Endothelin receptor antagonists for pulmonary arterial hypertension
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multi‐centre randomised placebo parallel double blind trial.Randomisation conducted centrally according to a computer‐generated random number table.Allocation concealment adequate.Blinding of treater, participants and assessors were all blinded.Dropout and withdraw were described. Intention to treat analysis was conducted. Study duration (12 weeks). | |
| Participants | Total n = 178 (60 in placebo group; 118 in sitaxsentan group) enrolled. Total 166 completed (55 in placebo group; 111 in sitaxsentan group). Women (47 in placebo group; 94 in sitaxsentan group)Men (13 in placebo group; 17 in sitaxsentan group)Age (48+/14 in placebo group; 45+/‐14 in sitaxsentan 100 mg once daily group; 44+/‐12 in 300 mg sitaxsentan daily group)PPH (37 in placebo group, 57 in sitaxsentan group) PAH secondary to collagen vascular disease (9 in placebo group; 33 in sitaxsentan group)PAH secondary to congenital heart disease (14 in placebo group; 28 in sitaxsentan group)NYHA functional class II (22 in placebo group, 37 in sitaxsentan group)NYHA functional class III (36 in placebo group; 81 in sitaxsentan group)NYHA functional class IV (2 in placebo group, 0 in sitaxsentan group) | |
| Interventions | Intervention group: 100 mg once daily or 300 mg once dailyControl group: placebo | |
| Outcomes | Primary outcome: Peak oxygen consumption (Peak O2) Sedentary outcomes:Exercise capacity (six minute walk distance)NYHA functional class (I for no symptoms on exertion, IV for symptoms at rest)Pulmonary artery pressure (PAP)Cardiac index (CI)Pulmonary vascular resistance (PVR) Cardiac index was cardiac output (L/min) divided by body surface area.Pulmonary vascular resistance was calculated by (mean pulmonary artery pressure [mm Hg]‐ pulmonary capillary wedge pressure [mm Hg])/cardiac output (L/min)*80. | |
| Notes | Data reported as mixed population, Specific data for PPH or PAH secondary to collagen vascular disease not available. Authors and Pharmaceutical company has been contacted, yet no detailed data obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | As above. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | As above. | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Multi‐centre randomised parallel trial comparing bosentan with placebo for 12 weeks. Randomisation conducted by computer using the Almedica Drug Labelling System. Allocation concealment adequate.Participants, treaters and outcome assessors were blinded.Dropout and withdraw were described. Intention to treat analysis was conducted. Study duration (12 weeks). | |
| Participants | Total n = 32 enrolled (30 completed protocol; 21 in bosentan group; 11 in placebo group). Men (4 in bosentan group, 0 in placebo group)Women (11 in placebo group, 17 in bosentan group)Age (47.4+/‐14.0 in placebo group; 52.2+/‐12.2 in bosentan group)All patients were in WHO functional class III. PPH (10 in placebo group, 17 in bosentan group).PAH secondary to scleroderma (1 in placebo group, 4 in bosentan group) | |
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg twice daily) unless drug related adverse events arose, or matching doses of placebo.Control group: placebo | |
| Outcomes | The primary outcome:Exercise capacity (six‐min walk test) at week of 12 and was measured the distance a patient could walk in 6 minutes.Secondary outcomes:Cardiopulmonary haemodynamics (pulmonary vascular resistance, cardiac index, mean pulmonary artery pressure, pulmonary capillary wedge pressure, and mean right atrial pressure)Cardiac index was cardiac output (L/min) divided by body surface area.Pulmonary vascular resistance was calculated by (mean pulmonary artery pressure [mm Hg]‐ pulmonary capillary wedge pressure [mm Hg])/cardiac output (L/min)*80.Borg dyspnoea index (0 for nothing at all; 10 for most difficult)WHO functional class (I for no symptoms on exertion, IV for symptoms at rest) | |
| Notes | All patients (n = 32) were entered into the meta‐analysis. Specific data for PPH or PAH secondary to scleroderma were not available.The article just provided mean and standard error; and we converted standard error into standard deviation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Multi‐centre randomised placebo parallel triple arms trial comparing bosentan with placebo.Randomisation method not clear. Allocation concealment not clear. Blinding of participants, treaters and outcome assessors not clear. Dropout and withdraw were described. Intention to treat was performed.Study duration (16 weeks). | |
| Participants | Total n = 85 enrolled (81 completed study; 26 in placebo group; 55 in bosentan group). Men (5 in placebo group; 8 in bosentan group)Women (24 in placebo group; 48 in bosentan group)Age (44.9 +/‐19.2 in placebo group; 45.1 +/‐ 16.2 in bosentan group)PAH secondary to scleroderma (4 in placebo group; 7 in bosentan group)Other (2 in placebo group; 1 in bosentan group)WHO functional class III (28 in placebo group; 49 in bosentan group)WHO functional class IV (1 in placebo group; 7 in bosentan group) | |
| Interventions | Same as study carried out by Rubin 2002. | |
| Outcomes | Cardiac index | |
| Notes | Data are from Rubin 2002. The study reports data from 13/27 study centres who volunteered to participate in an echocardiography sub‐study. The article provided mean and standard error only; we converted standard error into standard deviation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | Multi‐centre randomised placebo parallel triple arms trial comparing bosentan with placebo.Randomisation method not clear. Allocation concealment not clear. Blinding of participants, treaters and outcome assessors not clear. Dropout and withdraw were described. Intention to treat was performed.Study duration (16 weeks). | |
| Participants | Total n = 213 (69 in placebo group; 144 in bosentan group)Man (15 in placebo group, 30 in bosentan group)Women (54 in placebo group; 114 in bosentan group)Age (47.2+/‐16.2 in placebo group; 48.7+/‐15.8 in bosentan group)PPH (48 in placebo group; 102 in bosentan group)PAH secondary to collagen vascular disease (21 in placebo group; 42 in bosentan group)WHO functional class III (65 in placebo group; 130 in bosentan group) WHO functional class IV (4 in placebo group; 14 in bosentan group) | |
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg or 250 mg twice daily) for 12 weeks.Control group: placebo | |
| Outcomes | Primary outcomes:Exercise capacity by week of 16 measured by six‐min walk testThe secondary outcome: Borg dyspnoea index;WHO functional class | |
| Notes | The article just provided mean and standard error; and we converted standard error into standard deviation. All patients (n = 213) were entered into the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a RCT. | |
| Not a RCT. | |
| Non‐randomised assessment of the additive effects of ERAs to prostacyclin | |
| Trial participants had heart failure; not a RCT. | |
| Trial participants had heart failure. | |
| Not a RCT. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Six minute walk Show forest plot | 2 | Metres (Fixed, 95% CI) | 46.24 [23.96, 68.53] | |
| Analysis 1.1  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 1 Six minute walk. | ||||
| 2 Number of patients who improved their classification (WHO or NYHA functional class) Show forest plot | 3 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| Analysis 1.2  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 2 Number of patients who improved their classification (WHO or NYHA functional class). | ||||
| 2.1 Nonselective ERAs | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.02, 2.26] |
| 2.2 Selective ERAs | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.02, 3.84] |
| 3 Symptoms Show forest plot | 2 | Borg dyspnoea score (Random, 95% CI) | ‐0.83 [‐1.65, ‐0.01] | |
| Analysis 1.3  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 3 Symptoms. | ||||
| 4 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 4 Mortality. | ||||
| 5 Pulmonary artery pressure (change from baseline) Show forest plot | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐4.36 [‐6.77, ‐1.94] |
| Analysis 1.5  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 5 Pulmonary artery pressure (change from baseline). | ||||
| 5.1 Nonselective ERAs | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐12.67, ‐0.73] |
| 5.2 Selective ERAs | 2 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐6.54, ‐1.25] |
| 6 Pulmonary vascular resistance (change from baseline) Show forest plot | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐286.73 [‐369.10, ‐204.36] |
| Analysis 1.6  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 6 Pulmonary vascular resistance (change from baseline). | ||||
| 6.1 Nonselective ERAs | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐414.0 [‐595.77, ‐232.23] |
| 6.2 Selective ERAs | 2 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐253.84 [‐346.24, ‐161.44] |
| 7 Cardiac Index (change from baseline) Show forest plot | 4 | 289 | Mean Difference (IV, Random, 95% CI) | 0.51 [0.22, 0.81] |
| Analysis 1.7  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 7 Cardiac Index (change from baseline). | ||||
| 7.1 Nonselective ERAs | 2 | 111 | Mean Difference (IV, Random, 95% CI) | 0.68 [0.06, 1.30] |
| 7.2 Selective ERAs | 2 | 178 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.18, 0.52] |
| 8 Hepatic toxicity Show forest plot | 3 | 422 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [0.57, 4.55] |
| Analysis 1.8  Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 8 Hepatic toxicity. | ||||
| 8.1 Nonselective ERAs | 2 | 245 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.22, 9.71] |
| 8.2 Selective ERAs | 1 | 177 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.30, 7.81] |

Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 1 Six minute walk.

Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 2 Number of patients who improved their classification (WHO or NYHA functional class).
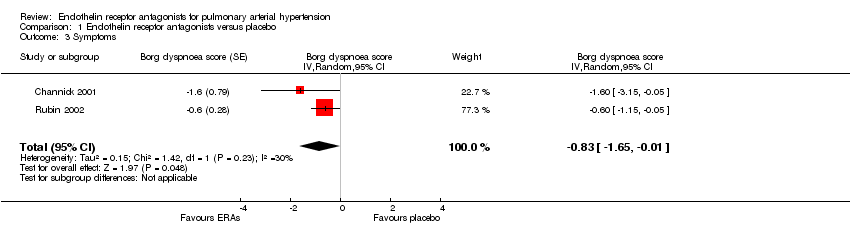
Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 3 Symptoms.

Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 4 Mortality.
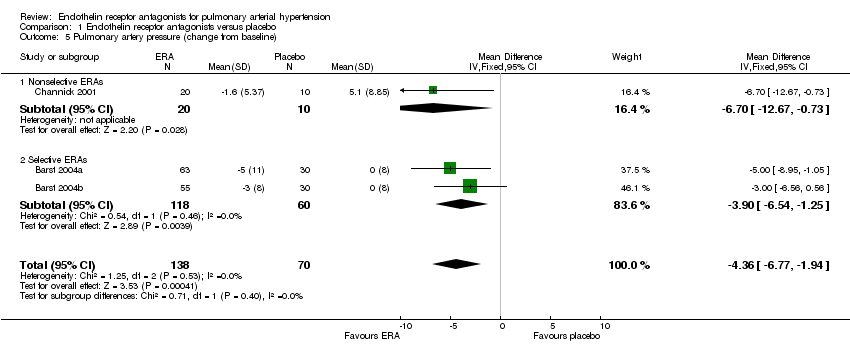
Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 5 Pulmonary artery pressure (change from baseline).
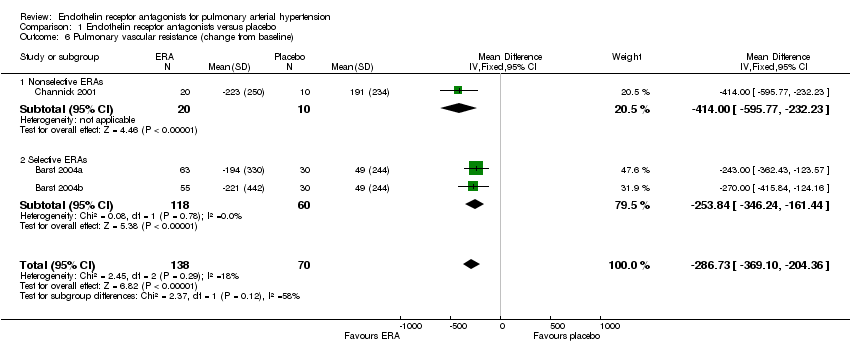
Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 6 Pulmonary vascular resistance (change from baseline).
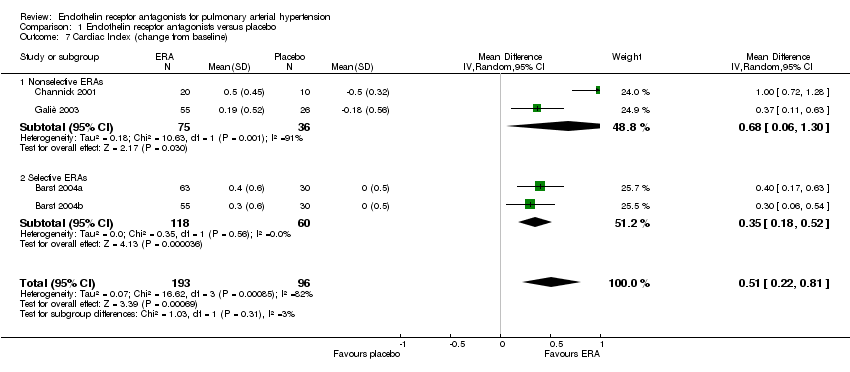
Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 7 Cardiac Index (change from baseline).

Comparison 1 Endothelin receptor antagonists versus placebo, Outcome 8 Hepatic toxicity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Six minute walk Show forest plot | 2 | Metres (Fixed, 95% CI) | 46.24 [23.96, 68.53] | |
| 2 Number of patients who improved their classification (WHO or NYHA functional class) Show forest plot | 3 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 2.1 Nonselective ERAs | 2 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.02, 2.26] |
| 2.2 Selective ERAs | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.02, 3.84] |
| 3 Symptoms Show forest plot | 2 | Borg dyspnoea score (Random, 95% CI) | ‐0.83 [‐1.65, ‐0.01] | |
| 4 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Pulmonary artery pressure (change from baseline) Show forest plot | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐4.36 [‐6.77, ‐1.94] |
| 5.1 Nonselective ERAs | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐12.67, ‐0.73] |
| 5.2 Selective ERAs | 2 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐6.54, ‐1.25] |
| 6 Pulmonary vascular resistance (change from baseline) Show forest plot | 3 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐286.73 [‐369.10, ‐204.36] |
| 6.1 Nonselective ERAs | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐414.0 [‐595.77, ‐232.23] |
| 6.2 Selective ERAs | 2 | 178 | Mean Difference (IV, Fixed, 95% CI) | ‐253.84 [‐346.24, ‐161.44] |
| 7 Cardiac Index (change from baseline) Show forest plot | 4 | 289 | Mean Difference (IV, Random, 95% CI) | 0.51 [0.22, 0.81] |
| 7.1 Nonselective ERAs | 2 | 111 | Mean Difference (IV, Random, 95% CI) | 0.68 [0.06, 1.30] |
| 7.2 Selective ERAs | 2 | 178 | Mean Difference (IV, Random, 95% CI) | 0.35 [0.18, 0.52] |
| 8 Hepatic toxicity Show forest plot | 3 | 422 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [0.57, 4.55] |
| 8.1 Nonselective ERAs | 2 | 245 | Odds Ratio (M‐H, Random, 95% CI) | 1.45 [0.22, 9.71] |
| 8.2 Selective ERAs | 1 | 177 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.30, 7.81] |

