Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis
Abstract
Background
Respiratory tract infection with Pseudomonas aeruginosa occurs in most people with cystic fibrosis. Once chronic infection is established, Pseudomonas aeruginosa is virtually impossible to eradicate and is associated with increased mortality and morbidity. Early infection may be easier to eradicate.
This is an update of a Cochrane review first published in 2003, and previously updated in 2006 and 2009.
Objectives
To determine whether antibiotic treatment of early Pseudomonas aeruginosa infection in children and adults with cystic fibrosis eradicates the organism, delays the onset of chronic infection, and results in clinical improvement. To evaluate whether there is evidence that a particular antibiotic strategy is superior to or more cost‐effective than other strategies and to compare the adverse effects of different antibiotic strategies (including respiratory infection with other micro‐organisms).
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings.
Most recent search: 08 September 2014.
Selection criteria
We included randomised controlled trials of people with cystic fibrosis, in whom Pseudomonas aeruginosa had recently been isolated from respiratory secretions. We compared combinations of inhaled, oral or intravenous antibiotics with placebo, usual treatment or other combinations of inhaled, oral or intravenous antibiotics. We excluded non‐randomised trials, cross‐over trials, and those utilising historical controls.
Data collection and analysis
Both authors independently selected trials, assessed risk of bias and extracted data.
Main results
The search identified 49 trials; seven trials (744 participants) with a duration between 28 days and 27 months were eligible for inclusion. Three of the trials are over 10 years old and their results may be less applicable today given the changes in standard treatment. Some of the trials had low numbers of participants and most had relatively short follow‐up periods; however, there was generally a low risk of bias from missing data. In most trials it was difficult to blind participants and clinicians to treatment given the interventions and comparators used. Two trials were supported by the manufacturers of the antibiotic used.
Evidence from two trials (38 participants) at the two‐month time‐point showed treatment of early Pseudomonas aeruginosa infection with inhaled tobramycin results in microbiological eradication of the organism from respiratory secretions more often than placebo, odds ratio 0.15 (95% confidence interval 0.03 to 0.65) and data from one of these trials, with longer follow up, suggested that this effect may persist for up to 12 months.
One randomised controlled trial (26 participants) compared oral ciprofloxacin and nebulised colistin versus usual treatment. Results after two years suggested treatment of early infection results in microbiological eradication of Pseudomonas aeruginosa more often than no anti‐pseudomonal treatment, odds ratio 0.12 (95% confidence interval 0.02 to 0.79).
One trial comparing 28 days to 56 days treatment with nebulised tobramycin solution for inhalation in 88 participants showed that both treatments were effective and well‐tolerated, with no notable additional improvement with longer over shorter duration of therapy. However, this trial was not powered to detect non‐inferiority or equivalence .
A trial of oral ciprofloxacin with inhaled colistin versus nebulised tobramycin solution for inhalation alone (223 participants) failed to show a difference between the two strategies, although it was underpowered to show this. A further trial of inhaled colistin with oral ciprofloxacin versus nebulised tobramycin solution for inhalation with oral ciprofloxacin also showed no superiority of the former, with increased isolation of Stenotrophomonas maltophilia in both groups.
A recent, large trial in 306 children aged between one and 12 years compared cycled nebulised tobramycin solution for inhalation to culture‐based therapy and also ciprofloxacin to placebo. The primary analysis showed no difference in time to pulmonary exacerbation or proportion of Pseudomonas aeruginosa positive cultures. An analysis performed in this review (not adjusted for age) showed fewer participants in the cycled therapy group with one or more isolates of Pseudomonas aeruginosa, odds ratio 0.51 (95% CI 0.31 to 0.28).
Authors' conclusions
We found that nebulised antibiotics, alone or in combination with oral antibiotics, were better than no treatment for early infection with Pseudomonas aeruginosa. Eradication may be sustained for up to two years. There is insufficient evidence to determine whether antibiotic strategies for the eradication of early Pseudomonas aeruginosa decrease mortality or morbidity, improve quality of life, or are associated with adverse effects compared to placebo or standard treatment. Four trials of two active treatments have failed to show differences in rates of eradication of Pseudomonas aeruginosa. There have been no published randomised controlled trials that investigate the efficacy of intravenous antibiotics to eradicate Pseudomonas aeruginosa in cystic fibrosis. Overall, there is still insufficient evidence from this review to state which antibiotic strategy should be used for the eradication of early Pseudomonas aeruginosa infection in cystic fibrosis.
PICO
Plain language summary
Different ways of giving antibiotics to eradicate Pseudomonas aeruginosa infection in people with cystic fibrosis
Review question
We reviewed the evidence for the effectiveness of antibiotics in getting rid of a lung infection with a germ called Pseudomonas aeruginosa in people with cystic fibrosis.
Background
Cystic fibrosis is an inherited condition where the airways often become blocked with mucus. It is associated with chest infections, which can lead to progressive breathing failure and death. A germ called Pseudomonas aeruginosa is a frequent cause of infection and is difficult to treat effectively, once infection has become established.
Search date
The evidence is current to September 2014.
Study characteristics
We wanted to compare different combinations of inhaled, oral and intravenous (IV) antibiotics for eliminating Pseudomonas aeruginosa in people with cystic fibrosis and find out if any single treatment works best and is more cost‐effective. We included seven trials with a total of 744 individuals, but the treatments were mostly different so we could not combine the results. Two trials compared tobramycin to placebo (a dummy treatment). Three trials used a combination of oral ciprofloxacin and inhaled colistin in one group of volunteers and compared this combination to no treatment in one trial, to inhaled tobramycin in a second trial and to oral ciprofloxacin with inhaled tobramycin in the third trial. Another trial compared 28 days of inhaled tobramycin to 56 days of inhaled tobramycin and the final included trial compared regular cycles of inhaled tobramycin (plus oral ciprofloxacin or placebo) to culture‐based inhaled tobramycin (plus oral ciprofloxacin or placebo). Trials included people with cystic fibrosis of both sexes, any age and both mild and more severe lung disease. The trials lasted from 28 days to 27 months.
Key results
Two small trials (38 volunteers) treating early infection showed that, after two months, nebulised antibiotics were better than no treatment and eliminated Pseudomonas aeruginosa in most people. One of these trials reported results over a longer period and these suggested that this effect may last for up to 12 months. Another small trial (26 volunteers) which lasted two years showed that treating early infection with a combination of nebulised and oral antibiotics was better than no treatment at eliminating Pseudomonas aeruginosa. A further trial (88 volunteers), which compared 28 days of nebulised tobramycin solution for inhalation to 56 days, showed both were equally tolerated and effective at eliminating Pseudomonas aeruginosa. Four direct comparisons of oral or inhaled antibiotics (or combinations of both), one of which reported on 223 volunteers, did not find a difference between different antibiotic combinations. A recent, large trial in 306 children (aged up to 12 years) compared a regular cycle of treatment to treatment only when it was shown that a child was infected with Pseudomonas aeruginosa, the treatment used was either an antibiotic or a placebo. When we analysed the data for this review, we found that when children were given a regular cycle of inhaled tobramycin (with either oral ciprofloxacin or placebo) fewer of them grew Pseudomonas aeruginosa from their sputum. The official published results from this trial made an adjustment for age and did not show any difference in the number of times Pseudomonas aeruginosa was grown from samples between the groups, nor was there any difference in the length of time until the patients had their next chest infection.
Quality of the evidence
Some of the trials were conducted between 10 and 20 years ago and the results may not be applicable to patients today. Some trials were small and all the trials had quite a short follow‐up period. Therefore, we could not show whether treatment made people with cystic fibrosis feel better or live longer. Given the types of treatment used in most of the trials, it would have been easy for the volunteers to guess which treatment they were receiving, which might have influenced some of the results. Two trials were supported by the pharmaceutical industry. Further research is still needed to see whether eliminating the bacteria completely improves the well‐being and quality of life in people with cystic fibrosis and to establish which antibiotic combination provides the best way of eliminating Pseudomonas aeruginosa.
Authors' conclusions
Background
Description of the condition
Cystic fibrosis (CF) is the most common life‐limiting, autosomal recessively inherited disease in Caucasian populations. Although this is a multisystem disease, the primary cause of death in CF is respiratory failure resulting from chronic pulmonary infection (FitzSimmons 1993). The most frequent cause of chronic pulmonary infection beyond infancy in people with CF is Pseudomonas aeruginosa (P. aeruginosa) and, once established, appears to be permanent in the majority of cases (Fitzsimmons 1996). A number of definitions have been used for chronic P. aeruginosa infection in CF.
-
The presence of P. aeruginosa in monthly specimens for six successive months or the development of precipitating antibodies to P. aeruginosa or both (Valerius 1991).
-
The culture of P. aeruginosa from the sputum or respiratory secretions, on two or more occasions extending over six months or a shorter period if accompanied by a sustained rise of anti‐pseudomonal antibodies (UK CF Trust 2004).
-
The isolation of P. aeruginosa in more than 50% of months over a 12‐month period (Lee 2003) ‐ the second and subsequent positive specimens in the same month do not count. Conversely, eradication is defined as all monthly specimens negative for P. aeruginosa over 12 months.
-
Three or more isolates of P. aeruginosa in a 12‐month period (UK CF Registry 2012).
We have used the first definition in this systematic review.
The age‐specific prevalence of P. aeruginosa in pre‐school children is under 5%, rising to 30% at aged 16 years (UK CF Registry 2013). Some authors have suggested that the use of prophylactic anti‐staphylococcal antibiotic therapy in early childhood may predispose to chronic P. aeruginosa infection (Ratjen 2001b; Stutman 2002). However, this effect was not seen in a systematic review of prophylactic antibiotic use, including over 400 participants (Smyth 2012).
In children who are too young to expectorate, cough swabs or oropharyngeal swabs are the only respiratory specimens which can be easily obtained. These do not reliably predict the presence of P. aeruginosa in the lower respiratory tract (Armstrong 1996; Rosenfeld 1999), whereas flexible fibreoptic bronchoscopy with bronchoalveolar lavage (BAL) may detect positive P. aeruginosa in children with negative cough swabs or oropharyngeal swabs (Douglas 2009; Hilliard 2007). Sputum cultures have been shown to accurately reflect lower respiratory tract organisms in expectorating children and adults (Iacocca 1963; Thomassen 1984). Over half of people with CF have chronic infection with P. aeruginosa by their early twenties (UK CF Registry 2013), although prior to chronic infection P. aeruginosa is often isolated intermittently from respiratory tract specimens. This may represent transient colonies of P. aeruginosa within the lower respiratory tract or alternatively it may reflect the difficulties in accurately detecting P. aeruginosa in the lungs of young people with CF (Burns 2001). The quantity and type of P. aeruginosa present in the lower respiratory tract changes as infection becomes established. It is known that P. aeruginosa has two major phenotypes ‐ mucoid and non‐mucoid. Following first isolation there is a progressive increase in the density of P. aeruginosa colonies in the lower respiratory tract (Rosenfeld 2001). Initial isolates often show a non‐mucoid phenotype; however, as infection progresses a mucoid phenotype may prevail and will be more difficult to eradicate. Douglas has reported a relatively high prevalence (18.2%) of mucoid P. aeruginosa) at first isolation in newborn screened infants, speculating that the notion of transformation from non‐mucoid to mucoid phenotype under environmental pressure may be inaccurate in young children. (Douglas 2009). It has been reported that P. aeruginosa provokes an inflammatory response of the lower respiratory tract (Muhlebach 1999) and there is a marked step up in this inflammatory response as the number of P. aeruginosa colonies increases (Armstrong 1996).
The presence of P. aeruginosa in respiratory secretions is a major predictor of mortality in children with CF (Emerson 2002). Individuals with CF infected with P. aeruginosa also suffer greater morbidity with a more rapid deterioration in lung function (Emerson 2002; Pamukcu 1995) and a more rapid decline in chest radiograph score (Kosorok 2001), poor growth, reduced quality of life, increased hospitalisation and increased need for antibiotic treatment (Ballman 1998; Nixon 2001; Winnie 1991). Some studies suggest there is a temporal relationship between the onset of chronic infection and increased morbidity (Abman 1991; Hudson 1993; Kosorok 2001; Parad 1999), whilst others do not support these findings (Kerem 1990; Rosenfeld 2001). On balance, there seems to be good evidence from well‐designed non‐experimental studies that clinical state deteriorates after first isolation of P. aeruginosa.
Description of the intervention
Several strategies exist to treat early infection with P. aeruginosa and include the use of the inhaled antibiotics such as colistin and tobramycin (Littlewood 1985; Ratjen 2001a), oral quinolones such as ciprofloxacin (Taccetti 2005) and intravenous antibiotics usually consisting of combination of an aminoglycoside with a beta‐lactam (Döring 2000; Douglas 2009).
How the intervention might work
As well as antibiotic treatment of P. aeruginosa given at the time of first isolation, other strategies have the potential to prevent or delay infection of the respiratory tract. These include avoidance of contact with people who carry P. aeruginosa (UK CF Trust 2004) and the development of vaccines against P. aeruginosa (Johansen 2013). Uncontrolled series have indicated that a variety of anti‐pseudomonal antibiotics either singly (Littlewood 1985; Ratjen 2001a) or in combination (Vazquez 1993) at first isolation may delay the onset of chronic infection. A trial using historical controls suggested that oral ciprofloxacin and nebulised colistin are effective in delaying or preventing chronic infection (Frederiksen 1997). An uncontrolled pilot study of intravenous therapy suggested that intravenous treatment alone was less effective in delaying the onset of chronic infection (Steinkamp 1989). There is also evidence supporting eradication therapy from long‐term observational studies of chronic infection with P. aeruginosa in CF clinics such as the study reported by Lee (Lee 2004).
Why it is important to do this review
There are differences in the approach to detection and management of first isolation of P. aeruginosa. Some CF centres advocate frequent microbiological surveillance with attempts to eradicate P. aeruginosa when it first appears in the lung (Döring 2000), whereas others treat only when clinical or radiological signs of pulmonary infection are present (Ramsey 1996). There is evidence that, when P. aeruginosa is cleared from respiratory secretions it is not simply suppressed because, when infection recurs, this is with a genetically distinct organism in most cases (Munck 2001). Evidence that eradication strategies result in increased survival or improved quality of life for people with CF are from observational studies alone. There are multiple different eradication regimens that have been described using different anti‐pseudomonal antibiotics in different combinations of intravenous, oral or nebulised (or both) and with varying doses and duration of therapy (Lee 2009). Given the expense of chronic anti‐pseudomonal suppressive therapy, there is a clear rationale for early eradication from a cost‐effectiveness perspective and this is supported by observational data (Taccetti 2005); however, there has not been any formal evaluation of cost effectiveness to date.
This is an update of a Cochrane review first published in 2003, and previously updated in 2006 and 2009 (Langton Hewer 2009; Wood 2003; Wood 2006).
Objectives
To determine whether antibiotic treatment of early P. aeruginosa infection in children and adults with CF alters clinical and microbiological outcome when compared to usual treatment.
To test the hypotheses that antibiotics against P. aeruginosa, given at the time of first isolation, reduce CF‐related mortality; improve quality of life; improve pulmonary function; nutritional status; and reduce the need for subsequent hospitalisation and consumption of antibiotics.
To investigate whether these antibiotics prevent or delay the onset of chronic infection of the respiratory tract with P. aeruginosa; increase the incidence of isolates of other micro‐organisms from the respiratory tract; and are associated with adverse effects which are either important to the individual with CF or have long‐term sequelae.
To investigate whether there is evidence of superior P. aeruginosa eradication efficacy or improved cost‐effectiveness between different antibiotic strategies.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Children and adults with CF, diagnosed clinically and by sweat or genetic testing (or both) with a first ever positive microbiological isolate of P. aeruginosa from a respiratory tract specimen. Trials will also be included where the participants have been proven to be free of P. aeruginosa for at least six months before a new isolation and should not be currently receiving Pseudomonas‐suppressing treatment such as daily inhaled antibiotic therapy. Participants should be enrolled into a trial within six months (post hoc change ‐ previously not more than two months) from isolation of P. aeruginosa. In a further post hoc change, we have also altered our eligibility criteria to allow trials where all participants receive some eradication therapy before randomisation (seeDifferences between protocol and review). People with CF of all ages and disease severity will be included.
Types of interventions
Combinations of inhaled, oral or intravenous antibiotics with the aim of eradicating first pulmonary isolates of P. aeruginosa compared with placebo or usual treatment (or both) or other combinations of inhaled, oral or intravenous antibiotics.
Types of outcome measures
Primary outcomes
-
Eradication of P. aeruginosa from the respiratory tract as defined by
-
clearance of P. aeruginosa from bronchoalveolar lavage (BAL), sputum or oropharyngeal cultures at 1, 2, 3, 6, 12 and 24 months after commencement of therapy
-
time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
-
Secondary outcomes
-
Mortality
-
Quality of life assessment
-
Spirometric lung function (e.g. forced expiratory volume in one second (FEV1) and forced vital capacity (FVC)) expressed as percentage predicted values for age, sex and height
-
Growth and nutritional status as measured by weight, height (children), body mass index (BMI) or z score
-
Frequency of respiratory exacerbations as defined by:
-
frequency of infective pulmonary exacerbations expressed as the number of exacerbations per patient year
-
time to next course of IV antibiotics from commencement of therapy
-
days in hospital expressed as days in hospital per patient year
-
days of antibiotic usage expressed as days of antibiotic usage per patient year
-
-
Isolation of other micro‐organisms from the respiratory tract expressed as the number of positive cultures per patient year (where available, the microbiology detection method will be described in view of the differences in sensitivity and specificity of oropharyngeal, sputum and BAL samples for bacteriology, mycology and non‐tuberculous mycobacteria)
-
Adverse effects to antibiotics, e.g. renal or auditory impairment and hypersensitivity reactions
Additional outcomes which have arisen during the review
-
Time to chronic infection (as defined above in Description of the condition)
-
Clinical and radiological scores
-
Cost effectiveness (trials looking at cost effectiveness will be compared, where possible)
Search methods for identification of studies
Relevant trials were identified from the Group's Cystic Fibrosis Trials Register using the terms: antibiotics AND (pseudomonas aeruginosa OR mixed infections) AND (eradication OR unknown).
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), quarterly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major CF conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the Trials Register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Group's trials register: 08 September 2014.
We have also searched the relevant clinical trials databases clinicaltrials.gov/ and ISRCTN. We used the search terms "cystic fibrosis" AND "Pseudomonas aeruginosa" AND "eradication".
Date of last search: 01 September 2014.
Data collection and analysis
Selection of studies
For the original review, two authors (DW, AS) independently selected the trials to be included in the review. From Issue 3, 2009 of The Cochrane Library two authors (SLH, AS) selected the trials to be included in the review. Where there was disagreement on the suitability of a trial for inclusion in the review, or on its risk of bias, the authors reached a consensus by discussion.
Data extraction and management
Each author independently extracted data using standard data acquisition forms. Where there was disagreement on the suitability of a trial for inclusion in the review, or on its risk of bias, the authors reached a consensus by discussion.
We planned to group outcome data into those measured at one, three, six, twelve months and annually thereafter. In addition, we previously stated that if outcome data were recorded at other time periods as well, that we would also consider examining these data. Some trials reported data at two months for some outcomes and we have included these data within the review. In the Taccetti trial, cumulative data were reported at six months and final follow‐up data at a median of 16 months (range 12 to 28 months); we have included both time points in our analysis (Taccetti 2012). In the Treggiari trial, cumulative data for pulmonary exacerbations and isolates of P. aeruginosa are presented for the 70‐week follow‐up period and these have been reported and analysed in this review (Treggiari 2011).
Assessment of risk of bias in included studies
For earlier versions of this review, in order to assess the risk of bias, each author independently assessed the methodological quality of each trial, based on the method described by Schulz (Schulz 1995). From the 2011 update, each author independently assessed the risk of bias using the tool recommended by the Cochrane Collaboration (Higgins 2011). The risk of bias was judged to be high, unclear or low for the domains of:
-
sequence generation;
-
allocation concealment;
-
blinding (risk of bias increased as the level of blinding decreased);
-
incomplete outcome data (the risk of bias increased if any withdrawals were not adequately described and reasons for withdrawals given, or if the withdrawals were not equal across groups);
-
selective outcome reporting (bias increased if stated outcome measures were only partially reported or not reported at all);
-
other potential sources of bias.
Where there was disagreement on the quality and risk of bias of a trial, the authors reached a consensus by discussion.
Measures of treatment effect
For binary outcome measures, in order to allow an intention‐to‐treat analysis, the authors sought data on the number of participants with each outcome event, by allocated treated group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow up. We calculated a pooled estimate of the treatment effect for each outcome across trials ‐ the odds ratio (OR) or the ratio of the odds of an outcome among treatment allocated participants to the corresponding odds among controls with 95% confidence intervals (CIs).
For continuous outcomes, in order to allow an intention‐to‐treat analysis, we sought outcome data by allocated treated group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow up. We recorded either mean change from baseline for each group or mean post‐treatment or intervention values and standard deviation (SD). We calculated a pooled estimate of treatment effect by calculating the mean difference (MD) with 95% CIs.
The authors have reported longitudinal data as individual time points. We realise that this method ignores any correlation between the participants; however, we have been unable to analyse these data using more appropriate methods as we do not have the correlation co‐efficient for these data. If in the future, we are able to obtain the correlation co‐efficient, we will analyse these data more appropriately.
In this version of the review, we entered time‐to‐event data into the meta‐analysis using the log hazard ratio. This was possible for the outcome 'time to pulmonary exacerbation' (severe or any), in the comparison of cycled versus. culture‐based therapy (Analysis 6.5; Analysis 6.7) and ciprofloxacin versus placebo (Analysis 7.5; Analysis 7.7). We presented binary data on clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures and occurrence of chronic infection with P. aeruginosa at multiple time points. We calculated the OR at each time point separately, thus ignoring the correlation between time points. For future updates of this review, for time‐to‐event data, such as time to next P. aeruginosa infection or time to chronic infection, the authors will attempt to obtain individual patient data (IPD). We will use these IPD to provide estimates of the log hazard ratio and its standard error and plan to combine time‐to‐event data from trials in a meta‐analysis. We used the generic inverse variance (GIV) to analyse the data for time to next isolation of P. aeruginosa from the Ratjen trial (Ratjen 2010).
Unit of analysis issues
Cross‐over trials are not eligible for inclusion within this review.
The natural history of infection with P. aeruginosa in CF comprises an initial infection with the organism usually in planktonic form, followed by chronic infection (in which the P. aeruginosa frequently exists in the mucoid state). In the planktonic form, antibiotics can eradicate the organism; however, persistent infection is associated with biofilm growth and adaptive evolution mediated by genetic variation. The development of mucoidy, hypermutability and the acquisition of mutational antibiotic resistance are important factors associated with persistent infection and are associated with increased difficulty in eradication (Ciofu 2012).
In a cross‐over trial comparing active treatment with placebo, given the progression of infections due to of P.aeruginosa, the group receiving the active treatment after placebo will be at a disadvantage compared with those receiving active treatment first. TheP. aeruginosa may form a biofilm during placebo treatment and so it would not be able to be eradicated during the active treatment phase. Hence, a cross‐over trial is an inappropriate design and the authors have not included cross‐over trials in this review.
Dealing with missing data
In trials where outcome data were unavailable for randomised participants, the authors performed an available‐case analysis. This available‐case analysis included data on only those participants whose results are known, using as a denominator the total number of people who completed the trial for the particular outcome in question.
When data were incomplete, the authors imputed the missing data to provide best‐case and worst‐case scenarios, in order to show the range of possible results for the combined analysis (seeAnalysis 1.4; Analysis 1.5). The best‐case scenario analysis is based on the assumption that all the missing data points represented beneficial clinical outcomes, whereas the worst‐case analysis assumes that all missing data points had a negative clinical outcome.
Assessment of heterogeneity
For future updates of this review, if we are able to combine a sufficient number of trials (at least four), we will test for heterogeneity using the I2 statistic (Higgins 2003). We will consider values of I2 up to 30% to indicate little or no heterogeneity, values between 30% and 60% to represent moderate heterogeneity, values from 60% to 90% to represent substantial heterogeneity and values over 90% to represent considerable heterogeneity. We accept that the importance of the observed value of I2 depends firstly on the magnitude and direction of effects and secondly on strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a confidence interval for I2). In a future version of this review (with more trials included in the meta‐analysis of individual treatment comparisons), if we find evidence of at least substantial clinical heterogeneity (as defined above) in the included trials, we will perform a random‐effects analysis.
Assessment of reporting biases
The authors sought evidence of reporting bias by comparison of the reported outcomes with those listed in the trial's methodological description. Where important outcomes have not been identified, we have requested the original trial protocol from the authors.
Data synthesis
The authors have analysed the data using a fixed‐effect model. If, in future updates of this review, we find evidence of at least substantial clinical heterogeneity (as defined above) in the included trials, we plan to perform a random‐effects analysis.
Subgroup analysis and investigation of heterogeneity
If we identify a moderate degree of heterogeneity or higher (Higgins 2003) and are able to combine a sufficient number of trials (at least 10), then we will investigate this with subgroup analyses. We plan to categorise participants if possible as P. aeruginosa‐free and P. aeruginosa‐naive according to the definition by Lee (Lee 2003). These subgroups will be analysed separately.
Sensitivity analysis
We also plan to test the robustness of our results with the following sensitivity analyses:
-
trials where participants receive treatment within three months of isolation of P. aeruginosa versus those where the interval is between three and 12 months;
-
trials with high risk of bias versus low risk of bias for generation of allocation sequence;
-
trials with a high risk of bias versus a low risk of bias for concealment of allocation;
-
multi‐centre versus single centre trials.
Results
Description of studies
Results of the search
Our search identified a total of 49 trials, none of these were cross‐over trials. Of these 49 trials, seven met our inclusion criteria (Gibson 2003; Proesmans 2013; Ratjen 2010; Taccetti 2012; Treggiari 2011; Valerius 1991; Wiesemann 1998); we identified one trial which is still ongoing and will include data from this trial in a future update of this review once they have been published (TORPEDO Trial); 40 trials were excluded and one trial is listed as 'Awaiting classification' (Noah 2010). We have contacted the investigators of this trial for further information to allow us to include or exclude it at a future update.
Included studies
The seven included trials enrolled a total of 744 participants (Gibson 2003; Proesmans 2013; Ratjen 2010; Taccetti 2012; Treggiari 2011; Valerius 1991; Wiesemann 1998).
Trial characteristics
All seven included trials were randomised controlled trials of parallel design. Two trials were placebo‐controlled (Gibson 2003; Wiesemann 1998); one trial compared active treatment to no treatment (Valerius 1991); and three trials were open‐label trials comparing different active treatments (Proesmans 2013; Ratjen 2010; Taccetti 2012). The design of the Treggiari trial was complex, with randomisation to cycled treatment with tobramycin solution for inhalation (TSI) or culture‐based treatment and further randomisation to additional oral ciprofloxacin or placebo (Treggiari 2011).
One trial reported stratification for age and participating centre (Gibson 2003), another trial reported stratification by age and FEV1 values as an expression of illness severity (Taccetti 2012); the remaining five trials did not use stratification (Proesmans 2013; Ratjen 2010; Treggiari 2011; Valerius 1991; Wiesemann 1998). Two trials stated that they were double‐blind (Gibson 2003; Wiesemann 1998); the Treggiari trial used placebo to blind for ciprofloxacin but not for TSI (Treggiari 2011); the other trials could not be blinded due to differing treatment regimens. Five trials were multicentre (Gibson 2003; Ratjen 2010; Taccetti 2012; Treggiari 2011; Wiesemann 1998) and the other two were single‐centre trials (Proesmans 2013; Valerius 1991). Five trials were based in Europe (Proesmans 2013; Ratjen 2010; Taccetti 2012; Valerius 1991; Wiesemann 1998) and two in North America (Gibson 2003; Treggiari 2011). The number of participants in each trial ranged from 21 to 306 and were as follows: 306 in the Treggiari trial (Treggiari 2011); 223 in the Taccetti trial (Taccetti 2012); 88 in the Ratjen trial (Ratjen 2010); 26 in the Proesmans trial (Proesmans 2013); 26 in the Valerius trial (Valerius 1991); 22 in the Wiesemann trial (Wiesemann 1998); and 21 in the Gibson trial (Gibson 2003). The Gibson trial reported that the planned sample size was 98 participants, but randomisation was stopped after an early interim analysis by the Data Monitoring Committee was undertaken due to poor accrual. This analysis showed a statistically significant treatment effect and so the trial was stopped (Gibson 2003). The duration of the trials varied and ranged from 28 days (Gibson 2003) to 27 months (Valerius 1991).
Participant characteristics
All seven trials had approximately equal numbers of males and females. Only two trials recruited adult participants; Taccetti recruited participants aged from 1 to 35 years (Taccetti 2012) and in the Ratjen trial any patient over six months of age was eligible for inclusion (Ratjen 2010). Two trials were restricted to younger children: six months to six years (Gibson 2003) and 1 to 12 years (Treggiari 2011). All seven trials specified that participants had to have microbiological evidence of recent onset of airway infection with P. aeruginosa. However, the interval allowed between isolation of P. aeruginosa and randomisation to study treatment varied greatly, from four weeks (Proesmans 2013; Wiesemann 1998) to as long as six months (Treggiari 2011). Two trials additionally specified that individuals with raised titres to anti‐pseudomonal antibodies were excluded from the trial (Ratjen 2010; Wiesemann 1998). Other data from the EPIC study have shown that raised antibodies to P. aeruginosa (anti‐alkaline protease and anti‐exotoxin A) are associated with an increased risk of recurrence in the 60 weeks following eradication treatment (Anstead 2013). Studies excluding participants with raised antibodies might therefore be expected to achieve higher eradication rates.
Intervention
Trials have used various combinations of inhaled tobramycin, inhaled colistin, oral ciprofloxacin, placebo and no treatment. The duration of the intervention has varied greatly, from three weeks (Valerius 1991) to one year (Wiesemann 1998). Two trials compared tobramycin to placebo (Gibson 2003; Wiesemann 1998). One trial used tobramycin solution for inhalation (TSI) TOBI® (now marketed by Novartis) at a dose of 300 mg twice‐daily for 28 days (Gibson 2003); the second trial used aerosolised tobramycin parenteral preparation (Eli Lilly, Bad Homburg, Germany) at a dose of 80 mg twice‐daily for 12 months (Wiesemann 1998). Ratjen evaluated a short (28 days) versus a longer (56 days) TSI course (Ratjen 2010). Participants in the Treggiari trial were randomised to receive either cycles of four weeks of treatment with nebulised TSI in every 12‐week period or TSI only when respiratory culture was positive for P. aeruginosa (Treggiari 2011). In this trial, participants were also randomised to receive either oral ciprofloxacin or placebo for two weeks, commencing at the same time as TSI. This design resulted in a four‐arm trial where all trial participants had an initial 28‐day course of TSI, with an additional 28 days given if the patient remained positive after initial treatment. Ciprofloxacin or placebo was not given with second course of TSI and follow up was for 18 months from randomisation and first treatment with TSI (Treggiari 2011). Three trials evaluated inhaled colistin in combination with oral ciprofloxacin (Proesmans 2013; Taccetti 2012; Valerius 1991). Proesmans compared colistin 2 million units (MU) twice daily for three months (in combination with oral ciprofloxacin 30 mg/kg/day) to TSI 300 mg twice daily for 28 days (Proesmans 2013). The Taccetti trial compared 28 days of inhaled colistin with 28 days TSI; both arms also had oral ciprofloxacin 30 mg/kg/day for 28 days (Taccetti 2012). Valerius compared colistin 1 MU (plus ciprofloxacin 250 mg to 750 mg), both given twice daily for three weeks, for initial and any subsequent isolate of P. aeruginosa to no treatment (Valerius 1991).
Outcome measures
The most widely used primary outcome measure was eradication of P. aeruginosa from respiratory secretions, though definitions of eradication differ considerably between trials. In the Gibson trial, the primary outcome was the change in P. aeruginosa density, on BAL, from baseline to 28 days (Gibson 2003). However, the trial also looked at eradication, defined as a density of P. aeruginosa in BAL of less than 20 colony forming units (CFU) at 28 days from baseline. Proesmans defined eradication as a negative culture result for P. aeruginosa (from sputum, cough swab or BAL) at 28 days or three months (depending on which intervention the participant received) (Proesmans 2013). Both trials based successful eradication on a single specimen (Gibson 2003; Proesmans 2013). In contrast, Taccetti used a more stringent definition of eradication as per guidance published by the UK CF Trust of three negative cultures in a six‐month period (Taccetti 2012; UK CF Trust 2004). Ratjen used the median time to recurrence of any strain of P. aeruginosa during a 27‐month follow‐up period (Ratjen 2010). In the oldest trial, the primary outcome measure was time to chronic infection with P. aeruginosa, defined as the presence of P. aeruginosa in monthly sputum samples for six consecutive months or the development of precipitating serum antibodies against P. aeruginosa or both (seeDescription of the condition for other definitions of chronic infection) (Valerius 1991). There were two primary outcomes in the Treggiari trial, time to pulmonary exacerbation requiring intravenous antibiotics and proportion of P. aeruginosa positive cultures over the 18‐month trial period (Treggiari 2011).
Other measured outcomes included less severe pulmonary exacerbations, Pseudomonas antibody levels, lung function, nutritional status, modified Shwachman score and monitoring for adverse clinical and microbiological effects.
Excluded studies
We excluded 40 trials from our analysis for a number of reasons. Three trials were excluded because therapy was not randomised (Gibson 2007; Postnikov 2000; Schelstraete 2010) and one trial was excluded because it was an observational study (Ballman 1998). Four trials were excluded as they did not have a control group (Heinzl 2002; Littlewood 1985; Ratjen 2001a; Steinkamp 1989) and a further five because they used a historical control group (Frederiksen 1997; Griese 2002; Kenny 2009; Taccetti 2005; Vazquez 1993). A total of 16 trials were excluded as they involved participants with chronic P. aeruginosa infection (Clancy 2013; Coates 2011; Goss 2009; Konstan 2010; Latzin 2008; Lenoir 2007; Mazurek 2012; Oermann 2009; Postnikov 2007; Prayle 2013; Ramsey 1999; Retsch‐Bogart 2008; Retsch‐Bogart 2009; Steinkamp 2007; Trapnell 2012; Wainwright 2011b). Two trials were excluded as they were designed to evaluate a diagnostic technique for P. aeruginosa (Brett 1992; Wainwright 2011a). A further two trials were excluded as they evaluated symptomatic rather than eradication treatment (Church 1997; Schaad 1997) and one was of a prophylactic antibiotic regimen to prevent infection with P. aeruginosa (Tramper‐Stranders 2009). Five trials looked at pharmacokinetics and drug tolerability (Alothman 2002; Alothman 2005; Geller 2007; Rietschel 2009; Schuster 2013) and the final trial was excluded as it looked at antibiotic sinonasal nebulisation aiming to eradicate from the sinuses only (Mainz 2014).
Risk of bias in included studies
Please see further information in the risk of bias sections of the tables (Characteristics of included studies) and the graphical risk of bias summary (Figure 1).
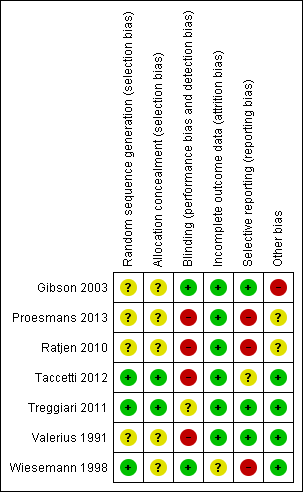
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All seven trials were described as randomised controlled trials; in four, the method of generation of allocation sequence was not stated; hence the trials are judged to have an unclear risk of bias (Gibson 2003; Proesmans 2013; Ratjen 2010; Valerius 1991). The remaining three trials are judged to have a low risk of bias (Taccetti 2012; Treggiari 2011; Wiesemann 1998). In the first of these, Taccetti describes a balanced randomisation sequence that was created using statistical software using permuted blocks of size 10 (Taccetti 2012). Treggiari employed a computer random number generator to assign treatments within permuted blocks of 12 (Treggiari 2011). In the Wiesemann trial, the allocation sequence was generated using a coin flip (Wiesemann 1998). There is no information as to who was responsible for the coin flip or what controls were in place to ensure validity of the result of the coin flip; however we have still judged this to have a low risk of bias.
In five trials it was not reported how allocation was concealed and we judged these to have an unclear risk of bias (Gibson 2003; Proesmans 2013; Ratjen 2010; Valerius 1991; Wiesemann 1998). In the Taccetti trial, the staff involved in randomisation and in treatment assignments were "kept separate" and we judged this trial to have a low risk of selection bias (Taccetti 2012). In the Treggiari trial, randomisation was remote and so allocation was concealed (Treggiari 2011).
Blinding
Two trials were reported as double‐blind trials (Gibson 2003; Wiesemann 1998). Gibson did not provide any details in the published paper regarding who was blinded or the method of blinding (Gibson 2003). However, in response to a request for further information, Dr Gibson confirmed that drugs and placebo were sufficiently masked that neither participants or clinicians were able to differentiate between them. Wiesemann reported that participants were blinded by providing a placebo inhalation with a similar taste to the treatment inhalation, but it is not clear whether the clinicians administering the treatment were blinded to treatment allocation; however, we still judged this to have a low risk of bias (Wiesemann 1998).
In the Treggiari trial, the oral ciprofloxacin treatment was blinded but the TSI was open label and so we judged this to have an unclear risk of bias (Treggiari 2011).
The remaining four trials did not utilise blinding and were judged to have a high risk of bias (Proesmans 2013; Ratjen 2010; Taccetti 2012; Valerius 1991). The Proesmans trial compared an inhaled intervention to a combination of inhaled and oral treatment, so blinding of participants and clinicians was not possible; there is no information available as to whether the outcome assessors were blinded (Proesmans 2013). The Ratjen trial was open label comparing 28 or 56 days of nebulised therapy and made no attempt at blinding (Ratjen 2010). The Taccetti trial was also open label comparing 28 days of inhaled colistin with 28 days of TSI, both groups also receiving 28 days of oral ciprofloxacin (Taccetti 2012). Valerius compared a combination of inhaled and oral treatment to no treatment, so again blinding of participants and clinicians was not possible and no information is available with regards to the outcome assessors (Valerius 1991).
Incomplete outcome data
Six trials were judged to have a low risk of bias (Gibson 2003; Proesmans 2013; Ratjen 2010; Taccetti 2012; Treggiari 2011; Valerius 1991). Four trials were analysed on an intention‐to‐treat basis; all four reported data on all participants who were randomised (Gibson 2003; Proesmans 2013; Taccetti 2012; Valerius 1991). In the Ratjen trial, 65 of the 88 randomised participants were included in the primary outcome (time to recurrence of P. aeruginosa). A total of 52 participants were prematurely withdrawn from the trial, but these were approximately evenly distributed across the two treatment groups and reasons were given for each withdrawal; we therefore judge there to be a low risk of bias (Ratjen 2010). In the Treggiari trial only two of 306 randomised participants were excluded from the analysis (because they did not receive treatment) (Treggiari 2011).
One trial had an unclear risk of bias (Wiesemann 1998). Five participants withdrew from this trial after randomisation and only baseline data at entry to the trial were presented for these participants; to date we have been unable to obtain further outcome data. The trial was therefore analysed on an available‐case basis and we judged it to have an unclear risk of bias (Wiesemann 1998).
Selective reporting
It is current practice for newer trials to publish a protocol, but we have not been able to locate a published protocol for the Taccetti trial and have therefore judged this trial to have an unclear risk of bias (Taccetti 2012).
We judged three trials to have a high risk of bias (Proesmans 2013; Ratjen 2010; Wiesemann 1998). Although a protocol for the Proesmans trial was available online and we were able to confirm from the full paper that all outcomes from the protocol were measured, the paper did not provide actual data for BMI z score, weight z score or frequency of exacerbations and simply reported that none of these changed significantly for trial participants (Proesmans 2013). Ratjen reported that there were no major short‐term (at three months) or long‐term (at 27 months) changes in spirometry, but did not record the figures for either of the two groups. In addition, only summary statements and no numerical data were provided for weight, height or BMI (Ratjen 2010). Wiesemann reported no change in spirometric pulmonary function during or after the treatment period, but again no data were given (Wiesemann 1998).
We judged three trials to have a low risk of bias from selective reporting (Gibson 2003; Treggiari 2011; Valerius 1991). The trial protocol for the EPIC trial was published as a separate paper; primary and secondary outcome measures were clearly described in the protocol and data on all of these outcomes were presented in either the main paper, related papers or in the online supplement (Treggiari 2011). We have compared the 'Methods' sections of the reports from the remaining trials with the 'Results' sections of the same and have not found any evidence of selective reporting (Gibson 2003; Valerius 1991).
Other potential sources of bias
We judged one trial to have a high risk due to other potential sources of bias (Gibson 2003). The Gibson trial planned to recruit to a sample size of 98 participants, but was stopped early by the Data Monitoring Committee after interim analysis of the first 21 participants showed a statistically significant microbiological effect in favour of the tobramycin‐treated group (Gibson 2003). This trial was supported in part by Chiron, the manufacturer of the inhaled tobramycin (Gibson 2003).
We judged the Ratjen trial to have an unclear risk due to other potential sources of bias (Ratjen 2010). This trial recruited fewer participants than planned, the total number of planned randomised participants was 100, but 35 from the recruited cohort of 123 were not randomised: 31 because of high P. aeruginosa antibody levels (which led the investigators to believe that they were chronically infected with P aeruginosa); one for an adverse event; one where consent was withdrawn; one for a protocol deviation; and one 'other' (unspecified) reason (Ratjen 2010). Therefore the trial investigators actually randomised 88 participants and the primary outcome was evaluable in 65 of these (Ratjen 2010). The trial results cannot be generalised to a population where anti‐Pseudomonas antibodies are not measured. Furthermore, like the earlier Gibson trial, this trial was initially supported by Chiron and later Novartis Pharma, the manufacturer of TSI (Ratjen 2010).
The Proesmans study was judged to have an unclear risk of bias in view of the different time‐points at which the primary outcome was measured ‐ at 28 days in the inhaled tobramycin group and three months in the colistin with ciprofloxacin group (Proesmans 2013).
In four trials no other potential source of bias was identified and these were judged to have a low risk of bias (Taccetti 2012; Treggiari 2011; Valerius 1991; Wiesemann 1998).
Effects of interventions
Inhaled tobramycin versus placebo
This comparison included two trials with 43 participants (Gibson 2003; Wiesemann 1998).
Primary outcome
1. Eradication of P. aeruginosa from the respiratory tract
a. Clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures
The intervention (inhaled tobramycin) was given in a very different dose in the Wiesemann trial (80 mg twice daily) compared to the Gibson trial (300 mg twice daily) (Gibson 2003; Wiesemann 1998). In the Gibson trial, significantly fewer children who received TSI had a positive BAL at one month, OR 0.01 (95% CI 0.00 to 0.30), but not at two months, OR 0.21 (95% CI 0.03 to 1.47) (Analysis 1.1). Results were not available for all the participants in the Wiesemann trial, thereby precluding an intention‐to‐treat analysis (Wiesemann 1998). Wiesemann was able to demonstrate a statistically significant reduction in the odds of a positive culture from the respiratory tract specimen only after six months, OR 0.06 (95% CI 0.00 to 0.92) and 12 months of treatment, OR 0.02 (95% CI 0.00 to 0.67) but not at one, two or three months (Analysis 1.2). An available‐case analysis of the data presented in the Wiesemann trial showed that, when combined with the data from the Gibson trial, there was a reduction in the odds of a positive culture in the treatment group compared to the placebo group at one month, OR 0.06 (95% CI 0.01 to 0.33); and two months, OR 0.15 (95% CI 0.03 to 0.65) both of which were statistically significant (Analysis 1.3).
A sensitivity analysis following imputation of the missing data to provide best‐case and worst case‐scenarios for the combined analysis showed a range of possible results. The best‐case scenario showed a reduction in the odds of a positive culture of P. aeruginosa in the treatment group at both one month, OR 0.06 (95% CI 0.01 to 0.30); and two months, OR 0.14 (95% CI 0.03 to 0.60) (Analysis 1.4). Furthermore, these imputed data also showed a significant difference in favour of tobramycin at six months, OR 0.04 (95% CI 0.00 to 0.48) and 12 months, OR 0.01 (95% CI 0.00 to 0.26), but not at three months (Analysis 1.4). In the worst‐case scenario the odds of a positive culture was reduced at one month, OR 0.08 (95% CI 0.02 to 0.38) and two months, OR 0.18 (95% CI 0.04 to 0.73), but was not statistically significant for Wiesemann alone at three, six or 12 months (Analysis 1.5).
b. Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
Neither trial assessed or reported on this outcome.
Secondary Outcomes
1. Mortality
Mortality was not included as an outcome in either trial, but there were no reported deaths during any of the trial periods (Gibson 2003; Wiesemann 1998).
2. Quality of life
Neither trial assessed or reported on this outcome.
3. Spirometric lung function
Wiesemann reported no change in spirometric pulmonary function during or after the treatment period, but no data were given (Wiesemann 1998). Gibson did not assess or report on spirometric lung function; most of the participants in this trial were too young to perform spirometry reliably (Gibson 2003).
4. Growth and nutritional status
Only the trial by Gibson presented data on weight (Gibson 2003). There was no significant difference found between the two groups in the change in weight from baseline (measured at trial entry) and subsequent weights measured at one month and two months (Analysis 1.6).
5. Frequency of respiratory exacerbations
Neither trial assessed or reported on this outcome.
6. Isolation of other micro‐organisms
Gibson reported no changes in the prevalence of other micro‐organisms, including multi‐resistant organisms, cultured from respiratory secretions (Gibson 2003). Wiesemann did not collect data on this outcome (Wiesemann 1998).
7. Adverse effects of antibiotics
Gibson reported cough in association with inhalation in seven out of eight participants in the treatment group and in 12 out of 13 in the placebo group, but this result was not statistically significant (Analysis 1.7) . There was no evidence of a difference in serum creatinine levels or auditory threshold between the groups, however the numbers of participants was small (Gibson 2003). Wiesemann reported one withdrawal from the placebo group because of cough, however the authors did not report on the presence or absence of cough in other participants (Wiesemann 1998).
Additional outcomes which have arisen during the review
1. Time to chronic infection
Neither trial assessed or reported on this outcome.
2. Clinical and radiological scores
Only the Gibson trial reported modified Shwachmann scores, which were recorded at one month and two months from enrolment and were expressed as both mean scores with SDs and mean change from baseline with SDs (Gibson 2003). There were no significant differences between the two groups in changes in either mean scores or modified Schwachman scores from baseline at either one month or two months (Analysis 1.8).
3. Cost
Neither trial reported a health economic analysis.
Oral ciprofloxacin and inhaled colistin versus no treatment
This intervention included only one trial with 26 participants (Valerius 1991).
Primary outcome
1. Eradication of P. aeruginosa from the respiratory tract
a. Clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures
The included trial did not report on this outcome.
b. Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
The included trial did not report on this outcome.
Secondary Outcomes
1. Mortality
The included trial did not report on this outcome.
2. Quality of life
The included trial did not report on this outcome.
3. Spirometric lung function
The included trial did not report on this outcome.
4. Growth and nutritional status
The included trial did not report on this outcome.
5. Frequency of respiratory exacerbations
The included trial did not report on this outcome.
6. Isolation of other micro‐organisms
The included trial did not report on this outcome.
7. Adverse effects of antibiotics
Valerius did not describe cough specifically, but reported that there were no adverse effects in either group (Valerius 1991).
Additional outcomes which have arisen during the review
1. Time to chronic infection
We defined this as the presence of P. aeruginosa in each monthly sputum sample for six consecutive months or the presence of precipitating antibodies to P. aeruginosa or both. In the Valerius trial, from the data provided, it was possible to calculate the proportion of participants in each group who were defined as chronically colonised with P. aeruginosa from respiratory secretions at 3, 6, 12 and 24 month time points (Valerius 1991). The odds of being chronically infected with P. aeruginosa were reduced in the treatment group compared to the placebo group after 24 months, OR 0.12 (95% CI 0.02 to 0.79) (Analysis 2.1). No significant difference was detected between the two groups at the other time points. No other trials in the review used this outcome measure to express their findings.
2. Clinical and radiological scores
The included trial did not report on this outcome.
3. Cost
The Valerius trial did not include a health economic analysis.
Oral ciprofloxacin and inhaled colistin versus inhaled tobramycin
This intervention comparing oral ciprofloxacin and inhaled colistin (three months) with inhaled tobramycin (28 days) included only one trial including 58 participants (29 in each treatment group) (Proesmans 2013).
Primary outcome
1. Eradication of P. aeruginosa from the respiratory tract
a. Clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures
At some point in the six months following treatment, P. aeruginosa was isolated in 10 out of 29 participants enrolled to the inhaled colistin with oral ciprofloxacin arm compared to 16 out of 29 in the TSI arm. The difference between groups was not statistically significant (Analysis 3.1).
b. Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
The included trial did not report on this outcome.
Secondary Outcomes
1. Mortality
There were no deaths in either arm (Proesmans 2013).
2. Quality of life
The included trial did not report on this outcome.
3. Spirometric lung function
The median change from baseline in FEV1 (% predicted) for all the participants was ‐1%. The changes are not reported separately for each treatment arm (Proesmans 2013).
4. Growth and nutritional status
Both BMI z score and weight z score were reported not to have changed significantly for trial participants as a whole, but numerical data are not reported (Proesmans 2013).
5. Frequency of respiratory exacerbations
The authors report that, during the first six months of follow up, there was no difference between the two treatment arms in number of oral antibiotic treatment days. These oral antibiotics were given for symptoms and not because of failed eradication. However, numerical data are not reported (Proesmans 2013).
6. Isolation of other micro‐organisms
The included trial did not report on this outcome.
7. Adverse effects of antibiotics
One participant is reported to have developed a severe cough with TSI, but this result was not statistically significant (Analysis 3.2). No other adverse effects are reported.
Additional outcomes which have arisen during the review
1. Time to chronic infection
The included trial did not report on this outcome.
2. Clinical and radiological scores
The included trial did not report on this outcome.
3. Cost
The included trial did not include a health economic analysis.
Inhaled tobramycin (28 days) versus inhaled tobramycin (56 days)
This intervention comparing 28 days TSI with 56 days TSI included one trial of 123 participants, of whom 88 were randomised (Ratjen 2010).
Primary outcome
1. Eradication of P. aeruginosa from the respiratory tract
a. Clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures
This outcome was not evaluated; data are presented in the paper at one month when participants were all given the initial 28 days of TSI before randomisation and were excluded if they had raised anti P. aeruginosa antibody levels (Ratjen 2010).
b. Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
The median time to recurrence was 26.12 months in the 28‐day treatment group and 25.82 months in the 56‐day treatment group. Differences between groups were not significant (Analysis 4.1).
Secondary Outcomes
1. Mortality
There were no deaths reported (Ratjen 2010).
2. Quality of life
The included trial did not report on this outcome.
3. Spirometric lung function
The paper presented data in a table for the median change from baseline to three months and to 27 months for FEV1% predicted, FVC % predicted and FEF25‐75 % predicted; however, presented data were combined for both the 28‐day and the 56‐day treatment groups. The paper states that there were "no major short‐ or long‐term changes in spirometric parameters observed during the study period" (Ratjen 2010).
4. Growth and nutritional status
No significant differences in weight, height or body mass index were reported. Only a summary statement was presented, indicating that the no significant difference was found in weight, height or body mass index. No data were provided (Ratjen 2010).
5. Frequency of respiratory exacerbations
Two participants allocated to the 56‐day treatment group were hospitalised on one occasion, each for a pulmonary exacerbation. One of these isolated P. aeruginosa and was treated with intravenous ceftazidime and tobramycin. When entered into the analysis this gave a non‐significant result (Analysis 4.2).
6. Isolation of other micro‐organisms
There were no consistent trends reported in the isolation of non‐P. aeruginosa organisms (one isolate only of Stenotrophomonas maltophilia (S. maltophilia) which was seen in the 28‐day arm).
7. Adverse effects of antibiotics
Adverse events up to three months that were considered possibly or probably related to treatment were reported by 14 participants in each treatment group, with the majority being related to dysphonia in both treatment groups (11% and 14%, respectively) and cough in the 28‐day group (9%). There were no significant differences between treatment groups for any of the reported adverse events at any time‐point (Analysis 4.3; Analysis 4.4).
Additional outcomes which have arisen during the review
1. Time to chronic infection
The included trial did not report on this outcome.
2. Clinical and radiological scores
The included trial did not report on this outcome.
3. Cost
The included trial did not have a health economic analysis.
Inhaled colistin plus oral ciprofloxacin versus inhaled tobramycin plus oral ciprofloxacin
This comparison of inhaled colistin plus oral ciprofloxacin with inhaled tobramycin plus oral ciprofloxacin included one trial with 223 participants (Taccetti 2012).
Primary outcome
1. Eradication of P. aeruginosa from the respiratory tract
a. Clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures
In the inhaled colistin plus oral ciprofloxacin group, P. aeruginosa was isolated within the first six months from 39 out of 105 participants (37.1%) and in the the inhaled tobramycin plus oral ciprofloxacin group from 41 out of 118 participants (34.7%) (Taccetti 2012). When data were analysed, the rate of isolation of P. aeruginosa between the two arms was not significantly different (Analysis 5.1). At a median follow‐up period of 16 months, P. aeruginosa had been isolated from 36 out of 97 participants in the colistin with ciprofloxacin arm for whom data were available and from 24 out of 108 participants in the tobramycin with ciprofloxacin arm; the two arms were not significantly different. The trial authors report that subgroup analyses by gender, age (one to five years; five to twelve years and over twelve years), lung function (FEV1 less than or greater than 70%) and participants with first ever isolation of P. aeruginosa failed to show any significant differences between groups (Taccetti 2012).
b. Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
The trial did not report on this outcome.
Secondary Outcomes
1. Mortality
No deaths were reported (Taccetti 2012).
2. Quality of life
The trial did not report on this outcome.
3. Spirometric lung function
Following treatment, after a mean (SD) time of observation of 54 (39) days, the mean (SD) relative change (percentage of predicted) in FEV1 from baseline was 2.15 (8.50)% in the inhaled colistin plus oral ciprofloxacin group compared to 4.55 (11.54)% in the the inhaled tobramycin plus oral ciprofloxacin group (not statistically significant) (Analysis 5.2).
4. Growth and nutritional status
The trial did not report these outcomes.
5. Frequency of respiratory exacerbations
The trial did not report this outcome.
6. Isolation of other micro‐organisms
There was an observation that S. maltophilia was isolated more frequently in the follow‐up period than before eradication treatment. There were no differences during follow up between the two groups for isolation of: S. maltophilia, Achromobacter xylosoxidans or Aspergillus species (Analysis 5.3).
7. Adverse effects to antibiotics
There were a total of 38 out of 223 randomised participants (17%) who discontinued treatment early; of these 17 were from the inhaled colistin plus oral ciprofloxacin group and 21 from the inhaled tobramycin plus oral ciprofloxacin group (Analysis 5.4). There were a number of reasons for these discontinuations including vomiting, photosensitivity, wheeze, pulmonary exacerbation and lack of compliance.
Additional outcomes which have arisen during the review
1. Time to chronic infection
The trial did not report on this outcome.
2. Clinical and radiological scores
The trial did not report on this outcome.
3. Cost
A health economic analysis was not undertaken in the included trial.
Cycled inhaled tobramycin versus culture‐based inhaled tobramycin
This comparison of cycled inhaled tobramycin (with oral ciprofloxacin or placebo) with culture‐based inhaled tobramycin (with oral ciprofloxacin or placebo) included one trial, with 306 participants randomised and data analysed on 304 participants who received treatment (Treggiari 2011).
Primary outcome
1. Eradication of P. aeruginosa from the respiratory tract
a. Clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures
Treggiari reported 43 out of 148 children on cycled therapy had one or more isolates of P. aeruginosa compared to 67 out of 150 children on culture‐based therapy giving a statistically significant effect in favour of cycled therapy, OR 0.51 (95% CI 0.31 to 0.82) (Analysis 6.1). The main trial publication reports an age‐adjusted OR, using generalised estimating equations, with robust variance, specifying a logit link and assuming an independence working correlation (Treggiari 2011). This may explain the difference between the trial publication and the findings of this review.
b. Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
This was not reported in the included trial.
Secondary Outcomes
1. Mortality
No deaths were reported in either arm (Treggiari 2011).
2. Quality of life
This outcome was not reported in the included trial.
3. Spirometric lung function
The included trial reports the mean 70‐week change in FEV1 % predicted, but the mean difference between the two arms was not significant (Analysis 6.2).
4. Growth and nutritional status
The Treggiari trial reports the mean 70‐week change from baseline in weight (kg) for each treatment arm, but the MD between arms was not significant (Analysis 6.3). The trial also reports data for the change from baseline in height (cm) for each arm at the same time point; again the MD between arms was not significant (Analysis 6.4).
5. Frequency of respiratory exacerbations
A primary outcome in the Treggiari trial was the time to a severe pulmonary exacerbation (i.e. an exacerbation requiring intravenous antibiotics or hospitalisation or both) (Treggiari 2011). The analysis shows no significant difference in time to a severe exacerbation (Analysis 6.5).
Data on the frequency of severe exacerbations, during the 70‐week follow‐up period, are also presented in the paper. From our analysis, the data indicate no significant difference between groups (Analysis 6.6).
Treggiari also reported a secondary outcome of time to pulmonary exacerbation of any severity (including any exacerbation treated with intravenous, inhaled or oral antibiotics or requiring hospitalisation). The results of our analysis are slightly different to those reported in the paper, but still show no statistically significant difference in time to any exacerbation (Analysis 6.7).
Finally, the number of exacerbations of any severity was also reported. In our analysis, the OR was not significantly different between cycled and culture‐based therapy (Analysis 6.8).
6. Isolation of other micro‐organisms
The Treggiari trial reported the number of participants in each arm with one or more isolates of the emerging pathogen Stenotrophomonas maltophilia (Treggiari 2011). There was no significant difference between cycled and culture‐based therapy (Analysis 6.9).
7. Adverse effects to antibiotics
With regards to the incidence of adverse effects, Treggiari did not report any significant difference between treatment arms, although adverse events attributable to antibiotic therapy were not recorded separately from adverse events which were unlikely to be related to the study intervention (Treggiari 2011). Our results were also not statistically significant (Analysis 6.10).
Additional outcomes which have arisen during the review
1. Time to chronic infection
This was not reported in the one included trial.
2. Clinical and radiological scores
These outcomes were not reported in the one included trial.
3. Cost
A health economic analysis was not undertaken in the one included trial.
Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin therapy
As discussed under Description of studies, the Treggari trial randomised participants to cycled versus culture‐based inhaled tobramycin therapy and then to oral ciprofloxacin versus placebo for two weeks with each 28‐day course of TSI (Treggiari 2011). This section presents the comparison of outcomes in the ciprofloxacin and placebo arms.
Primary outcome
1. Eradication of P. aeruginosa from the respiratory tract
a. Clearance of P. aeruginosa from BAL, sputum or oropharyngeal cultures
Treggiari reported that 49 out of 146 children on oral ciprofloxacin had one or more isolates of P. aeruginosa compared to 55 out of 150 children on placebo (Treggiari 2011). The data for this outcome show a non‐statistically significant effect. Age group–adjusted ORs are reported in the trial paper; in our analysis, we did not adjust for age.
b. Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures
This was not reported in the included trial.
Secondary Outcomes
1. Mortality
No deaths were reported in either arm (Treggiari 2011).
2. Quality of life
This outcome was not reported in the included trial.
3. Spirometric lung function
The included trial reports the mean 70‐week change in FEV1 % predicted, but the MD between the two arms was not significant (Analysis 7.2).
4. Growth and nutritional status
The Treggiari trial reports the mean 70‐week change from baseline in weight (kg) for each treatment arm; the MD between arms was not significant (Analysis 7.3). The trial also reports data for the change from baseline in height (cm) for each arm; again the MD between arms was not significant (Analysis 7.4).
5. Frequency of respiratory exacerbations
A primary outcome in the Treggiari trial was the time to a severe pulmonary exacerbation (i.e. an exacerbation requiring intravenous antibiotics or hospitalisation, or both) (Treggiari 2011). Again, for the ciprofloxacin versus placebo comparison, there was no significant difference in time to a severe exacerbation (Analysis 7.5).
Data on the frequency of severe exacerbations, during the 70‐week follow‐up period, are also presented in the paper. From our analysis, the OR is not significantly different between groups (Analysis 7.6).
We analysed the outcome 'time to pulmonary exacerbation (any severity)', including any exacerbation treated with intravenous, inhaled or oral antibiotics or requiring hospitalisation (Analysis 7.7). There was no difference between ciprofloxacin and placebo in time to exacerbation (any severity).
Finally, the number of exacerbations of any severity was not significantly different between ciprofloxacin and placebo (Analysis 7.8).
6. Isolation of other micro‐organisms
In the Treggiari trial, there was no significant difference between ciprofloxacin and placebo in the number of isolates of S. maltophilia (Analysis 7.9).
7. Adverse effects to antibiotics
Treggiari did not report any significant difference between treatment arms in the incidence of adverse events, although adverse events attributable to antibiotic therapy were not recorded separately from adverse events which were unlikely to be related to the trial intervention (Treggiari 2011). Our analysis showed no difference between arms in the number of participants with one or more serious adverse events (Analysis 7.10).
Additional outcomes which have arisen during the review
1. Time to chronic infection
This was not reported in the one included trial.
2. Clinical and radiological scores
These outcomes were not reported in the one included trial.
3. Cost
A health economic analysis was not undertaken in the one included trial.
Discussion
Summary of main results
Our review includes seven trials (with data from 744 participants) of antibiotic strategies for the eradication of P. aeruginosa infection in CF, conducted over a period of over 20 years. Each trial used a different intervention and only two trials could be combined in a meta‐analysis of the same treatment comparison. An early interim analysis was performed due to slow accrual in one trial (Gibson 2003).
Cumulative data from 43 participants in two of the seven included trials indicate that P. aeruginosa was more frequently eradicated from the respiratory secretions in the participants receiving antibiotics than from those receiving placebo (Gibson 2003; Wiesemann 1998). This reduction in the number of isolates of P. aeruginosa was noted at both one month and two months after the start of treatment. A further trial suggests that the onset of chronic infection with P. aeruginosa is delayed in those individuals who have received antibiotic therapy compared to those receiving no therapy (Valerius 1991). There was evidence from the trials by Valerius and Wiesemann that this effect may persist for up to 24 months (Valerius 1991; Wiesemann 1998). We found no difference in adverse events. In two trials, an increased incidence of the emerging pathogen S. maltophilia was reported following treatment, but no difference was found between eradication regimens in either of these trials (Taccetti 2012; Treggiari 2011) .
The trial by Proesmans, which randomised 58 participants, was associated with a low rate of short‐term eradication of P. aeruginosa from both groups of children treated with nebulised high‐dose tobramycin and a combination of oral ciprofloxacin with inhaled colistin (Proesmans 2013). The numbers of participants in each group were too low to allow comparisons of superiority between the two eradication regimens to be made. A further possible cause of the low eradication rate in this trial may be that participants were recruited if they had been free of P. aeruginosa for at least six months (with negative microbiology samples for at least six months), which is shorter than the 12‐month P. aeruginosa‐free interval used by the Leeds definition of 'Pseudomonas‐free' (Lee 2003).
The trial by Ratjen took place in 21 centres in Germany, France, The Netherlands, UK, Spain and Austria from November 2003 until January 2008. The trial has reported that 28‐day and 56‐day treatment with inhaled tobramycin to be effective and safe; with no additional advantage to 56 days of therapy over 28 days (Ratjen 2010). The authors of this trial concluded that head‐to‐head comparisons will clarify whether adding either oral ciprofloxacin or intravenous antibiotic therapy can further increase treatment success in people with CF with early P. aeruginosa infection.
The Taccetti trial randomised 223 participants and failed to demonstrate superiority of inhaled tobramycin with oral ciprofloxacin over inhaled colistin with oral ciprofloxacin, both treatments given over 28 days (Taccetti 2012). As in the Ratjen trial, the included participants had been free of P. aeruginosa for at least six months, again at variance with the Leeds definition of Pseudomonas‐free (Lee 2003).
The Treggiari trial was the largest trial of P. aeruginosa eradication in CF reported so far and randomised 306 children (aged 1 to 12 years) and reported data on 304 children (Treggiari 2011). The trial used a complex, four‐arm design, comparing cycled with culture‐based treatment with TSI and also additional oral ciprofloxacin versus placebo. The trial was adequately powered for its two primary outcome measures (time to severe pulmonary exacerbation and proportion of P. aeruginosa positive cultures) and found no difference between arms for either outcome. In contrast, our analysis (which was not corrected for age) did find a significant difference in the proportion of P. aeruginosa positive cultures in favour of cycled therapy. There was no difference between ciprofloxacin and placebo for this outcome.
No deaths were reported in any of the trials.
Overall completeness and applicability of evidence
The aim of antibiotic therapy for early P. aeruginosa infection in CF should be both eradication of the micro‐organism and improvement in (or slowing in the rate of decline of) clinical parameters, whilst minimising adverse effects and the isolation of new micro‐organisms. If P. aeruginosa is successfully eradicated, but there is no measurable clinical benefit, it is likely that current measures of clinical status are not sufficiently sensitive or that the duration of follow up is too short to show a difference.
There are differences in the type and dose of drug administered to the treatment groups in the two trials where nebulised tobramycin was compared to placebo. In the Wiesemann trial, tobramycin injectable solution was administered by nebuliser, at a low dose (80 mg twice daily) for a long duration (12 months) (Wiesemann 1998); whereas Gibson used TSI TOBI® (now marketed by Novartis) at a high dose (300 mg twice daily) for a short duration (28 days) (Gibson 2003). This is a potential source of heterogeneity between the trials. It is of interest that significant heterogeneity was detected between the trials at the one‐month time point but this was not detected at the two‐month time point.
The Proesmans and Taccetti trials included patients that were Pseudomonas‐naive and those that had not isolated P. aeruginosa for at least six months (Proesmans 2013; Taccetti 2012), which is at variance with the 12‐month definition proposed by Lee to define a patient as 'Pseudomonas‐free' (Lee 2003). It was not possible for the authors of this review to extract IPD for those patients that fit this longer definition of Pseudomonas‐free. The Treggiari trial required patients to have been free of P. aeruginosa for at least two years (Treggiari 2011).
Eradication of isolates of P. aeruginosa is easiest in people with CF with recent onset of P. aeruginosa infection of the respiratory tract, in particular in those who have non‐mucoid isolates of P. aeruginosa as they seem particularly susceptible to antibiotic therapy. Two trials included in this review have recruited adult patients suggesting that early P. aeruginosa infection can be eradicated from adults as well as from children and therefore adults should be included in future studies.
Finally, it should be noted that some of the trials were conducted between 10 and 20 years ago and the results may be less applicable to patients today.
Quality of the evidence
The quality of the trials was variable with important deficiencies identified in some. For example, in four out of the seven included trials we judged there to be a high risk from a lack of blinding of participants and clinicians; in two of these trials the different interventions used in the treatment and control groups precluded blinding (Proesmans 2013; Valerius 1991) and two trials were open label and no attempt was made at blinding. We also judged there to be a high risk of bias due to selective reporting in three of the trials, which only reported summary statements with no actual data for a number of outcomes (Proesmans 2013; Ratjen 2010; Wiesemann 1998).
While we generally judged there to be a low risk of bias due to incomplete outcome data, there was incomplete follow up of a number of participants in one of the older and smaller trials (Wiesemann 1998). The absence of these data has complicated the combined analysis of the two trials that compare inhaled tobramycin with placebo (Gibson 2003; Wiesemann 1998); one trial was analysed on an intention‐to‐treat basis (Gibson 2003) and another on an available‐case basis (Wiesemann 1998). A sensitivity analysis based on best‐ and worst‐case scenarios demonstrated similar results to the available‐case analysis. The available‐case analysis revealed a reduction in the odds of a positive culture for P. aeruginosa in the group treated with tobramycin inhalation when compared to the odds in the placebo group at both one and two months from the start of treatment (Wiesemann 1998).
The Gibson trial was stopped early because of evidence of significant treatment effect (Gibson 2003). It has been suggested that the results of randomised controlled trials stopped early for benefit should be interpreted with caution particularly when the number of events is small (Montori 2005).
In the Ratjen trial, the authors wished to enrol 120 participants in order to randomise 100 patients. They succeeded in recruiting 123 individuals of whom 88 were randomised and 65 could be included in the analysis of the primary outcome (time to recurrence of P. aeruginosa). This trial is potentially subject to bias because of the exclusion of non‐randomised individuals, including 31 people in whom there were elevated antibody titres to P. aeruginosa (Ratjen 2010).
The relationship between the presence of P. aeruginosa in secretions from the upper respiratory tract and the isolation of P. aeruginosa from the lower respiratory tract is inconsistent. Reporting of the presence of organisms in respiratory secretions is difficult to standardise, dependent on the sampling methods used and on the number of samples taken. The trials included in this review used a heterogeneous mix of methods to sample respiratory secretions from both the lower and upper respiratory tracts. No two trials used the same methods and more than one method was used in two trials. There was no subgroup analysis based on sampling method in any of the trials, probably owing to relatively small numbers of participants in individual trials. Wiesemann used a combination of oropharyngeal swabs and sputum samples, whereas Gibson used oropharyngeal swabs and BAL fluid (Gibson 2003; Wiesemann 1998). Proesmans used sputum, throat swab and BAL (Proesmans 2013). The Taccetti study did not describe the technique used for culture collection (Taccetti 2012). The Treggiari trial enrolled younger children and used oropharyngeal swabs or sputum samples (Treggiari 2011). Armstrong has shown that the results of oropharyngeal specimens are poorly predictive of the presence of organisms in the lower respiratory tract (Armstrong 1996). Valerius relied on sputum samples which can be of poor quality in younger children (Valerius 1991).
Potential biases in the review process
The original review stated that trials would be included only where eradication begins within two months of isolation of P. aeruginosa. In a post hoc change, this has now been altered to include those where eradication has been initiated up to six months since isolation. Whilst prompt treatment of new P. aeruginosa infection is recommended (Smyth 2014), there is no robust evidence for a specific time limit for initiation of treatment.
The review now also includes patients from two distinct groups, those that are Pseudomonas‐naive and those that are Pseudomonas‐free.
Agreements and disagreements with other studies or reviews
We are not aware of any other studies or reviews which recommend specific eradication treatment.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 1 Positive respiratory culture for P. aeruginosa (300 mg 2x daily).
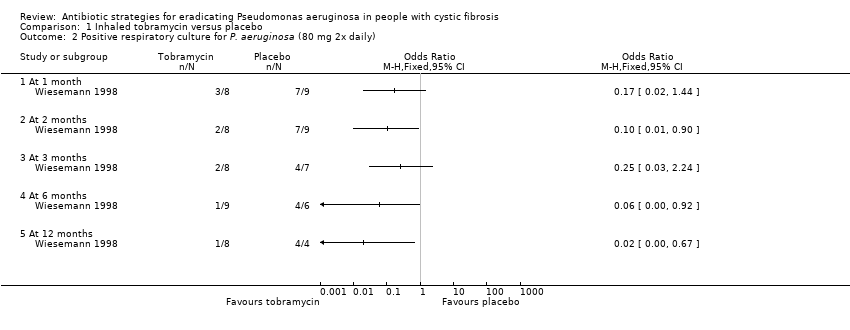
Comparison 1 Inhaled tobramycin versus placebo, Outcome 2 Positive respiratory culture for P. aeruginosa (80 mg 2x daily).
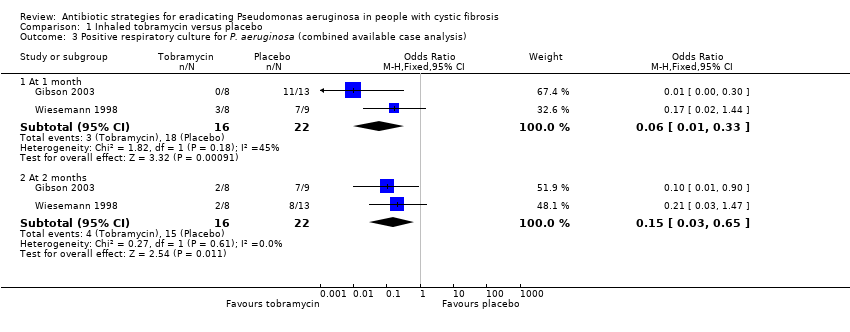
Comparison 1 Inhaled tobramycin versus placebo, Outcome 3 Positive respiratory culture for P. aeruginosa (combined available case analysis).

Comparison 1 Inhaled tobramycin versus placebo, Outcome 4 Positive respiratory culture for P. aeruginosa (combined) ‐ best case.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 5 Positive respiratory culture for P. aeruginosa (combined) ‐ worst case.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 6 Change in weight from baseline.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 7 Adverse events.
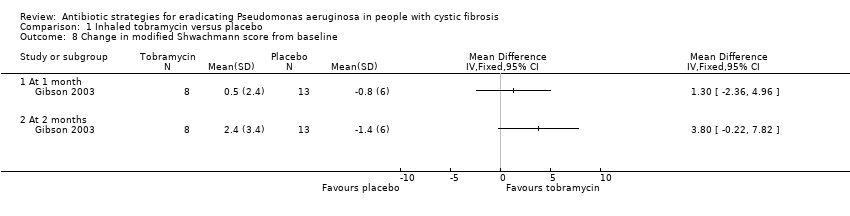
Comparison 1 Inhaled tobramycin versus placebo, Outcome 8 Change in modified Shwachmann score from baseline.

Comparison 2 Oral ciprofloxacin and inhaled colistin versus no treatment, Outcome 1 Proportion colonised with P. aeruginosa.

Comparison 3 Oral ciprofloxacin and inhaled colistin versus inhaled tobramycin, Outcome 1 Positive respiratory culture for P.aeruginosa.

Comparison 3 Oral ciprofloxacin and inhaled colistin versus inhaled tobramycin, Outcome 2 Adverse events.
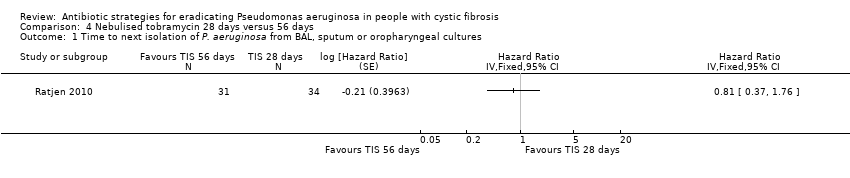
Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 1 Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures.

Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 2 Number of respiratory exacerbations.
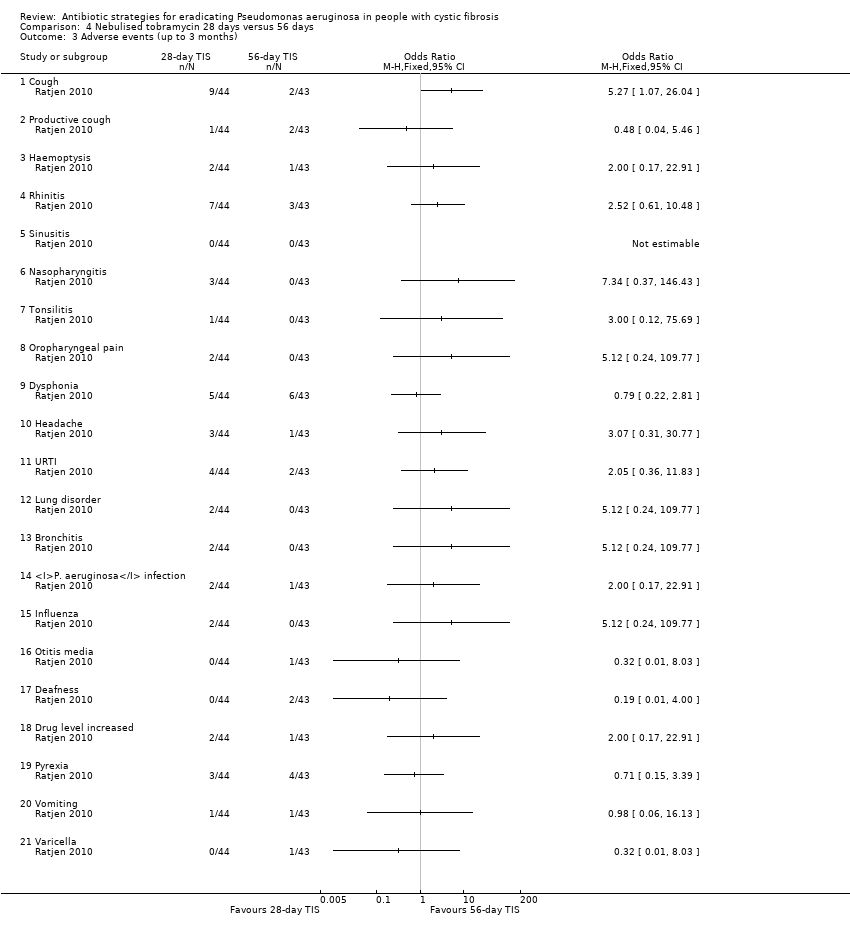
Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 3 Adverse events (up to 3 months).

Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 4 Adverse events (over 3 months).
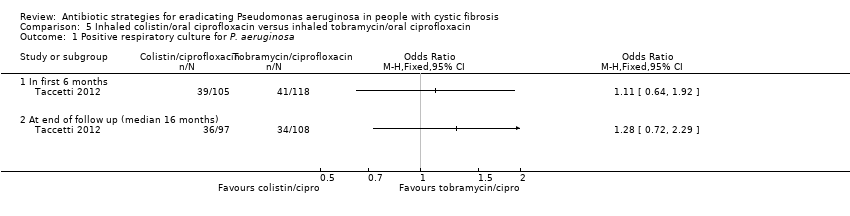
Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 1 Positive respiratory culture for P. aeruginosa.

Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 2 Relative change in % predicted FEV1 from baseline (to mean 54 days).
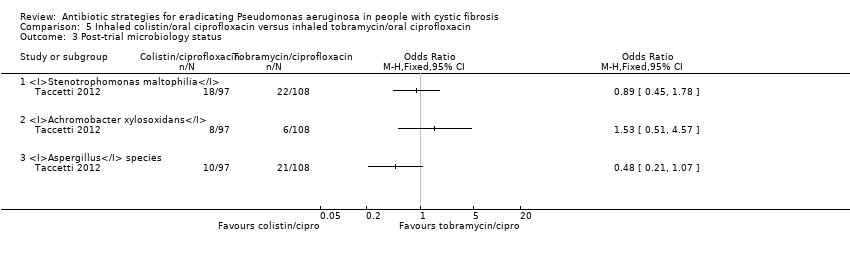
Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 3 Post‐trial microbiology status.

Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 4 Adverse events leading to trial discontinuation.
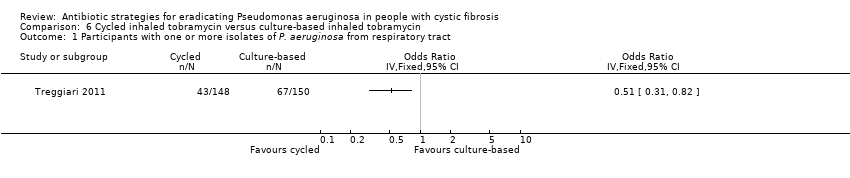
Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 1 Participants with one or more isolates of P. aeruginosa from respiratory tract.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 2 Mean 70‐week change in FEV1 % predicted.
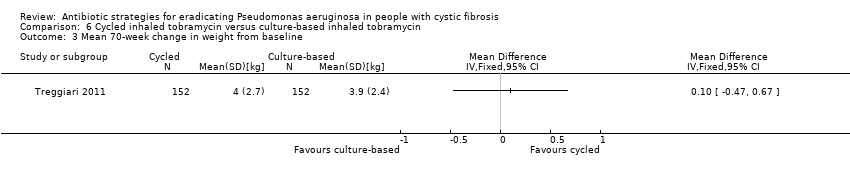
Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 3 Mean 70‐week change in weight from baseline.
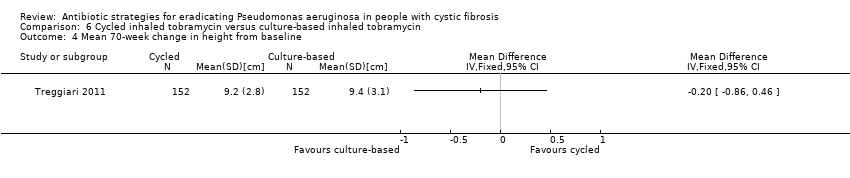
Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 4 Mean 70‐week change in height from baseline.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 5 Time to severe pulmonary exacerbation.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 6 Participants with one or more severe pulmonary exacerbations.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 7 Time to pulmonary exacerbation (any severity).

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 8 Participants with one or more pulmonary exacerbations (any severity).

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 9 Participants with new isolates of Stenotrophomonas maltophilia.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 10 Participants with one or more serious adverse event.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 1 Participants with one or more isolates of P. aeruginosa from respiratory tract.
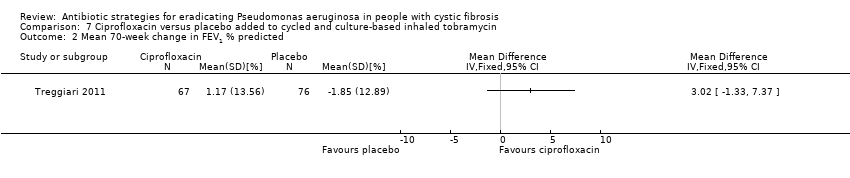
Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 2 Mean 70‐week change in FEV1 % predicted.
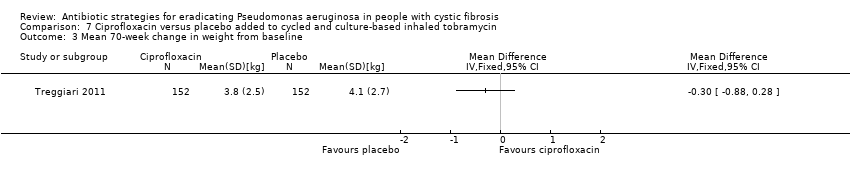
Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 3 Mean 70‐week change in weight from baseline.
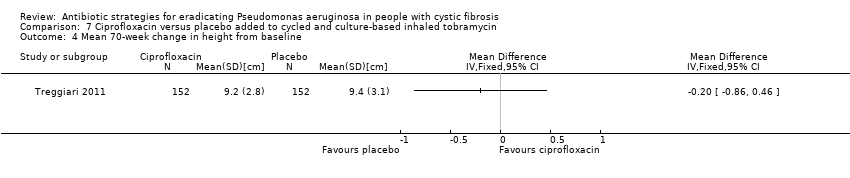
Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 4 Mean 70‐week change in height from baseline.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 5 Time to severe pulmonary exacerbation.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 6 Participants with one or more severe pulmonary exacerbations.
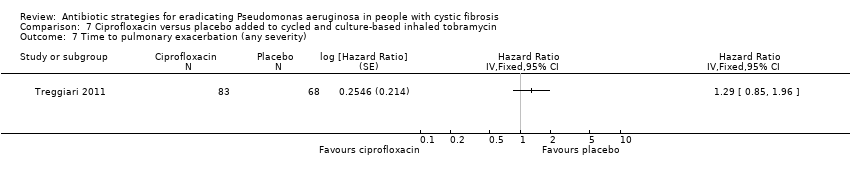
Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 7 Time to pulmonary exacerbation (any severity).

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 8 Participants with one of more pulmonary exacerbation (any severity).

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 9 Participants with new isolates of Stenotrophomonas maltophilia.
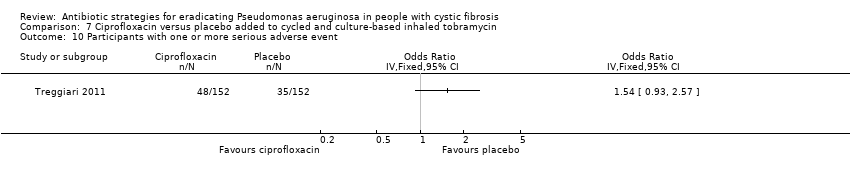
Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 10 Participants with one or more serious adverse event.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P. aeruginosa (300 mg 2x daily) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 2 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Positive respiratory culture for P. aeruginosa (80 mg 2x daily) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 2 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Positive respiratory culture for P. aeruginosa (combined available case analysis) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1 month | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.33] |
| 3.2 At 2 months | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.65] |
| 4 Positive respiratory culture for P. aeruginosa (combined) ‐ best case Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 1 month | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.30] |
| 4.2 At 2 months | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 4.3 At 3 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.16] |
| 4.4 At 6 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.48] |
| 4.5 At 12 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.26] |
| 5 Positive respiratory culture for P. aeruginosa (combined) ‐ worst case Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 1 month | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.38] |
| 5.2 At 2 months | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.73] |
| 5.3 At 3 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.05, 2.77] |
| 5.4 At 6 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 1.83] |
| 5.5 At 12 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.05, 2.77] |
| 6 Change in weight from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in modified Shwachmann score from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 At 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion colonised with P. aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P.aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 In first 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Severe cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to next isolation of P. aeruginosa from BAL, sputum or oropharyngeal cultures Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of respiratory exacerbations Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Until recurrence of P. aeruginosa | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events (up to 3 months) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Productive cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Haemoptysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Rhinitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Sinusitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Nasopharyngitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Tonsilitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Oropharyngeal pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Dysphonia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 URTI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Lung disorder | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Bronchitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 P. aeruginosa infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Influenza | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Otitis media | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Deafness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 Drug level increased | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.19 Pyrexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.20 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.21 Varicella | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events (over 3 months) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Productive cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Haemoptysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Rhinitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Sinusitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Nasopharyngitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Tonsilitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Oropharyngeal pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Dysphonia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 URTI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Lung disorder | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Bronchitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 P. aeruginosa infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 Influenza | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Otitis media | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Deafness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.18 Drug level increased | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.19 Pyrexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.20 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.21 Varicella | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P. aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 In first 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At end of follow up (median 16 months) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Relative change in % predicted FEV1 from baseline (to mean 54 days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Post‐trial microbiology status Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Stenotrophomonas maltophilia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Achromobacter xylosoxidans | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Aspergillus species | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events leading to trial discontinuation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Photosensitivity | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Wheeze | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Pulmonary exacerbation during early eradication treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Lack of compliance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with one or more isolates of P. aeruginosa from respiratory tract Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Mean 70‐week change in FEV1 % predicted Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean 70‐week change in weight from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Mean 70‐week change in height from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Time to severe pulmonary exacerbation Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 6 Participants with one or more severe pulmonary exacerbations Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Time to pulmonary exacerbation (any severity) Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 8 Participants with one or more pulmonary exacerbations (any severity) Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Participants with new isolates of Stenotrophomonas maltophilia Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Participants with one or more serious adverse event Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with one or more isolates of P. aeruginosa from respiratory tract Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Mean 70‐week change in FEV1 % predicted Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Mean 70‐week change in weight from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Mean 70‐week change in height from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Time to severe pulmonary exacerbation Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 6 Participants with one or more severe pulmonary exacerbations Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Time to pulmonary exacerbation (any severity) Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 8 Participants with one of more pulmonary exacerbation (any severity) Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Participants with new isolates of Stenotrophomonas maltophilia Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Participants with one or more serious adverse event Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |

