Aspirina para la fertilización in vitro
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004832.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 03 noviembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Ginecología y fertilidad
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
V Poustie devised the idea for the original review, which was further refined following discussions with A Drakeley and S Dodd. A Drakeley wrote the background, S Dodd wrote the statistical content of the methods of the review and V Poustie completed the remaining sections. All authors commented on the draft of the review prior to completion.
in 2011, C Siristatidis with A Drakeley updated the review. V Poustie was not involved in that update.
During the current update (2016), S Siristatidis with P Vogiatzi updated the review, G Basios commented on and edited the final text, while V Pergialiotis followed all steps for this update, this being his first participation in a Cochrane review.
Sources of support
Internal sources
-
None, Other.
External sources
-
MDSG, Trials Search Coordinator, New Zealand.
-
Susanna Dodd, UK.
We would like to help Susanna Dodd for her valuable help in her major contribution during the initial version and the first update of this review
-
George Basios, Greece.
We would like to thank George Basios for his valuable help in selecting the studies in the initial phase of the 2014 update.
Declarations of interest
Previous author V Poustie has undergone IVF treatment in the past and has previously taken low‐dose aspirin during this treatment.
Acknowledgements
We would like to thank Dr Ingrid Lok, Dr Ákos Várnagy, Dr Tahereh Madani and Prof. Antonio Colicchia for providing additional information on the trials (Hung Lok 2004; Várnagy 2010; Gourabi 2012; Pergolini 2013). We also would like to thank Dr Andrew Drakeley for his help in the initial selection of studies.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Nov 03 | Aspirin for in vitro fertilisation | Review | Charalampos S Siristatidis, George Basios, Vasilios Pergialiotis, Paraskevi Vogiatzi | |
| 2011 Aug 10 | Aspirin for in vitro fertilisation | Review | Charalambos S Siristatidis, Susanna R Dodd, Andrew J Drakeley | |
| 2007 Oct 17 | Low‐dose aspirin for in vitro fertilisation | Review | Vanessa J Poustie, Susanna R Dodd, Andrew J Drakeley | |
| 2004 Jul 19 | Low‐dose aspirin for in vitro fertilisation | Protocol | Vanessa Jane Poustie, Susanna R Dodd, Andrew J Drakeley | |
Differences between protocol and review
None
Notes
None
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Fertilization in Vitro;
- Anti‐Inflammatory Agents, Non‐Steroidal [*administration & dosage];
- Aspirin [*administration & dosage];
- Cyclooxygenase Inhibitors [*administration & dosage];
- Live Birth;
- Platelet Aggregation Inhibitors [*administration & dosage];
- Pregnancy Outcome;
- Pregnancy Rate;
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Adult; Female; Humans; Pregnancy;
PICO
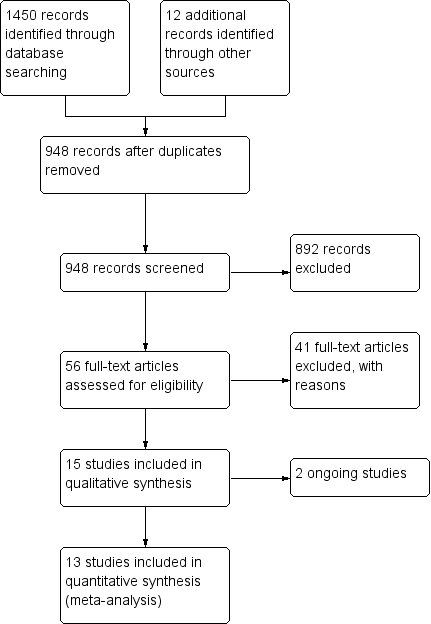
Study flow diagram
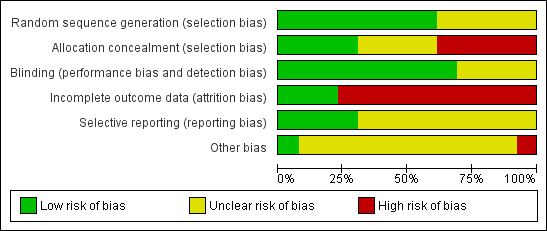
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
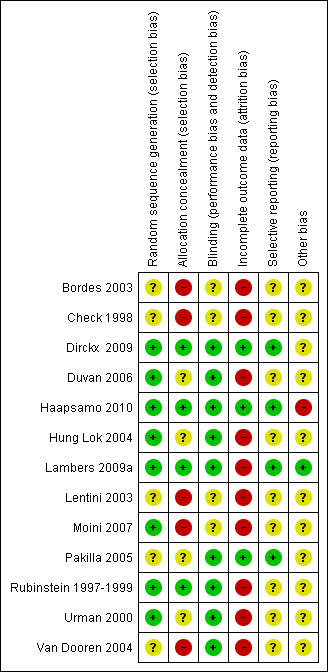
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.1 Live birth rate per woman or couple.

Forest plot of comparison: 1 Low‐dose aspirin versus placebo or no treatment, outcome: 1.2 Clinical pregnancy rate per woman or couple.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 1 Live birth rate per woman or couple.
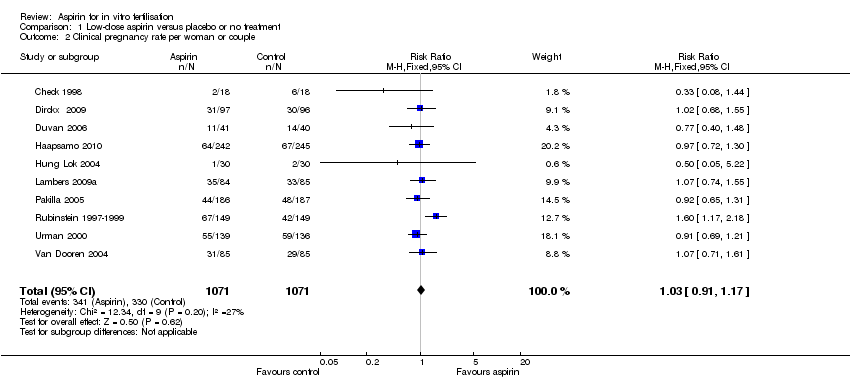
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 2 Clinical pregnancy rate per woman or couple.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 3 Ongoing pregnancy rate (beyond 12 weeks).
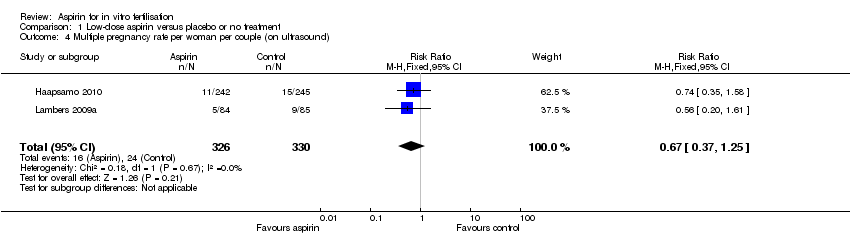
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 4 Multiple pregnancy rate per woman per couple (on ultrasound).
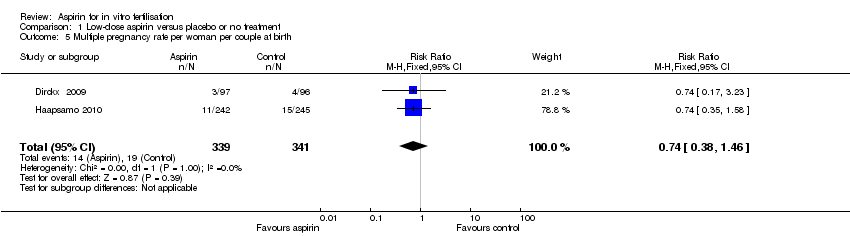
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 5 Multiple pregnancy rate per woman per couple at birth.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 6 Miscarriage rate per woman per couple.
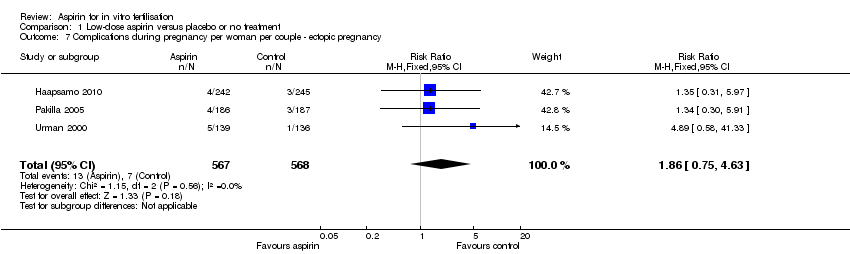
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy.
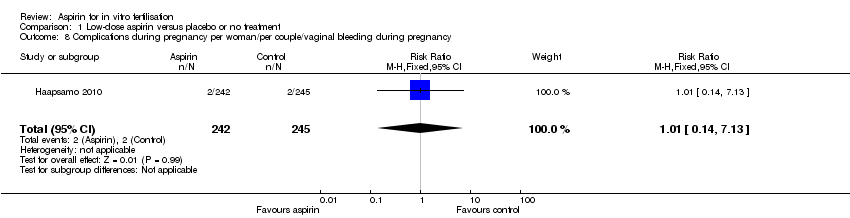
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy.

Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment.
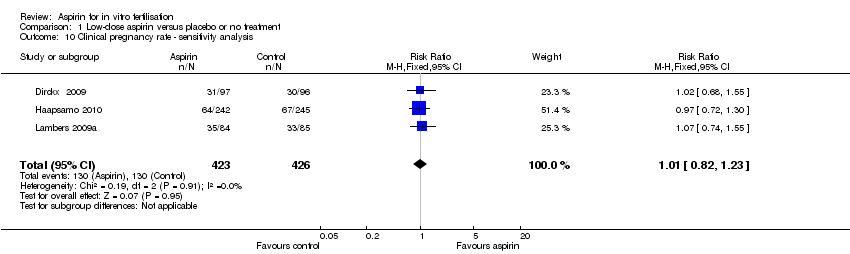
Comparison 1 Low‐dose aspirin versus placebo or no treatment, Outcome 10 Clinical pregnancy rate ‐ sensitivity analysis.
| Low‐dose aspirin compared to placebo or no treatment for women undergoing ART | ||||||
| Population: Women with subfertility | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Low‐dose aspirin | |||||
| Live birth | 225 per 1000 | 204 per 1000 | RR 0.91 | 1053 | ⊕⊕⊕⊝ | |
| Clinical pregnancy | 337 per 1000 | 347 per 1000 | RR 1.03 | 2142 | ⊕⊕⊕⊝ | |
| Multiple pregnancy (on ultrasound) | 84 per 1000 | 56 per 1000 | RR 0.67 (0.37 to 1.25) | 656 | ⊕⊕⊝⊝ | |
| Miscarriage rate per woman | 43 per 1000 | 47 per 1000 | RR 1.1 | 1497 | ⊕⊕⊝⊝ | |
| Ectopic pregnancy | 12 per 1000 | 23 per 1000 | RR 1.86 | 1135 | ⊕⊝⊝⊝ | |
| Vaginal bleeding | 8 per 1000 | 8 per 1000 (1 to 58) | RR 1.01 (0.14 to 7.13) | 487 (1 study) | ⊕⊝⊝⊝ | |
| Other adverse events | Other adverse events (such as preterm birth, antepartum haemorrhage, need for operative delivery) were not reported in the included studies | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious imprecision with low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in the control group. 6 Single study. Very serious imprecision with very low event rate. Confidence interval compatible with no effect from the intervention or with clinically meaningful benefit in either group. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman or couple Show forest plot | 3 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.15] |
| 2 Clinical pregnancy rate per woman or couple Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 3 Ongoing pregnancy rate (beyond 12 weeks) Show forest plot | 2 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.69, 1.27] |
| 4 Multiple pregnancy rate per woman per couple (on ultrasound) Show forest plot | 2 | 656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.25] |
| 5 Multiple pregnancy rate per woman per couple at birth Show forest plot | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.38, 1.46] |
| 6 Miscarriage rate per woman per couple Show forest plot | 5 | 1497 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.68, 1.77] |
| 7 Complications during pregnancy per woman per couple ‐ ectopic pregnancy Show forest plot | 3 | 1135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.75, 4.63] |
| 8 Complications during pregnancy per woman/per couple/vaginal bleeding during pregnancy Show forest plot | 1 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.14, 7.13] |
| 9 Clinical pregnancy rate ‐ subgroup analysis ‐ timing of treatment Show forest plot | 10 | 2142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.91, 1.17] |
| 9.1 Treatment started before down‐regulation | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.80, 1.38] |
| 9.2 Treatment started at down‐regulation | 4 | 1006 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.92, 1.31] |
| 9.3 Treatment started after down‐regulation | 4 | 774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.17] |
| 10 Clinical pregnancy rate ‐ sensitivity analysis Show forest plot | 3 | 849 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.23] |

