Profilaktyka antybiotykowa w przypadku porodu drogą naturalną ukończonego w sposób zabiegowy
Abstract
Background
Vacuum and forceps assisted vaginal deliveries are reported to increase the incidence of postpartum infections and maternal readmission to hospital compared to spontaneous vaginal delivery. Prophylactic antibiotics may be prescribed to prevent these infections. However, the benefit of antibiotic prophylaxis for operative vaginal deliveries is still unclear.
Objectives
To assess the effectiveness and safety of antibiotic prophylaxis in reducing infectious puerperal morbidities in women undergoing operative vaginal deliveries including vacuum or forceps deliveries, or both.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (12 July 2017), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (12 July 2017) and reference lists of retrieved studies.
Selection criteria
All randomised trials comparing any prophylactic antibiotic regimens with placebo or no treatment in women undergoing vacuum or forceps deliveries were eligible. Participants were all pregnant women without evidence of infections or other indications for antibiotics of any gestational age undergoing vacuum or forceps delivery for any indications. Interventions were any antibiotic prophylaxis (any dosage regimen, any route of administration or at any time during delivery or the puerperium) compared with either placebo or no treatment.
Data collection and analysis
Two review authors assessed trial eligibility and methodological quality. Two review authors extracted the data independently using prepared data extraction forms. Any discrepancies were resolved by discussion and a consensus reached through discussion with all review authors. We assessed methodological quality of the one included trial using the GRADE approach.
Main results
One trial, involving 393 women undergoing either vacuum or forceps deliveries, was included. The trial compared the antibiotic intravenous cefotetan after cord clamping compared with no treatment. This trial reported only two out of the nine outcomes specified in this review. Seven women in the group given no antibiotics had endomyometritis and none in prophylactic antibiotic group, the risk reduction was 93% (risk ratio (RR) 0.07; 95% confidence interval (CI) 0.00 to 1.21; low‐quality evidence). There was no difference in the length of hospital stay between the two groups (mean difference (MD) 0.09 days; 95% CI ‐0.23 to 0.41; low‐quality evidence). Overall, the risk of bias was judged to be unclear. The quality of the evidence using GRADE was low for both endometritis and maternal length of stay.
Authors' conclusions
One small trial was identified reporting only two outcomes. Evidence from this single trial suggests that antibiotic prophylaxis may lead to little or no difference in endometritis or maternal length of stay. There were no data on any other outcomes to evaluate the impact of antibiotic prophylaxis after operative vaginal delivery. Future research on antibiotic prophylaxis for operative vaginal delivery is needed to conclude whether it is useful for reducing postpartum morbidity.
PICO
Streszczenie prostym językiem
Czy profilaktyka antybiotykowa jest skuteczna lub bezpieczna u kobiet, u których poród drogą naturalną został ukończony w sposób zabiegowy?
Na czym polega problem?
Chcieliśmy ocenić, czy podawanie antybiotyków wszystkim kobietom, u których poród drogą naturalną zakończył się w sposób zabiegowy (z zastosowaniem kleszczy lub próżnociągu) zapobiega zakażeniom u matki bez zwiększania ryzyka wystąpienia innych ubocznych skutków zarówno u matki jak i dziecka.
Dlaczego problem jest istotny?
Kobiety, u których podczas porodu drogą naturalną zastosowano kleszcze lub próżnociąg, mogą być narażone na większe ryzyko zakażenia po porodzie lub ponownej hospitalizacji w porównaniu z kobietami, które rodziły drogą naturalną spontanicznie. Główne wskazania do porodu zabiegowego to przedłużony drugi okres porodu, podejrzenie wystąpienia problemów z dzieckiem, chęć skrócenia drugiego etapu porodu ze względu na matkę.
Profilaktyczna antybiotykoterapia może być zastosowana w celu zapobiegania lub zmniejszenia ryzyka zakażeń. Istnieją jednak wątpliwości co do korzyści z profilaktycznego stosowania antybiotyków w celu redukcji zakażeń po porodzie zabiegowym.
Jakie dane naukowe odnaleźliśmy?
Poszukiwaliśmy danych naukowych dostępnych w lipcu 2017 roku i znaleźliśmy tylko jedno badanie opublikowane w 1989 roku. Badanie obejmowało 393 kobiety, u których podczas porodu zastosowano kleszcze lub próżnociąg i z których część otrzymała antybiotyk (cefotetan), a część nie miała takiego leczenia. Nie zaobserwowano różnic w zakresie wieku, liczbie wcześniejszych ciąż oraz innych istotnych cechach pomiędzy grupami. W badaniu oceniano jedynie dwa elementy: wystąpienie zakażenia macicy (zakażenie błony śluzowej macicy) i czas pobytu w szpitalu. Wykazano, że u siedmiu kobiet nie otrzymujących żadnych antybiotyków wystąpiło zakażenie macicy (śluzówki macicy). W grupie kobiet otrzymujących antybiotyki nie zaobserwowano zakażeń. Podawanie antybiotyków nie miało wpływu na czas pobytu matki w szpitalu w żadnej z grup. Jakość danych naukowych dla obydwu uzyskanych wyników oceniona jako niską: dane naukowe pochodziły z pojedynczego badania, które obejmowało bardzo małą liczbę kobiet i oceniało tylko dwa punkty końcowe.
Co to oznacza?
Dane naukowe pochodzące z pojedynczego badania sugerują, że profilaktyka antybiotykowa może skutkować niewielką lub żadną różnicą w ryzyku wystąpienia zapalenia śluzówki macicy oraz czasu hospitalizacji matki. Nie było żadnych innych danych dotyczących wyników, które oceniałyby efekty profilaktyki antybiotykowej po porodzie zabiegowym. Do oceny skuteczności interwencji konieczne są dalsze badania dotyczące stosowania profilaktyki antybiotykowej w przypadku porodu ukończonego w sposób zabiegowy.
Authors' conclusions
Summary of findings
| Any antibiotics versus placebo or no treatment for operative vaginal delivery | ||||||
| Population: women undergoing operative vaginal delivery Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antibiotics versus placebo or no treatment | |||||
| Endometritis | Study population | RR 0.07 | 393 | ⊕⊕⊝⊝ | ||
| 35 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 35 per 1000 | 2 per 1000 | |||||
| Maternal length of hospital stay (days) | The mean maternal length of stay in the intervention groups was | 393 | ⊕⊕⊝⊝ | |||
| Fever | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Infected episiotomy/perineal/vaginal laceration | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Urinary tract infection | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Serious infectious complications | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Maternal adverse reactions | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, few events and a small sample size. | ||||||
Background
Description of the condition
Operative vaginal delivery is the term used to describe delivery of the fetal head assisted by either vacuum extractor or forceps. The commonest indications for operative vaginal delivery are prolonged second stage of labour, suspicion of immediate or potential fetal compromise and shortening of the second stage of labour for maternal benefit (ACOG 2001). The rates of operative vaginal delivery reported variously by centres in different countries (Cammu 2011; Hanley 2010; Hehir 2013; Janni 2002; Kabiru 2001; Lawani 2014; Mola 2011; Walsh 2013) range from 2.1% in Papua NewGuinea (Mola 2011) to 19.2% of all births in Northern Belgium (Cammu 2011).
The risk of postpartum infection is increased after operative vaginal birth because of higher rates of vaginal lacerations, routine bladder catheterisation, multiple vaginal examinations, insertion of instruments into the vagina and contamination (Chaim 2000; Pranchev 1993). Instrumental deliveries require additional vaginal examinations, a known risk factor for endometritis and febrile morbidity (Chang 1992; Dare 1998). Insertion of instruments and contamination is also assumed to be one of the risks of postpartum infection because of difficulties in adhering to aseptic practices during delivery (Dare 1998).
Description of the intervention
Antibiotic prophylaxis is one of the methods used to reduce the risk of postpartum infections. It has been widely studied in obstetrics and has shown to be effective in reducing postoperative puerperal morbidity after caesarean section in a Cochrane review (Smail 2014). However, there are still some doubts about the benefit of prophylactic antibiotics in reducing postpartum infection after operative vaginal delivery.
How the intervention might work
The reported incidence of postpartum infection or endometritis after operative vaginal delivery varied from 3.5% to 16% (Hagadorn‐Freathy 1991; Heitmann 1989; Kabiru 2001; Williams 1991). In addition, the outcomes of readmission within 60 days after delivery and maternal sepsis have been reported as increased in incidence following the use of operative vaginal delivery in comparison with spontaneous vaginal delivery (Acosta 2014; Liu 2005). Postpartum infection not only affects the physical status of the mother and prolongs hospital stay after birth but also significantly impacts on the psychological well‐being of the mother (ACOG 2001).
The most common micro‐organisms in the genitourinary tract causing postpartum morbidities such as fever, endometritis, infected episiotomy/vaginal laceration or urinary tract infection are Enterococci, Streptococci, Staphylococci, Gram‐negative bacilli and anaerobes (Kok 2000; Stray‐Pedersen 1988). Group B streptococcus, Enterococcus, Gardnerella vaginalis, Staphylococcus aureus and anaerobe bacteria were usually recovered from the cervix and endometrium among febrile postpartum women (Eschenbach 1986).
Why it is important to do this review
Previous studies (Janisch 1979; Rechlin 1988) have indicated that prophylactic antibiotics may not be necessary due to the relatively low risk of infectious morbidity, and uncertain effect on puerperal fever. Aseptic precautions during operative vaginal delivery may be enough to prevent postpartum infection (Janisch 1979). In contrast, other studies did suggest that antibiotic prophylaxis might reduce the risk of infection after normal vaginal delivery and operative vaginal delivery (Fernandez 1993; Heitmann 1989). Criscuolo 1990 suggested that the cost of prophylactic antibiotics could be much lower compared with the cost of treating the complications of infection related to procedures during delivery.
However, widespread use of antibiotics may contribute to the development of antibiotic‐resistant bacteria (Towers 1998; Weinstein 1996). A study in Vietnam found that 98% of women who gave birth vaginally received antibiotics (Ngoc 2005).
In addition, antibiotics may contaminate breast milk, as well as cause adverse reactions such as rash or antibiotic‐related diarrhoea (Dancer 2004).
There is also a concern that there may be a significantly increased risk of third or fourth degree tears, severe maternal morbidity and death, perinatal mortality and neonatal mortality in women with operative vaginal birth compared with normal birth (Angioli 2000; Lumbiganon 2010). The incidence of third‐ and fourth‐degree tears reported range from 1% to 36% of all births (Boucoiran 2010; Goldberg 2003; Johnson 2004; Nkwabong 2011; Panigrahy 2008; Prapas 2009). The rate of perineal wound complication measured at two weeks postpartum was found to be significantly lower with the use of antibiotic prophylaxis; however, loss to follow‐up was high and thus these results should be interpreted with caution (Buppasiri 2014; Duggal 2008).
Therefore, the effectiveness of antibiotic prophylaxis for operative vaginal delivery needs to be carefully evaluated.
Objectives
To assess the effectiveness and safety of antibiotic prophylaxis in reducing infectious puerperal morbidities in women undergoing operative vaginal deliveries including vacuum or forceps delivery, or both.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing any prophylactic antibiotic regimens with placebo or no treatment in women undergoing vacuum or forceps deliveries. Cluster‐trials were eligible for inclusion. Quasi‐randomised and cross‐over trials were not eligible for inclusion.
Types of participants
Pregnant women without evidence of infections or other indications for antibiotics of any gestational age undergoing vacuum or forceps deliveries for any indications.
Types of interventions
Any antibiotic prophylaxis (any dosage regimen, any route of administration or at any time during delivery or puerperium) compared with either placebo or no treatment.
Types of outcome measures
We considered the following clinical outcomes.
Primary outcomes
-
Fever (body temperature of 38 degrees celsius or higher) occurring on any two occasions in the first 10 days postpartum, exclusive of the first 24 hours
-
Infected episiotomy/perineal/vaginal laceration (oedematous, erythematous, wound edge with pain, serosanguineous or frankly purulent material or wound dehiscence)
-
Endometritis (fever and uterine tenderness or heavy bleeding)
-
Urinary tract infection (fever or dysuria and positive urine culture)
-
Serious infectious complications (such as bacteraemia, septic shock, septic thrombophlebitis, necrotising fasciitis or death attributed to infection)
Secondary outcomes
-
Maternal adverse reactions such as allergic reactions, anaphylaxis, antibiotic‐associated diarrhoea
-
Maternal length of stay
-
Costs
-
Neonatal adverse reactions such as such as jaundice, early neonatal infection, or any infant outcomes reported
Search methods for identification of studies
The following methods section of this review was based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (12 July 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (12 July 2017) using the search terms given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeLiabsuetrakul 2014b.
For this update, we planned to use the following methods. One new ongoing study was identified (ANODE 2015) and we will review it in the next update.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for the one included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for the included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for the one included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered in advance that studies would be at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for the included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for the included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for the included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for the one included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether the included study was at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, if more studies are included, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison between any antibiotic prophylaxis (any dosage regimen, any route of administration or at any time during delivery or puerperium) compared with either placebo or no treatment.
-
Fever (body temperature of 38 degrees celsius or higher) occurring on any two occasions in the first 10 days postpartum, exclusive of the first 24 hours
-
Infected episiotomy/perineal/vaginal laceration (oedematous, erythematous, wound edge with pain, serosanguineous or frankly purulent material or wound dehiscence)
-
Endometritis (fever and uterine tenderness or heavy bleeding)
-
Urinary tract infection (fever or dysuria and positive urine culture)
-
Serious infectious complications (such as bacteraemia, septic shock, septic thrombophlebitis, necrotising fasciitis or death attributed to infection)
-
Maternal adverse reactions such as allergic reactions, anaphylaxis, antibiotic‐associated diarrhoea
-
Maternal length of stay
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We planned to use the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods. As only one trial, was included, we used the mean difference.
Unit of analysis issues
Cluster‐randomised trials
In future updates, if cluster‐randomised trials are identified and included, we will adjust the sample errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC.
If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Not applicable.
Other unit of analysis issues
Not applicable.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in the included study was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
There was only one included study and so it was not necessary to assess heterogeneity. In future updates, we will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either a Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identify substantial heterogeneity (above 30%), we will explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). Only one study was included. In future updates, if more trials become available for inclusion, we will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar.
If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Only one trial was included and so it was not necessary to carry out subgroup analysis. In future updates if more studies are included and we identify substantial heterogeneity, we plan to investigate it using subgroup analyses and we will consider whether an overall summary is meaningful, and if it is, we will use a random‐effects analysis to produce it.
In future updates, we will carry out the following subgroup analyses for primary outcomes.
-
Vacuum or forceps deliveries
-
Different antibiotic regimens
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. As only one study was included, we were unable to carry out the planned sensitivity analysis.
Results
Description of studies
Results of the search
See: Figure 1.
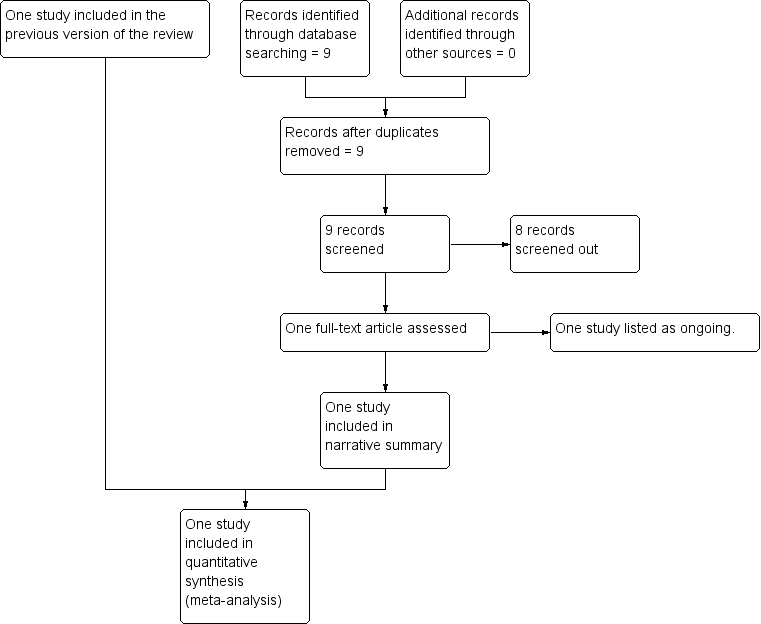
Study flow diagram.
The original search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved three trial reports, one trial was included and one trial (two reports) was excluded. An updated search in July 2017 retrieved no further reports from the Pregnancy and Childbirth Group's Trials Register and nine reports from ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP). Eight of these were screened out as not within the scope of the review and one study was added to Ongoing studies.
One ongoing trial ANODE 2015 met the inclusion criteria and was added to Ongoing studies. This trial was started in September 2009 and includes healthy women aged over 16 years having an operative vaginal delivery. The comparison is between a single intravenous dose of co‐amoxiclav (1 g amoxycillin/200 mg clavulanic acid in 20 mL water for injections for active drug) and placebo (20 mL 0.9% saline for placebo). The main outcome is confirmed or suspected maternal infection within six weeks of delivery. The trial is expected to finish in August 2017.
Included studies
For details of the one included trial, see the Characteristics of included studies table.
Heitmann 1989 met the inclusion criteria. The trial was carried out between September 1986 and February 1989, involving 393 women undergoing either vacuum or forceps delivery. The comparison was between 2 g of cefotetan (n = 192) versus no treatment (n = 201). The randomisation procedure and outcome assessment were not described in the trial report. Nevertheless, there were no differences between the two groups regarding age, parity, fourth‐degree laceration, length of first and second stage of labour, duration of ruptured membrane, number of documented vaginal examinations, total time in labour and delivery suite, type of monitoring used, type of anaesthesia or level of physician's expertise. The main outcome was endomyometritis. The funding sources of were not described and no declarations of interest among the primary researchers were reported.
Excluded studies
One trial was excluded (De Meeus 1991). The trial was only available as an abstract and there was insufficient information in order to assess fully for eligibility. We have contacted the authors for further information. For further details of the excluded study, see the Characteristics of excluded studies table.
Risk of bias in included studies
Details of the one included trial are in the Characteristics of included studies table. Each risk of bias domain in summarised in Figure 2 and Figure 3.
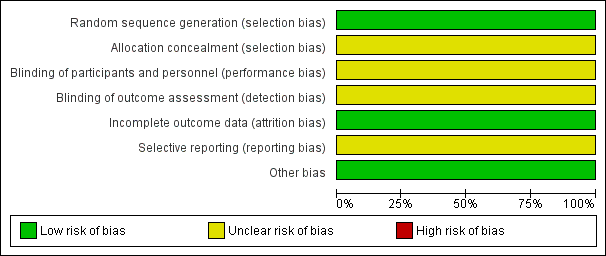
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Heitmann 1989 used a randomisation table to generate allocation sequence, but did not report concealment of randomisation. There were more vacuum deliveries than forceps deliveries but they were equally balanced between the two groups (vacuum rate was 57% in the cefotetan group and 59% in the no treatment group).
Blinding
There were no details of the blinding of intervention to either women or clinicians as well as the outcome measurements. It could be assumed that it was not blinded because the comparison was no treatment. However, the main outcome was objectively measured so it was unlikely to have been influenced by lack of blinding.
Incomplete outcome data
The number of samples given at intervention was the same as those at outcome measure; however, the intention‐to‐treat analysis was not clearly described.
Selective reporting
The study protocol was not available; therefore, there was insufficient information to permit judgement. There was no attempt to analyse the subgroups according to the type of delivery.
Other potential sources of bias
The study appeared to be free of other sources of bias.
Effects of interventions
One trial was included in the review comparing cefotetan given after cord clamping with no treatment group (Heitmann 1989). This trial recruited 393 women and reported only two out of the nine outcomes specified in this review (endometritis for primary outcome and maternal length of stay for secondary outcome).
There were no cases of endomyometritis in the cefotetan group compared with seven women (4%) in the no treatment group. The risk reduction was 93% (risk ratio (RR) 0.07; 95% confidence interval (CI) 0.00 to 1.21; low‐quality evidence) (Analysis 1.1). There was no difference in the length of hospital stay between the two groups in days (mean difference (MD) 0.09; 95% CI ‐0.23 to 0.41; low‐quality evidence) (Analysis 1.2). Both outcomes were assessed as being of low quality according to GRADE. The reasons for downgrading the quality of the evidence were because of a wide confidence interval crossing the line of no effect, few events and small sample size.
Discussion
Summary of main results
One trial, involving 393 women undergoing either vacuum or forceps deliveries, was included. The trial compared the antibiotic intravenous cefotetan after cord clamping compared with no treatment. This trial reported only two out of the nine outcomes specified in this review. Seven women in the group given no antibiotics had endomyometritis and none in prophylactic antibiotic group, the risk reduction was 93%. There was no difference in the length of hospital stay between the two groups.
Overall completeness and applicability of evidence
Only one small study reporting on only two outcomes with few events was identified for inclusion in this review included. This is a major limitation of this review. The results of this systematic review should therefore be interpreted with caution.
Quality of the evidence
Overall, the included study was at low risk of bias except for allocation concealment, blinding and selective reporting, the details for which were unclear. We could not assess fever, infected episiotomy/perineal/vaginal laceration, urinary tract infection, serious infectious complications and maternal adverse reactions because these outcomes were not reported in the trial. Therefore, we assessed endometritis and maternal length of stay in hospital. The quality of the evidence using GRADE was low for endometritis, and maternal length of stay in hospital. The reasons for downgrading the quality of the evidence were because of a wide confidence interval crossing the line of no effect, few events and small sample size.
Potential biases in the review process
We conducted an extensive search, but it remains possible that studies may have been missed. If we identify any such studies in future searches, we will assess them for potential inclusion in this review.
As the quality of the included trial is unclear, the potential for bias is present. Another significant source of potential bias is missing outcome data. Therefore study results should be interpreted with caution.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews on antibiotic prophylaxis for operative vaginal delivery published. No additional randomised controlled trials on this issue have been conducted. Although there was no evidence to support the use of antibiotic prophylaxis in operative vaginal delivery, it is widely used in clinical practice (Liabsuetrakul 2014a).
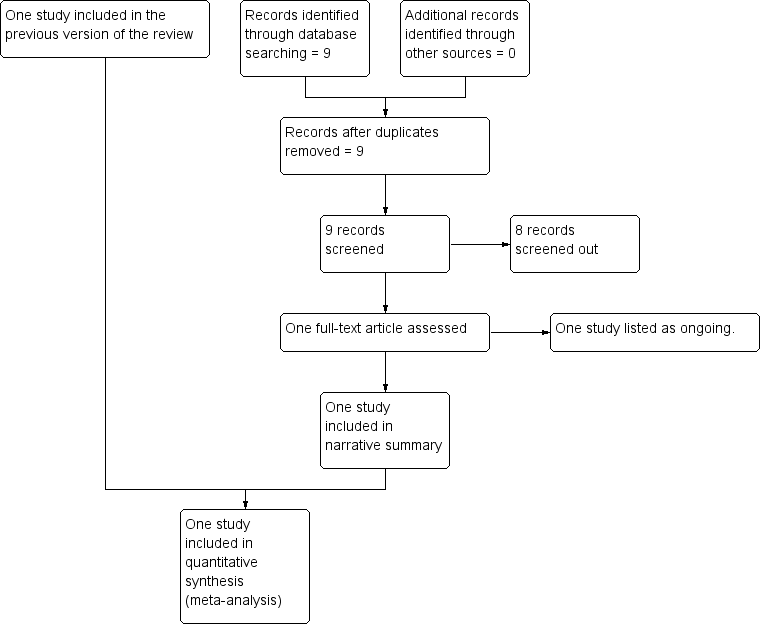
Study flow diagram.
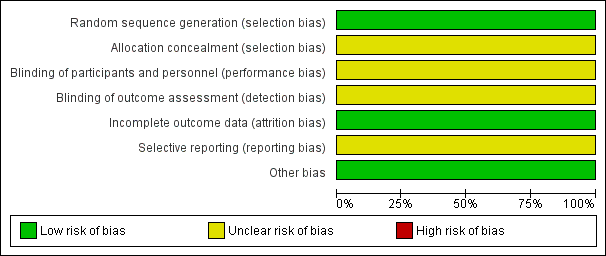
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
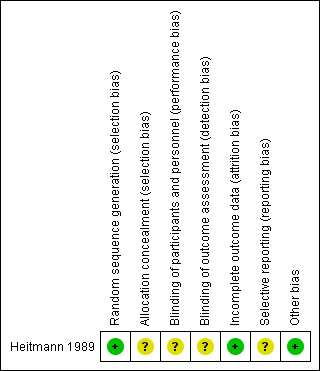
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 1 Endometritis.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 2 Maternal length of stay.
| Any antibiotics versus placebo or no treatment for operative vaginal delivery | ||||||
| Population: women undergoing operative vaginal delivery Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antibiotics versus placebo or no treatment | |||||
| Endometritis | Study population | RR 0.07 | 393 | ⊕⊕⊝⊝ | ||
| 35 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 35 per 1000 | 2 per 1000 | |||||
| Maternal length of hospital stay (days) | The mean maternal length of stay in the intervention groups was | 393 | ⊕⊕⊝⊝ | |||
| Fever | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Infected episiotomy/perineal/vaginal laceration | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Urinary tract infection | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Serious infectious complications | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Maternal adverse reactions | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, few events and a small sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometritis Show forest plot | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.21] |
| 2 Maternal length of stay Show forest plot | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |

