Single dose oral celecoxib for acute postoperative pain in adults
Información
- DOI:
- https://doi.org/10.1002/14651858.CD004233.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 22 octubre 2013see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
JB, JR, and RAM undertook searches, extracted and analysed the data for the first review.
SD and RAM carried out searches, data extraction, analysis, and writing for both earlier updates.
TW and SD carried out searches, data extraction and analysis, and updated this review. RAM checked all stages.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
No sources of support supplied
Declarations of interest
SD and TW have no interests to declare. RAM has consulted for various pharmaceutical companies and received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. SD and RAM have received research support from charities, government, and industry sources at various times; no such support was received for this work.
Acknowledgements
Tom Weir (work experience student) helped with all aspects of this update.
This update was supported by funds from the Oxford Pain Relief Trust. Earlier updates were supported by the NHS Cochrane Collaboration Programme Grant Scheme.
Jodie Barden, Jane Edwards, and Henry McQuay were involved in the earlier reviews.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Oct 22 | Single dose oral celecoxib for acute postoperative pain in adults | Review | Sheena Derry, R Andrew Moore | |
| 2012 Mar 14 | Single dose oral celecoxib for acute postoperative pain in adults | Review | Sheena Derry, R Andrew Moore | |
| 2008 Oct 08 | Single dose oral celecoxib for acute postoperative pain in adults | Review | Sheena Derry, Jodie Barden, Henry J McQuay, R Andrew Moore | |
| 2003 Jan 20 | Single dose oral celecoxib for postoperative pain | Review | Jodie Barden, Jayne Edwards, Henry HJ McQuay, R A Moore, Andrew Moore | |
Notes
At December 2016, this review has been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions. New searches in November 2016 did not identify any studies that would affect the conclusions of the review. In one single‐dose study in dental pain and two multiple‐dose studies in bunionectomy, celecoxib 400 mg was used as an active comparator for submicron formulations of diclofenac and indomethacin. Results for celecoxib versus placebo were consistent with the review findings.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Pain [*drug therapy];
- Administration, Oral;
- Celecoxib;
- Cyclooxygenase 2 Inhibitors [*administration & dosage];
- Cyclooxygenase Inhibitors [administration & dosage];
- Pain, Postoperative [*drug therapy];
- Pyrazoles [*administration & dosage];
- Randomized Controlled Trials as Topic;
- Sulfonamides [*administration & dosage];
Medical Subject Headings Check Words
Adult; Humans;
PICO

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
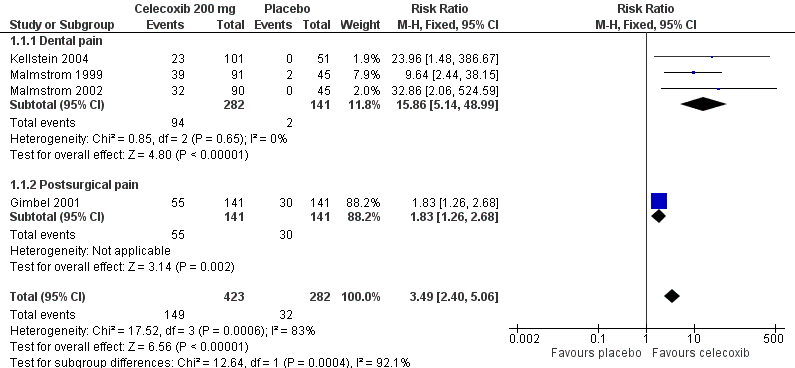
Forest plot of comparison: 1 Celecoxib 200 mg versus placebo, outcome: 1.1 At least 50% pain relief over 4‐6 hours.

L'Abbé plot of celecoxib 200 mg versus placebo for at least 50% pain relief. Size of circle is proportional to size of study (inset scale). Cream circles ‐ dental studies; pink circle ‐ orthopaedic study.

Forest plot of comparison: 2 Celecoxib 400 mg versus placebo, outcome: 2.1 At least 50% pain relief over 4‐6 hours dental pain.
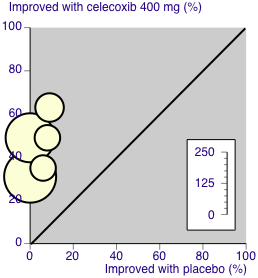
L'Abbé plot of celecoxib 400 mg versus placebo for at least 50% pain relief. Size of circle is proportional to size of study (inset scale). Cream circles ‐ dental studies

Comparison 1 Celecoxib 200 mg versus placebo, Outcome 1 At least 50% pain relief over 4‐6 hours.

Comparison 1 Celecoxib 200 mg versus placebo, Outcome 2 Use of rescue medication over 24 hours.

Comparison 1 Celecoxib 200 mg versus placebo, Outcome 3 Any adverse event.
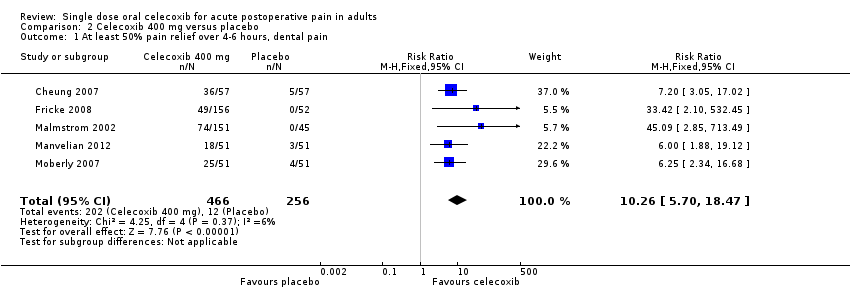
Comparison 2 Celecoxib 400 mg versus placebo, Outcome 1 At least 50% pain relief over 4‐6 hours, dental pain.
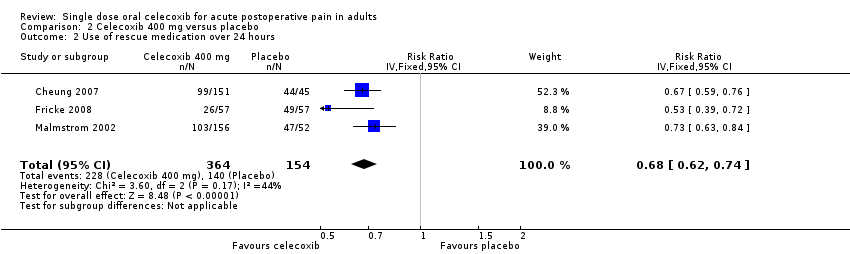
Comparison 2 Celecoxib 400 mg versus placebo, Outcome 2 Use of rescue medication over 24 hours.

Comparison 2 Celecoxib 400 mg versus placebo, Outcome 3 Any adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain relief over 4‐6 hours Show forest plot | 4 | 705 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [2.40, 5.06] |
| 1.1 Dental pain | 3 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.86 [5.14, 48.99] |
| 1.2 Postsurgical pain | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.26, 2.68] |
| 2 Use of rescue medication over 24 hours Show forest plot | 2 | 271 | Risk Ratio (IV, Fixed, 95% CI) | 0.78 [0.70, 0.86] |
| 3 Any adverse event Show forest plot | 4 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.63, 1.29] |
| 3.1 Dental | 3 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.49] |
| 3.2 Orthopaedic | 1 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.43, 1.48] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain relief over 4‐6 hours, dental pain Show forest plot | 5 | 722 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.26 [5.70, 18.47] |
| 2 Use of rescue medication over 24 hours Show forest plot | 3 | 518 | Risk Ratio (IV, Fixed, 95% CI) | 0.68 [0.62, 0.74] |
| 3 Any adverse event Show forest plot | 6 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.17] |


