Antibiotic prophylaxis for operative vaginal delivery
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004455.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 05 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Tippawan Liabsuetrakul (TL) gathered background information and wrote the first and final draft of the protocol. TL assessed trial eligibility and methodological quality, extracted data, wrote the first draft of the review and co‐ordinated the comments from the other review authors. For the 2008 update, TL reviewed the draft and final version of the updated review. For the 2012, 2014 and the current 2017 update, TL reviewed and updated the text.
Thanapan Choobun (TC) gathered background information and commented on the draft protocol. TC assessed trial eligibility and methodological quality, extracted data and commented on the draft review. For the 2008 update, TC approved the final version of the updated review. For the 2012, 2014 and the current 2017 update, TC approved the version for publication.
Krantarat Peeyananjarassri (KP) gathered background information and commented on the draft protocol. KP commented on the draft review. For the 2008 update, KP approved the final version of the updated review. For the 2012, 2014 and the current 2017 update, KP approved the version for publication.
Monir Islam (MI) supervised the development of the protocol and review. MI commented on the draft protocol and review. For the 2008 update, MI approved the final version of the updated review. For the 2012, 2014 and the current 2017 update, MI approved the version for publication.
Sources of support
Internal sources
-
Faculty of Medicine, Prince of Songkla University, Thailand.
External sources
-
Thailand Research Fund (Distinguished Research Professor Award), Thailand.
-
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Tippawan Liabsuetrakul: none known.
Thanapan Choobun: none known.
Krantarat Peeyananjarassri: none known.
Q Monir Islam: none known.
Acknowledgements
We thank Zarko Alfirevic, Eduardo Bergel, Mario Festin, Simon Gates and the Cochrane Pregnancy and Childbirth Group Consumer Panel for their helpful comments on the protocol and the first published version of this review. We also thank Lynn Hampson for the translation (De Meeus 1991) and Erika Ota for preparing the 'Summary of findings' table.
Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors are responsible for the views expressed in this publication (2014 update).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2020 Mar 26 | Antibiotic prophylaxis for operative vaginal delivery | Review | Tippawan Liabsuetrakul, Thanapan Choobun, Krantarat Peeyananjarassri, Q Monir Islam | |
| 2017 Aug 05 | Antibiotic prophylaxis for operative vaginal delivery | Review | Tippawan Liabsuetrakul, Thanapan Choobun, Krantarat Peeyananjarassri, Q Monir Islam | |
| 2014 Oct 13 | Antibiotic prophylaxis for operative vaginal delivery | Review | Tippawan Liabsuetrakul, Thanapan Choobun, Krantarat Peeyananjarassri, Q Monir Islam | |
| 2004 Jul 19 | Antibiotic prophylaxis for operative vaginal delivery | Review | Tippawan Liabsuetrakul, Thanapan Choobun, Krantarat Peeyananjarassri, Q Monir Islam | |
| 2003 Oct 20 | Antibiotic prophylaxis for operative vaginal delivery | Protocol | Tippawan Liabsuetrakul, Thanapan Choobun, Krantarat Peeyananjarassri, Monir Islam | |
Differences between protocol and review
Methods updated to current standard PCG methods (2017).
We added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Amoxicillin-Potassium Clavulanate Combination [therapeutic use];
- Anti-Bacterial Agents [therapeutic use];
- *Antibiotic Prophylaxis [adverse effects];
- Cefotetan [therapeutic use];
- Endometritis [prevention & control];
- Episiotomy [adverse effects];
- Extraction, Obstetrical [*adverse effects];
- Length of Stay;
- Obstetrical Forceps;
- Perineum [injuries];
- Puerperal Infection [*prevention & control];
- Randomized Controlled Trials as Topic;
- Surgical Wound Infection [prevention & control];
- Vacuum Extraction, Obstetrical [adverse effects];
- Vaginal Diseases [*prevention & control];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO
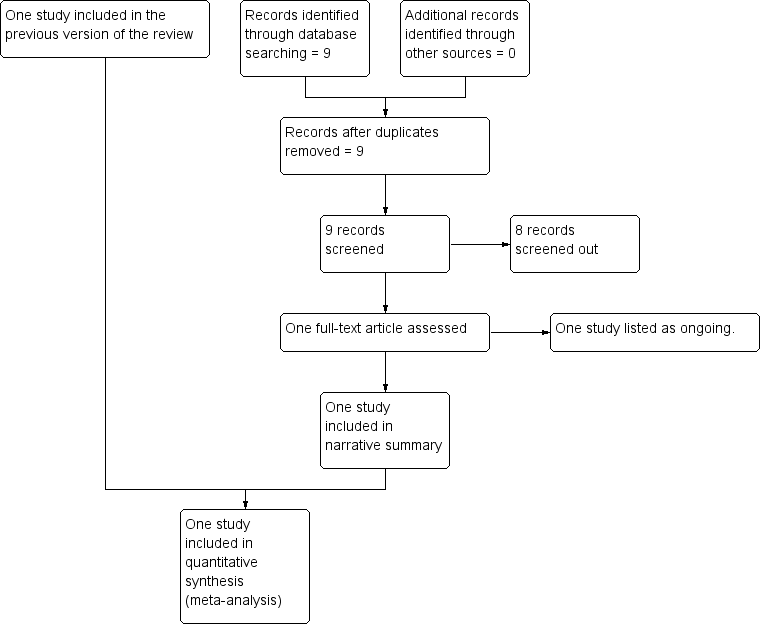
Study flow diagram.
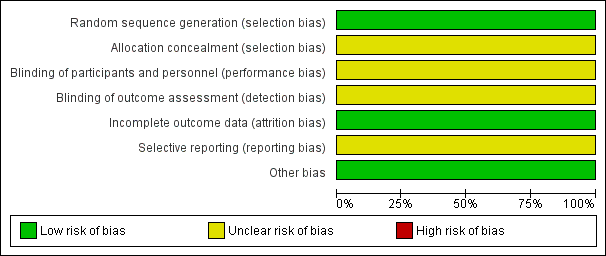
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
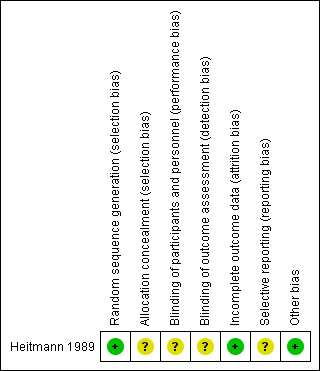
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 1 Endometritis.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 2 Maternal length of stay.
| Any antibiotics versus placebo or no treatment for operative vaginal delivery | ||||||
| Population: women undergoing operative vaginal delivery Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antibiotics versus placebo or no treatment | |||||
| Endometritis | Study population | RR 0.07 | 393 | ⊕⊕⊝⊝ | ||
| 35 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 35 per 1000 | 2 per 1000 | |||||
| Maternal length of hospital stay (days) | The mean maternal length of stay in the intervention groups was | 393 | ⊕⊕⊝⊝ | |||
| Fever | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Infected episiotomy/perineal/vaginal laceration | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Urinary tract infection | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Serious infectious complications | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Maternal adverse reactions | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, few events and a small sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometritis Show forest plot | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.21] |
| 2 Maternal length of stay Show forest plot | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |

