Profilaxis antibiótica para el parto vaginal instrumentado
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | A multi‐centre, randomised, blinded, controlled trial carried out at 27 hospital obstetric units in the UK. | |
| Participants | 3420 women undergoing all types of operative vaginal birth at 36 weeks or greater gestation. Women were excluded if they had any clinical indication for antibiotic administration after delivery, third‐degree or fourth‐degree perineal tears, receipt of antenatal or intrapartum antibiotics with ongoing antibiotics after delivery or a known allergy to penicillin or any of the components of amoxicillin and clavulanic acid, or who had a history of anaphylaxis to another β‐lactam agent. | |
| Interventions | A single dose of intravenous amoxicillin and clavulanic acid (1 g amoxicillin and 200 mg clavulanic acid) as soon as possible and no more than 6 hours after giving birth (n = 1715) or placebo group using 20 mL of intravenous sterile 0.9% saline within the same timeframe (n = 1705). | |
| Outcomes | Primary outcomes were a confirmed or suspected maternal infection within 6 weeks of delivery as defined as a new antibiotic prescription for a presumed perineal wound‐related infection, endometritis or uterine infection, urinary tract infection with systemic features (pyelonephritis or sepsis) or other systemic infection (clinical sepsis); confirmed systemic infection on culture; or endometritis required at least 1 of the following criteria to be met‐organisms were cultured from fluid (including amniotic fluid) or tissue from endometrium obtained during an invasive procedure or biopsy, or the woman exhibited at least 2 of fever (> 38 degrees Celsius), abdominal pain, uterine tenderness, or purulent drainage from uterus (with no other recognised cause for the latter 3 symptoms). Secondary outcomes assessed within 6 weeks of delivery were: systemic sepsis, perineal wound infection, perineal pain, use of pain relief, hospital bed stay until discharge, need for additional perineal care, dyspareunia, ability to sit comfortably to feed the baby, maternal health‐related quality of life, breastfeeding, wound breakdown, intervention side effects, healthcare resource use and costs, or adverse events. | |
| Notes | Dates study conducted: the trial was carried out between 13 March, 2016 and 13 June 2018. Funding sources for the study: NIHR Health Technology Assessment programme. Declarations of interest among primary researchers: 4 authors declared receipt of funding from NIHR outside the submitted work. All other authors have no competing interests to declare. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted blocks of variable size was used for random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially‐numbered, indistinguishable packs containing active drug or placebo as designated randomisation list performed by an independent trials programmer. |
| Blinding of participants and personnel (performance bias) | Low risk | Women and most clinicians including research midwives and those taking consent were masked to allocation either intervention or placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Midwives, nurses, or doctors collecting outcome information were masked to allocation either intervention or placebo. |
| Incomplete outcome data (attrition bias) | Low risk | Primary outcome data were complete. Secondary outcome data were incomplete with 24% loss to follow‐up; however, it was balanced between both groups. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as in the study protocol. |
| Other bias | Low risk | Study appeared to be free of other sources of bias. |
| Methods | Selected by randomisation table to receive treatment or no treatment; not blinded or placebo‐controlled. | |
| Participants | 393 women undergoing instrumental deliveries (either vacuum or forceps deliveries). Women were excluded if they had evidence of chorioamnionitis, or other infections, or if they were allergic to penicillin or cephalosporins. Setting: University Hospital of Jacksonville, USA; September 1986 to February 1988. | |
| Interventions | 2 g of cefotetan intravenously after cord clamping (n = 192) or no treatment (n = 201). | |
| Outcomes | Endomyometritis (at least 1 rise in oral temperature greater than 38.1 degrees Celsius after the first 24 hours of delivery and uterine tenderness or foul‐smelling lochia with no clinical or laboratory evidence confirming another source of the fever). | |
| Notes | Dates study conducted: the trial was carried out between September 1986 and February 1989. Funding sources for the study: the funding sources of an included study could not be identified. Declarations of interest among primary researchers: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation table was used for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment could not be interpreted. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not clearly mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Who measured the outcome was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | Same number of samples at intervention given and outcome measure. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, so there was insufficient information to permit judgement. |
| Other bias | Low risk | Study appeared to be free of other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Abstract only (translated). This randomised study included 200 women including, not only instrumental delivery but also women undergoing manual removal of placenta or uterine exploration, or both, premature rupture of the membranes of more than 6 hours and a labour of more than 8 hours. No details of the interventions were given for either the treatment or the control groups. The study outcomes of postpartum fever in both comparison groups were given but they were not described for subgroups; therefore, there were no data suitable for extraction. We could not find a published article. We have tried to contact the author but without success to date. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infected episiotomy/laceration (superficial perineal wound infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.40, 0.69] |
| Analysis 1.1  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 1 Infected episiotomy/laceration (superficial perineal wound infection). | ||||
| 2 Infected episiotomy/laceration (deep perineal wound infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.69] |
| Analysis 1.2  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 2 Infected episiotomy/laceration (deep perineal wound infection). | ||||
| 3 Infected episiotomy/laceration (organ or space infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
| Analysis 1.3  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 3 Infected episiotomy/laceration (organ or space infection). | ||||
| 4 Infected episiotomy/laceration (wound breakdown) Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.43, 0.63] |
| Analysis 1.4  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 4 Infected episiotomy/laceration (wound breakdown). | ||||
| 5 Endometritis Show forest plot | 2 | 3813 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.64] |
| Analysis 1.5  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 5 Endometritis. | ||||
| 6 Serious infectious complications Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.22, 0.89] |
| Analysis 1.6  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 6 Serious infectious complications. | ||||
| 7 Confirmed or suspected maternal infection Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.49, 0.69] |
| Analysis 1.7  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 7 Confirmed or suspected maternal infection. | ||||
| 8 Maternal adverse reactions Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 22.05] |
| Analysis 1.8  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 8 Maternal adverse reactions. | ||||
| 9 Maternal length of stay Show forest plot | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |
| Analysis 1.9  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 9 Maternal length of stay. | ||||
| 10 Perineal pain Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.78, 0.91] |
| Analysis 1.10  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 10 Perineal pain. | ||||
| 11 Use of pain relief for perineal pain Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.56, 0.92] |
| Analysis 1.11  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 11 Use of pain relief for perineal pain. | ||||
| 12 Need for additional perineal care Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.80] |
| Analysis 1.12  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 12 Need for additional perineal care. | ||||
| 13 Dyspareunia Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.23] |
| Analysis 1.13  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 13 Dyspareunia. | ||||
| 14 Breastfeeding at 6 weeks Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| Analysis 1.14  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 14 Breastfeeding at 6 weeks. | ||||
| 15 Perineum "ever too painful or uncomfortable" to feed baby Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.84] |
| Analysis 1.15  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 15 Perineum "ever too painful or uncomfortable" to feed baby. | ||||
| 16 Any primary care or home visits in relation to perineum Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.81] |
| Analysis 1.16  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 16 Any primary care or home visits in relation to perineum. | ||||
| 17 Any outpatient visits in relation to perineum Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.43, 0.70] |
| Analysis 1.17  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 17 Any outpatient visits in relation to perineum. | ||||
| 18 Maternal hospital re‐admission Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.03] |
| Analysis 1.18  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 18 Maternal hospital re‐admission. | ||||
| 19 Maternal health‐related quality of life Show forest plot | 1 | 2593 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| Analysis 1.19  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 19 Maternal health‐related quality of life. | ||||
| 20 Costs (£) Show forest plot | 1 | 2593 | Mean Difference (IV, Fixed, 95% CI) | ‐52.60 [‐97.26, ‐7.94] |
| Analysis 1.20  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 20 Costs (£). | ||||

Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 1 Infected episiotomy/laceration (superficial perineal wound infection).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 2 Infected episiotomy/laceration (deep perineal wound infection).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 3 Infected episiotomy/laceration (organ or space infection).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 4 Infected episiotomy/laceration (wound breakdown).

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 5 Endometritis.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 6 Serious infectious complications.
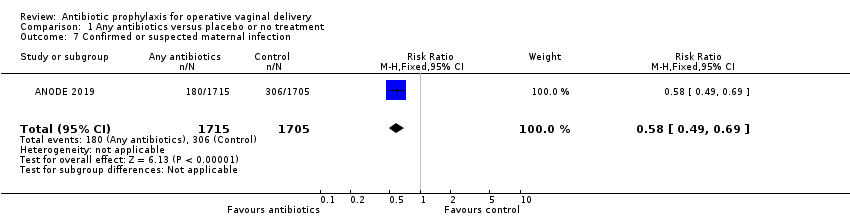
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 7 Confirmed or suspected maternal infection.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 8 Maternal adverse reactions.
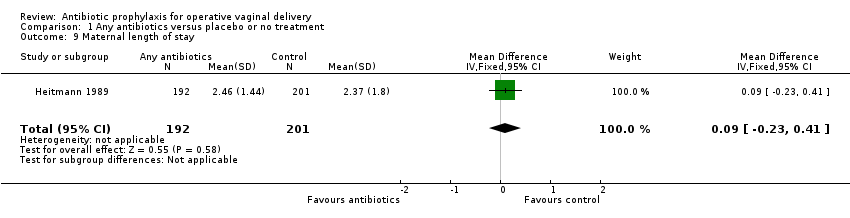
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 9 Maternal length of stay.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 10 Perineal pain.
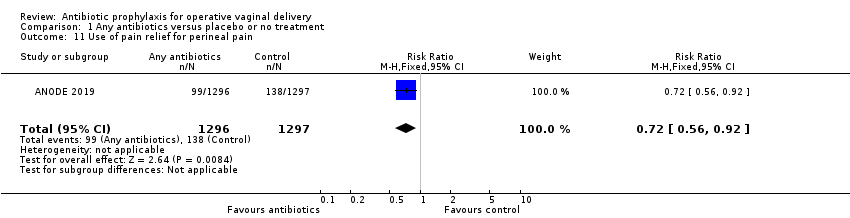
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 11 Use of pain relief for perineal pain.
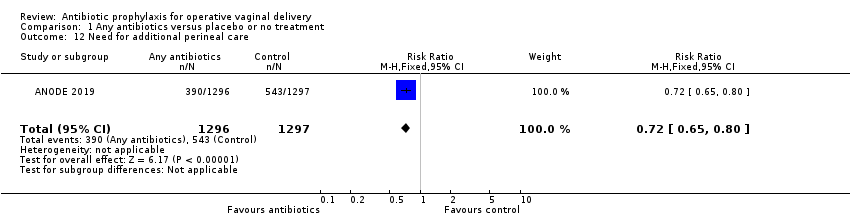
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 12 Need for additional perineal care.
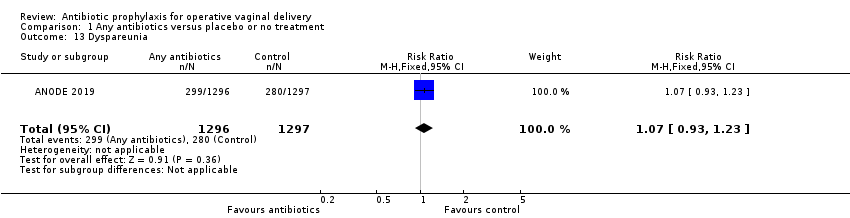
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 13 Dyspareunia.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 14 Breastfeeding at 6 weeks.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 15 Perineum "ever too painful or uncomfortable" to feed baby.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 16 Any primary care or home visits in relation to perineum.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 17 Any outpatient visits in relation to perineum.
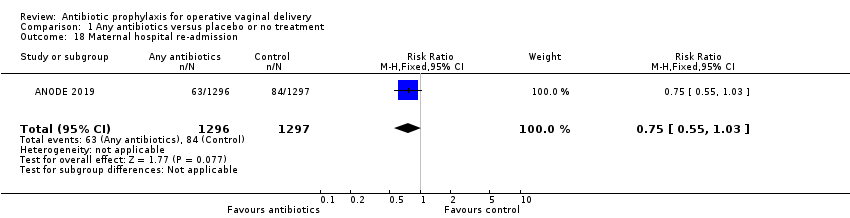
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 18 Maternal hospital re‐admission.
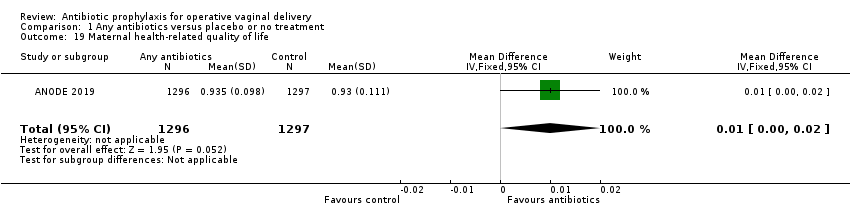
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 19 Maternal health‐related quality of life.
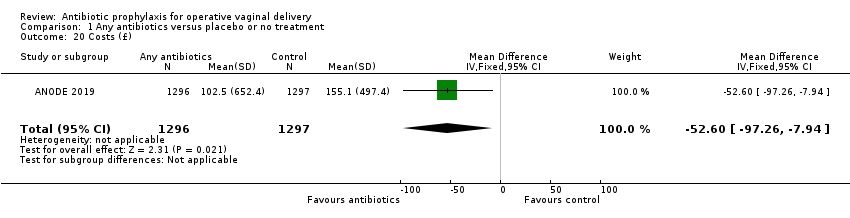
Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 20 Costs (£).
| Any antibiotics compared to placebo or no treatment for operative vaginal delivery | ||||||
| Patient or population: operative vaginal delivery | ||||||
| Outcomes | Relative effect | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
| Without any antibiotics | With any antibiotics | Difference | ||||
| Fever ‐ not measured | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the 2 included studies. | |
| Infected episiotomy/laceration (superficial perineal wound infection) | RR 0.53 | Study population | ⊕⊕⊕⊕ | |||
| 8.3% | 4.4% | 3.9% fewer | ||||
| Infected episiotomy/laceration (deep perineal wound infection | RR 0.46 | Study population | ⊕⊕⊕⊕ | |||
| 4.5% | 2.1% | 2.4% fewer | ||||
| Infected episiotomy/laceration (organ or space infection) | RR 0.11 | Study population | ⊕⊕⊝⊝ | |||
| 0.2% | 0.0% | 0.2% fewer | ||||
| Infected episiotomy/laceration (wound breakdown) | RR 0.52 | Study population | ⊕⊕⊕⊝ | |||
| 21.0% | 10.9% | 10.1% fewer | ||||
| Endometritis | RR 0.32 | Study population | ⊕⊕⊝⊝ | |||
| 1.6% | 0.5% | 1.1% fewer | ||||
| Urinary tract infection ‐ not measured | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the 2 included studies. | |
| Serious infectious complications | RR 0.44 | Study population | ⊕⊕⊕⊕ | |||
| 1.5% | 0.6% | 0.8% fewer | ||||
| Maternal adverse reactions | RR 2.00 | Study population | ⊕⊕⊝⊝ | |||
| 0.1% | 0.2% | 0.1% more | ||||
| Maternal length of stay | ‐ | The mean maternal length of stay without any antibiotics was 2.37 days. | The mean maternal length of stay with antibiotics was 0.09 days more (0.23 days less to 0.41 days more) | ⊕⊕⊝⊝ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 We downgraded (2) levels for very serious imprecision due to wide confidence interval crossing the line of no effect and small number of events. 2 We downgraded (1) level for serious limitation in study design due to loss to follow up for this outcome higher than 20%. 3 We downgraded (1) level for serious inconsistency due to unexplained substantial heterogeneity. 4 We downgraded (1) level for serious imprecision due to wide confidence interval. 5 We downgraded (1) level for serious limitations in study design due to many domains being at unclear risk of bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Infected episiotomy/laceration (superficial perineal wound infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.40, 0.69] |
| 2 Infected episiotomy/laceration (deep perineal wound infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.31, 0.69] |
| 3 Infected episiotomy/laceration (organ or space infection) Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.05] |
| 4 Infected episiotomy/laceration (wound breakdown) Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.43, 0.63] |
| 5 Endometritis Show forest plot | 2 | 3813 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.04, 2.64] |
| 6 Serious infectious complications Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.22, 0.89] |
| 7 Confirmed or suspected maternal infection Show forest plot | 1 | 3420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.49, 0.69] |
| 8 Maternal adverse reactions Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.18, 22.05] |
| 9 Maternal length of stay Show forest plot | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |
| 10 Perineal pain Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.78, 0.91] |
| 11 Use of pain relief for perineal pain Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.56, 0.92] |
| 12 Need for additional perineal care Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.80] |
| 13 Dyspareunia Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.23] |
| 14 Breastfeeding at 6 weeks Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 15 Perineum "ever too painful or uncomfortable" to feed baby Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.84] |
| 16 Any primary care or home visits in relation to perineum Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.65, 0.81] |
| 17 Any outpatient visits in relation to perineum Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.43, 0.70] |
| 18 Maternal hospital re‐admission Show forest plot | 1 | 2593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.03] |
| 19 Maternal health‐related quality of life Show forest plot | 1 | 2593 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.00, 0.02] |
| 20 Costs (£) Show forest plot | 1 | 2593 | Mean Difference (IV, Fixed, 95% CI) | ‐52.60 [‐97.26, ‐7.94] |

