Aspirina para la fertilización in vitro
Resumen
Antecedentes
La aspirina se utiliza con el fin de optimizar las posibilidades de tener un hijo vivo en las mujeres que se someten a técnicas de reproducción asistida (TRA), a pesar de la evidencia poco consistentes de su eficacia y seguridad (en cuanto a la hemorragia intraoperatoria durante la recuperación de los ovocitos y el riesgo de aborto espontáneo). Aún están por determinar el momento más adecuado para comenzar el tratamiento con aspirina y la duración necesaria del tratamiento. Esta es la segunda actualización de la revisión publicada por primera vez en 2007.
Objetivos
Evaluar la efectividad y la seguridad de la aspirina en pacientes sometidas a TRA.
Métodos de búsqueda
Se realizaron búsquedas en el registro de ensayos del Grupo Cochrane de Ginecología y Fertilidad (Cochrane Gynaecology and Fertility Group), en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL; 2016, número 4) en The Cochrane Library (búsqueda el 9 de mayo 2016); en las bases de datos MEDLINE (1946 a 9 de mayo 2016) y Embase (1974 a 9 de mayo 2016); y en registros de ensayos (ClinicalTrials.gov y el portal de búsquedas de la World Health Organization International Clinical Trials Registry Platform). También se examinaron las listas de referencia de todos los estudios primarios y artículos de revisión conocidos, las listas de citas de las publicaciones pertinentes y los resúmenes de las principales reuniones científicas, en combinación con la estrategia de búsqueda del Grupo Cochrane de Ginecología y Fertilidad.
Criterios de selección
Ensayos controlados aleatorizados sobre la aspirina para las mujeres que se someten a TRA.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente evaluaron la elegibilidad de los ensayos y el riesgo de sesgo y extrajeron los datos. El resultado primario de la revisión fueron los nacidos vivos. Los resultados secundarios incluyeron embarazo clínico, embarazo en curso, embarazo múltiple, aborto y otras complicaciones asociadas con la FIV/ICSI o con el embarazo y el parto. Se combinaron los datos para calcular los riesgos relativos (RR) (para los datos dicotómicos) y las diferencias de medias (DM) (para los datos continuos), y los intervalos de confianza (IC) del 95%. La heterogeneidad estadística se evaluó con la estadística I². La calidad general de la evidencia para las comparaciones principales se evaluó mediante criterios GRADE.
Resultados principales
La búsqueda identificó 13 ensayos para su inclusión en la revisión, con 2653 participantes con una media de edad de 35 años. Diez estudios utilizaron una dosis diaria de aspirina de 100 mg y tres utilizaron 80 mg. En la mayoría de ellos, la aspirina se empezó a tomar inmediatamente al comienzo del tratamiento de disminución de receptores, mientras que la duración del tratamiento varió mucho. Ocho estudios proporcionaron placebo para el grupo control.
No hubo evidencia de diferencias entre el grupo de aspirina y el grupo que no recibió tratamiento o placebo en cuanto a las tasas de nacidos vivos (RR 0,91; IC del 95%: 0,72 a 1,15; tres ECA, n = 1053; I² = 15%, evidencia de calidad moderada). Además, las tasas de embarazo clínico también fueron similares para los dos grupos (RR 1,03; IC del 95%: 0,91 a 1,17; diez ECA, n = 2142; I² = 27%, evidencia de calidad moderada); el análisis de sensibilidad, con la exclusión de los estudios de alto riesgo de sesgo, no modificó la estimación del efecto. No hubo evidencia de una diferencia entre los grupos en cuanto a los embarazos múltiples confirmados por ecografía (RR 0,67; IC del 95%: 0,37 a 1,25; dos ECA, n = 656; I² = 0%, evidencia de calidad baja), el aborto espontáneo (RR 1,10; IC del 95%: 0,68 a 1,77; cinco ECA, n = 1497; I² = 0%, evidencia de calidad baja), el embarazo ectópico (RR 1,86; IC del 95%: 0,75 a 4,63; tres ECA, n = 1135; I² = 0%, evidencia de calidad muy baja) o la hemorragia vaginal (RR 1,01; IC del 95%: 0,14 a 7,13; un ECA, n = 487; evidencia de calidad muy baja). Faltaron datos sobre otros efectos adversos.
La calidad general de la evidencia varió entre muy baja y moderada; las limitaciones fueron el informe deficiente sobre los métodos de los estudios y la sospecha de sesgo de publicación.
Conclusiones de los autores
Actualmente no hay evidencia a favor del uso sistemático de la aspirina para mejorar las tasas de embarazo en una población general de FIV. Lo anterior se basa en los datos disponibles de los ensayos controlados aleatorizados, en los que actualmente no hay evidencia de un efecto de la aspirina en las mujeres que se someten a TRA, ya que no hay una sola medida de resultado que demuestre un efecto beneficioso con su uso. Además, la evidencia actual no excluye la posibilidad de que se produzcan efectos adversos.
PICO
Resumen en términos sencillos
Aspirina para mujeres que se someten a técnicas de reproducción asistida (TRA).
Pregunta de la revisión
Los investigadores Cochrane examinaron la evidencia sobre la efectividad y la seguridad de la aspirina administrada con el objetivo de aumentar las posibilidades de tener un hijo vivo en las mujeres que se someten a técnicas de reproducción asistida (TRA).
Antecedentes
La aspirina se ha utilizado de manera habitual en un intento de aumentar las posibilidades de tener un hijo vivo en las mujeres que se someten a TRA. Sin embargo, hay evidencia contradictoria sobre la efectividad de este tratamiento y sobre el momento adecuado para comenzarlo. así como sobre su duración. Aunque fisiológicamente la aspirina ejerce un efecto beneficioso en algunos aspectos necesarios para el éxito del embarazo, la ingestión de aspirina también se ha asociado con aborto espontáneo y hemorragia vaginal. Por lo tanto, fue importante evaluar la evidencia actual sobre la efectividad de este tratamiento.
Características de los estudios
Se encontraron 13 ensayos controlados aleatorizados con grupos paralelos, que compararon la aspirina con placebo o ningún tratamiento, en 2653 mujeres que se sometieron a TRA. Los estudios se realizaron en los EE.UU. y en diversos países de Europa y Asia. Uno de los ensayos incluidos fue financiado en parte por una empresa farmacéutica relacionada con la intervención. En la mayoría de los estudios los grupos fueron comparables y la media de edad de las participantes en ambos grupos fue 32 años. En la mayoría de los estudios se administró una dosis idéntica de la intervención y la mayoría informó de un momento similar de inicio de la ingesta de aspirina. La duración del ensayo varió entre los estudios, pero fue suficiente para proporcionar datos sobre los resultados informados como investigados respectivamente por cada grupo. La evidencia está actualizada hasta el 9 de mayo 2016.
Resultados clave
No hubo evidencia de que hubiera diferencias entre los grupos en cuanto a las tasas de nacidos vivos, embarazos clínicos, embarazos ectópicos, embarazos múltiples, abortos espontáneos o hemorragias vaginales. El número de estudios fue limitado y la calidad de la evidencia varió de muy baja a moderada, mientras que los datos sobre las tasas de complicaciones, ya sea durante el procedimiento de FIV/ICSI o durante el embarazo y el parto, fueron muy limitados o faltaron. En esta segunda actualización no fue posible agregar nuevos datos de estudios adicionales, ya que no se encontraron nuevos ECA que informaran sobre estos resultados en las comparaciones preestablecidas. Sobre la base de la evidencia disponible, se llegó a la misma conclusión que en la versión inicial de la revisión: ninguna medida de resultado única demostró un efectos beneficioso con el uso de aspirina. Actualmente no hay evidencia que apoye la administración del tratamiento con aspirina para mejorar las tasas de embarazo de una población general de FIV.
Calidad de la evidencia
La evidencia fue de calidad moderada en el caso de los nacidos vivos y de calidad muy baja a moderada en el caso de otros resultados. Las principales limitaciones de la evidencia fueron el informe deficiente sobre los métodos de estudio, el sesgo de publicación y la falta de estudios que investigaran los resultados deseados.
Authors' conclusions
Summary of findings
| Low‐dose aspirin compared to placebo or no treatment for women undergoing ART | ||||||
| Population: Women with subfertility | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Low‐dose aspirin | |||||
| Live birth | 225 per 1000 | 204 per 1000 | RR 0.91 | 1053 | ⊕⊕⊕⊝ | |
| Clinical pregnancy | 337 per 1000 | 347 per 1000 | RR 1.03 | 2142 | ⊕⊕⊕⊝ | |
| Multiple pregnancy (on ultrasound) | 84 per 1000 | 56 per 1000 | RR 0.67 (0.37 to 1.25) | 656 | ⊕⊕⊝⊝ | |
| Miscarriage rate per woman | 43 per 1000 | 47 per 1000 | RR 1.1 | 1497 | ⊕⊕⊝⊝ | |
| Ectopic pregnancy | 12 per 1000 | 23 per 1000 | RR 1.86 | 1135 | ⊕⊝⊝⊝ | |
| Vaginal bleeding | 8 per 1000 | 8 per 1000 (1 to 58) | RR 1.01 (0.14 to 7.13) | 487 (1 study) | ⊕⊝⊝⊝ | |
| Other adverse events | Other adverse events (such as preterm birth, antepartum haemorrhage, need for operative delivery) were not reported in the included studies | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious imprecision with low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in the control group. 6 Single study. Very serious imprecision with very low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in either group. | ||||||
Background
Description of the condition
Assisted reproduction technologies (ART), and especially in vitro fertilization (IVF) and intra‐cytoplasmic sperm injection (ICSI), have allowed a high degree of intervention in the physiological course of reproduction. The potential of pregnancy requires an embryo with increased implantation potential, a receptive endometrium and highly coordinated signalling and precise crosstalk between these interlinked sides. Unfortunately, success rates remain low. The majority of women undergoing IVF reach the transfer stage with good‐quality embryos available for transfer, but only a small proportion of them ever achieve a live birth. More than 85% of the embryos placed into the uterine cavity fail to give a viable pregnancy. Different medical and non‐medical strategies have been proposed to prevent this.
Description of the intervention
Aspirin (acetylsalicylic acid) is widely known to reduce fever, pain and inflammation, but may cause stomach ulcers (Flower 2003). Long‐term use of aspirin, even at low doses, is associated with bleeding in the digestive tract (Derry 2000). Its main method of action is the inhibition of the synthesis of prostaglandin (a chemical in the body which promotes inflammation, fever and pain) by the enzyme cyclo‐oxygenase (Vane 1971); and subsequent reduction of platelet aggregation (making the blood less sticky and hence less likely to clot). There are two main types of cyclo‐oxygenase: COX 1 and COX 2. COX 1‐generated prostaglandins are required to maintain physiological functions (such as protection of the stomach lining, platelet aggregation) whereas COX 2 is involved in the production of an inflammatory response. Aspirin irreversibly inhibits both types, thus a single aspirin tablet can depress platelet aggregation for many days despite the short amount of time it takes for the body to break the drug down. Aspirin is broken down in the body by freeing the salicylate from the acetyl group, and salicylate may also inhibit cyclo‐oxygenase.
As well as reducing fever or pain, aspirin has been used to treat or prevent other diseases including some forms of cancer (Bosetti 2002). How these effects occur is unclear, though production of COX 2 is increased in many tumours and its suppression may restrict cancer cell growth or could activate the destruction of cancer cells (Thun 2002). Aspirin is widely used in the prevention and treatment of cardiovascular disease (ISIS‐3 1992); and its use has been linked to a reduction in Alzheimer's disease (Stewart 1997), again possibly by inhibiting COX 2.
How the intervention might work
Within the field of reproductive medicine, aspirin has been used to increase the weight of newborns in pregnant women with foetal growth retardation (Wallenburg 1987), to prevent recurrent unexplained foetal growth retardation (Yamaguchi 1985), and to improve placental and foetal blood flow in women with pre‐eclampsia (Jouppilsa 1985). Historically, aspirin has also been prescribed for women who had suffered recurrent miscarriages in the past, and for women with antiphospholipid antibody syndrome (APS), in combination with heparin (Rai 1997).
The role of aspirin in women with infertility is controversial and the evidence is inconsistent (RCOG 2003). Some papers have demonstrated a beneficial effect of aspirin in women undergoing assisted conception (Rubinstein 1999); whilst others have not (Urman 2000). Proposed benefits of aspirin include improvement in uterine and ovarian blood flow (Rubinstein 1999); and inhibition of platelet cyclo‐oxygenase, thus inhibiting thromboxane synthesis and so preventing thrombosis in the placental vasculature. It may also be a potent stimulator of interleukin‐3 (IL‐3) in low doses through its ability to raise leukotriene production (proteins associated with pregnancy success) (Fishman 1995). However, the pre‐conceptual use of aspirin has been associated with increased miscarriage rates in retrospective studies (Li 2003). As aspirin is thought to increase endometrial thickness, which is purported to increase implantation rates, aspirin therapy is often used to support intracytoplasmic sperm injection treatment cycles in addition to traditional IVF.
The anti‐platelet effect of aspirin lasts for about seven days after discontinuation and so consideration to excessive bleeding risk at the egg collection stage should be given, especially for empirical reasons. For women who are on anticoagulants for genuine reasons such as proven thrombophilias, previous thrombo‐embolic events and cardiac patients (Fatemi 2013), a bridging plan should be established with their haematologist so as to minimise bleeding risk at egg collection versus thrombotic risk from their predisposed state and the hyperoestrogenic — and so hypercoagulable — state induced by the gonadotrophins used in assisted conception.
If indicated at all, when aspirin should be prescribed in an IVF treatment cycle is unclear. Possible times are: during down‐regulation of the woman's own reproductive hormones; before administration of drugs to stimulate ovulation; after egg collection; or after confirmation of pregnancy either by a pregnancy test or when confirmed by ultrasound. Also unclear is when aspirin should be stopped, for example after 12 weeks gestation, at 34 weeks or at delivery.
Why it is important to do this review
Couples may feel desperate enough to try anything that may help in assisted conception. They may be aware of the use of aspirin in some assisted conception units but may also have heard of the association between aspirin use and risk of miscarriage and bleeding. It is essential that any medically prescribed drug is fully tested under rigorous trial conditions so that the potential benefits and risks are clear to both clinicians and the couples undergoing assisted conception treatment cycles. We updated a systematic review of the randomised controlled trial evidence on the use of aspirin in assisted conception. We intend that this will help guide both healthcare providers and consumers in their decision‐making processes.
Objectives
To evaluate the effectiveness and safety of aspirin in women undergoing ART.
Methods
Criteria for considering studies for this review
Types of studies
All parallel‐design randomised controlled trials (RCTs), published or unpublished, which addressed the objectives of the review. Non‐randomised and quasi‐randomised trials were excluded as they are not randomised and are therefore more prone to bias. From crossover trials, only data from the first phase were included in meta‐analyses.
Types of participants
Women undergoing in vitro fertilisation (IVF) or intra‐cytoplasmic sperm injection (ICSI), or both, and their partners.
Types of interventions
Aspirin, versus placebo, any other active intervention or no treatment, taken either pre‐conceptually or at different stages of the treatment cycle (for example during down‐regulation, during stimulation of ovulation, after oocyte collection or after confirmation of pregnancy by a pregnancy test or ultrasound). Aspirin treatment given for varying lengths of time was considered (for example up to different points in the treatment cycle; until confirmation of pregnancy; up to 12 weeks' gestation, 34 weeks' gestation or delivery). We defined low dose as a dose of 150 mg or less taken once per day. Trials reporting on the synchronous use of aspirin with any other treatment were excluded from the review.
Types of outcome measures
These were divided into primary and secondary outcomes.
Primary outcomes
Live birth rate (defined as delivery of a live foetus after 20 completed weeks of gestational age) per woman or couple randomised.
Secondary outcomes
1. Clinical pregnancy rate (confirmed by ultrasound at seven gestational weeks) per woman or couple randomised.
2. Ongoing pregnancy rate (number of pregnancies which continued beyond 12 gestational weeks) per woman or couple randomised.
3. Multiple pregnancy rate per woman or couple:
(a) confirmed by ultrasound;
(b) on delivery.
4. Miscarriage rate (confirmed by ultrasound and pregnancy test, or histology) per woman or couple randomised.
5. Complications with the IVF or ICSI procedure:
(a) intra‐operative haemorrhage (bleeding);
(b) vaginal or pelvic haematoma (painful swelling caused by the collection of blood between tissues);
(c) hospital admission;
(d) blood transfusion;
(e) ovarian hyperstimulation syndrome;
(f) gastritis (inflammation of the stomach lining);
(g) haematemesis (vomiting blood).
6. Gestational age of baby.
7. Complications during pregnancy or birth:
(a) threatened miscarriage;
(b) antepartum haemorrhage (bleeding during late pregnancy);
(c) need for operative delivery;
(d) postpartum haemorrhage (bleeding after delivery);
(e) ectopic pregnancy;
(f) preterm birth.
Search methods for identification of studies
We searched for all published and unpublished RCTs on aspirin in ART, without language restriction and in consultation with the Gynaecology and Fertility Group Information Specialist.
Electronic searches
All reports which appeared to describe RCTs of aspirin for women undergoing IVF were obtained using the following search strategies (from the inception of the databases to 9 May 2016):
(1) The Cochrane Gynaecology and Fertility Group Specialised Register of controlled trials was searched for any trials (Appendix 1).
(2) The Cochrane Central Register of Controlled Trials (CENTRAL CRSO) was searched in all fields (Appendix 2).
(3) The electronic databases MEDLINE (Appendix 3), Embase (Appendix 4), and PsycINFO (Appendix 5) were searched using search terms that included "(aspirin OR acetylsalicylic acid) AND (in‐vitro fertilisation OR intracytoplasmic sperm injection)". The MEDLINE search was combined with the Cochrane Menstrual Disorders and Subfertility Group's Highly Sensitive Search Strategy for identifying randomised controlled trials which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.2 chapter 6, 6.4.11).
(4) Published or ongoing trials in the trial registers for ongoing and registered trials: 'ClinicalTrials.gov', a service of the US National Institutes of Health (clinicaltrials.gov/ct2/home) and www.controlled‐trials.com, as well as the World Health Organization International Trials Registry Platform search portal (who.int/trialsearch/Default.aspx) (Appendix 6).
The latest search was run on 9 May 2016. All searches have been date limited from 1 January 2014 to 9 May 2016. following the same search strategy as mentioned above.
Searching other resources
The citation lists of any relevant publications identified (including review articles and included studies) were searched for further potentially eligible studies; as were articles in books identified through the citation lists of any relevant publications together with internal reports and conference proceedings.
Data collection and analysis
Selection of studies
During the update of this review, the search was largely supported by the Gynaecology and Fertility Group Information Specialist. C Siristatidis and P Vogiatzi independently selected trials among all potentially eligible studies and disagreements were resolved by discussion. The same two authors independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the update of the review. Further information was sought from the authors of studies where insufficient information was included in the papers to make a decision about eligibility; e‐mails were sent seeking the necessary clarifications. In order to allow an intention‐to‐treat analysis for binary outcome measures, data were sought on the number of participants with each outcome event by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up.
During the initial version, from the trials identified by the above search strategies, V Poustie and A Drakeley independently selected trials which were eligible for inclusion based on the criteria for considering studies for the review (as detailed above). Disagreements were resolved by discussion or through arbitration by S Dodd. As a large number of studies were identified, V Poustie and A Drakeley independently screened the titles and discarded those studies that were clearly ineligible. The abstracts of the remaining studies were reviewed independently by V Poustie and A Drakeley. The full papers of any studies which appeared to be eligible, or for which we could not confirm eligibility, were obtained and reviewed independently. Further information was sought from the authors of studies where insufficient information was included in the papers to make a decision about eligibility. During the current update of the review, the same strategy was followed, performed by C Siristatidis and P Vogiatzi.
Data extraction and management
Data were extracted independently by C Siristatidis and P Vogiatzi using a data extraction form designed and pilot‐tested by the authors and disagreements between the two aforementioned authors were resolved by consensus with G Basios. Where studies had multiple publications, the authors collated multiple reports of the same study, so that each study rather than each report is the unit of interest in the review, and such studies have a single study ID with multiple references. We sought additional information on trial methodology or actual original trial data from the principal author of trials which appeared to meet the eligibility criteria when aspects of methodology were unclear, or where the data were unpublished or in a form unsuitable for meta‐analysis. The following quality criteria and methodological details are presented in the table 'Characteristics of included studies' and help provide a context for discussing the reliability of the results.
Analysis was carried out using Review Manager 5 software.
Where rates were reported, such that women may be counted more than once (for example 'per embryo transfer' or 'per cycle'), or reporting in percentages, rates 'per woman or couple' were calculated and reported if sufficient data were available, either extracted from papers or obtained from the trial authors. Binary outcome measures for each study were expressed and pooled using relative risks (RR) with 95% confidence intervals (CI). We presented relative risks in preference to odds ratios (OR) as they are more easily understood where event rates are high, as may have been the case in these studies.
Assessment of risk of bias in included studies
The risk of bias of all studies that were deemed eligible for the review was assessed independently by C Siristatidis and G Basios.
The Cochrane 'Risk of bias' assessment tool (handbook.cochrane.org) was used to assess: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias. Disagreements were resolved by discussion. All judgements were fully described and presented in the conclusions through the 'Risk of bias' table.
Measures of treatment effect
Risk ratios (RR) and 95% confidence intervals (CIs) were calculated for all outcomes.
Unit of analysis issues
The primary analysis was per woman/couple randomised. Multiple live births (e.g. twins or triplets) were counted as one live birth event.
Only first‐phase data from crossover trials were included.
Dealing with missing data
Only the available data were analysed. In the cases that missing data were considered significant, the original investigators were contacted by e‐mail to request missing data or information. Live births were assumed not to have occurred in participants without a reported outcome. For other outcomes, we analysed only the available data.
Assessment of heterogeneity
Statistical heterogeneity between the results of different studies was examined by inspecting the scatter in the data points and the overlap in their confidence intervals in the forest plot and, more formally, by checking the results of the Chi² test for heterogeneity and the I² statistic.
Assessment of reporting biases
Funnel plots were used to explore the possibility of small study effects. A funnel plot was not presented due to the small number of studies reporting the primary outcome.
Data synthesis
Low‐dose aspirin was compared to placebo or no treatment and data for each outcome were pooled using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
If sufficient studies had been identified (five or more), we would have performed subgroup analyses on the results to look at the possible contribution of:
-
Differences in duration and timing of treatment.
-
Couples with/without infertility.
-
Aspirin dose ‒ higher or lower than 150 mg.
If heterogeneity was evident, it was investigated by carrying out the pre‐planned subgroup and sensitivity analyses stated below. If unexplained heterogeneity remained, it was incorporated by using random‐effects modelling. An I² value greater than 50% was predefined as the cut‐off for further exploration.
Exploratory subgroup analyses were undertaken to assess the effect of timing of treatment (started pre‐conceptually or at first confirmation of pregnancy). Further subgroup analysis would have been undertaken to assess the effects of duration of treatment, had sufficient studies been identified to allow this.
Sensitivity analysis
If sufficient studies had been identified (five or more), we intended to perform sensitivity analysis to determine whether the conclusions are robust to arbitrary decisions made regarding the eligibility and analysis, according to study risk of bias, including only studies at low risk of bias related to:
(1) sequence generation;
(2) allocation concealment;
(3) loss to follow‐up;
(4) blinding.
Overall quality of the body of evidence: Summary of findings table
For the assessment of the quality of evidence we prepared a 'Summary of findings' table using GRADEpro and Cochrane methods. This table evaluated the overall quality of the body of evidence for the main review outcomes (live birth, clinical pregnancy, multiple pregnancy, miscarriage, ectopic pregnancy, vaginal bleeding and other adverse events) for the main review comparison. We assessed the quality of the evidence using GRADE criteria. Two review authors (C Siristatidis and P Vogiatzi) independently conducted judgements about evidence quality, and resolved disagreements by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
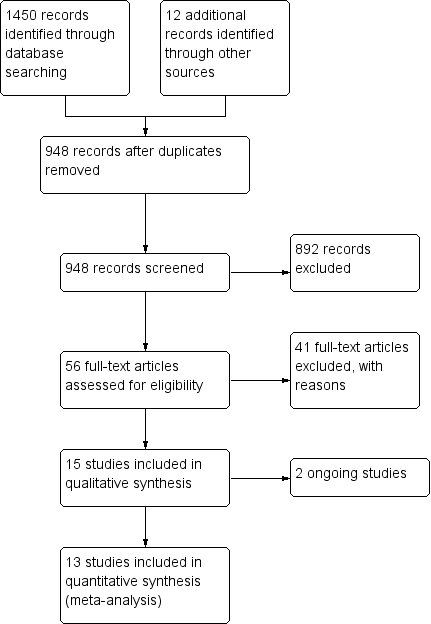
The search retrieved 1450 articles. Eighty‐six studies were potentially eligible and were retrieved in full text. Fifty‐six studies met our inclusion criteria. We excluded 41 studies, there were no studies awaiting further assessment and two studies are ongoing. Complete characteristics of the studies can be found in the respective tables, Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies, while a complete flow diagram of study selection process is presented in Figure 1.

Study flow diagram
Included studies
Thirteen studies involving a total of 2653 women met the criteria for inclusion in the review (Bordes 2003; Check 1998; Dirckx 2009; Duvan 2006; Haapsamo 2010; Hung Lok 2004; Lambers 2009a; Lentini 2003; Moini 2007; Pakilla 2005; Rubinstein 1997‐1999; Urman 2000; Van Dooren 2004).
Study design and setting
Thirteen RCTs were included, with parallel groups. Studies were conducted in ART clinics in the USA, the Netherlands, Finland, France, Belgium, Turkey, Iran and Hong Kong; four studies did not report the setting of the trial (Lentini 2003; Rubinstein 1997‐1999; Urman 2000; Van Dooren 2004). Three studies were large: one included 487 participants (Haapsamo 2010), another 374 participants (Pakilla 2005), one 300 (Urman 2000) and one 298 participants (Rubinstein 1997‐1999).
The other studies were smaller, including 36 (Check 1998), 84 (Lentini 2003), 62 (Hung Lok 2004), 138 (Bordes 2003), 145 (Moini 2007), 169 (Lambers 2009a), 170 (Van Dooren 2004), 193 (Dirckx 2009) and 197 (Duvan 2006) participants, respectively. One trial was partly funded by a pharmaceutical company that produces aspirin (Haapsamo 2010).
Participants
In most of the studies, the mean age of participants in both groups was 32 years. Two studies did not state the age of the participants (Bordes 2003; Check 1998). One study (Van Dooren 2004) described the overall age of study participants regardless of group allocation (median of 32 years, range 22 to 44 years).
Cause and duration of subfertility was not described in three of the studies (Bordes 2003; Check 1998; Lentini 2003).
Four studies stated whether the participants had previously undergone IVF or ICSI treatment. In one, 56.7% of the treatment group had undergone previous treatment, as had 60% of the control group (Hung Lok 2004); and in another the rates were reported as 40% and 42%, respectively (Haapsamo 2010). All participants in the third and fourth studies had previously undergone IVF treatment (Check 1998; Lambers 2009a).
One study involved only women undergoing their first IVF or ICSI cycle (Van Dooren 2004), ICSI cycle only (Duvan 2006), and one undergoing either their first or second IVF or ICSI cycle (Dirckx 2009).
Interventions
Ten studies used a dose of 100 mg of aspirin per day (Bordes 2003; Dirckx 2009; Duvan 2006; Haapsamo 2010; Lambers 2009a; Lentini 2003; Moini 2007; Pakilla 2005; Rubinstein 1997‐1999; Van Dooren 2004); two used 80 mg per day (Hung Lok 2004; Urman 2000); and in one the dose was 81 mg per day (Check 1998). In most of the studies aspirin was commenced immediately at the start of down‐regulation of the women's own reproductive hormones, or a short time before down‐regulation. The exception was a study commenced with frozen embryo cycles, where aspirin intake was initiated on the second day of the frozen cycle (Check 1998); while in another study, aspirin intake was initiated at the day of embryo transfer (Duvan 2006; ). Eight studies provided a placebo for the control group (Bordes 2003; Haapsamo 2010; Hung Lok 2004; Lambers 2009a; Moini 2007; Pakilla 2005; Rubinstein 1997‐1999; Van Dooren 2004). Duration of treatment varied from study to study and according to whether pregnancy was achieved. In one study aspirin was stopped prior to egg collection (Hung Lok 2004). In the other studies aspirin was continued in women with a positive pregnancy outcome until the pregnancy was confirmed (Dirckx 2009; Duvan 2006; Urman 2000); for 10 weeks after embryo transfer (Van Dooren 2004); until the pregnancy reached 12 weeks (Lambers 2009a; Rubinstein 1997‐1999) or for the duration of the pregnancy (Check 1998; Haapsamo 2010; Pakilla 2005). The remaining studies did not state the duration of the aspirin treatment (Bordes 2003; Lentini 2003; Moini 2007).
Outcomes
Only three studies provided data on live birth rate per woman or couple (Dirckx 2009; Haapsamo 2010; Pakilla 2005). All studies provided data on clinical pregnancy rate per woman or couple, except from one that provided data per embryo transfer (Moini 2007), and another two that gave data as percentages (Bordes 2003; Lentini 2003).
Five studies provided data on chemical pregnancy rate (Check 1998; Haapsamo 2010; Lentini 2003; Rubinstein 1997‐1999; Urman 2000). However, only two provided the data as per woman or couple (Haapsamo 2010; Lentini 2003); the remainder presented data as per embryo transferred. Multiple pregnancy rate was presented in four studies (Dirckx 2009; Haapsamo 2010; Lambers 2009a; Rubinstein 1997‐1999); and miscarriage rate was presented in five studies (Dirckx 2009; Haapsamo 2010; Lambers 2009a; Pakilla 2005; Urman 2000). Only three studies provided details of any complications during the pregnancies by presenting ectopic pregnancy rate (Haapsamo 2010; Pakilla 2005; Urman 2000); and only one by presenting hypertensive complications, vaginal bleeding, intrauterine growth restriction in singletons and blood loss at delivery (Haapsamo 2010). One study reported incidence of ovarian hyperstimulation syndrome (Moini 2007).
All other outcomes relevant to this review were either not investigated or not reported.
Excluded studies
Forty‐one studies were excluded from the review. Twelve of these investigated the use of aspirin alongside prednisone, heparin or terbutaline therapy (Geva 2000; Grandone 2014; Guan 2007; Hanevik 2012; Mollo 2002; Revelli 2008; Sher 1994; Stern 2001; Stern 2003; Strehler 2002; Ubaldi 2002; Zhu 2013), thirteen were not randomised studies (Akhtar 2013; Frattarelli 2008; Gizzo 2014; Hatasaka 2000; Hurst 2005; Kuo 1997; Kutteh 1997; Sher 1998; Stoval 1999; Villani 2015; Wada 1994; Wallace 2003; Ying 2012), four were quasi‐randomised, while in two studies participants were allocated to treatment group according to which day of the week they attended the clinic (Waldenström 2004); or on a weekly basis (Pergolini 2013). In another two, allocation was based on the paired or unpaired status of participants' social security number (Várnagy 2010; Weckstein 1997). Rabiee 2011 compared different regimens of aspirin in IVF. Haapsamo 2009 measured uterine artery haemodynamics after aspirin treatment; Lambers 2009 described the results after a questionnaire given to participants of the original study; and two studies did not refer to IVF or ICSI (Hsieh 2000; Kosar 2011). Another study was excluded because researchers investigated the impact of low‐dose aspirin on patients undergoing ovulation induction with a Chinese patent medicine and, thus, the outcomes were not relevant to IVF (Zhao 2014). Four studies were excluded as the authors failed to respond to our request for additional information concerning their study (Matassa 2001; Madani 2014; Lee 2001; Vandana 2014); two studies were conducted in the framework of an oocyte donation programme (Rubinstein 1999; Weckstein 1997); while in Zolghadr 2011 the outcomes investigated were number of follicles, serum estradiol and ovarian size, thus greatly differing from the outcomes of this analysis.
Two ongoing studies have been registered as clinical trials: one has completed recruitment with no results published to date (Gourabi 2012), and the second was due in December 2014 (Vandana 2013); however, to date, the authors have not provided their results. The authors of both studies have been contacted in order to provide some preliminary results to assess whether these are eligible to be included in the analysis, but no information is available to date.
Studies awaiting assessment
No studies are awaiting further classification.
Risk of bias in included studies
See the 'Characteristics of included studies' table Figure 2 and Figure 3
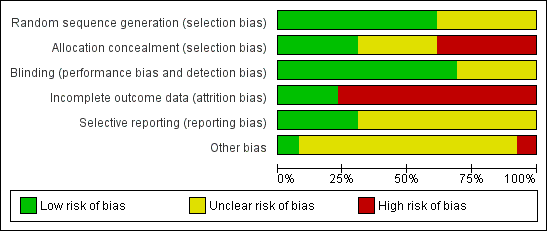
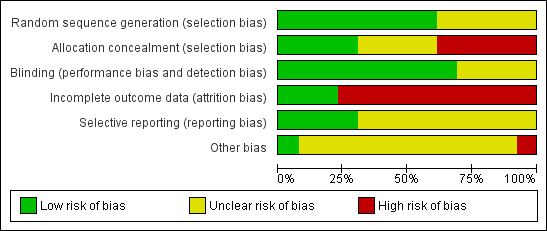
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
All studies were stated to be randomised trials, although five did not provide details of how the randomisation schedule was prepared (Bordes 2003; Check 1998; Lentini 2003; Pakilla 2005; Van Dooren 2004). The remaining studies used computer‐generated randomisation schedules.
Allocation concealment
Allocation concealment was assessed at low risk in four out of 13 studies, as it was stated that it was performed by an independent pharmacist in the hospital pharmacy (Lambers 2009a), performed by the central pharmacy of the hospital (Dirckx 2009), or opaque sealed envelopes were used (Rubinstein 1997‐1999; Haapsamo 2010).
In four studies risk of bias associated with allocation concealment was assessed as unclear, as sealed envelopes were used in two (Duvan 2006; Pakilla 2005), but it was unclear who prepared the envelopes and whether they were opaque and serially numbered; while in two, participants were randomised by a research nurse but it was unclear whether the nurse was involved in the study (Hung Lok 2004; Urman 2000).
Blinding
Nine out of 13 studies were double blind, with both the participants and treatment providers reported as blinded (Dirckx 2009; Duvan 2006; Haapsamo 2010; Hung Lok 2004; Lambers 2009a; Pakilla 2005; Rubinstein 1997‐1999; Van Dooren 2004; Moini 2007); one was at least single blind as the assessors were blinded (Check 1998); one reported to be double blind but no further information was given (Bordes 2003); and one study was single blind as the treatment providers were unaware of which group the participants belonged to (Urman 2000).
Eight trials were reported as placebo controlled (Bordes 2003; Haapsamo 2010; Hung Lok 2004; Lambers 2009a; Moini 2007; Pakilla 2005; Rubinstein 1997‐1999; Van Dooren 2004); in one study tablets of aspirin or placebo of identical appearance were given (Haapsamo 2010); in another a similar dose of 100 mg of placebo was reported (Lambers 2009a).
Incomplete outcome data
Three studies were assessed as at low risk of attrition bias, as they provided rates of the primary outcome (Dirckx 2009; Haapsamo 2010; Pakilla 2005). Three others provided pregnancy rates as a percentage rather than actual numbers (Bordes 2003; Lentini 2003; Moini 2007). These studies did not state how many women completed the study or withdrew from treatment, so that it is unknown whether the rates were calculated per woman, per transfer or per cycle. They have not been included in the analysis.
Intention‐to‐treat analysis was clearly stated in three studies (Haapsamo 2010; Lambers 2009a; Rubinstein 1997‐1999). Three of the studies did not specifically state that an intention‐to‐treat analysis was undertaken; however they presented results on all recruited participants and reported no withdrawals (Check 1998; Duvan 2006; Rubinstein 1997‐1999).
Withdrawals were reported in eight studies: they were stated either as none (Rubinstein 1997‐1999); or they represented less than 10% of the study population (Dirckx 2009; Haapsamo 2010; Hung Lok 2004; Lambers 2009a; Pakilla 2005; Urman 2000), while in one study cancellation rates were stated as more than this percentage at 16.7% and 23.3% of the study and control groups, respectively (Moini 2007).
Selective reporting
Four out of 13 studies were at low risk of reporting bias (Dirckx 2009; Haapsamo 2010; Lambers 2009a; Pakilla 2005), while the other nine were at unclear risk (Bordes 2003; Check 1998; Duvan 2006; Hung Lok 2004; Lentini 2003; Moini 2007; Rubinstein 1997‐1999; Urman 2000; Van Dooren 2004).
Other potential sources of bias
Inclusion and exclusion criteria were clearly described and treatment and control group participants appeared to be comparable in eight studies (Dirckx 2009; Check 1998; Duvan 2006; Haapsamo 2010; Hung Lok 2004; Lambers 2009a; Pakilla 2005; Urman 2000). The remaining studies provided insufficient detail to reach a decision on these factors. In most of the studies the general care programmes in the treatment and control groups were identical, although one study failed to provide this information (Lentini 2003).
Compliance to aspirin treatment was not assessed in all but one of the studies (Hung Lok 2004)
We found no other potential sources of within‐study bias in the 13 included studies.
Effects of interventions
Primary outcome measure
(1) Live birth rate per woman or couple
This outcome was reported in three studies involving 1053 participants (Dirckx 2009; Haapsamo 2010; Pakilla 2005).
No evidence of a difference was found (Analysis 1.1) between the treatment and control groups (RR 0.91, 95% CI 0.72 to 1.15, 3 RCTs, n = 1053, I² = 0%, moderate‐quality evidence) (Figure 4).

Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.1 Live birth rate per woman or couple.
Secondary outcome measures
(1) Clinical pregnancy rate (confirmed by ultrasound) per woman or couple
This outcome was reported in all of the included studies. Three studies provided pregnancy rates as a percentage rather than actual numbers (Bordes 2003; Lentini 2003; Moini 2007). These studies did not state how many women completed the study or withdrew from treatment, so that it is unknown whether the rates were calculated per woman, per transfer or per cycle. They have not been included in the analysis.
Based on data from 2142 participants in 10 studies (Analysis 1.2), no evidence of a difference was found in clinical pregnancy rate between the treatment and control groups (RR 1.03, 95% CI 0.91 to 1.17, 10 RCTs, n = 2142, I² = 27%, moderate‐quality evidence) (Figure 5).
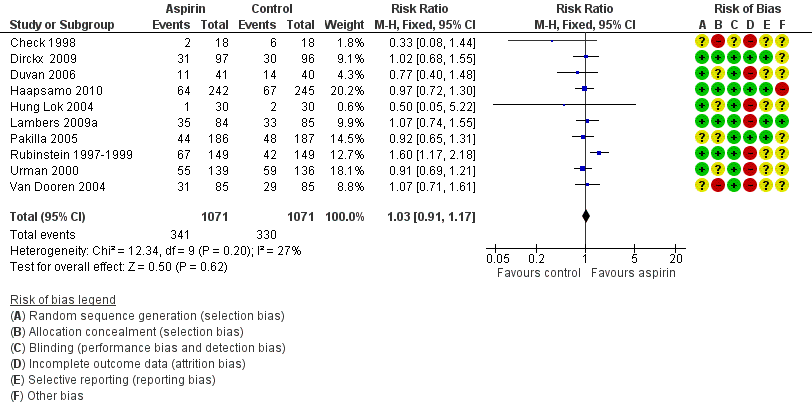
Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.2 Clinical pregnancy rate per woman or couple.
Subgroup analysis of nine studies on the timing of starting treatment showed no evidence of a difference in clinical pregnancy rates whether treatment started before, at or after down‐regulation (RR 1.03, 95% CI 0.90 to 1.17, 9 RCTs, n = 1972, I² = 35%, moderate‐quality evidence).
Sensitivity analysis by including only studies with low risk of bias in all domains listed ended up with three trials (Dirckx 2009; Haapsamo 2010; Lambers 2009a). Based on data from 849 participants no evidence of a difference was found in clinical pregnancy rate between the treatment and control groups (RR 1.01, 95% CI 0.82 to 1.23, 3 RCTs, n = 849, I² = 0%, high‐quality evidence) (Analysis 1.10).
(2) Ongoing pregnancy rate (number of pregnancies which continued beyond 12 weeks) per woman or couple
This outcome was reported in only two of the included studies (Lambers 2009a; Van Dooren 2004). No evidence of a difference was found between the intervention and control groups (RR = 0.94, 95% CI 0.69 to 1.27, 2 RCTs, n = 339, I² = 0%, low‐quality evidence).
(3) Multiple pregnancy rate per woman or couple
This outcome was reported in three of the included studies (Dirckx 2009; Haapsamo 2010; Lambers 2009a). There was no evidence of a difference in the number of multiple pregnancies found at ultrasound (RR 0.67, 95% CI 0.37 to 1.25, 2 RCTs, n = 656, I² = 0%, low‐quality evidence) (Haapsamo 2010; Lambers 2009a), or in the number of multiple births (RR 0.74, 95% CI 0.38 to 1.46, 2 RCTs, n = 680, I² = 0%, low‐quality evidence) (Dirckx 2009; Haapsamo 2010), between the treatment and control groups.
(4) Miscarriage rate (confirmed by ultrasound and pregnancy test, or histology) per woman or couple
This outcome was reported in five of the included studies (Dirckx 2009; Haapsamo 2010; Lambers 2009a; Pakilla 2005; Urman 2000). There was no evidence of a difference between the treatment and control groups (RR 1.10, 95% CI 0.68 to 1.77, 5 RCTs, n = 1497, I² = 0%, low‐quality evidence).
(5) Complications with the IVF or ICSI procedure
In one study ovarian hyperstimulation syndrome (OHSS) was reported as occurring in 5.6% of the intervention group and 23.3% of the control group (Moini 2007). No further analysis was possible given that neither the numerators nor denominators were provided, so that we could not add the necessary data into the analysis. No studies reported outcomes related to intra‐operative haemorrhage, formation of haematoma or the need for blood transfusion during the procedure. No cases of gastritis or haematemesis were reported.
(6) Gestational age of baby
This outcome was reported in one of the studies (Haapsamo 2010): 38 (31 to 42) versus 38 (28 to 41) in the study and control groups, respectively.
(7) Complications during pregnancy or birth
One study involving 487 participants reported the rate of vaginal bleeding during pregnancy (Haapsamo 2010). There was no evidence of a difference between the treatment and control groups (RR 1.01, 95% CI 0.14 to 7.13, 1 RCT, n = 487, very low quality evidence).
Three studies reported the rate of ectopic pregnancies (Haapsamo 2010; Pakilla 2005; Urman 2000). There was no evidence of a difference between the treatment and control groups (RR 1.86, 95% CI 0.75 to 4.63, 3 RCTs, n = 1135, I² = 0%, very low quality evidence).
Other potential adverse effects, including preterm birth, antepartum haemorrhage and need for operative delivery were not reported in any included study.
Discussion
Summary of main results
This review included a total of 2653 participants that were recruited in randomised controlled trials assessing aspirin versus control (no intervention) in women undergoing ART. We found no evidence that aspirin had an effect on live birth rate in this population. We also found no evidence that aspirin intake can influence rates of clinical or ongoing or multiple pregnancy or miscarriage. However, the number of studies was limited and the quality of the evidence ranged from very low to moderate. Furthermore, data on complication rates, either during the IVF/ICSI procedure (intra‐operative bleeding, vaginal/pelvic haematoma, blood transfusion, ovarian hyperstimulation syndrome, gastritis, haematemesis) or during pregnancy and childbirth (threatened miscarriage, antepartum haemorrhage, need for operative delivery, postpartum haemorrhage, ectopic pregnancy) were either very limited or missing; therefore, to date, definitive conclusions are precluded.
Medical personnel involved in ART are mostly committed to offering couples adequate management towards achieving a successful clinical outcome: a pregnancy and the birth of a healthy baby. It is imperative that before treatments become embedded in unit protocols they are subjected to rigorous, properly designed and adequately powered randomised controlled clinical trials. We must first do 'no harm', but it is also essential that purported treatments do actually convey benefit. Treatment with aspirin for IVF is one such treatment.
In an attempt to reassess whether aspirin administration is useful in increasing pregnancy rates in IVF and ICSI cycles, we performed a second update of a previously published Cochrane review on this issue. We were not able to add new data from additional studies, as we found no new RCTs reporting on these outcomes based on the desired comparisons. We have excluded 17 new studies. Based on the available evidence, we reached the same conclusion as the initial version of the review: no single outcome measure demonstrated a benefit with the use of aspirin.
Currently, there is no evidence to support the use of aspirin treatment in order to improve pregnancy rates for a general IVF population. Women undergoing IVF treatment are often prepared to undertake any number of measures to improve their chances of conceiving; therefore, recruitment targets in clinical trials of this treatment are likely to be achievable. Such women should be given the opportunity to participate in adequately powered, high‐quality studies which aim to improve the evidence base for all couples requiring IVF treatment.
Overall completeness and applicability of evidence
The relevance of the evidence sourcing from the identified studies to the review question was borderline. Very few of them reported on all the objectives of the review question, especially live birth rate, which is ultimately the outcome all couples undergoing IVF are most interested in. The difficulty of collecting data at birth and the possible reporting bias of outcomes could justify such a lack. The small number of eligible studies identified and variations across studies in dose of treatment provided, stage of treatment in terms of commencement and cessation and use of placebo was not adequate. Lack of sufficient data on miscarriage rates associated with aspirin treatment is also a concern, particularly in light of evidence suggesting that higher doses of aspirin can result in increased miscarriage rates in pregnancy. Finally, a limitation of this second update of the review was the difficulty in identifying the full text of three new possibly eligible RCTs, while in two cases there was difficulty in assessing the risk of bias, whereas no new data were obtained in our correspondence effort.
Quality of the evidence
For the primary outcome, live birth, the evidence was of moderate quality. For clinical pregnancy, the evidence was of moderate quality: three studies failed to provide specifications on randomisation methods; two studies did not specify how allocation concealment was performed; one study was performed with inadequate blinding. In ongoing pregnancy the evidence was of low quality, as one study failed to provide specifications on the method of randomisation and allocation concealment.
For other outcomes the quality of the evidence was low or very low. The main limitations were failure to report study methods in sufficient detail and imprecision.
Potential biases in the review process
The strength of the review in terms of preventing bias was maintained by having two independent review authors. The methods established to conduct the current review were agreed by all authors and any potential bias that could have been introduced was bypassed through independent screening, assessment, selection and data extraction with discrepancies resolved through team consensus. The search was supported by the CGFG Information Specialist. Importantly, relevant data were obtained by discussion between the three authors.
Agreements and disagreements with other studies or reviews
The conclusions of the second update of this Cochrane review are in agreement with the current literature.
The first published systematic review and meta‐analysis involved six trials (Gelbaya 2007). That review found no difference between patients who received aspirin and those who received placebo or no treatment in clinical pregnancy rate per embryo transfer (RR 1.09, 95% CI 0.92 to 1.29); and there were no differences in a variety of outcome measures studied, including live births. The authors concluded that aspirin had no substantial positive effect on likelihood of pregnancy and, therefore, it should not be routinely recommended for women undergoing IVF and ICSI.
Similarly, another meta‐analysis of randomised trials evaluating the effects of aspirin in IVF (Ruopp 2008), including 10 trials, found no difference between women taking aspirin and those who did not with regards to implantation and miscarriage rates but the clinical pregnancy rate per embryo transfer was reported to be significantly higher in the aspirin group (RR 1.15, 95% CI 1.03 to 1.27). This study commented on the difference in findings on the clinical pregnancy rate, as compared with the previous review (Gelbaya 2007), attributing the "divergent findings to different approaches to statistical modelling, study selection, and statistical inference" (Ruopp 2008).
In this second update of our review, we found two further systematic reviews (Groeneveld 2011; Dentali 2012).
The first group of authors performed an individual patient data (IPD) meta‐analysis; whereas the conventional meta‐analysis limited to studies that could provide IPD showed an OR of 0.89 (95% CI 0.69 to 1.2), the conventional meta‐analysis limited to the eight studies of which method of randomisation could be confirmed showed an OR of 0.94 (95% CI 0.76 to 1.17) and the conventional meta‐analysis including all 10 eligible RCTs identified changed the OR to 1.07 (95% CI 0.81 to 1.41): authors concluded that aspirin does not improve pregnancy rates after IVF.
The second group of authors using pooled odds ratios (ORs) and 95% confidence intervals (CIs), included 17 studies with 6403 patients: authors found that the use of aspirin did not improve live birth pregnancy rate compared with placebo or no treatment (OR 1.08, 95% CI 0.90 to 1.29), but pregnancy rates were significantly increased in patients randomised to low‐dose aspirin (OR 1.19, 95% CI 1.01 to 1.39), while miscarriage rates were not (OR 1.18, 95% CI 0.82 to 1.68); on the contrary, results of sensitivity analyses including high‐quality studies did not show statistically significant differences in all considered endpoints. The conclusion reached was that there was not a substantial efficacy of aspirin in women undergoing IVF/ICSI and does not support the use of low‐dose aspirin to improve the success of IVF/ICSI in terms of pregnancy outcomes.
Study flow diagram
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
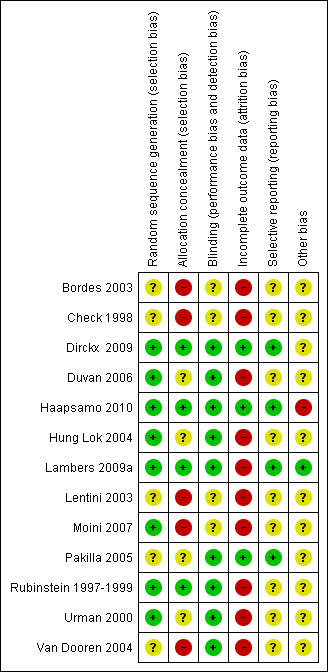
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.1 Live birth rate per woman or couple.

Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.2 Clinical pregnancy rate per woman or couple.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 1 Live birth rate per woman or couple.
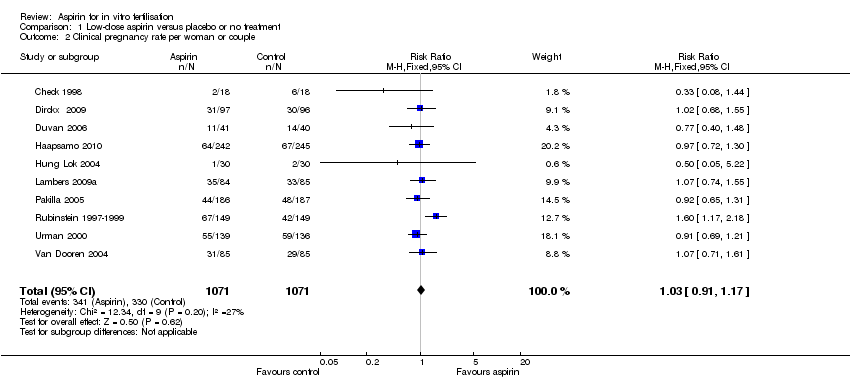
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 2 Clinical pregnancy rate per woman or couple.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 3 Ongoing pregnancy rate (beyond 12 weeks).
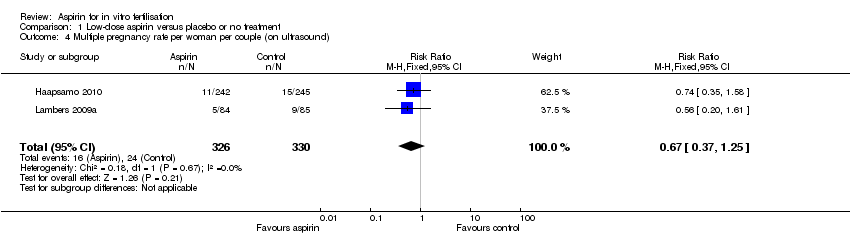
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 4 Multiple pregnancy rate per woman per couple (on ultrasound).
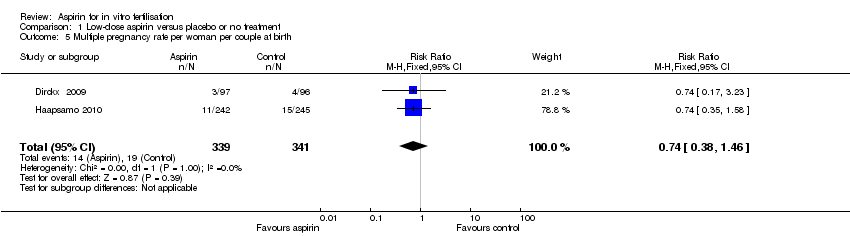
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 5 Multiple pregnancy rate per woman per couple at birth.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 6 Miscarriage rate per woman per couple.
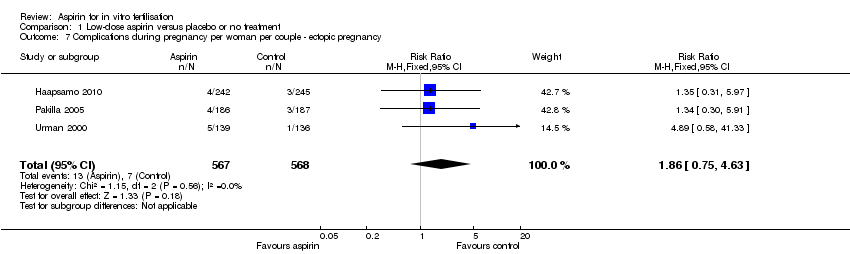
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy.
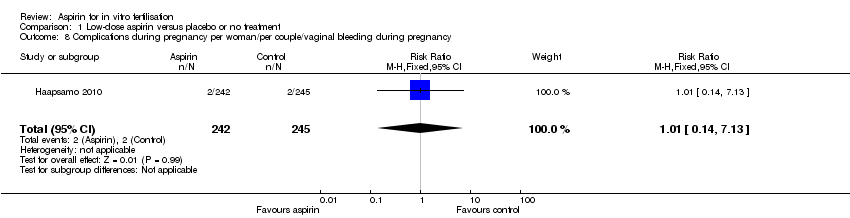
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment.
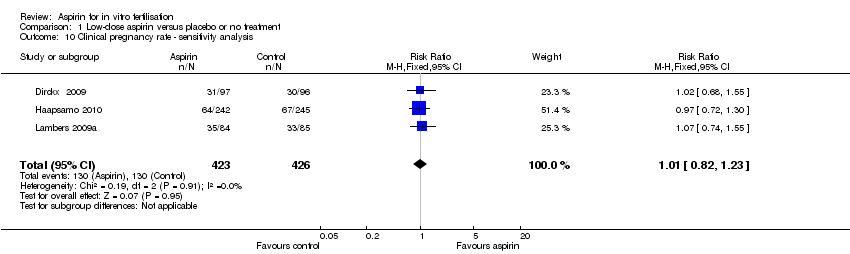
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 10 Clinical pregnancy rate ‐ sensitivity analysis.
| Low‐dose aspirin compared to placebo or no treatment for women undergoing ART | ||||||
| Population: Women with subfertility | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Low‐dose aspirin | |||||
| Live birth | 225 per 1000 | 204 per 1000 | RR 0.91 | 1053 | ⊕⊕⊕⊝ | |
| Clinical pregnancy | 337 per 1000 | 347 per 1000 | RR 1.03 | 2142 | ⊕⊕⊕⊝ | |
| Multiple pregnancy (on ultrasound) | 84 per 1000 | 56 per 1000 | RR 0.67 (0.37 to 1.25) | 656 | ⊕⊕⊝⊝ | |
| Miscarriage rate per woman | 43 per 1000 | 47 per 1000 | RR 1.1 | 1497 | ⊕⊕⊝⊝ | |
| Ectopic pregnancy | 12 per 1000 | 23 per 1000 | RR 1.86 | 1135 | ⊕⊝⊝⊝ | |
| Vaginal bleeding | 8 per 1000 | 8 per 1000 (1 to 58) | RR 1.01 (0.14 to 7.13) | 487 (1 study) | ⊕⊝⊝⊝ | |
| Other adverse events | Other adverse events (such as preterm birth, antepartum haemorrhage, need for operative delivery) were not reported in the included studies | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious imprecision with low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in the control group. 6 Single study. Very serious imprecision with very low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in either group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman or couple Show forest plot | 3 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.15] |
| 2 Clinical pregnancy rate per woman or couple Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 3 Ongoing pregnancy rate (beyond 12 weeks) Show forest plot | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| 4 Multiple pregnancy rate per woman per couple (on ultrasound) Show forest plot | 2 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.25] |
| 5 Multiple pregnancy rate per woman per couple at birth Show forest plot | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.38, 1.46] |
| 6 Miscarriage rate per woman per couple Show forest plot | 5 | 1497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.68, 1.77] |
| 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy Show forest plot | 3 | 1135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.75, 4.63] |
| 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy Show forest plot | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.13] |
| 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 9.1 Treatment started before down‐regulation | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 9.2 Treatment started at down‐regulation | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.92, 1.31] |
| 9.3 Treatment started after down‐regulation | 4 | 774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.17] |
| 10 Clinical pregnancy rate ‐ sensitivity analysis Show forest plot | 3 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.23] |

