Etosuksymid, walproinian sodu lub lamotrygina w leczeniu padaczki z napadami nieświadomości u dzieci i młodzieży
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Ir a:
| Methods | Randomised double‐blind response ‐ conditional cross‐over study. VPS with PCB for 6 weeks followed by ESM with PCB for 6 weeks for one group. The other group followed the same regimen in a reverse order. Follow‐up 3 months. | |
| Participants | 45 naive and drug resistant participants aged 3 to 18 years with absence seizures (not specified if typical or atypical). 18 male. | |
| Interventions | Drug naive participants were on monotherapy (ESM or VPS) while refractory to previous treatment participants were on polytherapy. | |
| Outcomes | Reduction in seizure frequency as judged by 12‐hour EEG telemetry, 100% for drug naive and 80% for drug‐resistant participants. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how patients were randomly assigned to treatments. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) | Unclear risk | Study was described as "double‐blinded" without further details. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study was described as "double‐blinded" without further details. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study was described as "double‐blinded" without further details. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Methods | Randomised, parallel open study designed to compare ESM with VPS treatment. Followed up for 18 months to 4 years, mean 3 years. | |
| Participants | 28 drug naive participants (13 male, 15 female), aged between 4 to 15 years. All participants with typical absence seizures. | |
| Interventions | Monotherapy with ESM or VPS. | |
| Outcomes | Complete or partial (50% to 90%) remission of seizures confirmed by 6 hours telemetry and observation by parents and teachers. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Methods | Participants randomly assigned to either ESM or VPS treatment. Parallel open design. All were followed up for 1 to 2 years. 6 participants did not co‐operate; they were not included in the analysis. | |
| Participants | 20 participants with recent (less than 6 weeks) onset of 'simple absences' only, other types of seizures observed in 4 out of 5 participants whose seizures were not completely controlled. Age: 5 to 8 years old, 5 were male. | |
| Interventions | Monotherapy with ESM or VPS. | |
| Outcomes | Number of seizures per day as observed by parents. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Methods | Randomised using 1:1 ratio, double‐blind, parallel design. This study was a second phase of a trial designed as 'responder‐enriched'. It followed an open‐label dose escalation trial. The LTG therapy was tapered over 2 weeks in the PCB group. The length of follow‐up for the randomised double‐blind study was 4 weeks. | |
| Participants | The individuals who became seizure free on LTG during a pre‐randomisation baseline randomised to continue LTG or to PCB. All participants who entered the preceding study were newly diagnosed children with typical absence seizures. 29 participants were randomised, 15 into LTG group and 14 into PCB. 1 person in the LTG group withdrew consent. In the PCB group the age was 8.8+/‐3.1 years, 36% boys. In the LTG group the age was 6.9+/‐2.3 years, 36% were boys. | |
| Interventions | Monotherapy with LTG or PCB. | |
| Outcomes | Proportion of participants that remained seizure free, as measured by hyperventilation EEG. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) | Low risk | Lamotrigine was and placebo were identically matched. |
| Blinding of participants and personnel (performance bias) | Low risk | Lamotrigine was and placebo were identically matched. |
| Blinding of outcome assessment (detection bias) | Low risk | Lamotrigine was and placebo were identically matched. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Methods | Randomised, parallel group unblinded study. Follow‐up for 12 months. | |
| Participants | 38 drug naive participants, all with typical absence seizures, age 3 to 13 years. | |
| Interventions | Monotherapy with VPS or LTG. | |
| Outcomes | Total seizures freedom defined by clinical reports, 24 hours EEG and hyperventilation test. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated using a randomisation code. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding (performance bias and detection bias) | High risk | No blinding. It is not stated whether tables of VPA and LTG were indistinguishable. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. It is not stated whether tables of VPA and LTG were indistinguishable. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. It is not stated whether tables of VPA and LTG were indistinguishable. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Methods | Randomised, open‐label, parallel group design. Follow‐up 12 months. | |
| Participants | 30 patients with typical absence seizures (males 16; females 14. Age between 5 and 14 years) 15 patients allocated to VPA and 15 to LTG. | |
| Interventions | No detailed information on drug dosages. The doses of both the drugs were escalated according to the clinical response, starting from a low dose. Lamotrigine was titrated very slowly at 2‐weekly intervals to avoid unwanted side effects (maximum 10 mg/kg/day). | |
| Outcomes | Seizure freedom. | |
| Notes | Results of this study were published as abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information to permit judgement. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to permit judgement. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Methods | Randomised, parallel group unblinded study. Follow‐up 12 months. | |
| Participants | 48 drug naive participants, all with typical absence seizures, age 6 to 10 years | |
| Interventions | Monotherapy with VPS or LTG. | |
| Outcomes | Seizure freedom at 1, 3, 6 and 12 months. Complete normalisation of EEG with seizure freedom. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Blinding (performance bias and detection bias) | High risk | No blinding. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
| Methods | Parallel, randomised, double‐blind study, with partial cross‐over to open‐label (at treatment failure only) with subsequent follow‐up. Follow‐up 12 months. | |
| Participants | 453 drug naive participants (193 male, 260 female), aged between 7 months to 12 years 11 months. All participants with typical absence seizures. | |
| Interventions | Monotherapy with LTG, VPS, or ESM. | |
| Outcomes | Freedom from treatment failure assessed 12 months after randomisation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated using permuted blocks. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding (performance bias and detection bias) | Low risk | Placebo and active drugs indistinguishable. |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo and active drugs indistinguishable. |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo and active drugs indistinguishable. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but it is clear that the published reports include all expected outcomes. |
EEG: electroencephalogram
ESM: ethosuximide
LTG: lamotrigine
PCB: placebo
VPA: valproic acid
VPS: sodium valproate
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No randomisation. | |
| No randomisation. | |
| No randomisation. | |
| Retrospective study. | |
| No randomisation. | |
| No patients with absence seizures included. | |
| No patients with childhood absence seizures included. | |
| Retrospective study. | |
| No randomisation. | |
| No randomisation. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 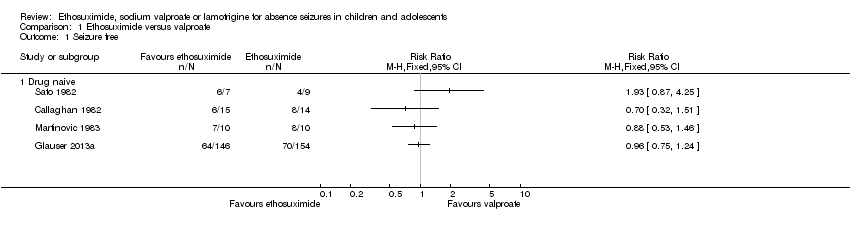 Comparison 1 Ethosuximide versus valproate, Outcome 1 Seizure free. | ||||
| 1.1 Drug naive | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 80% or greater reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Ethosuximide versus valproate, Outcome 2 80% or greater reduction in seizure frequency. | ||||
| 2.1 Previously treated | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 50% or greater reduction in seizure frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 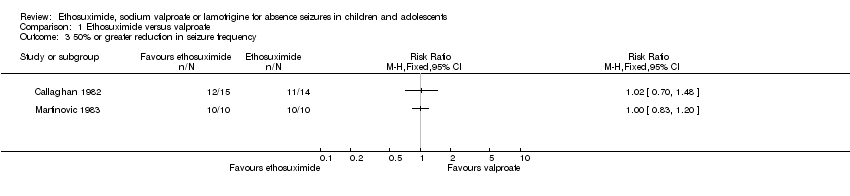 Comparison 1 Ethosuximide versus valproate, Outcome 3 50% or greater reduction in seizure frequency. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Lamotrigine versus valproate, Outcome 1 Seizure free. | ||||
| 1.1 Seizure free at 1 month | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [2.77, 25.59] | |
| 1.2 Seizure free at 3 months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.16, 2.31] | |
| 1.3 Seizure freedom at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.88, 2.28] | |
| 1.4 Seizure free at 12 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.32, 2.11] | |
| 2 Normalization fo the EEG Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Lamotrigine versus valproate, Outcome 2 Normalization fo the EEG. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 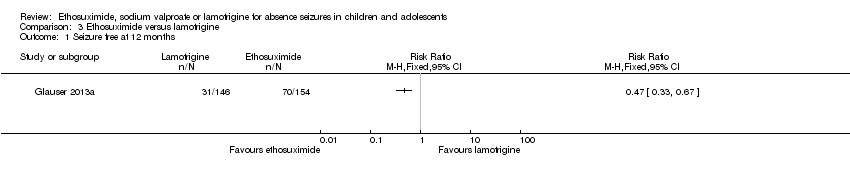 Comparison 3 Ethosuximide versus lamotrigine, Outcome 1 Seizure free at 12 months. | ||||
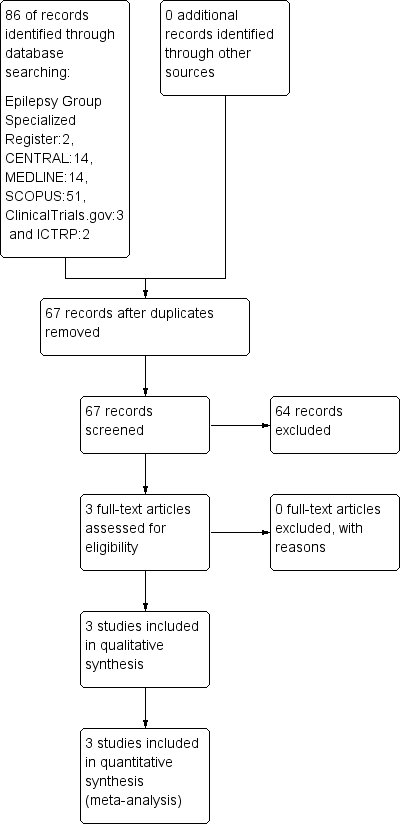
Study flow diagram (results refer only to the updated version of the review).
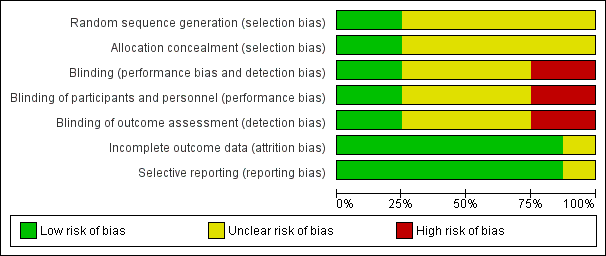
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
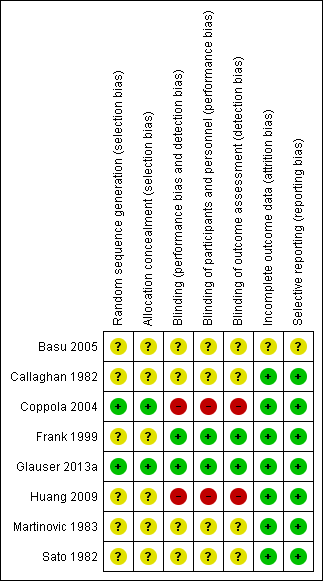
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
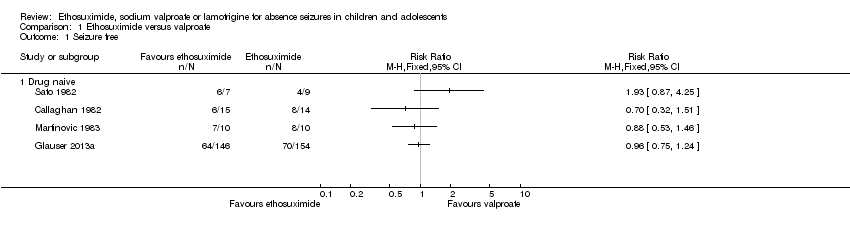
Comparison 1 Ethosuximide versus valproate, Outcome 1 Seizure free.
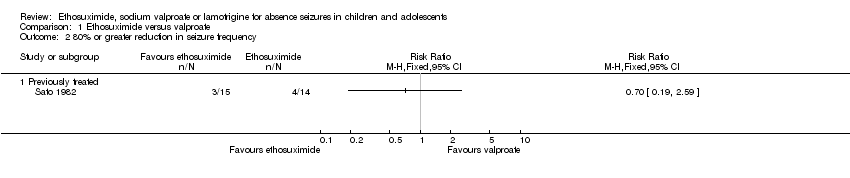
Comparison 1 Ethosuximide versus valproate, Outcome 2 80% or greater reduction in seizure frequency.

Comparison 1 Ethosuximide versus valproate, Outcome 3 50% or greater reduction in seizure frequency.

Comparison 2 Lamotrigine versus valproate, Outcome 1 Seizure free.
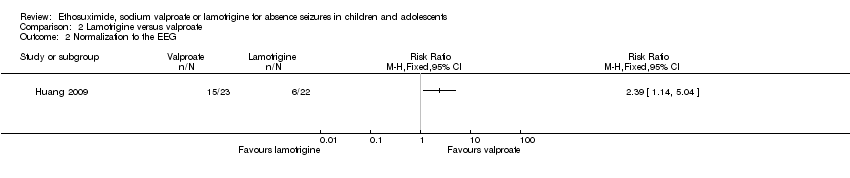
Comparison 2 Lamotrigine versus valproate, Outcome 2 Normalization fo the EEG.
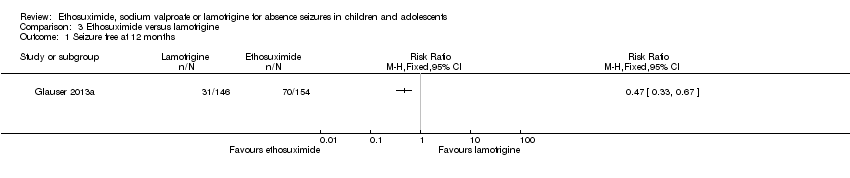
Comparison 3 Ethosuximide versus lamotrigine, Outcome 1 Seizure free at 12 months.
| Event | Callaghan 1982 | Sato 1982 | Martinovic 1983 | Coppola 2004 | Huang 2009 | Glauser 2013 |
| Acute pancreatitis | 1 | |||||
| Obesity/Weight gain | 1 | 1 | 14 | |||
| Drowsiness | 4 | |||||
| Nausea | 5 | 3 | 12* | |||
| Vomiting | 1 | 2 | 12* | |||
| Decreased platelet numbers | 2 | 4 | ||||
| Increased appetite | 15 | |||||
| Poor appetite | 1 | 8 | ||||
| Diarrhoea | 1 | 7 | ||||
| Dizziness | 1 | 2 | ||||
| Hyperactivity | 23 | |||||
| Attention problems | 24 | |||||
| Hostility | 22 | |||||
| Concentration decreased | 18 | |||||
| Personality change | 17 | |||||
| Sleep problem | 17 | |||||
| Depression | 11 | |||||
| Slow process speed | 11 | |||||
| Memory problem | 10 | |||||
| Apathy | 9 | |||||
| Fatigue | 27 | |||||
| Headache | 1 | 18 | ||||
| Leukopenia | 2 | |||||
| Elevated liver function tests | 1 | 7 | ||||
| Elevated LDH | 1 | |||||
| Rash | 2 | |||||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking valproate: 14 (Callaghan 1982), 22 (Sato 1982), 10 (Martinovic 1983), 19 (Coppola 2004), 23 (Huang 2009), 146 (Glauser 2013a). | ||||||
| Event | Callaghan 1982 | Sato 1982 | Martinovic 1983 | Glauser 2013 |
| Drowsiness | 1 | 5 | ||
| Tiredness | 2 | |||
| Nausea | 3 | 2 | 29* | |
| Vomiting | 3 | 29* | ||
| Increased appetite | 6 | |||
| Poor appetite | 1 | 10 | ||
| Diarrhoea | 9 | |||
| Dizziness | 1 | 10 | ||
| Headache | 2 | 23 | ||
| Leukopenia | 3 | |||
| Hiccups | 1 | |||
| Moodiness | 1 | |||
| Hyperactivity | 13 | |||
| Attention problems | 8 | |||
| Hostility | 4 | |||
| Concentration decreased | 6 | |||
| Personality change | 6 | |||
| Sleep problem | 11 | |||
| Depression | 4 | |||
| Slow process speed | 3 | |||
| Memory problem | 0 | |||
| Apathy | 4 | |||
| Fatigue | 26 | |||
| Rash | 6 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking ethosuximide: 14 (Callaghan 1982), 23 (Sato 1982), 10 (Martinovic 1983), 154 (Glauser 2013a). | ||||
| Event | Frank 1999 | Coppola 2004 | Huang 2009 | Glauser 2013 |
| Abdominal pain | 5 | |||
| Headache | 2 | 2 | 14 | |
| Nausea | 3 | 2* | ||
| Vomiting | 2* | |||
| Poor appetite | 2 | 9 | ||
| Increased appetite | 1 | 10 | ||
| Diarrhoea | 2 | |||
| Dizziness | 3 | 5 | 5 | |
| Hyperkinesia | 2 | |||
| Hyperactivity | 12 | |||
| Attention problems | 11 | |||
| Hostility | 11 | |||
| Concentration decreased | 9 | |||
| Personality change | 10 | |||
| Sleep problem | 5 | |||
| Depression | 11 | |||
| Slow process speed | 7 | |||
| Memory problem | 8 | |||
| Apathy | 3 | |||
| Fatigue | 1 | 18 | ||
| Rash | 10 | 1 | 2 | 6 |
| Nervousness | 1 | |||
| Diplopia | 1 | |||
| * Nausea, vomiting, or both Numbers of individuals within each study undertaking lamotrigine: 15 (Frank 1999), 19 (Coppola 2004), 24 (Huang 2009), 146 (Glauser 2013a). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Drug naive | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 80% or greater reduction in seizure frequency Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Previously treated | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 50% or greater reduction in seizure frequency Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Seizure free at 1 month | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.42 [2.77, 25.59] | |
| 1.2 Seizure free at 3 months | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.16, 2.31] | |
| 1.3 Seizure freedom at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.88, 2.28] | |
| 1.4 Seizure free at 12 months | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.32, 2.11] | |
| 2 Normalization fo the EEG Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Seizure free at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |

