Antibiotic prophylaxis for hernia repair.
Abstract
Background
The use of antibiotic prophylaxis for hernia repair is currently a controversial issue given the disparity among study results in this area.
Objectives
The objective of this systematic review was to clarify the effectiveness of antibiotic prophylaxis in reducing postoperative wound infection rates in elective open inguinal hernia repair.
Search methods
Searches in the Cochrane Colorectal Cancer Group specialized register were conducted crossing the terms herni* and inguinal or groin and the terms antimicr* or antibiot* , as free text and MeSH terms. A similar search in Medline (WebSPIRS from Silver Platter, January/1966 to March/2004) and Embase (1976 to December/2003) was conducted using the following terms: #1 antibiotic* or antimicrob* or anti infecti* or antiinfecti*; #2 prophyla* or prevent*; #3 #1 and #2; #4 clean and (surgery or tech* or proced*); #5 herni*; #6 (wound infection) and #4; #7 #3 and (#4 or #5 or #6). Reference lists of the included studies were checked to identify additional studies.
Selection criteria
Only randomized clinical trials were included.
Data collection and analysis
Eight randomized clinical trials were identified. Three of them used prosthetic material for hernia repair (hernioplasty) whereas the remaining studies did not (herniorraphy). Pooled and subgroup analysis were conducted depending on whether prosthetic material was or not used. A random effects model was used in the analysis.
Main results
The total number of patients included was 2907 (treatment group: 1421, control group: 1486). Overall infection rates were 2.88% and 4.3% in the prophylaxis and control groups, respectively (OR 0.65, 95%CI 0.35 ‐ 1.21).
The subgroup of patients with herniorrhaphy had infection rates of 3.78% and 4.87% in the prophylaxis and control groups, respectively (OR 0.84, 95%CI 0.53 ‐ 1.34).
The subgroup of patients with hernioplasty had infection rates of 1.2% and 3,3% in the prophylaxis and control groups, respectively (OR 0.28, 95%CI 0.02 ‐ 3.14).
Authors' conclusions
Based on the results of this meta‐analysis, there was no clear evidence that routine administration of antibiotic prophylaxis for elective inguinal hernia repair reduced infection rates.
PICO
Plain language summary
The use of antibiotic prophylaxis for elective hernia repair is currently a controversial issue. Although elective hernia repair is considered a clean procedure, the rate of postoperative wound infection in many countries exceeds the one expected for clean surgery, increasing discomfort in patients and health care expenses. Alternatively, antibiotics administration is not exempt of potential risks.
Controlled clinical trials on the use of antibiotic prophylaxis for hernia repair are scarce, the number of patients studied is low and the results are diverse. Based on the results of this meta‐analysis of randomized clinical trials, administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be firmly recommended nor discarded.
Authors' conclusions
Background
Wound infection is one of the most commonly occurring surgical complications. Infection of a wound may result from a number of factors both intrinsic and extrinsic to the patient. Although many of the intrinsic factors cannot be modified, the external ones can certainly be influenced. In particular those related to aseptic conditions, surgical technique and peri‐operative care. However even under the most scrupulous aseptic conditions and with a careful technique, post‐operative wound infection still presents a very serious problem.
The use of antibiotic prophylaxis to avoid infectious complications of surgery is very common in surgical practice. However, indiscriminate use of antibiotics can lead to problems including an increase in costs and the emergence of resistant micro‐organisms. The benefits of antibiotic prophylaxis either in clean‐contaminated, contaminated and dirty surgery are universally accepted. Antibiotic prophylaxis is generally accepted in clean surgery (i.e. surgery with no inflammation, no contact with septic material, or interruption of aseptic technique where hollow viscera is not opened) when the placement of prosthetic materials, or the presence of infection poses a significant risk to the patient. Nonetheless, controversy remains about the use of antibiotics in some types of clean surgery.
Surgery for inguinal hernia is one of the most common techniques performed in a general surgical service making up approximately a third of total interventions (Cainzos 1990). This type of surgery is considered clean and it has been estimated that the rate of postoperative infection should not be greater than 2% (Condon 1991; Page 1993; Dellinger 1994; Woods 1998).
Currently, the use of antibiotic prophylaxis is recommended for elective open mesh inguinal hernia repair (Condon 1991; Page 1993; Woods 1998). However, this treatment is not universally accepted. For hernia repair not involving prosthetic material, the antibiotic prophylaxis is not recommended in the absence of risk factors, but the controversy arises when wound infection rates exceed the expected figures (Bailey 1992; Ranaboldo 1993; Holmes 1994). Contradictory results from clinical trials investigating the effectiveness of antibiotic prophylaxis have complicated this situation (Wittmann 1995; Leaper 1998).
Objectives
The objective of this systematic review was to clarify the effectiveness of antibiotic prophylaxis in reducing postoperative wound infection rates in elective open inguinal hernia repair.
Methods
Criteria for considering studies for this review
Types of studies
Randomized clinical trials on antibiotic prophylaxis for hernia repair were included. Randomized clinical trials of antibiotic prophylaxis in patients subject to clean surgical techniques were also included in the review when the report allowed the extraction of data on hernia repair.
Types of participants
Adult patients undergoing open elective inguinal or femoral hernia repair, with or without the use of prosthetic material. Laparoscopic repairs were excluded from this review. We performed an overall analysis of the studies, stratified by whether herniorrhaphy (non‐mesh repair) or hernioplasty (mesh repair) was used.
Types of interventions
Treatment group: administration of prophylactic antibiotics, irrespective of the type of administered antibiotic or the route of administration.
Control group: placebo or no treatment.
Studies using antiseptics for prophylaxis were not included in the review.
Types of outcome measures
Wound infection rate assessed at least at 30 days after the prophylactic antibiotic treatment was given. The criteria of infection were as defined by the authors of each primary study:
discharge of pus from the wound; a wound that was opened and not reclosed; spreading erythema indicative of cellulitis or definitions established by associations such as Centers for Disease Control (Horan 1992).
Search methods for identification of studies
A search in the Cochrane Colorectal Cancer Group specialised register was conducted crossing the terms herni* and inguinal or groin and the terms antimicr* or antibiot* as free text and MeSH terms.
A similar search was conducted in Medline (WebSPIRS from Silver Platter, January/1966 to March/2004), and in Embase (1976 to December/2003) using the following terms:
#1 antibiotic* or antimicrob* or anti infecti* or antiinfecti*
#2 prophyla* or prevent*
#3 #1 and #2
#4 clean and (surgery or tech* or proced*)
#5 herni*
#6 (wound infection) and #4
#7 #3 and (#4 or #5 or #6)
Reference lists of the included studies were checked to identify further studies.
Controlled clinical trials were sought as a source of supplementary evidence.
Data collection and analysis
Data extraction
Both authors independently performed the selection of studies and data extraction. Disagreements were resolved through consensus, or by the input of a third party. The following data were extracted from each study: study design; type of allocation and allocation concealment; number of patients included; mesh or non‐mesh hernia repair; antibiotics used; dose, mode and timing of antibiotic administration; wound infection rates in prophylaxis and control groups, respectively.
Assessment of methodological quality
The methodological quality of the included studies was assessed by evaluating the allocation concealment, and by means of a methodological checklist developed by the first reviewer. Allocation concealment was classified as adequate (i.e. use of sealed envelopes, computer programs or similar methods); unclear (i.e. it is unknown how the allocation was conducted) and inadequate (i.e. use of open random numbers lists, alternate days, coin tossing, case record numbers or similar methods). The methodological checklist is presented in Table 1.
| Parameter | Score | ANDERSEN | EVANS | LAZORTHES | PLATT | TAYLOR | MORALES | YERDEL | OTEIZA |
| (A) Random allocation | 1 = explained 0 = not explained | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| (B) Blinded study | 1 = yes 0 = no | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| (C) Groups of patients are homogeneous | 1 = comparable in technique used, age, sex and comorbid factors 0,5 = one factor differs significantly 0 = more than one factor differs significantly or comparability not specified | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| (D) Statistical method adequate | 1 = yes 0 = no | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| (E) Incisional surgical site infection was definied | 1 = yes 0 = no | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| (F) Number of antibiotics | 1 = uses only one antibiotic 0,5 = uses more than one antibiotic | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (G) Outcome assesment | 1 = by personel no related to study 0,5 = by study personel 0 = not specified | 0 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 1 | 0.5 |
| (H) Route of administration | 1 = especified 0 = not especified | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (I) Number of doses | 1 = especified 0= not especified | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (J) Timing of administration | 1 = especified 0 = not especified | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| SCORE (%) | 7,5 (75%) | 6,5 (65%) | 5,5 (55%) | 9,5 (95%) | 10 (100%) | 9,5 (95%) | 10 (100%) | 8,5 (85%) |
Statistical analysis
Odds ratios and 95% confidence intervals were computed for included studies. Since heterogeneity between studies due to differences in study design and populations was expected, a random effects model was used in the analysis. Nevertheless, chi‐square tests for heterogeneity were conducted to test whether significant heterogeneity precluded a meta‐analysis. If significant heterogeneity was found to be present, the use of meta‐regression techniques was considered to assess the impact of prophylaxis on the following factors:
* Number of antibiotics used.
* Quality of study design.
We expected that a small number of events would be observed in the included studies. Therefore, a fixed‐effect meta‐analysis using Peto odds ratios was performed as a sensitivity analysis, as a tool to reduce bias in the analysis of scarce data (Deeks 1999). Sensitivity analyses were also conducted excluding highly influential studies, as well as poor quality studies.
Numbers needed to treat (NNT) with 95% confidence intervals were estimated for each study, for the herniorrhaphy and hernioplasty sub‐groups, and for the pooled results, if there was a statistically significant benefit with the use of antibiotic prophylaxis. Numbers needed to treat were computed as the inverse of risk differences.
Results of the controlled clinical trials were summarized in an additional table.
Results
Description of studies
From the initial search strategy we identified 39 potentially eligible studies, and after further analyses 7 studies met the inclusion criteria of the review. After updating the search strategy, one additional study was identified, so eight studies met the above criteria and were included in the current systematic review. The remaining studies were excluded.
Studies were described according to the antibiotic used, timing and route of administration, number of doses given, technique of hernia repair, criteria for the diagnosis of infection and follow‐up time.
In one study on the use of different surgical techniques including hernia repair, Evans 1973 used intra‐muscular (IM) cephaloridine 1 g in the anaesthesia induction and two further intra‐muscular doses in the postoperative period. Definition of infection was: purulent exudates at the wound site or need for pus drainage from the wound. Follow‐up was 4 weeks.
Andersen 1980 used a single dose of 1 g topical subfascial ampicillin before closure of non‐mesh hernia repair and colecystectomy. The criteria for wound infection considered in this study was the collection of pus in the wound site requiring revision. Patients in the study were followed for one year.
In the study on antimicrobial prophylaxis for mastectomy and non‐mesh hernia repair, Platt 1990 used a single dose of 1g cefonicid, administered intra‐venouosly 90 minutes before surgery. Wound infection was defined as the presence of erythema and drainage, purulent exudates, or non‐closing open wound. Probable infection was considered when erythema extending at least 2 cm to any direction was present, or when wound infection was diagnosed even if established infection criteria were not met.
Lazorthes 1992 combined cefamandole 750 mg to local anaesthesia for patients undergoing non‐mesh hernia repair. Wound abscess was defined as all wounds with discharge in which pathogens grew regardless of whether or not the discharge was purulent or serohematic. Discharge of pus, even when germs were not found, was also considered a wound abscess. Patients were assessed one month after the intervention.
Taylor 1997 administered amoxicillin‐clavulanic acid 1.2 g intra‐venouosly before the incision in patients undergoing non‐mesh hernia repair. Infection was defined as purulent wound discharge or spreading erythema indicative of cellulites; wound breakdown; or dehiscence with clinical evidence of infection. Patients were assessed 4 and 6 weeks after surgery.
Morales 2000 is a multicenter study that assessed the efficacy of a single dose of cefazolin 2 g administered intra‐venouosly during anaesthetic induction for patients undergoing mesh hernia repair. The authors defined the following criteria for wound infection: a) cutaneous erythema greater than 2 cm on both sides of the incision, b) purulent exudates through the wound, c) organism isolated from culture of non‐ purulent exudates, and d) an open wound that was not closed afterwards. The wound was assessed 30 days after the surgery.
Yerdel 2001 used ampicillin‐sulbactam 1.5 g IV before the incision in patients undergoing mesh hernia repair. The criteria of the Center for Disease Control (1992) for wound infection were applied. One year follow‐up period was considered.
Oteiza 2004 used amoxycilin‐clavulanic acid 2 g IV 15‐30 minutes before the incision in patients undergoing elective mesh hernia repair, Lichtenstein or plug‐mesh technique. Wound infection was defined as the purulent exudate or non purulent exudate with positive culture or the surgeon declares that incisional infection is present. A month follow‐up period was considered.
The remaining studies were excluded for several reasons:
Studies focused on several clean surgical techniques, with no consideration of hernia pathology (Bozzetti 1975; Gaskill 1989; Lord 1977; Lord 1983; Luke 1991; Menzies 1989; Wersink 1991);
Studies focused on clean surgical techniques including hernia pathology, but data for this subgroup of patients could not be collected (Houck 1989; Lewis 1995; Nundy 1983);
Final results are not yet available for one study (Karran 1992);
Studies reported results of patients series having no antibiotic prophylaxis (Hedawoo 1995; Wantz 1996), otherwise, they were non‐controlled studies of antibiotic prophylaxis (Angio 2001; Dazzi 1994; Gervino 2000; Massaioli 1995; Spallitta 1999; Van‐Damme 1981; Sultan 1989);
Both study arms received antibiotic prophylaxis (Musella 2001; Shwed 1991; Reggiori 1996);
Studies used historical controls (Abo‐Rahmy 1998), they were comparative retrospective or non‐randomized studies, or misbalances in patients and techniques used in both treatment groups were present (Barreca 2000; Escartín 1999; Gilbert 1993; Hair 2000; Platt 1992; Ryan 1967; Vara 1993);
Studies did not use antibiotic but local antiseptic treatment (Gilmore 1977).
Risk of bias in included studies
A data form including ten methodological parameters for the initial classification of the studies was designed (Table 1).
Two studies did not provide information on the random allocation concealment procedures (Andersen 1980; Lazorthes 1992). Consequently, they are considered as "unclear". The concealment procedure of Evans 1973 (coin tossing) was inappropriate. The remaining studies used appropriate concealment methods (sealed envelopes: Morales 2000, computer programs: Platt 1990; Taylor 1997; Yerdel 2001, Oteiza 2004).
Morales 2000; Platt 1990; Taylor 1997; Yerdel 2001 were blinded studies; Andersen 1980 was described by the author as a triple‐blinded trial despite the fact that the control group received no intervention, and Evans 1973,Lazorthes 1992 and Oteiza 2004 were not blinded studies.
The patient comparison groups were homogeneous in the majority of included studies, with respect to epidemiological characteristics, techniques used in the
hernia repair, and associated comorbilidities. However, the study Andersen 1980 differed with regard to one factor, and the study Evans 1973 differed by more than one factor.
Apart from Evans 1973 and Lazorthes 1992, the statistical methods were clearly reported in the studies.
All studies except Lazorthes 1992, clearly described a set of wound infection criteria.
All studies used a penicillin derivative antibiotic. The route of administration was intravenous in five studies, subcutaneous/subfascial in two studies (Andersen 1980; Lazorthes 1992) and intramuscularly in one study (Evans 1973). Lazorthes 1992; Morales 2000; Platt 1990; Taylor 1997; Yerdel 2001 and Oteiza 2004 used a single preoperative dose. Andersen 1980 administered subfascial antibiotic before closing the aponeurosis. Evans 1973 used one preoperative and two postoperative intramuscularly doses.
Andersen 1980; Evans 1973; Lazorthes 1992 and Oteiza 2004 included patients with no drug intervention as the control group. The remaining studies used placebo.
Effects of interventions
The total number of patients included in the meta‐analysis was 2907 (prophylaxis group: 1421, control group: 1486). There was no obvious evidence of significant heterogeneity when all the eight studies were pooled (p=0.18). The overall infection rates were 2.88% in the prophylaxis group, and 4.30% in the control group (OR 0.65, 95% CI 0.35 ‐ 1.21). Despite the odds ratio not being significant, the risk differences for this outcome were in fact of borderline significance. This result should be considered cautiously, as a sign of what the expected benefit could be, in case of reaching significance.
Analysis of the group of patients with herniorrhaphy showed no evidence of heterogeneity (p=0.42). The number of patients treated with prophylaxis was 924 and the infection rate for this group was 3.78%. The number of patients in the control group was 943 and the infection rate was 4.87% (OR 0.84, 95%CI 0.53 ‐ 1.34). As in the previous paragraph, the risk differences showed a borderline significative effect. This result should be considered cautiously, as a sign of what the expected benefit could be, in case of reaching significance.
Analysis of the group of patients with hernioplasty showed significant heterogeneity (p=0.07). The number of patients treated with prophylaxis was 497 and the infection rate for this group was 1.2%. The number of patients in the control group was 543 and the infection rate was 3.3% (OR 0.48; CI 95%: 0.07 ‐ 3.37). The risk differences were not significant for this outcome, and so the intervention studied should be viewed as able to produce a benefit or a harm; the confidence interval reflects this dual character by computing to which extent the intervention could be beneficial or harmful (Altman 1998).
Factors like number of antibiotics administered and methodological quality are not related to differences in the treatment effect between the included studies, as shown by the non significant results of the metaregression model (details can be sought from the authors).
Sensitivity analyses
Analysis of the Peto odds ratio under the fixed effect model provided statistically significant estimates for the global effect size (Peto OR 0.65, 95% CI 0.43‐0.96) and for the hernioplasty subgroup (Peto OR 0.37, 95% CI 0.15‐0.91). On the other hand, in the herniorrhaphy subgroup no significant results were obtained (Peto OR 0.76, 95%CI 0.48‐1.19). An explanation for these results, in contrast with the main analysis, could be the low rates of infection observed in all the studies but two (Taylor and Yerdel show an incidence around 9% while the other trials show incidences below 5%).
The subgroup analysis performed with the highest quality trials (Platt 1990; Taylor 1997; Yerdel 2001) showed no qualitative differences with respect to the main analyses.
None of the included studies is highly influential on the analysis, since the exclusion of any of them did not change the global qualitative conclusion. Global heterogeneity appeared when any of the following studies was excluded: Andersen 1980; Evans 1973; Platt 1990; Morales 2000.
Discussion
A systematic review is important, considering that hernia repair is a commonly used technique in any general hospital. As a clean procedure, the wound infection rate should not exceed 2%. However, follow‐up studies have shown figures as low as 0.1% (Wantz 1996; Rutkow 1993), and close to 10% (Bailey 1992). The mean wound infection rate in general hospitals has been estimated around 4% (Cainzos 1990; Holmes 1994). Surgeons do not usually assess wound infection after hernia repair because in most cases, the patient is discharged from the hospital under an outpatient‐based major surgical regimen, or in the first 48 hours after the procedure. Therefore, wound abscess drainage is usually performed in emergency rooms several days after discharge, without attaining any control. This gives the impression that infection rates are lower than the actual values. It has been calculated that 72% of patients are diagnosed after discharge during a 4‐6 week follow‐up period, once the intervention has taken place (Ranaboldo 1993).
Wound infection after hernia repair is not a devastating event as in other types of clean surgery (i.e. Neurosurgery), where antibiotic prophylaxis is given to avoid case fatality. In general, simple drainage with or without antibiotic therapy is enough to resolve the problem in such a way that vital risk is not a major problem for the patient. Nonetheless, wound infection can lead to significant discomfort and inconvenience, and leading to use of more potent antibiotics, to a higher risk of hernia relapse and even to reintervention, raising significantly the costs. Therefore, even though wound infection is not a severe condition, it is a common event that constitutes an important health problem. A study conducted to assess the postoperative infection‐related costs, found that annual expenses for infections after hernia repair (a very often performed procedure) were similar to those for colon surgery (a less frequent technique) (Davey 1998).
Several factors that may increase the infection rate after hernia repair have been analysed (NRC 1964; Haley 1985; Wittmann 1995; Porcu 1996). Although it may not be possible to modify the patient‐related factors, it could be possible to modify factors related to the environment and the surgical technique, in such a way that the administration of antibiotic prophylaxis does not involve the detriment of either sanitary conditions or the surgical technique. Current recommendations suggest the administration of antibiotic prophylaxis when prosthetic material is being used or when risk factors are present (Condon 1991; Page 1993; Woods 1998; Mangram 1999). Controversy arises when greater series using synthetic material show up infection rates around 0% (Gilbert 1993; Wantz 1996) whereas series without prosthetic material provide rates around 10% (Bailey 1992; Ranaboldo 1993; Taylor 1997). Alternatively, benefits of antibiotic prophylaxis to prevent infections after the first week from the intervention have been questioned, as it would not be covered by the prophylaxis administration of antibiotics (Sanderson 1999). Surveys conducted among surgeons have reported that about half of them use antibiotic prophylaxis for hernia repair (Mozillo 1988; Codina 1999; Heineck 1999).
There are several studies on the use of antibiotics for hernia repair, but most of them compare new antibiotics versus antibiotics whose efficacy has already been established. Other studies are conducted with too few patients and insufficient statistical power to draw firm conclusions. Many of them are retrospective series and in some instances there is a lack of control groups. In order to detect a 50% difference between both groups (reduction of the actual rate from 4% to an appropriate rate of 2% in clean surgical procedures) and to have sufficient statistical power, a prospective, randomized blinded study should include at least 800 patients in each treatment arm. This involves performing multicenter studies or studies with longer recruitment periods.
From those studies considered for further analysis after reading the abstracts, there were only eight studies that met the criteria to be included in the review. They were well‐designed comparative, randomized and often blinded studies. However, conclusions cannot be drawn due to disparity of results. Andersen 1980; Evans 1973; Morales 2000; Platt 1990; Taylor 1997 and Oteiza 2004 concluded that antibiotic prophylaxis is not efficacious, whereas Lazorthes 1992 and Yerdel 2001 did so. The latter study was finished early due to the incidence of high infection rates in the control group.
Meta‐analysis is a non‐perfect technique that is no substitute for a large and well‐designed randomized controlled study. Nonetheless, the technique is indicated for similar situations where the number of patients in the studies is low, or when results are conflicting, as it provides pooled estimates with narrower confidence intervals and greater statistical power (DerSimonian 1982; Sacks 1987; Sackett 1997; Imperiale 1999). Unlike the early version of this review (Sanchez‐Manuel 2001) where studies should be only controlled trials to meet the inclusion criteria, the current review has been restricted to randomized prospective trials to improve the quality of the review, reducing biases produced by lack of randomization, as well as the level of heterogeneity.
The pooled analysis of the eight trials included on the review has not shown evidence of statistical heterogeneity, and although the random effects meta‐analysis was not significant, a fixed effects analysis was. There are several reasons, widely known, that explain this phenomenon: under the random effects model, confidence intervals are wider than under a fixed effects one, and smaller studies have more weight. In addition, the incidence of infections in the populations studied is low, thus being more difficult to show a significant reduction caused by the prophylaxis. It is possible that this sum of factors is masking a small, significant benefit of antibiotic prophylaxis, that might be of interest to clinicians in settings of high incidence of infections.
The current meta‐analysis has only addressed the use of antibiotic (whatever the type) for prophylaxis. The antibiotics considered in the included studies were betalactamic agents, which are commonly used for antibiotic prophylaxis. They are able to attack Gram‐positive cocci, commonly responsible for infections after hernia repair. All the included studies used antibiotic prophylaxis according to clinical management norms (Condon 1991; Page 1993; Woods 1998; Mangram 1999). In every case, patients were followed‐up for longer than 30 days, the time required to follow up postoperative infections (Ranaboldo 1993).
Meta‐analysis is only as strong as the primary data on which it is based (Imperiale 1999). Therefore, non‐randomized studies, even not included in a statistical analysis, should be taken into account.
A separate analysis of studies that used non‐mesh hernia repair showed that it was possible to combine these (no statistically significant heterogeneity). Results showed that prophylaxis might reduce the postoperative wound infection, although significance was not achieved.
A separate analysis of the three studies that used mesh hernia repair was not possible because their results were quite diverse (significant heterogeneity).
When the analysis included all the comparative and controlled studies, the observed trend in the confidence intervals of the randomized clinical trials becomes more evident, with a higher statistical significance at the expense of greater heterogeneity (Table 2). That is to say, a greater benefit from antibiotic prophylaxis is detected, particularly when mesh repair is used. Heterogeneity in the set of comparative studies came from the herniorrhaphy group. Variations in the results among the studies were statistically significant, whereas they were absent in the hernioplasty group. However, caution should be exercised when interpreting these results since non‐randomized and unblinded studies tend to overestimate the effects of treatment or prophylaxis.
| AUTHOR/YEAR | PROPHYLAXIS n/N (%) | CONTROL n/N (%) | OR (95% CI Random) |
| Andersen 1980 | 5/137 (3,6%) | 6/150 (4%) | 0.91 (0.27,3.05) |
| Escartín 1999 | 0/14 (0%) | 13/184 (7%) | 0.44 (0.02,7.75) |
| Evans 1973 | 1/48 (2%) | 2/49 (4%) | 0.50 (0.04,5.70) |
| Lazorthes 1992 | 0/155 (0%) | 7/153 (4,5%) | 0.06 (0.00,1.11) |
| Platt 1990 | 4/301 (1,3%) | 6/311 (1,9%) | 0.68 (0.19,2.45) |
| Platt 1992 | 2/239 (0,8%) | 15/982 (1,5%) | 0.54 (0.12,2.40) |
| Reggiori 1996 | 0/123 (0%) | 8/106 (7,5%) | 0.05 (0.00,0.82) |
| Ryan 1967 | 2/1183 (0,1%) | 82/5335 (1,5%) | 0.11(0.03,0.44) |
| Taylor 1997 | 25/283 (8,8%) | 25/280 (8,9%) | 0.99 (0.55,1.77) |
| SUBTOTAL HERNIORRAPHIES (heterogeneity chi‐square=16.93 df=8 p=0.031) | 39/2483 (1,5%) | 164/7550 (2,1%) | 0.34 (0.20,0.91) |
| Barreca 2000 | 0/63 (0%) | 0/84 (0%) | Not Estimable |
| Escartín 1999 | 3/144 (2%) | 10/133 (7,5%) | 0.26 (0.07,0.97) |
| Gilbert 1993 | 10/1150 (0,8%) | 4/684 (0,6%) | 1.49 (0.47,4.77) |
| Morales 2000 | 4/237 (1,6%) | 6/287 (2,1%) | 0.80 (0.22,2.88) |
| Musella 2001 | 1/293 (0,3%) | 6/284 (2,1%) | 0.16(0.02,1.33) |
| Vara 1993 | 2/141 (1,4%) | 9/137 (6,5%) | 0.20 (0.04,0.97) |
| Yerdel 2001 | 1/136 (0,7%) | 12/133 (9%) | 0.07 (0.01,0.58) |
| Otaiza 2004 | 1/124 (0,8%) | 0/123 (0%) | Not Estimable |
| SUBTOTAL HERNIOPLASTIES (heterogeneity chi‐square=10.61 df=5 p=0.06) | 22/2288 (0,9%) | 47/1865 (2,5%) | 0.37 (0.15,0.90) |
| T O T A L (heterogeneity chi‐square=28.21 df=14 p=0.013) | 61/4771 (1,3%) | 211/9415 (2,2%) | 0.41 (0.24,0.71) |
In summary, the results of this meta‐analysis show a non‐significant reduction of wound infection when antibiotic prophylaxis is used in elective hernia repair. When assessing this results it is important to take into account the setting of the included studies. Neither individual patient risk factors nor hospital‐related risk factors (outpatient surgery, hospitals of different level) that might change the conclusions from this meta‐analysis were considered in the included studies.
These results should also be considered within their context; that is to say, the applicability of the results is related to the studies included in this meta‐analysis. Therefore, to make generalizations of the findings inclusion of studies conducted in other settings should be carefully considered.
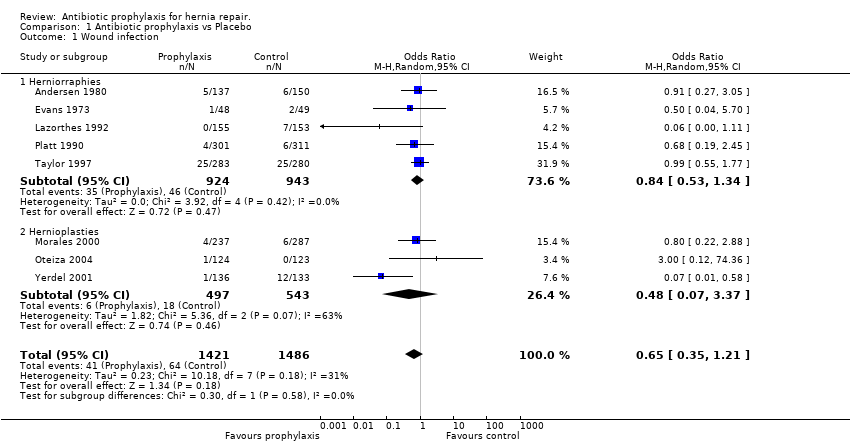
Comparison 1 Antibiotic prophylaxis vs Placebo, Outcome 1 Wound infection.
| Parameter | Score | ANDERSEN | EVANS | LAZORTHES | PLATT | TAYLOR | MORALES | YERDEL | OTEIZA |
| (A) Random allocation | 1 = explained 0 = not explained | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| (B) Blinded study | 1 = yes 0 = no | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 |
| (C) Groups of patients are homogeneous | 1 = comparable in technique used, age, sex and comorbid factors 0,5 = one factor differs significantly 0 = more than one factor differs significantly or comparability not specified | 0.5 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| (D) Statistical method adequate | 1 = yes 0 = no | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| (E) Incisional surgical site infection was definied | 1 = yes 0 = no | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| (F) Number of antibiotics | 1 = uses only one antibiotic 0,5 = uses more than one antibiotic | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (G) Outcome assesment | 1 = by personel no related to study 0,5 = by study personel 0 = not specified | 0 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 1 | 0.5 |
| (H) Route of administration | 1 = especified 0 = not especified | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (I) Number of doses | 1 = especified 0= not especified | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| (J) Timing of administration | 1 = especified 0 = not especified | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| SCORE (%) | 7,5 (75%) | 6,5 (65%) | 5,5 (55%) | 9,5 (95%) | 10 (100%) | 9,5 (95%) | 10 (100%) | 8,5 (85%) |
| AUTHOR/YEAR | PROPHYLAXIS n/N (%) | CONTROL n/N (%) | OR (95% CI Random) |
| Andersen 1980 | 5/137 (3,6%) | 6/150 (4%) | 0.91 (0.27,3.05) |
| Escartín 1999 | 0/14 (0%) | 13/184 (7%) | 0.44 (0.02,7.75) |
| Evans 1973 | 1/48 (2%) | 2/49 (4%) | 0.50 (0.04,5.70) |
| Lazorthes 1992 | 0/155 (0%) | 7/153 (4,5%) | 0.06 (0.00,1.11) |
| Platt 1990 | 4/301 (1,3%) | 6/311 (1,9%) | 0.68 (0.19,2.45) |
| Platt 1992 | 2/239 (0,8%) | 15/982 (1,5%) | 0.54 (0.12,2.40) |
| Reggiori 1996 | 0/123 (0%) | 8/106 (7,5%) | 0.05 (0.00,0.82) |
| Ryan 1967 | 2/1183 (0,1%) | 82/5335 (1,5%) | 0.11(0.03,0.44) |
| Taylor 1997 | 25/283 (8,8%) | 25/280 (8,9%) | 0.99 (0.55,1.77) |
| SUBTOTAL HERNIORRAPHIES (heterogeneity chi‐square=16.93 df=8 p=0.031) | 39/2483 (1,5%) | 164/7550 (2,1%) | 0.34 (0.20,0.91) |
| Barreca 2000 | 0/63 (0%) | 0/84 (0%) | Not Estimable |
| Escartín 1999 | 3/144 (2%) | 10/133 (7,5%) | 0.26 (0.07,0.97) |
| Gilbert 1993 | 10/1150 (0,8%) | 4/684 (0,6%) | 1.49 (0.47,4.77) |
| Morales 2000 | 4/237 (1,6%) | 6/287 (2,1%) | 0.80 (0.22,2.88) |
| Musella 2001 | 1/293 (0,3%) | 6/284 (2,1%) | 0.16(0.02,1.33) |
| Vara 1993 | 2/141 (1,4%) | 9/137 (6,5%) | 0.20 (0.04,0.97) |
| Yerdel 2001 | 1/136 (0,7%) | 12/133 (9%) | 0.07 (0.01,0.58) |
| Otaiza 2004 | 1/124 (0,8%) | 0/123 (0%) | Not Estimable |
| SUBTOTAL HERNIOPLASTIES (heterogeneity chi‐square=10.61 df=5 p=0.06) | 22/2288 (0,9%) | 47/1865 (2,5%) | 0.37 (0.15,0.90) |
| T O T A L (heterogeneity chi‐square=28.21 df=14 p=0.013) | 61/4771 (1,3%) | 211/9415 (2,2%) | 0.41 (0.24,0.71) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Wound infection Show forest plot | 8 | 2907 | Odds Ratio (M‐H, Random, 95% CI) | 0.65 [0.35, 1.21] |
| 1.1 Herniorraphies | 5 | 1867 | Odds Ratio (M‐H, Random, 95% CI) | 0.84 [0.53, 1.34] |
| 1.2 Hernioplasties | 3 | 1040 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.07, 3.37] |

