Profilaxis antibiótica para el parto vaginal instrumentado
Resumen
Antecedentes
Se informó que los partos vaginales asistidos con ventosa y fórceps aumentan la incidencia de las infecciones posparto y el reingreso materno al hospital en comparación con el parto vaginal espontáneo. Para prevenir estas infecciones se pueden recetar antibióticos profilácticos. Sin embargo, aún no queda claro cuál es el beneficio de la profilaxis antibiótica para los partos vaginales instrumentados. Esta es una actualización de una revisión publicada por última vez en 2017.
Objetivos
Evaluar la efectividad y la seguridad de la profilaxis antibiótica para reducir la morbilidad puerperal infecciosa en las pacientes que se someten a partos vaginales instrumentados, incluido el parto con ventosa o con fórceps, o ambos.
Métodos de búsqueda
Para esta actualización, se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth's Trials Register ), ClinicalTrials.gov, la WHO International Clinical Trials Registry Platform (ICTRP) (5 de julio 2019) y en las listas de referencias de los estudios recuperados.
Criterios de selección
Fueron elegibles todos los ensayos controlados aleatorizados que compararon cualquier régimen de antibióticos profilácticos con placebo o ningún tratamiento en pacientes que se sometieron a partos asistidos con ventosa o con fórceps. Todas las participantes eran embarazadas sin indicios de infecciones ni otras indicaciones de antibióticos en cualquier edad gestacional. Las intervenciones consistían en cualquier tipo de profilaxis antibiótica (cualquier régimen de dosis, cualquier vía de administración o en cualquier momento durante el parto o el puerperio).
Obtención y análisis de los datos
Dos autores de la revisión evaluaron la elegibilidad de los ensayos y el riesgo de sesgo. Dos autores de la revisión extrajeron los datos, de forma independiente, utilizando formularios de extracción de datos preparados. Todas las discrepancias se resolvieron mediante el debate y el consenso alcanzado en las deliberaciones con todos los autores de la revisión. Se evaluó la calidad metodológica de los dos estudios incluidos mediante los criterios GRADE.
Resultados principales
Se incluyeron dos estudios en los que participaron 3813 pacientes sometidas a partos con ventosa o con fórceps. Un estudio con 393 pacientes comparó el antibiótico cefotetán intravenoso después del pinzamiento del cordón umbilical con la ausencia de tratamiento. El otro estudio, en el que participaron 3420 pacientes, comparó una sola dosis de amoxicilina y ácido clavulánico por vía intravenosa con placebo, se utilizó 20 ml de solución salina intravenosa estéril al 0,9%.
La evidencia indica que los antibióticos profilácticos reducen la infección de la herida perineal superficial (riesgo relativo [RR] 0,53; intervalo de confianza [IC]del 95%: 0,40 a 0,69; pacientes = 3420; 1 estudio; evidencia de certeza alta), la infección de la herida perineal profunda (RR 0,46; IC del 95%: 0,31 a 0,69; pacientes = 3420; 1 estudio; evidencia de certeza alta) y probablemente reducir la dehiscencia de la herida (RR 0,52; IC del 95%: 0,43 a 0,63; mujeres = 2593; 1 estudio; evidencia de certeza moderada). No está claro qué efecto hay sobre la infección de las heridas de los órganos o del espacio perineal (RR 0,11; IC del 95%: 0,01 a 2,05; pacientes = 3420; 1 estudio) y la endometritis (RR promedio 0,32; IC del 95%: 0,04 a 2,64; 15/1907 versus 30/1906; pacientes = 3813; 2 estudios) en función de la evidencia de certeza baja con IC amplios que no incluyen ningún efecto. Los antibióticos profilácticos probablemente disminuyen las complicaciones infecciosas graves (RR 0,44; IC del 95%: 0,22 a 0,89; pacientes = 3420; 1 estudio; evidencia de certeza alta). También tienen un efecto importante en la reducción de la infección materna confirmada o presunta. Los dos estudios incluidos no informaron sobre fiebre ni infección urinaria.
No está claro, en función de la evidencia de certeza baja, si los antibióticos profilácticos tienen algún efecto en las reacciones adversas maternas (RR 2,00; IC del 95%: 0,18 a 22,05; pacientes = 2593; 1 estudio) y en la duración de la estancia materna (DM 0,09 días; IC del 95%: ‐0,23 a 0,41; pacientes = 393; 1 estudio), ya que los IC fueron amplios y no incluyeron ningún efecto. Los antibióticos profilácticos mejoran levemente el dolor perineal y las consecuencias para la salud del dolor perineal y probablemente reducen los costos. Los antibióticos profilácticos no tuvieron un efecto importante en la dispareunia (coito doloroso o dificultad para mantener relaciones) ni en la lactancia materna a las seis semanas. La profilaxis antibiótica puede mejorar levemente el reingreso materno al hospital y la calidad de vida relacionada con la salud materna. No se informó sobre reacciones adversas neonatales en ninguno de los ensayos incluidos.
Conclusiones de los autores
Los antibióticos profilácticos intravenosos son eficaces para reducir las morbilidades infecciosas puerperales, en términos de infección de las heridas perineales superficiales y profundas, ni de complicaciones infecciosas graves en las pacientes que se someten a partos vaginales instrumentados sin indicaciones clínicas para la administración de antibióticos después del parto. Los antibióticos profilácticos mejoran levemente el dolor perineal y las consecuencias para la salud del dolor perineal, probablemente reducen los costos, y pueden reducir levemente el reingreso materno al hospital y la calidad de vida relacionada con la salud. Sin embargo, no está claro qué efecto hay sobre la reducción de la endometritis, la infección de las heridas de los órganos o del espacio perineal, las reacciones adversas maternas y la duración de la estancia de la madre debido a la evidencia de certeza baja.
Como la evidencia provino principalmente de un solo estudio multicéntrico realizado en un contexto de ingresos altos, es necesario que se realicen ensayos aleatorizados adicionales diseñados en otros contextos, en particular en contextos de ingresos bajos y medios, para confirmar el efecto de la profilaxis antibiótica para el parto vaginal instrumentado.
PICO
Resumen en términos sencillos
¿La profilaxis antibiótica es efectiva o segura para las pacientes que se someten a un parto vaginal instrumentado?
Se propuso evaluar, a partir de estudios controlados aleatorizados, si la administración de antibióticos a todas las pacientes que se someten a partos vaginales instrumentados previene las infecciones en la madre sin aumentar los resultados adversos en la madre ni en el recién nacido. La extracción con ventosa o con fórceps se utiliza para extraer la cabeza del bebé en los partos vaginales instrumentados.
¿Cuál es el problema?
Las pacientes que se someten a partos vaginales asistidos por ventosas o por fórceps pueden tener más probabilidades de sufrir una infección después del parto en comparación con las pacientes que experimentan un parto vaginal espontáneo normal. También es más probable que sean reingresadas al hospital. Las pacientes corren un mayor riesgo de infección debido a la necesidad de un cateterismo vesical rutinario, varios tactos vaginales, la inserción de instrumental en la vagina y un mayor riesgo de cortes profundos o desgarros vaginales durante el parto instrumentado. La infección aparece como fiebre, infección del útero y de los tejidos circundantes, un desgarro vaginal o una episiotomía infectada, o una infección urinaria. Estos cuadros afectan el estado físico de la madre y pueden afectar su bienestar. La infección también podría ingresar en el torrente sanguíneo y afectar a todo el cuerpo.
¿Por qué esto es importante? La extracción con ventosa o con fórceps se utilizan para acortar el trabajo de parto desde el momento en que el cuello uterino se abre completamente hasta el parto (período expulsivo), especialmente si se prolonga o si el bebé presenta signos de sufrimiento fetal. Se pueden administrar antibióticos a las madres en el momento del parto para prevenir o reducir el riesgo de infección. Sin embargo, todavía hay algunas dudas sobre el beneficio de dichos antibióticos. Los antibióticos también pueden causar reacciones adversas como erupción o diarrea en la madre, y pueden estar presentes en la leche materna, de modo que el bebé amamantado se expone a ellos.
¿Qué evidencia se encontró?
Se actualizó la búsqueda de evidencia de estudios controlados aleatorizados en julio 2019. Se incluyeron dos estudios publicados antes de 1989 y de 2019. El estudio más antiguo se realizó en los EE.UU. y el estudio más reciente pertenecía a un número de unidades de obstetricia de un hospital del Reino Unido. Se incluyó un total de 3813 pacientes que se sometieron a parto vaginal instrumentado. El estudio de EE.UU. incluyó a 393 pacientes y se comparó 2 g de cefotetán intravenoso después del pinzamiento del cordón umbilical con ningún tratamiento. En el otro estudio participaron 3420 pacientes. Este estudio comparó la amoxicilina intravenosa y el ácido clavulánico con un placebo. La certeza de la evidencia varió de alta a baja, y la certeza baja se redujo debido a preocupaciones relacionadas con la imprecisión de los resultados, con pocos eventos y un solo estudio que informó sobre varios de los hallazgos.
Los antibióticos profilácticos administrados para reducir o prevenir la infección redujeron a la mitad el número de pacientes con episiotomías o laceraciones infectadas. Estos resultados incluyeron infecciones de las heridas perineales superficiales y profundas (un estudio, 3420 pacientes; evidencia de certeza alta) o dehiscencia de las heridas (un estudio, 2593 pacientes; evidencia de certeza moderada). También se redujeron las complicaciones infecciosas graves (1 estudio, 3420 pacientes; evidencia de certeza alta). Debido a evidencia de certeza baja, los antibióticos profilácticos tuvieron efectos inciertos en la endometritis, que se manifestó en forma de fiebre y sensibilidad uterina o hemorragia profusa (2 estudios, 3813 pacientes; evidencia de certeza baja) y en la episiotomía/laceración infectada que presentó una infección de los órganos o del espacio perineal (1 estudio, 3420 pacientes; evidencia de certeza baja).
El efecto sobre las reacciones adversas maternas (1 estudio, 2593 pacientes; evidencia de certeza baja) y la duración de la estancia de la madre en el hospital (1 estudio, 393 pacientes; evidencia de certeza baja) tampoco quedó claro debido a la certeza baja del estudio. Hubo una ligera reducción en el dolor perineal y en las consecuencias para la salud del dolor perineal. Los antibióticos profilácticos no tuvieron un efecto claro sobre el dolor durante el coito y la lactancia a las seis semanas. El reingreso de la madre al hospital y la calidad de vida relacionada con la salud materna podrían mejorar levemente. Con el uso de antibióticos profilácticos se redujeron los costos. Ninguno de los estudios midió específicamente la fiebre, la infección urinaria ni las reacciones adversas en los bebés.
¿Qué significa esto?
Los antibióticos profilácticos intravenosos son efectivos para reducir las enfermedades causadas por infecciones en pacientes que se someten a partos vaginales instrumentados y que no tienen indicaciones clínicas para la administración de antibióticos. La evidencia procedía principalmente de un único estudio realizado en un país de ingresos altos. Se necesitan estudios aleatorizados bien diseñados en otros contextos para confirmar este hallazgo.
Authors' conclusions
Summary of findings
| Any antibiotics compared to placebo or no treatment for operative vaginal delivery | ||||||
| Patient or population: operative vaginal delivery | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
| Without any antibiotics | With any antibiotics | Difference | ||||
| Fever ‐ not measured | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the 2 included studies. | |
| Infected episiotomy/laceration (superficial perineal wound infection) | RR 0.53 | Study population | ⊕⊕⊕⊕ | |||
| 8.3% | 4.4% | 3.9% fewer | ||||
| Infected episiotomy/laceration (deep perineal wound infection | RR 0.46 | Study population | ⊕⊕⊕⊕ | |||
| 4.5% | 2.1% | 2.4% fewer | ||||
| Infected episiotomy/laceration (organ or space infection) | RR 0.11 | Study population | ⊕⊕⊝⊝ | |||
| 0.2% | 0.0% | 0.2% fewer | ||||
| Infected episiotomy/laceration (wound breakdown) | RR 0.52 | Study population | ⊕⊕⊕⊝ | |||
| 21.0% | 10.9% | 10.1% fewer | ||||
| Endometritis | RR 0.32 | Study population | ⊕⊕⊝⊝ | |||
| 1.6% | 0.5% | 1.1% fewer | ||||
| Urinary tract infection ‐ not measured | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the 2 included studies. | |
| Serious infectious complications | RR 0.44 | Study population | ⊕⊕⊕⊕ | |||
| 1.5% | 0.6% | 0.8% fewer | ||||
| Maternal adverse reactions | RR 2.00 | Study population | ⊕⊕⊝⊝ | |||
| 0.1% | 0.2% | 0.1% more | ||||
| Maternal length of stay | ‐ | The mean maternal length of stay without any antibiotics was 2.37 days. | The mean maternal length of stay with antibiotics was 0.09 days more (0.23 days less to 0.41 days more) | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded (2) levels for very serious imprecision due to wide confidence interval crossing the line of no effect and small number of events. 2 We downgraded (1) level for serious limitation in study design due to loss to follow up for this outcome higher than 20%. 3 We downgraded (1) level for serious inconsistency due to unexplained substantial heterogeneity. 4 We downgraded (1) level for serious imprecision due to wide confidence interval. 5 We downgraded (1) level for serious limitations in study design due to many domains being at unclear risk of bias. | ||||||
Background
Description of the condition
Operative vaginal delivery is the term used to describe delivery of the fetal head assisted by either vacuum extractor or forceps. The commonest indications for operative vaginal delivery are prolonged second stage of labour, suspicion of immediate or potential fetal compromise and shortening of the second stage of labour for maternal benefit (RCOG 2011; ACOG 2015). The rates of operative vaginal delivery reported vary by centres in different countries (Cammu 2011; Hanley 2010; Hehir 2013; Janni 2002; Kabiru 2001; Lawani 2014; Mola 2011; Walsh 2013) with ranges from 2.1% in Papua NewGuinea (Mola 2011) to 19.2% of all births in northern Belgium (Cammu 2011).
The risk of postpartum infection is increased after operative vaginal birth because of higher rates of vaginal lacerations, routine bladder catheterisation, multiple vaginal examinations, insertion of instruments into the vagina and contamination (Chaim 2000; Pranchev 1993). Instrumental deliveries require additional vaginal examinations, a known risk factor for endometritis and febrile morbidity (ACOG 2015; Chang 1992; Dare 1998). Insertion of instruments and contamination is also assumed to be one of the risks of postpartum infection because of difficulties in adhering to aseptic practices during delivery (Dare 1998).
Description of the intervention
Antibiotic prophylaxis is one of the methods used to reduce the risk of postpartum infections. It has been widely studied in obstetrics and has shown to be effective in reducing postoperative puerperal morbidity after caesarean section in a Cochrane Review (Smail 2014). However, there are still some doubts about the benefit of prophylactic antibiotics in reducing postpartum infection after operative vaginal delivery. Due to the physiological change of gastric emptying time, plasma volume and renal function during pregnancy, the pharmacokinetics of antibiotics differ between pregnant and nonpregnant women. For these reasons careful consideration needs to be given to the types of antibiotics and antibiotic regimens given to pregnant women for prophylactic use (ACOG 2018).
How the intervention might work
The reported incidence of postpartum infection or endometritis after operative vaginal delivery in studies varies from 3.5% to 16% (Hagadorn‐Freathy 1991; Heitmann 1989; Kabiru 2001; Williams 1991). In addition, the outcomes of readmission within 60 days after delivery and maternal sepsis have been reported as increased in incidence following the use of operative vaginal delivery in comparison with spontaneous vaginal delivery (Acosta 2014; Liu 2005). Postpartum infection not only affects the physical status of the mother and prolongs hospital stay after birth, but also significantly impacts on the psychological well‐being of the mother (RCOG 2011).
The most common micro‐organisms in the genitourinary tract causing postpartum morbidities such as fever, endometritis, infected episiotomy/vaginal laceration or urinary tract infection are Enterococci, Streptococci, Staphylococci, Gram‐negative bacilli and anaerobes (Kok 2000; Stray‐Pedersen 1988). Group B streptococcus, Enterococcus, Gardnerella vaginalis, Staphylococcus aureus and anaerobe bacteria were usually recovered from the cervix and endometrium among febrile postpartum women (Eschenbach 1986). The types of antibiotics selected to be prophylactically used should be effective against these common micro‐organisms and include the following: ampicillin, cephazolin, clindamycin, vancomycin, azithromycin, and the aminoglycosides (ACOG 2018).
Why it is important to do this review
Previous studies (Janisch 1979; Rechlin 1988) have indicated that prophylactic antibiotics may not be necessary due to the relatively low risk of infectious morbidity, and uncertain effect on puerperal fever. Aseptic precautions during operative vaginal delivery may be enough to prevent postpartum infection (Janisch 1979). In contrast, other studies did suggest that antibiotic prophylaxis might reduce the risk of infection after normal vaginal delivery and operative vaginal delivery (Fernandez 1993; Heitmann 1989). Criscuolo 1990 suggested that the cost of prophylactic antibiotics could be much lower compared with the cost of treating the complications of infection related to procedures during delivery.
However, widespread use of antibiotics may contribute to the development of antibiotic‐resistant bacteria (Towers 1998; Weinstein 1996). A study in Vietnam found that 98% of women who gave birth vaginally received antibiotics (Ngoc 2005). The recently reported resistance patterns of isolated strains of Escherichia coli, Staphylococcus aureus, Enterococci , and Streptococcus pneumoniae are concerning (ACOG 2018). In addition, antibiotics may contaminate breast milk, as well as cause adverse reactions such as rash or antibiotic‐related diarrhoea (Dancer 2004).
There is also a concern that there may be a significantly increased risk of third‐ or fourth‐degree tears, severe maternal morbidity and death, perinatal mortality and neonatal mortality in women with operative vaginal birth compared with normal birth (Angioli 2000; Lumbiganon 2010). The incidence of third‐ and fourth‐degree tears reported range from 1% to 36% of all births (Boucoiran 2010; Goldberg 2003; Johnson 2004; Nkwabong 2011; Panigrahy 2008; Prapas 2009). The rate of perineal wound complication measured at two weeks postpartum was found to be significantly lower with the use of antibiotic prophylaxis; however, loss to follow‐up was high and thus these results should be interpreted with caution (Buppasiri 2014; Duggal 2008).
Therefore, the effectiveness of antibiotic prophylaxis for operative vaginal delivery needs to be carefully evaluated. This is an update of a review last published in 2017.
Objectives
To assess the effectiveness and safety of antibiotic prophylaxis in reducing infectious puerperal morbidities in women undergoing operative vaginal deliveries including vacuum or forceps delivery, or both.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing any prophylactic antibiotic regimens with placebo or no treatment in women undergoing vacuum or forceps delivery. Cluster‐trials were eligible for inclusion as were trials presented as an Abstract if sufficient information was reported in order to assess eligibility. Quasi‐randomised and cross‐over trials were not eligible for inclusion.
Types of participants
Pregnant women without evidence of infections or other indications for antibiotics of any gestational age undergoing vacuum or forceps delivery for any indication.
Types of interventions
Any antibiotic prophylaxis (any dosage regimen, any route of administration or at any time during delivery or puerperium) compared with either placebo or no treatment.
Types of outcome measures
We considered the following clinical outcomes.
Primary outcomes
-
Fever (body temperature of 38 degrees celsius or higher) occurring on any two occasions in the first 10 days postpartum, exclusive of the first 24 hours
-
Infected episiotomy/perineal/vaginal laceration (oedematous, erythematous, wound edge with pain, serosanguineous or frankly purulent material or wound dehiscence)
-
Endometritis (fever and uterine tenderness or heavy bleeding)
-
Urinary tract infection (fever or dysuria and positive urine culture)
-
Serious infectious complications (such as bacteraemia, systemic infection, septic shock, septic thrombophlebitis, necrotising fasciitis or death attributed to infection)
-
Confirmed or suspected maternal infection within six weeks of delivery, defined by a new prescription of antibiotics for presumed perineal wound‐related infection, endometritis or uterine infection, urinary tract infection with systemic features or infection; confirmed systemic infection on culture; or endometritis (non‐prespecified)
Secondary outcomes
-
Maternal adverse reactions such as allergic reactions, anaphylaxis, antibiotic‐associated diarrhoea
-
Maternal length of stay
-
Perineal pain (non‐prespecified)
-
Use of pain relief for perineal pain (non‐prespecified)
-
Need for additional perineal care (non‐prespecified)
-
Dyspareunia (difficult or painful sexual intercourse) (non‐prespecified)
-
Breastfeeding at six weeks (non‐prespecified)
-
Perineum "ever too painful or uncomfortable" to feed baby (non‐prespecified)
-
Any primary care or home visits in relation to perineum (non‐prespecified)
-
Any outpatient visit in relation to perineum (non‐prespecified)
-
Maternal hospital re‐admission (non‐prespecified)
-
Maternal health‐related quality of life (non‐prespecified)
-
Costs
-
Neonatal adverse reactions such as such as jaundice, early neonatal infection, or any infant outcomes reported
Search methods for identification of studies
The following methods section of this review was based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (5 July 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (5 July 2019) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeLiabsuetrakul 2017.
For this update, we used the following methods. The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for the two included studies the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for the two included studies the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the included studies the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered in advance that studies would be at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included studies the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for the two included studies, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for both included studies how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the studies' pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the studies' pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for both included studies any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether the included studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, if more studies are included, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the certainty of the evidence using the GRADE approach
We assessed the certainty of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparison between any antibiotic prophylaxis (any dosage regimen, any route of administration or at any time during delivery or puerperium) compared with either placebo or no treatment.
-
Fever (body temperature of 38 degrees celsius or higher) occurring on any two occasions in the first 10 days postpartum, exclusive of the first 24 hours
-
Infected episiotomy/perineal/vaginal laceration (oedematous, erythematous, wound edge with pain, serosanguineous or frankly purulent material or wound dehiscence)
-
Endometritis (fever and uterine tenderness or heavy bleeding)
-
Urinary tract infection (fever or dysuria and positive urine culture)
-
Serious infectious complications (such as bacteraemia, septic shock, septic thrombophlebitis, necrotising fasciitis or death attributed to infection)
-
Maternal adverse reactions such as allergic reactions, anaphylaxis, antibiotic‐associated diarrhoea
-
Maternal length of stay
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of certainty for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
In future updates, if cluster‐randomised trials are identified and included, we will adjust the standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC.
If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This study design not applicable for this review.
Other unit of analysis issues
Not applicable.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in the included study was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis. If any outcome includes only one study, the test for heterogeneity is not applicable.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar.
When substantial statistical heterogeneity was detected, we considered whether the clinical or methodological heterogeneity was influenced using subgroup or sensitivity analyses and we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary was treated and presented as the average of the range of treatment effects with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to investigate the substantial heterogeneity due to different clinical characteristics among included studies using subgroup analysis, if appropriate.
In future updates, we will carry out the following subgroup analyses for primary outcomes.
-
Vacuum or forceps deliveries
-
Different antibiotic regimens
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor‐quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
See: Figure 1.

Study flow diagram.
This review update includes two studies (ANODE 2019; Heitmann 1989). One of these (Heitmann 1989) was included in the previous version of this review (Liabsuetrakul 2017). For this update, we identified three new reports that were assessed, together with a report of an ongoing study that had been awaiting classification in the previous version of this review. All four reports relate to a new trial, which has been included in this update (ANODE 2019).
Included studies
We included two studies involving a total of 3813 women, 393 women in one study (Heitmann 1989) and 3420 women in another study (ANODE 2019). A total of seven women withdrew consent before the intervention was given comparing prophylactic antibiotics in women undergoing operative vaginal deliveries with placebo or no antibiotics (ANODE 2019).
(1) Study location and settings
One study was conducted in USA (Heitmann 1989), and the other study was conducted in 27 hospital obstetric units in the UK (ANODE 2019).
(2) Participants
A total of 3813 women in two included studies involving 1907 women undergoing operative vaginal delivery received prophylactic antibiotics versus 1906 women with no treatment or placebo. One trial involved 393 women, of which 43.2% delivered by forceps and 56.8% by vacuum extraction (Heitmann 1989). The other trial involved 3420 women. of which 20 sets of twins were included leading to a total of 3440 births, delivered by forceps in 2234 (65%) and vacuum extraction in 1196 (35%), but it was noted that less than 1% (10) were by spontaneous vaginal delivery (ANODE 2019).
(3) Interventions
One study (Heitmann 1989) compared 2 g of intravenous cefotetan after cord clamping (n = 192) with no treatment (n = 201). The other study (ANODE 2019) compared intravenous amoxicillin and clavulanic acid (1 g amoxicillin and 200 mg clavulanic acid) as soon as possible and no more than six hours after giving birth (n = 1715) with placebo using 20 mL of intravenous sterile 0·9% saline within the same timeframe (n = 1705).
(4) Outcomes
One study reported the outcomes of endomyometritis and maternal length of stay (Heitmann 1989). The other study (ANODE 2019) measured confirmed or suspected maternal infection, confirmed systemic infection on culture or endometritis as primary outcomes, and systemic sepsis, perineal wound infection, perineal pain, use of pain relief, hospital bed stay until discharge, need for additional perineal care, dyspareunia, breastfeeding at six weeks, perineum "ever too painful or uncomfortable" to feed baby, maternal health‐related quality of life, breastfeeding, wound breakdown, intervention side‐effects, healthcare resource use and costs, or adverse events as secondary outcomes. Perineal wound infections in terms of superficial incisional infection, deep incisional infection, or organ or space infection and wound breakdown were reported in ANODE 2019, which were considered as the infected episiotomy/perineal/vaginal laceration in this review. The measurement or scale used to assess perineal pain and perineum "ever too painful or uncomfortable" to feed baby was not described in the trial. However, in the trial protocol (Knight 2018), it was mentioned that pain was measured by the standard questions developed for the HOOP study (McCandlish 1998) and the PREVIEW study (Dudley 2017) where pain was rated as none/mild/moderate/severe.
The definitions of endomyometritis in Heitmann 1989 were as same as that of endometritis in ANODE 2019. Both studies did not specifically measure fever, urinary tract infection (fever or dysuria and positive urine culture) or neonatal adverse reactions.
(5) Dates of study, funding sources and declarations of interest
Dates when the studies were conducted were reported as: September 1986 to February 1989 (Heitmann 1989) and 13 March 2016 to 13 June 2018 (ANODE 2019).
Funding sources and declarations of interest among primary researchers were reported in one included study (ANODE 2019) and could not be identified in the other included study (Heitmann 1989).
For details of the two included trials, see the Characteristics of included studies table.
Excluded studies
One study was excluded (De Meeus 1991). The study was only available as an abstract and there was insufficient information in order to assess fully for eligibility. We had contacted the authors for further information. For further details of the excluded study, see the Characteristics of excluded studies table.
Risk of bias in included studies
According to the 'Risk of bias' tool, one study had unclear risk of bias overall (Heitmann 1989) and another study showed a low risk of bias overall (ANODE 2019).
Details of the two included studies are in the Characteristics of included studies table. Each risk of bias domain in summarised in Figure 2 and Figure 3.
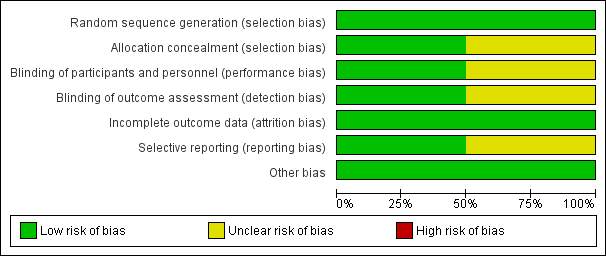
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Heitmann 1989 used a randomisation table to generate allocation sequence, but did not report concealment of randomisation. There were more vacuum deliveries than forceps deliveries but they were equally balanced between the two groups (vacuum rate was 57% in the cefotetan group and 59% in the no treatment group).
ANODE 2019 used permuted blocks of variable size for random sequence generation and the randomisation list was concealed by a designated independent trial programmer and sealed, sequentially numbered, indistinguishable packs containing the prepared drug or placebo.
Blinding
There were no details of the blinding of intervention to either women or clinicians as well as the outcome measurements in one study (Heitmann 1989). It could be assumed that it was not blinded because the comparison was no treatment. However, the main outcome was objectively measured so it was unlikely to have been influenced by lack of blinding (Heitmann 1989).
We assessed that the method of ANODE 2019 study for blinding of participant, caregiver and outcome assessors was of low risk of bias.
Incomplete outcome data
The number of samples given at intervention was the same as those at outcome measure; however, the intention‐to‐treat analysis was not clearly described in Heitmann 1989 For ANODE 2019, the number of primary outcomes were complete but secondary outcomes showed 24% loss to follow‐up; however, it was balanced between both groups thus it was assessed as being of low risk of bias.
Selective reporting
In Heitmann 1989, the study protocol was not available; therefore, there was insufficient information to permit judgement. Also, there was no attempt to analyse the subgroups according to the type of delivery (Heitmann 1989). Outcomes in the study of ANODE 2019 were reported as defined in the protocol.
Other potential sources of bias
Both studies appeared to be free of other sources of bias.
Effects of interventions
Two studies, involving 3813 women undergoing either vacuum or forceps deliveries, were included. One study involving 393 women was judged as unclear risk of bias in most domains comparing the antibiotic intravenous cefotetan after cord clamping compared with no treatment (Heitmann 1989). The other study involving 3420 women had low risk of bias comparing a single dose of intravenous amoxicillin and clavulanic acid with placebo using 20 mL of intravenous sterile 0.9% saline (ANODE 2019).
Primary outcome
Prophylactic antibiotics reduce infected episiotomy/laceration presenting with superficial perineal wound infection (risk ratio (RR) 0.53, 95% confidence interval (CI) 0.40 to 0.69; women = 3420; 1 study; high‐certainty evidence; Analysis 1.1), deep perineal wound infection (RR 0.46, 95% CI 0.31 to 0.69; women = 3420; 1 study; high‐certainty evidence; Analysis 1.2), and wound breakdown (RR 0.52, 95% CI 0.43 to 0.63; women = 2593; 1 study; moderate‐certainty evidence; Analysis 1.4). We are unclear of the effect on infected episiotomy/laceration presenting with organ or space infection (RR 0.11, 95% CI 0.01 to 2.05; women = 3420; 1 study; low‐certainty evidence; Analysis 1.3) or on endometritis (average RR 0.32, 95% CI 0.04 to 2.64; 15/1907 versus 30/1906; women = 3813; 2 studies; random‐effects model; I2 = 58%; low‐certainty evidence; Analysis 1.5) because the certainty of the evidence was low and the CIs were wide and included no effect. We could not perform subgroup or sensitivity analyses of endometritis because only two studies were included. Prophylactic antibiotics also probably lower serious infectious complications (RR 0.44, 95% CI 0.22 to 0.89; women = 3420; 1 study; high‐certainty evidence; Analysis 1.6). Prophylactic antibiotics have an important effect on reduction of confirmed or suspected maternal infection (Analysis 1.7). The two included studies did not report fever or urinary tract infection.
Six primary outcomes were assessed as being of low‐ to high‐certainty evidence according to GRADE. High‐certainty evidence on superficial or deep perineal wound infection and serious infectious complications were shown.
Secondary outcomes
It is unclear whether antibiotics have any impact on maternal adverse reactions (RR 2.00, 95% CI 0.18 to 22.05; women = 2593; 1 study; low‐certainty evidence; Analysis 1.8) and maternal length of stay (MD 0.09 days, 95% CI ‐0.23 to 0.41; women = 393; 1 study; low‐certainty evidence; Analysis 1.9), because the results for both of these were based on low‐certainty evidence with confidence intervals that included both benefit and harm. Secondary outcomes, not prespecified in the protocol, were perineal pain (Analysis 1.10), use of pain relief for perineal pain (Analysis 1.11), need for additional perineal care (Analysis 1.12), dyspareunia (Analysis 1.13), breastfeeding at six weeks (Analysis 1.14), perineum "ever too painful or uncomfortable" to feed baby (Analysis 1.15), any primary care or home visits in relation to perineum (Analysis 1.16), any outpatient visit in relation to perineum (Analysis 1.17), maternal hospital re‐admission (Analysis 1.18), maternal health‐related quality of life (Analysis 1.19) and costs (Analysis 1.20). Prophylactic antibiotics slightly improve perineal pain and health consequences of perineal pain, probably reduce the costs, and may slightly reduce the maternal hospital re‐admission and health‐related quality of life. Neonatal adverse reactions were not reported in any included trials.
Two outcomes, maternal adverse reactions and maternal length of stay, were assessed as being of low certainty according to GRADE. The reasons for downgrading the certainty of the evidence were because of very serious imprecision due to wide CIs crossing the line of no effect, a small number of events and serious limitations in study design with many domains being at unclear risk of bias.
Discussion
Summary of main results
Two studies, involving 3813 women undergoing either vacuum or forceps deliveries, were included. The studies compared the prophylactic antibiotics with intravenous cefotetan after cord clamping or a single dose of amoxicillin and clavulanic acid after giving birth compared with no treatment or placebo. One study published in 1989 reported only two out of the nine outcomes specified in this review. One recent study published in 2019 reported almost all outcomes specified, except fever, urinary tract infection and neonatal adverse reactions. In addition, perineal pain and its health consequences due to perineal pain, maternal hospital re‐admission, and health‐related quality of life, which were not prespecified in the protocol, were also reported.
Prophylactic antibiotics reduce infected episiotomy/laceration presenting with superficial or deep perineal wound infection and wound breakdown with high‐certainty and moderate‐certainty evidence, respectively. We are unclear about the effect of prophylactic antibiotics on endometritis or organ or space perineal wound infection due to low‐certainty evidence. Antibiotic prophylaxis probably lowers serious infectious complications with high‐certainty evidence and has an important effect on reduction of confirmed or suspected maternal infection. The two included studies did not report on fever or urinary tract infection. It is unclear whether prophylactic antibiotics have any impact on maternal adverse reactions and maternal length of stay with low‐certainty evidence, respectively. Prophylactic antibiotics slightly improve perineal pain and health consequences of perineal pain and probably reduce costs, and may slightly reduce the maternal hospital re‐admission and health‐related quality of life. Neonatal adverse reactions were not reported in either of the included trials. Prophylactic antibiotics did not have an important effect on dyspareunia or breastfeeding at six weeks. Prophylactic antibiotics may slightly reduce maternal hospital re‐admission and health‐related quality of life.
Overall completeness and applicability of evidence
There were only two included studies in this review (ANODE 2019; Heitmann 1989). The large multi‐centre trial involving 3420 women(ANODE 2019) dominates the analyses, with only two outcomes including data from the older, smaller trial (Heitmann 1989). The sample size of the first trial (n = 393 women) (Heitmann 1989) was one tenth that of the large, multi‐centre trial (ANODE 2019).
In a recent survey, although the evidence has been lacking to support the use of antibiotic prophylaxis in operative vaginal delivery, it was reported to be widely used in clinical practice (Liabsuetrakul 2014a). From our review, there were important effects of prophylactic antibiotics on reduction of infected episiotomy/laceration presenting with superficial or deep perineal wound infection and wound breakdown. Prophylactic antibiotics probably lower serious infectious complications. However, our review findings have been mainly due to the findings from a large, multi‐centre trial conducted in the UK using a single dose of amoxicillin and clavulanic acid after giving birth. The practice of operative vaginal deliveries and rate of postpartum infections are different across settings (ACOG 2015). A literature review of 10 studies conducted in the USA and the UK on postpartum infection in women undergoing operative vaginal delivery showed the incidence of infectious morbidities varying from 0.7% to 16%, which was higher in forceps delivery than vacuum extraction (Mohamed‐Ahmed 2019). More trials in different settings where the practice is more widespread are needed particularly in low‐ and middle‐income settings.
In addition, there was insufficient information on the scale used to assess the perineal pain in the ANODE 2019 trial. The pain results were presented as a binary outcome (ANODE 2019), which could be a limitation. In the trial protocol (Knight 2018), the pain tools referred to were described in two studies (Dudley 2017; McCandlish 1998), which defined pain as none, mild, moderate and severe. It is therefore unclear how the binary pain outcomes were calculated.
Quality of the evidence
Overall, the included studies were at low risk of bias. The certainty of the evidence using GRADE was low‐ to high‐certainty evidence. The reasons for downgrading the certainty of the evidence were due to limitations in study design, imprecision and inconsistency. High‐certainty evidence was found for prophylactic antibiotics on reducing infected episiotomy/laceration (superficial and deep perineal wound infection and serious infectious complications in operative vaginal deliveries). The certainty of evidence on infected episiotomy/laceration presenting with wound breakdown was downgraded due to high loss to follow‐up at six weeks postpartum. We downgraded two levels for very serious imprecision due to wide confidence intervals crossing the line of no effect and small number of events for infected episiotomy/laceration presenting with organ or space perineal wound infection and maternal adverse reactions. The certainty of evidence on endometritis was downgraded due to inconsistency and imprecision. We downgraded the certainty of evidence on maternal length of stay due to a small trial with unclear risk of bias (Heitmann 1989).
Potential biases in the review process
We conducted extensive search strategies with no language restriction and all potential studies were checked by two review authors to prevent the reporting biases. Independently blinding processes of study selection, assessing risk of biases, and data extraction using clearly‐defined criteria and checklists were performed to minimise potential biases.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews on antibiotic prophylaxis for operative vaginal delivery published. One additional randomised controlled trial on this issue has been conducted and was published in 2019 (ANODE 2019) and this is now included in this review.

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 1 Infected episiotomy/laceration (superficial perineal wound infection).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 2 Infected episiotomy/laceration (deep perineal wound infection).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 3 Infected episiotomy/laceration (organ or space infection).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 4 Infected episiotomy/laceration (wound breakdown).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 5 Endometritis.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 6 Serious infectious complications.
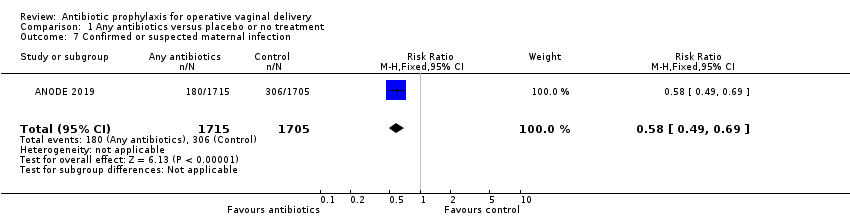
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 7 Confirmed or suspected maternal infection.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 8 Maternal adverse reactions.
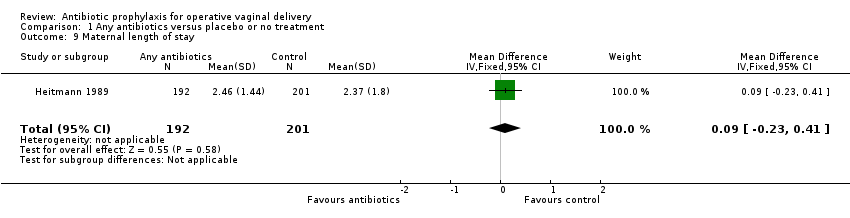
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 9 Maternal length of stay.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 10 Perineal pain.
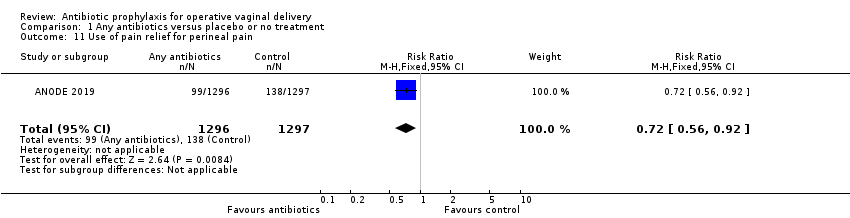
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 11 Use of pain relief for perineal pain.
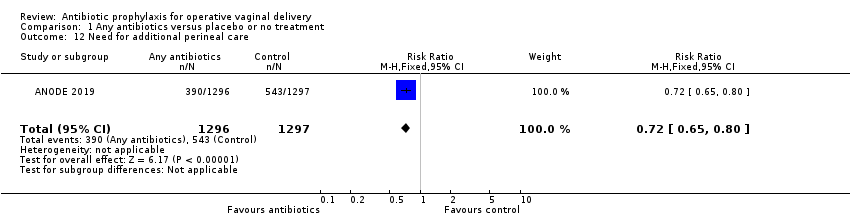
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 12 Need for additional perineal care.
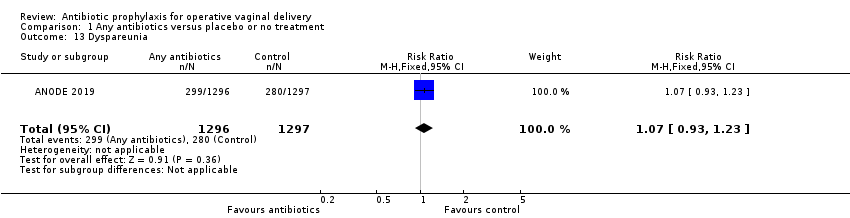
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 13 Dyspareunia.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 14 Breastfeeding at 6 weeks.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 15 Perineum "ever too painful or uncomfortable" to feed baby.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 16 Any primary care or home visits in relation to perineum.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 17 Any outpatient visits in relation to perineum.
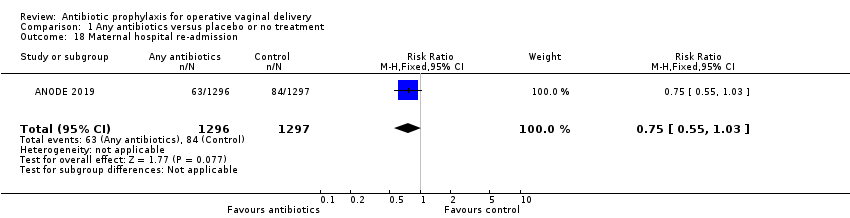
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 18 Maternal hospital re‐admission.
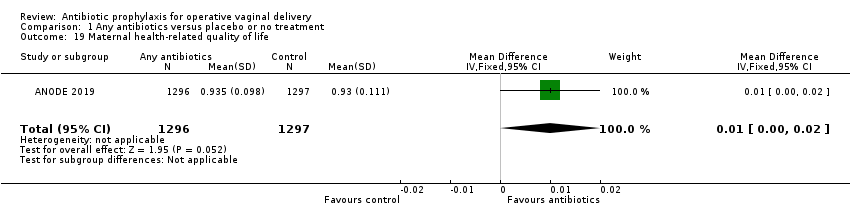
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 19 Maternal health‐related quality of life.
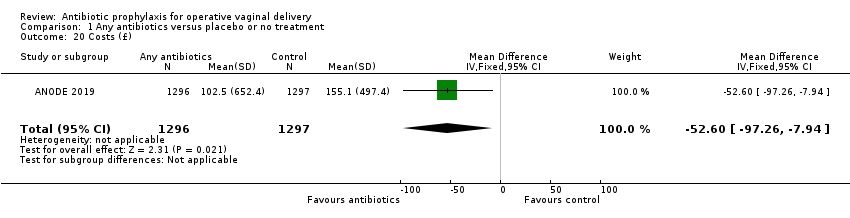
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 20 Costs (£).
| Any antibiotics compared to placebo or no treatment for operative vaginal delivery | ||||||
| Patient or population: operative vaginal delivery | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
| Without any antibiotics | With any antibiotics | Difference | ||||
| Fever ‐ not measured | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the 2 included studies. | |
| Infected episiotomy/laceration (superficial perineal wound infection) | RR 0.53 | Study population | ⊕⊕⊕⊕ | |||
| 8.3% | 4.4% | 3.9% fewer | ||||
| Infected episiotomy/laceration (deep perineal wound infection | RR 0.46 | Study population | ⊕⊕⊕⊕ | |||
| 4.5% | 2.1% | 2.4% fewer | ||||
| Infected episiotomy/laceration (organ or space infection) | RR 0.11 | Study population | ⊕⊕⊝⊝ | |||
| 0.2% | 0.0% | 0.2% fewer | ||||
| Infected episiotomy/laceration (wound breakdown) | RR 0.52 | Study population | ⊕⊕⊕⊝ | |||
| 21.0% | 10.9% | 10.1% fewer | ||||
| Endometritis | RR 0.32 | Study population | ⊕⊕⊝⊝ | |||
| 1.6% | 0.5% | 1.1% fewer | ||||
| Urinary tract infection ‐ not measured | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the 2 included studies. | |
| Serious infectious complications | RR 0.44 | Study population | ⊕⊕⊕⊕ | |||
| 1.5% | 0.6% | 0.8% fewer | ||||
| Maternal adverse reactions | RR 2.00 | Study population | ⊕⊕⊝⊝ | |||
| 0.1% | 0.2% | 0.1% more | ||||
| Maternal length of stay | ‐ | The mean maternal length of stay without any antibiotics was 2.37 days. | The mean maternal length of stay with antibiotics was 0.09 days more (0.23 days less to 0.41 days more) | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded (2) levels for very serious imprecision due to wide confidence interval crossing the line of no effect and small number of events. 2 We downgraded (1) level for serious limitation in study design due to loss to follow up for this outcome higher than 20%. 3 We downgraded (1) level for serious inconsistency due to unexplained substantial heterogeneity. 4 We downgraded (1) level for serious imprecision due to wide confidence interval. 5 We downgraded (1) level for serious limitations in study design due to many domains being at unclear risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infected episiotomy/laceration (superficial perineal wound infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.40, 0.69] |
| 2 Infected episiotomy/laceration (deep perineal wound infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.69] |
| 3 Infected episiotomy/laceration (organ or space infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
| 4 Infected episiotomy/laceration (wound breakdown) Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.43, 0.63] |
| 5 Endometritis Show forest plot | 2 | 3813 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.64] |
| 6 Serious infectious complications Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.22, 0.89] |
| 7 Confirmed or suspected maternal infection Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.49, 0.69] |
| 8 Maternal adverse reactions Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 22.05] |
| 9 Maternal length of stay Show forest plot | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |
| 10 Perineal pain Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.78, 0.91] |
| 11 Use of pain relief for perineal pain Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.56, 0.92] |
| 12 Need for additional perineal care Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.80] |
| 13 Dyspareunia Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.23] |
| 14 Breastfeeding at 6 weeks Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 15 Perineum "ever too painful or uncomfortable" to feed baby Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.84] |
| 16 Any primary care or home visits in relation to perineum Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.81] |
| 17 Any outpatient visits in relation to perineum Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.43, 0.70] |
| 18 Maternal hospital re‐admission Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.03] |
| 19 Maternal health‐related quality of life Show forest plot | 1 | 2593 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| 20 Costs (£) Show forest plot | 1 | 2593 | Mean Difference (IV, Fixed, 95% CI) | ‐52.60 [‐97.26, ‐7.94] |

