Antibióticos para la tos productiva prolongada en niños
Appendices
Appendix 1. Database search strategies
Cochrane Airways Trials Register search strategy
#1 MeSH descriptor Cough explode all trees
#2 MeSH descriptor Bronchitis explode all trees
#3 cough* or bronchiti*
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Anti‐Bacterial Agents explode all trees
#6 antibiot* or anti‐biot* or antimicrob* or anti‐microb* or antibacterial* or anti‐bacterial* or erythromycin or amoxycillin or ampicillin or doxycycline
#7 (#5 OR #6)
#8 MeSH descriptor Child explode all trees
#9 MeSH descriptor Infant explode all trees
#10 MeSH descriptor Adolescent explode all trees
#11 MeSH descriptor Pediatrics explode all trees
#12 child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or pre school* or pre‐school* or newborn* or new born* or new‐born* or neo‐nat* or neonat*
#13 (#8 OR #9 OR #10 OR #11 OR #12)
#14 (#4 AND #7 AND #13)
CENTRAL search strategy
#1 MeSH descriptor Cough explode all trees
#2 MeSH descriptor Bronchitis explode all trees
#3 cough* or bronchiti*
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Anti‐Bacterial Agents explode all trees
#6 antibiot* or anti‐biot* or antimicrob* or anti‐microb* or antibacterial* or anti‐bacterial* or erythromycin or amoxycillin or ampicillin or doxycycline
#7 (#5 OR #6)
#8 MeSH descriptor Child explode all trees
#9 MeSH descriptor Infant explode all trees
#10 MeSH descriptor Adolescent explode all trees
#11 MeSH descriptor Pediatrics explode all trees
#12 child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or pre school* or pre‐school* or newborn* or new born* or new‐born* or neo‐nat* or neonat*
#13 (#8 OR #9 OR #10 OR #11 OR #12)
#14 (#4 AND #7 AND #13)
MEDLINE search strategy
Topic search
1. exp COUGH/
2. exp Bronchitis/
3. (cough$ or bronchit$).mp.
4. 1 or 2 or 3
5. exp Anti‐Bacterial Agents/
6. (antibiot$ or anti‐biot$ or antimicrob$ or anti‐microb$ or antibacterial$ or anti‐bacterial$ or erythromycin or amoxycillin or ampicillin or doxycycline).mp.
7. 5 or 6
8. exp adolescent/ or exp child/ or exp infant/
9. exp Pediatrics/
10. (child$ or paediat$ or pediat$ or adolesc$ or infan$ or toddler$ or bab$ or young$ or preschool$ or pre school$ or pre‐school$ or newborn$ or new born$ or new‐born$ or neo‐nat$ or neonat$).mp.
11. 8 or 9 or 10
12. 4 and 7 and 11
RCT filter
1. (clinical trial or controlled clinical trial or randomised controlled trial).pt.
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
Embase search strategy
Topic search
1. exp Coughing/
2. exp Bronchitis/
3. (cough$ or bronchit$).mp.
4. 1 or 2 or 3
5. exp Antibiotic Agent/
6. (antibiot$ or anti‐biot$ or antimicrob$ or anti‐microb$ or antibacterial$ or anti‐bacterial$ or erythromycin or amoxycillin or ampicillin or doxycycline).mp.
7. 5 or 6
8. Child/
9. Adolescent/
10. Infant/
11. exp pediatrics/
12. (child$ or paediat$ or pediat$ or adolesc$ or infan$ or toddler$ or bab$ or young$ or preschool$ or pre school$ or pre‐school$ or newborn$ or new born$ or new‐born$ or neo‐nat$ or neonat$).mp.
13. or/8‐12
14. 4 and 7 and 13
RCT filter
1. Randomized Controlled Trial/
2. Controlled Study/
3. randomisation/
4. Double Blind Procedure/
5. Single Blind Procedure/
6. Clinical Trial/
7. Crossover Procedure/
8. follow up/
9. exp prospective study/
10. or/1‐9
11. (clinica$ adj3 trial$).mp.
12. ((singl$ or doubl$ or trebl$ or tripl$) adj5 (mask$ or blind$ or method$)).mp.
13. exp Placebo/
14. placebo$.mp.
15. random$.mp.
16. (latin adj3 square$).mp.
17. exp Comparative Study/
18. ((control$ or prospectiv$ or volunteer$) adj3 (trial$ or method$ or stud$)).mp.
19. (crossover$ or cross‐over$).mp.
20. or/11‐19
21. 10 or 20
22. exp ANIMAL/
23. Nonhuman/
24. Human/
25. 22 or 23
26. 25 not 24
27. 21 not 26
Appendix 2. Search history
| Year of search | Number of references retrieved |
| 2004 | 1550 |
| 2006 | 297 |
| 2008 | 365 |
| 2010 | 567 |
| 2014 | 443 |
| 2016 | 683 |
| 2017 | 386 |

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Antibiotics versus no antibiotics/placebo for wet cough in children, outcome: 1.1 Children not cured or not substantially improved at follow‐up (using intention‐to‐treat analysis).

In the control group, 75/100 people had not been cured over two weeks, compared to 31/100 (95% CI 17 to 48) for the active treatment group. Number needed to treat for an additional beneficial outcome 3 (95% CI 2 to 4).

Forest plot of comparison: 1 Antibiotics versus no antibiotics/placebo for wet cough in children, outcome: 1.4 Illness progression: participants with progression of disease resulting in additional medical therapy required.
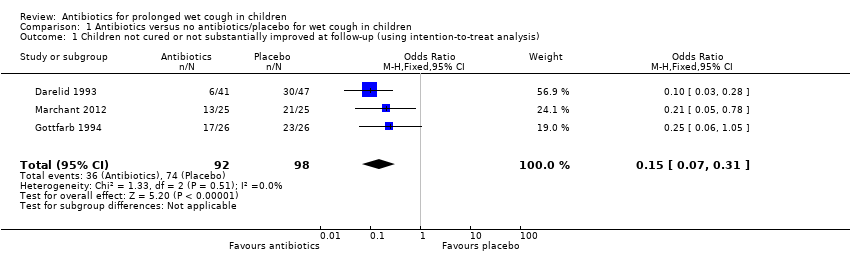
Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 1 Children not cured or not substantially improved at follow‐up (using intention‐to‐treat analysis).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 2 Illness progression: participants with progression of disease resulting in additional antibiotic therapy required (available data only).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 3 Children experiencing adverse effects of antibiotics (vomiting, diarrhoea, rash).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 4 Children not cured or not substantially improved at follow‐up (excluding those known to have B pertussis).

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 5 Subgroup analysis (placebo controlled): children not cured or substantially improved at follow‐up.
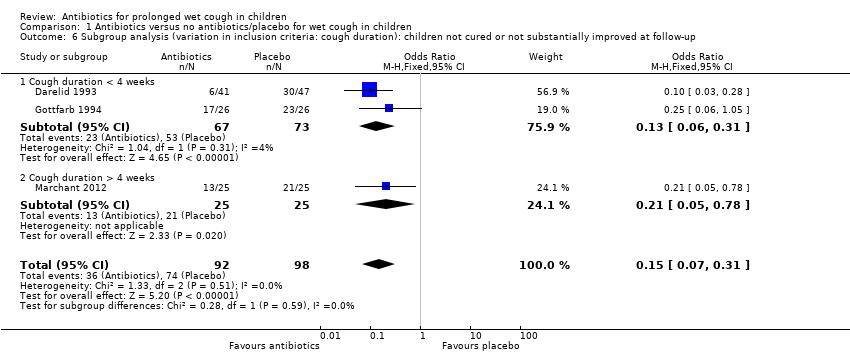
Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 6 Subgroup analysis (variation in inclusion criteria: cough duration): children not cured or not substantially improved at follow‐up.

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 7 Subgroup analysis (antibiotics used): children not cured or not substantially improved at follow‐up.

Comparison 1 Antibiotics versus no antibiotics/placebo for wet cough in children, Outcome 8 Sensitivity analysis: children not cured or not substantially improved at follow‐up (using available data only).
| Antibiotics compared to placebo for prolonged wet cough in children | ||||||
| Patient or population: prolonged wet cough in children | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with antibiotics | |||||
| Children not cured or substantially improved at follow‐up (using intention‐to‐treat analysis) (measured as proportion of participants) Follow‐up: range 7–14 days | 76 per 100 | 32 per 100 | OR 0.15 | 190 | ⊕⊕⊕⊝ | — |
| Children with progression of the disease resulting in additional medical therapy (complications) (measured as proportion of participants) Follow‐up: range 7–14 days | 36 per 100 | 5 per 100 | OR 0.10 | 125 | ⊕⊕⊕⊝ | — |
| Children experiencing adverse effects of antibiotics (e.g. diarrhoea, nausea, skin rash, allergic reactions (measured as proportion of participants)) | 5 per 100 | 10 per 100 | OR 1.88 | 190 | ⊕⊕⊝⊝ | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aOne study carrying 46% of the analysis weight was at high risk of bias due to lack of blinding. Downgraded one level for risk of bias (Darelid 1993). bOne study carrying 87% of the analysis weight was at high risk of bias due to lack of blinding. Downgraded one level for risk of bias (Darelid 1993). cOne study carrying 10% of the analysis weight was at high risk of bias due to lack of blinding and the result has been downgraded for imprecision due to wide CIs. Downgraded two levels for risk of bias and imprecision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Children not cured or not substantially improved at follow‐up (using intention‐to‐treat analysis) Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 2 Illness progression: participants with progression of disease resulting in additional antibiotic therapy required (available data only) Show forest plot | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| 3 Children experiencing adverse effects of antibiotics (vomiting, diarrhoea, rash) Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.62, 5.69] |
| 4 Children not cured or not substantially improved at follow‐up (excluding those known to have B pertussis) Show forest plot | 2 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.05, 0.30] |
| 5 Subgroup analysis (placebo controlled): children not cured or substantially improved at follow‐up Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 5.1 Placebo controlled | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.60] |
| 5.2 No placebo used | 1 | 88 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.28] |
| 6 Subgroup analysis (variation in inclusion criteria: cough duration): children not cured or not substantially improved at follow‐up Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 6.1 Cough duration < 4 weeks | 2 | 140 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.06, 0.31] |
| 6.2 Cough duration > 4 weeks | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.05, 0.78] |
| 7 Subgroup analysis (antibiotics used): children not cured or not substantially improved at follow‐up Show forest plot | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.31] |
| 7.1 Amoxicilin/clavulanic acid use | 2 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.08, 0.60] |
| 7.2 Other antibiotics used | 1 | 88 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.28] |
| 8 Sensitivity analysis: children not cured or not substantially improved at follow‐up (using available data only) Show forest plot | 3 | 171 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.07, 0.29] |

