Granulocyte‐Colony Stimulating Factor (G‐CSF) as an adjunct to antibiotics in the treatment of pneumonia in adults
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Multicentre, double blinded RCT | |
| Participants | Community‐acquired pneumonia in hospitalized adults. | |
| Interventions | G‐CSF 300mcg/d for 10 days vs placebo | |
| Outcomes | Time to resolution of morbidity, 28 day mortality, time to ICU and hospital discharge, adverse events including organ dysfunction. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Multicentre, double blinded RCT | |
| Participants | Community acquired multilobar pneumonia in hospitalized adults | |
| Interventions | G‐CSF 300mcg/d for 10 days vs placebo | |
| Outcomes | 28 day mortality, therapeutic failure, adverse events including organ dysfunction | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Multicentre, double blinded RCT | |
| Participants | Confirmed hospital acquired or community acquired pneumonia with severe sepsis | |
| Interventions | G‐CSF 300mcg/day for 5 days vs placebo | |
| Outcomes | 28 day mortality, time to ICU discharge, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | Double blinded RCT in 3 centres in US | |
| Participants | Confirmed hospital acquired or community acquired pneumonia with severe sepsis | |
| Interventions | G‐CSF 300mcg/d for 5 days vs placebo | |
| Outcomes | Non‐significant reduction in mortality. No difference in organ dysfunction | |
| Notes | Small study. heterogenous population. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Not a randomized controlled trial | |
| Clinical endpoints, such as mortality or rates of organ dysfunction, were not reported. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28 day mortality Show forest plot | 4 | 1955 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.77, 1.29] |
| Analysis 1.1  Comparison 1 Mortality, Outcome 1 28 day mortality. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event (including organ dysfunction) Show forest plot | 4 | 1955 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.14] |
| Analysis 2.1  Comparison 2 Adverse events, Outcome 1 Any adverse event (including organ dysfunction). | ||||
| 2 Adult respiratory distress syndrome Show forest plot | 3 | 1254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.54] |
| Analysis 2.2  Comparison 2 Adverse events, Outcome 2 Adult respiratory distress syndrome. | ||||
| 3 Disseminated intravascular coagulation Show forest plot | 3 | 1254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.22] |
| Analysis 2.3  Comparison 2 Adverse events, Outcome 3 Disseminated intravascular coagulation. | ||||
| 4 Acute renal failure Show forest plot | 3 | 1254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.27, 1.63] |
| Analysis 2.4  Comparison 2 Adverse events, Outcome 4 Acute renal failure. | ||||
| 5 Septic shock Show forest plot | 2 | 1236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.08] |
| Analysis 2.5  Comparison 2 Adverse events, Outcome 5 Septic shock. | ||||

Comparison 1 Mortality, Outcome 1 28 day mortality.
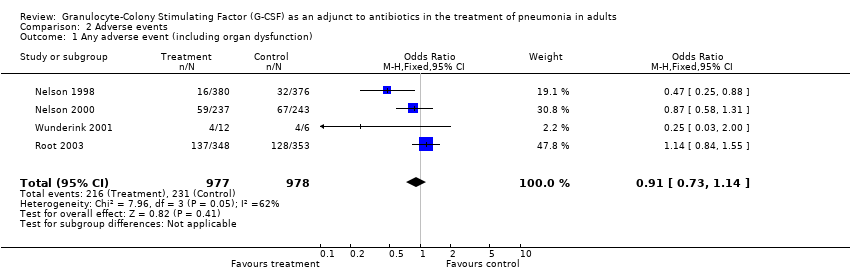
Comparison 2 Adverse events, Outcome 1 Any adverse event (including organ dysfunction).
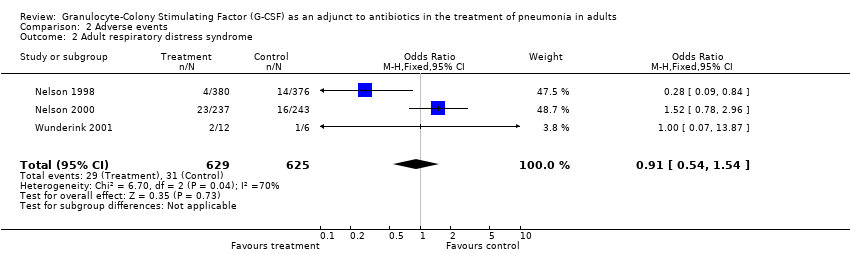
Comparison 2 Adverse events, Outcome 2 Adult respiratory distress syndrome.

Comparison 2 Adverse events, Outcome 3 Disseminated intravascular coagulation.
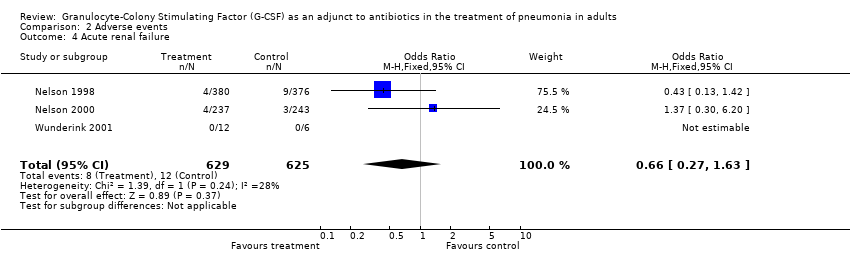
Comparison 2 Adverse events, Outcome 4 Acute renal failure.

Comparison 2 Adverse events, Outcome 5 Septic shock.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 28 day mortality Show forest plot | 4 | 1955 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.77, 1.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any adverse event (including organ dysfunction) Show forest plot | 4 | 1955 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.14] |
| 2 Adult respiratory distress syndrome Show forest plot | 3 | 1254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.54] |
| 3 Disseminated intravascular coagulation Show forest plot | 3 | 1254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.22] |
| 4 Acute renal failure Show forest plot | 3 | 1254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.27, 1.63] |
| 5 Septic shock Show forest plot | 2 | 1236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.08] |

