Single dose oral celecoxib for acute postoperative pain in adults
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, DB single oral dose, 3 parallel groups | |
| Participants | Impacted third molar extraction | |
| Interventions | Celecoxib 400 mg, n = 57 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization schedule, prepared before the start of the study" |
| Allocation concealment (selection bias) | Low risk | Medication supplied in patient‐specific carton. Identity of assignment contained in concealed section of label, which was removed at dispensing, and attached to patient case report form |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind method. Placebo capsules or tablets identical in number and appearance to active treatments |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (57 participants) |
| Methods | RCT, DB single oral and multiple oral dose, 3 parallel groups | |
| Participants | Impacted third molar extraction | |
| Interventions | Celecoxib 200 mg, n = 74 | |
| Outcomes | PI: 4‐point scale | |
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method. "The appearance, presentation and labelling of the placebo formulations were identical to those of the corresponding active drugs" |
| Incomplete outcome data (attrition bias) | Low risk | Participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (74 active, 26 placebo participants) |
| Methods | RCT, DB, double‐dummy, single oral dose, 3 parallel groups | |
| Participants | Impacted third molar extraction | |
| Interventions | Celecoxib 400 mg, n = 156 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R2, DB1, W1 Participants permitted to use rescue medication at any time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Remote, automated allocation to randomisation numbers |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (156 active, 52 placebo participants) |
| Methods | RCT, DB single oral and multiple oral dose, 3 parallel groups | |
| Participants | Orthopaedic surgery (uncomplicated) | |
| Interventions | Celecoxib 200 mg, n = 141 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R1, DB1, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (136 to 141 participants) |
| Methods | Randomised, double‐blind, single‐dose, parallel‐group, duration 8 h Medication given when pain ≥ moderate | |
| Participants | Surgical removal of ≥ 2 impacted third molars M and F, age 18 to 50 years N = 203 | |
| Interventions | Celecoxib 400 mg, n = 51 Indomethacin 20 mg, n = 50 Indomethacin 40 mg, n = 51 Placebo, n = 51 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Clinical trial summary Oxford Quality Score: R1, DB1, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how data from withdrawals were handled |
| Size | Unclear risk | Small treatment group size (50 to 51 participants) |
| Methods | RCT, DB, double‐dummy, single oral dose, 4 parallel groups | |
| Participants | Impacted third molar extraction | |
| Interventions | Celecoxib 200 mg, n = 101 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method. Placebo capsules and tablets matching corresponding active treatments |
| Incomplete outcome data (attrition bias) | Low risk | Participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (100 to 101 active, 51 placebo participants) |
| Methods | RCT, DB single oral dose and multiple oral dose, 4 parallel groups | |
| Participants | Impacted third molar extraction | |
| Interventions | Celecoxib 200 mg, n = 91 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated allocation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method, using marketed tablet or capsule formulations or matching placebos |
| Incomplete outcome data (attrition bias) | Low risk | Participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (90, 91 coxib, 45, 46 ibuprofen, and placebo participants) |
| Methods | RCT, DB single oral dose, 5 parallel groups | |
| Participants | Impacted third molar extraction | |
| Interventions | Celecoxib 400 mg, n = 151 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated allocation schedule" |
| Allocation concealment (selection bias) | Low risk | Participants allocated to next randomisation number (lowest for moderate pain, highest for severe pain) |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method. Each active treatment had matching placebo tablets or capsules |
| Incomplete outcome data (attrition bias) | Low risk | Participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (90 to 151 coxib, 45 to 50 ibuprofen, and placebo participants) |
| Methods | Randomised, double‐blind, single‐dose, parallel‐group, duration 12 h Medication given when pain ≥ moderate Pain assessed at 0, 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12 h | |
| Participants | Surgical removal of ≥ 2 impacted third molars M and F, age 18 to 50 years N = 202 | |
| Interventions | Celecoxib 400 mg, n = 51 Diclofenac, nano‐formulated 18 mg, n = 49 Diclofenac, nano‐formulated 35 mg, n = 51 Placebo, n = 51 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R1, D1, W0 Participants asked to refrain from rescue medication for 1h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how data from withdrawals were handled |
| Size | Unclear risk | Small treatment group size (49 to 51 participants) |
| Methods | RCT, DB single oral dose, 6 parallel groups | |
| Participants | Impacted third molar extraction | |
| Interventions | Celecoxib 400 mg, n = 51 | |
| Outcomes | PI: std 4‐point scale | |
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Investigator, all study staff and related personnel were unaware of treatment assignment |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy method. Matching tablets for CS‐706 and corresponding placebo. Celecoxib and corresponding placebo capsules differed in markings, so participant blindfolded and treatment administered by a third party. |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants accounted for; analysis appropriate for relevant time interval |
| Size | Unclear risk | Small treatment group size (50 to 51 participants) |
RCT ‐ randomised controlled trial; R ‐ randomisation; DB ‐ double blind; W ‐ withdrawals; PI ‐ pain intensity; PR ‐ pain relief; PGE ‐ patient global evaluation; std ‐ standard
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No single‐dose data | |
| No placebo group; included participants with musculoskeletal injuries, not postoperative pain | |
| Not established moderate to severe pain |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised, double‐blind, parallel‐group, duration 2 days Medication given when pain ≥ moderate |
| Participants | Postoperative pain M and F, age ≥ 20 years N = 616 |
| Interventions | Celecoxib Etodolac Placebo Doses not given |
| Outcomes | Patient impression Pain intensity, pain intensity difference Discontinuation due to lack of efficacy Adverse events |
| Notes | May not have single‐dose data Primary completion date November 2010 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Mentioned as "recently completed postoperative pain study" in ARRY‐797‐22 |
| Methods | Randomised, double‐blind, single‐dose, parallel‐group, duration 6 h (to second dose) Medication given when pain ≥ moderate |
| Participants | Surgical removal of ≥ 3 third molars (1 mandibular and impacted) M and F, age 18 to 50 years N = 250 |
| Interventions | Celecoxib 400 mg ARRY‐31797 200 mg ARRY‐31797 400 mg ARRY‐31797 600 mg Placebo |
| Outcomes | TOTPAR (dose 1) Use of rescue medication Adverse events |
| Notes | Primary completion June 2008 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting translation (Japanese) |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain relief over 4‐6 hours Show forest plot | 4 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [2.40, 5.06] |
| Analysis 1.1  Comparison 1 Celecoxib 200 mg versus placebo, Outcome 1 At least 50% pain relief over 4‐6 hours. | ||||
| 1.1 Dental pain | 3 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.86 [5.14, 48.99] |
| 1.2 Postsurgical pain | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.26, 2.68] |
| 2 Use of rescue medication over 24 hours Show forest plot | 2 | 271 | Risk Ratio (IV, Fixed, 95% CI) | 0.78 [0.70, 0.86] |
| Analysis 1.2  Comparison 1 Celecoxib 200 mg versus placebo, Outcome 2 Use of rescue medication over 24 hours. | ||||
| 3 Any adverse event Show forest plot | 4 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.29] |
| Analysis 1.3  Comparison 1 Celecoxib 200 mg versus placebo, Outcome 3 Any adverse event. | ||||
| 3.1 Dental | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.49] |
| 3.2 Orthopaedic | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.43, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain relief over 4‐6 hours, dental pain Show forest plot | 5 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.26 [5.70, 18.47] |
| Analysis 2.1  Comparison 2 Celecoxib 400 mg versus placebo, Outcome 1 At least 50% pain relief over 4‐6 hours, dental pain. | ||||
| 2 Use of rescue medication over 24 hours Show forest plot | 3 | 518 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.62, 0.74] |
| Analysis 2.2  Comparison 2 Celecoxib 400 mg versus placebo, Outcome 2 Use of rescue medication over 24 hours. | ||||
| 3 Any adverse event Show forest plot | 6 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.17] |
| Analysis 2.3  Comparison 2 Celecoxib 400 mg versus placebo, Outcome 3 Any adverse event. | ||||

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
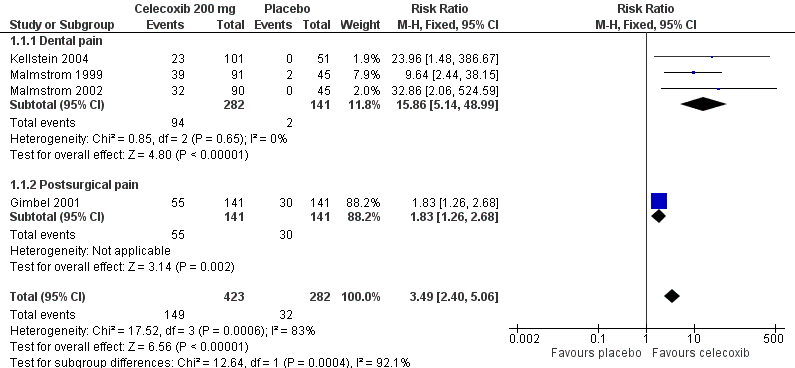
Forest plot of comparison: 1 Celecoxib 200 mg versus placebo, outcome: 1.1 At least 50% pain relief over 4‐6 hours.

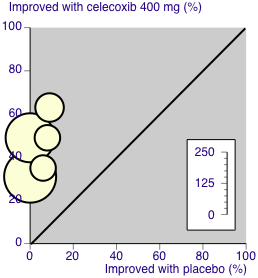
L'Abbé plot of celecoxib 200 mg versus placebo for at least 50% pain relief. Size of circle is proportional to size of study (inset scale). Cream circles ‐ dental studies; pink circle ‐ orthopaedic study.

Forest plot of comparison: 2 Celecoxib 400 mg versus placebo, outcome: 2.1 At least 50% pain relief over 4‐6 hours dental pain.
L'Abbé plot of celecoxib 400 mg versus placebo for at least 50% pain relief. Size of circle is proportional to size of study (inset scale). Cream circles ‐ dental studies

Comparison 1 Celecoxib 200 mg versus placebo, Outcome 1 At least 50% pain relief over 4‐6 hours.

Comparison 1 Celecoxib 200 mg versus placebo, Outcome 2 Use of rescue medication over 24 hours.

Comparison 1 Celecoxib 200 mg versus placebo, Outcome 3 Any adverse event.
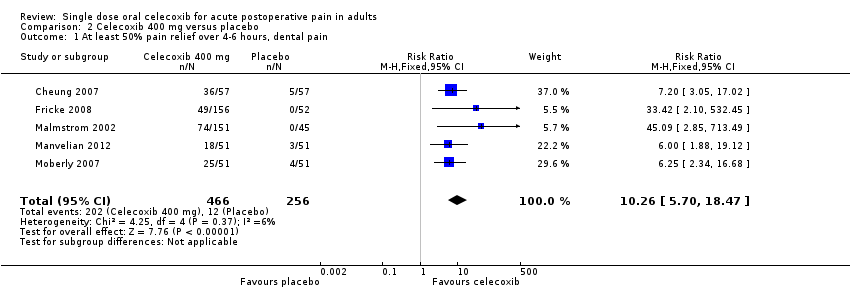
Comparison 2 Celecoxib 400 mg versus placebo, Outcome 1 At least 50% pain relief over 4‐6 hours, dental pain.
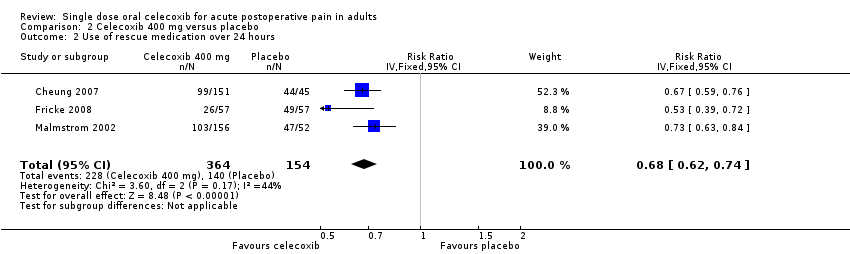
Comparison 2 Celecoxib 400 mg versus placebo, Outcome 2 Use of rescue medication over 24 hours.

Comparison 2 Celecoxib 400 mg versus placebo, Outcome 3 Any adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain relief over 4‐6 hours Show forest plot | 4 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [2.40, 5.06] |
| 1.1 Dental pain | 3 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.86 [5.14, 48.99] |
| 1.2 Postsurgical pain | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.26, 2.68] |
| 2 Use of rescue medication over 24 hours Show forest plot | 2 | 271 | Risk Ratio (IV, Fixed, 95% CI) | 0.78 [0.70, 0.86] |
| 3 Any adverse event Show forest plot | 4 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.29] |
| 3.1 Dental | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.49] |
| 3.2 Orthopaedic | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.43, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain relief over 4‐6 hours, dental pain Show forest plot | 5 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.26 [5.70, 18.47] |
| 2 Use of rescue medication over 24 hours Show forest plot | 3 | 518 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.62, 0.74] |
| 3 Any adverse event Show forest plot | 6 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.17] |


