| 1 Global effect: 1. No marked global improvement Show forest plot | 10 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 1.1 0‐6 weeks (clinician rated). | 3 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.31, 0.62] |
| 1.2 >6‐24 weeks (clinician rated) | 8 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.59, 0.81] |
| 1.3 >6‐24 weeks (nurse rated) | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.92] |
| 2 Global effect: 2. Not discharged from hospital (>6‐24 weeks) Show forest plot | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.47, 1.52] |
|
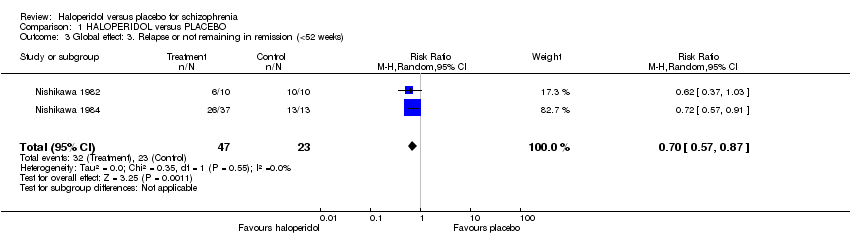
| 3 Global effect: 3. Relapse or not remaining in remission (<52 weeks) Show forest plot | 2 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.57, 0.87] |
|
| 4 Mental state: 1. No clinical improvement (<20% reduction in BPRS score, 0‐6 weeks) Show forest plot | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.08] |
|
| 5 Mental state: 2. Average end point BPRS score by 6 weeks (high = poor) Show forest plot | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐11.89 [‐17.04, ‐6.74] |
|
| 6 Mental state: 3. Change in BPRS total score by 3 weeks (high = good, data likely to be skewed) Show forest plot | | | Other data | No numeric data |
|
| 7 Leaving the study early Show forest plot | 20 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 7.1 0‐6 weeks | 12 | 898 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.73, 0.95] |
| 7.2 >6‐24 weeks | 8 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.04] |
| 7.3 < 52 weeks | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [0.14, 46.83] |
| 8 Adverse events: 1. Anticholinergic effects Show forest plot | 3 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 8.1 blurred vision | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 3.42 [0.91, 12.91] |
| 8.3 dry mouth | 2 | 73 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.71, 4.59] |
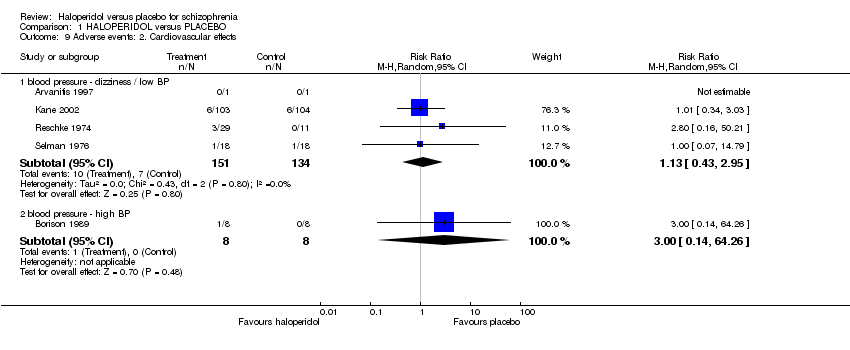
| 9 Adverse events: 2. Cardiovascular effects Show forest plot | 5 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 9.1 blood pressure ‐ dizziness / low BP | 4 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.43, 2.95] |
| 9.2 blood pressure ‐ high BP | 1 | 16 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 64.26] |
| 10 Adverse events: 3a. Movement disorders ‐ acute Show forest plot | 5 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 10.1 dystonia | 3 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 8.52 [1.66, 43.85] |
| 10.2 oculogyric crises | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.10, 8.89] |
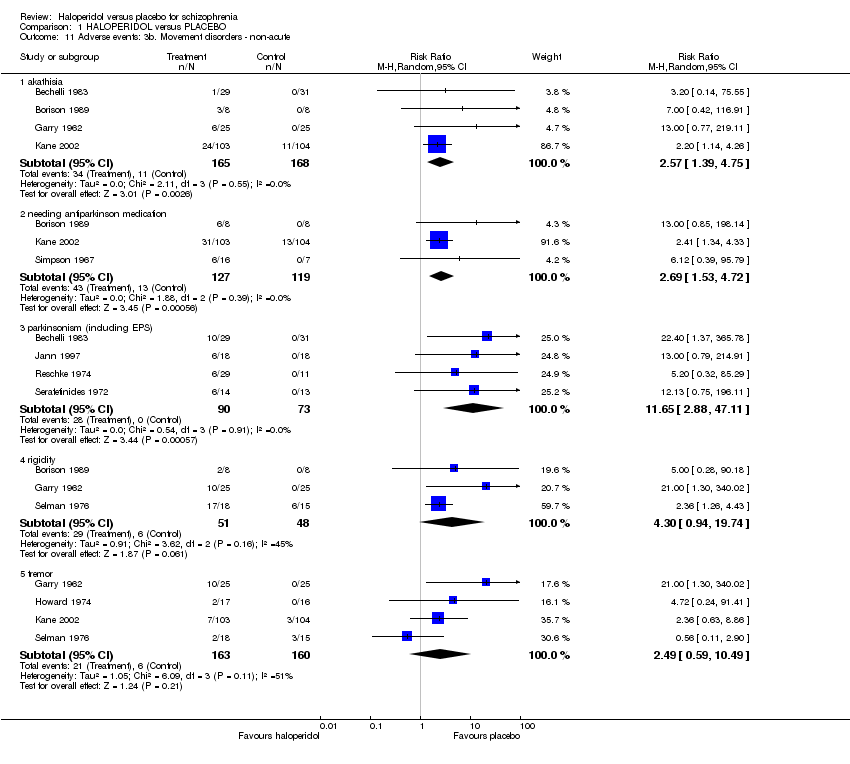
| 11 Adverse events: 3b. Movement disorders ‐ non‐acute Show forest plot | 10 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 11.1 akathisia | 4 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.39, 4.75] |
| 11.2 needing antiparkinson medication | 3 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 2.69 [1.53, 4.72] |
| 11.3 parkinsonism (including EPS) | 4 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 11.65 [2.88, 47.11] |
| 11.4 rigidity | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.94, 19.74] |
| 11.5 tremor | 4 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 2.49 [0.59, 10.49] |
| 12 Adverse events: 3c. Movement disorders ‐ chronic Show forest plot | 2 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 12.1 dyskinesia and tardive dyskinesia | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.12, 64.89] |
| 12.2 teeth grinding | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.11, 57.83] |
| 12.3 'thick' speech | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 5.89 [0.33, 105.81] |
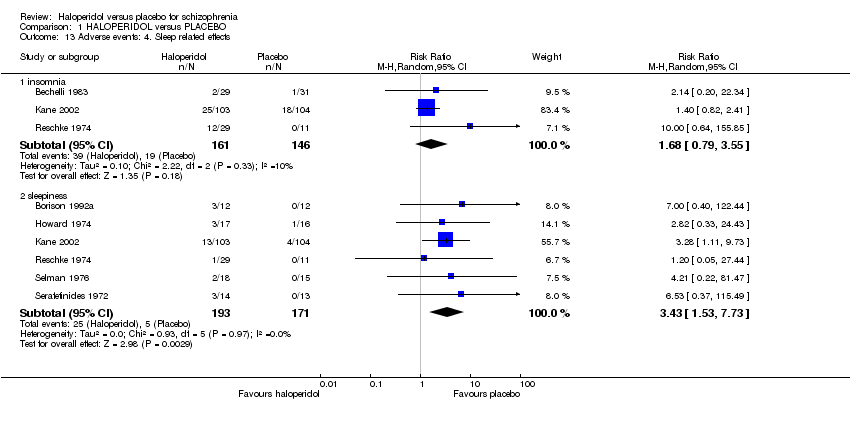
| 13 Adverse events: 4. Sleep related effects Show forest plot | 7 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 13.1 insomnia | 3 | 307 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.79, 3.55] |
| 13.2 sleepiness | 6 | 364 | Risk Ratio (M‐H, Random, 95% CI) | 3.43 [1.53, 7.73] |
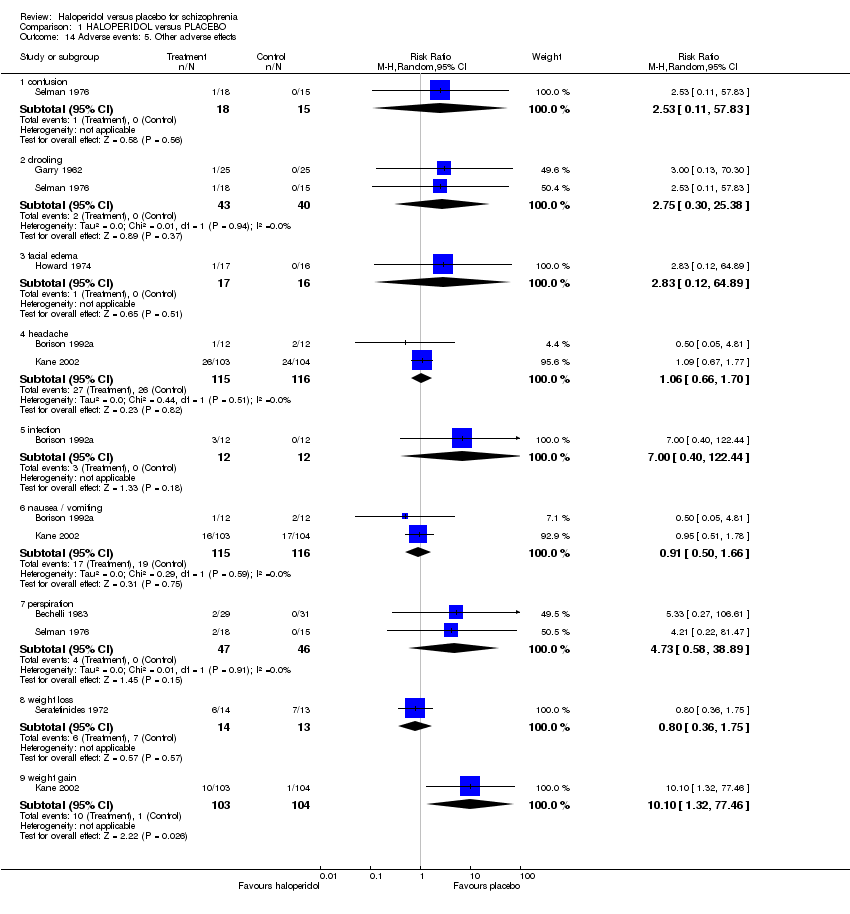
| 14 Adverse events: 5. Other adverse effects Show forest plot | 7 | | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
|
| 14.1 confusion | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 2.53 [0.11, 57.83] |
| 14.2 drooling | 2 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.75 [0.30, 25.38] |
| 14.3 facial edema | 1 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.12, 64.89] |
| 14.4 headache | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.66, 1.70] |
| 14.5 infection | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.40, 122.44] |
| 14.6 nausea / vomiting | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.50, 1.66] |
| 14.7 perspiration | 2 | 93 | Risk Ratio (M‐H, Random, 95% CI) | 4.73 [0.58, 38.89] |
| 14.8 weight loss | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.36, 1.75] |
| 14.9 weight gain | 1 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 10.10 [1.32, 77.46] |