Cirugía para mujeres con prolapso del compartimiento anterior
Resumen
Antecedentes
Para minimizar la tasa de prolapso recurrente después de la reparación tradicional con tejido nativo (colporrafia anterior), los médicos han utilizado diversas técnicas quirúrgicas.
Objetivos
Determinar la seguridad y efectividad de la cirugía para el prolapso del compartimiento anterior.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Especializado del Grupo Cochrane de Incontinencia (Cochrane Incontinence Group) incluido el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), MEDLINE, MEDLINE in process (23 de agosto de 2016), y se hicieron búsquedas manuales en revistas y actas de congresos (15 de febrero de 2016) y se buscó en los registros de ensayos (1 de agosto de 2016).
Criterios de selección
Ensayos controlados aleatorizados (ECA) que examinaron las intervenciones quirúrgicas para el prolapso del compartimento anterior.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente seleccionaron los ensayos, evaluaron el riesgo de sesgo y extrajeron los datos. Los resultados primarios fueron la conciencia del prolapso, la repetición de la cirugía y el prolapso recurrente al examen.
Resultados principales
Se incluyeron 33 ensayos (3332 mujeres). La calidad de la evidencia varió de muy baja a moderada. Las principales limitaciones fueron el riesgo de sesgo y la imprecisión. Se resumen los resultados para las comparaciones principales.
Tejido nativo versus injerto biológico
Conciencia del prolapso: La evidencia indicó poca o ninguna diferencia entre los grupos (riesgo relativo [RR] 0,98; intervalo de confianza [IC] del 95%: 0,52 a 1,82; cinco ECA; 552 mujeres; I2= 39%; evidencia de calidad baja), lo que indica que si el 12% de las mujeres tenían conciencia del prolapso después del injerto biológico, del 7% al 23% lo tendrían después de la reparación con tejido nativo.
Cirugía repetida para el prolapso: Los resultados no mostraron diferencias probables entre los grupos (RR 1,02; IC del 95%: 0,53 a 1,97; siete ECA; 650 mujeres; I2= 0%; evidencia de calidad moderada), lo que indica que si el 4% de las mujeres necesitaron de repetición de la cirugía después del injerto biológico, del 2% al 9% la necesitarían después de la reparación con tejido nativo.
Prolapso recurrente del compartimiento anterior: La reparación del tejido nativo probablemente aumentó el riesgo de recurrencia (RR 1,32; IC del 95%: 1,06 a 1,65; ocho ECA; 701 mujeres; I2 = 26%; evidencia de calidad moderada), lo que indica que si el 26% de las mujeres presentaban prolapso recurrente después del injerto biológico, entre el 27% y el 42% tendrían una recurrencia después de la reparación con tejido nativo.
Incontinencia urinaria de esfuerzo (IUE): Los resultados no mostraron diferencias probables entre los grupos (RR 1,44; IC del 95%: 0,79 a 2,64; dos ECA; 218 mujeres; I2 = 0%; evidencia de calidad moderada).
Dispareunia: La evidencia indicó poca o ninguna diferencia entre los grupos (RR 0,87; IC del 95%: 0,39 a 1,93; dos ECA; 151 mujeres; I2 = 0%; evidencia de calidad baja).
Tejido nativo versus malla de polipropileno
Conciencia del prolapso: Probablemente fue más probable después de la reparación con tejido nativo (RR 1,77; IC del 95%: 1,37 a 2,28; nueve ECA; 1133 mujeres; I2= 0%; evidencia de calidad moderada), lo que indica que si el 13% de las mujeres eran conscientes del prolapso después de la reparación con malla, entre el 18% y el 30% serían conscientes del prolapso después de la reparación con tejido nativo.
Cirugía repetida para el prolapso: Probablemente fue más probable después de la reparación con tejido nativo (RR 2,03; IC del 95%: 1,15 a 3,58; 12 ECA; 1629 mujeres; I2= 39%; evidencia de calidad moderada), lo que indica que si el 2% de las mujeres necesitaron cirugía repetida después de la reparación con malla, entre el 2% y el 7% la necesitarían después de la reparación con tejido nativo.
Prolapso recurrente del compartimiento anterior: Probablemente fue más probable después de la reparación con tejido nativo (RR 3,01; IC del 95%: 2,52 a 3,60; 16 ECA; 1976 mujeres; I2= 39%; evidencia de calidad moderada), lo que indica que si ocurrió un prolapso recurrente en el 13% de las mujeres después de la reparación con malla, entre el 32% y el 45% tendrían una recurrencia del prolapso después de la reparación con tejido nativo.
Cirugía repetida para el prolapso, incontinencia urinaria de esfuerzo o exposición de la malla (resultado compuesto): Puede haber sido más probable después de la reparación con tejido nativo (RR 0,59; IC del 95%: 0,41 a 0,83; 12 ECA; 1527 mujeres; I2= 45%; evidencia de calidad moderada), lo que indica que si el 10% de las mujeres necesitaron cirugía repetida después de la reparación con malla de polipropileno, entre el 4% y el 8% la necesitarían después de la reparación con tejido nativo.
IUE de novo: La evidencia indicó poca o ninguna diferencia entre los grupos (RR 0,67; IC del 95%: 0,44 a 1,01; seis ECA; 957 mujeres; I2 = 26%; evidencia de calidad baja). Ninguna evidencia indicó una diferencia en las tasas de cirugía repetida para la IUE.
Dispareunia (de novo): La evidencia indicó poca o ninguna diferencia entre los grupos (RR 0,54; IC del 95%: 0,27 a 1,06; ocho ECA; 583 mujeres; I2 = 0%; evidencia de calidad baja).
Tejido nativo versus malla absorbible
Conciencia del prolapso: No está claro si los resultados mostraron diferencias entre los grupos (RR 0,95; IC del 95%: 0,70 a 1,31; un ECA; n = 54; evidencia de calidad muy baja),
Cirugía repetida para el prolapso: No está claro si los resultados mostraron diferencias entre los grupos (RR 2,13; IC del 95%: 0,42 a 10,82; un ECA; n = 66; evidencia de calidad baja).
Prolapso recurrente del compartimiento anterior: Puede haber sido más probable después de la reparación con tejido nativo (RR 1,50; IC del 95%: 1,09 a 2,06; tres ECA; n = 268; I2= 0%; evidencia de calidad moderada), lo que indica que si el 27% de las mujeres tuvieron un prolapso recurrente después de la reparación con malla, entre el 29% y el 55% tendrían un prolapso recurrente después de la reparación con tejido nativo.
IUE: No está claro si los resultados mostraron diferencias entre los grupos (RR 0,72; IC del 95%: 0,50 a 1,05; un ECA; n = 49; evidencia de calidad baja).
Dispareunia: No se informaron datos.
Conclusiones de los autores
La reparación biológica del injerto o la malla absorbible proporciona una ventaja mínima en comparación con la reparación con tejido nativo.
La reparación con tejido nativo se asoció con una mayor conciencia del prolapso y un mayor riesgo de repetición de la cirugía para el prolapso y de recurrencia del prolapso del compartimento anterior, en comparación con la reparación con malla de polipropileno. Sin embargo, la reparación del tejido nativo se asoció con un menor riesgo de IUE de novo, menos lesiones vesicales y menores tasas de cirugía repetida para el prolapso, incontinencia urinaria de esfuerzo y exposición a la malla (resultado compuesto).
La evidencia actual no apoya el uso de la reparación con malla en comparación con la reparación con tejido nativo para el prolapso del compartimento anterior debido al aumento de la morbilidad.
Muchas mallas transvaginales de polipropileno se han retirado voluntariamente del mercado, y las mallas ttransvaginales ligeras más modernas que están disponibles no se han evaluado en ECA. Los médicos y las mujeres deben ser cautelosos al utilizar estos productos, ya que no se ha establecido su seguridad y eficacia.
PICOs
Resumen en términos sencillos
Tratamiento quirúrgico del prolapso de órganos pélvicos en las mujeres
Pregunta de la revisión
Determinar la seguridad y efectividad de la cirugía para el prolapso de la pared vaginal anterior.
Antecedentes
El prolapso de los órganos pélvicos ocurre en hasta el 50% de las mujeres que han tenido un parto. Puede suceder en diferentes sitios dentro de la vagina; el prolapso del compartimiento anterior es más difícil de reparar y las tasas de recurrencia son más altas que en otros sitios vaginales. Este desafío ha dado lugar al uso de una variedad de técnicas quirúrgicas e injertos para mejorar los resultados de la cirugía par el prolapso del compartimiento anterior. El objetivo fue evaluar las intervenciones quirúrgicas para el prolapso del compartimiento anterior.
Características de los estudios
Los autores de la revisión incluyeron 33 ensayos controlados aleatorizados (ECA) que evaluaron 3332 cirugías para comparar la reparación anterior con tejido nativo tradicional versus injertos biológicos (ocho ensayos), malla absorbible (tres ensayos), malla permanente (polipropileno) (16 ensayos) y reparación paravaginal abdominal (dos ensayos). Cuatro ensayos compararon un injerto transvaginal versus otro injerto transvaginal, y cuatro ensayos evaluaron la reparación con tejido nativo de los compartimentos anteriores o posteriores de la vagina versus la reparación con injerto. La evidencia está actualizada hasta el 23 de agosto de 2016.
Resultados clave
La reparación biológica del injerto o la malla absorbible proporciona una ventaja mínima en comparación con la reparación con tejido nativo. Los resultados no mostraron evidencia de diferencias entre el injerto biológico y la reparación con tejido nativo en las tasas de conciencia de prolapso o de repetición de la cirugía para el prolapso. Sin embargo, la tasa de prolapso anterior recurrente fue mayor después de la reparación con tejido nativo en comparación con cualquier injerto biológico. Lo anterior indica si la conciencia del prolapso después del injerto biológico ocurre en el 12% de las mujeres, entre el 7% y el 23% tendría conciencia del prolapso después de la reparación con tejido nativo.
La malla permanente dio lugar a tasas más bajas de conciencia del prolapso, prolapso recurrente de la pared anterior y cirugía repetida para el prolapso en comparación con la reparación con tejido nativo. Sin embargo, la reparación con tejido nativo se asoció con una reducción del riesgo de nueva incontinencia urinaria de esfuerzo. Otros efectos beneficiosos de la reparación con tejido nativo incluyeron la reducción de las lesiones vesicales y la reducción de las tasas de repetición de la cirugía para el prolapso, la incontinencia urinaria de esfuerzo y la exposición de la malla (como un resultado combinado).
Calidad de la evidencia
En general la calidad de los datos relacionados con la reparación anterior con tejido nativo tradicional en comparación con los injertos biológicos y la malla permanente es baja a moderada. Las principales limitaciones fueron el informe incompleto de los métodos de los estudios, incluido el ocultamiento de la asignación y el sesgo y la imprecisión en los resultados de los datos. Probablemente los datos relacionados con la eficacia de la malla absorbible son incompletos.
Authors' conclusions
Summary of findings
| Anterior prolapse repair: native tissue versus biological graft in women with anterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Comparison: biological graft | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Biological graft | Native tissue | |||||
| Awareness of prolapse (1‐2 years) | 124 per 1000 | 122 per 1000 | RR 0.98 | 552 | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse (1‐2 years) | 44 per 1000 | 45 per 1000 | RR 1.02 | 650 | ⊕⊕⊕⊝ | |
| Recurrent anterior compartment prolapse (1‐2 years) | 257 per 1000 | 340 per 1000 | RR 1.32 | 701 | ⊕⊕⊝⊝ | |
| Stress urinary incontinence (1‐2 years) | 130 per 1000 | 187 per 1000 | RR 1.44 | 218 | ⊕⊕⊕⊕ | Repeat surgery for SUI was not reported by any studies |
| Dyspareunia (1‐2 years) | 149 per 1000 | 129 per 1000 | RR 0.87 | 151 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRisk of bias: allocation concealment not reported in 2/5, downgraded one level. bSerious imprecision: wide confidence interval, greater than 25% increase in RR, downgraded one level. | ||||||
| Anterior prolapse repair: native tissue versus polypropylene mesh for women with anterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology Comparison: polypropylene mesh | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polypropylene mesh repair | Native tissue repair | |||||
| Awareness of prolapse (1‐3 years) | 130 per 1000 | 230 per 1000 | RR 1.77 | 1133 | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse (1‐3 years) | 18 per 1000 | 37 per 1000 | RR 2.03 | 1629 | ⊕⊕⊕⊝ | |
| Repeat surgery for stress urinary incontinence (1‐2 years) | 29 per 1000 | 35 per 1000 (17 to 69) | RR 1.19 (0.60 to 2.36) | 881 (5 studies) | Low3,4 | |
| Recurrent anterior compartment prolapse (1‐3 years) | 126 per 1000 | 379 per 1000 | RR 3.01 | 1976 | ⊕⊕⊝⊝ | |
| Stress urinary incontinence (de novo) (1‐3 years) | 102 per 1000 | 69 per 1000 | RR 0.67 | 957 | ⊕⊕⊝⊝ | |
| Dyspareunia (de novo) (1‐2 years) | 72 per 1000 | 39 per 1000 (19 to 76) | RR 0.54 (0.27 to 1.06) | 583 (8 studies) | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse, SUI or mesh exposure (1‐3 years) | 97 per 1000 | 54 per 1000 | RR 0.59 (0.41 to 0.83) | 1527 (12 studies) | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: allocation concealment not reported in 4/9, downgraded one level. 3Risk of bias: allocation concealment not reported in 2/5: downgraded one level. 4Serious imprecision: wide CI with lower RR (0.25), downgraded one level. 5Risk of bias: 11/15 trials did not report blinded outcome assessment, downgraded one level. 6Risk of bias: allocation concealment not reported in 7/15, downgraded one level. 7Risk of bias: poor methodological reporting of allocation concealment and/or blinding, downgraded one level. | ||||||
| Anterior prolapse repair: native tissue repair versus absorbable mesh for women with anterior and/or posterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Absorbable mesh | Native tissue repair | |||||
| Awareness of prolapse (2 years) | 760 per 1000 | 722 per 1000 | RR 0.95 | 54 | ⊕⊝⊝⊝ | |
| Repeat surgery for prolapse (stage 2 or greater) at 2 years | 59 per 1000 | 125 per 1000 | RR 2.13 | 66 | ⊕⊝⊝⊝ | |
| Recurrent anterior compartment prolapse | 267 per 1000 | 401 per 1000 | RR 1.50 | 268 | ⊕⊕⊝⊝ | |
| De novo dyspareunia | Not reported in the included studies | |||||
| Stress urinary incontinence (2 years) | 818 per 1000 | 589 per 1000 | RR 0.72 | 49 | ⊕⊝⊝⊝ | Repeat surgery for SUI was not reported by any studies |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRisk of bias: at 2 years, 18% lost to review, downgraded one level. dRisk of bias: blinded outcome assessment not reported in 2/3 trials, and high attrition in one, downgraded one level. | ||||||
Background
Description of the condition
Pelvic organ prolapse is common and is seen on examination in 40% to 60% of parous women (Handa 2004; Hendrix 2002). The annual aggregated rate of associated surgery in the United States is in the range of 10 to 30 per 10,000 women (Brubaker 2002). The anterior compartment of the vagina is the vaginal site most commonly affected by prolapse and is the most difficult to repair.
Pelvic organ prolapse is the descent of one or more of the pelvic organs (uterus, vagina, bladder or bowel). Different types of prolapse include:
-
upper vaginal prolapse (i.e. uterus, vaginal vault (after hysterectomy when the top of the vagina drops down));
-
anterior vaginal wall prolapse (i.e. cystocele (bladder descends), urethrocele (urethra descends), paravaginal defect (pelvic fascia defect)); and
-
posterior vaginal wall prolapse (i.e. enterocele (small bowel descends), rectocele (rectum descends), perineal deficiency).
A woman can present with prolapse of one or more of these sites.
The aetiology of pelvic organ prolapse is complex and multi‐factorial. Possible risk factors include pregnancy, childbirth, congenital or acquired connective tissue abnormalities, denervation or weakness of the pelvic floor, ageing, hysterectomy, menopause and factors associated with chronically raised intra‐abdominal pressure (Bump 1998; Gill 1998; MacLennan 2000).
Women with anterior compartment prolapse commonly have a variety of pelvic floor symptoms, only some of which are directly related to the prolapse. Generalised symptoms of prolapse include pelvic heaviness; a bulge, lump or protrusion coming down from the vagina; a dragging sensation in the vagina; and backache. Symptoms of bladder, bowel or sexual dysfunction are frequently present. For example, women may need to reduce the prolapse by using their fingers to push the prolapse up to aid urinary voiding or defecation. These symptoms may be directly related to the prolapsed organ, for example, poor urinary stream when a cystocele is present or obstructed defecation when a rectocele is present. Symptoms may also be independent of the prolapse, for example, symptoms of overactive bladder (urinary urgency) or urinary stress incontinence when a cystocele is present.
Description of the intervention
Treatment of a woman with prolapse depends on the severity of the prolapse, its symptoms, the woman's general health and surgeon preference and capabilities. Options for treatment include conservative, mechanical and surgical interventions.
Generally, conservative or mechanical treatments are considered for women with a mild degree of prolapse, those who wish to have more children, frail women and those unwilling to undergo surgery. Conservative and mechanical interventions have been considered in separate Cochrane reviews (Adams 2004; Hagen 2011). No good evidence was available to guide management in either of these reviews.
A wide variety of abdominal and vaginal surgical techniques are available for the treatment of patients with prolapse (Appendix 1). The most common and traditional procedure is the anterior repair (colporrhaphy) for anterior vaginal wall prolapse, which is frequently performed in conjunction with other interventions for prolapse and/or urinary stress incontinence. Together, anterior and posterior compartment surgeries account for more than 90% of all prolapse operations (Olsen 1997). Two main approaches can be used.
-
Vaginal approaches include vaginal hysterectomy, anterior or posterior vaginal wall repair (colporrhaphy), McCall culdoplasty, Manchester repair (amputation of the cervix with uterus suspension to the cardinal ligaments), prespinous and sacrospinous colpopexy, enterocele ligation, paravaginal repair, Le Fortes procedure and perineal reconstruction.
-
Abdominal approaches include hysterectomy, sacral colpopexy, paravaginal repair, vault suspending and uterosacral ligament plication, enterocele ligation and posterior vaginal wall repair. Abdominal surgery can be performed through an open incision or keyhole incisions via the laparoscope or robot.
The current review considers all surgical procedures for women with anterior compartment pelvic organ prolapse.
A combination of some of the above‐mentioned procedures may be employed concomitantly at the time of anterior compartment prolapse surgery. In addition to the variety of prolapse operations that can be performed, the surgeon must choose whether to use absorbable sutures such as polyglycolic acid‐based materials (e.g. polyglactin), delayed‐absorption sutures such as polydioxanone or non‐absorbable sutures such as polypropylene. Furthermore, to improve the anatomical outcomes of anterior compartment prolapse, some techniques employ grafts. Graft material can be synthetic (e.g. permanent polypropylene, absorbable polyglactin mesh) or biological. Biological grafts can be further divided into autologous (from a person's own tissue, such as fascial sheath), alloplastic (from animals, such as porcine dermis) and homologous (such as cadaveric fascia lata).
The choice of operation depends on several factors, which include the nature, site and severity of the prolapse; whether additional symptoms are affecting urinary, bowel or sexual function; the general health of the woman; and surgeon preference and capability. Concomitant procedures are often performed to treat or prevent urinary incontinence at the same time. Mid‐urethral slings and tapes that are utilised in continence surgery are not the topic of this review, and the reader is referred to Ford 2015 for a full review of continence surgery.
To aid in assessment of the surgery, clear preoperative and postoperative site‐specific vaginal grading, details of the operative intervention and impact of the surgery on functional aspects of bladder, bowel and sexual function should be recorded.
Over the past five years and following significant litigation regarding outcomes of prolapse surgery after transvaginal polypropylene mesh, many of the products evaluated in this review have been voluntarily removed from the market (Prolift ‐ Gynecare/Ethicon, Somerville, NJ, USA; Perigee American Medical Systems, Minnetonka, MN, USA; Avaulta® ‐ Bard, Covington, LA, USA), or companies have excluded transvaginal utilisation of the mesh product (Gynemesh PS, Gynecare/Ethicon). The reviewer needs to be mindful when reading this review that the data presented include some products that are no longer available for use.
How the intervention might work
The aim of surgery ‐ to improve quality of life ‐ is achieved by:
-
restoration of normal vaginal anatomy;
-
restoration or maintenance of normal bladder function;
-
restoration or maintenance of normal bowel function; or
-
restoration or maintenance of normal sexual function.
Why it is important to do this review
The wide variety of surgical treatments available for prolapse indicates lack of consensus as to the optimal treatment. No consensus guidelines have been published in the available literature, and treatment is based on evidence from studies of mixed type and quality (Carey 2001). Provided that a sufficient number of trials of adequate quality have been conducted, the most reliable evidence is likely to come from the findings of randomised controlled trials. We conducted this review to gather evidence that would help review authors identify optimal practice while highlighting areas in which further research is needed.
This review should be read as part of a series of six Cochrane reviews related to the surgical management of prolapse, including:
-
surgery for women with anterior compartment prolapse;
-
surgery for women with posterior compartment prolapse;
-
surgery for women with apical compartment prolapse;
-
continence outcomes in pelvic organ prolapse;
-
native tissue, biological grafts or mesh for transvaginal prolapse surgery (Maher 2016); and
-
perioperative interventions at prolapse surgery.
Objectives
To determine the safety and effectiveness of surgery for anterior compartment prolapse.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with 20 or more patients in each arm, in which at least one arm provided a surgical intervention for pelvic organ prolapse.
Types of participants
Adult women seeking treatment for symptomatic anterior compartment pelvic organ prolapse. Both primary prolapse and recurrent prolapse were considered.
Types of interventions
Anterior compartment pelvic organ prolapse includes cystocele, urethrocele and a paravaginal defect for which both vaginal and abdominal surgeries were the primary inclusion criteria. Included trials performed interventions solely for anterior compartment prolapse or for concomitant prolapse or incontinence. Comparison interventions included no treatment, conservative management and use of a mechanical device or an alternative approach to surgery.
Types of outcome measures
Primary outcomes
1. Awareness of prolapse: any affirmative response to questions related to awareness of prolapse or vaginal bulge, or any affirmative response to question three of the Pelvic Floor Distress Inventory (PFDI‐20): “Do you usually have a bulge or something falling out that you can see or feel in the vaginal area?”
2. Repeat surgery
2.1 Repeat surgery for prolapse
2.2 Repeat surgery for stress urinary incontinence
2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome), if relevant
3. Recurrent anterior prolapse ‐ defined as any stage 2 or greater anterior vaginal prolapse (Pelvic Organ Prolapse Quantification (POPQ): prolapse 1 cm above or below the hymen)
Secondary outcomes
4. Adverse events
Outcomes to be reported include but are not limited to:
4.1 death (related to surgery);
4.2 mesh exposure;
4.3 injury to bladder (cystotomy);
4.4 injury to bowel (enterotomy); or
4.5 repeat surgery for mesh exposure.
5. Prolapse outcomes
5.1 Objective failure
5.1.1 Stage 2 or greater apical compartment prolapse (point C at or beyond 1 cm inside the introitus)
5.1.2 Stage 2 or greater posterior vaginal compartment prolapse (point Bp at or beyond 1 cm inside the introitus)
5.1.3 POPQ system scores: POPQ scores include nine measurements of the vagina performed to quantify and describe vaginal prolapse. For simplicity, we report four of these basic measurements
5.1.3.a Point Ba on POPQ (range ‐3 to +10 cm): Point Ba is approximately mid‐point on the anterior vaginal wall
5.1.3.b Point Bp on POPQ (range ‐3 to +10 cm): Point Bp is approximately mid‐point on the posterior vaginal wall
5.1.3.c Point C on POPQ (range ‐10 cm to non‐determined limit): Point C describes the vaginal apex (upper vagina)
5.1.3.d Total vaginal length (TVL) in cm (range 0 to 14 cm): TVL is the length from the vaginal entrance to the apex (cervix or vaginal cuff)
6. Bladder function
6.1 Stress urinary incontinence
6.2 De novo stress urinary incontinence
6.3 Surgery for stress urinary incontinence
6.4 De novo bladder overactivity or urge incontinence
6.5 Urinary voiding dysfunction
7. Bowel function
7.1 De novo faecal incontinence
7.2 De novo obstructed defecation
7.3 Constipation
8. Sexual function
8.1 Dyspareunia
8.2 De novo dyspareunia
8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ‐12; range 0 to 48): Higher score indicates better sexual function
9. Quality of life and satisfaction
Outcomes to be reported include but are not limited to:
9.1 Patient Global Impression of Improvement (PGI‐I) questionnaire (data presented on 7‐point Likert scale): Responses of "much" or "very much" better were considered affirmative and are presented as dichotomous outcomes;
9.2 Prolapse Quality of Life (PQOL) questionnaire (range 0 to 100): Higher score indicates greater dysfunction;
9.3 Pelvic Floor Distress Inventory (PFDI‐20; range 0 to 300): Higher score indicates greater dysfunction;
9.4 Pelvic Floor Impact Questionnaire (PFIQ‐7; range 0 to 300): Higher score indicates greater dysfunction; and
9.5 International Consultation on Incontinence Modular Questionnaires (ICIQ; variable range): Higher score indicates greater dysfunction.
10. Measures associated with surgery
10.1 Operating time (minutes)
10.2 Length of hospital stay (days)
10.3 Blood transfusion (%)
Search methods for identification of studies
We imposed no language limits and no other limits on any of the searches detailed below.
Electronic searches
This review has drawn on the search strategy developed in consultation with the Cochrane Incontinence Review Group Trials Search Co‐ordinator. We identified relevant trials from the Incontinence Group Specialised Register of controlled trials, which is described, along with the Review Group search strategy, under the Group module in the Cochrane Library. This Register contains trials identified by a search of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and by a handsearch of journals and conference proceedings. We searched the Incontinence Group Specialised Register using the keyword system of the Group (all searches were based on the keyword field of Reference Manager 12, Thomson Reuters). Search terms used were as follows: ({design.cct*} OR {design.rct*}) AND ({topic.prolapse*}) AND ({intvent.surg*}), and the search date was 23 August 2016.
Trials in the Incontinence Group Specialised Register are also included in CENTRAL.
Review authors also undertook searches of health care‐related bibliographic databases such as clinicaltrials.gov (most recent, 1 August 2016), as per Appendix 2.
Searching other resources
We handsearched conference proceedings for the International Urogynecological Association (IUGA) and the International Continence Society (ICS) for podium presentations from 2012 to 15 February 2016. We searched the reference lists of relevant articles and contacted researchers in the field (Moher 2009).
We constructed a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) chart (Moher 2009) to present search flow.
Data collection and analysis
Selection of studies
Two review authors assessed titles and, if available, abstracts of all possibly eligible studies for compliance with the inclusion criteria for this review. Two or more review authors independently assessed full reports of each study likely to be eligible for inclusion. We have listed excluded studies along with reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two or more review authors independently extracted and compared data to ensure accuracy. We resolved discrepancies by discussion or by consultation with a third party. When trial data were not reported adequately, we attempted to acquire the necessary information from the trialist.
Assessment of risk of bias in included studies
Two review authors used the Cochrane risk of bias assessment tool (Higgins 2011) to independently examine studies for risk of bias by assessing selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. We planned to resolve disagreements by discussion or by consultation with a third review author. We described all judgements fully and presented our conclusions in the Risk of bias table, which we incorporated into our interpretation of review findings via sensitivity analyses (see below).
Measures of treatment effect
For dichotomous data, we used numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs). For continuous data, if all studies reported exactly the same outcomes, we calculated mean differences (MDs) between treatment groups. If similar outcomes were reported on different scales, we calculated the standardised mean difference (SMD). We presented 95% confidence intervals (CIs) for all outcomes, and we compared the magnitude and direction of effect reported by studies with how they are presented in the review, while taking account of legitimate differences.
Unit of analysis issues
All analyses were performed per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (once randomised to an intervention, participants were analysed because intervention and analysis include all randomised participants) and attempted to obtain missing data from the original trialist. When these could not be obtained, we analysed only available data.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measuring I2. We regarded an I2 measurement greater than 50% as indicating substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If we included 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small‐study effects (i.e. a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
If studies were sufficiently similar, we combined the data using RevMan software (RevMan 2014) and a fixed‐effect model for the following comparisons.
-
Native tissue versus biological graft.
-
Native tissue versus polypropylene mesh.
-
Native tissue versus absorbable mesh.
-
One graft versus another graft for anterior compartment prolapse.
-
Vaginal repair versus abdominal repair.
-
Native tissue repair versus graft repair for anterior and/or posterior prolapse.
Subgroup analysis and investigation of heterogeneity
When data were available, we conducted subgroup analyses to identify separate evidence for primary outcomes within the following subgroups.
-
Native tissue versus biological graft in studies using porcine dermis graft (compared with studies using other types of biological graft).
-
Native tissue versus polypropylene mesh in studies of mesh currently available on the market.
If we detected substantial heterogeneity, we explored possible explanations in sensitivity analyses. We took statistical heterogeneity into account when interpreting the results, especially if we noted variation in the direction of effect. When concern regarding heterogeneity persisted, we used a random‐effects model.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes, provided data were sufficient (five or more studies), to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether review conclusions would have differed if:
-
a random effects model had been adopted; or
-
the summary effect measure had been odds ratio rather than risk ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEPRO software (GRADEpro GDT 2014), in accordance with Cochrane methods (Higgins 2011). In this table, we evaluated the overall quality of the body of evidence for main review outcomes (awareness of prolapse, repeat surgery for prolapse or stress urinary incontinence, recurrent anterior compartment prolapse, dyspareunia) with regard to the main review comparisons (native tissue vs biological graft, native tissue vs polypropylene mesh, native tissue vs absorbable mesh) by using GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias) (Atkins 2004). We justified, documented and incorporated judgements about evidence quality (high, moderate, low) into reporting of results for each outcome. Two review authors working independently assessed GRADE ratings and resolved disagreements by discussion.
Results
Description of studies
Results of the search
We included in this report full reports on 33 studies (Ali 2006 abstract;Allahdin 2008;Altman 2011; Carey 2009;Colombo 2000;Dahlgren 2011; Delroy 2013; De Ridder 2004 abstract;De Tayrac 2013;El‐Nazer 2007;Farthmann 2012;Feldner 2010; Gandhi 2005; Guerette 2009;Gupta 2014;Hviid 2010; Lamblin 2014;Menefee 2011; Meschia 2007; Minassian 2010 abstract; Natale 2009;Nguyen 2008;Nieminen 2008; Robert 2014;Rudnicki 2014;Sand 2001; Sivaslioglu 2008; Tamanini 2015;Thijs 2010 abstract; Turgal 2013;Vollebregt 2011; Weber 2001;Withagen 2011). Two studies (Ek 2010; Ek 2011) are ancillary reports to Altman 2011, and three trials (Allahdin 2008; Dahlgren 2011; Menefee 2011) contributed to multiple comparisons.
The flow of literature through the assessment process is shown in the PRISMA flow chart (Figure 1).
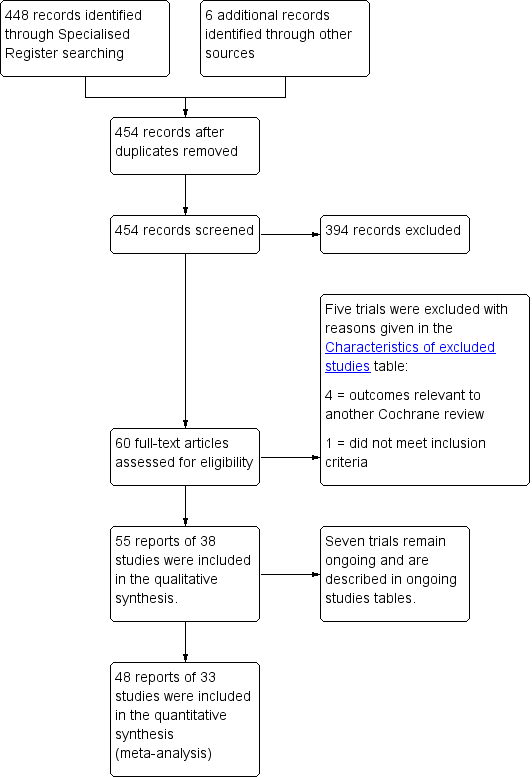
PRISMA study flow diagram for 2016 review.
No study authors reported median follow‐up of less than one year, and the following study authors reported extended reviews: 2 years ‐ Delroy 2013; De Ridder 2004 abstract;Guerette 2009; Meschia 2007;Minassian 2010 abstract;Tamanini 2015; 3 years ‐ Farthmann 2012; Natale 2009; Nieminen 2008; and 5 years ‐ Colombo 2000.
Included studies
Study design and setting
In total, 33 randomised controlled trials were conducted in 15 countries (Italy, USA, Argentina, Australia, Belgium, the Netherlands, Finland, India, Germany, Chile, France, Norway, Denmark, Sweden and Turkey). Fourteen trials were multi‐centre randomised trials (Altman 2011; Dahlgren 2011: De Tayrac 2013; Farthmann 2012; Guerette 2009; Lamblin 2014; Menefee 2011; Meschia 2007; Natale 2009; Nieminen 2008; Rudnicki 2014; Thijs 2010 abstract; Vollebregt 2011; Withagen 2011). All studies used a parallel design.
Participants
We evaluated in this review 33 randomised controlled trials evaluating 3332 women in the surgical management of anterior compartment prolapse.
Interventions
-
Eight articles (Dahlgren 2011; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Menefee 2011; Meschia 2007; Robert 2014) compared native tissue repair anterior colporrhaphy (n = 413) versus various biological grafts (n = 450) and assessed the following interventions. Porcine dermis (Pelvicol) was utilised in four trials (Dahlgren 2011; Hviid 2010; Menefee 2011; Meschia 2007), small intestine submucosa in two (Feldner 2010; Robert 2014), cadaveric fascia lata patch in one (Gandhi 2005) and bovine pericardium collagen in one (Guerette 2009). Meschia 2007 evaluated only primary anterior compartment prolapse, and Dahlgren 2011 included only those who had undergone at least one failed prior surgical intervention in the treated compartment. Hviid 2010 included only those with anterior compartment prolapse and excluded those undergoing concomitant surgery.
-
Sixteen trials (Ali 2006 abstract; Altman 2011; Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Gupta 2014; Lamblin 2014; Menefee 2011; Nieminen 2008; Nguyen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Thijs 2010 abstract; Turgal 2013; Vollebregt 2011) assessed anterior colporrhaphy (n = 926) versus permanent polypropylene mesh (n = 959). Each study evaluated a relatively similar cohort of women; however, the following exclusions were introduced in individual trials: prior graft surgery (Lamblin 2014; Nguyen 2008; Tamanini 2015), concomitant surgery (Altman 2011; Nieminen 2008; Rudnicki 2014), prior prolapse surgery (Rudnicki 2014; Turgal 2013; Vollebregt 2011), urinary incontinence (Turgal 2013) and bladder injury at surgery (De Tayrac 2013).
-
Many of the mesh products that we evaluated have been voluntarily removed from the market, including Prolift (Gynecare/Ethicon, Somerville, NJ, USA) (Altman 2011), Perigee (American Medical Systems, Minnetonka, MN, USA) (Lamblin 2014; Nguyen 2008; Thijs 2010 abstract) and Avaulta (Bard, Covington, LA, USA) (Rudnicki 2014; Vollebregt 2011). In addition, some companies have excluded transvaginal utilisation of mesh products, including Gynemesh PS and Gynecare/Ethicon (Ali 2006 abstract; Carey 2009; El‐Nazer 2007; Gupta 2014).
-
-
Three trials (Allahdin 2008; Sand 2001; Weber 2001) evaluated effects of absorbable polyglactin (Vicryl) mesh inlay used to augment prolapse repair. Data from two trials were aggregated in a meta‐analysis, as they included follow‐up of at least 12 months (Sand 2001; Weber 2001), and the non‐mesh arms from one trial (traditional anterior vaginal wall repair and ultra‐lateral anterior vaginal wall repair) were aggregated for comparison with the mesh arm in one trial (Weber 2001).
-
Four trials compared one type of graft versus another for management of anterior compartment prolapse. Biological graft (Pelvicol) was compared with polypropylene mesh by Menefee 2011 and Natale 2009, and with absorbable mesh (polyglactin) by De Ridder 2004 abstract. Natale included only those with recurrent prolapse and reported three‐year outcomes. Farthmann 2012 compared a conventional polypropylene mesh with lighter‐weight polypropylene mesh with an absorbable coating.
-
Two studies (Colombo 2000; Minassian 2010 abstract) are included in this subgroup. Both compared anterior colporrhaphy and abdominal paravaginal repair/Burch as the interventions. In Colombo 2000, vaginal interventions included vaginal hysterectomy and uterosacral colpopexy as compared with abdominal group abdominal hysterectomy and uterosacral suspension. In Minassian 2010 abstract, the non‐randomised surgery in both groups was a sacral colpopexy.
-
Four trials (Allahdin 2008; Carey 2009; Dahlgren 2011; Withagen 2011) were included in another subgroup. Prior prolapse surgery was an inclusion criterion for Withagen 2011, and prior prolapse surgery in the treated compartment was an inclusion criterion for Dahlgren 2011. Native tissue repair in the anterior or posterior compartment was compared with absorbable mesh in Allahdin 2008, permanent polypropylene mesh in Carey 2009 and Withagen 2011 and porcine dermis in Dahlgren 2011.
Outcomes
Trialists reported a wide variety of prolapse outcomes that broadly followed the outcomes listed under Methods. No studies reported all outcomes, and no studies reported no outcomes.
Fourteen trials (Altman 2011; Dahlgren 2011; Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Gandhi 2005; Gupta 2014; Hviid 2010; Lamblin 2014; Meschia 2007; Nieminen 2008; Robert 2014; Turgal 2013; Vollebregt 2011) reported on awareness of prolapse.
Twelve trials (Altman 2011; Delroy 2013; De Tayrac 2013; Lamblin 2014, Menefee 2011; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Thijs 2010 abstract; Vollebregt 2011) reported on repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome).
Seven trials (Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Feldner 2010; Lamblin 2014; Rudnicki 2014; Tamanini 2015) reported on repeat anterior compartment prolapse on objective examination.
Full details of the included trials are given in the Characteristics of included studies table.
Excluded studies
Overall, we excluded five studies from the review (Heinonen 2011; Kringel 2010; Tincello 2009; Van Der Steen 2011; Weemhoff 2011); details are given in the Characteristics of excluded studies table.
Risk of bias in included studies
Allocation
Fifteen of the RCTs (Allahdin 2008; Altman 2011; Dahlgren 2011; Delroy 2013; El‐Nazer 2007; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Meschia 2007; Minassian 2010 abstract; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Weber 2001) provided sufficient detail by adequately describing the randomisation process and by confirming that secure concealment of the randomisation process was used, for example, allocation by a remote person or by sealed envelopes.
Of the remainder, 11 trials (Carey 2009; De Ridder 2004 abstract; Farthmann 2012; Gupta 2014; Lamblin 2014; Menefee 2011; Sand 2001; Sivaslioglu 2008; Tamanini 2015; Vollebregt 2011; Withagen 2011) utilised computer‐generated number lists, but it was unclear whether allocation was concealed before assignment. Randomisation was performed by drawing lots in De Tayrac 2013, and Tamanini 2015 described a raffle used in the randomisation process. In Colombo 2000, randomisation was appropriate; however, the open randomisation list ensured high risk of allocation bias. Neither randomisation nor allocation concealment was reported in the final two reports presented as abstracts El‐Nazer 2007; Minassian 2010 abstract.
Review authors rated 28 RCTs as having low risk of bias related to sequence generation, four as unclear risk and one as high risk. We rated 14 trials as having low risk of bias related to allocation concealment, 15 as unclear risk and four as high risk.
Blinding
Women and surgeons could not be blinded to the procedure when different surgical routes were compared (Colombo 2000; Delroy 2013; Minassian 2010 abstract; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Tamanini 2015). Three trials (Allahdin 2008; Menefee 2011; Nguyen 2008) blinded participants and the postoperative reviewer. Non‐surgeons conducted outcome assessments in five trials (Allahdin 2008; El‐Nazer 2007; Feldner 2010; Natale 2009; Weber 2001). These findings, which are summarised in Figure 2 show that five trials were at low risk of performance bias, 17 at unclear risk and 11 at high risk. We rated eight trials as having low risk of detection bias, 16 unclear risk and nine high risk.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Incomplete outcome data
Loss to follow‐up was a variable problem, ranging from zero (Allahdin 2008) to 53% in Guerette 2009 (49/93) at two years. Weber 2001 reported a statistically significantly greater loss to follow‐up in one arm of the trial (ultra‐lateral anterior vaginal wall repair). Twenty‐four trials were at low risk of attrition bias, three at uncertain risk and six at high risk.
Selective reporting
Generally, the level of reporting was adequate for one trial (Altman 2011), which did not report the rate of mesh exposure. However, data were supplied as personal communication. We rated all trials as having low risk of bias in this domain.
Other potential sources of bias
Thirteen trials (Allahdin 2008; Altman 2011; Dahlgren 2011; Delroy 2013; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Lamblin 2014; Meschia 2007; Nguyen 2008; Nieminen 2008; Rudnicki 2014) provided a CONSORT flow diagram, randomisation techniques, allocation statements and sample size calculations. These findings are summarised in Figure 3.
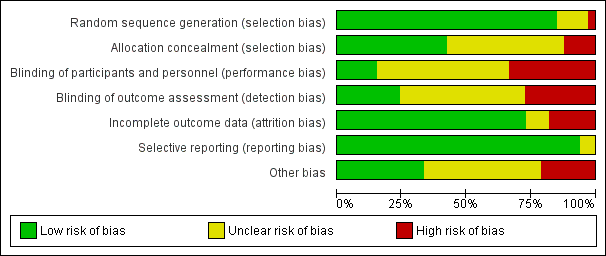
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
All trials except three reported baseline descriptive characteristics that were equally distributed. Sand 2001 reported that previous hysterectomy was more common in the mesh overlay group. Withagen 2011 reported that those in the native tissue group had a greater degree of prolapse than those in the mesh group at point A posterior (Ap), point B posterior (Bp) and genital hiatus (GH), and that prior sacral colpopexy was three times more frequent in the mesh group than in the native tissue group. Lamblin 2014 reported that the rate of hysterectomy performed as concomitant surgery was 77% in the native tissue group versus 33% in the transvaginal polypropylene mesh group (P < 0.001).
All trials but one (De Ridder 2004 abstract) reported preoperative prolapse status, and two trials (Ali 2006 abstract; Sand 2001) did not specifically report equal distribution and severity of prolapse between groups. In one trial (Weber 2001), 7% of women had stage 1 anterior vaginal wall prolapse preoperatively (at the time of inclusion), which would have been classified as a postoperative success.
Effects of interventions
See: Summary of findings for the main comparison Anterior prolapse repair: native tissue versus biological graft in women with anterior compartment pelvic organ prolapse; Summary of findings 2 Anterior prolapse repair: native tissue versus polypropylene mesh for women with anterior compartment pelvic organ prolapse; Summary of findings 3 Anterior prolapse repair: native tissue versus absorbable mesh for women with anterior and/or posterior compartment pelvic organ prolapse
1 Native tissue versus biological graft
Eight trials (Dahlgren 2011; Feldner 2010; Gandhi 2005; Guerette 2009; Hviid 2010; Menefee 2011; Meschia 2007; Robert 2014) compared native tissue repair (anterior colporrhaphy) (n = 384) versus biological grafts (n = 422).
Primary outcomes
1.1 Awareness of prolapse (1 to 2 years)
1.1.1 Native tissue repair (anterior colporrhaphy) versus any biological graft
Women showed no difference in awareness of prolapse between native tissue repair (anterior colporrhaphy) and any biological graft (average RR 0.98, 95% CI 0.52 to 1.82; five RCTs; n = 552; I2 = 39%; low‐quality evidence; Analysis 1.1; Figure 4). These data suggest that if awareness of prolapse after biological graft occurs in 12% of women, then 7% to 23% would be aware of prolapse after native tissue repair (anterior colporrhaphy).
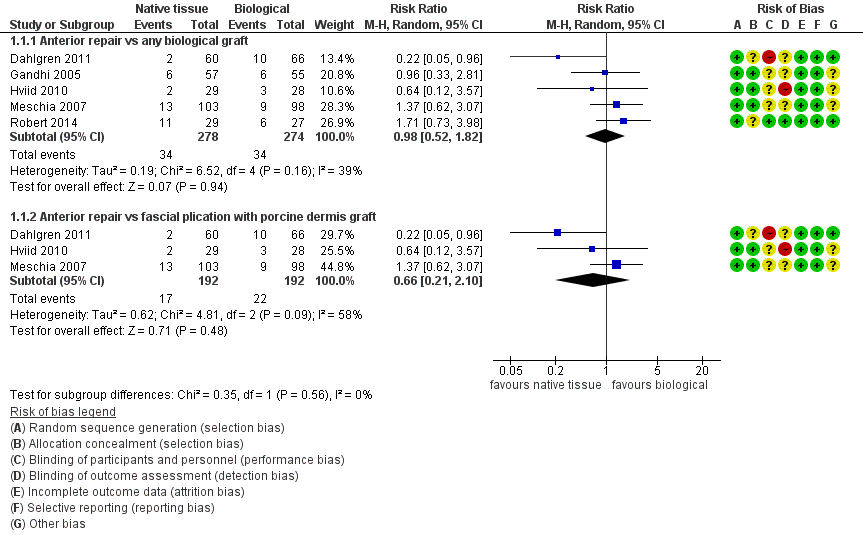
Forest plot of comparison: 1 Native tissue versus biological graft, outcome: 1.1 Awareness of prolapse.
1.1.2 Subgroup analysis by type of graft
Results showed no difference in awareness of prolapse between native tissue repair (anterior colporrhaphy) and biological graft with porcine dermis (average RR 0.66, 95% CI 0.21 to 2.10; three RCTs; n = 384; I2 = 58%; Analysis 1.1). The test for subgroup differences showed no evidence of differences between studies that used porcine dermis and those using other types of biological graft (test for subgroup differences: Chi² = 1.16; df = 1 (P = 0.28); I² = 13.6%).
1.2 Repeat surgery (1 to 2 years)
1.2.1 Repeat surgery for prolapse
We found no evidence of a difference between native tissue repair (anterior colporrhaphy) and biological graft (RR 1.02, 95% CI 0.53 to 1.97; seven RCTs; n = 650; I2 = 0%; moderate‐quality evidence; Analysis 1.2). Data suggest that if repeat prolapse surgery after biological graft was required in 4% of women, then 2% to 9% would undergo repeat prolapse surgery after native tissue repair (anterior colporrhaphy).
1.2.2 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
1.2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
1.3 Recurrent anterior wall prolapse (1 to 2 years)
1.3.1 Anterior compartment
Native tissue repair (anterior colporrhaphy) was associated with increased risk of recurrent anterior wall prolapse compared with biological graft (RR 1.32, 95% CI 1.06 to 1.65; eight RCTs; n = 701; I2 = 26%; moderate‐quality evidence; Analysis 1.3). These data suggest that if recurrent anterior wall prolapse after biological graft occurs in 26% of women, then 27% to 42% would have recurrence after native tissue repair (anterior colporrhaphy).
1.3.2 Subgroup analysis by type of graft
Studies provided no evidence of a difference in recurrent anterior wall prolapse between native tissue repair and porcine dermis repair (RR 1.29, 95% CI 0.98 to 1.70; four RCTs; n = 392; I2 = 51%; Analysis 1.3). The test for subgroup differences showed no evidence of a difference between studies that used porcine dermis and those using other types of biological graft (test for subgroup differences: Chi² = 0.05, df = 1 (P = 0.83); I² = 0%).
Secondary outcomes
1.4 Adverse events
1.4.1 Death (related to surgery)
Studies provided no data for this outcome.
1.4.2 Mesh exposure
Studies provided no data for this outcome.
1.4.3 Injury to bladder (cystotomy)
Studies provided no data for this outcome.
1.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
1.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
1.5 Objective failure
1.5.1 Stage 2 or greater apical compartment prolapse (point C at or beyond 1 cm inside the introitus)
Studies provided no data for this outcome.
1.5.2 Stage 2 or greater posterior vaginal compartment prolapse (point Bp at or beyond 1 cm inside the introitus)
Studies provided no data for this outcome.
1.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
1.5.3.1 Point Ba ‐ Native tissue repair was associated with less pronounced Ba score compared with biological graft as reported in a single trial (MD 0.50, 95% CI 0.02 to 0.98; one RCT; n = 56; Analysis 1.5)
1.5.3.2 Point Bp ‐ Studies provided no data for this outcome
1.5.3.3 Point C ‐ Studies reported no data for this outcome
1.5.3.4 Total vaginal length ‐ Studies provided no data for this outcome
1.6 Bladder function
1.6.1 Stress urinary incontinence (one to two years)
Studies provided no evidence of a postoperative difference in stress urinary incontinence between native tissue repair (anterior colporrhaphy) and biological graft (RR 1.44, 95% CI 0.79 to 2.64; two RCTs; n = 218; I2 = 0%; moderate‐quality evidence; Analysis 1.4). These data suggest that if the rate of stress urinary incontinence is 13% in patients receiving a biological graft, then 10% to 34% have stress urinary incontinence after a native tissue repair.
1.6.2 De novo stress urinary incontinence
Studies provided no data for this outcome.
1.6.3 De novo urge incontinence (one year)
Studies provided no evidence of a postoperative difference in urge incontinence between native tissue repair and biological graft (RR 1.14, 95% CI 0.61 to 2.14; one RCT; n = 201; Analysis 1.6).
1.6.4 Urinary voiding dysfunction (one year)
Studies provided no evidence of a postoperative difference between native tissue repair and biological graft in voiding dysfunction (RR 1.13, 95% CI 0.71 to 1.80; two RCTs; n = 155; I2 = 0%; Analysis 1.7).
1.7 Bowel function
1.7.1 De novo faecal incontinence
Studies provided no data for this outcome.
1.7.2 De novo obstructed defecation
Studies provided no data for this outcome.
1.7.3 Constipation
Studies provided no data for this outcome.
1.8 Sexual function
1.8.1 Dyspareunia (one to two years)
Studies provided no evidence of a difference between native tissue repair and biological graft (RR 0.87, 95% CI 0.39 to 1.93; two RCTs; n= 151; I2 = 0%; low‐quality evidence; Analysis 1.8). Data suggest that if the rate of dyspareunia is 15% after biological graft, then 6% to 29% would have dyspareunia after native tissue repair.
1.8.2 De novo dyspareunia
Studies provided no data for this outcome.
1.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ)
Studies provided no data for this outcome.
1.9 Quality of life and satisfaction
1.9.1 Patient Global Impression of Improvement (PG1‐1) ‐ Studies provided no data for this outcome
1.9.2 Prolapse Quality of Life Questionnaire (PQOL): Studies provided no evidence of a difference in quality of life outcomes between native tissue repair and biological graft when the PQOL was used in a single study (MD ‐1.00, 95% CI ‐6.01 to 4.01; one RCT; n = 56; Analysis 1.9)
1.9.3 Pelvic Floor Distress Inventory (PFDI‐20) ‐ Studies provided no data for this outcome
1.9.4 Pelvic Floor Impact Questionnaire (PFIQ‐7) ‐ Studies provided no data for this outcome
1.10. Measures associated with surgery
1.10.1 Operating time (minutes)
Native tissue repair was associated with reduced operating time compared with use of a biological graft (MD ‐10.35, 95% CI ‐14.45 to 6.24; two RCTs; n = 113; I2 = 42%; Analysis 1.10).
1.10.2 Length of hospital stay
Studies provided no evidence of a difference in length of hospital stay between native tissue repair and biological graft (MD 0.30 days, 95% CI ‐0.09 to 0.69; one RCT; n = 201; Analysis 1.11).
1.10.3 Blood transfusion
Studies provided no data for this outcome.
We have summarised data outcomes in summary of findings Table for the main comparison.
2 Native tissue versus polypropylene mesh
Fifteen trials (Ali 2006 abstract; Altman 2011; Delroy 2013; De Tayrac 2013; El‐Nazer 2007; Gupta 2014;Lamblin 2014;Menefee 2011; Nguyen 2008; Nieminen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Thijs 2010 abstract; Vollebregt 2011) assessed native tissue repair (anterior colporrhaphy) (n = 906) versus use of permanent polypropylene mesh (n = 949). Three studies (Allahdin 2008;Carey 2009;Withagen 2011) included both anterior and posterior prolapse.
Primary outcomes
2.1 Awareness of prolapse (one to three years)
Awareness of prolapse was more likely after native tissue repair (anterior colporrhaphy) than after mesh repair (RR 1.77, 95% CI 1.37 to 2.28; nine RCTs; n = 1133; I2 = 0%; moderate‐quality evidence; Analysis 2.1). This suggests that if awareness of prolapse after polypropylene mesh repair occurs in 13% of women, then 18% to 30% would develop awareness of prolapse after native tissue repair (anterior colporrhaphy) (Figure 5).

Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.1 Awareness of prolapse.
2.2 Repeat surgery (one to three years)
2.2.1 Repeat surgery for prolapse
Repeat surgery for prolapse was more likely after native tissue repair than after mesh repair (RR 2.03, 95% CI 1.15 to 3.58; 11 RCTs; n = 1461; I2 = 39%; moderate‐quality evidence; Analysis 2.2). This suggests that if 2% underwent repeat surgery for prolapse after polypropylene mesh repair, then 2% to 7% would require surgery after native tissue repair (anterior colporrhaphy).
2.2.2 Repeat surgery for stress urinary incontinence (one to three years)
Studies provided no evidence of a difference in the rate of repeat surgery for urinary stress urinary incontinence between native tissue repair (anterior colporrhaphy) and polypropylene mesh repair (RR 1.19, 95% CI 0.60 to 2.36; five RCTs; n = 881; I2 = 30%; Analysis 2.2).
2.2.3 Reoperation rate for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome) was less likely after native tissue repair than after polypropylene mesh repair (RR 0.59, 95% CI 0.41 to 0.83; 12 RCTs; n = 1527; I2 = 45%; Analysis 2.2). This suggests that if 10% underwent subsequent surgery after polypropylene mesh repair, then 4% to 8% would require subsequent surgery after native tissue repair.
2.3 Recurrent anterior wall prolapse (one to three years)
Recurrence of anterior wall prolapse was more likely after native tissue repair than after polypropylene mesh repair (RR 3.01, 95% CI 2.52 to 3.60; 16 RCTs; n = 1976; I2 = 39%; moderate‐quality evidence; Analysis 2.3;Figure 6). This suggests that if recurrent anterior wall prolapse occurred in 13% of women after polypropylene mesh repair, then 32% to 45% would have recurrence after native tissue repair.

Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.
Secondary outcomes
2.4 Adverse events
2.4.1 Death (related to surgery)
Studies provided no data for this outcome.
2.4.2 Mesh exposure
The mesh exposure rate after transvaginal polypropylene mesh was 11.3% (101/896) at one to three years (Table 1).
| Study ID | Mesh exposure | Mesh repairs |
| Al‐Nazer 2007 | 1 | 21 |
| Ali 2006 abstract | 3 | 46 |
| Altman 2011 | 21 | 183 |
| De Tayrac 2013 | 7 | 76 |
| Delroy 2013 | 2 | 40 |
| Gupta 2014 | 4 | 44 |
| Lamblin 2014 | 2 | 33 |
| Menefee 2011 | 5 | 28 |
| Nguyen 2008 | 2 | 37 |
| Nieminen 2008 | 18 | 104 |
| Rudnick 2014 | 12 | 78 |
| Sivaslioglu 2008 | 3 | 43 |
| Tamanini 2014 | 7 | 42 |
| Turgal 2014 | 3 | 20 |
| Thijs 2010 abstract | 9 | 48 |
| Vollebregt 2011 | 2 | 53 |
| Total | 101 | 896 |
| Anterior repair vs absorbable mesh | ||
| Sand 2001 | 0 | 73 |
| Weber 2001 | 1 | 26 |
2.4.3 Injury to bladder (cystotomy)
Intraoperative cystotomy was less likely after native tissue repair (1/416) than after polypropylene mesh repair (11/455) (RR 0.21, 95% CI 0.06 to 0.82; six RCTs; n = 871; I2 = 0%; Analysis 2.4). Wide confidence intervals reflect the low event rates for this outcome.
2.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
2.4.5 Repeat surgery for mesh exposure
The repeat surgery rate for mesh exposure was 7.3% (56/768) at one to three years (Table 2).
| Study ID | Surgery mesh exposure | Mesh repairs |
| Altman 2011 (1) | 6 | 183 |
| De Tayrac 2013 (2) | 4 | 76 |
| Delroy 2013 (3) | 2 | 40 |
| Gupta 2014 (4) | 2 | 44 |
| Nguyen 2008 (5) | 2 | 37 |
| Nieminen 2008 (6) | 14 | 104 |
| Rudnick 2014 (7) | 5 | 78 |
| Sivaslioglu 2008 (8) | 3 | 43 |
| Tamanini 2014 (9) | 7 | 42 |
| Turgal 2014 | 5 | 20 |
| Thijs 2010 abstract (10) | 4 | 48 |
| Vollebregt 2011 (11) | 2 | 53 |
| Total | 56 | 768 |
2.5 Objective failure
2.5.1 Stage 2 or greater apical compartment prolapse
Studies provided no data for this outcome.
2.5.2 Stage 2 or greater posterior vaginal compartment prolapse
Studies provided no data for this outcome. Subsequent prolapse in the posterior or apical compartment was less likely after native tissue repair than after polypropylene mesh repair (RR 0.54, 95% CI 0.30 to 0.99; two RCTs; n = 300; I2 = 0%; Analysis 2.5). This suggests that if 18% of women developed prolapse in the apical or posterior compartment on examination after polypropylene mesh repair, then 5% to 18% would develop apical or posterior compartment prolapse after native tissue repair.
2.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
2.5.3.1 Point Ba (one to two years) ‐ Anatomical assessment based on POPQ revealed less support at point Ba (mid‐anterior vaginal wall) after native tissue repair than after polypropylene mesh (average MD 0.55 cm, 95% CI 0.30 to 0.80; six RCTs; n = 568; I2 = 56%; Analysis 2.6).
2.5.3.2 Point Bp (one to two years) ‐ Studies provided no evidence of a difference between native tissue repair and mesh repair for anatomical assessment based on POPQ at point Bp (average MD ‐0.43 cm, 95% CI ‐0.92 to 0.06; four RCTs; n = 355; I2 = 70%; Analysis 2.6).
2.5.3.3 Point C (one to two years) ‐ Studies provided no evidence of a difference between native tissue repair and mesh repair for anatomical assessment based on POPQ at point C (vaginal apex) (MD 0.27 cm, 95% CI ‐0.47 to 1.01; four RCTs; n = 369; I2 = 82%; Analysis 2.6).
2.5.3.4 Total vaginal length (one to two years) ‐ Studies reported no difference between native tissue repair and mesh repair for anatomical assessment based on total vaginal length (MD ‐0.18, 95% CI ‐0.78 to 0.43; three RCTs; n = 366; I2 = 69%; Analysis 2.6).
2.6 Bladder function
2.6.1 Stress urinary incontinence (one to three years)
Studies provided no data for this outcome.
2.6.2 De novo stress urinary incontinence
Native tissue repair was associated with a reduction in de novo urinary stress incontinence compared with mesh repair (RR 0.67, 95% CI 0.44 to 1.01; six RCTs; n = 957; I2 = 26%; moderate‐quality evidence; Analysis 2.7). This suggests that if after mesh repair 10% developed de novo stress urinary incontinence, then 5% to 10% would develop de novo stress urinary incontinence after native tissue repair.
2.6.3 Urge incontinence (one year)
Studies provided no evidence of postoperative differences between groups in rate of urge incontinence (RR 2.20, 95% CI 0.33 to 14.68; two RCTs; n = 198; I2 = 0%; Analysis 2.10). Caution is advised in interpreting these results owing to low event rates and wide confidence intervals crossing the line of no effect, suggesting imprecision.
2.6.4 Urinary voiding dysfunction (one to two years)
Studies provided no evidence of postoperative differences between groups in rate of voiding dysfunction (RR 1.22, 95% CI 0.33 to 4.47; three RCTs; n = 277; I2 = 15%; Analysis 2.9). Caution is advised in interpreting these results owing to low event rates and wide confidence intervals crossing the line of no effect, suggesting imprecision.
2.7 Bowel function
Studies provided no data for this outcome.
2.8 Sexual function
2.8.1 De novo dyspareunia (one to three years)
Studies provided no evidence of differences between groups in rate of de novo dyspareunia between native tissue repair and mesh repair (RR 0.54, 95% CI 0.27 to 1.06; eight RCTs; n = 583; I2 = 0%; moderate‐quality evidence; Analysis 2.8). This suggests that if 7% developed de novo dyspareunia after mesh repair, then 2% to 8% would experience de novo dyspareunia after native tissue repair.
2.8.2 Dyspareunia (one to two years)
Studies provided no evidence of a postoperative difference in rate of dyspareunia between native tissue repair and mesh repair (RR 1.06, 95% CI 0.59 to 1.90; eight RCTs; n = 1096; I2 = 5%; Analysis 2.11).
2.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ) (one year)
Studies provided no evidence of differences between groups on the PISQ (MD ‐0.06, 95% CI ‐0.76 to 0.64; four RCTs; n = 741; I2 = 25%; Analysis 2.12.4).
2.9 Quality of life and satisfaction
Quality of life questionnaires: Only four of the 15 studies (Ali 2006 abstract; El‐Nazer 2007; Gupta 2014; Vollebregt 2011) did not report a validated quality of life outcome.
2.9.1 Prolapse Quality of Life questionnaire (PQOL) (one to two years)
Studies provided no evidence of differences in PQOL scores between groups (MD 1.09, 95% CI ‐1.19 to 3.37; two RCTs; n = 164; I2 = 0%; Analysis 2.12).
2.9.2 Pelvic Floor Impact Questionnaire (PFIQ‐7) (one to two years)
Studies provided no evidence of differences between groups on the PFIQ‐7 (average MD 1.90, 95% CI ‐7.78 to 11.59; three RCTs; n = 290; I2 = 69%; Analysis 2.12).
2.9.3 Pelvic Floor Distress Inventory (PFDI‐20) (one to two years)
Studies provided no evidence of differences between groups on the PFDI‐20 (average MD 3.89, 95% CI ‐12.82 to 20.61; three RCTs; n = 294; I2 = 86%; Analysis 2.12.3).
2.9.4 International Consultation on Incontinence Modular Questionnaire (ICIQ) (one to two years)
Studies provided no evidence of differences between groups on the ICIQ (MD 0.70, 95% CI ‐0.15 to 1.55; one RCT; n = 92; Analysis 2.12) or in ICIQ vaginal symptoms (MD 1.10, 95% CI ‐0.88 to 3.08; one RCT; n = 92; Analysis 2.12).
2.10 Perioperative outcomes
2.10.1 Operating time
Operating time was shorter after native tissue repair (anterior colporrhaphy) than after polypropylene mesh repair (MD ‐17.89 minutes, 95% CI ‐25.81 to ‐9.98; seven RCTs; n = 1099; I2 = 91%). Because of heterogeneity, we have not reported the data in a meta‐analysis (Analysis 2.14).
2.10.2 Hospital stay
Studies provided no evidence of differences between groups in hospital stay (MD 0.08 days, 95% CI ‐0.17 to 0.33; five RCTs; n = 707; I2 = 67%; Analysis 2.13).
2.10.3 Transfusion
Blood transfusion was less likely after native tissue repair than after mesh repair (RR 0.42, 95% CI 0.24 to 0.76; four RCTs; n = 486; I2 = 42%; Analysis 2.15).
We have summarised study findings in summary of findings Table 2.
Subgroup analysis by market availability
We conducted a post hoc subgroup analysis for our primary outcomes that was restricted to studies of meshes currently on the market. This did not change our main findings with respect to awareness of prolapse (RR 2.12, 95% CI 1.35 to 3.35; nine RCTs; n = 518; I2 = 0%; Analysis 3.1), repeat surgery for prolapse (RR 2.34, 95% CI 1.20 to 4.59; 12 RCTs; n = 815; I2 = 31%; Analysis 3.2.1) or prolapse on examination (RR 2.35, 95% CI 1.83 to 3.01; 16 RCTs; n = 970; I2 = 31%; Analysis 3.3). However, rates of repeat surgery for prolapse, stress urinary incontinence or mesh exposure were no longer significantly different between groups (RR 0.82, 95% CI 0.55 to 1.24; 12 RCTs; n = 648; I2 = 51%; Analysis 3.2.3).
3 Native tissue compared with absorbable mesh
Three trials (Allahdin 2008; Sand 2001; Weber 2001) evaluated the effects of using absorbable polyglactin (Vicryl) mesh inlay to augment prolapse repairs.
Primary outcomes
3.1 Awareness of prolapse (two years)
Studies provided no evidence of a difference in awareness of prolapse at the two‐year review between native tissue repair and absorbable mesh (RR 0.95, 95% CI 0.70 to 1.31; one RCT; n = 54; very low‐quality evidence). We downgraded evidence for attrition bias, imprecision and publication bias. Evidence suggests that if awareness of prolapse after absorbable mesh repair occurred in 76% of women, then 53.2% to 99.6% would develop awareness of prolapse after native tissue repair (anterior colporrhaphy). See summary of findings Table 3.
3.2 Repeat surgery (two years)
3.2.1 Repeat surgery for prolapse
Studies provided no evidence of a difference in the need for repeat surgery for prolapse at the two‐year review between native tissue repair and absorbable mesh (RR 2.13, 95% CI 0.42 to 10.82; one RCT; n = 66; very low‐quality evidence). We downgraded evidence for attrition bias, imprecision and publication bias. The evidence suggests that if repeat surgery for prolapse were required after absorbable mesh repair in 5.9% of women, then 2.5% to 63.6% would require repeat surgery after native tissue repair (anterior colporrhaphy). See summary of findings Table 3.
3.2.2 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
3.2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
3.3 Recurrent anterior prolapse (one to three years)
Recurrent anterior wall prolapse was more likely after native tissue repair than after absorbable mesh repair (RR 1.50, 95% CI 1.09 to 2.06; three RCTs; n = 268; I2 = 0%; moderate‐quality evidence; Analysis 4.3). We downgraded evidence for attrition bias. The evidence suggests that if the rate of anterior wall prolapse after absorbable mesh repair were 26.7%, then 29.1% to 55% would have recurrent anterior wall prolapse after native tissue repair. See summary of findings Table 3.
Secondary outcomes
3.4. Adverse events
3.4.1 Death (related to surgery)
Two trials (Allahdin 2008; Weber 2001) reported no events of death in native tissue or absorbable mesh groups (Analysis 4.4).
3.4.2 Mesh exposure
Two trials (Sand 2001; Weber 2001) reported one vaginal polyglactin mesh erosion (1/99; 1%; Table 1), and in Allahdin 2008, two women needed partial removal of mesh (2/32; 6.1%) for undisclosed reasons.
3.4.3 Injury to bladder (cystotomy)
Studies provided no data for this outcome.
3.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
3.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
3.5 Objective failure
3.5.1 Stage 2 or greater apical compartment prolapse
Studies provided no data for this outcome.
3.5.2 Stage 2 or greater posterior vaginal compartment prolapse
Studies provided no evidence of a difference between native tissue repair and absorbable mesh repair for posterior compartment prolapse (RR 0.88, 95% CI 0.31 to 2.49; one RCT; n = 132).
3.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
Studies provided no data for this outcome.
3.6 Bladder function
3.6.1 Stress urinary incontinence
Studies provided no evidence of a difference between native tissue repair and absorbable mesh for stress urinary incontinence (RR 0.72, 95% CI 0.50 to 1.05; one RCT; n = 49; very low‐quality evidence). We downgraded evidence for attrition bias, imprecision and publication bias. The evidence suggests that if stress urinary incontinence after absorbable mesh repair occurred in 81.8% of women, then 40.9% to 85.9% would have stress urinary incontinence after native tissue repair (anterior colporrhaphy). See summary of findings Table 3.
3.6.2 De novo stress urinary incontinence
Studies provided no data for this outcome.
3.6.3 De novo bladder overactivity or urge incontinence
Studies provided no data for this outcome.
3.6.4 Urinary voiding dysfunction
Studies provided no data for this outcome.
3.7 Bowel function
Studies provided no data for this outcome.
3.8 Sexual function
Studies provided no data for this outcome.
3.9 Quality of life and satisfaction
3.9.1 Non‐validated quality of life visual analogue scale (0 to 10)
Studies provided no evidence of a difference in quality of life visual analogue score between native tissue and absorbable mesh groups (MD 0.00, 95% CI ‐2.82 to 2.82; one RCT; n = 54; very low‐quality evidence). We downgraded the evidence for attrition bias, imprecision and publication bias. See summary of findings Table 3. We noted no difference in quality of life scores between the two groups.
3.10 Measures associated with surgery
Studies provided no data for this outcome.
4 One graft versus another graft for anterior compartment prolapse
Four trials compared one type of graft versus another for management of anterior compartment prolapse. Menefee 2011 and Natale 2009 compared biological graft (Pelvicol) versus polypropylene mesh, and De Ridder 2004 abstract compared biological graft versus absorbable mesh (polyglactin). Natale 2009 included only those with recurrent prolapse and reported three‐year outcomes. Farthmann 2012 compared a conventional polypropylene mesh versus a lighter‐weight polypropylene mesh with an absorbable coating.
Primary outcomes
4.1 Awareness of prolapse
Studies provided no evidence of a difference in awareness of prolapse after polypropylene mesh and biological graft in a single study (RR 0.98, 95% CI 0.20 to 4.73; one RCT; n = 190; very low‐quality evidence; Analysis 5.1). We downgraded the evidence for inadequate methodological reporting, imprecision and publication bias. Evidence suggests that if after a biological graft 3.2% of women were aware of prolapse, then 0.6% to 15.1% would be aware of prolapse after use of polypropylene mesh.
4.2 Repeat surgery (two years)
4.2.1 Repeat surgery for prolapse
Studies provided no evidence of differences in the rate of repeat surgery for prolapse between groups (RR 3.05, 95% CI 0.87 to 10.73; two RCTs; n = 315; I2 = 0%; very low‐quality evidence; Analysis 5.2). We downgraded the evidence for inadequate methodological reporting and imprecision. Evidence suggests that if repeat surgery for prolapse was required in 1.9% of women after a biological graft, then 1.7% to 20.5% would require repeat surgery after use of polypropylene mesh.
4.2.2 Surgery for stress urinary incontinence
Studies provided no data for this outcome.
4.2.3 Surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
4.3 Recurrent anterior prolapse
4.3.1 Recurrent anterior wall compartment prolapse
Overall, studies provided no evidence of a difference between mesh (permanent or absorbable) and a biological graft (RR 1.38, 95% CI 0.28 to 6.85; two RCTs; n = 315; very low‐quality evidence; Analysis 5.3). We downgraded the evidence for inadequate methodological reporting, imprecision and inconsistency. Evidence suggests that if recurrent anterior compartment prolapse occurred in 29.9% of women after biological graft, then 8.4% to 100% would have recurrent anterior compartment prolapse after use of polypropylene mesh.
The test for subgroup differences was significant (Chi² = 11.35, df = 1 (P = 0.0008), I² = 91.2%). Recurrent anterior wall prolapse after polypropylene mesh (32/124; 26%) was less than after porcine dermis (53/120; 44%) (RR 0.64, 95% CI 0.43 to 0.96; one RCT; n = 190; Analysis 5.3). The rate of recurrent anterior wall prolapse after absorbable mesh was higher than after a biological graft (RR 3.22, 95%CI 1.38 to 7,52; one RCT; n = 125; Analysis 5.3).
Secondary outcomes
4.4 Adverse events
4.4.1 Death (related to surgery)
Studies provided no data for this outcome.
4.4.2 Mesh exposure
Mesh exposure was less in the polypropylene mesh group (no events) than in the biological graft group (RR 0.09, 95% CI 0.01 to 0.69; two RCTs; n = 241; I2 = 0%; Analysis 5.4).
4.4.3 Injury to bladder (cystotomy)
Studies provided no data for this outcome.
4.4.4 Injury to bowel (enterotomy)
Studies provided no data for this outcome.
4.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
4.5 Objective failure
Studies provided no data for this outcome.
4.6 Bladder function
4.6.1 Stress urinary incontinence
Studies provided no evidence of a difference between polypropylene mesh and biological graft groups for stress urinary incontinence (RR 1.96, 95% CI 0.18 to 21.23; one RCT; n = 190; Analysis 4.5; very low‐quality evidence). We downgraded the evidence for inadequate methodological reporting, imprecision and publication bias. If stress urinary incontinence occurred in 1.1% of women after biological graft, then 0.2% to 22.6% would experience stress urinary incontinence after use of polypropylene mesh.
4.6.2 De novo stress urinary incontinence
Studies provided no data for this outcome.
4.6.3 De novo bladder overactivity or urge incontinence
Studies provided no evidence of a difference between polypropylene mesh and biological graft groups for this outcome (RR 0.47, 95% CI 0.05 to 4.78; one RCT; n = 37; Analysis 4.6).
4.6.4 Urinary voiding dysfunction
Studies provided no data for this outcome.
4.7 Bowel function
4.7.1 De novo faecal incontinence ‐ Studies provided no data for this outcome
4.7.2 De novo obstructed defecation ‐ Studies provided no data for this outcome
4.7.3 Constipation ‐ Studies provided no data for this outcome
4.8 Sexual function
4.8.1 Dyspareunia
Studies provided no evidence of a difference between polypropylene mesh and biological graft groups for this outcome (RR 0.82, 95% CI 0.37 to 1.80; one RCT; n = 190).
4.8.2 De novo dyspareunia
Studies provided no data for this outcome.
4.8.3 Prolapse and Incontinence Sexual Questionaire (PISQ)
Studies provided no data for this outcome.
4.9 Quality of life and satisfaction
Studies provided no data for this outcome.
4.10. Measures associated with surgery
Studies provided no data for this outcome.
5 Vaginal repair versus abdominal repair for anterior compartment prolapse
Two studies (Colombo 2000; Minassian 2010 abstract) were included in this subgroup. Both compared anterior colporrhaphy and abdominal paravaginal repair/Burch as the interventions. In Colombo 2000, vaginal interventions included vaginal hysterectomy and uterosacral colpopexy as compared with abdominal group abdominal hysterectomy and uterosacral suspension. In Minassian 2010 abstract, the non‐randomised surgery performed in both groups was a sacral colpopexy.
Primary outcomes
5.1. Awareness of prolapse
Studies provided no data for this outcome.
5.2. Repeat surgery
Studies provided no data for this outcome.
5.3 Recurrent anterior prolapse
Studies provided no evidence of a difference in recurrent anterior compartment prolapse between vaginal and abdominal surgery groups (average RR 0.31, 95% CI 0.03 to 3.46; two RCTs; n = 118; I2 = 81%; Analysis 6.1; very low‐quality evidence). We downgraded the evidence for high risk of bias, imprecision and inconsistency. Evidence suggests that if recurrent anterior compartment prolapse occurred in 36.7% of women in the abdominal repair group, then 1.1% to 100% of women in the vaginal repair group would have recurrent anterior compartment prolapse.
Secondary outcomes
5.4 Adverse events
5.4.1 Death (related to surgery)
Studies provided no data for this outcome.
5.4.2 Mesh exposure
Studies provided no data for this outcome.
5.4.3 Injury to bladder
Studies provided no evidence of a difference between vaginal and abdominal repair groups for bladder injury (RR 0.97, 95% CI 0.06 to 14.88; one RCT; n = 67; Analysis 6.2; very low‐quality evidence). We downgraded the evidence for high risk of bias, imprecision and publication bias. Evidence suggests that if bladder injury occurred in 3% of women after abdominal repair, then 0.2% to 45.1% of women in the vaginal repair group would have a bladder injury.
5.4.4 Injury to bowel
Studies provided no data for this outcome.
5.4.5 Repeat surgery for mesh exposure
Studies provided no data for this outcome.
5.5 Objective failure
5.5.1 Stage 2 or greater apical compartment prolapse (point C at or beyond 1 cm inside the introitus)
Studies provided no data for this outcome.
5.5.2 Stage 2 or greater posterior vaginal compartment prolapse (point Bp at or beyond 1 cm inside the introitus)
Studies provided no evidence of a difference in the rate of posterior compartment prolapse between groups (RR 1.81, 95% CI 0.17 to 19.65; two RCTs; n = 118; I2 = 65%; Analysis 6.3).
5.5.3 Pelvic Organ Prolapse Quantification (POPQ) system scores
5.5.3.1 Point Ba on POPQ ‐ Studies provided no evidence of a difference between vaginal and abdominal repair groups for point Ba (MD 0.90, 95% CI ‐0.15 to 1.95; one RCT; n = 50; Analysis 6.4)
5.5.3.2 Point Bp on POPQ ‐ Studies provided no data for this outcome
5.5.3.3 Point C on POPQ ‐ Studies provided no data for this outcome
5.5.3.4 Total vaginal length (TVL) in cm (range 0 to 14 cm): Total vaginal length was longer after vaginal repair than after abdominal repair (MD 3.20 cm, 95% CI 2.58 to 3.82; one RCT; n = 68; Analysis 6.4).
5.6 Bladder function
Studies provided no data for this outcome.
5.7. Bowel function
Studies provided no data for this outcome.
5.8 Sexual function
5.8.1 Dyspareunia
Dyspareunia was more likely after vaginal anterior colporrhaphy than after paravaginal/Burch (3/49; 6%) (RR 5.17, 95% CI 1.63 to 16.35; two RCTs; n = 97; I2 = 0%; Analysis 6.5).
5.8.2 De novo dyspareunia
Studies provided no data for this outcome.
5.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ)
Studies provided no evidence of a difference between vaginal and abdominal repair in PISQ score (MD ‐2.00, 95% CI ‐6.24 to 2.24; one RCT; n = 50; Analysis 6.6).
5.9 Quality of life and satisfaction
5.9.1 Pelvic Floor Impact Questionnaire (PFIQ‐7)
Studies provided no evidence of a difference between vaginal and abdominal repair in PFIQ‐7 scores (MD ‐9.00, 95% CI ‐52.11 to 34.11; one RCT; n = 50; Analysis 6.6).
5.10 Measures associated with surgery
5.10.1 Operating time (minutes)
Studies provided no evidence of a difference in operating time between vaginal and abdominal repair groups (MD 16.00 minutes, 95% CI ‐24.48 to 56.48; one RCT; n = 67; Analysis 6.7).
5.10.2 Length of hospital stay
Studies provided no data for this outcome.
5.10.3 Blood transfusion
Studies provided no evidence of differences in risk of needing a blood transfusion between vaginal and abdominal repair groups (RR 0.97, 95% CI 0.06 to 14.88; one RCT; n = 67; Analysis 6.8).
6 Native tissue repair versus graft repair for anterior and/or posterior compartment prolapse
Four trials (Allahdin 2008; Carey 2009; Dahlgren 2011; Withagen 2011) evaluated these interventions. Prior prolapse surgery was an inclusion criterion for Withagen 2011, and prior prolapse surgery in the treated compartment was an inclusion criterion for Dahlgren 2011. Allahdin 2008 compared native tissue repair in the anterior or posterior compartment versus absorbable mesh, Carey 2009 and Withagen 2011 versus permanent polypropylene mesh and Dahlgren 2011 versus porcine dermis.
Carey 2009 and Withagen 2011 were similar enough in inclusion criteria and interventions undertaken to be included in a meta‐analysis comparing native tissue repair in the anterior and/or posterior vagina versus use of transvaginal polypropylene mesh.
Primary outcomes
6.1 Awareness of prolapse
When anterior and/or posterior repair was compared with polypropylene mesh, studies provided no evidence of a difference between groups in awareness of prolapse (average RR 0.85, 95% CI 0.36 to 1.99; three RCTs; n = 406; I2 = 59%; very low‐quality evidence; Analysis 7.1). We downgraded the evidence for high risk of bias and inconsistency. Evidence suggests that if 15.5% of women had awareness of prolapse after mesh repair, then 5.6% to 30.9% would have awareness of prolapse after native tissue repair.
6.2 Repeat surgery
6.2.1 Repeat surgery for prolapse
When anterior and/or posterior repair was compared with polypropylene mesh, investigators reported no difference in repeat surgery rate for prolapse between groups (RR 2.09, 95% CI 0.06 to 5.48; three RCTs; n = 416; I2 = 18%; very low‐quality evidence; Analysis 7.2). We downgraded the evidence for high risk of bias and imprecision. Evidence suggests that if 2.4% of women had repeat surgery for prolapse after mesh repair, then 1.9% to 13.1% would require repeat surgery after native tissue repair.
6.2.2 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
6.2.3 Repeat surgery for prolapse, stress urinary incontinence or mesh exposure (composite outcome)
Studies provided no data for this outcome.
6.3 Recurrent anterior wall prolapse (stage 2 or higher)
When anterior colporrhaphy was compared with polypropylene mesh, studies provided no evidence of a difference between groups in rates of recurrent anterior wall prolapse on examination (RR 1.03, 95% CI 0.60 to 1.40; three RCTs; n = 367; I2 = 0%; low‐quality evidence; Analysis 7.3). We downgraded the evidence for high risk of bias. Evidence suggests that if 27% of women had recurrent anterior wall prolapse after mesh repair, then 20.5% to 37.8% would have recurrent anterior wall prolapse after native tissue repair.
Secondary outcomes
6.4 Adverse events
6.4.1 Death (related to surgery)
Studies provided no data for this outcome.
6.4.2 Mesh exposure
The mesh exposure rate was 12.3% (18/146; Table 3).
| Study ID | Mesh exposure | Mesh repairs |
| Carey 2009 | 4 | 63 |
| Withagen 2011 | 14 | 83 |
| Total | 18 | 146 |
6.4.3 Injury to bladder
Studies provided no evidence of a difference between groups in rate of cystotomy (bladder injury) (RR 0.20, 95% CI 0.01 to 4.01; one RCT; n = 166; Analysis 6.4).
6.4.4 Injury to bowel
Studies provided no data for this outcome.
6.4.5 Repeat surgery for mesh exposure
Repeat surgery for mesh exposure was undertaken in 5.6% (8/146; Table 4).
| Study ID | Reoperation mesh exposure | Mesh repairs |
| Carey 2009 | 3 | 63 |
| Withagen 2011 | 5 | 83 |
| Total | 8 | 146 |
6.5 Objective failure
A single study (Withagen 2011) demonstrated significantly improved outcomes for POPQ points Ba and Bp as compared with native tissue repair; however, these data were reported as median values and could not be included in the meta‐analysis.
6.6 Bladder function
6.6.1 Stress urinary incontinence
Studies provided no data for this outcome.
6.6.2 De novo stress urinary incontinence
Studies provided no evidence of a difference between groups for de novo stress urinary incontinence as reported in one study (RR 0.98, 95% CI 0.34 to 2.85; one RCT; n = 105; Analysis 7.5).
6.6.3 Repeat surgery for stress urinary incontinence
Studies provided no data for this outcome.
6.6.4 De novo bladder overactivity or urge incontinence
Studies provided no data for this outcome.
6.6.5 Urinary voiding dysfunction
Studies provided no data for this outcome.
6.7 Bowel function
Studies provided no data for this outcome.
6.8 Sexual function
6.8.1 Dyspareunia
Studies provided no evidence of a difference between groups in rate of persistent dyspareunia (RR 1.03, 95% CI 0.70 to 1.52; one RCT; n = 122; Analysis 7.6).
6.8.2 De novo dyspareunia
Studies reported no difference between groups in rate of de novo dyspareunia (RR 1.23, 95% CI 0.64 to 2.36; two RCTs; n = 188; I2 = 0%; low‐quality evidence; Analysis 7.6). We downgraded the evidence for high risk of bias. Evidence shows that if 14.1% of women experienced de novo dyspareunia after mesh repair, then 9.1% to 33.4% would experience de novo dyspareunia after native tissue repair.
6.8.3 Prolapse and Incontinence Sexual Questionnaire (PISQ)
Studies provided no evidence of a difference between groups in PISQ scores (MD 0.40, 95% CI ‐2.74 to 3.54; one RCT; n = 60; Analysis 7.7).
6.9 Quality of life and satisfaction
Studies provided no data for this outcome.
6.10 Measures associated with surgery
Studies provided no data for this outcome.
Additional analyses
We conducted our planned sensitivity analyses for the primary outcomes. These did not substantially differ from our main findings.
We constructed a funnel plot for analysis of data from more than 10 studies. We found no strong suggestion of publication bias. See Figure 7 for a funnel plot of Analysis 2.3.

Funnel plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.
Discussion
Summary of main results
Available information on the repair of anterior compartment prolapse is increasing. Evidence generally is not supportive of the use of absorbable mesh or biological grafts as compared with native tissue for repair (anterior colporrhaphy) of anterior compartment prolapse. Two trials (Colombo 2000; Minassian 2010 abstract) compared anterior colporrhaphy and retropubic suspensions (paravaginal/Burch colposuspension) for anterior compartment prolapse and demonstrated similar outcomes for recurrent prolapse; however, the rate of dyspareunia was lower after the abdominal approach was used. These results should be viewed with caution, as the baseline non‐randomised intervention was different in each trial.
Sixteen trials demonstrated advantages of using a polypropylene mesh as compared with native tissue for repair, including lower rates of awareness of prolapse, of anterior wall prolapse on examination and of rate of repeat surgery for prolapse. Disadvantages of mesh utilisation include longer operating time, higher rates of cystotomy, the need for transfusion, de novo stress urinary incontinence and subsequent prolapse of apical or posterior vaginal compartments. The rate of mesh erosion was 11.3%, and 7% required surgery to manage the mesh exposure. The total rate of repeat surgery for prolapse, stress urinary incontinence, mesh exposure or pain was significantly higher after use of transvaginal permanent mesh (10.0%) than after native tissue repair (5.7%). The rate of postoperative dyspareunia was similar in the two groups. Investigators noted no differences between groups on validated pelvic floor dysfunction questionnaires, including the Prolapse and Incontinence Sexual Questionnaire (PISQ), the Prolapse Quality of Life questionnaire (PQOL), the Pelvic Floor Distress Inventory (PFDI‐20), the Urinary Distress Inventory (UDI) and the Pelvic Floor Impact Questionnaire (PFIQ). The quality of evidence supporting these findings ranged from low to moderate.
In conclusion, data demonstrate a clear risk/benefit analysis that can help to guide clinicians and patients in deciding whether to utilise transvaginal polypropylene mesh as compared with native tissue for anterior compartment prolapse repair. Since 2011, some of the mesh products evaluated in this section, including Avaulta (Bard), the Gynemesh overlay, Prolift (Ethicon) and the AMS mesh kit (Perigee), have been removed from the market. However, separate evaluation of primary outcomes with products that remain available for use reveals little change in outcome measures except that rates of repeat surgery for prolapse, stress urinary incontinence and mesh exposure have been evaluated under the auspices of randomised controlled trials. Newer, lighter‐weight mesh implants available for the management of anterior wall prolapse have not been evaluated under the auspices of a randomised controlled trial.
Overall completeness and applicability of evidence
The quality of the 33 trials that addressed the surgical management of anterior vaginal compartment prolapse was variable but is improving. All trials published over the past four years included a CONSORT flow statement, and all reported some form of objective evaluation of anterior vaginal support for the specific pelvic floor defect that was repaired, but full vaginal site‐specific outcomes were available for only 12 trials (Altman 2011; Colombo 2000; Delroy 2013; De Tayrac 2013; Farthmann 2012; Menefee 2011; Nguyen 2008; Rudnicki 2014; Sivaslioglu 2008; Tamanini 2015; Vollebregt 2011; Weber 2001). No trials reported outcomes for less than 12 months. Delroy 2013; De Ridder 2004 abstract;Guerette 2009; Lamblin 2014;Meschia 2007;Minassian 2010 abstract and Tamanini 2015 reported two‐year data; Farthmann 2012; Natale 2009 and Nieminen 2008 three‐year data; and Colombo 2000 five‐year data.
Although investigators generally performed randomisation by using a computer‐generated randomisation list, recent trials have reported drawing lots (De Tayrac 2013) and conducting a raffle (Tamanini 2015). Nineteen trials (Allahdin 2008; Altman 2011; Dahlgren 2011; Delroy 2013; El‐Nazer 2007; Feldner 2010; Gandhi 2005; Guerette 2009; Gupta 2014; Hviid 2010; Lamblin 2014; Meschia 2007; Minassian 2010 abstract; Natale 2009; Nguyen 2008; Nieminen 2008; Rudnicki 2014;Sivaslioglu 2008; Weber 2001) reported allocation concealment. Few trials have reported clearly on whether participants and/or reviewers were blinded.
Generally, reporting on the impact of surgery on bladder and sexual function is improving; however, great variation is evident in trialists' choice of outcomes measures. Recent studies (Altman 2011; Delroy 2013; Farthmann 2012;Feldner 2010; Lamblin 2014;Nguyen 2008;Rudnicki 2014;Sivaslioglu 2008; Tamanini 2015;Thijs 2010 abstract; Vollebregt 2011; Withagen 2011) usually include a validated pelvic floor quality of life outcome and report data suitable for meta‐analysis (mean and standard deviation). No trialists have provided a cost analysis.
To minimise risk of bias, it is preferable if surgeons who design studies do not have a financial relationship with the company whose product is being evaluated. Unfortunately, for several studies in this review (Altman 2011; Carey 2009; De Tayrac 2013; Farthmann 2012; Guerette 2009; Withagen 2011), this conflict was feasible and was exacerbated by lack of assurance that reviewers were blinded, resulting in possibly heightened risk of bias for reported outcomes.
Although the rate of mesh exposure reported in this review is consistent with previous reports, adverse events of vaginal pain and/or dyspareunia were commonly reported to the Food and Drug Administration (FDA) in America, and this ultimately triggered the FDA 2011 transvaginal mesh alert that resulted in voluntary removal of many permanent meshes from the market. Among the 15 included trials that evaluated permanent transvaginal mesh only De Tayrac 2013 reported the outcome of vaginal pain on examination at one year, at a rate of 18% in the mesh group as compared with 9% in the native tissue repair group. No reports among the nearly 1000 cases of transvaginal permanent anterior mesh repair described in this review described subsequent surgery performed for vaginal pain and/or dyspareunia.
Quality of the evidence
The quality of evidence in comparisons of native tissue repair versus use of biological grafts is low to moderate owing to risk of bias and imprecision, with lack of reporting of study methods, especially blinding status of reviewers and participants, and unclear risk of data attrition. Low event rate was problematic for both stress urinary incontinence and dyspareunia.
The quality of evidence in comparisons of native tissue repair versus use of polypropylene mesh is generally low to moderate owing to serious risk of bias and imprecision. Bias was related to lack of reporting of allocation concealment and reports that reviewers were unblinded. Findings for stress urinary incontinence and dyspareunia were imprecise.
The quality of evidence in comparisons of native tissue repair versus absorbable mesh is generally low to very low, reflecting smaller, older studies with lack of reporting of study methods, risk of bias due to lack of blinding and high rates of attrition and imprecision.
The quality of evidence related to native tissue repair and use of polypropylene mesh for anterior and/or posterior compartment prolapse is generally low owing to risk of bias (with neither trial reporting methods of allocation concealment and reports that reviewers were unblinded) and imprecision.
Potential biases in the review process
We are sure that we have avoided bias during the review process. We have systematically searched multiple electronic databases for published and unpublished evidence, regardless of language or date of publication. We have adhered to Cochrane methods for selection of studies and extraction of data for inclusion in this review.
Agreements and disagreements with other studies or reviews
The Medicines and Healthcare Products Regulatory Agency (MHRA) presented a report in late 2014 (MHRA 2014) after reviewing literature described in the York report in 2012, commissioning another literature review in 2012, taking submissions from support groups and reviewing adverse event reports submitted to MHRA, engaging with professional organisations and regulatory bodies in the European Union and the USA and participating in the European Commission (EC) Task Force Group on vaginal mesh implants. The full report was extensive and on the basis of reported data concluded that:
-
for most women, the use of vaginal mesh implants is safe and effective; and
-
when these products are used correctly, they can help alleviate the very distressing symptoms of stress urinary incontinence (SUI) and pelvic organ prolapse (POP), thus resulting in benefit outweighing risk.
We have demonstrated advantages associated with transvaginal mesh related to utilisation in the anterior compartment, where permanent mesh resulted in decreased awareness of prolapse, prolapse on examination and reoperation for prolapse; however, transvaginal permanent mesh was associated with increased morbidity, including increased operating time, blood loss, transfusion and cystotomy, and higher rates of de novo stress urinary incontinence and de novo dyspareunia, as compared with native tissue. The rate of mesh exposure was 11.3%, and surgery for mesh exposure was required in 7%.
We have concluded, in contrast to the MHRA 2014 report, that although mesh utilisation may be warranted in individual cases of anterior compartment prolapse, it cannot be considered a first‐line treatment option for women with anterior compartment prolapse because of the not insignificant morbidity surrounding transvaginal mesh usage.
These differences in findings and conclusions between the MHRA 2014 report and our report can be explained by the fact that many new randomised controlled trials were not included in the MHRA 2014 review, which was informed by literature reviews published in 2012.
It is interesting to note that in our review of nearly 1000 cases of anterior transvaginal mesh, we were unable to identify a single case in which further surgical intervention was undertaken for pain and/or dyspareunia, although these were among the leading adverse events reported to the FDA in the USA in 2011. In the MHRA 2014 report, adverse events were reported separately by healthcare professionals and members of the public. In reports from healthcare professionals, mesh exposure accounted for 42% of complaints, and pain accounted for 13%. In contrast, in reports made by the public, pain was the leading complaint in 15%, and mesh exposure was described in 12% of reports. The disparity between the facts that pain accounted for many complaints to regulatory authorities in the United Kingdom and the USA but it did not account for a single surgical intervention in nearly 1000 transvaginal mesh surgeries included in this review remains difficult to explain.
Furthermore, and in contrast to the MHRA 2014 report, we have highlighted that most of the data informing our report were derived from transvaginal mesh products that were voluntarily removed from the market in 2012, and that transvaginal mesh products currently available for use have not been evaluated under the auspices of randomised controlled trials. We believe it is prudent that until data on currently available transvaginal mesh products become available, these products should be utilised under the discretion of the local ethics committee.

PRISMA study flow diagram for 2016 review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Forest plot of comparison: 1 Native tissue versus biological graft, outcome: 1.1 Awareness of prolapse.

Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.1 Awareness of prolapse.

Forest plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.

Funnel plot of comparison: 2 Native tissue versus polypropylene mesh, outcome: 2.3 Recurrent anterior compartment prolapse.
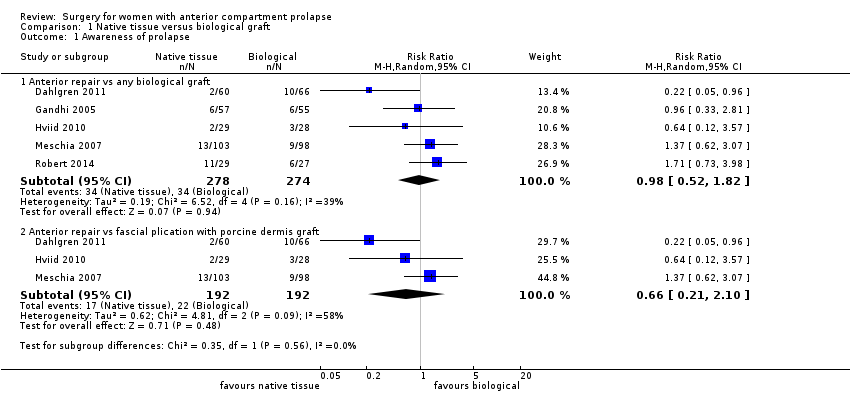
Comparison 1 Native tissue versus biological graft, Outcome 1 Awareness of prolapse.

Comparison 1 Native tissue versus biological graft, Outcome 2 Repeat surgery.

Comparison 1 Native tissue versus biological graft, Outcome 3 Recurrent anterior compartment prolapse.
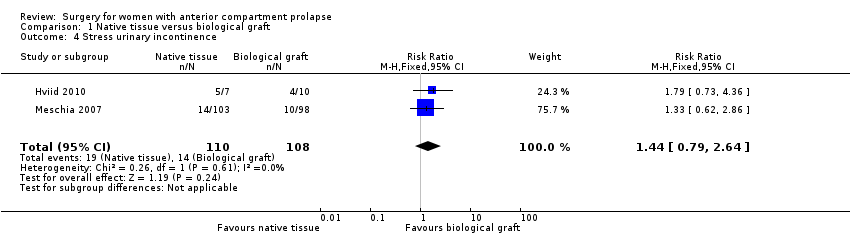
Comparison 1 Native tissue versus biological graft, Outcome 4 Stress urinary incontinence.

Comparison 1 Native tissue versus biological graft, Outcome 5 POPQ assessment.

Comparison 1 Native tissue versus biological graft, Outcome 6 Urge incontinence.

Comparison 1 Native tissue versus biological graft, Outcome 7 Voiding dysfunction.

Comparison 1 Native tissue versus biological graft, Outcome 8 Dyspareunia.

Comparison 1 Native tissue versus biological graft, Outcome 9 Quality of life PROLAPSE.

Comparison 1 Native tissue versus biological graft, Outcome 10 Operating time (minutes).

Comparison 1 Native tissue versus biological graft, Outcome 11 Hospital stay.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 1 Awareness of prolapse.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 2 Repeat surgery.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 3 Recurrent anterior compartment prolapse.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 4 Bladder injury.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 5 Apical or posterior compartment prolapse.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 6 POPQ assessment.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 7 Stress urinary incontinence (de novo).
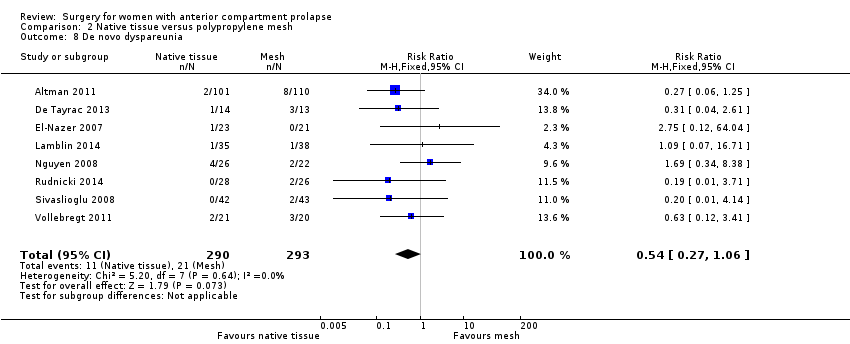
Comparison 2 Native tissue versus polypropylene mesh, Outcome 8 De novo dyspareunia.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 9 Voiding dysfunction.
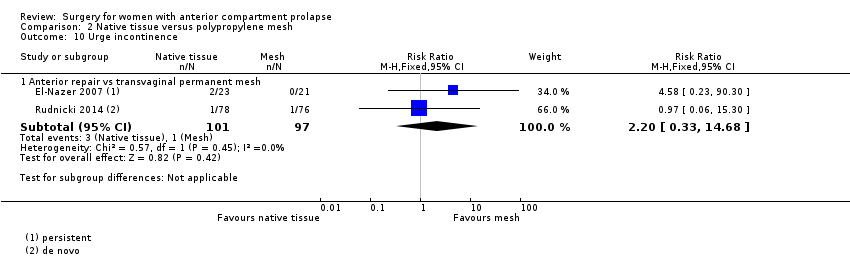
Comparison 2 Native tissue versus polypropylene mesh, Outcome 10 Urge incontinence.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 11 Dyspareunia.
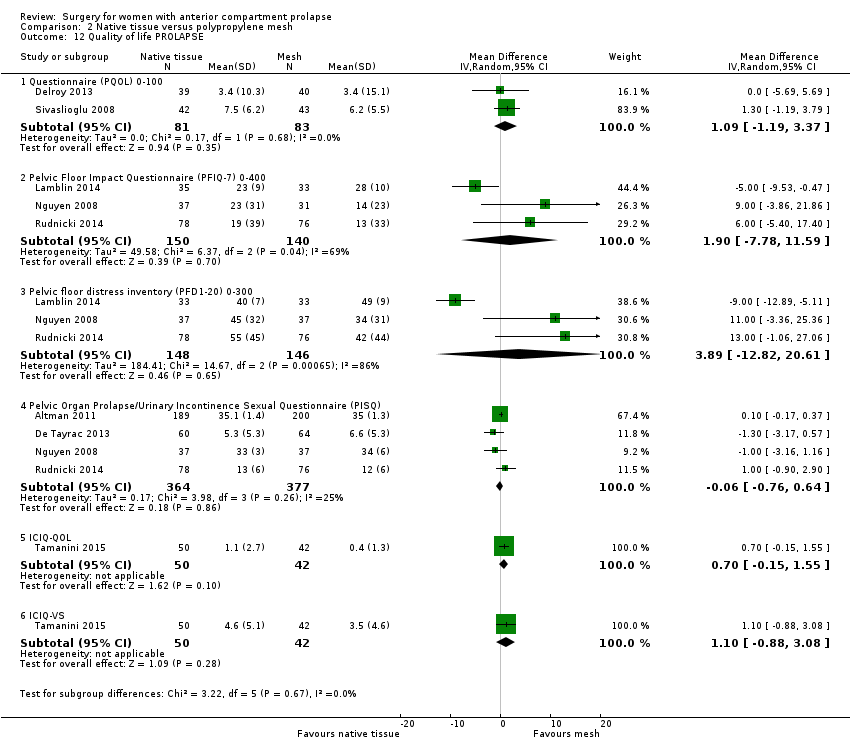
Comparison 2 Native tissue versus polypropylene mesh, Outcome 12 Quality of life PROLAPSE.

Comparison 2 Native tissue versus polypropylene mesh, Outcome 13 Hospital stay (days).

Comparison 2 Native tissue versus polypropylene mesh, Outcome 14 Operating time (minutes).

Comparison 2 Native tissue versus polypropylene mesh, Outcome 15 Transfusion.

Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 1 Awareness of prolapse.

Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 2 Repeat surgery.

Comparison 3 Subgroup analysis: native tissue versus polypropylene mesh available for use, Outcome 3 Recurrent anterior compartment prolapse.

Comparison 4 Native tissue versus absorbable mesh, Outcome 1 Awareness of prolapse (2‐year review).
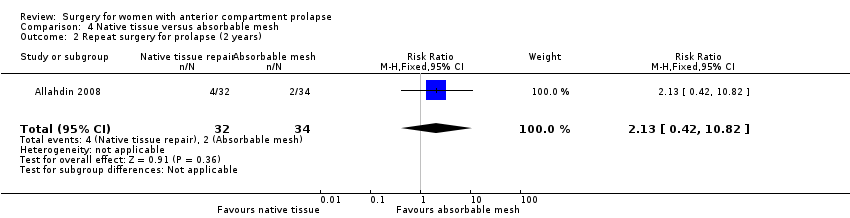
Comparison 4 Native tissue versus absorbable mesh, Outcome 2 Repeat surgery for prolapse (2 years).

Comparison 4 Native tissue versus absorbable mesh, Outcome 3 Anterior compartment prolapse (3 months‐2 years).

Comparison 4 Native tissue versus absorbable mesh, Outcome 4 Death.
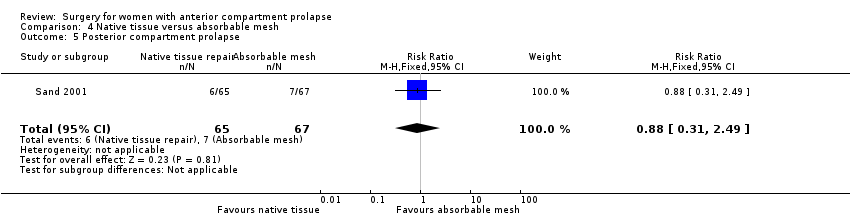
Comparison 4 Native tissue versus absorbable mesh, Outcome 5 Posterior compartment prolapse.
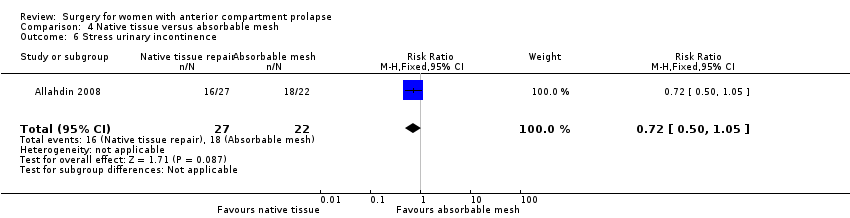
Comparison 4 Native tissue versus absorbable mesh, Outcome 6 Stress urinary incontinence.
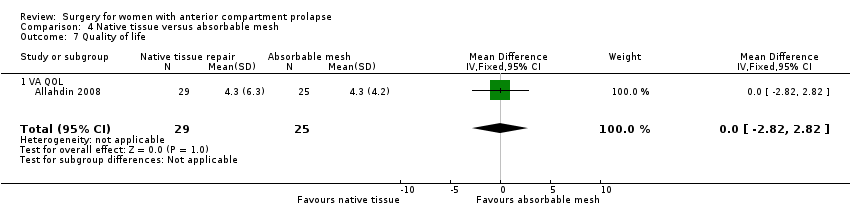
Comparison 4 Native tissue versus absorbable mesh, Outcome 7 Quality of life.

Comparison 5 Mesh versus biological graft, Outcome 1 Awareness of prolapse.

Comparison 5 Mesh versus biological graft, Outcome 2 Repeat surgery.

Comparison 5 Mesh versus biological graft, Outcome 3 Recurrent anterior wall compartment prolapse (stage 2 or greater).

Comparison 5 Mesh versus biological graft, Outcome 4 Mesh exposure.

Comparison 5 Mesh versus biological graft, Outcome 5 Stress urinary incontinence (de novo).

Comparison 5 Mesh versus biological graft, Outcome 6 Urgency, detrusor overactivity or overactive bladder (de novo).

Comparison 5 Mesh versus biological graft, Outcome 7 Dyspareunia (persistent).

Comparison 6 Vaginal repair versus abdominal repair, Outcome 1 Recurrent anterior wall prolapse.

Comparison 6 Vaginal repair versus abdominal repair, Outcome 2 Injury.

Comparison 6 Vaginal repair versus abdominal repair, Outcome 3 Posterior compartment prolapse.
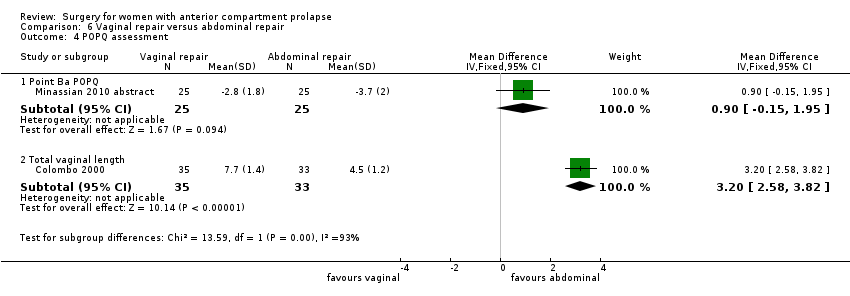
Comparison 6 Vaginal repair versus abdominal repair, Outcome 4 POPQ assessment.
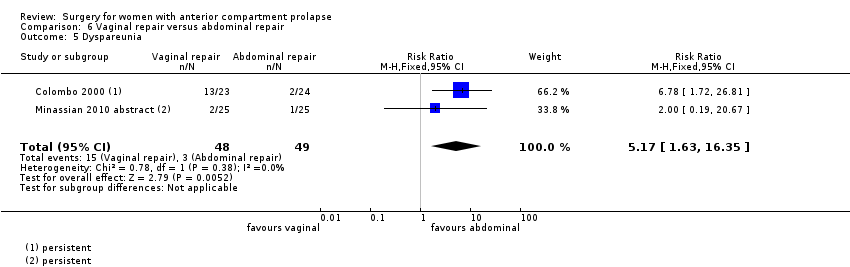
Comparison 6 Vaginal repair versus abdominal repair, Outcome 5 Dyspareunia.
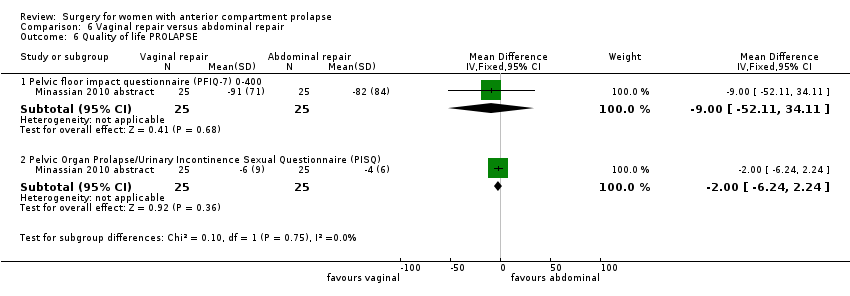
Comparison 6 Vaginal repair versus abdominal repair, Outcome 6 Quality of life PROLAPSE.

Comparison 6 Vaginal repair versus abdominal repair, Outcome 7 Operating time (minutes).

Comparison 6 Vaginal repair versus abdominal repair, Outcome 8 Transfusion.

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 1 Awareness of prolapse.
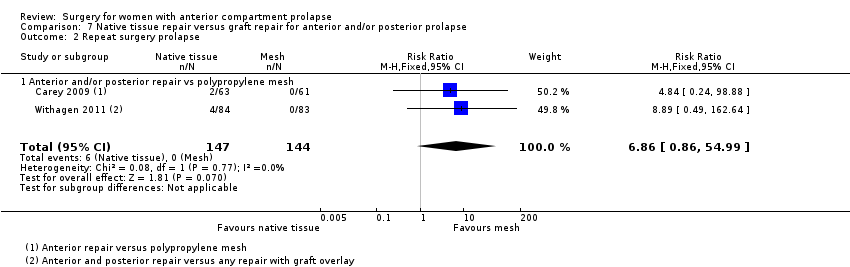
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 2 Repeat surgery prolapse.
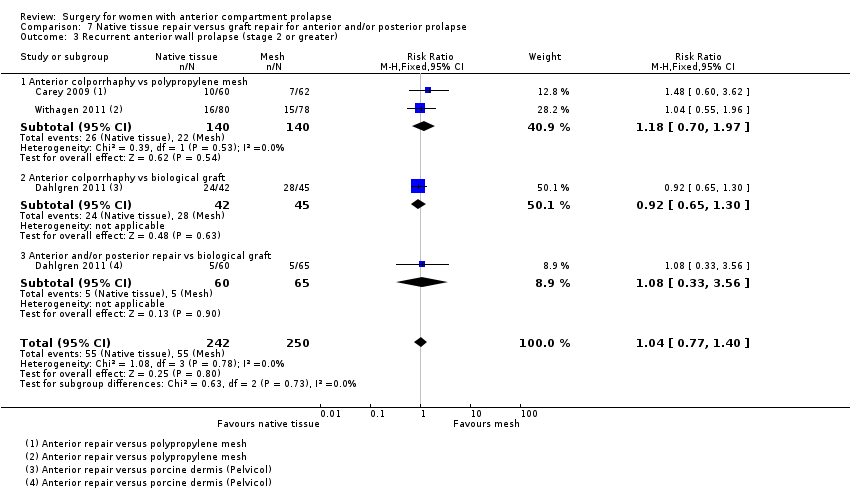
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 3 Recurrent anterior wall prolapse (stage 2 or greater).

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 4 Bladder injury.

Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 5 Stress urinary incontinence (de novo).
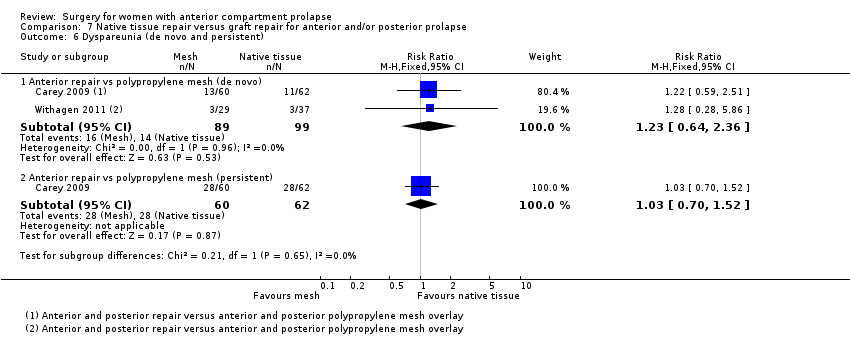
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 6 Dyspareunia (de novo and persistent).
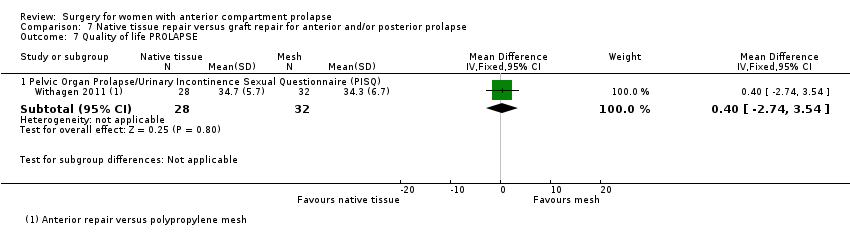
Comparison 7 Native tissue repair versus graft repair for anterior and/or posterior prolapse, Outcome 7 Quality of life PROLAPSE.
| Anterior prolapse repair: native tissue versus biological graft in women with anterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Comparison: biological graft | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Biological graft | Native tissue | |||||
| Awareness of prolapse (1‐2 years) | 124 per 1000 | 122 per 1000 | RR 0.98 | 552 | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse (1‐2 years) | 44 per 1000 | 45 per 1000 | RR 1.02 | 650 | ⊕⊕⊕⊝ | |
| Recurrent anterior compartment prolapse (1‐2 years) | 257 per 1000 | 340 per 1000 | RR 1.32 | 701 | ⊕⊕⊝⊝ | |
| Stress urinary incontinence (1‐2 years) | 130 per 1000 | 187 per 1000 | RR 1.44 | 218 | ⊕⊕⊕⊕ | Repeat surgery for SUI was not reported by any studies |
| Dyspareunia (1‐2 years) | 149 per 1000 | 129 per 1000 | RR 0.87 | 151 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRisk of bias: allocation concealment not reported in 2/5, downgraded one level. bSerious imprecision: wide confidence interval, greater than 25% increase in RR, downgraded one level. | ||||||
| Anterior prolapse repair: native tissue versus polypropylene mesh for women with anterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology Comparison: polypropylene mesh | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Polypropylene mesh repair | Native tissue repair | |||||
| Awareness of prolapse (1‐3 years) | 130 per 1000 | 230 per 1000 | RR 1.77 | 1133 | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse (1‐3 years) | 18 per 1000 | 37 per 1000 | RR 2.03 | 1629 | ⊕⊕⊕⊝ | |
| Repeat surgery for stress urinary incontinence (1‐2 years) | 29 per 1000 | 35 per 1000 (17 to 69) | RR 1.19 (0.60 to 2.36) | 881 (5 studies) | Low3,4 | |
| Recurrent anterior compartment prolapse (1‐3 years) | 126 per 1000 | 379 per 1000 | RR 3.01 | 1976 | ⊕⊕⊝⊝ | |
| Stress urinary incontinence (de novo) (1‐3 years) | 102 per 1000 | 69 per 1000 | RR 0.67 | 957 | ⊕⊕⊝⊝ | |
| Dyspareunia (de novo) (1‐2 years) | 72 per 1000 | 39 per 1000 (19 to 76) | RR 0.54 (0.27 to 1.06) | 583 (8 studies) | ⊕⊕⊕⊝ | |
| Repeat surgery for prolapse, SUI or mesh exposure (1‐3 years) | 97 per 1000 | 54 per 1000 | RR 0.59 (0.41 to 0.83) | 1527 (12 studies) | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Risk of bias: allocation concealment not reported in 4/9, downgraded one level. 3Risk of bias: allocation concealment not reported in 2/5: downgraded one level. 4Serious imprecision: wide CI with lower RR (0.25), downgraded one level. 5Risk of bias: 11/15 trials did not report blinded outcome assessment, downgraded one level. 6Risk of bias: allocation concealment not reported in 7/15, downgraded one level. 7Risk of bias: poor methodological reporting of allocation concealment and/or blinding, downgraded one level. | ||||||
| Anterior prolapse repair: native tissue repair versus absorbable mesh for women with anterior and/or posterior compartment pelvic organ prolapse | ||||||
| Patient or population: women with anterior compartment pelvic organ prolapse Setting: hospital departments of obstetrics and gynaecology | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Absorbable mesh | Native tissue repair | |||||
| Awareness of prolapse (2 years) | 760 per 1000 | 722 per 1000 | RR 0.95 | 54 | ⊕⊝⊝⊝ | |
| Repeat surgery for prolapse (stage 2 or greater) at 2 years | 59 per 1000 | 125 per 1000 | RR 2.13 | 66 | ⊕⊝⊝⊝ | |
| Recurrent anterior compartment prolapse | 267 per 1000 | 401 per 1000 | RR 1.50 | 268 | ⊕⊕⊝⊝ | |
| De novo dyspareunia | Not reported in the included studies | |||||
| Stress urinary incontinence (2 years) | 818 per 1000 | 589 per 1000 | RR 0.72 | 49 | ⊕⊝⊝⊝ | Repeat surgery for SUI was not reported by any studies |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aRisk of bias: at 2 years, 18% lost to review, downgraded one level. dRisk of bias: blinded outcome assessment not reported in 2/3 trials, and high attrition in one, downgraded one level. | ||||||
| Study ID | Mesh exposure | Mesh repairs |
| Al‐Nazer 2007 | 1 | 21 |
| Ali 2006 abstract | 3 | 46 |
| Altman 2011 | 21 | 183 |
| De Tayrac 2013 | 7 | 76 |
| Delroy 2013 | 2 | 40 |
| Gupta 2014 | 4 | 44 |
| Lamblin 2014 | 2 | 33 |
| Menefee 2011 | 5 | 28 |
| Nguyen 2008 | 2 | 37 |
| Nieminen 2008 | 18 | 104 |
| Rudnick 2014 | 12 | 78 |
| Sivaslioglu 2008 | 3 | 43 |
| Tamanini 2014 | 7 | 42 |
| Turgal 2014 | 3 | 20 |
| Thijs 2010 abstract | 9 | 48 |
| Vollebregt 2011 | 2 | 53 |
| Total | 101 | 896 |
| Anterior repair vs absorbable mesh | ||
| Sand 2001 | 0 | 73 |
| Weber 2001 | 1 | 26 |
| Study ID | Surgery mesh exposure | Mesh repairs |
| Altman 2011 (1) | 6 | 183 |
| De Tayrac 2013 (2) | 4 | 76 |
| Delroy 2013 (3) | 2 | 40 |
| Gupta 2014 (4) | 2 | 44 |
| Nguyen 2008 (5) | 2 | 37 |
| Nieminen 2008 (6) | 14 | 104 |
| Rudnick 2014 (7) | 5 | 78 |
| Sivaslioglu 2008 (8) | 3 | 43 |
| Tamanini 2014 (9) | 7 | 42 |
| Turgal 2014 | 5 | 20 |
| Thijs 2010 abstract (10) | 4 | 48 |
| Vollebregt 2011 (11) | 2 | 53 |
| Total | 56 | 768 |
| Study ID | Mesh exposure | Mesh repairs |
| Carey 2009 | 4 | 63 |
| Withagen 2011 | 14 | 83 |
| Total | 18 | 146 |
| Study ID | Reoperation mesh exposure | Mesh repairs |
| Carey 2009 | 3 | 63 |
| Withagen 2011 | 5 | 83 |
| Total | 8 | 146 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Anterior repair vs any biological graft | 5 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.52, 1.82] |
| 1.2 Anterior repair vs fascial plication with porcine dermis graft | 3 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.21, 2.10] |
| 2 Repeat surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 7 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.53, 1.97] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Anterior repair vs any biological graft | 8 | 701 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |
| 3.2 Anterior repair vs fascial plication with porcine dermis graft | 4 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.98, 1.70] |
| 4 Stress urinary incontinence Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.79, 2.64] |
| 5 POPQ assessment Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| 5.1 Point Ba POPQ | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [0.02, 0.98] |
| 6 Urge incontinence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Voiding dysfunction Show forest plot | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.71, 1.80] |
| 8 Dyspareunia Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.39, 1.93] |
| 9 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Questionnaire (P‐QOL) 0‐100 | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.01, 4.01] |
| 10 Operating time (minutes) Show forest plot | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐10.35 [‐14.45, ‐6.24] |
| 11 Hospital stay Show forest plot | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.09, 0.69] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 9 | 1133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.37, 2.28] |
| 2 Repeat surgery Show forest plot | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 12 | 1629 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.15, 3.58] |
| 2.2 Reoperation for stress urinary incontinence (1‐3 years) | 5 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.36] |
| 2.3 Surgery for prolapse, SUI or mesh exposure | 12 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.83] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| 3.1 Permanent mesh vs native tissue repair | 16 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.52, 3.60] |
| 4 Bladder injury Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Anterior repair vs any transvaginal polypropylene mesh | 6 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.82] |
| 5 Apical or posterior compartment prolapse Show forest plot | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.99] |
| 6 POPQ assessment Show forest plot | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Point Ba POPQ | 6 | 568 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.30, 0.80] |
| 6.2 Point Bp POPQ | 3 | 276 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 6.3 Point C POPQ | 4 | 369 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.47, 1.01] |
| 6.4 Total vaginal length | 3 | 366 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.78, 0.43] |
| 7 Stress urinary incontinence (de novo) Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Polypropylene mesh vs native tissue (de novo) | 6 | 957 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.01] |
| 8 De novo dyspareunia Show forest plot | 8 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.06] |
| 9 Voiding dysfunction Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Anterior repair vs polypropylene mesh (persistent) | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.33, 4.47] |
| 10 Urge incontinence Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Anterior repair vs transvaginal permanent mesh | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.33, 14.68] |
| 11 Dyspareunia Show forest plot | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 11.1 Anterior repair vs any transvaginal polypropylene mesh | 8 | 1096 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.59, 1.90] |
| 12 Quality of life PROLAPSE Show forest plot | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Questionnaire (PQOL) 0‐100 | 2 | 164 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐1.19, 3.37] |
| 12.2 Pelvic Floor Impact Questionnaire (PFIQ‐7) 0‐400 | 3 | 290 | Mean Difference (IV, Random, 95% CI) | 1.90 [‐7.78, 11.59] |
| 12.3 Pelvic floor distress inventory (PFD1‐20) 0‐300 | 3 | 294 | Mean Difference (IV, Random, 95% CI) | 3.89 [‐12.82, 20.61] |
| 12.4 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 4 | 741 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.76, 0.64] |
| 12.5 ICIQ‐QOL | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐0.15, 1.55] |
| 12.6 ICIQ‐VS | 1 | 92 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.88, 3.08] |
| 13 Hospital stay (days) Show forest plot | 5 | 707 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.17, 0.33] |
| 14 Operating time (minutes) Show forest plot | 7 | 1099 | Mean Difference (IV, Random, 95% CI) | ‐17.89 [‐25.81, ‐9.98] |
| 15 Transfusion Show forest plot | 4 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.24, 0.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 5 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.35, 3.35] |
| 2 Repeat surgery Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 7 | 815 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.20, 4.59] |
| 2.2 Repeat surgery for stress urinary incontinence (1‐3 years) | 2 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.60, 4.10] |
| 2.3 Repeat surgery for prolapse, SUI or mesh exposure | 6 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.55, 1.24] |
| 3 Recurrent anterior compartment prolapse Show forest plot | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
| 3.1 Permanent mesh vs native tissue repair | 8 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.83, 3.01] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse (2‐year review) Show forest plot | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.70, 1.31] |
| 2 Repeat surgery for prolapse (2 years) Show forest plot | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.42, 10.82] |
| 3 Anterior compartment prolapse (3 months‐2 years) Show forest plot | 3 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.09, 2.06] |
| 4 Death Show forest plot | 2 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Posterior compartment prolapse Show forest plot | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.31, 2.49] |
| 6 Stress urinary incontinence Show forest plot | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 7 Quality of life Show forest plot | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| 7.1 VA QOL | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.82, 2.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Polypropylene mesh (Gynemesh) vs porcine dermis | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.73] |
| 2 Repeat surgery Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prolapse | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.87, 10.73] |
| 3 Recurrent anterior wall compartment prolapse (stage 2 or greater) Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Permanent mesh vs biological graft | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.96] |
| 3.2 Absorbable mesh vs biological graft | 1 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 3.22 [1.38, 7.52] |
| 4 Mesh exposure Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Polypropylene mesh vs porcine dermis | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.69] |
| 5 Stress urinary incontinence (de novo) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.18, 21.23] |
| 6 Urgency, detrusor overactivity or overactive bladder (de novo) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.05, 4.78] |
| 7 Dyspareunia (persistent) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Armed polypropylene mesh (Gynemesh) vs Pelvicol | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.37, 1.80] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Recurrent anterior wall prolapse Show forest plot | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 3.46] |
| 2 Injury Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Bladder | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 14.88] |
| 3 Posterior compartment prolapse Show forest plot | 2 | 118 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.17, 19.65] |
| 4 POPQ assessment Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Point Ba POPQ | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.15, 1.95] |
| 4.2 Total vaginal length | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [2.58, 3.82] |
| 5 Dyspareunia Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [1.63, 16.35] |
| 6 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Pelvic floor impact questionnaire (PFIQ‐7) 0‐400 | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐52.11, 34.11] |
| 6.2 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐6.24, 2.24] |
| 7 Operating time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Transfusion Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Awareness of prolapse Show forest plot | 3 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.36, 1.99] |
| 1.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.70, 1.96] |
| 1.2 Anterior and/or posterior repair vs porcine dermis (Pelvicol) | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.96] |
| 2 Repeat surgery prolapse Show forest plot | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| 2.1 Anterior and/or posterior repair vs polypropylene mesh | 2 | 291 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.86 [0.86, 54.99] |
| 3 Recurrent anterior wall prolapse (stage 2 or greater) Show forest plot | 3 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.40] |
| 3.1 Anterior colporrhaphy vs polypropylene mesh | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.70, 1.97] |
| 3.2 Anterior colporrhaphy vs biological graft | 1 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.30] |
| 3.3 Anterior and/or posterior repair vs biological graft | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.33, 3.56] |
| 4 Bladder injury Show forest plot | 1 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.01] |
| 5 Stress urinary incontinence (de novo) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.34, 2.85] |
| 6 Dyspareunia (de novo and persistent) Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Anterior repair vs polypropylene mesh (de novo) | 2 | 188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.64, 2.36] |
| 6.2 Anterior repair vs polypropylene mesh (persistent) | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.52] |
| 7 Quality of life PROLAPSE Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐2.74, 3.54] |

