Clorhexidina vaginal durante el trabajo de parto para la prevención de la infección neonatal por estreptococos del grupo B de inicio temprano
Resumen
Antecedentes
Aunque la infección por estreptococo β‐hemolítico del grupo B (EGB) de inicio temprano es poco frecuente, representa aproximadamente el 30% de las infecciones neonatales, tiene una tasa de mortalidad elevada y se adquiere mediante la transmisión vertical a partir de madres colonizadas. Varios ensayos han demostrado la eficacia de la profilaxis intraparto con antibióticos para prevenir la enfermedad de inicio temprano. La desinfección vaginal con clorhexidina durante el trabajo de parto se ha propuesto como otra estrategia para prevenir la enfermedad de inicio temprano por EGB en el neonato prematuro y a término. Se ha encontrado que la clorhexidina no tiene repercusión sobre la resistencia a los antibióticos, es de bajo coste y aplicable en lugares donde se realizan partos con equipamiento deficiente.
Objetivos
Determinar la efectividad de la desinfección vaginal con clorhexidina durante el trabajo de parto en pacientes colonizadas por EGB para prevenir la infección por EGB de inicio temprano en los neonatos prematuros y a término.
Métodos de búsqueda
Se hicieron búsquedas en el registro de ensayos del Grupo Cochrane de Embarazo y Parto (Cochrane Pregnancy and Childbirth Group) (31 de octubre de 2014) y en las listas de referencias de los estudios recuperados.
Criterios de selección
Ensayos aleatorios y cuasialeatorios que compararan la desinfección vaginal con clorhexidina (lavado vaginal o gel / crema) versus placebo o ningún tratamiento.
Obtención y análisis de los datos
Tres revisores evaluaron de forma independiente los ensayos para la inclusión y el riesgo de sesgo, extrajeron los datos y verificaron su exactitud.
Resultados principales
No se identificaron nuevos ensayos elegibles para su inclusión en esta actualización. Un estudio que en la versión anterior de la revisión estaba entre los estudios incluidos se trasladó a los estudios excluidos. Cuatro estudios con 1125 neonatos prematuros y a término cumplieron los criterios de inclusión e informaron al menos uno de los resultados de interés. Para la comparación de clorhexidina (lavado vaginal o gel) versus placebo o ningún tratamiento se agruparon dos estudios (n = 987). Al comparar clorhexidina (lavado vaginal o gel / crema) versus placebo o ningún tratamiento, no hubo diferencias estadísticamente significativas en la enfermedad por EGB (sepsis o meningitis) de inicio temprano; cociente de riesgos (CR) 2,32 (intervalo de confianza [IC] del 95%: 0,34 a 15,63); I cuadrado (I²) = 0% ni en la neumonía por EGB; CR 0,35 (IC del 95%: 0,01 a 8,6); prueba de heterogeneidad no aplicable. El resultado de la colonización del neonato por EGB se informó en tres estudios (n = 328); CR 0,64 (IC del 95%: 0,40 a 1,01; hubo heterogeneidad significativa entre los estudios (ji² = 3,19; p = 0,20; I² = 37%). Los efectos secundarios maternos leves (ardor o irritación local) (tres ensayos, 1066 pacientes) se observaron con más frecuencia en las pacientes tratadas con clorhexidina (CR 8,50 [IC del 95%: 1,60 a 45,28]); no hubo heterogeneidad (ji² = 0,01; gl = 1 [p = 0,91]; I² = 0%). No se informaron efectos secundarios entre los neonatos.
En la comparación lavado vaginal con clorhexidina versus lavado mecánico con placebo o ningún tratamiento (un estudio, n = 79) hubo una reducción significativa de la colonización neonatal por EGB; CR 0,32 (IC del 95%: 0,12 a 0,90). Las pruebas de heterogeneidad no fueron aplicables. No hubo otros resultados significativos para esta comparación.
En la comparación gel o crema de clorhexidina versus placebo o ningún tratamiento no hubo resultados estadísticamente significativos entre los resultados presentados.
La calidad de los ensayos varió y el riesgo general de sesgo se calificó de incierto o alto. La calificación de calidad de las pruebas con el uso de GRADE fue muy baja para los resultados de la comparación de clorhexidina (lavado vaginal o gel / crema) versus placebo o ningún tratamiento. Estos resultados incluyeron: enfermedad de inicio temprano por EGB (sepsis o meningitis), neumonía por EGB, colonización neonatal por EGB, mortalidad neonatal debida a infección de inicio temprano por EGB y efectos adversos (leves) en la madre y el neonato.
Conclusiones de los autores
La calidad de los cuatro ensayos incluidos varió, así como el riesgo de sesgo, y la calidad de las pruebas al utilizar GRADE fue muy baja. La clorhexidina vaginal no se asoció con reducciones en cualquiera de los resultados primarios de enfermedad de inicio temprano por EGB (sepsis o meningitis) ni neumonía por EGB. La clorhexidina vaginal puede reducir la colonización por EGB de los neonatos. La intervención se asoció con un mayor riesgo de efectos adversos maternos leves. Actualmente la revisión no apoya el uso de la desinfección vaginal con clorhexidina en el trabajo de parto para prevenir la enfermedad de inicio temprano. Los resultados deben interpretarse con cautela, ya que la calidad metodológica de los estudios fue deficiente. Como la enfermedad de inicio temprano por EGB es una afección poco frecuente se necesitan ensayos con tamaños de la muestra muy grandes para evaluar la efectividad de la clorhexidina vaginal para reducir su aparición. En la era de la profilaxis intraparto con antibióticos dichos ensayos pueden ser difíciles de justificar, especialmente en los países desarrollados.
PICO
Resumen en términos sencillos
Clorhexidina antibacteriana aplicada a la vagina durante el trabajo de parto para prevenir la infección de inicio temprano por estreptococo del grupo B en el recién nacido
No hay pruebas que indiquen que el lavado de la vagina con clorhexidina líquida antibacteriana o utilizar un gel de clorhexidina durante el trabajo de parto reduzca las infecciones por estreptococo β‐hemolítico del grupo B (EGB) en los recién nacidos.
La vagina de la mujer habitualmente contiene numerosas bacterias que en general no provocan problemas a los recién nacidos. Sin embargo, ocasionalmente el recién nacido adquiere una infección durante el parto. La infección por EGB puede causar enfermedad grave en los recién nacidos y es muy poco probable que el recién nacido pueda morir como resultado de la infección. En esta revisión sistemática se estudió el lavado de la vagina con clorhexidina o la aplicación de gel o crema de clorhexidina durante el trabajo de parto como posibles maneras de reducir las infecciones. La revisión de cuatro ensayos incluyó a pacientes colonizadas por vía vaginal o rectal con EGB y sus 1125 recién nacidos prematuros y a término. Esta revisión mostró que aunque la clorhexidina puede reducir el número de bacterias que pasan de las madres a los recién nacidos cuando estos pasan a través del canal del parto o al aspirar líquido amniótico contaminado, los estudios no fueron suficientemente grandes para determinar si la clorhexidina redujo las infecciones por EGB o no.
La desinfección vaginal con clorhexidina no dio lugar a una reducción de la enfermedad de inicio temprano por EGB en los recién nacidos como sepsis, neumonía, meningitis o muertes causadas por la infección. Puede reducir la colonización por EGB de los recién nacidos en comparación con el lavado mecánico con placebo (a partir de un estudio).
En la comparación gel o crema de clorhexidina versus placebo o ningún tratamiento no hubo resultados significativos en los resultados informados.
Los efectos secundarios maternos leves como ardor o irritación local fueron más frecuentes en las pacientes tratadas con clorhexidina. Se utilizaron diferentes preparaciones, dosis, frecuencias de dosis y resultados informados. No se informaron efectos secundarios entre los recién nacidos.
El escaso número de estudios que informaron cada uno de los resultados de interés y el tamaño de la muestra relativamente pequeño (1125 recién nacidos) debido a la baja incidencia de infección por EGB (uno a tres por 1000 nacidos vivos) en la población general, significa que las pruebas fueron limitadas.
Es necesario realizar un ensayo aleatorio grande y bien diseñado que examine la eficacia de la desinfección vaginal con clorhexidina para reducir la infección por EGB en los recién nacidos a término y prematuros y supere las limitaciones metodológicas de los estudios incluidos. Los costos asociados con los tratamientos con antibióticos y la falta de personal capacitado han limitado la disponibilidad de tratamiento preventivo para las pacientes en las áreas más pobres del mundo. La clorhexidina es barata y no tiene repercusiones sobre la aparición de resistencia a los antibióticos.
Authors' conclusions
Summary of findings
| Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment | ||||||
| Population: women with vaginal or rectal colonization with GBS during labour and their preterm/term infants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment | |||||
| Early onset GBS disease (sepsis and/or meningitis within the first seven days of life)) | Study population | RR 2.32 | 987 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 2 per 1000 | |||||
| GBS pneumonia within the first seven days of life | Study population | RR 0.35 | 987 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 0 per 1000 | |||||
| Neonatal colonization with GBS within the first seven days of life | Study population | RR 0.64 | 328 | ⊕⊝⊝⊝ | ||
| 225 per 1000 | 144 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 213 per 1000 | |||||
| Neonatal mortality due to early‐onset GBS infection | See comment | See comment | Not estimable | 190 | See comment | The outcome was reported with no events. |
| Adverse (mild) effects in the mother | Study population | RR 8.5 | 1066 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse (mild) effects in the neonate | See comment | See comment | Not estimable | 1066 | See comment | The outcome was reported with no events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations. | ||||||
Background
Description of the condition
During labour an infant may be exposed to a wide spectrum of infectious organisms including group B ß‐hemolytic streptococcus (GBS), or Streptococcus agalactiae. The maternal gastrointestinal tract, vagina and urethra serve as reservoirs for GBS. Since the 1970s, GBS has been a leading bacterial cause of illness and death in newborns (Keenan 1998; Shah 2000).
Although approximately 15% to 35% of all pregnant women are colonized with GBS in the vagina and/or rectum, approximately 65% of the infants born to GBS positive mothers will be colonized with the bacteria, and approximately one to two infants per 1000 births will become infected (Schuchat 1998). An overview in 1992, reported maternal colonization rates from 19 studies in the years from 1980 to 1991 ranging from 1.6% in Israel, to 28% in England (Ohlsson 1992). The transmission rate for GBS colonization from mother to infant varied from 35% in England, to 69% in Brazil. The incidence of early‐onset GBS disease varied from 0.2 per 1000 live births in Israel, to 5.0 per 1000 live births in the US (Ohlsson 1992). In a systematic review on the prevalence of maternal GBS colonization in European countries, Barcaite 2008 identified 21 studies published between 1996 and 2006, that reported on 24,093 women. Group B streptococcus vaginal colonization rates ranged from 6.5% to 36%. The carriage rates were reported to be 19.7% to 29.3% in Eastern Europe, 11% to 21% in Western Europe, 24.3% to 36% in Scandinavia, and 6.5% to 32% in Southern Europe (Barcaite 2008). Similar colonization rates have been found in developing countries (17.8%) (Stoll 1998).
Two forms of GBS disease in infants are well recognized. Early‐onset disease (EOD) is defined as isolation of GBS from a normally sterile site (i.e., blood or cerebrospinal fluid, or both) in an infant less than seven days of age with clinical signs compatible with a systemic infection (Schuchat 1998). The three most common clinical presentations include sepsis, pneumonia, and meningitis with bacteria present in the blood stream, the lungs and/or around the membranes covering the brain. Preverbal infants cannot report symptoms but clinical signs include; hypo‐ or hyperthermia, irritability, crying, breathing difficulties, feeding difficulties, neck stiffness, tense fontanel and dehydration. Group B streptococcus EOD accounts for approximately 30% of neonatal infections; has a high mortality rate and is acquired through vertical transmission from colonized mothers (Freij 1999; Stoll 1996). Exposure of the fetus/neonate to the organism can also occur from aspiration of contaminated amniotic fluid leading to invasive disease in some infants. Perinatal transmission can occur through intact membranes or by acquisition during passage through the birth canal (Schuchat 1998; Schuchat 1999). Infants presenting with meningitis may suffer permanent neurological damage including cognitive delay, cerebral palsy, and sensory damage (Baker 1995).
Late‐onset disease (LOD) usually occurs in infants between one week and up to three months of age, with meningitis being the most common clinical presentation (85% of cases) (Baker 1995). Late‐onset disease is acquired either by vertical transmission (delayed infection after early colonization) (Dillon 1987), or by horizontal transmission due to cross‐infection in the hospital by healthcare workers, or in the community (Noya 1987). The pathogenesis of LOD is less well understood than EOD (Schuchat 1998).
The rapid onset of postnatal disease and its associated high mortality, the morbidity of long‐term neurological sequelae among survivors, and the rising cost of comprehensive healthcare to treat GBS infection has led to numerous strategies to prevent EOD (Adriaanse 1995b; Schuchat 1999). One strategy evaluated extensively over the last two decades is intrapartum antibiotic prophylaxis (IAP) with penicillin or alternative antibiotics such as erythromycin or clindamycin (Ohlsson 1994; Ohlsson 2014; Smaill 1996). The most recent update of the Cochrane review on 'Intrapartum antibiotics for known maternal Group B streptococcal colonization' (Ohlsson 2014) includes three trials (involving 500 women) (Boyer 1986; Matorras 1990; Tuppurainen 1989). The authors conclude that there is lack of evidence from well‐designed and conducted trials to recommend IAP to reduce EOD (Ohlsson 2014). Despite the possible benefits of IAP, increasing antibiotic resistance to erythromycin and clindamycin recommended for women allergic to penicillin is an increasing health concern (Fernandez 1998). In addition, costs associated with treatment have limited the availability of IAP to women in poorer areas of the world. In a review of the relevant cost literature, Benitz and others (Benitz 1999) found that the costs of GBS prevention and treatment ranged from US $9,720 to $22,215 per case prevented. The authors found that the variation in costs was due to the specific screening and IAP protocol used.
The first guidelines for GBS prevention were published in the US in 1992 (AAP 1992; ACOG 1992). Since then numerous different guidelines have been published by various healthcare organizations (AAP 1997; ACOG 1996; CDC 1996; CDC 2002; CDC 2010; Money 2013 (for SOGC); RCOG 2003; Shah 2001; SOGC 1994; SOGC 1997; SOGC 2004). The same literature has been interpreted differently by different professional organizations. Although these current guidelines are based on studies of poor quality (Ohlsson 1994; Ohlsson 2014), there seems to be a temporal association between the introduction of guidelines and a decline in the EOD rate (CDC 2005; CDC 2007; Schrag 2002). The incidence of invasive EOD decreased from 1.8 cases per 1000 live births in the early 1990s to 0.26 cases per 1000 live births in 2010 (Schrag 2013). Mortality has decreased but all cases of EOD cannot be prevented. There has been no reduction in LOD in infants (CDC 2007; Schrag 2013).
Several GBS vaccine candidates have been developed against the nine currently identified GBS serotypes (Johri 2006) and a type III conjugate vaccine has been found to be safe and immunogenic in pregnant women (Johri 2006).
Description of the intervention
Vaginal disinfection with chlorhexidine during labour has been proposed as another strategy for preventing GBS early‐onset infection in the preterm and term neonate (Christensen 1983; Dykes 1987; Schuchat 1999). Chlorhexidine can be applied as a vaginal wash/douching or as a gel/cream application. Chlorhexidine gel is applied around the portio and onto the fornices or by examination gloves lubricated with chlorhexidine cream. Chlorhexidine has been described as a powerful mucous membrane disinfectant, which can result in suppression of GBS organisms.
How the intervention might work
Christensen and others (Christensen 1983) found that the minimum inhibitory concentration of chlorhexidine for GBS is 0.5 to 1 mg/L. Dykes and colleagues (Dykes 1983) demonstrated that chlorhexidine results in a greater than 100‐fold reduction of urethral and vaginal counts of the organism in carriers within one hour of application and for a duration of many hours. Chlorhexidine has been found to have no impact on antibiotic resistance, is inexpensive and simple, and applicable to poorly equipped delivery sites (Schuchat 1999). In general, the disinfectant has been associated with only minor adverse effects such as skin rashes and dermatological hypersensitivity reactions (Garland 1996; Thune 1998). However, adverse reactions such as severe anaphylactic reaction in the adult (Autegarden 1999; Pham 2000) and bradycardia in the neonate have been reported with topical application of chlorhexidine (Quinn 1989).
Why it is important to do this review
Clavisi and others (Clavisi 2000) conducted a systematic review to examine the efficacy of vaginal disinfection with chlorhexidine during labour for reducing infection rates and mortality. However, the review did not focus on the efficacy of the intervention for reducing early‐onset GBS infection in neonates, but examined the effectiveness of chlorhexidine for reducing all types of infection in both the mother and baby. Six randomized controlled trials and two comparative and not randomized or quasi‐randomized controlled trials were included. The time frame of the search strategy was limited, and included trials from January 1990 to October 1998. The authors concluded that "well designed randomised controlled trials do not support the use of chlorhexidine for reducing postnatal infection rates in mothers and babies" (Clavisi 2000).
Lumbiganon and co‐workers (Lumbiganon 2014) in a Cochrane review that was updated in Issue 9, 2014, assessed the effectiveness and side effects of chlorhexidine vaginal douching during labour in reducing maternal and neonatal infections (excluding GBS and human immunodeficiency virus (HIV)). The review is complimentary to the present review. They included three studies (3012 participants) and found no evidence of an effect of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. Although the data suggested a trend in reducing postpartum endometritis, the difference was not statistically significant. The review authors concluded that "there is no evidence to support the use of vaginal chlorhexidine during labour in preventing maternal and neonatal infections". Although all three included trials were of high quality, one trial used only 20 mL of chlorhexidine or sterile water for vaginal irrigation, while the other two trials used 200 mL of chlorhexidine or sterile saline solution. The effectiveness of vaginal chlorhexidine might depend on the volume of the solution used for irrigation. "There is a need for a well‐designed randomized controlled trial using appropriate concentration and volume of vaginal chlorhexidine irrigation solution and with adequate sample size" (Lumbiganon 2014).
Goldenberg and co‐workers conducted a systematic review to determine the potential for chlorhexidine, used as a vaginal and neonatal wash, to reduce adverse outcomes of pregnancy, especially in developing countries (Goldenberg 2006). They summarized the results of every study that they identified (randomized controlled trials and observational studies) in which chlorhexidine was used as a vaginal treatment, with or without wash of the neonate. All pregnancy outcomes except mother‐to‐child transmission of HIV were included. They found that in developed countries in general, although chlorhexidine when used as a vaginal or newborn disinfectant reduced bacterial load including transmission of GBS from mother to the fetus/infant, it has not been shown to reduce life‐threatening maternal or neonatal infections (Goldenberg 2006). They referred to two large non‐randomized studies conducted in Malawi (Taha 1997 ) and Egypt (Bakr 2005) that suggested that important reductions in maternal and neonatal sepsis and neonatal mortality may be achievable with vaginal or neonatal chlorhexidine treatment (Goldenberg 2006). They concluded that further study of this highly promising treatment is indicated (Goldenberg 2006).
The current systematic review attempted to overcome the limitations of the earlier reviews, and conforms to the methods and criteria of The Cochrane Collaboration. The aim of this review was to combine all trials (randomized controlled trials and quasi‐randomized trials) comparing vaginal disinfection with chlorhexidine versus placebo, or to no treatment, to determine the efficacy of the intervention in preventing early‐onset GBS infection in preterm and term neonates.
Objectives
The primary objective of this review was to determine the effectiveness of vaginal disinfection with chlorhexidine (vaginal wash or gel/cream) during labour in women who were colonized with GBS for the prevention of early‐onset group B ß‐hemolytic streptococcus (GBS) infection in preterm and term neonates.
The secondary objectives were to: 1) describe any benefits or adverse effects to the mother when chlorhexidine was used as a vaginal disinfectant (vaginal wash or gel/cream) during labour, and 2) to describe any adverse effects to the neonate when vaginal chlorhexidine was used.
We carried out a primary analysis of all included trials (vaginal wash or gel/cream versus placebo or no treatment) for the primary and secondary objectives. It was hypothesized that mechanical washing of the vagina during labour, irrespective of the solution used, may result in suppression of GBS. Thus, we conducted comparative analyses a priori to compare the effectiveness of mechanical washing with chlorhexidine to mechanical washing with placebo (saline or water) and of chlorhexidine obstetrical gel/cream versus placebo or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials that compared the effectiveness of vaginal disinfection with chlorhexidine during labour versus placebo or no treatment for the prevention of early‐onset disease (EOD) in preterm and term neonates.
Types of participants
Women with vaginal or rectal colonization with GBS during labour and their preterm/term infants. Colonization with GBS was demonstrated by culture or rapid screening test.
Types of interventions
Vaginal disinfection with chlorhexidine (vaginal wash or gel/cream application) during, but not before, onset of labour of women colonized with GBS, compared with placebo or no intervention for the prevention of EOD in preterm and term neonates.
Types of outcome measures
Studies that reported on the incidence or occurrence of one or more of the following outcomes amongst all randomized trials.
Primary outcomes
1. Early‐onset GBS disease (sepsis and/or meningitis), defined as a positive blood and/or cerebrospinal fluid (CSF) culture with GBS in an infant less than seven days of age with clinical signs consistent with a systemic infection.
2. GBS pneumonia within the first seven days of life. This was added in this update as a non‐prespecified outcome.
Secondary outcomes
3. Neonatal colonization with GBS within the first seven days of life.
4. Neonatal mortality due to early‐onset GBS infection within the first 28 days of life.
5. Adverse effects in the mother related to vaginal disinfection with chlorhexidine, including dermatological hypersensitivity reactions, anaphylactic shock, and others.
6. Adverse effects in the neonate related to maternal vaginal disinfection including dermatological hypersensitivity reactions, bradycardia, and others.
7. Benefits to the mother related to vaginal disinfection with chlorhexidine, such as decreased incidence of endometritis, urinary infections, and others.
8. Long‐term neurological sequelae, which may include cognitive delay, cerebral palsy, cortical blindness, deafness, and/or hydrocephalus.
Search methods for identification of studies
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 October 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (OVID);
-
weekly searches of Embase (OVID);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional author searching carried out for the previous version of this review (Stade 2004), please see Appendix 1.]
Searching other resources
We searched cited references from retrieved articles for additional studies. We reviewed abstracts and letters to the editor to identify randomized controlled trials which have not been published. If a randomized controlled trial was identified, we attempted to contact the primary investigator directly to obtain further data. We reviewed editorials, indicating expert opinion, to identify and ensure that no key studies were missed for inclusion in this review.
We did not apply language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review (Stade 2004), please see Appendix 2.
For this update the following methods were used. These methods are based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Three review authors (AO, VS, BS) independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We used a form designed by the review group to extract data. For eligible studies, two review authors (AO, VS) extracted the data using the agreed form. We resolved discrepancies through discussion. One review author (AO) entered data into Review Manager software (RevMan 2014) and another review author (VS) checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (AO, VS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor (BS).
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We consider that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following key outcomes for the main comparison Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment:
-
Early onset neonatal infection (GBS sepsis and or meningitis) defined as a positive blood and/or CSF culture with GBS within the first seven days.
-
GBS pneumonia within the first seven days.
-
Neonatal colonization with GBS within the first seven days.
-
Neonatal mortality due to early‐onset GBS infection within the first 28 days of life. There was no neonatal mortality reported in any of the studies so this outcome was not included in the GRADE assessment.
-
Adverse effects (mild, as defined by the authors) in the mother.
-
Adverse effects (mild, as defined by the authors) in the neonate.
GRADEprofiler (GRADE 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we present results as summary (typical) risk ratio (RR) with 95% confidence intervals.
Continuous data
For continuous data, we would have used the mean difference (MD) if outcomes were measured in the same way between trials. We would have used the standardized mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
We would have included cluster‐randomized trials in the analyses along with individually‐randomized trials. We would have adjusted their sample sizes using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population (Higgins 2011). If we had used ICCs from other sources, we would have reported this and conducted sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomized trials and individually‐randomized trials, we planned to synthesize the relevant information. We would have considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit was considered to be unlikely.
We would have acknowledged heterogeneity in the randomization unit and would have performed a sensitivity analysis to investigate the effects of the randomization unit.
No cluster‐randomized trials were identified for this 2014 update.
Cross‐over trials
It was unlikely that cross‐over designs would have been a valid study design for this review. No cross‐over trials were identified.
Other unit of analysis issues
This review did not focus on multiple pregnancies. If that had been the case, special methods would have been needed to analyze data relating to multiple pregnancies (see the Pregnancy and Childbirth Group Methodological Guidelines and theHandbook sections 9.3.7 and 16.3) (Higgins 2011). In the study by Adriaanse 1995a and co‐workers 1.9% of the total of 1020 participating women (n = 19) carried a twin pregnancy. The authors did not specify how many of these women were GBS carriers and how many were randomized into each of the three treatment groups. No specific information related to the twins was provided. Twin or multiple pregnancies were excluded in the study by Burman 1992. Hennequin 1995 and co‐workers did not provide any information on whether multiple pregnancies were included or not. The study by Stray‐Pedersen 1999 an co‐workers included 13 pairs of twins among 2002 liveborn infants (1.2%). No information related to twin pregnancies was provided regarding the 79 mothers who were GBS carriers. Becasue of the lack of information related to twin pregnancies we did not apply any special statistical methods related to multiple pregnancies.
Dealing with missing data
For included studies, we noted levels of attrition. We would have explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We report statistical heterogeneity in each meta‐analysis using the I‐squared (I²) and Chi² statistics using a fixed‐effect model. We report heterogeneity as substantial if the I² was greater than 30% and, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. We did not use the random‐effects model and therefore we do not report on the Tau² statistic (See Handbook [Section 9.5.4]) (Higgins 2011).
Assessment of reporting biases
Had there been 10 or more studies in a meta‐analysis we would have investigated reporting biases (such as publication bias) using funnel plots. We would have assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we would have performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used the fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we would have used a random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary would have been treated as the average of the range of possible treatment effects and we would have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we would not combine trials.
Had we used random‐effects analyses, the results would have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we had identified substantial heterogeneity, we would have investigated it using subgroup analyses and sensitivity analyses. We would have considered whether an overall summary was meaningful, and if it was, we would have used random‐effects analysis to produce it.
For the two primary outcomes 'early‐onset GBS infection (sepsis and/or meningitis)', and 'pneumonia' within the first seven days of life) there was no substantial (I² > than 30%) heterogeneity and therefore no subgroup analyses were undertaken. The only result with substantial heterogeneity was for the outcome 'Neonatal colonization with GBS within the first seven days of life' in Comparison 01 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment. Using the fixed‐effect model the results were: risk (RR) 0.64 (95% confidence interval (CI) 0.40 to 1.01); the I² value was 37%. Using the random‐effects model the CI widened but the point estimate was essentially the same RR 0.65 (95% CI 0.36 to 1.18); the I² value was 37%. In the discussion section we provide a possible explanation for the heterogeneity in this analysis (See Discussion).
In future updates we will attempt to carry out subgroup analyses for analyses with substantial heterogeneity based on how laboratory samples for GBS were collected, handled, plated and analyzed.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Because of the paucity of included trials no sensitivity analyses were conducted. In the future if more trials become available we will perform sensitivity analyses based on perceived study quality and study location (industrialized versus non‐industrialized countries). We will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, with poor‐quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
For this update in 2014 the search of the literature in August 2014 identified three studies for potential inclusion (Cutland 2009; Pereira 2011; Saleem 2010), but all were excluded as they included a co‐intervention of washing the infant with chlorhexidine. One study (Calkin 1996) was moved from included to excluded studies as maternal GBS status during labour was not known. We also retrieved one further report (Rouse 1997) by searching the reference lists of retrieved studies. This was subsequently excluded.
Included studies
We have provided details of the included studies in the table Characteristics of included studies.
Four studies, including 1125 term and preterm infants, met the inclusion criteria and reported on at least one of the outcomes of interest for this systematic review (Adriaanse 1995a; Burman 1992; Hennequin 1995; Stray‐Pedersen 1999). These studies were performed in four countries (the Netherlands, Sweden, Belgium, Norway).
Different methods of administration and preparations of chlorhexidine were used; 0.3 % chlorhexidine digluconate gel (Adriaanse 1995a); lubricated glove with 1 % chlorhexidine digluconate cream (Hennequin 1995); and 0.2 % chlorhexidine acetate/diacetate vaginal wash (Burman 1992; Stray‐Pedersen 1999).
For one study (Adriaanse 1995a), we had made an error in data abstraction in the previous review and when corrected the outcome of 'Neonatal colonization with GBS within the first seven days' no longer showed a statistically significant reduction..
Excluded studies
We have provided details of the excluded studies in the table Characteristics of excluded studies. For this update of the review in 2014, a total of 18 studies were excluded. We excluded studies that lacked randomization (Christensen 1985a; Christensen 1985b; Christensen 1987; Coppens 2000; Dykes 1987; Kaihura 2000; Kollee 1989; Sanderson 1985); included interventions that did not meet inclusion criteria (vaginal chlorhexidine versus ampicillin) (Facchinetti 2002); did not meet the inclusion criteria related to types of intervention (Henrichsen 1994); or GBS status was not ascertained during labour (Rouse 1997). One study (Calkin 1996) was moved from included to excluded studies as maternal GBS status during labour was not known. For the previous update of this review (Stade 2004), we identified four potential randomized controlled trials; one published in abstract form (Mushangwe 2006;) and two ongoing trials (Madhi 2007; Moss 2007) on the use of vaginal chlorhexidine in labour. None of the trials were eligible for inclusion as they did not have as an entry criterion 'maternal colonization with GBS'. According to www.clinicaltrials.gov the recruitment status of the Madhi 2007 is unknown since 2007 and the study by Moss 2007 is no longer listed in the trials registry. As stated above three newly identified trials (Cutland 2009; Pereira 2011; Saleem 2010) were excluded as they included a co‐intervention of washing the infant with chlorhexidine.
Risk of bias in included studies
We have presented the assessment of individual studies in the table of Characteristics of included studies. See Figure 1 and Figure 2 for a summary of the 'Risk of bias' assessments.
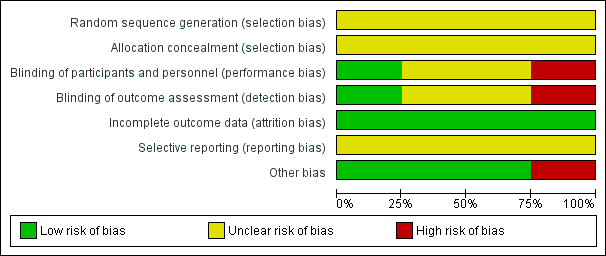
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
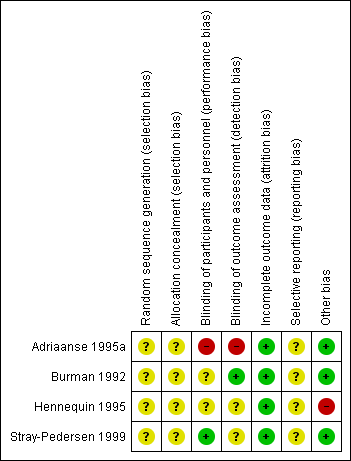
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
In the four included studies the overall risk of bias was unclear (Burman 1992; Stray‐Pedersen 1999) or high (Adriaanse 1995a; Hennequin 1995).
Allocation
We assessed the risk as unclear for random sequence generation and for allocation concealment in all studies (Adriaanse 1995a; Burman 1992; Hennequin 1995; Stray‐Pedersen 1999).
Blinding
We assessed the risk as high for performance and detection bias in the study by Adriaanse 1995a; as unclear for the study by Burman 1992 for performance bias but low for detection bias (one neonatologist made the final decision on infants' diagnoses from their charts before breaking the treatment code); as unclear for both performance and detection bias for the study by Hennequin 1995; and as low risk for performance bias and unclear risk for detection bias for the study by Stray‐Pedersen 1999.
Incomplete outcome data
We assessed the risk of attrition bias as low for all four studies as outcomes (at least one in each study) were reported on all randomized women/infants.
Selective reporting
We assessed the risk as unclear for the four included studies (Adriaanse 1995a; Burman 1992; Hennequin 1995; Stray‐Pedersen 1999) as the protocols for the studies were not available to us and therefore we could not judge if there were any deviations from the protocol in the execution and reporting of the trials.
Other potential sources of bias
We assessed three trials (Adriaanse 1995a; Burman 1992; Stray‐Pedersen 1999) as being free of other bias and assigned a rating of 'Low risk'. We assigned a rating of 'High risk" for bias to the rial by Hennequin 1995 as the study was reported in letter format only and no baseline characteristics were presented so we cannot judge if there were imbalances.
Effects of interventions
For the update of this review, we identified no additional eligible trials. Four studies met the inclusion criteria. These included a total of 1125 infants, and reported on at least one of the outcomes of interest for this systematic review. We report the findings in three separate comparisons 1) Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment (Comparison 01), 2) Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment (Comparison 02) and 3) Chlorhexidine digluconate gel or cream versus placebo or no treatment (Comparison 03).
Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment (Comparison 01)
Primary outcomes
Early onset GBS disease (sepsis and/or meningitis) within the first seven days of life (Outcome 01.01)Analysis 1.1
Two studies (including 987 infants) reported on the outcome of early‐onset GBS infection following chlorhexidine vaginal disinfection. None of the studies reported a statistically significant difference for this outcome. When the studies were combined, there was no statistically significant difference in this outcome; risk ratio (RR) 2.32 (95% confidence interval (CI) 0.34 to 15.63). There was no substantial between‐study heterogeneity for RR (Chi² = 0.65; P = 0.42, I² = 0%).
GBS pneumonia within the first seven days of life (Outcome 01.02)Analysis 1.2
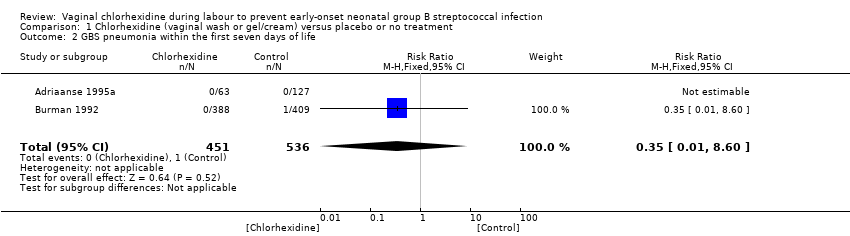
Two studies (including 987 infants) reported on the outcome of early‐onset GBS pneumonia following chlorhexidine vaginal disinfection. In one study (Adriaanse 1995a) there was no case of pneumonia in either group. The studies combined showed no statistically significant difference in this outcome; RR 0.35 (95% CI 0.01 to 8.60). Tests for heterogeneity not applicable for RR.
Secondary outcomes
Neonatal colonization with GBS within the first seven days of life (Outcome 01.03)Analysis 1.3
Three studies (including 328 infants) reported on the outcome of infants colonized with GBS. When the studies were combined there was no statistically significant difference in colonization; average RR 0.65 (95% CI 0.36 to 1.18). There was substantial between‐study heterogeneity for RR (Chi² = 3.19; P = 0.20; I² = 37%).
Neonatal mortality due to early‐onset GBS infection (Outcome 01.04)Analysis 1.4
One study (including 190 infants) reported on mortality due to the early‐onset GBS infection. No deaths due to early‐onset GBS infection was reported in this study, and the RR was not estimable. Tests for heterogeneity were not applicable.
Adverse (mild) effects in the mother (Outcome 01.05)Analysis 1.5
Three studies (including 1066 mothers) reported on this outcome. When the studies were combined, there was a statistically significant increase in the incidence of adverse events following chlorhexidine vaginal disinfection; RR 8.50 (95% CI 1.60 to 45.28). Adverse effects were minor and included stinging or local irritation. There were no major adverse effects reported. There was no substantial between‐study heterogeneity for RR (Chi² = 0.01; P = 0.91; I² = 0%).
Adverse effects in the neonate (Outcome 01.06)Analysis 1.6
Three studies (including 1066 infants) reported on adverse effects to the neonate. No adverse effects in the neonates were noted; RR not estimable. Tests for heterogeneity not applicable for RR.
Benefits to the mother
No study reported on any benefits to the mother.
Long‐term neurological sequelae in the infants
Long‐term follow‐up was not reported in any of the studies.
Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment (Comparison 02)
Early onset GBS disease (sepsis and or meningitis within the first seven days of life) (Outcome 02.01)
One study (including 797 infants) reported on GBS sepsis within the first seven days of life. There was no statistically significant effect of the intervention; RR 1.05 (95% CI 0.07 to 16.79). Tests for heterogeneity not applicable.
GBS pneumonia within the first seven days of life (Outcome 02.02)Analysis 2.2
One study (including 797 infants) reported on GBS pneumonia. There was no statistically significant effect of the intervention; RR 0.35 (95% CI 0.01 to 8.60). Tests for heterogeneity not applicable.
Neonatal colonization with GBS within the first seven days of life (Outcome 02.03)Analysis 2.3
One study (including 79 infants) reported on infants colonized with GBS. It showed a statistically significant reduction in GBS colonization; RR 0.32 (95% CI 0.12 to 0.90). Test for heterogeneity not applicable.
Neonatal mortality due to early‐onset GBS infection
No study reported on this outcome.
Adverse (mild) effects in the mother (Outcome 02.05)Analysis 2.4
Two studies (including 876 mothers) reported on adverse (mild) effects in the mother. There was a statistically significantly increased risk for adverse effects in the mother with the use of vaginal wash with chlorhexidine compared to placebo or no treatment; RR 8.50 (95% CI 1.60 to 45.28). There was no substantial heterogeneity for RR (Chi² = 0.01; P = 0.91; I² = 0 %).
Adverse (mild) effects in the neonate (Outcome 02.06)Analysis 2.5
Two studies (including 876 neonates) reported on adverse (mild) effects in the neonates. There were no adverse events noted in any of the neonates; RR not estimable. Tests for heterogeneity not applicable for RR.
Chlorhexidine digluconate gel or cream versus placebo or no treatment (Comparison 03)
Early onset GBS disease (sepsis and or meningitis within the first seven days of life) (Outcome 03.01)Analysis 3.1
One study (including 190 neonates) reported on early‐onset GBS disease (sepsis and/or meningitis within the first seven days of life). There was no statistically significant difference between the groups; RR 6.0 (95% CI 0.25 to 145.22). Tests for heterogeneity not applicable.
GBS pneumonia within the first seven days of life (Outcome 03.02)Analysis 3.2
One study (including 190 neonates) reported on GBS pneumonia within the first seven days of life. There was no case of pneumonia in either group. There was no statistically significant difference between groups; RR not estimable. Tests for heterogeneity not applicable.
Neonatal colonization with GBS within the first seven days (Outcome 03.03)Analysis 3.3
Two studies (including 249 infants) reported on the outcome of infants colonized with GBS within the first seven days. When the studies were combined, there was no statistically significant reduction in colonization for RR 0.81 (95% CI 0.48 to 1.35). There was no statistically significant between‐study heterogeneity for RR (Chi² = 0.39; P = 0.53), I² = 0%).
Neonatal mortality due to early‐onset GBS infection (Outcome 03.04)Analysis 3.4
One study (including 190 infants) reported on this outcome. There was no mortality in either group. The RR was not estimable. Tests for heterogeneity not applicable.
Adverse (mild) effects in the mother (Outcome 03.05)Analysis 3.5
One study (including 190 mothers) reported on this outcome. There were no cases of adverse effects in either group; RR not estimable. Tests for heterogeneity not applicable.
Adverse (mild) effects in the neonate (Outcome 03.06)Analysis 3.6
One study (including 190 neonates) reported on this outcome. There were no cases of adverse effects in either group; RR not estimable. Tests for heterogeneity not applicable.
Discussion
Summary of main results
The quality of the evidence was very low (see Quality of the evidence below).
For the main comparison Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment (Comparison 01) there were no statistically significant reductions in early‐onset GBS sepsis, early‐onset GBS pneumonia, early‐onset GBS meningitis, neonatal colonization with GBS within the first seven days of life, or mortality due to early‐onset GBS infection. There was no statistically significant between‐study heterogeneity for these outcomes. Adverse effects to the mother were statistically significantly higher for the intervention group. The adverse effects were minor: stinging or local irritation. No adverse effects to the infant were noted. There was no statistically significant between‐study heterogeneity for any outcomes except for neonatal colonization with GBS within the first seven days of life (three studies, 328 infants) (I² = 37%). For the comparison Chlorhexidine digluconate gel or cream versus placebo or no treatment (Comparison 03) there was no heterogeneity for the outcome neonatal colonization with GBS within the first seven days of life (two studies 249 infants). In Comparison 01 Analysis 1.3 the study by Stray‐Pedersen 1999 appears to be a clear outlier with a significant reduction in neonatal colonization with GBS within the first seven days of life. In Comparison 02 Chlorhexidine wash versus mechanical washing with placebo or no treatment, the study by Stray‐Pedersen 1999 is the only study that reports on the outcome and there is a statistically significant reduction in GBS neonatal colonization; RR 0.32 (95% CI 0.12 to 0.90). The heterogeneity noted when all studies are combined could be due to the different methods of administering chlorhexidine or by different methods of obtaining cultures for GBS, or processing the samples.
Vaginal disinfection with chlorhexidine did not result in a reduction of early‐onset GBS morbidity. This finding may be due to the methodological quality of the studies, particularly the lack of clarity around incidence of morbidity outcomes; the limited number of studies that report on each of the outcomes of interest; and the relatively small sample size (n = 1125 infants) given the low incidence of GBS infection (one to three per 1000 live births) in the general population (Schuchat 1999). There is a need to conduct a large, multi‐centre, double‐blinded randomized trial that examines the efficacy of vaginal disinfection with chlorhexidine for reducing GBS infection in term and preterm infants and overcomes the methodological limitations of the studies reviewed. Such a study may particularly benefit settings where costs associated with treatment and lack of skilled personnel have limited the availability of intrapartum chemoprophylaxis to women in poorer areas of the world.
We examined the effectiveness of vaginal disinfection for the prevention of group B ß‐hemolytic streptococcus (GBS) infection in term and preterm infants in this systematic review. The review addressed the limitations of two earlier systematic reviews (Clavisi 2000; Goldenberg 2006). Specifically, the current review focused on the efficacy of the intervention for reducing early‐onset GBS infection in neonates and did not include other types of neonatal infection; included only randomized or quasi‐randomized controlled trials; and ensured that the time frame of the search strategy was not limited, but rather extended over 30 years.
Overall completeness and applicability of evidence
This systematic review includes 1125 infants born to mothers, who were enrolled in trials in four different countries of the world. As early‐onset GBS disease is a rare condition in newborn infants very large cohorts of GBS carrier mothers need to be enrolled in trials to be able to ascertain if vaginal chlorhexidine applications do have any impact on the condition or not.
Quality of the evidence
The methodological quality of the included trials varied. No study was of high quality and elements of bias could not be excluded in any of the studies, mainly due to the fact that information was lacking regarding 'random sequence generation' and 'allocation concealment'. In addition the intervention was performed unblinded to group assignment and there was lack of clarity around blinding of outcomes. The quality of the evidence using GRADE was very low for early‐onset neonatal infection, GBS pneumonia within the first seven days, neonatal colonization with GBS within the first seven days and adverse effects in the mother of comparison chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment. These outcomes were downgraded due to the design limitations and wide confidence interval crossing the line of no effect with few events, or small sample size.
Potential biases in the review process
We are not aware of any bias in our review process.
Agreements and disagreements with other studies or reviews
In a recent randomized controlled study (n = 108), Facchinetti 2002 found similar rates of neonatal GBS colonization when intrapartum ampicillin versus chlorhexidine vaginal flushing was used. This finding suggests the need for further research comparing the effectiveness of chlorhexidine, ampicillin, and placebo or no treatment for reduction of neonatal GBS colonization.
Clavisi and others (Clavisi 2000) conducted a systematic review to examine the efficacy of vaginal disinfection with chlorhexidine during labour for reducing infection rates and mortality. However, the review did not focus on the efficacy of the intervention for reducing early‐onset GBS infection in neonates, but examined the effectiveness of chlorhexidine for reducing all types of infection in both the mother and baby. Six randomized controlled trials and two comparative and not randomized or quasi‐randomized controlled trials were included. The time frame of the search strategy was limited, and included trials from January 1990 to October 1998. The authors concluded that "well designed randomised controlled trials do not support the use of chlorhexidine for reducing postnatal infection rates in mothers and babies" (Clavisi 2000).
In a Cochrane review that excluded GBS and HIV infections Lubiganon and co‐workers (Lumbiganon 2014) found no evidence to support the use of vaginal chlorhexidine during labour in preventing maternal and neonatal infections. They concluded like we did that there is a need for a well‐designed randomized controlled trial using appropriate concentration and volume of vaginal chlorhexidine irrigation solution and with an adequate sample size.
Goldenberg and co‐workers conducted a systematic review to determine the potential for chlorhexidine, used as a vaginal and neonatal wash, to reduce adverse outcomes of pregnancy, especially in developing countries (Goldenberg 2006). They summarized the results of every study that they identified (randomized controlled trials and observational studies) in which chlorhexidine was used as a vaginal treatment, with or without wash of the neonate. All pregnancy outcomes except mother‐to‐child transmission of HIV were included. They found that in developed countries in general, although chlorhexidine when used as a vaginal or newborn disinfectant reduced bacterial load including transmission of GBS from mother to the fetus/infant, it has not been shown to reduce life‐threatening maternal or neonatal infections (Goldenberg 2006). They referred to two large non‐randomized studies conducted in Malawi (Taha 1997) and Egypt (Bakr 2005) that suggested that important reductions in maternal and neonatal sepsis and neonatal mortality may be achievable with vaginal or neonatal chlorhexidine treatment (Goldenberg 2006). They concluded that further study of this highly promising treatment is indicated (Goldenberg 2006).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life)).
Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 2 GBS pneumonia within the first seven days of life.

Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 3 Neonatal colonization with GBS within the first seven days of life.

Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 4 Neonatal mortality due to early‐onset GBS infection.

Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 5 Adverse (mild) effects in the mother.

Comparison 1 Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment, Outcome 6 Adverse (mild) effects in the neonate.

Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 1 Early onset GBS disease (sepsis and/or meningitis) within the first seven days of life.

Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 2 GBS pneumonia within in the first seven days of life.

Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 3 Neonatal colonization with GBS within the first seven days of life.

Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 4 Adverse effects (mild) in the mother.

Comparison 2 Chlorhexidine vaginal wash versus mechanical washing with placebo or no treatment, Outcome 5 Adverse effects (mild) in the neonate.

Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life).

Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 2 GBS pneumonia within the first seven days of life.

Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 3 Neonatal colonization with GBS within the first seven days of life.

Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 4 Neonatal mortality due to early‐onset GBS infection.

Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 5 Adverse (mild) effects in the mother.

Comparison 3 Chlorhexidine digluconate gel or cream versus placebo or no treatment, Outcome 6 Adverse (mild) effects in the neonate.
| Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment | ||||||
| Population: women with vaginal or rectal colonization with GBS during labour and their preterm/term infants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Chlorhexidine (vaginal wash or gel/cream) versus placebo or no treatment | |||||
| Early onset GBS disease (sepsis and/or meningitis within the first seven days of life)) | Study population | RR 2.32 | 987 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 4 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 2 per 1000 | |||||
| GBS pneumonia within the first seven days of life | Study population | RR 0.35 | 987 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 1 per 1000 | |||||
| Moderate | ||||||
| 1 per 1000 | 0 per 1000 | |||||
| Neonatal colonization with GBS within the first seven days of life | Study population | RR 0.64 | 328 | ⊕⊝⊝⊝ | ||
| 225 per 1000 | 144 per 1000 | |||||
| Moderate | ||||||
| 333 per 1000 | 213 per 1000 | |||||
| Neonatal mortality due to early‐onset GBS infection | See comment | See comment | Not estimable | 190 | See comment | The outcome was reported with no events. |
| Adverse (mild) effects in the mother | Study population | RR 8.5 | 1066 | ⊕⊝⊝⊝ | ||
| 2 per 1000 | 15 per 1000 | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 | |||||
| Adverse (mild) effects in the neonate | See comment | See comment | Not estimable | 1066 | See comment | The outcome was reported with no events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life)) Show forest plot | 2 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.34, 15.63] |
| 2 GBS pneumonia within the first seven days of life Show forest plot | 2 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] |
| 3 Neonatal colonization with GBS within the first seven days of life Show forest plot | 3 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.36, 1.18] |
| 4 Neonatal mortality due to early‐onset GBS infection Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse (mild) effects in the mother Show forest plot | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.50 [1.60, 45.28] |
| 6 Adverse (mild) effects in the neonate Show forest plot | 3 | 1066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early onset GBS disease (sepsis and/or meningitis) within the first seven days of life Show forest plot | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.79] |
| 2 GBS pneumonia within in the first seven days of life Show forest plot | 1 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.60] |
| 3 Neonatal colonization with GBS within the first seven days of life Show forest plot | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.90] |
| 4 Adverse effects (mild) in the mother Show forest plot | 2 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.50 [1.60, 45.28] |
| 5 Adverse effects (mild) in the neonate Show forest plot | 2 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Early onset GBS disease (sepsis and/or meningitis within the first seven days of life) Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.25, 145.22] |
| 2 GBS pneumonia within the first seven days of life Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Neonatal colonization with GBS within the first seven days of life Show forest plot | 2 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.35] |
| 4 Neonatal mortality due to early‐onset GBS infection Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adverse (mild) effects in the mother Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adverse (mild) effects in the neonate Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

