Antibióticos para la tos productiva prolongada en niños
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004822.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 31 julio 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vías respiratorias
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For the review:
-
all reviewed the manuscript.
For update:
-
JM and HP selection of articles from search, data extraction, data analysis and writing of review.
-
PM: review of data and review of manuscript.
-
AC: review of articles for inclusion, data extraction, double data entry, data analysis, writing of review.
-
Justin Gaffney (author of original review) was removed as author from this update.
Sources of support
Internal sources
-
The authors declared no internal funding was received for this systematic review update, Other.
External sources
-
Australian Cochrane Airways Group Scholarship 2004, Australia.
-
Asthma Australia, Australia.
Supporting HP through an early career fellowship
Declarations of interest
JM: none
HP: Employed as a senior lecturer at Griffith University, received a post doctoral fellowship from National Health Medical Research Council (ID. 1040830), received an early career fellowship from Asthma Australia
PM: Member of Expert Advisory Group on chronic suppurative otitis media and conjugate pneumococcal vaccines in Australia for GlaxoSmithKline
AC: Unrestricted, investigator‐initiated grant from GlaxoSmithKline in an area unrelated to review topic. Consultancy work for Merck on an unrelated topic. Merck does not produce a drug relevant to this review.
Three of the authors (JM, AC, HP) have conducted an RCT on this topic, which was included in this updated review (Marchant 2012).
Acknowledgements
We are grateful to Toby Lasserson, Chris Cates and Michael McKean from the Cochrane Airways Group for their advice and support in reviewing the original protocol and review. In the updated review, we thank all the editorial members of the Cochrane Airways Group in particular Chris Cates, Emma Dennett, Rebecca Normansell and Emma Jackson.
We also thank Elizabeth Stovold for conducting the relevant searches.
We are grateful to Johan Darelid for providing the additional information on his original study needed to complete this review.
We thank Justin Gaffney who was an author in a previous version of this review.
Finally, we thank the Cochrane Airways Australia Group and Scholarship for providing funding for JMM to complete the original review and present its findings at a national Australian meeting.
The Background and Methods sections of this review were based on a standard template used by the Cochrane Airways Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Jul 31 | Antibiotics for prolonged wet cough in children | Review | Julie M Marchant, Helen L Petsky, Peter S Morris, Anne B Chang | |
| 2005 Oct 19 | Antibiotics for prolonged moist cough in children | Review | Julie M Marchant, Peter S Morris, Justin Gaffney, Anne B Chang | |
| 2004 Apr 19 | Antibiotics for prolonged moist cough in children | Protocol | Julie M Marchant, Justin Gaffney, Peter Morris, Anne B Chang | |
Differences between protocol and review
October 2004: during the review process the selection criteria were changed from RCTs comparing antibiotics to placebo medication to also include studies comparing antibiotics with a 'no treatment' control group. This was done because there were an insufficient number of placebo‐controlled trials to be sure that the information obtained from unblinded studies would not be clinically useful. The inclusion criteria were changed to allow studies of children who had prolonged wet cough for more than 10 days. We planned to assess the impact of antibiotics on children with prolonged wet cough of more than three weeks (our preferred definition of chronic cough) as an a priori subgroup analysis. We also decided to include studies that were not exclusively limited to children with "wet sounding" cough if subgroup data were not available and more than 50% of children had a wet cough or other clinical features consistent with diagnosis (e.g. sputum production, excess secretions, etc.). The review used a summary weighted odds ratio rather than risk ratio as was stated in the protocol. This decision was made as it appeared the most clinically relevant analysis method particularly when converted to number needed to treat.
September 2017: the original protocol was written in 2004. Since this time, Cochrane methodology has become more rigorous. Therefore, this updated review has considered these changes and adapted as necessary. Helen Petsky was added as an author for the update in 2017.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Amoxicillin‐Potassium Clavulanate Combination [adverse effects, *therapeutic use];
- Anti‐Bacterial Agents [adverse effects, *therapeutic use];
- Chronic Disease;
- Cough [classification, *drug therapy];
- Disease Progression;
- Erythromycin [adverse effects, *therapeutic use];
- Intention to Treat Analysis;
- Randomized Controlled Trials as Topic;
- Sputum [metabolism];
Medical Subject Headings Check Words
Child; Child, Preschool; Humans; Infant;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Antibiotics versus no antibiotics/placebo for wet cough in children, outcome: 1.1 Children not cured or not substantially improved at follow‐up (using intention‐to‐treat analysis).

In the control group, 75/100 people had not been cured over two weeks, compared to 31/100 (95% CI 17 to 48) for the active treatment group. Number needed to treat for an additional beneficial outcome 3 (95% CI 2 to 4).

Forest plot of comparison: 1 Antibiotics versus no antibiotics/placebo for wet cough in children, outcome: 1.4 Illness progression: participants with progression of disease resulting in additional medical therapy required.
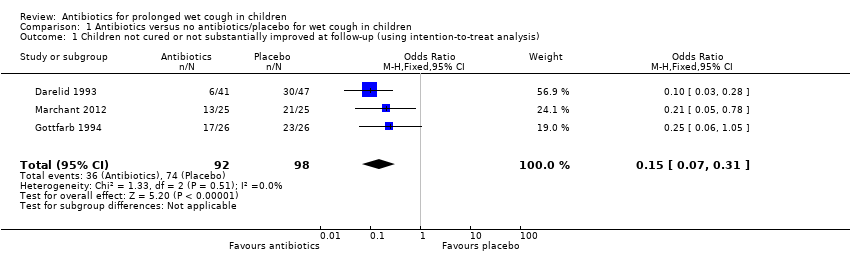
Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 1 Children not cured or not substantially improved at follow‐up (using intention‐to‐treat analysis).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 2 Illness progression: participants with progression of disease resulting in additional antibiotic therapy required (available data only).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 3 Children experiencing adverse effects of antibiotics (vomiting, diarrhoea, rash).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 4 Children not cured or not substantially improved at follow‐up (excluding those known to have B pertussis).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 5 Subgroup analysis (placebo controlled): children not cured or substantially improved at follow‐up.
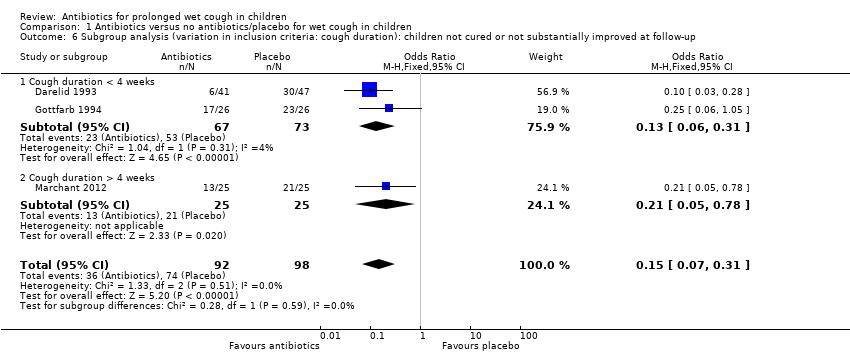
Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 6 Subgroup analysis (variation in inclusion criteria: cough duration): children not cured or not substantially improved at follow‐up.

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 7 Subgroup analysis (antibiotics used): children not cured or not substantially improved at follow‐up.

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 8 Sensitivity analysis: children not cured or not substantially improved at follow‐up (using available data only).
| Antibiotics compared to placebo for prolonged wet cough in children | ||||||
| Patient or population: prolonged wet cough in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with antibiotics | |||||
| Children not cured or substantially improved at follow‐up (using intention‐to‐treat analysis) (measured as proportion of participants) Follow‐up: range 7–14 days | 76 per 100 | 32 per 100 | OR 0.15 | 190 | ⊕⊕⊕⊝ | — |
| Children with progression of the disease resulting in additional medical therapy (complications) (measured as proportion of participants) Follow‐up: range 7–14 days | 36 per 100 | 5 per 100 | OR 0.10 | 125 | ⊕⊕⊕⊝ | — |
| Children experiencing adverse effects of antibiotics (e.g. diarrhoea, nausea, skin rash, allergic reactions (measured as proportion of participants)) | 5 per 100 | 10 per 100 | OR 1.88 | 190 | ⊕⊕⊝⊝ | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOne study carrying 46% of the analysis weight was at high risk of bias due to lack of blinding. Downgraded one level for risk of bias (Darelid 1993). bOne study carrying 87% of the analysis weight was at high risk of bias due to lack of blinding. Downgraded one level for risk of bias (Darelid 1993). cOne study carrying 10% of the analysis weight was at high risk of bias due to lack of blinding and the result has been downgraded for imprecision due to wide CIs. Downgraded two levels for risk of bias and imprecision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Children not cured or not substantially improved at follow‐up (using intention‐to‐treat analysis) Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 2 Illness progression: participants with progression of disease resulting in additional antibiotic therapy required (available data only) Show forest plot | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| 3 Children experiencing adverse effects of antibiotics (vomiting, diarrhoea, rash) Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.62, 5.69] |
| 4 Children not cured or not substantially improved at follow‐up (excluding those known to have B pertussis) Show forest plot | 2 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.05, 0.30] |
| 5 Subgroup analysis (placebo controlled): children not cured or substantially improved at follow‐up Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 5.1 Placebo controlled | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.60] |
| 5.2 No placebo used | 1 | 88 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.28] |
| 6 Subgroup analysis (variation in inclusion criteria: cough duration): children not cured or not substantially improved at follow‐up Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 6.1 Cough duration < 4 weeks | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.06, 0.31] |
| 6.2 Cough duration > 4 weeks | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.05, 0.78] |
| 7 Subgroup analysis (antibiotics used): children not cured or not substantially improved at follow‐up Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 7.1 Amoxicilin/clavulanic acid use | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.60] |
| 7.2 Other antibiotics used | 1 | 88 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.28] |
| 8 Sensitivity analysis: children not cured or not substantially improved at follow‐up (using available data only) Show forest plot | 3 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.07, 0.29] |

