Memantina para la demencia
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised | |
| Participants | ||
| Interventions | ||
| Outcomes | 'Efficacy, safety and tolerability' | |
| Notes | Started in 2002 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Randomized | |
| Participants | Country: UK | |
| Interventions | Route: Oral | |
| Outcomes | Primary end points: ADAS‐cog, CGI‐C | |
| Notes | ITT Population: 548 (95%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: Latvia | |
| Interventions | Route: Oral | |
| Outcomes | Primary end points: | |
| Notes | ITT Population: 166 (98%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: France, Belgium and Switzerland | |
| Interventions | Route: Oral | |
| Outcomes | ‐ Primary endpoints: | |
| Notes | ITT population: 288 (90%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: USA | |
| Interventions | Route: oral | |
| Outcomes | Primary end points: NYU CIBIC‐plus; Modified; Modified ADCS‐ADL Inventory | |
| Notes | ITT Population: 236 (94%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized, double‐blind, parallel‐group, placebo‐controlled | |
| Participants | Mild to moderate AD 65 European sites | |
| Interventions | 20 mg monotherapy | |
| Outcomes | Primary end points: CIBIC+, ADAS‐Cog; Secondary: ADCS‐ADL23, NPI | |
| Notes | ITT population: 461/470 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: Germany | |
| Interventions | Route: Oral | |
| Outcomes | Physician's global impression,, SCAG, The Syndrom‐ Kurtztest, ADL test. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: Germany | |
| Interventions | Route: Oral | |
| Outcomes | SCAG, CGI, GBS, ADL behaviour investigation, Tapping test, trace test. | |
| Notes | No. not included in the analysis: 83 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized | |
| Participants | Country: US | |
| Interventions | Route: oral | |
| Outcomes | Primary end points: SIB, ADCS‐ADL19. | |
| Notes | ITT Population: 336 (96%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: USA | |
| Interventions | Route: oral | |
| Outcomes | Primary end points: SIB, ADCS‐ADL19. | |
| Notes | ‐ ITT Population: 395 (98%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Randomized | |
| Participants | Country: USA | |
| Interventions | Route: oral | |
| Outcomes | Primary end points: ADAS‐Cog, CIBIC‐plus | |
| Notes | ‐ ITT Population: 394 (98%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: US | |
| Interventions | Route: oral | |
| Outcomes | ADAS‐Cog; Secondary: ADCS‐ADL23, NPI | |
| Notes | ITT population: 427/433 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Randomized | |
| Participants | Country: France | |
| Interventions | 13 weeks 20 mg memantine monotherapy | |
| Outcomes | ||
| Notes | No results available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Randomized | |
| Participants | Country: Portugal | |
| Interventions | 12 weeks monotherapy 20 mg memantine | |
| Outcomes | ||
| Notes | No results available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Randomized | |
| Participants | 56 participants | |
| Interventions | Monotherapy 20 mg memantine 14 weeks | |
| Outcomes | ||
| Notes | No results available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Randomized | |
| Participants | Country: Germany | |
| Interventions | Route: Oral | |
| Outcomes | Global assessment of clinical efficacy, SCAG, BGP, NOSIE‐Index, Physician's global rating of tolerability | |
| Notes | No. not included in the analysis: 59? | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Included patients suffering from any severe chronic disease of the Central Nervous System. | |
| Single‐blind trial | |
| Open clinical trial | |
| Not placebo controlled | |
| Open label study | |
| Not AD but frontotemporal dementia |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A Randomized, Placebo‐Controlled, 52‐Week Clinical Trial in patients with AD |
| Methods | |
| Participants | ‐N=20 |
| Interventions | memantine versus placebos |
| Outcomes | ‐MRS measures of brain |
| Starting date | ‐recruiting at 110106 |
| Contact information | ‐[email protected] |
| Notes | blinding unclear |
| Trial name or title | MEDUSA: randomized controlled trial in patients with AD |
| Methods | |
| Participants | ‐N=75 (15 in each arm of the trial) |
| Interventions | 1.ChEi as usual |
| Outcomes | what evidence is there that altering therapy, after initial treatment starts to fail, will benefit the patient? |
| Starting date | unknown |
| Contact information | ISRCTN55568578 |
| Notes |
| Trial name or title | randomised placebo controlled double‐blind trial in patients with mild to moderate AD taking donopezil |
| Methods | |
| Participants | ‐N=840 |
| Interventions | 1. alpha‐tocopherol plus memantine placebo |
| Outcomes | ‐ADCS |
| Starting date | Jan 2006: not yet open for recruitment |
| Contact information | ClinicalTrials.gov Identifier: NCT00235716 |
| Notes |
| Trial name or title | A randomised, double‐blind, placebo‐controlled evaluation of the effectiveness and safety of memantine in nursing home residents with moderate to severe AD |
| Methods | |
| Participants | ‐three months trial ‐USA |
| Interventions | memantine versus placebo |
| Outcomes | |
| Starting date | 5 October 2004 |
| Contact information | www.forestclinicaltrials.com |
| Notes | recruitment finished in March 2005 |
| Trial name or title | A randomised, double‐blind, placebo‐controlled evaluation of the effectiveness and safety of memantine in non‐institutionalised agitated patients with moderate to severe AD |
| Methods | |
| Participants | USA |
| Interventions | |
| Outcomes | |
| Starting date | 27 October 2004 |
| Contact information | www.forestclinicaltrials.com |
| Notes |
| Trial name or title | Open‐Label Evaluation of the Safety of Memantine in Patients with Moderate‐to‐Severe Dementia of the Alzheimer's Type |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | safety and tolerability of memantine in outpatients |
| Starting date | Recruitment: Open (at August 2005) |
| Contact information | http://www.forestclinicaltrials.com/CTR/CTRController/CTROngoingListStudies |
| Notes |
| Trial name or title | A Randomized, Double‐Blind, Placebo‐Controlled Evaluation of the Safety and Efficacy of Memantine in Patients with Moderate‐to‐Severe Dementia of the Alzheimer's Type |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | safety |
| Starting date | Start Date: 19‐MAY‐2005 //recruiting. |
| Contact information | http://www.forestclinicaltrials.com/CTR/CTRController/CTROngoingListStudies |
| Notes |
| Trial name or title | randomized, controlled, single blind trial |
| Methods | |
| Participants | ‐N=20 |
| Interventions | comprehensive individualized management approach and memantine versus ??? |
| Outcomes | Clinician Interview‐Based Assessment of Change Plus Caregiver Input (CIBIC‐Plus)//Alzheimer's Disease Cooperative Study Activities of Daily Living Inventory modified for severe dementia (ADCS‐ADLsev)//Severe Impairment Battery//Mini‐Mental State Examination (MMSE)//Functional Assessment Staging//Global Deterioration Scale//Behavioral Pathology in Alzheimer's Disease‐Frequency Weighted//Memory and Behavior Problems Checklist |
| Starting date | ‐Begin date: August 2005 |
| Contact information | ClinicalTrials.gov Identifier: NCT00120874 |
| Notes | DH: Difficult to make out from info available if it is memantine which is on trial or the management approach. On balance it is probably the latter and if so this trial does NOT belong here. |
| Trial name or title | memantine versus placebo for severe to moderately severe AD |
| Methods | |
| Participants | Japan |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | http://www.dsup.co.jp/eg/research/index.html |
| Notes | Daiichi Suntori Pharma |
| Trial name or title | memantine versus placebo for mild to moderate AD |
| Methods | |
| Participants | Japan |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | http://www.dsup.co.jp/eg/research/index.html |
| Notes | Daiichi Suntori Pharma |
| Trial name or title | randomized controlled trial of memantine versus placebo for moderate to severe AD |
| Methods | |
| Participants | ‐N= ? |
| Interventions | memantine versus placebo |
| Outcomes | behavioural aspects of moderate to advanced Alzheimer Disease, such as moodiness, irritability, indifference or apathy, pacing or wandering, changing eating habits or types of food preferred |
| Starting date | ‐duration 6 months |
| Contact information | |
| Notes | Is Gauthier involved in this trial? |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: CIBIC+ (24‐28 weeks) Show forest plot | 3 | 964 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.15, 0.41] |
| Analysis 1.1  Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 1 Clinical Global: CIBIC+ (24‐28 weeks). | ||||
| 2 Cognitive function: SIB (change from baseline at 24‐28 weeks) Show forest plot | 3 | 976 | Mean Difference (IV, Fixed, 95% CI) | 2.97 [1.68, 4.26] |
| Analysis 1.2  Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 2 Cognitive function: SIB (change from baseline at 24‐28 weeks). | ||||
| 3 Activities of daily living: ADCS‐ADLsev19 (change from baseline at 24‐28 weeks) Show forest plot | 3 | 978 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [0.44, 2.09] |
| Analysis 1.3  Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 3 Activities of daily living: ADCS‐ADLsev19 (change from baseline at 24‐28 weeks). | ||||
| 4 Behaviour and mood: NPI total (change from baseline at 24‐28 weeks) Show forest plot | 3 | 936 | Mean Difference (IV, Fixed, 95% CI) | 2.76 [0.88, 4.63] |
| Analysis 1.4  Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 4 Behaviour and mood: NPI total (change from baseline at 24‐28 weeks). | ||||
| 5 Number of dropouts Show forest plot | 3 | 1006 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.49, 0.88] |
| Analysis 1.5  Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 5 Number of dropouts. | ||||
| 6 Number suffering at least one adverse event Show forest plot | 3 | 1005 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.84, 1.52] |
| Analysis 1.6  Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 6 Number suffering at least one adverse event. | ||||
| 7 Number suffering agitation as an adverse event Show forest plot | 3 | 1005 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.42, 0.86] |
| Analysis 1.7  Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 7 Number suffering agitation as an adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical global: CIBIC+ (at 24 weeks) Show forest plot | 3 | 1281 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.01, 0.25] |
| Analysis 2.1  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 1 Clinical global: CIBIC+ (at 24 weeks). | ||||
| 2 Cognitive function: ADAS‐Cog (change from baseline at 24 weeks) Show forest plot | 3 | 1279 | Mean Difference (IV, Fixed, 95% CI) | 0.99 [0.21, 1.78] |
| Analysis 2.2  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 2 Cognitive function: ADAS‐Cog (change from baseline at 24 weeks). | ||||
| 3 Activities of daily living: ADCS‐ADL23 (change from baseline at 24 weeks) Show forest plot | 3 | 1271 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.87, 1.27] |
| Analysis 2.3  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 3 Activities of daily living: ADCS‐ADL23 (change from baseline at 24 weeks). | ||||
| 4 Mood and behaviour: NPI total (change from baseline at 24 weeks) Show forest plot | 3 | 1252 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.48, 0.98] |
| Analysis 2.4  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 4 Mood and behaviour: NPI total (change from baseline at 24 weeks). | ||||
| 5 Number of dropouts Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.60] |
| Analysis 2.5  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 5 Number of dropouts. | ||||
| 6 Number suffering agitation as an adverse event Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.57, 1.46] |
| Analysis 2.6  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 6 Number suffering agitation as an adverse event. | ||||
| 7 Number suffering fall as an adverse event Show forest plot | 2 | 836 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| Analysis 2.7  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 7 Number suffering fall as an adverse event. | ||||
| 8 Number suffering somnolence as an adverse event Show forest plot | 1 | 403 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.49 [1.68, 33.38] |
| Analysis 2.8  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 8 Number suffering somnolence as an adverse event. | ||||
| 9 Number suffering confusion as an adverse event Show forest plot | 2 | 836 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.72, 2.70] |
| Analysis 2.9  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 9 Number suffering confusion as an adverse event. | ||||
| 10 Number suffering at least one adverse event Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.33] |
| Analysis 2.10  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 10 Number suffering at least one adverse event. | ||||
| 11 Number suffering depression as an adverse event Show forest plot | 2 | 836 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.32] |
| Analysis 2.11  Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 11 Number suffering depression as an adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: CGI (at 28 weeks) ITT‐LOCF or OC Show forest plot | 2 | 775 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.13, 0.19] |
| Analysis 3.1  Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 1 Clinical Global: CGI (at 28 weeks) ITT‐LOCF or OC. | ||||
| 2 Cognitive function ADAS‐Cog (change from baseline at 28 weeks) ITT‐LOCF Show forest plot | 2 | 815 | Mean Difference (IV, Fixed, 95% CI) | 1.85 [0.88, 2.83] |
| Analysis 3.2  Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 2 Cognitive function ADAS‐Cog (change from baseline at 28 weeks) ITT‐LOCF. | ||||
| 3 Activities of daily living : NOSGER self care subscale (change from baseline at 28 weeks) OC Show forest plot | 2 | 542 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.43, 0.67] |
| Analysis 3.3  Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 3 Activities of daily living : NOSGER self care subscale (change from baseline at 28 weeks) OC. | ||||
| 4 Behaviour: NOSGER disturbing behaviour subscale (change from baseline at 28 weeks) OC Show forest plot | 2 | 541 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.06, 0.91] |
| Analysis 3.4  Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 4 Behaviour: NOSGER disturbing behaviour subscale (change from baseline at 28 weeks) OC. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities of daily living (change from baseline at 12 weeks) Show forest plot | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.65, 1.65] |
| Analysis 4.1  Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 1 Activities of daily living (change from baseline at 12 weeks). | ||||
| 1.1 Dose 10 mg/day, BGP subscore care dependence (change from baseline at 12 weeks) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.65, 1.65] |
| 2 CGIC (numbers improved at 12 weeks) Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.72, 6.33] |
| Analysis 4.2  Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 2 CGIC (numbers improved at 12 weeks). | ||||
| 2.1 Dose 10mg/day | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.72, 6.33] |
| 3 Number of drop‐outs Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.25, 4.25] |
| Analysis 4.3  Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 3 Number of drop‐outs. | ||||
| 4 Number suffering at least one adverse event Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.16] |
| Analysis 4.4  Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 4 Number suffering at least one adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global Show forest plot | 3 | 213 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.04, ‐0.48] |
| Analysis 5.1  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 1 Global. | ||||
| 1.1 Dose 30mg/day, Physicians global impression (6 weeks) | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.33, ‐0.32] |
| 1.2 Dose 30mg/day, SCAG Clinical global impression of disturbances (4 weeks) | 1 | 59 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.58, ‐0.49] |
| 1.3 Dose 20mg/day, CGI (6 weeks) | 1 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.98, ‐0.13] |
| 2 Cognition Show forest plot | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐3.04 [‐5.68, ‐0.40] |
| Analysis 5.2  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 2 Cognition. | ||||
| 2.1 Dose 30mg/day, SKT (change from baseline at 6 weeks) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐3.04 [‐5.68, ‐0.40] |
| 3 Activities of daily living Show forest plot | 2 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐1.73, ‐0.94] |
| Analysis 5.3  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 3 Activities of daily living. | ||||
| 3.1 Dose 30mg/day, BGP care dependence subsscale (change from baseline at 6 weeks) | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐2.68, ‐1.42] |
| 3.2 Dose 30mg/day, ADL‐test total time (6 weeks) | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.39, ‐0.37] |
| 4 Mood and behaviour Show forest plot | 3 | 208 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.46, ‐0.86] |
| Analysis 5.4  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 4 Mood and behaviour. | ||||
| 4.1 Dose 30mg/day, NOSIE (change from baseline at 4 weeks) | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.02 [‐2.65, ‐1.39] |
| 4.2 Dose 30mg/day, SCAG total (change from baseline at 6 weeks) | 2 | 148 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.25, ‐0.57] |
| 5 Number of dropouts Show forest plot | 2 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.27, 2.67] |
| Analysis 5.5  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 5 Number of dropouts. | ||||
| 6 Number suffering at least one adverse event Show forest plot | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.83, 4.81] |
| Analysis 5.6  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 6 Number suffering at least one adverse event. | ||||
| 7 Number suffering agitation as an adverse event Show forest plot | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.48, 15.19] |
| Analysis 5.7  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 7 Number suffering agitation as an adverse event. | ||||
| 8 Number suffering restlessness as an adverse event Show forest plot | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.5 [2.71, 67.18] |
| Analysis 5.8  Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 8 Number suffering restlessness as an adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: CIBIC+ or CGI‐C Show forest plot | 8 | 3020 | Stand. Tx effect (Fixed, 95% CI) | 0.15 [0.07, 0.23] |
| Analysis 6.1  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 1 Clinical Global: CIBIC+ or CGI‐C. | ||||
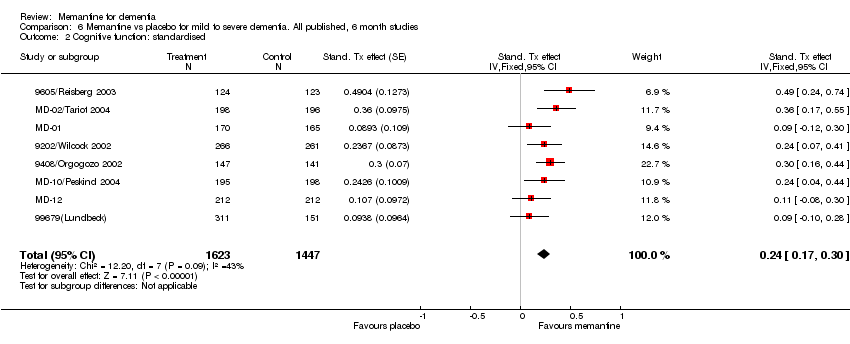
| 2 Cognitive function: standardised Show forest plot | 8 | 3070 | Stand. Tx effect (Fixed, 95% CI) | 0.24 [0.17, 0.30] |
| Analysis 6.2  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 2 Cognitive function: standardised. | ||||
| 3 Activities of daily living: standardised Show forest plot | 8 | 2791 | Stand. Tx effect (Fixed, 95% CI) | 0.08 [0.01, 0.15] |
| Analysis 6.3  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 3 Activities of daily living: standardised. | ||||
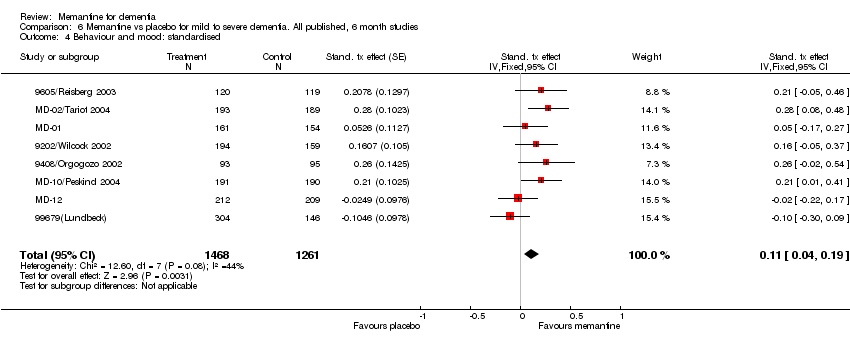
| 4 Behaviour and mood: standardised Show forest plot | 8 | 2729 | Stand. tx effect (Fixed, 95% CI) | 0.11 [0.04, 0.19] |
| Analysis 6.4  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 4 Behaviour and mood: standardised. | ||||
| 5 Number of dropouts Show forest plot | 8 | 3212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.09] |
| Analysis 6.5  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 5 Number of dropouts. | ||||
| 6 Number suffering at least one adverse event Show forest plot | 8 | 3211 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.27] |
| Analysis 6.6  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 6 Number suffering at least one adverse event. | ||||
| 7 Number suffering agitation as an adverse event Show forest plot | 8 | 3612 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.99] |
| Analysis 6.7  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 7 Number suffering agitation as an adverse event. | ||||
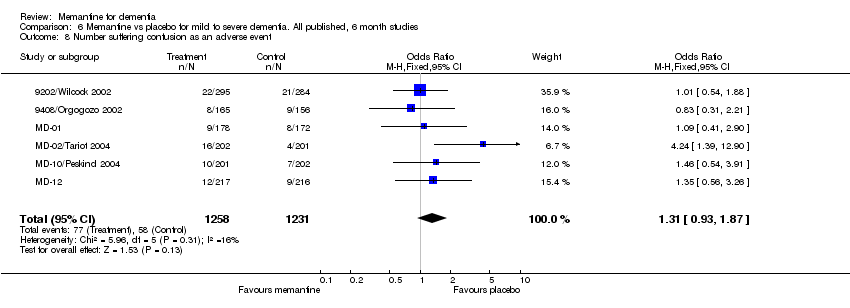
| 8 Number suffering confusion as an adverse event Show forest plot | 6 | 2489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.93, 1.87] |
| Analysis 6.8  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 8 Number suffering confusion as an adverse event. | ||||
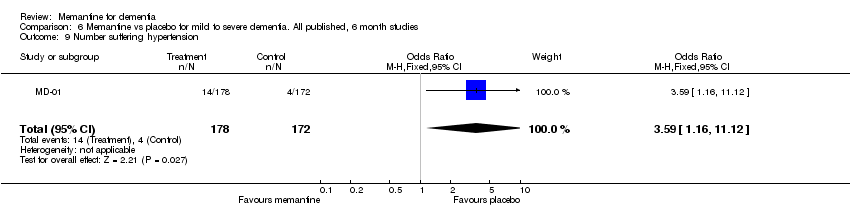
| 9 Number suffering hypertension Show forest plot | 1 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.59 [1.16, 11.12] |
| Analysis 6.9  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 9 Number suffering hypertension. | ||||
| 10 Number suffering dizziness as an adverse event Show forest plot | 6 | 2489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.86, 1.60] |
| Analysis 6.10  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 10 Number suffering dizziness as an adverse event. | ||||
| 11 Number suffering headache as an adverse event Show forest plot | 4 | 1765 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.56] |
| Analysis 6.11  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 11 Number suffering headache as an adverse event. | ||||
| 12 Number suffering fall as an adverse event Show forest plot | 6 | 2420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| Analysis 6.12  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 12 Number suffering fall as an adverse event. | ||||
| 13 Number suffering insomnia as an adverse event Show forest plot | 4 | 1614 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.43] |
| Analysis 6.13  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 13 Number suffering insomnia as an adverse event. | ||||
| 14 Number suffering accidental injury as an adverse event Show forest plot | 6 | 2308 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.98] |
| Analysis 6.14  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 14 Number suffering accidental injury as an adverse event. | ||||
| 15 Number suffering urinary incontinence as an adverse event Show forest plot | 3 | 1234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.67, 1.85] |
| Analysis 6.15  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 15 Number suffering urinary incontinence as an adverse event. | ||||
| 16 Number suffering from diarrhoea as an adverse event Show forest plot | 4 | 1584 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| Analysis 6.16  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 16 Number suffering from diarrhoea as an adverse event. | ||||
| 17 Number suffering from influenza like symptoms as an adverse event Show forest plot | 4 | 1589 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.72, 1.63] |
| Analysis 6.17  Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 17 Number suffering from influenza like symptoms as an adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: standardised Show forest plot | 4 | 377 | Stand. Tx effect (Fixed, 95% CI) | 0.62 [0.41, 0.82] |
| Analysis 7.1  Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 1 Clinical Global: standardised. | ||||
| 2 Cognitive function: standardised Show forest plot | 1 | 59 | Stand. Tx effect (Fixed, 95% CI) | 0.59 [0.11, 1.07] |
| Analysis 7.2  Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 2 Cognitive function: standardised. | ||||
| 3 Activities of daily living: standardised Show forest plot | 3 | 292 | Stand. Tx effect (Fixed, 95% CI) | 0.73 [0.50, 0.96] |
| Analysis 7.3  Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 3 Activities of daily living: standardised. | ||||
| 4 Mood and behaviour: standardised Show forest plot | 3 | 208 | Stand. Tx effect (Fixed, 95% CI) | 1.27 [0.99, 1.54] |
| Analysis 7.4  Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 4 Mood and behaviour: standardised. | ||||
| 5 Number of drop‐outs Show forest plot | 3 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.38, 2.23] |
| Analysis 7.5  Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 5 Number of drop‐outs. | ||||
| 6 Number suffering at least one adverse event Show forest plot | 2 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.77, 2.38] |
| Analysis 7.6  Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 6 Number suffering at least one adverse event. | ||||
| 7 Number suffering agitation as an adverse event Show forest plot | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.48, 15.19] |
| Analysis 7.7  Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 7 Number suffering agitation as an adverse event. | ||||

Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 1 Clinical Global: CIBIC+ (24‐28 weeks).
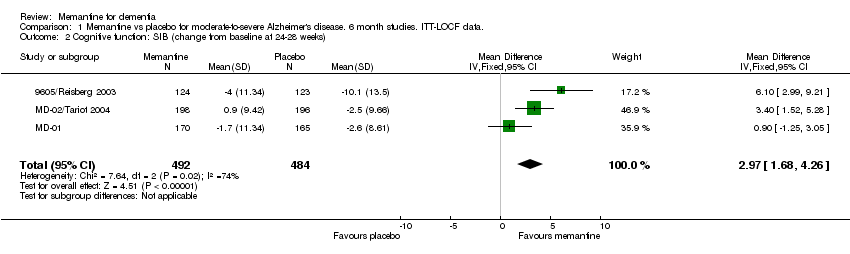
Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 2 Cognitive function: SIB (change from baseline at 24‐28 weeks).
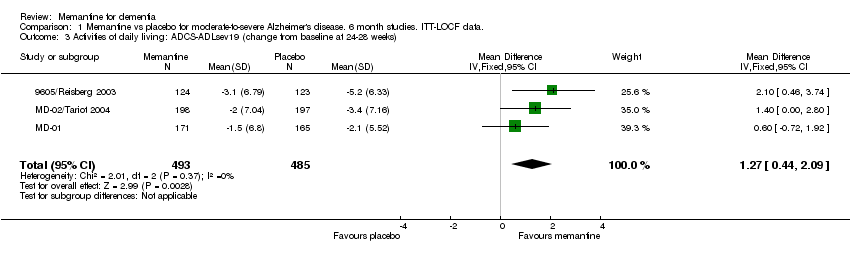
Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 3 Activities of daily living: ADCS‐ADLsev19 (change from baseline at 24‐28 weeks).

Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 4 Behaviour and mood: NPI total (change from baseline at 24‐28 weeks).
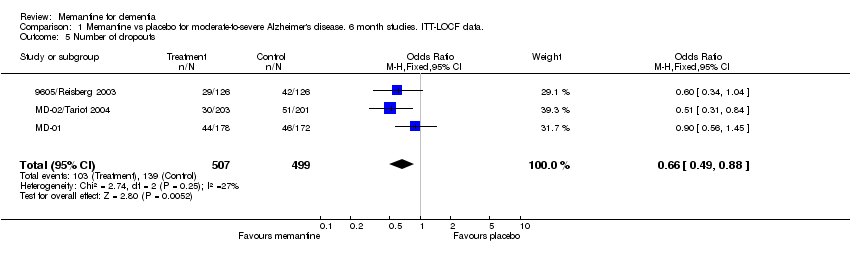
Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 5 Number of dropouts.

Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 6 Number suffering at least one adverse event.
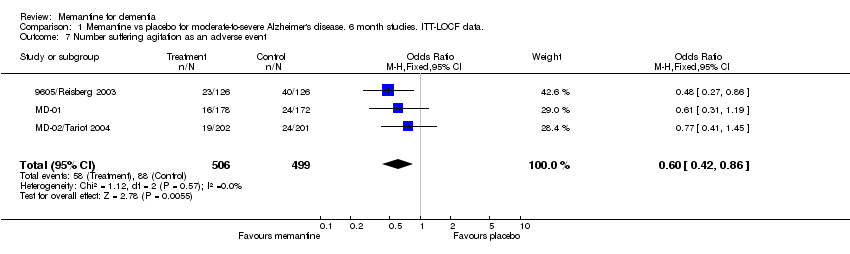
Comparison 1 Memantine vs placebo for moderate‐to‐severe Alzheimer's disease. 6 month studies. ITT‐LOCF data., Outcome 7 Number suffering agitation as an adverse event.

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 1 Clinical global: CIBIC+ (at 24 weeks).

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 2 Cognitive function: ADAS‐Cog (change from baseline at 24 weeks).
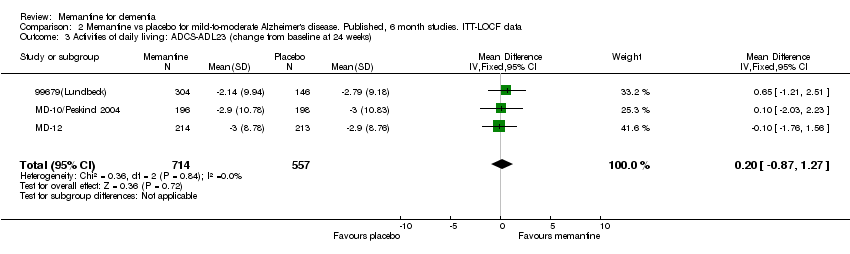
Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 3 Activities of daily living: ADCS‐ADL23 (change from baseline at 24 weeks).

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 4 Mood and behaviour: NPI total (change from baseline at 24 weeks).

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 5 Number of dropouts.

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 6 Number suffering agitation as an adverse event.

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 7 Number suffering fall as an adverse event.

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 8 Number suffering somnolence as an adverse event.

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 9 Number suffering confusion as an adverse event.

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 10 Number suffering at least one adverse event.

Comparison 2 Memantine vs placebo for mild‐to‐moderate Alzheimer's disease. Published, 6 month studies. ITT‐LOCF data, Outcome 11 Number suffering depression as an adverse event.

Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 1 Clinical Global: CGI (at 28 weeks) ITT‐LOCF or OC.
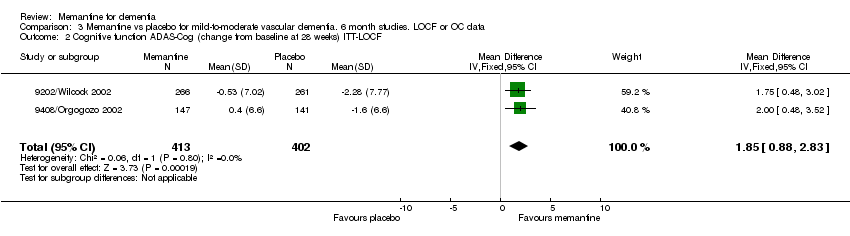
Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 2 Cognitive function ADAS‐Cog (change from baseline at 28 weeks) ITT‐LOCF.

Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 3 Activities of daily living : NOSGER self care subscale (change from baseline at 28 weeks) OC.

Comparison 3 Memantine vs placebo for mild‐to‐moderate vascular dementia. 6 month studies. LOCF or OC data, Outcome 4 Behaviour: NOSGER disturbing behaviour subscale (change from baseline at 28 weeks) OC.
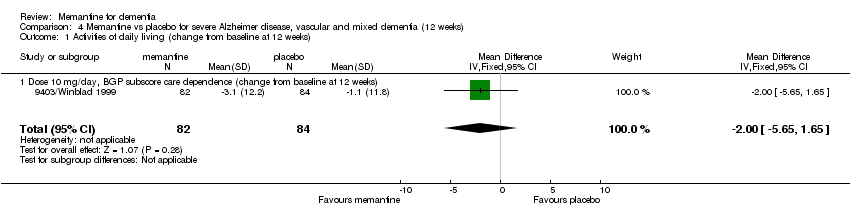
Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 1 Activities of daily living (change from baseline at 12 weeks).
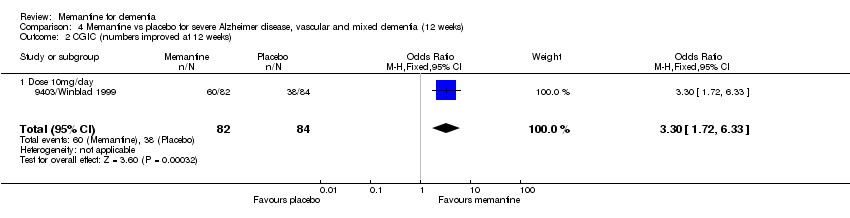
Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 2 CGIC (numbers improved at 12 weeks).
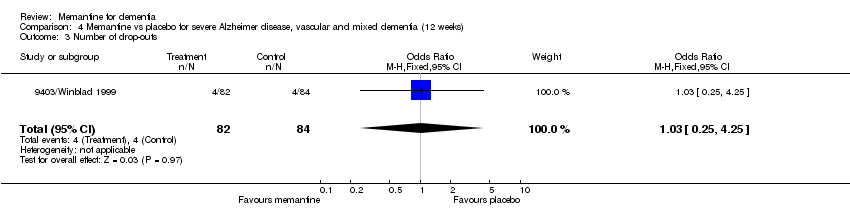
Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 3 Number of drop‐outs.
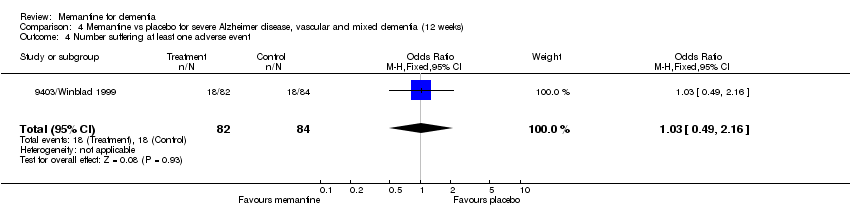
Comparison 4 Memantine vs placebo for severe Alzheimer disease, vascular and mixed dementia (12 weeks), Outcome 4 Number suffering at least one adverse event.

Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 1 Global.

Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 2 Cognition.

Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 3 Activities of daily living.

Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 4 Mood and behaviour.

Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 5 Number of dropouts.

Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 6 Number suffering at least one adverse event.

Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 7 Number suffering agitation as an adverse event.
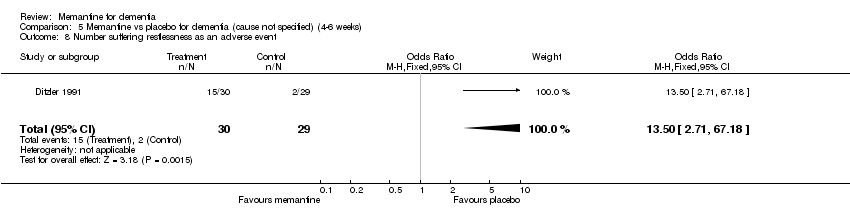
Comparison 5 Memantine vs placebo for dementia (cause not specified) (4‐6 weeks), Outcome 8 Number suffering restlessness as an adverse event.
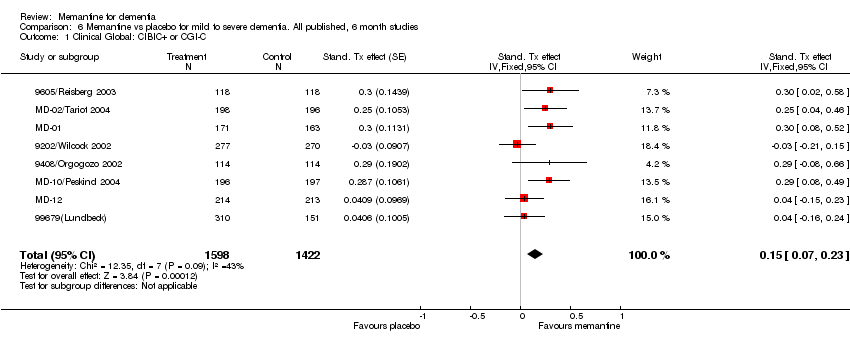
Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 1 Clinical Global: CIBIC+ or CGI‐C.
Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 2 Cognitive function: standardised.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 3 Activities of daily living: standardised.
Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 4 Behaviour and mood: standardised.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 5 Number of dropouts.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 6 Number suffering at least one adverse event.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 7 Number suffering agitation as an adverse event.
Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 8 Number suffering confusion as an adverse event.
Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 9 Number suffering hypertension.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 10 Number suffering dizziness as an adverse event.
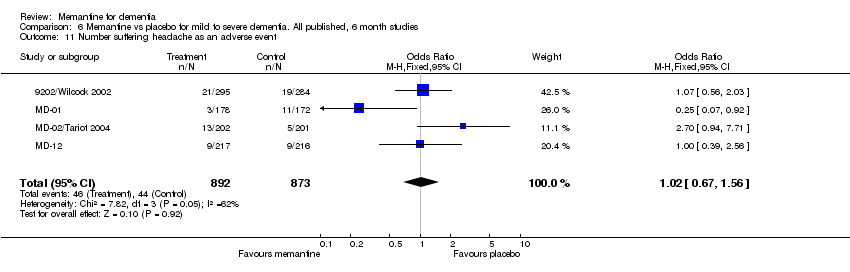
Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 11 Number suffering headache as an adverse event.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 12 Number suffering fall as an adverse event.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 13 Number suffering insomnia as an adverse event.
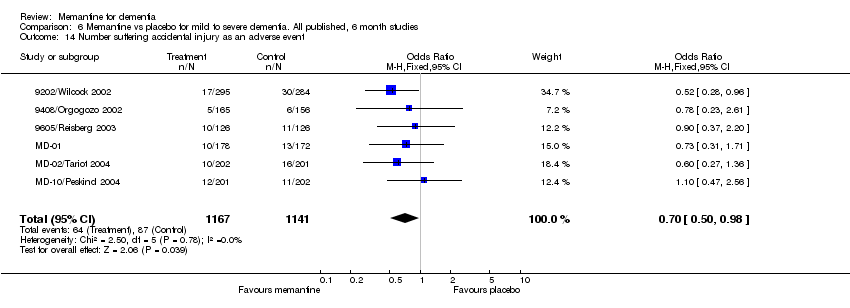
Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 14 Number suffering accidental injury as an adverse event.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 15 Number suffering urinary incontinence as an adverse event.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 16 Number suffering from diarrhoea as an adverse event.

Comparison 6 Memantine vs placebo for mild to severe dementia. All published, 6 month studies, Outcome 17 Number suffering from influenza like symptoms as an adverse event.

Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 1 Clinical Global: standardised.
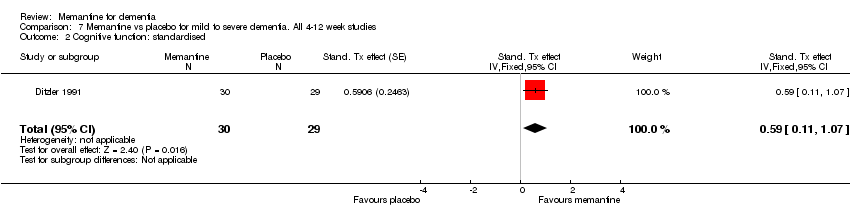
Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 2 Cognitive function: standardised.
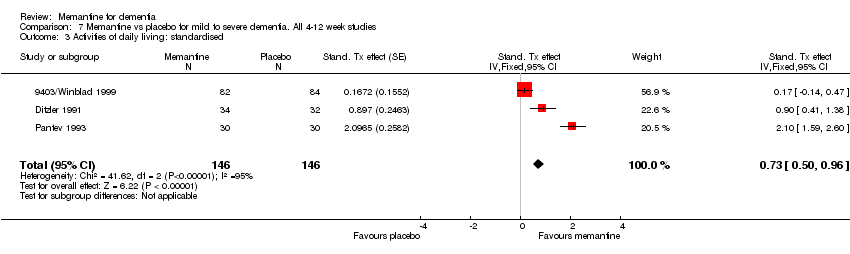
Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 3 Activities of daily living: standardised.
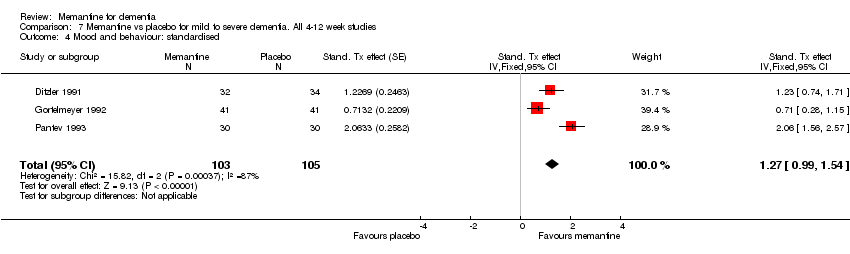
Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 4 Mood and behaviour: standardised.

Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 5 Number of drop‐outs.

Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 6 Number suffering at least one adverse event.
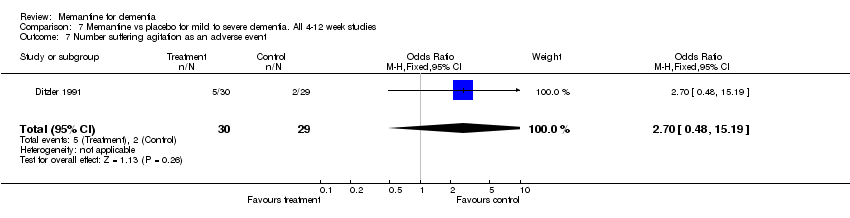
Comparison 7 Memantine vs placebo for mild to severe dementia. All 4‐12 week studies, Outcome 7 Number suffering agitation as an adverse event.
| Study | Number randomized | Diagnosis | Severity of disease | Mean age (s.e.) | Mean MMSE (s.e.) | Mean SCAG (s.e.) | Mean ADAS‐Cog (s.e.) | % female | duration (weeks) |
| Ditzler 1991 | 66 | AD (6%), VD (79%), MD (15%) | mild to moderate | 72.2 | 63.3 | 65 | 6 | ||
| Gortelmeyer 1992 | 88 | AD (9%), VD (76%), MD (15%) | mild to moderate | 71.5 | 24.1 | 64.2 | 68 | 6 | |
| 9408/Orgogozo 2002 | 321 | VD | mild to moderate | 76.4 (6.7) | 16.9 (2.5) | 21.0 (9.1) | 47 | 28 | |
| Pantev 1993 | 60 | all dementias | mild to moderately severe | 72.4 (5.7) | 85.3 (3.8) | 75 | 4 | ||
| 9605/Reisberg 2003 | 252 | AD | moderately severe to severe | 76.1(8.07) | 7,9 (3,64) | 67 | 28 | ||
| 9202/Wilcock 2002 | 579 | VD | mild to moderate | 77 | 17.6 (3.25) | 48 | 28 | ||
| Winblad 1999 | 167 | AD (48%), VD + MD (52%) | severe | 71.6 (5.6) | 6.3 (2.7) | 58 | 12 | ||
| MD‐02/Tariot 2004 | 404 | AD | moderate to severe | 75.5 | 9,9 (3,13) | 64,8 | 24 | ||
| MD‐10/Peskind 2004 | 403 | AD | mild to moderate | 77.5 (7.8) | 17.1 (3.6) | 27.3 (10.6) | 58.8 | 24 | |
| MD‐01 | 350 | AD | moderate to severe | 78.2 (7.9) | 71.4 | 24 | |||
| MD‐12 | 432 | AD | mild to moderate | 24 | |||||
| 99679 | 470 | AD | mild to moderate | 26 | |||||
| MRZ‐9104 | 56 | AD | 13 | ||||||
| MRZ‐9105 | 27 | primary dementia | mild to severe | 12 | |||||
| MRZ‐9206 | 56 | VD | moderate to severe | 77.5 (7.8) | M: 17.2 (3.4), P: 17.4 (3.7) | M: 27.3 (9.7) P: 27.2 (11) | 58.8 | 14 |
| Study | ADAS‐Cog | SIB | ADCS‐ADL | ADL | BGP | CIBIC‐Plus | CGIC | NPI | Other |
| 9202/Wilcock 2002 | X | X | NOSGER | ||||||
| 9408/Orgogozo 2000 | X | X | X | GBS, MMSE, NOSGER | |||||
| 9403/Winblad 1999 | X | X | |||||||
| 9605/Reisberg 2003 | X | X | X | X | FAST, SIB | ||||
| Ditzler 1991 | X | Physician's Global Impression, Syndrom Kurztest, SCAG | |||||||
| Gortelmeyer 1992 | X | CGI, GBS, Tapping test, Trace test, SCAG | |||||||
| 99679 (Lundbeck) | |||||||||
| MD‐01 | X | X | X | X | X | FAST, SIB | |||
| MD‐02/Tariot 2004 | X | X | X | X | X | SIB | |||
| MD‐10/Peskind 2004 | X | X | X | X | |||||
| MD‐12 | |||||||||
| MRZ‐9104 | |||||||||
| MRZ‐9105 | |||||||||
| MRZ‐9206 | |||||||||
| Pantev 1993 | X | Global assessment of clinical efficacy, NOSIE‐Index, Physician's global rating of tolerability, SCAG |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: CIBIC+ (24‐28 weeks) Show forest plot | 3 | 964 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.15, 0.41] |
| 2 Cognitive function: SIB (change from baseline at 24‐28 weeks) Show forest plot | 3 | 976 | Mean Difference (IV, Fixed, 95% CI) | 2.97 [1.68, 4.26] |
| 3 Activities of daily living: ADCS‐ADLsev19 (change from baseline at 24‐28 weeks) Show forest plot | 3 | 978 | Mean Difference (IV, Fixed, 95% CI) | 1.27 [0.44, 2.09] |
| 4 Behaviour and mood: NPI total (change from baseline at 24‐28 weeks) Show forest plot | 3 | 936 | Mean Difference (IV, Fixed, 95% CI) | 2.76 [0.88, 4.63] |
| 5 Number of dropouts Show forest plot | 3 | 1006 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.49, 0.88] |
| 6 Number suffering at least one adverse event Show forest plot | 3 | 1005 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.84, 1.52] |
| 7 Number suffering agitation as an adverse event Show forest plot | 3 | 1005 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.42, 0.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical global: CIBIC+ (at 24 weeks) Show forest plot | 3 | 1281 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.01, 0.25] |
| 2 Cognitive function: ADAS‐Cog (change from baseline at 24 weeks) Show forest plot | 3 | 1279 | Mean Difference (IV, Fixed, 95% CI) | 0.99 [0.21, 1.78] |
| 3 Activities of daily living: ADCS‐ADL23 (change from baseline at 24 weeks) Show forest plot | 3 | 1271 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.87, 1.27] |
| 4 Mood and behaviour: NPI total (change from baseline at 24 weeks) Show forest plot | 3 | 1252 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.48, 0.98] |
| 5 Number of dropouts Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.60] |
| 6 Number suffering agitation as an adverse event Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.57, 1.46] |
| 7 Number suffering fall as an adverse event Show forest plot | 2 | 836 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.41, 1.67] |
| 8 Number suffering somnolence as an adverse event Show forest plot | 1 | 403 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.49 [1.68, 33.38] |
| 9 Number suffering confusion as an adverse event Show forest plot | 2 | 836 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.72, 2.70] |
| 10 Number suffering at least one adverse event Show forest plot | 3 | 1306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.33] |
| 11 Number suffering depression as an adverse event Show forest plot | 2 | 836 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: CGI (at 28 weeks) ITT‐LOCF or OC Show forest plot | 2 | 775 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.13, 0.19] |
| 2 Cognitive function ADAS‐Cog (change from baseline at 28 weeks) ITT‐LOCF Show forest plot | 2 | 815 | Mean Difference (IV, Fixed, 95% CI) | 1.85 [0.88, 2.83] |
| 3 Activities of daily living : NOSGER self care subscale (change from baseline at 28 weeks) OC Show forest plot | 2 | 542 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.43, 0.67] |
| 4 Behaviour: NOSGER disturbing behaviour subscale (change from baseline at 28 weeks) OC Show forest plot | 2 | 541 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.06, 0.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Activities of daily living (change from baseline at 12 weeks) Show forest plot | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.65, 1.65] |
| 1.1 Dose 10 mg/day, BGP subscore care dependence (change from baseline at 12 weeks) | 1 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.65, 1.65] |
| 2 CGIC (numbers improved at 12 weeks) Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.72, 6.33] |
| 2.1 Dose 10mg/day | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.72, 6.33] |
| 3 Number of drop‐outs Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.25, 4.25] |
| 4 Number suffering at least one adverse event Show forest plot | 1 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global Show forest plot | 3 | 213 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.04, ‐0.48] |
| 1.1 Dose 30mg/day, Physicians global impression (6 weeks) | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.82 [‐1.33, ‐0.32] |
| 1.2 Dose 30mg/day, SCAG Clinical global impression of disturbances (4 weeks) | 1 | 59 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐1.58, ‐0.49] |
| 1.3 Dose 20mg/day, CGI (6 weeks) | 1 | 88 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.98, ‐0.13] |
| 2 Cognition Show forest plot | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐3.04 [‐5.68, ‐0.40] |
| 2.1 Dose 30mg/day, SKT (change from baseline at 6 weeks) | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐3.04 [‐5.68, ‐0.40] |
| 3 Activities of daily living Show forest plot | 2 | 126 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐1.73, ‐0.94] |
| 3.1 Dose 30mg/day, BGP care dependence subsscale (change from baseline at 6 weeks) | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐2.68, ‐1.42] |
| 3.2 Dose 30mg/day, ADL‐test total time (6 weeks) | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.39, ‐0.37] |
| 4 Mood and behaviour Show forest plot | 3 | 208 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐1.46, ‐0.86] |
| 4.1 Dose 30mg/day, NOSIE (change from baseline at 4 weeks) | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.02 [‐2.65, ‐1.39] |
| 4.2 Dose 30mg/day, SCAG total (change from baseline at 6 weeks) | 2 | 148 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.91 [‐1.25, ‐0.57] |
| 5 Number of dropouts Show forest plot | 2 | 154 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.27, 2.67] |
| 6 Number suffering at least one adverse event Show forest plot | 1 | 82 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.83, 4.81] |
| 7 Number suffering agitation as an adverse event Show forest plot | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.48, 15.19] |
| 8 Number suffering restlessness as an adverse event Show forest plot | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 13.5 [2.71, 67.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: CIBIC+ or CGI‐C Show forest plot | 8 | 3020 | Stand. Tx effect (Fixed, 95% CI) | 0.15 [0.07, 0.23] |
| 2 Cognitive function: standardised Show forest plot | 8 | 3070 | Stand. Tx effect (Fixed, 95% CI) | 0.24 [0.17, 0.30] |
| 3 Activities of daily living: standardised Show forest plot | 8 | 2791 | Stand. Tx effect (Fixed, 95% CI) | 0.08 [0.01, 0.15] |
| 4 Behaviour and mood: standardised Show forest plot | 8 | 2729 | Stand. tx effect (Fixed, 95% CI) | 0.11 [0.04, 0.19] |
| 5 Number of dropouts Show forest plot | 8 | 3212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.09] |
| 6 Number suffering at least one adverse event Show forest plot | 8 | 3211 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.27] |
| 7 Number suffering agitation as an adverse event Show forest plot | 8 | 3612 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.99] |
| 8 Number suffering confusion as an adverse event Show forest plot | 6 | 2489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.93, 1.87] |
| 9 Number suffering hypertension Show forest plot | 1 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.59 [1.16, 11.12] |
| 10 Number suffering dizziness as an adverse event Show forest plot | 6 | 2489 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.86, 1.60] |
| 11 Number suffering headache as an adverse event Show forest plot | 4 | 1765 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.67, 1.56] |
| 12 Number suffering fall as an adverse event Show forest plot | 6 | 2420 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.30] |
| 13 Number suffering insomnia as an adverse event Show forest plot | 4 | 1614 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.43] |
| 14 Number suffering accidental injury as an adverse event Show forest plot | 6 | 2308 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.98] |
| 15 Number suffering urinary incontinence as an adverse event Show forest plot | 3 | 1234 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.67, 1.85] |
| 16 Number suffering from diarrhoea as an adverse event Show forest plot | 4 | 1584 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 17 Number suffering from influenza like symptoms as an adverse event Show forest plot | 4 | 1589 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.72, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical Global: standardised Show forest plot | 4 | 377 | Stand. Tx effect (Fixed, 95% CI) | 0.62 [0.41, 0.82] |
| 2 Cognitive function: standardised Show forest plot | 1 | 59 | Stand. Tx effect (Fixed, 95% CI) | 0.59 [0.11, 1.07] |
| 3 Activities of daily living: standardised Show forest plot | 3 | 292 | Stand. Tx effect (Fixed, 95% CI) | 0.73 [0.50, 0.96] |
| 4 Mood and behaviour: standardised Show forest plot | 3 | 208 | Stand. Tx effect (Fixed, 95% CI) | 1.27 [0.99, 1.54] |
| 5 Number of drop‐outs Show forest plot | 3 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.38, 2.23] |
| 6 Number suffering at least one adverse event Show forest plot | 2 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.77, 2.38] |
| 7 Number suffering agitation as an adverse event Show forest plot | 1 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.48, 15.19] |

