from Fan 2012 on a comprehensive care management programme that included action plans. Investigators in Fan 2012 stopped the study prematurely because a higher number of deaths in the intervention group versus the control group could not be explained satisfactorily by study authors.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Action plan versus usual care, outcome: 1.18 Mortality (all cause) 12 months.

Comparison 1 Action plan versus usual care, Outcome 1 Hospitalizations for COPD /100 patient years.

Comparison 1 Action plan versus usual care, Outcome 2 At least 1 hospital admission (12 months).

Comparison 1 Action plan versus usual care, Outcome 3 at least 1 Hospital Admission (6 months).

Comparison 1 Action plan versus usual care, Outcome 4 Hospital admission (12 months).
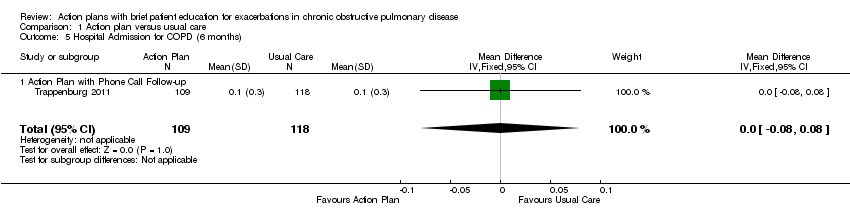
Comparison 1 Action plan versus usual care, Outcome 5 Hospital Admission for COPD (6 months).
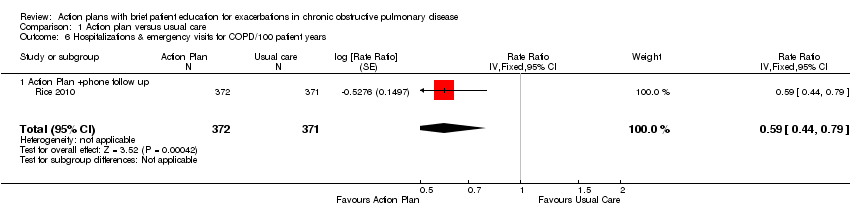
Comparison 1 Action plan versus usual care, Outcome 6 Hospitalizations & emergency visits for COPD/100 patient years.

Comparison 1 Action plan versus usual care, Outcome 7 At Least 1 Hospital or Emergency Department Visit for COPD.

Comparison 1 Action plan versus usual care, Outcome 8 Emergency department visits for COPD /100 patient years.

Comparison 1 Action plan versus usual care, Outcome 9 Emergency department visit for COPD (12 months).

Comparison 1 Action plan versus usual care, Outcome 10 At least 1 emergency department visit (12 months).

Comparison 1 Action plan versus usual care, Outcome 11 Emergency Department Visits for COPD (6 months).
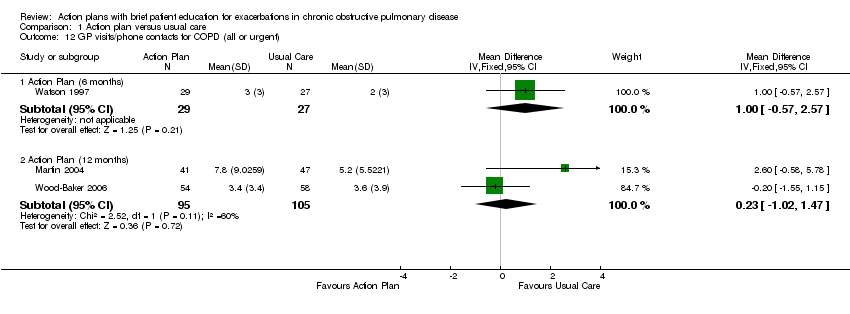
Comparison 1 Action plan versus usual care, Outcome 12 GP visits/phone contacts for COPD (all or urgent).
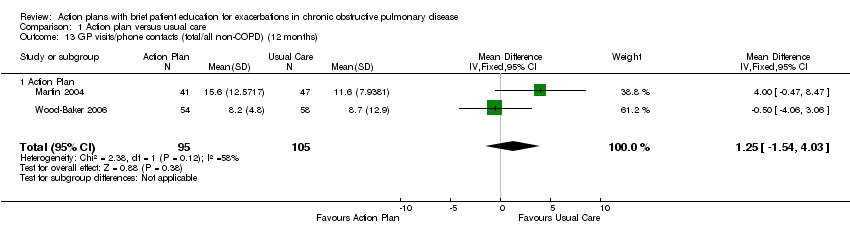
Comparison 1 Action plan versus usual care, Outcome 13 GP visits/phone contacts (total/all non‐COPD) (12 months).

Comparison 1 Action plan versus usual care, Outcome 14 Unscheduled Physician Visits (6 months).

Comparison 1 Action plan versus usual care, Outcome 15 Ambulance calls (total).

Comparison 1 Action plan versus usual care, Outcome 16 Total Hospital Days (12 months).

Comparison 1 Action plan versus usual care, Outcome 17 Total ICU Days (12 months).
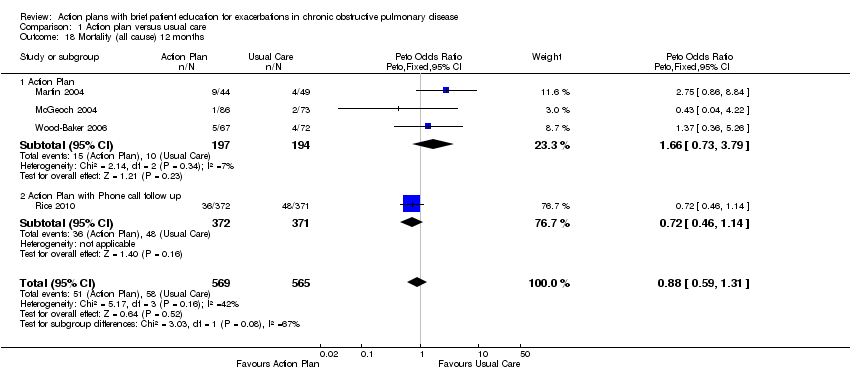
Comparison 1 Action plan versus usual care, Outcome 18 Mortality (all cause) 12 months.

Comparison 1 Action plan versus usual care, Outcome 19 Mortality (all cause) per 100 Patient‐Years (12 months).
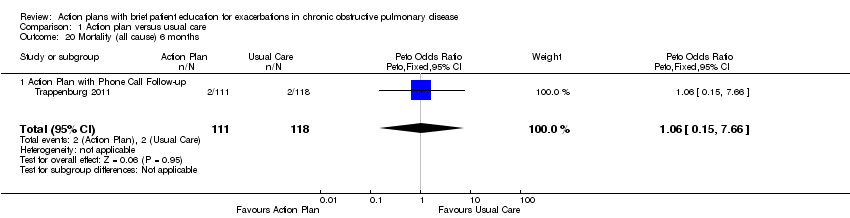
Comparison 1 Action plan versus usual care, Outcome 20 Mortality (all cause) 6 months.

Comparison 1 Action plan versus usual care, Outcome 21 At least 1 course oral steroids for exacerbation.

Comparison 1 Action plan versus usual care, Outcome 22 Courses of oral corticosteroids (12 months).

Comparison 1 Action plan versus usual care, Outcome 23 Courses of Corticosteroids (6 months).

Comparison 1 Action plan versus usual care, Outcome 24 Days on corticosteroids (6 months).

Comparison 1 Action plan versus usual care, Outcome 25 Prednisolone mg (12 months).

Comparison 1 Action plan versus usual care, Outcome 26 At least 1 course antibiotics for exacerbation.

Comparison 1 Action plan versus usual care, Outcome 27 Courses of antibiotics (12 months).

Comparison 1 Action plan versus usual care, Outcome 28 Courses of Antibiotics (6 months).

Comparison 1 Action plan versus usual care, Outcome 29 Days on antibiotics (6 months).

Comparison 1 Action plan versus usual care, Outcome 30 SGRQ overall score (12 months).

Comparison 1 Action plan versus usual care, Outcome 31 SGRQ overall score (6 months).
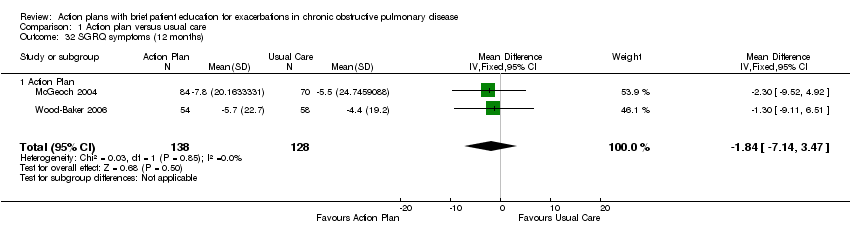
Comparison 1 Action plan versus usual care, Outcome 32 SGRQ symptoms (12 months).

Comparison 1 Action plan versus usual care, Outcome 33 SGRQ symptoms (6 months).

Comparison 1 Action plan versus usual care, Outcome 34 SGRQ activity limitation (12 months).

Comparison 1 Action plan versus usual care, Outcome 35 SGRQ activity limitation (6 months).
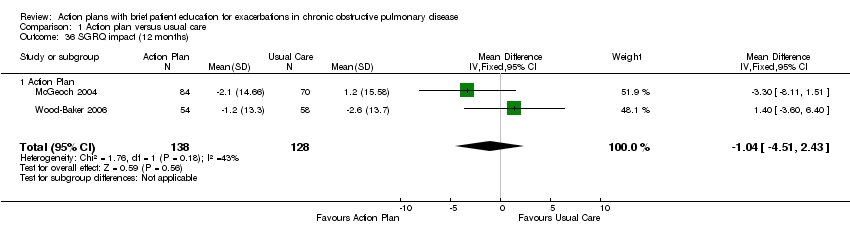
Comparison 1 Action plan versus usual care, Outcome 36 SGRQ impact (12 months).

Comparison 1 Action plan versus usual care, Outcome 37 SGRQ impact score (6 months).

Comparison 1 Action plan versus usual care, Outcome 38 SF36 physical function (6 months).

Comparison 1 Action plan versus usual care, Outcome 39 SF36 role limitation physical (6 months).
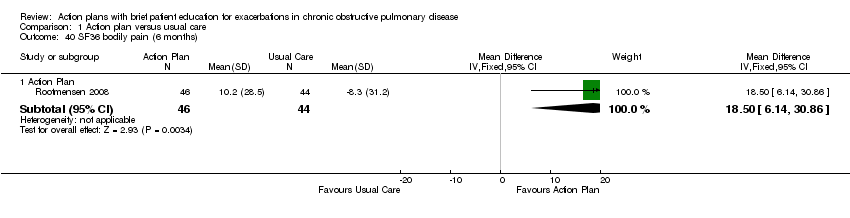
Comparison 1 Action plan versus usual care, Outcome 40 SF36 bodily pain (6 months).

Comparison 1 Action plan versus usual care, Outcome 41 SF36 general health (6 months).

Comparison 1 Action plan versus usual care, Outcome 42 SF36 vitality (6 months).

Comparison 1 Action plan versus usual care, Outcome 43 SF36 mental health (6 months).

Comparison 1 Action plan versus usual care, Outcome 44 SF36 role limitation emotional (6 months).
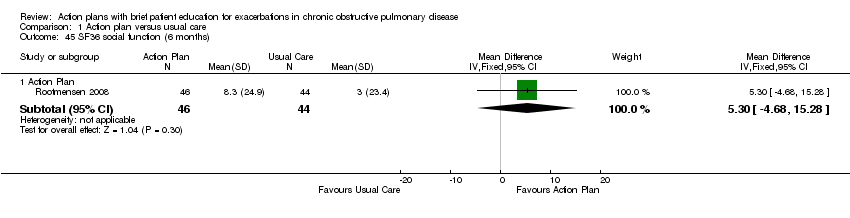
Comparison 1 Action plan versus usual care, Outcome 45 SF36 social function (6 months).

Comparison 1 Action plan versus usual care, Outcome 46 HADS ‐ depression score (12 months).
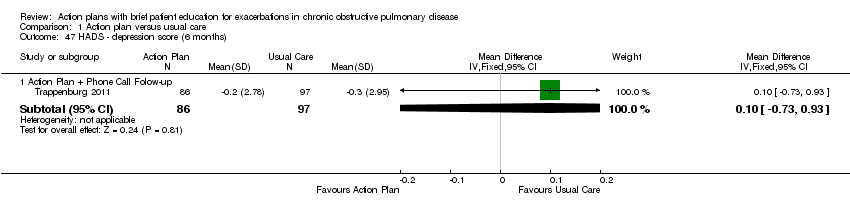
Comparison 1 Action plan versus usual care, Outcome 47 HADS ‐ depression score (6 months).

Comparison 1 Action plan versus usual care, Outcome 48 HADS ‐ anxiety score (12 months).

Comparison 1 Action plan versus usual care, Outcome 49 HADS ‐ anxiety score (6 months).

Comparison 1 Action plan versus usual care, Outcome 50 Exacerbation knowledge when well (12 months).

Comparison 1 Action plan versus usual care, Outcome 51 Exacerbation actions when well (12 months).

Comparison 1 Action plan versus usual care, Outcome 52 Early exacerbation knowledge (12 months).
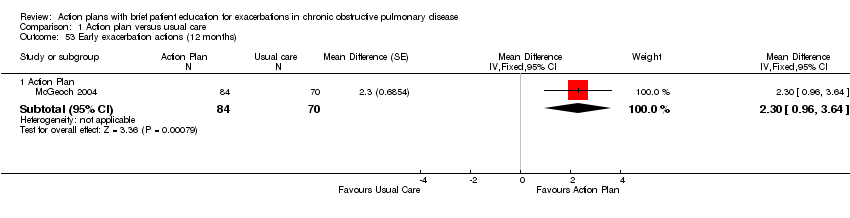
Comparison 1 Action plan versus usual care, Outcome 53 Early exacerbation actions (12 months).
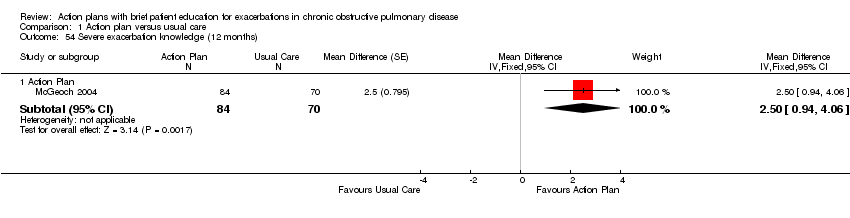
Comparison 1 Action plan versus usual care, Outcome 54 Severe exacerbation knowledge (12 months).

Comparison 1 Action plan versus usual care, Outcome 55 Severe exacerbation actions (12 months).

Comparison 1 Action plan versus usual care, Outcome 56 Self‐management exacerbation actions (6 months).

Comparison 1 Action plan versus usual care, Outcome 57 Self‐efficacy for Exacerbation Recognition (6 months).

Comparison 1 Action plan versus usual care, Outcome 58 Self‐efficacy for Exacerbation Prevention/Action (6 months).

Comparison 1 Action plan versus usual care, Outcome 59 FEV1 % predicted.
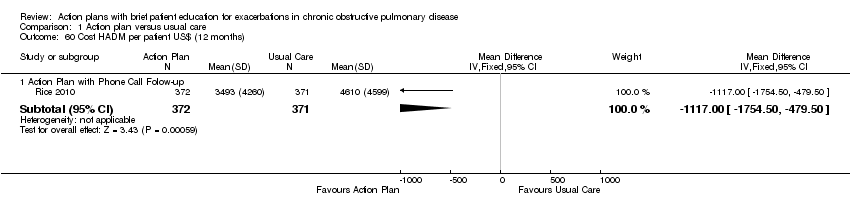
Comparison 1 Action plan versus usual care, Outcome 60 Cost HADM per patient US$ (12 months).

Comparison 1 Action plan versus usual care, Outcome 61 Cost EDV Per Patient US$ (12 months).

Comparison 1 Action plan versus usual care, Outcome 62 Cost Pulmonary Drug Prescriptions per Patient US$ (12 months).
| Do action plans improve patient outcomes in acute exacerbations of chronic obstructive pulmonary disease | ||||||
| Patient or population: individuals with exacerbations of chronic obstructive pulmonary disease | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with usual care | Risk with action plan | |||||
| Hospitalisations for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.69 | 743 | ⊕⊕⊕⊝ | |||
| Hospitalisations and emergency visits for COPD/100 patient‐years (action plan + phone follow‐up) | Rate ratio 0.59 | 743 | ⊕⊕⊕⊕ | |||
| At least 1 hospital admission | 209 per 1000 | 154 per 1000 | Odds ratio 0.69 | 897 | ⊕⊕⊕⊝ | |
| Mortality (all‐cause) | 103 per 1000 | 91 per 1000 | Odds ratio 0.88 | 1134 | ⊕⊕⊕⊝ | |
| Courses of oral corticosteroids | Mean courses of oral corticosteroids were 1.05 | Mean courses of oral corticosteroids in the intervention group were 0.74 more (0.12 more to 1.35 more) | ‐ | 200 | ⊕⊕⊕⊝ | |
| Courses of antibiotics | Mean courses of antibiotics ranged from 1.6 to 3.2 | Mean courses of antibiotics in the intervention group were 2.26 more (1.82 more to 2.7 more) | ‐ | 943 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Respiratory‐related quality of life: SGRQ overall score | Mean respiratory‐related quality of life: SGRQ overall score ranged from ‐2 to +6 units | Mean respiratory‐related quality of life: SGRQ overall score in the intervention group was 2.82 units lower (0.83 lower to 4.81 lower) | ‐ | 1009 | ⊕⊕⊕⊝ | Not downgraded for presence of substantial heterogeneity, which is explicable by differences in study design |
| Depression score | Mean depression score was ‐0.04 | Mean depression score in the intervention group was 0.25 lower (1.14 lower to 0.64 higher) | ‐ | 154 | ⊕⊕⊝⊝ | |
| *Risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aWide confidence interval; effect size includes null. bUnclear risk of bias for two studies for allocation and blinding of assessors. cUnclear risk of bias for three studies for allocation and blinding of assessors. dUnclear risk of bias for one study for allocation and blinding of assessors. | ||||||
| Study ID | Dates | Recruitment/Randomisation unit | Follow‐up | Length SME (educator) | RAN, n/WD, n | Age*, years/ % male | % current smokers | FEV1 % pred* INT‐CONT | QoL INT‐CONT |
| Not known | Consortium practices, New Zealand/participants | 12 months | Single interview, length not stated (respiratory nurse) | 96/26 | 70/51 | n/a | 35‐34 | 57‐51 | |
| 7/2002‐ 12/2003 | 2 groups of practices, New Zealand/practice | 12 months | 1 hour (practice nurse or respiratory educator) | 159/9 | 71/59 | 28 | 55‐53 | 43‐37 | |
| Rootmensen 2008 (all participants) | Not known | 1 hospital pulmonary outpatient clinic, Netherlands/ participants | 6 months | 45 minutes (pulmonary nurse) | 157 (111 COPD)/17 | 60/55 | 12 | 57‐64 | n/a |
| 07/2004‐ 07/2008 | Centralised electronic medical record database/participants | 12 months | 1 to 1.5‐hour group educational session (case manager) | 743/84 | 70/98 | 22 | 36.1‐38.1 | n/a | |
| 12/2008‐ 12/2010 | 8 regional hospitals and 5 general practices/participants (stratified by gender and centre) | 6 months | Single interview, length not stated (nurse case manager). | 233/41 | 66/57 | 29 | 56.7‐56.5 | n/a | |
| 1993‐ 07/1994 | 12 practices, 22 GPs, New Zealand/participants | 6 months | Single interview, length not stated (practice nurse) | 69/13 | 68/65 | 28 | 37‐36 | 43‐39 | |
| 2002‐2003 | 54 GPs, 31 practices, Australia/practice | 12 months | 1 hour (respiratory research nurse) | 139/27 | 70/76 | 42 | 46‐44 | 47‐47 | |
| *: mean; AP: action plan; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in one second; GP: general practitioner; INT‐CONT: intervention group‐control group; QoL: % impairment quality of life 0‐100; RAN: randomisation; SME: self‐management education; WD: withdrawal or death. | |||||||||
| Individualised AP | Standard written AP | Support for AP during study period | SME (individual/group) | Prescription /supply OCS | Prescription /supply ABS | Written COPD educational component | Comparison | |
| Written | 3‐Monthly visit regarding use of AP | Individual interview with respiratory nurse, length not stated, individualised action plan according to current treatment and symptoms | All had 7‐day supply | All had 7‐day supply | No | Usual care by own GP | ||
| Yes | No | Individual session by practice nurse or respiratory educator in association with GP 1 hour, covering major points of COPD self‐management plan, and use of validated sputum colour charts | Prescription | Prescription | Educational package | Non‐standard education on COPD according to practice standards | ||
| Written | Monthly phone call from nurse | Group 1‐1.5 hours, individualised action plan with respiratory nurse | Yes | Prescription | Usual care + 1‐page summary of principles of COPD care according to published guidelines. No AP | |||
| Oral | No | Individual protocol‐based educational session covering disease, medications, vaccination, smoking cessation and exacerbation management, 45 minutes in length | Oral medication provided to some, % unknown | Oral medication provided to some, % unknown | No | Usual care | ||
| Written | Standardised phone calls at 1 and 4 months | Individualised action plan education, length of session not stated | 2%' | 22% | ✓ COPD information | Usual care ‐ pharmacological and non‐pharmacological care according to most recent evidence‐based guidelines, specifically AP denied. All included participants seen by respiratory nurse, who systematically checked and discussed aspects of COPD care, including vaccination, optimisation of medication, inhalation techniques, exercise, nutritional aspects, smoking (cessation) and exacerbation management. | ||
| Yes | No | Individual session education about use of the action plan with COPD booklet by a senior respiratory outreach nurse; length not stated | Prescription | Prescription | ✓ Guide to living positively with COPD | Usual care by GP, specifically denied access to AP and booklet | ||
| Written | No | Individual educational session with respiratory nurse, covering COPD, smoking cessation, immunisation, nutrition, exercise, sputum clearance, breathing, medication, inhaler use. Individualised action plan developed with GP input. Length not known | 2% | 22% | COPD information booklet | Usual care, COPD information booklet and individual educational session with nurse, but no AP | ||
| ABS: antibiotics; AP: action plan; COPD: chronic obstructive pulmonary disease; GP: general practitioner; OCS: oral corticosteroids; SME: self‐management education. | ||||||||
| Outcome | SF‐36 domain | Mean difference | 95% CI |
| Physical function | 0.30 | ‐7.13 to 7.73 | |
| Role limitation | 9.00 | ‐8.07 to 26.07 | |
| Bodily pain | 18.50 | 6.14 to 30.86 | |
| General health | 2.60 | ‐3.71 to 8.91 | |
| Vitality | 1.60 | ‐4.73 to 7.93 | |
| Social function | 5.30 | ‐4.68 to 15.28 | |
| Role limitation | 7.50 | ‐8.56 to 23.56 | |
| Mental health | 6.30 | 0.64 to 11.96 |
| Outcome | Domain | Follow‐up: months | MD | 95% CI | n |
| Depression | 12 | ‐0.25 | ‐1.14 to 0.64 | 154 | |
| Depression | 6 | 0.10 | ‐0.73 to 0.93 | 183 | |
| Anxiety | 12 | 0.14 | ‐1.38 to 1.66 | 154 | |
| Anxiety | 6 | 0.00 | ‐0.83 to 0.83 | 183 |
| Outcome | Study | Item | Direction improvement | Months | MD | 95% CI | n |
| Self‐management knowledge when well | + | 12 | 1.10 | 0.46 to 1.74 | 154 | ||
| Self‐management actions when well | + | 12 | 0.50 | ‐0.24 to 1.24 | 154 | ||
| Self‐management knowledge early exacerbation | + | 12 | 1.80 | 0.75 to 2.85 | 154 | ||
| Self‐management actions early exacerbation | + | 12 | 2.30 | 0.96 to 3.64 | 154 | ||
| Self‐management knowledge severe exacerbation | + | 12 | 2.50 | 0.94 to 4.06 | 154 | ||
| Self‐management action severe exacerbation | + | 12 | 1.50 | 0.47 to 2.53 | 154 | ||
| Self‐management exacerbation actions | + | 6 | ‐5.10 | ‐15.26 to 5.06 | 90 | ||
| Self‐efficacy for exacerbation recognition | ‐ | 6 | ‐0.70 | ‐0.98 to ‐0.42 | 183 | ||
| Self‐efficacy for exacerbation prevention/action | ‐ | 6 | ‐0.90 | ‐1.18 to ‐0.62 | 183 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hospitalizations for COPD /100 patient years Show forest plot | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 1.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.47, 1.01] |
| 2 At least 1 hospital admission (12 months) Show forest plot | 2 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.97] |
| 2.1 Action Plan | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.31, 3.03] |
| 2.2 Action Plan + Phonecall Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.95] |
| 3 at least 1 Hospital Admission (6 months) Show forest plot | 1 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.30, 2.31] |
| 3.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.30, 2.31] |
| 4 Hospital admission (12 months) Show forest plot | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.03, 0.49] |
| 4.1 Action Plan | 2 | 205 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.03, 0.49] |
| 5 Hospital Admission for COPD (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 5.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.08, 0.08] |
| 6 Hospitalizations & emergency visits for COPD/100 patient years Show forest plot | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.44, 0.79] |
| 6.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.59 [0.44, 0.79] |
| 7 At Least 1 Hospital or Emergency Department Visit for COPD Show forest plot | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 7.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.80] |
| 8 Emergency department visits for COPD /100 patient years Show forest plot | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 8.1 Action Plan +phone follow up | 1 | 743 | Rate Ratio (Fixed, 95% CI) | 0.49 [0.33, 0.73] |
| 9 Emergency department visit for COPD (12 months) Show forest plot | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 9.1 Action Plan | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.50, 1.24] |
| 10 At least 1 emergency department visit (12 months) Show forest plot | 2 | 897 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.78] |
| 10.1 Action Plan | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.25, 1.66] |
| 10.2 Action Plan + Phone Call Follow‐up | 1 | 743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.36, 0.78] |
| 11 Emergency Department Visits for COPD (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 11.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 12 GP visits/phone contacts for COPD (all or urgent) Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Action Plan (6 months) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.57, 2.57] |
| 12.2 Action Plan (12 months) | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.02, 1.47] |
| 13 GP visits/phone contacts (total/all non‐COPD) (12 months) Show forest plot | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.54, 4.03] |
| 13.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.54, 4.03] |
| 14 Unscheduled Physician Visits (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 14.1 Action Plan with Phonecall Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.36, 0.36] |
| 15 Ambulance calls (total) Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.17, 3.23] |
| 15.1 Action Plan | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [0.17, 3.23] |
| 16 Total Hospital Days (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [0.00, ‐0.20] |
| 16.1 Action Plan + Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [0.00, ‐0.20] |
| 17 Total ICU Days (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| 17.1 Action Plan + Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.60, ‐0.00] |
| 18 Mortality (all cause) 12 months Show forest plot | 4 | 1134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 18.1 Action Plan | 3 | 391 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [0.73, 3.79] |
| 18.2 Action Plan with Phone call follow up | 1 | 743 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.46, 1.14] |
| 19 Mortality (all cause) per 100 Patient‐Years (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐8.86, 1.46] |
| 19.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐8.86, 1.46] |
| 20 Mortality (all cause) 6 months Show forest plot | 1 | 229 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.15, 7.66] |
| 20.1 Action Plan with Phone Call Follow‐up | 1 | 229 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [0.15, 7.66] |
| 21 At least 1 course oral steroids for exacerbation Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Action Plan (6 months) | 1 | 56 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.58 [1.29, 33.62] |
| 21.2 Action Plan (12 months) | 1 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.34, 4.69] |
| 22 Courses of oral corticosteroids (12 months) Show forest plot | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.12, 1.35] |
| 22.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.12, 1.35] |
| 23 Courses of Corticosteroids (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.23, 0.23] |
| 23.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.23, 0.23] |
| 24 Days on corticosteroids (6 months) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.53, 17.53] |
| 24.1 Action Plan | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.53, 17.53] |
| 25 Prednisolone mg (12 months) Show forest plot | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 779.0 [533.23, 1024.77] |
| 25.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 779.0 [533.23, 1024.77] |
| 26 At least 1 course antibiotics for exacerbation Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 26.1 Action Plan (6 months) | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.51 [2.02, 21.05] |
| 26.2 Action Plan (12 months) | 2 | 293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.01, 2.69] |
| 27 Courses of antibiotics (12 months) Show forest plot | 3 | 943 | Mean Difference (IV, Fixed, 95% CI) | 2.26 [1.82, 2.70] |
| 27.1 Action Plan | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.78 [‐0.24, 1.79] |
| 27.2 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 2.6 [2.12, 3.08] |
| 28 Courses of Antibiotics (6 months) Show forest plot | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 28.1 Action Plan with Phone Call Follow‐up | 1 | 227 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.26, 0.26] |
| 29 Days on antibiotics (6 months) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [1.40, 10.60] |
| 29.1 Action Plan | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [1.40, 10.60] |
| 30 SGRQ overall score (12 months) Show forest plot | 3 | 1009 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐4.77, ‐0.82] |
| 30.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐2.70, 3.34] |
| 30.2 Action Plan + Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐5.10 [‐7.70, ‐2.50] |
| 31 SGRQ overall score (6 months) Show forest plot | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐2.93, 1.27] |
| 31.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐3.03, 2.37] |
| 31.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐1.6 [‐4.94, 1.74] |
| 32 SGRQ symptoms (12 months) Show forest plot | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐7.14, 3.47] |
| 32.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐7.14, 3.47] |
| 33 SGRQ symptoms (6 months) Show forest plot | 4 | 448 | Mean Difference (IV, Fixed, 95% CI) | ‐2.55 [‐6.92, 1.83] |
| 33.1 Action Plan | 3 | 265 | Mean Difference (IV, Fixed, 95% CI) | ‐2.07 [‐8.34, 4.20] |
| 33.2 Action Plan + Phone Call Follow‐up (change from baseline) | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐9.10, 3.10] |
| 34 SGRQ activity limitation (12 months) Show forest plot | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 2.87 [‐1.26, 7.00] |
| 34.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | 2.87 [‐1.26, 7.00] |
| 35 SGRQ activity limitation (6 months) Show forest plot | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.90, 3.67] |
| 35.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | 1.41 [‐1.99, 4.82] |
| 35.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐5.05, 4.65] |
| 36 SGRQ impact (12 months) Show forest plot | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.51, 2.43] |
| 36.1 Action Plan | 2 | 266 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.51, 2.43] |
| 37 SGRQ impact score (6 months) Show forest plot | 4 | 452 | Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐3.47, 0.95] |
| 37.1 Action Plan | 3 | 269 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐4.45, 1.39] |
| 37.2 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.9 [‐4.27, 2.47] |
| 38 SF36 physical function (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 38.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐7.13, 7.73] |
| 39 SF36 role limitation physical (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 39.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐8.07, 26.07] |
| 40 SF36 bodily pain (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 40.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 18.5 [6.14, 30.86] |
| 41 SF36 general health (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 41.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐3.71, 8.91] |
| 42 SF36 vitality (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 42.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 1.6 [‐4.73, 7.93] |
| 43 SF36 mental health (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 43.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 6.3 [0.64, 11.96] |
| 44 SF36 role limitation emotional (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 44.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 7.5 [‐8.56, 23.56] |
| 45 SF36 social function (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 45.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | 5.30 [‐4.68, 15.28] |
| 46 HADS ‐ depression score (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 46.1 Action Plan | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.14, 0.64] |
| 47 HADS ‐ depression score (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 47.1 Action Plan + Phone Call Folow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.73, 0.93] |
| 48 HADS ‐ anxiety score (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 48.1 Action Plan | 1 | 154 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐1.38, 1.66] |
| 49 HADS ‐ anxiety score (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 49.1 Action Plan + Phone Call Follow‐up (change from baseline) | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.83, 0.83] |
| 50 Exacerbation knowledge when well (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 50.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.1 [0.46, 1.74] |
| 51 Exacerbation actions when well (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 51.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 0.5 [‐0.24, 1.24] |
| 52 Early exacerbation knowledge (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 52.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.80 [0.75, 2.85] |
| 53 Early exacerbation actions (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 53.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 2.3 [0.96, 3.64] |
| 54 Severe exacerbation knowledge (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 54.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 2.5 [0.94, 4.06] |
| 55 Severe exacerbation actions (12 months) Show forest plot | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 55.1 Action Plan | 1 | 154 | Mean Difference (Fixed, 95% CI) | 1.5 [0.47, 2.53] |
| 56 Self‐management exacerbation actions (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 56.1 Action Plan | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐5.1 [‐15.26, 5.06] |
| 57 Self‐efficacy for Exacerbation Recognition (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 57.1 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐0.98, ‐0.42] |
| 58 Self‐efficacy for Exacerbation Prevention/Action (6 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 58.1 Action Plan + Phone Call Follow‐up | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.18, ‐0.62] |
| 59 FEV1 % predicted Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 59.1 6 months | 2 | 179 | Mean Difference (IV, Fixed, 95% CI) | 1.83 [‐1.05, 4.71] |
| 59.2 12 months | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐1.89, 5.89] |
| 60 Cost HADM per patient US$ (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 60.1 Action Plan with Phone Call Folow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐1117.0 [‐1754.50, ‐479.50] |
| 61 Cost EDV Per Patient US$ (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 61.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | ‐141.0 [‐234.31, ‐47.69] |
| 62 Cost Pulmonary Drug Prescriptions per Patient US$ (12 months) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 62.1 Action Plan with Phone Call Follow‐up | 1 | 743 | Mean Difference (IV, Fixed, 95% CI) | 15.00 [‐6.32, 36.32] |

