Betamimetics for inhibiting preterm labour
Abstract
Background
Preterm birth is a major contributor to perinatal mortality and morbidity worldwide. Tocolytic agents are drugs used to inhibit uterine contractions. The most widely used tocolytic agents are betamimetics especially in resource‐poor countries.
Objectives
To assess the effects of betamimetics given to women with preterm labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (May 2006) without language restrictions. We updated this search on 1 October 2009 and added the results to the awaiting classification section.
Selection criteria
Randomised controlled trials of betamimetics, administered by any route or any dose, in the treatment of women in preterm labour where betamimetics are compared with other betamimetics, placebo or no treatment.
Data collection and analysis
Two review authors evaluated independently methodological quality and extracted the data. We sought additional information to enable assessment of methodology and conduct intention‐to‐treat analyses. We present the results using the relative risk for categorical data and the weighted mean difference for continuous data.
Main results
Seventeen randomised controlled trials are included. Eleven trials, involving 1320 women, compared betamimetics with placebo. Betamimetics decreased the number of women in preterm labour giving birth within 48 hours (relative risk (RR) 0.63; 95% confidence interval (CI) 0.53 to 0.75) but there was no decrease in the number of births within seven days after carrying out a sensitivity analysis of studies with adequate allocation of concealment. No benefit was demonstrated for betamimetics on perinatal death (RR 0.84; 95% CI 0.46 to 1.55, seven trials, n = 1332), or neonatal death (RR 1.00; 95% CI 0.48 to 2.09, five trials, n = 1174). No significant effect was demonstrated for respiratory distress syndrome (RR 0.87; 95% CI 0.71 to 1.08, eight trials, n = 1239). A few trials reported the following outcomes, with no difference detected: cerebral palsy, infant death and necrotizing enterocolitis. Betamimetics were significantly associated with the following: withdrawal from treatment due to adverse effects; chest pain; dyspnea; tachycardia; palpitation; tremor; headaches; hypokalemia; hyperglycemia; nausea or vomiting; and nasal stuffiness; and fetal tachycardia. Other betamimetics were compared with ritodrine in five trials (n = 948) and hexoprenaline compared with salbutamol in one trial (n = 140). Trials were small, varied and of insufficient quality to delineate any consistent patterns of effect.
Authors' conclusions
Betamimetics help to delay delivery for women transferred to tertiary care or completed a course of antenatal corticosteroids. However, multiple adverse effects must be considered. The data are too few to support the use of any particular betamimetics.
[Note: The 14 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
PICO
Plain language summary
Betamimetics for inhibiting preterm labour
Betamimetics are effective in delaying birth for 48 hours in women with preterm labour but they increase side‐effects.
Short delays in preterm birth can enable women to reach specialist care and receive 'corticosteroid' drugs that are given to women before birth to improve their babies' lung function. The review includes 17 randomised controlled trials testing the effect of betamimetics for inhibiting preterm labour in 2408 women. Eleven small studies found that women in preterm labour who received 'betamimetic' drugs were less likely to give birth in 48 hours than women who did not have these drugs. However, no overall benefit for the babies was found. The studies did find increased risk for various side‐effects in mothers, including chest pain, breathing difficulties, heart irregularities, headaches, and shaking.
Authors' conclusions
Background
Preterm birth, defined as birth occurring before 37 complete weeks (WHO 1977), accounts for 5% to 11% of births in the world: 9% to 10% in low‐income countries ($735 or less); and 6% to 11.9% in high‐income countries ($9076 or more). Preterm birth is a leading cause of perinatal morbidity and mortality (Guyer 1996), and is associated with 60% to 80% of deaths of infants without congenital abnormalities. Preterm birth can cause respiratory distress syndrome (a condition in which the baby's lungs are not developed enough to take in the air they need), bronchopulmonary dysplasia (a chronic lung disease, which can follow respiratory distress syndrome), intraventricular haemorrhage (bleeding into the normal fluid spaces (ventricles) within the brain and also used to refer to bleeding in areas near the ventricles even if the blood is not within them), sepsis (generalized infection or infection of the blood stream), cerebral palsy (an injury to the brain resulting in children being unable to use some of the muscles to walk, talk, eat or play in the normal way), intellectual impairment (limitations in mental function and in skills such as communicating, taking care of oneself, and social skills), blindness and deafness. Preterm infants often require intensive care. This is associated with emotional and economic costs to both the family and society (Petrou 2001): difficulty with care; uncertainty for the future; and long‐term costs for child care, education, and paid or unpaid work in order to spend time with them. It would seem beneficial to be able to delay birth until the fetus is more mature, when it may be possible to reduce the risks of short‐ and long‐term morbidity for the infant.
The mechanisms or pathogenesis of preterm labour are not well understood. However, the causes of spontaneous preterm labour may involve genetic factors, stress, inflammatory response, mechanical factors and haemorrhage (Lockwood 2001; Savitz 1999). Current strategies to prevent preterm birth are largely ineffective, and the rate of preterm birth has not decreased for many decades (Joseph 1998; Martin 2002).
Tocolytic agents (aimed at inhibiting contractions of the uterus) have been used to prolong pregnancy. This may improve perinatal outcomes by allowing (a) the fetus to mature more before being born, (b) enhance lung maturation by antenatal corticosteroid administration (Crowley 1996), and (c) allow time for in‐utero transfer to a tertiary care centre with neonatal intensive care facilities (Powell 1995). Several tocolytic agents have been used including betamimetics (Gyetvai 1999; King 1988a), calcium channel blockers (King 2003b), prostaglandin synthase inhibitors, magnesium sulphate (Crowther 2002), nitric oxide donor (Duckitt 2002), and oxytocin receptor antagonists.
Betamimetics (isoxsuprine, hexoprenaline, orciprenaline, ritodrine, terbutaline, and salbutamol) have been used extensively in the past 20 years (Barden 1980b). Beta‐adrenergic agonists activate adenyl cyclase to form cyclic adenosine 3',5' monophosphate (cAMP). The increased cellular levels of cAMP decrease myosin light‐chain kinase activity, both by phosphorylation of the myosin light‐chain kinase itself, and by reducing intracellular calcium through increasing calcium uptake by sarcoplasmic reticulum (Gabor 1982).
The ideal tocolytic agent is one which is effective in prolonging pregnancy but has no side‐effects for the woman or the infant. Betamimetics stimulate beta‐adrenergic receptors and cause palpitations, tremor, nausea, vomiting, headaches, nervousness, anxiety, chest pain, shortness of breath, and a range of biochemical disturbances such as hyperglycaemia (high blood sugar level) and hypokalaemia (low serum potassium level). Moreover, pulmonary edema (fluid accumulation in the lungs) may occur in about 3% of women (Goldenberg 2002) and has been associated with maternal death. The occurrence of side‐effects may necessitate some women stopping the medication. Betamimetics cross the placenta and may cause fetal tachycardia (increase fetal heart rate), hypoglycaemia (low blood sugar level) and hyperinsulinism after birth. When considering whether or not to use tocolytic agents in preterm labour, consideration needs to be given to the risks and benefits for both mother and infant, including the side‐effects of any medication used.
A meta‐analysis of randomised controlled trials of betamimetics compared with placebo or no treatment has shown them to be effective in delaying delivery by up to seven days and longer, although no impact has yet been shown on perinatal outcomes (Gyetvai 1999; King 1988a). However, the first review does not include one study conducted in Canada (CPLG 1992). The second review was done by Gyetvai to evaluate any tocolytic compared with a placebo or no treatment for preterm labour on prolongation of pregnancy, perinatal outcomes and maternal side‐effects. However, this review did not compare betamimetics with other betamimetics. Also, the types of outcome measures were limited. Moreover, the searching strategy used was limited to English language publications with American spelling only and the inclusion of unpublished data was not mentioned. The method for assessing validity was not formally reported. It is not clear whether statistical pooling was appropriate.
We have conducted a fresh systematic review of betamimetics. Also, antenatal corticosteroids use in preterm labour has probably become more routine since the dissemination of the Cochrane review on the topic (Crowley 1996). However, the improvement of perinatal morbidity and mortality rate may have been influenced by the corticosteroids themselves or confounded by prolonging the pregnancy due to tocolytic agents or the improvement of neonatal care facilities.
Currently, betamimetics have been used as the first‐line drug for inhibiting preterm labour in many countries, especially resource‐poor countries. We aim to review the evidence for benefits and harms of betamimetics.
Objectives
To assess the effects of betamimetics in the treatment of preterm labour.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials that report data that compare outcomes for women and infants in which betamimetics were used by any route or any dose to inhibit preterm labour with outcomes in controls with betamimetics, placebo or no treatment.
Types of participants
Pregnant women assessed as being in spontaneous preterm labour and considered suitable for tocolytic agents.
For the purpose of the review, preterm labour was defined by trial authors (with intact or rupture membranes) unless they contradict the gestational age criterion.
Types of interventions
Betamimetics, administered by any route or any dose, in the treatment of women in preterm labour where betamimetics are compared with other betamimetics, placebo or no treatment.
Types of outcome measures
Primary outcomes
Four primary outcomes were chosen as being most representative of the clinically important measures of effectiveness:
(1) birth within 48 hours of treatment;
(2) perinatal death (at seven days);
(3) neonatal morbidity:
(a) respiratory distress syndrome;
(b) chronic lung disease or bronchopulmonary dysplasia as defined by oxygen dependency at 28 days or 36 weeks postmenstrual age, or both;
(c) severe neuroradiological abnormality, for example, cystic periventricular leukomalacia (softening of the brain near the ventricles because brain tissue in this area has died) or grade III to IV periventricular haemorrhage, or both;
(d) abnormal long‐term neurodevelopmental status at more than 12 months corrected age (defined as moderate to severe developmental delay and/or cerebral palsy, and/or sensory impairment, for example, blind and deaf);
(4) neonatal length of hospital stay.
Secondary outcomes
(1) Time to delivery (interval between randomisation and delivery):
(a) delivery within seven days;
(b) delivery before 28 completed weeks;
(c) delivery before 34 completed weeks;
(d) delivery before 37 completed weeks.
(2) Adverse events:
(a) cessation of treatment due to adverse drug reaction;
(b) serious maternal outcomes, for example, maternal death, cardiac arrest, respiratory arrest, admission to intensive care unit;
(c) maternal adverse drug reactions (all), for example, palpitation, tachycardia, cardiac arrythmias, pulmonary edema, myocardial ischemia, chest pain, dyspnea, tremor, hypotension, hyperglycaemia, hypokalaemia, nausea, vomiting, nasal stuffiness, headaches, nervousness, anxiety;
(d) fetal and neonatal side‐effects (all), for example, hypoglycaemia, fetal tachycardia.
(3) Neonatal morbidity:
(a) neonatal sepsis;
(b) necrotizing enterocolitis (an inflammation causing injury to the bowel);
(c) retinopathy of prematurity (abnormal growth of blood vessels in the baby's eye).
(4) Neonatal death (at 28 days).
(5) Infant death (at 12 months of age).
A priori subgroup analyses
We therefore conducted a subgroup analysis of trials where antenatal corticosteroids are given routinely to see if they have a different effect size. Studies that explicitly state that both the intervention and control participants received corticosteroids concurrently with the betamimetics, placebo or no treatment compared with studies that do not mention the use of steroids routinely or steroid administration rate was reported as 50% or less.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (20 May 2006). We updated this search on 1 October 2009 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE;
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We scanned the reference lists of all articles found.
We did not apply any language restrictions.
Data collection and analysis
S Anotayanonth and N Subhedar independently assessed the trials against the eligibility criteria, using a standardized form. We resolved disagreement by discussion or by consulting P Garner and J Neilson. The trials that did not meet the inclusion criteria were excluded and the reasons given in the Characteristics of excluded studies table.
The methodological quality of each trial was assessed by at least two review authors. This process was completed without consideration of trial results and with no blinding of authorship. We would have resolved any differences of opinion by discussion. The information would have been displayed in an additional table and described in the section 'Methodological quality of included studies'.
The generation of the random sequence and the method of allocation concealment were assessed separately. This was then judged as adequate or inadequate using the criteria described in Section VI of the Cochrane Reviewers' Handbook (Clarke 2002): A = adequate, B = unclear, C = inadequate, D = not used. Studies rated as a D were excluded.
In addition, quality scores were assigned to each trial for use of placebo, completeness of follow up and blinding of outcome assessment as follows.
Use of placebo
(a) Placebo used;
(b) attempt at a placebo (not identical);
(c) no placebo;
(d) unclear.
Completeness of follow up
(a) Less than 3% of participants excluded;
(b) 3% to 9.9% of participants excluded;
(c) 10% to 19.9% of participants excluded;
(d) 20% or more excluded;
(e) unclear.
Blinding
Blinding was examined with regards to who was blinded in the trials, that is, participant, caregiver, outcome assessor or analyst. All levels were sought and categorized as below.
(a) Double blind: neither investigator nor participant knew or were likely to guess the allocated treatment.
(b) Single blind: either the investigator or the participant knew the allocation. Or, the trial is described as double blind, but side‐effects of one or other treatments mean that it is likely that for a significant proportion (at least 20%) of participants the allocation could be correctly identified.
(c) No blinding: both investigator and participant knew (or were likely to guess) the allocated treatment.
(d) Unclear.
We developed a standardised data extraction form and then piloted it for consistency and completeness. Data were extracted independently by two authors and double entered. Any disagreements were resolved by discussion or consulting P Garner and J Neilson. If data from the trial reports were insufficient or missing, we contacted the study authors for additional information. The data were analysed using the Review Manager computer software (RevMan 2000). For continuous data, the weighted mean difference was used. For binary data, relative risks and 95% confidence intervals were calculated, and in the absence of heterogeneity (using the chi‐square test for heterogeneity with a 10% level of statistical significance), results were pooled using a fixed‐effect model. If significant heterogeneity was found a random‐effects model was used. A funnel plot was done to assess publication bias, quality or heterogeneity.
A priori it was decided that all eligible trials would be included in the initial analysis and sensitivity analyses carried out to evaluate the effect of trial quality. This was done by excluding trials given a B or C rating for quality of allocation concealment, then B, C or D for use of a placebo, then D or E for completeness of follow up and then C or D for blinding. There was only one trial left for inclusion in the analysis after applying all criteria of sensitivity analysis for betamimetics compared with placebo (CPLG 1992). For betamimetics compared with other betamimetics, there were only one trial for each comparison. Therefore, all trials were included in the analysis.
Subgroup analysis was planned to explore steroid administration as a possible source of heterogeneity in subgroup analyses by stratifying all studies (inside and outside US):
-
studies before and after 1994 (year of the National Institutes of Health consensus development conference on the use of corticosteroids for fetal maturation and their effect on perinatal outcomes);
-
studies, which reported that steroids were routinely given, or the steroid administration rate was reported as higher than 50%.
We were unable to undertake the subgroup analysis since there was no antenatal corticosteroids usage at least 50% in any trials and all the trials were conducted before 1990.
Results
Description of studies
Eighty‐five articles were identified from the literature search. There were three additional excluded studies and one additional included study. Therefore, sixty‐three trials were excluded and a further five studies are unable to be included until additional information is provided by the authors. This review includes 17 randomised controlled trials testing the effect of betamimetics for inhibiting preterm labour. (Nine reports from an updated search in October 2009 have been added to Studies awaiting classification.)
Excluded studies
Sixty‐three trials were excluded for the following reasons: method of generation of allocation was not truly randomised or inadequate (Calder 1985; Caritis 1991; Castren 1975; Chhabra 1998; Csapo 1977; Das 1969; Gummerus 1981; Karlsson 1980; Kim 1983; Kosasa 1985; Lenz 1985; Park 1982; Ritcher 1975; Ryden 1977; Sirohiwal 2001; Sivasamboo 1972; Spatling 1989; Weisbach 1986); participants were not in preterm labour (Dunlop 1986; Guinn 1997; Hallak 1992; Hallak 1993; Leake 1983; Levy 1985; Lipshitz 1976; Sanchez 1972; Tarnow‐Mordi 1988); the interventions were not consistent with the protocol (Ally 1992; Bedoya 1972; Besinger 1991; Ferguson 1987; Francioli 1988; Gamissans 1978a; Gamissans 1978b; Gamissans 1982; Hatjis 1987; Herzog 1995; Ieda 1991; Katz 1983; Raymajhi 2003; Reynolds 1978; Rios‐Anez 2001; Ritcher 1979; Ross 1983); loss to follow up exceeded 20% (Beall 1985); study did not report any useable outcomes (Braden 1997; Castillo 1988; Gonik 1988; Kovacs 1987; Kullander 1985; Merkatz 1980; Muller‐Holve 1987; Penney 1980; Philipsen 1981; Sciscione 1993; Spellacy 1974; Spellacy 1978; Thoulon 1982).
Some studies, which were included in the previous review (Gyetvai 1999; King 1988a) were excluded due to many reasons: loss to follow up 20% or more (Garite 1987; Howard 1982; Larsen 1986); conducted in preterm premature rupture of membranes without preterm labour (Christensen 1980); and data were reported by episode of preterm labour (Wesselius‐De 1971).
Please see table of Characteristics of excluded studies for further details.
Included studies
Seventeen randomised controlled trials testing the effect of betamimetics for inhibiting preterm labour are included.
A total of 1320 women participated in the 11 included trials comparing betamimetics with placebo or no treatment (Adam 1966; Barden 1980a; Cotton 1984; CPLG 1992; Hobel 1980; Ingemarsson 1976; Larsen 1980; Leveno 1986a; Mariona 1980; Scommegna 1980; Spellacy 1979). The data of four trials (Barden 1980a; Hobel 1980; Mariona 1980; Scommegna 1980) referred to data from the previous review (King 1988a) from personal communication with the authors. Also, 948 women participated in the five trials of comparing other betamimetics with ritodrine (Caritis 1984; Essed 1978; Gummerus 1983; Holleboom 1996; Lipshitz 1988; Von Oeyen 1990). We included two trials with abstracts only (Lipshitz 1988; Von Oeyen 1990), and one trial of 140 women compared hexoprenaline and salbutamol (Gummerus 1983) due to useful data for the review.
(1) Study location and settings
All included studies were conducted in tertiary care or University hospitals in high‐income countries such as Canada, Sweden, Denmark, but mostly were in USA. Six trials were multicenter studies.
(2) Participants
The participants included in these trials were reasonably homogeneous. The gestational age at inclusion varied from 20 to 37 weeks. Preterm labour was reasonably consistently defined across the trials as presence of uterine contractions documented by external tocography or cervical dilatation up to 4 cm to 5 cm, or both, except for three trials, which did not state the definition (Gummerus 1983; Lipshitz 1988; Von Oeyen 1990). Three trials included women admitted for preterm labour with preterm premature rupture of membranes (Caritis 1984; Cotton 1984; CPLG 1992) and three trials included twins (Adam 1966; Cotton 1984; CPLG 1992). Four trials did not state the exclusion criteria (Essed 1978; Gummerus 1983; Lipshitz 1988; Von Oeyen 1990). Most trials excluded those women with chorioamnionitis, pregnancy‐induced hypertension, vaginal bleeding, intrauterine compromise or death, and contraindications to use betamimetics.
(3) Interventions
Eight trials compared ritodrine with placebo (Barden 1980a; CPLG 1992; Hobel 1980; Larsen 1980; Leveno 1986a; Mariona 1980; Scommegna 1980; Spellacy 1979). Intravenous ritodrine was used initially and maintained orally in all trials except one (Larsen 1980), which used intramuscular ritodrine initially. The dosage was consistent both intravenously and orally. Ritodrine was usually started at 100 mcg/minute and increased by 50 mcg/minute up to a maximum of 350 mcg/minute, or until contractions ceased, or there were intolerable adverse effects. Two trials compared terbutaline with placebo (Cotton 1984; Ingemarsson 1976); the same regimen for intravenous infusion was used but oral terbutaline was only given in one trial (Ingemarsson 1976). One trial compared isoxsuprine with placebo (Adam 1966). Two trials compared terbutaline with ritodrine (Caritis 1984; Von Oeyen 1990). Only one trial compared other betamimetics with ritodrine: fenoterol with ritodrine (Essed 1978); ritodrine loading dose with incremental dose (Holleboom 1996); and hexoprenaline with ritodrine (Lipshitz 1988). An additional study compared hexoprenaline with salbutamol (Gummerus 1983).
(4) Outcomes
There was some inconsistency across the trials with regards to the way in which maternal outcomes were reported. Only three common primary outcomes were consistently reported: birth within 48 hours, perinatal death, and respiratory distress syndrome. However, the definition of respiratory distress syndrome was not stated. There were six trials comparing ritodrine or terbutaline with placebo and two trials comparing other betamimetics with ritodrine with birth reported within 48 hours. Perinatal death or respiratory distress syndrome was reported in eight trials. Only the biggest trial reported neonatal outcomes both in the short‐ and long‐term (CPLG 1992). Secondary outcomes were less consistently reported and definitions were not stated. Mainly cardiovascular adverse effects from betamimetics were reported particularly when treatment was stopped due to adverse reactions. Other adverse effects were less consistently reported.
Please see table of 'Characteristics of included studies' for further details.
Risk of bias in included studies
Five of the included trials reported adequate concealment of allocation (Caritis 1984; CPLG 1992; Holleboom 1996; Ingemarsson 1976; Leveno 1986a). The method of concealment of allocation was unclear in 12 trials (Adam 1966; Barden 1980a; Cotton 1984; Essed 1978; Gummerus 1983; Hobel 1980; Larsen 1980; Lipshitz 1988; Mariona 1980; Scommegna 1980; Spellacy 1979; Von Oeyen 1990). All these trials were conducted before 1990, mostly more than 20 years ago. More information on allocation of concealment could not be obtained from the authors. We decided to include these papers. Method of blinding (double‐blind and single‐bind) was reported in eight trials (Adam 1966; Barden 1980a; CPLG 1992; Hobel 1980; Ingemarsson 1976; Mariona 1980; Scommegna 1980; Spellacy 1979), and in one trial (Larsen 1980) that compared betamimetics with placebo. However, placebo was used in two other trials (Cotton 1984; Larsen 1980). Only one trial comparing other betamimetics with ritodrine used double‐blinding (Caritis 1984). Other trials did not discuss blinding. However, we included those studies because the route of administration for both groups was similar. In this review, we have attempted to conduct an intention‐to‐treat analysis for all outcomes. All the participants in 11 trials completed the study. There are three trials reporting loss to follow up of less than 3%. Two trials, reported as abstracts only, did not mention loss to follow up (Lipshitz 1988; Von Oeyen 1990).
We have asked for further information on methods and outcomes from trial investigators and we will include this information in future updates when available.
Effects of interventions
(1) Betamimetics compared with placebo
This review included data from 11 trials with a total of 707 women in placebo groups and 613 women in betamimetics group. One trial (Larsen 1980) randomised the participants into four groups: long ritodrine infusion group, short ritodrine infusion group, intramuscular group and placebo. Data from all women on ritodrine regimens (three groups) were pooled together to compare with placebo‐treated women.
Maternal outcomes
The use of betamimetics significantly decreased the number of women giving birth within 48 hours (relative risk (RR) 0.63; 95% confidence interval (CI) 0.53 to 0.75) and within seven days (RR 0.78; 95% CI 0.68 to 0.90) but did not reduce the risk of delivering prior to 37 weeks' gestation (RR 0.95; 95% CI 0.88 to 1.03). The effect of betamimetics on reduction of birth within 48 hours was not changed after sensitivity analysis, retaining only studies with adequate concealment (CPLG 1992; Ingemarsson 1976; Leveno 1986a) (RR 0.56; 95% CI 0.46 to 0.70). However, the reduction of birth within seven days was not demonstrated after sensitivity analysis (RR 0.67; CI 0.48 to 1.01), using a random‐effects model. No subgroup data based on delivery before 28 and before 34 completed weeks were reported because most participants were 32 weeks' gestation or more.
Maternal adverse effects were significantly increased with betamimetics compared to placebo: cessation of treatment due to adverse drug reaction (RR 11.38; 95% CI 5.21 to 24.86); chest pain (RR 11.29; 95% CI 3.81 to 33.46); dyspnea (RR 3.86; 95% CI 2.21 to 6.77); tachycardia (RR 4.08; 95% CI 1.55 to 10.73); palpitation (RR 10.11; 95% CI 6.56 to 15.58); tremor (RR 10.74; 95% CI 6.20 to 18.59); headaches (RR 4.07; 95% CI 2.60 to 6.35); hypokalaemia (RR 6.07; 95% CI 4.00 to 9.20); hyperglycaemia (RR 2.90; 95% CI 2.05 to 4.09); nausea or vomiting (RR 1.76; 95% CI 1.29 to 2.42); and nasal stuffiness (RR 2.90; 95% CI 1.64 to 5.12). However, no maternal deaths were reported in two trials to explicitly mention this (CPLG 1992; Larsen 1980); also, other serious maternal outcomes, for example, cardiac arrest, respiratory arrest and admission to intensive care unit, were not reported in any trial. Only one case of pulmonary edema was reported in all three trials (Cotton 1984; CPLG 1992; Leveno 1986a) that did not show any statistical difference (RR 3.03; 95% CI 0.12 to 74.23).
Neonatal outcomes
Betamimetics were not shown to reduce perinatal deaths (RR 0.84; 95% CI 0.46 to 1.55), respiratory distress syndrome (RR 0.87; 95% CI 0.71 to 1.08), cerebral palsy (RR 0.19; 95% CI 0.02 to 1.63), neonatal death (RR 1.00; 95% CI 0.48 to 2.09), infant death (RR 0.51; 95% CI 0.05 to 5.64) and necrotizing enterocolitis (RR 0.42; 95% CI 0.06 to 2.78). Significant heterogeneity was identified for two outcomes: sepsis and hypoglycaemia. No significant difference was seen for either outcome, using a random‐effects model.
Only one trial reported the mean neonatal length of hospital stay (Leveno 1986a) with total length of hospital stay being 24.5 days in the ritodrine group and 24.8 days in the placebo group. The mean neonatal length of intensive care stay was 14.3 days in the ritodrine group and 12.4 days in the placebo group. No data on chronic lung disease, bronchopulmonary dysplasia, severe neuroradiological abnormality, and retinopathy of prematurity were reported. Only one trial reported abnormal long‐term neurodevelopmental status after follow up of infants at 18 months (CPLG 1992). Bayley Psychomotor Development Index was 110.9 (standard deviation (SD) = 16.8) in the ritodrine group and 108.7 (SD = 15.5) in the placebo group. The average score on the Bayley Mental Developmental Index was 100.3 (SD = 18.3) in the ritodrine group and 95.1 (SD = 18.8) in the placebo group. Fetal tachycardia associated with betamimetics was significantly increased compared with placebo (RR 2.40; 95% CI 1.12 to 5.13) in one trial (Ingemarsson 1976).
(2) Other betamimetics compared with ritodrine
(a) Terbutaline compared with ritodrine
There were only two trials in this group involving 183 women: 90 in the terbutaline group and 93 in the ritodrine group (Caritis 1984; Von Oeyen 1990). Only 19 outcomes were reported.
Maternal and neonatal outcomes
Hyperglycaemia was statistically significantly increased in the terbutaline group (RR 1.78; 95% CI 1.05 to 3.03). However, this outcome was reported in one trial with 100 women only (Caritis 1984). Other maternal and neonatal outcomes were not statistically significantly different between the two groups: birth within 48 hours; birth within seven days; birth before 28 completed weeks; perinatal death; respiratory distress syndrome; cessation of treatment due to adverse drug reactions; any maternal side‐effects; chest pain; shortness of breath or dyspnea; arrhythmia; tachycardia; palpitation; hypotension; and headache.
(b) Fenoterol compared with ritodrine
There was only one trial in this group, involving 48 women in each group (Essed 1978). Only six outcomes were reported.
Maternal and neonatal outcomes
Neonatal death was statistically significantly decreased in the fenoterol group (RR 0.13; 95% CI 0.02 to 0.96). However, the sample size was small and the methodological quality was poor. There were no statistically significant differences between the groups for other maternal and neonatal outcomes: perinatal death; respiratory distress syndrome; tachycardia; fetal bradycardia; and hypoglycaemia.
(c) Ritodrine loading dose with conventional incremental dose
There was only one trial in this group involving 203 women: 101 in the ritodrine loading dose group and 102 in the incremental dose group (Holleboom 1996). Only 12 outcomes were reported.
There was no statistically significant differences between the groups for maternal and neonatal outcomes: birth within 48 hours; birth before 34 completed weeks; birth before 37 completed weeks; respiratory distress syndrome; any maternal adverse effects; tachycardia; nausea or vomiting; palpitation; headache; neonatal death; periventricular haemorrhage grade three to four; and sepsis.
(d) Hexoprenaline with ritodrine
There was only one trial in this group involving 466 women: 314 in the hexoprenaline group and 152 in the ritodrine group (Lipshitz 1988). Only six outcomes were reported.
Maternal and neonatal adverse effects were statistically significantly decreased in the hexoprenaline group: cessation of treatment due to adverse reaction (RR 0.28; 95% CI 0.08 to 0.93); any maternal adverse effects (RR 0.83; 95% CI 0.76 to 0.91); nausea or vomiting (RR 0.63; 95% CI 0.45 to 0.89); palpitation (RR 0.75; 95% CI 0.60 to 0.94); hypotension (RR 0.77; 95% CI 0.61 to 0.96); increased fetal heart rate (RR 0.74; 95% CI 0.56 to 0.98). However, the quality of this trial was poor (abstract only).
(3) Hexoprenaline compared with salbutamol
There was only one trial in this group involving 140 women: 70 in the hexoprenaline group and 70 in the salbutamol group (Gummerus 1983). Only six outcomes were reported.
Maternal adverse effects were statistically significantly decreased in the hexoprenaline group: any maternal adverse effects (RR 0.38; 95% CI 0.18 to 0.80) and tachycardia (RR 0.11; 95% CI 0.01 to 0.85). However, the methodological quality of this trial was poor. There was no statistically significant differences between the groups for respiratory distress syndrome, cessation of treatment due to adverse reactions, nausea or vomiting, headache, and tremor.
Subgroup analysis based on adjunctive steroid treatment was not done because of the low incidence of corticosteroid usage in all trials.
Discussion
The additional study did not change the previous results. Betamimetics were shown to be effective in delaying delivery within 48 hours. This is sufficient time to allow transfer of the woman to a tertiary care unit or to complete a course of antenatal corticosteroid. However, there was no evidence that this delay in the timing of birth translated into any improvements in neonatal outcomes, and the side‐effects for the woman are considerable.
It may be that betamimetics are ineffective, or that actual effectiveness has not been demonstrated for several reasons.
-
Time gain in‐utero: most participants were 32 weeks' gestation or more. Therefore, increased gestational age might have little or no effect on perinatal outcomes leading to no statistical significance in the pooled estimate of important outcomes such as respiratory distress syndrome, perinatal death, neonatal death and infant death. Also, prolongation of pregnancy itself may be disadvantage to the baby. If the eligibility criteria had been restricted to lower gestation groups, and had the number of participants been greater, an effect on clinical outcomes might have been detected.
-
Referral to higher care level: all trials in this review took place in tertiary care or University hospitals, that should have decent neonatal intensive care facilities. If the trials had involves women who needed to be transferred to distant referral hospitals, again a difference in outcome might have been seen.
-
Antenatal corticosteroids administration: all trials were conducted before 1990, when antenatal corticosteroids were not widely used. Even in CPLG 1992, antenatal corticosteroids were prescribed only to one third of all women in each arm. Therefore, subgroup analysis to evaluate effect of betamimetics to allow a complete course of antenatal corticosteroids (betamimetics alone and betamimetics combined with corticosteroids) on neonatal outcomes couldn't be assessed.
There is not enough evidence to suggest that one betamimetic agent is superior to another.
Calcium channel blockers and magnesium sulfate are also available as tocolytic drugs. Calcium channel blockers when compared with betamimetics have demonstrated less birth within seven days and before 34 completed weeks with reduction in some neonatal outcomes such as respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage, and jaundice and maternal side‐effects (King 2003b). Also, there is the advantage of oral formulation. However, there are no placebo controlled trials of calcium channel blockers. The variation in dosage and formulation needs to be assessed. Safety issues regarding their possible adverse effects on fetal or placental circulation has concerned clinicians. Magnesium sulfate is ineffective at delaying preterm birth (Crowther 2002). Also, the margin between tocolytic effect and loss of deep tendon reflexes and respiratory depression is small. Therefore, the choice of tocolysis should be based on maternal conditions, potential adverse effects, gestational age, and hospital facilities.

Comparison 1 All betamimetics versus placebo, Outcome 1 Birth within 48 hours of treatment.

Comparison 1 All betamimetics versus placebo, Outcome 2 Perinatal death (7 days).

Comparison 1 All betamimetics versus placebo, Outcome 3 Respiratory distress syndrome.
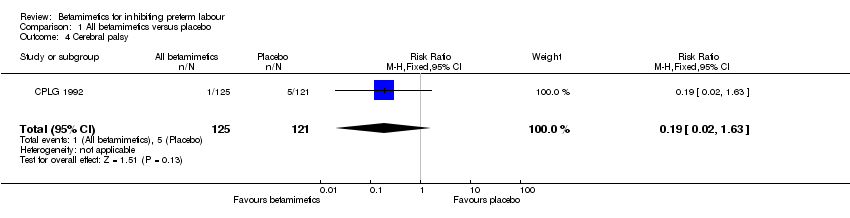
Comparison 1 All betamimetics versus placebo, Outcome 4 Cerebral palsy.
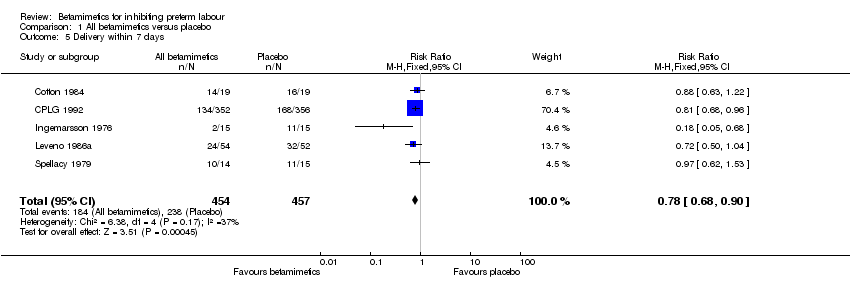
Comparison 1 All betamimetics versus placebo, Outcome 5 Delivery within 7 days.

Comparison 1 All betamimetics versus placebo, Outcome 6 Delivery less than 37 weeks' gestation.

Comparison 1 All betamimetics versus placebo, Outcome 7 Cessation of treatment due to adverse drug reaction.

Comparison 1 All betamimetics versus placebo, Outcome 8 Maternal death.
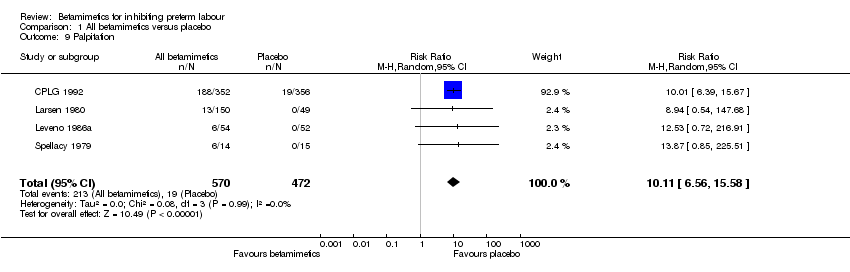
Comparison 1 All betamimetics versus placebo, Outcome 9 Palpitation.

Comparison 1 All betamimetics versus placebo, Outcome 10 Tachycardia.
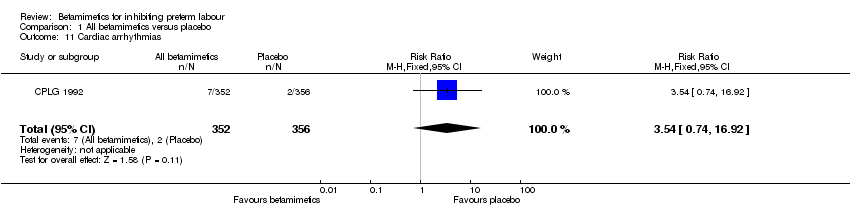
Comparison 1 All betamimetics versus placebo, Outcome 11 Cardiac arrhythmias.

Comparison 1 All betamimetics versus placebo, Outcome 12 Pulmonary edema.

Comparison 1 All betamimetics versus placebo, Outcome 13 Myocardial ischemia.

Comparison 1 All betamimetics versus placebo, Outcome 14 Chest pain.

Comparison 1 All betamimetics versus placebo, Outcome 15 Dyspnea.

Comparison 1 All betamimetics versus placebo, Outcome 16 Tremor.

Comparison 1 All betamimetics versus placebo, Outcome 17 Hypotension.

Comparison 1 All betamimetics versus placebo, Outcome 18 Hyperglycemia.
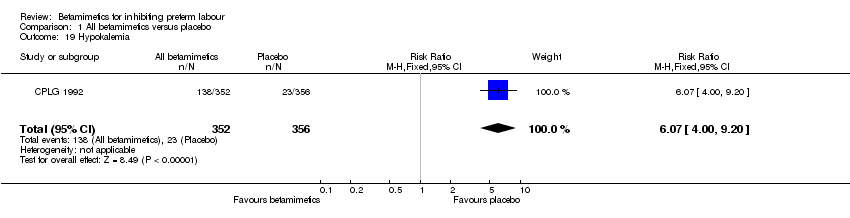
Comparison 1 All betamimetics versus placebo, Outcome 19 Hypokalemia.

Comparison 1 All betamimetics versus placebo, Outcome 20 Nausea or vomiting.
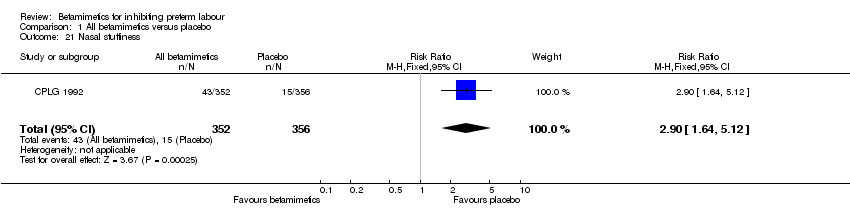
Comparison 1 All betamimetics versus placebo, Outcome 21 Nasal stuffiness.
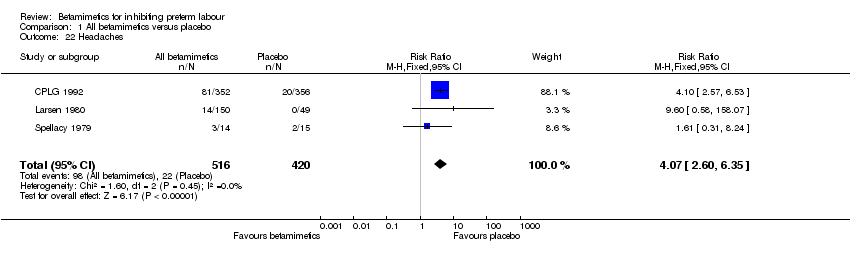
Comparison 1 All betamimetics versus placebo, Outcome 22 Headaches.
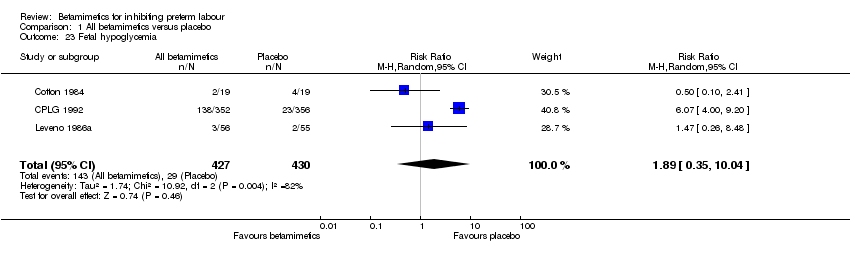
Comparison 1 All betamimetics versus placebo, Outcome 23 Fetal hypoglycemia.

Comparison 1 All betamimetics versus placebo, Outcome 24 Fetal tachycardia.
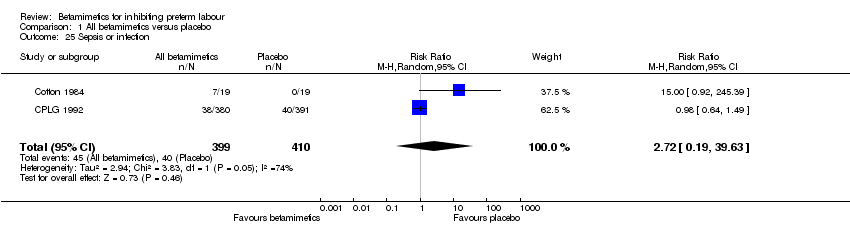
Comparison 1 All betamimetics versus placebo, Outcome 25 Sepsis or infection.
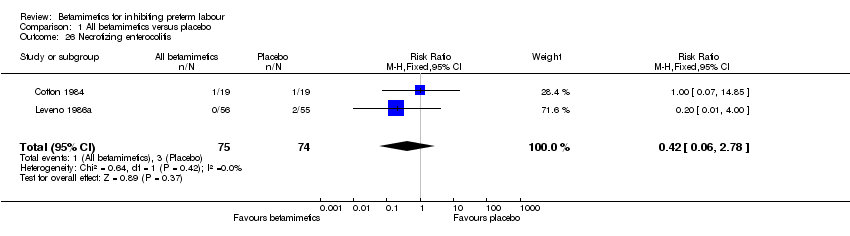
Comparison 1 All betamimetics versus placebo, Outcome 26 Necrotizing enterocolitis.

Comparison 1 All betamimetics versus placebo, Outcome 27 Neonatal death.
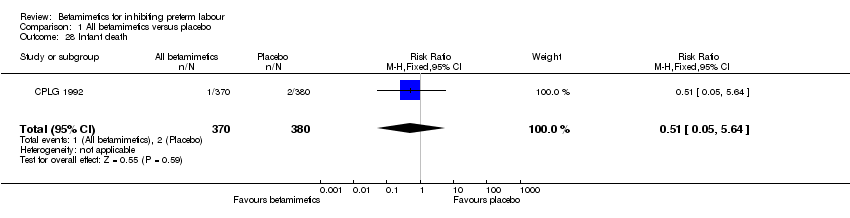
Comparison 1 All betamimetics versus placebo, Outcome 28 Infant death.

Comparison 2 Terbutaline versus ritodrine, Outcome 1 Birth within 48 hours.

Comparison 2 Terbutaline versus ritodrine, Outcome 2 Perinatal death.
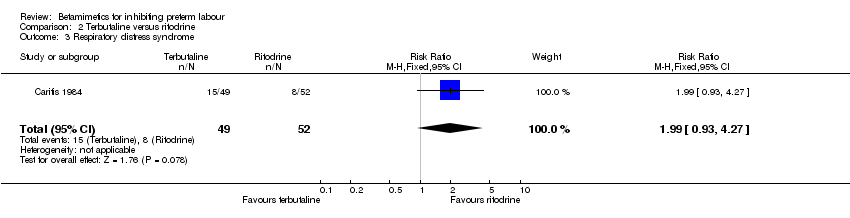
Comparison 2 Terbutaline versus ritodrine, Outcome 3 Respiratory distress syndrome.

Comparison 2 Terbutaline versus ritodrine, Outcome 4 Delivery within 7 days.
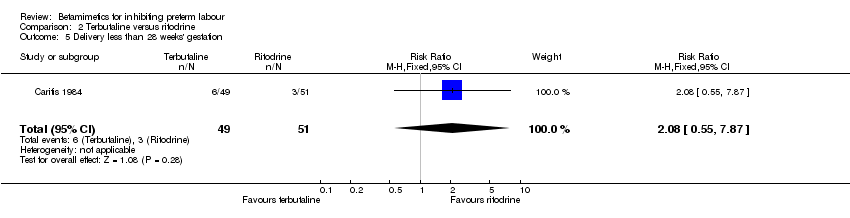
Comparison 2 Terbutaline versus ritodrine, Outcome 5 Delivery less than 28 weeks' gestation.

Comparison 2 Terbutaline versus ritodrine, Outcome 6 Cessation of treatment due to adverse drug reactions.
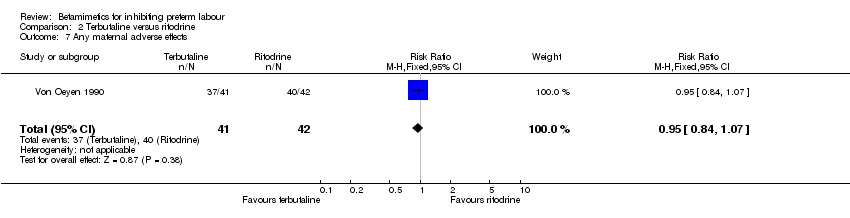
Comparison 2 Terbutaline versus ritodrine, Outcome 7 Any maternal adverse effects.

Comparison 2 Terbutaline versus ritodrine, Outcome 8 Chest pain.

Comparison 2 Terbutaline versus ritodrine, Outcome 9 Shortness of breath or dyspnea.
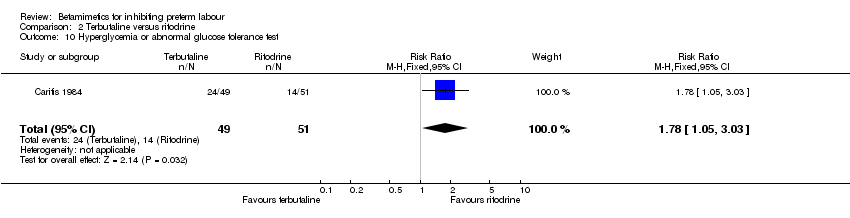
Comparison 2 Terbutaline versus ritodrine, Outcome 10 Hyperglycemia or abnormal glucose tolerance test.

Comparison 2 Terbutaline versus ritodrine, Outcome 11 Palpitations.

Comparison 2 Terbutaline versus ritodrine, Outcome 12 Tachycardia.

Comparison 2 Terbutaline versus ritodrine, Outcome 13 Arrhythmia.
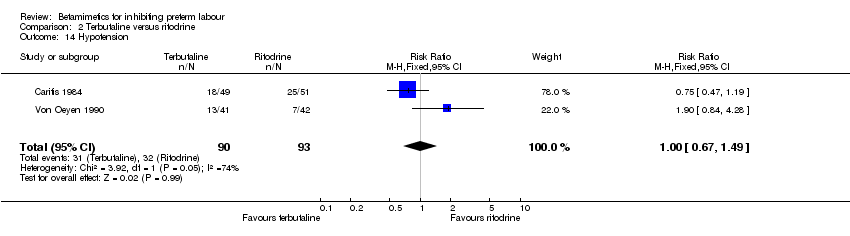
Comparison 2 Terbutaline versus ritodrine, Outcome 14 Hypotension.
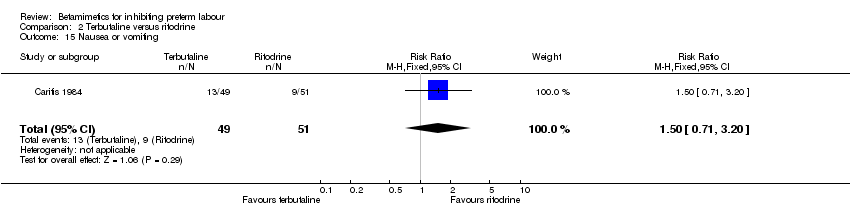
Comparison 2 Terbutaline versus ritodrine, Outcome 15 Nausea or vomiting.
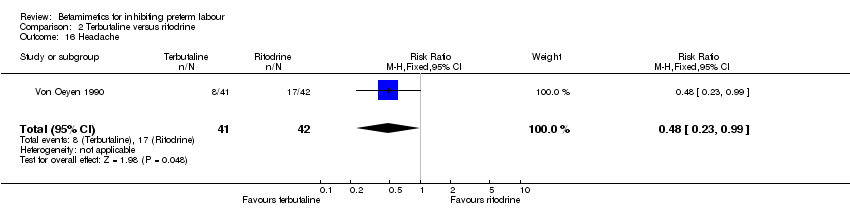
Comparison 2 Terbutaline versus ritodrine, Outcome 16 Headache.

Comparison 2 Terbutaline versus ritodrine, Outcome 17 Anxiety.
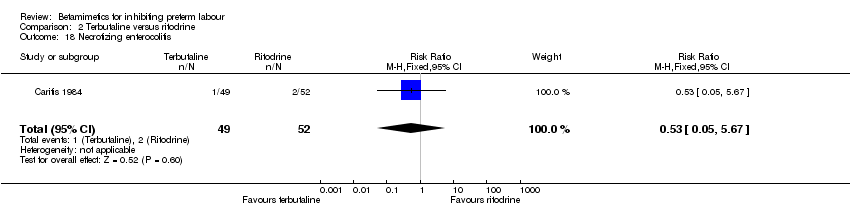
Comparison 2 Terbutaline versus ritodrine, Outcome 18 Necrotizing enterocolitis.
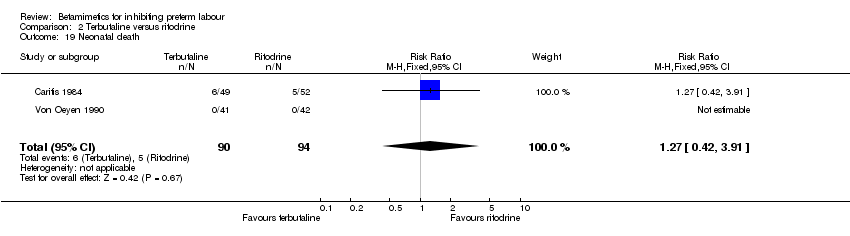
Comparison 2 Terbutaline versus ritodrine, Outcome 19 Neonatal death.

Comparison 3 Fenoterol versus ritodrine, Outcome 1 Perinatal death.

Comparison 3 Fenoterol versus ritodrine, Outcome 2 Respiratory distress syndrome.
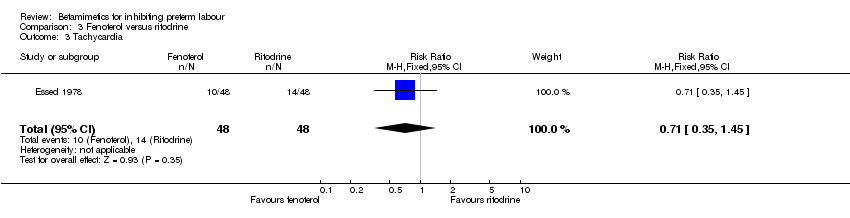
Comparison 3 Fenoterol versus ritodrine, Outcome 3 Tachycardia.

Comparison 3 Fenoterol versus ritodrine, Outcome 4 Hypoglycemia.

Comparison 3 Fenoterol versus ritodrine, Outcome 5 Fetal bradycardia.
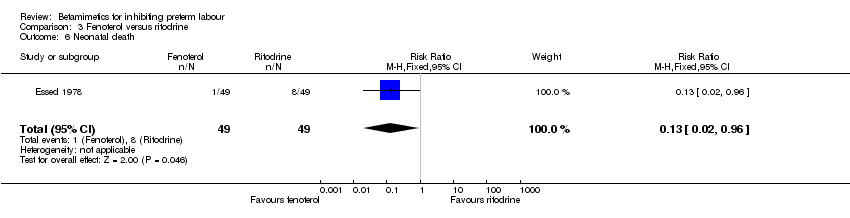
Comparison 3 Fenoterol versus ritodrine, Outcome 6 Neonatal death.

Comparison 4 Hexoprenaline versus ritodrine, Outcome 1 Cessation of treatment due to adverse drug reactions.
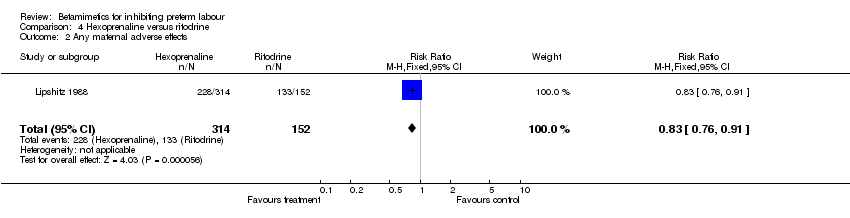
Comparison 4 Hexoprenaline versus ritodrine, Outcome 2 Any maternal adverse effects.
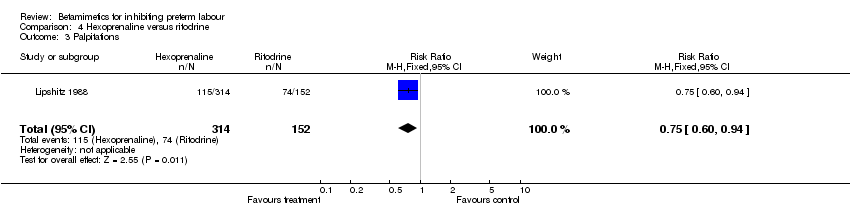
Comparison 4 Hexoprenaline versus ritodrine, Outcome 3 Palpitations.
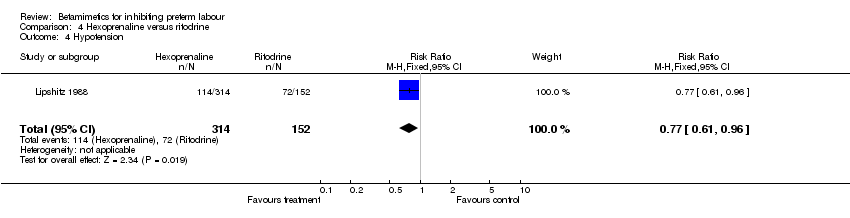
Comparison 4 Hexoprenaline versus ritodrine, Outcome 4 Hypotension.

Comparison 4 Hexoprenaline versus ritodrine, Outcome 5 Nausea or vomiting.

Comparison 4 Hexoprenaline versus ritodrine, Outcome 6 Increase in fetal heart rate.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 1 Birth within 48 hours.
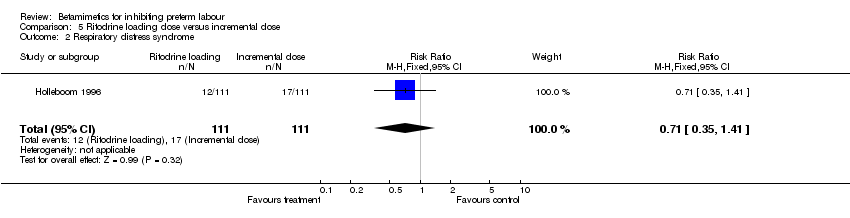
Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 2 Respiratory distress syndrome.
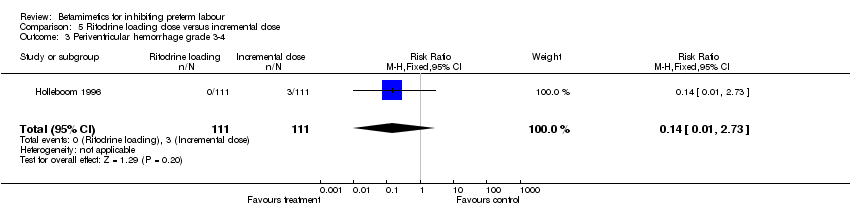
Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 3 Periventricular hemorrhage grade 3‐4.
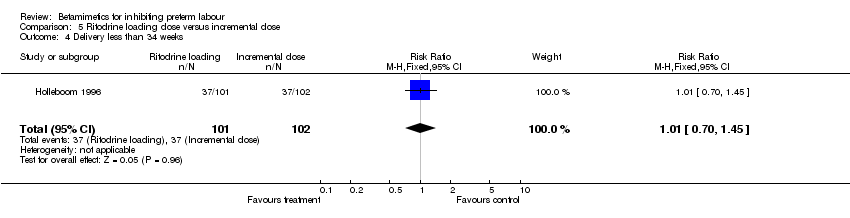
Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 4 Delivery less than 34 weeks.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 5 Delivery less than 37 weeks.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 6 Any maternal adverse effects.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 7 Palpitations.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 8 Tachycardia.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 9 Nausea or vomiting.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 10 Headache.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 11 Sepsis.

Comparison 5 Ritodrine loading dose versus incremental dose, Outcome 12 Neonatal death.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 1 Respiratory distress syndrome.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 2 Cessation of treatment due to adverse drug reactions.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 3 Any maternal adverse effects.
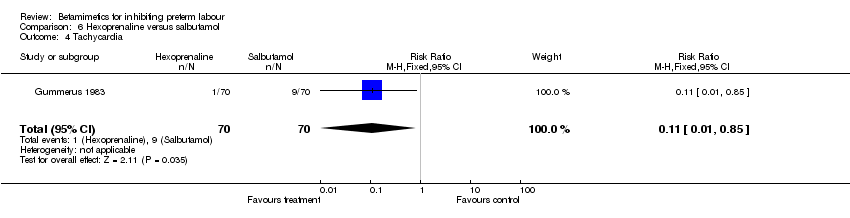
Comparison 6 Hexoprenaline versus salbutamol, Outcome 4 Tachycardia.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 5 Nausea or vomiting.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 6 Headache.

Comparison 6 Hexoprenaline versus salbutamol, Outcome 7 Tremor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours of treatment Show forest plot | 10 | 1209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.53, 0.75] |
| 2 Perinatal death (7 days) Show forest plot | 11 | 1332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.46, 1.55] |
| 3 Respiratory distress syndrome Show forest plot | 8 | 1239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.08] |
| 4 Cerebral palsy Show forest plot | 1 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.63] |
| 5 Delivery within 7 days Show forest plot | 5 | 911 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.68, 0.90] |
| 6 Delivery less than 37 weeks' gestation Show forest plot | 10 | 1212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.88, 1.03] |
| 7 Cessation of treatment due to adverse drug reaction Show forest plot | 5 | 1081 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.38 [5.21, 24.86] |
| 8 Maternal death Show forest plot | 2 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Palpitation Show forest plot | 4 | 1042 | Risk Ratio (M‐H, Random, 95% CI) | 10.11 [6.56, 15.58] |
| 10 Tachycardia Show forest plot | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [1.55, 10.73] |
| 11 Cardiac arrhythmias Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.54 [0.74, 16.92] |
| 12 Pulmonary edema Show forest plot | 3 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.23] |
| 13 Myocardial ischemia Show forest plot | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.53 [0.72, 216.91] |
| 14 Chest pain Show forest plot | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.29 [3.81, 33.46] |
| 15 Dyspnea Show forest plot | 2 | 814 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.86 [2.21, 6.77] |
| 16 Tremor Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.74 [6.20, 18.59] |
| 17 Hypotension Show forest plot | 2 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.39, 8.06] |
| 18 Hyperglycemia Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [2.05, 4.09] |
| 19 Hypokalemia Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.07 [4.00, 9.20] |
| 20 Nausea or vomiting Show forest plot | 3 | 932 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.29, 2.42] |
| 21 Nasal stuffiness Show forest plot | 1 | 708 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.64, 5.12] |
| 22 Headaches Show forest plot | 3 | 936 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.07 [2.60, 6.35] |
| 23 Fetal hypoglycemia Show forest plot | 3 | 857 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [0.35, 10.04] |
| 24 Fetal tachycardia Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.12, 5.13] |
| 25 Sepsis or infection Show forest plot | 2 | 809 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [0.19, 39.63] |
| 26 Necrotizing enterocolitis Show forest plot | 2 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.06, 2.78] |
| 27 Neonatal death Show forest plot | 6 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.48, 2.09] |
| 28 Infant death Show forest plot | 1 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.77, 5.48] |
| 2 Perinatal death Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Respiratory distress syndrome Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.93, 4.27] |
| 4 Delivery within 7 days Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.57, 1.10] |
| 5 Delivery less than 28 weeks' gestation Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [0.55, 7.87] |
| 6 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.92] |
| 7 Any maternal adverse effects Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| 8 Chest pain Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.25] |
| 9 Shortness of breath or dyspnea Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| 10 Hyperglycemia or abnormal glucose tolerance test Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.05, 3.03] |
| 11 Palpitations Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.78, 1.79] |
| 12 Tachycardia Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.43, 1.00] |
| 13 Arrhythmia Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| 14 Hypotension Show forest plot | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.67, 1.49] |
| 15 Nausea or vomiting Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.71, 3.20] |
| 16 Headache Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.99] |
| 17 Anxiety Show forest plot | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.67, 1.75] |
| 18 Necrotizing enterocolitis Show forest plot | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.05, 5.67] |
| 19 Neonatal death Show forest plot | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.42, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Perinatal death Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
| 2 Respiratory distress syndrome Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.38, 10.42] |
| 3 Tachycardia Show forest plot | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.45] |
| 4 Hypoglycemia Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.65] |
| 5 Fetal bradycardia Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.54] |
| 6 Neonatal death Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.93] |
| 2 Any maternal adverse effects Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.76, 0.91] |
| 3 Palpitations Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.60, 0.94] |
| 4 Hypotension Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.96] |
| 5 Nausea or vomiting Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.89] |
| 6 Increase in fetal heart rate Show forest plot | 1 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Birth within 48 hours Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.60, 1.91] |
| 2 Respiratory distress syndrome Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.41] |
| 3 Periventricular hemorrhage grade 3‐4 Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.73] |
| 4 Delivery less than 34 weeks Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.70, 1.45] |
| 5 Delivery less than 37 weeks Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.13] |
| 6 Any maternal adverse effects Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.43, 1.11] |
| 7 Palpitations Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.23, 1.13] |
| 8 Tachycardia Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.33, 2.35] |
| 9 Nausea or vomiting Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.38, 3.84] |
| 10 Headache Show forest plot | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 15.93] |
| 11 Sepsis Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 12 Neonatal death Show forest plot | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Respiratory distress syndrome Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.54, 5.71] |
| 2 Cessation of treatment due to adverse drug reactions Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.67] |
| 3 Any maternal adverse effects Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.80] |
| 4 Tachycardia Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 0.85] |
| 5 Nausea or vomiting Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.95] |
| 6 Headache Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.13] |
| 7 Tremor Show forest plot | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |

