Profilaktyka antybiotykowa w przypadku porodu drogą naturalną ukończonego w sposób zabiegowy
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Selected by randomisation table to receive treatment or no treatment; not blinded or placebo‐controlled. | |
| Participants | 393 women undergoing instrumental deliveries (either vacuum or forceps deliveries). | |
| Interventions | 2 g of cefotetan intravenously after cord clamping (n = 192) or no treatment (n = 201). | |
| Outcomes | Endomyometritis (at least 1 rise in oral temperature greater than 38.1 degrees Celsius after the first 24 hours of delivery and uterine tenderness or foul‐smelling lochia with no clinical or laboratory evidence confirming another source of the fever). | |
| Notes | Dates study conducted: the trial was carried out between September 1986 and February 1989. Funding sources for the study: the funding sources of an included study could not be identified. Declarations of interest among primary researchers: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation table was used for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment could not be interpreted. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Information not clearly mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Who measured the outcome was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | Same number of samples at intervention given and outcome measure. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, so there was insufficient information to permit judgement. |
| Other bias | Low risk | Study appeared to be free of other sources of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Abstract only (translated). This randomised study included 200 women including, not only instrumental delivery but also women undergoing manual removal of placenta or uterine exploration, or both, premature rupture of the membranes of more than 6 hours and a labour of more than 8 hours. No details of the interventions were given for either the treatment or the control groups. The study outcomes of postpartum fever in both comparison groups were given but they were not described for subgroups; therefore, there were no data suitable for extraction. We could not find a published article. We have tried to contact the author but without success to date. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | ANODE: Prophylactic antibiotics for the prevention of infection following operative delivery. |
| Methods | Participants are randomly allocated into two groups. |
| Participants | Healthy women aged 16 years and over who have had an operative vaginal delivery. |
| Interventions | Co‐amoxiclav versus placebo, A single intravenous dose (1 g amoxycillin/200 mg clavulanic acid in 20 mL water for injections for active drug, 20 mL 0.9% saline for placebo). |
| Outcomes | Confirmed or suspected maternal infection within 6 weeks of delivery. |
| Starting date | September 2009. |
| Contact information | Mrs Shan Gray. NPEU Clinical Trials Unit, Oxford (UK) |
| Notes | Expected to finish in August 2017. EudraCT number: 2015‐000872‐89. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometritis Show forest plot | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.21] |
| Analysis 1.1  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 1 Endometritis. | ||||
| 2 Maternal length of stay Show forest plot | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |
| Analysis 1.2  Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 2 Maternal length of stay. | ||||
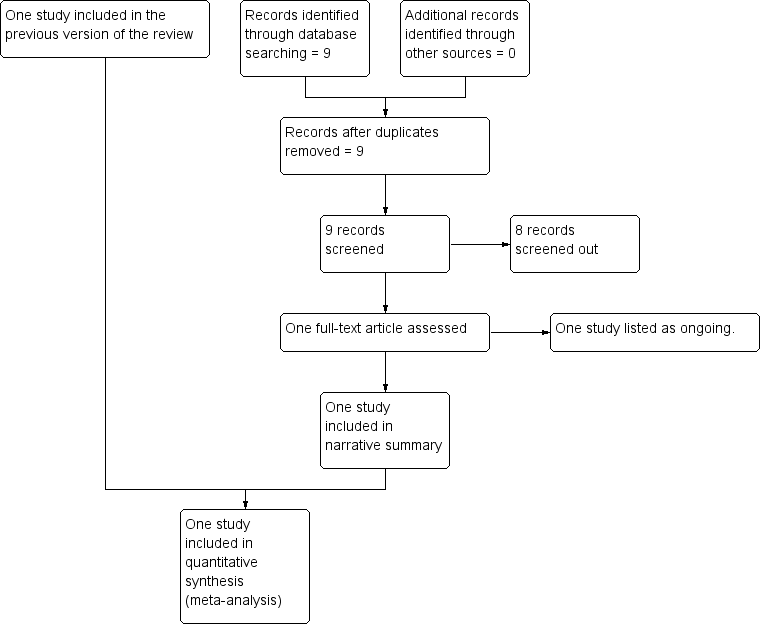
Study flow diagram.
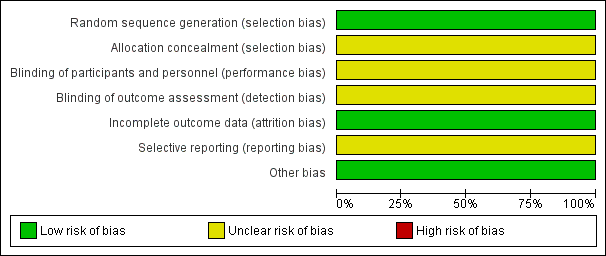
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
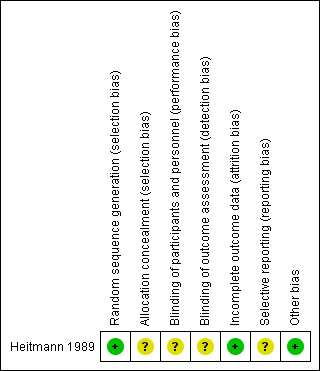
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 1 Endometritis.

Comparison 1 Any antibiotics versus placebo or no treatment, Outcome 2 Maternal length of stay.
| Any antibiotics versus placebo or no treatment for operative vaginal delivery | ||||||
| Population: women undergoing operative vaginal delivery Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any antibiotics versus placebo or no treatment | |||||
| Endometritis | Study population | RR 0.07 | 393 | ⊕⊕⊝⊝ | ||
| 35 per 1000 | 2 per 1000 | |||||
| Moderate | ||||||
| 35 per 1000 | 2 per 1000 | |||||
| Maternal length of hospital stay (days) | The mean maternal length of stay in the intervention groups was | 393 | ⊕⊕⊝⊝ | |||
| Fever | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Infected episiotomy/perineal/vaginal laceration | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Urinary tract infection | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Serious infectious complications | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| Maternal adverse reactions | Not estimable | 0 (no study) | See comment | This outcome was not reported in the one included study. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Wide confidence interval crossing the line of no effect, few events and a small sample size. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometritis Show forest plot | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.21] |
| 2 Maternal length of stay Show forest plot | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.41] |

