Endothelin receptor antagonists for pulmonary arterial hypertension
Abstract
Background
Pulmonary arterial hypertension is a devastating disease that leads to right heart failure and premature death. Endothelin receptor antagonists have shown efficacy in the treatment of pulmonary arterial hypertension.
Objectives
To evaluate the efficacy of endothelin receptor antagonists (ERAs) in pulmonary arterial hypertension.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and the reference sections of retrieved articles. The searches are current as of 4 November 2020.
Selection criteria
We included randomised trials and quasi‐randomised trials involving participants with pulmonary arterial hypertension.
Data collection and analysis
Two of five review authors selected studies, extracted data and assessed study quality according to established criteria. We used standard methods expected by Cochrane. The primary outcomes were exercise capacity (six‐minute walk distance, 6MWD), World Health Organization (WHO) or New York Heart Association (NYHA) functional class, Borg dyspnoea scores and dyspnoea‐fatigue ratings, and mortality.
Main results
We included 17 randomised controlled trials involving a total of 3322 participants. Most trials were of relatively short duration (12 weeks to six months). Sixteen trials were placebo‐controlled, and of these nine investigated a non‐selective ERA and seven a selective ERA.
We evaluated two comparisons in the review: ERA versus placebo and ERA versus phosphodiesterase type 5 (PDE5) inhibitor. The abstract focuses on the placebo‐controlled trials only and presents the pooled results of selective and non‐selective ERAs.
After treatment, participants receiving ERAs could probably walk on average 25.06 m (95% confidence interval (CI) 17.13 to 32.99 m; 2739 participants; 14 studies; I2 = 34%, moderate‐certainty evidence) further than those receiving placebo in a 6MWD. Endothelin receptor antagonists probably improved more participants' WHO functional class (odds ratio (OR) 1.41, 95% CI 1.16 to 1.70; participants = 3060; studies = 15; I2 = 5%, moderate‐certainty evidence) and probably lowered the odds of functional class deterioration (OR 0.43, 95% CI 0.26 to 0.72; participants = 2347; studies = 13; I2 = 40%, moderate‐certainty evidence) compared with placebo. There may be a reduction in mortality with ERAs (OR 0.78, 95% CI 0.58, 1.07; 2889 participants; 12 studies; I2 = 0%, low‐certainty evidence), and pooled data suggest that ERAs probably improve cardiopulmonary haemodynamics and may reduce Borg dyspnoea score in symptomatic patients. Hepatic toxicity was not common, but may be increased by ERA treatment from 37 to 67 (95% CI 34 to 130) per 1000 over 25 weeks of treatment (OR 1.88, 95% CI 0.91 to 3.90; moderate‐certainty evidence). Although ERAs were well tolerated in this population, several cases of irreversible liver failure caused by sitaxsentan have been reported, which led the licence holder for sitaxsentan to withdraw the product from all markets worldwide.
As planned, we performed subgroup analyses comparing selective and non‐selective ERAs, and with the exception of mean pulmonary artery pressure, did not detect any clear subgroup differences for any outcome.
Authors' conclusions
For people with pulmonary arterial hypertension with WHO functional class II and III, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, result in favourable changes in cardiopulmonary haemodynamic variables compared with placebo. However, they are less effective in reducing dyspnoea and mortality. The efficacy data were strongest in those with idiopathic pulmonary hypertension. The irreversible liver failure caused by sitaxsentan and its withdrawal from global markets emphasise the importance of hepatic monitoring in people treated with ERAs. The question of the effects of ERAs on pulmonary arterial hypertension has now likely been answered. The combined use of ERAs and phosphodiesterase inhibitors may provide more benefit in pulmonary arterial hypertension; however, this needs to be confirmed in future studies.
PICO
Plain language summary
Endothelin receptor antagonists for pulmonary arterial hypertension
Review question
Do endothelin receptor antagonists increase how much a person is capable of exercising (exercise capacity), improve symptoms, or reduce death in people with pulmonary arterial hypertension (PAH)?
Background
Pulmonary arterial hypertension is a devastating disease characterised by an increase in pulmonary vascular resistance which leads to right heart failure and ultimately death.
Endothelin receptor antagonists are a class of strong vasodilators (medications that open (dilate) blood vessels) capable of stopping the process of cell division, which could dilate and result in a favourable pulmonary arterial structural alteration.
Study characteristics
We reviewed the evidence from randomised studies (studies in which people are assigned to one of two or more treatment groups using a random method). After a thorough search and assessment of the medical literature, we identified 17 studies with a total of 3322 participants for inclusion in the review. A vast majority of the participants had PAH without known cause (idiopathic). The evidence is current to November 2020.
Key results
Endothelin receptor antagonists probably increase exercise capacity, improve World Health Organization functional class (a measurement of how severe a person's pulmonary hypertension symptoms are), and may improve death rates and symptoms in people with PAH; however they may also increase the risk of liver damage, although this was rare. The question of the effects of endothelin receptor antagonists on PAH has now likely been answered.
Certainty of the evidence
Overall, the evidence presented is of moderate certainty due to the high occurrence of missing data.
Authors' conclusions
Summary of findings
| Endothelin receptor antagonists compared to placebo for pulmonary arterial hypertension | ||||||
| Participant or population: pulmonary arterial hypertension | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with endothelin receptor antagonists | |||||
| Change from baseline in 6MWD (m) mean duration of study 16.3 weeks | The weighted mean change on control was −4.56 m. | MD 25.06 higher | ‐ | 2739 | ⊕⊕⊕⊝ | Higher is better for 6MWD. |
| Proportion of participants with improved functional class mean duration of study 16.8 weeks | 175 per 1000 | 230 per 1000 | OR 1.41 | 3060 | ⊕⊕⊕⊝ | Participants with high OR are more likely to achieve functional improvement. |
| Change from baseline in BDI mean duration of study 14.3 weeks | The weighted mean change on control was 0.25 higher. | MD 0.43 lower | ‐ | 788 | ⊕⊕⊝⊝Low2 | Symptoms are worse with higher score of BDI. |
| Mortality mean duration of study 30.2 weeks | 73 per 1000 | 58 per 1000 | OR 0.78 | 2889 | ⊕⊕⊝⊝ | Participants with lower OR are less likely to die. |
| Change from baseline in mean PAP (mmHg) mean duration of study 17.1 weeks | The weighted mean change on control was 0.53 higher. | MD 4.65 lower | ‐ | 729 | ⊕⊕⊕⊝ | Participants are worse with higher pulmonary artery pressure. |
| Change from baseline in PVR (dyn/s/cm5) mean duration of study 15.7 weeks | The weighted mean change on control was 63.55 higher. | MD 236.24 lower | ‐ | 586 | ⊕⊕⊕⊝ | Participants are worse with higher pulmonary vascular resistance. |
| Hepatic toxicity mean duration of study 25 weeks | 37 per 1000 | 67 per 1000 | OR 1.88 | 2250 | ⊕⊕⊕⊝ | Participants with higher OR are more likely to suffer hepatic toxicity. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Incomplete outcome data (attrition bias) due to missing data imbalanced between intervention and control groups in most of the included studies (−1 level). | ||||||
Background
Description of the condition
Pulmonary arterial hypertension (PAH) is a rare disease of the distal pulmonary arteries characterised by vasoconstriction, vascular proliferation, obstructive remodelling of the pulmonary vessel wall, inflammation, and thrombosis, which lead to a progressive increase in pulmonary vascular resistance, and, ultimately, right ventricular failure and death (Galiè 2015; Thenappan 2018). PAH includes the following subcategories (Simonneau 2019): 1. idiopathic PAH; 2. heritable PAH; 3 drug‐ and toxin‐induced PAH; 4. PAH associated with (4.1 connective tissue disease; 4.2 HIV infection; 4.3 portal hypertension; 4.4 congenital heart disease; 4.5 schistosomiasis); 5. PAH long‐term responders to calcium channel blockers; 6. PAH with overt features of venous/capillaries involvement; and 7. persistent pulmonary hypertension of the newborn (PPHN) syndrome.
Considerable progress has been made in identifying the causes of PAH in recent decades. Mutations in the type II bone morphogenetic protein receptor (BMPR2) gene contributes to heritable PAH (Thenappan 2018). Epigenetic dysregulation of DNA methylation, histone acetylation, and microRNAs play a key role in disease pathogenesis (Galiè 2015). Complex changes in cytokines, cellular immunity, and autoantibodies indicate that PAH is in part an autoimmune, inflammatory disease (Guignabert 2013). Endothelial dysfunction characterised by overexpression of vasoconstrictors and downexpression of vasoactive mediators occurs in PAH as a result. Reduced production of vasoactive mediators such as nitric oxide (NO) and prostacyclin, along with overexpression of vasoconstrictors such as endothelin‐1 (ET‐1) and thromboxane A2, increase pulmonary vascular tone and promote pulmonary vascular remodelling in people with PAH. The substances targeting pulmonary vasoactive mechanisms (Galiè 2015; Klinger 2019; Thenappan 2018), used alone or in combination, therefore improve functional capacity and haemodynamics and reduce hospital admissions.
Description of the intervention
Endothelin receptor antagonists (ERAs) are a class of potent vasodilators and antimitotic substances that could specifically dilate and remodel the pulmonary arterial system (Horinouchi 2013). Two separate receptors for ET have been identified: endothelin type A (ET‐A) receptors, which are most commonly found on vascular smooth muscle cells and which induce vasoconstriction by increasing intracellular calcium; and endothelin type B (ET‐B) receptors located on endothelial cells, which stimulate the release of vasodilating agents such as NO and prostacyclin (Madonna 2015). However, ET‐B receptors also appear on vascular smooth muscle cells, where they stimulate vasoconstriction. Theoretically, either selective blocking of ET‐A receptors alone, or non‐selective blocking of both ET‐A and ET‐B receptors together, could dilate local pulmonary vessels (Klinger 2019; Thenappan 2018).
How the intervention might work
Endothelin is a potent vasoconstrictor and smooth muscle mitogen. It is overexpressed in the lungs of people with PAH, and elevated plasma endothelin concentrations are correlated with poorer prognosis (Chester 2014). Furthermore, an overproduction of endothelin may also lead to pulmonary vascular remodelling in PAH (Shao 2011). ERAs have shown efficacy in the treatment of PAH in clinical trials (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; COMPASS‐2; SERAPHIN; STRIDE‐1; STRIDE‐2).
Why it is important to do this review
Since bosentan, the first ERA, was approved by the US Food and Drug Administration (FDA) to treat PAH, many new agents, both selective and non‐selective ERAs, have been developed and tested in clinical trials. Importantly, the efficacy and safety of combined use of ERAs and phosphodiesterase (PDE) inhibitors have been investigated in population with PAH (AMBITION; COMPASS‐2). However, the efficacy of ERAs was not consistent in these clinical trials; a systematic review to determine the efficacy of ERAs in the treatment of PAH was thus warranted.
Objectives
To evaluate the efficacy of endothelin receptor antagonists in pulmonary arterial hypertension.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials or quasi‐randomised clinical trials.
Types of participants
We included trials involving adults and children (≥ 2 years) with PAH. The diagnosis of PAH should have been made according to European Respiratory Society/European Society of Cardiology/World Health Organization (ERS/ESC/WHO) guidelines. We included studies involving participants with PAH secondary to other diseases if data assessing the outcomes specific to the PAH component of their syndrome were available.
Types of interventions
We considered trials in which participants took an ERA alone or in combination against any comparator.
We included the following co‐interventions.
-
Phosphodiesterase type 5 (PDE5) inhibitors
-
Soluble guanylate cyclase (sGC)
-
Prostanoids
-
Nitrates
-
Calcium channel blockers
-
Non‐pulmonary hypertension‐specific medications including diuretics, anticoagulants, and oxygen
Types of outcome measures
Primary outcomes
-
Exercise capacity (as measured by a six‐minute walk distance (6MWD)).
-
World Health Organization (WHO) functional class or New York Heart Association (NYHA) functional class (WHO/NYHA).
-
Borg dyspnoea scores and dyspnoea‐fatigue ratings.
-
Mortality.
Secondary outcomes
-
Cardiopulmonary haemodynamics including mean pulmonary artery pressure (PAP); pulmonary vascular resistance (PVR), cardiac index, cardiac output (CO), systemic arterial oxygen saturation and systemic oxygen transport.
-
Pulmonary function tests.
-
Adverse events (e.g. hepatic toxicity).
Search methods for identification of studies
Electronic searches
The Cochrane Airways Group Information Specialist undertook literature searches on 4 November 2020 using the following sources: the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 11 of 12, November 2020) (from 1966 to November 2020), which contains the Cochrane Airways Group Register of Trials, MEDLINE Ovid SP (from 1966 to November 2020), Embase Ovid SP (from 1980 to November 2020), the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/), and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/). We applied no language restrictions. The database search strategies are shown in Appendix 1.
Searching other resources
We handsearched the reference lists of retrieved articles for additional trials. We also searched documents released from the FDA and European Medicines Agency (EMA).
Data collection and analysis
Selection of studies
Three review authors (LC, CJ, and DB) independently screened the titles and abstracts of records identified by the search for potential eligibility, categorising them as 'retrieve' or 'do not retrieve'. We retrieved all available full‐text publications. Three review authors (LC, CJ, and DB) independently assessed the full‐text reports as either eligible for inclusion, or identified and reported the reasons for exclusion (Figure 1). Any disagreements were resolved by consensus or by involving a third person/review author (GY). One final review author (LK) analysed the included and excluded texts to ensure uniform enforcement of the study protocol. We excluded duplicates and collated multiple reports of the same study (Figure 1). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and 'Characteristics of excluded studies' table.
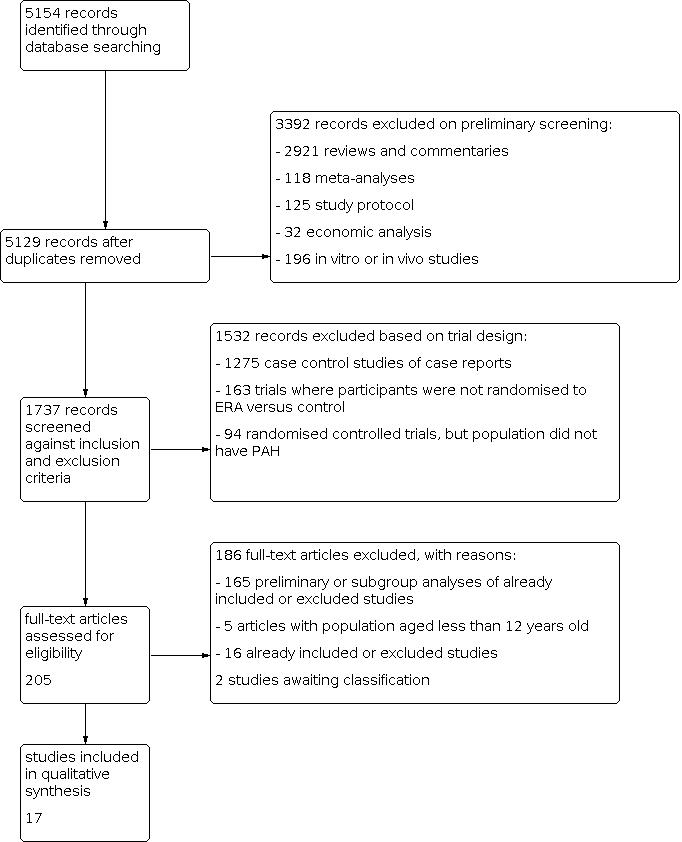
Study flow diagram.
Data extraction and management
We used Covidence to collect study characteristics and outcome data (Covidence). Three review authors (LC, CJ, and GY) extracted the following study characteristics from the included studies.
-
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
-
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
-
Interventions: intervention, comparison, concomitant medications, and excluded medications.
-
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
-
Notes: funding for studies and notable conflicts of interest of trial authors.
Three review authors (LC, CJ, and GY) independently extracted the outcome data from included studies. Any disagreements were resolved through discussion or by consulting a third review author (DB) if required. One review author (LC) transferred data into the Review Manager 5 file (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (LK) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
We independently assessed the methodological quality of included trials against the following criteria.
-
Was the study described as randomised?
-
Was the allocation concealment adequate?
-
Were the participants blinded?
-
Was there blinded outcome assessment?
-
Was there a description of withdrawals and dropouts?
-
Were the results analysed according to intention‐to‐treat?
-
Was there any other bias?
We graded each potential source of bias as 'low', 'high', or 'unclear', and provided a justification for our judgement in the 'Risk of bias' table. We took into account the risk of bias when considering treatment effects.
Measures of treatment effect
We treated short ordinal scales (e.g. WHO functional class, NYHA class) as dichotomous measures, arranging the data into two categories: improvement or deterioration in class. We analysed linear scales (e.g. Borg dyspnoea scores) as continuous data.
Unit of analysis issues
For studies where more than one eligible treatment arm was included, we split the data between two treatment‐control comparisons by splitting the control group and halving the sample sizes. The total numbers in the meta‐analysis thus added up to the original size of the group.
Dealing with missing data
For missing data on prespecified primary outcomes, we contacted the study investigators to request the data. If the data were not available, we performed sensitivity analyses to assess how sensitive the results were to reasonable changes in the assumptions made.
Assessment of heterogeneity
We compared the Chi2 statistic with its degrees of freedom to identify heterogeneity. We considered a statistic bigger than its degrees of freedom and I2 > 30% as evidence of heterogeneity.
Assessment of reporting biases
We conducted standard funnel plots for the primary outcome (i.e. 6MWD) to investigate the potential for the influence of publication bias on the analysis.
Data synthesis
In the absence of significant heterogeneity, we combined results using a mean difference (MD) for continuous data, and odds ratio (OR) for dichotomous data. If there was significant heterogeneity (i.e. the ratio of Chi2 statistic and its degrees of freedom was bigger than 1.0, and the I2 > 30%), we used the random‐effects model to assess treatment effect.
A number of articles reported studies where more than one eligible treatment arm was included. For these studies, we split the data between two treatment‐control comparisons by splitting the control group and halving the sample sizes. For example, STRIDE‐1 was a triple‐arm clinical study that did not provide combined 'sitaxsentan' data versus placebo on secondary outcomes such as mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR). For relevant outcomes, we entered data from both intervention arms separately for the meta‐analysis, denoting the dosage distinction in footnotes. Data for placebo participants were entered with the number of participants in each of the placebo 'groups' halved. The total numbers in the meta‐analysis thus added up to the original size of the group. The same method was used in STRIDE‐2, STRIDE‐4, and SERAPHIN (see Characteristics of included studies). STRIDE‐2 compared sitaxsentan with both a placebo arm and an open‐label bosentan arm. For this study, we used the data from participants treated with bosentan and placebo for reference in the notes, and did not pool the data with those of other studies.
The AMBITION study had triple arms, that is combination therapy with 10 mg of ambrisentan plus 40 mg of tadalafil (combination‐therapy group), 10 mg of ambrisentan plus placebo (ambrisentan monotherapy group), or 40 mg of tadalafil plus placebo (tadalafil‐monotherapy group). We pooled the data from the combination‐therapy groups versus tadalafil‐monotherapy group in the review, and compared ambrisentan monotherapy versus tadalafil in the subgroup analysis of ERAs versus phosphodiesterase inhibitors.
Subgroup analysis and investigation of heterogeneity
-
Non‐selective ERA (antagonising both ET‐A and ET‐B) versus selective ERA (antagonising ET‐A) only.
-
Participants with idiopathic PAH versus those with PAH related to other conditions.
Sensitivity analysis
When there was a significant difference in the characteristics of participants, intervention, comparator, or outcome, we conducted a sensitivity analysis to assess how the results were affected by reasonable changes to the assumptions made.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the following outcomes (summary of findings Table 1): change in 6MWD, improvement in WHO functional class, Borg dyspnoea scores, mortality, PAP, PVR, and risk of hepatic toxicity. We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), employing GRADEpro GDT software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes and made comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
The literature search identified 5154 titles and abstracts. Of these, we identified 17 randomised controlled trials for inclusion in this version of the review (Table 1) (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPH; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). We divided four trials based on the doses given (SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4); see Data synthesis for details. The process of identifying studies eligible for inclusion in the analysis is summarised in a flow diagram (Figure 1). We obtained the full‐text versions of the 17 included studies from published papers or the FDA and EMA websites. Galiè 2003 reported data from a subset of participants from the BREATHE‐1 study. Studies referenced in multiple sources were assessed for completeness of study details. When there was a discrepancy in the data between published journal papers and documents from the FDA and EMA, we used data from the FDA or EMA. We identified two potentially eligible studies in abstract form. We contacted the authors for detailed data, but received no response, therefore we assessed these studies as awaiting classification (for details see Characteristics of studies awaiting classification).
| Study | N | Country | Intervention | Control | Outcomes |
|---|---|---|---|---|---|
| AMBITION | 747 | International | Ambrisentan + tadalafil or ambrisentan | Tadalafil + placebo | Primary outcome: time to the first event of clinical failure Secondary outcomes: change from baseline in NT‐proBNP level, 6MWD, WHO FC, and Borg dyspnoea index |
| ARIES‐1 | 201 | International | Ambrisentan (5 mg/day or 10 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 physical functioning scale |
| ARIES‐2 | 192 | International | Ambrisentan (2.5 mg/day or 5 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 |
| BREATHE‐1 | 213 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD |
| BREATHE‐2 | 33 | International | Bosentan | Placebo | Primary outcome: change from baseline to week 16 in TPR |
| BREATHE‐5 | 54 | International | Bosentan | Placebo | Primary outcome: change from baseline in SpO2 and PVR |
| Channick 2001 | 32 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD |
| COMPASS‐2 | 334 | International | Bosentan | Placebo | Primary outcome: time to the first morbidity/mortality event Secondary outcomes: change in 6MWD, WHO FC, time to the first occurrence of death from any cause, hospitalisation for PAH or start of intravenous prostanoid therapy, atrial septostomy, or lung transplant. |
| EARLY | 185 | International | Bosentan | Placebo | Primary outcomes: PVR and change from baseline in 6MWD |
| EDITA | 38 | Germany | Ambrisentan | Placebo | Primary outcome: change in mPAP Secondary outcomes: change in WHO FC, change in cardiac index, change in PVR, symptoms of SSc, quality of life (SF‐36), lung function tests, right heart dimensions and function, NT‐proBNP, measures of disease‐related progression |
| MAESTRO | 150 | International | Macitentan | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC and Borg dyspnoea index |
| PORTICO | 85 | International | Macitentan | Placebo | Primary outcome: change from baseline to PVR Secondary outcomes: change from baseline in RAP, mPAP, cardiac index, total pulmonary resistance, SvO2, NT‐proBNP, 6MWD, and WHO FC |
| SERAPH | 26 | British | Bosentan | Sildenafil | Primary outcome: change in right ventricle mass from baseline |
| SERAPHIN | 742 | International | Macitentan | Placebo | Primary outcome: time from the initiation of treatment to the first event related to pulmonary arterial hypertension Secondary outcomes: change from baseline in 6MWD, percentage of participants with an improvement in WHO FC, death due to PAH or hospitalisations for PAH, and death from |
| STRIDE‐1 | 178 | International | Sitaxsentan | Placebo | Primary outcome: peak oxygen consumption |
| STRIDE‐2 | 245 | International | Sitaxsentan | Placebo | Primary outcome: change from baseline in 6MWD |
| STRIDE‐4 | 98 | International | Sitaxsentan | Placebo | Primary efficacy endpoint was the change in 6MWD from baseline to week 18. Secondary outcomes: changes in WHO FC from baseline at each assessment and time to clinical worsening, Borg dyspnoea index |
6MWD: 6‐minute walk distance; BNP: B‐type natriuretic peptide; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; MVO2: mixed venous oxygen saturation; NT‐proBNP: N‐terminal pro–brain natriuretic peptide; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PVR: pulmonary vascular resistance; SF‐36: 36‐item Short Form Health Survey; SpO2: oxygen saturation; SSc: systemic sclerosis; SvO2: mixed venous oxygen saturation; TPR: total pulmonary resistance; WHO FC: World Health Organization functional class
Included studies
Methods
Sixteen studies were randomised and placebo‐controlled (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). One study was a randomised head‐to‐head study of bosentan (an ERA) and sildenafil (a PDE5 inhibitor) (SERAPH). A full description of each study with clinical characteristics at baseline, inclusion/exclusion criteria, methods of randomisation, and outcomes is provided in Characteristics of included studies.
Interventions
Nine trials compared a non‐selective ERA (bosentan and macitentan) against placebo (BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; MAESTRO; PORTICO; SERAPHIN). Seven trials compared selective ERAs (sitaxsentan or ambrisentan) against placebo (AMBITION; ARIES‐1; ARIES‐2; EDITA; STRIDE‐1; STRIDE‐2; STRIDE‐4). Three studies tested the efficacy of combination therapy versus monotherapy in participants with PAH (AMBITION; BREATHE‐2; COMPASS‐2). SERAPH compared bosentan against sildenafil (a PDE5 inhibitor).
For the purposes of the review, we specified two comparisons: comparison 1: ERA versus placebo (where ERAs are subgrouped into selective versus non‐selective); and comparison 2: ERA versus PDE5 inhibitor.
Participants
Thirteen trials recruited participants with idiopathic PAH and PAH secondary to connective tissue disease and congenital heart disease (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; Channick 2001; COMPASS‐2; EARLY; SERAPH; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). Two trials tested the efficacy of ERAs in participants with Eisenmenger syndrome (BREATHE‐5; MAESTRO). One study investigated the effect of macitentan on participants with portopulmonary hypertension (PORTICO). One study investigated systemic sclerosis patients with mildly elevated mean pulmonary arterial pressure (mPAP) at rest between 21 and 24 mmHg and/or > 30 mmHg during low‐dose exercise (EDITA).
Outcomes
Seven trials used the 6MWD as the primary outcome measure (ARIES‐1; ARIES‐2; BREATHE‐1; Channick 2001; MAESTRO; STRIDE‐2; STRIDE‐4), whilst one trial used change from baseline in peripheral oxygen saturation (SpO2) and PVR as the primary outcome (BREATHE‐5). STRIDE‐1 used peak exercise VO2 as its primary outcome. EARLY and PORTICO used PVR as the primary outcome. The primary efficacy parameter in BREATHE‐2 was change from baseline to week 16 in total pulmonary resistance (TPR). SERAPH used change in right ventricle mass from baseline as its primary endpoint. Three studies used time to first event of clinical worsening as the primary endpoint (AMBITION; COMPASS‐2; SERAPHIN). The EDITA study used change in mPAP at rest between groups as the primary endpoint. Additional outcome measures used in the included studies were WHO or NYHA functional class status, Borg dyspnoea score, cardiopulmonary haemodynamics, and drug safety and tolerability. For details, see Characteristics of included studies.
Excluded studies
We excluded 101 studies, with reasons provided in the Characteristics of excluded studies table.
Risk of bias in included studies
All but two trials, EDITA; SERAPH, were reported as international, multicentre, double‐blind, randomised, placebo‐controlled trials. Both SERAPH and EDITA are single‐centre, double‐blind, randomised, placebo‐controlled trials. Randomisation in all trials was conducted according to computer‐generated random number programs. We judged allocation concealment to be adequate in all trials. All trials described dropouts and withdrawals. All but two trials, EARLY; EDITA, were statistically analysed according to the intention‐to‐treat principle. See Characteristics of included studies for a full description of the risk of bias in included studies, and Figure 2 for a 'Risk of bias' summary.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the included trials used computer‐generated random numbers and were placebo controlled, therefore we judged allocation concealment to be adequate.
Blinding
Eight trials reported detailed descriptions of how treating clinicians, participants, and assessors were sufficiently blinded and were therefore judged as at low risk of bias (AMBITION; BREATHE‐5; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN). Other trials were reported to be double‐blind, but as no further information was available we judged them to be at unclear risk of bias.
Incomplete outcome data
All studies described details of dropouts and withdrawals. All trials except for two, EARLY; EDITA, performed statistical analysis based on intention‐to‐treat. However, due to the high occurrence of missing data, 13 included trials were categorized as at high risk of attrition bias. For details, see Characteristics of included studies.
Selective reporting
Funnel plots for trials comparing ERAs versus placebo (outcome of change from baseline in 6MWD) were qualitatively symmetrical and did not suggest the presence of publication bias. Nine trials reported their prespecified primary outcomes according to pre‐published protocols in ClinicalTrials.gov and were therefore considered to be at low risk of reporting bias (AMBITION; ARIES‐1; ARIES‐2; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN). We did not have access to protocols for the other studies.
Other potential sources of bias
It should be noted that most (16 out of 17) included studies were sponsored by the drug manufacturer.
Effects of interventions
Only outcomes specified in the protocol were included in the meta‐analysis. Unless otherwise stated, data used in the review were from published material or documents released from the FDA and EMA.
Comparison 1: Endothelin receptor antagonists versus placebo
Sixteen studies reported ERA versus placebo; these have been subgrouped into selective and non‐selective ERAs.
Primary outcomes
Exercise capacity
Sixteen trials assessed change from baseline in 6MWD (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4). The pooled data showed that participants treated with ERAs could walk 25 metres further than those treated with placebo (mean difference (MD) 25.06 m, 95% confidence interval (CI) 17.13 to 32.99; participants = 2739; studies = 14; I2 = 34%; Analysis 1.1, moderate‐certainty evidence). We observed significant heterogeneity between the trials.
Subgroup analysis showed that the therapeutic effect of the non‐selective ERA (bosentan or macitentan) on 6MWD was 20.51 metres (95% CI 10.03 to 31.00; participants = 1860; studies = 8; I2 = 42%), and the selective ERAs was 33.48 metres (95% CI 23.12 to 43.83; participants = 879; studies = 6; I2 = 0%) compared to placebo. The test for subgroup differences was not significant (Chi2 = 2.97, df = 1 (P = 0.08), I2 = 66.4%).
Data from BREATHE‐2 and AMBITION were insufficient to evaluate the exercise capacity test. The BREATHE‐2 study showed a remarkable increase in the 6MWD in both treatment groups (68 metres (median) in the bosentan/prostacyclin group versus 74 metres (median) in the placebo/prostacyclin group). However, no statistical difference was observed between the two groups in the 6MWD.
AMBITION showed the median change from baseline to week 24 in 6MWD to be 48.98 (interquartile range (IQR) 4.63 to 85.75) in the combined ambrisentan plus tadalafil group and 22.70 (IQR −8.25 to 66.00) in the tadalafil‐monotherapy group, favouring the combination therapy group (P< 0.01).
WHO or NYHA functional class
Fifteen trials including a total of 3184 participants reported detailed numbers of participants with improved or deteriorated WHO/NYHA functional class (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; COMPASS‐2; EARLY; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2; STRIDE‐4).
ERAs improved more participants' functional class than placebo (odds ratio (OR) 1.41, 95% CI 1.16 to 1.70; participants = 3060; studies = 15; I2 = 5%; Analysis 1.2, moderate‐certainty evidence) and lowered the odds of functional class deterioration (OR 0.43, 95% CI 0.26 to 0.72; participants = 2347; studies = 13; I2 = 40%; Analysis 1.3, moderate‐certainty evidence).
For non‐selective ERAs, 248 of 1118 participants in the ERA group improved their functional class versus 117 to 778 participants in the placebo group (OR 1.45, 95% CI 1.13 to 1.87; participants = 1896; studies = 9; I2 = 29%; Analysis 1.2), favouring non‐selective ERAs. For selective ERAs, 260 of 880 participants in the ERA group improved their functional class versus 90 of 408 in the placebo group (OR 1.35, 95% CI 1.01 to 1.80; participants = 1164; studies = 6; I2 = 0%), favouring selective ERAs (Analysis 1.2). The test for subgroup differences was not significant (Chi2 = 0.13, df = 1 (P = 0.72), I2 = 0%).
Both non‐selective ERA and selective ERAs significantly lowered the occurrence of WHO/NYHA function deterioration in participants with PAH: non‐selective ERAs (OR 0.65, 95% CI 0.30 to 1.42; participants = 1121; studies = 7; I2 = 32%) and selective ERAs (OR 0.31, 95% CI 0.17 to 0.60; participants = 1226; studies = 6; I2 = 36%; Analysis 1.3) compared to placebo. The test for subgroup differences was not significant (Chi2 = 1.98, df = 1 (P = 0.16), I2 = 49.6%).
Borg dyspnoea score
Considering symptoms such as dyspnoea is an important aspect of assessing response to therapy. In the most commonly used Borg dyspnoea scale in PAH, lower is better, and the minimal clinically important difference in PAH is 1 unit (Khair 2016). Data from seven trials included in this review addressed this outcome (ARIES‐1; ARIES‐2; BREATHE‐1; Channick 2001; EDITA; SERAPH; STRIDE‐2). We found heterogeneity in the selective ERA trials that could not be explained in terms of clinical characteristics and methodological quality, so we pooled the data using a random‐effects model. Overall, ERAs may mildly reduce Borg dyspnoea scores by 0.43 units (MD −0.43, 95% CI −0.90 to 0.04; participants = 788; studies = 7; I2 = 49%; Analysis 1.4, low‐certainty evidence).
Compared with placebo, both the non‐selective ERA bosentan (MD −0.27, 95% CI −1.58 to 1.03; participants = 240; studies = 3; I2 = 60%) and selective ERAs (MD −0.43, 95% CI −1.01 to 0.14; participants = 548; studies = 4; I2 = 55%; Analysis 1.4) may reduce Borg dyspnoea score. The test for subgroup differences was not significant (Chi2 = 0.05, df = 1 (P = 0.83), I2 = 0%).
MAESTRO reported that no relevant trends in change from baseline to week 16 in the Borg dyspnoea index existed between the macitentan and placebo groups, but did not provide detailed data. BREATHE‐5 reported Borg score as an outcome with limited detail.
AMBITION reported the treating effects in the study portal (ClinicalTrials.gov: NCT01178073) as median IQR: the change from baseline in Borg dyspnoea score was −1.00 (−2.00 to 0.50) in the combination therapy group; and −0.50 (−2.00 to 0.88) in the tadalafil‐monotherapy group.
Mortality
Twelve trials including 2889 participants reported a total of 193 deaths (AMBITION; ARIES‐1; ARIES‐2; BREATHE‐1; BREATHE‐2; COMPASS‐2; EARLY; MAESTRO; SERAPHIN; STRIDE‐1; STRIDE‐2), of which 113 were on active treatment and 80 on placebo. There was a non‐significant trend towards ERAs lowering mortality in participants with PAH (OR 0.78, 95% CI 0.58 to 1.07; participants = 2889; studies = 12; I2 = 0%; Analysis 1.5, low‐certainty evidence).
Subgroup analysis from the pooled data showed that there was no significant difference in mortality between non‐selective ERAs and selective ERAs and placebo (OR 0.88, 95% CI 0.62 to 1.23; participants = 1759; studies = 7; I2 = 0%), whereas selective ERAs were found to significantly lower mortality (OR 0.45, 95% CI 0.21 to 0.94; participants = 1130; studies = 5; I2 = 0%). The test for subgroup differences was not significant (Chi2 = 2.62, df = 1 (P = 0.11), I2 = 61.8%).
To compare the treatment effects of subgroups (selective ERAs versus non‐selective ERAs) on mortality, we calculated the interaction between the two subgroups using a method previously described by Altman 2003. The estimated interaction effect is a relative odds ratio 0.51 (95% CI 0.22 to 1.16), indicating that there is no clear evidence of a different treatment effect between non‐selective ERAs and selective ERAs on mortality.
Secondary outcomes
Cardiopulmonary haemodynamics
Ten studies measured haemodynamics variables (BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1): mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (mPVR), and cardiac index. Haemodynamic variables were measured by right‐heart catheterisation in seven studies (BREATHE‐2; Channick 2001; EARLY; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1), compared with BREATHE‐1, which used an echocardiological method. Data from BREATHE‐1 were reported as a subgroup analysis in Galiè 2003.
Mean pulmonary artery pressure
Nine studies assessed mPAP (BREATHE‐2; BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1). We observed significant heterogeneity between the studies, therefore we pooled them using random‐effects modelling. Pooled data from eight studies showed that ERAs lowered mPAP by 4.65 mmHg (95% CI −6.05 to −3.26; participants = 729; I2 = 19%; Analysis 1.6, moderate‐certainty evidence) more than placebo (BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1).
The non‐selective ERA (bosentan) lowered mPAP by 5.79 mmHg (95% CI −7.30 to −4.27; participants = 519; studies = 6; I2 = 0%; Analysis 1.6) more than placebo, whereas the selective ERA lowered mPAP by 2.65 mmHg (95% CI −5.31 to 0.00; participants = 210; studies = 2; I2 = 36%; Analysis 1.6) more than placebo. There was a significant difference in the test for subgroup differences (Chi2= 4.04, df = 1 (P = 0.04), I2 = 75.2%).
BREATHE‐2 did not report data for change from baseline in mPAP, but instead as an end‐of‐treatment score: mean ± standard error (SE) was 59.2 ± 3.2 mmHg in the placebo group and 52.5 ± 2.4 mmHg in the bosentan group, which was not statistically significant (P = 0.3).
Pulmonary vascular resistance
Seven studies on 586 participants reported data of change from baseline in PVR, all of them showing that ERA significantly improved PVR compared with placebo (BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; STRIDE‐1).
We observed significant heterogeneity between studies and therefore pooled them using random‐effects modelling. Data from seven trials showed that ERAs could significantly reduce PVR by 236.24 dyn/s/cm5 (95% CI −333.21 to −139.26; participants = 586; studies = 7; I2 = 14.7%; Analysis 1.7, moderate‐certainty evidence) more than placebo (BREATHE‐5; Channick 2001; EARLY; EDITA; MAESTRO; PORTICO; STRIDE‐1).
BREATHE‐2 and SERAPHIN reported an end‐of‐treatment score, as mean and standard deviation. The pooled data showed that participants treated with ERAs had much lower PVR than those treated with placebo (MD −288.59 dyn/s/cm5, 95% CI −472.18 to −104.99; participants = 175; studies = 2; I2 = 0%; Analysis 1.8).
Three studies reported ratio of geometric mean PVR. The pooled data showed that participants receiving ERAs had a much lower ratio of geometric mean PVR (0.69, 95% CI 0.60 to 0.80; participants = 124; Analysis 1.9), favouring ERAs (MAESTRO; PORTICO; SERAPHIN).
Cardiac index
Seven studies on 718 participants reported cardiac index data (Channick 2001; EARLY; EDITA; Galiè 2003; PORTICO; SERAPHIN; STRIDE‐1). We observed significant heterogeneity between studies and therefore pooled them using a random‐effects model. Pooled data from these studies showed that ERAs could significantly increase cardiac index compared with placebo (MD 0.50 L/min/m2, 95% CI 0.35 to 0.65; participants = 718; studies = 7; I2 = 59%; Analysis 1.10) (Channick 2001; EARLY; EDITA; Galiè 2003; PORTICO; SERAPHIN; STRIDE‐1). The placebo‐corrected therapeutic effect of the non‐selective ERA on cardiac index was 0.55 L/min/m2 (95% CI 0.34 to 0.77; participants = 509; studies = 5; I2 = 70%), whilst for the selective ERA (sitaxsentan) it was 0.39 L/min/m2 (95% CI 0.23 to 0.54; participants = 209; studies = 2; I2 = 0%). The test for subgroup differences was not significant (Chi2 = 1.50, df = 1 (P = 0.22), I2 = 33.2%).
Adverse events
Eleven trials including 2250 participants reported hepatic toxicity in detail (AMBITION; BREATHE‐1; BREATHE‐2; BREATHE‐5; Channick 2001; EARLY; MAESTRO; PORTICO; SERAPHIN; STRIDE‐1; STRIDE‐2). We observed heterogeneity between studies and therefore pooled them using a random‐effects model. Overall, 102 of 1357 participants receiving ERA had hepatic toxicity events compared with 33 of 893 participants receiving placebo, which was not significantly different (OR 1.88, 95% CI 0.91 to 3.90; participants = 2250; studies = 11; I2 = 53%, moderate‐certainty evidence), though the direction of effect favoured placebo.
ARIES‐1 and ARIES‐2 reported reassuring combined data for hepatic toxicity. None of the 261 participants receiving ambrisentan developed serum aminotransferase concentrations greater than three times the upper limit of normal, compared with three participants (2.3%) receiving placebo displaying elevated levels.
Subgroup analysis
Pulmonary arterial hypertension associated with connective tissue disease
The subgroup analysis of non‐selective ERAs versus selective ERAs for mortality is described above. We could not perform a subgroup analysis comparing participants with idiopathic PAH versus those with PAH related to other conditions due to insufficient information. However, Denton 2006 reported the combined data from two trials, BREATHE‐1; Channick 2001, comparing bosentan to placebo for PAH secondary to connective tissue disease (PAH‐CTD). There was no significant difference between the bosentan and placebo groups.
Seibold 2005 reported the combined data of sitaxsentan from STRIDE‐1, STRIDE‐2, and STRIDE‐4 for PAH‐CTD, which included sitaxsentan at 50 mg, 100 mg, and 300 mg once daily; 110 of 512 participants had PAH‐CTD, including 63 with systemic sclerosis (SSc), 22 with overlap/mixed connective tissue disease, and 25 with systemic lupus erythematosus. The data showed a change from baseline in 6MWD of −16 ± 15.0 metres (mean ± SE) for placebo (N = 28); −2 ± 13.4 metres for sitaxsentan 50 mg daily (N = 26); 21 ± 10.4 metres for sitaxsentan 100 mg daily (N = 39); and 2 ± 14.1 metres for sitaxsentan 300 mg daily (N = 17). Only participants treated with 100 mg daily (the dose authorised by the EMA) significantly improved in 6MWD, which was comparable to participants with idiopathic PAH treated with sitaxsentan.
Badesch 2007 reported the pooled data for ambrisentan therapy in idiopathic PAH (n = 251) and PAH‐CTD (n = 124). At week 12, the placebo‐adjusted increase in 6MWD was 58 metres (95% CI 36 to 79) in participants with idiopathic PAH and 19 metres (95% CI −10 to 48) in participants with PAH‐CTD; the placebo‐adjusted decrease in Borg dyspnoea score was −0.8 (95% CI −1.4 to −0.19) in the idiopathic PAH group and −1.0 (95% CI −1.7 to −0.18) in the PAH‐CTD group. WHO functional class deterioration was 2.4% in participants with idiopathic PAH and 3.7% in participants with PAH‐CTD in the ambrisentan group, compared with 16.5% in participants with idiopathic PAH and 20.9% in participants with PAH‐CTD in the placebo group.
In the AMBITION study, 187 participants had CTD associated with PAH, of whom 118 had SSc associated with PAH. Initial combination therapy reduced the risk of clinical failure when compared with pooled monotherapy in each subgroup: CTD‐PAH (hazard ratio 0.43, 95% CI 0.24 to 0.77) and SSc‐PAH (0.44, 95% CI 0.22 to 0.89).
In the EDITA study, 38 participants had SSc associated with mildly elevated mPAP between 21 and 24 mmHg at rest and/or > 30 mmHg during low‐dose exercise were randomly assigned to treatment with either ambrisentan 5 to 10 mg/day or placebo. After six months, the two groups did not differ in the primary endpoint (i.e. change in mPAP). However, the ambrisentan group showed significant improvements in the secondary endpoints, that is cardiac index and PVR, at rest or at peak exercise.
Pulmonary arterial hypertension associated with portopulmonary hypertension
PORTICO first reported the effect of the non‐selective ERA macitentan on portopulmonary hypertension. The study's primary outcome (i.e. PVR at the end of study) was improved in the macitentan group, but not exercise capacity or WHO functional class at the end of study.
Pulmonary arterial hypertension associated with congenital heart disease
Two trials investigated the efficacy of non‐selective ERAs on Eisenmenger syndrome (BREATHE‐5; MAESTRO). The pooled data showed that non‐selective ERAs had no impact on exercise capacity, WHO functional class, or mortality. However, ERAs did improve mPAP (MD −4.63 mmHg, 95% CI −8.03 to −1.23; participants = 90; studies = 2; I2 = 0%; Analysis 3.5) and PVR (MD −480.07 dyn/s/cm5, 95% CI −753.34 to −206.79; participants = 93; studies = 2; I2 = 0%; Analysis 3.6).
Comparison 2: Endothelin receptor antagonists versus phosphodiesterase‐5 inhibitors
Primary outcomes
Exercise capacity
Two trials tested the efficacy of ERA versus PDE5 inhibitors (AMBITION; SERAPH).
SERAPH reported that mean 6MWD increased with both treatments compared to baseline. Sildenfafil may increase exercise capacity (MD 55 m, 95% CI 0.1 to 109.9; Analysis 2.1) when compared with bosentan in participants with PAH.
AMBITION showed a median change from baseline to week 24 in 6MWD of 27.00 (IQR −14.00 to 63.25) in the ambrisentan‐monotherapy group and 22.70 (IQR −8.25 to 66.00) in the tadalafil‐monotherapy group, with no difference between groups.
WHO or NYHA functional class
Only AMBITION reported the effect of ambrisentan versus tadalafil on WHO functional class. There was no difference between groups in either WHO functional improvement or deterioration (Analysis 2.2; Analysis 2.3).
Borg dyspnoea score
Only the SERAPH study reported detailed data on Borg dyspnoea score. A trend showed that sildenafil may reduce Borg dyspnoea score compared with bosentan in participants with PAH (Analysis 2.4).
Mortality
A trend showed that ERAs and PDE‐5 inhibitors may reduce mortality in participants with PAH (OR 0.32, 95% CI 0.07 to 1.36; participants = 273; studies = 2; I2 = 0%; Analysis 2.5).
Secondary outcomes
Cardiac index
Only the SERAPH study reported data on cardiac index. There was no difference between treatments for this outcome (MD 0 L/min/m2, 95% CI −0.14 to 0.14; participants = 25; studies = 1; Analysis 2.6).
Sensitivity analysis
Including or excluding combination therapy did not make a difference in the effect of ERAs on the primary outcomes (i.e. exercise capacity, functional class, and mortality) (Table 2).
| Outcome | Including combination therapy | Excluding combination therapy |
|---|---|---|
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 25.65 (16.80 to 34.49) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.52 (1.22 to 1.91) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.82 (0.58 to 1.17) |
6MWD: 6‐minute walk distance; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; WHO FC: World Health Organization functional class
Including or excluding PAH participants with PAH associated with congenital heart disease and portal hypertension had little impact on the effect of ERAs on primary outcomes such as exercise capacity, functional class, and mortality (Table 3).
| Outcome | Including combination therapy | Excluding combination therapy |
|---|---|---|
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 27.90 (20.96 to 34.83) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.46 (1.19 to 1.78) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.77 (0.57 to 1.05) |
6MWD: 6‐minute walk distance; CHD: congenital heart disease; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; PAH: pulmonary arterial hypertension; SSc: systemic sclerosis; WHO FC: World Health Organization functional class
Discussion
The pooled data show that ERAs probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, reduce dyspnoea, and improve cardiopulmonary haemodynamic variables, and may improve mortality in participants with PAH. However, they may also increase the risk of liver damage, although this was rare.
Clinical efficacy
Minimal clinically important differences (MCID) are patient‐derived scores that reflect changes in a clinical intervention that are meaningful for the patient. The six‐minute walk test (6MWD), as a reliable tool for the assessment of exercise capacity in participants with PAH, is an independent predictor of mortality (Miyamoto 2000; Sitbon 2002). Although the pooled data showed that ERAs statistically significantly increased 6MWD by 25.06 m in participants with PAH, it is less than MCID estimated by Mathai 2012. Additionally, ERAs reduced the pooled Borg dyspnoea score by 0.43, where the MCID for PAH is 1 unit (Khair 2016).
Mortality events across the included trials were low, and we did not find a significant difference for these comparisons despite the trend favouring ERAs. The included studies were underpowered for evaluating mortality, particularly given their short duration of study period and the use of time to first event of related to PAH as primary endpoint, which led to censoring of participants earlier (BREATHE‐5; Channick 2001; STRIDE‐4). There is insufficient certainty around the observed result to exclude either a reduction in mortality or no difference between groups. Transplantation or epoprostenol remains the preferred therapy with a major reduction in mortality in PAH participants with WHO functional class IV (Barst 1996).
The exact mechanism of action of ERAs on the pulmonary vascular bed remains unclear. Vasodilatation is likely to be just a part of the mechanism since usually 70% to 80% of participants with idiopathic PAH do not respond acutely to vasodilators (Rich 1992; Sitbon 1998). PAH‐targeted therapies (e.g. prostaglandins, PDE inhibitors, ERAs, and soluble guanylate cyclase stimulators), used alone or in combination, improve exercise capacity and haemodynamics and reduce hospitalisations. However, these pulmonary vasodilators do not target key features of PAH pathogenesis and have not been shown to reduce mortality (Galiè 2015; Klinger 2019; Thenappan 2018).
Using ERAs as part of combination therapy is an attractive option to target the multiple pathophysiological mechanisms in PAH. Combination therapy might be pursued by the simultaneous initiation of two (or more) treatments or by the addition of a second (or third) treatment to a previous therapy that is considered insufficient. Several combination studies of ERAs and PDE inhibitors have been carried out in participants with PAH (AMBITION; COMPASS‐2; Zhuang 2014). One randomised controlled trial used bosentan plus sildenafil (COMPASS‐2), and another two randomised controlled trials used ambrisentan plus tadalafil (AMBITION; Zhuang 2014). The evidence consistently showed that participants with PAH receiving combination therapy could walk longer than those receiving monotherapy.
The BREATHE‐2 trials, with small sample sizes, explored the efficacy and safety of combination therapy (bosentan in combination with intravenous prostacyclin), showing a trend without statistical significance towards haemodynamic and clinical improvement of this combination in participants with PAH. There is clearly an interaction between bosentan and prostacyclin in the BREATHE‐2 trial. However, in line with the predefined subgroups in the protocol for this review, the BREATHE‐2 trial was included in the meta‐analysis of ERAs versus placebo. Nevertheless, excluding BREATHE‐2 from the meta‐analysis made little difference to the results.
Subgroup analysis
Both a non‐selective ERA and a selective ERA can dilate constricted pulmonary pre‐capillary arterioles and improve exercise capacity and symptoms. Determining the relative benefit of these agents requires head‐to‐head comparisons. A post hoc analysis of the STRIDE‐2 trial that was neither designed nor powered to detect non‐inferiority or equivalence showed a mean difference in the 18‐week change from baseline between sitaxsentan and bosentan of 1.9 metres (95% CI −22.7 to 26.5; P = 0.9). Also, the AMBITION trial reported that there was no difference on 6MWD between ambrisentan‐monotherapy group and tadalafil‐monotherapy group.
ERAs showed efficacy in participants with PAH associated with CTD in post hoc analysis. ERAs did improve cardiopulmonary haemodynamic in participants with congenital heart disease and portal hypertension, but had little impact on 6MWD and functional class.
Safety and tolerability
One major concern regarding ERAs is hepatotoxicity and its capacity to cause substantial damage to the liver. This review did not show a high occurrence of hepatic toxicity. However, there were three reported cases of irreversible liver failure induced by sitaxsentan, one in the UK in 2009 and two cases from clinical trials in India and Ukraine in 2010. The reported fatal hepatic toxicity in association with sitaxsentan was idiosyncratic, unlikely to be detected by monthly monitoring, and irreversible when sitaxsentan was discontinued. Consequently, sitaxsentan was withdrawn from the market voluntarily by its licence holder. These cases emphasise the importance of dosing and hepatic monitoring in individuals with PAH.
Summary of main results
For participants with pulmonary arterial hypertension with WHO functional class II and III, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, result in favourable changes in cardiopulmonary haemodynamic variables. However, they are less effective in reducing dyspnoea and mortality.
Overall completeness and applicability of evidence
All the trials identified in the current review addressed our main outcomes (i.e. 6MWD, WHO/NYHA functional class, and mortality). All relevant types of participants, interventions, and outcomes have been investigated (Table 1). The evidence we collected is highly relevant to the review questions. For people with pulmonary arterial hypertension with WHO functional class II and III, endothelin receptor antagonists probably increase exercise capacity, improve WHO functional class, prevent WHO functional class deterioration, result in favourable changes in cardiopulmonary haemodynamic variables. However, they are less effective in reducing dyspnoea and mortality.
Certainty of the evidence
All data included in the meta‐analysis were from randomised, placebo‐controlled clinical trials, and the participants, treating clinicians, and assessors were blinded. All but two trials, EARLY; EDITA, performed analyses based on intention‐to‐treat for their prespecified primary outcomes. However, the risk of attrition bias is high in the included trials due to the high occurrence of missing data (Table 1). On this basis we consider the overall certainty of the evidence to be moderate. Overall, the results of the studies included in the review are consistent, confirming the internal validity of the results of the review.
Potential biases in the review process
Many studies used Borg dyspnoea score as an outcome but provided limited data, thus bias may be introduced into the review. We analysed hepatic toxicity as an adverse event because this was listed in the protocol and approved. However, there are other serious adverse events related to ERAs which limit its use in clinical practice.
Agreements and disagreements with other studies or reviews
Our results are consistent with recent overviews (Duo‐Ji 2017; Wang 2018). Our review is more current than these overviews and provides more robust data which further confirm the clinical efficacy of ERAs in participants with PAH.
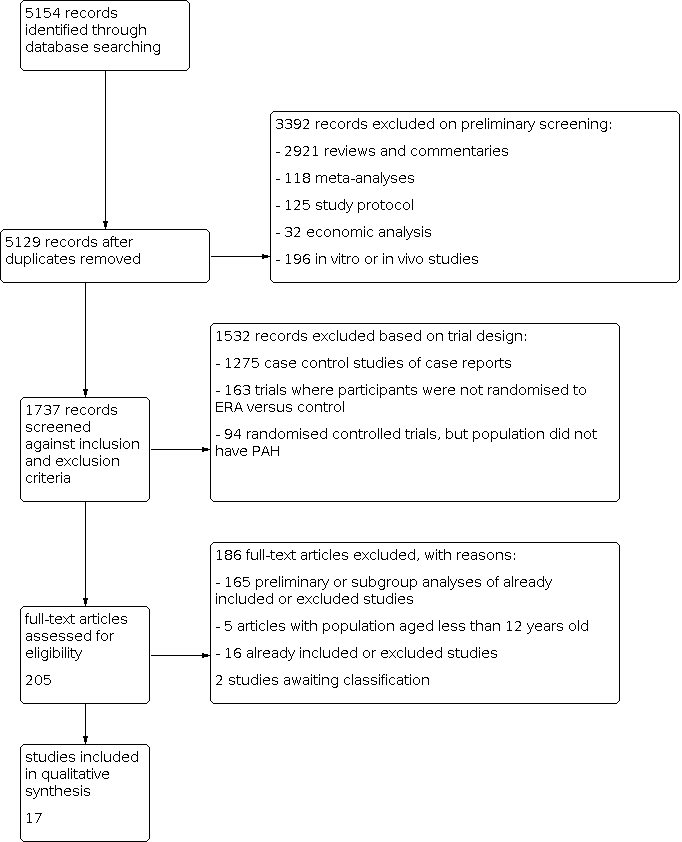
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 1: Change from baseline in 6‐minute walk

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 2: Proportion of participants with improved functional class

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 3: Proportion of participants with deteriorated functional class

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 4: Change from baseline in Borg dyspnoea index

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 5: Mortality

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 6: Change from baseline in mean pulmonary artery pressure

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 7: Change from baseline in pulmonary vascular resistance

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 8: Pulmonary vascular resistance

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 9: Ratio of geometric mean PVR

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 10: Change from baseline in cardiac index

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 11: Change from baseline in SpO 2

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 12: Hepatic toxicity

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 1: 6‐minute walk

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 2: Proportion of participants with improved functional class

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 3: Proportion of participants with deteriorated functional class

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 4: Symptoms

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 5: Mortality

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 6: Cardiac index

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 1: Change from baseline in 6‐minute walk

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 2: Proportion of participants with improved functional class

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 3: Proportion of participants with deteriorated functional class

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 4: Mortality

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 5: Change from baseline in mean pulmonary arterial pressure

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 6: Change from baseline in pulmonary vascular resistance

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 7: Change from baseline in SpO 2
| Endothelin receptor antagonists compared to placebo for pulmonary arterial hypertension | ||||||
| Participant or population: pulmonary arterial hypertension | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with endothelin receptor antagonists | |||||
| Change from baseline in 6MWD (m) mean duration of study 16.3 weeks | The weighted mean change on control was −4.56 m. | MD 25.06 higher | ‐ | 2739 | ⊕⊕⊕⊝ | Higher is better for 6MWD. |
| Proportion of participants with improved functional class mean duration of study 16.8 weeks | 175 per 1000 | 230 per 1000 | OR 1.41 | 3060 | ⊕⊕⊕⊝ | Participants with high OR are more likely to achieve functional improvement. |
| Change from baseline in BDI mean duration of study 14.3 weeks | The weighted mean change on control was 0.25 higher. | MD 0.43 lower | ‐ | 788 | ⊕⊕⊝⊝Low2 | Symptoms are worse with higher score of BDI. |
| Mortality mean duration of study 30.2 weeks | 73 per 1000 | 58 per 1000 | OR 0.78 | 2889 | ⊕⊕⊝⊝ | Participants with lower OR are less likely to die. |
| Change from baseline in mean PAP (mmHg) mean duration of study 17.1 weeks | The weighted mean change on control was 0.53 higher. | MD 4.65 lower | ‐ | 729 | ⊕⊕⊕⊝ | Participants are worse with higher pulmonary artery pressure. |
| Change from baseline in PVR (dyn/s/cm5) mean duration of study 15.7 weeks | The weighted mean change on control was 63.55 higher. | MD 236.24 lower | ‐ | 586 | ⊕⊕⊕⊝ | Participants are worse with higher pulmonary vascular resistance. |
| Hepatic toxicity mean duration of study 25 weeks | 37 per 1000 | 67 per 1000 | OR 1.88 | 2250 | ⊕⊕⊕⊝ | Participants with higher OR are more likely to suffer hepatic toxicity. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Incomplete outcome data (attrition bias) due to missing data imbalanced between intervention and control groups in most of the included studies (−1 level). | ||||||
| Study | N | Country | Intervention | Control | Outcomes |
|---|---|---|---|---|---|
| AMBITION | 747 | International | Ambrisentan + tadalafil or ambrisentan | Tadalafil + placebo | Primary outcome: time to the first event of clinical failure Secondary outcomes: change from baseline in NT‐proBNP level, 6MWD, WHO FC, and Borg dyspnoea index |
| ARIES‐1 | 201 | International | Ambrisentan (5 mg/day or 10 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 physical functioning scale |
| ARIES‐2 | 192 | International | Ambrisentan (2.5 mg/day or 5 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 |
| BREATHE‐1 | 213 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD |
| BREATHE‐2 | 33 | International | Bosentan | Placebo | Primary outcome: change from baseline to week 16 in TPR |
| BREATHE‐5 | 54 | International | Bosentan | Placebo | Primary outcome: change from baseline in SpO2 and PVR |
| Channick 2001 | 32 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD |
| COMPASS‐2 | 334 | International | Bosentan | Placebo | Primary outcome: time to the first morbidity/mortality event Secondary outcomes: change in 6MWD, WHO FC, time to the first occurrence of death from any cause, hospitalisation for PAH or start of intravenous prostanoid therapy, atrial septostomy, or lung transplant. |
| EARLY | 185 | International | Bosentan | Placebo | Primary outcomes: PVR and change from baseline in 6MWD |
| EDITA | 38 | Germany | Ambrisentan | Placebo | Primary outcome: change in mPAP Secondary outcomes: change in WHO FC, change in cardiac index, change in PVR, symptoms of SSc, quality of life (SF‐36), lung function tests, right heart dimensions and function, NT‐proBNP, measures of disease‐related progression |
| MAESTRO | 150 | International | Macitentan | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC and Borg dyspnoea index |
| PORTICO | 85 | International | Macitentan | Placebo | Primary outcome: change from baseline to PVR Secondary outcomes: change from baseline in RAP, mPAP, cardiac index, total pulmonary resistance, SvO2, NT‐proBNP, 6MWD, and WHO FC |
| SERAPH | 26 | British | Bosentan | Sildenafil | Primary outcome: change in right ventricle mass from baseline |
| SERAPHIN | 742 | International | Macitentan | Placebo | Primary outcome: time from the initiation of treatment to the first event related to pulmonary arterial hypertension Secondary outcomes: change from baseline in 6MWD, percentage of participants with an improvement in WHO FC, death due to PAH or hospitalisations for PAH, and death from |
| STRIDE‐1 | 178 | International | Sitaxsentan | Placebo | Primary outcome: peak oxygen consumption |
| STRIDE‐2 | 245 | International | Sitaxsentan | Placebo | Primary outcome: change from baseline in 6MWD |
| STRIDE‐4 | 98 | International | Sitaxsentan | Placebo | Primary efficacy endpoint was the change in 6MWD from baseline to week 18. Secondary outcomes: changes in WHO FC from baseline at each assessment and time to clinical worsening, Borg dyspnoea index |
| 6MWD: 6‐minute walk distance; BNP: B‐type natriuretic peptide; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; MVO2: mixed venous oxygen saturation; NT‐proBNP: N‐terminal pro–brain natriuretic peptide; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PVR: pulmonary vascular resistance; SF‐36: 36‐item Short Form Health Survey; SpO2: oxygen saturation; SSc: systemic sclerosis; SvO2: mixed venous oxygen saturation; TPR: total pulmonary resistance; WHO FC: World Health Organization functional class | |||||
| Outcome | Including combination therapy | Excluding combination therapy |
|---|---|---|
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 25.65 (16.80 to 34.49) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.52 (1.22 to 1.91) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.82 (0.58 to 1.17) |
| 6MWD: 6‐minute walk distance; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; WHO FC: World Health Organization functional class | ||
| Outcome | Including combination therapy | Excluding combination therapy |
|---|---|---|
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 27.90 (20.96 to 34.83) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.46 (1.19 to 1.78) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.77 (0.57 to 1.05) |
| 6MWD: 6‐minute walk distance; CHD: congenital heart disease; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; PAH: pulmonary arterial hypertension; SSc: systemic sclerosis; WHO FC: World Health Organization functional class | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Change from baseline in 6‐minute walk Show forest plot | 14 | 2739 | Mean Difference (IV, Random, 95% CI) | 25.06 [17.13, 32.99] |
| 1.1.1 Non‐selective ERA | 8 | 1860 | Mean Difference (IV, Random, 95% CI) | 20.51 [10.03, 31.00] |
| 1.1.2 Selective ERA | 6 | 879 | Mean Difference (IV, Random, 95% CI) | 33.48 [23.12, 43.83] |
| 1.2 Proportion of participants with improved functional class Show forest plot | 15 | 3060 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.16, 1.70] |
| 1.2.1 Non‐selective ERAs | 9 | 1896 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.13, 1.87] |
| 1.2.2 Selective ERAs | 6 | 1164 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.01, 1.80] |
| 1.3 Proportion of participants with deteriorated functional class Show forest plot | 13 | 2347 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.72] |
| 1.3.1 Non‐selective ERA | 7 | 1121 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.42] |
| 1.3.2 Selective ERAs | 6 | 1226 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.60] |
| 1.4 Change from baseline in Borg dyspnoea index Show forest plot | 7 | 788 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.90, 0.04] |
| 1.4.1 Non‐selective ERAs | 3 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.58, 1.03] |
| 1.4.2 Selective ERAs | 4 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.01, 0.14] |
| 1.5 Mortality Show forest plot | 12 | 2889 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.07] |
| 1.5.1 Non‐selective ERAs | 7 | 1759 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.23] |
| 1.5.2 Selective ERAs | 5 | 1130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.94] |
| 1.6 Change from baseline in mean pulmonary artery pressure Show forest plot | 8 | 729 | Mean Difference (IV, Random, 95% CI) | ‐4.65 [‐6.05, ‐3.26] |
| 1.6.1 Non‐selective ERAs | 6 | 519 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐7.30, ‐4.27] |
| 1.6.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐5.31, 0.00] |
| 1.7 Change from baseline in pulmonary vascular resistance Show forest plot | 7 | 586 | Mean Difference (IV, Random, 95% CI) | ‐236.24 [‐333.21, ‐139.26] |
| 1.7.1 Non‐selective ERAs | 5 | 376 | Mean Difference (IV, Random, 95% CI) | ‐281.74 [‐395.85, ‐167.63] |
| 1.7.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐173.73 [‐332.52, ‐14.94] |
| 1.8 Pulmonary vascular resistance Show forest plot | 2 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐288.59 [‐472.18, ‐104.99] |
| 1.9 Ratio of geometric mean PVR Show forest plot | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 Selective ERAs | 0 | Ratio of Geometric mean (IV, Random, 95% CI) | Not estimable | |
| 1.9.2 Non‐selective ERAs | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | 0.69 [0.60, 0.80] | |
| 1.10 Change from baseline in cardiac index Show forest plot | 7 | 718 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.35, 0.65] |
| 1.10.1 Non‐selective ERAs | 5 | 509 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.34, 0.77] |
| 1.10.2 Selective ERAs | 2 | 209 | Mean Difference (IV, Random, 95% CI) | 0.39 [0.23, 0.54] |
| 1.11 Change from baseline in SpO 2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12 Hepatic toxicity Show forest plot | 11 | 2250 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.91, 3.90] |
| 1.12.1 Non‐selective ERAs | 9 | 1888 | Odds Ratio (M‐H, Random, 95% CI) | 2.33 [0.98, 5.56] |
| 1.12.2 Selective ERAs | 2 | 362 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 6‐minute walk Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Proportion of participants with improved functional class Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Proportion of participants with deteriorated functional class Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Mortality Show forest plot | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.36] |
| 2.6 Cardiac index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Change from baseline in 6‐minute walk Show forest plot | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| 3.1.1 Non‐selective ERA | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| 3.1.2 Selective ERA | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.2 Proportion of participants with improved functional class Show forest plot | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| 3.2.1 Non‐selective ERAs | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| 3.2.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Proportion of participants with deteriorated functional class Show forest plot | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| 3.3.1 Non‐selective ERA | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| 3.3.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.4 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 Non‐selective ERAs | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 Selective ERAs | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5 Change from baseline in mean pulmonary arterial pressure Show forest plot | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| 3.5.1 Non‐selective ERAs | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| 3.5.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.6 Change from baseline in pulmonary vascular resistance Show forest plot | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| 3.6.1 Non‐selective ERAs | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| 3.6.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.7 Change from baseline in SpO 2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

