Endothelin receptor antagonists for pulmonary arterial hypertension
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group | |
| Participants | Baseline characteristics Combination (ambrisentan plus tadalafil) therapy
Ambrisentan monotherapy
Tadalafil monotherapy
| |
| Interventions | Intervention characteristics Combination (ambrisentan plus tadalafil) therapy
Ambrisentan monotherapy
Tadalafil monotherapy
| |
| Outcomes | 6MWD Mortality WHO FC improved WHO FC deteriorated | |
| Identification | Sponsorship source: Gilead Sciences and GlaxoSmithKline Country: Italy Setting: International, multicentre study Comments: p. 843, the end of study Author's name: Nazzareno Galiè Institution: Department of Experimental, Diagnostic, and Specialty Medicine, University of Bologna Email: [email protected] Address: Massarenti 9, Bologna 40138, Italy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Randomisation was performed centrally by the study sponsors using an interactive voice‐response system. p. 836, the first paragraph in section "study procedures" |
| Allocation concealment (selection bias) | Low risk | "Matching placebo tablets were administered to maintain blinding" in appendix materials. p. 22 |
| Blinding (performance bias and detection bias) | Low risk | Judgement comment: "Matching placebo tablets were administered to maintain blinding" in appendix materials. p. 22 |
| Incomplete outcome data (attrition bias) | High risk | Outcome data were missing in 3 intervention groups and missing data were not balanced. 21.7% (55/253) participants discontinued from the combination therapy group, 34.1% (43/126) participants from the ambrisentan group, and 24.0% (29/121) participants from the tadalafil group. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol published at ClinicalTrials.gov. |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants | Baseline characteristics Placebo
Ambrisentan (5 mg/day)
Ambrisentan (10 mg/day)
| |
| Interventions | Participants were randomised to receive placebo or ambrisentan 5 or 10 mg orally daily for 12 weeks.
| |
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP and 36‐item Short Form Health Survey (SF‐36) physical functioning scale | |
| Identification | ‐ | |
| Notes | The combined data of ARIES‐1 and ARIES‐2 showed that 21 participants discontinued in the placebo group in ARIES‐1 and ARIES‐2, 6 participants in the ambrisentan 2.5 mg group, 9 participants in the ambrisentan 5 mg group, and 5 participants in the ambrisentan 10 mg group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A central randomisation scheme stratified by PAH cause (idiopathic versus other PAH causes) was used to assign participants." |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | Outcome data were missing in the intervention groups, and missing data were not balanced. A total of 41 participants in ARIES‐1 and ARIES‐2 studies discontinued prematurely during the 12‐week treatment period: 21 (15.9%) receiving placebo and 20 (7.6%) receiving |
| Selective reporting (reporting bias) | Low risk | Prespecified primary outcome was reported in the results. ClinicalTrials.gov Identifier: NCT00091598 |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants | Baseline characteristics Placebo
Ambrisentan (5 mg/day)
Ambrisentan (5 mg/day)
| |
| Interventions | Participants were randomised to receive placebo or ambrisentan 2.5 or 5 mg daily for 12 weeks.
| |
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP and 36‐item Short Form Health Survey (SF‐36) physical functioning scale | |
| Identification | ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A central randomisation scheme stratified by PAH cause (idiopathic versus other PAH causes) was used to assign participants." |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | Outcome data were missing in the intervention groups, and missing data were not balanced. A total of 41 participants in ARIES‐1 and ARIES‐2 studies discontinued prematurely during the 12‐week treatment period: 21 (15.9%) receiving placebo and 20 (7.6%) receiving |
| Selective reporting (reporting bias) | Low risk | Prespecified primary outcome was reported in the results. ClinicalTrials.gov Identifier: NCT00091598 |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted according to a computer‐generated random number. | |
| Participants | Baseline characteristics Placebo
Bosentan
| |
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg or 250 mg twice daily) for 12 weeks Control group: placebo
| |
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: Borg dyspnoea index; WHO FC | |
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation by pharmacy‐controlled randomisation |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | 9% participants discontinued from the placebo group due to suffering clinical worsening of symptoms of PAH or syncope occurred, as compared with no participants in the bosentan group. The missing data were not balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted according to a computer‐generated random number. | |
| Participants | Baseline characteristics Placebo/epoprostenol
Bosentan/epoprostenol
| |
| Interventions | 33 participants with PAH started prostacyclin treatment (2 ng/kg/min starting dose, up to 14 ± 2 ng/kg/min at week 16) and were randomised for 16 weeks in a 2:1 ratio to bosentan (62.5 mg twice daily for 4 weeks then 125 mg twice daily) or placebo.
| |
| Outcomes | Primary outcomes: change from baseline to week 16 in TPR | |
| Identification | ‐ | |
| Notes | The standard deviations of change from baseline in 6MWD were estimated from figures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation by pharmacy‐controlled randomisation |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | Outcome data were missing (1/11 in the placebo group versus 4/22 in the bosentan group), and missing data were not balanced. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted according to a computer‐generated random number. | |
| Participants | Baseline characteristics Placebo
Bosentan
| |
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg twice daily) for 12 weeks. Participants who did not tolerate the target dose of 125 mg twice daily could be down titrated to the starting dose (62.5 mg twice daily). Control group: placebo
| |
| Outcomes | Primary outcomes: change from baseline in SpO2 and PVR index Secondary outcomes: change from baseline in 6MWD, WHO FC, cardiac index, PVR, PAP, and RAP | |
| Identification | ‐ | |
| Notes | Standard deviations were estimated from standard errors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was controlled by study medication packaging (Almedica HPS AG, Reinach, Switzerland)." |
| Allocation concealment (selection bias) | Low risk | "The investigators, participants, monitors, and sponsor personnel remained blinded to the treatment until closure of the clinical database." p. 49 |
| Blinding (performance bias and detection bias) | Low risk | "The investigators, participants, monitors, and sponsor personnel remained blinded to the treatment until closure of the clinical database." p. 49 |
| Incomplete outcome data (attrition bias) | High risk | Outcome data were missing. 2/17 (11.8%) in the placebo group versus 2/37 (5.4%) in the bosentan group discontinued from the study. Missing data were balanced. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Multicentre, randomised, parallel trial comparing bosentan with placebo for 12 weeks. Randomisation was computer generated with a block size of 3. | |
| Participants | Baseline characteristics Placebo
Bosentan
| |
| Interventions | Intervention group: 62.5 mg bosentan twice daily for the first 4 weeks followed by the target dose (125 mg twice daily) Control group: placebo
| |
| Outcomes | Primary outcomes: 6MWD Secondary outcomes: cardiopulmonary haemodynamics (cardiac index, PVR, mPAP, and mRAP), WHO FC | |
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was computer generated using Drug Labelling System with a block size of three." |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | Outcome data were missing (2/11) in the placebo group, but not in the bosentan group (0/21). Missing data were not balanced. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group | |
| Participants | Baseline characteristics Bosentan
Placebo
| |
| Interventions | Intervention characteristics Bosentan
Placebo
| |
| Outcomes | Mortality WHO FC improved WHO FC worsened Change in 6MWD Hepatic toxicity | |
| Identification | Sponsorship source: Actelion Pharmaceuticals Ltd. Setting: International, multicentre, university‐based hospital Author's name: Vallerie McLaughlin Institution: University of Michigan Health System Email: [email protected] Address: Department of Internal Medicine, Division of Cardiovascular Medicine, University of Michigan Health System, Ann Arbor, MI 48109‐5853, USA | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "COMPASS‐2 was a prospective, international, randomised, double‐blind, placebo‐controlled, event‐driven trial" |
| Allocation concealment (selection bias) | Low risk | "COMPASS‐2 was a prospective, international, randomised, double‐blind, placebo‐controlled, event‐driven trial (www.clinicaltrials.gov identifier number NCT00303459)." |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | 25.7% participants were discontinued from the placebo group and 31.4% from the bosentan group. |
| Selective reporting (reporting bias) | Low risk | Reported according to the protocol published at ClinicalTrials.gov. |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial | |
| Participants | Baseline characteristics Placebo
Bosentan
| |
| Interventions | Participants treated with either bosentan at an initial dose of 62.5 mg twice daily, uptitrating to 125 mg twice daily after 4 weeks, or remaining at 62.5 mg twice daily if bodyweight < 40 kg, or placebo for a 6‐month double‐blind treatment period.
| |
| Outcomes | Primary outcomes: PVR and change from baseline in 6MWD Secondary outcomes: time to clinical worsening, change from baseline to month 6 in WHO FC, Borg dyspnoea index, mPAP, cardiac index, RAP, and mixed venous oxygen saturation | |
| Identification | ‐ | |
| Notes | Standard deviations were estimated from confidence intervals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned in a one to one ratio to receive either bosentan using a centralised integrated voice recognition system." |
| Allocation concealment (selection bias) | Low risk | "This system assigned a unique randomisation number to each participant and designated the correct blinded study medication to be dispensed, both at the start of study treatment and at each scheduled visit. This code was accessible only to authorised individuals who were not involved in the conduct or analysis of the study, until the time of unblinding." |
| Blinding (performance bias and detection bias) | Low risk | "This system assigned a unique randomisation number to each participant and designated the correct blinded study medication to be dispensed, both at the start of study treatment and at each scheduled visit. This code was accessible only to authorised individuals who were not involved in the conduct or analysis of the study, until the time of unblinding." |
| Incomplete outcome data (attrition bias) | Low risk | 12.9% (12/93) participants discontinued bosentan, and 10.8% (10/92) participants discontinued placebo. Missing data were balanced. |
| Selective reporting (reporting bias) | Low risk | Prespecified primary outcome reported in the results. ClinicalTrials.gov Identifier: NCT00091715 |
| Study characteristics | ||
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group | |
| Participants | Baseline characteristics Placebo
Ambrisentan (10 mg/day)
| |
| Interventions | Intervention characteristics Placebo
Ambrisentan (10 mg/day)
| |
| Outcomes | Change in mean PAP Change in WHO FC Change in cardiac index Change in PVR Symptoms of SSc Quality of life (SF‐36) Lung function tests, right NT‐proBNP Measures of disease‐related progression | |
| Identification | Sponsorship source: GlaxoSmithKline Setting: Single‐centre, university‐based hospital Author's name: Ekkehard Grünig Institution: Centre for Pulmonary Hypertension, Thoraxklinik at Heidelberg University Hospital Email: [email protected]‐heidelberg.de Address: Röntgenstrasse 1, 69126 Heidelberg, Germany | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: "The EDITA study was a single‐center (PH Center, Thoraxklinik at Heidelberg University Hospital, Heidelberg, Germany) investigator‐initiated trial using a prospective, randomised, double‐blind (patient and investigator), parallel group, placebo‐controlled, phase IIA clinical study design. Patients were randomised 1:1 to either ambrisentan or placebo by simple randomisation." p. 3 in the study section |
| Allocation concealment (selection bias) | Low risk | "Placebo tablets with the same color and shape as ambrisentan were provided by GlaxoSmithKline." p. 3 in the study section |
| Blinding (performance bias and detection bias) | Low risk | "Placebo tablets with the same color and shape as ambrisentan were provided by GlaxoSmithKline." p. 3 in the study section |
| Incomplete outcome data (attrition bias) | High risk | Outcome data were missing in the intervention group and placebo group, and missing data were not balanced. 2/19 participants discontinued from the intervention group, and 4/19 participants discontinued from the placebo group. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol published at ClinicalTrials.gov. |
| Study characteristics | ||
| Methods | This trial report is on a subset of participants from the BREATHE‐1 study. | |
| Participants | Baseline characteristics Placebo
Bosentan
| |
| Interventions | Same as BREATHE‐1 | |
| Outcomes | Cardiac index | |
| Identification | ‐ | |
| Notes | This is a subgroup analysis of BREATHE‐1 study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See BREATHE‐1. |
| Allocation concealment (selection bias) | Low risk | See BREATHE‐1. |
| Blinding (performance bias and detection bias) | Low risk | See BREATHE‐1. |
| Incomplete outcome data (attrition bias) | High risk | 2 out of 29 participants in the placebo group and 1 out of 56 in the |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group | |
| Participants | Baseline characteristics Macitentan
Placebo
| |
| Interventions | Intervention characteristics Macitentan
Placebo
| |
| Outcomes | 6MWD Mortality WHO FC improved WHO FC deteriorated mPAP PVR Change from baseline in 6MWD Change in SPO2 Change in PAP Change in PVR | |
| Identification | Sponsorship source: Actelion Pharmaceuticals Ltd. Country: International study Setting: Tertiary referral hospital Author's name: Michael A Gatzoulis Institution: The Royal Brompton Hospital Email: [email protected] Address: Sydney Street, London, SW3 6NP, UK | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was based on a prespecified randomisation schedule using randomisation lists generated by an independent Contract Research Organization (Almac Clinical Technologies) and their centralized randomisation system, via an Interactive Voice Response System or Interactive Web Response System." |
| Allocation concealment (selection bias) | Low risk | "Participants and sites remained blinded to their previous treatment allocation." |
| Blinding (performance bias and detection bias) | Low risk | "Participants and sites remained blinded to their previous treatment allocation." |
| Incomplete outcome data (attrition bias) | Low risk | 97% participants in the macitentan group and 98% participants in the placebo group completed the study. |
| Selective reporting (reporting bias) | Low risk | Reported as per protocol published at ClinicalTrials.gov. |
| Study characteristics | ||
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group | |
| Participants | Baseline characteristics Macitentan (10 mg)
Placebo
| |
| Interventions | Intervention characteristics Macitentan (10 mg)
Placebo
| |
| Outcomes | Change in 6MWD Mortality WHO FC improved Change in mPAP Change in cardiac index Change in PVR WHO FC deteriorated | |
| Identification | Sponsorship source: Actelion Pharmaceuticals Ltd. Country: International study Author's name: Olivier Sitbon Email: olivier.sitbon@u‐psud.fr | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned in a 1:1 ratio, with block sizes of four, via an interactive voice and web response system (by independent contract research organisation Almac) to receive either macitentan 10 mg or matching placebo orally once a day." Judgement comment: p. 596, the first paragraph in section "randomisation and masking" |
| Allocation concealment (selection bias) | Low risk | "Participants were randomly assigned in a 1:1 ratio, with block sizes of four, via an interactive voice and web response system (by independent contract research organisation Almac) to receive either macitentan 10 mg or matching placebo orally once a day." Judgement comment: p. 596, the first paragraph in section "randomisation and masking" |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | 9.4% participants in the macitentan group and 2.4% participants in the placebo group discontinued the study. Missing data were observed and imbalanced between groups. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: there was no obvious selective outcome reporting based on the protocol reported in ClinicalTrials.gov. |
| Study characteristics | ||
| Methods | Single‐centre, randomised, head‐to‐head, double‐blind trial | |
| Participants | Baseline characteristics Sildenafil
Bosentan
| |
| Interventions | Intervention group: bosentan (62.5 mg twice daily for first 4 weeks, then uptitrated to 125 mg twice daily)
Control group: sildenafil (50 mg twice daily during the first 4 weeks, then uptitrated to 50 mg 3 times daily)
| |
| Outcomes | Primary outcome: change in right ventricle mass from baseline | |
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The medication was blinded in identical‐looking gelatin capsules and randomised using a computer generated random list by the Hammersmith Hospital pharmacy." |
| Allocation concealment (selection bias) | Low risk | "The medication was blinded in identical‐looking gelatin capsules and randomised using a computer generated random list by the Hammersmith Hospital pharmacy." |
| Blinding (performance bias and detection bias) | Low risk | "The medication was blinded in identical‐looking gelatin capsules and randomised using a computer generated random list by the Hammersmith Hospital pharmacy." |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant discontinued sildenafil because of death, and none discontinued bosentan during the study. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Study design: Randomised controlled trial Study grouping: Parallel group | |
| Participants | Baseline characteristics Placebo
Macitentan (3 mg)
Macitentan (10 mg)
| |
| Interventions | Intervention characteristics Placebo
Macitentan (3 mg)
Macitentan (10 mg)
| |
| Outcomes | Change in 6MWD Mortality WHO FC improved Change in mPAP Change in cardiac index Change in PVR | |
| Identification | Sponsorship source: Actelion Pharmaceuticals Setting: Muticentre, international, university‐based hospital Author's name: Tomás Pulido Institution: Ignacio Chávez National Heart Institute Email: [email protected] Address: Ignacio Chávez National Heart Institute, Juan Badiano 1, 4th Fl., Mexico City, 14080 Mexico | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned via an interactive voice and web response system. |
| Allocation concealment (selection bias) | Low risk | Confirmed with the study sponsor: a central randomisation scheme was used to assign participants, and matching placebo tablets were administered to maintain blinding. |
| Blinding (performance bias and detection bias) | Low risk | "An independent clinical event committee adjudicated, in a blinded fashion" |
| Incomplete outcome data (attrition bias) | High risk | 59.2% participants discontinued placebo versus 45.7% participants discontinued macitentan. The missing data were not balanced. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: clinicaltrials.gov/ct2/show/NCT00660179 |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants | Baseline characteristics Placebo
Sitaxsentan 100 mg
Sitaxsentan 300 mg
| |
| Interventions | Intervention group: 100 mg once daily or 300 mg once daily Control group: placebo
| |
| Outcomes | Primary outcomes: peak oxygen consumption Secondary outcomes: 6MWD, NYHA FC, PAP, cardiac index, and PVR | |
| Identification | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | Low risk | 8.3% (5/60) participants discontinued placebo versus 5.9% (7/118) participants sitaxsentan. The missing data was few and balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial. Randomisation was conducted centrally according to a computer‐generated random number. | |
| Participants | Baseline characteristics Placebo
Sitaxsentan 50 mg
Sitaxsentan 100 mg
Bosentan
| |
| Interventions | Participants were randomised to receive placebo, sitaxsentan 50 mg or 100 mg orally once daily, or open‐label bosentan for 18 weeks.
| |
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening | |
| Identification | ‐ | |
| Notes | STRIDE‐2 trial compared sitaxsentan with both a placebo arm and an open‐label bosentan arm, therefore we have listed data for participants treated with bosentan and placebo for reference; however, we did not pool these data with the other data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally according to a computer‐generated random number table. Randomization was 1:1:1:1." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | High risk | 17.7% (5/60) participants discontinued placebo versus 8.2% (20/245) sitaxsentan. The missing data were imbalanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
| Study characteristics | ||
| Methods | Multicentre, randomised, placebo‐controlled, parallel, double‐blind trial | |
| Participants | Baseline characteristics Placebo
Sitaxsentan 50 mg
Sitaxsentan 100 mg
| |
| Interventions | Participants were randomised to receive placebo, sitaxsentan 50 mg, or sitaxsentan 100 mg orally once daily for 18 weeks.
| |
| Outcomes | Primary outcomes: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening | |
| Identification | ‐ | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally and stratified by centre in blocks according to a computer‐generated random number table." |
| Blinding (performance bias and detection bias) | Low risk | Confirmed with the study sponsor: matching placebo tablets were administered to maintain blinding. |
| Incomplete outcome data (attrition bias) | Low risk | 12.5% (4/32) participants discontinued placebo versus 10.9% (7/64) sitaxsentan. The missing data were balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol. |
6MWD: 6‐minute walk distance; BNP: B‐type natriuretic peptide; CAD: coronary artery disease; CHD: congenital heart disease; CTD: connective tissue disease; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PAP: pulmonary artery pressure; PDE5: phosphodiesterase type 5; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; RAP: right atrial pressure; SD: standard deviation; SF‐36: 36‐item Short Form Health Survey; SpO2: oxygen saturation; SSc: systemic sclerosis; WHO FC: World Health Organization functional class
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| RCT, but populations did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants were not randomised to an ERA. | |
| Long‐term follow‐up data of RCT using sitaxsentan | |
| Participants were not randomised to an ERA. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Mixed population used. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Duplicate publications | |
| Participants were not randomised to an ERA. | |
| Participants were not randomised to an ERA. | |
| Participants were not randomised to an ERA. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Long‐term follow‐up data of RCT using sitaxsentan | |
| Long‐term follow‐up data of RCT using sitaxsentan | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants did not have PAH. | |
| Participants were not randomised to an ERA. | |
| Participants did not have PAH. |
ERA: endothelin receptor antagonists; PAH: pulmonary arterial hypertension; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
| Methods | Randomisation method was not clear. |
| Participants | Pulmonary arterial hypertension in systemic sclerosis Inclusion criteria:
Exclusion criteria:
|
| Interventions | Sildenafil 20 mg and bosentan 62.5 mg versus sildenafil 20 mg and placebo |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Notes |
| Methods | Randomisation method was not clear. |
| Participants | PAH patients with WHO FC II or III |
| Interventions | Intervention characteristics Combination (ambrisentan plus tadalafil) therapy
Ambrisentan monotherapy
Tadalafil monotherapy
|
| Outcomes | Change in mPAP Change in RAP Change in PVR Change from baseline in 6MWD Change in MVO2 |
| Notes |
6MWD: 6‐minute walk distance; ERA: endothelin receptor antagonist; mPAP: mean pulmonary artery pressure; MVO2: mixed venous oxygen saturation; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PAP: pulmonary artery pressure; PDE5: phosphodiesterase type 5; PVR: pulmonary vascular resistance; RAP: right atrial pressure; NT‐proBNP; N‐terminal pro B‐type natriuretic peptide; SSc: systemic sclerosis; TPR: total pulmonary resistance; WHO FC: World Health Organization functional class
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Change from baseline in 6‐minute walk Show forest plot | 14 | 2739 | Mean Difference (IV, Random, 95% CI) | 25.06 [17.13, 32.99] |
| Analysis 1.1  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 1: Change from baseline in 6‐minute walk | ||||
| 1.1.1 Non‐selective ERA | 8 | 1860 | Mean Difference (IV, Random, 95% CI) | 20.51 [10.03, 31.00] |
| 1.1.2 Selective ERA | 6 | 879 | Mean Difference (IV, Random, 95% CI) | 33.48 [23.12, 43.83] |
| 1.2 Proportion of participants with improved functional class Show forest plot | 15 | 3060 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.16, 1.70] |
| Analysis 1.2  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 2: Proportion of participants with improved functional class | ||||
| 1.2.1 Non‐selective ERAs | 9 | 1896 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.13, 1.87] |
| 1.2.2 Selective ERAs | 6 | 1164 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.01, 1.80] |
| 1.3 Proportion of participants with deteriorated functional class Show forest plot | 13 | 2347 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.72] |
| Analysis 1.3  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 3: Proportion of participants with deteriorated functional class | ||||
| 1.3.1 Non‐selective ERA | 7 | 1121 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.42] |
| 1.3.2 Selective ERAs | 6 | 1226 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.60] |
| 1.4 Change from baseline in Borg dyspnoea index Show forest plot | 7 | 788 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.90, 0.04] |
| Analysis 1.4  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 4: Change from baseline in Borg dyspnoea index | ||||
| 1.4.1 Non‐selective ERAs | 3 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.58, 1.03] |
| 1.4.2 Selective ERAs | 4 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.01, 0.14] |
| 1.5 Mortality Show forest plot | 12 | 2889 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.07] |
| Analysis 1.5  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 5: Mortality | ||||
| 1.5.1 Non‐selective ERAs | 7 | 1759 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.23] |
| 1.5.2 Selective ERAs | 5 | 1130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.94] |
| 1.6 Change from baseline in mean pulmonary artery pressure Show forest plot | 8 | 729 | Mean Difference (IV, Random, 95% CI) | ‐4.65 [‐6.05, ‐3.26] |
| Analysis 1.6  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 6: Change from baseline in mean pulmonary artery pressure | ||||
| 1.6.1 Non‐selective ERAs | 6 | 519 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐7.30, ‐4.27] |
| 1.6.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐5.31, 0.00] |
| 1.7 Change from baseline in pulmonary vascular resistance Show forest plot | 7 | 586 | Mean Difference (IV, Random, 95% CI) | ‐236.24 [‐333.21, ‐139.26] |
| Analysis 1.7  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 7: Change from baseline in pulmonary vascular resistance | ||||
| 1.7.1 Non‐selective ERAs | 5 | 376 | Mean Difference (IV, Random, 95% CI) | ‐281.74 [‐395.85, ‐167.63] |
| 1.7.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐173.73 [‐332.52, ‐14.94] |
| 1.8 Pulmonary vascular resistance Show forest plot | 2 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐288.59 [‐472.18, ‐104.99] |
| Analysis 1.8  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 8: Pulmonary vascular resistance | ||||
| 1.9 Ratio of geometric mean PVR Show forest plot | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 9: Ratio of geometric mean PVR | ||||
| 1.9.1 Selective ERAs | 0 | Ratio of Geometric mean (IV, Random, 95% CI) | Not estimable | |
| 1.9.2 Non‐selective ERAs | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | 0.69 [0.60, 0.80] | |
| 1.10 Change from baseline in cardiac index Show forest plot | 7 | 718 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.35, 0.65] |
| Analysis 1.10  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 10: Change from baseline in cardiac index | ||||
| 1.10.1 Non‐selective ERAs | 5 | 509 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.34, 0.77] |
| 1.10.2 Selective ERAs | 2 | 209 | Mean Difference (IV, Random, 95% CI) | 0.39 [0.23, 0.54] |
| 1.11 Change from baseline in SpO 2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.11  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 11: Change from baseline in SpO 2 | ||||
| 1.12 Hepatic toxicity Show forest plot | 11 | 2250 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.91, 3.90] |
| Analysis 1.12  Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 12: Hepatic toxicity | ||||
| 1.12.1 Non‐selective ERAs | 9 | 1888 | Odds Ratio (M‐H, Random, 95% CI) | 2.33 [0.98, 5.56] |
| 1.12.2 Selective ERAs | 2 | 362 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 6‐minute walk Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 1: 6‐minute walk | ||||
| 2.2 Proportion of participants with improved functional class Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 2: Proportion of participants with improved functional class | ||||
| 2.3 Proportion of participants with deteriorated functional class Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 3: Proportion of participants with deteriorated functional class | ||||
| 2.4 Symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4  Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 4: Symptoms | ||||
| 2.5 Mortality Show forest plot | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.36] |
| Analysis 2.5  Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 5: Mortality | ||||
| 2.6 Cardiac index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 6: Cardiac index | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Change from baseline in 6‐minute walk Show forest plot | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| Analysis 3.1  Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 1: Change from baseline in 6‐minute walk | ||||
| 3.1.1 Non‐selective ERA | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| 3.1.2 Selective ERA | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.2 Proportion of participants with improved functional class Show forest plot | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| Analysis 3.2  Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 2: Proportion of participants with improved functional class | ||||
| 3.2.1 Non‐selective ERAs | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| 3.2.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Proportion of participants with deteriorated functional class Show forest plot | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| Analysis 3.3  Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 3: Proportion of participants with deteriorated functional class | ||||
| 3.3.1 Non‐selective ERA | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| 3.3.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.4 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 4: Mortality | ||||
| 3.4.1 Non‐selective ERAs | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 Selective ERAs | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5 Change from baseline in mean pulmonary arterial pressure Show forest plot | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| Analysis 3.5  Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 5: Change from baseline in mean pulmonary arterial pressure | ||||
| 3.5.1 Non‐selective ERAs | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| 3.5.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.6 Change from baseline in pulmonary vascular resistance Show forest plot | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| Analysis 3.6  Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 6: Change from baseline in pulmonary vascular resistance | ||||
| 3.6.1 Non‐selective ERAs | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| 3.6.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.7 Change from baseline in SpO 2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.7  Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 7: Change from baseline in SpO 2 | ||||
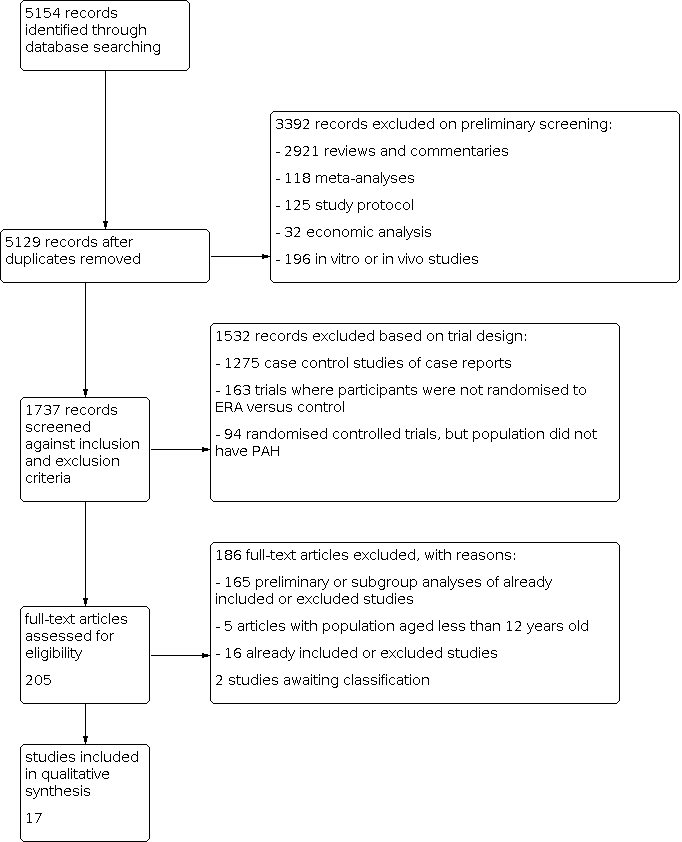
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 1: Change from baseline in 6‐minute walk

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 2: Proportion of participants with improved functional class

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 3: Proportion of participants with deteriorated functional class

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 4: Change from baseline in Borg dyspnoea index

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 5: Mortality

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 6: Change from baseline in mean pulmonary artery pressure

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 7: Change from baseline in pulmonary vascular resistance

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 8: Pulmonary vascular resistance

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 9: Ratio of geometric mean PVR

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 10: Change from baseline in cardiac index

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 11: Change from baseline in SpO 2

Comparison 1: Endothelin receptor antagonists versus placebo, Outcome 12: Hepatic toxicity

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 1: 6‐minute walk

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 2: Proportion of participants with improved functional class

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 3: Proportion of participants with deteriorated functional class

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 4: Symptoms

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 5: Mortality

Comparison 2: Endothelin receptor antagonists versus PDE5 inhibitor, Outcome 6: Cardiac index

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 1: Change from baseline in 6‐minute walk

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 2: Proportion of participants with improved functional class

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 3: Proportion of participants with deteriorated functional class

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 4: Mortality

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 5: Change from baseline in mean pulmonary arterial pressure

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 6: Change from baseline in pulmonary vascular resistance

Comparison 3: Endothelin receptor antagonists in Eisenmenger syndrome, Outcome 7: Change from baseline in SpO 2
| Endothelin receptor antagonists compared to placebo for pulmonary arterial hypertension | ||||||
| Participant or population: pulmonary arterial hypertension | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with placebo | Risk with endothelin receptor antagonists | |||||
| Change from baseline in 6MWD (m) mean duration of study 16.3 weeks | The weighted mean change on control was −4.56 m. | MD 25.06 higher | ‐ | 2739 | ⊕⊕⊕⊝ | Higher is better for 6MWD. |
| Proportion of participants with improved functional class mean duration of study 16.8 weeks | 175 per 1000 | 230 per 1000 | OR 1.41 | 3060 | ⊕⊕⊕⊝ | Participants with high OR are more likely to achieve functional improvement. |
| Change from baseline in BDI mean duration of study 14.3 weeks | The weighted mean change on control was 0.25 higher. | MD 0.43 lower | ‐ | 788 | ⊕⊕⊝⊝Low2 | Symptoms are worse with higher score of BDI. |
| Mortality mean duration of study 30.2 weeks | 73 per 1000 | 58 per 1000 | OR 0.78 | 2889 | ⊕⊕⊝⊝ | Participants with lower OR are less likely to die. |
| Change from baseline in mean PAP (mmHg) mean duration of study 17.1 weeks | The weighted mean change on control was 0.53 higher. | MD 4.65 lower | ‐ | 729 | ⊕⊕⊕⊝ | Participants are worse with higher pulmonary artery pressure. |
| Change from baseline in PVR (dyn/s/cm5) mean duration of study 15.7 weeks | The weighted mean change on control was 63.55 higher. | MD 236.24 lower | ‐ | 586 | ⊕⊕⊕⊝ | Participants are worse with higher pulmonary vascular resistance. |
| Hepatic toxicity mean duration of study 25 weeks | 37 per 1000 | 67 per 1000 | OR 1.88 | 2250 | ⊕⊕⊕⊝ | Participants with higher OR are more likely to suffer hepatic toxicity. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Incomplete outcome data (attrition bias) due to missing data imbalanced between intervention and control groups in most of the included studies (−1 level). | ||||||
| Study | N | Country | Intervention | Control | Outcomes |
|---|---|---|---|---|---|
| AMBITION | 747 | International | Ambrisentan + tadalafil or ambrisentan | Tadalafil + placebo | Primary outcome: time to the first event of clinical failure Secondary outcomes: change from baseline in NT‐proBNP level, 6MWD, WHO FC, and Borg dyspnoea index |
| ARIES‐1 | 201 | International | Ambrisentan (5 mg/day or 10 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 physical functioning scale |
| ARIES‐2 | 192 | International | Ambrisentan (2.5 mg/day or 5 mg/day) | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC, Borg dyspnoea index and time to clinical worsening, plasma BNP, and SF‐36 |
| BREATHE‐1 | 213 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD |
| BREATHE‐2 | 33 | International | Bosentan | Placebo | Primary outcome: change from baseline to week 16 in TPR |
| BREATHE‐5 | 54 | International | Bosentan | Placebo | Primary outcome: change from baseline in SpO2 and PVR |
| Channick 2001 | 32 | International | Bosentan | Placebo | Primary outcome: change from baseline in 6MWD |
| COMPASS‐2 | 334 | International | Bosentan | Placebo | Primary outcome: time to the first morbidity/mortality event Secondary outcomes: change in 6MWD, WHO FC, time to the first occurrence of death from any cause, hospitalisation for PAH or start of intravenous prostanoid therapy, atrial septostomy, or lung transplant. |
| EARLY | 185 | International | Bosentan | Placebo | Primary outcomes: PVR and change from baseline in 6MWD |
| EDITA | 38 | Germany | Ambrisentan | Placebo | Primary outcome: change in mPAP Secondary outcomes: change in WHO FC, change in cardiac index, change in PVR, symptoms of SSc, quality of life (SF‐36), lung function tests, right heart dimensions and function, NT‐proBNP, measures of disease‐related progression |
| MAESTRO | 150 | International | Macitentan | Placebo | Primary outcome: change from baseline in 6MWD Secondary outcomes: change from baseline in WHO FC and Borg dyspnoea index |
| PORTICO | 85 | International | Macitentan | Placebo | Primary outcome: change from baseline to PVR Secondary outcomes: change from baseline in RAP, mPAP, cardiac index, total pulmonary resistance, SvO2, NT‐proBNP, 6MWD, and WHO FC |
| SERAPH | 26 | British | Bosentan | Sildenafil | Primary outcome: change in right ventricle mass from baseline |
| SERAPHIN | 742 | International | Macitentan | Placebo | Primary outcome: time from the initiation of treatment to the first event related to pulmonary arterial hypertension Secondary outcomes: change from baseline in 6MWD, percentage of participants with an improvement in WHO FC, death due to PAH or hospitalisations for PAH, and death from |
| STRIDE‐1 | 178 | International | Sitaxsentan | Placebo | Primary outcome: peak oxygen consumption |
| STRIDE‐2 | 245 | International | Sitaxsentan | Placebo | Primary outcome: change from baseline in 6MWD |
| STRIDE‐4 | 98 | International | Sitaxsentan | Placebo | Primary efficacy endpoint was the change in 6MWD from baseline to week 18. Secondary outcomes: changes in WHO FC from baseline at each assessment and time to clinical worsening, Borg dyspnoea index |
| 6MWD: 6‐minute walk distance; BNP: B‐type natriuretic peptide; mPAP: mean pulmonary artery pressure; mRAP: mean right atrial pressure; MVO2: mixed venous oxygen saturation; NT‐proBNP: N‐terminal pro–brain natriuretic peptide; NYHA FC: New York Heart Association functional class; PAH: pulmonary arterial hypertension; PVR: pulmonary vascular resistance; SF‐36: 36‐item Short Form Health Survey; SpO2: oxygen saturation; SSc: systemic sclerosis; SvO2: mixed venous oxygen saturation; TPR: total pulmonary resistance; WHO FC: World Health Organization functional class | |||||
| Outcome | Including combination therapy | Excluding combination therapy |
|---|---|---|
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 25.65 (16.80 to 34.49) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.52 (1.22 to 1.91) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.82 (0.58 to 1.17) |
| 6MWD: 6‐minute walk distance; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; WHO FC: World Health Organization functional class | ||
| Outcome | Including combination therapy | Excluding combination therapy |
|---|---|---|
| Change in 6MWD, mean with 95% CI | 25.06 (17.13 to 32.99) | 27.90 (20.96 to 34.83) |
| WHO/NYHA FC improvement, OR with 95% CI | 1.41 (1.16 to 1.70) | 1.46 (1.19 to 1.78) |
| Mortality, OR with 95% CI | 0.78 (0.58 to 1.07) | 0.77 (0.57 to 1.05) |
| 6MWD: 6‐minute walk distance; CHD: congenital heart disease; CI: confidence interval; NYHA FC: New York Heart Association functional class; OR: odds ratio; PAH: pulmonary arterial hypertension; SSc: systemic sclerosis; WHO FC: World Health Organization functional class | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Change from baseline in 6‐minute walk Show forest plot | 14 | 2739 | Mean Difference (IV, Random, 95% CI) | 25.06 [17.13, 32.99] |
| 1.1.1 Non‐selective ERA | 8 | 1860 | Mean Difference (IV, Random, 95% CI) | 20.51 [10.03, 31.00] |
| 1.1.2 Selective ERA | 6 | 879 | Mean Difference (IV, Random, 95% CI) | 33.48 [23.12, 43.83] |
| 1.2 Proportion of participants with improved functional class Show forest plot | 15 | 3060 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.16, 1.70] |
| 1.2.1 Non‐selective ERAs | 9 | 1896 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.13, 1.87] |
| 1.2.2 Selective ERAs | 6 | 1164 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.01, 1.80] |
| 1.3 Proportion of participants with deteriorated functional class Show forest plot | 13 | 2347 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.26, 0.72] |
| 1.3.1 Non‐selective ERA | 7 | 1121 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.30, 1.42] |
| 1.3.2 Selective ERAs | 6 | 1226 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.17, 0.60] |
| 1.4 Change from baseline in Borg dyspnoea index Show forest plot | 7 | 788 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.90, 0.04] |
| 1.4.1 Non‐selective ERAs | 3 | 240 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.58, 1.03] |
| 1.4.2 Selective ERAs | 4 | 548 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.01, 0.14] |
| 1.5 Mortality Show forest plot | 12 | 2889 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.07] |
| 1.5.1 Non‐selective ERAs | 7 | 1759 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.62, 1.23] |
| 1.5.2 Selective ERAs | 5 | 1130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.21, 0.94] |
| 1.6 Change from baseline in mean pulmonary artery pressure Show forest plot | 8 | 729 | Mean Difference (IV, Random, 95% CI) | ‐4.65 [‐6.05, ‐3.26] |
| 1.6.1 Non‐selective ERAs | 6 | 519 | Mean Difference (IV, Random, 95% CI) | ‐5.79 [‐7.30, ‐4.27] |
| 1.6.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐2.65 [‐5.31, 0.00] |
| 1.7 Change from baseline in pulmonary vascular resistance Show forest plot | 7 | 586 | Mean Difference (IV, Random, 95% CI) | ‐236.24 [‐333.21, ‐139.26] |
| 1.7.1 Non‐selective ERAs | 5 | 376 | Mean Difference (IV, Random, 95% CI) | ‐281.74 [‐395.85, ‐167.63] |
| 1.7.2 Selective ERAs | 2 | 210 | Mean Difference (IV, Random, 95% CI) | ‐173.73 [‐332.52, ‐14.94] |
| 1.8 Pulmonary vascular resistance Show forest plot | 2 | 175 | Mean Difference (IV, Fixed, 95% CI) | ‐288.59 [‐472.18, ‐104.99] |
| 1.9 Ratio of geometric mean PVR Show forest plot | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 Selective ERAs | 0 | Ratio of Geometric mean (IV, Random, 95% CI) | Not estimable | |
| 1.9.2 Non‐selective ERAs | 3 | Ratio of Geometric mean (IV, Random, 95% CI) | 0.69 [0.60, 0.80] | |
| 1.10 Change from baseline in cardiac index Show forest plot | 7 | 718 | Mean Difference (IV, Random, 95% CI) | 0.50 [0.35, 0.65] |
| 1.10.1 Non‐selective ERAs | 5 | 509 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.34, 0.77] |
| 1.10.2 Selective ERAs | 2 | 209 | Mean Difference (IV, Random, 95% CI) | 0.39 [0.23, 0.54] |
| 1.11 Change from baseline in SpO 2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12 Hepatic toxicity Show forest plot | 11 | 2250 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.91, 3.90] |
| 1.12.1 Non‐selective ERAs | 9 | 1888 | Odds Ratio (M‐H, Random, 95% CI) | 2.33 [0.98, 5.56] |
| 1.12.2 Selective ERAs | 2 | 362 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 6‐minute walk Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Proportion of participants with improved functional class Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.3 Proportion of participants with deteriorated functional class Show forest plot | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4 Symptoms Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Mortality Show forest plot | 2 | 273 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.36] |
| 2.6 Cardiac index Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Change from baseline in 6‐minute walk Show forest plot | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| 3.1.1 Non‐selective ERA | 2 | 280 | Mean Difference (IV, Random, 95% CI) | 21.49 [‐31.23, 74.21] |
| 3.1.2 Selective ERA | 0 | 0 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 3.2 Proportion of participants with improved functional class Show forest plot | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| 3.2.1 Non‐selective ERAs | 2 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.90] |
| 3.2.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3 Proportion of participants with deteriorated functional class Show forest plot | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| 3.3.1 Non‐selective ERA | 2 | 280 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.85] |
| 3.3.2 Selective ERAs | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | Not estimable |
| 3.4 Mortality Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 Non‐selective ERAs | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 Selective ERAs | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5 Change from baseline in mean pulmonary arterial pressure Show forest plot | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| 3.5.1 Non‐selective ERAs | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐4.63 [‐8.03, ‐1.23] |
| 3.5.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.6 Change from baseline in pulmonary vascular resistance Show forest plot | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| 3.6.1 Non‐selective ERAs | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐480.07 [‐753.34, ‐206.79] |
| 3.6.2 Selective ERAs | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3.7 Change from baseline in SpO 2 Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |

