Cirugía para mujeres con prolapso del compartimiento anterior
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre RCT Inclusion grade 3 or 4 cysto‐urethrocele (BW halfway system) No exclusion No power Randomisation and concealment, blinding NS 6/12 follow‐up | |
| Participants | No CONSORT N = 108 Inclusion: women with grade 3 or 4 cysto‐urethrocele (BW halfway system) No significant differences between groups regarding preoperative storage symptoms, urodynamics and degree of prolapse | |
| Interventions | A (54): anterior colporrhaphy alone B (54): anterior colporrhaphy with tension‐free polypropylene (Gynemesh PS) overlay | |
| Outcomes | Assessed at 6 months postop Reported the following review outcomes
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Significant attrition: group AC: 46/54: mesh 43/54 completed 6/12‐month review |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Unclear risk | Not stated |
| Methods | Single‐centre RCT comparing vaginal fascial repair with or without polyglactin mesh and with polydioxanone or polyglactin sutures; 2 × 2 factorial design PC randomisation, "secure" remote concealment Blinded participants, ward staff and follow‐up assessor Follow‐up at 3 months with exam, at 6 months with non‐validated questionnaire, at 2 years with validated questionnaire | |
| Participants | 73 randomised, 7 ineligible after randomisation, 66 included in trial Lost to follow‐up: 8 at 3 months, 4 at 6 months, 12 at 2 years Inclusion: grade 2 or greater prolapse (unclear examination technique), anterior and/or posterior prolapse Concomitant procedures: vaginal hysterectomy 14, cervical amputation (Manchester) 18, TVT 13 | |
| Interventions | A (32): repair with polyglactin mesh overlay B (34): repair without mesh C (33): repair of fascia with polydioxanone sutures D (33): repair of fascia with polyglactin sutures | |
| Outcomes | Assessed at 3 months, 6 months and 2 years postop Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Secure method of concealment of randomisation (remote computer allocation) |
| Blinding of participants and personnel (performance bias) | Low risk | Allocation concealed from women |
| Blinding of outcome assessment (detection bias) | Low risk | Reviewers blinded; participant‐completed questionnaires; data entry blinded to randomisation |
| Incomplete outcome data (attrition bias) | Low risk | Equal non‐response between groups, medical records seen for all non‐responders: 1 year ‐ Vicryl mesh 29/32, no mesh 32/34, PDS 29/33, Vicryl suture 33/33 |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | Unfunded study |
| Methods | Multi‐centre RCT: 53 centres, 58 surgeons 90% powered to detect 20% differences between groups with 1% type 1 error, central randomisation PC Participants blinded Reviews conducted for 2 and 12 months by surgeon 1/3, non‐surgeon 2/3 Completed before and at 1 year: Urogenital Distress Inventory (UDI) and Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ‐12) | |
| Participants | 1685 screened, 389 randomised Underwent surgery: A 182, B 191 Lost to follow‐up: A 7, B 14 (1 year: A 182, B 186) Inclusion: > 18 years, ≥ stage 2 symptomatic cystocele POPQ Exclusion: previous cancer of any pelvic organ, systemic glucocorticoid treatment, insulin‐treated diabetes, inability to participate or provide consent, need for concomitant surgery | |
| Interventions | A (182): anterior colporrhaphy, slow absorption monofilament thread, sham skin markings, excessive trimming of vagina discouraged B (191): Gynecare transvaginal anterior mesh (Prolift), absorbable sutures, excessive vaginal trimming discouraged, catheter care at discretion of surgeon
| |
| Outcomes | Assessed at 1 year postop Reported the following review outcomes at 1 year
Sexual function: dyspareunia, PISQ (end scores with 95% CI) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Secure concealment with remote computer |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded (sham skin markings) |
| Blinding of outcome assessment (detection bias) | High risk | Reviewers: surgeon 1/3, non‐surgeon 2/3 Participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | Participant flow accounted for completely in both groups: at 1 year ‐ 186/206 AC, 182/204 mesh |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported with exception of mesh exposure (personal communication) |
| Other bias | High risk | Funded by Karolinska Institute and Ethicon: Conflict of interest statements from members of Nordic transvaginal mesh group who were reviewers of surgery were not provided |
| Methods | Single‐centre RCT CONSORT: no Randomisation computer generated Allocation concealment NS Participants, surgeons and reviewers not blinded 12‐Month follow‐up | |
| Participants | Inclusion criteria: women recommended for vaginal surgery for anterior and posterior compartment with ≥ grade 2 prolapse Exclusion criteria: requiring only anterior or posterior compartment surgery with apical prolapse beyond the hymen, those requiring abdominal mesh surgery Randomised: 139 (A 70, B 69); 10 women breached study protocol and 11 more were recruited. All were analysed. Lost to follow‐up: A 6, B 9 Analysed at 12 months: A 63, B 61 | |
| Interventions | A (70): traditional anterior and posterior fascial plication with polydioxanone sutures B (69): anterior and posterior repair with Gynemesh PS augmentation | |
| Outcomes | Assessed at 6 months and 1 year postop Reported the following review outcomes at 1 year
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | No information on allocation concealment. Significant preoperative data missing, as above |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | Clear follow‐up of participants in both groups: 1 year ‐ no mesh 62/78 (89%), mesh 61/69 (88%) |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | High risk | |
| Methods | Single‐centre RCT (computer‐generated open number list ) | |
| Participants | 71 randomised | |
| Interventions | A (35): Burch group: total abdominal hysterectomy and vault to uterosacral ligament, Moschcowitz, Burch with 3‐4 Ethibond | |
| Outcomes | Definition of cure: no subjective stress urinary incontinence, no positive stress test | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Inadequate: computer‐generated randomisation by an open list |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | AC group 33/34 (97%), Burch colposuspension 35/37 (95%) |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Unclear risk | Not stated |
| Methods | Multi‐centre (8), Swedish open RCT Computer‐generated block randomisation stratified for each centre Allocation concealment by opaque sealed envelopes SS 160 would allow 90% power to detect 15% difference between groups with 5% alpha error and dropout rate of 10% 3‐Year review Intention to treat and CONSORT not stated | |
| Participants | Inclusion: recurrent (prior surgery on prolapsing site) POP in anterior and/or posterior compartment No exclusion criteria 135 randomised Gp A native tissue repair 66, 3 years 60/66 Gp B porcine dermis repair 65, 3 years 65/68 | |
| Interventions | Standardised surgery with 2 meeting workshops before the study Native tissue repair; midline fascial plication with interrupted polydioxanone suture, vagina closed with polyglactin absorbable suture Porcine: porcine dermal implant (Pelvicol, Bard, Sweden) as inlay with no fascial plication: inlay anchored to vaginal wall and fascia with 6‐8 polydioxanone suture, vagina closed with polyglactin suture Concomitant MUS, apical support and levator plication performed as required | |
| Outcomes | Assessed at 3 months and 3 years Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated blocked randomisation list stratified for each centre |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes (unclear if consecutive) |
| Blinding of participants and personnel (performance bias) | High risk | Nil |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Gp A 60/68, Gp B 65/68 completed 3‐year review |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | Low risk | No COI; funded by local research institutes |
| Methods | RCT (unclear randomisation and concealment) | |
| Participants | 134 included | |
| Interventions | A (65): Raz 4 defect cystocele repair reinforced with porcine dermis overlay (Pelvicol) | |
| Outcomes | Primary outcome: recurrence of cystocele stage 2: A 6/63, B 19/62 (P = 0.002) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Unclear method |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Outcome data reported |
| Other bias | Unclear risk | Not stated |
| Methods | Multi‐centre (12 French hospitals) RCT 12‐Month review Randomisation by drawing lots, stratified by centre Allocation concealment not discussed Intention to treat stated yes, but participants already randomised were removed if cystotomy occurred during surgery CONSORT guidelines Sample size of 194 provided 80% power to detect 20% difference with 5% alpha error and dropout rate of 10% Assessors not clear | |
| Participants | Inclusion criteria: symptomatic stage 2 anterior wall prolapse, 60 years of age or older Exclusion criteria: steroids, poorly controlled diabetes, prior pelvic radiation, untreated vaginal or urinary infection, ascites, bladder injury during procedure All used preoperative oestrogen therapy 163 included, 162 randomised Gp A (82): 1 year 67/82 Gp B (80): 1 year 66/60 Preop demographics and potential confounders similar in both groups, except colorectal impact greater in AC group | |
| Interventions | Gp A anterior colporrhaphy (AC): no mesh (plication of fascia with 2.0 polyglactin absorbable suture), uterosacral colpopexy and hysterectomy as required Gp B anterior polypropylene: macroporous mesh (Ugtex, Sofradim, Covidien), 4‐armed transobturator mesh, fixed with 2 × 2.0 permanent polypropylene sutures to uterine isthmus or uterosacral ligaments and 2 × 2.0 polyglactin sutures to inferior edge of pubic rami, vaginal trimming minimised Concomitant surgery: MUS, hysterectomy and any native tissue repair, but no other transvaginal mesh intervention included | |
| Outcomes | Assessed at 1‐year follow‐up Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by drawing lots? |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | Gp A 82, 1 year 67/82 Gp B 80, 1 year 66/80, 20% attrition |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | High risk | Study author COI with Sofradim, which provided partial funding and whose product was being evaluated |
| Methods | Single‐centre non‐inferiority RCT Computer‐generated random number list Allocation at inclusion with surgeon aware only in OT Envelope allocation Sample size; 35 in each group, 80% power to detect 5% significant change with 10% dropout Intention‐to‐treat analysis Assessors blinded Participants unblinded | |
| Participants | Any anterior POP point Ba ≥ +1 on POPQ Excluded malignant urogenital disease, prior radiation, acute genitourinary infection, connective tissue disorders, steroid treatments, insulin‐dependent diabetes | |
| Interventions | All procedures performed under spinal by 3 experienced surgeons AC: Plicate fascia pursestring Vicryl 0, vaginal trimming, transvaginal Trocar‐guided polypropylene mesh (kits donated by Promedon), Nazca TC (Promedon, Corboda, Argentina), prepubic and 2 transobturator macroporous monofilaments, vagina closed in overlapping fashion 355 accessed, 79 randomised AC: 39 completed 1‐year review Anterior mesh: 40 randomised, 40 completed 1‐year review Concomitant surgery as required | |
| Outcomes | Assessed at 1 year Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation tables |
| Allocation concealment (selection bias) | Unclear risk | Envelopes (opaque?, sealed?) |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded assessors |
| Incomplete outcome data (attrition bias) | Low risk | 79 randomised; all completed 1‐year review |
| Selective reporting (reporting bias) | Low risk | Most outcome data reported |
| Other bias | Low risk | Funded by Federal university of Sao Paulo, Brazil; Promedon contributed product free of charge No author COI reported |
| Methods | Single‐centre RCT for stage 2 POPQ prolapse PC‐generated randomisation 2‐Year follow‐up No CONSORT statement Blinding not stated Power of 80%, need sample size of 20 in each arm if subsequent prolapse surgery in 1 group 11% and 44% in mesh group | |
| Participants | 40 randomised Inclusion criteria: stage 2 POPQ cystocele with no plans for pregnancy in 12 months Exclusion criteria: contemplating pregnancy, patients with paravaginal defects, need for continence surgery, prior colposuspension or vaginal surgery, immunocompromised, diabetic | |
| Interventions | A (23): anterior colporrhaphy AC 0 polyglactin Vicryl suture B (21): self‐styled armless soft polypropylene (Gynemesh) mesh without AC | |
| Outcomes | Assessed at 6 weeks, 3 months, then every 6 months to 2 years postop Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | PC‐generated randomised number tables |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes to ensure allocation concealment: as not consecutive, rated as unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Reviewers blinded |
| Incomplete outcome data (attrition bias) | Low risk | At 1 year, completed review; no mesh 20/23, mesh 20/21 |
| Selective reporting (reporting bias) | Low risk | Main outcome reported |
| Other bias | Unclear risk | Funding not stated; study authors reported no COI |
| Methods | Prospective open‐label RCT, multi‐centre (n = 6) 3 years Randomisation and allocation concealment not stated CONSORT and intention to treat, no | |
| Participants | Inclusion: cystocele greater than or equal to stage 2, with risk factors of recurrent prolapse, overweight, COPD, chronic obstipation Exclusion: younger than 18 years, not completed family, allergy to polypropylene, prior mesh; prior cancer of lower urinary tract, genital organs, rectosigmoid 200 randomised, 177/200 at 3 years | |
| Interventions | GP A: partially absorbable polypropylene mesh (Seratom, Germany), coated in polyglycolic acid and caprolactone, which is absorbed at 120 days, leaving light weight of 17 g/m2 GP B: polypropylene mesh, 6 arms, 29 g/m2 Concomitant surgery performed | |
| Outcomes | Review outcomes at 3 years
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation, computer generated, stratified for each centre |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | High risk | Not performed |
| Blinding of outcome assessment (detection bias) | High risk | Not performed |
| Incomplete outcome data (attrition bias) | Low risk | 175/200 at 3 years, groups Gp A: partially absorbable polypropylene mesh 89/97 (92%); Gp B: polypropylene mesh 86/102 (84%) |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | High risk | Study sponsored by company whose product was evaluated; unblinded reviewers had COI with the company whose product was being evaluated (Serag‐Wiessner, Naila, Germany) |
| Methods | Single‐centre RCT Randomisation and allocation concealment described Evaluated 1 year after anterior colporrhaphy (AC) as compared with small intestine submocosa graft Blinded reviewers | |
| Participants | Inclusion criteria: women with point Ba ≥ ‐1 Exclusion criteria: those with hypertension, prior radiation, pelvic sepsis, diabetes and chronic illness Concomitant surgery allowed, including vaginal hysterectomy, if greater than stage 2 uterine prolapse | |
| Interventions | Gp A (27): anterior colporrhaphy with interrupted 0 Vicryl sutures Gp B (29): non‐cross‐linked xenograft porcine small intestine submucosa 7 × 10 cm, with dissection to suprapubic arch, fixed with 0 prolene ×3 each side | |
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Allocation concealment appropriate |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded reviewers and participant‐completed validated questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | Data well described: 1 year AC 27/27, SIS 29/29 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data reported |
| Other bias | Low risk | No COI and no external funding |
| Methods | Single‐centre RCT (computer generated, opaque envelopes, adequate concealment) | |
| Participants | 162 signed consent form | |
| Interventions | A (76): "ultra‐lateral" midline plication of anterior endopelvic connective tissue using Vicryl buttress sutures (as described by Weber 2001), plus additional cadaveric fascia lata patch (Tutoplast) anchored at the lateral limits of the colporrhaphy | |
| Outcomes | Assessed at 1 year Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Data complete at 1 year: AC 76/78 (97%), biological 76/76 |
| Selective reporting (reporting bias) | Low risk | Main outcome reported |
| Other bias | Unclear risk | No COI and no funding statement |
| Methods | Multi‐centre RCT 24‐Month follow‐up Randomisation computer generated Allocation concealment without blinding of participants or surgeon Not according to CONSORT | |
| Participants | Randomised: Gp A 47, Gp B 47 2 years: Gp A 33, Gp B 26 Examination: A 27, B 17 Inclusion criteria: point Ba ≥ ‐1, Exclusion criteria: TVL < 6 cm, severe atrophy, isolated paravaginal defect, allergic bovine material prior vaginal implant surgery, those with ulceration | |
| Interventions | A (46): anterior colporrhaphy B (44): anterior colporrhaphy with bovine pericardium collagen matrix graft reinforcement | |
| Outcomes | Assessed at 6 months, 1 year and 2 years Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated whether assessors were blinded, participant‐completed questionnaire |
| Incomplete outcome data (attrition bias) | High risk | Equal losses in both groups, only 50% at 2‐year review: AC 33/47: biological 26/47 |
| Selective reporting (reporting bias) | Low risk | Main outcome data reported |
| Other bias | High risk | Extensive COI reported: study funded in part by Synovis Life Technology, whose product was being evaluated ‐ bovine pericardium |
| Methods | Single‐centre RCT, India; computer‐generated randomisation Allocation concealment ‐ not stated Blinding of participants and reviewers ‐ not stated Sample size 106, with 80% power to detect 21% difference between groups, with 5% type 1 error | |
| Participants | Inclusion: stage 2 or greater anterior compartment prolapse Exclusion: SUI, dominant post vaginal prolapse; suspected malignancy; vaginal infection | |
| Interventions | Group A: anterior colporrhaphy, 2.0 Vicryl (n = 54), 1 year (n = 41) Group B: self‐styled, 4 arms, monofilament polypropylene mesh (Vypro mesh, J&J) (n = 52), 1 year (n = 44) | |
| Outcomes | Assessed at 6 months, 1 year Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | Gp A 41/54 (76%), Gp B 44/52 (87%), at 1 year |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
| Methods | Single‐centre RCT Computer‐generated randomisation and allocation concealment were appropriate, with sealed envelopes opened in operating room Reviews by non‐blinded surgeon No concomitant surgery 80% power to detect 20% difference, 5% type 1 error | |
| Participants | Inclusion criteria: symptomatic prolapse, point Ba ≥ ‐1; defects in posterior or apical compartment; prior pelvic surgery; Exclusion: history of collagen or endocrine disorders Allocated: Gp A 31, Gp B 30 1 year: A 26, B 28 | |
| Interventions | A (31): 2.0 interrupted Vicryl plication B (30): no plication, Pelvicol porcine dermis 4 × 7 cm anchored with 2.0 Vicryl sutures No concomitant surgery | |
| Outcomes | Assessed at 1 year Reported the following review outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Sealed non‐transparent sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Reviewers non‐blinded, participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | At 1 year: AC 26/31 (84%), biological 28/30 (93%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No COI declared, no statement on funding |
| Methods | Single‐centre RCT, France Computer‐generated 6‐block randomisation Allocation concealment not stated Blinding ‐ no participants or reviewers Intention to treat not stated | |
| Participants | Inclusion: stage 3 or greater anterior compartment prolapse Exclusion: pregnancy, family not completed, prior cancer or radiation, poorly controlled DM, polypropylene sensitivity, immunocompromised Concomitant surgery performed | |
| Interventions | Gp A: AC with bilateral vaginal colposuspension (Ethibond suture) (n = 35), at 2 years (n = 32) Gp B: polypropylene transobturator mesh (Perigee AMS) (n = 33), at 2 years (n = 31) More women underwent hysterectomy in the colposuspension group (77%) than in the mesh group (33%), P < 0.001 | |
| Outcomes | Assessed at 3 months, 1 year and 2 years Reported the following review outcomes at 2 years
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | At 2 years: Gp A 32/35 (91%), Gp B 31/33 (94%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | Funded by the Claude Bernard University. Study authors reported no COI. |
| Methods | Double‐blinded triple‐arm RCT Randomisation, allocation concealment, NS power, 33 in each group, 80% power to detect 35% difference with 5% type 2 error 2‐Year review | |
| Participants | Inclusion: women ≥ 18 years of age with a POPQ point Ba ≥ 0 Exclusion: NS Concomitant surgery: hysterectomy, colpopexy, posterior repair, continence at surgeon's discretion | |
| Interventions | 99 randomised A (32): standard anterior colporrhaphy using midline plication with delayed absorbable suture B (31): vaginal paravaginal repair using free‐hand formed porcine dermis graft (Pelvicol) C (36): vaginal/paravaginal repair using free‐formed polypropylene mesh (M). All graft material was secured to the arcus tendineus fascia pelvis by a Capio device with permanent monofilament suture | |
| Outcomes | Assessed at 2 years Reported the following review outcomes at 2 years
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | Moderate attrition 1 year: AC 24/32 (75%) ‐ porcine 26/31 (84%), mesh 28/36 (77%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | High risk | Study authors reported COI with companies producing product evaluated; funding by Boston Scientific, whose product Capio was being evaluated |
| Methods | Multi‐centre RCT (computer generated) on primary surgery, anterior vaginal wall prolapse Power calculation: 90 in each arm required Follow‐up: 2 years Intention‐to‐treat analysis: yes, including women with missing data at 2 years but with 1‐year follow up completed | |
| Participants | 206 randomised No differences between the 2 groups with respect to demographic and clinical characteristics At 2 years, number available for analysis: 176 (A 91, B 85) Intention‐to‐treat analysis: 201 analysed (A 103, B 98) | |
| Interventions | A (100): interrupted fascial plication Vicryl 00 WITH Pelvicol overlay, fixed with PDS suburethrally and uterosacral cardinal ligament distally | |
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | 1 year: AC 101/106, 98/100 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
| Methods | Single‐centre 2‐surgeon RCT Randomisation list PC generated and sealed opaque envelopes 32 in each group, 80% power to to detect 25% difference with 5% type 1 error Participants and surgeons unblinded, along with who reviewed NS 2‐Year review Intention‐to‐treat analysis | |
| Participants | Inclusion criteria: women over the age of 18 with symptomatic cystoceles scheduled for reconstructive surgery Exclusion criteria: pregnant or planning to have a future pregnancy, 2 previous failed anterior vaginal wall repairs 90 screened, 70 randomised AC (34): 2 years (n = 25) Paravaginal (33): 2 years (n = 25) | |
| Interventions | A (34): AC ‐ plication of the cystocele in the midline was performed with 0‐polydioxanone interrupted mattress sutures over a polyglactin 910 (Vicryl) mesh within the imbricated fold of vaginal muscularis and adventitia B (33): paravaginal defect repair, 0‐polydioxanone sutures were used to attach the pubovesical fascia to that of the obturator and pubococcygeus muscle, also over a Vicryl mesh 2 surgeons Concomitant POP and continence surgery allowed Most undergoing sacral colpopexy, 74% both groups, MUS 76% hysterectomy, AC group 25/34 (74%), paravaginal group 14/34 (42%); P = 0.01 | |
| Outcomes | 2‐Year review
Perioperative outcomes
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | High risk | AC group: 34 randomised, 25 at 2 years (73%) Paravaginal repair: 33 randomised, 25 at 2 years (76%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | No COI |
| Methods | CONSORT statement: no Power calculation: 100 in each arm Type of randomisation: computer generated Blinding strategy: not specified Allocation concealment: not specified Definition of cure: point Ba < ‐1 (i.e. stage 0 or 1 according to the POPQ system) Follow‐up: 24 months Prolapse assessment: POPQ Update of Cervigni 2005 abstract | |
| Participants | Inclusion: recurrent symptomatic stage 2 or greater anterior vaginal wall prolapse (point Ba ≥ ‐1), planning to undergo secondary pelvic reconstructive surgery Exclusion: need for concomitant anti‐incontinence procedure, diabetes mellitus or collagen disease Randomised: 190 Analysed: 190 Women were comparable at baseline in terms of demographic data, degree of POP and clinical or urodynamic findings. Previous hysterectomy: A 60/96, B 54/94 | |
| Interventions | A (96): cystocele repair with armed monofilament polypropylene mesh (Gynemesh) B (94): cystocele repair with armed porcine dermis graft (Pelvicol) Concomitant surgery: not specified. Prophylactic antibiotic cover All underwent tension‐free cystocele repair (TCR) and levator myorrhaphy and vaginal hysterectomy, if required Sheets of both Pelvicol graft and synthetic mesh were trimmed to an identical rounded shape, with 2 lateral wings/arms. In each operation, the central, rounded portion of the graft was positioned under the urinary bladder in a tension‐free fashion, while its arms were inserted deep into the periurethral tissue on both sides towards the pubic bone. A single fixating Monocryl 2/0 suture was performed at the base of 1 wing of mesh, at the periurethral level | |
| Outcomes | 2‐Year outcomes (3‐year abstract for objective failure rate)
| |
| Notes | Trialists concluded that Gynemesh was not statistically significantly superior to porcine graft in the management of anterior compartment prolapse at 2 years. Sexuality and PQOL were superior in the porcine graft group as compared with the Gynemesh PS group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 2‐Year mesh 96/96, Pelvicol 94/94 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
| Methods | Single‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 38 in each arm Type of randomisation: computer generated Blinding strategy: primary surgeon ‐ until the surgery day; patients, research nurse and medical assistant remained blinded Allocation concealment: sealed opaque envelopes Definition of cure
Follow‐up: 12 months (full publication) and 24 months (abstract only) Prolapse assessment: POPQ | |
| Participants | Inclusion: 21 years old and older with POPQ stage 2 or greater anterior prolapse requiring surgical correction Exclusion: pregnancy (present or contemplated), prior repair with graft, systemic infection, compromised immune system, uncontrolled diabetes mellitus, previous pelvic irradiation/cancer, polypropylene allergy, scheduled for concomitant Burch or pubovaginal sling Randomised: 76 Withdrawal: 1 Lost to follow‐up: 1 Analysed: 76 | |
| Interventions | A (38): anterior colporrhaphy (AC) with delayed absorbable (PDS) sutures B (38): AC + polypropylene 4‐armed mesh kit repair (Perigee, American Medical Systems) Concomitant surgery: vaginal hysterectomy, bilateral salpingo‐oophorectomy, uterosacral suspension, mid‐urethral tape, site‐specific rectocele repair, perineoplasty, Apogee mesh kit repair Concomitant prolapse and suburethral tape surgeries were performed in both groups | |
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
| |
| Notes | Data regarding study methods were obtained from the full published article, with follow‐up at 12 months PFDI ‐ Pelvic Floor Distress Inventory (quality of life measure) PFIQ ‐ Pelvic Floor Incontinence Questionnaire (quality of life measure) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Assessors blinded; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Unclear risk | Data set complete: AC 37/38, mesh 37/38 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
| Methods | Muti‐centre RCT on anterior vaginal prolapse CONSORT statement: yes Power calculation: 101 in each arm Type of randomisation: computer generated Allocation concealment: opaque envelopes Blinding strategy: not specified, but lack of a non‐surgical blinded outcome reviewer Definition of cure: less than stage 2 prolapse at Aa or Ba Follow‐up: 24 months Prolapse assessment: POPQ | |
| Participants | Inclusion: postmenopausal women with symptomatic anterior vaginal wall prolapse to the hymen or beyond Exclusion: apical defect indicating vaginal fixation or stress urinary incontinence necessitating surgery, or main symptomatic prolapse component in the posterior vaginal wall. Also, gynaecological tumour or malignancy calling for laparotomy or laparoscopy and untreated vaginal infection Randomised: 202 Withdrawal: 1 Lost to follow‐up: 1 Analysed: 200 No significant differences in baseline demographics, prior hysterectomy or prolapse surgeries between the 2 groups | |
| Interventions | A (96): anterior colporrhaphy (AC) with a 0 or 2/0 multi‐filament suture B (104): AC + self‐tailored (from a 6 × 11 cm mesh patch), 4‐armed low‐weight polypropylene mesh Type of mesh: non‐absorbable monofilament polypropylene (Parietene Light, Sofradim, France) Sutures for AC: absorbable 0 or 2/0 multi‐filament suture Concomitant surgery: vaginal hysterectomy, posterior repair, culdoplasty as required, no concomitant continence surgeries | |
| Outcomes | Assessed at 2 months and 1, 2 and 3 years Reported the following review outcomes at 3 years
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 2‐Year AC 85/96 (88%), mesh 97/104 (93%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | Not clear |
| Methods | Parallel‐group RCT | |
| Participants | Included: women with a cystocele requiring surgical management Excluded: allergy to graft material, immunocompromised, non‐English speaking, unavailable for follow‐up Concomitant surgery and previous non‐anterior prolapse surgery were not exclusion criteria | |
| Interventions | Small intestine mesh‐augmented procedure vs same anterior repair without mesh | |
| Outcomes | Assessed at 1 year Reported the following review outcomes at 1 year
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation through data manager |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded to treatment allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Follow‐up performed by blinded clinician blinded to allocation, with no involvement in participant care |
| Incomplete outcome data (attrition bias) | Low risk | 55/57 women randomised (96%) and included in analysis for objective cure, 57/57 (100%) for subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Reported expected outcomes |
| Other bias | Low risk | Supplier of product (Cook) partially funded the study, but double‐blinding overcame potential biases |
| Methods | Multi‐centre (6) international RCT, Nordic countries ‐ Norway, Sweden, Denmark and Finland Block computer‐generated randomisation list Allocation concealment, opaque sealed envelopes Intention‐to‐treat analysis Sample size: 130 participants allowed, 80% power to detect 20% difference with an alpha error of 5% and a dropout rate of 15% Assessors: surgeons Participants: unblinded Surgeons trained (?) to ensure that uniform surgery was performed | |
| Participants | Inclusion criteria: ≥ 55 years, anterior wall prolapse stage 2, POPQ Aa or Ba ≥ ‐1 Exclusion criteria: previous major pelvic surgery, with the exception of a hysterectomy for reasons other than genital prolapse; previous vaginal surgery or hysterectomy for POP; concomitant prolapse of the uterus or an enterocele of stage 1 or greater; previous incontinence sling surgery performed through the obturator membrane; current treatment with corticosteroids; history of genital or abdominal cancer All surgery covered with intraoperative antibiotics and presurgical and postsurgical local oestrogens Concomitant surgery allowed posterior repair | |
| Interventions | AC group: interrupted absorbable suture fascial plication, vaginal trimming and closure with running unlocked absorbable suture Mesh: biosynthetic system monofilament polypropylene mesh with central portion coated in absorbable hydrophilic porcine collagen film (Bard, Avulta Plus anterior) 169 available for randomisation, with 161 randomised AC (79): randomised, 1 year ‐ 76 Mesh (82): randomised, 1 year ‐ 78 | |
| Outcomes | Assessed at 3 months, 1 year and 3 years Reported the following review outcomes at 1 year
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked computer‐generated randomisation list for each of 4 countries |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Surgeons evaluated |
| Incomplete outcome data (attrition bias) | Low risk | 1‐Year evaluation, randomised AC 76/79 (96%), mesh 78/82 (95%) |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No COI |
| Methods | Single‐centre RCT (computer‐generated number table) | |
| Participants | 143 women | |
| Interventions | A (70): no mesh ‐ Vicryl plication of anterior endopelvic fascia | |
| Outcomes | Assessed at 2, 6 and 12 weeks and at 1 year Reported the following review outcomes at 1 year
| |
| Notes | No subjective success | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | 143/170 (84%) completed 1‐year review |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
| Other bias | Unclear risk | No conflict of interest statement |
| Methods | Single‐centre RCT comparing polypropylene mesh surgery with site‐specific surgery in the treatment of patients with cystocoele CONSORT statement: yes Power calculation: 45 in each arm Type of randomisation: computer generated Blinding strategy: no (assessment was performed by non‐blinded reviewers) Allocation concealment: not specified Definition of cure/failure: 'Acceptable cure' defined as cystocele less than ‐1 cm (stage 1 POPQ) Follow‐up: mean 12 months (range 8 to 16) Prolapse assessment: POPQ | |
| Participants | Inclusion: primary cystocele Exclusion: stress urinary incontinence, concomitant rectocele or enterocoele or recurrent cystocoele Randomised: 90 (45 to each arm) Analysed: 85 Lost to follow‐up: 5 | |
| Interventions | A (42): site‐specific polyglactin 910 anterior repair B (43): self‐styled 4‐armed polypropylene (Parietene, Sofradim, France) mesh, no anterior repair Concomitant surgery not standardised, management of concomitant apical prolapse not specified in either group | |
| Outcomes | Assessed at 6 weeks, 6 months and annually Reported the following review outcomes at mean follow‐up of 1 year (range 8 to 16 months)
| |
| Notes | Sivaslioglu and colleagues evaluated a site‐specific polyglactin 910 repair and self‐styled 4‐armed polypropylene (Parietene, Sofradim) mesh Management of concomitant apical prolapse was not specified in either group, and assessment was performed by non‐blinded reviewers. Three patients in the AC group developed de novo SUI, and 2 in the mesh group developed de novo dyspareunia. Operating time and blood loss were not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded reviewers' objective assessment, participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | AC 42/45, mesh 43/45 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | No funding and no COI |
| Methods | Single‐unit raffle randomisation before surgery No allocation concealment described Surgeons and participants unblinded Unclear who performed assessments (blinded?) Sample size: 100 women, 80% power to detect 26% difference between groups with alpha error of 5% and 20% loss to follow‐up at 2 years | |
| Participants | 122 reviewed, 100 randomised AC (55): 1 year 54, 2 year 50 Mesh (45): 1 year 43, 2 year 42 Inclusion criteria: 45 years old or older, AVWP ≥ 2 (POPQ stage) (7) without previous surgical correction or with previous surgical treatment of AVWP without the use of PM Exclusion criteria: previous treatment (due to AVWP or SUI) with PM, receiving oncological treatment, altered Papanicolau smear exam or uterine bleeding, genital or acute urinary infection, lack of commitment to ambulatory follow‐up, refusal to sign informed consent All preop urodynamics | |
| Interventions | Spinal anaesthesia with antibiotics NAZCA TC Kit (Promedon, Cordoba, Argentina), monofilament macroporous, 4 arms (1 prepubic and 1 transobturator each side), concomitant surgery as required: hysterectomy, apical or posterior repair AC group: 2.0 Vicryl fascial plication mid‐urethral sling if SUI on preop UDS (14/55) | |
| Outcomes | Assessed at 1 year and 2 years Reported the following review outcomes at 2 years
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Raffle randomisation: 55 in AC, 45 in mesh |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded |
| Incomplete outcome data (attrition bias) | High risk | AC group: 42/55 at 2 years (76%) Mesh group: 42/45 completed (93%), high risk due to disparity between groups |
| Selective reporting (reporting bias) | Unclear risk | Significant outcome data |
| Other bias | Low risk | No COI |
| Methods | Multi‐centre and multi‐national RCT Randomisation and allocation concealment NS 90% power to detect 20% difference, urinary distress inventory prolapse domain at 1 year with 5% type 1 error, with 38 in each group | |
| Participants | A (48): anterior colporrhaphy B (48): Perigee transobturator polypropylene mesh A (35): AC only, 5 SSF, 5 hysterectomy, 6 mid‐urethral sling B (34): Perigee only, 4 SSF, 8 hysterectomy, 1 mid‐urethral sling | |
| Interventions | Inclusion: stage 2 or greater cystocele Exclusion: anterior not the leading prolapse Concomitant surgery allowed Stage 2 or greater uterine prolapse hysterectomy or sacrospinous ligament fixation (SSF) SUI mid‐urethral sling | |
| Outcomes | A, median 50; B, median 100 Blood loss > 500 mL: A 1, B 1 UDI: A vs B at baseline Discomfort: 27 (24), 27 (23) Overactive bladder: 34 (30), 41 (33) Obstructive micturition: 28 (32), 19 (20) Prolapse: 56 (30), 58 (35) Incontinence: 23 (24), 19 (20) UDI: A vs B at 1 year Discomfort: 13 (19), 8 (12) Overactive bladder: 16 (25), 15 (23) Obstructive micturition: 15 (23), 11 (19) Prolapse: 12 (22), 1 (4) Incontinence: 18 (29), 16 (23) B mesh erosion: 9/48 B surgery mesh exposure: 4/48 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not clear |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | No statement |
| Methods | Parallel‐group RCT | |
| Participants | Inclusion criteria: grade 2 or greater cystocele Exclusion: urinary incontinence, prior gynaecological surgery, concomitant rectocele or enterocele, recurrent cystocele | |
| Interventions | Polypropylene mesh (00000.3, Sofradim, Parieten) (20 women) vs AC (20 women) | |
| Outcomes | Assessed at 6 weeks, 6 months, 1 year Reported the following review outcomes at 1 year
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocated by computer programme" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | All 40/40 randomised women were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Reports main review outcomes |
| Other bias | Low risk | Reports "no conflict of interest". No other potential bias identified |
| Methods | Mutli‐centre RCT Randomisation was computerised, and stratification was performed for the presence of uterine descent ≥ 2. No blinding to group assignment was performed. Allocation concealment NS Power 80 to detect 25% difference between groups with 5% type 1 error from sample size of 50 in each group | |
| Participants | Inclusion: ≥ stage 2 cystocele Exclusions: history of urogynaecological surgery for pelvic organ prolapse or incontinence, cancer or COPD, concomitant urinary stress incontinence with an indication for surgical correction, recurrent lower urinary tract infection (> 3 culture‐proven infections/y), maximum bladder capacity < 300 mL, indication for hysterectomy, childbearing potential and inadequate birth control measures Randomised: A 64, B 61 Withdrawals before surgery: A 2, B 2 12 months: A 51, B 53 | |
| Interventions | A: AC; B: trocar‐guided transobturator synthetic mesh (AVULTA) | |
| Outcomes | Assessed at 6 months and 1 year Reported the following review outcomes at 1 year
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Research nurse from online list |
| Blinding of participants and personnel (performance bias) | High risk | No |
| Blinding of outcome assessment (detection bias) | Low risk | Reviewers blinded by strapping of thighs before review |
| Incomplete outcome data (attrition bias) | Low risk | AC 55/56, mesh 55/58 ‐ 1 year |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Low risk | No funding and no COI |
| Methods | RCT with computer‐generated random number tables. Sealed envelopes concealed assignment. Investigators compared 3 surgical techniques | |
| Participants | 83 women | |
| Interventions | A (33): anterior repair: midline plication without tension, 0 PDS | |
| Outcomes | Assessed at 6 months, 1 year and 2 years Reported the following review outcomes at median follow‐up 23 months (range 4.5 to 44.4 months)
| |
| Notes | Number and level of surgeons unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | High risk | 2‐Year review: AC 33/37 (89%); ultra‐lateral AC 24/39 (62%); Vicryl mesh 28/36 (78%) ‐ high risk due to disparity between groups |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | Unclear risk | NS |
| Methods | Multi‐centre randomised controlled trial 13 centres, 22 surgeons Randomisation list computer generated for each of 13 centres. Allocation concealment was not discussed, and participants, surgeon and assessor (surgeon) were not blinded. Surgeons underwent specific Prolift mesh training. Full‐power calculation was completed. | |
| Participants | Randomised: GP A 99, Gp B 95 1‐Year examination: A 84, B 83 Inclusion criteria: recurrent stage 2 or higher anterior and/or posterior wall prolapse Exclusion criteria: pregnancy, future pregnancy, prior vaginal mesh repair, compromised immune system or any other condition that would compromise healing, previous pelvic irradiation or cancer, blood coagulation disorders, renal failure, upper urinary tract obstruction, renal failure and upper urinary tract obstruction, presence of large ovarian cysts or myomas | |
| Interventions | Gp A: Conventional surgery was performed at the discretion of the surgeon, although absorbable sutures were specified and hysterectomies permitted Gp B: standardised and structured in the tension‐free vaginal mesh; performed as described by Fatton (Fatton 2007) previously, and no hysterectomies performed nor T incisions allowed | |
| Outcomes | Assessed at 6 months and at 1 year Reported the following review outcomes at 1 year
Duration of surgery: reported median and range Definition of success is unorthodox and different in Methods (≥ grade 2 prolapse in the treated site) and Results sections (≥ grade 2 POP in treated compartment or subsequent prolapse surgery). Furthermore, definition of treated compartment varies in each group. A includes all surgical sites; B excludes sites at which mesh was not utilised. | |
| Notes | Study authors concluded that at 12 months, anatomical failure was less in Gp B (Prolift mesh) as compared with Gp A. These findings were overshadowed by the fact that the 2 groups were significantly different before intervention in terms of important findings. Lack of allocation concealment in the randomisation process, variability in and unorthodox definitions of success, non‐blinded surgeons reviewing their own surgery ‐ significant limitations of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not described. Preoperatively, group A was significantly different from the mesh group B, as demonstrated by a greater degree of prolapse at Ap, Bp and GH in Table 4, and a significantly greater number with ≥ stage 2 apical compartment prolapse among those in Table 1 undergoing prior apical surgery: 36% (16/45) in the non‐mesh group vs 18% (10/56) in the mesh group (P = 0.04, OR 2.54); finally, prior sacral colpopexy was 3 times as frequent in the mesh group. |
| Blinding of participants and personnel (performance bias) | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded reviewers; participant‐completed questionnaires |
| Incomplete outcome data (attrition bias) | Low risk | 1 year ‐ AC 90/95, mesh 96/99 |
| Selective reporting (reporting bias) | Low risk | Significant outcome data |
| Other bias | High risk | Funded university research: All authors reported financial support from Ethicon, the company manufacturing the product being evaluated by non‐blinded reviewers |
AC = anterior colporrhaphy
AVWP = anterior vaginal wall prolapse
BW = Baden‐Walker
CI = confidence interval
COI = conflict of interest
COPD = chronic obstructive pulmonary disease
DM = diabetes mellitus
GH = genital hiatus
ICS = International Continence Society
IVS = intravaginal slingplasty
MUCP = maximum urethral catheter pressure
MUS = Mid‐urethral sling
NS = Not stated
OAB = overactive bladder
OR = odds ratio
OT = Operating time
PC = personal computer
PDS = absorbable polydioxanone surgical suture (PDS)
PFDI = Pelvic Floor Distress Inventory
PFIQ = Pelvic Floor Impact Questionnaire
PGI‐I = Patient Global Impression of Improvement
PISQ = Prolapse and Incontinence Sexual Questionnaire
POP = pelvic organ prolapse
POPQ = Pelvic Organ Prolapse Quantification (according to ICS)
PQOL = Prolapse Quality of Life Questionnaire
RCT = randomised controlled trial
SD = standard deviation
SIS = Small intestine submucosa
SS = statistically significant
SSF = sacrospinous (ligament) fixation
SUI = stress urinary incontinence (symptom diagnosis)
TCR = tension‐free cystocele repair
TVT = tension‐free vaginal tape
UDI = Urogenital Distress Inventory
UI = urinary incontinence
USI = urinary stress incontinence
UTI = urinary tract infection
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Heinonen and Nieminen evaluated outcomes of anterior vaginal wall mesh augmentation with concomitant sacrospinous ligament fixation (SSLF) (n = 14) or with concomitant posterior intravaginal slingplasty (IVS) (n = 8) for uterovaginal or vaginal vault prolapse. On the basis of a predetermined decision that papers with fewer than 20 individuals in each treatment group would not be included in the review, we excluded the manuscript. | |
| Kringel and colleagues compared interventions in a 3‐arm RCT (indwelling urinary catheter for 24 hours or 96 hours or suprapubic catheter for 96 hours) after an anterior colporrhaphy. Study authors concluded that optimal removal of an indwelling urinary catheter took place after 24 hours. We excluded this study from this review and will review catheter issues only at the time of prolapse surgery as a separate subgroup analysis within the surgical management of pelvic organ prolapse. | |
| Tincello and associates reported a pilot randomised patient preference study that compared colposuspension or TVT for urinary incontinence at the time of anterior repair for prolapse. Although 31 women were recruited, only 4 (2 in each arm) were randomised. On the basis of a predetermined decision that papers with fewer than 20 individuals in each treatment group would not be included in the review, we excluded this manuscript. | |
| In a prospective randomised controlled trial, Van Der Steen compared 1‐day and 3‐day suprapubic catheters in women undergoing anterior colporrhaphy to determine the optimal duration of catheterisation. A total of 179 participants were randomly allocated to the 2 groups. We excluded this study from this review and will review catheter issues only at the time of prolapse surgery as a separate subgroup analysis within the surgical management of pelvic organ prolapse. | |
| Weemhoff and colleagues compared the numbers of temporary catheter replacements and urinary tract infections after indwelling catheterisation for 2 vs 5 days after an anterior colporrhaphy. A total of 246 participants were randomly assigned to 2 or 5 days of indwelling catheterisation. We excluded this study from this review and will review catheter issues only at the time of prolapse surgery as a separate subgroup analysis within the surgical management of pelvic organ prolapse. |
IVS = intravaginal slingplasty.
RCT = randomised controlled trial.
SSLF = sacrospinous ligament fixation.
TVT = tension‐free vaginal tape.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Anterior Pelvic Organ Prolapse Surgery: A randomised controlled trial of Xenform anterior repair versus anterior colporrhaphy |
| Methods | Patients will be recruited directly by participating surgeons. Randomisation will occur prior to surgery, with a central office co‐ordinating block randomisation with sealed envelopes |
| Participants | Women of age greater than 40 years, who are symptomatic anterior POP at or beyond hymen (point Ba greater than or equal to 0) AND have a desire for surgery |
| Interventions | Xenform anterior repair versus anterior colporrhaphy. |
| Outcomes | Primary
Secondary
|
| Starting date | Anticipated date of first participant enrolment: 11 February 2016 |
| Contact information | PI: Dr Todd Ladanchuk, King Edward Memorial Hospital 374 Bagot Road, Subiaco, Western Australia 6008 +61(8)93402222; [email protected] |
| Notes |
| Trial name or title | ATHENA |
| Methods | RCT |
| Participants | Women with occult UI |
| Interventions | POP + SUI surgery vs POP surgery alone |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | PROSPECT (PROlapse Surgery: Pragmatic Evaluaiton and Randomised Controlled Trials) |
| Methods | RCT |
| Participants | Women having prolapse surgery |
| Interventions | Anterior and posterior repair (colporrhaphy) with or without non‐absorbable or biological mesh inlay, or mesh kit |
| Outcomes | Prolapse symptoms (POP‐SS), prolapse stage (POP‐Q), economic outcomes |
| Starting date | 01‐09‐2009 |
| Contact information | |
| Notes | HTA‐funded study in UK |
| Trial name or title | Prosthetic Pelvic Organ Prolapse Repair (Prospere) |
| Methods | RCT |
| Participants | Cystocele |
| Interventions | Lap sacral colpopexy vs vaginal mesh procedure unspecified |
| Outcomes | |
| Starting date | 2012 |
| Contact information | http://clinicaltrials.gov/show/NCT01637441 |
| Notes | Study has been completed but outcomes relevant to this review have not yet been reported |
| Trial name or title | Trial of Small Intestine Submucosa (SIS) Mesh for Anterior Repair |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair vs SIS biograft (Cook) |
| Outcomes | |
| Starting date | 2009 |
| Contact information | http://clinicaltrials.gov/show/NCT00955448 |
| Notes | Study completed, but unable to identify publication |
| Trial name or title | The Elegant Trial: Elevate Transvaginal Mesh Versus Anterior Colporrhaphy |
| Methods | RCT |
| Participants | Anterior prolapse |
| Interventions | Anterior repair vs elevate (AMS) anterior repair |
| Outcomes | |
| Starting date | 2011 |
| Contact information | http://clinicaltrials.gov/show/NCT01497171 |
| Notes | Study terminated owing to funding termination |
| Trial name or title | Porcine Dermis vs Vicryl Plug in Raz Cystocele Repair |
| Methods | |
| Participants | 79 women (76 with concomitant prolapse) |
| Interventions | RCT, porcine dermis vs Vicryl |
| Outcomes | UDI, IIQ, urinary urgency, recurrent cystocele |
| Starting date | 2003? |
| Contact information | Dr P Verleyen, University Hospitals, Gassthuisberg |
| Notes |
AMS = American Medical Systems
HTA = Health Technology Assessment
IIQ = impact of urinary incontinence on activities, roles, and emotional states
POP = pelvic organ prolapse
POP‐Q = prolapse stage
POP‐SS = prolapse symptoms
RCT = randomised controlled trial
SIS = small intestine submucosa
SUI = stress urinary incontinence
TVT = tension‐free vaginal tape
UDI = Urinary Distress Inventory
UI = urinary infection
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.1 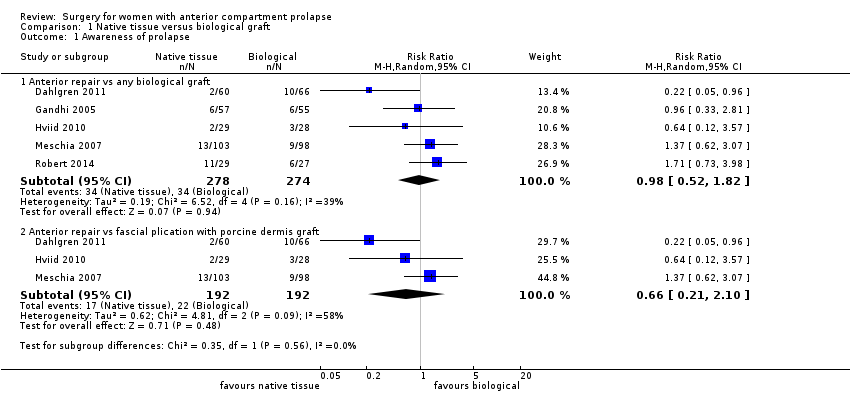 Comparison 1 Native tissue versus biological graft, Outcome 1 Awareness of prolapse. | ||||
| 1.1 Anterior repair vs any biological graft | 5 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.82] |
| 1.2 Anterior repair vs fascial plication with porcine dermis graft | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.21, 2.10] |
| 2 Repeat surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Native tissue versus biological graft, Outcome 2 Repeat surgery. | ||||
| 2.1 Prolapse | 7 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.53, 1.97] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Native tissue versus biological graft, Outcome 3 Recurrent anterior compartment prolapse. | ||||
| 3.1 Anterior repair vs any biological graft | 8 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |
| 3.2 Anterior repair vs fascial plication with porcine dermis graft | 4 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.98, 1.70] |
| 4 Stress urinary incontinence Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.79, 2.64] |
| Analysis 1.4 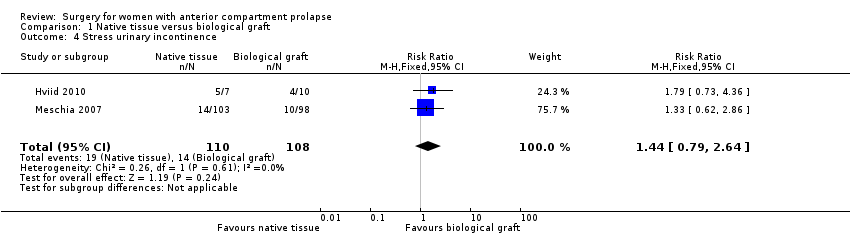 Comparison 1 Native tissue versus biological graft, Outcome 4 Stress urinary incontinence. | ||||
| 5 POPQ assessment Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| Analysis 1.5  Comparison 1 Native tissue versus biological graft, Outcome 5 POPQ assessment. | ||||
| 5.1 Point Ba POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| 6 Urge incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Native tissue versus biological graft, Outcome 6 Urge incontinence. | ||||
| 7 Voiding dysfunction Show forest plot | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.71, 1.80] |
| Analysis 1.7  Comparison 1 Native tissue versus biological graft, Outcome 7 Voiding dysfunction. | ||||
| 8 Dyspareunia Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.39, 1.93] |
| Analysis 1.8  Comparison 1 Native tissue versus biological graft, Outcome 8 Dyspareunia. | ||||
| 9 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Native tissue versus biological graft, Outcome 9 Quality of life PROLAPSE. | ||||
| 9.1 Questionnaire (P‐QOL) 0‐100 | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.01, 4.01] |
| 10 Operating time (minutes) Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐10.35 [‐14.45, ‐6.24] |
| Analysis 1.10  Comparison 1 Native tissue versus biological graft, Outcome 10 Operating time (minutes). | ||||
| 11 Hospital stay Show forest plot | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.09, 0.69] |
| Analysis 1.11  Comparison 1 Native tissue versus biological graft, Outcome 11 Hospital stay. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 9 | 1133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.37, 2.28] |
| Analysis 2.1  Comparison 2 Native tissue versus polypropylene mesh, Outcome 1 Awareness of prolapse. | ||||
| 2 Repeat surgery Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Native tissue versus polypropylene mesh, Outcome 2 Repeat surgery. | ||||
| 2.1 Prolapse | 12 | 1629 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.15, 3.58] |
| 2.2 Reoperation for stress urinary incontinence (1‐3 years) | 5 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.36] |
| 2.3 Surgery for prolapse, SUI or mesh exposure | 12 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.83] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| Analysis 2.3  Comparison 2 Native tissue versus polypropylene mesh, Outcome 3 Recurrent anterior compartment prolapse. | ||||
| 3.1 Permanent mesh vs native tissue repair | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| 4 Bladder injury Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Native tissue versus polypropylene mesh, Outcome 4 Bladder injury. | ||||
| 4.1 Anterior repair vs any transvaginal polypropylene mesh | 6 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.82] |
| 5 Apical or posterior compartment prolapse Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.99] |
| Analysis 2.5  Comparison 2 Native tissue versus polypropylene mesh, Outcome 5 Apical or posterior compartment prolapse. | ||||
| 6 POPQ assessment Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.6  Comparison 2 Native tissue versus polypropylene mesh, Outcome 6 POPQ assessment. | ||||
| 6.1 Point Ba POPQ | 6 | 568 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.30, 0.80] |
| 6.2 Point Bp POPQ | 3 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 6.3 Point C POPQ | 4 | 369 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.47, 1.01] |
| 6.4 Total vaginal length | 3 | 366 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.78, 0.43] |
| 7 Stress urinary incontinence (de novo) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.7  Comparison 2 Native tissue versus polypropylene mesh, Outcome 7 Stress urinary incontinence (de novo). | ||||
| 7.1 Polypropylene mesh vs native tissue (de novo) | 6 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.01] |
| 8 De novo dyspareunia Show forest plot | 8 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.06] |
| Analysis 2.8 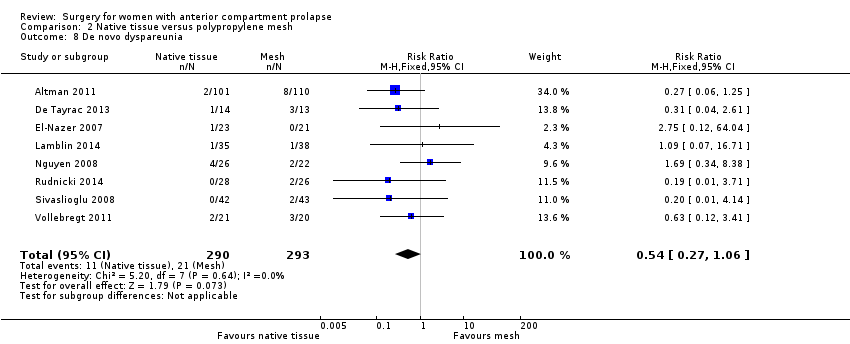 Comparison 2 Native tissue versus polypropylene mesh, Outcome 8 De novo dyspareunia. | ||||
| 9 Voiding dysfunction Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.9  Comparison 2 Native tissue versus polypropylene mesh, Outcome 9 Voiding dysfunction. | ||||
| 9.1 Anterior repair vs polypropylene mesh (persistent) | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.33, 4.47] |
| 10 Urge incontinence Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.10 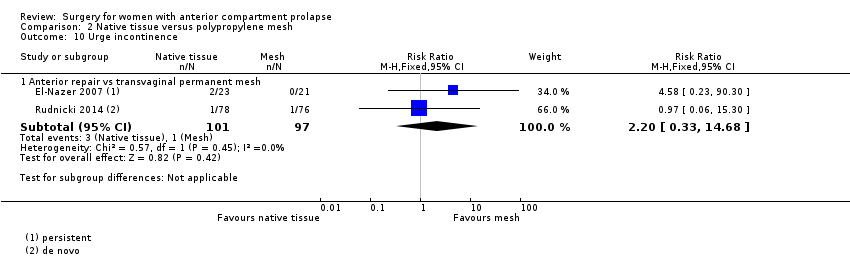 Comparison 2 Native tissue versus polypropylene mesh, Outcome 10 Urge incontinence. | ||||
| 10.1 Anterior repair vs transvaginal permanent mesh | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.33, 14.68] |
| 11 Dyspareunia Show forest plot | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| Analysis 2.11  Comparison 2 Native tissue versus polypropylene mesh, Outcome 11 Dyspareunia. | ||||
| 11.1 Anterior repair vs any transvaginal polypropylene mesh | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 12 Quality of life PROLAPSE Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 2.12 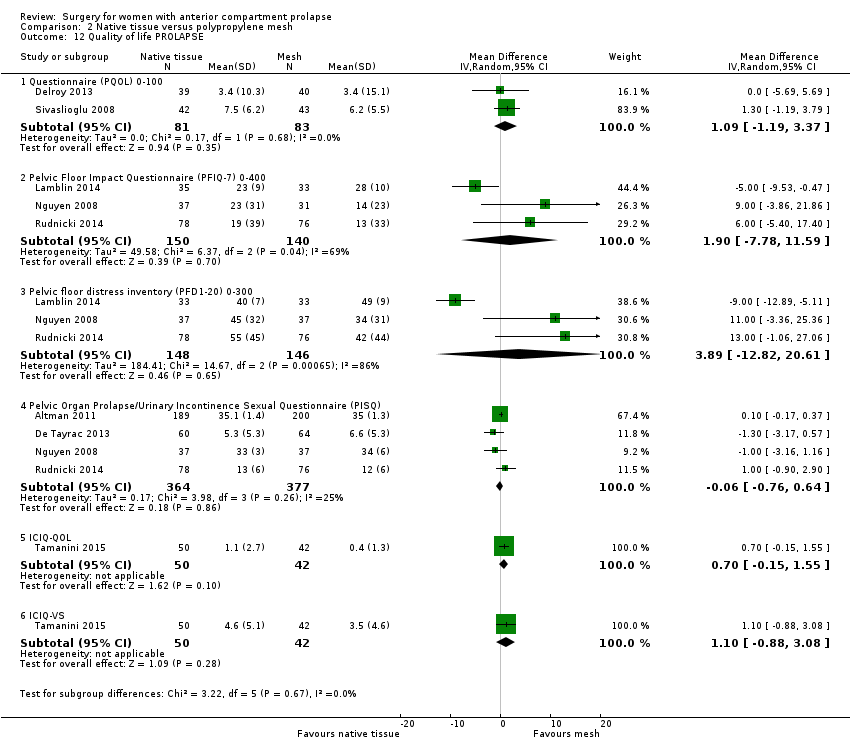 Comparison 2 Native tissue versus polypropylene mesh, Outcome 12 Quality of life PROLAPSE. | ||||
| 12.1 Questionnaire (PQOL) 0‐100 | 2 | 164 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐1.19, 3.37] |
| 12.2 Pelvic Floor Impact Questionnaire (PFIQ‐7) 0‐400 | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐7.78, 11.59] |
| 12.3 Pelvic floor distress inventory (PFD1‐20) 0‐300 | 3 | 294 | Mean Difference (IV, Random, 95% CI) | 3.89 [‐12.82, 20.61] |
| 12.4 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 4 | 741 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.76, 0.64] |
| 12.5 ICIQ‐QOL | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.15, 1.55] |
| 12.6 ICIQ‐VS | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.88, 3.08] |
| 13 Hospital stay (days) Show forest plot | 5 | 707 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.17, 0.33] |
| Analysis 2.13  Comparison 2 Native tissue versus polypropylene mesh, Outcome 13 Hospital stay (days). | ||||
| 14 Operating time (minutes) Show forest plot | 7 | 1099 | Mean Difference (IV, Random, 95% CI) | ‐17.89 [‐25.81, ‐9.98] |
| Analysis 2.14  Comparison 2 Native tissue versus polypropylene mesh, Outcome 14 Operating time (minutes). | ||||
| 15 Transfusion Show forest plot | 4 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.76] |
| Analysis 2.15  Comparison 2 Native tissue versus polypropylene mesh, Outcome 15 Transfusion. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 5 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.35, 3.35] |
| Analysis 3.1  Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 1 Awareness of prolapse. | ||||
| 2 Repeat surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 2 Repeat surgery. | ||||
| 2.1 Prolapse | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.20, 4.59] |
| 2.2 Repeat surgery for stress urinary incontinence (1‐3 years) | 2 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.60, 4.10] |
| 2.3 Repeat surgery for prolapse, SUI or mesh exposure | 6 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.24] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
| Analysis 3.3  Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 3 Recurrent anterior compartment prolapse. | ||||
| 3.1 Permanent mesh vs native tissue repair | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (2‐year review) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.31] |
| Analysis 4.1  Comparison 4 Native tissue versus absorbable mesh, Outcome 1 Awareness of prolapse (2‐year review). | ||||
| 2 Repeat surgery for prolapse (2 years) Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.42, 10.82] |
| Analysis 4.2  Comparison 4 Native tissue versus absorbable mesh, Outcome 2 Repeat surgery for prolapse (2 years). | ||||
| 3 Anterior compartment prolapse (3 months‐2 years) Show forest plot | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.09, 2.06] |
| Analysis 4.3  Comparison 4 Native tissue versus absorbable mesh, Outcome 3 Anterior compartment prolapse (3 months‐2 years). | ||||
| 4 Death Show forest plot | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 4.4  Comparison 4 Native tissue versus absorbable mesh, Outcome 4 Death. | ||||
| 5 Posterior compartment prolapse Show forest plot | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.31, 2.49] |
| Analysis 4.5  Comparison 4 Native tissue versus absorbable mesh, Outcome 5 Posterior compartment prolapse. | ||||
| 6 Stress urinary incontinence Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| Analysis 4.6  Comparison 4 Native tissue versus absorbable mesh, Outcome 6 Stress urinary incontinence. | ||||
| 7 Quality of life Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| Analysis 4.7 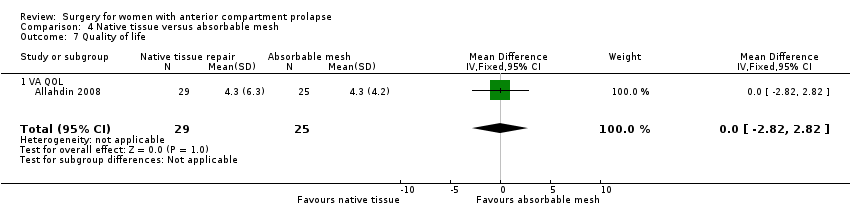 Comparison 4 Native tissue versus absorbable mesh, Outcome 7 Quality of life. | ||||
| 7.1 VA QOL | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1  Comparison 5 Mesh versus biological graft, Outcome 1 Awareness of prolapse. | ||||
| 1.1 Polypropylene mesh (Gynemesh) vs porcine dermis | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.73] |
| 2 Repeat surgery Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Mesh versus biological graft, Outcome 2 Repeat surgery. | ||||
| 2.1 Prolapse | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.87, 10.73] |
| 3 Recurrent anterior wall compartment prolapse (stage 2 or greater) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 5.3  Comparison 5 Mesh versus biological graft, Outcome 3 Recurrent anterior wall compartment prolapse (stage 2 or greater). | ||||
| 3.1 Permanent mesh vs biological graft | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 3.2 Absorbable mesh vs biological graft | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 3.22 [1.38, 7.52] |
| 4 Mesh exposure Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.4  Comparison 5 Mesh versus biological graft, Outcome 4 Mesh exposure. | ||||
| 4.1 Polypropylene mesh vs porcine dermis | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 5 Stress urinary incontinence (de novo) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.5  Comparison 5 Mesh versus biological graft, Outcome 5 Stress urinary incontinence (de novo). | ||||
| 5.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.18, 21.23] |
| 6 Urgency, detrusor overactivity or overactive bladder (de novo) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.6  Comparison 5 Mesh versus biological graft, Outcome 6 Urgency, detrusor overactivity or overactive bladder (de novo). | ||||
| 6.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.78] |
| 7 Dyspareunia (persistent) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.7  Comparison 5 Mesh versus biological graft, Outcome 7 Dyspareunia (persistent). | ||||
| 7.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent anterior wall prolapse Show forest plot | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.46] |
| Analysis 6.1  Comparison 6 Vaginal repair versus abdominal repair, Outcome 1 Recurrent anterior wall prolapse. | ||||
| 2 Injury Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Vaginal repair versus abdominal repair, Outcome 2 Injury. | ||||
| 2.1 Bladder | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 14.88] |
| 3 Posterior compartment prolapse Show forest plot | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.17, 19.65] |
| Analysis 6.3  Comparison 6 Vaginal repair versus abdominal repair, Outcome 3 Posterior compartment prolapse. | ||||
| 4 POPQ assessment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.4 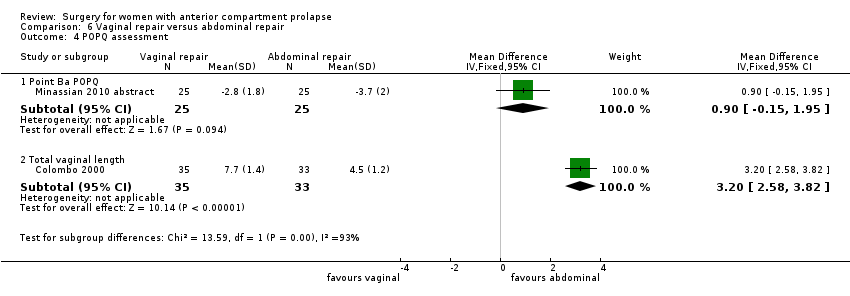 Comparison 6 Vaginal repair versus abdominal repair, Outcome 4 POPQ assessment. | ||||
| 4.1 Point Ba POPQ | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.15, 1.95] |
| 4.2 Total vaginal length | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [2.58, 3.82] |
| 5 Dyspareunia Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [1.63, 16.35] |
| Analysis 6.5 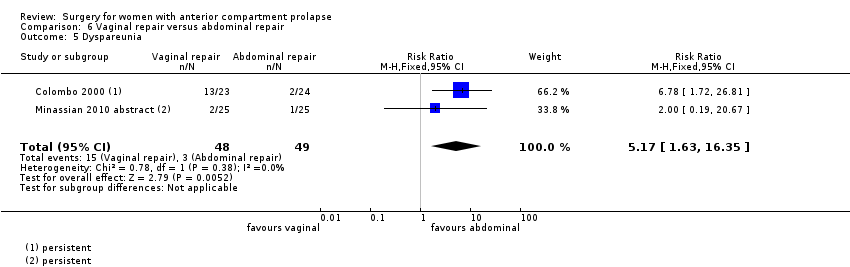 Comparison 6 Vaginal repair versus abdominal repair, Outcome 5 Dyspareunia. | ||||
| 6 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.6 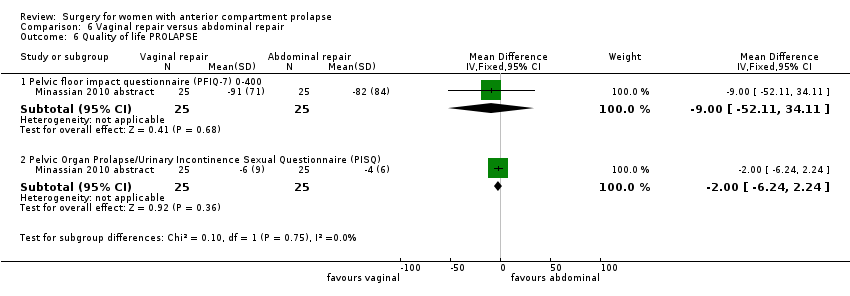 Comparison 6 Vaginal repair versus abdominal repair, Outcome 6 Quality of life PROLAPSE. | ||||
| 6.1 Pelvic floor impact questionnaire (PFIQ‐7) 0‐400 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐52.11, 34.11] |
| 6.2 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐6.24, 2.24] |
| 7 Operating time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.7  Comparison 6 Vaginal repair versus abdominal repair, Outcome 7 Operating time (minutes). | ||||
| 8 Transfusion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.8  Comparison 6 Vaginal repair versus abdominal repair, Outcome 8 Transfusion. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 3 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.36, 1.99] |
| Analysis 7.1  Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 1 Awareness of prolapse. | ||||
| 1.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.70, 1.96] |
| 1.2 Anterior and/or posterior repair vs porcine dermis (Pelvicol) | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.96] |
| 2 Repeat surgery prolapse Show forest plot | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| Analysis 7.2 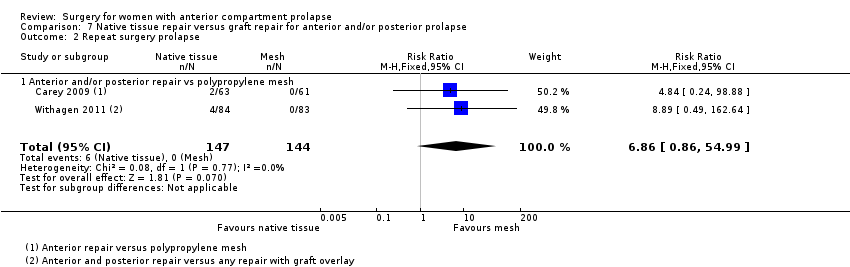 Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 2 Repeat surgery prolapse. | ||||
| 2.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| 3 Recurrent anterior wall prolapse (stage 2 or greater) Show forest plot | 3 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| Analysis 7.3 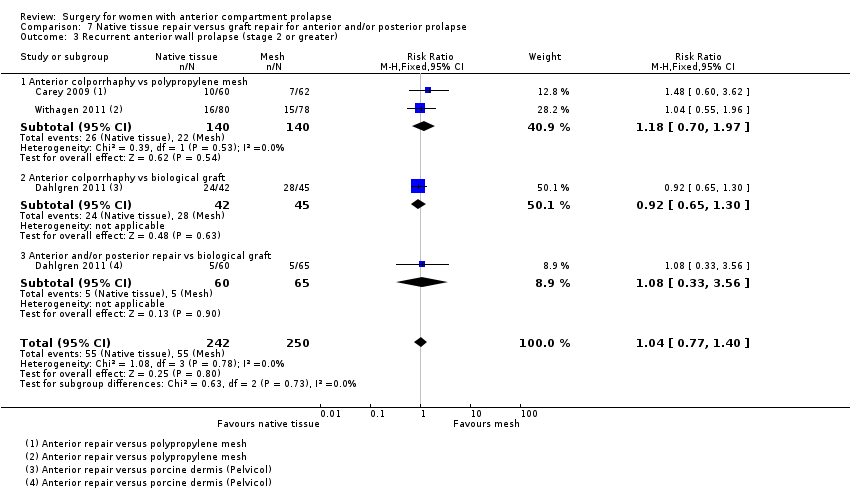 Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 3 Recurrent anterior wall prolapse (stage 2 or greater). | ||||
| 3.1 Anterior colporrhaphy vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.70, 1.97] |
| 3.2 Anterior colporrhaphy vs biological graft | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.30] |
| 3.3 Anterior and/or posterior repair vs biological graft | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.56] |
| 4 Bladder injury Show forest plot | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.01] |
| Analysis 7.4  Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 4 Bladder injury. | ||||
| 5 Stress urinary incontinence (de novo) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.34, 2.85] |
| Analysis 7.5  Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 5 Stress urinary incontinence (de novo). | ||||
| 6 Dyspareunia (de novo and persistent) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.6 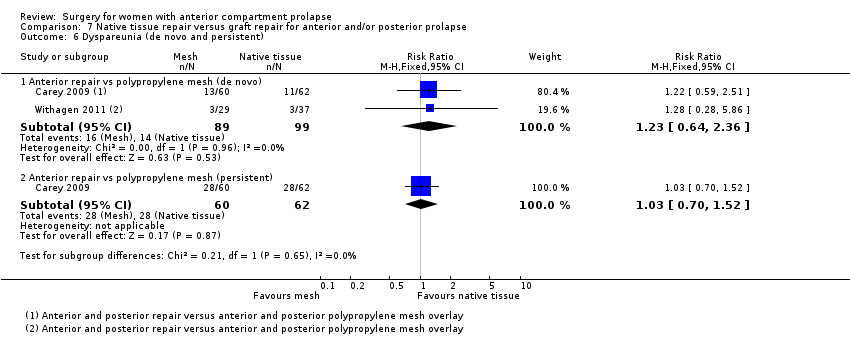 Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 6 Dyspareunia (de novo and persistent). | ||||
| 6.1 Anterior repair vs polypropylene mesh (de novo) | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.64, 2.36] |
| 6.2 Anterior repair vs polypropylene mesh (persistent) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 7 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.7 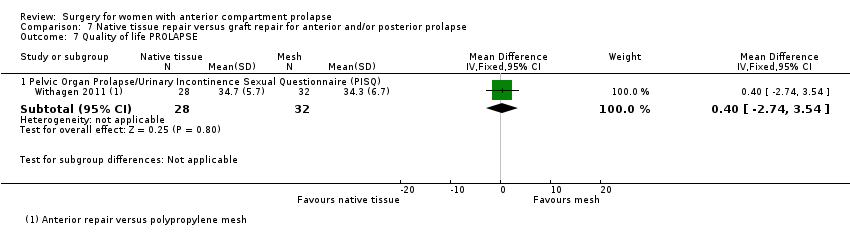 Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 7 Quality of life PROLAPSE. | ||||
| 7.1 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.74, 3.54] |
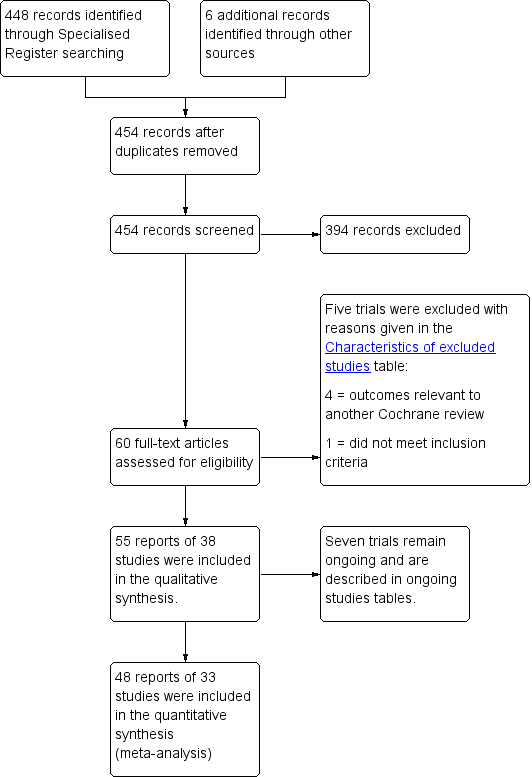
PRISMA study flow diagram for 2016 review.
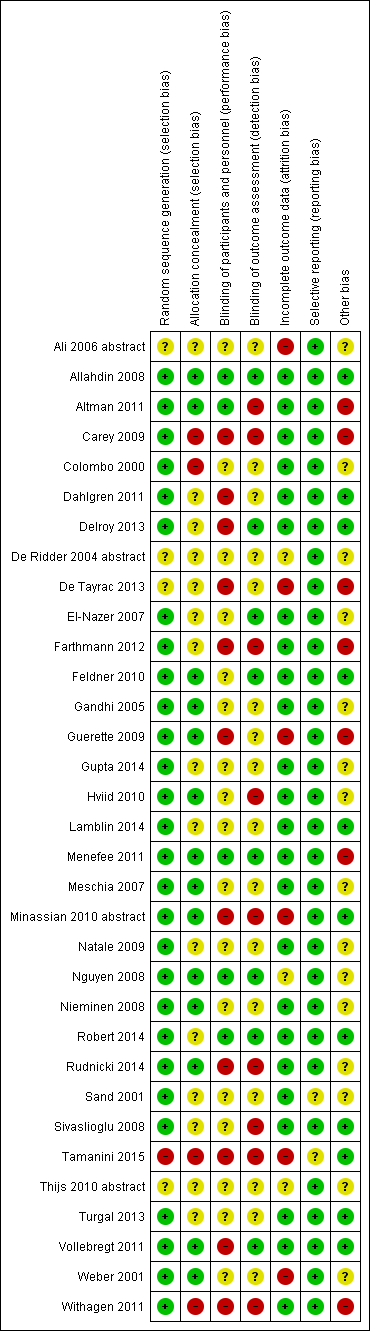
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
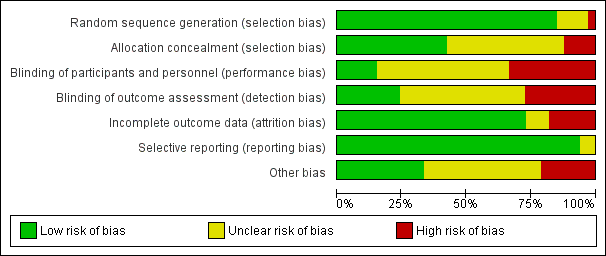
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
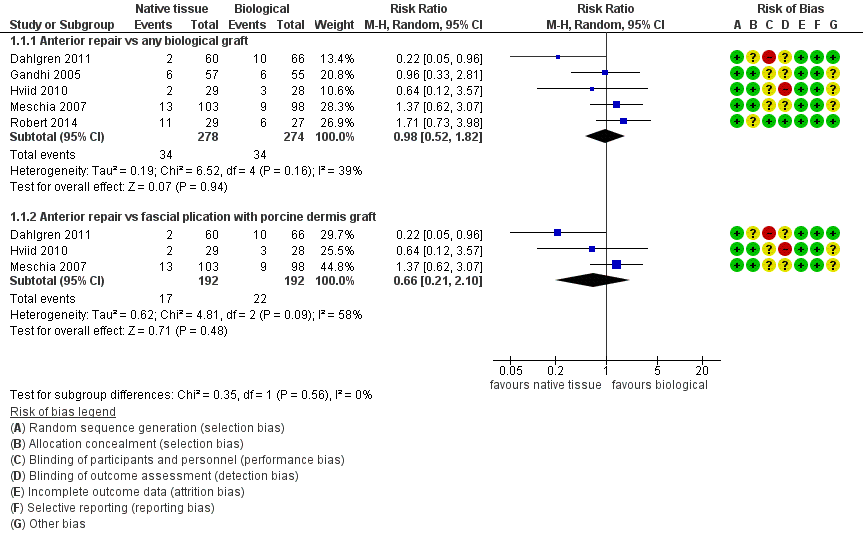
Forest plot of comparison: 1 Native tissue versus biological graft, outcome: 1.1 Awareness of prolapse.

Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.1 Awareness of prolapse.
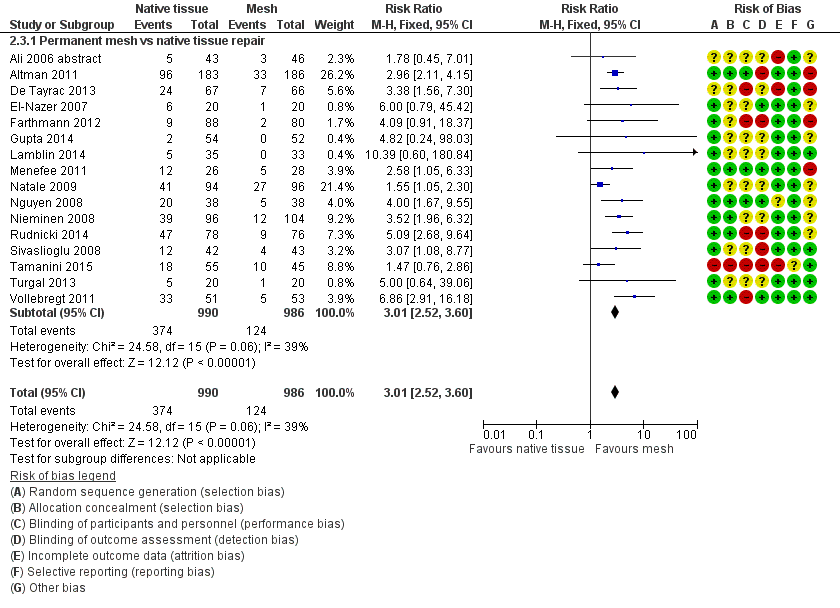
Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.

Funnel plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.

Comparison 1 Native tissue versus biological graft, Outcome 1 Awareness of prolapse.

Comparison 1 Native tissue versus biological graft, Outcome 2 Repeat surgery.
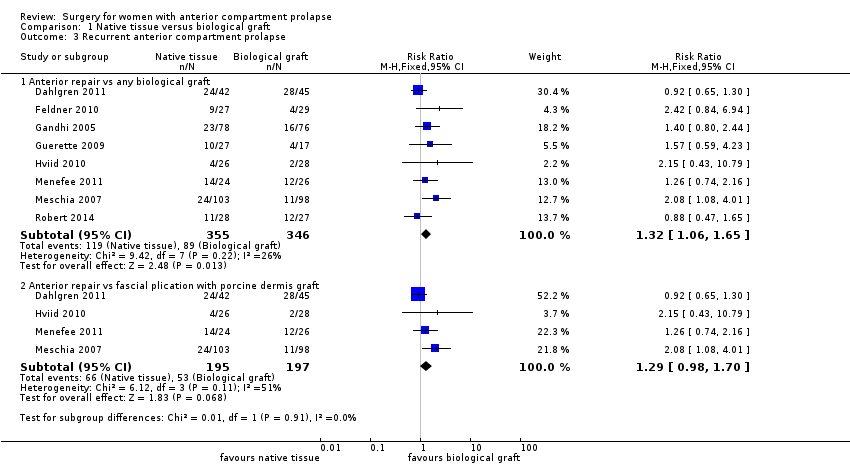
Comparison 1 Native tissue versus biological graft, Outcome 3 Recurrent anterior compartment prolapse.

Comparison 1 Native tissue versus biological graft, Outcome 4 Stress urinary incontinence.
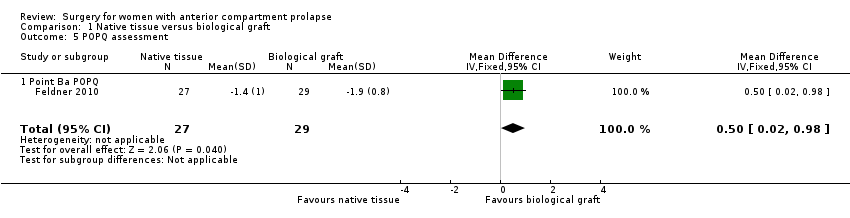
Comparison 1 Native tissue versus biological graft, Outcome 5 POPQ assessment.

Comparison 1 Native tissue versus biological graft, Outcome 6 Urge incontinence.
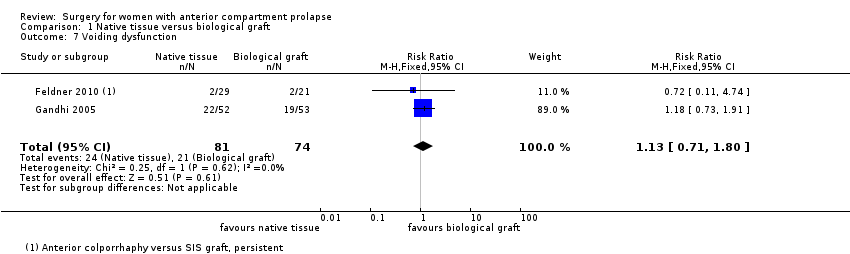
Comparison 1 Native tissue versus biological graft, Outcome 7 Voiding dysfunction.

Comparison 1 Native tissue versus biological graft, Outcome 8 Dyspareunia.
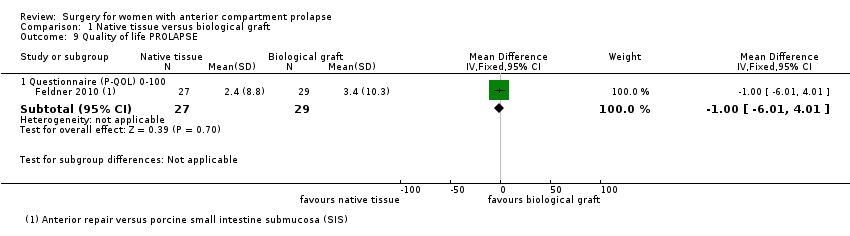
Comparison 1 Native tissue versus biological graft, Outcome 9 Quality of life PROLAPSE.
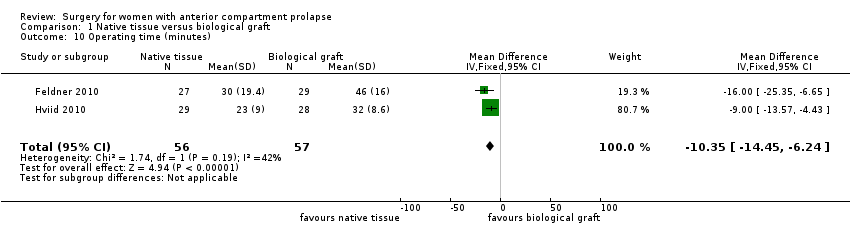
Comparison 1 Native tissue versus biological graft, Outcome 10 Operating time (minutes).

Comparison 1 Native tissue versus biological graft, Outcome 11 Hospital stay.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 1 Awareness of prolapse.
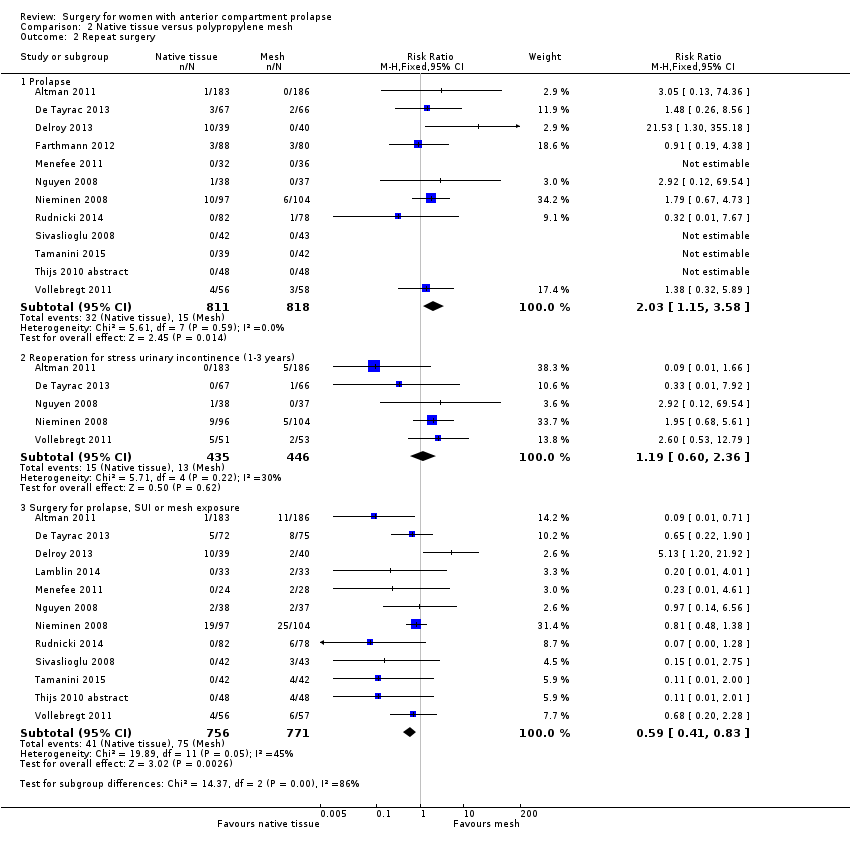
Comparison 2 Native tissue versus polypropylene mesh, Outcome 2 Repeat surgery.
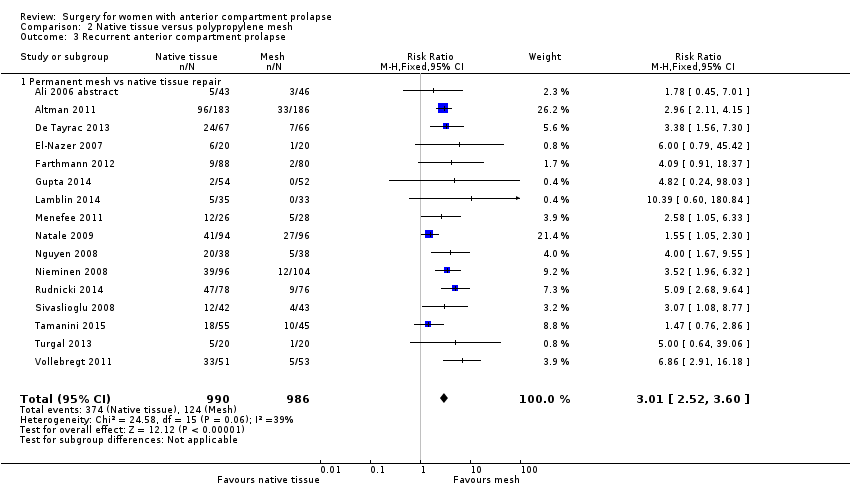
Comparison 2 Native tissue versus polypropylene mesh, Outcome 3 Recurrent anterior compartment prolapse.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 4 Bladder injury.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 5 Apical or posterior compartment prolapse.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 6 POPQ assessment.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 7 Stress urinary incontinence (de novo).
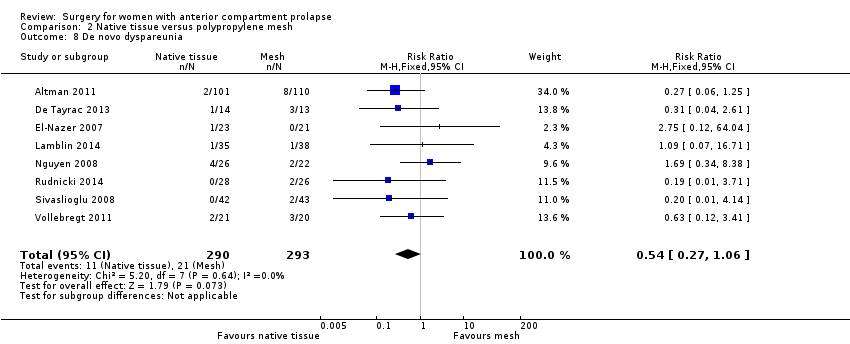
Comparison 2 Native tissue versus polypropylene mesh, Outcome 8 De novo dyspareunia.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 9 Voiding dysfunction.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 10 Urge incontinence.
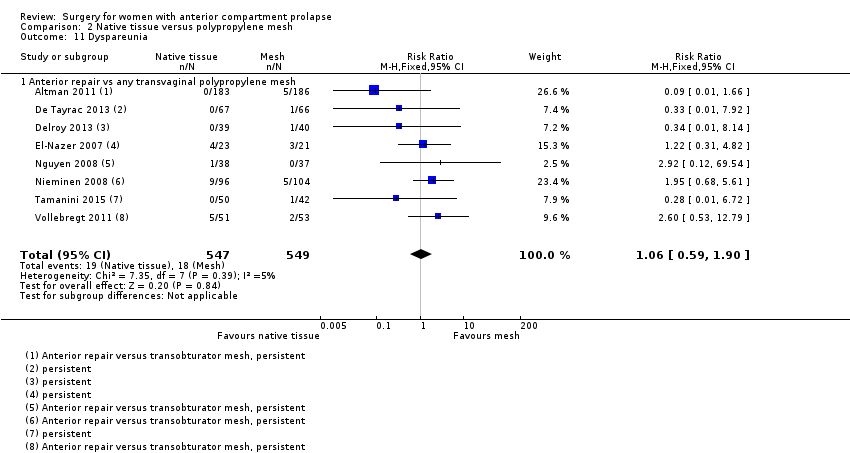
Comparison 2 Native tissue versus polypropylene mesh, Outcome 11 Dyspareunia.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 12 Quality of life PROLAPSE.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 13 Hospital stay (days).
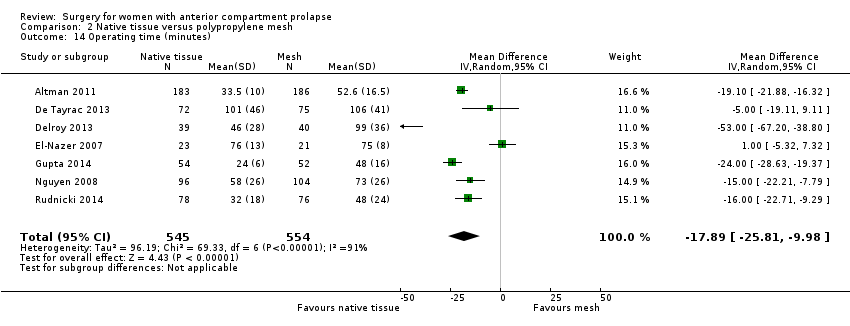
Comparison 2 Native tissue versus polypropylene mesh, Outcome 14 Operating time (minutes).

Comparison 2 Native tissue versus polypropylene mesh, Outcome 15 Transfusion.
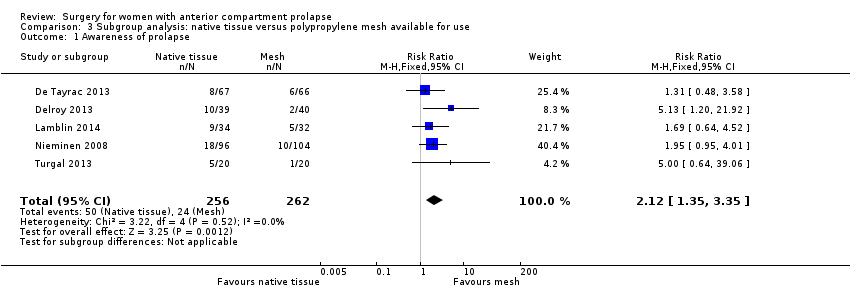
Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 1 Awareness of prolapse.

Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 2 Repeat surgery.

Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 3 Recurrent anterior compartment prolapse.
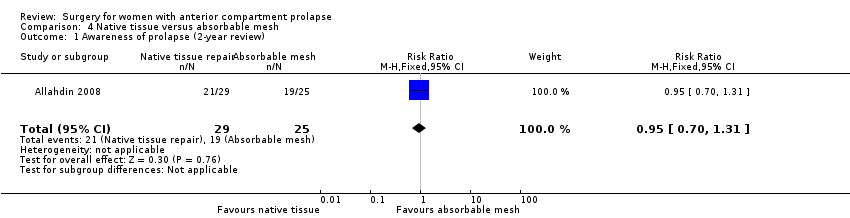
Comparison 4 Native tissue versus absorbable mesh, Outcome 1 Awareness of prolapse (2‐year review).
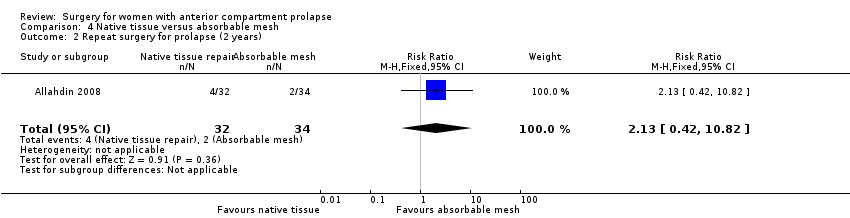
Comparison 4 Native tissue versus absorbable mesh, Outcome 2 Repeat surgery for prolapse (2 years).

Comparison 4 Native tissue versus absorbable mesh, Outcome 3 Anterior compartment prolapse (3 months‐2 years).

Comparison 4 Native tissue versus absorbable mesh, Outcome 4 Death.

Comparison 4 Native tissue versus absorbable mesh, Outcome 5 Posterior compartment prolapse.

Comparison 4 Native tissue versus absorbable mesh, Outcome 6 Stress urinary incontinence.
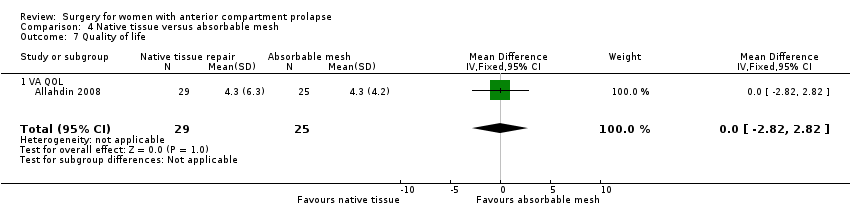
Comparison 4 Native tissue versus absorbable mesh, Outcome 7 Quality of life.
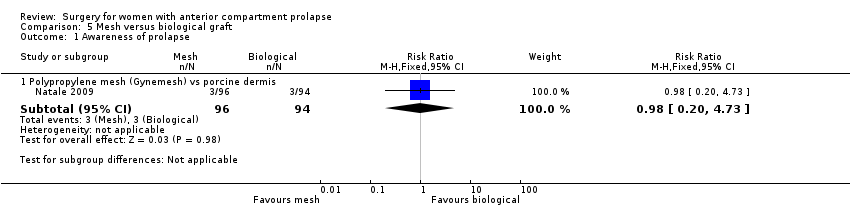
Comparison 5 Mesh versus biological graft, Outcome 1 Awareness of prolapse.

Comparison 5 Mesh versus biological graft, Outcome 2 Repeat surgery.

Comparison 5 Mesh versus biological graft, Outcome 3 Recurrent anterior wall compartment prolapse (stage 2 or greater).

Comparison 5 Mesh versus biological graft, Outcome 4 Mesh exposure.

Comparison 5 Mesh versus biological graft, Outcome 5 Stress urinary incontinence (de novo).
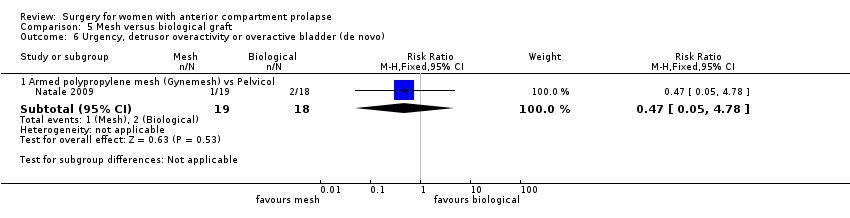
Comparison 5 Mesh versus biological graft, Outcome 6 Urgency, detrusor overactivity or overactive bladder (de novo).

Comparison 5 Mesh versus biological graft, Outcome 7 Dyspareunia (persistent).
Comparison 6 Vaginal repair versus abdominal repair, Outcome 1 Recurrent anterior wall prolapse.
Comparison 6 Vaginal repair versus abdominal repair, Outcome 2 Injury.

Comparison 6 Vaginal repair versus abdominal repair, Outcome 3 Posterior compartment prolapse.
Comparison 6 Vaginal repair versus abdominal repair, Outcome 4 POPQ assessment.

Comparison 6 Vaginal repair versus abdominal repair, Outcome 5 Dyspareunia.
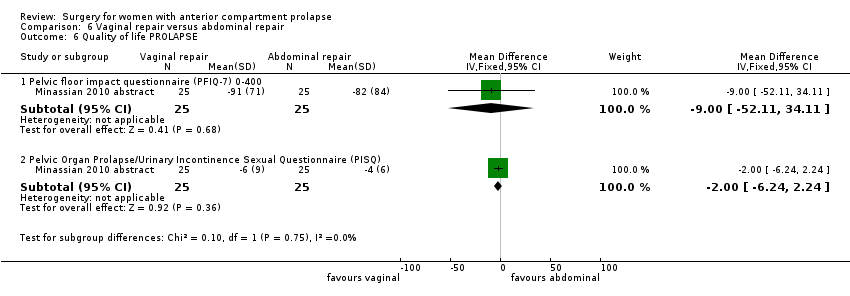
Comparison 6 Vaginal repair versus abdominal repair, Outcome 6 Quality of life PROLAPSE.
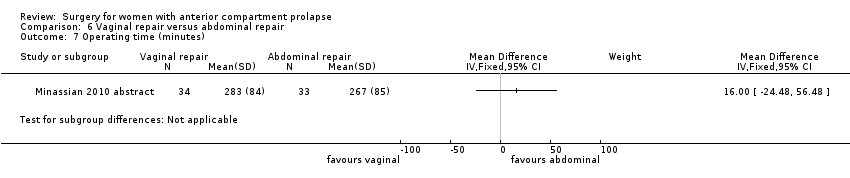
Comparison 6 Vaginal repair versus abdominal repair, Outcome 7 Operating time (minutes).
Comparison 6 Vaginal repair versus abdominal repair, Outcome 8 Transfusion.

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 1 Awareness of prolapse.

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 2 Repeat surgery prolapse.

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 3 Recurrent anterior wall prolapse (stage 2 or greater).

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 4 Bladder injury.

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 5 Stress urinary incontinence (de novo).

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 6 Dyspareunia (de novo and persistent).

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 7 Quality of life PROLAPSE.
| Anterior prolapse repair: native tissue versus biological graft in women with anterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Comparison: biological graft | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Biological graft | Native tissue | |||||
| Awareness of prolapse (1‐2 years) | 124 per 1000 | 122 per 1000 | RR 0.98 | 552 | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse (1‐2 years) | 44 per 1000 | 45 per 1000 | RR 1.02 | 650 | ⊕⊕⊕⊝ | |
| Recurrent anterior compartment prolapse (1‐2 years) | 257 per 1000 | 340 per 1000 | RR 1.32 | 701 | ⊕⊕⊝⊝ | |
| Stress urinary incontinence (1‐2 years) | 130 per 1000 | 187 per 1000 | RR 1.44 | 218 | ⊕⊕⊕⊕ | Repeat surgery for SUI was not reported by any studies |
| Dyspareunia (1‐2 years) | 149 per 1000 | 129 per 1000 | RR 0.87 | 151 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRisk of bias: allocation concealment not reported in 2/5, downgraded one level. bSerious imprecision: wide confidence interval, greater than 25% increase in RR, downgraded one level. | ||||||
| Anterior prolapse repair: native tissue versus polypropylene mesh for women with anterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology Comparison: polypropylene mesh | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polypropylene mesh repair | Native tissue repair | |||||
| Awareness of prolapse (1‐3 years) | 130 per 1000 | 230 per 1000 | RR 1.77 | 1133 | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse (1‐3 years) | 18 per 1000 | 37 per 1000 | RR 2.03 | 1629 | ⊕⊕⊕⊝ | |
| Repeat surgery for stress urinary incontinence (1‐2 years) | 29 per 1000 | 35 per 1000 (17 to 69) | RR 1.19 (0.60 to 2.36) | 881 (5 studies) | Low3,4 | |
| Recurrent anterior compartment prolapse (1‐3 years) | 126 per 1000 | 379 per 1000 | RR 3.01 | 1976 | ⊕⊕⊝⊝ | |
| Stress urinary incontinence (de novo) (1‐3 years) | 102 per 1000 | 69 per 1000 | RR 0.67 | 957 | ⊕⊕⊝⊝ | |
| Dyspareunia (de novo) (1‐2 years) | 72 per 1000 | 39 per 1000 (19 to 76) | RR 0.54 (0.27 to 1.06) | 583 (8 studies) | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse, SUI or mesh exposure (1‐3 years) | 97 per 1000 | 54 per 1000 | RR 0.59 (0.41 to 0.83) | 1527 (12 studies) | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: allocation concealment not reported in 4/9, downgraded one level. 3Risk of bias: allocation concealment not reported in 2/5: downgraded one level. 4Serious imprecision: wide CI with lower RR (0.25), downgraded one level. 5Risk of bias: 11/15 trials did not report blinded outcome assessment, downgraded one level. 6Risk of bias: allocation concealment not reported in 7/15, downgraded one level. 7Risk of bias: poor methodological reporting of allocation concealment and/or blinding, downgraded one level. | ||||||
| Anterior prolapse repair: native tissue repair versus absorbable mesh for women with anterior and/or posterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Absorbable mesh | Native tissue repair | |||||
| Awareness of prolapse (2 years) | 760 per 1000 | 722 per 1000 | RR 0.95 | 54 | ⊕⊝⊝⊝ | |
| Repeat surgery for prolapse (stage 2 or greater) at 2 years | 59 per 1000 | 125 per 1000 | RR 2.13 | 66 | ⊕⊝⊝⊝ | |
| Recurrent anterior compartment prolapse | 267 per 1000 | 401 per 1000 | RR 1.50 | 268 | ⊕⊕⊝⊝ | |
| De novo dyspareunia | Not reported in the included studies | |||||
| Stress urinary incontinence (2 years) | 818 per 1000 | 589 per 1000 | RR 0.72 | 49 | ⊕⊝⊝⊝ | Repeat surgery for SUI was not reported by any studies |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRisk of bias: at 2 years, 18% lost to review, downgraded one level. dRisk of bias: blinded outcome assessment not reported in 2/3 trials, and high attrition in one, downgraded one level. | ||||||
| Study ID | Mesh exposure | Mesh repairs |
| Al‐Nazer 2007 | 1 | 21 |
| Ali 2006 abstract | 3 | 46 |
| Altman 2011 | 21 | 183 |
| De Tayrac 2013 | 7 | 76 |
| Delroy 2013 | 2 | 40 |
| Gupta 2014 | 4 | 44 |
| Lamblin 2014 | 2 | 33 |
| Menefee 2011 | 5 | 28 |
| Nguyen 2008 | 2 | 37 |
| Nieminen 2008 | 18 | 104 |
| Rudnick 2014 | 12 | 78 |
| Sivaslioglu 2008 | 3 | 43 |
| Tamanini 2014 | 7 | 42 |
| Turgal 2014 | 3 | 20 |
| Thijs 2010 abstract | 9 | 48 |
| Vollebregt 2011 | 2 | 53 |
| Total | 101 | 896 |
| Anterior repair vs absorbable mesh | ||
| Sand 2001 | 0 | 73 |
| Weber 2001 | 1 | 26 |
| Study ID | Surgery mesh exposure | Mesh repairs |
| Altman 2011 (1) | 6 | 183 |
| De Tayrac 2013 (2) | 4 | 76 |
| Delroy 2013 (3) | 2 | 40 |
| Gupta 2014 (4) | 2 | 44 |
| Nguyen 2008 (5) | 2 | 37 |
| Nieminen 2008 (6) | 14 | 104 |
| Rudnick 2014 (7) | 5 | 78 |
| Sivaslioglu 2008 (8) | 3 | 43 |
| Tamanini 2014 (9) | 7 | 42 |
| Turgal 2014 | 5 | 20 |
| Thijs 2010 abstract (10) | 4 | 48 |
| Vollebregt 2011 (11) | 2 | 53 |
| Total | 56 | 768 |
| Study ID | Mesh exposure | Mesh repairs |
| Carey 2009 | 4 | 63 |
| Withagen 2011 | 14 | 83 |
| Total | 18 | 146 |
| Study ID | Reoperation mesh exposure | Mesh repairs |
| Carey 2009 | 3 | 63 |
| Withagen 2011 | 5 | 83 |
| Total | 8 | 146 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Anterior repair vs any biological graft | 5 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.82] |
| 1.2 Anterior repair vs fascial plication with porcine dermis graft | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.21, 2.10] |
| 2 Repeat surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 7 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.53, 1.97] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Anterior repair vs any biological graft | 8 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |
| 3.2 Anterior repair vs fascial plication with porcine dermis graft | 4 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.98, 1.70] |
| 4 Stress urinary incontinence Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.79, 2.64] |
| 5 POPQ assessment Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| 5.1 Point Ba POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| 6 Urge incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Voiding dysfunction Show forest plot | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.71, 1.80] |
| 8 Dyspareunia Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.39, 1.93] |
| 9 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Questionnaire (P‐QOL) 0‐100 | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.01, 4.01] |
| 10 Operating time (minutes) Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐10.35 [‐14.45, ‐6.24] |
| 11 Hospital stay Show forest plot | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.09, 0.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 9 | 1133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.37, 2.28] |
| 2 Repeat surgery Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 12 | 1629 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.15, 3.58] |
| 2.2 Reoperation for stress urinary incontinence (1‐3 years) | 5 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.36] |
| 2.3 Surgery for prolapse, SUI or mesh exposure | 12 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.83] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| 3.1 Permanent mesh vs native tissue repair | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| 4 Bladder injury Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Anterior repair vs any transvaginal polypropylene mesh | 6 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.82] |
| 5 Apical or posterior compartment prolapse Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.99] |
| 6 POPQ assessment Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Point Ba POPQ | 6 | 568 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.30, 0.80] |
| 6.2 Point Bp POPQ | 3 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 6.3 Point C POPQ | 4 | 369 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.47, 1.01] |
| 6.4 Total vaginal length | 3 | 366 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.78, 0.43] |
| 7 Stress urinary incontinence (de novo) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Polypropylene mesh vs native tissue (de novo) | 6 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.01] |
| 8 De novo dyspareunia Show forest plot | 8 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.06] |
| 9 Voiding dysfunction Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Anterior repair vs polypropylene mesh (persistent) | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.33, 4.47] |
| 10 Urge incontinence Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Anterior repair vs transvaginal permanent mesh | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.33, 14.68] |
| 11 Dyspareunia Show forest plot | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 11.1 Anterior repair vs any transvaginal polypropylene mesh | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 12 Quality of life PROLAPSE Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Questionnaire (PQOL) 0‐100 | 2 | 164 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐1.19, 3.37] |
| 12.2 Pelvic Floor Impact Questionnaire (PFIQ‐7) 0‐400 | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐7.78, 11.59] |
| 12.3 Pelvic floor distress inventory (PFD1‐20) 0‐300 | 3 | 294 | Mean Difference (IV, Random, 95% CI) | 3.89 [‐12.82, 20.61] |
| 12.4 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 4 | 741 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.76, 0.64] |
| 12.5 ICIQ‐QOL | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.15, 1.55] |
| 12.6 ICIQ‐VS | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.88, 3.08] |
| 13 Hospital stay (days) Show forest plot | 5 | 707 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.17, 0.33] |
| 14 Operating time (minutes) Show forest plot | 7 | 1099 | Mean Difference (IV, Random, 95% CI) | ‐17.89 [‐25.81, ‐9.98] |
| 15 Transfusion Show forest plot | 4 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 5 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.35, 3.35] |
| 2 Repeat surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.20, 4.59] |
| 2.2 Repeat surgery for stress urinary incontinence (1‐3 years) | 2 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.60, 4.10] |
| 2.3 Repeat surgery for prolapse, SUI or mesh exposure | 6 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.24] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
| 3.1 Permanent mesh vs native tissue repair | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (2‐year review) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.31] |
| 2 Repeat surgery for prolapse (2 years) Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.42, 10.82] |
| 3 Anterior compartment prolapse (3 months‐2 years) Show forest plot | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.09, 2.06] |
| 4 Death Show forest plot | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Posterior compartment prolapse Show forest plot | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.31, 2.49] |
| 6 Stress urinary incontinence Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 7 Quality of life Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| 7.1 VA QOL | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Polypropylene mesh (Gynemesh) vs porcine dermis | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.73] |
| 2 Repeat surgery Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.87, 10.73] |
| 3 Recurrent anterior wall compartment prolapse (stage 2 or greater) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Permanent mesh vs biological graft | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 3.2 Absorbable mesh vs biological graft | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 3.22 [1.38, 7.52] |
| 4 Mesh exposure Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Polypropylene mesh vs porcine dermis | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 5 Stress urinary incontinence (de novo) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.18, 21.23] |
| 6 Urgency, detrusor overactivity or overactive bladder (de novo) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.78] |
| 7 Dyspareunia (persistent) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent anterior wall prolapse Show forest plot | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.46] |
| 2 Injury Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bladder | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 14.88] |
| 3 Posterior compartment prolapse Show forest plot | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.17, 19.65] |
| 4 POPQ assessment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Point Ba POPQ | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.15, 1.95] |
| 4.2 Total vaginal length | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [2.58, 3.82] |
| 5 Dyspareunia Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [1.63, 16.35] |
| 6 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Pelvic floor impact questionnaire (PFIQ‐7) 0‐400 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐52.11, 34.11] |
| 6.2 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐6.24, 2.24] |
| 7 Operating time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Transfusion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 3 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.36, 1.99] |
| 1.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.70, 1.96] |
| 1.2 Anterior and/or posterior repair vs porcine dermis (Pelvicol) | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.96] |
| 2 Repeat surgery prolapse Show forest plot | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| 2.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| 3 Recurrent anterior wall prolapse (stage 2 or greater) Show forest plot | 3 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| 3.1 Anterior colporrhaphy vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.70, 1.97] |
| 3.2 Anterior colporrhaphy vs biological graft | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.30] |
| 3.3 Anterior and/or posterior repair vs biological graft | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.56] |
| 4 Bladder injury Show forest plot | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.01] |
| 5 Stress urinary incontinence (de novo) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.34, 2.85] |
| 6 Dyspareunia (de novo and persistent) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Anterior repair vs polypropylene mesh (de novo) | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.64, 2.36] |
| 6.2 Anterior repair vs polypropylene mesh (persistent) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 7 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.74, 3.54] |

